10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2025; 21(2):823-841. doi:10.7150/ijbs.101055 This issue Cite
Research Paper
Toll-like receptor adaptor protein TIRAP has specialized roles in signaling, metabolic control and leukocyte migration upon wounding in zebrafish larvae
1. Institute of Biology Leiden, Animal Science and Health, Leiden University, Einsteinweg 55, 2333 CC Leiden, The Netherlands.
2. Present address: Center for Thrombosis and Hemostasis (CTH), Johannes Gutenberg-University Medical Center, Langenbeckstraße 1, Bldg. 70855131 Mainz, Germany.
† These authors contributed equally to this work.
‡ These authors contributed equally to this work.
Received 2024-7-17; Accepted 2024-11-18; Published 2025-1-1
Abstract

The TIRAP protein is an adaptor protein in TLR signaling which links TLR2 and TLR4 to the adaptor protein Myd88. The transcriptomic profiles of zebrafish larvae from a tirap, myd88 and tlr2 mutant and the corresponding wild type controls under unchallenged developmental conditions revealed a specific involvement of tirap in calcium homeostasis and myosin regulation. Metabolomic profiling showed that the tirap mutation results in lower glucose levels, whereas a tlr2 mutation leads to higher glucose levels. A tail-wounding zebrafish larval model was used to identify the role of tirap in leukocyte migration to tissue wounding. We found that more neutrophils were recruited to the wounded region in the tirap mutant larvae compared to the wild type controls, whereas there was no difference in macrophage recruitment. In contrast, published data show that tlr2 and myd88 mutants recruit fewer neutrophils and macrophages to the wounds. Based on cell tracking analysis, we demonstrate that the neutrophil migration speed is increased in the tirap mutant in contrast to neutrophil behavior in myd88 and tlr2 mutants. In conclusion, we show that tirap plays specialized roles distinct from tlr2 and myd88 in signaling, metabolic control, and in regulating neutrophil migration speed upon wounding.
Keywords: tirap, Mal, transcriptome, metabolome, neutrophil migration, tissue wounding
1. Introduction
Membrane-localized pattern recognition receptors (PRRs) are required by leukocytes during perception and recognition processes facing invasive infection and tissue damage[1, 2]. Toll-like receptors (TLRs) are among the most extensively studied PRRs and are the crucial recognition factors for pathogen-associated molecular patterns (PAMPs) derived from invading pathogens and damage-associated molecular patterns (DAMPs) released by stressed, damaged or dead cells[3]. Upon interaction with PAMPs and DAMPs, TLRs can bind to some adapters to initiate downstream signaling cascades. The Toll-interleukin-1 receptor (TIR) domain-containing adaptor protein (TIRAP), also known as MyD88-associated ligand (MAL), is an adaptor protein of TLR2 or TLR4[4, 5]. As a key intracellular signaling transducer, TIRAP contains a TIR domain that can bind to the TIR domain of TLR2 and then recruits MyD88[6]. TIRAP thereby activates the downstream cascade of MyD88-dependent TLR signaling, ultimately leading to the production of proinflammatory cytokines and in this way is involved in the regulation of the innate immune responses of leukocytes[7, 8]. It is therefore crucial to understand the underlying signaling mechanisms mediated by the TLRs pathway and the modulatory roles of TIRAP within it.
TLR signaling has been recently shown to be involved in the control of migration of leukocytes towards tissue damage and wounding. Leukocytes can be rapidly recruited to the site of injury by inflammatory mediators and chemokines, and play a crucial role in immune clearance and damage repair upon tissue wounding[9]. As first responders to wounded tissues, the recruitment and migration of leukocytes to injured tissues is particularly important in the acute inflammatory response[10]. In our previous study on TLR signaling influencing leukocyte migration in a zebrafish larval model, it was found that both tlr2 and myd88 mutants recruited less neutrophils and macrophages to the wounding area than their wild type controls[11]. However, the role that TIRAP plays in this process has not been studied.
Since the first discovery of TIRAP in 2001, the function of TIRAP in diverse immune responses as an adaptor molecule has gradually been revealed[12, 13]. Studies in lung diseases have demonstrated the important role of TIRAP in immune modulation by affecting cytokines and chemokines. For example, when TIRAP is deficient, the lipopolysaccharide (LPS) induced expression of cytokines and chemokines such as tumor necrose factor-α (TNF-α), interleukine-6 (IL-6) and IL-8 in alveolar macrophages is attenuated[14]. Also, macrophages from TIRAP mutant mice were defective in the early burst of proinflammatory cytokine production and in the ability to limit intracellular growth of pathogen Bordetella pertussis[15]. Studies in inflammatory signaling have further revealed that the regulated activation of TIRAP is an important part of preventing excessive inflammatory damage to the host[8]. Tyrosine kinases such as Bruton's tyrosine kinase (BTK) can tyrosine phosphorylate the TIR domain of TIRAP, therefore transiently activating TIRAP, and suppressor of cytokine signaling 1 (SOCS1) can mediate the ubiquitin-dependent degradation of phosphorylated TIRAP[16]. The transient activation of TIRAP and subsequent SOCS-1-mediated degradation leads to a rapid and balanced inflammatory response[16]. The normal function of TIRAP is thereby essential to avoid contributing to chronic inflammation and related diseases[16]. There is also evidence that TIRAP can be recruited by the receptor for advanced glycation end products (RAGE) and such engagement allows for the activation of downstream effector molecules that mediate various cellular processes including inflammatory responses and apoptotic cell migration and death[17, 18]. Mutations in the TIR domain of TIRAP lead to the inhibition of downstream inflammatory signaling, as well as affecting the role of RAGE in inducing proinflammatory immune responses during disease[17, 18]. TIRAP enables interaction with various intracellular signaling molecules and influences leukocyte immune function, indicating its central role in diverse immune responses. However, current research implies that the role of TIRAP in the resolution of inflammation is a double-edged sword[19] and that the impact on the immune response still needs further study, as does the function of TIRAP in the migratory behavior of immune cells.
Current advances in live imaging and the application of new model systems have contributed to revealing the motility behavior of leukocyte cells in wounded and infected tissues. As the small size and transparency of zebrafish larvae are useful features for screening and imaging transgenic reporter strains, zebrafish larvae are considered an ideal model for visualizing and tracking fluorescent leukocytes in the resolution of inflammation such as bacterial infection and tail wounding[20, 21]. Some signals have been defined to influence leukocyte motility behavior in the resolution of inflammation through the application of zebrafish models. For example, the chemokine receptors C-X-C Motif Chemokine Receptor 1(Cxcr1) and Cxcr2 can regulate the recruitment of neutrophils to the sites of tissue damage in zebrafish larvae[22]. In our previous study, also using a tail-wounding zebrafish larval model, the importance of Tlr2 and its adaptor protein Myd88 in the regulation of leukocyte migration in response to tail wounding has been revealed. This confirmed the involvement of TLR signaling in controlling the migration behavior of neutrophils and macrophages during wounding[11].
In order to explore the specific role of Tirap, we systematically investigated the transcriptional and metabolic profiles using RNAseq and 1H nuclear magnetic resonance (NMR) techniques in tirap mutant and also in myd88 mutant fish lines. We compared our results with data from the tlr2 mutant from our previous study[23]. We found that significantly differentially expressed genes in the tirap mutant are different from myd88 and tlr2 mutants. In addition, fewer differentially altered metabolites were found in the tirap mutant than in the tlr2 mutant. This implies that tirap is different from tlr2 and myd88 in terms of transcriptional level and metabolic regulation. To investigate the regulatory role of tirap in the innate immune process in cellular responses to inflammation, we used live fluorescent imaging to study the response after mycobacterial infection and leukocyte migration after tail wounding in larvae. In the infection experiments, tirap mutant larvae had a higher bacterial burden after infection with Mycobacterium marinum strain Mma20 than the wild type control. In the tail wounding experiments, tirap mutant larvae recruited significantly more neutrophils to the wounds than the wild type control, whereas there was no significant difference in the recruitment for macrophages. Further studies of neutrophil motility showed that tirap controls neutrophil migration speed, but not directional persistence upon tail wounding. Finally, we summarize the results of all our zebrafish larval test systems with mutants of tirap, myd88 and tlr2.
2. Materials and Methods
2.1. Zebrafish maintenance and mutant line construction
The maintenance of all adult and larval zebrafish and all animal experiments described in this study were conducted at Leiden University in accordance with the standard protocols (zfin.org) and adhered to the international guidelines specified by the EU Animal Protection Directive 2010/63/EU. The culture of adult fish was approved by the university's local animal welfare committee (DEC) (License number: protocol 14,198) and no adult zebrafish were sacrificed in this study. Eggs and larvae were grown in laboratory-manufactured egg water (containing 60 mg/l instant ocean sea salts) at 28.5℃. For infection, wounding and live imaging assays, the experiments were all performed on larvae up to 5 days post fertilization (dpf) and therefore prior to the free-feeding stage, which is not covered by the animal experimentation law according to the EU Animal Protection Directive 2010/63/EU. 27 and 28 hours post fertilization (hpf) larvae during injection experiments and 3 dpf larvae during wounding experiments were anesthetized with egg water containing 0.02% buffered 3-aminobenzoic acid ethyl ester (Tricaine, Sigma-Aldrich, Netherlands).
The tirapsa182 mutant (further referred as tirap-/- or tirap mutant) line (ENU-mutagenized) was obtained from the Sanger Institute Zebrafish Mutation Resource (Hinxton, Cambridge, UK) and shipped by the zebrafish resource Center of the Karlsruche Institute of Technology. All mutant alleles were identified by sequencing and the homozygote carriers of the mutations were outcrossed more than three times to wild type (AB/TL strains). Homozygote mutants and their wild type siblings (further referred as tirap+/+) were used to generate models in this study. To investigate the effect of the tirap mutation on the cell migration behavior, fluorescent transgenic lines tirap+/+ Tg (mpeg1: mCherry-F), tirap+/+ Tg (mpx: EGFP), tirap-/- Tg (mpeg1: mCherry-F) and tirap-/- Tg (mpx: EGFP) were used. The generation of transgenic lines and myd88 mutant zebrafish line refer to previous studies[11, 21].
2.2. LPS injection and qRT-PCR detecting
In order to determine whether the tirap mutation results in a loss of function of Tirap and blocked the downstream pathway, we detected the downstream gene expression in zebrafish larvae after injection with lipopolysaccharide (LPS)[24]. 1 nl purified LPS (100 μg/ml) solutions were injected into the blood stream through the blood island at 27 hpf for each larva using a Femtojet microinjector (Eppendorf, the Netherlands) equipped with a capillary glass needle. 1 nl sterile water was injected as the control experiment. At 1 hour post injection (hpi), the injected larvae were collected for the quantitative real-time PCR (qRT-PCR) analysis. qRT-PCR were performed on a CFX96TM Touch Real-Time PCR Detection (Bio-Rad Laboratories, Inc, USA). The expression of target gene was normalized to the expression of ppial as a reference gene. The primer sequences used in this study are shown in Supplementary Table 1. The qRT-PCR reaction procedure was performed according to the following protocol: 95℃ 3 min, 40 cycles real time of 95℃ 15 sec, 68℃ 30 sec and 72℃ 30 sec, and final melting curve of 95℃ 1 min and 55℃ 10 sec. The quantitative qRT-PCR assay was biologically repeated for three times and the relative expression level was determined by the comparative 2-ΔΔCt method[25].
2.3. RNAseq processing and analysis
For the purpose of RNA sequencing, the extraction of total RNA from 5 dpf tirap mutant zebrafish larvae and wild type controls was performed using TRIzol Reagent (Life Technologies), following the manufacturer's instructions. DNase treatment was then conducted by using the kit (Thermo Scientific) to remove the DNA contamination. RNA sequencing of unchallenged tirap mutant and wild type zebrafish larvae was conducted by GenomeScan B.V. (Leiden, The Netherlands) as previously described[26]. Sequencing data of three biological replicates for the tirap mutants and wild type controls were aligned and mapped to the zebrafish genome GRCz11 using Salmon v1.2.1[27]. Differential gene expression was analyzed by using DESeq2 v1.24.0[28]. Statistical significance was determined by a padj value of ≤ 0.05[29]. KEGG pathway enrichment analysis was performed in DAVID Bioinformatics Resources 6.8 (https://david.Ncifcrf.Gov/). For unchallenged myd88 mutant and wild type zebrafish larvae, consistent sequencing process and analysis were performed.
2.4. NMR sample preparation and measurement
The extraction of metabolites from zebrafish larvae in wild type and mutant groups was based on our previous research method[30, 31]. Each group contained 120 zebrafish larvae at 5 dpf in a mixture of methanol : water (1:1, v/v) with 1 ml of chloroform. The mixture was sonicated for 15 min and then centrifuged at 5,000 rpm for 5 min. After centrifugation, two layers were formed. The methanol and water from the upper layer was collected and then dried with nitrogen. The dried methanol : water layer containing metabolites was dissolved in 1 ml of 100 mM deuterated phosphate (KD2PO4, pH: 7.0) containing 0.02% trimethylsilyl propionate (TSP) as internal standard and then filtered using a Millipore filter (Millex-HV0.45-lmFilterUnit). Metabolites in zebrafish larvae were measured using a Bruker DMX 600 MHz NMR spectrometer at 4°C. The spectrometer was equipped with a 5 mm inverted triple high resolution probe with an actively shielded gradient coil. In order to obtain a satisfactory signal-to-noise ratio, the 1H NMR spectra were accumulated with 65,000 data points, a relaxation delay of 2 s, a scan width of 12.4 kHz and 256 scans.
One-dimensional (1-D) 1H NMR spectra obtained from both the wild type and mutant groups were corrected for baseline and phase shifts with MestReNova software version 11.0 (Mestrelab Research S.L., Santiago de Compostela, Spain). The spectra were then subdivided into buckets of 0.04 ppm in the range of 0 to 10.00 ppm. For removal of the water peak, the range from 4.30 to 6.00 ppm was excluded from the analysis. The spectra from chemical shifts 0.80 to 4.30 ppm were assigned to specific metabolites. Metabolite quantification was performed using Chenomx NMR Suite 8.3, which allows both qualitative and quantitative analysis of the NMR spectra by fitting spectral features from the HMDB database to the spectra. The data matrix obtained was exported to Microsoft Office Excel (Microsoft Corporation). These data were then simultaneously imported into MetaboAnalyst 4.0 for the Partial least squares-discriminant analysis (PLS-DA) and heat map analysis. A correlation coefficient of P < 0.05 was considered to be statistically significant.
2.5. Infection modelling
In the present study, a bacterial strain Mycobacterium marinum m20 (Mma20) expressing mCherry fluorescent protein was used for infection experiments. The preparation of bacteria was performed as previously described[32]. The infection inoculum was prepared in 2% polyvinylpyrrolidone 40 solution (Calbiochem, the Netherlands), and approximately 150 colony-forming units (CFU) of bacteria were injected into the blood stream through the blood island at 28 hpf for each larva[26]. At 4 days post infection (dpi), pools of larvae were collected and imaged using the MZ16FA Fluorescence Stereo Microscope (Leica Microsystems, Wetzlar Germany). The statistical analysis of bacterial burden of the whole body of the larvae was conducted respectively in a dedicated pixel counting software QuantiFish 2.1 program[33].
2.6. Tail wounding modelling and qRT-PCR detecting
The caudal fin wounding model in this study was applied based on previous studies[11, 21]. 3 dpf tirap mutant zebrafish larvae and their wild type controls were anesthetized in egg water with 0.02% tricaine (Sigma Aldrich). The inactive larvae were then placed in a 2% agarose covered petri dish and the caudal fins of larvae were wounded by using a 1 mm sterile sapphire blade scalpel (World Precision Instruments) under a MZ16FA Fluorescence Stereo Microscope (Leica Microsystems, Wetzlar Germany) equipped with a DFC420C color camera (Leica Microsystems). After the wounding, the wounded larvae were placed back into untreated egg water and incubated at 28.5 ℃ for the subsequent imaging experiments. We performed qRT-PCR on a CFX96TM Touch Real-Time PCR Detection (Bio-Rad Laboratories, Inc, USA) to quantify the immune related gene expression profiles from tirap wild type and mutant larvae after tail wounding. Target gene expression was normalized to the expression of ppial as a reference gene. The primer sequences used in this study are shown in Supplementary Table 1. The qRT-PCR reaction procedure and condition were the same as described in section 2.2.
2.7. Imaging and quantification
At 1, 3, and 6 hours post wounding (hpw), larvae were collected and fixed with 4% paraformaldehyde (PFA). The wounded tail area of each fixed specimen was imaged by using the Leica MZ16FA fluorescence stereo microscope. The number of neutrophils and macrophages recruited to the wounded area after tail wounding was manually quantified. Cells localized within an area of 200 µm from the wounding edge toward the body trunk were counted as recruited cells[11].
2.8. Live imaging and quantification
Time lapse imaging of 3 dpf zebrafish larvae were visualized under a Leica TCS SP8 confocal laser scanning microscope (CLSM, Leica Microsystems, Wetzlar Germany) with 1 min time interval for 2 h using a 20× objective (N.A.0.75). For unchallenged condition, the caudal hematopoietic tissue (CHT) of zebrafish larvae was imaged using the CLSM. For tail wounding condition, the wounded tail area of zebrafish larvae was imaged from 1 hpw to 3 hpw under the CLSM. The quantification for leukocyte cell behavior was conducted in IMARIS x64 7.4 Software program (Bitplane), which loaded with an automated 3D cell tracking algorithm was employed to establish neutrophil trajectories in live imaging of zebrafish larvae. Using the IMARIS software, data of the number, mean speed, and meandering index of recruited neutrophils in the wounded tail region were obtained.
2.9. Statistical analysis
The statistical analysis was performed using the Graphpad Prism software (Version 8.1.1; GraphPad Software, San Diego, CA, USA). All experimental data in this study are shown as mean ± SD. D'Agostino-Pearson and Shapiro-Wilk normality test was performed to determine the Gaussian distribution of the data. The statistical significant differences between wild type and tirap mutant larvae were determined based on the Gaussian distribution of data using either Mann-Whitney and Kolmogorov-Smirnov (non-parametric tests) or unpaired t test with Welch's correction (parametric tests). The significance was established as * P < 0.05, ** P < 0.01 and *** P < 0.001.
3. Results
3.1. Characterization of tirap mutant zebrafish line
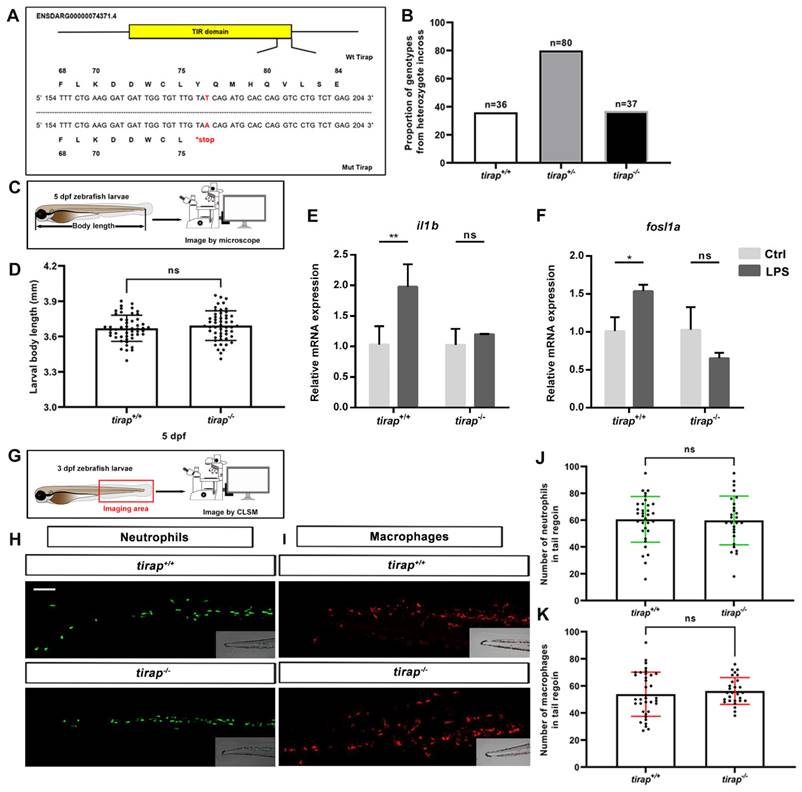
The tirap mutant was generated by a point mutation from thymine (T) to adenine (A) that leads to a stop codon located in its TIR domain, resulting in a truncated protein (Figure 1A). This condition resulted in mutant zebrafish lacking the intact Tirap protein, thereby preventing the dimerization with Myd88 in the signaling cascade[19]. The genotype proportion from heterozygote cross revealed that this mutation is non-lethal, as the ratio of genotype was the expected 1:2:1 (Figure 1B). The development of tirap mutant zebrafish larvae was further investigated. The results revealed no significant difference in terms of the whole body length of tirap mutant larvae at 5 dpf compared to their wild type controls (Figure 1C, D). It is expected that tirap mutation in zebrafish embryos affects their ability to respond to microbial PAMPs. To confirm this hypothesis, we injected LPS into the blood island of tirap+/+ and tirap-/- embryos at 27 hpf[24]. Subsequently, we performed a qRT-PCR experiment to determine the expression of il1b and fosl1a in the 1-hpi tirap+/+ and tirap-/- embryos. The il1b gene encodes a typical pro-inflammatory cytokine in the downstream TLR signaling cascade during the stimulation by LPS[24]. In a previous study, we have shown that fosl1a is specifically regulated by Tlr2 signaling[26]. We found that both il1b and fosl1a can be significantly induced in tirap+/+ embryos upon LPS infection, while no different expression was found in tirap-/- embryos compared to mock injection control (Figure 1E, F). The results show that the recognition of LPS in zebrafish embryos requires tirap, which is consistent with a previous study in a myd88 mutant[24]. The results confirm that the tirap-/- embryos generated in this study have lost the function of Tirap. To investigate whether the tirap mutation affects the number of leukocytes, the transgenic lines tirap+/+ Tg (mpeg1: mCherry-F), tirap+/+ Tg (mpx: EGFP), tirap-/- Tg (mpeg1: mCherry-F) and tirap-/- Tg (mpx: EGFP) were imaged at 3 dpf to count the number of neutrophils and macrophages in their tail region under unchallenged condition (Figure 1G, H, I). The counting results found no significant difference in the number of neutrophils (Figure 1J) and macrophages (Figure 1K) in tirap mutants compared to their wild type controls under unchallenged condition.
3.2. Transcriptomic profiling of tirap and myd88 mutant zebrafish
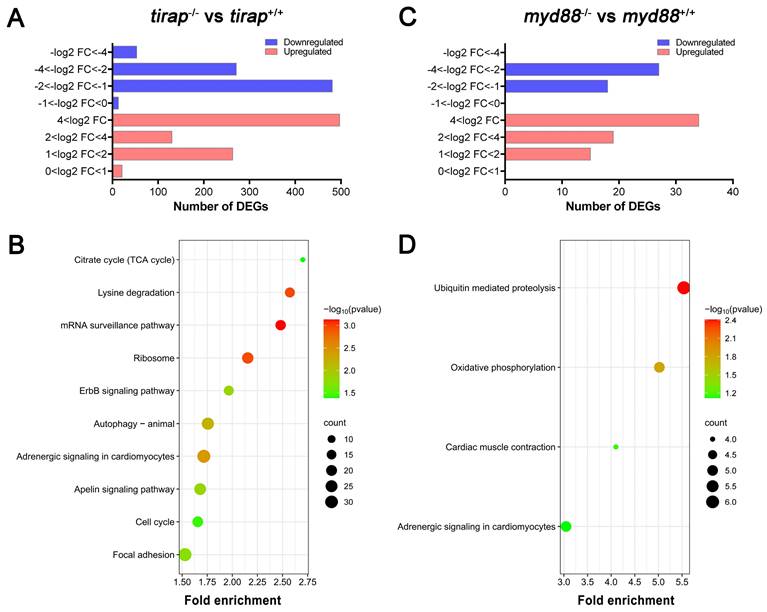
In order to compare systemic effects of the knock out of the tirap and myd88 genes on transcriptional pathway regulation, we performed whole-larval level transcriptomic studies on mutants and their corresponding sibling wild type controls under unchallenged conditions. For the tirap mutant, the number of significantly differentially expressed genes (DEGs) based on the padj value less than 0.05 in the tirap mutants compared to the wild type controls was 1729, including 911 upregulated genes and 818 downregulated genes (Figure 2A). Then for comparing the transcriptional characteristics between the tirap mutant and myd88 mutant, we also performed RNAseq of myd88 mutant zebrafish larvae and their corresponding wild type controls. 113 DEGs were found in myd88 mutant larvae, including 68 upregulated genes and 45 downregulated genes, compared with the wild type controls based on the padj value less than 0.05 (Figure 2B). For gene enrichment analysis of DEGs, KEGG pathway enrichment analysis was performed using the DAVID bioinformatics program (http://david.abcc.ncifcrf.gov/). In tirap mutant larvae, significantly enriched KEGG pathways include citrate cycle (TCA cycle), lysine degradation, mRNA surveillance pathway, ribosome, ErbB signaling pathway, autophagy, adrenergic signaling in cardiomyocytes, apelin signaling pathway, cell cycle and focal adhesion (Figure 2C). KEGG pathways that are significantly enriched in myd88 mutant larvae include ubiquitin mediated proteolysis, oxidative phosphorylation, cardiac muscle contraction and adrenergic signaling in cardiomyocytes (Figure 2D). The gene annotation of these pathways indicated a common link to various calcium signaling pathways that underlie the changes in the adrenergic signaling and cardiac muscle contraction.
Characterization of tirap mutant zebrafish larvae. (A) Mutant DNA and protein sequence of Tirap. A point mutation (T to A) in the TIR domain of zebrafish tirap results in a stop codon. (B) Proportion of genotypes. The proportion of genotypes after the incross of tirap+/- heterozygous. (C) Experimental scheme of body length measurement. (D) Development. The body measurement of tirap+/+ and tirap-/- zebrafish at 5 days post fertilization (5 dpf). The measurements are based on 3 independent experiments with tirap+/+ (n=52) and tirap-/- (n=52). (E, F) qPCR of tirap+/+ and tirap-/- zebrafish embryos. Tirap+/+ and tirap-/- embryos were injected with purified LPS (100 µg/ml) or sterile water as a control at 27 hour post fertilization (hpf). The expression level of il1b and fosl1a were analyzed at 1 hour post injection (hpi) by qRT-PCR. Data (mean ± SD) are combined with 3 biological replicates (n=15 embryos/group). Statistical significance was determined by two-way ANOVA with Tukey's multiple comparison method as a post-hoc test. (G, H, I) Experimental scheme and representative image of macrophages and neutrophils in zebrafish larvae (tirap+/+ and tirap-/-). Scale bar: 50 µm. (J, K) Quantification of neutrophils and macrophages number in tirap mutant and wild-type zebrafish larvae. Number of neutrophils and macrophages in zebrafish larvae under unchallenged conditions. The number of neutrophils for tirap+/+ (n=33) and tirap-/- (n=27) and the number of macrophages for tirap+/+ (n=36) and tirap-/- (n=28) are based on two independent experiments. Statistical significant difference was determined by unpaired t-test, ns, non-significant.
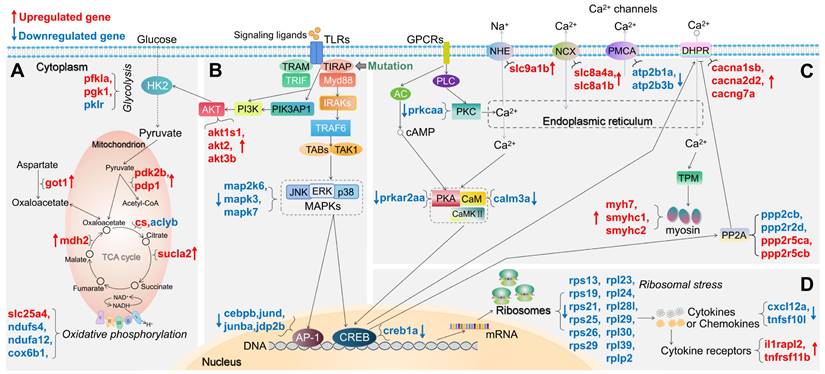
For both tirap mutant and myd88 mutant, we summarized the changes in expression of DEGs in a number of pathways associated with TLR and calcium signaling and the TCA cycle in Figures 3 and 4, respectively. For example, in glycolysis and gluconeogenesis and TCA cycle pathway, in the tirap mutant, pfkla, pgk1, got1, pdk2b, pdp1, cs, mdh2 and sucla2 are upregulated, while only pklr and aclyb are downregulated (Figure 3A). In oxidative phosphorylation pathway, only slc25a4 is upregulated while ndufs4, ndufa12 and cox6b1 are downregulated in the tirap mutant (Figure 3A). In addition, in the TLR-associated signaling and cytokine production pathway, akt1s1, akt2, akt3b, il1rapl2 and tnfrsf11b are upregulated. In contrast, map2k6, mapk3, mapk7, cebpb, jund, junba, jdp2b, nkap, cxcl12a, c1qtnf12 and tnfsf10l are downregulated in the tirap mutant compared with the wild type controls (Figure 3B,D). In calcium regulation-related pathways, cacna1sb, cacna2d2b, cacng7a, ppp2r5ca, ppp2r5cb, slc8a1b, slc8a4a, slc9a1b, myh7, smyhc1 and smyhc2 are upregulated, while ppp2cb, ppp2r2d, atp2b1a, atp2b3b, calm3a, prkcaa and creb1a are downregulated in the tirap mutant (Figure 3C). In addition, we see a large group of ribosomal genes that are downregulated (rps13, rps19, rps21, rps25, rps26, rps29, rpl23, rpl24, rpl28l, rpl29, rpl30, rpl39 and rplp2) in the tirap mutant (Figure 3D). The details of these DEGs from the representative pathways in tirap-/- versus tirap+/+ zebrafish larvae groups are shown in Supplementary Table 2.
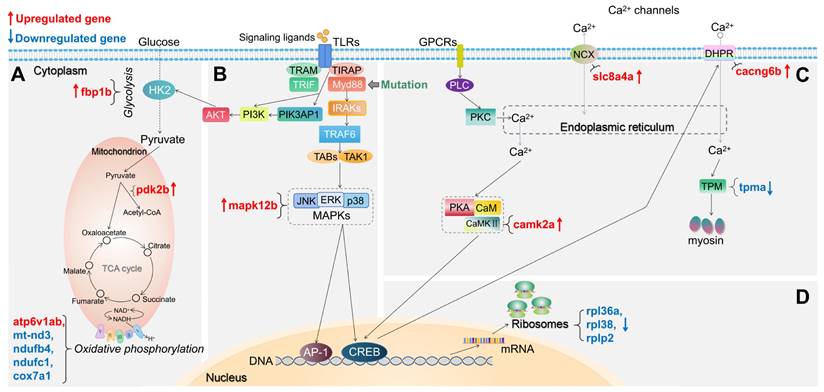
For the myd88 mutant, we also summarized the changes in expression of DEGs in representative pathways associated with TLR signaling in Figure 4. In glycolysis and gluconeogenesis and TCA cycle pathway, in the myd88 mutant, fbp1b and pdk2b are upregulated (Figure 4A). In oxidative phosphorylation pathway, only atp6v1ab is upregulated while mt-nd3, ndufb4, ndufc1 and cox7a1 are downregulated in the myd88 mutant (Figure 4A). In the TLR-associated signaling and cytokine production pathway, only mapk12b is upregulated in the myd88 mutant compared with wild type controls (Figure 4B). In calcium regulation-related pathways, slc8a4a, camk2a and cacng6b are upregulated, while only tpma is downregulated in the myd88 mutant (Figure 4C). Ribosomal genes that are downregulated are rpl36a, rpl38 and rplp2 in the myd88 mutant (Figure 4D). The details of these DEGs from the representative pathways in myd88-/- versus myd88+/+ zebrafish larvae groups are shown in Supplementary Table 3.
Transcriptomic profiles of tirap mutant and myd88 mutant zebrafish larvae measured by RNAseq. (A) Overview of the distribution of differentially expressed genes (DEGs) log2 fold change in tirap-/- versus tirap+/+ zebrafish larvae. (B) Overview of the distribution of DEGs log2 fold change in myd88-/- versus myd88+/+ zebrafish larvae. DEGs were assessed by padj value less than 0.05. Down-regulated gene sets are shown in blue, and up-regulated gene sets are shown in red. (C) KEGG pathway enrichment analysis of tirap-/- versus tirap+/+. (D) KEGG pathway enrichment analysis of myd88-/- versus myd88+/+. Significantly enriched KEGG pathway terms for DEGs of tirap-/- versus tirap+/+ groups were determined by using the hypergeometric test/Fisher's exact test, with a threshold of p value < 0.05, which were adjusted using the Benjamini and Hochbery FDR correction. For myd88-/- versus myd88+/+ group, significantly enriched KEGG pathway terms for DEGs were determined by using the hypergeometric test/Fisher's exact test, with a threshold of p value < 0.1.
Schematic diagram of significantly differentially expressed genes (DEGs) in the representative pathways in tirap mutant zebrafish larvae compared to the wild type controls. (A) Glycolysis and gluconeogenesis, TCA cycle and oxidative phosphorylation related pathways. (B) TLR-associated signaling pathways. (C) Calcium regulation-related pathways. (D) Ribosome-associated pathway. The significance was established as padj value less than 0.05. Red arrows represent significantly upregulated DEGs, blue arrows represent significantly downregulated DEGs. The link between TIRAP and PIK3AP1 refers to Luo, et al.[59]. The link between CREB and DHPR refers to Antony, et al.[44]. The link between CREB and PP2A refers to Chen, et al.[60]. Other links refer to KEGG Pathway Database (https://www.genome.jp/kegg/pathway.html).
Schematic diagram of significantly differentially expressed genes (DEGs) in the representative pathways in myd88 mutant zebrafish larvae compared to the wild type controls. (A) Glycolysis and gluconeogenesis, TCA cycle and oxidative phosphorylation related pathways. (B) TLR-associated signaling pathways. (C) Calcium regulation-related pathways. (D) Ribosome-associated pathway. The significance was established as padj value less than 0.05. Red arrows represent significantly upregulated DEGs, blue arrows represent significantly downregulated DEGs. The link between TIRAP and PIK3AP1 refers to Luo, et al.[59]. The link between CREB and DHPR refers to Antony, et al.[44]. Other links refer to KEGG Pathway Database (https://www.genome.jp/kegg/pathway.html).
In conclusion, the effects of knocking out the tirap and myd88 genes are very different both quantitatively and in the affected pathways as discussed below. Most notably, there are significant differences in pathways related to metabolism.
3.3. Metabolomic profiling of tirap and myd88 mutant zebrafish
The tirap mutation caused greater differences at the transcriptional level compared to its wild type control, as evidenced by a much higher number of DEGs than the myd88 mutation under unchallenged conditions. To compare the effects of mutations in tirap and myd88 on a broader level, we used 1-D 1H NMR spectroscopy to investigate the differences in metabolite levels between tirap mutant and wild type zebrafish larvae at 5 dpf, and also between myd88 mutant and wild type control at 5 dpf. Representative 1-D 1H NMR spectra of tirap mutant and wild type groups are shown in Figure 5A. The chemical shifts of the 1-D 1H NMR spectra were assigned according to the chemical shifts of the reference metabolites from the Chenomx 600 MHz library (version 11). Subsequently the 1-D 1H-NMR spectra of tirap wild type and mutant groups were examined by multivariate analysis using the Partial least squares-discriminant analysis (PLS-DA) modeling to investigate whether these two experimental groups can be well discriminated. PLS-DA results showed clustering of tirap wild-type and mutant groups. This suggests that tirap deficiency in zebrafish larvae leads to metabolic changes (Figure 5B). For the tirap mutants, six significantly downregulated metabolites including proline, 2-Hydroxyglutarate, asparagine, glucose, serine and citrulline were detected compared to wild-type controls (Figure 5C). The details of these significantly altered metabolites in tirap-/- versus tirap+/+ zebrafish larvae groups are shown in Supplementary Table 4.
Metabolic profiles of tirap mutant and myd88 mutant zebrafish larvae measured by 1H NMR and analysis of PLS-DA and heat map. (A) The representative one-dimensional 1H NMR spectra of tirap wild type and mutant zebrafish larvae measured by NMR spectrometry. Spectra from chemical shift 0.8 to 4.3 were assigned to specific metabolites. (B) PLS-DA analysis of tirap wild type and mutant groups. n = 3, each replicate represents 120 pooled larvae. (C) Heat map analysis of significantly altered metabolites detected in tirap mutants (P < 0.05). n = 3, each replicate represents 120 pooled larvae. (D) The representative one-dimensional 1H NMR spectra of myd88 wild type and mutant zebrafish larvae measured by NMR spectrometry. Spectra from chemical shift 0.8 to 4.3 were assigned to specific metabolites. (E) PLS-DA analysis of myd88 wild type and mutant groups. n = 3, each replicate represents 120 pooled larvae. (F) Heat map analysis of significantly altered metabolites detected in myd88 mutants (P < 0.05). Thr threonine, Lac lactate, Asp aspartate, Ser Serine, tCr total creatine (creatine + phosphocreatine), Glc Glucose, 2-Pho 2-Phosphoglycerate, Ala alanine, Gln glutamine, Arg arginine, Leu leucine, Val valine, Gly glycine, Tau taurine, His Histidine, Tyr tyrosine, Mal Malate, Met Methionine, Suc Succinate, Pyr Pyruvate, Glu glutamate, Acet Acetate, Lys lysine, Mel Melatonin, Eth Ethanol, Isol Isoleucine, 2h3m 2-Hydroxy-3-methylvalerate, 2-Hyd 2-Hydroxyglutarate.
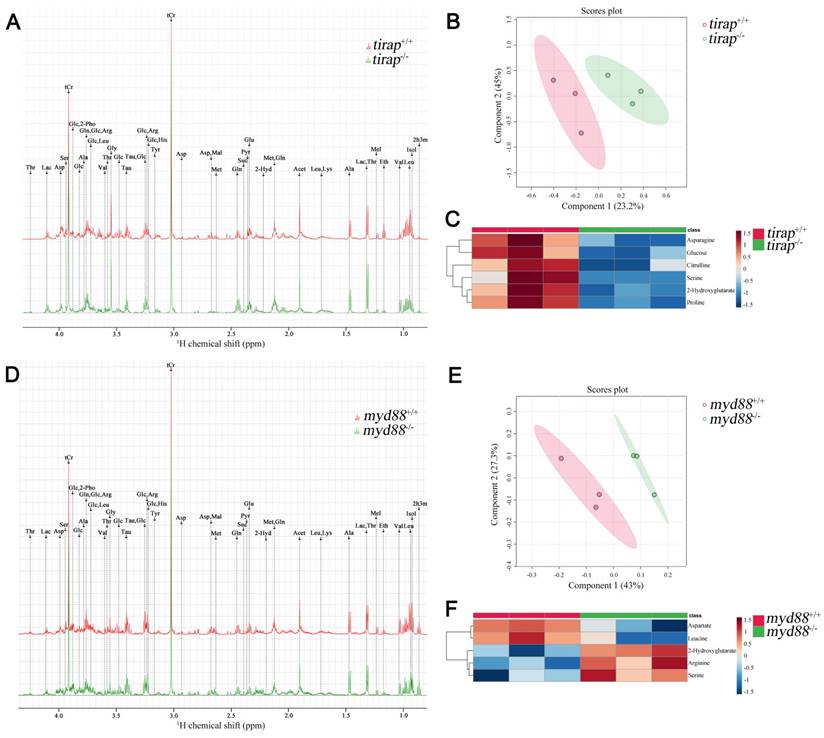
Quantification of bacterial burden after M. marinum infection in tirap mutant and wild type zebrafish larvae. (A) tirap+/+ and tirap-/- zebrafish were infected with mCherry-labeled M. marinum strain Mma20 by caudal vein injection at 28 hours post fertilization (hpf). (B) Representative images were taken after 4 days of infection (dpi), respectively. (C) Bacterial burdens at 4 dpi were quantified using bacterial fluorescence pixels. Data were combined from 3 independent experiments, for tirap+/+, n=67, for tirap-/-, n=65. ****, P <0.0001. Scale bar: 500 µm.
Similarly, representative 1-D 1H NMR spectra of myd88 mutant and wild type groups are shown in Figure 5D. PLS-DA results of myd88 mutants showed clustering of myd88 wild-type and mutant groups (Figure 5E). Five significantly altered metabolites were detected in the myd88 mutants compared to wild-type controls, with aspartate and leucine being downregulated and serine, arginine and 2-Hydroxyglutarate being upregulated (Figure 5F). The details of these significantly altered metabolites in myd88-/- versus myd88+/+ zebrafish larvae groups are shown in Supplementary Table 5.
3.4. tirap mutant larvae have higher bacterial burden after M. marinum infection
To explore the effect of tirap on immune response after mycobacterial infection, the strain Mma20 was injected into the blood island of tirap mutant larvae and their wild type controls at 28 hpf. Quantitative results after imaging at 4 dpi showed that tirap mutant larvae have significantly higher bacterial burden compared to their wild type controls (Figure 6).
3.5. Tirap mediates neutrophil recruitment to wounds, but not macrophages
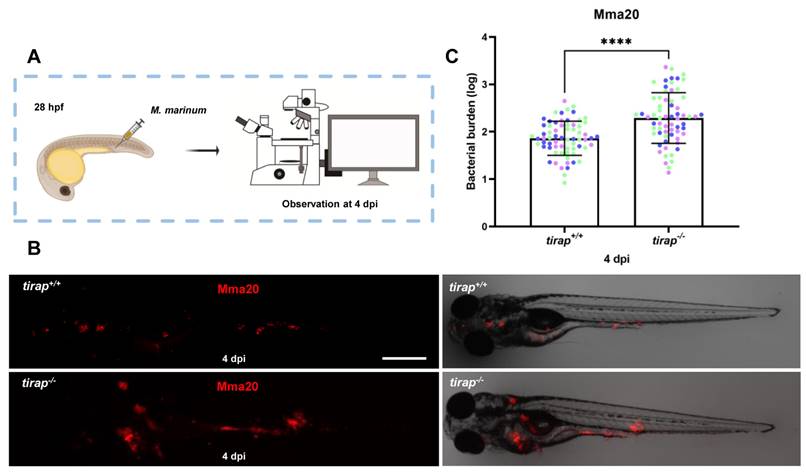
After observing no difference in neutrophil numbers between tirap mutant larvae and their wild type controls under the unchallenged condition (Figure 1), the tail wounding model was next used to investigate whether tirap had an effect in inflammation resolution. In 3 dpf zebrafish larvae, after tail wounding, the number of recruited neutrophils to the wounded area (in a range close to 200 µm from the tail wound edge) was counted at 1, 3 and 6 hpw (Figure 7A). The results showed no difference in the number of neutrophils recruited between tirap mutant and wild type larvae at 1 hpw. However, at 3 hpw and 6 hpw, tirap mutant larvae recruited significantly more neutrophils than wild type controls, indicating that tirap is involved in regulating neutrophil recruitment in inflammatory resolution (Figure 7B-C). For macrophage recruitment at the wounding part, there was no significant difference in the number of macrophages recruited between tirap mutant and wild type larvae at 1, 2, 4 and 6 hpw, suggesting that tirap has no effect on macrophage recruitment after wounding (Supplementary Figure 1).
3.6. Tirap affects neutrophil migration speed but not directional persistence upon tail wounding
Having established that tirap can regulate neutrophil recruitment after wounding, we further investigated neutrophil behaviour in tirap mutant larvae, such as neutrophil migration speed and directional persistence. Firstly, we investigated the migratory behaviour of neutrophils from tirap mutant larvae under unchallenged condition. Therefore, live imaging of the caudal haematopoietic tissue (CHT) region of zebrafish larvae was performed. The neutrophil mean speed and meandering index were specified as two indicators to assess the migratory behaviour of neutrophils. Mean speed is obtained by dividing the total displacement by the travel time. Meandering index corresponds to the neutrophil distance between first and the last frame (net displacement) divided by their total distance (total displacement) and can be used to indicate the direction of migration[10]. The results revealed no significant differences between tirap mutant and wild type larvae in terms of neutrophil migration speed and direction under unchallenged conditions (Supplementary Figure 2). Subsequently, the role of tirap in neutrophil motility behaviour upon tail wounding was investigated. Live imaging of the caudal region of wounded zebrafish larvae was performed from 1 hpw to 3 hpw for 2 hours in total (Figure 8A-B). In terms of migration speed, the results showed that the mean speed of neutrophils in the tirap mutant larvae was significantly faster compared to the wild type controls (Figure 8C). As for the direction of migration, the results showed no significant difference in the meandering index of neutrophils in the tirap mutant larvae compared to the wild type (Figure 8D). These results suggest that tirap deficiency affects the speed of neutrophil migration at the caudal wound area, but not the direction of migration.
The number of recruited neutrophils upon tail wounding in zebrafish larvae. (A) Schematic representation of experiment. The wounded zebrafish larvae were imaged at designated times 1 hour post wounding (hpw), 3 hpw and 6 hpw. (B) Representative images of tirap+/+ and tirap-/- at 1 hpw and 6 hpw. Scale bar: 100 µm. (C) Number of neutrophils recruited to tail region upon tail wounding at 1, 3, 6 hpw. Red stars indicate the representative images shown in the panel B. The number of neutrophils at 1 hpw from one independent experiment for tirap+/+ (n=23) and tirap-/- (n=20), at 3 hpw for tirap+/+ (n= 52) and tirap-/- (n= 53) and at 6 hpw for tirap+/+ (n=72) and tirap-/- (n=57) are based on three independent experiments. Statistically significant difference was determined by nonparametric tests: Mann-Whitney and Kolmogorov-Smirnov, ns, non-significant; **, P < 0.01, ***, P < 0.001.
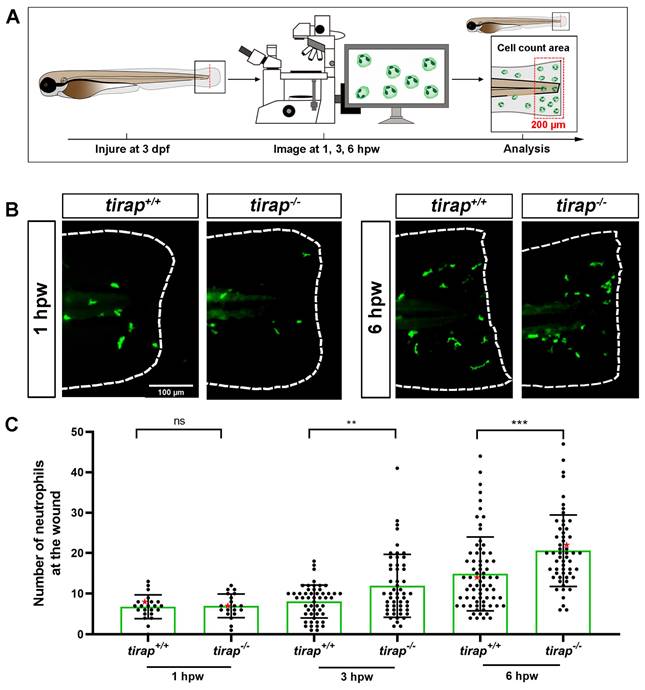
Quantification of neutrophil behaviour upon tail wounding in zebrafish larvae. (A) Schematic representation of experiment. (B) Representative image of neutrophil migration at frame 120 (3 hpw) (C-D) Quantification of neutrophil migratory capability of 3 dpf tirap larvae upon tail wounding. Mean speed (C) and meandering index (D) were quantified using IMARIS Software. The mean speed of neutrophils is measured as total displacement of neutrophils divided by the number of frames. Data consists of 3 tirap+/+ and 3 tirap-/- larvae. Statistically significant difference was determined by unpaired t test with Welch's correction, ns, non-significant; ***, P < 0.001.
3.7. Gene expression profiles of immune-related genes upon tail wounding
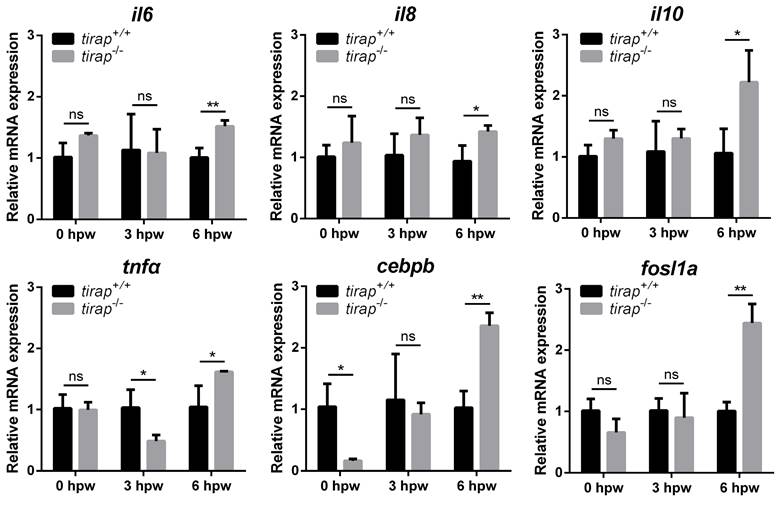
To further investigate the molecular mechanisms by which tirap affects post-injury neutrophil migration, the relative expression of downstream genes associated with TLR signaling was examined in zebrafish larvae after tail wounding. These genes include il6, il8, il10, tnfa, cebpb and fosl1a. The results showed no significant difference in the relative expression of the genes examined in the tirap mutant versus wild type controls before tail wounding, except for cebpb, which was expressed lower in the tirap mutant than in wild types. At 3 hours post wounding (hpw), with the exception of tnfa, there was no significant difference in the relative expression of other examined genes in the tirap mutants and wild type controls. However, at 6 hpw, all tested genes showed significantly elevated relative expression in the tirap mutant larvae compared to the wild type controls (Figure 9).
Relative expression of immune-related genes upon tail wounding in tirap mutant zebrafish larvae. hpw, hour post wounding; ns, non-significant; *, P < 0.05; **, P < 0.01.
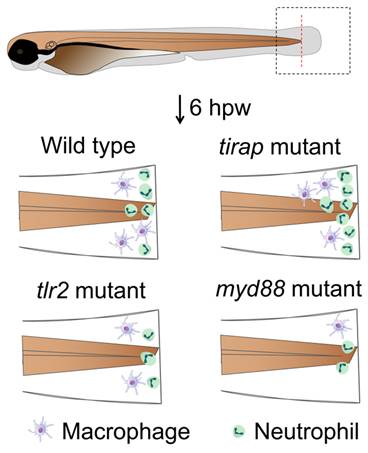
Graphical summary of tirap mutant compared to tlr2 and myd88 mutants in affecting leukocyte migration behavior upon tail wounding in zebrafish larvae. hpw, hour post wounding.
4. Discussion
In this study, we have investigated the function of tirap and myd88 in transcriptomic and metabolomic regulation under unchallenged conditions by obtaining RNAseq and NMR profiles in mutant zebrafish larvae and their wild type controls. We made further comparisons with our previously published tlr2 mutant data[23]. In Table 1, we systematically summarize the transcriptomic and metabolomic characteristics of three mutants, tirap, myd88 and tlr2, as compared to the three sibling control lines under unchallenged conditions. In terms of transcriptomic characteristics, the number of DEGs in tirap-/- versus tirap+/+ group is much higher than in the myd88-/- versus myd88+/+ group and in the tlr2-/- versus tlr2+/+ group. No overlapping DEGs were found in the three mutants (Supplementary Figure 3). Also, among the DEGs, there are more genes involved in TLR signaling-related pathways in tirap-/- versus tirap+/+ group than in the other two mutants (Table 1A). For example, akt1s1, akt2 and akt3b are only upregulated in tirap mutant larvae. Interestingly, it was shown previously that TIRAP and MyD88 are both required for TLR2/6-mediated PI3K-dependent mouse macrophage membrane ruffling at the leading edge and PIP3 formation, whereas only TIRAP is essential for Akt phosphorylation[34]. Therefore, a specialized function of TIRAP is also apparent in mammalian cells.
Simplified phenomics comparison of three mutants
| A. Transcriptome | tirap-/- vs tirap+/+ | myd88-/- vs myd88+/+ | tlr2-/- vs tlr2+/+ [23] | ||
|---|---|---|---|---|---|
| DEG number (padj value) | 1729* | 113* | 7 | ||
| DEG number (s value) | 1632 | 155 | 149* | ||
| TLRs-associated signaling and cytokine production | ↑ | akt1s1, akt2, akt3b, il1rapl2, tnfrsf11b | mapk12b | ― | |
| ↓ | map2k6, mapk3, mapk7, cebpb, jund, junba, jdp2b, cxcl12a, tnfsf10l | ― | map3k4 | ||
| Glycolysis and gluconeogenesis | ↑ | pfkla, pgk1, got1 | fbp1b | ― | |
| ↓ | pklr | ― | gpib, pfkma | ||
| TCA cycle | ↑ | pdk2b, pdp1, cs, mdh2, sucla2 | pdk2b | ― | |
| ↓ | aclyb | ― | pck2 | ||
| Ribosome | ↑ | ― | ― | rpl10, rpl13, rrp7a | |
| ↓ | rps13, rps19, rps21, rps25, rps26, rps29, rpl23, rpl24, rpl28l, rpl29, rpl30, rpl39, rplp2 | rpl36a, rpl38, rplp2 | ― | ||
| Oxidative phosphorylation | ↑ | slc25a4 | atp6v1ab | ndufa6, mt-nd3, ndufs4, ndufv3 | |
| ↓ | ndufs4, ndufa12, cox6b1 | mt-nd3, ndufb4, ndufc1, cox7a1 | ― | ||
| Calcium regulation | ↑ | cacna1sb, cacna2d2b, cacng7a, ppp2r5ca, ppp2r5cb, slc8a1b, slc8a4a, slc9a1b, myh7, smyhc1, smyhc2, | slc8a4a, camk2a, cacng6b | ― | |
| ↓ | ppp2cb, ppp2r2d, atp2b1a, atp2b3b, calm3a, prkcaa, creb1a | tpma | ― | ||
| B. Metabolism | tirap-/- vs tirap+/+ | myd88-/- vs myd88+/+ | tlr2-/- vs tlr2+/+ [23] | ||
| Number of significantly altered metabolites | 6 | 5 | 29 | ||
| Amino acids and amines | ↑ | ― | Serine, arginine | Glycine, 2-Aminobutyrate, kynurenine, 4-Aminobutyrate, leucine, asparagine, aspartate, threonine, valine, alanine, glutamine, tyrosine, ethanolamine | |
| ↓ | Proline, asparagine, serine, citrulline | Leucine, aspartate | Melatonin | ||
| Carbohydrates and organic acids | ↑ | ― | 2-Hydroxyglutarate | Succinate, 2-phosphoglycerate, glucose, indole-3-acetate, O-phosphoethanolamine, acetate, formate, galactose, glutamate, lactate, creatine, malate, taurine | |
| ↓ | 2-Hydroxyglutarate, glucose | ― | Glycerate | ||
| C. Leukocyte migration | tirap-/- vs tirap+/+ | myd88-/- vs myd88+/+ | tlr2-/- vs tlr2+/+ [23] | ||
| Under unchallenged condition | No effect | No effect | No effect | ||
| Wounding response | Neutrophil | Recruitment | Increased | Reduced | Reduced |
| Migration speed | Increased | No effect | No effect | ||
| Directional persistence | No effect | Decreased meandering index | Decreased meandering index | ||
| Macrophage | Recruitment | No effect | Reduced | Reduced | |
| Migration speed | No effect | Reduced | Reduced | ||
| Directional persistence | No effect | Decreased meandering index | Decreased meandering index | ||
The analysis of transcriptomic and metabolomic data for the tirap and myd88 mutants is summarised from the current study, and that of tlr2 mutant from previous study [22]. Leukocyte migration studies for the tirap mutant were derived from the current study, and for myd88 and tlr2 from previous study [10]. “↑” represents upward regulation or increased status compared with corresponding wild type control, “↓” represents downward regulation or reduced status compared with corresponding wild type control, “-” represents that no genes or metabolites were found. * represents that transcriptomic analysis of tirap and myd88 mutants is based on padj value less than 0.05, whereas that of tlr2 mutant is based on the s value less than 0.005
In addition, the tirap mutant is the only one of the three mutants that causes numerous down-regulations in the expression of transcripts of ribosomal proteins. Since ribosomal protein genes are under cotranslational regulation upon ribosomal stress[35], the cohort of down-regulated ribosomal protein genes might represent ribosomal stress. It has been shown that down regulation of ribosomal proteins such as RPS19 leads to ribosomal stress and severe proteomic changes for instance in proteins related to cytoskeletal reorganisation[36]. Previous studies have also shown that ribosomal proteins have functions in resolving physiological inflammation. For instance, ribosomal protein L13a depletion abrogates the endogenous translation control of several chemokines in macrophages[37]. Furthermore, ribosomal protein L13a-dependent translational silencing suppresses the synthesis of a series of inflammatory proteins in monocytes and macrophages[38]. In our study, we also found some downregulated chemokine (cxcl12a) and cytokine (tnfsf10l) genes and upregulated cytokine receptor genes (il1rapl2 and tnfrsf11b) in the tirap mutant. This suggests that Tirap is associated with the regulation of the inflammatory response, which might be the result of altered expression of ribosomal proteins.
The down-regulation of inflammatory cytokine and chemokine expression observed in the tirap mutant larvae may also be attributed the result of a defect in p38 mitogen-activated protein kinase (p38 MAPK) signaling. The downstream p38 MAPK signaling axis of TLR signaling controls AP-1-mediated proinflammatory cytokines expression[39, 40]. In the tirap mutant, map2k6, mapk3 and mapk7 were downregulated and AP-1 transcription factors, sush as cebpb, jund, junba and jdp2b, were also downregulated. However, in myd88 and tlr2 mutants, no changes in the expression of AP-1-related genes were detected. Notably, previous studies have shown that TIRAP brings PKCδ and p38 in close proximity to each other to form a complex and this complex is required for AP-1 mediated inflammatory responses[41]. Further studies are needed to identify a specific function of TIRAP also in mammalian inflammation responses related to AP-1 activation.
Except for AP-1, TLRs can also trigger activation of other transcription factors, such as cAMP responsive element binding protein (CREB)[42]. Our results show that the creb1a gene is only downregulated in the tirap mutant larvae. Interestingly, TIRAP was shown in a previous study to function as a key upstream regulator of CREB to control both pro- and anti-inflammatory gene expression[43]. In addition, it was found that CREB mediated CACNA1S expression in the context of Mycobacterium tuberculosis (Mtb) infection, which is involved in the regulation of calcium homeostasis[44]. In the tirap mutant, we found several DEGs related to calcium channels regulation. In contrast only one different calcium channel protein was differentially expressed in the myd88 mutant whereas no calcium channel genes were differentially expressed in the tlr2 mutant (Table 1A). This suggests that Tirap has specific roles in maintaining calcium homeostasis.
Although the tirap and myd88 mutations do not affect the growth of zebrafish larvae (Figure 1)[24], we show that both the tirap-/- versus tirap+/+ group and myd88-/- versus myd88+/+ group showed several differentially expressed genes associated with the glycolysis and gluconeogenesis and TCA cycle. In the tirap mutant, we found the up-regulation of the pfkla gene encoding a phosphofructokinase and pgk1 encoding a phosphoglycerate kinase 1 and down-regulation of the gene pklr encoding a pyruvate kinase (Figure 3). This suggests that Tirap is involved in regulating the glycolytic pathway. However, in the myd88 mutant, only the gene fbp1b which encodes a fructose-1,6-bisphosphatase was found to be upregulated (Figure 4). In our previous study of the transcriptome of tlr2 mutant zebrafish larvae, the expression of the gpib and pfkma genes encoding a glucose-6-phosphate isomerase and phosphofructokinase, respectively, were downregulated[23]. These results indicate different roles of Tirap, Myd88 and Tlr2 in regulating glucose metabolism.
To further explore how the tirap mutant and myd88 mutant affect metabolism, we performed NMR spectroscopy for metabolomic analysis. In the tirap mutant, we found a decrease in the contents of proline, asparagine, serine and citrulline, and also a decrease in 2-hydroxyglutarate and glucose (Table 1B). However, the myd88 mutant was found to have an unaltered glucose level (Table 1B). Combined with the transcriptomic data, we speculate that the reduced glucose in the tirap mutant may result from the elevated expression of genes involved in the glycolytic process. In contrast, our published data about tlr2 mutant zebrafish larvae, showed that higher glucose levels were detected in the tlr2 mutant, which was associated with lower gene expression in glycolysis pathway[23]. These results suggest that Tirap and Tlr2 control glucose metabolism in a different way under unchallenged conditions.
Our transcriptional and metabolic results illustrate that the alterations triggered by the three mutants are quite different under unchallenged conditions, with the tirap mutation leading to a broader range of alterations of genes related the modulation of the inflammatory response. Therefore, we further explored the role of tirap in response to M. marinum infection. The higher bacterial burden detected in the tirap mutant shows a protective role of Tirap against mycobacterial infection. Previous studies have shown that tlr2 and myd88 mutations also lead to a higher bacterial burden using the same infection system[26, 45]. TLR signaling has been reported to play a role in host defense against mycobacteria, such as Mtb[46, 47]. However, the function of the tirap gene in mycobacterial infection has not been much studied. Surprisingly, in a mouse infection model of tuberculosis it was shown that Tirap heterozygous mouse mutants were more resistant to infection[48]. This negative function of Tirap was linked to a Cish-dependent acidification of macrophages. Considering that the function of Cish is tightly linked with T-cell functions[49], it is possible that the absence of adaptive immune components in the zebrafish larval model explains the difference with the results of the mouse infection model. However, the protective function of Tirap would not be surprising, considering its function in inflammation and metabolism in the unchallenged conditions. It has been shown that metabolic regulation such as the shift to glycolysis is an important component of host defense in a mouse Mtb model[50]. The switch to glycolysis in Mtb-exposed cells relies on TLR2 recognition and is partly dependent on the intracellular AKT-mTOR axis[51]. Our metabolomic data show that tirap has a regulatory role in glycolytic process, and transcriptomic data show that tirap mutation leads to an increased expression of several akt genes. It is therefore interesting to further study the function of Tirap during infection and inflammation in more detail in other model systems which also reflect adaptive immune components.
In this study, we demonstrated that mutation of the tirap gene does not affect the number of neutrophils and macrophages in vivo under the unchallenged condition (Figure 1). However, live imaging in a zebrafish larval tail wounding model revealed that the tirap mutant larvae recruited more neutrophils at the wounds (Figure 7). In our previous study on tlr2 and myd88 in TLR signaling affecting leukocyte migration in zebrafish, it was similarly found that tlr2 and myd88 mutation could affect the migratory behaviour of leukocytes towards the wounds[11]. However, in the present study we show that tirap has a very a different function in the wounding response compared to tlr2 and myd88. When tirap was absent, more neutrophils were recruited to the wound compared to the wild type control (Figure 7), whereas both tlr2 and myd88 mutant larvae recruited fewer neutrophils compared to their respective wild type controls (Figure 10, Table 1C). We can speculate that the deficiency of tirap may have resulted in impaired reverse migration resulting in a higher number of neutrophils at the wounds at later time points. Future studies using transgenic lines in which Tg(mpx:Dendra) reporters are crossed in our mutant will be essential to further study the function of tirap in reverse neutrophil migration. Based on our transcriptome and metabolome study, there are several possible explanations why the tirap mutant behaves very different than the myd88 and tlr2 mutants in wounding.
In the first place, the tirap mutant shows an effect on the transcription of many genes involved in calcium-dependent cytoskeletal regulation. In our results, up-regulation of calcium channels and myosin-related gene expression was specifically found in the tirap mutant. Calcium ions serve as the main signal, which induces activation of actin-myosin interactions not only in muscle cells but also in many other cell types[52-54]. Myosin activity has been shown to be an important component of defense responses in myeloid cells[55]. It has also been shown that reactive oxygen species play a negative role in Mtb-mediated CACNA1S expression. Since reactive oxygen species play a major role in cell migration during wounding[56]. The effect of the tirap mutation on expression of cacna1a might also be relevant in understanding its function in neutrophil migration. Therefore, the specific function of tirap in regulation of calcium-dependent myosin function could explain a higher speed of neutrophils migrating to the wounds in the tirap mutant.
Another explanation for the very different effect on cell migration in the tirap mutant as compared to myd88 and tlr2 mutants might be related to its different function in cytokine or chemokine regulation. Considering that the tirap mutant is unique in its down-regulation of cxcl12a, this relates to the various observations of the function of this chemokine in neutrophil responses to inflammation. Previous study shows increased neutrophil recruitment to wounds in cxcl12a mutant zebrafish larvae[57]. Therefore, our observation that the tirap mutant recruited more neutrophils to wounds may result from a down-regulation of cxcl12a. However, we have no clue yet as why tirap has no function in macrophage migration in contrast to the other mutants. We will need single cell analysis methods for further studies to obtain insights in the specific functions of Tirap in various cell types such as macrophages, neutrophils and T-cells.
Considering the specialized functions of tirap compared to myd88, it might be relevant to further study whether it can be used as a therapeutic target. Recent studies have demonstrated that the intervention in reverse migration may be a therapeutic target to facilitate resolution of neutrophil inflammation[22, 58]. Therefore, strategies to enhance tirap expression might be useful to combat hyper inflammatory responses. Yet, since there is still a great lack of knowledge of the function of TIRAP in organismal or cellular models, many further experiments are still needed to unravel the mechanism of interactions between tirap and its upstream and downstream partners in resolving inflammation.
5. Conclusions
From our study of zebrafish larvae with mutations in tirap and myd88, we conclude that Tirap has specialized roles in signaling, metabolic control and leukocyte migration upon wounding in zebrafish larvae. The transcriptome results indicate a specialized function in calcium homeostasis and myosin regulation under unchallenged conditions. The metabolomic studies show that tirap, like myd88 and tlr2, has a function in control of metabolism under unchallenged conditions. However, the metabolic function of tirap is very different from myd88 and tlr2. For instance, the tirap mutation leads to lower glucose levels, whereas a tlr2 mutation leads to higher glucose levels at the systems level. The results of the tail-wound model show that the tirap mutant larvae recruited significantly more neutrophils to the wounds than the wild type control, in contrast to myd88 and tlr2 mutants. This could be related to a specialized function in the regulation of calcium-channel dependent myosin-actin dynamics, or in the specific regulation of chemokine such as cxcr12a. The specialized function of tirap as an adapter of TLR signaling makes it an extractive target for therapeutic studies aimed at modulating neutrophil inflammatory responses.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
We would like to thank Guus van der Velden, Ulrike Nehrdich and Natasha Montiadi for helping us take care of adult zebrafish.
Funding
L. Liu is funded by China Scholarship Council.
Author contributions
Initialization of study, H.P.S.; Conceptualization, H.P.S. and W.H.; sample collection, W.H. and L.L.; RNAseq analysis, L.L. and W.H.; NMR analysis, L.L.; tail-wounding experiment, W.H. and F.D.K.; infection experiment, W.H.; qRT-PCR validation, L.L.; writing—original draft preparation, L.L., H.P.S. and W.H.; writing—review and editing, H.P.S. and W.H.; supervision, H.P.S.; All authors have read and agreed to the published version of the manuscript.
Institutional review board statement
The culture of zebrafish with mutations in immune genes was approved by the local animal welfare committee (DEC) of the University of Leiden (protocol 14,198). All protocols adhered to the international guidelines specified by the EU Animal Protection Directive 2010/63/EU.
Data availability statement
The RNAseq data is under submission in the NCBI GEO database. The GEO accession number is GSE270453.
Competing Interests
The authors have declared that no competing interest exists.
References
1. de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016;16:378-91
2. Hato T, Dagher PC. How the Innate Immune System Senses Trouble and Causes Trouble. Clin J Am Soc Nephrol. 2015;10:1459-69
3. Vijay K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int Immunopharmacol. 2018;59:391-412
4. Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T. et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324-9
5. Lannoy V, Cote-Biron A, Asselin C, Rivard N. TIRAP, TRAM, and Toll-Like Receptors: The Untold Story. Mediators Inflamm. 2023;2023:2899271
6. Sampaio NG, Kocan M, Schofield L, Pfleger KDG, Eriksson EM. Investigation of interactions between TLR2, MyD88 and TIRAP by bioluminescence resonance energy transfer is hampered by artefacts of protein overexpression. PLoS One. 2018;13:e0202408
7. Bernard NJ, O'Neill LA. Mal, more than a bridge to MyD88. IUBMB Life. 2013;65:777-86
8. Rajpoot S, Wary KK, Ibbott R, Liu D, Saqib U, Thurston TLM. et al. TIRAP in the Mechanism of Inflammation. Front Immunol. 2021;12:697588
9. Filep JG. Leukocytes in Inflammation, Resolution of Inflammation, Autoimmune Diseases and Cancer. Cells. 2021;10:1735
10. Li L, Yan B, Shi YQ, Zhang WQ, Wen ZL. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J Biol Chem. 2012;287:25353-60
11. Hu W, van Steijn L, Li C, Verbeek FJ, Cao L, Merks RMH. et al. A Novel Function of TLR2 and MyD88 in the Regulation of Leukocyte Cell Migration Behavior During Wounding in Zebrafish Larvae. Front Cell Dev Biol. 2021;9:624571
12. Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies C A, Mansell AS, Brady G, Brint E. et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78-83
13. Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329-33
14. Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ. et al. Toll-IL-1 receptor domain-containing adaptor protein is critical for early lung immune responses against Escherichia coli lipopolysaccharide and viable Escherichia coli. J Immunol. 2005;175:7484-95
15. Bernard NJ, Finlay CM, Tannahill GM, Cassidy JP, O'Neill LA, Mills KH. A critical role for the TLR signaling adapter Mal in alveolar macrophage-mediated protection against Bordetella pertussis. Mucosal Immunol. 2015;8:982-92
16. Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ. et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7:148-55
17. Putranto EW, Murata H, Yamamoto K, Kataoka K, Yamada H, Futami J. et al. Inhibition of RAGE signaling through the intracellular delivery of inhibitor peptides by PEI cationization. Int J Mol Med. 2013;32:938-44
18. Sakaguchi M, Murata H, Yamamoto K, Ono T, Sakaguchi Y, Motoyama A. et al. TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS One. 2011;6:e23132
19. Belhaouane I, Hoffmann E, Chamaillard M, Brodin P, Machelart A. Paradoxical Roles of the MAL/Tirap Adaptor in Pathologies. Front Immunol. 2020;11:569127
20. Meijer AH, Spaink HP. Host-Pathogen Interactions Made Transparent with the Zebrafish Model. Curr Drug Targets. 2011;12:1000-17
21. Renshaw SA, Loynes CA, Trushell DM, Elworthy S, Ingham PW, Whyte MK. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108:3976-8
22. Powell D, Tauzin S, Hind LE, Deng Q, Beebe DJ, Huttenlocher A. Chemokine Signaling and the Regulation of Bidirectional Leukocyte Migration in Interstitial Tissues. Cell Rep. 2017;19:1572-85
23. Hu W, Liu L, Forn-Cuni G, Ding Y, Alia A, Spaink HP. Transcriptomic and Metabolomic Studies Reveal That Toll-like Receptor 2 Has a Role in Glucose-Related Metabolism in Unchallenged Zebrafish Larvae (Danio rerio). Biology (Basel). 2023;12:323
24. van der Vaart M, van Soest JJ, Spaink HP, Meijer AH. Functional analysis of a zebrafish myd88 mutant identifies key transcriptional components of the innate immune system. Dis Model Mech. 2013;6:841-54
25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-8
26. Hu W, Yang S, Shimada Y, Munch M, Marin-Juez R, Meijer AH. et al. Infection and RNA-seq analysis of a zebrafish tlr2 mutant shows a broad function of this toll-like receptor in transcriptional and metabolic control and defense to Mycobacterium marinum infection. BMC Genomics. 2019;20:878
27. Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14:417-9
28. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550
29. Zhu A, Ibrahim JG, Love MI. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics. 2019;35:2084-92
30. Ding Y, Haks MC, Forn-Cuni G, He J, Nowik N, Harms AC. et al. Metabolomic and transcriptomic profiling of adult mice and larval zebrafish leptin mutants reveal a common pattern of changes in metabolites and signaling pathways. Cell Biosci. 2021;11:126
31. Ding Y, Haks MC, van den Eeden SJF, Ottenhoff THM, Harms AC, Hankemeier T. et al. Leptin mutation and mycobacterial infection lead non-synergistically to a similar metabolic syndrome. Metabolomics. 2022;18:67
32. Benard EL, van der Sar AM, Ellett F, Lieschke GJ, Spaink HP. Infection of Zebrafish Embryos with Intracellular Bacterial Pathogens. Journal of visualized experiments: JoVE. 2012;61:3781
33. Stirling DR, Suleyman O, Gil E, Elks PM, Torraca V, Noursadeghi M. et al. Analysis tools to quantify dissemination of pathology in zebrafish larvae. Sci Rep. 2020;10:3149
34. Santos-Sierra S, Deshmukh SD, Kalnitski J, Kuenzi P, Wymann MP, Golenbock DT. et al. Mal connects TLR2 to PI3Kinase activation and phagocyte polarization. EMBO J. 2009;28:2018-27
35. Luan Y, Tang N, Yang J, Liu S, Cheng C, Wang Y. et al. Deficiency of ribosomal proteins reshapes the transcriptional and translational landscape in human cells. Nucleic Acids Res. 2022;50:6601-17
36. Caterino M, Corbo C, Imperlini E, Armiraglio M, Pavesi E, Aspesi A. et al. Differential proteomic analysis in human cells subjected to ribosomal stress. Proteomics. 2013;13:1220-7
37. Poddar D, Basu A, Baldwin WM 3rd, Kondratov RV, Barik S, Mazumder B. An extraribosomal function of ribosomal protein L13a in macrophages resolves inflammation. J Immunol. 2013;190:3600-12
38. Basu A, Poddar D, Robinet P, Smith JD, Febbraio M, Baldwin WM 3rd. et al. Ribosomal protein L13a deficiency in macrophages promotes atherosclerosis by limiting translation control-dependent retardation of inflammation. Arterioscler Thromb Vasc Biol. 2014;34:533-42
39. Slomiany BL, Slomiany A. Involvement of p38 MAPK-dependent activator protein (AP-1) activation in modulation of gastric mucosal inflammatory responses to Helicobacter pylori by ghrelin. Inflammopharmacology. 2013;21:67-78
40. Guo RM, Xu WM, Lin JC, Mo LQ, Hua XX, Chen PX. et al. Activation of the p38 MAPK/NF-kappaB pathway contributes to doxorubicin-induced inflammation and cytotoxicity in H9c2 cardiac cells. Mol Med Rep. 2013;8:603-8
41. Baig MS, Liu D, Muthu K, Roy A, Saqib U, Naim A. et al. Heterotrimeric complex of p38 MAPK, PKCdelta, and TIRAP is required for AP1 mediated inflammatory response. Int Immunopharmacol. 2017;48:211-8
42. Sanin DE, Prendergast CT, Mountford AP. IL-10 Production in Macrophages Is Regulated by a TLR-Driven CREB-Mediated Mechanism That Is Linked to Genes Involved in Cell Metabolism. J Immunol. 2015;195:1218-32
43. Mellett M, Atzei P, Jackson R, O'Neill LA, Moynagh PN. Mal mediates TLR-induced activation of CREB and expression of IL-10. J Immunol. 2011;186:4925-35
44. Antony C, Mehto S, Tiwari BK, Singh Y, Natarajan K. Regulation of L-type Voltage Gated Calcium Channel CACNA1S in Macrophages upon Mycobacterium tuberculosis Infection. PLoS One. 2015;10:e0124263
45. Hosseini R, Lamers GEM, Bos E, Hogendoorn PCW, Koster AJ, Meijer AH. et al. The adapter protein Myd88 plays an important role in limiting mycobacterial growth in a zebrafish model for tuberculosis. Virchows Arch. 2021;479:265-75
46. Reiling N, Holscher C, Fehrenbach A, Kroger S, Kirschning CJ, Goyert S. et al. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol. 2002;169:3480-4
47. Drennan MB, Nicolle D, Quesniaux VJ, Jacobs M, Allie N, Mpagi J. et al. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol. 2004;164:49-57
48. Belhaouane I, Pochet A, Chatagnon J, Hoffmann E, Queval CJ, Deboosere N. et al. Tirap controls Mycobacterium tuberculosis phagosomal acidification. PLoS Pathog. 2023;19:e1011192
49. Sobah ML, Liongue C, Ward AC. SOCS Proteins in Immunity, Inflammatory Diseases, and Immune-Related Cancer. Front Med (Lausanne). 2021;8:727987
50. Gleeson LE, Sheedy FJ, Palsson-McDermott EM, Triglia D, O'Leary SM, O'Sullivan MP. et al. Cutting Edge: Mycobacterium tuberculosis Induces Aerobic Glycolysis in Human Alveolar Macrophages That Is Required for Control of Intracellular Bacillary Replication. J Immunol. 2016;196:2444-9
51. Lachmandas E, Beigier-Bompadre M, Cheng SC, Kumar V, van Laarhoven A, Wang X. et al. Rewiring cellular metabolism via the AKT/mTOR pathway contributes to host defence against Mycobacterium tuberculosis in human and murine cells. Eur J Immunol. 2016;46:2574-86
52. Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol. 2007;176:573-80
53. Doyle AD, Kutys ML, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Micro-environmental control of cell migration-myosin IIA is required for efficient migration in fibrillar environments through control of cell adhesion dynamics. J Cell Sci. 2012;125:2244-56
54. Bera K, Kiepas A, Godet I, Li Y, Mehta P, Ifemembi B. et al. Extracellular fluid viscosity enhances cell migration and cancer dissemination. Nature. 2022;611:365-73
55. Pillon M, Doublet P. Myosins, an Underestimated Player in the Infectious Cycle of Pathogenic Bacteria. Int J Mol Sci. 2021;22:615
56. Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126-67
57. Paredes-Zuniga S, Morales RA, Munoz-Sanchez S, Munoz-Montecinos C, Parada M, Tapia K. et al. CXCL12a/CXCR4b acts to retain neutrophils in caudal hematopoietic tissue and to antagonize recruitment to an injury site in the zebrafish larva. Immunogenetics. 2017;69:341-9
58. Robertson AL, Holmes GR, Bojarczuk AN, Burgon J, Loynes CA, Chimen M. et al. A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Sci Transl Med. 2014;6:225ra29
59. Luo L, Lucas RM, Liu L, Stow JL. Signalling, sorting and scaffolding adaptors for Toll-like receptors. J Cell Sci. 2019;133:jcs239194
60. Chen HG, Han WJ, Deng M, Qin J, Yuan D, Liu JP. et al. Transcriptional regulation of PP2A-A alpha is mediated by multiple factors including AP-2alpha, CREB, ETS-1, and SP-1. PLoS One. 2009;4:e7019
Author contact
![]() Corresponding authors: w.huleidenuniv.nl (W.H.); h.p.spainkleidenuniv.nl (H.P.S.).
Corresponding authors: w.huleidenuniv.nl (W.H.); h.p.spainkleidenuniv.nl (H.P.S.).

 Global reach, higher impact
Global reach, higher impact