Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(2):133-150. doi:10.7150/ijbs.6.133 This issue Cite
Review
Does the difference between physically active and couch potato lie in the dopamine system?
1. Department of Kinesiology, University of North Carolina, Charlotte NC, USA
2. Department of Health, Leisure, and Exercise Science, Appalachian State University, North Carolina Research Campus, Kannapolis NC, USA
Received 2009-12-14; Accepted 2010-3-2; Published 2010-3-9
Abstract
Obesity and other inactivity related diseases are increasing at an alarming rate especially in Western societies. Because of this, it is important to understand the regulating mechanisms involved in physical activity behavior. Much research has been done in regard to the psychological determinants of physical activity behavior; however, little is known about the underlying genetic and biological factors that may contribute to regulation of this complex trait. It is true that a significant portion of any trait is regulated by genetic and biological factors. In the case of voluntary physical activity behavior, these regulating mechanisms appear to be concentrated in the central nervous system. In particular, the dopamine system has been shown to regulate motor movement, as well as motivation and reward behavior. The pattern of regulation of voluntary physical activity by the dopamine system is yet to be fully elucidated. This review will summarize what is known about the dopamine system and regulation of physical activity, and will present a hypothesis of how this signaling pathway is mechanistically involved in regulating voluntary physical activity behavior. Future research in this area will aid in developing personalized strategies to prevent inactivity related diseases.
Keywords: Physical activity, behavior, dopamine, dopamine receptors, dopamine signaling, wheel running, motivation
Introduction
Voluntary physical activity is important to human health for many reasons, including the prevention of obesity [22, 138]. The rate of obesity has steadily increased over the last 30 years [184], while at the same time the amount of voluntary physical activity has decreased [1]. Increases in sedentary lifestyles in Western cultures has led to an increase in inactivity related diseases such as obesity, cardiovascular disease, Type II Diabetes, and certain types of cancer [118]. Research has shown the benefits of physical activity to human health and its importance in increasing resting metabolic rate [154], prevention of certain types of cancer [9], prevention of age related muscle loss, or sarcopenia [33], and treatment of depression and anxiety [30]. Although the physiology of exercise has been well studied, the factors controlling voluntary physical activity levels in humans are not fully understood. Thus, the main goal of this review is to highlight what is currently understood about the biological regulating factors of voluntary physical activity, in addition to providing a novel hypothesis of the role of the dopamine system in regulating voluntary physical activity levels. Understanding the regulation of physical activity will lead to better understanding of inactivity related diseases and lead to improved human health.
Biological Influence on Physical Activity
The manifestation of a particular phenotype (in this case voluntary physical activity level) is traditionally thought to be determined by the following equation:
Phenotype = environment + genetics/biological factor + environment/genetic interaction).
The relative contribution of each of these components differs depending on the phenotype in question. Several recent genetic studies have investigated the level of genetic association with physical activity in humans and in animal models. The estimated genetic component for physical activity from these studies ranges from 20-80% [42, 73, 82, 87, 91, 92, 114, 159, 165]. Additional support for the genetic component of voluntary physical activity can be found in mice selectively bred for high wheel running activity [161]. Even after just 10 generations of selective breeding for high wheel running, selected animals exhibited a 75% increase in wheel running activity [161], and after 35 generations selected animals ran 170% more than controls [120]. Recently, Lightfoot et al. (2008) conducted single-gene quantitative trait loci (QTL) analysis to determine the genetic locations possibly involved in regulation of physical activity. QTL analysis allows for the investigation of specific areas of the genome that are associated with a given trait. Using three wheel running indices in mice as indicative of physical activity, one significant QTL for distance (Chr. 13), one significant QTL for duration (Chr. 13), and two significant QTL for speed (Chr. 13 and 9) were found, confirming a genetic component to the regulation of voluntary physical activity in mice [92]. Further work from this group [84], in combination with the initial QTL analysis, showed that in the inbred F2 model used, the single-gene and epistatic [gene-gene inte ractions] QTL together accounted for 84-100% of the genetically-related phenotypic variance. Although these studies provide strong evidence of a genetic component to physical activity regulation, in order to fully understand the exact mechanisms regulating this broad behavior it becomes necessary to investigate the numerous components such as other biological (non-genetic) factors, and the interactions between these components (both gene and environment), that indeed contribute to the manifestation of this complex phenotype.
Where does the genetic/biological regulation occur?
The site of action of possible genetic/biological components affecting physical activity may include either peripheral mechanisms (e.g. fiber type, number of mitochondria, cell metabolism components, oxygen consumption etc.), and/or central mechanisms (e.g. brain signaling, neurotransmitters, motivational behaviors etc.). Interestingly, work done with animals selectively bred for high wheel running, has shown very few and/or minimal peripheral differences between mice selected for high wheel running, compared to control mice [35, 74, 119, 120, 162, 163, 167, 168]. Peripheral differences alone cannot explain the huge differences in wheel running between selectively-bred high active mice and control mice suggesting that a significant portion of the genetic/biological component affecting physical activity likely comes from central factors. This hypothesis is supported by several studies. First, mice selectively bred for high activity have increased Brain Derived Neurotrophic Factor (BDNF) in the hippocampal area of the brain compared to control mice [72]. Rhodes and colleagues also showed that mice selected for high wheel running had increased activity as measured by Fos immunoreactivity in specific areas of the brain including the mid-brain [123]. Finally, Bronikowski et al. (2004) showed that mice selected for high wheel running had a 20% increase in dopamine 2 (D2) and dopamine 4 (D4) receptors in the hippocampus as compared to control line mice [16]. The gene array used in this study did not contain the D1-like receptors, and the hippocampus is not known as a brain region mediating dopaminergic mediated motivation and reward; however, the authors still suggested the data indicate a possible role of the dopamine system to an increased motivation to run in selected mice [16]. Furthermore, given the fact that selected mice and control line mice respond similarly to D2-like antagonists [122], but respond differentially to D1-like antagonists suggests the D1-like receptors, and not the D2-like receptors, in certain areas of mid-brain are important in activity regulation in selectively bred high active mice [122, 123]. The results from studies on the central nervous system in the selectively bred mice are summarized in Table 1.
Summary of dopaminergic findings in selectively bred mice for high WR. Evidence from studies in selectively bred mice for high wheel running suggest the central regulation of physical activity likely involves the dopamine system.
| Area of Brain | Methods | Finding | Conclusions | Reference |
|---|---|---|---|---|
| Hipocampus | Gene Array | 24% ↑ D4 receptors 19% ↑ D2 receptors | small changes in gene expression in the brain can cause large phenotypic changes. D1 receptors were not analyzed. | Bronikowski et al., 2004 |
| Lateral Hypothalamus, Medial Frontal Cortex, Striatum | Fos expression in selected mice blocked from wheel | ↑ Fos expression | Different brain regions in control of intensity of running vs. motivation for running | Rhodes et al., 2003 |
| N/A | Agonists, Antagonists, re-uptake inhibitor | Differential responses in WR in selected mice vs. controls | D1-like receptors likely involved in mediating high WR in selected mice | Rhodes and Garland, 2003 |
Supporting the hypothesis that the dopaminergic system is an appropriate genetic/biological candidate in the central control of voluntary physical activity are studies that have implicated dopamine functioning in the control of motor movement [131], reward [139], learning, motivation [111], and emotion [147]. Thus, the dopamine system would be a likely candidate to help control voluntary physical activity because this is a motivated and rewarding behavior that involves motor control. However, to this point, the majority of studies investigating physical activity in humans have treated changes in neurotransmitter systems, such as dopamine, as a dependent factor that responds to physical activity stimuli such as intensity or duration of exercise. States another way, most investigations treat dopaminergic changes as a consequence of physical activity. Similarly, work done in animals has for the most part employed research designs focusing on neurotransmitter systems and “locomotion” in relation to diseases such as Parkinson's disease. However, extensive recent evidence presented by Garland and colleagues [16, 121-124] with mice selectively bred for high voluntary activity indicated a strong central component that may act in an independent fashion; i.e. the central component may control physical activity levels as part of a genetic/biological regulation scheme (e.g. physical activity may be the consequence of dopaminergic function). This current examination will review the literature implicating the dopaminergic system as a possible independent regulator of physical activity (as a separate form of locomotion and energy expenditure) in animals, as well as the emerging effort to understand the role the dopamine system plays in the regulation of motivation for voluntary physical activity. Based on the current literature, a novel interpretation of the central biological regulation of voluntary physical activity with respect to the dopaminergic system will also be presented.
The Dopaminergic System
While an exhaustive review of the structure and function of the dopaminergic system is beyond the scope of this review, in order to place the potential function of the dopamine system within the context of the central regulation of physical activity, a short overview of the dopamine system is necessary.
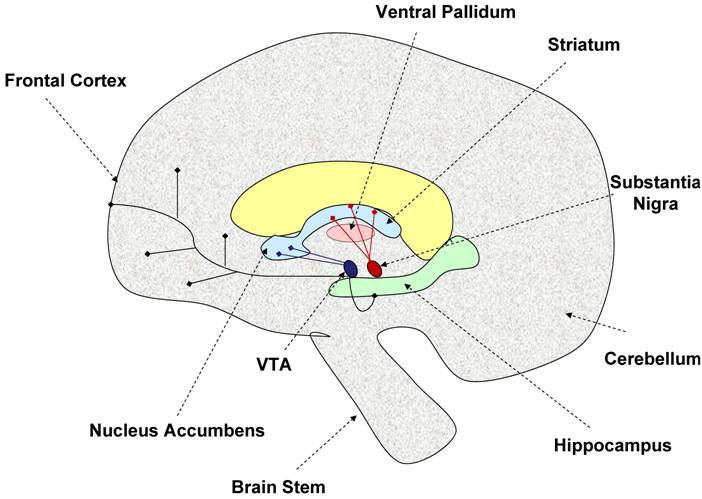
The dopaminergic neurons in the brain originate from two distinct areas. The neurons originating from the substantia nigra pars compacta project into the dorsal striatum via the nigrostriatal tract [60], while those neurons originating from the ventral tegmental area project into the cortex and ventral striatum (nucleus accumbens) via the mesolimbic tract [36, 89]. The dopaminergic neurons interconnect with many areas of the brain leading to the implication of the dopaminergic system in many central functions including reward, learning, motivation, response to stimuli, and movement [153]. Figure 1 illustrates the important dopaminergic pathways in the brain. Potentially important for the regulation of physical activity is the striatum/nucleus accumbens area given this area is involved in motivation, reward, and motor movement. Also highlighted as part of the basal ganglia, is the ventral pallidum because this area may be important in integrating dopaminergic signals from both motivational/reward centers and motor movement centers in the brain [152].
There are two evolutionarily and genetically different subtypes of receptors for dopamine within the dopaminergic system, and a total of five known distinct receptors [18, 153]. The dopamine D1-like receptor family includes the dopamine one (D1) and dopamine five (D5) receptors. These receptors contain no introns, act by way of Gs-proteins, and activate adenylyl cyclase, thus increasing cAMP production [85, 169]. The D-2 like receptor family includes the dopamine two (D2), dopamine three (D3), and dopamine four (D4) receptors. These receptors contain introns, act via Gi-proteins, inhibit adenylyl cyclase activity, and thus decrease cAMP activity [85, 105]. The two dopamine receptor families do not appear to act in isolation however, because it has been shown that activation of D1 receptors in the rat striatum causes D2 receptors to shift to a “low binding state” for dopamine [143]. Likewise, D1 and D2 receptors have been shown to physically interact in certain areas of the brain, possibly working synergistically to affect downstream signaling [36]. Dopaminergic signals also interact with GABA interneurons [2] and other neurotransmitter signaling, highlighting the many levels of control of the resultant neuronal signaling, and downstream effects.
Model of brain dopaminergic tracts. This figure illustrates the known dopaminergic neuronal tracts discussed in this review. The nigro-striatal tract (shown in red) consists of dopaminergic neurons originating from the substantia nigra, and projecting into the striatum. This tract is thought to be involved in control of motor movement. The mesolimbic tract (shown in deep purple) is made of dopaminergic neurons projecting from the ventral tegmental area (VTA) into the nucleus accumbens, frontal cortex, and hippocampus. This area is thought to be involved in motivation, reward, and learning. The ventral pallidum acts as a limbi-somatic motor interface. Thus, the striatum and nucleus accumbens may play an important role in regulating the motivation for physical activity. Dashed arrows indicate specific brain regions, while blunt ended solid line arrows indicate dopaminergic neuronal tracts.
Dopamine receptors differ in their anatomical locations on specific neurons, vary in density in specific regions of the brain, and can be found either presynaptically or postsynaptically depending on the type of tissue and/or neuron [105]. Dopamine receptor expression is found in nearly all areas of the brain, but receptors are most highly expressed in nigrostriatal and mesolimbic regions including the striatum, VTA, and cortex [27, 68]. The distribution of dopamine receptors in the brain is diverse; however, specific dopamine receptors are differentially expressed at higher or lower levels in particular areas of brain [36]. The five known dopamine receptors differ in their affinity for dopamine, natural ligands, receptor activity, anatomical locations, genetic sequence, and thus, physiological activity [18]; however, the dopamine receptors work in concert with each other to produce integrated responses and signals in the brain and body.
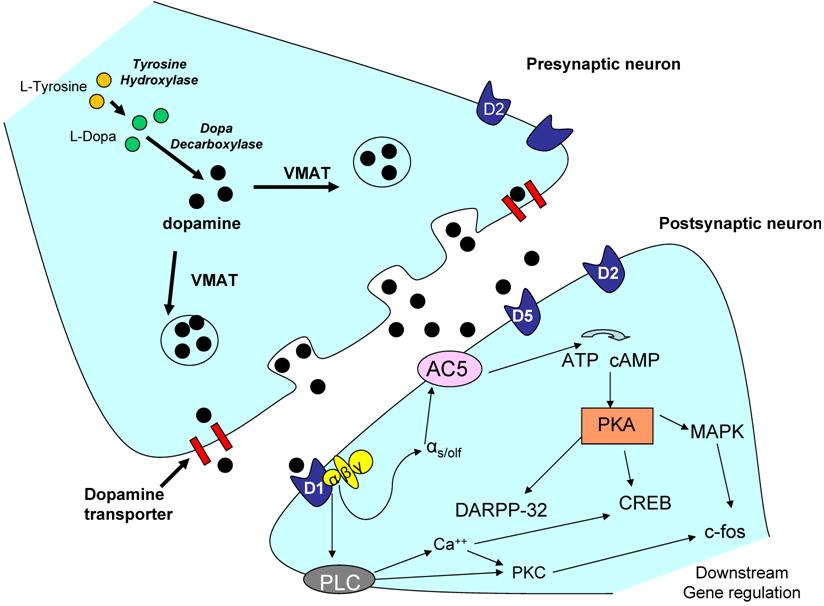
Expression levels of the dopamine receptors may be important in mediating downstream behavioral responses including voluntary activity. Dopamine receptor expression can be affected by the levels of dopamine in the system [55], level and length of treatment of pharmacological agents [17], as well as other external stimuli mediated through rewarding behavior such as sexual activity [102], or exercise [46]. However, overall dopaminergic responses and signaling are also dependent on other factors such as the electrical response produced (dopamine signaling can act in both an excitatory manner, as well as an inhibitory manner depending on the circumstance) [20, 64, 89], as well as interactions with other neurotransmitters and signaling molecules. For example, the dopamine system has been shown to interact with glutamate [145], GABA [57], acetylcholine [137], and serotonin [39]. Depending on the receptor involved and the anatomical location, dopamine receptors activate or repress a variety of signaling cascades including ERK/MAPK [95], CREB [125], and CAMKII [67], by affecting calcium and/or potassium channels in the nerve cell [105]. A representative dopaminergic synapse is shown in Figure 2. Only possible signaling pathways for the D1-like receptors are illustrated. Possible signaling pathways in the dopaminergic neurons are extensively reviewed by Neve and colleagues (2004) [107], and these downstream signaling pathways may be important in future investigations of the role of the dopamine system and regulation of voluntary physical activity.
Representative dopaminergic synapse. The above illustration is a representative dopaminergic synapse. The signaling pathways in the postsynaptic neuron are only representative of D1-like receptor signaling (which increases cAMP). D2-like receptors are known to have opposite affects on cAMP activity, and thus slightly different downstream signaling cascades. Dopaminergic signaling effects on ion channels and membrane permeability are not shown however, may be important in the regulation of behavior such as physical activity. For a full review of the signaling cascades proposed to be involved in D1-like and D2-like receptor signaling please refer to Neve et al. 2004 [107]. Abbreviations: AC5 - adenylate cyclase 5; ATP - adenylyl tri-phosphate; CREB - cyclic AMP response element binding protein; DARPP-32 - dopamine and cyclic AMP-regulated phosphoprotein (thought to be important in positive feedback signaling); D1 - dopamine receptor 1; MAPK - mitogen-activated protein kinase; PKA - protein kinase A; PKC - protein kinase C; PLC - phospholipase C; VMAT -- vesicular monoamine transporter; c-fos - downstream early gene.
Important in mediating downstream signaling are gene expression changes, and dopamine receptor signaling has been shown to affect gene expression [105]. Several immediate early genes that are activated in dopaminergic neurons following stimulation include those of the Fos family [65, 106, 123, 178]. Fos is a transcription factor that is up-regulated in the mid-brain in response to stimulation from drugs, or other natural rewarding stimuli such as sexual behavior or exercise [123, 150]. Fos is the product of the immediate early gene c-Fos, and Fos expression has been shown to be regulated by dopamine signaling [127]. Pharmacological studies show that Fos immunoreactivity in the striatum and other key regions of the brain is increased following administration of D1 and D2 agonists [58, 66, 69, 109, 126], suggesting Fos may be important as a downstream gene regulated by dopaminergic signaling. ΔFosB, a transcription factor and also a member of the Fos family of proteins, is likewise up-regulated in response to drugs of abuse and exercise. The expression of ΔFosB is usually longer lasting than Fos, and is thought to be involved in long term changes in behavior [106, 178]. Brain Derived Neurotrophic Factor (BDNF) also appears to be regulated in part by dopamine signaling and has been shown to increase as a result of physical exercise [41]. Additionally, it is thought that the antidepressant effect of exercise is mediated through the dopamine system, and increased expression of BDNF [38].
Thus, while Fos and BDNF are two examples of downstream transcription factors regulated by dopamine signaling, the dopamine system potentially affects a large number of downstream genes and signaling pathways that may ultimately be important in the understanding of the genetic mechanisms involved in regulation of physical activity levels in animals and humans. For example, dopamine signaling has also been shown to have direct affects on expression levels of certain neuropeptides including substance P (SP) [54], dynorphin [7, 48, 157], enkephalin [83, 158], and orexin [79]. In addition to other functions, these neuropeptides can in-turn also modulate other gene expression and downstream signaling, highlighting the possible indirect effects of dopamine signaling on downstream gene expression changes. Thus, the point should be made that any regulation of voluntary physical activity by dopamine signaling may be mediated through not only dopamine receptor expression levels, but also downstream signaling pathways including those that affect expression of transcription factors and other neuropeptides known to affect transcription and gene expression. The current literature, highlighted from this point forward, clearly indicate the dopamine system has an affect on the regulation of physical activity; however, these studies are limited to receptor expression level changes, pharmacological interventions, and other genetic interventions (e.g. knock-out models). Further research into the downstream mechanisms of control of voluntary physical activity will be needed in order to dig deeper into the exact method in which this regulation occurs. As explained in later sections, reconciling dopamine and reward literature, with the exercise science aspect of regulation of physical activity may be a good method of investigating the regulation of this complex behavior.
Dopaminergic Regulation of “Locomotion” and “motor movement”
Extensive studies have been conducted to assess the role of the dopamine receptors and the dopamine system in various behavioral functions [70, 183]. Literature investigating disease states such as Parkinson's disease is available which emphasizes the role of the dopamine system in regulation of raw motor movement. Here, it is important to make the distinction between “locomotion” and “physical activity”. The term locomotion in scientific literature generally refers to any act of movement, which depending on methodology, can operationally differ significantly between studies. Conversely, physical activity is generally defined as purposeful exercise and/or movement that expends a significant amount of energy. While there are slight differences between operational definitions of locomotion and physical activity which are highlighted later in this review, it is still important to point out the known dopaminergic involvement in locomotion and raw motor movement to understand the possible duel faceted role the dopamine system might play in regulating physical activity, especially since the preponderance of the available literature deals with 'locomotion' in disease states rather than voluntary physical activity.
Parkinson 's disease is a good example to highlight the role of the dopamine system in regulation of motor movement control. Common characteristics of Parkinson's disease include resting tremors, bradykinesia, rigidity, and overall difficulty in motor movement as a result of degradation and subsequent loss of dopaminergic neurons in the substantia nigra area of the brain [3, 185]. One particular animal model of Parkinson's symptoms gives insight into the importance of the dopamine system in locomotor behavior and motor movement. Toxin-induced models of Parkinson's commonly involve the use of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a toxin which when administered causes malfunction and loss of dopaminergic neurons in the brain. When MPTP is administered to mice, reduced locomotor function is evident through various tests including open field [142], and rotarod assessment [129]. Interestingly, there appear to be strain differences in susceptibility to MPTP and this may be caused by genetic differences in the dopamine system between different strains of mice [62, 142], suggesting genetic differences in the dopamine system may determine susceptibility to locomotor defects.
Another important line of evidence supporting the involvement of the dopaminergic system in regulation of locomotion and/or motor movement is its well studied role in Attention Deficit Hyperactivity Disorder (ADHD) [4, 88]. ADHD usually presents in childhood, but can also persist into adulthood [156], indicating that the central functioning mediating the symptoms may sometimes be irreversible. Genetic alterations of both the D4 and D5 receptors have been implicated as primary mechanisms in ADHD. Drd4 polymorphisms have been found in both human and animal models of ADHD [104]. Additionally, inheritance studies suggest an increased risk of ADHD associated with particular alleles of DRD4 and DRD5 [4, 40, 90]. Moreover, inheritance and allelic variant studies show an association between DAT, the dopamine transporter gene which is involved in transporting dopamine back into the neuron after it has been released into the synapse, and ADHD [40, 50]. However, the most compelling evidence regarding dopaminergic involvement with ADHD comes from pharmacological studies. Stimulants which block DAT, resulting in increased synaptic dopamine levels, have been shown to significantly reduce the hyperactive symptoms of ADHD [98, 179-181]. A complete review of the role of DAT in locomotion and parkinsonism can be found by GR Uhl, Movement Disorders, 2003 [166]. Intriguingly, mice exhibiting high amounts of wheel running after many generations of selective breeding have been suggested as a potential model of ADHD [122, 170]. Garland and colleagues have shown that these selectively-bred mice have altered dopamine profiles compared to control line mice, as well as respond more profoundly to dopaminergic acting drugs such as dopamine transporter inhibitors, suggesting similar mechanistic pathways as ADHD [123, 124]. Whether this model will provide mechanistic insight into the dopaminergic regulation of voluntary physical activity and/or ADHD is still unknown.
Human studies using positron emission tomography (PET) imaging also provide insight into the role of the dopamine system in regulation of motor tasks [6, 56, 110]. Lappin and colleagues [81] have shown that [11C]-raclopride binding potentials are significantly decreased in the sensorimotor striatum are of the brain, indicative of increased dopamine release, when subjects completed an active motor control task (such as a learned sequence of key presses) compared to rest conditions, suggesting the dopamine release and binding are subject to behavior conditions. What is hard to study in humans however is whether a priori genetic differences in regional dopamine functioning in the brain could affect motor control inter-individual differences? Relative to this review, it is important to point out that human brain imaging studies also show increased dopamine release in the striatum in response to reward tasks, further suggestive of the dopamine system acting as a cognitive-motor integration center.
Dopaminergic Regulation of Motivated Behavior
Voluntary physical activity essentially has two relevant components: a motor movement component and a motivational/rewarding component. This motivational component sets voluntary physical exercise apart from general “locomotion” studies. The role of the dopamine system in control of motor movement was explained above with the examples of Parkinson's Disease and ADHD. However, a role of the dopamine system in regulating motivation for physical activity can be implied from studies of addiction. It is well accepted that the dopamine system is a major mediator of addiction to drugs (reviewed extensively in Vetulani, 2001; Peirce and Kumaresan, 2006; and Di Chiara, 2007) [29, 116, 171]. Specifically, the dopamine reward centers are known to involve the neurons in the ventral tegmental area which project into the nucleus accumbens and other forebrain regions. It has been hypothesized that people who are addicted to such things as risky behavior, drugs, and gambling may have genetic differences in their dopamine system that predispose them to such behavior [173]. This hypothesis has been supported by results investigating the administration of methylphenidate (a psychoactive drug) to non-drug users whose D2 receptor expression was high in the brain. The administration of methylphenidate to these subjects produced a feeling of aversion, as opposed to what happened when methylphenidate was administered to people with low levels of D2 receptor expression; in these subjects the drug produced a pleasure feeling [174]. Additional evidence in rodents has suggested both D1-like and D2-like dopamine receptors, and the dopamine transporter gene may be a mediator in addictive behavior [53, 61, 155]. These results can be used to hypothesize that the dopaminergic system may play a role in the pleasurable/rewarding feelings associated with voluntary physical activity in humans and thus, might contribute to the observed variation in animals and humans in motivation for physical activity.
Additionally, studies of feeding behavior and food intake in animals suggest the dopamine system is important in motivated behavior, specifically motivation for physical activity in order to obtain food [112]. Recent studies have begun to investigate the increase in activity that results from the starvation characteristics of anorexia nervosa, which is sometimes labeled the “drive for activity” [19]. Typically, reported symptoms of semi-starvation include slowing of motor movement and lethargy; however, in a significant percentage of anorexia nervosa patients quite the opposite is observed with anorexic patients exhibiting increased physical activity levels [13, 71]. Several monoamine neurotransmitters including norepinephrine, serotonin [5], as well as dopamine have been suggested to play a role in this increased motivation for activity in anorexia nervosa [117]. In animal models of “activity induced anorexia” the dopaminergic system is suggested as a mediator of the increased physical activity seen in this disorder [52, 117]. Although the exact mechanism is still unclear, it has been shown that exercising intensely increases dopaminergic reward signaling [15], and subjects with anorexia may exercise excessively in order to relieve the “anhedonic state” created by insufficient nutrition [26, 47].
Thus, although there is much evidence in regard to the role of the dopamine system in movement disorders such as Parkinson's Disease and ADHD, as well as behavioral/motivational diseases such as addiction and anorexia; there is still a lack of mechanistic evidence on how the dopamine system may mediate motivation for physical activity in general. How this regulation occurs in the brain will have long lasting effects on the prevention of inactivity related diseases such as obesity.
An overview of the role of the dopamine system in these four disease states is outlined in Table 2.
The dopamine system in regulation of motor movement and addictive behaviors.
| Disease | Parkinson's Disease | ADHD | Anorexia | Addiction |
|---|---|---|---|---|
| Possible Mechanism | loss of DA neurons | DRD4/DRD5 and DAT | D2/D3? Interactions with other neuropeptides (orexin) | D1/D2, DAT, altered signaling |
| locomotor outcome | lack of motor control | Hyperactive Phenotype | ↑ drive for activity (other OCD tendencies) | mediates motivation for pleasure/reward seeking |
Dopamine signaling plays a prominent role in several disease states having to do with either motor movement or motivational behavior. The four disease states listed in this table are examples of how the dopamine system plays an important role in both motor movement as well as behavior. This is meant to highlight the multifaceted role the dopamine system likely plays in regulating motivation for physical activity in healthy populations. Possible mechanisms of regulation are listed based on the described literature. Abbreviations: DA - dopamine; DAT - dopamine transporter; OCD - obsessive compulsive disorder.
Dopaminergic regulation of Physical Activity: Evidence from animal models in wheel running studies
There is wealth of literature concerning animal models and “locomotion” with respect to the dopamine system. However, “locomotion” is a very broad term that can refer to several different types of movement including novelty induced, open field, drug induced, wheel running, and/or food seeking. It is contended that motivated physical activity in the form of exercise is also a type of locomotion with possibly very different regulatory mechanisms than for example, novelty induced locomotion. Eikelboom and colleagues [37] have contested that wheel running models in animals is the best parallel to human voluntary physical activity. Thus, because wheel running in animals is an innately controlled behavior that is completely voluntary, this section will only focus on those studies involving dopaminergic control of wheel running levels in animals.
Wheel Running Studies
Evidence for involvement of the dopamine system with physical activity levels can be implied from wheel running studies conducted in animals. Again, a strong case has been made that wheel running in animals is an appropriate model of voluntary physical activity in humans [37, 148]. Thus, as opposed to the drug induced locomotion studies, wheel running studies may give more accurate insights into the involvement of the dopamine system in general physical activity levels in humans.
Inbred mice strain differences in both dopaminergic anatomy and wheel running may prove useful in elucidating how genetic differences in dopaminergic signaling may differentially regulate voluntary physical activity in inbred mice. Lightfoot and colleagues screened 13 strains of mice for distance, duration, and speed on a running wheel, and found significant differences between strains in all running wheel indices, indicating a significant genetic component to regulation of wheel running behavior [91]. Additionally, strain differences in dopamine anatomy and function have also been shown by various authors [8, 100, 103, 108, 141, 146, 160]. For example, Fink and Reis, 1981, showed that BALB/cJ mice have more dopamine activity in both the nigrostriatal, and mesolimbic pathways in the brain compared to CBA/J mice [43]. Combining the knowledge that CBA/J and Balb/cJ mice differ in dopaminergic anatomy in the mid-brain [43], as well as differ in wheel running indices [91], it is reasonable to suggest that genetic differences in the dopamine system between inbred strains of mice may translate into behavioral differences, including voluntary wheel running. Similarly, work done recently in our lab [75] suggests expression differences of D1-like receptors as well and tyrosine hydroxylase between differentially active inbred strains may be important in mediating behavior differences in running wheel activity in differentially active inbred mice.
Supporting the hypothesis that genetic differences in the dopamine system may mediate physical activity behavioral differences in animal models is work done using selective breeding. Bronikowski and colleagues (2004) investigated gene expression changes in the hippocampus region of the brain and found that mice selectively bred for high wheel running had a 20% increase in D2 and D4 receptor expression (D1-like receptors were not analyzed in this study) compared to control line mice [16]. Also, Rhodes et al. (2003) investigated patterns of brain activity in mice selected for high wheel running, and found that certain areas of the brain exhibited increased activity (as measured by Fos expression) in selected animals compared to the control animals [123]. Several of the regions identified in this research, including the nucleus accumbens, striatum, prefrontal cortex, and lateral hypothalamus are regions associated with high dopaminergic activity. Another study by Waters et al. (2008) in rats selectively bred for high aerobic capacity showed that the high capacity rats exhibited increased wheel running activity compared to controls while also exhibiting increased dopaminergic activity in the striatum area of the brain compared to low aerobic capacity rats [177]. The authors suggested that artificial selection may have acted upon the dopamine system because the dopamine system is involved in motivation and that wheel running activity is a motivated behavior [177]. Given the knowledge from genetic studies of dopamine and wheel running in both inbred and selectively bred mice, it is warranted to investigate further the connection between the dopamine system and wheel running in animals.
Further elucidation of the role of the dopamine system in wheel running comes from investigations of the effects of pharmacological interventions (specifically psychoactive drugs) on wheel running in mice. The selectively bred mice mentioned above (see Garland et al. 2006 for a complete description of these selectively bred mice) [51] responded differently (wheel running amounts) than controls to several dopaminergic acting drugs including D1-like and D2-like agonists and antagonists, suggesting a dopaminergic involvement in regulation of wheel running in these selected animals [122, 124]. Specifically, selected animals significantly reduced their wheel running by decreasing their speed as compared to control animals in response to cocaine and GBR 12909 [124]. Both of these drugs act by inhibiting DAT which effectively increases the amount of dopamine in the synapse. In another study, Rhodes and colleagues (2003) showed that a DAT inhibitor (Ritalin, 15mg/Kg and 30mg/Kg) decreased wheel running in selected animals, but increased wheel running in control animals. A non-selective dopamine agonist (apomorphine, 0.25mg/Kg and 0.5mg/Kg) decreased wheel running more in control animals compared to selected animals at higher doses. Additionally, a selective D1-like antagonist (SCH-23390, 0.025-0.1mg/Kg) decreased wheel running in the control animals more than selected animals, while a selective D2-like antagonist (raclopride, 0.5-2.0mg/Kg) had similar effects on both selected and control animals [122]. These results suggest that D1-like receptors and DAT were involved in mediating the differences seen in wheel running between the selected animals compared to controls, but not the D2-like receptors.
Earlier studies by Schumacher and colleagues (1994) using mice classified as high active, or low active based on performance in a running wheel test, also showed differential locomotor responses to dopamine agonists such as apomorphine, bromocriptine, and amphetamine between the high active and low active mice. Specifically, bromocriptine and amphetamine stimulated physical activity more in the low active mice compared to the high active mice, suggesting a decreased functioning of the mesolimbic dopamine system in the high active mice [140]. A study conducted in 2004 by Leng and colleagues showed that C57Bl/6 mice, after pre-treatment with MPTP (a dopaminergic neurotoxin), exhibited significantly reduced wheel running after treatment with a tyrosine hydroxylase inhibitor which effectively reduced dopamine synthesis, highlighting the importance of dopamine itself, in addition to individual dopamine receptors, in the regulation of locomotion in the form of wheel running in mice [86]. Additionally, C57L/J mice (previously shown to be high active in the form of wheel running) [91] significantly reduce wheel running in response to a D1-like agonist, but do not significantly change wheel running behavior in response to a D1-like antagonist, dopamine re-uptake inhibitor, or a tyrosine hydroxylase inhibitor [76]. C3H/HeJ mice (previously shown to be low active) [91] did not respond to the D1-like agonist or antagonist, but did significantly increase wheel running in response to a dopamine re-uptake inhibitor [76]. Genetic differences in the dopamine system between C57L/J mice and C3H/HeJ mice could explain the differential response to dopaminergic acting drugs. Specifically, it appears that signaling through D1-like receptors is important in mediating the high activity observed in C57L/J mice, while dopamine half-life and presence in the synapse may be more important in mediating wheel running behavior in low active C3H/HeJ mice.
As is apparent, a preponderance of evidence suggests that the dopamine system is involved in the regulation of wheel running behavior in mice. From a genetic aspect, studies suggest inbred strains of mice, as well as mice selectively bred for high amounts of wheel running differ not only in the amount of physical activity performed, but also in dopaminergic anatomy, and thus function, in the mid-brain. Similarly, pharmacological studies provide insight into the possible role of the dopamine system in regulation of wheel running behavior. However, it is still unclear whether the dopamine system is acting in an independent fashion to control physical activity or if there are possible dependent changes in the dopamine system due to physical activity which is in-turn mediating activity behavior.
Going Further: Linking the Dopamine System and Regulation of Physical Activity in Humans
It is known that exercise acts as an independent agent to cause changes in various neurotransmitter systems, specifically the dopamine system, noradrenergic systems, and the serotonergic system [101]. For example, exercise increases the amount of dopamine released and metabolized in certain areas of the brain [176]. In this respect, changes in the dopamine system act in a dependent fashion in response to exercise (e.g. the exercise itself caused a change in dopaminergic signaling). However, this dependent change in the dopamine system is usually accompanied by a positive reinforcing response in which the dopamine system in-turn acts in an independent fashion causing changes in behavior to seek rewarding and/or pleasurable responses [183]. Even though we can postulate that seeking rewarding and/or pleasurable responses in humans leads to increased physical activity, evidence is still lacking as to whether the dopamine system is actually working in an independent role in influencing voluntary physical activity (e.g. can dopaminergic differences cause changes in motivated physical activity in humans?). It has been shown that dopamine neurons in the striatum are primarily responsible for changes in motor activity [136], while dopaminergic function in the nucleus accumbens is involved in anticipatory behavior (anticipation of a reward or “motivation”) [12, 115, 134]. From an anatomical perspective, it is important to point out that locomotor control areas of the brain (striatum, nigro-striatal pathways), and reward/motivational areas of the brain (nucleus accumbens, ventra tegmental area), are integrated by neural connections through regions such as the ventral pallidum. Thus, although collectively the basal ganglia neurons control distinct areas of the brain, these regions do not act in isolation, and it is certainly likely that motivation for exercise involves an integrated control from several of these regions. Dopamine depletion studies in the nucleus accumbens of rodents show a decreased motor activity response to certain drugs [25], and dopamine depleted animals showed lack of motivation for more effortful tasks [24, 133]. Thus, there is overlap between the motivational aspects and motor control aspects of brain neurology [130], with the dopamine system mediating both portions. This multifaceted role of the dopamine system provides reason to investigate the relationship between dopaminergic activity in the brain and amount of voluntary physically activity that the organism undertakes.
The fact that exercise is often used as a treatment in depression illustrates the dependent role of the dopamine system in response to physical activity. It has been shown that exercise alleviates symptoms of depression, most likely mediated through changes in the central nervous system in the brain [34]. Along this same line of thought, the benefits of physical activity on the brain seem to be primarily mediated through catecholamine systems. Exercise and/or physical activity is known to increase neurotransmitter production and metabolism [30, 32, 96], which are thought to lead to changes at the molecular and cellular level that improve neuronal plasticity [45, 101], cognitive functioning [151], learning [182], and overall mood [32], all aspects that protect brain function. Mice that perform voluntary physical activity in the form of wheel running produce more brain-derived neurotrophic factor (BDNF), causing an increase in synaptogenesis and neurogenesis, neuron survival, and increased learning capacity, all leading to possible protection from cognitive decline [23]. Similarly, it has been shown that moderate physical activity decreases the risk of Parkinson's Disease [94, 164], as well as helps alleviate and slow the progression of symptoms of the disease [44, 80].
Training studies have also shed light on the dependent changes in the dopamine system in response to exercise in the form of training. Rats who undergo endurance training show increased D2 receptor binding over the lifespan compared to control animals, suggesting that endurance training provides some protection from age related loss of D2 receptor functioning [97]. Likewise, rats exposed to treadmill running have increased Fos expression in the striatum area of the brain mediated through D1 receptors [93]. Similarly human exercise training studies show dependent changes in neurotransmitter systems, including the dopamine system [10, 14, 21, 63, 77, 113], in response to exercise, and these cause and effect changes are likely due to dopamine's involvement in control of sympathetic nervous activity [99]. In these particular studies dopamine was treated as the dependent variable in response to exercise, or training.
However, some research suggests that not only is dopaminergic functioning altered in response to exercise, but perhaps the dopaminergic system also acts in an independent fashion to regulate physical activity levels. For example, a study in humans using PET imaging showed no changes in dopamine D2 receptor availability in the caudate putamen after treadmill running (submax); however, the subjects used in this study were already persons with a history of regular exercise [175]. It is plausible to assume that one reason no difference was seen from baseline, is that dopamine release in the striatum may not have been the true dependent variable in this methodology. It would be interesting to compare PET imaging of regular exercisers to non-exercisers in the case that dopamine signaling may work in an independent manner in relation to physical activity, and even training in some circumstances. Further support for an independent role of dopamine and physical activity comes from genetic studies linking single nucleotide polymorphisms in the DRD4 [59], and DRD2 genes [149], with physical activity levels in humans. Similarly, aging studies suggest an independent mechanism of action for the dopamine system and regulation of physical activity levels. It is known that a decline in physical activity over the lifespan is most likely due in part to a decline in the functioning of the dopaminergic system [128]. However, as mentioned, studies show that physical activity in the form of exercise can slow the rate of decline in functioning of the dopamine system, and increase quality of life. Thus, the benefits of physical activity on central nervous system functioning suggests that the dopamine system can have both a dependent and independent mechanism of action in regulation of physical activity levels.
It is clear that the dopaminergic system is affected by physical activity (dopaminergic function = dependent variable), and it is plausible that the amount of voluntary physical activity is regulated at least in part by the dopamine system (dopaminergic function = independent variable). The mechanisms behind this correlation are yet to be fully understood.
Dopamine, Reward, and possible implications for Physical Activity Regulation
A full neurobiological discussion of the role of the dopamine system in reinforcement and reward is outside the scope of this review; however, a brief discussion of the reward pathways is necessary to relate the proposed relationship of the dopamine system to regulation of physical activity. In the past several decades it has become increasingly clear from studies in drug addiction that dopaminergic signaling mediates behavioral responses to rewarding stimuli [139]. Rewards, in and of themselves, provide three basic functions including eliciting a behavior, providing reinforcement (or positive feedback so as to increase the frequency or intensity of the behavior), and provision of some type of pleasurable feeling or response [139]. With the context of these three basic functions, it is clear that drugs of abuse are “addictive” because they provide all three functions of a “reward”. It is generally accepted that the dopamine system is implicated in reward and reinforcing mechanisms as evidenced by the results of psychostimulant administration [28, 183]. Specifically, the administration of psychostimulant drugs increases dopamine release and signaling in the mesolimbic areas of the brain, while withdrawal of these drugs causes a decrease in dopamine signaling in these areas and this response appears to be mediated by both D1 and D2 receptors [49, 78]. Studies suggest that D2 receptors are responsible for mediating the self-reinforcing effect of drugs, while the D1 receptors act in a permissive fashion to facilitate the response. Cocaine self-administration studies suggest the D2 receptors are responsible for mediating further motivation to seek cocaine, while the D1 receptors may mediate a reduced drive to seek further cocaine reinforcement [144].
More recent evidence has led researchers to suggest that the dopamine system is specifically involved in the motivational aspect of reward for natural stimuli such as food. Dopamine depletion and dopamine antagonist studies in the nucleus accumbens of animals show that appetite for food is not reduced under these conditions; however, the motivation to engage in effortful tasks for food is significantly reduced [132]. Thus, the dopamine system appears to regulate certain aspects of the “wanting” instead of the “liking” of natural rewards such as food [11]. Drugs of abuse are typically thought of as artificial rewards, while actions such as sexual behavior, food, and/or exercise can be termed “natural rewards.” Traditionally, it has been assumed that drugs of abuse initiate the natural reward system in the brain, mainly the dopamine system, and thus act in a similar fashion as natural rewards. This theory, which is based on the notion that the dopaminergic system mediates the reinforcing properties of natural rewarding stimuli, has been known as the “General Anhedonia Model” [135]. As stated, this theory may not be the entire picture as it appears that the dopamine system may mediate the motivation for natural rewards, and not necessarily the reinforcement mechanism at least in the case of food rewards. Thus, the dopamine system and its role in mediating reward is complex, and the exact mechanisms through which the dopamine system mediates reward signaling to natural rewards such as physical activity is not known. However, it is increasingly clear from genetic studies involving locomotion and wheel running, as well as evidence from reward signaling in response to naturally rewarding behavior that the dopamine system plays a role in the regulation of physical activity in regard to mediating the natural rewarding properties of this behavior. The mechanism for dopaminergic regulation of a complex behavior such as motivation for physical activity is likely to be multifaceted, but as this review highlights, many potential avenues of study including but not limited to dopaminergic interaction with downstream signaling molecules, other neurotransmitters and neuropeptides, and the relative role of genetics vs. environment in this regulation could shed light on this important question.
Proposed Model for Dopaminergic Regulation of Physical Activity
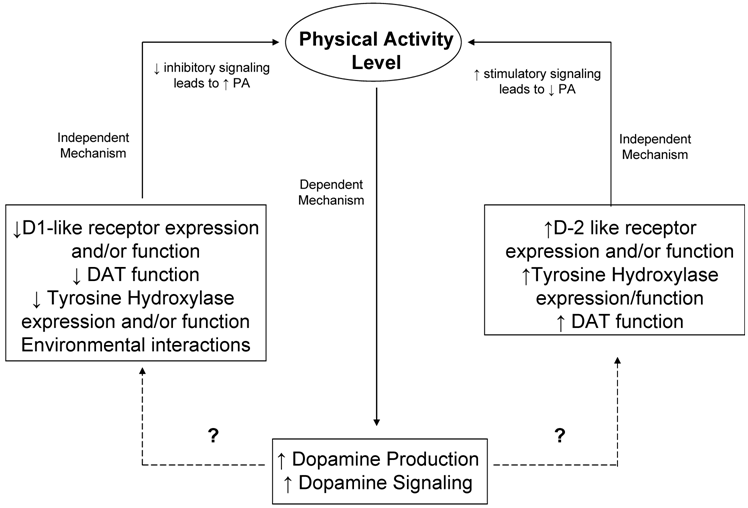
As already outlined in this review, it is well known that exercise induces changes in neurotransmitter systems as well as endorphin release and signaling. These changes typically depend on intensity and duration of exercise. To date, most studies investigating changes in neurotransmitters due to exercise treat the neurotransmitter changes as the dependent variable. Studies involving motor movement and/or locomotion, wheel running, and addiction however, provide evidence for a regulatory role of the dopaminergic system on voluntary physical activity. Furthermore, it is warranted to propose a dual role for the dopamine system in the genetic and biological regulation of physical activity. First, it appears that physical activity in the form of exercise itself and/or training produces beneficial changes in the dopamine system including increased dopamine signaling as well as increased BDNF levels in the brain. In this role, dopamine signaling is acting in a dependent fashion to mediate central changes in response to physical activity. Second, it is also apparent from the growing amount of literature on the role of the dopamine system in motivation for natural rewards, that the dopamine system creates a positively reinforcing condition in which the dopamine system acts in an independent fashion controlling the “wanting” and/or motivation for natural rewarding stimuli such as physical activity. Thus, it is proposed that dopaminergic signaling acts in both a dependent and independent fashion in the regulation of physical activity (proposed schematic outlined in Figure 3).
Going back to the equation mentioned in the first part of this review, any phenotype is affected by both genetic and environmental components, as well as biological interactions:
Phenotype = environment + genetics/biological factor + environment/genetic interaction).
Genetic studies involving dopamine and locomotion outlined in this review provide a solid basis for genetic differences in the dopamine system mediating behavioral differences in regard to physical activity in animals. A metanalysis of genetic alterations which produce increased “locomotor activity” was elegantly described by Viggiano [172] in which he describes the number of genes involved in regulating “hyperactivity” in animals is likely to be numerous, and include those involved in regulation of chatecholamines such as dopamine. Similarly, Dishman [31] reviewed and described a multifaceted approach to understanding the gene/environment interactions that must be understood in order to research the role of inactivity in the development of obesity. Here too, it was suggested that candidate genes be searched for in the area of “motivational systems” of energy expenditure and energy intake [31]. Thus, the current review narrows the focus of these previous reviews to suggest genes and gene/environment interactions within the dopamine system are indeed the major contributor to the regulation of voluntary physical activity levels in animals and humans due to its role in locomotor behavior as well as motivation. Not covered in this review, but still very important, are the biological interactions that may also be playing a role in dopaminergic regulation of physical activity. The dopamine system does not act in isolation, and is affected by interaction with other neurotransmitter systems such as serotonin. Other biological, epigenetic, and/or environmental factors such as hormonal influences may also play an important role in this regulation. A proposed model for this regulation is outlined in Figure 3. The dopamine system appears to be a central component determining the phenotype of voluntary physical activity in that dopaminergic signaling is determined in part by genetics, is also influenced by the environment, and can interact with the environment and other biological components. Thus, it is proposed that the dopamine system acts in a dual role - both dependently and independently to regulate levels of physical activity performed by a given animal. As a result, it is important to take a multifaceted yet systematic and narrowed approach in future research to seek out the underlying mechanisms of this genetic/biological regulation of physical activity in order to improve human health and prevent inactivity related diseases.
Proposed Schematic of the role of dopamine system in the central regulation of physical activity. It is proposed that the dopamine system can act in both an independent and dependent manner in regard to regulation of physical activity. Both genetic factors, and biological factors that interact with the genetic machinery, are important in second messenger signaling, and downstream gene expression changes to dopaminergic neuronal signaling. Likewise, it is also possible that physical activity (i.e. intensity and duration of exercise) can cause changes in neuronal signaling as well, possibly mediating a reinforcing behavioral mechanism. Proposed differential effects on physical activity of D1-like vs. D2-like receptor expression, DAT function, and Tyrosine Hydroxylase function are included. Dashed lines indicate unknown signaling pathways or environmental interactions.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. CDC. Prevalence of no leisure-time physical activity--35 States and the District of Columbia, 1988-2002. MMWR Morb Mortal Wkly Rep. 2004;53:82-86
2. Acosta-Garcia J, Hernandez-Chan N, Paz-Bermudez F, Sierra A, Erlij D, Aceves J, Floran B. D4 and D1 dopamine re-ceptors modulate [(3)H]GABA release in the substantia nigra pars reticulata of the rat. Neuropharmacology. 2009
3. Albin R.L, Young A.B, Penney J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366-375
4. Arnsten A.F. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 67 Suppl. 2006;8:7-12
5. Atchley D.P, Eckel L.A. Treatment with 8-OH-DPAT at-tenuates the weight loss associated with activity-based anorexia in female rats. Pharmacol Biochem Behav. 2006;83:547-553
6. Badgaiyan R.D, Fischman A.J, Alpert N.M. Striatal dopa-mine release in sequential learning. Neuroimage. 2007;38:549-556
7. Bailey A, Yoo J.H, Racz I, Zimmer A, Kitchen I. Prepro-dynorphin mediates locomotion and D2 dopamine and mu-opioid receptor changes induced by chronic 'binge' cocaine administration. J Neurochem. 2007;102:1817-1830
8. Baker H, Joh T.H, Reis D.J. Genetic control of number of midbrain dopaminergic neurons in inbred strains of mice: rela-tionship to size and neuronal density of the striatum. Proc Natl Acad Sci U S A. 1980;77:4369-4373
9. Basterfield L, Reul J.M, Mathers J.C. Impact of physical activity on intestinal cancer development in mice. J Nutr. 2005;135:3002S-3008S
10. Bauer B.A, Rogers P.J, Miller T.D, Bove A.A, Tyce G.M. Exercise training produces changes in free and conjugated catecholamines. Med Sci Sports Exerc. 1989;21:558-562
11. Berridge K.C, Robinson T.E. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive sali-ence? Brain Res Brain Res Rev. 1998;28:309-369
12. Blackburn J.R, Phillips A.G, Jakubovic A, Fibiger H.C. Dopamine and preparatory behavior: II. A neurochemical analysis. Behav Neurosci. 1989;103:15-23
13. Bouten C.V, van Marken Lichtenbelt W.D, Westerterp K.R. Body mass index and daily physical activity in anorexia ner-vosa. Med Sci Sports Exerc. 1996;28:967-973
14. Bove A.A, Dewey J.D, Tyce G.M. Increased conjugated dopamine in plasma after exercise training. J Lab Clin Med. 1984;104:77-85
15. Brene S, Bjornebekk A, Aberg E, Mathe A.A, Olson L, Werme M. Running is rewarding and antidepressive. Physiol Behav. 2007;92:136-140
16. Bronikowski A.M, Rhodes J.S, Garland TJr, Prolla T.A, Awad T.A, Gammie S.C. The evolution of gene expression in mouse hippocampus in response to selective breeding for in-creased locomotor activity. Evolution Int J Org Evolution. 2004;58:2079-2086
17. Buckland P.R, O'Donovan M.C, McGuffin P. Changes in dopamine D1, D2 and D3 receptor mRNA levels in rat brain following antipsychotic treatment. Psychopharmacology (Berl). 1992;106:479-483
18. Callier S, Snapyan M, Le Crom S, Prou D, Vincent J.D, Vernier P. Evolution and cell biology of dopamine receptors in vertebrates. Biol Cell. 2003;95:489-502
19. Casper R.C. The 'drive for activity' and "restlessness" in ano-rexia nervosa: potential pathways. J Affect Disord. 2006;92:99-107
20. Centonze D, Bracci E, Pisani A, Gubellini P, Bernardi G, Calabresi P. Activation of dopamine D1-like receptors excites LTS interneurons of the striatum. Eur J Neurosci. 2002;15:2049-2052
21. Christensen N.J, Mathias C.J, Frankel H.L. Plasma and urinary dopamine: studies during fasting and exercise and in tetraplegic man. Eur J Clin Invest. 1976;6:403-409
22. Connelly J.B, Duaso M.J, Butler G. A systematic review of controlled trials of interventions to prevent childhood obesity and overweight: a realistic synthesis of the evidence. Public Health. 2007;121:510-517
23. Cotman C.W, Engesser-Cesar C. Exercise enhances and protects brain function. Exerc Sport Sci Rev. 2002;30:75-79
24. Cousins M.S, Atherton A, Turner L, Salamone J.D. Nu-cleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res. 1996;74:189-197
25. Cousins M.S, Sokolowski J.D, Salamone J.D. Different effects of nucleus accumbens and ventrolateral striatal dopa-mine depletions on instrumental response selection in the rat. Pharmacol Biochem Behav. 1993;46:943-951
26. Davis C, Woodside D.B. Sensitivity to the rewarding effects of food and exercise in the eating disorders. Compr Psychiatry. 2002;43:189-194
27. Dearry A, Gingrich J.A, Falardeau P, Fremeau RTJr, Bates M.D, Caron M.G. Molecular cloning and expression of the gene for a human D1 dopamine receptor. Nature. 1990;347:72-76
28. Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38:95-137
29. Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn't do. Curr Opin Pharmacol. 2007;7:69-76
30. Dishman R.K. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc. 1997;29:63-74
31. Dishman R.K. Gene-physical activity interactions in the etiol-ogy of obesity: behavioral considerations. Obesity (Silver Spring). 16 Suppl. 2008;3:S60-65
32. Dishman R.K, Berthoud H.R, Booth F.W, Cotman C.W, Edgerton V.R, Fleshner M.R, Gandevia S.C, Gomez-Pinilla F, Greenwood B.N, Hillman C.H, Kramer A.F, Levin B.E, Moran T.H, Russo-Neustadt A.A, Salamone J.D, Van Hoo-missen J.D, Wade C.E, York D.A, Zigmond M.J. Neuro-biology of exercise. Obesity (Silver Spring). 2006;14:345-356
33. Doherty T.J. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95:1717-1727
34. Duman C.H, Schlesinger L, Russell D.S, Duman R.S. Voluntary exercise produces antidepressant and anxiolytic be-havioral effects in mice. Brain Res. 2008;1199:148-158
35. Dumke C.L, Rhodes J.S, Garland TJr, Maslowski E, Swallow J.G, Wetter A.C, Cartee G.D. Genetic selection of mice for high voluntary wheel running: effect on skeletal muscle glucose uptake. J Appl Physiol. 2001;91:1289-1297
36. Dziedzicka-Wasylewska M. Brain dopamine recep-tors--research perspectives and potential sites of regulation. Pol J Pharmacol. 2004;56:659-671
37. Eikelboom R. Human parallel to voluntary wheel running: exercise. Anim Behav. 1999;57:F11-F12
38. Ernst C, Olson A.K, Pinel J.P, Lam R.W, Christie B.R. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J Psychiatry Neurosci. 2006;31:84-92
39. Esposito E, Di Matteo V, Di Giovanni G. Sero-tonin-dopamine interaction: an overview. Prog Brain Res. 2008;172:3-6
40. Faraone S.V, Perlis R.H, Doyle A.E, Smoller J.W, Goralnick J.J, Holmgren M.A, Sklar P. Molecular genetics of atten-tion-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313-1323
41. Ferris L.T, Williams J.S, Shen C.L. The effect of acute exer-cise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39:728-734
42. Festing M.F. Wheel activity in 26 strains of mouse. Lab Anim. 1977;11:257-258
43. Fink J.S, Reis D.J. Genetic variations in midbrain dopamine cell number: parallel with differences in responses to dopa-minergic agonists and in naturalistic behaviors mediated by central dopaminergic systems. Brain Res. 1981;222:335-349
44. Fisher B.E, Wu A.D, Salem G.J, Song J, Lin C.H, Yip J, Cen S, Gordon J, Jakowec M, Petzinger G. The effect of exercise training in improving motor performance and corticomotor ex-citability in people with early Parkinson's disease. Arch Phys Med Rehabil. 2008;89:1221-1229
45. Foley T.E, Fleshner M. Neuroplasticity of dopamine cir-cuits after exercise: implications for central fatigue. Neuro-molecular Med. 2008;10:67-80
46. Foley T.E, Greenwood B.N, Day H.E, Koch L.G, Britton S.L, Fleshner M. Elevated central monoamine receptor mRNA in rats bred for high endurance capacity: implications for cen-tral fatigue. Behav Brain Res. 2006;174:132-142
47. Frank G.K, Bailer U.F, Henry S.E, Drevets W, Meltzer C.C, Price J.C, Mathis C.A, Wagner A, Hoge J, Ziolko S, Bar-barich-Marsteller N, Weissfeld L, Kaye W.H. Increased dopamine D2/D3 receptor binding after recovery from ano-rexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry. 2005;58:908-912
48. Frankel P.S, Alburges M.E, Bush L, Hanson G.R, Kish S.J. Striatal and ventral pallidum dynorphin concentrations are markedly increased in human chronic cocaine users. Neuro-pharmacology. 2008;55:41-46
49. Franklin K.B, Vaccarino F.J. Differential effects of am-phetamine isomers on SN self-stimulation: evidence for DA neuron subtypes. Pharmacol Biochem Behav. 1983;18:747-751
50. Friedel S, Saar K, Sauer S, Dempfle A, Walitza S, Renner T, Romanos M, Freitag C, Seitz C, Palmason H, Scherag A, Windemuth-Kieselbach C, Schimmelmann B.G, Wewetzer C, Meyer J, Warnke A, Lesch K.P, Reinhardt R, Her-pertz-Dahlmann B, Linder M, Hinney A, Remschmidt H, Schafer H, Konrad K, Hubner N, Hebebrand J. Associa-tion and linkage of allelic variants of the dopamine transporter gene in ADHD. Mol Psychiatry. 2007;12:923-933
51. Garland TJr, Kelly S.A. Phenotypic plasticity and experi-mental evolution. J Exp Biol. 2006;209:2344-2361
52. Gelegen C, van den Heuvel J, Collier D.A, Campbell I.C, Oppelaar H, Hessel E, Kas M.J. Dopaminergic and brain-derived neurotrophic factor signalling in inbred mice exposed to a restricted feeding schedule. Genes Brain Behav. 2008;7:552-559
53. George S.R, Fan T, Ng G.Y, Jung S.Y, O'Dowd B.F, Na-ranjo C.A. Low endogenous dopamine function in brain pre-disposes to high alcohol preference and consumption: reversal by increasing synaptic dopamine. J Pharmacol Exp Ther. 1995;273:373-379
54. Gerfen C.R, Engber T.M, Mahan L.C, Susel Z, Chase T.N, Monsma F.JJr, Sibley D.R. D1 and D2 dopamine recep-tor-regulated gene expression of striatonigral and striatopalli-dal neurons. Science. 1990;250:1429-1432
55. Giros B, Jaber M, Jones S.R, Wightman R.M, Caron M.G. Hyperlocomotion and indifference to cocaine and ampheta-mine in mice lacking the dopamine transporter. Nature. 1996;379:606-612
56. Goerendt I.K, Messa C, Lawrence A.D, Grasby P.M, Piccini P, Brooks D.J. Dopamine release during sequential finger movements in health and Parkinson's disease: a PET study. Brain. 2003;126:312-325
57. Gonzalez-Hernandez T, Rodriguez M. Compartmental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. J Comp Neurol. 2000;421:107-135
58. Graybiel A.M, Moratalla R, Robertson H.A. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990;87:6912-6916
59. Guo G, North K.E, Gorden-Larsen P, Bulik C.M, Choi S. Body mass, DRD4, physical activity, sedentary behavior, and family socioeconomic status: the add health study. Obesity (Silver Spring). 2007;15:1199-1206
60. Haber S.N, Fudge J.L, McFarland N.R. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369-2382
61. Haile C.N, Kosten T.R, Kosten T.A. Genetics of dopamine and its contribution to cocaine addiction. Behav Genet. 2007;37:119-145
62. Hamre K, Tharp R, Poon K, Xiong X, Smeyne R.J. Dif-ferential strain susceptibility following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) admini-stration acts in an autosomal dominant fashion: quantitative analysis in seven strains of Mus musculus. Brain Res. 1999;828:91-103
63. Hartling O.J, Kelbaek H, Gjorup T, Nielsen M.D, Trap-Jensen J. Plasma concentrations of adrenaline, noradrena-line and dopamine during forearm dynamic exercise. Clin Physiol. 1989;9:399-404
64. Henze D.A, Gonzalez-Burgos G.R, Urban N.N, Lewis D.A, Barrionuevo G. Dopamine increases excitability of py-ramidal neurons in primate prefrontal cortex. J Neurophysiol. 2000;84:2799-2809
65. Hiroi N, Martin A.B, Grande C, Alberti I, Rivera A, Moratalla R. Molecular dissection of dopamine receptor sig-naling. J Chem Neuroanat. 2002;23:237-242
66. Hope B, Kosofsky B, Hyman S.E, Nestler E.J. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A. 1992;89:5764-5768
67. Hou X.Y, Zhang G.Y. Inhibitory effect of dopamine on Ca(2+)-calmodulin-dependent protein kinase II activity in rat hippocampal slices. Zhongguo Yao Li Xue Bao. 1999;20:902-906
68. Hurley M.J, Jenner P. What has been learnt from study of dopamine receptors in Parkinson's disease? Pharmacol Ther. 2006;111:715-728
69. Jaber M, Cador M, Dumartin B, Normand E, Stinus L, Bloch B. Acute and chronic amphetamine treatments differently regulate neuropeptide messenger levels RNA and Fos immu-noreactivity in rat striatal neurons. Neuroscience. 1995;65:1041-1050
70. Jackson D.M, Westlind-Danielsson A. Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol Ther. 1994;64:291-370
71. Jacobs B.W, Isaacs S. Pre-pubertal anorexia nervosa: a retrospective controlled study. J Child Psychol Psychiatry. 1986;27:237-250
72. Johnson R.A, Rhodes J.S, Jeffrey S.L, Garland TJr, Mitchell G.S. Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience. 2003;121:1-7
73. Kaprio J, Koskenvuo M, Sarna S. Cigarette smoking, use of alcohol, and leisure-time physical activity among same-sexed adult male twins. Prog Clin Biol Res. 69 Pt C: 37-46. 1981
74. Kelly S.A, Czech P.P, Wight J.T, Blank K.M, Garland T. Jr. Experimental evolution and phenotypic plasticity of hindlimb bones in high-activity house mice. J Morphol. 2006;267:360-374
75. Knab A.M, Bowen R.S, Hamilton A.T, Gulledge A.A, Lightfoot J.T. Altered dopaminergic profiles: implications for the regulation of voluntary physical activity. Behav Brain Res. 2009;204:147-152
76. Knab A.M, Bowen R.S, Hamilton A.T, Lightfoot J.T. Pharmacological manipulation of the dopaminergic system af-fects wheel running activity in differentially active mice. Behav Brain Res. 2009 submitted
77. Koch G, Johansson U, Arvidsson E. Radioenzymatic de-termination of epinephrine, norepinephrine and dopamine in 0.1 ml plasma samples: plasma catecholamine response to submaximal and near maximal exercise. J Clin Chem Clin Bio-chem. 1980;18:367-372
78. Kornetsky C, Esposito R.U. Reward and detection thresh-olds for brain stimulation: dissociative effects of cocaine. Brain Res. 1981;209:496-500
79. Korotkova T.M, Brown R.E, Sergeeva O.A, Ponomarenko A.A, Haas H.L. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677-2685
80. Kurtais Y, Kutlay S, Tur B.S, Gok H, Akbostanci C. Does treadmill training improve lower-extremity tasks in Parkinson disease? A randomized controlled trial. Clin J Sport Med. 2008;18:289-291
81. Lappin J.M, Reeves S.J, Mehta M.A, Egerton A, Coulson M, Grasby P.M. Dopamine release in the human striatum: motor and cognitive tasks revisited. J Cereb Blood Flow Metab. 2009;29:554-564
82. Lauderdale D.S, Fabsitz R, Meyer J.M, Sholinsky P, Rama-krishnan V, Goldberg J. Familial determinants of moderate and intense physical activity: a twin study. Med Sci Sports Ex-erc. 1997;29:1062-1068
83. Le Moine C, Normand E, Guitteny A.F, Fouque B, Teoule R, Bloch B. Dopamine receptor gene expression by enkephalin neurons in rat forebrain. Proc Natl Acad Sci U S A. 1990;87:230-234
84. Leamy L.J, Pomp D, Lightfoot J.T. An epistatic genetic basis for physical activity traits in mice. J Hered. 2008;99:639-646
85. Lee D, Huang W, Lim A.T. Dopamine induces a biphasic modulation of hypothalamic ANF neurons: a ligand concentra-tion-dependent effect involving D5 and D2 receptor interaction. Mol Psychiatry. 2000;5:39-48
86. Leng A, Mura A, Hengerer B, Feldon J, Ferger B. Effects of blocking the dopamine biosynthesis and of neurotoxic do-pamine depletion with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on vol-untary wheel running in mice. Behav Brain Res. 2004;154:375-383
87. Lerman I, Harrison B.C, Freeman K, Hewett T.E, Allen D.L, Robbins J, Leinwand L.A. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. Journal of Applied Physiology. 2002;92:2245-2255
88. Levy F. What do dopamine transporter and cate-chol-o-methyltransferase tell us about attention defi-cit-hyperactivity disorder? Pharmacogenomic implications. Aust N Z J Psychiatry. 2007;41:10-16
89. Lewis B.L, O'Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential 'up' states in pyramidal neurons via D(1) dopamine receptors. Cereb Cortex. 2000;10:1168-1175
90. Li D, Sham P.C, Owen M.J, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Hum Mol Genet. 2006;15:2276-2284
91. Lightfoot J.T, Turner M.J, Daves M, Vordermark A, Kleeberger S.R. Genetic influence on daily wheel running ac-tivity level. Physiol Genomics. 2004;19:270-276
92. Lightfoot J.T, Turner M.J, Pomp D, Kleeberger S.R, Leamy L.J. Quantitative trait loci for physical activity traits in mice. Physiol Genomics. 2008;32:401-408
93. Liste I, Guerra M.J, Caruncho H.J, Labandeira-Garcia J.L. Treadmill running induces striatal Fos expression via glutamate NMDA and dopamine receptors. Exp Brain Res. 1997;115:458-468
94. Logroscino G, Sesso H.D, Paffenbarger R.SJr, Lee I.M. Physical activity and risk of Parkinson's disease: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2006;77:1318-1322
95. Luo Y, Kokkonen G.C, Hattori A, Chrest F.J, Roth G.S. Dopamine stimulates redox-tyrosine kinase signaling and p38 MAPK in activation of astrocytic C6-D2L cells. Brain Res. 1999;850:21-38
96. Ma Q. Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neurosci Bull. 2008;24:265-270
97. MacRae P.G, Spirduso W.W, Walters T.J, Farrar R.P, Wilcox R.E. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolites in prese-nescent older rats. Psychopharmacology (Berl). 1987;92:236-240
98. Madras B.K, Miller G.M, Fischman A.J. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1397-1409
99. Mannelli M, Ianni L, Lazzeri C, Castellani W, Pupilli C, La Villa G, Barletta G, Serio M, Franchi F. In vivo evidence that endogenous dopamine modulates sympathetic activity in man. Hypertension. 1999;34:398-402
100. McNamara R.K, Levant B, Taylor B, Ahlbrand R, Liu Y, Sul-livan J.R, Stanford K, Richtand N.M. C57BL/6J mice ex-hibit reduced dopamine D3 receptor-mediated locomo-tor-inhibitory function relative to DBA/2J mice. Neuroscience. 2006;143:141-153
101. Meeusen Exercise R and the brain. insight in new therapeutic modalities. Ann Transplant. 2005;10:49-51
102. Melis M.R, Argiolas A. Dopamine and sexual behavior. Neurosci Biobehav Rev. 1995;19:19-38
103. Michaluk J, Antkiewicz-Michaluk L, Rokosz-Pelc A, Sansone M, Oliverio A, Vetulani J. Dopamine receptors in the striatum and limbic system of various strains of mice: relation to differences in responses to apomorphine. Pharmacol Bio-chem Behav. 1982;17:1115-1118
104. Mill J, Caspi A, Williams B.S, Craig I, Taylor A, Polo-Tomas M, Berridge C.W, Poulton R, Moffitt T.E. Prediction of heterogeneity in intelligence and adult prognosis by genetic polymorphisms in the dopamine system among children with attention-deficit/hyperactivity disorder: evidence from 2 birth cohorts. Arch Gen Psychiatry. 2006;63:462-469
105. Missale C, Nash S.R, Robinson S.W, Jaber M, Caron M.G. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189-225
106. Nestler E.J, Barrot M, Self D.W. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98:11042-11046
107. Neve K.A, Seamans J.K, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165-205
108. Ng G.Y, O'Dowd B.F, George S.R. Genotypic differences in brain dopamine receptor function in the DBA/2J and C57BL/6J inbred mouse strains. Eur J Pharmacol. 1994;269:349-364
109. Nguyen T.V, Kosofsky B.E, Birnbaum R, Cohen B.M, Hyman S.E. Differential expression of c-fos and zif268 in rat striatum after haloperidol, clozapine, and amphetamine. Proc Natl Acad Sci U S A. 1992;89:4270-4274
110. Ouchi Y, Yoshikawa E, Futatsubashi M, Okada H, Torizuka T, Sakamoto M. Effect of simple motor performance on regional dopamine release in the striatum in Parkinson disease patients and healthy subjects: a positron emission tomography study. J Cereb Blood Flow Metab. 2002;22:746-752
111. Owesson-White C.A, Cheer J.F, Beyene M, Carelli R.M, Wightman R.M. Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc Natl Acad Sci U S A. 2008;105:11957-11962
112. Palmiter R.D. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375-381
113. Peronnet F, Cleroux J, Perrault H, Thibault G, Cousineau D, de Champlain J, Guilland J.C, Klepping J. Plasma nore-pinephrine, epinephrine, and dopamine beta-hydroxylase activity during exercise in man. Med Sci Sports Exerc. 1985;17:683-688
114. Perusse L, Tremblay A, Leblanc C, Bouchard C. Genetic and environmental influences on level of habitual physical ac-tivity and exercise participation. Am J Epidemiol. 1989;129:1012-1022
115. Pfaus J.G, Damsma G, Nomikos G.G, Wenkstern D.G, Blaha C.D, Phillips A.G, Fibiger H.C. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530:345-348
116. Pierce R.C, Kumaresan V. The mesolimbic dopamine sys-tem: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215-238
117. Pirke K.M, Broocks A, Wilckens T, Marquard R, Schweiger U. Starvation-induced hyperactivity in the rat: the role of endocrine and neurotransmitter changes. Neurosci Biobehav Rev. 1993;17:287-294
118. Powell K.E, Blair S.N. The public health burdens of seden-tary living habits: theoretical but realistic estimates. Med Sci Sports Exerc. 1994;26:851-856
119. Rezende E.L, Gomes F.R, Malisch J.L, Chappell M.A, Garland T. Jr. Maximal oxygen consumption in relation to subordinate traits in lines of house mice selectively bred for high voluntary wheel running. J Appl Physiol. 2006;101:477-485
120. Rezende E.L, Kelly S.A, Gomes F.R, Chappell M.A, Gar-land T. Jr. Effects of size, sex, and voluntary running speeds on costs of locomotion in lines of laboratory mice selectively bred for high wheel-running activity. Physiol Biochem Zool. 2006;79:83-99
121. Rhodes J.S, Gammie S.C, Garland T. Neurobiology of Mice Selected for High Voluntary Wheel-running Activity. In-tegr. Comp. Biol. 2005;45:438-455
122. Rhodes J.S, Garland T. Differential sensitivity to acute administration of Ritalin, apomorphine, 23390 SCH, but not ra-clopride in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology (Berl). 2003;167:242-250
123. Rhodes J.S, Garland TJr, Gammie S.C. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav Neurosci. 2003;117:1243-1256
124. Rhodes J.S, Hosack G.R, Girard I, Kelley A.E, Mitchell G.S, Garland T. Jr. Differential sensitivity to acute administra-tion of cocaine, 12909 GBR, and fluoxetine in mice selectively bred for hyperactive wheel-running behavior. Psychopharma-cology (Berl). 2001;158:120-131
125. Roberson E.D, English J.D, Adams J.P, Selcher J.C, Kondratick C, Sweatt J.D. The mitogen-activated protein kinase cas-cade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neu-rosci. 1999;19:4337-4348
126. Robertson G.S, Fibiger H.C. Neuroleptics increase c-fos expression in the forebrain: contrasting effects of haloperidol and clozapine. Neuroscience. 1992;46:315-328
127. Robertson G.S, Vincent S.R, Fibiger H.C. D1 and D2 do-pamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992;49:285-296
128. Roth G.S, Joseph J.A. Cellular and molecular mechanisms of impaired dopaminergic function during aging. Ann N Y Acad Sci. 1994;719:129-135
129. Rozas G, Lopez-Martin E, Guerra M.J, Labandeira-Garcia J.L. The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J Neurosci Methods. 1998;83:165-175
130. Salamone J.D. Behavioral pharmacology of dopamine systems: a new synthesis. In: (ed.) Scheel-Kruger PW. The Mesolimbic Dopamine System: from Motivation to Action. Cambridge: Campbridge University Press. 1991:598-613
131. Salamone J.D. Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes. Psychopharmacology (Berl). 1992;107:160-174
132. Salamone J.D, Correa M. Motivational views of reinforce-ment: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3-25
133. Salamone J.D, Cousins M.S, Bucher S. Anhedonia or aner-gia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221-229
134. Salamone J.D, Cousins M.S, McCullough L.D, Carriero D.L, Berkowitz R.J. Nucleus accumbens dopamine release in-creases during instrumental lever pressing for food but not free food consumption. Pharmacol Biochem Behav. 1994;49:25-31
135. Salamone J.D, Cousins M.S, Snyder B.J. Behavioral func-tions of nucleus accumbens dopamine: empirical and concep-tual problems with the anhedonia hypothesis. Neurosci Biobe-hav Rev. 1997;21:341-359
136. Salamone J.D, Keller R.W, Zigmond M.J, Stricker E.M. Behavioral activation in rats increases striatal dopamine me-tabolism measured by dialysis perfusion. Brain Res. 1989;487:215-224
137. Sanz A.G, Badia A, Clos M.V. Role of calcium on the modulation of spontaneous acetylcholine efflux by the D2 do-pamine receptor subtype in rat striatal synaptosomes. Brain Res. 2000;854:42-47
138. Schrauwen P, Westerterp K.R. The role of high-fat diets and physical activity in the regulation of body weight. Br J Nutr. 2000;84:417-427
139. Schultz W. Reward signaling by dopamine neurons. Neuro-scientist. 2001;7:293-302
140. Schumacher H.E, Oehler J, Jaehkel M. Individual motor activity--relationships to dopaminergic responses. Pharmacol Biochem Behav. 1994;48:839-844
141. Seale T.W, McLanahan K, Johnson P, Carney J.M, Rennert O.M. Systematic comparison of apomorphine-induced behav-ioral changes in two mouse strains with inherited differences in brain dopamine receptors. Pharmacol Biochem Behav. 1984;21:237-244
142. Sedelis M, Hofele K, Auburger G.W, Morgan S, Huston J.P, Schwarting R.K. MPTP susceptibility in the mouse: be-havioral, neurochemical, and histological analysis of gender and strain differences. Behav Genet. 2000;30:171-182
143. Seeman P, Tallerico T. Link between dopamine D1 and D2 receptors in rat and human striatal tissues. Synapse. 2003;47:250-254
144. Self D.W, Barnhart W.J, Lehman D.A, Nestler E.J. Oppo-site modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586-1589
145. Sesack S.R, Carr D.B, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36-52
146. Severson J.A, Randall P.K, Finch C.E. Genotypic influences on striatal dopaminergic regulation in mice. Brain Res. 1981;210:201-215
147. Sevy S, Hassoun Y, Bechara A, Yechiam E, Napolitano B, Burdick K, Delman H, Malhotra A. Emotion-based deci-sion-making in healthy subjects: short-term effects of reducing dopamine levels. Psychopharmacology (Berl). 2006;188:228-235
148. Sherwin C.M. Voluntary wheel running: a review and novel interpretation. Anim Behav. 1998;56:11-27
149. Simonen R.L, Rankinen T, Perusse L, Leon A.S, Skinner J.S, Wilmore J.H, Rao D.C, Bouchard C. A dopamine D2 re-ceptor gene polymorphism and physical activity in two family studies. Physiol Behav. 2003;78:751-757
150. Simpson C.S, Morris B.J. Induction of c-fos and zif/268 gene expression in rat striatal neurons, following stimulation of D1-like dopamine receptors, involves protein kinase A and protein kinase C. Neuroscience. 1995;68:97-106
151. Singh-Manoux A, Hillsdon M, Brunner E, Marmot M. Effects of physical activity on cognitive functioning in middle age: evidence from the Whitehall II prospective cohort study. Am J Public Health. 2005;95:2252-2258
152. Smith K.S, Tindell A.J, Aldridge J.W, Berridge K.C. Ven-tral pallidum roles in reward and motivation. Behav Brain Res. 2009;196:155-167
153. Smythies J. Section II. The dopamine system. Int Rev Neurobiol. 2005;64:123-172
154. Speakman J.R, Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc. 2003;62:621-634
155. Spielewoy C, Biala G, Roubert C, Hamon M, Betancur C, Giros B. Hypolocomotor effects of acute and daily d-amphetamine in mice lacking the dopamine transporter. Psychopharmacology (Berl). 2001;159:2-9
156. Staller J.A, Faraone S.V. Targeting the dopamine system in the treatment of attention-deficit/hyperactivity disorder. Ex-pert Rev Neurother. 2007;7:351-362
157. Steiner H, Gerfen C.R. Dynorphin regulates D1 dopamine receptor-mediated responses in the striatum: relative contribu-tions of pre- and postsynaptic mechanisms in dorsal and ven-tral striatum demonstrated by altered immediate-early gene induction. J Comp Neurol. 1996;376:530-541
158. Steiner H, Gerfen C.R. Enkephalin regulates acute D2 do-pamine receptor antagonist-induced immediate-early gene ex-pression in striatal neurons. Neuroscience. 1999;88:795-810
159. Stubbe J.H, Boomsma D.I, De Geus E.J. Sports participa-tion during adolescence: a shift from environmental to genetic factors. Med Sci Sports Exerc. 2005;37:563-570
160. Sved A.F, Baker H.A, Reis D.J. Dopamine synthesis in inbred mouse strains which differ in numbers of dopamine neurons. Brain Res. 1984;303:261-266
161. Swallow J.G, Carter P.A, Garland T. Jr. Artificial selection for increased wheel-running behavior in house mice. Behav Genet. 1998;28:227-237
162. Swallow J.G, Garland TJr, Carter P.A, Zhan W.Z, Sieck G.C. Effects of voluntary activity and genetic selection on aero-bic capacity in house mice (Mus domesticus). J Appl Physiol. 1998;84:69-76
163. Swallow J.G, Koteja P, Carter P.A, Garland TJr. Food consumption and body composition in mice selected for high wheel-running activity. J Comp Physiol [B]. 2001;171:651-659
164. Thacker E.L, Chen H, Patel A.V, McCullough M.L, Calle E.E, Thun M.J, Schwarzschild M.A, Ascherio A. Recreational physical activity and risk of Parkinson's disease. Mov Disord. 2008;23:69-74
165. Turner M.J, Kleeberger S.R, Lightfoot J.T. Influence of genetic background on daily running-wheel activity differs with aging. Physiol Genomics. 2005;22:76-85
166. Uhl G.R. Dopamine transporter: basic science and human variation of a key molecule for dopaminergic function, loco-motion, and parkinsonism. Mov Disord. 18 Suppl. 2003;7:S71-80
167. Vaanholt L.M, Meerlo P, Garland TJr, Visser G.H, van Dijk G. Plasma adiponectin is increased in mice selectively bred for high wheel-running activity, but not by wheel running per se. Horm Metab Res. 2007;39:377-383
168. Vaanholt L.M, Speakman J.R, Garland TJr, Lobley G.E, Visser G.H. Protein synthesis and antioxidant capacity in aging mice: effects of long-term voluntary exercise. Physiol Biochem Zool. 2008;81:148-157
169. Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000;24:125-132
170. van der Kooij M.A, Glennon J.C. Animal models concern-ing the role of dopamine in attention-deficit hyperactivity dis-order. Neurosci Biobehav Rev. 2007;31:597-618
171. Vetulani J. Drug addiction. Part II. Neurobiology of addiction. Pol J Pharmacol. 2001;53:303-317
172. Viggiano D. The hyperactive syndrome: metanalysis of genetic alterations, pharmacological treatments and brain lesions which increase locomotor activity. Behav Brain Res. 2008;194:1-14
173. Volkow N.D, Fowler J.S, Wang G.J. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol. 1999;13:337-345
174. Volkow N.D, Wang G.J, Fowler J.S, Logan J, Gatley S.J, Gif-ford A, Hitzemann R, Ding Y.S, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440-1443
175. Wang G.J, Volkow N.D, Fowler J.S, Franceschi D, Logan J, Pappas N.R, Wong C.T, Netusil N. PET studies of the ef-fects of aerobic exercise on human striatal dopamine release. J Nucl Med. 2000;41:1352-1356
176. Waters R.P, Emerson A.J, Watt M.J, Forster G.L, Swallow J.G, Summers C.H. Stress induces rapid changes in central catecholaminergic activity in Anolis carolinensis: restraint and forced physical activity. Brain Res Bull. 2005;67:210-218
177. Waters R.P, Renner K.J, Pringle R.B, Summers C.H, Britton S.L, Koch L.G, Swallow J.G. Selection for aerobic capacity affects corticosterone, monoamines and wheel-running activity. Physiol Behav. 2008;93:1044-1054
178. Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler E.J, Brene S. Delta FosB regulates wheel running. J Neu-rosci. 2002;22:8133-8138
179. Wilens T.E. Drug therapy for adults with attention-deficit hy-peractivity disorder. Drugs. 2003;63:2395-2411
180. Wilens T.E. Effects of methylphenidate on the catecholaminer-gic system in attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2008;28:S46-53
181. Wilens T.E, Klint T, Adler L, West S, Wesnes K, Graff O, Mikkelsen B. A randomized controlled trial of a novel mixed monoamine reuptake inhibitor in adults with ADHD. Behav Brain Funct. 2008;4:24
182. Winter B, Breitenstein C, Mooren F.C, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S. High impact running improves learning. Neurobiol Learn Mem. 2007;87:597-609
183. Wise R.A. Dopamine, learning and motivation. Nat Rev Neu-rosci. 2004;5:483-494
184. Wyatt S.B, Winters K.P, Dubbert P.M. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci. 2006;331:166-174
185. Zhang J, Goodlett D.R. Proteomic approach to studying Parkinson's disease. Mol Neurobiol. 2004;29:271-288
Author contact
![]() Corresponding author: Amy Knab, knabamedu, (704) 250-5352, Address: Appalachian State University, Human Performance Laboratory, Plants for Human Health Institute, North Carolina Research Campus, 600 Laureate Way, Kannapolis, NC 28081
Corresponding author: Amy Knab, knabamedu, (704) 250-5352, Address: Appalachian State University, Human Performance Laboratory, Plants for Human Health Institute, North Carolina Research Campus, 600 Laureate Way, Kannapolis, NC 28081

 Global reach, higher impact
Global reach, higher impact