10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2025; 21(5):2012-2026. doi:10.7150/ijbs.107195 This issue Cite
Review
Regulated Cell Death in Lenvatinib Resistance of Hepatocellular Carcinoma: from Molecular Mechanisms to Therapeutic Strategies
1. Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310003, China.
2. NHC Key Laboratory of Combined Multi-organ Transplantation, Hangzhou 310003, China.
3. Zhejiang University School of Medicine, Hangzhou 310058, China.
4. The Fourth School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou 310053, China.
5. School of Clinical Medicine, Hangzhou Medical College, Hangzhou 310059, China.
6. Institute of Translational Medicine, Zhejiang University, Hangzhou 310000, China.
*These authors contributed equally to this paper.
Received 2024-11-19; Accepted 2025-2-8; Published 2025-2-18
Abstract
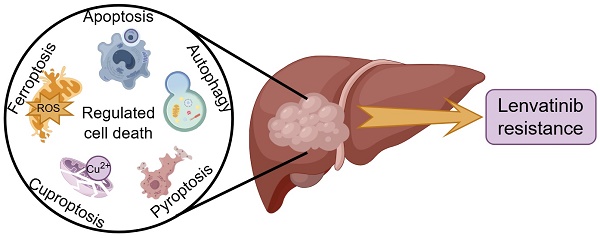
Lenvatinib, a multi-target tyrosine kinase inhibitor (TKI), has been established as the first-line treatment for advanced hepatocellular carcinoma (HCC) because of its superior efficacy when in comparison with sorafenib. However, the inevitable development of drug resistance is a significant barrier to achieve a curative outcome and negatively impacts the prognosis. Therefore, it is imperative to delve into the mechanisms underlying lenvatinib resistance (LR) and to identify potential strategies for rational combination treatments. Regulated cell death (RCD) refers to the process by which cells undergo demise when the adaptive responses are insufficient to maintain homeostasis, and RCD takes a crucial part in the disease progression and response to therapeutic agents including TKI of cancer. Resisting cell death is one of the fundamental hallmarks and the major reasons contributing to drug resistance in cancer. Particularly, numerous studies have demonstrated that RCD (including apoptosis, autophagy, ferroptosis, cuproptosis and pyroptosis) plays a significant role in the emergence of LR in HCC. This article offers an in-depth review of recent discoveries concerning the mechanisms of LR in relation to RCD and proposes potential strategies to boost the effectiveness of lenvatinib by incorporating RCD modulators.
Keywords: Lenvatinib, Hepatocellular carcinoma, Drug resistance, Combination treatments, Regulated cell death
Introduction
Hepatocellular carcinoma (HCC), the leading type of primary liver cancer, is the sixth most frequently diagnosed cancer and the third leading cause of cancer-related deaths worldwide [1]. At present, the therapeutic options for HCC include liver transplantation (LT), surgical resection, radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE), stereotactic body radiotherapy (SBRT), and systemic therapy. Up until now, LT, surgical resection and RFA are the only curative treatments available for HCC patients [2]. Regrettably, the majority of HCC patients are usually diagnosed at advanced stages, making them ineligible for curative interventions [3, 4]. Systemic therapy constitutes the mainstay of treatment for HCC patients who are in the advanced stages of the disease [5, 6]. Systemic therapy for HCC encompasses conventional chemotherapy, targeted therapies specific to the disease, and immunotherapy [7]. Kinase inhibitors are the preferred option for targeted therapy in HCC, and the first-line agents contain sorafenib and lenvatinib. In addition, new targets in HCC and their corresponding treatment approaches are continuously evolving, yet there remains significant progress needed before they can be effectively utilized in clinical settings [8, 9].
In 2007, sorafenib, a pioneering multi-target kinase inhibitor, received official approval for the treatment of advanced HCC. The approval was based on the SHARP trial, which showed a significant increase in progression-free survival (PFS) from 2.8 months to 5.5 months, as well as an improvement in overall survival (OS) from 7.9 months to 10.7 months [10]. Despite the statistically significant results, the objective response rate (ORR) to sorafenib was a modest 2%, with the majority of patients experiencing only stable disease (SD) during the treatment period. In 2018, lenvatinib became the second first-line targeted therapy for advanced HCC to receive approval, based on the outcomes of the REFLECT trial [11]. Lenvatinib demonstrated notable enhancements over sorafenib in secondary outcome measures, including a higher ORR, extended PFS, and a longer time to progression (TTP). Similar to sorafenib, lenvatinib is an oral small molecule tyrosine kinase inhibitor (TKI) that exhibits its anti-cancer effects by inhibiting a variety of targets, such as vascular endothelial growth factor receptor (VEGFR) 1-3, fibroblast growth factor receptor (FGFR) 1-4, platelet-derived growth factor receptor α, KIT, and RET [12]. While the introduction of lenvatinib has led to promising progress in the treatment of advanced HCC, it has only resulted in an average survival extension of 2 to 3 months [13]. Drug resistance significantly diminishes the clinical effectiveness of lenvatinib. Therefore, it is imperative to investigate the mechanisms underlying lenvatinib resistance (LR) and to identify potential targets for rational combination therapies. Studies on LR in HCC have identified potential mechanisms such as dysfunctional pathway activation, drug transport variations, regulated cell death (RCD), epigenetic alterations, influences from the tumor microenvironment (TME), the role of cancer stem cells (CSC), epithelial-mesenchymal transition (EMT), and others [14]. Our previous study revealed that phosphorylated non-muscle myosin heavy chain 9 (p-MYH9) at Ser1943, could deubiquitinate and stabilize HIF-1α by recruiting (ubiquitin-specific protease 22) USP22 in HCC, which led to the development of LR [15]. The employment of a casein kinase-2 (CK2) inhibitor alongside a USP22 inhibitor successfully overcame LR, suggesting a promising treatment strategy for HCC patients who experienced LR.
RCD refers to the process by which cells undergo demise as a result of molecular mechanisms triggered by either intracellular or extracellular factors, and this occurs when the cell's adaptive responses are insufficient to maintain homeostasis [16]. RCD encompasses a spectrum of distinct forms of cell demise, each characterized by unique mechanisms, including apoptosis, autophagy, ferroptosis, cuproptosis, pyroptosis, necroptosis, parthanatos, lysozincrosis and disulfidptosis [17]. RCD plays a crucial role in the development of organisms and the maintenance of homeostasis, and various lethal subroutines in the process of RCD affect disease progression and response to therapeutic agents in cancer [18, 19]. Resisting cell death is one of the several fundamental hallmarks and the major reasons contributing to drug resistance in cancer [20, 21]. In particular, extensive studies have shown that RCD is involved in the development of resistance to lenvatinib in HCC (Figure 1). This research provides a comprehensive overview of the latest findings regarding the mechanisms underlying LR in the context of RCD, and proposes potential strategies aimed at enhancing the efficacy of lenvatinib with RCD modulators.
Apoptosis
Apoptosis, widely recognized as the most renowned form of regulated cell death, functions as a critical physiological process that curbs the cell proliferation [22]. It plays a dual role in either preserving tissue homeostasis or eliminating cells that could pose a threat to the organism [23]. Efficient cancer therapies frequently target the regulation of apoptotic signaling pathways, and blocking these pathways may result in resistance to anti-cancer treatments [24, 25]. Up until now, extensive researches have indicated that apoptosis signaling is crucial for triggering LR in HCC, and therefore targeting the pathways involved in apoptosis might serve as a potential approach to reverse LR.
Zhao et al. reported that overexpressed fibroblast FGFR1, along with the activation of its downstream pathways AKT/mTOR and ERK, could cause LR by inhibiting apoptosis in HCC [26]. Tan's study revealed that glutathione peroxidase (GPX) 2 exerted a significant role in reactive oxygen species (ROS) related apoptosis induced by lenvatinib in HCC cells, and over-expression of GPX2 could induce LR through impairing apoptosis by acting as a downstream gene of β-catenin [27]. NAD(P)H:quinone oxidoreductase 1 (NQO1) was up-regulated in LR HCC cells, and high levels of NQO1 suppressed the ROS-associated apoptosis induced by lenvatinib, which led to LR [28].
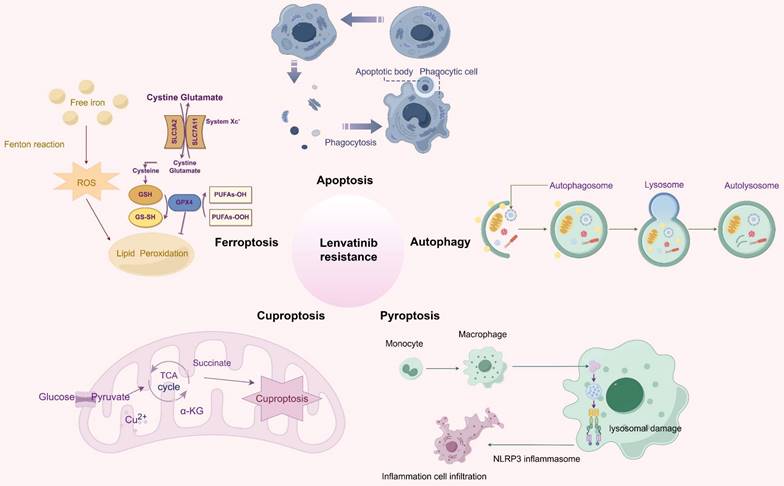
An overview of the molecular mechanisms of RCD in relation to LR. Apoptosis is the most renowned form of RCD characterized by the formation of apoptotic bodies; Autophagy refers to the process in which intracellular components including proteins and organelles are conveyed to lysosomes, degraded and recycled; Ferroptosis, driven by iron-dependent phospholipid peroxidation, leads to inactivation of GPX4 or system xc-cystine/glutamate antiporter, and producing excessive ROS; Cuproptosis is triggered by excessive Cu2+ ions, which bind to TCA cycle proteins, leading to mitochondrial aggregation; Pyroptosis is characterized by its lytic and inflammatory nature, typically initiated by inflammasomes, marked by cellular swelling, membrane rupture, and the release of intracellular contents. Abbreviations: LR: lenvatinib resistance; RCD: regulated cell death; ROS: reactive oxygen species; TCA: tricarboxylic acid.
Previous researches have pinpointed certain compounds that possess the ability to modulate pathways associated with apoptosis. Among these compounds are members of the Bcl-2 protein family, caspase family, c-Myc, tumor necrosis factor-related ligands and their receptors, Apaf-1, Cytochrome C, the NF-κB signaling, and the p53 protein. Genetic alterations or the activation of specific pathways can suppress the process of apoptosis and foster resistance to lenvatinib by modifying the behavior of these molecular components. Yu et al. demonstrated that HCC cells with high levels of lncMT1JP expression could acquire resistance to lenvatinib via triggering the inhibition of apoptosis [29]. The underlying mechanism involved lncMT1JP modulating the miR-24-3p/BCL2L2 pathway, which resulted in the suppression of apoptosis. Xu's study showed that down-regulation of miR-128-3p contributed to LR through inhibiting apoptosis by attenuating the cleavage of caspase-9 and caspase-3 [30]. Mechanistically, miR-128-3p negatively regulated the expression of c-Met by binding to the 3'-UTR of c-Met mRNA, and resulted in activation of AKT pathway. Guo et al. reported that elevated levels of interferon regulatory factor 2 (IRF2) were linked to LR in HCC through the modulation of the Wnt/β-catenin signaling, which in turn affected the regulation of proteins involved in apoptosis (e.g., Bcl-2 and caspase-3) [31]. Another study proved that the expressions of USP22 and Jumonji domain-containing protein 8 (JMJD8) were elevated in HCC cells resistant to lenvatinib, and USP22 and JMJD8 formed a regulatory axis that regulated LR by altering apoptosis-related protein c-Myc [32].
Methylation, a significant epigenetic mechanism, encompasses the enzymatic process of transferring methyl group from active methyl donors to various other molecules, including DNA, RNA and histones [33-35]. Abnormal methylation could result in progression and drug resistance in HCC [36]. Methylation modifications of RNA mainly include m6A, m7G, m5C, m1A [37]. m7G modification, prevalent in the 5' caps of eukaryotic mRNA or within rRNA and tRNA across all species, is linked to tumorigenesis and multiple cancer-related biological behaviors [38]. Recently, Huang et al. reported that the m7G tRNA modification, catalyzed by the methyltransferase-like protein (METTL) 1, specifically modulated the EGFR signaling pathway, enhancing LR via impairing the apoptosis capacity of HCC cells [39]. Given that EGFR is a downstream target of METTL3, Wang et al. discovered that METTL3 was identified as the most notably increased protein among these m6A regulators in HCC cells that are resistant to lenvatinib, and inhibition of METTL3 increased cell apoptosis upon lenvatinib treatment, which overrode LR [40]. In addition, they found that LR HCC patients exhibit elevated levels of METTL3, and patients with reduced METTL3 levels had better survival outcomes. N4-acetocytidine (ac4C), an mRNA acetylation modification, is implicated in the pathogenesis of various human diseases, including cancer [41]. Pan's research demonstrated that NAT10-mediated mRNA ac4C modification of HSP90AA1 regulated tumor metastasis and LR in HCC [42]. Thus, identifying new therapeutic targets in the realm of translational regulation holds great promise for improving the prognosis of individuals with HCC.
Accordingly, deepening our comprehension of the link between apoptosis and LR is of significant clinical relevance, particularly for strategies aimed at reducing drug resistance.
Autophagy
Autophagy refers to the process in which intracellular components including proteins and organelles are conveyed to lysosomes, degraded and recycled, thereby meeting the metabolic demands and supporting the self-renewal of cells [43]. Various cancer treatments can trigger autophagy, and enhanced autophagy acts as an acquired resistance mechanism during exposition to stresses encountered from metabolic demands and therapeutic interventions [44]. For instance, in prostate cancer, breast cancer and gastrointestinal stromal tumors, inhibiting autophagy has been shown to overcome resistance to chemotherapy drugs [45-47]. To date, research indicates that autophagy is crucially involved in the development of LR in HCC.
Autophagy exhibits a dual-function in modulating the drug resistance of lenvatinib in HCC (Figure 2). Using CRISPR-Cas9 screen along with database analysis, Pan et al. pinpointed lysosomal protein transmembrane 5 (LAPTM5) as a crucial contributor to LR in HCC, and discovered that LAPTM5 could enhance the intrinsic autophagic flux by promoting the formation of autolysosomes [48]. Importantly, in clinical HCC samples, lenvatinib-sensitive patients showed lower LAPTM5 levels than LR patients, and recurrent patients after surgery had higher LAPTM5 expressions than non-recurrent ones. Similar results were obtained in various studies which demonstrated up-regulated autophagy may promote LR in HCC. Syntaxin-6 (STX6), negatively regulated by the upstream stimulatory factor 2 (USF2), facilitated the fusion of autophagosomes with lysosomes and subsequently accelerated autophagic flux, which led to LR [49]. Gu's study indicated that the suppression of the long non-coding RNA HOTAIRM1 led to a reduction in autophagy levels within lenvatinib-resistant HCC cells via miR-34a-Beclin-1 regulatory axis, and resulted in an enhanced responsiveness to lenvatinib treatment [50]. Moreover, the expression of HOTAIRM1 was considerably elevated in HCC patients resistant to lenvatinib compared to those who were sensitive to the agent. However, contradictory findings were obtained by other researches, showing through experiments that increasing autophagy could potentially boost the effectiveness of lenvatinib and reduce LR in HCC. Eva-1 homolog A (EVA1A) loss, which prevented the autophagosome formation, was proved to drive LR via inhibiting mutant p53 degradation through suppressing the PI3K/AKT signaling pathway [51]. Palanca's study indicated that Neuropilin-1 (NRP1), upregulated by HIF-1α in hypoxic environments, could negate the therapeutic impact of lenvatinib, resulting in LR. This occured because lenvatinib's antitumor efficacy was mediated by the autophagy-dependent degradation of NRP1, which involved the assembling of autolysosome [52]. Wang et al. reported that forkhead box protein A2 (FOXA2) overexpression was found to amplify the effectiveness of lenvatinib on HCC cells by upregulating the AMPK-mTOR-autophagy signaling pathway [53]. Further investigation is warranted to explore the potential of modulating autophagy, either through inhibition or enhancement, as a strategy to potentiate the antitumor effects of lenvatinib in HCC treatment.
Autophagy is categorized into selective and non-selective forms based on its substrate degradation mechanism [54]. In selective autophagy, specific autophagic pathways are employed to target and recycle unwanted or damaged cellular components such as mitochondria, ribosomes, peroxisomes, lysosomes, endoplasmic reticulum (ER), nuclei, lipid droplets and proteasomes. Impairments in selective autophagy are intimately associated with a range of pathologies, including cancer, metabolic disorders, neurodegenerative diseases and heart failure [55-58]. Mitochondrial autophagy (i.e., mitophagy), a specialized type of selective autophagy, targets and removes impaired mitochondria to maintain the integrity of the mitochondrial network [59]. Studies have shown that mitophagy takes a vital part in regulating LR in HCC. CRISPR activation screen was applied in Zhang's study, and they identified TMX2 (thioredoxin related transmembrane protein 2) as an essential gene for HCC cell survival [60]. Further experiments found that TMX2 relieved LR and amplified the anti-tumor impact of lenvatinib in HCC by suppressing autophagy and mitophagy, which was achieved through the inhibition of karyopherin subunit beta (KPNB) 1's nuclear export and transcription factor EB (TFEB) 's nuclear import. LINC01607, was proved to act as a competitive endogenous RNA (ceRNA), antagonize miR-892b and thereby stimulate the upregulation of P62. The increase in P62 activity induced protective mitophagy, contributing to the development of LR in HCC [61]. Zheng's research revealed that under cellular stress, Stomatin-like protein 2 (STOML2) was capable of interacting with and stabilizing PTEN-induced putative kinase 1 (PINK1), which significantly amplified the PINK1-Parkin-mediated mitophagy process in HCC. This enhancement of mitophagy was identified as a key factor contributing to the development of LR [62]. Wang et al. reported that in lenvatinib-resistant HCC cells, the excessive activation of BCL2 interacting protein 3 (BNIP3)-driven mitophagy enhanced glycolytic flux, resulting in constantly sustained competitive edge of lenvatinib-resistant cells over their sensitive counterparts, which maintained LR [63]. This was achieved by shifting energy metabolism from mitochondrial oxidative phosphorylation to glycolysis, which was regulated through the AMPK-enolase 2 (ENO2) signaling pathway. In forthcoming studies, it will be crucial to investigate the strategies targeting selective autophagy with the aim of amplifying the therapeutic effects of lenvatinib. Simultaneously, ensuring that the essential functions of autophagy within the cellular context are preserved will be of paramount importance to avoid unintended side effects.
Ferroptosis
The study of ferroptosis has experienced a dramatic surge in interest and development since the concept was first introduced in 2012. This distinctive form of cell death, propelled by the peroxidation of phospholipids that is reliant on iron, is governed by a variety of cellular metabolic processes, which include the maintenance of redox balance, the management of iron levels, the functioning of mitochondria, and the metabolism of amino acids, sugars and lipids [64, 65]. Furthermore, it is influenced by numerous signaling pathways that are pertinent to various diseases, such as cancers, autoimmune diseases, cardiovascular diseases, chronic liver diseases and metabolic diseases [66-70]. Intriguingly, cancer cells that are resistant to therapy, especially those in a mesenchymal state and inclined to spread, exhibit a remarkable susceptibility to ferroptosis [71-73]. So far, studies have shown that ferroptosis plays a significant role in the induction of LR in HCC.
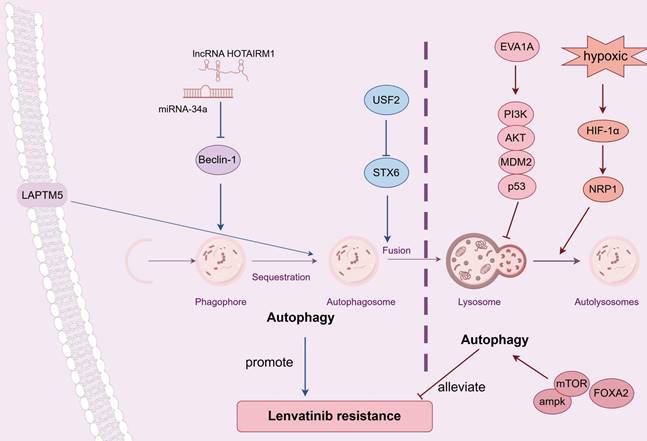
Dual effect of autophagy on LR. LAPTM5 could enhance the intrinsic autophagic flux, which contributed to LR; Silencing TMX2 relieved LR by suppressing autophagy through KPNB1/TFEB pathway; STX6, negatively regulated by USF2, accelerated autophagic flux, which led to LR; The suppression of lncRNA HOTAIRM1 led to a reduction in autophagy levels via miR-34a/Beclin-1 axis, which counteracted LR. FOXA2 overexpression impared LR by upregulating the AMPK-mTOR-autophagy pathway; NRP1, upregulated by HIF-1α in hypoxic conditions, could amplify LR by diminishing autophagy; EVA1A loss drove LR via inhibiting autophagy through suppressing PI3K/AKT/MDM2/p53 signaling. Abbreviations: LR: lenvatinib resistance; LAPTM5: lysosomal protein transmembrane 5; TMX2: thioredoxin related transmembrane protein 2; STX6: Syntaxin-6; USF2: upstream stimulatory factor 2; FOXA2: forkhead box protein A2; NRP1: Neuropilin-1; EVA1A: Eva-1 homolog A.
It was reported that lenvatinib might trigger ferroptosis by suppressing the FGFR/system xc-/GPX4 pathway [74]. An increased expression of the nuclear factor erythrocyte 2-related factor 2 (Nrf2), a crucial transcription factor for regulating intracellular ROS, could suppress lenvatinib-induced ferroptosis and cause LR in HCC. Additionally, low FGFR4 expression and high P-Nrf2 expression in HCC tissues are associated with LR and shorter PFS. Zeng's study showed that serine beta-lactamase-like protein (LACTB) was identified as a downstream target of lenvatinib, and overexpression of LACTB triggered ferroptosis through (solute carrier family 7 member 11) SLC7A11/GSH/GPX4 signalling pathway, resulting in the enhancement of lenvatinib's anti-tumor efficacy and attenuation of LR [75]. Lai et al. reported that heme oxygenase-1 (HO-1) knockdown up-regulated GPX4 in HCC, leading to reduction of ferroptosis and LR [76]. Another study revealed that GPX4 transcriptionally inhibited ferroptosis via grainyhead-like transcription factor 3 (GRHL3)/PTEN/PI3K/AKT axis, which accentuated LR [77]. Hiromatsu et al. reported that transferrin receptor (TR) knockdown abolished lenvatinib-induced ferroptosis, which contributed to the development of LR in HCC cell lines [78]. Hypoxia is a common characteristic of the TME, and it is linked to the pathological features, prognosis, and therapeutic outcomes in HCC patients [79]. Peroxisome proliferator-activated receptor-gamma coactivator-1α (PPARGC1A) was down-regulated in lenvatinib-resistant HCC cells, and under hypoxia, overexpression of PPARGC1A improved sensitivity to ferroptosis and counteracted LR by controlling bone morphogenetic protein and activin membrane-bound inhibitor (BAMBI)/ACSL5 signaling [80]. PPARGC1A/BAMBI/ACLS5 pathway was hypoxia-responsive and WTAP-mediated m6A modification modulated PPARGC1A mRNA under hypoxic conditions.
Noncoding RNAs, encompassing microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and circular RNAs (circRNAs), have been implicated in the biological processes associated with ferroptosis and the development of drug resistance in a range of cancers [81, 82]. lncRNA HAND2-AS1 was down-regulated in lenvatinib-resistant HCC cell lines, and overexpressed HAND2-AS1 promoted ferroptosis to reverse LR by competing endogenous miR-219a-1-3p in HCC cells [83]. In recent developments, it has been found that there is cross-signaling between ferroptosis and selective autophagy, with ferroptosis being shown to be heavily reliant on selective autophagy, such as ER-phagy, lipophagy and ferritinophagy [84]. Bi's research uncovered that ER-phagy-driven ferroptosis was involved in HCC cells treated with lenvatinib, and they discovered that the reduction of circFAM134B specifically directed ER-phagy to enhance ferroptosis induced by lenvatinib [85]. Mechanically, circFAM134B competitively interacted with poly (A) binding protein cytoplasmic 4 (PABPC4), consequently affecting the nonsense decay of family with sequence similarity 134, member B (FAM134B) mRNA. Zhang's study demonstrated that the overexpression of circPIAS1 suppressed ferroptosis by sponging mir455-3p, which in turn led to the upregulation of Nuclear Protein 1 (NUPR1). Additionally, NUPR1 facilitated the transcription of ferritin heavy chain 1 (FTH1), increasing iron storage within HCC cells and thus providing resistance to ferroptosis and lenvatinib treatment [86].
In conclusion, there is a strong association between ferroptosis and the development of resistance to lenvatinib. Further investigation into new mechanisms and potential countermeasures is warranted.
Other forms of RCD
Cuproptosis
Cuproptosis represents a novel type of regulated cell death that is activated by an overabundance of Cu2+ ions [87]. Intracellular copper targets and attaches to lipoylated elements in the tricarboxylic acid (TCA) cycle. The clumping of copper-bound lipoylated proteins in the mitochondria, along with the subsequent decrease in iron-sulfur cluster, induce protein misfolding stress and eventual cell death [88]. Cuproptosis has been garnering increasing attention, particularly after its link to cancer was established, prompting many researchers to delve into the connection between cuproptosis and various types of cancer [89, 90]. Yang et al. reported that cuproptosis-related gene Dihydrolipoamide S-acetyltransferase (DLAT) was up-regulated in the drug-resistant group treated with lenvatinib, and the mechanism study showed that DLAT might trigger LR by inhibiting cuproptosis [91]. Li's study showed that a decrease in the expression of anti-cuproptotic proteins (DLAT, LIAS, and FDX1) and an increase in the expression of the pro-cuproptotic protein CDKN2A were observed in LR HCC cells, indicating cuproptosis took a vital part in drug resistance of lenvatinib [92].
Pyroptosis
Pyroptosis is a form of regulated cell death characterized by its lytic and inflammatory nature, typically initiated by inflammasomes and carried out by gasdermin proteins [93]. Key features of pyroptosis include cellular swelling, the breaching of the cell membrane, and the subsequent release of intracellular contents [94]. Appealingly, pyroptosis, being a form of immunogenic cell death, offers a novel approach to cancer elimination by triggering pyroptotic cell demise and stimulating a robust antitumor immune response [95, 96]. Fu's research reported that treatment with lenvatinib increased the mRNA and protein levels of pyroptosis-related gene GSDME, and up-regulated active GSDME N-terminal in HCC cell lines, implying that down-regulation of GSDME might induce LR by inhibiting pyroptosis [97].
Strategies to overcome LR with RCD modulators
The overall response rate of lenvatinib in HCC patients is approximately 40%, yet its clinical utility is frequently constrained by the development of drug resistance. Therefore, there is an imperative to enhance the potency of lenvatinib in clinical practice. Up to this point, we have explored various pathways and cellular mechanisms that contribute to the development of drug resistance to lenvatinib within the realm of RCD. We further provide a summary of the potential strategies to overcome LR with RCD modulators (Table 1).
Targeting apoptosis
Targeting apoptosis is a promising way to overcome LR (Figure 3). Oxysophocarpine, an alkaloid derived from the plant Siphocampylus verticillatus, possesses a range of therapeutic properties, including anti-tumor effects, anti-inflammatory capabilities, and antiviral activities [98-100]. As mentioned earlier, overexpressed FGFR1 together with activated AKT/mTOR and ERK pathways could induce LR in HCC, and oxysophocarpine could reduce the expression of FGFR1 and concurrently suppress the downstream signaling, thereby enhancing the sensitivity of lenvatinib [26]. High levels of NQO1 contributed to LR by suppressing ROS-associated apoptosis, and the concurrent administration of lenvatinib with the NQO1 inhibitor, dicoumarol, counteracted drug resistance [28]. IRF2/β-catenin pathway was stimulated in HCC LR cells, and inhibiting β-catenin with the use of XAV-939 effectively significantly enhanced the efficacy of lenvatinib by promoting cell apoptosis [31]. Cheng et al. reported that metformin, a commonly utilized medication for diabetes, worked synergistically to boost the antitumor effects of lenvatinib in HCC by promoting apoptosis and triggering cell cycle arrest through the modulation of the AKT-Forkhead box protein O3 (FOXO3) pathway [101]. Yan's research demonstrated the combined use of lenvatinib and SAHA, an HDAC inhibitor, works synergistically to suppress HCC cell proliferation and to trigger cell apoptosis by modulating the PTEN/AKT pathway [102]. Moreover, Class IIa HDACI [103], Sophoridine [104], elacridar, gefitinib and copanlisib [105], were proven to ameliorate LR via regulating apoptosis-related pathway in HCC.
Drugs overcome LR via regulating RCD
| Drugs | Target | Mechanism | Phase | NCT number | Refs. |
|---|---|---|---|---|---|
| Oxysophocarpine | FGFR1/AKT/mTOR | apoptosis | - | - | [26] |
| Dicoumarol | NQO1 | apoptosis | - | - | [28] |
| XAV-939 | IRF2/β-catenin | apoptosis | - | - | [31] |
| Metformin | AKT/FOXO3 | apoptosis | - | - | [101] |
| SAHA | PTEN/PI3K/AKT | apoptosis | - | - | [102] |
| TMP269 | FGFR | apoptosis | - | - | [103] |
| Sophoridine | VEGFR2/RAS/ERK | apoptosis | - | - | [104] |
| Elacridar | MDR1 and BCRP | apoptosis | - | - | [105] |
| Gefitinib | EGFR/PI3K/AKT | apoptosis | Post-market | NCT04642547 | [105] |
| Copanlisib | PI3K/AKT | apoptosis | - | - | [105] |
| STM2457 | METTL3/EGFR | apoptosis | - | - | [40] |
| Reserpine | METTL3/SMAD3 | apoptosis | - | - | [107] |
| HCQ | - | autophagy | - | - | [48] |
| CQ | - | mitophagy | - | - | [62] |
| 3-MA | - | autophagy | - | - | [50] |
| Olomoucine | BNIP3-AMPK-ENO2 | mitophagy | - | - | [63] |
| CP | - | autophagy | - | - | [111] |
| Metformin | METTL3/PPARGC1A | ferroptosis | - | - | [80] |
| ZZW-115 | circPIAS1/miR-455-3p/NUPR1 | ferroptosis | - | - | [86] |
| AKT-IN3 | GPX4/GRHL3/ PTEN/PI3K/AKT | ferroptosis | - | - | [77] |
| Solanum torvum | HO-1/GPX4 | ferroptosis | - | - | [76] |
| Artesunate | TFRC | ferroptosis | - | - | [78] |
| DSF | DLAT, LIAS, CDKN2A, FDX1 | cuproptosis | - | - | [92] |
Abbreviations: LR: lenvatinib resistance; RCD: regulated cell death; HCQ: hydroxychloroquine;
CQ: chloroquine; 3-MA: 3-Methyladenine; CP: Compound Phyllanthus urinaria; DSF: disulfiram.
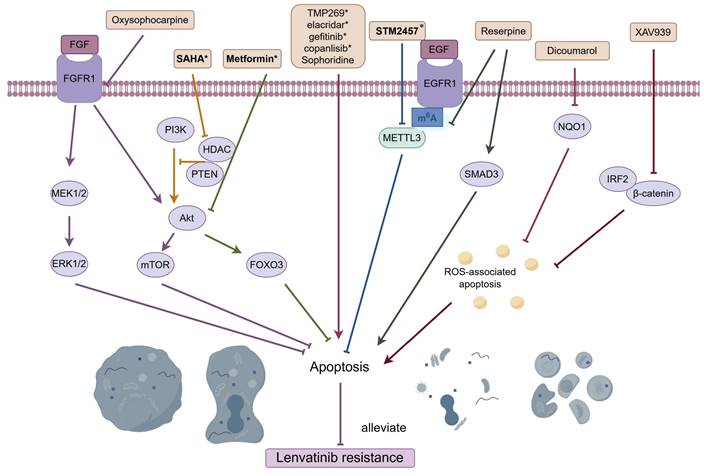
Combined treatments that reverse LR by targeting apoptosis. Oxysophocarpine vanquished LR by promoting apoptosis via FGFR1 and its downstream signaling; Dicoumarol, the NQO1 inhibitor, counteracted LR by enhancing ROS-associated apoptosis; Inhibiting β-catenin with the use of XAV-939 overpowered LR via fostering apoptosis; Metformin weakened LR by the promotion of apoptosis through the modulation of the AKT-FOXO3 pathway; SAHA, an HDAC inhibitor, mitigated LR by triggering cell apoptosis via modulating the PTEN/AKT pathway; METTL3 inhibitor STM2457 surmounted LR via increasing cell apoptosis through METTL3-m6A-EGFR-axis; Reserpine reversed LR by inducing apoptosis through the suppression of m6A and the activation of SMAD3; TMP269, Sophoridine, elacridar, gefitinib and copanlisib, were all proven to ameliorate LR via regulating apoptosis-related pathway. Abbreviations: LR: lenvatinib resistance; FGFR1: fibroblast growth factor receptor; NQO1: NAD(P)H:quinone oxidoreductase 1; ROS: reactive oxygen species; METTL3: methyltransferase-like protein 3. Medications that exhibit a synergistic effect with lenvatinib are indicated by an asterisk (*).
Increasing evidence has suggested that m6A methylation influences the stem-like properties of cancer cells and their resistance to a range of treatments, encompassing chemotherapeutic agents and targeted drugs [106]. As noted before, METTL3-m6A-EGFR-axis was responsible for acquired resistant to lenvatinib, and the particular METTL3 inhibitor STM2457 amplified tumor response to lenvatinib in HCC animal models via increasing cell apoptosis [40]. Another study reported that LR in HCC was associated with elevated levels of m6A and increased METTL3 expression, and the plant-derived hypertension drug Reserpine could potentially revert a resistant phenotype to a sensitive one by inducing apoptosis through the suppression of m6A and the activation of SMAD3 [107].
Targeting autophagy
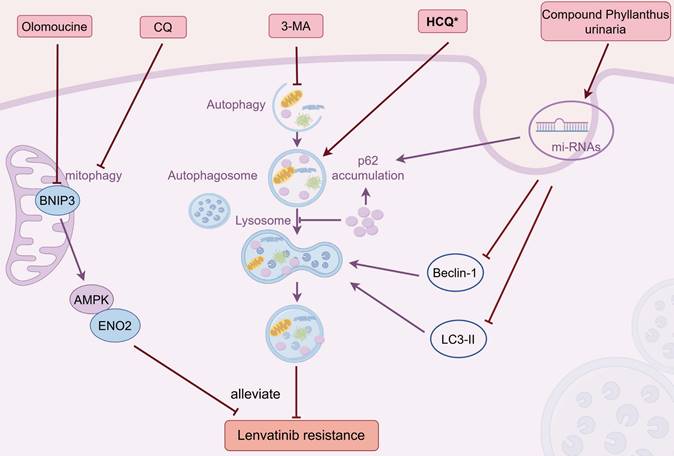
Targeting autophagy represents a potential strategy to conquer LR (Figure 4). Hydroxychloroquine (HCQ) and chloroquine (CQ) are the sole pharmaceuticals currently accessible for clinical use that specifically aim to modulate autophagy [108]. In LR HCC cells, HCQ combined with lenvatinib could work synergistically to suppress tumor growth and restore the sensitivity of lenvatinib by inhibiting the process of autophagy [48]. Another study showed that blocking mitophagy with CQ sensitized HCC cells to lenvatinib treatment, which reversed LR [62].
Currently, an array of small molecule autophagy inhibitors has been crafted specifically for the purposes of scientific investigation, including 3-Methyladenine (3-MA). The concurrent administration of 3-MA heightened the effectiveness of lenvatinib and surmounted LR in HCC cells through autophagy modulation [50]. Furthermore, several other agents were reported to conquer the drug resistance of lenvatinib via altering autophagy pathway. As mentioned before, the BNIP3-AMPK-ENO2 signaling pathway played a crucial role in sustaining the competitive advantage of LR HCC cells via regulating mitophagy-driven energy metabolism reprogramming, and co-treatment of BNIP3 inhibiter olomoucine substantially enhanced the antitumor effectiveness of lenvatinib [63]. Compound Phyllanthus urinaria (CP), a traditional Chinese medicine (TCM), is effective across the spectrum of liver disease treatment, from viral hepatitis to liver fibrosis/cirrhosis and HCC, showcasing significant therapeutic potential [109, 110]. Liao et al. reported that the synergistic administration of CP and lenvatinib potently suppressed HCC and counteracted LR by regulating autophagy-associated microRNAs in the exosomes, which is achieved by modulating the expression levels of Beclin-1, LC3-II, and P62 [111]. Icaritin, another TCM and derived from epimedium genus, has been documented as a potent anticancer substance against a spectrum of cancers, including HCC [112, 113]. In a Phase I clinical trial, the safety profiles and initial evidence of sustained survival benefits of icaritin were observed in patients with advanced HCC, potentially attributed to its immunomodulatory effects [114]. Of the 15 patients who were assessed, 7 (which is 46.7%) showed a positive outcome, including 1 showing partial response (PR, 6.7%) and 6 cases of SD (40%). The median TTP was calculated as 141 days (range: 20-343 days), and the median OS was estimated to be 192 days. Yu's study demonstrated that the targeted codelivery of icaritin and doxorubicin via PLGA-PEG-AEAA nanoparticles, in conjunction with lenvatinib, has been found to exert a synergistic effect by enhancing both mitophagy and apoptosis to provoke immunogenic cell death (ICD) in advanced HCC [115].
Targeting ferroptosis
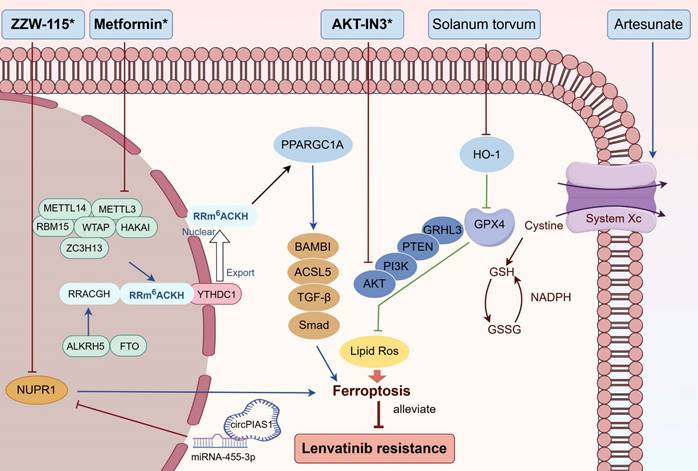
Targeting ferroptosis emerges as a feasible approach to triumph over LR (Figure 5). As previously stated, overexpression of PPARGC1A counteracted LR by promoting ferroptosis, and metformin combated LR by restoring the expression of PPARGC1A and diminishing its m6A methylation through the suppression of METTL3 [80]. The circPIAS1/miR-455-3p/NUPR1 pathway has been documented to regulate LR by influencing ferroptosis. The administration of ZZW-115, an inhibitor of NUPR1, reversed the proliferative impact of circPIAS1 and enhanced the sensitivity of HCC cells to lenvatinib [86]. GPX4/GRHL3/PTEN/PI3K/AKT axis was crucial in lenvatinib induced ferroptosis, and AKT-IN3, the AKT inhibitor, synergized with lenvatinib to reduce HCC metastasis [77]. Another study showed that HO-1 knockdown up-regulated GPX4 in HCC, leading to reduction of ferroptosis and LR. The combination of Solanum torvum and lenvatinib demonstrated an additive effect by advancing ferroptosis through the HO-1/GPX4 pathway [76]. Artesunate, a drug used to treat malaria, was found to enhance the ferroptosis-triggering effects of lenvatinib in HCC cell lines [78].
Combination therapies that overcome LR by targeting autophagy. Combined with HCQ or CQ counteracted LR by inhibiting autophagy or mitophagy, respectively; Concurrent administration of 3-MA surmounted LR through suppressing autophagy; Olomoucine reversed LR by inhibiting mitophagy via BNIP3-AMPK-ENO2 pathway; CP mitigated LR by modulating exosomal autophagy microRNAs and altering Beclin-1, LC3-II, and P62 levels. Abbreviations: LR: lenvatinib resistance; HCQ: hydroxychloroquine; CQ: chloroquine; 3-MA: 3-Methyladenine; CP: Compound Phyllanthus urinaria. Medications that exhibit a synergistic effect with lenvatinib are indicated by an asterisk (*).
Combined treatments that combate LR by targeting ferroptosis. Metformin diminished LR by promoting ferroptosis via METTL3/ PPARGC1A/BAMBI/ACSL5 axis; ZZW-115 reversed LR by enhancing ferroptosis through circPIAS1/miR-4553p/NUPR1 pathway; AKT-IN3 conquered LR by improving ferroptosis via GPX4/GRHL3/PTEN/PI3K/AKT signalling; Solanum torvum weakened LR by boosting ferroptosis through the HO-1/GPX4 pathway; Artesunate suppressed LR through the promotion of ferroptosis. Abbreviations: LR: lenvatinib resistance; PPARGC1A: Peroxisome proliferator-activated receptor-gamma coactivator-1α; HO-1: heme oxygenase-1. Medications that exhibit a synergistic effect with lenvatinib are indicated by an asterisk (*).
Targeting cuproptosis
Disulfiram (DSF), a medication used for over sixty years to treat alcohol dependence, has demonstrated established pharmacokinetic properties, safety, and tolerability at dosages recommended by the US Food and Drug Administration (FDA) [116]. Currently, DSF has attracted attention due to its potential to counteract drug resistance in cancer therapy [117]. Li et al. reported that DSF, acting as a copper ionophore, was capable of coordinating with Cu2+ to combat LR in HCC by suppressing cuproptosis and cancer cell stemness [92]. To mitigate systemic toxicity, DSF and CuO nanoparticles were encapsulated together to create an oil-in-water Pickering emulsion, which then merged with sodium alginate, leading to the formation of a DSF@CuO Gel through in situ gelation with Ca2+.
Summary and Future Perspectives
Although lenvatinib demonstrates considerable therapeutic benefits, many patients eventually develop resistance to the medication, resulting in cancer progression and poor prognosis. In this review, we have conducted a thorough analysis of the pathways and cellular mechanisms that contribute to the onset of LR within the framework of RCD in HCC. Moreover, we additionally offer an overview of potential approaches to counteract LR by targeting RCD-associated pathways, utilizing either combined therapeutic strategies or nanotechnology-aided drug delivery systems.
In our review, it was demonstrated that while autophagy exhibited a dual-function in modulating the drug resistance of lenvatinib, the disruption of apoptosis, ferroptosis, cuproptosis and pyroptosis all contributed to the development of LR. Generally, up-regulation of autophagy, an adaption mechanism for tumor cells to survival under stresses encountered from metabolic demands and therapeutic interventions, promotes LR in HCC [48-50]. However, modulating the autophagy of particular molecules or pathways might augment lenvatinib sensitivity. For instance, FOXA2 overexpression triggered the AMPK-mTOR axis, which resulted in activation of autophagy and attenuation of LR [53]. Additionally, promoting the autophagy-dependent degradation of NRP1 [52] or mutant p53 [51] could potentially counteract LR. Given the autophagy's dualistic influence on LR, forthcoming studies must comprehensively consider the choice of experimental methodologies and animal models to ensure the acquisition of precise and dependable outcomes. According to the existing literature, inhibition of mitophagy sensitized HCC cells to lenvatinib treatment and overcomed LR [60-63].
Over the past decade, cutting-edge scientific methods, such as single-cell RNA sequencing (scRNA-seq), artificial intelligence (AI) models, and gene editing technologies like CRISPR have revolutionized biomedical research, offering novel approaches to dissect various aspects of tumor biology. Utilizing scRNA-seq and AI, researchers can reveal the complex interactions between LR cancer cells and non-malignant stromal cells, further analyzing cellular composition, heterogeneity, and developmental trajectories, thereby systematically mapping the TME [118, 119]. Zhou et al. discovered that Mucosal-associated invariant T cells can confer resistance to the combination therapy of lenvatinib and anti-PD1 antibodies in HCC via the TNF-TNFRSF1B pathway, as identified by scRNA-seq [120]. Additionally, novel molecular typing and prediction models based on sequencing and machine learning have brought new hope for the precision and personalized treatment of malignant tumors. In a recent study, Zhang et al. employed a machine-learning-based approach to identify a panel of 13 signature genes that served as predictive biomarkers for lenvatinib response, achieving an area under the receiver operating characteristic curve of 0.86 [121]. The CRISPR system, a revolutionary genomic editing tool, is fundamentally changing cancer research paradigms and treatment modalities. It can comprehensively screen and precisely manipulate genes that drive tumor growth and resistance, opening up new possibilities for the development of more effective and personalized cancer therapies [122]. Pan et al.'s research, using genomic CRISPR-Cas9 screening, identified LAPTM5 as a key factor of LR in HCC, with the role of LAPTM5 being to enhance the formation of autolysosomes [48].
RCD was also reported to contribute substantially to the development of drug resistance in other targeted therapy and immunotherapy for HCC, and corresponding therapeutic strategies were proposed. Sorafenib is another first-line targeted therapy for advanced HCC. Lai's study revealed that the resistance of EGFR-overexpressing HCC cells to sorafenib was linked to inadequate autophagic activation, and metformin administration could enhance sorafenib sensitivity and autophagy via the activation of AMPK [123]. Elevated expression of fatty acid synthase (FASN) counteracted SLC7A11-related ferroptosis and therefore fostered resistance to sorafenib, and Orlistat, an inhibitor of FASN, could effectively improve the efficacy of sorafenib by promoting ferroptosis [124]. Immune checkpoint inhibitors (ICIs), containing anti-PD-1 treatment, have emerged as promising immunotherapies for advanced HCC. Gao's research demonstrated that anti-PD-1 treatment elevated autophagy and the expression of Yes-associated protein 1 (YAP1), and Yap1 knockout enhanced the effectiveness of anti-PD-1 treatment by inhibiting autophagy in HCC [125]. Zheng et al. showed that inhibition of phosphoglycerate mutase 1 (PGAM1) could enhance the efficacy of anti-PD-1 treatment in HCC by stimulating ferroptosis and the infiltration of CD8+ T-cells [126].
Despite the promising nature of studies regarding combination therapy or nanomaterials to boost the effectiveness of lenvatinib and reverse LR in HCC cell lines and animal models, few have been granted approval for clinical use. After conducting an exhaustive literature review and searching the ClinicalTrials.gov website, we identified one clinical trial, that focused on combating LR with the mentioned RCD modulators. It is a prospective clinical study aiming to test the safety and efficacy of lenvatinib in combination with gefitinib in people with lenvatinib resistant hepatocellular carcinoma. The inclusion criteria includes: (1) Unlimited gender, aged 18-75 years; (2) Meets American Association for the Study of Liver Diseases or European Association for the Study of the Liver clinical diagnostic criteria of HCC; (3) progressing after standard treatment; (4) Unresponsive or resistant to Lenvatinib; (5) Child-Pugh A or scored 7 B; (6) Eastern Cooperative Oncology Group performance status score <=1; (7) Platelet count >=60x10^9/L, Prothrombin time prolonged <=6 seconds. The results are as follows: following approximately 4 weeks of lenvatinib plus gefitinib therapy, 12 treated HCC patients exhibited a reduction in total tumor burden, as evidenced by MRI images, which was indicative of partial response. In addition to further investigating the molecular mechanism of LR, translating these insights into tangible clinical applications is a critical next step. Hence, corresponding clinical trials are urgently needed to test the safety and efficacy of these therapeutic strategies in real-world scenarios.
Abbreviations
ac4C: N4-acetocytidine; AI: artificial intelligence; BAMBI: bone morphogenetic protein and activin membrane-bound inhibitor; BNIP3: BCL2 interacting protein 3; ceRNA: competitive endogenous RNA; circRNAs: circular RNAs; CK2: casein kinase-2; CP: Compound Phyllanthus urinaria; CQ: chloroquine; CSC: cancer stem cell; DLAT: Dihydrolipoamide S-acetyltransferase; DSF: disulfiram; EMT: epithelial-mesenchymal transition; ENO2: enolase 2; ER: endoplasmic reticulum; EVA1A: eva-1 homolog A; FAM134B: family with sequence similarity 134, member B; FASN: fatty acid synthase; FDA: Food and Drug Administration; FGFR: fibroblast growth factor receptor; FOXA2: forkhead box protein A2; FOXO3: Forkhead box protein O3; FTH1: ferritin heavy chain 1; GPX: glutathione peroxidase; GRHL3: grainyhead-like transcription factor 3; HCC: hepatocellular carcinoma; HCQ: hydroxychloroquine; HO-1: heme oxygenase-1; ICD: immunogenic cell death; ICIs: immune checkpoint inhibitors; IRF2: interferon regulatory factor 2; JMJD8: Jumonji domain-containing protein 8; KPNB1: karyopherin subunit beta 1; LACTB: serine beta-lactamase-like protein; LAPTM5: lysosomal protein transmembrane 5; lncRNAs: long noncoding RNAs; LR: lenvatinib resistance; LT: liver transplantation; METTL: methyltransferase-like protein; miRNAs: microRNAs; NQO1: NAD(P)H:quinone oxidoreductase 1; Nrf2: nuclear factor erythrocyte 2-related factor 2; NRP1: neuropilin-1; NUPR1: nuclear protein 1; ORR: objective response rate; OS: overall survival; PABPC4: poly (A) binding protein cytoplasmic 4; PFS: progression-free survival; PINK1: PTEN-induced putative kinase 1; PGAM1: phosphoglycerate mutase 1; p-MYH9: phosphorylated non-muscle myosin heavy chain 9; PPARGC1A: peroxisome proliferator-activated receptor-gamma coactivator-1α; RCD: regulated cell death; RFA: radiofrequency ablation; ROS: reactive oxygen species; SBRT: stereotactic body radiotherapy; scRNA-seq: single-cell RNA sequencing; SD: stable disease; SLC7A11: solute carrier family 7 member 11; STOML2: stomatin-like protein 2; STX6: syntaxin-6; TACE: transcatheter arterial chemoembolization; TCA: tricarboxylic acid; TCM: traditional Chinese medicine; TFEB: transcription factor EB; TKI: tyrosine kinase inhibitor; TME: tumor microenvironment; TMX2: thioredoxin related transmembrane protein 2; TR: transferrin receptor; TTP: time to progression; USF2: upstream stimulatory factor 2; USP22: ubiquitin-specific protease 22; VEGFR: vascular endothelial growth factor receptor; YAP1: Yes-associated protein 1; 3-MA: 3-methyladenine.
Acknowledgements
This study was supported by Youth Fund of the National Natural Science Foundation of China (no.82303379) and the Natural Science Foundation of Zhejiang Province (no. LQ23H160030).
All illustrations were generated using Figdraw.
Author contributions
Conceptualization, Ronggao Chen and Shusen Zheng; writing the original draft, Ronggao Chen and Xin Hu; visualization, Yingchen Huang and Yao Jiang; Data curation, Guanrong Chen and Qiaonan Shan; writing the review and editing, Xiao Xu and Shusen Zheng; funding acquisition, Ronggao Chen and Shusen Zheng; supervision, Xiao Xu and Shusen Zheng. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
2. Tabrizian P, Abdelrahim M, Schwartz M. Immunotherapy and transplantation for hepatocellular carcinoma. J Hepatol. 2024;80:822-5
3. Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. 2023;20:864-84
4. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604
5. Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni A, Kamel IR, Cloyd JM. et al. Management of Hepatocellular Carcinoma: A Review. JAMA Surg. 2023;158:410-20
6. Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5:146
7. Wu TK, Hui RW, Mak LY, Fung J, Seto WK, Yuen MF. Hepatocellular carcinoma: Advances in systemic therapies. F1000Res. 2024;13:104
8. Liu Y, Mao Z, Ding Y, Wang W. Macrophages as Targets in Hepatocellular Carcinoma Therapy. Mol Cancer Ther. 2024;23:780-790
9. Cao LQ, Xie Y, Fleishman JS, Liu X, Chen ZS. Hepatocellular carcinoma and lipid metabolism: Novel targets and therapeutic strategies. Cancer Lett. 2024;597:217061
10. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF. et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-90
11. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-73
12. Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: A Review in Hepatocellular Carcinoma. Drugs. 2019;79:665-74
13. Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-62
14. Wang J, Yu H, Dong W, Zhang C, Hu M, Ma W. et al. N6-Methyladenosine-Mediated Up-Regulation of FZD10 Regulates Liver Cancer Stem Cells' Properties and Lenvatinib Resistance Through WNT/beta-Catenin and Hippo Signaling Pathways. Gastroenterology. 2023;164:990-1005
15. Shan Q, Yin L, Zhan Q, Yu J, Pan S, Zhuo J. et al. The p-MYH9/USP22/HIF-1alpha axis promotes lenvatinib resistance and cancer stemness in hepatocellular carcinoma. Signal Transduct Target Ther. 2024;9:249
16. Newton K, Strasser A, Kayagaki N, Dixit VM. Cell death. Cell. 2024;187:235-56
17. Yuan J, Ofengeim D. A guide to cell death pathways. Nat Rev Mol Cell Biol. 2024;25:379-95
18. Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347-64
19. Peng F, Liao M, Qin R, Zhu S, Peng C, Fu L. et al. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduction and Targeted Therapy. 2022;7:286
20. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74
21. Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31-46
22. D'Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43:582-92
23. Kari S, Subramanian K, Altomonte IA, Murugesan A, Yli-Harja O, Kandhavelu M. Programmed cell death detection methods: a systematic review and a categorical comparison. Apoptosis. 2022;27:482-508
24. Kulbay M, Paimboeuf A, Ozdemir D, Bernier J. Review of cancer cell resistance mechanisms to apoptosis and actual targeted therapies. J Cell Biochem. 2022;123:1736-61
25. Morana O, Wood W, Gregory CD. The Apoptosis Paradox in Cancer. Int J Mol Sci. 2022;23:1328
26. Zhao Z, Song J, Zhang D, Wu F, Tu J, Ji J. Oxysophocarpine suppresses FGFR1-overexpressed hepatocellular carcinoma growth and sensitizes the therapeutic effect of lenvatinib. Life Sci. 2021;264:118642
27. Tan W, Zhang K, Chen X, Yang L, Zhu S, Wei Y. et al. GPX2 is a potential therapeutic target to induce cell apoptosis in lenvatinib against hepatocellular carcinoma. J Adv Res. 2023;44:173-83
28. Xue W, Wang T, Tian WJ, Pang SQ, Zhang HF, Jia WD. NQO1 Mediates Lenvatinib Resistance by Regulating ROS-induced Apoptosis in Hepatocellular Carcinoma. Curr Med Sci. 2024;44:168-79
29. Yu T, Yu J, Lu L, Zhang Y, Zhou Y, Zhou Y. et al. MT1JP-mediated miR-24-3p/BCL2L2 axis promotes Lenvatinib resistance in hepatocellular carcinoma cells by inhibiting apoptosis. Cellular Oncology. 2021;44:821-34
30. Xu X, Jiang W, Han P, Zhang J, Tong L, Sun X. MicroRNA-128-3p Mediates Lenvatinib Resistance of Hepatocellular Carcinoma Cells by Downregulating c-Met. J Hepatocell Carcinoma. 2022;9:113-26
31. Guo Y, Xu J, Du Q, Yan Y, Geller DA. IRF2 regulates cellular survival and Lenvatinib-sensitivity of hepatocellular carcinoma (HCC) through regulating beta-catenin. Transl Oncol. 2021;14:101059
32. Guo J, Zhao J. USP22-JMJD8 axis promotes Lenvatinib resistance in hepatocellular carcinoma. Biochim Biophys Acta Mol Cell Res. 2024;1871:119617
33. Mattei AL, Bailly N, Meissner A. DNA methylation: a historical perspective. Trends Genet. 2022;38:676-707
34. Yang B, Wang JQ, Tan Y, Yuan R, Chen ZS, Zou C. RNA methylation and cancer treatment. Pharmacol Res. 2021;174:105937
35. Neganova ME, Klochkov SG, Aleksandrova YR, Aliev G. Histone modifications in epigenetic regulation of cancer: Perspectives and achieved progress. Semin Cancer Biol. 2022;83:452-71
36. Nagaraju GP, Dariya B, Kasa P, Peela S, El-Rayes BF. Epigenetics in hepatocellular carcinoma. Semin Cancer Biol. 2022;86:622-32
37. Tang Q, Li L, Wang Y, Wu P, Hou X, Ouyang J. et al. RNA modifications in cancer. Br J Cancer. 2023;129:204-21
38. Luo Y, Yao Y, Wu P, Zi X, Sun N, He J. The potential role of N(7)-methylguanosine (m7G) in cancer. J Hematol Oncol. 2022;15:63
39. Huang M, Long J, Yao Z, Zhao Y, Zhao Y, Liao J. et al. METTL1-Mediated m7G tRNA Modification Promotes Lenvatinib Resistance in Hepatocellular Carcinoma. Cancer Res. 2023;83:89-102
40. Wang L, Yang Q, Zhou Q, Fang F, Lei K, Liu Z. et al. METTL3-m(6)A-EGFR-axis drives lenvatinib resistance in hepatocellular carcinoma. Cancer Lett. 2023;559:216122
41. Zhang Y, Lei Y, Dong Y, Chen S, Sun S, Zhou F. et al. Emerging roles of RNA ac4C modification and NAT10 in mammalian development and human diseases. Pharmacol Ther. 2024;253:108576
42. Pan Z, Bao Y, Hu M, Zhu Y, Tan C, Fan L. et al. Role of NAT10-mediated ac4C-modified HSP90AA1 RNA acetylation in ER stress-mediated metastasis and lenvatinib resistance in hepatocellular carcinoma. Cell Death Discov. 2023;9:56
43. Liu S, Yao S, Yang H, Liu S, Wang Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023;14:648
44. Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24:560-75
45. Samaddar JS, Gaddy VT, Duplantier J, Thandavan SP, Shah M, Smith MJ. et al. A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol Cancer Ther. 2008;7:2977-87
46. Nguyen HG, Yang JC, Kung HJ, Shi XB, Tilki D, Lara PN Jr. et al. Targeting autophagy overcomes Enzalutamide resistance in castration-resistant prostate cancer cells and improves therapeutic response in a xenograft model. Oncogene. 2014;33:4521-30
47. Gupta A, Roy S, Lazar AJ, Wang WL, McAuliffe JC, Reynoso D. et al. Autophagy inhibition and antimalarials promote cell death in gastrointestinal stromal tumor (GIST). Proc Natl Acad Sci U S A. 2010;107:14333-8
48. Pan J, Zhang M, Dong L, Ji S, Zhang J, Zhang S. et al. Genome-Scale CRISPR screen identifies LAPTM5 driving lenvatinib resistance in hepatocellular carcinoma. Autophagy. 2023;19:1184-98
49. Zhou L, Wang Z, Chen X, Li X, Ge C, Min X. et al. Syntaxin-6 promotes the progression of hepatocellular carcinoma and alters its sensitivity to chemotherapies by activating the USF2/LC3B axis. Int J Biol Sci. 2023;19:3892-907
50. Gu D, Tong M, Wang J, Zhang B, Liu J, Song G. et al. Overexpression of the lncRNA HOTAIRM1 promotes lenvatinib resistance by downregulating miR-34a and activating autophagy in hepatocellular carcinoma. Discov Oncol. 2023;14:66
51. Liu X, Gao X, Yang Y, Yang D, Guo Q, Li L. et al. EVA1A reverses lenvatinib resistance in hepatocellular carcinoma through regulating PI3K/AKT/p53 signaling axis. Apoptosis. 2024;29:1161-84
52. Fernandez-Palanca P, Payo-Serafin T, San-Miguel B, Mendez-Blanco C, Tunon MJ, Gonzalez-Gallego J. et al. Hepatocellular carcinoma cells loss lenvatinib efficacy in vitro through autophagy and hypoxia response-derived neuropilin-1 degradation. Acta Pharmacol Sin. 2023;44:1066-82
53. Wang Z, Shen J, Chen C, Wen T, Li C. FOXA2 plays a critical role in hepatocellular carcinoma progression and lenvatinib-associated drug resistance. Biosci Trends. 2023;17:136-47
54. Vargas JNS, Hamasaki M, Kawabata T, Youle RJ, Yoshimori T. The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol. 2023;24:167-85
55. Liu J, Wu Y, Meng S, Xu P, Li S, Li Y. et al. Selective autophagy in cancer: mechanisms, therapeutic implications, and future perspectives. Mol Cancer. 2024;23:22
56. Zhang S, Peng X, Yang S, Li X, Huang M, Wei S. et al. The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders. Cell Death Dis. 2022;13:132
57. Choi I, Zhang Y, Seegobin SP, Pruvost M, Wang Q, Purtell K. et al. Microglia clear neuron-released alpha-synuclein via selective autophagy and prevent neurodegeneration. Nat Commun. 2020;11:1386
58. Wang B, Nie J, Wu L, Hu Y, Wen Z, Dong L. et al. AMPKalpha2 Protects Against the Development of Heart Failure by Enhancing Mitophagy via PINK1 Phosphorylation. Circ Res. 2018;122:712-29
59. Li A, Gao M, Liu B, Qin Y, Chen L, Liu H. et al. Mitochondrial autophagy: molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022;13:444
60. Zhang W, Tang Y, Yang P, Chen Y, Xu Z, Qi C. et al. TMX2 potentiates cell viability of hepatocellular carcinoma by promoting autophagy and mitophagy. Autophagy. 2024;20:2146-63
61. Zhang Y, Zhang Y, Tao H, Zhu J, Lu Y, Cheng F. et al. Targeting LINC01607 sensitizes hepatocellular carcinoma to Lenvatinib via suppressing mitophagy. Cancer Lett. 2023;576:216405
62. Zheng Y, Huang C, Lu L, Yu K, Zhao J, Chen M. et al. STOML2 potentiates metastasis of hepatocellular carcinoma by promoting PINK1-mediated mitophagy and regulates sensitivity to lenvatinib. J Hematol Oncol. 2021;14:16
63. Wang S, Cheng H, Li M, Gao D, Wu H, Zhang S. et al. BNIP3-mediated mitophagy boosts the competitive growth of Lenvatinib-resistant cells via energy metabolism reprogramming in HCC. Cell Death Dis. 2024;15:484
64. Dixon SJ, Olzmann JA. The cell biology of ferroptosis. Nat Rev Mol Cell Biol. 2024;25:424-42
65. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266-82
66. Zhou Q, Meng Y, Li D, Yao L, Le J, Liu Y. et al. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct Target Ther. 2024;9:55
67. Lai B, Wu CH, Wu CY, Luo SF, Lai JH. Ferroptosis and Autoimmune Diseases. Front Immunol. 2022;13:916664
68. Li N, Jiang W, Wang W, Xiong R, Wu X, Geng Q. Ferroptosis and its emerging roles in cardiovascular diseases. Pharmacol Res. 2021;166:105466
69. Cui S, Ghai A, Deng Y, Li S, Zhang R, Egbulefu C. et al. Identification of hyperoxidized PRDX3 as a ferroptosis marker reveals ferroptotic damage in chronic liver diseases. Mol Cell. 2023;83:3931-9 e5
70. Xie L, Fang B, Zhang C. The role of ferroptosis in metabolic diseases. Biochim Biophys Acta Mol Cell Res. 2023;1870:119480
71. Lei G, Zhuang L, Gan B. The roles of ferroptosis in cancer: Tumor suppression, tumor microenvironment, and therapeutic interventions. Cancer Cell. 2024;42:513-34
72. Zhang C, Liu X, Jin S, Chen Y, Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. 2022;21:47
73. Wang Y, Wu X, Ren Z, Li Y, Zou W, Chen J. et al. Overcoming cancer chemotherapy resistance by the induction of ferroptosis. Drug Resist Updat. 2023;66:100916
74. Iseda N, Itoh S, Toshida K, Tomiyama T, Morinaga A, Shimokawa M. et al. Ferroptosis is induced by lenvatinib through fibroblast growth factor receptor-4 inhibition in hepatocellular carcinoma. Cancer Sci. 2022;113:2272-87
75. Zeng K, Huang N, Liu N, Deng X, Mu Y, Zhang X. et al. LACTB suppresses liver cancer progression through regulation of ferroptosis. Redox Biol. 2024;75:103270
76. Lai HC, Weng JC, Huang HC, Ho JX, Kuo CL, Cheng JC. et al. Solanum torvum induces ferroptosis to suppress hepatocellular carcinoma. J Ethnopharmacol. 2024;335:118670
77. Pan R, Zhao Z, Xu D, Li C, Xia Q. GPX4 transcriptionally promotes liver cancer metastasis via GRHL3/PTEN/PI3K/AKT axis. Transl Res. 2024;271:79-92
78. Hiromatsu M, Toshida K, Itoh S, Harada N, Kohashi K, Oda Y. et al. Transferrin Receptor is Associated with Sensitivity to Ferroptosis Inducers in Hepatocellular Carcinoma. Ann Surg Oncol. 2023;30:8675-89
79. Zhang Q, Qiao L, Liao J, Liu Q, Liu P, Liu L. A novel hypoxia gene signature indicates prognosis and immune microenvironments characters in patients with hepatocellular carcinoma. J Cell Mol Med. 2021;25:3772-84
80. Zhang Q, Xiong L, Wei T, Liu Q, Yan L, Chen J. et al. Hypoxia-responsive PPARGC1A/BAMBI/ACSL5 axis promotes progression and resistance to lenvatinib in hepatocellular carcinoma. Oncogene. 2023;42:1509-23
81. Zuo YB, Zhang YF, Zhang R, Tian JW, Lv XB, Li R. et al. Ferroptosis in Cancer Progression: Role of Noncoding RNAs. Int J Biol Sci. 2022;18:1829-43
82. Chen B, Dragomir MP, Yang C, Li Q, Horst D, Calin GA. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct Target Ther. 2022;7:121
83. Song Z, Zhang Y, Luo W, Sun C, Lv C, Wang S. et al. HAND2-AS1 Promotes Ferroptosis to Reverse Lenvatinib Resistance in Hepatocellular Carcinoma by TLR4/NOX2/DUOX2 Axis. Curr Cancer Drug Targets. 2024;25:144-158
84. Chen X, Song X, Li J, Zhang R, Yu C, Zhou Z. et al. Identification of HPCAL1 as a specific autophagy receptor involved in ferroptosis. Autophagy. 2023;19:54-74
85. Bi T, Lu Q, Pan X, Dong F, Hu Y, Xu Z. et al. circFAM134B is a key factor regulating reticulophagy-mediated ferroptosis in hepatocellular carcinoma. Cell Cycle. 2023;22:1900-20
86. Zhang XY, Li SS, Gu YR, Xiao LX, Ma XY, Chen XR. et al. CircPIAS1 promotes hepatocellular carcinoma progression by inhibiting ferroptosis via the miR-455-3p/NUPR1/FTH1 axis. Mol Cancer. 2024;23:113
87. Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022;7:378
88. Tian Z, Jiang S, Zhou J, Zhang W. Copper homeostasis and cuproptosis in mitochondria. Life Sci. 2023;334:122223
89. Xie J, Yang Y, Gao Y, He J. Cuproptosis: mechanisms and links with cancers. Mol Cancer. 2023;22:46
90. Tang D, Kroemer G, Kang R. Targeting cuproplasia and cuproptosis in cancer. Nature Reviews Clinical Oncology. 2024;21:370-88
91. Yang Q, Zeng S, Liu W. Roles of cuproptosis-related gene DLAT in various cancers: a bioinformatic analysis and preliminary verification on pro-survival autophagy. PeerJ. 2023;11:e15019
92. Li X, Tang C, Ye H, Fang C. Injectable Hydrogel-Encapsulating Pickering Emulsion for Overcoming Lenvatinib-Resistant Hepatocellular Carcinoma via Cuproptosis Induction and Stemness Inhibition. Polymers (Basel). 2024;16:2418
93. Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduction and Targeted Therapy. 2021;6:128
94. Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao C. et al. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12:4310-29
95. Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T. et al. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. 2022;19:971-92
96. Zou Z, Zhao M, Yang Y, Xie Y, Li Z, Zhou L. et al. The role of pyroptosis in hepatocellular carcinoma. Cell Oncol (Dordr). 2023;46:811-23
97. Fu XW, Song CQ. Identification and Validation of Pyroptosis-Related Gene Signature to Predict Prognosis and Reveal Immune Infiltration in Hepatocellular Carcinoma. Front Cell Dev Biol. 2021;9:748039
98. Wang J, Wei W, Tang Q, Lu L, Luo Z, Li W. et al. Oxysophocarpine suppresses hepatocellular carcinoma growth and sensitizes the therapeutic blockade of anti-Lag-3 via reducing FGL1 expression. Cancer Med. 2020;9:7125-36
99. Liu R, Peng J, Wang H, Li L, Wen X, Tan Y. et al. Oxysophocarpine Retards the Growth and Metastasis of Oral Squamous Cell Carcinoma by Targeting the Nrf2/HO-1 Axis. Cell Physiol Biochem. 2018;49:1717-33
100. Yang Y, Li YX, Wang HL, Jin SJ, Zhou R, Qiao HQ. et al. Oxysophocarpine Ameliorates Carrageenan-induced Inflammatory Pain via Inhibiting Expressions of Prostaglandin E2 and Cytokines in Mice. Planta Med. 2015;81:791-7
101. Cheng Y, Zhan P, Lu J, Lu Y, Luo C, Cen X. et al. Metformin synergistically enhances the antitumour activity of Lenvatinib in hepatocellular carcinoma by altering AKT-FOXO3 signalling pathway. Liver Int. 2023;43:1577-92
102. Yan S, Chen L, Zhuang H, Yang H, Yang Y, Zhang N. et al. HDAC Inhibition Sensitize Hepatocellular Carcinoma to Lenvatinib via Suppressing AKT Activation. Int J Biol Sci. 2024;20:3046-60
103. Ito R, Miyanishi K, Kubo T, Hamaguchi K, Osuga T, Tanaka S. et al. Synergistic antitumor effect of histone deacetylase class IIa inhibitor with lenvatinib in hepatocellular carcinoma. Hepatology International. 2023;17:735-44
104. Zhao Z, Zhang D, Wu F, Tu J, Song J, Xu M. et al. Sophoridine suppresses lenvatinib-resistant hepatocellular carcinoma growth by inhibiting RAS/MEK/ERK axis via decreasing VEGFR2 expression. J Cell Mol Med. 2021;25:549-60
105. Sun D, Liu J, Wang Y, Dong J. Co-administration of MDR1 and BCRP or EGFR/PI3K inhibitors overcomes lenvatinib resistance in hepatocellular carcinoma. Front Oncol. 2022;12:944537
106. Zeng C, Huang W, Li Y, Weng H. Roles of METTL3 in cancer: mechanisms and therapeutic targeting. J Hematol Oncol. 2020;13:117
107. Zhao L, Ma H, Jiang Y, Li Y, Guo N, Chen Y. et al. Reserpine, a novel N6-methyladenosine regulator, reverses Lenvatinib resistance in hepatocellular carcinoma. Phytomedicine. 2024;135:156002
108. Chen JL, Wu X, Yin D, Jia XH, Chen X, Gu ZY. et al. Autophagy inhibitors for cancer therapy: Small molecules and nanomedicines. Pharmacol Ther. 2023;249:108485
109. Liu L, Wang B, Ma Y, Sun K, Wang P, Li M. et al. A review of Phyllanthus urinaria L. in the treatment of liver disease: viral hepatitis, liver fibrosis/cirrhosis and hepatocellular carcinoma. Front Pharmacol. 2024;15:1443667
110. Huang D, Yang B, Yao Y, Liao M, Zhang Y, Zeng Y. et al. Autophagic Inhibition of Caveolin-1 by Compound Phyllanthus urinaria L. Activates Ubiquitination and Proteasome Degradation of beta-catenin to Suppress Metastasis of Hepatitis B-Associated Hepatocellular Carcinoma. Front Pharmacol. 2021;12:659325
111. Liao M, Qin M, Liu L, Huang H, Chen N, Du H. et al. Exosomal microRNA profiling revealed enhanced autophagy suppression and anti-tumor effects of a combination of compound Phyllanthus urinaria and lenvatinib in hepatocellular carcinoma. Phytomedicine. 2024;122:155091
112. Tan HL, Chan KG, Pusparajah P, Saokaew S, Duangjai A, Lee LH. et al. Anti-Cancer Properties of the Naturally Occurring Aphrodisiacs: Icariin and Its Derivatives. Front Pharmacol. 2016;7:191
113. Luo P, An Y, He J, Xing X, Zhang Q, Liu X. et al. Icaritin with autophagy/mitophagy inhibitors synergistically enhances anticancer efficacy and apoptotic effects through PINK1/Parkin-mediated mitophagy in hepatocellular carcinoma. Cancer Lett. 2024;587:216621
114. Fan Y, Li S, Ding X, Yue J, Jiang J, Zhao H. et al. First-in-class immune-modulating small molecule Icaritin in advanced hepatocellular carcinoma: preliminary results of safety, durable survival and immune biomarkers. BMC Cancer. 2019;19:279
115. Yu Z, Guo J, Hu M, Gao Y, Huang L. Icaritin Exacerbates Mitophagy and Synergizes with Doxorubicin to Induce Immunogenic Cell Death in Hepatocellular Carcinoma. ACS Nano. 2020;14:4816-28
116. Lei Y, Tang L, Chen Q, Wu L, He W, Tu D. et al. Disulfiram ameliorates nonalcoholic steatohepatitis by modulating the gut microbiota and bile acid metabolism. Nat Commun. 2022;13:6862
117. Zeng M, Wu B, Wei W, Jiang Z, Li P, Quan Y. et al. Disulfiram: A novel repurposed drug for cancer therapy. Chinese Medical Journal. 2024;137:1389-98
118. Van de Sande B, Lee JS, Mutasa-Gottgens E, Naughton B, Bacon W, Manning J. et al. Applications of single-cell RNA sequencing in drug discovery and development. Nat Rev Drug Discov. 2023;22:496-520
119. Wu X, Li W, Tu H. Big data and artificial intelligence in cancer research. Trends Cancer. 2024;10:147-60
120. Zhou C, Sun BY, Zhou PY, Yang ZF, Wang ZT, Liu G. et al. MAIT cells confer resistance to Lenvatinib plus anti-PD1 antibodies in hepatocellular carcinoma through TNF-TNFRSF1B pathway. Clin Immunol. 2023;256:109770
121. Yang H, Cheng J, Zhuang H, Xu H, Wang Y, Zhang T. et al. Pharmacogenomic profiling of intra-tumor heterogeneity using a large organoid biobank of liver cancer. Cancer Cell. 2024;42:535-51 e8
122. Katti A, Diaz BJ, Caragine CM, Sanjana NE, Dow LE. CRISPR in cancer biology and therapy. Nat Rev Cancer. 2022;22:259-79
123. Lai HY, Tsai HH, Yen CJ, Hung LY, Yang CC, Ho CH. et al. Metformin Resensitizes Sorafenib-Resistant HCC Cells Through AMPK-Dependent Autophagy Activation. Front Cell Dev Biol. 2020;8:596655
124. Li Y, Yang W, Zheng Y, Dai W, Ji J, Wu L. et al. Targeting fatty acid synthase modulates sensitivity of hepatocellular carcinoma to sorafenib via ferroptosis. J Exp Clin Cancer Res. 2023;42:6
125. Gao Y, Peng Q, Li S, Zheng K, Gong Y, Xue Y. et al. YAP1 suppression inhibits autophagy and improves the efficacy of anti-PD-1 immunotherapy in hepatocellular carcinoma. Exp Cell Res. 2023;424:113486
126. Zheng Y, Wang Y, Lu Z, Wan J, Jiang L, Song D. et al. PGAM1 Inhibition Promotes HCC Ferroptosis and Synergizes with Anti-PD-1 Immunotherapy. Adv Sci (Weinh). 2023;10:e2301928
Author contact
![]() Corresponding authors: Shusen Zheng, shusenzhengedu.cn; Xiao Xu, zjxuedu.cn.
Corresponding authors: Shusen Zheng, shusenzhengedu.cn; Xiao Xu, zjxuedu.cn.

 Global reach, higher impact
Global reach, higher impact