Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(12):4838-4852. doi:10.7150/ijbs.90798 This issue Cite
Review
Potential Roles and Mechanisms of Curcumin and its Derivatives in the Regulation of Ferroptosis
1. State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China.
2. Department of Pharmacology, Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu 610041, China.
Received 2023-10-6; Accepted 2024-8-25; Published 2024-9-9
Abstract
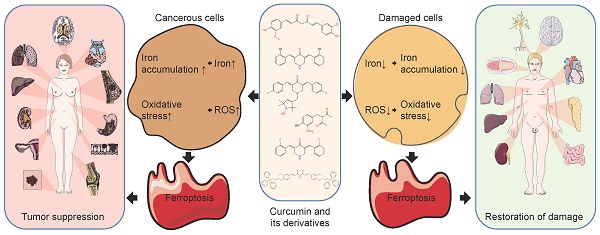
Ferroptosis is a recently discovered iron-dependent mode of oxidatively regulated cell death. It is not only associated with a wide range of diseases, but it is also a key component of many signaling pathways. In general, ferroptosis is a double-edged sword. On one hand, it induces nonapoptotic destruction of cancer cells, but on the other, it may lead to organ damage. Therefore, ferroptosis can be drug-targeted as a novel means of therapy. The properties of curcumin have been known for many years. It has a positive impact on the treatment of diseases such as cancer and inflammation. In this review, we focus on the regulation of ferroptosis by curcumin and its derivatives and review the main mechanisms by which curcumin affects ferroptosis. In conclusion, curcumin is a ferroptosis inducer with excellent anticancer efficacy, although it also exhibits organ protective and reparative effects by acting as a ferroptosis inhibitor. The differential regulation of ferroptosis by curcumin may be related to dose and cell type.
Keywords: Curcumin, Ferroptosis, Iron, Oxidation, Anticancer effect, Organ protective effect
1. Introduction
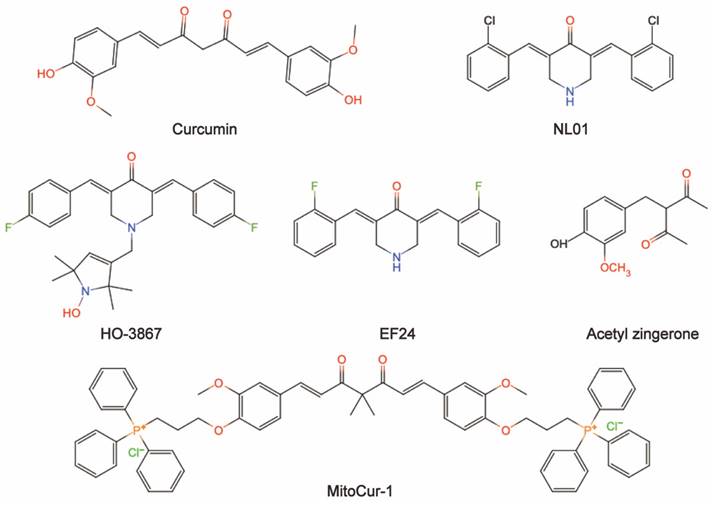
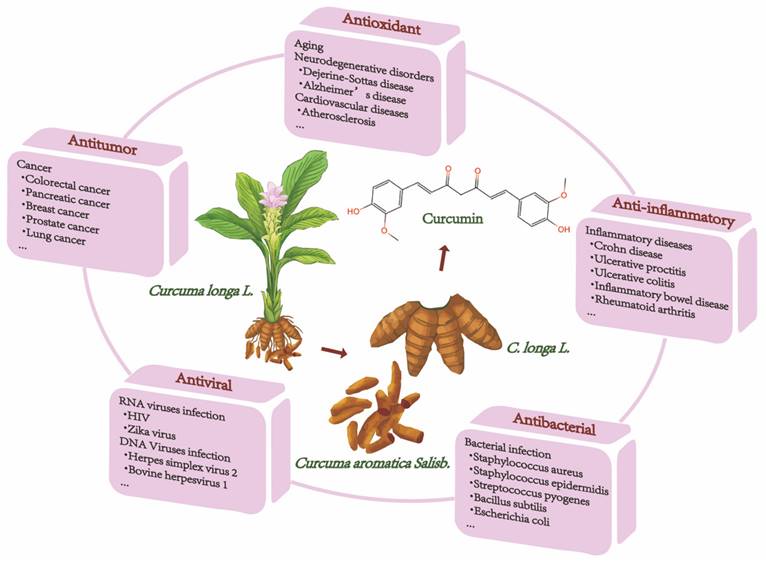
Natural ingredients extracted from traditional Chinese medicine (TCM) are immeasurable and have been used clinically for the treatment of various diseases[1,2]. Curcumin is among these molecules. Historically, curcumin was isolated in 1815 by Vogel and Pelletier and was derived from the root tuber and the rhizome of Curcuma longa L.[3]. This herb has traditionally been known to improve blood circulation, eliminate blood stasis, and relieve pain, an effect also attributed to the presence of its phenolic constituent curcumin[4]. Modern studies have shown that curcumin has a wide range of pharmacological activities, such as antioxidant, antitumor, anti-inflammatory, and antiviral properties and has shown promising therapeutic potential in preclinical and clinical studies[5-7]. Moreover, curcumin is being "Generally Recognized as Safe" by the US Food and Drug Administration (FDA)[8]. Chemically, curcumin is insoluble in water and is soluble in organic solvents such as acetic acid, ketone, alkali and chloroform. Due to its hydrophobicity, instability, rapid metabolism in vivo, and poor intestinal absorption[9,10], curcumin has inherent drawbacks, such as its low bioavailability, poor pharmacokinetic/pharmacodynamic properties, and poor efficacy in certain disease models[11-13]. Several efforts have been made to improve these properties. First, it is worthwhile to combine curcumin with photodynamic therapy. Recent findings have indicated that curcumin combined with photodynamic therapy prolonged the action time and increased the bioavailability of curcumin, resulting in a more efficient effect on cancer with a broad spectrum of targets[3]. Second, curcumin encapsulation using special polymers, such as liposomes and nanomaterials, has the advantages of high drug loading, high encapsulation rate, and high safety to improve curcumin bioavailability[14-16]. Furthermore, synthesized derivatives that are structurally similar to curcumin can be targeted to overcome these limitations and achieve good therapeutic prospects[17]. The chemical structures and other chemical information of curcumin and some of its derivatives are summarized in Fig. 1 and Table 1. The sources and biological activities of curcumin are displayed in Fig. 2.
Dixon first introduced the concept of ferroptosis, a new modality of cell death, to the world in 2012[18]. Since then, research on ferroptosis has been growing exponentially over the past few years. Unlike apoptosis, autophagy, and necroptosis, ferroptosis is a distinctive programmed cell death mechanism[19], as well as a new type of oxidatively regulated cell death driven by iron-dependent lipid peroxidation[20,21]. Specifically, when intracellular levels of lipid reactive oxygen species (L-ROS) exceed the antioxidant activity of glutathione peroxidase 4 (GPX4), this leads to a breakdown of cellular redox homeostasis[22]. Interestingly, ferroptosis appears to be more of a cellular "sabotage" than an active "suicide"[23]. In other words, ferroptosis refers to an iron-dependent, oxidative form of non-apoptotic cell death. Unlike apoptosis or autophagy, which appears to occur as a consequence of specialized molecular events taken on the initiative of cells for altruistic benefit, ferroptosis can be triggered by the depletion of the amino acid cysteine or the inhibition of GPX4, which is associated with the consumption of ATP or the production of lipid hydroperoxides with cell destruction, leading to catastrophic damage[24]. Mitochondria are the main intracellular generators of reactive oxygen species (ROS)[25], and the focal point of iron metabolism and homeostasis[26]. Ferroptotic cells show characteristic morphological changes including reduction in mitochondrial volume, decrease or even disappearance of mitochondrial crista, increase in the density of the mitochondrial membrane, and rupture of outer mitochondrial membranes[27,28].
The chemical information of curcumin and some of its derivatives.
| Chemical name | Formula | Molecular weight (g/ mol) | Type |
|---|---|---|---|
| Curcumin | C21H20O6 | 368.38 | Natural polyphenol compound |
| NL01 | - | - | - |
| HO-3867 | C28H30F2N2O2 | 464.55 | Synthesized diarylidenylpieperidone compound |
| EF24 | C19H16ClF2NO | 347.79 | Synthesized monoketone compound |
| Acetyl zingerone | C13H16O4 | 236.26 | - |
| MitoCur-1 | C65H64Cl2O6P2 | 1074.05 | - |
The chemical structures of curcumin and some of its derivatives (drawing by InDraw).
The sources and biological activities of curcumin.
Ferroptosis can be triggered by a variety of physiological conditions and pathological stresses in humans and animals and is increasingly recognized as an adaptive feature for the elimination of malignant cells[18]. Experimental compounds or drugs are capable of inhibiting ferroptosis in both cancer cells and certain normal cells[29-31]. Meanwhile, activation of mitochondrial voltage-dependent anion channels and mitogen-activated protein kinases, up-regulation of endoplasmic reticulum stress, and inhibition of cystine/glutamate resistant formate play key roles in the induction of ferroptosis[32]. Surprisingly, treatment-resistant cancer cells, especially those in a mesenchymal state and prone to metastasis, are highly susceptible to ferroptosis[33]. It is critical for suppressing tumorigenesis by removing cells that are deficient in key nutrients in the environment or damaged by infection or environmental stress[34]. In addition, many organ injuries and degenerative diseases are driven by ferroptosis[35-37]. Thus, pharmacological modulation of ferroptosis, through its induction and inhibition, has great potential in the treatment of drug-resistant cancers, ischemic organ damage, and other degenerative diseases associated with lipid peroxidation.
The key to ferroptosis is the iron-catalyzed peroxidation of polyunsaturated fatty acid (PUFA)-containing phospholipids (PUFA-PLs), which exceeds the buffering capacity of the defense system and ultimately leads to cell death. Accumulated intracellular iron is the trigger for ferroptosis as it can produce highly reactive free radicals via the Fenton reaction, which can lead to ferroptosis[38]. It has been shown that elevated intracellular ROS due to oxidative stress can accelerate the onset of ferroptosis or increase cellular susceptibility to ferroptosis inducers[39]. Intracellular iron accumulation and oxidation are the two central biochemical events causing ferroptosis. The ability of curcumin to chelate iron and regulate oxidation predicts that curcumin may have a role in regulating ferroptosis, which was confirmed in recent studies[40,41]. Herein, we review these activities and analyze the possible underlying mechanisms involved, for which there has been little information to date, in the hope of informing and contributing to the screening of natural medicines.
2. Curcumin and its derivatives promoted ferroptosis in cancerous cells
2.1 Regulation of iron metabolism
Iron's role in the redox cycle where it can act as an electron carrier allows it to catalyze the Fenton reaction with H2O2, followed by the production of highly reactive hydroxyl radicals (•OH), which, as part of ROS, can damage macromolecules such as lipids, proteins, and DNA, as well as cellular organelles such as lysosomes and mitochondria, and has been linked to various signaling pathway impairments[42]. Iron homeostasis is tightly regulated in healthy cells to balance systemic absorption and distribution as well as cellular uptake, storage and export[43,44]. A distinctive hallmark of cancer is dysregulation of iron homeostasis, including overexpression of genes involved in iron metabolism and increased intracellular labile iron[45,46]. Of particular note, tumor cell growth and survival cannot occur without an increase in iron concentration, and an increase in intracellular labile iron pools is also essential for cancer cell metastasis. However, increased iron levels can also lead to ferroptosis via the Fenton reaction[38]. Cancer management is becoming as diverse as the disease itself. Targeting iron metabolism in cancer cells is a powerful and promising therapeutic area.
Cellular iron metabolism involves the regulation of labile iron in the cell membrane, which is a minor but vital part of the total amount of redox-active iron in the cell[19]. Convincing evidence has shown that curcumin and its derivatives can induce iron accumulation by increasing the concentration of Fe2+ in a wide range of tumor cells and tissues[47-51]. Furthermore, Yin et al. developed a cascade catalytic nanoplatform (CaO2/Tf /CUR) for ion interference therapy. CaO2/Tf/CUR with tumor-targeting action was internalized in tumor cells and decomposed to release Ca2+ and curcumin. CaO2/Tf/CUR activated the mitochondrial apoptotic signaling pathway by inducing Ca2+ overload which further led to cellular damage. Conversely, the generated H2O2 disrupted the structure of transferrin (TF) thus releasing Fe3+. Ferroptosis is triggered by conversion to hydroxyl radicals via trivalent iron ion-mediated Fenton reaction[52]. DMT1 acts as a proton pump and utilizes the cell membrane potential for active iron transport. Ling et al. found that HO-3867 was able to upregulate DMT1 expression by regulating the level of p53[51].
Iron uptake through the transferrin receptor 1 (TFR1) and storage in ferritin (FT) is vital for regulating the labile iron pool in the cytoplasm. Among the many factors that affect iron metabolism, FT has been investigated to understand the mechanisms of release/accumulation of reactive iron as an important regulatory point for iron metabolism homeostasis and ferroptosis[53]. Activation of iron metabolism-related proteins promotes ferroptosis[54]. However, another argument suggests that the regulation of labile iron by overexpression of FT decreases the production of reactive free radicals, whereas the down-regulation of FT increases oxidative damage[55]. Curcumin has been proven to modulate TFR1 levels, as well as the two subunits of FT, in cancer cells, ferritin heavy chain (FTH1) and ferritin light chain (FTL), which in turn influences intracellular iron transport and storage functions and alters labile iron levels[50,56-58]. The suppression of IREB2, a prominent transcription factor involved in the regulation of iron metabolism, resulted in a notable increase in the expression of FTL and FTH1, consequently preventing the occurrence of erastin-induced ferroptosis[59]. Meanwhile, siIREB2 interference could reduce curcumin-induced cell death in A549 and H1299 cells, indirectly indicating that curcumin could participate in the ferroptosis process in lung cancer cells by targeting IREB2. Furthermore, curcumin can down-regulate the level of NCOA4 in clear cell renal cell carcinoma by upregulating the ADAMTS18 gene, which contributes to cell death and reverses resistance to sunitinib. This is because the delivery of FT to lysosomes requires NCOA4, which is highly aggregated in autophagosomes. In other words, cells lacking NCOA4 do not degrade FT, leading to a decrease in bioavailable intracellular iron[60].
The enzyme known as heme oxygenase-1 (HO-1) converts heme into free iron, carbon monoxide, and bilirubin. Under typical physiological circumstances, HO-1 scavenges ROS and offers cellular defense[61]. Yet, curcumin-induced HO-1 overactivation raises intracellular ferric ions, MDA, and ROS levels in cancer cells, surpassing FT's buffering ability and leading to the uncontrollably high release of iron and the consequent disturbance of iron metabolism[48,56,58,62,63]. Remarkably, while inhibiting the expression of GPX4, curcumin not only directly activates HO-1, but also upregulates its expression by activating Nrf2, promoting ferroptosis[56].
2.2 Inhibition of the antioxidant system
Organisms have developed a variety of antioxidant regulators, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX), as a means to protect against oxidative damage[64]. However, when these defense mechanisms are disrupted, resulting in an imbalance between the production and removal of ROS, cells experience impaired functionality or even death due to oxidative stress[65]. Cancer cells exhibit intrinsic oxidative stress, which makes them more susceptible to further ROS production by pro-oxidant anticancer agents (PAAs). However, PPAs inevitably generate ROS in normal cells, resulting in a narrow therapeutic window and high toxicity, which greatly limits their clinical application[66]. In the tumor microenvironment (TME), tumor cells evolve to gradually be insensitive to oxidative stress or other harmful forces, leading to their resistance to stress-inducing agents such as chemotherapy and radiotherapy[67]. In this context, ferroptosis could be a robust tool for developing a novel strategy by amplifying oxidative stress or inhibiting antioxidant molecules in tumor cells.
2.2.1 GPX4/GSH
Ferroptosis inducers such as glutamate and erastin can drain GSH and inactivate the enzyme activity of GPX4 by blocking the import of cystine by the cystine/glutamate antiporter (system Xc-)[68,69]. GPX4 is an essential regulator of ferroptosis in cancer cells as well as a key factor in maintaining cellular redox homeostasis[70]. GSH, as an important iron inhibitor and non-enzymatic antioxidant, provides an important defense system that protects cells from different types of oxidative stress[71]. GPX4 converts GSH to oxidized glutathione (GSSG) and the cytotoxicity of lipid peroxides (L-OOH) to the corresponding alcohol (L-OH)[72-74]. The ratio between GSH and GSSG generally indicates the level of cellular oxidative stress. However, L-OOHs are unstable and can be broken down into reactive compounds such as MDA, which acts as a "second messenger of oxidative stress" due to its long half-life and its ability to diffuse out of the formation site[75,76]. Together, GPX4 and GSH appear to be the major determinants of the balance between cell proliferation and death. Inactivation of GPX4 or depletion of GSH in cells may lead to ferroptosis[77]. ALZ003-induced AR ubiquitination improves glioblastoma resistance to temozolomide by disrupting GPX4-mediated redox homeostasis and promoting subsequent ferroptosis[78]. Curcumin inhibits GPX4 levels by upregulating Nrf2 expression, triggering the molecular and cytological characteristics of ferroptosis in breast cancer cells[56]. Curcumin and its derivative EF24 repress GPX4 expression and increase MDA and ROS levels to exert a pro-ferroptosis effect in osteosarcoma cells[48,79]. Similarly, a notable increase in MDA content was detected in tumor tissues of non-small cell lung carcinoma mice treated with curcumin, accompanied by a decrease in SOD activity[49]. It is well known that SOD is an indispensable constituent of the antioxidant enzyme system in biological systems. Meanwhile, a reduction in GSH content and L-OOH levels was measured in curcumin-treated TNBC cells[58]. Liu et al. designed a hypoxia-responsive nanodelivery system based on angelica polysaccharides and used curcumin as a model drug. When such curcumin-loaded micelles were employed in hepatocellular carcinoma mice, ferroptosis in solid tumors could be selectively enhanced by reducing GSH under hypoxic conditions[80]. Zhong et al. constructed a photodynamic therapy/photothermal therapeutic system by loading curcumin onto Au NRs, taking advantage of the varying pH and ROS levels of tumors and normal tissues to promote the production of lipid peroxide in melanoma[81].
2.2.2 System Xc-
System Xc- consists of two subunits, SLC7A11 and SLC3A2, and is an amino acid reverse transporter protein. Cystine and glutamate are exchanged intracellularly and extracellularly through System Xc- in a 1:1 ratio[18]. Absorbed cystine is reduced in the cell to cysteine, which continues to be involved in GSH synthesis and influences GPX activity. Interestingly, curcumin induced a decrease in SLC7A11 levels in tumor tissues from homozygous Lewis lung carcinoma mice, and this phenomenon was similarly observed in several lung cancer cells[49,82]. Furthermore, recent studies found that curcumin also negatively regulated the expression of SLC7A11 in colorectal cancer cells through PI3K/Akt/mTOR and p53 signaling, which selectively caused ferroptosis and suppressed cancer cell proliferation[83,84].
2.2.3 Glutamine
Glutamine is a conditionally essential amino acid for rapidly proliferating tumor cells[85]. Studies have shown that aberrant glutamine metabolism can promote cellular ferroptosis by enhancing the accumulation of lipid peroxides[54,86,87]. curcumin facilitates glutamine consumption by upregulating the expression of solute carrier family 1 member 5 (SLC1A5), a critical glutamine transporter, and exerts its antitumor effects against breast cancer in vitro and in vivo[50].
2.2.4 FSP1-CoQ10- NAD(P)H
Several studies have revealed that inhibition of GPX4 does not initiate ferroptosis in some cancer cell lines, suggesting the existence of alternative antiferroptosis regulators in cancer cells[88-90]. In line with this hypothesis, recent studies have confirmed the presence of the FSP1-CoQ10- NAD(P)H pathway as an independent parallel system involved in the curbing of lipid peroxidation and ferroptosis in cooperation with GPX4/GSH[88]. FSP1, previously known as apoptosis-inducing factor mitochondria-associated protein 2 (AIFM2), was identified as a GPX4-independent ferroptosis inhibitory protein[89]. As a lipophilic free radical adsorbing antioxidant, FSP1 prevents the propagation of lipid peroxides. More specifically, inositolized FSP1 is recruited to plasma membranes and uses NAD(P)H to catalyze the reduction of ubiquinone (CoQ10), forming ubiquinol as a free radical trapping antioxidant to terminate serum lipid peroxidation (LPO) and ultimately inhibit ferroptosis[74]. Curcumin downregulates the levels of FSP1, CoQ10, and NAD+/NADH proteins in tumor cells. Meanwhile, the positive expression of FSP1 in tumor tissues was also obviously downregulated by curcumin. Further studies revealed that an inhibitor of ferroptosis (Fer-1) significantly suppressed these curcumin-mediated effects[91]. Pharmacological inhibition of FSP1 synergized with inhibition of GPX4 to induce ferroptosis in many cancer cells. Thus, dual repression of GPX4 and FSP1 by curcumin is considered promising cancer therapy[92].
2.2.5 Thioredoxin reductase
Thioredoxin reductase (TrxR) catalyzes the reduction of disulfide bonds in thioredoxin (Trx) with the help of NAD(P)H. Subsequently, Trx interacts with a series of downstream proteins through thiol-disulfide exchange to regulate redox signaling events and protect cells from ROS-induced oxidative damage[93,94]. Overall, TrxR, together with Trx and NAD(P)H, constitutes a sulfur-oxygen reduction protein system that maintains cellular redox homeostasis[95]. oxidative stress, cancer cells typically overexpress TrxR[96], making the enzyme an attractive cancer-specific target[97-99]. The curcumin derivative 2c was able to selectively cause ROS-dependent apoptosis and ferroptosis in human non-small cell lung cancer cells, but not in human normal lung cells, by covalently modifying the Sec-498 residue of intracellular TrxR and generating ROS. Of interest, curcumin derivative 2c also dramatically arrested the growth of transplanted tumors in nude mice with non-small cell lung cancer cells without obvious toxicity to the liver or kidneys[66].
2.3 Other mechanisms
Wang et al. validated in vivo that silencing of circFOXP1 enhanced the expression of ferroptosis markers, establishing elevated levels of circFOXP1 in tumors and supporting its potential prognostic role in lung cancer. The specific mechanism involved circFOXP1 enhancing SLC7A11 expression in cancer cells by direct sponge adsorption of miR-520a-5p. Curcumin and quercetin inhibited the expression of circFOXP1 in lung cancer cells by regulating the miR-520a-5p/SLC7A11 axis, which in turn affected cell growth, migration and invasion as well as ferroptosis[47]. Curcumin can induce ferroptosis in colorectal cancer cells by down-regulating JNK signaling[100], as well as affecting a variety of ferroptosis-related genes[101]. MitoCur-1 reversed melanoma cell resistance to vemurafenib by inhibiting USP14 and promoting ferroptosis[102]. A growing number of findings suggest that ferroptosis frequently interferes with the immune response, leading to inflammation-associated immunosuppression[103,104]. During the development of alternative herbal medicines for the treatment of gastric cancer based on transcriptomic analysis of immune infiltration and ferroptosis, Li et al. discovered that TLR4 and KRAS, as common genes for immune infiltration and ferroptosis, play a major role in the progression of gastric cancer. Based on the prediction of these two key genes, several herbal components, including curcumin, provide research directions and alternative therapies for immunomodulation in the TME and ferroptosis of gastric cancer[105]. A recent study demonstrated that NL01 induced ferroptosis in two types of ovarian cancer cells. Intriguingly, this new derivative of curcumin was 13-times more potent than curcumin in curbing the growth of cancer cells. Further studies revealed that the mechanism by which NL01 contributes to ferroptosis is associated with lactate metabolism. It can reduce lactate uptake from the extracellular environment by decreasing the expression of hydroxycarboxylic acid receptor 1 (HCAR1)/monocarboxylic acid transporter protein 1 (MCT1), and activate the AMPK/ SREBP1 pathway to lower glucose uptake and lactate production to improve energy metabolism. Knockdown of HCAR1 expression revealed phenotypic and pathway alterations similar to those of NL01 treatment, which inversely validated the rationale for targeting lactate metabolism[106].
Last but not least, curcumin also affects the expression of proteins related to endoplasmic reticulum stress and autophagy pathways in cancer cells undergoing ferroptosis[56]. It is reasonable to infer that endoplasmic reticulum stress and autophagy may also be involved in the modulation of ferroptosis in cancer cells by curcumin, which requires further experimental verification. The mechanism of action of curcumin and its derivatives in cancer cells is summarized in Fig. 3 and Table 2.
Curcumin and its derivatives exert antitumor effects by modulating the ferroptosis pathway.

Curcumin and its derivatives promoted ferroptosis in cancerous cells.
| Disease | Experimental model | Concentration | Major mechanism | Effects | Reference |
|---|---|---|---|---|---|
| Glioblastoma | U87MG/A172 cells | 0.5-10 μM | AR↓, GPX4↓, ROS↑ | Inhibited cell survival | [78] |
| Follicular thyroid cancer Lung cancer | FTC-133/FTC-238 cells | 1-128 μM | HO-1↑, GPX4↓, MDA↑, GSH↓, ROS↑ | Inhibited tumorigenesis | [62] |
| HT29 cells xenograft mice | - | - | Inhibited cell growth, migration and invasion | [47] | |
| Lewis cells xenograft mice | 100 mg/kg | MDA↑, SOD↓, GSH↓, Fe2+↑, ACSL4↑, SLC7A11↑, GPX4↑ | Inhibited tumor growth and promoted cell death | [49] | |
| A549/H1299 cells | 3.1-100 μM | MDA↑, SOD↓, GSH↓, Iron↑, ACSL4↑, SLC7A11↓, GPX4↓ | Inhibited cell proliferation and promoted cell death | [49] | |
| A549 CD133+ cells | 0.01-0.08 μmol/ml | ROS↑, GSH↓, CoQ10↓, NAD+/NADH↓, GPX4↓, FSP1↓ | Inhibited cellular self-renewal capacity | [91] | |
| A549 CD133+ cells xenograft mice | 100 mg/kg | GPX4↓, FSP1↓ | Inhibited tumor growth | [91] | |
| H460/PC-9/H1975/A549/H1299/A549 p53 KO/H460 p53 KO cells | 5-80 μM | p53↑, DMT1↑, ROS↑, GPX4↓ | Inhibited cell viability and promoted cell death | [51] | |
| NCI-H460/A549/HepG2/HT-1080 cells | 0.5-2 μM | TrxR↓, ROS↑ | Promoted cell death | [66] | |
| NCI-H460 cells xenograft mice | 5-15 mg/kg | TrxR↓, GPX4↓ | Inhibited tumor growth | [66] | |
| LK-2/H1650 cells | 10-40 μM | DMRT3↓, SLC7A11↓ | Inhibited cell proliferation, tumorigenesis and induced apoptosis | [82] | |
| LK-2 cells xenograft mice | 50 mg/kg | Inhibited tumor growth | |||
| Liver cancer | HepG2/HUVECs cells | - | GSH↓ | Inhibited cell proliferation and promoted cell death | [80] |
| KMCH/Huh7/PLC cells | 25 μM | HO-1↑ | Promoted cell death | [63] | |
| Breast cancer | MCF7/MDA-MB-231 cells | 14-50 μM | Nrf2↑, HO-1↑, GPX4↓ | Promoted cell death | [56] |
| MCF-7 cells | - | - | Promoted cellular damage | [52] | |
| MDA-MB-453/ MCF-7 cells | 1-50 μM | ROS↑, MDA↑, Fe2+↑, SLC1A5↑, GPX4↓, FTL↓, ACSL4↑, NOX1↑ | Promoted cell death | [50] | |
| MCF-7 cells xenograft mice | 30 mg/kg | MDA↑, Fe2+↑, SLC1A5↑, GSH↓ | Inhibited tumorigenesis | [50] | |
| MCF-7/MDA-MB-231 cells | 5-50 μM | HO-1↑, GPX4↓, FHC↑, Fe2+↑, LOOH↑ | Inhibited cell viability | [58] | |
| Gastric cancer | - | - | TLR4, KRAS | - | [105] |
| Colorectal cancer | HCT-8 cells | 1-100 μM | Iron↑, MDA↑, ROS↑, GSH↓, GPX4↓, SLC7A11↓, p-PI3K↓, p-Akt↓, p-mTOR↓ | Inhibited cell proliferation | [83] |
| SW480/HCT116 cells | 1-5 μg/ml | GPX4↓, FSP-1↓ | Inhibited cell proliferation, clone formation and induced apoptosis | [92] | |
| SW620/LoVo cells | 10-80 μM | p53↑, GPX4↓, SLC7A11↓ | Inhibited cell proliferation, migration and clone formation | [84] | |
| SW620 cells xenograft mice | 100 mg/kg | Inhibited tumor proliferation | |||
| SW480 cells | 0-100 μM | JNK↓ | Inhibited cell proliferation | [100] | |
| SW480 cells | 5-50 μM | - | Inhibited cell proliferation | [101] | |
| Clear cell renal cell cancer | A498/786-O cells | 2-10 μM | ADAMTS18↑, NCOA4↓, FTH1↓, p53↓ | Inhibited cell proliferation | [57] |
| Ovarian cancer | Anglne/HO8910PM cells | 1-8 μM | HCAR1↓, MCT1↓ | Inhibited cell growth | [106] |
| HO8910PM cells xenograft mice | 5 mg/kg | Inhibited tumor proliferation | [106] | ||
| Osteosarcoma | U2os/Saos-2 cells | 0.5-4 μM | HO-1↑, GPX4↓, MDA↑, ROS↑, Iron↑ | Inhibited cell viability and promoted cell death | [48] |
| MNNG/HOS/MG-63 cells | - | Nrf2↓, GPX4↓ | Inhibited cell proliferation and invasion, induced apoptosis and G0/G1 phase arrest | [79] | |
| MNNG/HOS xenograft mice | - | Inhibited tumor proliferation | |||
| Melanoma | A375/B16 cells | - | LPO↑ | Promoted cell death | [81] |
| A375/SKMEL28 cells | 1-4 μM | USP14↓, GPX4↓, SLC7A11↓, GSH↓ | Inhibited cell proliferation and migration, induced apoptosis and cell cycle arrest | [102] |
3. Curcumin and its derivatives inhibited ferroptosis in tissue-damaged models
3.1 Brain
Curcumin is one of the few polyphenols that exhibit dramatic protective effects against ferroptosis-induced damage to cells[107]. Through activation of the Nrf2/HO-1 pathway, curcumin can both restrict high glucose-induced neuronal (N2a) cell injury[108] and promote clearance of intracranial hematomas, reduce perihematoma brain edema as well as promote neurological recovery after intracerebral hemorrhage (ICH)[109]. The main underlying mechanisms that produce this event are closely related to the antioxidant system and the iron metabolism regulatory system of curcumin. Curcumin pretreatment also effectively attenuated oxidative stress and neural ferroptosis in the ICH model by upregulating the antioxidant activity of mesenchymal stem cells (OM-MSCs)[110]. In addition, encapsulation of curcumin in nanoparticles (Cur-NPs) can better facilitate the delivery of curcumin to the brain through the physiological barrier[111]. Yoko et al. applied hybrid molecules consisting of the oxidized indole backbone of neuroprotective compounds and the polyphenol backbone of curcumin to mouse hippocampal HT22 cells for experimental purposes and noted that these preparations possessed superior neuroprotection and lower cytotoxicity compared to curcumin. In particular, they scavenge ROS to shield cells from endogenous oxidative stress as well as ferroptosis through stimulation of antioxidant-responsive elements and chelation of ferrous ions, and finally foster neuronal survival[112-115].
3.2 Heart
Combining various modern techniques, Feng et al. identified the key gene TGFBR1 from the efficient screening of immunity and ferroptosis-related biomarkers and immunomodulatory ability of herbal ingredients. TGFBR1 was found to dock well with curcumin, which was further validated to substantially attenuate myocardial fibrosis for the management of valvular atrial fibrillation[116]. Diabetes disordered the arrangement of cardiomyocytes and significantly enlarged the degree of myocardial fibrosis and collagen expression in cardiomyocytes. Curcumin treatment increases the nuclear translocation of Nrf2 and the expression of GPX4 and HO-1, alleviates glucose-induced cardiomyocyte injury, and reverses erastin-induced ferroptosis in cardiomyocytes[117]. Furthermore, curcumin mitigates oxidative stress, ferroptosis, and liver, pancreas, and heart injury after myocardial ischemia-reperfusion injury by modulating cellular lipid composition[118].
3.3 Liver
Curcumin can help promote the excretion of excess Cu2+ in a concentration-dependent manner, diminish the accumulation of Cu2+, reduce intracellular Cu2+ content in hepatolenticular degeneration (HLD) hepatocytes, and protect the copper-injured HLD model from oxidative stress based on the Nrf2/HO-1/GPX4 signaling pathway to achieve a protective function in normal rat hepatocytes[119]. Parallel to this, in liver-injured heterozygous silver crucian carp, curcumin relieved ammonia-induced oxidative stress and ferroptosis by inhibiting ROS and MDA levels along with activation of the Nrf2 pathway[120].
3.4 Kidney
There is no specific treatment for kidney damage caused by rhabdomyolysis. Ferroptosis is involved in cellular wounding and inflammation induced by rhabdomyolysis in vivo and in vitro. Curcumin dampened the characteristic changes in ferroptosis, which subsequently improved renal injury and inflammation[41,121]. HO-1 is a key pathway involved in the protective properties of curcumin[122]. The hydrophobic core of ferritin nanocages can load curcumin and specifically deliver it to the site of renal injury, improving bioavailability. More importantly, curcumin and ferritin nanocages can synergize their antioxidant activities to reduce ferroptosis and invert the pathological process of ischemia-reperfusion acute kidney injury (IR-AKI) by reducing ROS and absorbing overloaded iron, respectively[123].
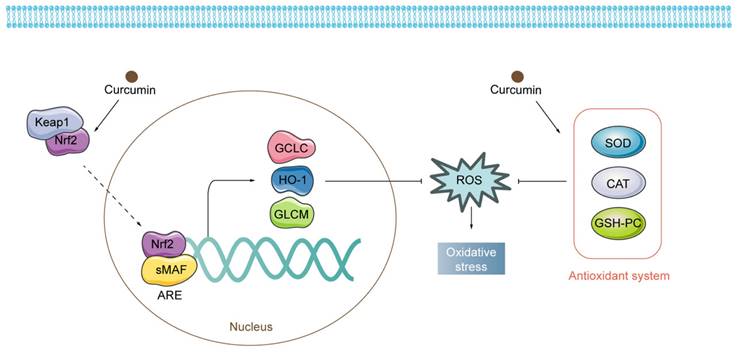
Regulatory mechanisms of curcumin on oxidative stress. Curcumin exerts antioxidant properties by activating both Nrf2-related pathways and the antioxidant system.
3.5 Other properties
Curcumin improves functional and histological lung damage from cigarette smoke and eases pulmonary ferroptosis, suggesting that curcumin may play a beneficial role in patients with COPD by limiting ferroptosis[124]. Encapsulation of cerium oxide nanoparticles (CeO2) and curcumin in mannose-modified chitosan (MCS) enhanced the therapeutic efficacy of inflammatory bowel disease (IBD), on the one hand, by increasing the expression of GSH and GPX4 to protect intestinal cells from ferroptosis, and, on the other hand, it could leverage the targeting of macrophages to minimize effects beyond the site of colonic inflammation[125]. Recent studies have demonstrated this phenomenon of curcumin suppression of ferroptosis in a mouse model of periodontal tissue injury in periodontitis, in which lipid peroxidation and System Xc- are involved and exert a crucial role[126]. Curcumin and its derivative acetyl zingerone can ameliorate osteoarthritis (OA) via the Nrf2 pathway[127,128]. In testicular tissue, curcumin upregulated SP1 and PRDX6 to stimulate self-protection against damage from ferroptosis[129]. The efficacy of curcumin and its derivatives in tissue-damaged models is shown in Fig. 4 and Table 3.
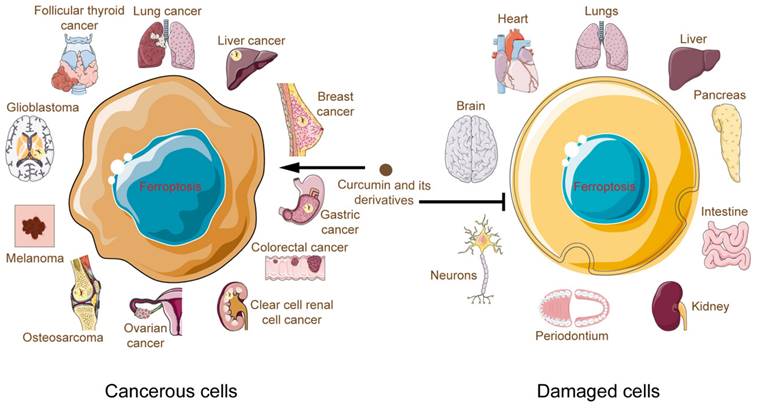
4. Conclusions and perspectives
Curcumin and its derivatives induced death in cancer cells, and bioinformatics analyses have revealed that the ferroptosis pathway was enriched more than other cell death pathways[56,105]. Moreover, inhibitors of apoptosis, necrosis, and autophagy failed to counteract this death outcome[48]. This fully justifies the importance of ferroptosis in the process of curcumin potency. For the first time, in this review, we comprehensively summarize the connection between curcumin and ferroptosis. We found that applying curcumin to different disease types and tissues causes ferroptosis to develop differently. In cancer, curcumin, on the one hand, directly or indirectly regulates cellular iron levels and, on the other hand, disrupts the antioxidant system by modulating pathways such as GPX4/GSH, FSP1-CoQ10- NAD(P)H. In contrast, curcumin exerts its iron-chelating effects in noncancer cells and ameliorates oxidative stress, curbing damage associated with ferroptosis in the brain, heart, liver, kidney, and other systems (Fig. 5). These hints point to a complicated curcumin regulation in various cell types that has to be elucidated.
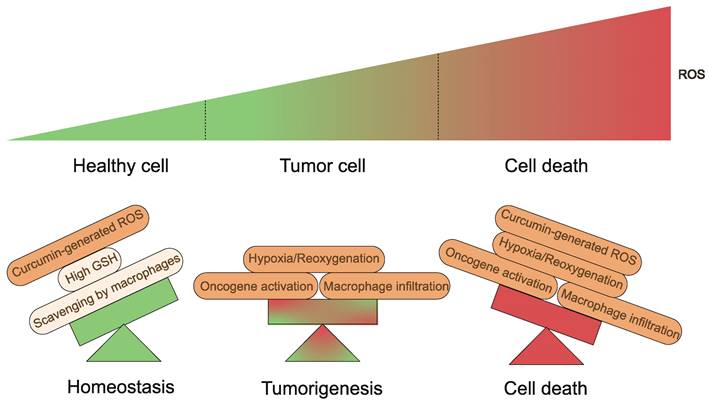
Fundamentally, a variety of elements appear to influence the results produced by curcumin and its derivatives on organisms. To begin with, the TME is a complex system with multiple levels and scales[130,131]. Tumor tissues are characterized by different properties than normal tissues, including slightly low pH and ROS overproduction, which lead to cancer cells with intrinsic oxidative stress, which is a key biochemical characteristic that distinguishes cancer cells from normal cells[132,133]. Curcumin-generated ROS become the last straw (Fig. 6). Conversely, higher levels of GSH are present in normal cells, which serves as a cellular defense system against ROS[134]. What's more, macrophages are capable of scavenging additional ROS produced in response to curcumin in vivo, thus preventing ferroptosis[83,135]. Equally important is that iron in cells is a central factor in cancer progression[136]. Tumor cells contain more iron than normal cells and proteins related to the regulation of iron metabolism are highly expressed in tumor tissues[137]. Therefore, the iron dependence of cancer cells makes them more susceptible to ferroptosis than normal cells[45,138-142]. Second, at the cellular level, the mechanism of action of curcumin and its derivatives is complex and multifactorial. A recent study found that HO-3867 caused downregulation of p53 in ovarian cancer cells[143]. However, Ling et al. presented experimental results showing that p53 levels tended to increase in NSCLC cells treated with HO-3867[51]. These variations are most likely due to the different doses of curcumin used. Curcumin stimulates HO-1 expression at low concentrations but seems to be less effective at higher concentrations[144]. The hormonal effects of curcumin have also been demonstrated in several studies[145-150]. It is a great antioxidant at low doses and has excellent pro-oxidant activity at high doses (≥20 μM)[147]. This also emphasizes the importance of having a proper dosage of the drug in the hands of the clinician.
Curcumin and its derivatives inhibited ferroptosis in tissue-damaged models.
| Disease | Experimental model | Concent-ration | Major mechanism | Effects | Reference |
|---|---|---|---|---|---|
| Diabetic neuropathy | N2a cells | 0.005 μmol/ ml | Fe2+↓, GPX4↑, SLC7A11↑, FTH1↑, TFR-1↓, Nrf2↑, HO-1↑ | Inhibited nerve cell death and promoted nerve cell viability | [108] |
| Intracerebral hemorrhage | ICH rats | 0.001-100 μM | Nrf2↑, HO-1↑ | Promoted the clearance of intracranial hematoma, reduced perihematomal brain edema, and promoted the recovery of neurological functions | [109] |
| Neurons | 10 μM | Fe2+↓, Iron↓, GPX4↑, FTH1↑, SLC7A11↑, ACSL4↓ | Reduced cell damage and nerve death | [110] | |
| ICH rats | - | Reduced blood-brain barrier dysfunction in brain tissue surrounding hematoma | [110] | ||
| HT22 cells | 2.5-320 μM | ROS↓, Nrf2↑, HO-1↑ | Inhibited hippocampal cell death | [111] | |
| Neurodegenerative disorders | HT22 cells | 10-50 μM | ARE↑, HO-1↑ | Protected nerves | [112] |
| 10-25 μM | GCLC↑, Sp1↑ | Promoted neuronal survival | [113] | ||
| 0.1-10 μM | ROS↓, Fe2+↓ | Inhibitd oxidative apoptosis and protected dopaminergic neurons | [114] | ||
| 0.1-10 μM | ROS↓ | Inhibited hippocampal cell death | [115] | ||
| Valvular atrial fibrillation | HL-1 cells | 0.005-1 μmol/ ml | TGFBR1↓ | Reduced myocardial fibrosis | [116] |
| Dabetic cardiomyopat-hy | Diabetic rabbits | 300 mg/kg | - | Improved myocardial structure | [117] |
| H9C2 cells | 0.001-0.018 μmol/ ml | Nrf2↑, HO-1↑, GPX4↑ | Alleviated the injury of cardiac myocytes and reversed the death of cardiac myocytes | [117] | |
| Ischemia/repe-rfusion injury | Ischemia/repe-rfusion-damaged rats | 100 mg/kg | ACSL↓, GPX4↑ | Reduced damage to the heart, liver and pancreas | [118] |
| Hepatolenticul-ar degeneration | TX mice | 50-100 mg/kg | - | Inhibited liver damage | [119] |
| BRL-3A cellls | 2.5-10 μM | Nrf2↑, HO-1↑, GPX4↑ | |||
| Liver injury | Gibel carp with liver injury | - | ROS↓, MDA↓, Nrf2↑, ACSL4↓, PTGS2↓, SLC7A11↑ | Improved mitochondrial morphology | [120] |
| Acute kidney injury | Mice with rhabdomyolys-is | 1000 mg/kg | HO-1↑, MDA↓, GSH↑ | Improved the function and histology of renal damage | [122] |
| MCTs/HK-2 cells | 10 μM | ||||
| HK-2 cells | 1-40 μg/ml | ROS↓, Iron↓ | Improved renal function and reversed the pathological process of IR-AKI | [123] | |
| Human renal tubular epithelial cells | 5-40 μM | p62↑, Keap1↑, Nrf2↑ | Promoted cell proliferation | [121] | |
| Mice with kidney injury | 50 mg/kg | Reduce the histopathological lesions in the kidney | |||
| Ducks with kidney injury | 400 mg/kg | NCOA4↓ | Alleviated growth retardation and renal distorted structure | [41] | |
| COPD | BEAS-2B cells | 5-20 μM | MDA↓, Iron↓, ROS↓, GSH↑, SLC7A11↑, GPX4↑, FTH1↑, TFR1↓ | Improved lung injury and inflammation | [124] |
| Rats with lung epithelial injury | 100 mg/kg | MDA↓, Iron↓, SLC7A11↑, GPX4↑, FTH1↑, TFR1↓ | |||
| Inflammatory bowel disease | IEC-6 cells | 0.125/ 1.25 μM | GSH↑, GPX4↑, MDA↓ | Improved mitochondrial morphology | [125] |
| IBD mice | 4 mg/kg | Improved typical features of ulcerative colitis, restored the histological structure of the colon, and reduced the destruction of colonic tissue | [125] | ||
| Periodontitis | Mice with periodontitis | 50-200 mg/kg | SOD↓, GSH↑, MDA↓, LC7A11↑, GPX4↑, ACSL4↓, TfR1↓ | Reduced periodontal tissue damage | [126] |
| - | MIN6 pancreatic cells | 5-20 μM | Iron↓, MDA↓, GSH↑, GPX4↑ | Inhibited MIN6 cell death | [107] |
| Osteoarthritis | Mouse chondrocytes | 0.5-32 μM | Nrf2↑ | Promoted cell proliferation | [127] |
| Knee OA mice | 50 mg/kg | Attenuated cartilage degeneration, cartilage erosion and matrix los | |||
| Rat chondrocytes | 20-100 μM | GPX4↑ | Inhibited apoptosis | [128] | |
| Knee OA mice | 0.5-1 mg/kg/ body weight | Nrf2↑, HO-1↑ | Attenuated articular cartilage degeneration | ||
| Testicular damage | Leydig/sertoli cells | 10-30 μM | SP1↑, PRDX6↑ | - | [129] |
| Rat with testicular damage | 300 mg/kg | Attenuated testicular damage |
Multifaceted roles of curcumin and its derivatives in ferroptosis as the antiferroptosis or pro-ferroptosis agent.
Generation and regulation of cellular ROS. Healthy cells have developed adequate adaptations to overcome the damaging effects of ROS. Balanced generation of ROS, sufficient antioxidant activity and scavenging by macrophages result in low concentrations of ROS. Tumorigenic events including oncogene activation, macrophage infiltration or hypoxia/reoxygenation processes in tissues yield high ROS concentrations. Curcumin-generated ROS become the last straw.
Natural products, with their wide chemical diversity, have been one of the most valuable avenues for the screening of novel clinical drugs. Izzo et al. demonstrated a new pharmacological practice guideline for the study of natural products, which could be beneficial for the reproducibility of studies on natural products[151]. The majority of research on curcumin presented in this review, however, does not refer to the methodology in the guidelines and suffers from a lack of standardization. In terms of the mechanisms investigated, the FSP1-CoQ10- NAD(P)H pathway is linked to the endosomal sorting complex required for transport III (ESCRT-III), as FSP1 is able to inhibit ferroptosis through a membrane repair process that involves the transportation of the ESCRT-III[152]. Additionally, spermidine/spermine N1-acetyltransferase 1 (SAT1) is a transcriptional target of P53, and activation of SAT1 promotes ROS-induced lipid peroxidation and ferroptosis, which is closely related to the expression of arachidonate lipoxygenase 15 (ALOX-15)[153]. Regrettably, no reports have described curcumin regulation of ferroptosis through modulation of the SAT1, ALOX-15, and ESCRT-III. Likewise, the epigenetic regulation of ferroptosis has been poorly studied. What are the roles of DNA methylation, RNA methylation, and post-translational modifications in the regulation of ferroptosis? How can epigenome editing be used to manipulate tumor cell sensitivity? These questions signal the need for additional pharmacological studies to explore the underlying mechanisms of ferroptosis mediated by curcumin.
Abbreviations
AIFM2: apoptosis-inducing factor mitochondria-associated protein 2; Akt: protein kinase B; ALOX-15: arachidonate lipoxygenase 15; AMPK: AMP-activated protein kinase; CAT: catalase; COPD: chronic obstructive pulmonary disease; CoQ10: coenzyme Q10; DMT1: recombinant divalent metal transporter 1; ECM: extracellular matrix; ESCRT-III: endosomal sorting complex required for transport III; FDA: Food and Drug Administration; FSP1: ferroptosis suppressor protein 1; FT: ferritin; FTH1: ferritin heavy chain; FTL: ferritin light chain; Gox: glucose oxidase; GPX: glutathione peroxidase; GPX4: glutathione peroxidase 4; GSH: glutathione; GSSG: oxidized glutathione; HCAR1: hydroxycarboxylic acid receptor 1; HLD: hepatolenticular degeneration; HO-1: heme oxygenase 1; IBD: inflammatory bowel disease; ICH: intracerebral hemorrhage; IR-AKI: ischemia-reperfusion acute kidney injury; IREB2: iron-responsive element binding protein 2; JNK: c-Jun N-terminal kinase; KRAS: Kirsten rat sarcoma viral oncogene; LPO: lipid peroxide; L-ROS: lipid reactive oxygen species; MCT1: monocarboxylic acid transporter protein 1; MDA: malondialdehyde; mTOR: mechanistic target of rapamycin; NAD(P)H: nicotinamide adenine dinucleotide phosphate hydrogen; NCOA4: nuclear receptor coactivator 4; Nrf2: nuclear factor-E2-related factor 2; OA: Osteoarthritis; OM-MSCs: mesenchymal stem cells; p53: transformation related protein 53; PAAs: pro-oxidant anticancer agents; PI3K: phosphatidylinositol 3-kinase; PRDX6: peroxiredoxin 6; PUFA: polyunsaturated fatty acid; PUFA-PLs: polyunsaturated fatty acid-containing phospholipids; REBP1: sterol regulatory element-binding protein 1; ROS: reactive oxygen species; SAT1: spermidine/spermine N1-acetyltransferase 1; SLC1A5: recombinant solute carrier family 1, member 5; SLC3A2: recombinant solute carrier family 3, member 2; SLC7A11: recombinant solute carrier family 7, member 11; SOD: superoxide dismutase; SP1: specific protein 1; TF: transferrin; TFR1: transferrin receptor 1; TGFBR1: transforming growth factor beta receptor 1; TLR4: toll-like receptor 4; TME: tumor microenvironment; Trx: thioredoxin; TrxR: thioredoxin reductase; USP14: ubiquitinspecific protease 14.
Acknowledgements
Parts of the figures were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
Funding
This work was supported by the National Natural Science Foundation of China (Nos.81891012; U19A2010), the Youth Talent Promotion Project of China Association for Science and Technology (No. CACM-2020-QNRC1-01), the National Interdisciplinary Innovation Team of Traditional Chinese Medicine (No.ZYYCXTD-D-202209), the Project of Science and Technology Department of Sichuan Province (Nos.2023NSFSC1928; 2023NSFSC1992), the Multidimensional Evaluation of Specialty Chinese Medicine Resources and Product Development Innovation Team (No.2022C001), and the Fundamental Research Funds for the central Universities.
Author contributions
Yuan Zhang: Conceptualization, Investigation, Visualization, Writing - original draft. Chenghao Yu: Conceptualization, Investigation, Visualization. Fu Peng: Conceptualization, Writing - review & editing, Supervision. Cheng Peng: Conceptualization, Writing - review & editing, Supervision.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wu Q, Chen Z, Ding Y, Tang Y, Cheng Y. Protective effect of traditional Chinese medicine on non-alcoholic fatty liver disease and liver cancer by targeting ferroptosis. Front Nutr. 2022Oct18;9:1033129
2. Cao X, Wang Y, Chen Y, Zhao M, Liang L, Yang M. et al. Advances in traditional Chinese medicine for the treatment of chronic obstructive pulmonary disease. Journal of Ethnopharmacology. 2023May;307:116229
3. Xie L, Ji X, Zhang Q, Wei Y. Curcumin combined with photodynamic therapy, promising therapies for the treatment of cancer. Biomedicine & Pharmacotherapy. 2022Feb;146:112567
4. Akaberi M, Sahebkar A, Emami SA. Turmeric and Curcumin: From Traditional to Modern Medicine. In: Guest PC, editor. Studies on Biomarkers and New Targets in Aging Research in Iran [Internet]. Cham: Springer International Publishing; 2021 [cited 2023 Aug 23]. p. 15-39. (Advances in Experimental Medicine and Biology; vol. 1291). Available from:https://link.springer.com/10.1007/978-3-030-56153-6_2
5. Peng Y, Ao M, Dong B, Jiang Y, Yu L, Chen Z. et al. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. DDDT. 2021;15:4503-25
6. Ming T, Tao Q, Tang S, Zhao H, Yang H, Liu M. et al. Curcumin: An epigenetic regulator and its application in cancer. Biomedicine & Pharmacotherapy. 2022Dec;156:113956
7. Zia A, Farkhondeh T, Pourbagher-Shahri AM, Samarghandian S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomedicine & Pharmacotherapy. 2021Feb;134:111119
8. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of Curcumin: Problems and Promises. Mol Pharmaceutics. 2007Dec1;4(6):807-18
9. Shi Q, Shih C, Lee K. Novel Anti-Prostate Cancer Curcumin Analogues That Enhance Androgen Receptor Degradation Activity. ACAMC. 2009Oct1;9(8):904-12
10. Heger M, Van Golen RF, Broekgaarden M, Michel MC. The Molecular Basis for the Pharmacokinetics and Pharmacodynamics of Curcumin and Its Metabolites in Relation to Cancer. Sibley DR, editor. Pharmacol Rev. 2014Jan;66(1):222-307
11. Burgos-Morón E, Calderón-Montaño JM, Salvador J, Robles A, López-Lázaro M. The dark side of curcumin. Int J Cancer. 2010;126:1771-5
12. Subramaniam D, May R, Sureban SM, Lee KB, George R, Kuppusamy P. et al. Diphenyl Difluoroketone: A Curcumin Derivative with Potent In vivo Anticancer Activity. Cancer Research. 2008Mar15;68(6):1962-9
13. Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS. et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895-900
14. Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM. et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006Mar17;6:10
15. Zhang Y, Xia Q, Li Y, He Z, Li Z, Guo T. et al. CD44 Assists the Topical Anti-Psoriatic Efficacy of Curcumin-Loaded Hyaluronan-Modified Ethosomes: A New Strategy for Clustering Drug in Inflammatory Skin. Theranostics. 2019Jan1;9(1):48-64
16. Zhang Y, He Z, Li Y, Xia Q, Li Z, Hou X. et al. Tumor cell membrane-derived nano-Trojan horses encapsulating phototherapy and chemotherapy are accepted by homologous tumor cells. Materials Science and Engineering: C. 2021Jan;120:111670
17. Joshi P, Bisht A, Paliwal A, Dwivedi J, Sharma S. Recent updates on clinical developments of curcumin and its derivatives. Phytotherapy Research. 2023Aug3;37:5109-5158
18. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE. et al. Ferroptosis: An Iron-Dependent Form of Non-Apoptotic Cell Death. Cell. 2012May25;149(5):1060-72
19. Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019May;29(5):347-64
20. Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F. Ferroptosis and Cancer: Mitochondria Meet the “Iron Maiden” Cell Death. Cells. 2020Jun20;9(6):1505
21. Wang Y, Zhang Z, Sun W, Zhang J, Xu Q, Zhou X. et al. Ferroptosis in colorectal cancer: Potential mechanisms and effective therapeutic targets. Biomedicine & Pharmacotherapy. 2022Sep;153:113524
22. Han C, Liu Y, Dai R, Ismail N, Su W, Li B. Ferroptosis and Its Potential Role in Human Diseases. Front Pharmacol. 2020Mar17;11:239
23. Green DR, Victor B. THE PANTHEON OF THE FALLEN. Trends Cell Biol. 2012Nov;22(11):555-6
24. Dixon SJ. Ferroptosis: bug or feature? Immunol Rev. 2017May;277(1):150-7
25. Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015Sep10;6:472-85
26. Paul BT, Manz DH, Torti FM, Torti SV. Mitochondria and Iron: Current Questions. Expert Rev Hematol. 2017Jan;10(1):65-79
27. Mou Y, Wang J, Wu J, He D, Zhang C, Duan C. et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019Dec;12(1):34
28. Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017Apr;21(4):648-57
29. Tang D, Kang R. From Oxytosis to Ferroptosis: 10 Years of Research on Oxidative Cell Death. Antioxidants & Redox Signaling. 2023Jul1;39(1-3):162-5
30. Costa I, Barbosa DJ, Benfeito S, Silva V, Chavarria D, Borges F. et al. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacology & Therapeutics. 2023Apr;244:108373
31. Yin L, Liu P, Jin Y, Ning Z, Yang Y, Gao H. Ferroptosis-related small-molecule compounds in cancer therapy: Strategies and applications. European Journal of Medicinal Chemistry. 2022Dec;244:114861
32. Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X. et al. Ferroptosis: process and function. Cell Death Differ. 2016Mar;23(3):369-79
33. Lee J, Roh JL. Targeting GPX4 in human cancer: Implications of ferroptosis induction for tackling cancer resilience. Cancer Letters. 2023Apr;559:216119
34. Fearnhead HO, Vandenabeele P, Vanden Berghe T. How do we fit ferroptosis in the family of regulated cell death? Cell Death Differ. 2017Dec;24(12):1991-8
35. Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R. et al. Activation of the p62-Keap1-NRF2 Pathway Protects against Ferroptosis in Hepatocellular Carcinoma Cells. Hepatology. 2016Jan;63(1):173-84
36. Abdalkader M, Lampinen R, Kanninen KM, Malm TM, Liddell JR. Targeting Nrf2 to Suppress Ferroptosis and Mitochondrial Dysfunction in Neurodegeneration. Front Neurosci. 2018Jul10;12:466
37. Zhang X, Yu Y, Lei H, Cai Y, Shen J, Zhu P. et al. The Nrf-2/HO-1 Signaling Axis: A Ray of Hope in Cardiovascular Diseases. Cardiol Res Pract. 2020Jan30;2020:5695723
38. Forciniti S, Greco L, Grizzi F, Malesci A, Laghi L. Iron Metabolism in Cancer Progression. Int J Mol Sci. 2020Mar24;21(6):2257
39. Li K, Fan C, Chen J, Xu X, Lu C, Shao H. et al. Role of oxidative stress-induced ferroptosis in cancer therapy. J Cell Mol Med. 2024May17;28(10):e18399
40. Lei L, Yuan J, Yang Q, Tu Q, Yu H, Chu L. et al. Curcumin-polydopamine nanoparticles alleviate ferroptosis by iron chelation and inhibition of oxidative stress damage. RSC Adv. 2024;14(21):14934-41
41. Liu H, He Y, Gao X, Li T, Qiao B, Tang L. et al. Curcumin alleviates AFB1-induced nephrotoxicity in ducks: regulating mitochondrial oxidative stress, ferritinophagy, and ferroptosis. Mycotoxin Res. 2023Nov;39(4):437-51
42. Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol. 2016Jan;90(1):1-37
43. Liang D, Minikes AM, Jiang X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell. 2022Jun16;82(12):2215-27
44. Andrews NC. Disorders of Iron Metabolism. The New England Journal of Medicine. 1999;341:1968-95
45. Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell. 2019Jun;35(6):830-49
46. Morales M, Xue X. Targeting iron metabolism in cancer therapy. Theranostics. 2021Jul25;11(17):8412-29
47. Wang W, Xie Y, Malhotra A. Potential of Curcumin and Quercetin in Modulation of Premature Mitochondrial Senescence and Related Changes during Lung Carcinogenesis. J Environ Pathol Toxicol Oncol. 2021;40(4):53-60
48. Lin H, Chen X, Zhang C, Yang T, Deng Z, Song Y. et al. EF24 induces ferroptosis in osteosarcoma cells through HMOX1. Biomedicine & Pharmacotherapy. 2021Apr;136:111202
49. Tang X, Ding H, Liang M, Chen X, Yan Y, Wan N. et al. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac Cancer. 2021Apr;12(8):1219-30
50. Cao X, Li Y, Wang Y, Yu T, Zhu C, Zhang X. et al. Curcumin suppresses tumorigenesis by ferroptosis in breast cancer. PLoS One. 2022Jan18;17(1):e0261370
51. Wu L, Xu G, Li N, Zhu L, Shao G. Curcumin Analog, HO-3867, Induces Both Apoptosis and Ferroptosis via Multiple Mechanisms in NSCLC Cells with Wild-Type p53. Evid Based Complement Alternat Med. 2023Feb13;2023:8378581
52. Yin Y, Jiang T, Hao Y, Zhang J, Li W, Hao Y. et al. Cascade catalytic nanoplatform based on ions interference strategy for calcium overload therapy and ferroptosis. International Journal of Pharmaceutics. 2021Sep;606:120937
53. Zuo S, Yu J, Pan H, Lu L. Novel insights on targeting ferroptosis in cancer therapy. Biomark Res. 2020Oct2;8:50
54. Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell. 2015Jul16;59(2):298-308
55. Lee J, Roh JL. Targeting Iron-Sulfur Clusters in Cancer: Opportunities and Challenges for Ferroptosis-Based Therapy. Cancers (Basel). 2023May10;15(10):2694
56. Li R, Zhang J, Zhou Y, Gao Q, Wang R, Fu Y. et al. Transcriptome Investigation and In Vitro Verification of Curcumin-Induced HO-1 as a Feature of Ferroptosis in Breast Cancer Cells. Saso L, editor. Oxidative Medicine and Cellular Longevity. 2020Nov19;2020:1-18
57. Xu B, Zhu WJ, Peng YJ, Cheng SD. Curcumin reverses the sunitinib resistance in clear cell renal cell carcinoma (ccRCC) through the induction of ferroptosis via the ADAMTS18 gene. Transl Cancer Res. 2021Jul;10(7):3158-67
58. Consoli V, Sorrenti V, Pittalà V, Greish K, D'Amico AG, Romeo G. et al. Heme Oxygenase Modulation Drives Ferroptosis in TNBC Cells. Int J Mol Sci. 2022May20;23(10):5709
59. Gammella E, Recalcati S, Rybinska I, Buratti P, Cairo G. Iron-Induced Damage in Cardiomyopathy: Oxidative-Dependent and Independent Mechanisms. Oxid Med Cell Longev. 2015;2015:230182
60. Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014May1;509(7498):105-9
61. Angeli JPF, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014Dec;16(12):1180-91
62. Chen H, Li Z, Xu J, Zhang N, Chen J, Wang G. et al. Curcumin Induces Ferroptosis in Follicular Thyroid Cancer by Upregulating HO-1 Expression. Oxid Med Cell Longev. 2023Jan14;2023:6896790
63. Liu Z, Ma H, Lai Z. The Role of Ferroptosis and Cuproptosis in Curcumin against Hepatocellular Carcinoma. Molecules. 2023Feb8;28(4):1623
64. Kajarabille N, Latunde-Dada GO. Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators. Int J Mol Sci. 2019Oct8;20(19):4968
65. Florean C, Song S, Dicato M, Diederich M. Redox biology of regulated cell death in cancer: A focus on necroptosis and ferroptosis. Free Radical Biology and Medicine. 2019Apr;134:177-89
66. Liu X, Cui H, Li M, Chai Z, Wang H, Jin X. et al. Tumor killing by a dietary curcumin mono-carbonyl analog that works as a selective ROS generator via TrxR inhibition. European Journal of Medicinal Chemistry. 2023Mar;250:115191
67. Zhu J, Xiong Y, Zhang Y, Wen J, Cai N, Cheng K. et al. The Molecular Mechanisms of Regulating Oxidative Stress-Induced Ferroptosis and Therapeutic Strategy in Tumors. Yi X, editor. Oxidative Medicine and Cellular Longevity. 2020Dec21;2020:1-14
68. Stockwell BR, Angeli JPF, Bayir H, Bush AI, Conrad M, Dixon S. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017Oct5;171(2):273-85
69. Qiu Y, Cao Y, Cao W, Jia Y, Lu N. The Application of Ferroptosis in Diseases. Pharmacological Research. 2020Sep;159:104919
70. Jia M, Qin D, Zhao C, Chai L, Yu Z, Wang W. et al. Redox homeostasis maintained by GPX4 facilitates STING activation. Nat Immunol. 2020Jul;21(7):727-35
71. Maher P. The effects of stress and aging on glutathione metabolism. Ageing Research Reviews. 2005May;4(2):288-314
72. Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021Feb;31(2):107-25
73. Li J, Cao F, Yin H liang, Huang Z jian, Lin Z tao, Mao N. et al. Ferroptosis: past, present and future. Cell Death Dis. 2020Feb3;11(2):88
74. Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G. et al. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021Jul26;7:193
75. Barrera G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncol. 2012Oct17;2012:137289
76. Barrera G, Pizzimenti S, Dianzani MU. Lipid peroxidation: control of cell proliferation, cell differentiation and cell death. Molecular Aspects of Medicine. 2008Feb;29(1-2):1-8
77. Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radical Biology and Medicine. 2020May;152:175-85
78. Chen TC, Chuang JY, Ko CY, Kao TJ, Yang PY, Yu CH. et al. AR ubiquitination induced by the curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting GPX4-Mediated redox homeostasis. Redox Biol. 2019Dec26;30:101413
79. Yuan C, Fan R, Zhu K, Wang Y, Xie W, Liang Y. Curcumin induces ferroptosis and apoptosis in osteosarcoma cells by regulating Nrf2/GPX4 signaling pathway. Exp Biol Med (Maywood). 2023Dec;248(23):2183-97
80. Liu X, Wu Z, Guo C, Guo H, Su Y, Chen Q. et al. Hypoxia responsive nano-drug delivery system based on angelica polysaccharide for liver cancer therapy. Drug Deliv. 2022;29(1):138-48
81. Zhong Y, Zhang X, Yang L, Liang F, Zhang J, Jiang Y. et al. Hierarchical dual-responsive cleavable nanosystem for synergetic photodynamic/photothermal therapy against melanoma. Materials Science and Engineering: C. 2021Dec;131:112524
82. Xu B, Zhou L, Zhang Q. Curcumin Inhibits the Progression of Non-small Cell Lung Cancer by Regulating DMRT3/SLC7A11 Axis. Mol Biotechnol. 2024
83. Chen M, Tan A hui, Li J. Curcumin Represses Colorectal Cancer Cell Proliferation by Triggering Ferroptosis via PI3K/Akt/mTOR Signaling. Nutrition and Cancer. 2023Feb7;75(2):726-33
84. Ming T, Lei J, Peng Y, Wang M, Liang Y, Tang S. et al. Curcumin suppresses colorectal cancer by induction of ferroptosis via regulation of p53 and solute carrier family 7 member 11/glutathione/glutathione peroxidase 4 signaling axis. Phytotherapy Research. 2024Jun4;38:3954-3972
85. Scalise M, Pochini L, Console L, Losso MA, Indiveri C. The Human SLC1A5 (ASCT2) Amino Acid Transporter: From Function to Structure and Role in Cell Biology. Front Cell Dev Biol. 2018Sep4;6:96
86. Luo M, Wu L, Zhang K, Wang H, Zhang T, Gutierrez L. et al. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018Aug;25(8):1457-72
87. Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W. et al. Molecular mechanisms of ferroptosis and its role in cancer therapy. J Cell Mol Med. 2019Aug;23(8):4900-12
88. Doll S, Freitas FP, Shah R, Aldrovandi M, Da Silva MC, Ingold I. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019Nov28;575(7784):693-8
89. Bersuker K, Hendricks J, Li Z, Magtanong L, Ford B, Tang PH. et al. The CoQ oxidoreductase FSP1 acts in parallel to GPX4 to inhibit ferroptosis. Nature. 2019Nov;575(7784):688-92
90. Zou Y, Palte MJ, Deik AA, Li H, Eaton JK, Wang W. et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019Apr8;10:1617
91. Zhou J, Zhang L, Yan J, Hou A, Sui W, Sun M. Curcumin Induces Ferroptosis in A549 CD133 + Cells through the GSH-GPX4 and FSP1-CoQ10-NAPH Pathways. Discovery Medicine. 2023;35(176):251
92. Miyazaki K, Xu C, Shimada M, Goel A. Curcumin and Andrographis Exhibit Anti-Tumor Effects in Colorectal Cancer via Activation of Ferroptosis and Dual Suppression of Glutathione Peroxidase-4 and Ferroptosis Suppressor Protein-1. Pharmaceuticals (Basel). 2023Mar2;16(3):383
93. Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radical Biology and Medicine. 2014Jan;66:75-87
94. Sobotta MC, Liou W, Stöcker S, Talwar D, Oehler M, Ruppert T. et al. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat Chem Biol. 2015Jan;11(1):64-70
95. Lu J, Holmgren A. Thioredoxin System in Cell Death Progression. Antioxidants & Redox Signaling. 2012Dec15;17(12):1738-47
96. Tonissen KF, Di Trapani G. Thioredoxin system inhibitors as mediators of apoptosis for cancer therapy. Mol Nutr Food Res. 2009Jan;53(1):87-103
97. Cai W, Zhang L, Song Y, Wang B, Zhang B, Cui X. et al. Small molecule inhibitors of mammalian thioredoxin reductase. Free Radical Biology and Medicine. 2012Jan;52(2):257-65
98. Zhang J, Li X, Han X, Liu R, Fang J. Targeting the Thioredoxin System for Cancer Therapy. Trends in Pharmacological Sciences. 2017Sep;38(9):794-808
99. Bian M, Fan R, Zhao S, Liu W. Targeting the Thioredoxin System as a Strategy for Cancer Therapy: Miniperspective. J Med Chem. 2019Aug22;62(16):7309-21
100. Xin W, Zhang Y. Curcumin activates the JNK signaling pathway to promote ferroptosis in colon cancer cells. Chem Biol Drug Des. 2024Mar;103(3):e14468
101. Firouzjaei AA, Aghaee-Bakhtiari SH, Tafti A, Sharifi K, Abadi MHJN, Rezaei S. et al. Impact of curcumin on ferroptosis-related genes in colorectal cancer: Insights from in-silico and in-vitro studies. Cell Biochemistry & Function. 2023Dec;41(8):1488-502
102. Li G, Zhou C, Wang L, Zheng Y, Zhou B, Li G. et al. MitoCur-1 induces ferroptosis to reverse vemurafenib resistance in melanoma through inhibition of USP14. Pigment Cell Melanoma Res. 2024Mar;37(2):316-28
103. Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer. 2019Jul;19(7):405-14
104. Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021May;18(5):280-96
105. Li M, Tao J, Qian R, Jiang F, Song Y, Zeng Z. et al. Development of alternative herbals remedy for gastric cancer based on transcriptomic analysis of immune infiltration and ferroptosis. Front Genet. 2023Mar3;14:1086368
106. Shi M, Zhang MJ, Yu Y, Ou R, Wang Y, Li H. et al. Curcumin derivative NL01 induces ferroptosis in ovarian cancer cells via HCAR1/MCT1 signaling. Cellular Signalling. 2023Sep;109:110791
107. Kose T, Vera-Aviles M, Sharp PA, Latunde-Dada GO. Curcumin and (-)- Epigallocatechin-3-Gallate Protect Murine MIN6 Pancreatic Beta-Cells against Iron Toxicity and Erastin-Induced Ferroptosis. Pharmaceuticals (Basel). 2019Feb6;12(1):26
108. Wang yan, Sun Z, Ren X, Zhao Z. Curcumin inhibits high glucose-induced ferroptosis in Neuro-2a cells via the Nrf2/HO-1 pathway. Journal of Fujian Medical University. 2023;57(2):79-88
109. Duan C, Wang H, Jiao D, Geng Y, Wu Q, Yan H. et al. Curcumin Restrains Oxidative Stress of After Intracerebral Hemorrhage in Rat by Activating the Nrf2/HO-1 Pathway. Front Pharmacol. 2022Apr27;13:889226
110. Huang Y, Liu J, He J, Tan F, Lu M, Yuan F. et al. Curcumin preconditioning enhances the neuroprotective effects of olfactory mucosa-derived mesenchymal stem cells on experimental intracerebral hemorrhage. Heliyon. 2023Jul3;9(7):e17874
111. Yang C, Han M, Li R, Zhou L, Zhang Y, Duan L. et al. Curcumin Nanoparticles Inhibiting Ferroptosis for the Enhanced Treatment of Intracerebral Hemorrhage. Int J Nanomedicine. 2021Dec14;16:8049-65
112. Hirata Y, Ito Y, Takashima M, Yagyu K, Oh-hashi K, Suzuki H. et al. Novel Oxindole-Curcumin Hybrid Compound for Antioxidative Stress and Neuroprotection. ACS Chem Neurosci. 2020Jan2;11(1):76-85
113. Ikawa T, Sato M, Oh-hashi K, Furuta K, Hirata Y. Oxindole-curcumin hybrid compound enhances the transcription of γ-glutamylcysteine ligase. European Journal of Pharmacology. 2021Apr;896:173898
114. Hirata Y, Tsunekawa Y, Takahashi M, Oh-hashi K, Kawaguchi K, Hayazaki M. et al. Identification of novel neuroprotective N,N-dimethylaniline derivatives that prevent oxytosis/ferroptosis and localize to late endosomes and lysosomes. Free Radical Biology and Medicine. 2021Oct;174:225-35
115. Hirata Y, Okazaki R, Sato M, Oh-hashi K, Takemori H, Furuta K. Effect of ferroptosis inhibitors oxindole-curcumin hybrid compound and N,N-dimethylaniline derivatives on rotenone-induced oxidative stress. European Journal of Pharmacology. 2022Aug;928:175119
116. Jiang F, Zhang W, Lu H, Tan M, Zeng Z, Song Y. et al. Prediction of herbal medicines based on immune cell infiltration and immune- and ferroptosis-related gene expression levels to treat valvular atrial fibrillation. Front Genet. 2022Sep28;13:886860
117. Wei Z, Shaohuan Q, Pinfang K, Chao S. Curcumin Attenuates Ferroptosis-Induced Myocardial Injury in Diabetic Cardiomyopathy through the Nrf2 Pathway. Cardiovasc Ther. 2022Jul15;2022:3159717
118. Kar F, Yıldız F, Hacioglu C, Kar E, Donmez DB, Senturk H. et al. LoxBlock-1 or Curcumin attenuates liver, pancreas and cardiac ferroptosis, oxidative stress and injury in Ischemia/reperfusion-damaged rats by facilitating ACSL/GPx4 signaling. Tissue and Cell. 2023Jun;82:102114
119. Sun X, Zhang X, Yan H, Wu H, Cao S, Zhao W. et al. Protective effect of curcumin on hepatolenticular degeneration through copper excretion and inhibition of ferroptosis. Phytomedicine. 2023May;113:154539
120. Wu L, Dong B, Chen Q, Wang Y, Han D, Zhu X. et al. Effects of Curcumin on Oxidative Stress and Ferroptosis in Acute Ammonia Stress-Induced Liver Injury in Gibel Carp (Carassius gibelio). Int J Mol Sci. 2023Mar29;24(7):6441
121. Zhai J, Chen Z, Zhu Q, Guo Z, Sun X, Jiang L. et al. Curcumin inhibits PAT-induced renal ferroptosis via the p62/Keap1/Nrf2 signalling pathway. Toxicology. 2024Aug;506:153863
122. Guerrero-Hue M, García-Caballero C, Palomino-Antolín A, Rubio-Navarro A, Vázquez-Carballo C, Herencia C. et al. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB j. 2019Aug;33(8):8961-75
123. Lou X, Lu J, Wang C, Song Y, Zhu L, You Y. et al. Self-oriented ferritin nanocages mitigate iron overload-induced oxidative stress for acute kidney injury. Chemical Engineering Journal. 2023Jun;466:143227
124. Tang X, Li Z, Yu Z, Li J, Zhang J, Wan N. et al. Effect of curcumin on lung epithelial injury and ferroptosis induced by cigarette smoke. Hum Exp Toxicol. 2021Dec;40(12_suppl):S753-62
125. Yang J, Bai Y, Shen S, Tao X, Ma C, Fu B. et al. An oral nano-antioxidant for targeted treatment of inflammatory bowel disease by regulating macrophage polarization and inhibiting ferroptosis of intestinal cells. Chemical Engineering Journal. 2023Jun;465:142940
126. Wang Y, Lin H, Huang W, Liu Z, Chen Z, Zhao X. et al. Curcumin Attenuates Periodontal Injury via Inhibiting Ferroptosis of Ligature-Induced Periodontitis in Mice. Int J Mol Sci. 2023Jun7;24(12):9835
127. Zhou Y, Jia Z, Wang J, Huang S, Yang S, Xiao S. et al. Curcumin reverses erastin-induced chondrocyte ferroptosis by upregulating Nrf2. Heliyon. 2023Oct;9(10):e20163
128. Chen X, Chen J, Miao C, Yin G, Zhang Z, Sun R. et al. Acetyl zingerone ameliorates osteoarthritis by inhibiting chondrocyte programmed cell death. Mol Med Rep. 2023Sep11;28(5):202
129. Bu H, Wang B, Wu Y, Li P, Cui Y, Jiang X. et al. Curcumin strengthens a spontaneous self-protective mechanism-SP1/PRDX6 pathway, against di-n-butyl phthalate-induced testicular ferroptosis damage. Environ Sci Pollut Res. 2023Nov15;30(58):122165-81
130. An L, Li M, Jia Q. Mechanisms of radiotherapy resistance and radiosensitization strategies for esophageal squamous cell carcinoma. Mol Cancer. 2023Aug19;22(1):140
131. Sherman MH, Beatty GL. Tumor Microenvironment in Pancreatic Cancer Pathogenesis and Therapeutic Resistance. Annu Rev Pathol Mech Dis. 2023Jan24;18(1):123-48
132. Policastro LL, Ibañez IL, Notcovich C, Duran HA, Podhajcer OL. The Tumor Microenvironment: Characterization, Redox Considerations, and Novel Approaches for Reactive Oxygen Species-Targeted Gene Therapy. Antioxidants & Redox Signaling. 2013Sep10;19(8):854-95
133. Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009Jul;8(7):579-91
134. Lv H, Zhen C, Liu J, Yang P, Hu L, Shang P. Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy. Oxid Med Cell Longev. 2019Jun10;2019:3150145
135. Rendra E, Riabov V, Mossel DM, Sevastyanova T, Harmsen MC, Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019Mar;224(2):242-53
136. Wu Y, Yu C, Luo M, Cen C, Qiu J, Zhang S. et al. Ferroptosis in Cancer Treatment: Another Way to Rome. Front Oncol. 2020Sep25;10:571127
137. Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013May;13(5):342-55
138. W J, C A P, J L S. Novel surface antigen expressed on dividing cells but absent from nondividing cells. J Exp Med. 1980Nov1;152(5):1430-5
139. Aulbert E, Disselhoff W, Sörje H, Schulz E, Gericke D. Lysosomal accumulation of 67Ga-transferrin in malignant tumors in relation to their growth rate. European Journal of Cancer (1965). 1980Sep;16(9):1217-32
140. Sutherland R, Delia D, Schneider C, Newman R, Kemshead J, Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981Jul;78(7):4515-9
141. Gatter KC, Brown G, Trowbridge IS, Woolston RE, Mason DY. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J Clin Pathol. 1983May;36(5):539-45
142. Shterman N, Kupfer B, Moroz C. Comparison of Transferrin Receptors, Iron Content and Isoferritin Profile in Normal and Malignant Human Breast Cell Lines. Pathobiology. 1991;59(1):19-25
143. Devor EJ, Schickling BM, Lapierre JR, Bender DP, Gonzalez-Bosquet J, Leslie KK. The Synthetic Curcumin Analog HO-3867 Rescues Suppression of PLAC1 Expression in Ovarian Cancer Cells. Pharmaceuticals (Basel). 2021Sep21;14(9):942
144. Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R. et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003May1;371(Pt 3):887-95
145. Kim JY, Cho TJ, Woo BH, Choi KU, Lee CH, Ryu MH. et al. Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Archives of Oral Biology. 2012Aug;57(8):1018-25
146. Li B, Takeda T, Tsuiji K, Wong TF, Tadakawa M, Kondo A. et al. Curcumin Induces Cross-Regulation Between Autophagy and Apoptosis in Uterine Leiomyosarcoma Cells. Int J Gynecol Cancer. 2013Jun;23(5):803-8
147. Rainey N, Motte L, Aggarwal BB, Petit PX. Curcumin hormesis mediates a cross-talk between autophagy and cell death. Cell Death Dis. 2015Dec3;6(12):e2003-e2003
148. Moustapha A, Pérétout P, Rainey N, Sureau F, Geze M, Petit JM. et al. Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events. Cell Death Discovery. 2015Oct26;1(1):15017
149. Guo S, Long M, Li X, Zhu S, Zhang M, Yang Z. Curcumin activates autophagy and attenuates oxidative damage in EA.hy926 cells via the Akt/mTOR pathway. Molecular Medicine Reports. 2016Mar;13(3):2187-93
150. Moghaddam NSA, Oskouie MN, Butler AE, Petit PX, Barreto GE, Sahebkar A. Hormetic effects of curcumin: What is the evidence? Journal Cellular Physiology. 2019Jul;234(7):10060-71
151. Izzo AA, Teixeira M, Alexander SPH, Cirino G, Docherty JR, George CH. et al. A practical guide for transparent reporting of research on natural products in the British Journal of Pharmacology: Reproducibility of natural product research. Br J Pharmacol. 2020May;177(10):2169-78
152. Dai E, Zhang W, Cong D, Kang R, Wang J, Tang D. AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochemical and Biophysical Research Communications. 2020Mar;523(4):966-71
153. Ou Y, Wang SJ, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci U S A. 2016Nov1;113(44):E6806-12
Author contact
![]() Corresponding author: E-mail address: pengchengedu.cn (P. C); pengfedu.cn (P. F).
Corresponding author: E-mail address: pengchengedu.cn (P. C); pengfedu.cn (P. F).

 Global reach, higher impact
Global reach, higher impact