10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2025; 21(3):1242-1258. doi:10.7150/ijbs.108858 This issue Cite
Review
Updated review of research on the role of the gut microbiota and microbiota-derived metabolites in acute pancreatitis progression and inflammation-targeted therapy
1. Department of Gastroenterology, Affiliated Hangzhou First People's Hospital, Westlake University School of Medicine, Hangzhou 310058, China.
2. Key Laboratory of Integrated Traditional Chinese and Western Medicine for Biliary and Pancreatic Diseases of Zhejiang Province, Hangzhou 310006, China.
3. Hangzhou Institute of Digestive Diseases, Hangzhou, Hangzhou 310006, China.
4. Zhejiang University School of Medicine, Hangzhou 310058, China.
# Authors contributed equally
Received 2024-12-16; Accepted 2025-1-10; Published 2025-1-20
Abstract
Acute pancreatitis (AP) is characterized by autodigestion of the pancreas, and some patients may rapidly progress to systemic inflammation, pancreatic necrosis, and multi-organ failure. Numerous studies have detailed the bidirectional communication networks between the pancreas and the intestinal microbiota, as well as its metabolites. Such crosstalk affects the progression of AP and recovery through intestinal barrier disruption. Furthermore, advances in experimental research and clinical studies have indicated that gut microorganisms exhibit distinct alterations in response to different levels of severity and etiologies of AP. This information has greatly expanded our knowledge of the role of the gut microflora and microbial metabolites in the pathology of disease and has reinforced the basis of therapeutic approaches that target candidate intestinal microbiota. In this review, we aim to provide an overview of the composition and diversity of the gut microbial community, to highlight the candidate bacteria and microbiota-derived metabolites responsible for AP, and to elucidate their interactions with and regulation of immune-relevant receptors in intestinal epithelial cells (IECs) in the host. Future research should focus on identifying and characterizing AP-associated bacterial strains, elucidating their distinct pathogenic mechanisms across different etiologies and stages of AP, and leveraging these insights to develop preventive and therapeutic strategies.
Keywords: intestinal microbiota dysbiosis, intestinal barrier integrity, immunomodulation, crosstalk, therapeutics, immune homeostasis
1. Introduction
Acute pancreatitis (AP) is a serious inflammatory disease with multiple complications and a high fatality rate, with an annual incidence of 34 cases per 100,000 people worldwide [1]. Nearly 80% of patients are diagnosed with mild AP (MAP), which is self-limiting for two weeks with minimal supportive caret; however, patients with severe AP (SAP) experience rapid aggravation, with a high mortality rate of nearly 30% [2]. However, the pathophysiological mechanisms underlying SAP remain elusive, and precise pharmacological therapeutic strategies with reliable and stable efficacy for the treatment of SAP are lacking. Currently, accumulating evidence suggests that the diversity and composition of the gut microbial ecosystem are strongly associated with the initiation, progression and treatment response of SAP [3, 4]. Numerous studies with diverse approaches have investigated reversing microbiota dysbiosis; these strategies have favorable therapeutic outcomes in AP patients [5, 6].
The early stage of SAP induces gut microbiota dysbiosis and destruction of the intestinal barrier, which in turn exacerbates the severity of AP in the advanced stage [7-9]. Gut microorganisms are involved in various immunopathological mechanisms and impact gut immune homeostasis by regulating the production of metabolites and interacting closely with immune-related receptors and transporters, which are also present in the gut [10]. However, the impact of gut bacterial dysbiosis and dysfunction on SAP, as well as the role of specific intestinal bacterial species and their metabolites in the etiology of SAP, remains largely unclear. In this review, we systematically describe the distinct spectrum and alterations of SAP-related intestinal microbiota (excluding viruses, bacteriophages, fungi and parasites) and microbiota-derived metabolites to explore potential therapeutic strategies related to reversing the intestinal microbiota dysbiosis.
2. Roles of the intestinal microbiome and metabolome in the pathogenesis, disease progression, and recovery of AP
Because of the connections among the pancreas, the duodenal papilla via the pancreatic duct, intestinal dysmotility, ischemia‒reperfusion and oxidative stress during the aggravation of AP, enterobacterial dysbiosis and translocation are thought to promote secondary infection and sepsis in AP patients and increase the risk of multiple organ failure (MOF), mortality, and the length of hospital stay [6]. Further studies on the role of microorganisms in AP progression have shown that infected pancreatic necrosis originates from the translocation of commensal gut bacteria into the pancreas and the pathogen-associated molecular patterns (PAMPs) of AP-associated tissue injury via the binding of intestinal bacteria with pattern recognition receptors, which aggravate symptoms [11, 12]. These studies indicate that gut bacteria play important roles in the progression of SAP; thus, revealing the role and mechanism of microbial targets and their metabolites in different stages and etiologies of SAP is necessary.
2.1 Bacterial taxa associated with AP in animal studies
Various studies with diverse approaches, such as those that utilize antibiotic intervention [13], germ-free mice [14], fecal microbiota transplantation (FMT) [15], and the genetic modification of mice [16], have been conducted to investigate the effectiveness of targeting the gut microflora in the treatment of inflammatory diseases, including AP. Although many microbial alterations have been identified by comparing microbiomes between patients with AP progression and normal controls, there is no consensus on the causal relationship between the gut microbiota and AP. To eliminate concerns about potential false-positive results and gain a comprehensive spectrum of the intestinal microbiome in AP, we focused primarily on the microbiota that underwent significant changes and consistently impacted AP in at least two independent metagenomic datasets from animal and clinical studies. Through this research, links between fourteen commensal bacteria and the progression of AP in animal models were established (Table 1).
Among the fourteen AP-relevant flora, the abundances of Escherichia-Shigella, Enterococcus, Proteobacteria, and Firmicutes were increased in AP patients, and the Prevotella, Bacteroides, Bifidobacterium, Lachnospiraceae, Muribaculaceae, Parabacteroides, Helicobacter, Lactobacillus, Akkermansia, and Actinobacillus were decreased, reflecting their consistent effectiveness in mediating AP progression [16-22]. Escherichia-Shigella and Enterococcus are the most widely reported opportunistic pathogens associated with inflammation and intestinal barrier disruption in AP [23, 24]. Several studies reported that the abundance of Escherichia-Shigella in AP patients was approximately 50- and 100-fold greater than that in healthy controls, which was strongly correlated with local or systematic inflammation, the invasion of intestinal epithelial cells (IECs) and the exacerbation of AP [4, 15]. Interestingly, dysbiosis of the gut microbiota, triggered by Escherichia-Shigella or Enterococcus, stimulates massive differentiation of Tregs, causing to a Treg/Th17 imbalance and inhibiting Teffs and IELs, which provides an opportunity for secondary pancreatic necrotic tissue infections [12, 25]. The antioxidant activity of chitosan oligosaccharides can significantly reduce the number of Escherichia-Shigella and Enterococcus bacteria to alleviate the progression of AP [26]. An increased abundance of Escherichia-Shigella in acute necrotizing pancreatitis (ANP) patients augments the endoplasmic reticulum stress response and disrupts intestinal tight junctions (TJs) through activation of the TLR4/MyD88/p38/NF-κB signaling pathway [27]. In addition, high levels of Escherichia-Shigella in the gut increase the serum levels of proinflammatory cytokines and aggravate intestinal permeability. Interestingly, Enterococcus was once considered to have low virulence, while it was strongly associated with new/persistent organ failure and increased mortality in ANP [28]. Moreover, vancomycin-resistant Enterococcus strains isolated from the feces of SAP patients presented a high degree of homology with peripancreatic effusion samples, indicating the occurrence of parenteral transmission [29, 30]. A recent study revealed that Enterococcus secretes protease gelatinase, which mediates virulence through regulating host tissue degradation and the immune response [31]. Therefore, we hypothesized that the transfer of Enterococcus from the intestinal tract to damaged tissues and systemic circulation is a causal factor in the aggravation of AP.
Representative animal studies demonstrating the effectiveness and mechanism of candidate gut bacteria in AP progression
| Tested sample | AP-related Bacteria | Rodent strains | Methods of AP model establishment | Sequencing methods | Main finding | Ref | |
|---|---|---|---|---|---|---|---|
| Fecal | (↑): Escherichia-Shigella, Enterococcus, Proteobacteria (↓): Prevotella, Bacteroides, Bifidobacterium, Lachnospiraceae | Male C57BL/6 mouse (n = 40) | Intraperitoneally, 100 μg/kg body weight, 10 times at 1h intervals, administrate with LPS (10 mg/kg) after final injection of caerulein. | 16S rRNA sequencing | The disturbed microbiota was closely correlated with AP severity; the antibiotic-treated mice and germ-free mice exhibited alleviated pancreatic injury after AP induction and FMT in turn exacerbated the disease. | [4] | |
| Fecal | (↑): Escherichia-Shigella, Enterococcus, Firmicutes (↓): Muribaculaceae, Bacteroides | Male C57BL/6 mouse (n = 28) | Intraperitoneally, 100 μg/kg body weight, 10 times at 1h intervals, and administrate with LPS (5 mg/kg) after final injection of caerulein. | Administration with chitosan oligosaccharides alleviated SAP by suppressed the oxidative stress and restoring intestinal homeostasis in the pancreas and ileums. | [26] | ||
| Fecal | (↑): Firmicutes (↓): Bacteroides, Parabacteroides | C57BL/6 mouse (n = 10) Hpa-Tg mouse (n = 10) | Intraperitoneally, 50 μg/kg body weight, 8 times at 1h intervals. | Parabacteroides produced acetate to alleviate AP by reducing neutrophil infiltration. | [10] | ||
| Fecal | (↓): Helicobacter, Lachnospiraceae | C57BL/6 mouse (n = 42) rIL-22 mice (n = 12) | 5% Taurocholic acid induced AP model: 10 μl/mice, 5μl/min, and the bile duct was occluded by noninvasive microslip. | Early stage decreased colonic IL-22 aggravates intestinal mucosal barrier dysfunction and microbiota dysbiosis in SAP. | [22] | ||
| Fecal | (↓): Muribaculacea, Lactobacillus | Male C57BL/6 mouse (n = 42) TLR4ΔIEC mouse (n = 12). | 8% L-arginine induced AP model: intraperitoneally, 4.0 g/kg body weight, 2 times at 1h intervals. | Loss of intestinal epithelial TLR4 exacerbated pancreatic and intestinal damage during AP via gut microbiota dysbiosis; L. reuteri maintained intestinal homeostasis via Paneth cells modulation. | [16] | ||
| Fecal | (↑): Escherichia-Shigella, Enterococcus (↓): Lachnospiraceae | C57BL/6 mouse (n = 34) DEREG moues (n = 16) | Intraperitoneally, 50 μg/kg body weight, 1 time, and partial pancreatic duct ligation. | Treg-activation disturbed the duodenal barrier function and permited translocation of commensal bacteria into pancreatic necrosis. | [12] | ||
| Duodenal mucosa | (↓): Prevotella, Actinobacillus | C57BL/6 mouse (n = 150) | 1) MAP: intraperitoneally, Caerulein, 25 μg/kg body weight, 10 times at 1h intervals; 2) SAP: 8% L-arginine, 4.5 g/kg body weight, 2 times at 2h intervals. | MAP mild duodenal barrier dysfunction and slight change in duodenal mucosal microbiota. | [17] | ||
| Fecal | (↑): Escherichia-Shigella, Enterococcu (↓): Prevotella, Akkermansia, Bacteroide, Parabacteroides | C57BL/6 mouse (n = 28) | Intraperitoneally, 100 μg/kg body weight, 10 times at 1h intervals, and administrate with LPS (10 mg/kg) after final injection of caerulein. | AMPK/NF-κB/NLRP3 pathway mediated by SCFAs along the gut-lung axis play an essential role in preventing the pathogenesis of SAP. | [14] | ||
| Fecal | (↑): Firmicutes (↓): Bacteroides, Actinobacillus | Male C57BL/6 mouse (n = 12) | Intraperitoneally, 100 μg/kg body weight, 7 times at 1h intervals, and administrate with LPS (10 mg/kg) after final injection of caerulein. | Metagenomics | Identified the relationship between the gut microbiome and metabolite levels during AP. | [45] | |
| Fecal | (↑): Escherichia-Shigella (↓): Lactobacillus, Lachnospiraceae | C57BL/6 mouse (n = 20) Rftn1 deficient mouse (n = 20) | 5% Taurocholic acid induced AP model: 0.1ml/100g body weight, retrograde injection. | 16S rDNA sequencing | Inhibiting M1 macrophages activation, reduce pancreatic lesions and reduce intestinal damage | [19] | |
| Fecal | (↑): Enterococcus (↓): Akkermansia, Bifidobacterium | C57BL/6 mouse (n = 42) TLR4 deficient mouse (n = 12) Nlrp3 deficient mouse (n = 12) Myd88 deficient mouse (n = 12) | 1) MAP: intraperitoneally, 25 μg/kg body weight, 7 times at 1h intervals; 2) SAP: intraperitoneally, 25 μg/kg body weight, 10 times at 1h intervals, and administrate with LPS (7.5 mg/kg) after final injection of caerulein. | B. animalis relieved macrophage-associated local and systemic inflammation of AP in a TLR4/MyD88- and NLRP3/Caspase1-dependent manner through its metabolite lactate. | [20] | ||
| Fecal | (↑): Helicobacter (↓): Muribaculaceae | Sprague-Dawley rat (n = 64) | 3.5% Taurocholic acid induced AP model: 0.1ml/100g body weight, retrograde injection. | Qingyi granules modulated the gut microbiota and metabolic abnormalities to ameliorate SAP. | [5] |
Notes: 1) Hpa-Tg mice: Heparanase transgenic mice; 2) rIL-22 mice: Administration of recombinant IL-22 mice; 3) TLR4ΔIEC mice: Intestinal epithelial TLR4 knockout mice; 4) MAP: mild acute pancreatitis; 5) SAP: severe acute pancreatitis.
Representative clinical human studies demonstrating the effectiveness and mechanism of candidate gut bacteria in AP progression
| Tested sample | AP-related bacteria | Subjects | Sequencing methods | Main finding | Ref |
|---|---|---|---|---|---|
| Duodenal mucosa | (↑): Streptococcus (↓): Prevotella | MAP patients (n = 16), Healthy controls (n = 16). | 16S rRNA sequencing | MAP patients with mild duodenal barrier dysfunction and slight changes in duodenal mucosal microbiota. | [17] |
| Fecal | (↑): Escherichia-Shigella, Enterococcus, Proteobacteria (↓): Blautia, Prevotella, Bacteroides, Faecalibacterium | MAP patients (n = 41), MSAP patients (n = 59), SAP patients (n = 30), Healthy controls (n = 35). | The disturbed microbiota was closely correlated with AP severity; the antibiotic-treated mice and germ-free mice exhibited alleviated pancreatic injury after AP induction and FMT in turn exacerbated the disease. | [4] | |
| Fecal | (↑): Escherichia-Shigella, Enterococcus (↓): Blautia, Bifidobacterium | ANP patients (n = 19), non-ANP patients (n = 39), Healthy control (n = 20). | Healthy, ANP and non-ANP patients presented distinct gut microorganism composition | [21] | |
| Fecal | (↑): Escherichia-Shigella, Enterococcus, Proteobacteria, Blautia, Bifidobacterium, Ruminococcus | AP patients (n = 65), Healthy controls (n = 20). | Healthy, AP-ARDS and AP-nonARDS patients presented distinct gut microorganism composition and functions. | [37] | |
| Fecal | (↑): Enterococcus (↓): Blautia, Ruminococcus, Faecalibacterium | MAP patients (n = 43), MSAP patients (n = 9), SAP patients (n = 2), Healthy controls (n = 46). | 16S rDNA sequencing | AP patients with lower gut microbiome diversity and a higher abundance of sulfidogenic bacteria. | [18] |
Note: 1) AP: Acute pancreatitis; 2) MAP: Mild acute pancreatitis; 3) SAP: Severe acute pancreatitis;4) HTGP: Hypertriglyceridemia-induced acute pancreatitis; 5) AP-ARDS: Acute pancreatitis patients with acute respiratory distress syndrome; 6) NETs: neutrophil extracellular traps.
Enteral nutrition and probiotics are thought to significantly modulate inflammation in both mice and rats, indicating that the gut microbiota serves as a mediator of these therapeutic effects. Tang et al. reported that colonization with Bifidobacteria, particularly B. animalis, and the administration of its metabolite lactate can alleviate inflammation and improve the survival rate, thereby highlighting the potential therapeutic value of modulating the commensal gut flora and microbiota-derived metabolites [20]. Additionally, Li et al. reported that a decreased abundance of endogenous Bacteroides in the gut microbiota impaired taurine production and increased inflammatory factor release in the colon, which triggered neutrophil extracellular trap formation [13]. Furthermore, the abundance of the core gut microbiota Lachnospiraceae [32], which are the main producers of short-chain fatty acids (SCFAs), was significantly decreased in patients with ANP and SAP [22]. Qingyi decoction, a commonly utilized traditional Chinese medicine for the treatment of SAP, can alleviate inflammation by increasing the abundance of SCFA-producing bacteria, such as Bacteroides [14]. However, the changes in the abundances of several bacteria are still controversial. For example, the relative abundance of Lactobacillus, which is extensively used in a variety of commercial products [33], is strongly associated with the amelioration of pancreatic and intestinal damage [16, 19]. Beneficial bacteria (e.g., Lactobacillus reuteri and Bifidobacterium) contribute to the proliferation and renewal of intestinal stem cells and the restoration of TJs by activating the Wnt/β-catenin pathway; this activation eventually attenuates SAP-mediated disruption of intestinal homeostasis and the pancreatic inflammatory response [34-36]. Paradoxically, two independent studies indicated that the abundance of Lactobacillus was increased in duodenal mucosal biopsy samples from C57BL/6 mice [17] and feces from AP rats [5]. Hence, to elucidate the association between different severity or etiology of AP and alterations in intestinal microflora, it is imperative to conduct further studies using animal models, which will enable a detailed exploration of the dynamic changes in microflora throughout the progression of AP.
2.2 Bacterial taxa associated with AP in human studies
Compared with those in healthy donors, significant structural shifts in the gut microbiota of AP patients have also been observed, as well. Among the variations in microorganisms associated with AP, ten gut microflora with consistent effects on AP were identified, and for additional information on the effects of dysbiosis of candidate bacteria in AP patients, please refer to Table 2. Interestingly, six candidate bacteria for AP, namely Escherichia-Shigella, Proteobacteria, Enterococcus, Bacteroides, Bifidobacterium, and Prevotella, have been found to be closely related to disease progression in both AP patients and experimental models.
In addition to the common features of intestinal microbial dysbiosis in AP, patients have distinct views of intestinal metagenomics that are related to the different levels of severity and pathogenic factors of AP [37]. Alterations in the gut microbiota may contribute to the severity of AP. For example, patients with SAP presented higher levels of AP-enriched species than did patients with MAP or MSAP, and the degree of microbiota alterations revealed distinctive patterns among different etiologies [38]. Tan et al. categorized AP patients into two groups on the basis of the similarity of their gut microorganisms to those of healthy donors, and the dissimilar group had higher incidences of MOF, infection-related complications, and APACHE scores, which indicated that altered fecal microbial composition in patients is a pivotal mechanism that contributes to the progression from MAP to SAP [39]. In addition, SAP patients, but not MAP patients, presented increased abundances of Acinetobacter and Stenotrophomonas and decreased abundances of Acteroides, Bifidobacterium and Gemella [4, 18]. Furthermore, alcoholic AP increased the abundance of Actinobacteria and decreased the abundance of Bacteroidetes [40]. Additionally, compared with biliary AP patients, HTGP patients presented greater abundances of Escherichia-Shigella and Enterococcus, and the variation in these bacteria was correlated with the severity of disease and patient prognosis [41]. These findings indicate a strong association between disrupted gut microbiota and the varying severity and etiological factors of AP, although the causal relationship still warrants further investigation.
2.3 Animal and human metabolomics studies revealed that the inflammatory response to AP is precisely orchestrated by microbiota-derived metabolites
Alterations in the levels of intestinal microorganisms, as primary determinants of metabolite production, are typically accompanied by changes in metabolomic profiles, contributing to the modulation of the physiological effects on the host organism [42]. As shown in Table 3, we reviewed the differentially abundant metabolites in serum [43], pancreas [44] and fecal [45] metabolomics matrices from murine AP models; these results may elucidate the relationships between metabolites and AP.
SCFAs produced by anaerobic bacteria, such as Bifidobacterium and Lactobacillus, act as "peacekeepers" in AP by maintaining TJs proteins in IECs, suppressing the production of proinflammatory factors [46], regulating commensal resistance 3, and restoring intestinal barrier function [47]. Lactulose effectively alleviated the inflammatory response in MSAP patients by restoring intestinal function and increasing the production of SCFAs [48]. In agreement with the clinical metabolome data, both oral and systemic butyrate supplementation decreased bacterial dissemination and reversed the microbiota alterations in the ANP model [15]. Butyrate, a core metabolite produced by Clostridium, drives the differentiation of monocytes into macrophages by inhibiting histone deacetylase 3 activity to augment the antimicrobial barrier in the host [49]. SCFAs inhibited the expression of HDAC and NF-κB in innate immune cells and activated PPAR-γ, leading to increased expression of ILC3s and the secretion of anti-inflammatory factors such as IL-10, IL-22, and TGF-β. SCFAs influence T and B cells through interactions with GRP41, GRP43, and GRP109A; stimulate the expression of mTOR, Foxp3, and Ahr; reduce the numbers of Th1 and Th17 cells; and thereby preserving homeostasis and mitigating inflammation [50, 51]. Additionally, SCFAs trigger the generation of intestinal TJs, such as ZO-1 and claudin-3, by activating the PI3K/AKT/mTOR pathway in IECs, which results in increased secretion of RegIIIγ and β-defensins to improve the intestinal barrier [52, 53]. Although increasing evidence has demonstrated that SCFAs exert beneficial effects on multiple diseases, including ameliorating AP, a recent study revealed that all abundant species in SAP belong to SCFA-producing taxonomic families, which might provide an explanation for the failure of SCFA-containing probiotics in SAP [54]. Thus, further studies are needed to fully understand the dynamics and functions of SCFA-producing species, perform targeted metabolomics, and explore the effects of probiotic interventions in AP [55].
Representative studies demonstrating therapeutic effects of probiotics in AP
| Probiotics | Subjects or Rodent strains | Treatments | Main findings | Ref |
|---|---|---|---|---|
| B.animalis | C57BL/6 mouse, TLR4 deficient mouse, Nlrp3 deficient mouse, Myd88 deficient mouse (n = 92) | Oral gavage, 1 × 1010 CFUs | B. animalis relieved macrophage-associated local and systemic inflammation of AP in a TLR4/MyD88 and NLRP3/Caspase1 dependent manner through its metabolite lactate. | [20] |
| plantarum B7/ L. rhamnosus L34 and L. paracasei B13 | Male ICR mouse (n = 24) | Oral gavage 6 days, 1 × 108 CFUs | Reducing inflammation and restoring the maintenance of intestinal integrity | [117] |
| L. plantarum FLPL05 | C57BL/6 mouse (n = 20) | Oral gavage, 1 × 107 CFUs | Partially reversing pancreatitis | [135] |
| Lactobacillus mixture | Male SPF Sprague-Dawley rats (n = 24) | Oral gavage, 4.5 × 109 CFUs | Exhibiting potent antioxidant and anti-inflammatory effects | [136] |
| Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus salivarius, Lactococcus lactis, Bifidobacterium bifidum, Bifidobacterium lactis | Predicted SAP patients (n = 152) | Enteral administration twice a day for 28 days, 1×1010 CFUs | Infectious complications, number of deaths, the risk of bowel ischemia occurred higher; Increasing the risk of infectious complications and risk of mortality | [121] |
| B. bifidum, B. acidophilus, E. faecalis, B. cereus | SAP patients (n = 120) | Enteral nutrition | Reducing the incidence of multiple organ dysfunction syndrome; shortening the duration of abdominal pain and hospital stay; the death rate was not significantly changed | [120] |
Note: a) ICR: Institute of Cancer Research; b) CFU: Colony-Forming Units; c) SPF: Specific pathogen-free.
In addition, tryptophan metabolites, which are specific ligands that bind to the aryl hydrocarbon receptor (AhR), stimulate the expression of IL-22, which further induces AhR activation and the formation of a positive feedback loop to ameliorate pancreatic injury and promote IEC homeostasis [56]. Lactobacillus enhances tryptophan metabolism by increasing the production of AhR ligands, resulting in the promotion of intestinal barrier functions and increased secretion of incretin hormone [57]. Notably, there is crosstalk between arginine metabolism and tryptophan metabolism. Lactobacillus promotes the synthesis of L-ornithine by promoting arginine metabolism, and this metabolite facilitates the activation of tryptophan metabolism in IECs, leads to the production of the AhR ligand L-kynurenine, and induces RORγt+IL-22+ILC3 cell differentiation [58]. Moreover, supplementation with tryptophan metabolites to restore the level of tryptophan metabolism in AP reduces the severity of AP via anti-inflammatory effects and a reduction in macrophage and neutrophil infiltration [19, 59].
Numerous studies have shown that intestinal bacterial and microbiota-derived metabolites are involved in functional and intestinal barrier integrity, but their exact mechanism in AP is still unclear. Hence, given the distinctive nature of intestinal bacterial and microbiota-derived metabolites and their unique functional roles, delving deeper into their potential mechanisms and contributions is imperative.
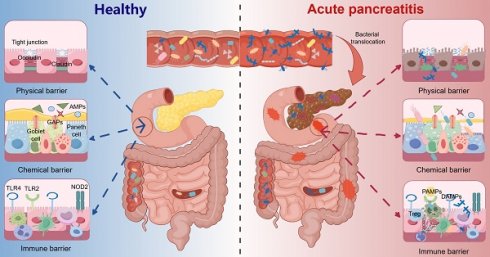
3. The severity of AP is closely associated with intestinal barrier injury
3.1 Physical intestinal barrier integrity
The physical intestinal barrier is composed of a layer of IECs, including enterocytes, enteroendocrine cells (ECs), goblet cells (GCs), and Paneth cells (PCs), which play essential roles in mediating intestinal permeability; maintaining the equilibrium of the intestinal barrier; and impeding invasion by bacteria, viruses, and endotoxins [60]. The permeability of the pore pathway is regulated by claudin, which modulates the formation of channels or barriers to respond to distinct gut conditions at TJs (Figure 1).
Effects of intestinal bacteria and their metabolites on physical barriers. The intercellular gaps between IECs are blocked by TJs, AJs and desmosomes. TJs are primarily composed of ZO-1, claudins, and occludins. Pathogenic bacteria and their cytotoxic byproduct, LPS, suppress occludin expression and induce ZO-1 aggregation by activating the TLR4/MyD88/p38 signaling pathway, upregulating Cdkn1a, and triggering NF‒κB signaling, ultimately disrupting intercellular junctions. C. jejuni-secreted HtrA compromises the epithelial barrier by inhibiting Cldn8 expression, thereby increasing paracellular permeability. In addition, IL-1, IL-6, and IL-13 further increase paracellular permeability by promoting the expression of Cldn2. The invasion of Fusobacterium nucleatum and the secretion of TNF-α, IL-1β, and IFN-γ activate MLCK1, leading to occludin internalization via myosin light chain phosphorylation, which increases intestinal permeability and facilitates gut microbiota translocation. Divertin, a small molecule, can specifically bind to the IgCAM domain in MLCK1 to inhibit its function. Conversely, probiotics such as L. reuteri and Bifidobacterium activate the Wnt/β-catenin pathway, promoting the proliferation of intestinal stem cells and restoring intestinal TJs. (Cdkn1a: cyclin-dependent kinase inhibitor; IECs: intestinal epithelial cells; TJs: tight junction; AJs: adherens junction; LPS: lipopolysaccharide; ZO-1: ZO1 tight junction protein; MLCK1: myosin light chain kinase).
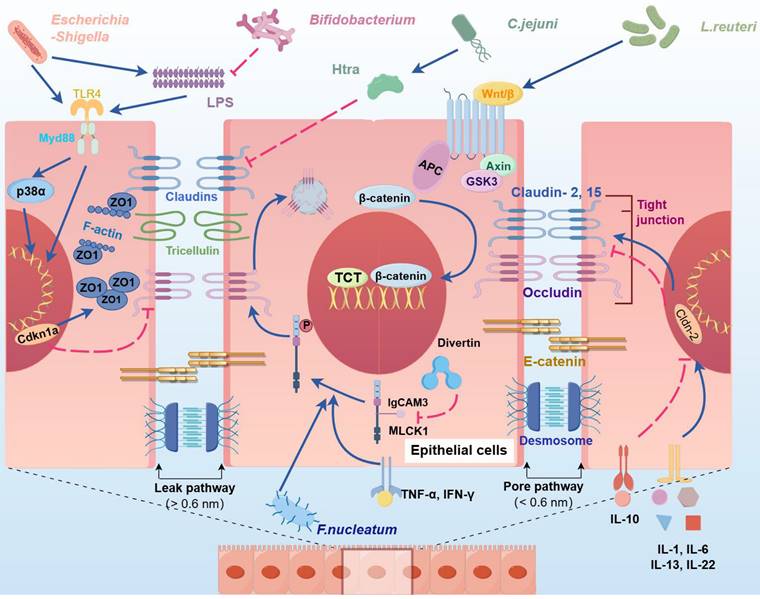
The physical barrier of the intestine is disrupted during AP aggravation, resulting in increased intestinal permeability and contributing to bacterial and toxin invasion and the translocation of bacteria from the bowel lumen to the portal circulation and mesenteric lymphatics [12, 17, 48]. The extensive proliferation of pathogenic bacteria suppresses the expression of TJ-related proteins through PAMPs, thereby mediating the translocation of harmful bacteria and exacerbating inflammatory responses [61]. For example, Campylobacter jejuni and Helicobacter pylori secrete a series of virulence factors, such as high-temperature requirement A (HtrA), to specifically inhibit claudin-8 in TJs; purified HtrA can cleave claudin-8 and occludin in vitro, thus promoting the paracellular translocation of the microorganism [62, 63]. Moreover, several bacteria secrete lipopolysaccharide (LPS) to inhibit the expression of ZO-1 and occludin in IECs, which can induce the aggregation of ZO-1 at cell junctions and alter its spatial distribution, resulting in a substantial increase in paracellular permeability and epithelial barrier damage [64].
3.2 Chemical intestinal barrier integrity
The chemical barrier of the intestine is primarily composed of the mucus layer on the surface of IECs, which serves as the first line of defense against pathogens. This barrier includes digestive fluids and mucus secreted by GCs, as well as antimicrobial peptides (AMPs) produced by PCs. The intestinal microbial composition and structure are closely related to the formation of intestinal mucus, and they interactively and cooperatively impact the physiology of the host. Excessive inflammatory responses damage the mucus layer, leading to a reduction in AMPs and an increased likelihood of pathogen penetration through the mucus barrier, resulting in secondary infections and complications in patients with AP.
On one hand, GCs serve as the primary cellular source within the mucus layer, which constitutes the initial barrier of the intestine and is also involved in modulating the intestinal immune response. A recent study revealed that, compared with ANP mice, MUC2-deficient mice experienced more severe pancreatic injury, inhibited colonic antimicrobial peptide β-defensin-2 expression (βD2), and increased bacterial translocation, with MUC2 supplementation partially reversing the damage and emphasizing the importance of intestinal mucus integrity for pancreatic function [65]. Under inflammatory conditions, MUC2 maintains intestinal homeostasis by promoting the expression of the antimicrobial peptide βD2, which effectively affects gram-negative bacteria and Candida [66]. Commensal intestinal bacteria and their metabolites can directly or indirectly regulate GCs through various mechanisms, contributing to the maintenance and regulation of the intestinal mucosal barrier. Lactobacillus rhamnosus induces the expression of 5-HT4R and MUC2 to promote the functions and differentiation of GCs and modulate the microbial community in the gut [67]. SCFA-producing bacteria, such as Akkermansia muciniphila, Bacteroides, and Parabacteroides, generate a diverse range of sulfatases, which allow them to interact with host mucins via the metabolism of the O-glycan domain of MUC2 as an endogenous energy source [68]. However, findings concerning the relationship between Akkermansia muciniphila and AP are conflicting. In SAP models, a significant increase in Akkermansia muciniphila was observed, with a 20-fold increase in the cecal mucosa and a 100-fold increase in the colonic mucosa; however, its abundance decreased following sodium butyrate supplementation [69, 70]. In contrast, several studies have reported that the abundance of Akkermansia muciniphila significantly increases after treatment for SAP, alleviating inflammation by specifically secreting the membrane protein Amuc_1100, which regulates host tryptophan metabolism and the composition of the gut microbiota [65, 71]. The dual role of Akkermansia muciniphila in AP appears to be strain specific; for example, the substrain FSDLZ20M4 exacerbates intestinal inflammation, whereas FSDLZ36M5 alleviates it by regulating immune defenses and protein synthesis [72].
On the other hand, PCs act as major protective agents for the intestinal milieu by producing a diverse array of AMPs, such as α-defensins, lysozyme, and regenerating islet-derived proteins [73]. Like those in GCs, which exhibit region specificity, PCs levels and the production of α-defensins are significantly greater in the ileum than in the duodenum [74], indicating that the duodenum exhibits inferior resistance to microorganisms, thus increasing susceptibility to infection in AP patients. During the malignant progression of AP, the differentiation of PCs is commonly suppressed, leading to the disruption of intestinal homeostasis and exacerbation of pancreatic injury. Fu et al. revealed that a PCs dysfunction was evident in both AP patients and experimental AP mice, and that lysozyme supplementation partially restored PCs function, reducing the severity of AP and gut microbiota dysbiosis [75]. Additionally, the relative abundance of Escherichia-Shigella bacteria and the severity of histopathological pancreatic injury are negatively correlated with a decrease in lysozyme levels in AP [76]. The administration of dithizone or other drugs to deplete intestinal PCs and exacerbate pancreatic and intestinal injury led to a significant increase in the abundance of pathogenic bacteria (e.g., Firmicutes, Helicobacter pylori, and Escherichia-Shigella) and a decrease in beneficial bacteria (e.g., Bacteroidetes and Blautia) in the intestinal tract of AP model mice [77]. Treatment of AP mice with L. reuteri restored intestinal barrier function and ameliorated pancreatic injury by stimulating the differentiation of PCs in a NOD2-dependent manner [16]. In addition, the secretion of AMPs by PCs plays a crucial role in the maintenance of gut homeostasis. Huang and colleagues reported that the levels of lysozyme and α-defensins secreted by PCs were significantly decreased, whereas the abundance of Escherichia-Shigella was increased in ANP patients, and supplementation with AMPs partially ameliorated pancreatic and intestinal inflammation and injury [78]. These findings indicate that maintaining the abundance of PCs in the intestine protects against SAP.
3.3 Immune intestinal barrier integrity
The immune barrier represents the ultimate line of defense in intestinal protection. In the early stage of AP, systemic inflammatory response syndrome (SIRS) is dominant, with excessive inflammatory activation and cascade amplification injuring the pancreas and intestines; subsequently, SIRS enters a remission period, where anti-inflammatory responses gradually take over, leading to compensatory anti-inflammatory response syndrome (CARS), allowing intestinal bacterial translocation to other organs and resulting in secondary infections, thus transforming the intestine from a victim into an accomplice [12, 79, 80]. The gut microbiota and its derivatives can act as PAMPs, which are specifically recognized by PRRs, participating in innate and adaptive immunity and amplifying the inflammatory response in AP (Figure 2).
Immune imbalance in AP progression. In AP, macrophages often shift towards the M1 phenotype, but this trend can be countered with supplements such as Bifidobacterium, lactate, TMAO and butyrate. The FFAR2 on ILC3 recognizes SCFAs, leading to IL-22 secretion which promotes epithelial growth, mucus production, and antimicrobial peptide secretion. Gut microbiota signals facilitate differentiation of specific ILC3 cells, which in turn inhibit the growth of Th17 cells. ILC3 cells also defend against infections like C. rodentium and S. typhimurium and help maintain the balance between Treg and Th17 cells, and contributing to immune homeostasis. FOXP3+RORγt+ Treg cells help reduce inflammation by pushing macrophages towards the M2 phenotype, increasing CTLA-4 expression, and releasing anti-inflammatory molecules such as IL-10 and IL-35. Probiotics and bacterial metabolites like isoallo-LCA also boost gut microbiota diversity and increase these Treg cells. However, in advanced AP, excessive Treg cells can overly suppress the immune response, hindering other immune cells in the gut and weakening the intestinal barrier, allowing potential pathogens to infiltrate. (TMAO: trimethylamine-N-oxide; SCFAs: short-chain fatty acids; FFAR2: free fatty acid receptor 2; ILC3: type 3 innate lymphoid cells; AHR: aryl hydrocarbon receptor; CTLA-4: cytotoxic T lymphocyte-associated antigen 4; Treg: Regulatory T cells).
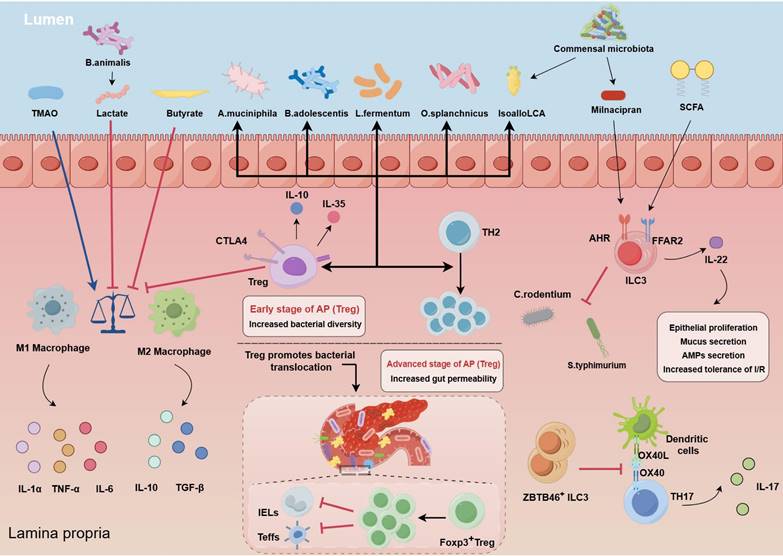
Intestinal macrophages and neutrophils are essential for regulating immune homeostasis. In the initial phases of AP, the pancreas is infiltrated by innate immune cells, including neutrophils, macrophages, and ILCs [49]. Among these cells, macrophages and neutrophils are pivotal components of the microbiota‒gut‒pancreas axis and are closely associated with excessive inflammatory activation and cascade amplification in AP. Parabacteroides can alleviate the inflammatory response in an AP experimental model by producing acetate to limit neutrophil infiltration [10]. Bifidobacterium and its metabolite lactic acid inhibited the aberrant differentiation of M1 macrophages, which was induced by inflammatory responses, in the pancreas and spleen [20]; the metabolite trimethylamine N-oxide promoted macrophage inflammatory polarization by stimulating NLRP3 expression [49]. The membrane protein Amuc_1100, which is secreted by Akkermansia muciniphila, alleviates the severity of AP through its anti-inflammatory properties, reducing the infiltration of Ly6C+ macrophages and neutrophils by modulating the composition of the gut microbiota and tryptophan metabolism [59]. The administration of GV-971 (sodium oligomannate) significantly inhibited macrophage M1 polarization and subsequent lethal inflammation by blocking the MAPK pathway while also enhancing both the peripheral and intestinal immune systems through the modulation of the gut microbiota [81]. Conversely, an increased abundance of pathogenic bacteria in the gut was positively correlated with pancreatic and systemic immune cell infiltration.
Dysregulation of the Treg/Th17 balance exacerbates AP. The Treg/Th17 balance is essential for maintaining intestinal barrier function and tissue homeostasis, and alterations in this balance are intricately linked to the collaboration between immune cells and commensal intestinal bacteria [82, 83]. Treg cells influence the differentiation and functionality of immune cells via diverse mechanisms and have potent anti-inflammatory effects. A significant increase in the number of circulating CD4+CD25+CD127low Tregs was associated with an increased risk of infectious complications and mortality in SAP patients [84]. In contrast, patients with SAP who develop MOF tend to have lower levels of circulating CD4+CD25+CD127high Tregs [85]. Immunosuppression, increased Tregs, and related inflammatory cytokines contribute to the complex interactions that lead to inflammation and infection in patients with SAP, primarily due to intestinal mucosal barrier dysfunction [86]. During the period of AP exacerbation, there is a gradual increase in Treg cells in the gut, which might be interpreted as an adaptive response to counteract inflammation. In addition, dysbiosis of the gut microbiota can influence the progression of acute pancreatitis by regulating the differentiation of Tregs. It has been demonstrated that parthenolide improves intestinal inflammation by modulating the Treg/Th17 balance in a gut microbiota-dependent manner [87]. Additionally, B. adolescentis is able to ameliorate chronic colitis by inducing a protective Treg/Th2 response and remodeling the gut microbiota [88]. Overactivation of Treg cells led to immunosuppressive responses and inhibited the differentiation of effector T cells and CD8α+γδTCR+ intraepithelial lymphocytes within the lamina propria, disrupting the intestinal barrier and facilitating the translocation of facultative pathogens, which might have contributed to ANP [12]. However, few studies have investigated the interplay between alterations in the intestinal microflora and Treg cells during the progression of AP. We need to further investigate the relationship between the dynamic evolution of regulatory Tregs and changes in intestinal flora, with the objective of potentially regulating the immune imbalance in AP.
4. Potential mechanisms underlying the disruption of intestinal homeostasis induced by SAP
4.1 The mechanism of bacterial transmission in SAP
More than half of AP patients exhibit intestinal barrier dysfunction, which is significantly associated with poorer clinical outcomes and prolonged hospital stays [89]. The intestinal microbiota breaks the compromised gut barrier, exacerbating systemic or local inflammation and leading to secondary infections, resulting in a “second strike” for AP patients. An increased abundance of Escherichia-Shigella, Enterococcus, or Staphylococcus stimulates Treg differentiation, leading to a Treg/Th17 imbalance, while also increasing the levels of proinflammatory intestinal factors that compromise intestinal permeability and result in secondary infections of necrotic pancreatic tissue [4, 12]. The precise mechanisms of bacterial metastasis processes in AP remain largely unexplored, but significant impairment of intestinal barrier function is a critical prerequisite for bacterial translocation.
One of the possible mechanisms of bacterial translocation involves bacteria or their endotoxins breaching the intestinal mucosal barrier, infiltrating the mesenteric lymph node and/or portal vein system, and disseminating to injured organs via the systemic circulation [90]. High concentrations of pathogenic bacteria, such as E. coli and Enterococcus, have been detected in the peripheral blood and the drainage fluid of SAP patients, and shifts in the abundances of these bacteria are significantly correlated with the levels of neutrophils and inflammatory factors in patients [4, 25, 91]. Furthermore, the retrograde transport of duodenal bacteria to the damaged pancreas via the pancreatic duct may constitute another potential process of bacterial metastasis in AP [70, 92]. In SAP, the microbiota in the duodenum is dramatically altered compared with that in the cecum and colon, and an imbalance in intestinal immunity results in Escherichia-Shigella translocation from the duodenum to the pancreas [12]. In addition, colonization by gut bacteria in the pancreas has also been observed in pancreatic ductal adenocarcinoma, which has been confirmed by colocalization analysis and fluorescence-labeled bacteria [93]. Owing to its anatomical structure, the pancreatic duct and bile duct converge and open at the large duodenal papilla, which obviously provides the opportunity for the intestinal flora to retrograde, especially the duodenal bacteria, which have more opportunities to colonize the pancreas through the pancreatic duct.
Although the mechanisms underlying gut microbiota dysbiosis and bacterial translocation require further exploration, there is a consensus that repairing intestinal damage is crucial for improving the progression of AP. Supplementation with biochanin A significantly mitigated AP-associated barrier damage by increasing the expression of TJ proteins, decreasing the translocation of E. coli to the pancreas, inhibiting TLR4-MAPK/NF-κB and NLRP3 inflammasome activation, and alleviating AP-associated tissue damage [94]. Additionally, increased lysozyme secretion from Paneth cells or mucin-2 secretion from goblet cells can activate the Wnt pathway and the expression of Lgr5 in intestinal stem cells, promoting intestinal homeostasis and contributing to the remission of AP [65, 75]. Similarly, the inhibition of phosphoenolpyruvate kinase 1 expression in IECs leads to the upregulation of TJs and the restoration of lysozyme and MUC2 secretion, ultimately alleviating AP [95]. Therefore, future studies aimed at alleviating AP should focus on repairing intestinal mucosal damage, adjusting intestinal motility, implementing selective digestive decontamination, inhibiting small intestinal bacterial overgrowth, and utilizing bacterial tracers to further elucidate the role of bacterial translocation in AP.
4.2 Gut microorganisms and metabolites intricately modulate multiple signaling pathways during AP progression
There is bidirectional crosstalk between the pancreas and the intestinal flora. On the one hand, alterations in pancreatic function result in significant shifts in the intestinal microbial composition [96]. AMPs produced by the pancreas can modify the gut microbiota profile upon entry into the intestine. The absence of the Ca2+ channel Orai1 in pancreatic acinar cells led to a decrease in AMP secretion, consequently fostering the proliferation and dysbiosis of intestinal microbes, which could be reversed by supplementation with cathelicidin-related antimicrobial peptides [97]. Research involving mouse models, porcine models and humans has consistently demonstrated that exocrine pancreatic insufficiency precipitates disturbances in the gut microbiota [98]. Furthermore, the administration of pancreatic enzymes enriched populations of beneficial bacteria, such as Akkermansia muciniphila and Lactobacillus reuteri, diminished the presence of pathogenic species such as Escherichia/Shigella, Acinetobacter, and Stenotrophomonas, and influenced the profile of bacterial metabolites detected in the bloodstream [99-101]. Contrarily, the gut microbiota and its metabolites regulate numerous signaling pathways during AP progression. Infected or colonized bacteria in humans can be recognized by the pattern recognition receptors (PRRs) of innate immune cells or ILCs through physical contact, toxin production, metabolite synthesis, or protein secretion, referred to as microbe-associated molecular patterns, to mediate signal transduction and immune responses in the host (Figure 3). PRRs primarily consist of extracellular TLRs or intracellular NOD-like receptors (NLRs), which fulfill the host's requirements for bacterial recognition and interaction [102]. If PRRs are activated by pathogens, they orchestrate the immune responses of the host against these pathogens, whereas in the presence of intestinal commensal bacteria, PRRs are responsible for maintaining immune tolerance. The classification and recognition of these bacteria strongly influence intestinal defense capabilities.
Gut microbes and metabolites mediate various signaling pathways related to AP progression. TLRs identify signals from gut bacteria, activating the MyD88/NF-κB signaling pathway, promoting inflammation and autophagy in AP. Supplementing beneficial probiotics such as B. animalis, L. reuteri, or B. cereus can reduce inflammation-induced damage by inhibiting the MyD88/NF-κB signaling pathway. The NOD1 receptor inside cells connects to DAP from gram-negative bacteria, activating the NOD1/RIP2/NF-κB pathway to drive inflammation. NOD2, detects MDP generated by DL-endopeptidase, thereby activating the NF-κB and MAPK signaling pathways and promoting the expression of inflammatory genes. Interestingly, NOD2 activation also counters inflammation by suppressing the TLR-mediated NF-κB and MAPK pathways. Probiotics generating MDP can reduce inflammation, and supplements like L. salivarius or mifamurtide can compensate for lacking DL-endopeptidase. The NLRP3 inflammasome, influenced by these pathways, promotes inflammatory cytokine release and cell death by activating caspase-1. The compound MCC950 and B. cereus can suppress NLRP3, inhibiting harmful cell death processes. (TLRs: toll like receptors 2; NOD1: nucleotide binding oligomerization domain containing 1; NOD2: nucleotide binding oligomerization domain containing 2; DAP: diaminopimelic acid; MDP: muramyl dipeptide).
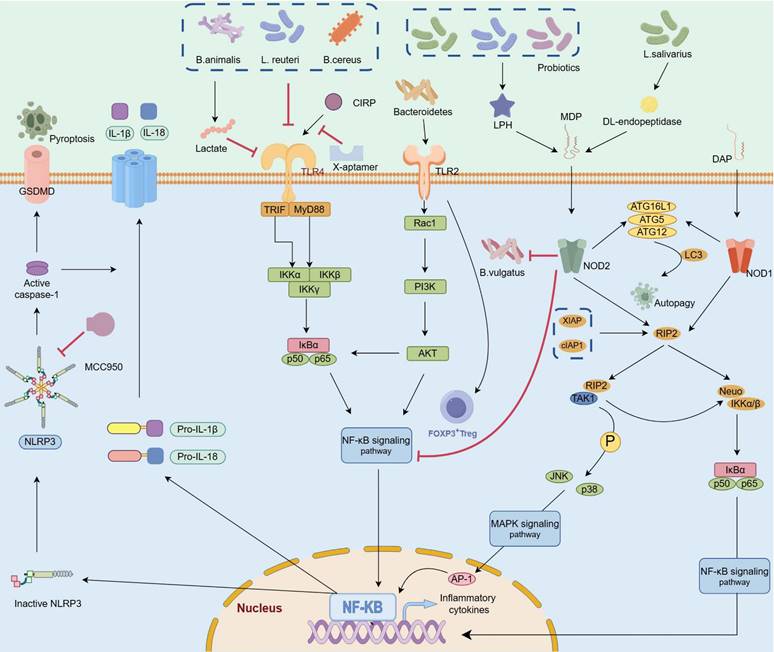
TLRs. TLRs are single transmembrane noncatalytic proteins that are widely expressed in IECs and innate immune cells, among which TLR2, TLR4 and TLR9 are involved in bacterial recognition. TLR4, a pivotal membrane receptor in AP, protects against pancreatic and lung injury by promoting NLRP3 activation and TLR4/MyD88/NF-κB signaling transmission [94, 103]. The colonization of Bifidobacterium animalis and reinforcement of the biosynthesis of its metabolite lactate acid in the intestinal tract of mice attenuated TLR4/MyD88 signal activation, resulting in reduced release of proinflammatory factors, inhibition of M1 macrophage infiltration in the pancreas, and alleviation of macrophage-associated local and systemic inflammatory responses [20]. In contrast, compared with wild-type AP model mice, TLR4ΔIEC model mice presented more pronounced pancreatic injury and systemic inflammatory responses, accompanied by dramatic dysbiosis of the intestinal microbiota, whereas L. reuteri administration ameliorated tissue injury and inflammatory responses in TLR4ΔIEC mice [16]. These findings indicate that unlike in other tissues, sustaining an inflammatory response in the intestine during the advanced stage of AP may mitigate the risk of complications such as pancreatic necrosis caused by ectopic flora.
Although the involvement of TLR4 in AP deterioration has been widely established, the role of TLR2 in AP remains controversial. For example, a study revealed that the expression of TLR2 in the pancreas and ileum did not significantly change during AP progression [16]. Additionally, Awla and colleagues induced AP in wild-type, Tlr2-deficient and Tlr4-deficient mice and reported that Tlr4, but not Tlr2, regulated chemokine formation, neutrophil recruitment and tissue damage in a SAP mouse model [104]. However, recent studies demonstrated that Tlr2 deficiency in Tlr2-/- mice significantly ameliorated pancreatic and pulmonary injury, increased the infiltration of neutrophils and macrophages, and markedly reduced the expression levels of NF-κB and NLRP3 in both MAP and SAP models [105, 106]. Microorganisms can specifically activate TLR2 and induce the recruitment of FoxP3+ Treg cells to the colonic lamina propria, suggesting that the recognition of the microbiota by TLR2 may serve as a potential mechanism for altering mucosal tolerance [107]. Hence, further investigation into the role of TLR2 is imperative, particularly in terms of elucidating the underlying biological mechanisms governing its interaction with the intestinal microbiota across different disease stages.
NLRs. TLRs primarily recognize extracellular pathogens and related substances, whereas intracellular bacteria and viruses are predominantly recognized by the cytoplasmic innate immune receptor NLR, which stimulates the NF-κB- and MAPK-related pathways and facilitates proinflammatory cytokine secretion [108]. NOD1, the major subtype of NLR, is widely expressed in a variety of cells and tissues in humans. NOD1 is specifically activated by the diaminopimelic acid fragment in gram-negative bacteria and subsequently mediates NOD1/RIP2/NF-kB signaling to promote inflammation. Interestingly, the expression levels of NOD1 are significantly increased in SAP-induced intestinal injury [109, 110], and interventions targeting this pathway significantly ameliorate pancreatic and intestinal injury and reduce the severity of SIRS [111].
Notably, NOD2 plays dual roles in inflammatory diseases. On the one hand, NOD2 specifically recognizes muramyl dipeptide, which activates the NF-kB and MAPK signaling pathways and promotes the expression of inflammatory-related genes and cytokines [112]; on the other hand, NOD2 suppresses the TLR-mediated NF-kB and MAPK pathways and exerts anti-inflammatory effects [108]. A previous study reported that Bacteroides vulgatus could inhibit CD82 activity, promote BRCC3-dependent K63 deubiquitination and increase the activation of the NLRP3 inflammasome, thereby alleviating intestinal injury induced by an overstimulated inflammatory response in the colon [113]. However, the interactive mechanisms between the intestinal microbiota and NOD2 in AP are largely unclear and deserve further exploration.
Anti-inflammatory and proinflammatory responses are simultaneously stimulated in AP, and NLRP3 is a key cytosolic immune factor that responds to cellular stress signals and is involved in orchestrating immune responses and establishing intestinal homeostasis. Convincing evidence has demonstrated that NLRP3 is involved in the activation of adaptive immunity in AP. Treatment with MCC950 (an NLRP3 inhibitor) reduces the infiltration of neutrophils and macrophages and dampens pancreatic and systemic inflammatory responses [79, 105]. Additionally, prophylactic treatment with antibiotics prevents the amplification of AP via the intestinal‒pancreatic axis by restraining the activation of TLR4/NLRP3 signaling in the colon, downregulating the expression of proinflammatory factors, promoting the expression of intestinal TJ-related proteins, improving the morphology of the intestine, and reducing the translocation of intestinal bacteria [114]. Previous studies have demonstrated that Bifidobacterium animalis[20] and Bacillus cereus[115] exert anti-inflammatory effects by suppressing TLR4/NF-κB/NLRP3 signaling, resulting in a reduction in intestinal barrier damage, weakened local and systemic inflammatory responses and decreased incidence of pancreatic edema, hemorrhage, and necrosis complications. Despite the proinflammatory effects of NLRP3 in diseases, it also contributes to the activation and functions of anti-inflammatory immune cells. Sendler et al. reported a significant decrease in Treg cells and an attenuated Th2-mediated immune response in NLRP3-deficient AP model mice [79].
Moreover, alterations in the interactions among the gut microbiota, the metabolic community, and the expression of crucial genes in the host are key in the cooperative regulation of AP progression. During the recovery stage of AP, NLRP3 activity decreases in antibiotic-treated and germ-free AP mice, whereas reactivation of NLRP3 occurs in mice receiving FMT from AP patients, leading to exacerbated systemic inflammation [25]. Despite numerous studies demonstrating the role of NLRP6 in various inflammatory disease processes, few studies have elucidated its role in AP.
5. Exploring novel strategies for improving the prognosis of patients with AP by regulating the gut microbiota
5.1 Probiotics
Currently, numerous experimental and clinical studies have indicated that changes in the gut microbiome can have a broad range of effects throughout the body. Hence, as shown in Table 3, probiotics have been employed as adjunctive therapies for various diseases; probiotics modulate the gut microbiota and alleviate symptoms [116]. In an AP mouse model, supplementation with Bifidobacterium animalis ameliorated macrophage-related inflammatory responses and attenuated acinar cell necrosis [20]; furthermore, Lactobacillus can modulate innate immune homeostasis, preserve PCs functionality, promote AMP secretion through increased tryptophan metabolism, and ameliorate pancreatic and intestinal edema and necrosis [117, 118]. The commensal flora Bifidobacterium is widely used to evaluate its therapeutic effects on inflammatory diseases in experimental and clinical trials. Researchers have administered artificial-enzyme-armed Bifidobacterium longum to mice, and the treatment increased the amount of reactive oxygen species scavenged, attenuated the level of inflammatory mediators, and modulated the intestinal microbiota [119]. Additionally, an RCT was performed to assess the effectiveness of Bifidobacterium quadruple living bacterium in 60 SAP patients; the incidence of MOF was significantly reduced and the duration of abdominal pain and hospital stay were significantly shortened, but the death rate was not significantly different [120]. However, in a multicenter randomized, double-blind, placebo-controlled trial with 298 predicted SAP patients, patients who were administered a multispecies probiotic preparation presented higher rates of infectious complications, deaths, and bowel ischemia than did those in the placebo group [121]. A meta-analysis indicated that the treatment of SAP patients with probiotics slightly influences infection or mortality rates during advanced disease stages [122]. We believe that these inconsistent outcomes might be the result of each study applying a unique starting time, dose, mode, duration of probiotic administration, and specific degree of clinical adjuvant therapy. Therefore, subsequent studies should define the use of probiotics more strictly.
5.2 Prebiotics
Prebiotics are indigestible carbohydrate substrates that improve intestinal function by promoting the proliferation of beneficial bacteria, modulating adaptive immunity, and enhancing mineral absorption. As shown in Table 4, lactulose has garnered significant attention because it can increase the abundance of beneficial bacteria such as Bifidobacterium, Lactobacillus, and Muribaculum, to regulate intestinal immune homeostasis [123, 124]. In addition, lactose is metabolized into lactic acid in the intestine in a dose-dependent manner to decrease serum amylase levels, promote the proliferation of intestinal stem cells and PCs and alleviate intestinal mucosal injury [125, 126]. Interestingly, in a randomized controlled trial (RCT) involving MSAP patients complicated with gut dysfunction, participants treated with lactose had lower serum cytokine levels and gut permeability indices than patients treated with the Chinese herb rhubarb [48].
5.3 Synbiotics
Synbiotics, which consist of a combination of probiotics and prebiotics, are formulated to increase their efficacy and durability. The combination of Bifidobacterium and lactulose increases the metabolism of tryptophan in the intestine to help maintain gut homeostasis [127], and the administration of Bacillus coagulans and lactulose significantly ameliorated intestinal barrier permeability damage and inhibited apoptotic cell death [128]. However, a prospective and double-blind RCT indicated that the administration of synbiotics to SAP patients did not significantly reduce complications, mortality, or intervention rates; however, the length of hospital stay was reduced [129].
Representative studies demonstrating therapeutic effects of prebiotics in AP
| Category | Prebiotics/metabolites | Subjects or Rodent strains | Treatments | Main findings | Ref |
|---|---|---|---|---|---|
| SCFAs | Butyrate | C57BL/6 mouse and GPR109A deficient mouse (n = 40) | Oral gavage 7 days, 200mg/kg | Ameliorating pancreatic inflammation; suppression of NLRP3 inflammasome activation and modulation of immune cell infiltration | [137] |
| Butyrate | Male C57BL/6 mouse (n = 32) | Oral gavage7 days, 200mg/kg or 500mg/kg | Inhibiting pancreatic inflammation by maintaining the intestinal barrier and regulating gut microbiota | [69] | |
| Butyric acid | AP patient (n = 35); C57BL/6 mouse | Drinking 4 weeks, 100mM | Ameliorating progression towards necrotizing pancreatitis | [15] | |
| Butyric acid | C57BL/6 mouse (n = 32) | Drinking 4 weeks, 100mM | Mitigating acute pancreatitis through amelioration of intestinal barrier dysfunction | [47] | |
| Oligosaccharides | Chitosan oligosaccharide | C57BL/6 mouse (n = 28) | Oral gavage, 200 mg/kg | Reducing oxidative stress and restoring intestinal homeostasis | [26] |
| Lactulose | MSAP patients (n = 73) | Administration of oral solution twice daily; 50 mL,10 mL | Restoring intestinal function, regulating gut microbiota and promoting the production of SCFAs | [48] | |
| Tryptophan metabolites | Norharman | C57BL/6 mouse (n = 20) Rftn1 deficient mouse (n = 20) | Oral gavage 3 times for 1 day, 100 mg/kg | Inhibiting M1 macrophages activation, reduce pancreatic lesions and reduce intestinal damage | [19] |
5.4 FMT
A broad range of clinical and animal experiments indicate that FMT is a potential therapeutic strategy for limiting inflammation and organ damage in various diseases FMT is achieved by implanting fecal material from healthy donors into the patient's intestine to alleviate gut microbiota dysbiosis [130-132]. However, the therapeutic efficacy of FMT remains controversial due to the heterogeneity of the fecal microbiota, variations in receptor reactivity across different samples, and unique inflammatory characteristics at different stages of AP [133]. Liu et al. reported that FMT in AP mice ameliorated the dysbiosis of gut microbiota communities, decreased mitochondrial dysfunction and the oxidative stress response, and reshaped the intestinal ecological balance [134]. Li et al. demonstrated that pancreatic injury and systemic inflammation were alleviated and that the intestinal NLRP3 inflammasome was stimulated in antibiotic-treated and germ-free mice. Nevertheless, FMT can reactivate the NLRP3 inflammasome and exacerbate AP [25]. After FMT in ANP mice, the mortality rate nearly doubled, accompanied by a significant increase in the number of pancreatic colony-forming units [15]. Thus, FMT is not currently recommended for the treatment of AP in clinical practice, and further studies are needed to assess the effect of FMT and decipher the underlying mechanisms of FMT as a treatment for AP.
6. Future perspectives
Significant advancements have been made in the investigation of the interactions between the gut microbiota and the pancreas via the “microbiota‒gut‒pancreas” axis. The intestinal flora and pancreatic tissue-resident microbes present unique spectrum in AP patients with different etiologies and severities, and novel therapeutic approaches targeting the gut microbiota generate differing outcomes owing to variations in clinical implementation protocols. Well-designed clinical trials are urgently needed to evaluate the dosage and formation of the multispecies probiotic preparation and the duration of treatment for AP.
Abbreviations
AP: acute pancreatitis; MAP: mild AP; SAP: severe AP; FMT: fecal microbiota transplantation; PAMPs: pathogen-associated molecular patterns; MOF: multiple organ failure; HTGP: hypertriglyceridemic pancreatitis; ANP: acute necrotizing pancreatitis; SCFAs: short chain fatty acids; IECs: intestinal epithelial cells; GCs: goblet cells; AhR: aryl hydrocarbon receptor; Rftn1: Raftlin 1; FXR: Farnesoid X receptor; TGR5: Takeda G protein-coupled receptor 5; TLR: Toll-like receptor; ECs: enteroendocrine cells; PCs: paneth cells; TJs: tight junctions; MLCK1: long myosin light chain kinase splice variant 1; MUC2: Mucin 2; FCGBP: IgGFc-binding protein; CLCAl: calcium-activated chloride channel regulator 1; GAPs: antigen passages; IgA: immunoglobulin A; Tregs: regulatory T cells; AMPs: antimicrobial peptides; SIRS: systemic inflammatory response syndrome; CARS: compensatory anti-inflammatory response syndrome; ILCs: innate lymphoid cells; RCT: randomized controlled trial; FMN: formononetin; ZO-1: ZO1 tight junction protein.
Acknowledgements
Figures were created under the academic license of Figdraw.com.
Funding
This study was supported in part by the National Natural Science Foundation of China (Grant No. 82000516 and 82472352); Zhejiang Province's Key R&D Plan Project (Grant No. 2023C03054 and 2024C03048); the Natural Science Foundation of Zhejiang Province (Grant No. LQ24H030008); Construction Fund of Medical Key Disciplines of Hangzhou (OO20190001).
Author contributions
QL and KR conceived and designed the study and wrote the manuscript. ZA and YL were involved in paper collection. JY and XZ conceived the current study and reviewed the manuscript. All authors reviewed previous versions and read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386:85-96
2. Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA. et al. Acute pancreatitis. Lancet (London, England). 2020;396:726-34
3. Wang Z, Liu J, Li F, Luo Y, Ge P, Zhang Y. et al. The gut-lung axis in severe acute Pancreatitis-associated lung injury: The protection by the gut microbiota through short-chain fatty acids. Pharmacol Res. 2022;182:106321
4. Zhu Y, He C, Li X, Cai Y, Hu J, Liao Y. et al. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. Journal of gastroenterology. 2019;54:347-58
5. Jiao J, Liu J, Luo F, Shang M, Pan C, Qi B. et al. Qingyi granules ameliorate severe acute pancreatitis in rats by modulating the gut microbiota and serum metabolic aberrations. Pharmaceutical biology. 2023;61:927-37
6. Zhu Y, Mei Q, Fu Y, Zeng Y. Alteration of gut microbiota in acute pancreatitis and associated therapeutic strategies. Biomed Pharmacother. 2021;141:111850
7. de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71:1020-32
8. Pontarollo G, Kollar B, Mann A, Khuu MP, Kiouptsi K, Bayer F. et al. Commensal bacteria weaken the intestinal barrier by suppressing epithelial neuropilin-1 and Hedgehog signaling. Nat Metab. 2023;5:1174-87
9. Paik D, Yao L, Zhang Y, Bae S, D'Agostino GD, Zhang M. et al. Human gut bacteria produce Tau(Eta)17-modulating bile acid metabolites. Nature. 2022;603:907-12
10. Lei Y, Tang L, Liu S, Hu S, Wu L, Liu Y. et al. Parabacteroides produces acetate to alleviate heparanase-exacerbated acute pancreatitis through reducing neutrophil infiltration. Microbiome. 2021;9:115
11. Wolbrink DRJ, Kolwijck E, Ten Oever J, Horvath KD, Bouwense SAW, Schouten JA. Management of infected pancreatic necrosis in the intensive care unit: a narrative review. Clin Microbiol Infect. 2020;26:18-25
12. Glaubitz J, Wilden A, Frost F, Ameling S, Homuth G, Mazloum H. et al. Activated regulatory T-cells promote duodenal bacterial translocation into necrotic areas in severe acute pancreatitis. Gut. 2023;72:1355-69
13. Li G, Liu L, Lu T, Sui Y, Zhang C, Wang Y. et al. Gut microbiota aggravates neutrophil extracellular traps-induced pancreatic injury in hypertriglyceridemic pancreatitis. Nature communications. 2023;14:6179
14. Wang Z, Liu J, Li F, Ma S, Zhao L, Ge P. et al. Mechanisms of Qingyi Decoction in Severe Acute Pancreatitis-Associated Acute Lung Injury via Gut Microbiota: Targeting the Short-Chain Fatty Acids-Mediated AMPK/NF-kappaB/NLRP3 Pathway. Microbiology spectrum. 2023;11:e0366422
15. van den Berg FF, van Dalen D, Hyoju SK, van Santvoort HC, Besselink MG, Wiersinga WJ. et al. Western-type diet influences mortality from necrotising pancreatitis and demonstrates a central role for butyrate. Gut. 2021;70:915-27
16. Qi-Xiang M, Yang F, Ze-Hua H, Nuo-Ming Y, Rui-Long W, Bin-Qiang X. et al. Intestinal TLR4 deletion exacerbates acute pancreatitis through gut microbiota dysbiosis and Paneth cells deficiency. Gut microbes. 2022;14:2112882
17. Zhao MQ, Cui MY, Jiang QL, Wang JJ, Fan MY, Lu YY. Characterization of Duodenal Microbiota in Patients with Acute Pancreatitis and Healthy Controls. Digestive diseases and sciences. 2023;68:3341-53
18. Yazici C, Thaker S, Castellanos KK, Al Rashdan H, Huang Y, Sarraf P. et al. Diet, Gut Microbiome, and Their End Metabolites Associate With Acute Pancreatitis Risk. Clinical and translational gastroenterology. 2023;14:e00597
19. Zhou Q, Tao X, Guo F, Wu Y, Deng D, Lv L. et al. Tryptophan metabolite norharman secreted by cultivated Lactobacillus attenuates acute pancreatitis as an antagonist of histone deacetylases. BMC medicine. 2023;21:329
20. Li H, Xie J, Guo X, Yang G, Cai B, Liu J. et al. Bifidobacterium spp. and their metabolite lactate protect against acute pancreatitis via inhibition of pancreatic and systemic inflammatory responses. Gut microbes. 2022;14:2127456
21. Zou M, Yang Z, Fan Y, Gong L, Han Z, Ji L. et al. Gut microbiota on admission as predictive biomarker for acute necrotizing pancreatitis. Frontiers in immunology. 2022;13:988326
22. Jin M, Zhang H, Wu M, Wang Z, Chen X, Guo M. et al. Colonic interleukin-22 protects intestinal mucosal barrier and microbiota abundance in severe acute pancreatitis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2022;36:e22174
23. Wang L, Gou X, Ding Y, Liu J, Wang Y, Wang Y. et al. The interplay between herbal medicines and gut microbiota in metabolic diseases. Frontiers in pharmacology. 2023;14:1105405
24. Zhou B, Yuan Y, Zhang S, Guo C, Li X, Li G. et al. Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Frontiers in immunology. 2020;11:575
25. Li X, He C, Li N, Ding L, Chen H, Wan J. et al. The interplay between the gut microbiota and NLRP3 activation affects the severity of acute pancreatitis in mice. Gut Microbes. 2020;11:1774-89
26. Mei QX, Hu JH, Huang ZH, Fan JJ, Huang CL, Lu YY. et al. Pretreatment with chitosan oligosaccharides attenuate experimental severe acute pancreatitis via inhibiting oxidative stress and modulating intestinal homeostasis. Acta pharmacologica Sinica. 2021;42:942-53
27. Zheng J, Lou L, Fan J, Huang C, Mei Q, Wu J. et al. Commensal Escherichia coli Aggravates Acute Necrotizing Pancreatitis through Targeting of Intestinal Epithelial Cells. Appl Environ Microbiol. 2019;85:e00059-19
28. Timmerhuis HC, van den Berg FF, Noorda PC, van Dijk SM, van Grinsven J, Sperna Weiland CJ. et al. Overuse and Misuse of Antibiotics and the Clinical Consequence in Necrotizing Pancreatitis: An Observational Multicenter Study. Ann Surg. 2023;278:e812-e9
29. Zhang Y, Gao SL, Zhang SY, Liang ZY, Yu WQ, Liang TB. Six cases of severe acute pancreatitis complicated with vancomycin-resistant enterococcus enteritis. Shock. 2014;42:400-6
30. Hanna EM, Hamp TJ, McKillop IH, Bahrani-Mougeot F, Martinie JB, Horton JM. et al. Comparison of culture and molecular techniques for microbial community characterization in infected necrotizing pancreatitis. J Surg Res. 2014;191:362-9
31. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266-78
32. Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms. 2020;8:573
33. Goldstein EJ, Tyrrell KL, Citron DM. Lactobacillus species: taxonomic complexity and controversial susceptibilities. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;60(Suppl 2):S98-107
34. Wu H, Xie S, Miao J, Li Y, Wang Z, Wang M. et al. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes. 2020;11:997-1014
35. Yang N, Zhang Y, Fu Y, Li Y, Yang S, Chen J. et al. Transmissible Gastroenteritis Virus Infection Promotes the Self-Renewal of Porcine Intestinal Stem Cells via Wnt/β-Catenin Pathway. J Virol. 2022;96:e0096222
36. Qi Y, He J, Zhang Y, Ge Q, Wang Q, Chen L. et al. Heat-inactivated Bifidobacterium adolescentis ameliorates colon senescence through Paneth-like-cell-mediated stem cell activation. Nat Commun. 2023;14:6121
37. Hu X, Han Z, Zhou R, Su W, Gong L, Yang Z. et al. Altered gut microbiota in the early stage of acute pancreatitis were related to the occurrence of acute respiratory distress syndrome. Frontiers in cellular and infection microbiology. 2023;13:1127369
38. Liu J, Yan Q. Integrative metagenomic and metabolomic analyses reveal the potential of gut microbiota to exacerbate acute pancreatitis. NPJ Biofilms Microbiomes. 2024;10:29
39. Tan C, Ling Z, Huang Y, Cao Y, Liu Q, Cai T. et al. Dysbiosis of Intestinal Microbiota Associated With Inflammation Involved in the Progression of Acute Pancreatitis. Pancreas. 2015;44:868-75
40. Philips CA, Phadke N, Ganesan K, Rajesh S, Padsalgi G, Ahamed R. et al. Gut Microbiota in Alcoholic Hepatitis is Disparate from Those in Acute Alcoholic Pancreatitis and Biliary Disease. Journal of clinical and experimental hepatology. 2019;9:690-8
41. Hu X, Gong L, Zhou R, Han Z, Ji L, Zhang Y. et al. Variations in Gut Microbiome are Associated with Prognosis of Hypertriglyceridemia-Associated Acute Pancreatitis. Biomolecules. 2021;11:695
42. Liu Q, Yu Z, Tian F, Zhao J, Zhang H, Zhai Q. et al. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb Cell Fact. 2020;19:23
43. Huang Y, Wen Y, Wang R, Hu L, Yang J, Yang J. et al. Temporal metabolic trajectory analyzed by LC-MS/MS based targeted metabolomics in acute pancreatitis pathogenesis and Chaiqin Chengqi decoction therapy. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2022;99:153996
44. Yang J, Wang M, Qiu Q, Huang Y, Wang Y, Pu Q. et al. Time-Course Lipidomics of Ornithine-Induced Severe Acute Pancreatitis Model Reveals the Free Fatty Acids Centered Lipids Dysregulation Characteristics. Metabolites. 2023;13:993
45. Zhou Q, Tao X, Guo F, Zhu Y, Wu Y, Xiang H. et al. The crosstalk between microbiota and metabolites in AP mice: an analysis based on metagenomics and untargeted metabolomics. Frontiers in cellular and infection microbiology. 2023;13:1134321
46. Yan X, Li J, Wu D. The Role of Short-Chain Fatty Acids in Acute Pancreatitis. Molecules. 2023;28:4985
47. Xia H, Guo J, Shen J, Jiang S, Han S, Li L. Butyrate ameliorated the intestinal barrier dysfunction and attenuated acute pancreatitis in mice fed with ketogenic diet. Life sciences. 2023;334:122188
48. Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H. et al. Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2023;163:114769
49. Peng C, Li Z, Yu X. The Role of Pancreatic Infiltrating Innate Immune Cells in Acute Pancreatitis. Int J Med Sci. 2021;18:534-45
50. Kim CH. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol Immunol. 2021;18:1161-71
51. Eslick S, Williams EJ, Berthon BS, Wright T, Karihaloo C, Gately M. et al. Weight Loss and Short-Chain Fatty Acids Reduce Systemic Inflammation in Monocytes and Adipose Tissue Macrophages from Obese Subjects. Nutrients. 2022;14:765
52. Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ, Yao S. et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11:752-62
53. Tang G, Du Y, Guan H, Jia J, Zhu N, Shi Y. et al. Butyrate ameliorates skeletal muscle atrophy in diabetic nephropathy by enhancing gut barrier function and FFA2-mediated PI3K/Akt/mTOR signals. Br J Pharmacol. 2022;179:159-78
54. Ammer-Herrmenau C, Antweiler KL, Asendorf T, Beyer G, Buchholz SM, Cameron S. et al. Gut microbiota predicts severity and reveals novel metabolic signatures in acute pancreatitis. Gut. 2024;73:485-95
55. van den Berg FF, Besselink MG. Short-chain fatty acids in patients with severe acute pancreatitis: friend or foe? Gut. 2024;15:332129
56. Mar JS, Ota N, Pokorzynski ND, Peng Y, Jaochico A, Sangaraju D. et al. IL-22 alters gut microbiota composition and function to increase aryl hydrocarbon receptor activity in mice and humans. Microbiome. 2023;11:47
57. Natividad JM, Agus A, Planchais J, Lamas B, Jarry AC, Martin R. et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018;28:737-49.e4
58. Qi H, Li Y, Yun H, Zhang T, Huang Y, Zhou J. et al. Lactobacillus maintains healthy gut mucosa by producing L-Ornithine. Commun Biol. 2019;2:171
59. Wang LJ, Jin YL, Pei WL, Li JC, Zhang RL, Wang JJ. et al. Amuc_1100 pretreatment alleviates acute pancreatitis in a mouse model through regulating gut microbiota and inhibiting inflammatory infiltration. Acta pharmacologica Sinica. 2024;45:570-80
60. Horowitz A, Chanez-Paredes SD, Haest X, Turner JR. Paracellular permeability and tight junction regulation in gut health and disease. Nat Rev Gastroenterol Hepatol. 2023;20:417-32
61. Shu LZ, Ding YD, Xue QM, Cai W, Deng H. Direct and indirect effects of pathogenic bacteria on the integrity of intestinal barrier. Therap Adv Gastroenterol. 2023;16:17562848231176427
62. Sharafutdinov I, Esmaeili DS, Harrer A, Tegtmeyer N, Sticht H, Backert S. Campylobacter jejuni Serine Protease HtrA Cleaves the Tight Junction Component Claudin-8. Front Cell Infect Microbiol. 2020;10:590186
63. Harrer A, Bücker R, Boehm M, Zarzecka U, Tegtmeyer N, Sticht H. et al. Campylobacter jejuni enters gut epithelial cells and impairs intestinal barrier function through cleavage of occludin by serine protease HtrA. Gut Pathog. 2019;11:4
64. Bein A, Zilbershtein A, Golosovsky M, Davidov D, Schwartz B. LPS Induces Hyper-Permeability of Intestinal Epithelial Cells. J Cell Physiol. 2017;232:381-90
65. Huang Z, Wu H, Fan J, Mei Q, Fu Y, Yin N. et al. Colonic mucin-2 attenuates acute necrotizing pancreatitis in rats by modulating intestinal homeostasis. FASEB J. 2023;37:e22994
66. Cobo ER, Kissoon-Singh V, Moreau F, Chadee K. Colonic MUC2 mucin regulates the expression and antimicrobial activity of β-defensin 2. Mucosal immunology. 2015;8:1360-72
67. Gu Y, Qin X, Zhou G, Wang C, Mu C, Liu X. et al. Lactobacillus rhamnosus GG supernatant promotes intestinal mucin production through regulating 5-HT4R and gut microbiota. Food Funct. 2022;13:12144-55
68. Luis AS, Hansson GC. Intestinal mucus and their glycans: A habitat for thriving microbiota. Cell Host Microbe. 2023;31:1087-100
69. Xiong Y, Ji L, Zhao Y, Liu A, Wu D, Qian J. Sodium Butyrate Attenuates Taurocholate-Induced Acute Pancreatitis by Maintaining Colonic Barrier and Regulating Gut Microorganisms in Mice. Frontiers in physiology. 2022;13:813735
70. van den Berg FF, Hugenholtz F, Boermeester MA, Zaborina O. Spatioregional assessment of the gut microbiota in experimental necrotizing pancreatitis. BJS open. 2021;5:zrab061
71. Wang Z, Liu J, Li F, Ma S, Zhao L, Ge P. et al. Mechanisms of Qingyi Decoction in Severe Acute Pancreatitis-Associated Acute Lung Injury via Gut Microbiota: Targeting the Short-Chain Fatty Acids-Mediated AMPK/NF-κB/NLRP3 Pathway. Microbiol Spectr. 2023;11:e0366422
72. Liu Q, Lu W, Tian F, Zhao J, Zhang H, Hong K. et al. Akkermansia muciniphila Exerts Strain-Specific Effects on DSS-Induced Ulcerative Colitis in Mice. Frontiers in cellular and infection microbiology. 2021;11:698914
73. Shin JH, Bozadjieva-Kramer N, Seeley RJ. Reg3γ: current understanding and future therapeutic opportunities in metabolic disease. Exp Mol Med. 2023;55:1672-7
74. Nakamura K, Yokoi Y, Fukaya R, Ohira S, Shinozaki R, Nishida T. et al. Expression and Localization of Paneth Cells and Their α-Defensins in the Small Intestine of Adult Mouse. Front Immunol. 2020;11:570296
75. Fu Y, Mei Q, Yin N, Huang Z, Li B, Luo S. et al. Paneth Cells Protect against Acute Pancreatitis via Modulating Gut Microbiota Dysbiosis. mSystems. 2022;7:e0150721
76. Chen J, Huang C, Wang J, Zhou H, Lu Y, Lou L. et al. Dysbiosis of intestinal microbiota and decrease in paneth cell antimicrobial peptide level during acute necrotizing pancreatitis in rats. PloS one. 2017;12:e0176583
77. Guo Y, Huang C, Liu L, Fu X, Lu Y, Zheng J. et al. Paneth Cell Ablation Aggravates Pancreatic and Intestinal Injuries in a Rat Model of Acute Necrotizing Pancreatitis after Normal and High-Fat Diet. Mediators Inflamm. 2019;2019:8474523
78. Huang C, Chen J, Wang J, Zhou H, Lu Y, Lou L. et al. Dysbiosis of Intestinal Microbiota and Decreased Antimicrobial Peptide Level in Paneth Cells during Hypertriglyceridemia-Related Acute Necrotizing Pancreatitis in Rats. Front Microbiol. 2017;8:776
79. Sendler M, van den Brandt C, Glaubitz J, Wilden A, Golchert J, Weiss FU. et al. NLRP3 Inflammasome Regulates Development of Systemic Inflammatory Response and Compensatory Anti-Inflammatory Response Syndromes in Mice With Acute Pancreatitis. Gastroenterology. 2020;158:253-69 e14
80. Wang Z, Li F, Liu J, Luo Y, Guo H, Yang Q. et al. Intestinal Microbiota - An Unmissable Bridge to Severe Acute Pancreatitis-Associated Acute Lung Injury. Front Immunol. 2022;13:913178
81. Chen X, Chen X. GV-971 prevents severe acute pancreatitis by remodeling the microbiota-metabolic-immune axis. Nat Commun. 2024;15:8278
82. Xu M, Pokrovskii M, Ding Y, Yi R, Au C, Harrison OJ. et al. Author Correction: c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature. 2019;566:E7
83. Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng R. et al. Microbiotas from Humans with Inflammatory Bowel Disease Alter the Balance of Gut Th17 and RORγt(+) Regulatory T Cells and Exacerbate Colitis in Mice. Immunity. 2019;50:212-24.e4
84. Minkov GA, Yovtchev YP, Halacheva KS. Increased Circulating CD4+CD25+CD127low/neg Regulatory T-cells as a Prognostic Biomarker in Acute Pancreatitis. Pancreas. 2017;46:1003-10
85. Wang W, Xiang HP, Wang HP, Zhu LX, Geng XP. CD4 + CD25 + CD127 high cells as a negative predictor of multiple organ failure in acute pancreatitis. World journal of emergency surgery: WJES. 2017;12:7
86. Li JP, Yang J, Huang JR, Jiang DL, Zhang F, Liu MF. et al. Immunosuppression and the infection caused by gut mucosal barrier dysfunction in patients with early severe acute pancreatitis. Frontiers in bioscience (Landmark edition). 2013;18:892-900
87. Liu YJ, Tang B, Wang FC, Tang L, Lei YY, Luo Y. et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics. 2020;10:5225-41
88. Fan L, Qi Y, Qu S, Chen X, Li A, Hendi M. et al. B. adolescentis ameliorates chronic colitis by regulating Treg/Th2 response and gut microbiota remodeling. Gut microbes. 2021;13:1-17
89. Wu LM, Sankaran SJ, Plank LD, Windsor JA, Petrov MS. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. The British journal of surgery. 2014;101:1644-56
90. Liu J, Huang L, Luo M, Xia X. Bacterial translocation in acute pancreatitis. Crit Rev Microbiol. 2019;45:539-47
91. Li Q, Wang C, Tang C, He Q, Li N, Li J. Bacteremia in patients with acute pancreatitis as revealed by 16S ribosomal RNA gene-based techniques*. Crit Care Med. 2013;41:1938-50
92. Widdison AL, Karanjia ND, Reber HA. Routes of spread of pathogens into the pancreas in a feline model of acute pancreatitis. Gut. 1994;35:1306-10
93. Guan SW, Lin Q, Yu HB. Intratumour microbiome of pancreatic cancer. World J Gastrointest Oncol. 2023;15:713-30
94. Pan X, Ye L, Ren Z, Li J, Li B, Pan LL. et al. Biochanin A ameliorates caerulein-induced acute pancreatitis and associated intestinal injury in mice by inhibiting TLR4 signaling. The Journal of nutritional biochemistry. 2023;113:109229
95. Yin N, Xu B. Inhibition of Pck1 in intestinal epithelial cells alleviates acute pancreatitis via modulating intestinal homeostasis. FASEB J. 2024;38:e23618
96. Thomas RM, Jobin C. Microbiota in pancreatic health and disease: the next frontier in microbiome research. Nat Rev Gastroenterol Hepatol. 2020;17:53-64
97. Ahuja M, Schwartz DM, Tandon M, Son A, Zeng M, Swaim W. et al. Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity. Cell Metab. 2017;25:635-46
98. Guo Y, Cao F, Li F. Impacts of pancreatic exocrine insufficiency on gut microbiota. J Zhejiang Univ Sci B. 2024;25:271-9
99. Nishiyama H, Nagai T, Kudo M, Okazaki Y, Azuma Y, Watanabe T. et al. Supplementation of pancreatic digestive enzymes alters the composition of intestinal microbiota in mice. Biochem Biophys Res Commun. 2018;495:273-9
100. Ritz S, Hahn D, Wami HT, Tegelkamp K, Dobrindt U, Schnekenburger J. Gut microbiome as a response marker for pancreatic enzyme replacement therapy in a porcine model of exocrine pancreas insufficiency. Microb Cell Fact. 2020;19:221
101. Pietzner M, Budde K, Rühlemann M, Völzke H, Homuth G, Weiss FU. et al. Exocrine Pancreatic Function Modulates Plasma Metabolites Through Changes in Gut Microbiota Composition. J Clin Endocrinol Metab. 2021;106:e2290-e8
102. Vaz J, Akbarshahi H, Andersson R. Controversial role of toll-like receptors in acute pancreatitis. World J Gastroenterol. 2013;19:616-30
103. Wen Y, Han C, Liu T, Wang R, Cai W, Yang J. et al. Chaiqin chengqi decoction alleviates severity of acute pancreatitis via inhibition of TLR4 and NLRP3 inflammasome: Identification of bioactive ingredients via pharmacological sub-network analysis and experimental validation. Phytomedicine. 2020;79:153328
104. Awla D, Abdulla A, Regner S, Thorlacius H. TLR4 but not TLR2 regulates inflammation and tissue damage in acute pancreatitis induced by retrograde infusion of taurocholate. Inflamm Res. 2011;60:1093-8
105. Li L, Liu Q, Le C, Zhang H, Liu W, Gu Y. et al. Toll-like receptor 2 deficiency alleviates acute pancreatitis by inactivating the NF-κB/NLRP3 pathway. Int Immunopharmacol. 2023;121:110547
106. Liu Q, Li L, Xu D, Zhu J, Huang Z, Yang J. et al. Identification of novel immune-related targets mediating disease progression in acute pancreatitis. Frontiers in cellular and infection microbiology. 2022;12:1052466
107. Spindler MP, Siu S, Mogno I, Li Z, Yang C, Mehandru S. et al. Human gut microbiota stimulate defined innate immune responses that vary from phylum to strain. Cell Host Microbe. 2022;30:1481-98.e5
108. Oviedo-Boyso J, Bravo-Patiño A, Baizabal-Aguirre VM. Collaborative action of Toll-like and NOD-like receptors as modulators of the inflammatory response to pathogenic bacteria. Mediators Inflamm. 2014;2014:432785
109. Yan Y, Lu B, Li P, Wang J. NOD receptor and TLR9 modulation in severe acute pancreatitis-induced intestinal injury. Mol Med Rep. 2017;16:8471-6
110. Xu S, Wei S, Guo Y, Cui D, Yao J. Involvement of Nucleotide-Binding and Oligomerization Domain-Like Receptors in the Intestinal Injury of Severe Acute Pancreatitis in Rats. Pancreas. 2018;47:245-51
111. Jiao J, Liu J, Li Q, Zhang G, Pan C, Luo F. et al. Gut Microbiota-Derived Diaminopimelic Acid Promotes the NOD1/RIP2 Signaling Pathway and Plays a Key Role in the Progression of Severe Acute Pancreatitis. Front Cell Infect Microbiol. 2022;12:838340
112. Griffin ME, Hespen CW, Wang YC, Hang HC. Translation of peptidoglycan metabolites into immunotherapeutics. Clin Transl Immunology. 2019;8:e1095
113. Kim JS, Kim HK, Lee J, Jang S, Cho E, Mun SJ. et al. Inhibition of CD82 improves colitis by increasing NLRP3 deubiquitination by BRCC3. Cell Mol Immunol. 2023;20:189-200
114. Jia L, Chen H, Yang J, Fang X, Niu W, Zhang M. et al. Combinatory antibiotic treatment protects against experimental acute pancreatitis by suppressing gut bacterial translocation to pancreas and inhibiting NLRP3 inflammasome pathway. Innate Immun. 2020;26:48-61
115. Sheng K, Xu Y, Kong X, Wang J, Zha X, Wang Y. Probiotic Bacillus cereus Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice through Improvement of the Intestinal Barrier Function, Anti-Inflammation, and Gut Microbiota Modulation. J Agric Food Chem. 2021;69:14810-23
116. Snigdha S, Ha K, Tsai P, Dinan TG, Bartos JD, Shahid M. Probiotics: Potential novel therapeutics for microbiota-gut-brain axis dysfunction across gender and lifespan. Pharmacol Ther. 2022;231:107978
117. Werawatganon D, Vivatvakin S, Somanawat K, Tumwasorn S, Klaikeaw N, Siriviriyakul P. et al. Effects of probiotics on pancreatic inflammation and intestinal integrity in mice with acute pancreatitis. BMC complementary medicine and therapies. 2023;23:166
118. Kobayashi K, Honme Y, Sashihara T. Lactobacillus delbrueckii subsp. bulgaricus 2038 and Streptococcus thermophilus 1131 Induce the Expression of the REG3 Family in the Small Intestine of Mice via the Stimulation of Dendritic Cells and Type 3 Innate Lymphoid Cells. Nutrients. 2019;11:2998
119. Cao F, Jin L, Gao Y, Ding Y, Wen H, Qian Z. et al. Artificial-enzymes-armed Bifidobacterium longum probiotics for alleviating intestinal inflammation and microbiota dysbiosis. Nat Nanotechnol. 2023;18:617-27
120. Wu P, Yu Y, Li L, Sun WJ. Effect and safety of probiotics combined early enteral nutrition on severe acute pancreatitis patients. Biomed Res-India. 2017;28:1403-7
121. Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM. et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2008;371:651-9
122. Gou S, Yang Z, Liu T, Wu H, Wang C. Use of probiotics in the treatment of severe acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18:R57
123. Duysburgh C, Van den Abbeele P, Franckenstein D, Westphal M, Kuchinka-Koch A, Marzorati M. Co-Administration of Lactulose Crystals with Amoxicillin Followed by Prolonged Lactulose Treatment Promotes Recovery of the Human Gut Microbiome In Vitro. Antibiotics (Basel). 2022;11:962
124. Hiraishi K, Zhao F, Kurahara LH, Li X, Yamashita T, Hashimoto T. et al. Lactulose Modulates the Structure of Gut Microbiota and Alleviates Colitis-Associated Tumorigenesis. Nutrients. 2022;14:649
125. Pan LL, Deng YY, Wang R, Wu C, Li J, Niu W. et al. Lactose Induces Phenotypic and Functional Changes of Neutrophils and Macrophages to Alleviate Acute Pancreatitis in Mice. Front Immunol. 2018;9:751
126. Lee YS, Kim TY, Kim Y, Lee SH, Kim S, Kang SW. et al. Microbiota-Derived Lactate Accelerates Intestinal Stem-Cell-Mediated Epithelial Development. Cell Host Microbe. 2018;24:833-46.e6
127. Hashikura N, Murakami R, Sakurai T, Horigome A, Toda K, Xiao JZ. et al. Synbiotics of Bifidobacterium breve MCC1274 and lactulose enhances production of tryptophan metabolites in fermented human fecal communities. Food Res Int. 2023;163:112308
128. Zheng W, Zhao Z, Yang Y, Ding L, Yao W. The synbiotic mixture of lactulose and Bacillus coagulans protects intestinal barrier dysfunction and apoptosis in weaned piglets challenged with lipopolysaccharide. J Anim Sci Biotechnol. 2023;14:80
129. Rohith G, Sureshkumar S, Anandhi A, Kate V, Rajesh BS, Abdulbasith KM. et al. Effect of Synbiotics in Reducing the Systemic Inflammatory Response and Septic Complications in Moderately Severe and Severe Acute Pancreatitis: A Prospective Parallel-Arm Double-Blind Randomized Trial. Digestive diseases and sciences. 2023;68:969-77
130. Paramsothy S, Nielsen S, Kamm MA, Deshpande NP, Faith JJ, Clemente JC. et al. Specific Bacteria and Metabolites Associated With Response to Fecal Microbiota Transplantation in Patients With Ulcerative Colitis. Gastroenterology. 2019;156:1440-54 e2
131. Wang M, Xie X, Zhao S, Ma X, Wang Z, Zhang Y. Fecal microbiota transplantation for irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Frontiers in immunology. 2023;14:1136343
132. Wang JW, Kuo CH, Kuo FC, Wang YK, Hsu WH, Yu FJ. et al. Fecal microbiota transplantation: Review and update. Journal of the Formosan Medical Association = Taiwan yi zhi. 2019;118(Suppl 1):S23-S31
133. Zhang C, Li G, Lu T, Liu L, Sui Y, Bai R. et al. The Interaction of Microbiome and Pancreas in Acute Pancreatitis. Biomolecules. 2023;14:59
134. Liu LW, Xie Y, Li GQ, Zhang T, Sui YH, Zhao ZJ. et al. Gut microbiota-derived nicotinamide mononucleotide alleviates acute pancreatitis by activating pancreatic SIRT3 signalling. Br J Pharmacol. 2023;180:647-66
135. Yu X, Zhang Y, Pei K, Tan J, Tian H, Xu T. et al. Lactiplantibacillus plantarum attenuates Coxsackievirus B3-induced pancreatitis through the BAX/BCL2/CASP3 signaling pathway. Food & function. 2023;14:4129-42
136. Mores MG, Fikry EM, El-Gendy AO, Mohamed WR, Badary OA. Probiotics mixture and taurine attenuate L-arginine-induced acute pancreatitis in rats: Impact on transient receptor potential vanilloid-1 (TRPV-1)/IL-33/NF-κB signaling and apoptosis. Tissue & cell. 2023;85:102234
137. Pan X, Fang X, Wang F, Li H, Niu W, Liang W. et al. Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. British journal of pharmacology. 2019;176:4446-61
Author contact
![]() Corresponding authors: Xiaofeng Zhang, Ph.D. Department of Gastroenterology, Affiliated Hangzhou First People's Hospital, Westlake University School of Medicine, Hangzhou 310006, China. E-mail: 837837edu.cn. Jianfeng Yang, Ph.D. Department of Gastroenterology, Affiliated Hangzhou First People's Hospital, Westlake University School of Medicine, Hangzhou 310006, China. E-mail: yjf-1976com.
Corresponding authors: Xiaofeng Zhang, Ph.D. Department of Gastroenterology, Affiliated Hangzhou First People's Hospital, Westlake University School of Medicine, Hangzhou 310006, China. E-mail: 837837edu.cn. Jianfeng Yang, Ph.D. Department of Gastroenterology, Affiliated Hangzhou First People's Hospital, Westlake University School of Medicine, Hangzhou 310006, China. E-mail: yjf-1976com.

 Global reach, higher impact
Global reach, higher impact