10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2025; 21(4):1410-1435. doi:10.7150/ijbs.96155 This issue Cite
Review
Bispecific antibodies as powerful immunotherapeutic agents for urological cancers: Recent innovations based on preclinical and clinical evidence
1. Nephrology and Urology Research Center, Clinical Sciences Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran.
2. Department of Pharmacology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
3. NUS Center for Cancer Research (N2CR), Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
4. Department of Biological, Chemical and Pharmaceutical Sciences and Technologies, University of Palermo, 90123 Palermo, Italy.
5. Department of Cell Systems and Anatomy, UT Health San Antonio, Long School of Medicine, San Antonio, Texas USA.
6. NanoBio High-Tech Materials Research Center, Department of Biological Sciences and Bioengineering, Inha University, 100 Inha-ro, Incheon 22212, Republic of Korea.
7. Department of Vitro Vision, DeepkinetiX Inc., Boston, MA, USA.
8. Department of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
Received 2024-3-10; Accepted 2024-8-25; Published 2025-1-27
Abstract
Conventional immunotherapy has emerged as a key option for cancer treatment. However, its efficacy has been limited in urological cancers, especially prostate cancer, because of the immunosuppressive tumor microenvironment (TME), difficulty in drug delivery, aberrant immune response, and damage to normal cells. Bispecific antibodies (BsAbs) are engineered proteins with two different antigen-binding domains, designed using different technologies and in various formats. BsAb-based tumor immunotherapy has yielded optimistic results in preclinical and clinical investigations of many tumor types, including urological cancers. However, a series of challenges, including tumor heterogeneity, TME, Ab immunogenicity, adverse effects, serum half-life, low response rates, and drug resistance, hamper the application of BsAbs. In this review, we provide insights into the most common BsAb platforms with different mechanisms of action, which are under preclinical and clinical research, along with ways to overcome the challenges in BsAb administration for treating urological cancer.
Keywords: Urologic neoplasms, Bispecific antibodies, Immunotherapy, Drug delivery
1. Background
Common urological cancers include prostate, kidney, bladder, testis, and penile cancers. While prostate, testicular, and penile cancers occur only in men, kidney and bladder cancers can affect both men and women, with men being more susceptible. Many novel therapeutic strategies have been introduced in recent years to manage cancer and increase the survival of patients; however, treating these cancer types, especially when they acquire a metastatic state, remains challenging, particularly in prostate carcinoma (1-3).
In recent decades, therapeutic antibodies (Abs) have been developed as important components of cancer treatment because of their specificity and sensitivity (4). Approved in 1986 by the Food and Drug Administration (FDA), muromonab is considered the pioneer of monoclonal Ab (mAb)-based therapeutic strategies (5). Despite the success of mAbs in cancer therapy, various factors and pathways are involved in cancer pathophysiology. Thus, achieving the desired goal is difficult because of drug resistance and toxicity (6-8). The introduction of bispecific antibodies (BsAbs) has addressed the problem of drug resistance and improved the efficacy of cancer therapy (9-11). BsAbs recognize two distinct antigens or antigenic epitopes and target specific points; they also redirect effector T cells and other immune cells to the tumor site (12). While most BsAbs under development are used to treat cancers, others are focused on chronic inflammatory conditions, hematological disorders, and infectious diseases (13).
The concept of BsAbs was initially proposed by Nisonoff et al. in 1961 and was based on coupling the Fab fragments of two different rabbit antibodies using reoxidation (14). In 1975, Milstein and Kohler introduced hybridoma technology by fusing splenic B lymphocytes and myeloma cells of immunized mice to solve the problem of producing pure mAbs (15). In 1983, Milstein et al. succeeded in producing the first BsAb with an IgG structure by fusing two different hybridoma cells, known as hybrid-hybridoma (quadroma) technology (16). In 1985, Perez et al. identified the anti-tumor function of BsAbs, which could bind to T cells and tumor-associated antigens (TAA), resulting in T cell recruitment to the malignant site and tumor killing (17). In 1988, Huston et al. developed a single-chain variable fragment (scFv), with fewer refolding problems (18). Emergence of the knobs-into-holes technology in 1996 significantly advanced BsAb production (19). With improvements in biotechnology and antibody engineering, various platforms were developed and BsAbs gradually entered marketing. In 2009, the European Medicines Agency (EMA) approved catumaxomab as the first therapeutic BsAb in Europe. Catumaxomab targets CD3 and the epithelial cell adhesion molecule (EpCAM), and is used to treat patients with malignant ascites; however, it was withdrawn from the market in 2017 owing to commercial reasons (July 10, 2017; EMA (428877/2017). However, it is still under investigation. Since 2014, the FDA has approved nine BsAbs for treating cancer and hematologic and ocular diseases.
In 2014, the FDA approved blinatumomab, an antibody targeting CD3 and CD19, which was developed for treating acute lymphoblastic leukemia. Emicizumab, amivantamab, tebentafusp, faricimab, teclistamab, mosunetuzumab, epcoritamab, and glofitamab are other BsAbs, approved by the FDA since 2021, mostly for treating hematologic cancers and disorders (20).
Considering the promising and effective results from the clinical application of BsAbs, they have become a top issue in Ab drug research. Recently, more than 200 clinical and preclinical studies have been reported in the field of BsAbs.
Therapeutic options available for urological cancer treatment show limited efficacy, and the development of novel and more specific therapies is a medical necessity. Several preclinical and clinical studies have evaluated the anti-tumor effects of BsAbs against urological cancers. Most of these studies provide valuable data and warrant further investigation in larger cohorts. This review describes the different types and mechanisms of action of BsAbs, advances in BsAb design, and the preclinical and clinical trial developments of BsAbs in urological cancers. In addition, it discusses the challenges and potential solutions for BsAb-based therapy, and future perspectives.
2. The landscape of bispecific antibodies
A conventional antibody (Ab) is a dual-valent but monospecific molecule comprising two antigen-binding (Fab) segments and one crystallizable fragment (Fc), which together form the characteristic Y configuration. Bispecific antibodies (BsAbs) that target two separate antigens can overcome the constraints of monospecific Abs, leading to reduced adverse effects and lower frequency of administration (21,22). However, the greater variety and complexity of BsAb structures compared with those of mAbs necessitate more sophisticated techniques for their synthesis and refinement (23,24). The BsAb sector is advancing rapidly and numerous structural variations are being explored. Initially, BsAbs were synthesized using quadroma technology; however, with the advent of genetic engineering, recombinant DNA methodologies have become paramount for crafting BsAbs with diverse attributes such as size, penetrability, serum longevity, and valency (25,26).
Based on the presence of the Fc region, BsAbs are typically classified into two groups: IgG-like (IgG-based) and non-IgG-like (fragment-based) (Table 1).
2.1. IgG-like BsAbs
IgG-like BsAbs resemble standard antibodies as they possess an Fc region and are composed of complete IgG molecules. The presence of an Fc region offers numerous benefits, including enhanced solubility and stability owing to improved purification, extended serum half-life, and involvement in immune effector functions, such as antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis, and complement-dependent cytotoxicity (40,41). However, these BsAb variants have drawbacks such as off-target binding of the active Fc region to Fc receptors (FcRs), which can lead to adverse effects and issues related to chain association (42). Coexpression of two distinct heavy (H) and light (L) chains poses initial challenges in BsAb development, reducing the likelihood of producing a functional BsAb from an array of potential combinations (43). Strategies have been devised recently to address these challenges. The main IgG-like BsAb formats include Triomab quadroma, Knobs-into-holes, CrossMab, Orthogonal, Duobody, XmAb, DVD-Ig, and FIT-Ig.
The Triomab quadroma approach relies on the somatic fusion of two disparate hybridomas, yielding a structure similar to that of classical Abs with two unique antigen-binding sites. However, this method is prone to mispairing the H and L chains. Creation of a chimeric rat/mouse quadroma mitigates this issue (27,42). For example, catumaxomab is a BsAb comprising murine IgG2a anti-CD3 and rat IgG2b anti-EpCAM (44).
Despite modifications to the quadroma platform, heavy-chain mispairing challenges have persisted. The knobs-into-holes technique, which involves engineering the CH3 domain of each H chain, was developed to minimize mismatches and enhance Fc heterodimerization (45). This strategy, which entails replacing a smaller amino acid with a larger one to create a 'knob' and vice versa to form a 'hole,' has resolved the issue of H chain mismatches; however, the L chain problem remains (29). Solutions such as the use of a common L chain, additional mutations in the VH-VL and CH-CL interfaces, and CrossMab formatting have been proposed to address this issue (30,46).
The CrossMab format, derived from the knobs-into-holes concept, involves domain swapping within one Fab of an Ab (CrossMabVH-VL) or exchanging the CL domain of the L chain with the CH1 domain of the H chain (CrossMabCH1-CL), thereby ensuring proper chain pairing (47,48). Faricimab, a CrossMab-based BsAb that targets ang-2 and VEGF, is currently FDA-approved for treating diabetic macular edema and nAMD (42).
The Orthogonal Fab interface, which introduces mutations into the variable region of Fab, allows the production of BsAbs in mammalian cells (31,49). LY3164530, which targets epidermal growth factor receptor (EGFR) and c-MET, is an example of this format (50).
The Duobody platform, achieved through controlled Fab arm exchange (cFAE), consistently produces the desired BsAb with high efficiency (42,51,52). Amivantamab, which targets MET and EGFR, is designed for the treatment of advanced non-small cell lung cancer and is based on this format (53,54).
The XmAb platform, developed by Moore et al., incorporates four mutations at the CH3-CH3 interface to enhance heavy chain heterodimerization, thus increasing FcR affinity and the potential for immune cell activation, as well as improving serum half-life through FcRn binding (33,55). XmAb20717, which targets PD-1 and CTLA-4, is currently in phase 2 clinical trials for advanced prostate cancer (NCT05032040).
Tetravalent BsAbs such as DVD-Ig and FIT-Ig, with four antigen-binding sites for two different targets, have also been developed. DVD-Ig features two variable domains per Fab arm, linked by short, flexible connectors, ensuring proper H and L chain pairing (34,56,57). Lutikizumab, a DVD-Ig-based BsAb, targeting IL-1α and IL-1β, is in phase 2 clinical trials for treating knee osteoarthritis synovitis (58).
FIT-Ig, comprising two sets of Fab domains, was introduced in 2017 (36). EpimAb, an FIT-Ig BsAb, targets EGFR and c-MET for treating advanced/metastatic solid tumors (59).
2.2. Non-IgG-like BsAbs
This category of BsAbs lacks the Fc segment and comprises variable light and heavy domains from two antibodies, or Fab units, linked with connectors such as disulfide or non-covalent bonds (60,61). Non-IgG-like BsAbs are characterized by their straightforward design and benefits including small size, reduced immunogenicity, cost-effectiveness, high production yield, enhanced tumor infiltration, and resolution of chain-related complications (26,46). However, they exhibit a brief serum lifespan, necessitating frequent dosing. Further, they are prone to reduced structural integrity and a heightened risk of aggregation, which are notable drawbacks.
Among these, the bispecific T cell engager (BiTE), also referred to as tandem scFv, is a BsAb with two scFv regions from two monoclonal antibodies connected by a supply peptide linker. One scFv in BiTE is invariably directed against CD3, whereas the other targets a tumor-associated antigen (TAA), facilitating the linkage of CD3+ T cells with specific tumor cells without the need for costimulation signals (62,63).
Blinatumomab is the inaugural FDA-sanctioned BiTE molecule (62,63). Additionally, a CD3 × prostate-specific membrane antigen (PSMA), BiTE BsAb was developed to treat PSMA-expressing tumors (64). Owing to the ephemeral half-life of BiTE BsAbs (approximately 2-4 hours), an advanced BiTE variant was conceived by combining BiTE with the Fc region, thereby prolonging its serum half-life to seven days. CD3 × CD19 half-life extended BiTE (HLE-BiTE) (AMG562) has demonstrated efficacy in treating CD19+ malignancies (65,66).
Dual-affinity retargeting molecules (DARTs) are synthesized by linking the VL and VH (scFv) domains of one antibody with those of another to form heterodimeric polypeptide chains (38). DARTs surpass BiTEs in terms of stability and efficacy in triggering T-cell responses against cancer cells. For instance, flotetuzumab, a DART targeting CD3ϵ and CD123, is accessible in Japan and Europe for treating refractory acute myeloid leukemia (67). Integration of the Fc fragment into DART, termed DART-Fc, enables FcRn-mediated recycling of the molecule (68).
Tandem diabodies (TandAbs) are quadrivalent entities comprising two peptide chains arranged inversely, each bearing two antigen-binding sites for distinct antigens (in the VL-1, VH-2, VL-2, and VH-1 configurations). TandAbs offer greater stability than that of their predecessors and can redirect T and NK cells to tumors (26,69). AFM11, which targets CD3 and CD19, has shown promising results in constraining Raji tumors in vivo (70). Furthermore, AFM13, another TandAb that binds to CD30 and CD16A on NK cells, has been utilized for the treatment of Hodgkin's lymphoma (71).
The bi-nanobody architecture consists solely of a heavy chain devoid of light chains. By connecting the VH domains of two or more antibodies, a bi- or multi-specific antibody is formed (39). This framework is notable for its simplicity, robustness, and superior tissue penetration; however, it suffers from a short serum half-life, which can be mitigated by conjugation with serum albumin (26,72). Ozoralizumab, a bi-nanobody-based BsAb featuring two anti-TNFα nanobodies and one anti-HAS nanobody, is currently undergoing phase 2 clinical trials for treating rheumatoid arthritis (72).
3. Mechanisms of action and design principles of bispecific antibodies
3.1. Mechanisms of action of BsAbs
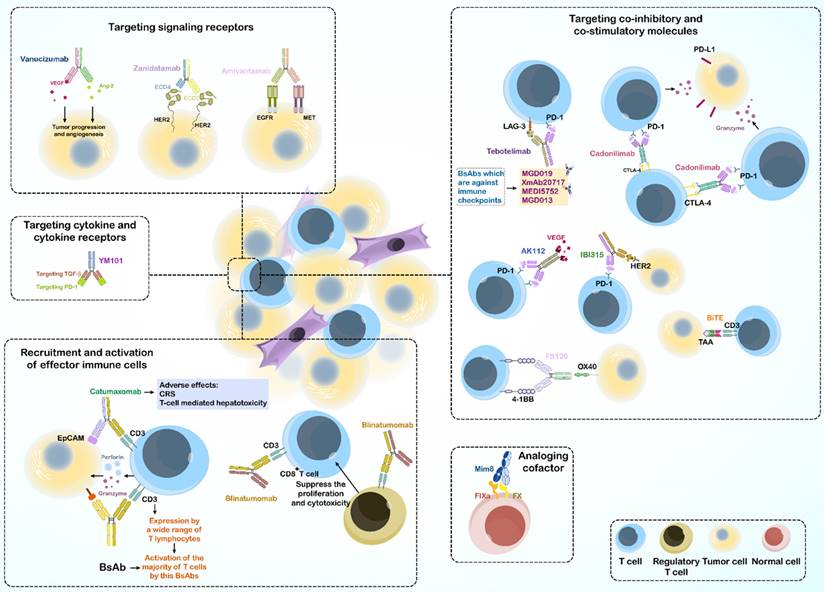
Targeting of two different antigens by BsAbs provides a variety of functional pathways; thus, BsAbs are used to treat diseases through multiple mechanisms. Some BsAbs play a role in recruitment and activation of innate and adaptive immune cells. Accordingly, they can connect immune cells with tumor cells to kill the tumor. Some BsAbs suppress co-inhibitory receptors, whereas others activate costimulatory molecules to activate immune cells. Other mechanisms include activation or inhibition of signaling receptors, targeting cytokines and their receptors, analogous cofactors, and using a target to transport them (Figure 1).
3.1.1. Recruitment and activation of effector immune cells
The initial application of BsAbs in cancer treatment focused on T cells recruitment to the site of tumor T cell-engaging BsAbs (T-BsAbs), which can connect T cells to specific cancer cells and ultimately improve tumor killing (73,74).
Owing to the fact that BsAbs mostly activate T cells through binding of the ε subunit of CD3 in the TCR complex, they bypass the major histocompatibility complex (MHC) restriction and induce activation without antigen presentation (75). This characteristic also circumvents tumor evasion strategies such as loss of MHC expression or defects in antigen presentation (76).
Different subtypes of bispecific antibodies
| Format | Date | Company | Design strategy | Ref. |
|---|---|---|---|---|
| Triomab quadroma | 1995 | Fresenius and Trion Pharma | Somatic fusion of mouse and rat hybridomas | (27,28) |
| Knobs-into-holes | 1996 | Genentech | Forming a knob by substituting a smaller amino acid with a larger one in the CH3 domain of an Ab chain Forming a hole by replacing a larger amino acid in the other chain with a smaller amino acid | (29) |
| CrossMab | 2011 | Roche | CrossMabVH-VL format: swapping heavy and light chain domains within one Fab of the Ab CrossMabCH1-CL format: swapping CL of the light chain with the CH1 domain of the heavy chain | (30) |
| Orthogonal | 2014 | Indicating mutation in the variable region of Fab in the antibodies | (31) | |
| Duobody | Genmab | Exchanging Fab arm between two antibodies | (32) | |
| XmAb | 2019 | Xencor, Inc. | Introducing mutations at the CH3-CH3 interface | (33) |
| DVD-Ig | 2007 | Abbott | Connecting two variable domains in each Fab arm with short flexible linkers and creating a tetravalent bispecific antibody with four antigen binding sites | (34,35) |
| FIT-Ig | 2017 | Epimab Biotherapeutics, Inc. | Similar to DVD-Ig but the FIT-Ig structure contains two pairs of Fab domains | (36) |
| BiTE | 1995 | Amgen | Two ScFv regions of two monoclonal antibodies bound together by a flexible peptide linker | (37) |
| DART | Macrogenics | Connecting VL and VH (ScFv) domains of one antibody with the VL and VH (ScFv) of another antibody, forming heterodimer polypeptide chains | (38) | |
| TandAb | Affimed | Pairing two chains in opposite direction to create a tetravalent molecule and each chain has two antigen binding sites | (26) | |
| Bi-Nanobody | 2018 | Ablynx | The VH domains of two or more antibodies connect together and a bispecific antibody is achieved | (39) |
Further, activated T cells can induce tumor cell necrosis or apoptosis by producing perforin and granzyme or stimulating death ligands, such as the Fas-FasL pathway (75). Blinatumomab induces cancerous cell removal in Philadelphia chromosome-negative B cell ALL by redirecting human T cells (77).
However, using BsAbs in cancer immunotherapy may also cause severe adverse effects like the cytokine release syndrome and cell T cell mediated cell cytotoxicity related to off-target binding of the active Fc domain to the FcγR of immune cells (78-81).
As CD3 is expressed by a wide range of T lymphocytes, CD3 binding can activate the majority of the T cell population, including regulatory T cells (82,83). An in vitro study reported that human regulatory T cells activated by blinatumomab suppressed the proliferation and cytotoxic effects of CD8+ cytotoxic T lymphocytes (CTLs) (84). Targeting specific T-cell subsets circumvents the activation of immunosuppressive regulatory T cells and decreases the risk of side effects.
Activation of cytotoxic γδ T cells was successful as BsAb was used to target the γδTCR and a human ovarian cancer antigen in vitro (85). 7D12-5 GS-6H4 is a novel BsAb targeting Vγ9 chain of the Vγ9Vδ2 TCR and of epidermal growth factor receptor (EGFR). 7D12-5 GS-6H4 induces activation of Vγ9Vδ2 T cells, a small subpopulation of γδ T cells, and increases apoptosis of colorectal cancer cells in a mouse xenograft model (86).
In addition to T cells, BsAbs can target specific antigens on the surface of NK cells [called Bispecific killer cell engager (BiKE)] and macrophages. For instance, AFM13, a TandAb that recognizes CD16A on NK cells and CD30 on cancerous lymphocytes, is used for treating Hodgkin's lymphoma, and is in phase I clinical trials (71). Overall, AFM13 can selectively activate NK cells and provide anti-tumor immunity (87).
3.1.2. Targeting co-inhibitory and co-stimulatory molecules
Immune cells express multiple regulatory molecules on their surface, including a group of immune checkpoint proteins that regulate cell activation, proliferation, and anti-tumor activity (88). Stronger anti-tumor immunity is achieved by inhibiting or stimulating the relevant pathways (89,90).
In cancer immunotherapy using immune checkpoint inhibitors (ICIs), immune cell activation in the tumor microenvironment (TME) is enhanced, resulting in the apoptosis of tumor cells, making ICIs an important treatment option for cancer (91,92). The therapeutic idea of blocking two inhibitory immune checkpoints came from the promising results of combinational therapy. For example, a clinical trial demonstrated the 5-year outcomes of nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4) combination therapy in advanced melanoma, which resulted in long-term progression-free and overall survival compared to those with monotherapy using each of the Abs (93).
Tebotelimab (MGD013) is a tetravalent DART BsAb, targeting PD-1 and LAG-3 and is used to control solid tumors and hematologic malignancy in phase 1 clinical trials (94). Recent studies have shown that tebotelimab is more effective than PD-1 antibody monotherapy (95).
Cadonilimab is a tetrabody BsAb that targets both PD-1 and CTLA-4. While infiltrated lymphocytes in the TME showed increased co-expression of CTLA-4 and PD-1 compared with that in peripheral T cells, cadonilimab enrichment was enhanced in the TME to activate exhausted T cells. This BsAb has been used in China since 2022 for treating recurrent or metastatic cervical cancer (R/M CC) (96).
Other BsAbs targeting dual immune checkpoints include MGD019, XmAb20717, MEDI5752, and MGD013, which are dual-target (6).
Further, BsAbs can target immune checkpoints and TAA simultaneously, such as AK112 (PD1 ˟ VEGF) and IBI315 (PD1 ˟ HER2). The mechanism of action in some BsAbs is based on the combination of T-cell redirection and immune checkpoint blockade, such as an anti-CD33 ˟ CD3 BiTE, which links to the extracellular domain of PD-1, known as checkpoint inhibitory T-cell engager (CiTE). The CiTE Ab leads to enhanced T cell cytotoxic effects toward CD33+ PD-L1+ cancer cells in preclinical models of acute myeloid leukemia (6,97).
Co-stimulatory molecules expressed by T cells through the TCR, are critical for T cell activation, survival, proliferation, cytokine production, and release (98).
FS120 is a BsAb on a tetravalent platform that targets 4-1BB and OX40. FS120 activates the 4-1BB pathway and subsequently induces T-cell activation and proliferation. To increase specificity and reduce the side effects of FS120, the binding arm targeting 4-1BB is activated only after Ab binding to OX40 (99,100). Further, many TAA-targeted 4-1BB-agonistic BsAbs, which are Fc-free or Fc-silenced, show acceptable anti-tumor activity without toxicity in mouse and primate models, and some of these are currently in clinical development (101-103).
3.1.3. Targeting signaling receptors
Targeting two tumor receptors using BsAbs ensures specific binding of the Ab to tumor cells, even if one of the desired TAAs is also expressed by normal cells, and low toxicity is observed. Many studies have reported dysregulation of multiple signaling pathways and proteins in tumor cells. Thus, targeting them provides a better approach for treating and overcoming drug resistance.
3.1.3.1. inhibition of signaling receptors
The inhibition of signaling pathways can suppress tumor development and angiogenesis in the TME. The HER family and VEGFR are two well-known receptor tyrosine kinases (RTKs) that play key roles in cell proliferation and the execution of cell programs (104). Mutations in RTKs and abnormal activation of their signaling in cells are associated with cancer development (105). Single targeting of RTKs in cancer seems to be effective in treatment; however, other RTKs in tumor cells can escape inhibition, resulting in drug resistance. Overall, BsAbs target multiple RTKs or their ligands and block two or more signaling pathways to reduce tumor cell escape.
Amivantamab, approved by the FDA on May 21, 2021, targets EGFR and MET simultaneously and circumvents drug resistance in non-small cell lung cancer (106-109).
Another example is a BsAb that recognizes two epitopes on the same antigen. Zanidatamab (ZW25) is a BsAb against two non-overlapping domains of HER2 antigen (ECD4 and ECD2) and has yielded promising results in treating HER2-positive breast cancer and gastroesophageal adenocarcinoma (GEA) in a phase 1 clinical trial (110,111). By targeting angiogenesis, vanucizumab provides a potential therapy for advanced solid tumors, as it targets VEGF and Ang-2, both of which are involved in tumor growth and angiogenesis (112).
3.1.3.2. Activation of signaling receptors
In contrast to inhibition of the desired signaling by inhibitory BsAbs, some therapeutic processes require activation of receptor signaling using agonistic antibodies. This mechanism of action of BsAbs is useful for treating diseases other than cancer. BFKB8488A, a bispecific antibody against fibroblast growth factor receptor 1 (FGFR1) and KLB has been used to treat obesity-related metabolic defects by activating FGFR1/KLB (113,114).
3.1.4. Targeting cytokine and cytokine receptors
Abnormal cytokine regulation is associated with tumor progression, and targeting these cytokines improves treatment efficacy. Although the high immunogenicity and short half-life of cytokine antagonists limit their use, combination therapies may overcome this limitation, which sparked the idea of BsAb designing (115,116).
A combination using TGF-β inhibitors with ICIs increases anti-tumor activity (117). For example, YM101, a BsAb targeting TGF-β and PD-1 based on the Checkbody platform, promoted the formation of hot tumor by increasing the attracting lymphocytes and dendritic cells to the tumor site, elevating the ratio of M1/M2, increasing cytokine production in T cells, and finally destroying the tumor (118).
In addition to cancer immunotherapy, BsAbs can also be used to treat inflammatory diseases such as rheumatoid arthritis. ABT122, designed in the DVD-Ig format, targets TNF-α and IL-17 to treat rheumatoid arthritis (119).
3.1.5. Acting as analogous cofactors
BsAbs can also act as enzymes or as a cofactor for an enzyme. The Mim8 BsAb targets activating factor IX (FIXa) and factor X (FX), as an activated factor VIII (FVIII) mimetic. which is important for achieving hemostasis. This BsAb can be used to treat hemophilia A in patients with congenital factor VIII deficiency (120).
3.1.6. Using target to transport
BsAbs can use the specificity of their first target to transport their second specific target, also known as hijacking. For example, in neurological diseases, BsAbs transport the transferrin receptor from their first antigen-binding domain across the blood-brain barrier (BBB) in immune-privileged brain regions and target pathogenic mediators with their second antigen-binding site (121,122). This process is also useful in treating viral and bacterial infection (123,124). Finally, targeting CD63 (also known as LAMP3), which is involved in lysosomal trafficking, and HER2, a TAA, using a BsAb can improves the intracellular delivery of an antibody-drug conjugate to the lysosome (125).
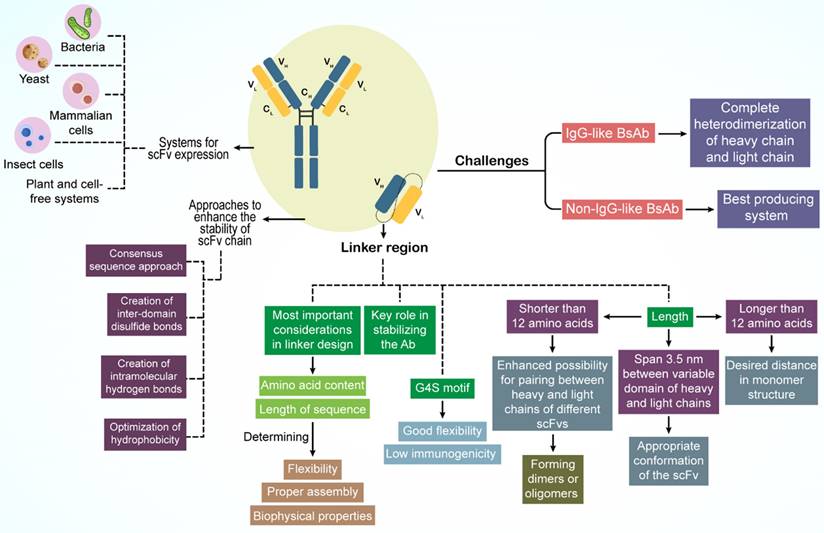
3.2. The design principle of BsAbs
Creating a high-quality BsAb with minimal contamination is a significant hurdle. For non-IgG BsAbs, the optimal production system depends on factors such as BsAb size, amino acid sequence, protein configuration, solubility, stability, and purification processes. Conversely, for IgG-like BsAbs, complete heterodimerization of the heavy and light chains is crucial for the production of pure antibodies (Figure 2).
For non-IgG-like BsAbs, the linker region connecting the light and heavy chain domains is vital for antibody stabilization and scFv optimization (126). The composition and sequence length of the amino acids in the linker are pivotal; they govern the flexibility, assembly accuracy, and biophysical characteristics of BsAbs (26). The G4S motif (G: glycine; S: serine), a commonly used linker, demonstrates notable flexibility and minimal immunogenicity (60).
The linker length is critical, ideally extending 3.5 nm between the variable domains of the heavy and light chains to ensure that scFv adopts the correct structure (26). Moreover, scFv stability is essential for BsAb design, and directly influences the biological function of the antibody. Stable scFv regions also serve as foundational elements for BsAbs (127). Various methods exist to bolster scFv chain stability, such as the consensus sequence approach, in which a mutation is introduced into the most prevalent amino acid in the homologous Fv domain to enhance stability. Other techniques include the formation of interdomain disulfide bonds, intramolecular hydrogen bonds, and refinement of hydrophobic interactions (26).
Diverse platforms are available for the expression of non-IgG-like BsAbs, including bacterial, yeast, mammalian, insect, plant, and cell-free systems, each of which is suitable for different BsAbs (128,129). Escherichia coli is a favored host for scFv expression owing to its rapid growth and ease of genetic manipulation despite its lack of chaperones and post-translational modification processes, which necessitate further scFv modifications. In contrast, mammalian cells offer a promising alternative, providing protein folding pathways and post-translational modifications to prevent misfolding (129-131).
Addressing chain-associated issues is a primary concern for IgG-like BsAbs (23). Several strategies have been devised to modify the CH3 domain for proper heavy chain assembly, as detailed in Section 2.1. Advanced methods for enhancing heavy- and light-chain interactions are discussed in the same section.
Bispecific antibodies: Mechanisms of action.
Design principles of BsAbs.
An innovative solution to light-chain mispairing involves the production of BsAbs through the combination of half-antibodies from two distinct cell lines, a co-culture approach that reduces contamination risks and costs (132). This method involves inoculating two separate E. coli strains, each harboring a plasmid for one half antibody, into a single vessel, facilitating the production of a broader range of stable antibodies (132). CHO cells are also employed in co-culture techniques with variations in plasmid design, inoculation, and harvesting processes (133).
Given the complexity of producing IgG-like BsAbs, which typically require two plasmids for heterodimerized heavy chains and one for a shared light chain or two separate light-chain plasmids, mammalian cells are predominantly used (43). CHO and KEK293 cell lines are considered optimal for this purpose (134,135).
The design of BsAbs is also influenced by their affinity and valency. Affinity affects the cytokine release, drug distribution, and overall tolerability of BsAbs. For instance, a CD3 arm with high affinity can trigger excessive cytokine release and reduce the ability to target disease sites (136,137). Research has shown that PSMA˟ CD3 BsAbs with lower CD3 affinity are more effective at eliminating tumor cells and reducing the incidence of cytokine release syndrome (CRS) in prostate cancer than BsAbs with higher CD3 affinity (138).
Valency, or the number of antigen binding sites, also plays a role in the efficacy of BsAbs. For example, glofitamab, with a 2:1 valency ratio against CD20 and CD3, exhibits an anti-tumor activity that is 40 times greater than that of BsAbs with a 1:1 valency (139).
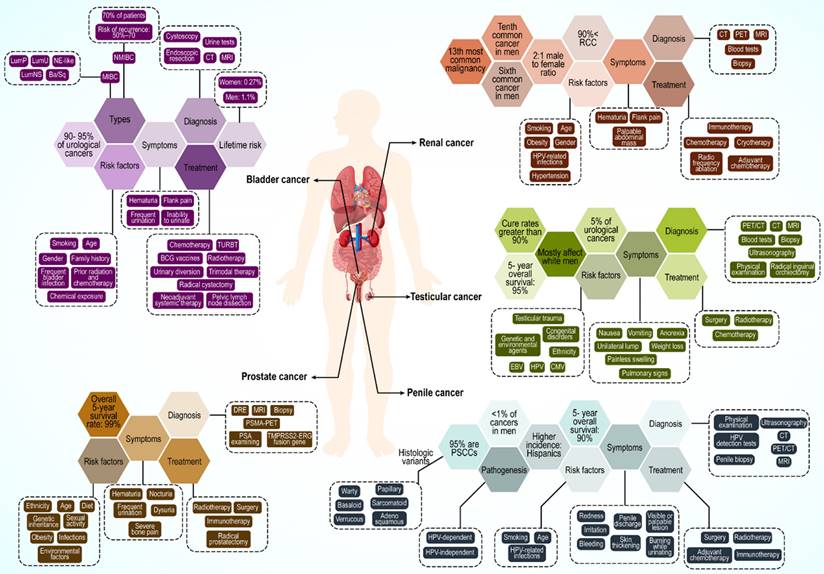
4. Urological cancers: A brief description
Urological cancer is a general term for prostate, kidney, bladder, testicular, and penile cancers (Figure 3). Among these, prostate, testicular, and penile cancers are male specific. Prostate cancer is the most common cancer across all racial and ethnic groups. Bladder and kidney cancers are the second and third most common, respectively, whereas penile cancers are very rare. However, this order can change because of racial differences.
4.1. Prostate cancer
Prostate cancer remains a significant public health concern for men globally, particularly in Western countries. In the US, prostate cancer is the most common cancer in males (ages 45-60) and a leading cause of cancer-related death across races (140-142). Its global incidence is projected to exceed 1.5 million new cases by 2030 owing to the aging population (143). Black men have a notably higher global incidence.
Prostate cancer originates as an adenocarcinoma of the prostate gland. These cancers can spread beyond the prostate and metastasize to the lymph nodes and bones (144,145). Early stages often lack specific symptoms and potentially mimic benign conditions (146). Metastatic disease commonly presents as intense bone pain in the back, pelvis, hips, or ribs (147). Genetics plays a major role, especially in men with a family history (148). Specific gene mutations (ATM, BRCA1/2, RNase L, MSR1, and ELAC2) and single-nucleotide polymorphisms (SNPs) are major contributors (149,150). Age, obesity, diet, sexual habits, infections, and environmental exposure also influence risk (151-153).
Like other neoplasms, early detection is crucial, and prostate-specific antigen (PSA) testing is a common screening method, with newer biomarkers such as PHI and TMPRSS2-ERG fusion genes gaining traction (154,155). Additional diagnostic tools include digital rectal examination (DRE), biopsy, magnetic resonance imaging (MRI), TRUS, and PSMA-PET scans (156,157).
Treatment depends on the stage and risk. Active surveillance, radical prostatectomy, and radiotherapy are standard treatments for low-risk and stage I-III cancer. Androgen ablation (surgical or pharmacological) is preferred for stage IV and high-risk stage III (158). Sipuleucel-T (Provenge) immunotherapy is used in advanced and hormone-resistant cases (159). Future directions include combination therapies, gene therapies, and strategies to overcome drug resistance (160,161).
4.2. Kidney cancer
Kidney cancer is the 13th most common malignancy worldwide, the sixth most common cancer in men, and the tenth most common in women (162,163). Renal cancer mostly occurs in European and North American populations, with a lower incidence in Asian individuals.
More than 90% of kidney cancers are renal cell carcinomas (RCC) originating from renal tubular epithelial cells (164). Other subsets of kidney cancers include urothelial carcinomas, sarcomas, and mesenchymal tumors, which occur at lower rates (165). RCC is usually diagnosed at the age of 50-70 years, with an approximate 2:1 male to female ratio. A continuously increasing incidence has been reported, particularly in developed countries. Mortality has dropped by approximately 1% every year since 2008; however, RCC remains the most lethal urological cancer in comparison to bladder and prostate cancers, with a mortality rate of 30-40% (166,167).
Typical symptoms of RCC include hematuria, flank pain, and a palpable abdominal mass, which is seen in only a small percentage of patients (168). Approximately, 30-50% of patients progress to metastasis with local disease, and 40% with localized RCC have distant metastases involving the lungs, bones, brain, adrenal glands, other kidney, and liver (169).
RCC is a heterogeneous cancer classified based on histological differences that affect the prognosis and treatment choice. Clear cell RCC (70-90%), papillary RCC (10-15%), and chromophore (3-5%) are the most common RCC subtypes. Age and sex are the most important risk factors for RCC (170). Ethnicity, smoking history, use of tobacco products, hypertension (HTN), and obesity are other possible risk factors (171-173).
Approximately 3% of RCC cases have a familial background with an autosomal-predominant pattern. Some of the genes involved in RCC incidence are VHL, MET, FH, BHD, and HRPT2, with most mutations in the VHL gene causing hereditary clear cell RCC (174). In addition to laboratory tests, contrast-enhanced ultrasound (CEUS), computed tomography (CT), MRI, positron emission tomography (PET), and tissue biopsy are used for RCC diagnosis (175,176).
Surgical treatment remains crucial for RCC. Further, active surveillance, cryotherapy, radio frequency ablation, and adjuvant therapy are other available forms of treatment. In metastatic RCC, immunotherapy using immunosuppressive factors such as IFN-α and IL-2 is common. Recently, nivolumab, an anti-PD-1 immune checkpoint inhibitor, has emerged as a promising treatment (177-179).
4.3. Bladder cancer
Bladder cancer is among the most prevalent cancers worldwide, ranging from nonaggressive and usually noninvasive tumors to aggressive or advanced-stage disease with high mortality. Approximately 90% of individuals with bladder cancer are older than 55 years (180). The lifetime risk of bladder cancer is approximately 1.1% in men and 0.27% in women. The incidence rate is higher in Western countries, largely because of carcinogen exposure. It is the eighth most common cause of cancer-related deaths in men in the US, but its mortality rate has decreased from 2016 to 2020 (181). Bladder cancer is a carcinoma of urothelial cells that accounts for 90-95% of all urothelial carcinomas (182). It can present as non-muscle-invasive bladder cancer (NMIBC), considered as stage Ta and T1, and muscle-invasive bladder cancer (MIBC) or a metastatic form of the disease which includes T2-T4 stages. Furthermore, there is a distinct phenotype of noninvasive lesions with a high rate of recurrence, known as carcinoma in situ (CIS) (182,183).
Approximately 70% of patients with bladder cancer have NMIBC, with a high risk of recurrence (50-70%); this progresses to MIBC form in 10-20% of cases (184).
The most obvious symptom of bladder cancer is hematuria, which may only be visible or detectable by microscopy. Other reported symptoms are nonspecific and include frequent urination, pain during urination, flank pain, and inability to urinate (185).
Age is the greatest risk factor for bladder cancer owing to increased exposure to carcinogens and a reduced ability to repair DNA. Other risk factors include smoking and tobacco use, sex, family history, frequent bladder infection, prior radiation and chemotherapy, and chemical exposure (186-189). Men are diagnosed with bladder cancer three to four times more often than women (185). Bladder cancer is a cancer with many mutations, with 70-80% of patients having mutations in the promoter of the gene encoding telomerase reverse transcriptase (TERT) (190). Moreover, in NMIBC cases, the most frequent phenotype, other DNA abnormalities have been noted, such as deletions in chromosome 9 and mutations in genes encoding FGFR3 and PI3K, which could be an early evidence for malignancy (191,192). Strategies for bladder cancer diagnosis include cystoscopy and endoscopic resection, cross-sectional urography (i.e., CT or MRI), urine tests, and urine cytology tests (193-195).
The best method for managing bladder cancer is selected based on the cancer type. In perioperative chemotherapy for patients with NMIBC, Bacille Calmette-Guérin (BCG) vaccines and resection of the bladder tumor (TURBT) are treatment options (196). Neoadjuvant systemic therapy, radical cystectomy, pelvic lymph node dissection, urinary diversion, and trimodal therapy are used to treat MIBC. In the case of metastasis, cytotoxic chemotherapy and immunotherapy, particularly with immune checkpoint inhibitor mAbs against PD-1 and its ligand, PD-L1, are used (197,198).
4.4. Testicular cancer
Testicular cancer is a rare disease accounting for 5% of urological cancers and the most common malignant tumor in young men aged 15 to 34 years, with an increasing incidence worldwide in the past two decades (198-200). Excellent outcomes have been reported for testicular cancer, with cure rates greater than 90% for all stages and 95% five-year survival rates (201). Testicular cancer mostly affects white men; therefore, an increase in the incidence rate of the disease is expected in European countries until 2035 (202,203).
The transformation of primordial germ cells (PGCs) and formation of germ cell neoplasia in situ (GCNIS) are regarded as precursor lesions to malignant testicular germ cell tumors (TGCTs) (204,205). These can progress to 1) seminoma, 2) embryonal carcinoma cells, 3) choriocarcinomas and yolk-sac tumors, and 4) teratomas. Embryonal carcinoma cells, choriocarcinomas, yolk-sac tumors, and teratomas are also known as nonseminomas (201). Approximately 50% of patients with testicular cancer worldwide are diagnosed with seminoma, whereas the others may have various types of non-seminomas or mixed TGCTs (206).
Testicular cancer typically appears as a unilateral lump, and painless swelling with pain is noted in approximately 10% of the patients. Symptoms or signs of metastatic disease such as weight loss, nausea, vomiting, anorexia, pulmonary and lymphatic involvement (shortness of breath and lymphadenopathy), bulky retroperitoneal disease, vascular obstruction or thrombosis, and gastrointestinal hemorrhage are rarely present in patients (207).
Both genetic and environmental factors are involved in testicular cancer development. Congenital disorders, such as cryptorchidism (one or both testicles absent in the scrotum), hypospadias (the urethral opening not at the head of the penis), and low sperm count, are associated with higher testicular cancer occurrence (208,209). Family history, ethnicity, infections such as human papillomavirus (HPV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV), testicular trauma, and carcinoma in situ are other risk factors (210-212). Anomaly of the short arm of chromosome 12 is pathognomonic for all types of adult germ cell tumors and is an example of genetic risk factors for testicular cancer (213).
Disease evaluation is achieved by physical examination, blood tests (AFP, bHCG, and LDH), CT, PET/CT, MRI, scrotal ultrasonography, radical inguinal orchiectomy, and biopsy (214,215). Germinal cell testicular cancer is managed based on tumor stage, similar to other urological cancers. Treatment strategies include surgery (orchiectomy), radiation therapy, chemotherapy, and stem cell transplantation. Occasionally, more than one type of treatment is used (215).
4.5. Penile cancer
Penile cancer is a rare malignancy accounting for less than 1% of cancers in men (216). The incidence of this cancer has been increasing over the past decade but varies between different populations depending on the risk factors (217,218). No significant difference is seen between white and black men; however, a higher incidence is noted among Hispanics (219). Penile cancer is usually diagnosed in men over 60 years of age, but can also manifest in younger patients (220,221).
Approximately 95% of penile cancers are penile squamous cell carcinomas (PSCCs), which can be subdivided into several histologic variants such as basaloid, warty, papillary, verrucous, sarcomatoid, adenosquamous, and other rare types. Basaloid, adenosquamous, and sarcomatoid subtypes are aggressive tumors (222). Moreover, mucosal melanoma, sarcoma, and extramammary Paget's disease can also occur in the penis (223).
Penile cancer pathogenesis can be classified as HPV-dependent- or HPV-independent. In the HPV-dependent type, by integrating HPV DNA into the host genome with expression of the E6 and E7 oncogenes, host cells transform into a malignant phenotype, resulting in malignant HPV-related lesions (224). HPV-related lesions in penile cancer appear as low-grade squamous hyperplasia while high-grade lesions appear as invasive carcinomas of the penis (225).
In HPV-independent penile cancers, a premalignant precursor lesion is involved, which is associated with chronic inflammation. In some chronic penile conditions, including balanoposthitis, phimosis and lichen sclerosus, an increase in cyclooxygenase 2 (COX2) expression drives the overproduction of prostaglandins (226), resulting in activation of EGFR, b-catenin, and PI3K, as key players in carcinogenesis. Furthermore, the loss of heterozygosity in p16, a tumor suppressor gene, is frequently reported in penile cancer (224). Finally, the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) during persistent inflammation induces DNA damage and genomic instability (227).
Penile cancer symptoms include redness and irritation on the penis, skin thickening, and a visible or palpable lesion manifesting as a painless lump or ulcer on the glans, coronal sulcus, or foreskin. Penile cancer is accompanied by bleeding from the penis, penile discharge, or burning sensation during urinating (228,229).
Smoking is one of the main risk factors for penile cancer (230). Age and some infections such as HPV (especially strains 16, 18, 31and 33) and HIV infection, circumcision status, chronic inflammatory conditions, and poor hygiene are other risk factors for penile cancer (230, 231). Early diagnosis and staging are crucial because lymphatic spread is strongly associated with poor prognosis (232). Physical examination of the penis and inguinal lymph nodes, HPV detection tests, ultrasonography, CT and PET/CT, MRI, and penile biopsy are common methods for diagnosing this cancer type (233,234). Treatment of penile cancer varies depending on the clinical stage. Treatments include surgery, radiotherapy, adjuvant chemotherapy and chemotherapy, and immune checkpoint blockade (229,235,236).
5. Targeting BsAbs in urological malignancies
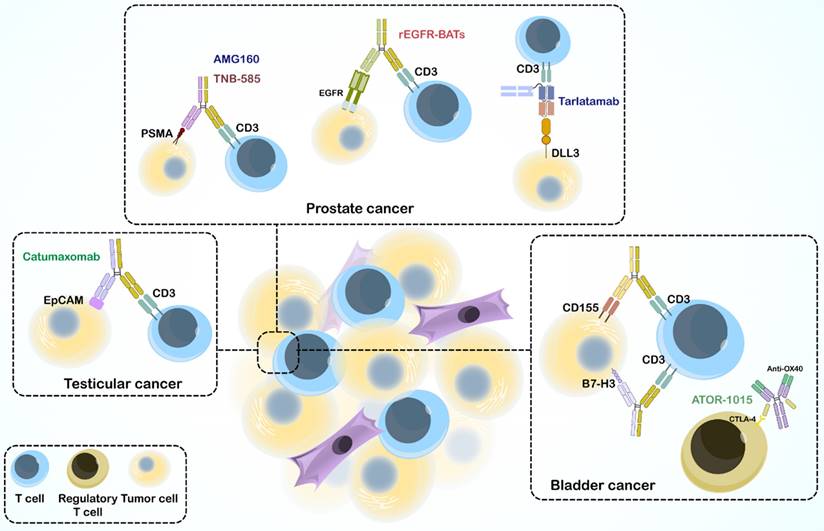
The immune system, especially cell-mediated immunity, plays a vital role in defense against cancer and cancer prevention. Recent immunotherapeutic drugs, including mAbs, BsAbs, immune checkpoint inhibitors, vaccines, CAR-T cell therapy, and immune cell transfer combat different mechanisms and affect different stages of cancer. Ag-specific immunotherapy stimulates the immune system to recognize tumors that express AgNPs to mediate tumor killing. An interesting focus in immunotherapy is the appearance of engineered BsAbs, which serve as a linker between immune cells and tumors but also many other defensive mechanisms discussed in Section 3, with an increasing ability to overcome drug resistance and decrease adverse effects. Over the past decades, immunotherapy has emerged as a treatment option for various urological malignancies, promising to change the field of urologic oncology. Numerous trials have used BsAbs to treat solid tumors and hematological malignancies. Here, we review the preclinical and clinical studies on BsAbs for specific urological cancers (Figure 4).
5.1. Preclinical studies using BsAbs in urological cancer models
The challenges posed by the prostate tumor environment have spurred research on novel immunotherapies. BsAbs are emerging as promising tools because of their ability to precisely target immune cells, tumor cells, and cancer-associated pathways (237,238).
Several preclinical studies have highlighted the potential of BsAbs in prostate cancer treatment; 10B3 BsAb, a novel BsAb targeting PSMA and CD3 (in both IgG-like and non-IgG-like forms), has demonstrated significant T cell activation and tumor cell reduction in LNCaP cells (a human prostate cancer cell line). The non-IgG-like form elicited a stronger inflammatory cytokine response, whereas the IgG-like form resulted in complete tumor regression in vivo (239). TNB-585 BsAb is another BsAb that combines an anti-PSMA arm with a low-affinity anti-CD3 arm, targeting PSMA+ tumor cells and patient-derived prostate cancer cells. Compared to high-affinity anti-CD3 BsAbs, TNB-585 leads to less cytokine release and regulatory T cell activation, while effectively destroying tumor cells (136-138). AMG160, as a BiTE antibody, has been found to target PSMA and CD3, effectively inducing T cell-mediated cytotoxicity against PSMA-expressing prostate cancer cells in preclinical studies. Encouraged by these results, AMG160 is currently undergoing clinical trials (NCT03792841) (240,241). Another BiTE is AMG 757, also known as tarlatamab, an extended half-life BiTE that targets CD3 and delta-like ligand 3 (DLL3), demonstrated potential in preclinical models for treating neuroendocrine prostate cancer, particularly when DLL3 is overexpressed (242). BC261 BsAb can also target CD3 and STEAP-1, with significant potential for immune cell infiltration and anti-tumor effects in prostate cancer cell lines, suggesting its therapeutic potential (243). Beyond targeting PSMA and CD3, bispecific antibody-armed activated T-cells (BATs) are another promising approach. Huang et al. demonstrated enhanced cytotoxicity and anti-tumor activity of T cells armed with a recombinant anti-EGFR and anti-CD3 BsAb (244).
The success of BsAbs extends beyond prostate cancer. Studies have demonstrated their potential in bladder cancer treatment, as ATOR-1015 BsAb, a human CTLA-4 ˟ OX40 IgG-like BsAb, activates T cells, suppresses regulatory T cells, and reduces tumor growth in bladder cancer models (245). BsAb anti-CD3 ˟ B7-H3 BATs have exhibited significant anti-cancer activity against bladder cancer cell lines, including chemoresistant lines (246). Moreover, CD155 Bi-armed activated T cells, as armed T cells, display increased cytotoxic activity and cytokine secretion compared to unarmed activated T cells, suggesting a novel therapeutic strategy (247). Finally, catumaxomab has shown good tolerance and potential efficacy against EpCAM-positive recurrent bladder cancer (248). Furthermore, catumaxomab has demonstrated potential for treating testicular cancer by engaging T cells and promoting NK cell-dependent cytotoxicity against various GCT cell lines (249).
Multiple types of Urological cancers.
Bispecific antibodies in treating urological cancers.
Although these preclinical studies provide a promising picture for BsAbs in urological cancers, further investigations are crucial. Large-scale clinical trials are necessary to determine their efficacy and safety profiles in real-world patients. Additionally, research on optimizing BsAb design and identifying the most effective treatment combinations can unlock their full potential to revolutionize urological cancer treatment.
5.2. Clinical trials and treatment landscape in urological cancers
By the end of 2023, a search of the clinicaltrials.gov website revealed over 20 active clinical trials utilizing BsAbs for treating urological cancers, but no BsAbs have been specifically approved by the FDA for this purpose.
BiTEs represent a notable category of BsAbs subjected to both preclinical and clinical evaluation. A phase 1 clinical trial reported at the ESMO meeting in 2020 demonstrated that AMG160 (PSMA ˣ CD3) exerted a disease-suppressive effect in patients with metastatic castration-resistant prostate cancer (mCRPC). PSA levels were reduced by more than 50% from the baseline in 34.3% (12 out of 35) of the cases, and 23.1% of patients experienced disappearance of circulating tumor cells during treatment. However, CRS occurred in 66% of the patients, albeit in a manageable form (NCT03792841) (250).
In another phase 1 clinical trial, CC-1 BsAb combined with tocilizumab, an IL-6 receptor (IL-6R) antagonist—was used prophylactically in 14 patients with prostate carcinoma. Although CRS was observed in 79% of patients treated with IgG-based BsAbs targeting PSMA and CD3, all heavily pretreated patients experienced a rapid decrease in elevated PSA levels (up to 60%). T-cell activation was also observed (NCT04104607) (251,252).
A phase 2 clinical trial assessed the safety and efficacy of HER2Bi-armed activated T cells (HER2 BATs) in combination with pembrolizumab, a PD-1 inhibitor, in mCRPC. This trial was the first comprehensive report to combine a checkpoint inhibitor with targeted T-cell therapy to evaluate its clinical efficacy. The primary endpoint of 6-month progression-free survival (PFS) was met by 5 of the 14 patients, with a median PFS of 5 months and a median survival of 31.6 months. A decrease in PSA levels of 25% or more was observed in six patients, and five patients (38.4%) were progression-free at six months. Post-infusion immunophenotyping showed significantly increased cytotoxic activity in PBMCs (CTL and KN cells) and a marked decrease in the regulatory T-cell population, indicating the efficacy of the pembrolizumab and HER2 BATs combination, and encouraging further investigation (NCT03406858) (253).
Amgen has recently introduced AMG340, an alternative anti-PSMA × CD3 BiTE to AMG160, for use in mCRPC. The phase 1 multi-center study of AMG340 is currently ongoing (NCT04740034). Additional ongoing trials evaluating the efficacy of BsAbs in prostate cancer treatment include a phase 1/2 study of REGN4336 (a PSMA × CD3 BsAb) administered alone or with cemiplimab in mCRPC (NCT05125016) and a phase 1 trial combining JNJ-87189401 (PSMA × CD28) with JNJ-78278343 (KLK2 × CD3) in advanced prostate cancer (NCT06095089). All clinical trials of BsAbs in prostate cancer treatment are summarized in Table 2. BsAbs target various kidney cancer markers, particularly renal cell carcinoma (RCC). These include monotherapy with AK104 (cadonilimab), a tetravalent bispecific IgG1 targeting PD-1 and CTLA-4 (NCT06035224), and in combination with axitinib, a tyrosine kinase inhibitor (TKI), in advanced and metastatic RCC (NCT05256472). Other targets include ENPP3 and CD3 via XmAb819 in advanced clear cell RCC (NCT05433142), HER2 and HER3 by MCLA-128 (zenocutuxumab) in patients with RCC or prostate cancer (NCT04100694), and PD-L1 and CD27 via CDX-527, a tetravalent PD-L1 × CD27 IgG1-scFv BsAb in patients with advanced malignancies, including RCC and bladder urothelial carcinoma (NCT04440943).
An ongoing Phase 1 dose-escalation study is investigating the therapeutic potential of catumaxomab, administered as an intravesical instillation, in patients with non-muscle invasive bladder cancer (NMIBC) at high and intermediate risk for progression. Catumaxomab mediates antibody-dependent cellular cytotoxicity against human epithelial tumor cells, including bladder cancer (NCT04819399). Several clinical trials related to various stages of urological cancer have been completed; however, their results are yet to be published. These include a phase 1 and 2 study on the safety of GEN1044 (DuoBody anti-CD3x5T4 BsAb) in patients with malignant solid tumors such as prostate and bladder cancer (NCT04424641), a phase 1 trial of GEM3PSCA in patients with renal and prostate cancer (NCT03927573), and two separate phase 1 studies evaluating the safety of XmAb20717 (BsAb targeting PD-1 and CTLA-4) (NCT03517488) and XmAb22841 (anti-CTLA-4 × LAG-3) monotherapy and in combination with pembrolizumab (NCT03849469) in urothelial carcinoma, renal cell carcinoma, castration-resistant prostate carcinoma, and squamous cell carcinoma of the penis. All related clinical trials are listed in Table 2.
6. Efficacy and safety profiles of BsAbs in urological cancers
Clinical trials assessing BsAbs for urological cancer are currently in phases 1 and 2, marking their inaugural use in patient treatment. Typically, first-in-human (FIH) studies aim to determine the safety, tolerability, and initial efficacy of BsAbs in patients with any form of urological cancer (Figure 5). These studies generally consisted of two segments: a dose-escalation phase to identify any dose-limiting toxicities, culminating in the establishment of the maximum dose if no such toxicities were observed, and a dose-expansion phase in which additional participants were administered the established maximum tolerated dose (MTD) (251). BsAbs are administered via continuous infusion, and patient safety is closely monitored throughout the process. During the dose-escalation phase, the Data Safety Monitoring Board (DSMB) reviews the safety reports for each patient whose dose has been increased to provide further recommendations or approvals.
The trials were structured around primary and secondary endpoints. The primary endpoints were the incidence and severity of adverse events. Secondary endpoints encompass a range of factors including safety, defined by the incidence and severity of adverse events (CTCAE V.5.0), immunogenicity as measured by the frequency of human-anti-human antibodies (HAHA) in patients, cytokine induction via serum cytokine level measurements, pharmacokinetics reflected in serum BsAb concentrations, anti-tumor activity, changes in tumor markers and responses, survival rates indicated by overall survival (OS) and progression-free survival (PFS), and quality of life as assessed by the European Organization for Research and Treatment of Cancer core quality of life questionnaire scores.
The objective tumor response, or objective response rate (ORR), was determined by the proportion of participants achieving a confirmed complete response (CR) or partial response (PR) according to RECIST 1.1 Criteria (254). In addition to monitoring adverse events, particular attention was paid to adverse events of special interest (AESIs), such as allergic reactions, development of HAHA, and cytokine release syndrome (CRS), with grade 3-5 events classified as severe. The efficacy and safety of BsAbs, which are influenced by structural factors such as affinity, valency, immunogenicity, and the presence or absence of an Fc region, were also evaluated in relation to these secondary endpoints.
Clinical trials using bispecific antibodies in urological cancer therapy
| Antibody | Targets | Platform/ Format | Sponsor | Type of cancer | Status | Phase | location | Start date | Finishing date | NTC number |
|---|---|---|---|---|---|---|---|---|---|---|
| CC-1 | CD3 PMSA | University Hospital Tüebingen | Castration resistant prostate carcinoma | Recruiting | 1 | Germany | 2019- 11-15 | 2024- 12-31 | NCT04104607 | |
| JNJ-87189401 + JNJ-78278343 | CD28 PMSA + CD3 KLK2 | Janssen Research & development, LLC | Advanced prostate cancer | Recruiting | 1 | United states | 2023- 11-01 | 2027- 06-21 | NCT06095089 | |
| BAY2010112 (Pasotuxizumab) | CD3 PMSA | BiTE | Bayer | Castration-resistant prostate cancer | Completed | 1 | Austria + Germany | 2012- 11-02 | 2018- 09-26 | NCT01723475 |
| CC-1 | CD3 PMSA | University Hospital Tuebingen | Recurrence of prostate cancer | Recruiting | 1 | Germany | 2022- 11-11 | 2025-12 | NCT05646550 | |
| AMG 340 | CD3 PMSA | BiTE | Amgen | Metastatic castration-resistant prostate carcinoma | Active, not recruiting | 1 | United states | 2021- 04-29 | 2024- 09-30 | NCT04740034 |
| ES414 | CD3 PMSA | Aptevo Therapeutics | Metastatic castration-resistant prostate cancer | Completed | 1 | United states + Australia | 2015-01 | 2019- 02-18 | NCT02262910 | |
| GEM3PSCA | CD3 PSCA | ATAC | AvenCell Europe GmbH | Solid tumors including prostate cancer + Renal cancer | Terminated | 1 | Germany | 2019- 04-15 | 2023- 06-28 | NCT03927573 |
| HER2Bi-armed activated T cells | CD3 HER2 | Barbara Ann Karmanos Cancer Institute | Metastatic castration resistant prostate cancer | Completed | 2 | United states | 2018- 06-07 | 2022- 11-07 | NCT03406858 | |
| GEN1044 | CD3 5T4 | Duobody | Genmab | Advanced or metastatic solid tumor including prostate cancer + Bladder cancer | Terminated | 1/2 | United sates + Denmark + Israel | 2020- 07-15 | 2021- 10-29 | NCT04424641 |
| XmAb20717 | PD-1 CTLA-4 | XmAb | Xencor, Inc | Metastatic castration-resistant prostate cancer + Renal cell carcinoma + Urothelial carcinoma + Squamous cell carcinoma of penis | Completed | 1 | United sates | 2018- 07-10 | 2022- 09-06 | NCT03517488 |
| XmAb20717 | PD-1 CTLA-4 | XmAb | Xencor, Inc | Metastatic castration-resistant prostate cancer | Recruiting | 2 | United sates | 2022- 07-21 | 2025- 05-30 | NCT05032040 |
| REGN4336 | CD3 PMSA | Regeneron pharmaceutical | Metastatic castration-resistant prostate cancer | Recruiting | 1/2 | United sates | 2021- 11-30 | 2026- 08-04 | NCT05125016 | |
| MCLA-128 (Zenocutuzumab) | HER2 HER3 | IgG1-based | Merus N.V. | Metastatic castration-resistant prostate cancer | Recruiting | 2 | United sates | 2022- 11-17 | 2026-03 | NCT05588609 |
| PT217 | HuCD47 HuDLL3 | Phanes Therapeutics | Advanced refractory cancers including neuro-endocrine prostate cancer | Recruiting | 1 | United sates | 2023-06 | 2025-06 | NCT05652686 | |
| XmAb20717 (Vudalimab) Combination therapy | PD-1 CTLA-4 | Emory University | Metastatic castration-sensitive prostate cancer | Recruiting | 1 | United sates | 2023- 08-03 | 2027- 12-16 | NCT05733351 | |
| XmAb808 | CD28 B7-H3 | XmAb 2+1 | Xencor, Inc | Advanced solid tumors including castration-resistant prostate cancer + Urothelial carcinoma | Recruiting | 1 | United sates | 2022- 12-14 | 2027-12 | NCT05585034 |
| MGC018 ADC (vobramitamab duocarmazine) | CD3 B7-H3 | MacroGenics | Advanced solid tumors including castration-resistant prostate cancer + Renal cell carcinoma | Recruiting | 1 | United sates | 2022- 04-19 | 2025-03 | NCT05293496 | |
| XmAb22841 | LAG-3 CTLA-4 | XmAb | Xencor, Inc. | Advanced solid tumors | Completed | 1 | United states | 2019- 05-29 | 2023- 02-16 | NCT03849469 |
| anti-CD3-MUC1 armed CIK | CD3 MUC-1 | Fuda Cancer Hospital, Guangzhou | Advanced kidney cancer | Withdrawn | 2 | China | 2018- 04-10 | 2018- 04-10 | NCT03540199 | |
| XmAb819 | CD3 ENPP3 | XmAb | Xencor, Inc. | Clear cell renal cell carcinoma | Recruiting | 1 | United states | 2022- 06-13 | 2027-03 | NCT05433142 |
| SI-B003 | PD-1 CTLA-4 | Sichuan Baili Pharmaceutical Co., Ltd | Solid tumors including kidney cancer | Recruiting | 1 | China | 2020- 11-10 | 2023-12 | NCT04606472 | |
| JNJ-78306358 | CD3 HLA-G | Janssen Research & Development, LLC | Advanced solid tumors including renal cell carcinoma | Completed | 1 | Israel + Spain | 2021- 10-24 | 2023- 02-09 | NCT04991740 | |
| CDX-527 | CD27 PDL-1 | IgG1-based | Celldex Therapeutics | Solid tumors including renal cell carcinoma + Bladder urothelial carcinoma | Completed | 1 | United states | 2020- 08-04 | 2023- 04-06 | NCT04440943 |
| AK104 (Cadonilimab) | PD-1 CTLA-4 | IgG1-based | RenJi Hospital | Advanced or metastatic clear cell carcinoma | Recruiting | 2 | China | 2023- 08-23 | 2026- 07-31 | NCT06035224 |
| CDX-585 | PD-1 ILT4 | IgG1-based | Celldex Therapeutics | Advanced malignancies including renal cell carcinoma + Bladder urothelial carcinoma | Recruiting | 1 | United states | 2023- 05-11 | 2026-02 | NCT05788484 |
| MCLA-128 (Zenocutuzumab) | HER2 HER3 | IgG1-based | Merus N.V | Advanced NRG1-fusion positive solid tumor including renal cell carcinoma + Prostate cancer | Available | Not provided | NCT04100694 | |||
| XmAb23104 | PD-1 ICOS | XmAb | Xencor, Inc. | Advanced solid tumors including renal cell carcinoma + Urothelial carcinoma | Recruiting | 1 | United states | 2019- 05-01 | 2025-09 | NCT03752398 |
| XmAb22841 | LAG-3 CTLA-4 | XmAb | Xencor, Inc | Advanced solid tumors including renal cell carcinoma + Prostate carcinoma + Urothelial carcinoma + Squamous cell carcinoma of penis | Completed | 1 | United states | 2019- 05-29 | 2023- 02-16 | NCT03849469 |
| Catumaxomab | CD3 EpCAM | Triomab quadroma | Lindis Biotech GmbH | Non-muscle invasive bladder cancer (NMIBC) | Unknown | 1 | Germany | 2020- 07-07 | 2023- 05-30 | NCT04819399 |
Diagram of efficacy and safety profiles of BsAbs in urological cancers.
In phase 2 trials, the primary goal was to estimate the clinical efficacy of BsAb infusions at specific doses and intervals by measuring the proportion of patients who remained clinically progression-free at predetermined time points post-registration. Secondary goals involve analyzing immune cell phenotypes and functions, cytokine profiles before and after immunotherapy, and their association with clinical outcomes including response rates, PFS, and OS (251,253).
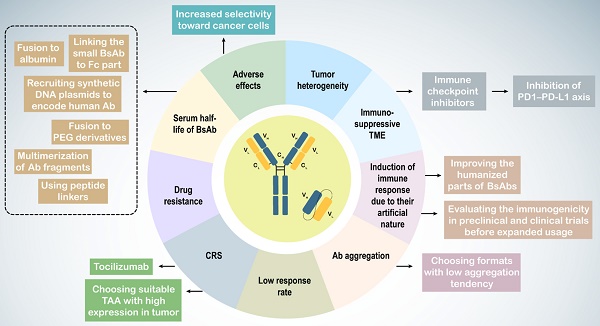
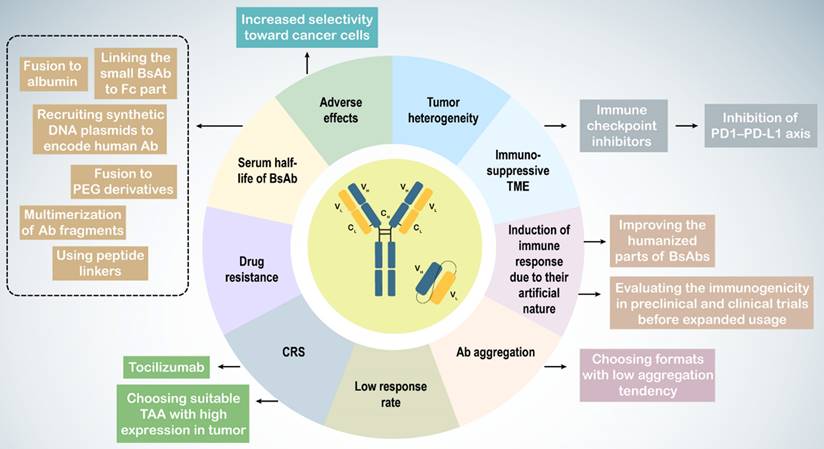
7. Challenging and promising solutions in BsAb therapy
Immunotherapy with BsAbs has shown potential for combating tumors in both preclinical and clinical research. However, challenges such as tumor heterogeneity, the tumor microenvironment (TME), Ab immunogenicity, adverse effects including CRS, short serum half-life, low response rates, and drug resistance complicate their use (Table 3). Tumor heterogeneity refers to the diverse phenotypic and genotypic characteristics of cancer cells, leading to subpopulations with varying behaviors and responses to immunotherapy.
In urological cancers, particularly prostate cancer, the immunosuppressive nature of the TME weakens immune responses, characterized by an increase in regulatory T cells, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) with an M2 anti-inflammatory phenotype, along with the production of anti-inflammatory cytokines and heightened immune checkpoint expression (256,257,270,271). M2 TAMs are associated with higher Gleason grades, increased PSA levels, and poor prognoses in patients with prostate cancer (272). To counteract the immunosuppressive TME and drug resistance, BsAbs may be used alongside other therapies, such as immune checkpoint inhibitors, to enhance immune cell responses (273). Blockade of the PD1-PD-L1 axis improves the activity of blinatumomab and the CD33 × CD3 BiTE AMG330 in acute lymphoblastic leukemia (274,275).
Because of their artificial molecular structure, BsAbs can trigger immune responses, leading to the production of anti-drug antibodies (ADAs) that form BsAb/ADA immune complexes, resulting in drug-related toxicities and reduced efficacy. Factors influencing Ab immunogenicity include BsAb impurities, Ab origin, dosage, and target molecules. Less than 1% of patients treated with blinatumomab develop ADAs (259,260). Strategies to mitigate this issue include enhancing the humanization of BsAbs and assessing their immunogenicity in preclinical and clinical trials to ensure safety and efficacy. Such results have not been reported for BsAbs in urological cancers.
BsAb treatment can cause adverse effects owing to nonspecific T cell activation. CRS, with IL-6 as a critical factor, can be severe or even fatal (276,277). Thus, BsAb therapy can lead to on-target on-tumor activation, which is essential for treatment success, as well as off-target activation, which occurs without target cells, such as when the BsAb antigen-binding site (e.g., anti-CD3 arm) binds with high affinity to its antigen (e.g., CD3), or because of other factors, such as antibody aggregation and Fc receptor (FcR) binding (278).
Slaga et al. developed a CD3 × HER2 T-cell-dependent BsAb with low-affinity HER2 arms, demonstrating selective binding to cells with high HER2 expression, reducing adverse effects and improving therapeutic outcomes (261). Alternative administration methods such as subcutaneous injection, may help mitigate these limitations.
Intravenous administration of catumaxomab led to local cytokine release and T cell-mediated hepatotoxicity owing to its active Fc region binding to FcγR-expressing immune cells (279). Engineered Fc domains may reduce FcγR binding in IgG-like CD3-targeting BsAbs. Other approaches include inducing mutations to suppress FcγR binding and using non-IgG-like BsAbs without an Fc region (263,280). Selecting BsAbs with low aggregation tendencies can also address antibody aggregation issues (239).
On-target off-tumor activation, caused by targeting antigens also expressed on non-tumor cells, limits safe dosages and can lead to CRS. Choosing tumor-associated antigens (TAAs) with high tumor expression and low or no expression on normal cells can help overcome this challenge.
Many BsAbs in urological cancer trials target PSMA, which is also expressed at low levels in healthy tissues, such as prostate cells, kidney proximal tubules, and gastrointestinal cells, posing a potential toxicity risk (281,282).
To prevent on-target off-tumor T-cell activation, researchers have utilized the TME characteristic of increased proteolysis. Preclinical studies have designed BsAbs that recruit T cells but are activated via proteolytic cleavage within the tumor, releasing the anti-CD3 binding moiety at the tumor site and avoiding off-target activation and toxicity (264,265). Tocilizumab, a humanized monoclonal antibody against the IL-6R, can be used prophylactically to control CRS (262).
Challenges and promising solutions in bispecific antibody-based immunotherapies
| Challenge | Definition | Solution | Ref. | |
|---|---|---|---|---|
| Tumor heterogenicity | Distinct phenotypic and genotypic profile of cancer cells | Personalized and combination therapy | (255) | |
| Tumor microenvironment | Immunosuppressive environment such as increased population of regulatory T cells, MDSCs, TAMs, anti-inflammatory cytokine production, immune checkpoint expression | Combination therapy, esp. with immune checkpoint inhibitors | (256-258) | |
| Antibody immunogenicity | Molecular structures of BsAbs are artificial and do not exist in nature | Improving humanized BsAbs and evaluating their immunogenicity in preclinical and clinical studies prior to large-scale usage | (259,260) | |
| Adverse effects (Cytokine release syndrome) | Off-target activation of T cell | The Ag-binding domain of BsAb binds to its target with high affinity | Designing a BsAb with a lower affinity binding site for Ag and alternative administration routes other than intravenous. Prophylactic treatment with tocilizumab | (261,262) |
| BsAb aggregation in non-IgG-like formats | Choosing IgG-like BsAbs with low aggregation tendency Prophylactic treatment with tocilizumab | (261-263) | ||
| Binding of the Fc part of IgG-like BsAbs to the Fc receptor of immune cells or Kupffer cells | Using engineered Fc domains and indicating mutations to suppress FcγR binding Consuming non-IgG like BsAbs instead Prophylactic treatment with tocilizumab | (239,262) | ||
| On-target off-tumor activation of T cells, because of targeting Ags which also have high expression on non-tumor cells | Selecting a TAA with high expression in tumor and no/low expression in normal cells Intratumor proteolytic cleavage of anti-CD3 binding moiety Prophylactic treatment with tocilizumab. | (262,264,265) | ||
| Serum half-life | Rapid clearance of non-IgG-like BsAbs from circulation | Fusion of non-IgG-like BsAbs to the Fc part, albumin, or PEG derivatives Multimerization of BsAb fragments Use of peptide linkers Recruitment of synthetic DNA plasmids to encode human BsAb In vivo production of BsAbs | (262,266-269) | |
| Drug resistance | Tumor escape from targeted therapy | Combination therapy | (258) | |
The short serum half-life of non-IgG-like BsAbs presents another clinical challenge (283). Multiple dosing can overcome rapid clearance from circulation. Extended half-life versions of non-IgG-like BsAbs include linking small BsAbs to the Fc part, fusion to albumin, polyethylene glycol (PEG) derivatives, carbohydrates, and dextran, and multimerization of antibody fragments, peptide linkers, and synthetic DNA plasmids encoding human antibodies (74,266,267,284).
In vivo production of BsAbs may prevent the rapid renal clearance of non-IgG-like platforms and maintain effective antibody concentrations. Direct gene delivery or inoculation of ex vivo genetically modified cells are the two main strategies for in vivo production (268,269,285,286). The in vivo production of BsAbs has been described in a review by Blanco et al. (85).
8. Future directions and novel strategies
Research on BsAbs has revealed numerous benefits, including increased specificity and effectiveness. With advancements in their design and manufacture, BsAbs have emerged as pivotal advances in cancer treatment, augmenting traditional therapies. BsAbs are optimally suited for integration into antibody-drug conjugates (ADCs) and chimeric antigen receptor (CAR) T-cell therapies, heralding a potential shift in personalized and combination therapeutic strategies. The advent of multi-specific antibodies, such as tri-specific and tetra-specific variants, can revolutionize cancer immunotherapy. The core principle of ADCs involves coupling the selectivity of BsAbs with the potency of cytotoxic agents, thereby enhancing targeted drug delivery and mitigating side effects. An example is the bispecific HER2 ˟ CD63his-ADC, which recognizes HER2 and CD63, facilitating improved internalization and anti-tumor efficacy of HER2-targeted ADCs. This antibody binds to HER2-expressing cancer cells through its anti-HER2 arm, whereas its second arm attaches to CD63, directing endocytosis-mediated transport of the drug payload directly to tumor cells, resulting in their destruction (125).
Multi-specific antibodies are considered to offer superior therapeutic promise compared to that of BsAbs and mAbs in clinical settings. SAR-442257, a tri-specific antibody, is currently undergoing phase I clinical trials to evaluate its effectiveness in treating relapsed/refractory multiple myeloma (R/R MM) and nonclassical Hodgkin lymphoma (R/R NHL) (NCT04401020). It simultaneously targets CD3, CD28, and CD38, leading to sustained T-cell activation and specific targeting of myeloma cells (287). Additionally, HPN424, another trispecific antibody comprising domains for T-cell engagement, tumor cell targeting, and half-life extension, has demonstrated tolerability and manageable adverse events in phase 1 and 2 clinical trials. A decrease in PSA levels in patients with metastatic castration-resistant prostate cancer (mCRPC) indicates the antitumor activity of HPN424(NCT03577028) (288,289). The first tetraspecific antibody to enter clinical trials was GNC-038, which binds to CD3, CD19, PD-L1, and 4-1BB. Its dual T-cell activation arms and tumor-targeting sites have been assessed for efficacy in lymphomas (NCT05192486) (290).
Addressing cancer, a multifaceted disease, using monospecific monoclonal antibodies (mAbs) often leads to drug resistance and tumor evasion. BsAb-based therapies have shown potential in overcoming these challenges and have shown promising clinical outcomes. However, BsAbs are still in the investigative phase and require further trials to validate the existing data (291).
Future developments in BsAbs will likely involve the identification of more precise targets, novel formats, and innovative combinations of established treatments and other immunotherapeutic approaches.
9. Conclusions
Recent breakthroughs in genetic engineering and antibody design have led to the development of BsAbs using various platforms. These platforms allow the creation of BsAbs with different structures and functionalities, primarily focusing on cancer treatment. Success in this field depends on precise design and target selection. BsAbs offer a unique advantage by maintaining the effectiveness of combination therapy while reducing the associated toxicity. Currently, over 200 BsAbs are being evaluated in clinical and preclinical trials, with nine already approved by the FDA for various conditions beyond cancer, including hemophilia A, macular degeneration, and diabetic macular edema.
However, extensive research on BsAbs has revealed several challenges that limit their applications. Overcoming these hurdles begins with an optimal design and rigorous target identification. BsAbs represent a major leap forward in drug development, enabling the development of novel drugs that can simultaneously target multiple pathways. Their high specificity makes BsAbs ideal candidates for antibody-drug conjugates (ADCs) to improve cancer cell elimination. Additionally, BsAbs can be used to activate T cells and potentially boost anti-tumor responses. Exploration of multi-specific antibodies holds significant promise for enhancing the ability to fight cancer. In the context of urological cancers, BsAbs are still in the early stages of clinical trials to determine their optimal dosages and initial clinical effectiveness. Nevertheless, preclinical studies using BsAbs in laboratory models of urological cancers, particularly prostate cancer, have shown positive results, providing a basis for further investigation and development.
Abbreviations
Ab: antibody
ADC: antibody drug conjugates
ADCC: antibody-dependent cell-mediated cytotoxicity
ADCP: antibody-dependent cellular phagocytosis
AESI: adverse events of special interest
Ag: antigen
BBB: blood-brain barrier
BCG: Bacille Calmette-Guérin
BiKE: Bispecific killer cell engager
BiTE: bispecific T cell engager
BsAb: bispecific antibody
CAR T-cell: chimeric antigen receptor T cell
CDC: complement-dependent cytotoxicity
CDR: complementarity-determining region
CEUS: contrast-enhanced ultrasound
cFAE: controlled Fab arm exchange
CiTE: checkpoint inhibitory T-cell engager
COX2: cyclooxygenase 2
CR: complete response
CRS: cytokine release syndrome
CTL: cytotoxic T lymphocytes
CTLA-4: cytotoxic T lymphocyte antigen 4
DART: dual-affinity retargeting molecule
DCT: distal connecting tubules
DRE: digital rectal examination
DSMB: data safety monitoring board
DVD-Ig: daul-variable-domain immunoglobulin
EGFR: epidermal-growth factor receptor
EMA: european medicines agency
EpCAM: Epithelial cell adhesion molecule
Fab: fragments of antigen-binding
Fc: crystallizable fragment
FcRs: Fc receptors
FIH: first in human
FIT-Ig: Fabs-in-tandem immunoglobulin
FDA: Food and Drug Administration
GEA: gastroesophageal adenocarcinoma
GCNIS: germ cell neoplasia in situ
HAHA: human-anti-human-Abs
HLE-BiTE: half-life extended BiTE
ICIs: immune checkpoint inhibitors
LAG3: lymphocyte activation gene 3
mAb: monoclonal antibody
mCRPC: metastatic castration-resistant prostate cancer
MDSC: myeloid-derived suppressor cell
MHC: major histocompatibility complex
MRI: magnetic resonance imaging
MTD: maximum tolerated dose
NMIBC: non-muscle invasive bladder cancer
NSCLC: non-small cell lung cancer
ORR: objective response rate
OS: overall survival
PD-1: programmed cell death 1
PD-L1: programmed cell death 1 ligand 1
PHI: prostate health index
PSA: prostate-specific Ag
PSMA: prostate-specific membrane antigen
PSMA-PET: prostate-specific membrane antigen positron emission tomography
RA: rheumatoid arthritis
RCC: renal cell carcinoma
PFS: progression free survival
PGC: primordial germ cell
PR: partial response
PSCC: penile squamous cell carcinoma
RECIST: response evaluation criteria in solid tumors
R/M CC: recurrent or metastatic cervical cancer
R/R ALL: refractory precursor B-cell acute lymphoblastic leukemia
R/R MM: relapsed/refractory multiple myeloma
R/R NHL: relapsed/refractory non-classical Hodgkin's lymphoma
RTK: receptor tyrosine kinases
scFv: single-chain variable fragment
TAA: tumor-associated Ag
TAM: tumor associated macrophage
TandAb: Tandem diabody
T-BsAb: T cell engaging bispecific antibodies
TERT: telomerase reverse transcriptase
TIM3: T-cell immunoglobulin mucin 3
TME: tumor microenvironment
TRUS: transrectal ultrasound
TNFRSF: TNF receptor superfamily
VEGF: Vascular-endothelial-growth factor
Acknowledgements
The authors would like to express sincere gratitude to Wiley Editing Services for their expert language editing, which significantly improved the clarity and professionalism of this manuscript.
Funding
A.P.K. was supported by grants from the Singapore Ministry of Education (MOE-T2EP30120-0016) and the National University of Singapore Seed Fund (NUHSRO/2023/039/RO5+6/Seed-Mar/04).
Consent for publication
All authors have read the manuscript and consented to its publication.
Author contributions
A.P.K., K.H., and B.E. conceptualized this review and collated the review materials; EHC.L., R.S., A.G., K.H., and B.E. collated the materials for revision and revised the manuscript; K.H., B.E., R.J.R., S.H.S., M.R.F., Y.S.H., and A.R.A. wrote the original review and assisted in editing the revised manuscript; S.S. and A.P.K. edited the original manuscript and the revised manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. (CDC). cfdcap. prostate cancer; 2023 [updated 06/17/2023; cited. 2024 01/07/2024]. Center for Disease Control and Prevention (CDC). Available from: https://www.cdc.gov/cancer/prostate/index.htm
2. (CDC) cfdcap. Male urologic cancers; 2020 [updated 11/17/2020; cited 2024 01/07/2024]. Center for Disease Control and Prevention (CDC). Available from: https://www.cdc.gov/cancer/uscs/about/data-briefs/no21-male-urologic-cancers.htm.
3. Downes MR. Urologic Cancers: exon puplication; 2022
4. Goulet DR, Atkins WM. Considerations for the design of antibody-based therapeutics. J Pharm Sci. 2020;109(1):74-103
5. Kung PC, Goldstein G, Reinherz EL, Schlossman SF. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979;206(4416):347-9
6. Ma J, Mo Y, Tang M, Shen J, Qi Y, Zhao W, Huang Y, Xu Y, Qian C. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616
7. Rezaee M, Mohammadi F, Keshavarzmotamed A, Yahyazadeh S, Vakili O, Milasi YE, Veisi V, Dehmordi RM, Asadi S, Ghorbanhosseini SS, Rostami M, Alimohammadi M, Azadi A, Moussavi N, Asemi Z, Aminianfar A, Mirzaei H, Mafi A. The landscape of exosomal non-coding RNAs in breast cancer drug resistance, focusing on underlying molecular mechanisms. Front Pharmacol. 2023;14:1152672
8. Taghvimi S, Vakili O, Soltani Fard E, Khatami SH, Karami N, Taheri-Anganeh M, Salehi M, Negahdari B, Ghasemi H, Movahedpour A. Exosomal microRNAs and long noncoding RNAs: novel mediators of drug resistance in lung cancer. J Cell Physiol. 2022;237(4):2095-106
9. Torka P, Barth M, Ferdman R, Hernandez-Ilizaliturri FJ. Mechanisms of resistance to monoclonal antibodies (mAbs) in lymphoid malignancies. Curr Hematol Malig Rep. 2019;14(5):426-38
10. Movahedpour A, Khatami SH, Khorsand M, Salehi M, Savardashtaki A, Mirmajidi SH, Negahdari B, Khanjani N, Naeli P, Vakili O, Taheri-Anganeh M. Exosomal noncoding RNAs: key players in glioblastoma drug resistance. Mol Cell Biochem. 2021;476(11):4081-92
11. Salehi M, Vafadar A, Khatami SH, Taheri-Anganeh M, Vakili O, Savardashtaki A, Negahdari B, Naeli P, Behrouj H, Ghasemi H, Movahedpour A. Gastrointestinal cancer drug resistance: the role of exosomal miRNAs. Mol Biol Rep. 2022;49(3):2421-32
12. Dahlén E, Veitonmäki N, Norlén P. Bispecific antibodies in cancer immunotherapy. Ther Adv Vaccines Immunother. 2018;6(1):3-17
13. Chen RP, Shinoda K, Rampuria P, Jin F, Bartholomew T, Zhao C, Yang F, Chaparro-Riggers J. Bispecific antibodies for immune cell retargeting against cancer. Expert Opin Biol Ther. 2022;22(8):965-82
14. Nisonoff A MM, Rivers MM. Recombination of a mixture of univalent antibody fragments of different specificity. Arch Biochem Biophys. 1961;93:460-2
15. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495-7
16. Milstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature. 1983;305(5934):537-40
17. Perez P, Hoffman RW, Shaw S, Bluestone JA, Segal DM. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature. 1985;316(6026):354-6
18. Huston JS, Levinson D, Mudgett-Hunter M, Tai MS, Novotný J, Margolies MN, Ridge RJ, Bruccoleri RE, Haber E, Crea R. et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988;85(16):5879-83
19. Merchant AM, Zhu Z, Yuan JQ, Goddard A, Adams CW, Presta LG, Carter P. An efficient route to human bispecific IgG. Nat Biotechnol. 1998;16(7):677-81
20. (FDA). USfada. Bispecific Antibodies: An Area of Research and Clinical Applications: U.S; 2023 [updated 08/02/2023; cited. 2024 01/05/2024]. Food and Drug Administration (FDA). Available from: https://www.fda.gov/drugs/news-events-human-drugs/bispecific-antibodies-area-research-and-clinical-applications
21. Dhimolea E, Reichert JM, editors. World Bispecific Antibody Summit, September 27-28, 2011, Boston, MA. mAbs. World bispecific antibody summit. Taylor & Francis. 2012;4(1):4-13
22. Ghalamfarsa F, Khatami SH, Vakili O, Taheri-Anganeh M, Tajbakhsh A, Savardashtaki A, Fazli Y, Uonaki LR, Shabaninejad Z, Movahedpour A, Ghalamfarsa G. Bispecific antibodies in colorectal cancer therapy: recent insights and emerging concepts. Immunotherapy. 2021;13(16):1355-67
23. Brinkmann U, Kontermann RE, editors. The making of bispecific antibodies. mAbs. Taylor & Francis. 2017;9(2):182-212
24. Godar M, de Haard H, Blanchetot C, Rasser J. Therapeutic bispecific antibody formats: a patent applications review (1994-2017). Expert Opin Ther Pat. 2018;28(3):251-76
25. Hosseini SS, Khalili S, Baradaran B, Bidar N, Shahbazi M-A, Mosafer J, Hashemzaei M, Mokhtarzadeh A, Hamblin MR. Bispecific monoclonal antibodies for targeted immunotherapy of solid tumors: recent advances and clinical trials. Int J Biol Macromol. 2021;167:1030-47
26. Wang Q, Chen Y, Park J, Liu X, Hu Y, Wang T, McFarland K, Betenbaugh MJ. Design and production of bispecific antibodies. Antibodies (Basel). 2019;8(3):43
27. Lindhofer H, Mocikat R, Steipe B, Thierfelder S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J Immunol. 1995;155(1):219-25
28. Zhang X, Yang Y, Fan D, Xiong D. The development of bispecific antibodies and their applications in tumor immune escape. Exp Hematol Oncol. 2017;6:12
29. Ridgway JB, Presta LG, Carter P. “Knobs-into-holes” engineering of antibody. Protein Eng. 1996;9(7):617-21
30. Schaefer W, Regula JT, Bähner M, Schanzer J, Croasdale R, Dürr H, Gassner C, Georges G, Kettenberger H, Imhof-Jung S, Schwaiger M, Stubenrauch KG, Sustmann C, Thomas M, Scheuer W, Klein C. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A. 2011;108(27):11187-92
31. Lewis SM, Wu X, Pustilnik A, Sereno A, Huang F, Rick HL, Guntas G, Leaver-Fay A, Smith EM, Ho C, Hansen-Estruch C, Chamberlain AK, Truhlar SM, Conner EM, Atwell S, Kuhlman B, Demarest SJ. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat Biotechnol. 2014;32(2):191-8
32. Labrijn AF, Meesters JI, de Goeij BECG, van den Bremer ETJ, Neijssen J, van Kampen MD, Strumane K, Verploegen S, Kundu A, Gramer MJ, van Berkel PHC, van de Winkel JGJ, Schuurman J, Parren PWHI. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci U S A. 2013;110(13):5145-50
33. Moore GL, Bernett MJ, Rashid R, Pong EW, Nguyen DT, Jacinto J, Eivazi A, Nisthal A, Diaz JE, Chu SY, Muchhal US, Desjarlais JR. A robust heterodimeric Fc platform engineered for efficient development of bispecific antibodies of multiple formats. Methods. 2019;154:38-50
34. Jakob CG, Edalji R, Judge RA, DiGiammarino E, Li Y, Gu J, Ghayur T. Structure reveals function of the dual variable domain immunoglobulin (DVD-Ig TM) molec;5(3). Austin: MABS. 2013
35. Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, Bose S, McCarthy D, Zhu RR, Santora L, Davis-Taber R, Kunes Y, Fung E, Schwartz A, Sakorafas P, Gu J, Tarcsa E, Murtaza A, Ghayur T. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol. 2007;25(11):1290-7
36. Gong S, Ren F, Wu D, Wu X, Wu C, editors. Fabs-in-tandem immunoglobulin is a novel and versatile bispecific design for engaging multiple therapeutic targets. mAbs. Taylor & Francis. 2017;9(7):1118-28
37. Baeuerle PA, Kufer P, Bargou R. BiTE: teaching antibodies to engage T-cells for cancer therapy. Curr Opin Mol Ther. 2009;11(1):22-30
38. Johnson S, Burke S, Huang L, Gorlatov S, Li H, Wang W, Zhang W, Tuaillon N, Rainey J, Barat B, Yang Y, Jin L, Ciccarone V, Moore PA, Koenig S, Bonvini E. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol. 2010;399(3):436-49
39. Salvador J-P, Vilaplana L, Marco M-P. Nanobody: outstanding features for diagnostic and therapeutic applications. Anal Bioanal Chem. 2019;411(9):1703-13
40. Nuñez-Prado N, Compte M, Harwood S, Álvarez-Méndez A, Lykkemark S, Sanz L, Álvarez-Vallina L. The coming of age of engineered multivalent antibodies. Drug Discov Today. 2015;20(5):588-94
41. Kontermann RE. Strategies to extend plasma half-lives of recombinant antibodies. BioDrugs. 2009;23(2):93-109
42. Sun Y, Yu X, Wang X, Yuan K, Wang G, Hu L, Zhang G, Pei W, Wang L, Sun C, Yang P. Bispecific antibodies in cancer therapy: target selection and regulatory requirements. Acta Pharm Sin B. 2023;13(9):3583-97
43. Kang J, Sun T, Zhang Y. Immunotherapeutic progress and application of bispecific antibody in cancer. Front Immunol. 2022;13:1020003
44. Frampton JE. Catumaxomab: in malignant ascites. Drugs. 2012;72(10):1399-410
45. Xu Y, Lee J, Tran C, Heibeck TH, Wang WD, Yang J, Stafford RL, Steiner AR, Sato AK, Hallam TJ, Yin G, editors. Production of bispecific antibodies in 'knobs-into-holes' using a cell-free expression system. mAbs. Taylor & Francis. 2015;7(1):231-42
46. Fan G, Wang Z, Hao M, Li J. Bispecific antibodies and their applications. J Hematol Oncol. 2015;8:130
47. Klein C, Schaefer W, Regula JT, Dumontet C, Brinkmann U, Bacac M, Umaña P. Engineering therapeutic bispecific antibodies using CrossMab technology. Methods. 2019;154:21-31
48. Li H, Er Saw P, Song E. Challenges and strategies for next-generation bispecific antibody-based antitumor therapeutics. Cell Mol Immunol. 2020;17(5):451-61
49. Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20(7):838-47
50. Patnaik A, Gordon M, Tsai F, Papadopoulos KP, Rasco D, Beeram M, Fu S, Janku F, Hynes SM, Gundala SR, Willard MD, Zhang W, Lin AB, Hong D. A phase I study of LY3164530, a bispecific antibody targeting MET and EGFR, in patients with advanced or metastatic cancer. Cancer Chemother Pharmacol. 2018;82(3):407-18
51. Goulet DR, Orcutt SJ, Zwolak A, Rispens T, Labrijn AF, de Jong RN, Atkins WM, Chiu ML. Kinetic mechanism of controlled Fab-arm exchange for the formation of bispecific immunoglobulin G1 antibodies. J Biol Chem. 2018;293(2):651-61
52. Chen Z, Qian Y, Song Y, Xu X, Tao L, Mussa N, Ghose S, Li ZJ, editors. Design of next-generation therapeutic IgG4 with improved manufacturability and bioanalytical characteristics. mAbs. Taylor & Francis. 2020;12(1):1829338
53. Hofmann T, Krah S, Sellmann C, Zielonka S, Doerner A. Greatest hits—innovative technologies for high throughput identification of bispecific antibodies. Int J Mol Sci. 2020;21(18):6551
54. Cho BC, Simi A, Sabari J, Vijayaraghavan S, Moores S, Spira A. Amivantamab, an epidermal growth factor receptor (EGFR) and mesenchymal-epithelial transition factor (MET) bispecific antibody, designed to enable multiple mechanisms of action and broad clinical applications. Clin Lung Cancer. 2023;24(2):89-97
55. Chen S, Li L, Zhang F, Wang Y, Hu Y, Zhao L. Immunoglobulin gamma-like therapeutic bispecific antibody formats for tumor therapy. J Immunol Res. 2019;2019:4516041
56. Dong F, Ghayur T. Recent advancements in DVD-Ig based therapeutic development. Drug Discov Today Technol. 2019;34:1-7
57. DiGiammarino E, Ghayur T, Liu J. Design and generation of DVD-Ig™ molecules for dual-specific targeting. Methods Mol Biol. 2012;899:145-56
58. Fleischmann RM, Bliddal H, Blanco FJ, Schnitzer TJ, Peterfy C, Chen S, Wang L, Feng S, Conaghan PG, Berenbaum F, Pelletier JP, Martel-Pelletier J, Vaeterlein O, Kaeley GS, Liu W, Kosloski MP, Levy G, Zhang L, Medema JK, Levesque MC. A phase II trial of lutikizumab, an anti-interleukin-1α/β dual variable domain immunoglobulin, in knee osteoarthritis patients with synovitis. Arthritis Rheumatol. 2019;71(7):1056-69
59. Ren F, Wu X, Yang D, Wu D, Gong S, Zhang Y, Lensky S, Wu C. EMB-01: an innovative bispecific antibody targeting EGFR and cMet on tumor cells mediates a novel mechanism to improve antitumor efficacy. Cancer Res. 2020;80(16_Supplement):528 -
60. Ahamadi-Fesharaki R, Fateh A, Vaziri F, Solgi G, Siadat SD, Mahboudi F, Rahimi-Jamnani F. Single-chain variable fragment-based bispecific antibodies: hitting two targets with one sophisticated arrow. Mol Ther Oncolytics. 2019;14:38-56
61. Krah S, Sellmann C, Rhiel L, Schröter C, Dickgiesser S, Beck J, Zielonka S, Toleikis L, Hock B, Kolmar H, Becker S. Engineering bispecific antibodies with defined chain pairing. New Biotechnol. 2017;39(B):167-73
62. Yuraszeck T, Kasichayanula S, Benjamin JE. Translation and clinical development of bispecific T-cell engaging antibodies for cancer treatment. Clin Pharmacol Ther. 2017;101(5):634-45
63. Einsele H, Borghaei H, Orlowski RZ, Subklewe M, Roboz GJ, Zugmaier G, Kufer P, Iskander K, Kantarjian HM. The BiTE (bispecific T-cell engager) platform: development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer. 2020;126(14):3192-201
64. Friedrich M, Raum T, Lutterbuese R, Voelkel M, Deegen P, Rau D, Kischel R, Hoffmann P, Brandl C, Schuhmacher J, Mueller P, Finnern R, Fuergut M, Zopf D, Slootstra JW, Baeuerle PA, Rattel B, Kufer P. Regression of human prostate cancer xenografts in mice by AMG 212/BAY2010112, a novel PSMA/CD3-Bispecific BiTE antibody cross-reactive with non-human primate antigens. Mol Cancer Ther. 2012;11(12):2664-73
65. Subklewe M, Stein A, Walter RB, Bhatia R, Wei AH, Ritchie D, Bücklein V, Vachhani P, Dai T, Hindoyan A, Agarwal S, Anderson A, Khaldoyanidi S, Ravandi F. Preliminary results from a phase 1 first-in-human study of AMG 673, a novel half-life extended (HLE) anti-CD33/CD3 BiTE®(bispecific T-cell engager) in patients with relapsed/refractory (R/R) acute myeloid leukemia (AML). Blood. 2019;134(Supplement 1):833
66. Lorenczewski G, Friedrich M, Kischel R, Dahlhoff C, Anlahr J, Balazs M. et al. Generation of a half-life extended anti-CD19 BiTE® antibody construct compatible with once-weekly dosing for treatment of CD19-positive malignancies. Blood. 2017;130:2815
67. Maschmeyer G, Bullinger L, Garcia-Vidal C, Herbrecht R, Maertens J, Menna P, Pagano L, Thiebaut-Bertrand A, Calandra T. Infectious complications of targeted drugs and biotherapies in acute leukemia. Clinical practice guidelines by the European Conference on Infections in Leukemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the International Immunocompromised Host Society (ICHS) and the European Leukemia Net (ELN). Leukemia. 2022;36(5):1215-26
68. Wei J, Yang Y, Wang G, Liu M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front Immunol. 2022;13:1035276
69. Suurs FV, Lub-de Hooge MN, de Vries EGE, de Groot DJA. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol Ther. 2019;201:103-19
70. Reusch U, Duell J, Ellwanger K, Herbrecht C, Knackmuss SH, Fucek I, Eser M, McAleese F, Molkenthin V, Gall FL, Topp M, Little M, Zhukovsky EA, editors. A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19+ tumor cells. mAbs. Taylor & Francis. 2015;7(3):584-604
71. Wu J, Fu J, Zhang M, Liu D. AFM13: a first-in-class tetravalent bispecific anti-CD30/CD16A antibody for NK cell-mediated immunotherapy. J Hematol Oncol. 2015;8:96
72. Ishiwatari-Ogata C, Kyuuma M, Ogata H, Yamakawa M, Iwata K, Ochi M, Hori M, Miyata N, Fujii Y. Ozoralizumab, a humanized Anti-TNFα NANOBODY® compound, exhibits efficacy not only at the onset of arthritis in a human TNF transgenic mouse but also during secondary failure of administration of an Anti-TNFα IgG. Front Immunol. 2022;13:853008
73. Zhou Y, Zong H, Han L, Xie Y, Jiang H, Gilly J, Zhang B, Lu H, Chen J, Sun R, Pan Z, Zhu J. A novel bispecific antibody targeting CD3 and prolactin receptor (PRLR) against PRLR-expression breast cancer. J Exp Clin Cancer Res. 2020;39(1):87
74. Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585-608
75. Kamakura D, Asano R, Kawai H, Yasunaga M. Mechanism of action of a T cell-dependent bispecific antibody as a breakthrough immunotherapy against refractory colorectal cancer with an oncogenic mutation. Cancer Immunol Immunother. 2021;70(1):177-88
76. Garrido F. HLA class-I expression and cancer immunotherapy. Adv Exp Med Biol. 2019;1151:79-90
77. Combariza JF, Arango M, Díaz L, Agudelo C, Hernandez S, Madera AM, León G, Avila V, Bautista L, Valdés J, Orduz R, Mejía F, Moreno L, Ramirez C. Measurable residual disease assessment and allogeneic transplantation as consolidation therapy in adult acute lymphoblastic leukemia in Colombia. Clin Lymphoma Myeloma Leuk. 2021;21(4):e365-72
78. Linke R, Klein A, Seimetz D, editors. Catumaxomab: clinical development and future directions. mAbs. Taylor & Francis. 2010;2(2):129-36
79. Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, Diedrich H, Topp MS, Brüggemann M, Horst HA, Havelange V, Stieglmaier J, Wessels H, Haddad V, Benjamin JE, Zugmaier G, Nagorsen D, Bargou RC. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-31
80. Wu Z, Cheung NV. T cell engaging bispecific antibody (T-BsAb): from technology to therapeutics. Pharmacol Ther. 2018;182:161-75
81. Garber K. Bispecific antibodies rise again. Nat Rev Drug Discov. 2014;13(11):799-801
82. Kuhn C, Weiner HL. Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy. 2016;8(8):889-906
83. Ganesan R, Chennupati V, Ramachandran B, Hansen MR, Singh S, Grewal IS. Selective recruitment of γδ T cells by a bispecific antibody for the treatment of acute myeloid leukemia. Leukemia. 2021;35(8):2274-84
84. Duell J, Dittrich M, Bedke T, Mueller T, Eisele F, Rosenwald A, Rasche L, Hartmann E, Dandekar T, Einsele H, Topp MS. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody Blinatumomab in patients with B-precursor ALL. Leukemia. 2017;31(10):2181-90
85. Blanco B, Domínguez-Alonso C, Alvarez-Vallina L. Bispecific immunomodulatory antibodies for cancer immunotherapy. Clin Cancer Res. 2021;27(20):5457-64
86. de Bruin RCG, Veluchamy JP, Lougheed SM, Schneiders FL, Lopez-Lastra S, Lameris R, Stam AG, Sebestyen Z, Kuball J, Molthoff CFM, Hooijberg E, Roovers RC, Santo JPD, van Bergen En Henegouwen PMP, Verheul HMW, de Gruijl TD, van der Vliet HJ. A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vγ9Vδ2-T cells. Oncoimmunology. 2017;7(1):e1375641
87. Rothe A, Sasse S, Topp MS, Eichenauer DA, Hummel H, Reiners KS, Dietlein M, Kuhnert G, Kessler J, Buerkle C, Ravic M, Knackmuss S, Marschner JP, Pogge von Strandmann E, Borchmann P, Engert A. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(26):4024-31
88. Zhang Y, Zheng J. Functions of immune checkpoint molecules beyond immune evasion. Adv Exp Med Biol. 2020;1248:201-26
89. Wang Y, Zhang X, Wang Y, Zhao W, Li H, Zhang L, Li X, Zhang T, Zhang H, Huang H, Liu C. Application of immune checkpoint targets in the anti-tumor novel drugs and traditional Chinese medicine development. Acta Pharm Sin B. 2021;11(10):2957-72
90. Kiaie SH, Sanaei MJ, Heshmati M, Asadzadeh Z, Azimi I, Hadidi S, Jafari R, Baradaran B. Immune checkpoints in targeted-immunotherapy of pancreatic cancer: New Hope for clinical development. Acta Pharm Sin B. 2021;11(5):1083-97
91. Sun C, Yin M, Cheng Y, Kuang Z, Liu X, Wang G, Wang X, Yuan K, Min W, Dong J, Hou Y, Hu L, Zhang G, Pei W, Wang L, Sun Y, Yu X, Xiao Y, Deng H, Yang P. Novel small-molecule PD-L1 inhibitor induces PD-L1 internalization and optimizes the immune microenvironment. J Med Chem. 2023;66(3):2064-83
92. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86
93. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Márquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535-46
94. Luke JJ, Patel MR, Blumenschein GR, Hamilton E, Chmielowski B, Ulahannan SV. et al. The PD-1-and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: a phase 1 trial. Nat Med. 2023:1-11
95. Woo S-R, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DAA. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917-27
96. NMPA. The NMPA approved the marketing of cadunilimumab injection with conditions: Center for Drug Evaluation, NMPA; 2022 [updated 06/29/2022; cited 2024; 1/5/2024. Available from: https://www.nmpa.gov.cn/yaowen/ypjgyw/20220629135936153.html.
97. Herrmann M, Krupka C, Deiser K, Brauchle B, Marcinek A, Ogrinc Wagner A. et al. Bifunctional PD-1× αCD3× αCD33 fusion protein reverses adaptive immune escape in acute myeloid leukemia. Blood. J Am Soc Hematol. 2018;132(23):2484-94
98. Duraiswamy J, Turrini R, Minasyan A, Barras D, Crespo I, Grimm AJ, Casado J, Genolet R, Benedetti F, Wicky A, Ioannidou K, Castro W, Neal C, Moriot A, Renaud-Tissot S, Anstett V, Fahr N, Tanyi JL, Eiva MA, Jacobson CA, Montone KT, Westergaard MCW, Svane IM, Kandalaft LE, Delorenzi M, Sorger PK, Färkkilä A, Michielin O, Zoete V, Carmona SJ, Foukas PG, Powell DJ, Rusakiewicz S, Doucey MA, Dangaj Laniti D, Coukos G. Myeloid antigen-presenting cell niches sustain antitumor T cells and license PD-1 blockade via CD28 costimulation. Cancer Cell. 2021;39(12):1623-1642.e20
99. Lakins M, Liao W, McConnell E, Kaka Q, Ofoedu J, Gradinaru C. et al. 573 FS120, an OX40/CD137 tetravalent bispecific dual agonist antibody, synergistically increases the antitumor activity of anti-PD-1 in preclinical studies. J Immunother Cancer. 2021;9(Supplement 2):A602-A
100. Kim HD, Park S, Jeong S, Lee YJ, Lee H, Kim CG, Kim KH, Hong SM, Lee JY, Kim S, Kim HK, Min BS, Chang JH, Ju YS, Shin EC, Song GW, Hwang S, Park SH. 4-1BB delineates distinct activation status of exhausted tumor-infiltrating CD8+ T cells in hepatocellular carcinoma. Hepatology. 2020;71(3):955-71
101. Compte M, Harwood SL, Muñoz IG, Navarro R, Zonca M, Perez-Chacon G, Erce-Llamazares A, Merino N, Tapia-Galisteo A, Cuesta AM, Mikkelsen K, Caleiras E, Nuñez-Prado N, Aznar MA, Lykkemark S, Martínez-Torrecuadrada J, Melero I, Blanco FJ, Bernardino de la Serna J, Zapata JM, Sanz L, Alvarez-Vallina L. A tumor-targeted trimeric 4-1BB-agonistic antibody induces potent anti-tumor immunity without systemic toxicity. Nat Commun. 2018;9(1):4809
102. Compte M, Harwood SL, Erce-Llamazares A, Tapia-Galisteo A, Romero E, Ferrer I, Garrido-Martin EM, Enguita AB, Ochoa MC, Blanco B, Oteo M, Merino N, Nehme-Álvarez D, Hangiu O, Domínguez-Alonso C, Zonca M, Ramírez-Fernández A, Blanco FJ, Morcillo MA, Muñoz IG, Melero I, Rodriguez-Peralto JL, Paz-Ares L, Sanz L, Alvarez-Vallina L. An Fc-free EGFR-specific 4-1BB-agonistic trimerbody displays broad antitumor activity in humanized murine cancer models without toxicity. Clin Cancer Res. 2021;27(11):3167-77
103. Hinner MJ, Aiba RSB, Jaquin TJ, Berger S, Dürr MC, Schlosser C, Allersdorfer A, Wiedenmann A, Matschiner G, Schüler J, Moebius U, Rothe C, Matis L, Olwill SA. Tumor-localized costimulatory T-cell engagement by the 4-1BB/HER2 bispecific antibody-anticalin fusion PRS-343. Clin Cancer Res. 2019;25(19):5878-89
104. Neben CL, Lo M, Jura N, Klein OD. Feedback regulation of RTK signaling in development. Dev Biol. 2019;447(1):71-89
105. Zhang N, Li Y. Receptor tyrosine kinases: biological functions and anticancer targeted therapy. Med. 2023;4(6):e446
106. Tan L, Zhang J, Wang Y, Wang X, Wang Y, Zhang Z, Shuai W, Wang G, Chen J, Wang C, Ouyang L, Li W. Development of dual inhibitors targeting epidermal growth factor receptor in cancer therapy. J Med Chem. 2022;65(7):5149-83
107. Remon J, Hendriks LEL, Cardona AF, Besse B. EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat Rev. 2020;90:102105
108. Vijayaraghavan S, Lipfert L, Chevalier K, Bushey BS, Henley B, Lenhart R, Sendecki J, Beqiri M, Millar HJ, Packman K, Lorenzi MV, Laquerre S, Moores SL. Amivantamab (JNJ-61186372), an Fc enhanced EGFR/cMet bispecific antibody, induces receptor downmodulation and antitumor activity by monocyte/macrophage trogocytosis. Mol Cancer Ther. 2020;19(10):2044-56
109. Park K, Haura EB, Leighl NB, Mitchell P, Shu CA, Girard N, Viteri S, Han JY, Kim SW, Lee CK, Sabari JK, Spira AI, Yang TY, Kim DW, Lee KH, Sanborn RE, Trigo J, Goto K, Lee JS, Yang JCH, Govindan R, Bauml JM, Garrido P, Krebs MG, Reckamp KL, Xie J, Curtin JC, Haddish-Berhane N, Roshak A, Millington D, Lorenzini P, Thayu M, Knoblauch RE, Cho BC. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391-402
110. Meric-Bernstam F, Hanna D, Beeram M, Lee K-W, Kang Y-K, Chaves J, Lee J, Goodwin R, Vaklavas C, Oh D-Y, Rha SY, Elimova E, Mayordomo J, Ferrario C, Cobleigh MA, Fortenberry A, Rowse G, Gray T, Lai R, Hamilton E. Safety, antitumour activity, and biomarker results of the HER2-targeted bispecific antibody ZW25. In: Ann Oncol. 2019;30:v167-8
111. Zymeworks. Clinical data demonstrating promising antitumor activity with zanidatamab in 1L setting of HER2-positive breast and gastroesophageal cancers to be presented at ASCO 2022: Zymeworks; 2022 [updated 04/26/2022; cited 2024; 01/05/2024. Available from: https://ir.zymeworks.com/news-releases/news-release-details/clinical-data-demonstratingpromising-antitumor-activity/.
112. Qi S, Deng S, Lian Z, Yu K. Novel drugs with high efficacy against tumor angiogenesis. Int J Mol Sci. 2022;23(13):6934
113. Kliewer SA, Mangelsdorf DJ. A dozen years of discovery: insights into the physiology and pharmacology of FGF21. Cell Metab. 2019;29(2):246-53
114. Baruch A, Wong C, Chinn LW, Vaze A, Sonoda J, Gelzleichter T, Chen S, Lewin-Koh N, Morrow L, Dheerendra S, Boismenu R, Gutierrez J, Wakshull E, Wilson ME, Arora PS. Antibody-mediated activation of the FGFR1/Klothoβ complex corrects metabolic dysfunction and alters food preference in obese humans. Proc Natl Acad Sci U S A. 2020;117(46):28992-9000
115. Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, Rodríguez-Ruiz ME, Ponz-Sarvise M, Castañón E, Melero I. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120(1):6-15
116. Montfort A, Filleron T, Virazels M, Dufau C, Milhès J, Pagès C, Olivier P, Ayyoub M, Mounier M, Lusque A, Brayer S, Delord JP, Andrieu-Abadie N, Levade T, Colacios C, Ségui B, Meyer N. Combining nivolumab and ipilimumab with infliximab or certolizumab in patients with advanced melanoma: first results of a phase Ib clinical trial. Clin Cancer Res. 2021;27(4):1037-47
117. Gulley JL, Schlom J, Barcellos-Hoff MH, Wang XJ, Seoane J, Audhuy F, Lan Y, Dussault I, Moustakas A. Dual inhibition of TGF-β and PD-L1: a novel approach to cancer treatment. Mol Oncol. 2022;16(11):2117-34
118. Yi M, Zhang J, Li A, Niu M, Yan Y, Jiao Y, Luo S, Zhou P, Wu K. The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-β and PD-L1. J Hematol Oncol. 2021;14(1):27
119. Genovese MC, Weinblatt ME, Aelion JA, Mansikka HT, Peloso PM, Chen K, Li Y, Othman AA, Khatri A, Khan NS, Padley RJ. ABT-122, a bispecific dual variable domain immunoglobulin targeting tumor necrosis factor and interleukin-17a, in patients with rheumatoid arthritis with an inadequate response to methotrexate: a randomized, double-blind study. Arthritis Rheumatol. 2018;70(11):1710-20
120. Østergaard H, Lund J, Greisen PJ, Kjellev S, Henriksen A, Lorenzen N. et al. A factor VIIIa-mimetic bispecific antibody, Mim8, ameliorates bleeding upon severe vascular challenge in hemophilia A mice. Blood. J Am Soc Hematol. 2021;138(14):1258-68
121. Stanimirovic D, Kemmerich K, Haqqani AS, Farrington GK. Engineering and pharmacology of blood-brain barrier-permeable bispecific antibodies. Adv Pharmacol. 2014;71:301-35
122. Dorostgou Z, Yadegar N, Dorostgou Z, Khorvash F, Vakili O. Novel insights into the role of circular RNAs in Parkinson disease: an emerging renaissance in the management of neurodegenerative diseases. J Neurosci Res. 2022;100(9):1775-90
123. Wec AZ, Nyakatura EK, Herbert AS, Howell KA, Holtsberg FW, Bakken RR, Mittler E, Christin JR, Shulenin S, Jangra RK, Bharrhan S, Kuehne AI, Bornholdt ZA, Flyak AI, Saphire EO, Crowe JE, Aman MJ, Dye JM, Lai JR, Chandran K. A 'Trojan horse' bispecific-antibody strategy for broad protection against ebolaviruses. Science. 2016;354(6310):350-4
124. DiGiandomenico A, Keller AE, Gao C, Rainey GJ, Warrener P, Camara MM, Bonnell J, Fleming R, Bezabeh B, Dimasi N, Sellman BR, Hilliard J, Guenther CM, Datta V, Zhao W, Gao C, Yu XQ, Suzich JA, Stover CK. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci Transl Med. 2014;6(262):262ra155
125. de Goeij BECG, Vink T, Ten Napel H, Breij ECW, Satijn D, Wubbolts R, Miao D, Parren PWHI. Efficient payload delivery by a bispecific antibody-drug conjugate targeting HER2 and CD63. Mol Cancer Ther. 2016;15(11):2688-97
126. Pantoliano MW, Bird RE, Johnson S, Asel ED, Dodd SW, Wood JF, Hardman KD. Conformational stability, folding, and ligand-binding affinity of single-chain Fv immunoglobulin fragments expressed in Escherichia coli. Biochemistry. 1991;30(42):10117-25
127. Miller BR, Demarest SJ, Lugovskoy A, Huang F, Wu X, Snyder WB, Croner LJ, Wang N, Amatucci A, Michaelson JS, Glaser SM. Stability engineering of scFvs for the development of bispecific and multivalent antibodies. Protein Eng Des Sel. 2010;23(7):549-57
128. Vaks L, Benhar I. Production of stabilized scFv antibody fragments in the E. coli bacterial cytoplasm. Methods Mol Biol. 2014;1060:171-84
129. Frenzel A, Hust M, Schirrmann T. Expression of recombinant antibodies. Front Immunol. 2013;4:217
130. Jäger V, Büssow K, Wagner A, Weber S, Hust M, Frenzel A, Schirrmann T. High level transient production of recombinant antibodies and antibody fusion proteins in HEK293 cells. BMC Biotechnol. 2013;13(1):52
131. Wang R, Xiang S, Feng Y, Srinivas S, Zhang Y, Lin M, Wang S. Engineering production of functional scFv antibody in E. coli by co-expressing the molecule chaperone Skp. Front Cell Infect Microbiol. 2013;3:72
132. Spiess C, Merchant M, Huang A, Zheng Z, Yang N-Y, Peng J, Ellerman D, Shatz W, Reilly D, Yansura DG, Scheer JM. Bispecific antibodies with natural architecture produced by co-culture of bacteria expressing two distinct half-antibodies. Nat Biotechnol. 2013;31(8):753-8
133. Shatz W, Ng D, Dutina G, Wong AW, Dunshee DR, Sonoda J, Shen A, Scheer JM, editors. An efficient route to bispecific antibody production using single-reactor mammalian co-culture. mAbs. Taylor & Francis. 2016;8(8):1487-97
134. Rajendra Y, Peery RB, Hougland MD, Barnard GC, Wu X, Fitchett JR, Bacica M, Demarest SJ. Transient and stable CHO expression, purification and characterization of novel hetero-dimeric bispecific I g G antibodies. Biotechnol Prog. 2017;33(2):469-77
135. Gong S, Wu C. Efficient production of bispecific antibodies—optimization of transfection strategy leads to high-level stable cell line generation of a Fabs-in-tandem immunoglobin. Antibody Ther. 2023;6(3):170-9
136. Singh A, Dees S, Grewal IS. Overcoming the challenges associated with CD3+ T-cell redirection in cancer. Br J Cancer. 2021;124(6):1037-48
137. Staflin K, Zuch de Zafra CLZ, Schutt LK, Clark V, Zhong F, Hristopoulos M, Clark R, Li J, Mathieu M, Chen X, Johnston J, Low J, Ybarra R, Slaga D, Yang J, Ovacik M, Dybdal NO, Totpal K, Junttila MR, Ellerman D, Lee G, Dennis MS, Prell R, Junttila TT. Target arm affinities determine preclinical efficacy and safety of anti-HER2/CD3 bispecific antibody. JCI Insight. 2020;5(7):e133757
138. Dang K, Castello G, Clarke SC, Li Y, Balasubramani A, Boudreau A, Davison L, Harris KE, Pham D, Sankaran P, Ugamraj HS, Deng R, Kwek S, Starzinski A, Iyer S, van Schooten W, Schellenberger U, Sun W, Trinklein ND, Buelow R, Buelow B, Fong L, Dalvi P. Attenuating CD3 affinity in a PSMAxCD3 bispecific antibody enables killing of prostate tumor cells with reduced cytokine release. J Immunother Cancer. 2021;9(6):e002488
139. Hutchings M, Morschhauser F, Iacoboni G, Carlo-Stella C, Offner FC, Sureda A, Salles G, Martínez-Lopez J, Crump M, Thomas DN, Morcos PN, Ferlini C, Bröske AE, Belousov A, Bacac M, Dimier N, Carlile DJ, Lundberg L, Perez-Callejo D, Umaña P, Moore T, Weisser M, Dickinson MJ. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J Clin Oncol. 2021;39(18):1959-70
140. Steele CB, Li J, Huang B, Weir HK. Prostate cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD-2 study. Cancer. 2017;123(Supplement 24):5160-77
141. (CDC)Aktary ML, Shewchuk B, Wang Q, Hyndman E, Shack L, Robson PJ, Kopciuk KA cfdcap. Health-Related and Psychosocial Factors Associated with Prostate Cancer Stage at Diagnosis among Males Participating in Alberta's Tomorrow Project. Prostate Cancer. Center for Disease Control and Prevention (CDC); [updated 07/17/2023; cited 2024. 2023 Available from: https://www.cdc.gov/cancer/prostate/index.htm;2023:4426167
142. Movahedpour A, Khatami SH, Karami N, Vakili O, Naeli P, Jamali Z, Shabaninejad Z, Tazik K, Behrouj H, Ghasemi H. Exosomal noncoding RNAs in prostate cancer. Clin Chim Acta. 2022;537:127-32
143. Taitt HE. Global trends and prostate cancer: a review of incidence, detection, and mortality as influenced by race, ethnicity, and geographic location. Am J Mens Health. 2018;12(6):1807-23
144. Costello LC, Franklin RB. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch Biochem Biophys. 2016;611:100-12
145. Braunhut BL, Punnen S, Kryvenko ON. Updates on grading and staging of prostate cancer. Surg Pathol Clin. 2018;11(4):759-74
146. Wang G, Zhao D, Spring DJ, DePinho RA. Genetics and biology of prostate cancer. Genes Dev. 2018;32(17-18):1105-40
147. Suzman DL, Boikos SA, Carducci MA. Bone-targeting agents in prostate cancer. Cancer Metastasis Rev. 2014;33(2-3):619-28
148. Rebbeck TR, editor. Prostate cancer genetics: variation by race, ethnicity, and geography. Semin Radiat Oncol. Elsevier. 2017;27(1):3-10
149. Moran A, O'Hara C, Khan S, Shack L, Woodward E, Maher ER, Lalloo F, Evans DG. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2012;11(2):235-42
150. Sekhoacha M, Riet K, Motloung P, Gumenku L, Adegoke A, Mashele S. Prostate cancer review: genetics, diagnosis, treatment options, and alternative approaches. Molecules. 2022;27(17):5730
151. Sato H, Narita S, Ishida M, Takahashi Y, Mingguo H, Kashima S, Yamamoto R, Koizumi A, Nara T, Numakura K, Saito M, Yoshioka T, Habuchi T. Specific gut microbial environment in lard diet-induced prostate cancer development and progression. Int J Mol Sci. 2022;23(4):2214
152. Shah SR, Freedland SJ, Aronson WJ, Kane CJ, Presti Jr JC, Amling CL, Terris MK. Exposure to Agent Orange is a significant predictor of prostate-specific antigen (PSA)-based recurrence and a rapid PSA doubling time after radical prostatectomy. BJU Int. 2009;103(9):1168-72
153. Hjelmborg JB, Scheike T, Holst K, Skytthe A, Penney KL, Graff RE, Pukkala E, Christensen K, Adami HO, Holm NV, Nuttall E, Hansen S, Hartman M, Czene K, Harris JR, Kaprio J, Mucci LA. The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2303-10
154. Ilic D, Djulbegovic M, Jung JH, Hwang EC, Zhou Q, Cleves A, Agoritsas T, Dahm P. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018;362:k3519
155. Alford AV, Brito JM, Yadav KK, Yadav SS, Tewari AK, Renzulli J. The use of biomarkers in prostate cancer screening and treatment. Rev Urol. 2017;19(4):221-34
156. Lamy P-J, Allory Y, Gauchez A-S, Asselain B, Beuzeboc P, De Cremoux P, Fontugne J, Georges A, Hennequin C, Lehmann-Che J, Massard C, Millet I, Murez T, Schlageter M-H, Rouvière O, Kassab-Chahmi D, Rozet F, Descotes J-L, Rébillard X. Prognostic biomarkers used for localised prostate cancer management: a systematic review. Eur Urol Focus. 2018;4(6):790-803
157. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, Briganti A, Budäus L, Hellawell G, Hindley RG, Roobol MJ, Eggener S, Ghei M, Villers A, Bladou F, Villeirs GM, Virdi J, Boxler S, Robert G, Singh PB, Venderink W, Hadaschik BA, Ruffion A, Hu JC, Margolis D, Crouzet S, Klotz L, Taneja SS, Pinto P, Gill I, Allen C, Giganti F, Freeman A, Morris S, Punwani S, Williams NR, Brew-Graves C, Deeks J, Takwoingi Y, Emberton M, Moore CM, PRECISION Study Group Collaborators. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767-77
158. Lima ZS, Ghadamzadeh M, Arashloo FT, Amjad G, Ebadi MR, Younesi L. Recent advances of therapeutic targets based on the molecular signature in breast cancer: genetic mutations and implications for current treatment paradigms. J Hematol Oncol. 2019;12(1):38
159. Molina A, Belldegrun A. Novel therapeutic strategies for castration resistant prostate cancer: inhibition of persistent androgen production and androgen receptor mediated signaling. J Urol. 2011;185(3):787-94
160. Jan R, Chaudhry GES. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv Pharm Bull. 2019;9(2):205-18
161. Altwaijry N, Somani S, Dufès C. Targeted nonviral gene therapy in prostate cancer. Int J Nanomedicine. 2018;13:5753-67
162. Miller KD, Goding Sauer A, Ortiz AP, Fedewa SA, Pinheiro PS, Tortolero-Luna G, Martinez-Tyson D, Jemal A, Siegel RL. Cancer statistics for hispanics/latinos, 2018. CA Cancer J Clin. 2018;68(6):425-45
163. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34
164. Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3(1):17009
165. Williamson SR. Renal cell cancer: pathology and genetics. Semin Cancer Biol. 2019;23:3-9
166. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30
167. Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, Gore JL, Sun M, Wood C, Russo P. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74-84
168. Menko FH, Maher ER, Schmidt LS, Middelton LA, Aittomäki K, Tomlinson I, Richard S, Linehan WM. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13(4):637-44
169. Bahadoram S, Davoodi M, Hassanzadeh S, Bahadoram M, Barahman M, Mafakher L. Renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment. G Ital Nefrol. 2022;39(3):2022-vol3
170. Nabi S, Kessler ER, Bernard B, Flaig TW, Lam ET. Renal cell carcinoma: a review of biology and pathophysiology. F1000Res. 2018;7:307
171. Gansler T, Fedewa S, Amin MB, Lin CC, Jemal A. Trends in reporting histological subtyping of renal cell carcinoma: association with cancer center type. Hum Pathol. 2018;74:99-108
172. Scelo G, Larose TL. Epidemiology and risk factors for kidney cancer. J Clin Oncol. 2018;36(36):JCO2018791905
173. Kim CS, Han K-D, Choi HS, Bae EH, Ma SK, Kim SW. Association of hypertension and blood pressure with kidney cancer risk: a nationwide population-based cohort study. Hypertension. 2020;75(6):1439-46
174. Maher ER. Hereditary renal cell carcinoma syndromes: diagnosis, surveillance and management. World J Urol. 2018;36(12):1891-8
175. Agnello F, Albano D, Micci G, Di Buono G, Agrusa A, Salvaggio G, Pardo S, Sparacia G, Bartolotta TV, Midiri M, Lagalla R, Galia M. CT and MR imaging of cystic renal lesions. Insights Imaging. 2020;11(1):5
176. Farolfi A, Koschel S, Murphy DG, Fanti S. PET imaging in urology: a rapidly growing successful collaboration. Curr Opin Urol. 2020;30(5):623-7
177. Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernández-Pello S, Giles RH, Hofmann F, Hora M, Kuczyk MA, Kuusk T, Lam TB, Marconi L, Merseburger AS, Powles T, Staehler M, Tahbaz R, Volpe A, Bex A. European Association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75(5):799-810
178. Carmen S, Corominas D, Mireia M, Blanca P, Tarek A, Nicolau C. Active surveillance of small renal masses. Insights Imaging. 2020;11(1):63
179. Sawhney P, Suyanto S, Michael A, Pandha H. Adjuvant therapy for renal cell carcinoma. J Cancer Metastasis Treat. 2021;7:48
180. Quek ML, Stein JP, Daneshmand S, Miranda G, Thangathurai D, Roffey P, Skinner EC, Lieskovsky G, Skinner DG. A critical analysis of perioperative mortality from radical cystectomy. J Urol. 2006;175(3 Pt 1):886-9 discussion 889
181. CANCER.net. Bladder cancer: statistics; 2023 [updated 03/2023; cited 2024 01/05/2024]. Available from: https://www.cancer.net/cancer-types/bladder-cancer/statistics.
182. Lobo N, Shariat SF, Guo CC, Fernandez MI, Kassouf W, Choudhury A, Gao J, Williams SB, Galsky MD, Taylor JA, Roupret M, Kamat AM. What is the significance of variant histology in urothelial carcinoma? Eur Urol Focus. 2020;6(4):653-63
183. Matulay JT, Kamat AM. Advances in risk stratification of bladder cancer to guide personalized medicine. F1000Res. 2018;7:F1000 Faculty Rev-1137
184. Crabb SJ, Douglas J. The latest treatment options for bladder cancer. Br Med Bull. 2018;128(1):85-95
185. Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: a review. JAMA. 2020;324(19):1980-91
186. Wang Y, Chang Q, Li Y. Racial differences in urinary bladder cancer in the United States. Sci Rep. 2018;8(1):12521
187. Gild P, Wankowicz SA, Sood A, von Landenberg N, Friedlander DF, Alanee S, Chun FKH, Fisch M, Menon M, Trinh QD, Bellmunt J, Abdollah F, editors. Racial disparity in quality of care and overall survival among black vs. white patients with muscle-invasive bladder cancer treated with radical cystectomy: A national cancer database analysis. Urol Oncol Semin Orig Investig. Elsevier. 2018;36(10):469.e1-469.e11
188. Ishida K, Hsieh MH. Understanding urogenital schistosomiasis-related bladder cancer: an update. Front Med (Lausanne). 2018;5:223
189. Moschini M, Zaffuto E, Karakiewicz PI, Andrea DD, Foerster B, Abufaraj M, Soria F, Mattei A, Montorsi F, Briganti A, Shariat SF. External beam radiotherapy increases the risk of bladder cancer when compared with radical prostatectomy in patients affected by prostate cancer: a population-based analysis. Eur Urol. 2019;75(2):319-28
190. Leão R, Lee D, Figueiredo A, Hermanns T, Wild P, Komosa M, Lau I, Mistry M, Nunes NM, Price AJ, Zhang C, Lipman T, Poyet C, Valtcheva N, Oehl K, Coelho H, Sayyid R, Gomes AM, Prado E Castro L, Sweet J, Vinagre J, Apolónio J, Stephens D, Faleiro I, Fadaak K, Richard PO, Kulkarni G, Zlotta AR, Hamilton RJ, Castelo-Branco P, Tabori U. Combined genetic and epigenetic alterations of the TERT promoter affect clinical and biological behavior of bladder cancer. Int J Cancer. 2019;144(7):1676-84
191. Tran L, Xiao J-F, Agarwal N, Duex JE, Theodorescu D. Advances in bladder cancer biology and therapy. Nat Rev Cancer. 2021;21(2):104-21
192. Degeorge KC, Holt HR, Hodges SC. Bladder cancer: diagnosis and treatment. Am Fam Physician. 2017;96(8):507-14
193. Xiong Y, Li J, Ma S, Ge J, Zhou L, Li D, Chen Q. A meta-analysis of narrow band imaging for the diagnosis and therapeutic outcome of non-muscle invasive bladder cancer. PLOS One. 2017;12(2):e0170819
194. Luo C, Huang B, Wu Y, Chen J, Chen L. Use of Vesical Imaging-Reporting and Data System (VI-RADS) for detecting the muscle invasion of bladder cancer: a diagnostic meta-analysis. Eur Radiol. 2020;30(8):4606-14
195. Faiena I, Rosser CJ, Chamie K, Furuya H. Diagnostic biomarkers in non-muscle invasive bladder cancer. World J Urol. 2019;37(10):2009-16
196. Pettenati C, Ingersoll MA. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol. 2018;15(10):615-25
197. Freshwater T, Li H, Valiathan C, Li M, Perini R, Bracco OL, Frenkl T, Keefe S. Systematic literature review and meta-analysis of response to first-line therapies for advanced/metastatic urothelial cancer patients who are cisplatin ineligible. Am J Clin Oncol. 2019;42(10):802-9
198. Wołącewicz M, Hrynkiewicz R, Grywalska E, Suchojad T, Leksowski T, Roliński J, Niedźwiedzka-Rystwej P. Immunotherapy in bladder cancer: current methods and future perspectives. Cancers. 2020;12(5):1181
199. Znaor A, Skakkebaek NE, Rajpert-De Meyts E, Laversanne M, Kuliš T, Gurney J, Sarfati D, McGlynn KA, Bray F. Testicular cancer incidence predictions in Europe 2010-2035: A rising burden despite population ageing. Int J Cancer. 2020;147(3):820-8
200. Bresciani M, Boarin M, Facconi I, Manara DF, Villa G. Awareness of testicular cancer among young men: A literature review. Int J Urol Nurs. 2021;15(1):5-11
201. Lehnen TL. Testicular cancer. https://irishpracticenurses.ie/wp-content/uploads/2022/02/Testicular-Cancer.pdf.
202. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M. et al. Global cancer observatory: cancer today. Lyon: International Agency for Research on Cancer; 2020. Cancer Tomorrow. 2021
203. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-49
204. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs—part A: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93-105
205. Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update. 2006;12(3):303-23
206. Daugaard G, Gundgaard MG, Mortensen MS, Agerbæk M, Holm NV, Rørth M, von der Maase H, Christensen IJ, Lauritsen J. Surveillance for stage I nonseminoma testicular cancer: outcomes and long-term follow-up in a population-based cohort. J Clin Oncol. 2014;32(34):3817-23
207. Motzer RJ, George J. 82 testicular cancer. Harrison's Princ Intern Med. 2005:550-3
208. Purdue MP, Devesa SS, Sigurdson AJ, McGlynn KA. International patterns and trends in testis cancer incidence. Int J Cancer. 2005;115(5):822-7
209. Lymperi S, Giwercman A. Endocrine disruptors and testicular function. Metabolism. 2018;86:79-90
210. Del Risco Kollerud R, Ruud E, Haugnes HS, Cannon-Albright LA, Thoresen M, Nafstad P, Vlatkovic L, Blaasaas KG, Næss Ø, Claussen B. Family history of cancer and risk of paediatric and young adult's testicular cancer: A Norwegian cohort study. Br J Cancer. 2019;120(10):1007-14
211. Garolla A, Vitagliano A, Muscianisi F, Valente U, Ghezzi M, Andrisani A, Ambrosini G, Foresta C. Role of viral infections in testicular cancer etiology: evidence from a systematic review and meta-analysis. Front Endocrinol. 2019;10:355
212. Grasso C, Zugna D, Fiano V, Robles Rodriguez N, Maule M, Gillio-Tos A, Ciuffreda L, Lista P, Segnan N, Merletti F, Richiardi L. Subfertility and risk of testicular cancer in the EPSAM case-control study. PLOS One. 2016;11(12):e0169174
213. Boccellino M, Vanacore D, Zappavigna S, Cavaliere C, Rossetti S, D'Aniello C, Chieffi P, Amler E, Buonerba C, Di Lorenzo G, Di Franco R, Izzo A, Piscitelli R, Iovane G, Muto P, Botti G, Perdonà S, Caraglia M, Facchini G. Testicular cancer from diagnosis to epigenetic factors. Oncotarget. 2017;8(61):104654-63
214. Oldenburg J, Berney DM, Bokemeyer C, Climent MA, Daugaard G, Gietema JA, De Giorgi U, Haugnes HS, Huddart RA, Leão R, Sohaib A, Gillessen S, Powles T, ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org, EURACAN. Testicular seminoma and non-seminoma: ESMO-EURACAN clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(4):362-75
215. Chovanec M, Cheng L. Advances in diagnosis and treatment of testicular cancer. BMJ. 2022;379:e070499
216. Cardona CEM, García-Perdomo HA. Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev Panam Salud Publ. 2018;41:e117
217. Douglawi A, Masterson TA. Penile cancer epidemiology and risk factors: a contemporary review. Curr Opin Urol. 2019;29(2):145-9
218. Olesen TB, Sand FL, Rasmussen CL, Albieri V, Toft BG, Norrild B, Munk C, Kjær SK. Prevalence of human papillomavirus DNA and p16INK4a in penile cancer and penile intraepithelial neoplasia: a systematic review and meta-analysis. Lancet Oncol. 2019;20(1):145-58
219. Hernandez BY, Barnholtz-Sloan J, German RR, Giuliano A, Goodman MT, King JB, Negoita S, Villalon-Gomez JM. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008 113(10)(Supplement):2883-91
220. Favorito LA, Nardi AC, Ronalsa M, Zequi SC, Sampaio FJB, Glina S. Epidemiologic study on penile cancer in Brazil. Int Braz J Urol. 2008;34(5):587-91 discussion 591
221. institute nc. Penile Cancer Treat (PDQ®)-Health Professional. version 2023 [updated 06/02/2023; cited. 2024 01/06/2024. Available from: https://www.cancer.gov/types/penile/hp/penile-treatment-pdq#section/all
222. Marchionne E, Perez C, Hui A, Khachemoune A. Penile squamous cell carcinoma: a review of the literature and case report treated with Mohs micrographic surgery. An Bras Dermatol. 2017;92(1):95-9
223. Thomas A, Necchi A, Muneer A, Tobias-Machado M, Tran ATH, Van Rompuy AS, Spiess PE, Albersen M. Penile cancer. Nat Rev Dis Primers. 2021;7(1):11
224. Chipollini J, Chaing S, Azizi M, Kidd LC, Kim P, Spiess PE. Advances in understanding of penile carcinogenesis: the search for actionable targets. Int J Mol Sci. 2017;18(8):1777
225. Douglawi A, Masterson TA. Updates on the epidemiology and risk factors for penile cancer. Transl Androl Urol. 2017;6(5):785-90
226. De Paula AAP, Motta ED, Alencar R, Saddi VA, da Silva RC, Caixeta GN, Almeida Netto JC, Carneiro MAS. The impact of cyclooxygenase-2 and vascular endothelial growth factor C immunoexpression on the prognosis of penile carcinoma. J Urol. 2012;187(1):134-40
227. Zuo L, Prather ER, Stetskiv M, Garrison DE, Meade JR, Peace TI, Zhou T. Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments. Int J Mol Sci. 2019;20(18):4472
228. CANCER.net. Penile Cancer: symptoms and Signs 2023 [updated 02/2023; cited 2024 01/06/2024]. Available from: https://www.cancer.net/cancer-types/penile-cancer/symptoms-and-signs.
229. Stecca CE, Alt M, Jiang DM, Chung P, Crook JM, Kulkarni GS, Sridhar SS. Recent advances in the management of penile cancer: a contemporary review of the literature. Oncol Ther. 2021;9(1):21-39
230. CANCER.net. Penile cancer: risk factors and prevention; 2023 [updated 02/2023; cited 2024 01/06/2024]. Available from: https://www.cancer.net/cancer-types/penile-cancer/risk-factors-and-prevention.
231. Thomas A, Vanthoor J, Vos G, Tsaur I, Albersen M, in collaboration with the European Reference Network for rare urogenital diseases, complex conditions (eUROGEN). Risk factors and molecular characterization of penile cancer: impact on prognosis and potential targets for systemic therapy. Curr Opin Urol. 2020;30(2):202-7
232. Zekan DS, Dahman A, Hajiran AJ, Luchey AM, Chahoud J, Spiess PE. Prognostic predictors of lymph node metastasis in penile cancer: a systematic review. Int Braz J Urol. 2021;47(5):943-56
233. Canete-Portillo S, Velazquez EF, Kristiansen G, Egevad L, Grignon D, Chaux A, Cubilla AL. Report From the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers V: Recommendations on the Use of Immunohistochemical and Molecular Biomarkers in Penile Cancer. Am J Surg Pathol. Report from the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers. 2020;44(7):e80-6
234. Peyraud F, Allenet C, Gross-Goupil M, Domblides C, Lefort F, Daste A, Yacoub M, Haaser T, Ferretti L, Robert G, Ravaud A. Current management and future perspectives of penile cancer: an updated review. Cancer Treat Rev. 2020;90:102087
235. Udager AM, Liu TY, Skala SL, Magers MJ, McDaniel AS, Spratt DE, Feng FY, Siddiqui J, Cao X, Fields KL, Morgan TM, Palapattu GS, Weizer AZ, Chinnaiyan AM, Alva A, Montgomery JS, Tomlins SA, Jiang H, Mehra R. Frequent PD-L1 expression in primary and metastatic penile squamous cell carcinoma: potential opportunities for immunotherapeutic approaches. Ann Oncol. 2016;27(9):1706-12
236. Ottenhof SR, Djajadiningrat RS, de Jong J, Thygesen HH, Horenblas S, Jordanova ES. Expression of programmed death ligand 1 in penile cancer is of prognostic value and associated with HPV status. J Urol. 2017;197(3 Pt 1):690-7
237. Gaspar M, Pravin J, Rodrigues L, Uhlenbroich S, Everett KL, Wollerton F, Morrow M, Tuna M, Brewis N. CD137/OX40 bispecific antibody induces potent antitumor activity that is dependent on target coengagement. Cancer Immunol Res. 2020;8(6):781-93
238. Ordóñez-Reyes C, Garcia-Robledo JE, Chamorro DF, Mosquera A, Sussmann L, Ruiz-Patiño A, Arrieta O, Zatarain-Barrón L, Rojas L, Russo A, de Miguel-Perez D, Rolfo C, Cardona AF. Bispecific antibodies in cancer immunotherapy: a novel response to an old question. Pharmaceutics. 2022;14(6):1243
239. Zekri L, Vogt F, Osburg L, Müller S, Kauer J, Manz T, Pflügler M, Maurer A, Heitmann JS, Hagelstein I, Märklin M, Hörner S, Todenhöfer T, Calaminus C, Stenzl A, Pichler B, la Fougère C, Schneider MA, Rammensee HG, Zender L, Sipos B, Salih HR, Jung G. An IgG-based bispecific antibody for improved dual targeting in PSMA-positive cancer. EMBO Mol Med. 2021;13(2):e11902
240. Bailis J, Deegen P, Thomas O, Bogner P, Wahl J, Liao M. et al. Preclinical evaluation of AMG;160, Anext-generation bispecific T cell engager (BiTE) targeting the prostate-specific membrane antigen PSMA for metastatic castration-resistant prostate cancer (mCRPC). American Society of Clinical Oncology. 2019
241. Deegen P, Thomas O, Nolan-Stevaux O, Li S, Wahl J, Bogner P, Aeffner F, Friedrich M, Liao MZ, Matthes K, Rau D, Rattel B, Raum T, Kufer P, Coxon A, Bailis JM. The PSMA-targeting half-life extended BiTE therapy AMG 160 has potent antitumor activity in preclinical models of metastatic castration-resistant prostate cancer. Clin Cancer Res. 2021;27(10):2928-37
242. Chou J, Egusa EA, Wang S, Badura ML, Lee F, Bidkar AP, Zhu J, Shenoy T, Trepka K, Robinson TM, Steri V, Huang J, Wang Y, Small EJ, Chan E, Stohr BA, Ashworth A, Delafontaine B, Rottey S, Cooke KS, Hashemi Sadraei N, Yu B, Salvati M, Bailis JM, Feng FY, Flavell RR, Aggarwal R. Immunotherapeutic targeting and PET imaging of DLL3 in Small-Cell Neuroendocrine Prostate Cancer. In: Cancer Res. 2023;83(2):301-15
243. Lin T-Y, Park JA, Long A, Guo H-F, Cheung NV. Novel potent anti-STEAP1 bispecific antibody to redirect T cells for cancer immunotherapy. J Immunother Cancer. 2021;9(9):e003114
244. Huang MTF, Sharma V, Mendelsohn A, Wei Q, Li J, Yu B, Larrick JW, Lum LG. Broad reactivity and enhanced potency of recombinant anti-EGFR× anti-CD3 bispecific antibody-armed activated T cells against solid tumours. Ann Med. 2022;54(1):1047-57
245. Kvarnhammar AM, Veitonmäki N, Hägerbrand K, Dahlman A, Smith KE, Fritzell S, von Schantz L, Thagesson M, Werchau D, Smedenfors K, Johansson M, Rosén A, Åberg I, Winnerstam M, Nyblom E, Barchan K, Furebring C, Norlén P, Ellmark P. The CTLA-4 x OX40 bispecific antibody ATOR-1015 induces anti-tumor effects through tumor-directed immune activation. J Immunother Cancer. 2019;7(1):103
246. Ma W, Ma J, Ma P, Lei T, Zhao M, Zhang M. Targeting immunotherapy for bladder cancer using anti-CD 3× B7-H3 bispecific antibody. Cancer Med. 2018;7(10):5167-77
247. Ma W, Ma J, Lei T, Zhao M, Zhang M. Targeting immunotherapy for bladder cancer by using anti-CD3× CD155 bispecific antibody. J Cancer. 2019;10(21):5153-61
248. Ruf P, Bauer HW, Schoberth A, Kellermann C, Lindhofer H. First time intravesically administered trifunctional antibody catumaxomab in patients with recurrent non-muscle invasive bladder cancer indicates high tolerability and local immunological activity. Cancer Immunol Immunother. 2021;70(9):2727-35
249. Schönberger S, Kraft D, Nettersheim D, Schorle H, Casati A, Craveiro RB, Mohseni MM, Calaminus G, Dilloo D. Targeting EpCAM by a bispecific trifunctional antibody exerts profound cytotoxic efficacy in germ cell tumor cell lines. Cancers. 2020;12(5):1279
250. Miyahira AK, Soule HR. The 27th annual Prostate Cancer Foundation scientific retreat report. Prostate. 2021;81(15):1107-24
251. Heitmann JS, Walz JS, Pflügler M, Kauer J, Schlenk RF, Jung G, Salih HR. Protocol of a prospective, multicentre phase I study to evaluate the safety, tolerability and preliminary efficacy of the bispecific PSMAxCD3 antibody CC-1 in patients with castration-resistant prostate carcinoma. BMJ Open. 2020;10(10):e039639
252. Heitmann JS, Walz JS, Pflügler M, Marconato M, Tegeler CM, Reusch J, Labrenz J, Schlenk R, Jung G, Salih H. Abstract CT141: CC-1, a bispecific PSMAxCD3 antibody for treatment of prostate carcinoma: results of the ongoing phase I dose escalation trial. Cancer Res. 2022;82(12_Supplement):CT141 -:Abstract CT141
253. Vaishampayan UN, Thakur A, Chen W, Deol A, Patel M, Dobson K, Dickow B, Schalk D, Schienschang A, Whitaker S, Polend A, Fontana JA, Heath EI, Lum LG. Phase II trial of pembrolizumab and anti-CD3 x anti-HER2 bispecific antibody-armed activated T cells in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2023;29(1):122-33
254. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. et al. New response evaluation criteria in solid tumours: revised RECIST guideline. version 1.1. Eur J Cancer. 2009 45(2). p. 228-47
255. Meng L, Yang Y, Mortazavi A, Zhang J. Emerging immunotherapy approaches for treating prostate cancer. Int J Mol Sci. 2023;24(18):14347
256. Kwon JTW, Bryant RJ, Parkes EE. The tumor microenvironment and immune responses in prostate cancer patients. Endocr Relat Cancer. 2021;28(8):T95-T107
257. Jiao S, Subudhi SK, Aparicio A, Ge Z, Guan B, Miura Y, Sharma P. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell. 2019;179(5):1177-1190.e13
258. Miller DR, Ingersoll MA, Teply BA, Lin M-F. Combination treatment options for castration-resistant prostate cancer. Exon Publications. 2021 p. 59-80
259. Krishna M, Nadler SG. Immunogenicity to biotherapeutics-the role of antidrug immune complexes. Front Immunol. 2016;7:21
260. Franquiz MJ, Short NJ. Blinatumomab for the treatment of adult B-cell acute lymphoblastic leukemia: toward a new era of targeted immunotherapy. Biologics. 2020;14:23-34
261. Slaga D, Ellerman D, Lombana TN, Vij R, Li J, Hristopoulos M, Clark R, Johnston J, Shelton A, Mai E, Gadkar K, Lo AA, Koerber JT, Totpal K, Prell R, Lee G, Spiess C, Junttila TT. Avidity-based binding to HER2 results in selective killing of HER2-overexpressing cells by anti-HER2/CD3. Sci Transl Med. 2018;10(463):eaat5775
262. Ahmed N, Caimi P, Reese JS, Otegbeye F, Patel S, Schneider D, Boughan KM, Cooper B, Gallogly M, Kruger W, Worden A, Kadan M, Lopes FBTP, Sharma A, Malek E, Metheny L, Tomlinson B, Wald D, Sekaly RP, Orentas R, Dropulic B, de Lima M. Prophylactic tocilizumab in patients with relapsed refractory lymphoma treated with anti CD19 chimeric antigen receptor T-cell therapy. Biol Blood Marrow Transplant. 2020;26(3):S275-6
263. Schlothauer T, Herter S, Koller CF, Grau-Richards S, Steinhart V, Spick C, Kubbies M, Klein C, Umaña P, Mössner E. Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Eng Des Sel. 2016;29(10):457-66
264. Panchal A, Seto P, Wall R, Hillier BJ, Zhu Y, Krakow J, Datt A, Pongo E, Bagheri A, Chen TT, Degenhardt JD, Culp PA, Dettling DE, Vinogradova MV, May C, DuBridge RB, editors. COBRA™: a highly potent conditionally active T cell engager engineered for the treatment of solid tumors. mAbs. Taylor & Francis. 2020;12(1):1792130
265. Sim B-C. AMX-168, a long-acting, tumor protease-sensitive bispecific precursor for the treatment of solid malignancies. Cancer Res. 2017;77(13):3638 -
266. Lemon B, Aaron W, Austin R, Baeuerle P, Barath M, Jones A. et al. HPN424, a half-life extended, PSMA/CD3-specific TriTAC for the treatment of metastatic prostate cancer. Cancer Res. 2018;78(13):1773 -
267. Zaman R, Islam RA, Ibnat N, Othman I, Zaini A, Lee CY, Chowdhury EH. Current strategies in extending half-lives of therapeutic proteins. J Control Release. 2019;301:176-89
268. Pang X, Ma F, Zhang P, Zhong Y, Zhang J, Wang T, Zheng G, Hou X, Zhao J, He C, Chen ZY. Treatment of human B-cell lymphomas using minicircle DNA vector expressing anti-CD3/CD20 in a mouse model. Hum Gene Ther. 2017;28(2):216-25
269. Perales-Puchalt A, Duperret EK, Yang X, Hernandez P, Wojtak K, Zhu X, Jung SH, Tello-Ruiz E, Wise MC, Montaner LJ, Muthumani K, Weiner DB. DNA-encoded bispecific T cell engagers and antibodies present long-term antitumor activity. JCI Insight. 2019;4(8):e126086
270. Subudhi SK, Vence L, Zhao H, Blando J, Yadav SS, Xiong Q, Reuben A, Aparicio A, Corn PG, Chapin BF, Pisters LL, Troncoso P, Tidwell RS, Thall P, Wu CJ, Zhang J, Logothetis CL, Futreal A, Allison JP, Sharma P. Neoantigen responses, immune correlates, and favorable outcomes after ipilimumab treatment of patients with prostate cancer. Sci Transl Med. 2020;12(537):eaaz3577
271. Abida W, Cyrta J, Heller G, Prandi D, Armenia J, Coleman I, Cieslik M, Benelli M, Robinson D, Van Allen EM, Sboner A, Fedrizzi T, Mosquera JM, Robinson BD, De Sarkar N, Kunju LP, Tomlins S, Wu YM, Nava Rodrigues D, Loda M, Gopalan A, Reuter VE, Pritchard CC, Mateo J, Bianchini D, Miranda S, Carreira S, Rescigno P, Filipenko J, Vinson J, Montgomery RB, Beltran H, Heath EI, Scher HI, Kantoff PW, Taplin ME, Schultz N, deBono JS, Demichelis F, Nelson PS, Rubin MA, Chinnaiyan AM, Sawyers CL. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A. 2019;116(23):11428-36
272. Dai J, Lu Y, Roca H, Keller JM, Zhang J, McCauley LK, Keller ET. Immune mediators in the tumor microenvironment of prostate cancer. Chin J Cancer. 2017;36(1):29
273. Dannah R, Miller MAI, Teply BA, Lin MF. Combination treatment options for castration-resistant prostate cancer [Internet]; 2021 [cited 2024 01/06/2024]. Exon Publications. Available from: https://www.ncbi.nlm.nih.gov/books/NBK571328/.
274. Feucht J, Kayser S, Gorodezki D, Hamieh M, Döring M, Blaeschke F, Schlegel P, Bösmüller H, Quintanilla-Fend L, Ebinger M, Lang P, Handgretinger R, Feuchtinger T. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget. 2016;7(47):76902-19
275. Krupka C, Kufer P, Kischel R, Zugmaier G, Lichtenegger FS, Köhnke T, Vick B, Jeremias I, Metzeler KH, Altmann T, Schneider S, Fiegl M, Spiekermann K, Bauerle PA, Hiddemann W, Riethmüller G, Subklewe M. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: reversing a T-cell-induced immune escape mechanism. Leukemia. 2016;30(2):484-91
276. Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56
277. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188-95
278. Heitmann JS, Pfluegler M, Jung G, Salih HR. Bispecific antibodies in prostate cancer therapy: current status and perspectives. Cancers. 2021;13(3):549
279. Borlak J, Länger F, Spanel R, Schöndorfer G, Dittrich C. Immune-mediated liver injury of the cancer therapeutic antibody catumaxomab targeting EpCAM, CD3 and Fcγ receptors. Oncotarget. 2016;7(19):28059-74
280. Vafa O, Gilliland GL, Brezski RJ, Strake B, Wilkinson T, Lacy ER, Scallon B, Teplyakov A, Malia TJ, Strohl WR. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods. 2014;65(1):114-26
281. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3(1):81-5
282. Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, Penetrante R. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology. 2007;50(4):472-83
283. Datta-Mannan A, Brown R, Key S, Cain P, Feng Y. Pharmacokinetic developability and disposition profiles of bispecific antibodies: a case study with two molecules. Antibodies (Basel). 2021;11(1):2
284. Muthumani K, Marnin L, Kudchodkar SB, Perales-Puchalt A, Choi H, Agarwal S, Scott VL, Reuschel EL, Zaidi FI, Duperret EK, Wise MC, Kraynyak KA, Ugen KE, Sardesai NY, Joseph Kim J, Weiner DB. Novel prostate cancer immunotherapy with a DNA-encoded anti-prostate-specific membrane antigen monoclonal antibody. Cancer Immunol Immunother. 2017;66(12):1577-88
285. Stadler CR, Bähr-Mahmud H, Celik L, Hebich B, Roth AS, Roth RP, Karikó K, Türeci Ö, Sahin U. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nat Med. 2017;23(7):815-7
286. Blanco B, Ramírez-Fernández Á, Alvarez-Vallina L. Engineering immune cells for in vivo secretion of tumor-specific T cell-redirecting bispecific antibodies. Front Immunol. 2020;11:1792
287. Wu L, Seung E, Xu L, Rao E, Lord DM, Wei RR, Cortez-Retamozo V, Ospina B, Posternak V, Ulinski G, Piepenhagen P, Francesconi E, El-Murr N, Beil C, Kirby P, Li A, Fretland J, Vicente R, Deng G, Dabdoubi T, Cameron B, Bertrand T, Ferrari P, Pouzieux S, Lemoine C, Prades C, Park A, Qiu H, Song Z, Zhang B, Sun F, Chiron M, Rao S, Radošević K, Yang ZY, Nabel GJ. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat Cancer. 2020;1(1):86-98
288. Bendell JC, Fong L, Stein MN, Beer TM, Ross A, Gao X. et al. First-in-human phase I study of HPN424, a tri-specific half-life extended PSMA-targeting T-cell engager in patients with metastatic castration-resistant prostate cancer (mCRPC). American Society of Clinical Oncology. 2020
289. De Bono JS, Fong L, Beer TM, Gao X, Geynisman DM, Burris III HA. et al. Results of an ongoing phase 1/2a dose escalation study of HPN424, a tri-specific half-life extended PSMA-targeting T-cell engager, in patients with metastatic castration-resistant prostate cancer (mCRPC). Wolters Kluwer Health. 2021
290. Systimmune S. GNC-038: Systimmune Systimmune; 2022 [updated 12/01/2022; cited 2024; 01/06/2024. Available from: https://systimmune.com/gnc-038.
291. Huang S, van Duijnhoven SMJ, Sijts AJAM, van Elsas A. Bispecific antibodies targeting dual tumor-associated antigens in cancer therapy. J Cancer Res Clin Oncol. 2020;146(12):3111-22
Author contact
![]() Corresponding authors: Alan Prem Kumar (apkumaredu.sg); Kiavash Hushmandi (houshmandi.kia7ac.ir); Behzad Einollahi (Einollahibac.ir).
Corresponding authors: Alan Prem Kumar (apkumaredu.sg); Kiavash Hushmandi (houshmandi.kia7ac.ir); Behzad Einollahi (Einollahibac.ir).

 Global reach, higher impact
Global reach, higher impact