10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2011; 7(2):244-252. doi:10.7150/ijbs.7.244 This issue Cite
Research Paper
Glibenclamide or Metformin Combined with Honey Improves Glycemic Control in Streptozotocin-Induced Diabetic Rats
1. Department of Pharmacology, School of Medical Sciences, Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia.
2. Department of Chemical Pathology, School of Medical Sciences, Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia.
3. Department of Pathology, School of Medical Sciences, Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia.
4. School of Medicine and Health Sciences, Monash University Sunway Campus, Jalan Lagoon Selatan, 46150, Bandar Sunway, Selangor, Malaysia.
Received 2010-5-13; Accepted 2010-10-2; Published 2011-3-14
Abstract
Diabetes mellitus is associated with deterioration of glycemic control and progressive metabolic derangements. This study investigated the effect of honey as an adjunct to glibenclamide or metformin on glycemic control in streptozotocin-induced diabetic rats. Diabetes was induced in rats by streptozotocin. The diabetic rats were randomized into six groups and administered distilled water, honey, glibenclamide, glibenclamide and honey, metformin or metformin and honey. The animals were treated orally once daily for four weeks. The diabetic control rats showed hypoinsulinemia (0.27 ± 0.01 ng/ml), hyperglycemia (22.4 ± 1.0 mmol/L) and increased fructosamine (360.0 ± 15.6 µmol/L). Honey significantly increased insulin (0.41 ± 0.06 ng/ml), decreased hyperglycemia (12.3 ± 3.1 mmol/L) and fructosamine (304.5 ± 10.1 µmol/L). Although glibenclamide or metformin alone significantly (p < 0.05) reduced hyperglycemia, glibenclamide or metformin combined with honey produced significantly much lower blood glucose (8.8 ± 2.9 or 9.9 ± 3.3 mmol/L, respectively) compared to glibenclamide or metformin alone (13.9 ± 3.4 or 13.2 ± 2.9 mmol/L, respectively). Similarly, glibenclamide or metformin combined with honey produced significantly (p < 0.05) lower fructosamine levels (301.3 ± 19.5 or 285.8 ± 22.6 µmol/L, respectively) whereas glibenclamide or metformin alone did not decrease fructosamine (330.0 ± 29.9 or 314.6 ± 17.9 µmol/L, respectively). Besides, these drugs or their combination with honey increased insulin levels. Glibenclamide or metformin combined with honey also significantly reduced the elevated levels of creatinine, bilirubin, triglycerides, and VLDL cholesterol. These results indicate that combination of glibenclamide or metformin with honey improves glycemic control, and provides additional metabolic benefits, not achieved with either glibenclamide or metformin alone.
Keywords: Diabetes mellitus, streptozotocin, fructosamine, glibenclamide, metformin, tualang honey
Introduction
An intensive treatment of hyperglycemia is known to lower the risks of microvascular complications such as retinopathy, nephropathy, and neuropathy as well as the incidence of cardiovascular events in both type 1 and type 2 diabetic patients [1, 2]. Unfortunately, many diabetics hardly achieve and/or maintain the recommended 7% glycated hemoglobin (HbA1c) goal for blood glucose control and are therefore exposed to increasing risks of diabetic complications as glycemic control deteriorates over time [3]. Studies have shown that diabetes mellitus is a progressive disorder which cannot be effectively managed with drug monotherapy [4-5]. Regardless of drug management, the pancreatic β-cells in type 2 diabetic patients continue to deteriorate leading to worsening glycemic control and consequent requirement for multiple therapies or exogenous insulin [6].
Increased generation of reactive oxygen species (ROS) resulting from metabolism of excessive glucose and/or free fatty acid has been identified as a contributor to the deterioration of pancreatic β-cell function [7, 8]. Due to the relatively low expression of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase in the pancreas [9], the pancreatic β-cells may be highly vulnerable to damage when exposed to oxidative stress. Thus, oxidative stress may play a major role in β-cell deterioration frequently observed in type 2 diabetes [10].
Antioxidants have been suggested to afford protection to the pancreas against oxidative stress in diabetes mellitus [11]. We had reported earlier that, in experimental rat models of diabetes mellitus, Malaysian tualang honey exhibited glucose lowering effect and ameliorated oxidative stress in the kidney and pancreas [12, 13] and that protection of the pancreas against oxidative stress might contribute to its hypoglycemic effect. In the present study, we have investigated how the addition of tualang honey as an adjunct to glibenclamide or metformin affects glycemic control in streptozotocin-induced diabetic rats.
Materials and Methods
Animals
The use of rats and all the experimental procedures were approved by the Animal Ethics Committee of Universiti Sains Malaysia and were performed in accordance with the Institutional Guidelines for the Care and Use of Animals for Scientific Purposes and in accordance with the Recommendations from Helsinki Declaration. A total of sixty male Sprague-Dawley rats aged 12-14 weeks were obtained from the Laboratory Animal Research Unit of Universiti Sains Malaysia, Health Campus, Kelantan, Malaysia. The animals were acclimatized to the animal room condition for at least a week at 25±20C with 12-hour light/12-hour dark cycles prior to the experiment. All animals were supplied with commercial pellet food and water ad libitum.
Preparation of tualang honey, glibenclamide and metformin
Tualang honey (AgroMas®, Malaysia) was supplied by Federal Agricultural Marketing Authority (FAMA), Kedah, Malaysia. This honey has the following composition: total reducing sugar (67.5%) [fructose (29.6 %), glucose (30.0 %), maltose (7.9 %); fructose/glucose ratio (0.99)], sucrose (0.6 %) and water (20.0 %). Tualang honey (1.0 g/kg body weight) was freshly diluted with distilled water just before each administration. Glibenclamide (0.6 mg/kg body weight) and metformin (100 mg/kg body weight) were dissolved in dimethyl sulfoxide and distilled water, respectively before they were administered.
Induction of diabetes
Diabetes was induced in overnight fasted rats by a single intraperitoneal injection of streptozotocin (60 mg/kg body weight) in a 0.1M sodium citrate buffer (pH 4.5). The age-matched control rats received an equivalent amount of citrate buffer. Food and water intake were closely monitored daily after streptozotocin (STZ) administration. The development of hyperglycemia in rats was confirmed by fasting (16 hour) blood glucose measurement in the tail vein blood, 48 hours after STZ administration, with a portable glucometer (Accu-Chek, Roche, Germany). The animals with fasting blood glucose level ≥ 14.0 mmol/L with other symptoms of diabetes mellitus such as polyphagia, polydipsia, polyuria, and weight loss were considered diabetic and included in the study.
Treatment
The animals were randomly divided into eight groups. Each group comprised five to seven rats. Distilled water, tualang honey, glibenclamide, and metformin were administered once daily by oral gavage. The treatment of animals began on the 3rd day after STZ injection and this was considered as 1st day of treatment. The animals were treated for 4 weeks as follows:
- Group 1: Non-diabetic + Distilled water (0.5 ml)
- Group 2: Non-diabetic + Tualang honey (1.0 g/kg body weight)
- Group 3: Diabetic + Distilled water (0.5 ml)
- Group 4: Diabetic + Tualang honey (1.0 g/kg body weight)
- Group 5: Diabetic + Glibenclamide (0.6 mg/kg body weight)
- Group 6: Diabetic + Glibenclamide (0.6 mg/kg body weight) + Tualang honey (1.0 g/kg body weight)
- Group 7: Diabetic + Metformin (100 mg/kg body weight)
- Group 8: Diabetic + Metformin (100 mg/kg body weight) + Tualang honey (1.0 g/kg body weight)
At the end of the treatment period, the animals were fasted for at least 16 hours and sacrificed by decapitation. Blood samples were collected in centrifuge tubes without anticoagulants and allowed to clot. The clotted blood was then centrifuged at 3000 x g for 20 min. Serum was separated and then quickly stored at -80°C for biochemical analyses.
Biochemical analyses
Serum glucose was determined by the glucose oxidase method as described by Barham and Trinder [14]. Serum concentrations of fructosamine were determined by a colorimetric method (FRUC kit, Roche Diagnostics, Mannheim, Germany). Serum insulin was determined using a rat insulin enzyme-linked immunosorbent assay kit (Crystal Chem, Chicago, IL) with rat antibody. Serum high density lipoprotein cholesterol (HDL-C) was estimated by the method of Warnick [15]. Total cholesterol (TC) and triglycerides (TG) were estimated by the methods of Siedel et al. [16] and Foster and Dunn [17], respectively. Low density lipoprotein cholesterol (LDL-C) and very low density lipoprotein cholesterol (VLDL-C) were calculated by Friedwald's formula [18]. Serum creatinine and urea were estimated using diagnostic kits based on the methods of Tomas [19]. Total bilirubin, albumin and globulin levels were determined using commercially available kits.
Statistical analysis
Statistical analysis was carried out using SPSS 12.0.1. The data are expressed as mean ± SEM. Groups were compared by the Kruskal-Wallis H test followed by Mann-Whitney U test to identify significance of difference between two groups. P value < 0.05 was considered statistically significant.
Results
Body weight and food intake
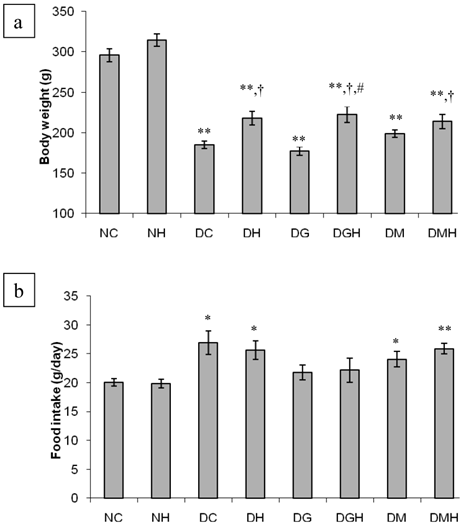
Figure 1 summarizes the results of the body weight and food intake of different groups of rats. There was no significant difference in body weight among the groups before the commencement of the study (results not shown). The diabetic control rats had significantly (p < 0.01) reduced body weight compared to non-diabetic control rats. Tualang honey significantly (p < 0.05) improved body weight in diabetic rats compared to diabetic control rats. Neither glibenclamide nor metformin prevented weight loss in diabetic rats. However, the diabetic groups that received glibenclamide or metformin in combination with honey showed significant (p < 0.05) improvement in body weight compared to diabetic control rats. Besides, the diabetic rats that received glibenclamide in combination with honey showed significantly (p < 0.05) higher body weight compared to the diabetic rats that received glibenclamide alone.
The diabetic control rats consumed significantly (p < 0.01) more pellets than the non-diabetic control rats. Although the amount of pellets consumed by the diabetic rats treated with glibenclamide, metformin, tualang honey or their combinations was less compared to that consumed by the diabetic control rats, there was no significant difference.
Serum glucose, serum fructosamine and serum insulin
Table 1 summarizes the results of serum glucose, serum fructosamine and serum insulin in different groups of animals at the end of the four-week treatment period. The serum glucose concentrations of the diabetic control rats were significantly higher (22.4 ± 1.0 mmol/L) than those of the non-diabetic control rats (5.7 ± 0.3 mmol/L). Treatment with glibenclamide, metformin, or honey significantly decreased the glucose levels (13.9 ± 3.4, 13.2 ± 2.9 or 12.3 ± 3.1 mmol/L, respectively) in diabetic rats. Combination of glibenclamide or metformin with honey further reduced the glucose concentrations (8.8 ± 2.9 or 9.9 ± 3.3 mmol/L, respectively) in diabetic rats. No significant effect of tualang honey in non-diabetic rats was observed (Table 1).
The diabetic control rats showed significantly (p < 0.01) elevated levels of fructosamine (360.0 ± 15.6 µmol/L) compared to non-diabetic control rats (145.3 ± 4.3 µmol/L). The diabetic rats that received tualang honey showed a significant (p < 0.05) decrease in fructosamine concentrations (304.5 ± 10.1 µmol/L). On the contrary, administration of glibenclamide or metformin to diabetic rats did not produce any significant difference on fructosamine levels compared to diabetic control rats. However, when glibenclamide or metformin was combined with tualang honey, the diabetic rats showed significantly (p < 0.05) reduced fructosamine levels compared to diabetic control rats (Table 1).
The diabetic control rats had significantly (p < 0.01) reduced insulin level (0.27 ± 0.01 ng/ml) compared to non-diabetic rats (0.60 ± 0.12 ng/ml). Treatment of diabetic rats with tualang honey, glibenclamide, metformin as well as their combinations produced a significant increase in insulin levels compared to diabetic controls (Table 1).
Serum creatinine, urea, bilirubin, albumin and albumin/globulin ratio
Table 2 depicts the levels of creatinine, urea, bilirubin, albumin and albumin/globulin ratio in the serum of control and streptozotocin-induced diabetic rats. Significantly increased serum levels of creatinine, urea and bilirubin were observed in the diabetic control rats compared to non-diabetic rats. Serum levels of creatinine and bilirubin remained elevated in the diabetic rats treated with glibenclamide or metformin. However, glibenclamide or metformin combined with tualang honey significantly (p < 0.05) decreased serum creatinine and bilirubin in diabetic rats compared to diabetic control rats. The diabetic control rats showed significantly (p < 0.05) reduced albumin and albumin/globulin compared to non-diabetic control rats. The diabetic rats that received honey or metformin showed significantly (p < 0.05) increased albumin and albumin/globulin compared to diabetic control rats.
Serum triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol and VLDL cholesterol
Table 3 shows the serum levels of triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol and VLDL cholesterol of control and streptozotocin-induced diabetic rats. Insignificantly increased levels of TG and VLDL cholesterol with no much change in total cholesterol, HDL cholesterol and LDL cholesterol levels were observed in diabetic control rats compared to non-diabetic rats. Administration of tualang honey, glibenclamide or metformin significantly (p < 0.05) decreased the levels of TG and VLDL cholesterol in diabetic rats compared to diabetic control rats. Besides, combination of glibenclamide or metformin with tualang honey further reduced the levels of TG and VLDL cholesterol in diabetic rats. Glibenclamide alone as well as combination of glibenclamide or metformin with tualang honey significantly decreased TC in diabetic rats.
Effects of tualang honey, glibenclamide, metformin or their combinations on (a) body weight and (b) food intake in streptozotocin-induced diabetic rats. NC (Non-diabetic control); NH (Non-diabetic + Honey); DC (Diabetic control); DH (Diabetic + Honey); DG (Diabetic + Glibenciamide); DGH (Diabetic + Glibenciamide + Honey); DM (Diabetic + Metformin); DMH (Diabetic + Metformin + Honey). Data are expressed as mean ± SEM. Each group consisted of five to seven animals. Values are statistically significant at * p < 0.05, ** p < 0.01 compared to NC; † p < 0.05 compared to DC; # p < 0.05 compared to DG.
Effects of tualang honey, glibenclamide, metformin or their combinations on serum glucose, serum fructosamine and serum insulin in streptozotocin-induced diabetic rats.
| Group | Serum glucose (mmol/L) | Serum fructosamine (µmol/L) | Serum insulin (ng/ml) |
|---|---|---|---|
| Non-diabetic control | 5.7 ± 0.3 | 145.3 ± 4.3 | 0.60 ± 0.12 |
| Non-diabetic + Honey | 5.6 ± 0.1 | 149.2 ± 2.6 | 0.57 ± 0.13 |
| Diabetic control | 22.4 ± 1.0** | 360.0 ± 15.6** | 0.27 ± 0.01** |
| Diabetic + Honey | 12.3 ± 3.1† | 304.5 ± 10.1**,† | 0.41 ± 0.06†† |
| Diabetic + Glibenclamide | 13.9 ± 3.4*,† | 330.0 ± 29.9** | 0.39 ± 0.02†† |
| Diabetic + Glibenclamide + Honey | 8.8 ± 2.9† | 301.3 ± 19.5**,† | 0.32 ± 0.02† |
| Diabetic + Metformin | 13.2 ± 2.9† | 314.6 ± 17.9** | 0.36 ± 0.03† |
| Diabetic + Metformin + Honey | 9.9 ± 3.3† | 285.8 ± 22.6**,† | 0.33 ± 0.02*,† |
Data are expressed as mean ± SEM. Each group consisted of five to seven animals.
Values are statistically significant at * p < 0.05, ** p < 0.01 compared to non-diabetic control;
† p < 0.05, †† p < 0.01 compared to diabetic control.
Effects of tualang honey, glibenclamide, metformin or their combinations on creatinine, urea, bilirubin, albumin and albumin/globulin ratio in the serum of streptozotocin-induced diabetic rats.
| Group | Creatinine (µmol/L) | Urea (mmol/L) | Bilirubin (µmol/L) | Albumin (g/L) | Albumin/ Globulin |
|---|---|---|---|---|---|
| Non-diabetic control | 27.8 ± 3.3 | 5.2 ± 0.3 | 1.50 ± 0.22 | 30.3 ± 1.2 | 0.80 ± 0.03 |
| Non-diabetic + Honey | 34.8 ± 3.3 | 5.9 ± 0.4 | 1.60 ± 0.40 | 31.2 ± 0.4 | 0.88 ± 0.02 |
| Diabetic control | 37.0 ± 1.4* | 20.3 ± 2.4** | 3.33 ± 0.42** | 23.0 ± 1.8** | 0.66 ± 0.03* |
| Diabetic + Honey | 25.6 ± 4.0 | 17.5 ± 2.6** | 2.00 ± 0.37† | 27.6 ± 0.9† | 0.81 ± 0.04† |
| Diabetic + Glibenclamide | 32.0 ± 4.7 | 17.7 ± 1.1** | 2.60 ± 0.69 | 27.8 ± 1.9 | 0.71 ± 0.06 |
| Diabetic + Glibenclamide + Honey | 26.7 ± 3.9† | 15.5 ± 1.5** | 1.67 ± 0.42† | 27.6 ± 1.6 | 0.73 ± 0.05 |
| Diabetic + Metformin | 32.7 ± 2.9 | 19.0 ± 2.4** | 2.29 ± 0.52 | 28.6 ± 1.4† | 0.80 ± 0.03† |
| Diabetic + Metformin + Honey | 25.2 ± 3.8† | 20.0 ± 1.2** | 1.60 ± 0.24† | 25.7 ± 1.8 | 0.82 ± 0.03† |
Data are expressed as mean ± SEM. Each group consisted of five to seven animals. Values are statistically significant at * p < 0.05, ** p < 0.01 compared to non-diabetic control; † p < 0.05 compared to diabetic control.
Effects of tualang honey, glibenclamide, metformin or their combinations on triglycerides (TG), total cholesterol (TC), high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol and very low density lipoprotein (VLDL) cholesterol in the serum of streptozotocin-induced diabetic rats.
| Group | TG (mg/dL) | TC (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | VLDL-C (mg/dL) |
|---|---|---|---|---|---|
| Non-diabetic control | 0.44 ± 0.04 | 1.50 ± 0.15 | 0.81 ± 0.08 | 0.42 ± 0.07 | 0.20 ± 0.02 |
| Non-diabetic + Honey | 0.51 ± 0.02 | 1.60 ± 0.11 | 0.88 ± 0.03 | 0.49 ± 0.08 | 0.23 ± 0.01 |
| Diabetic control | 0.71 ± 0.21 | 1.52 ± 0.09 | 0.75 ± 0.09 | 0.39 ± 0.04 | 0.32 ± 0.10 |
| Diabetic + Honey | 0.25 ± 0.04*,† | 1.42 ± 0.12 | 0.89 ± 0.09† | 0.38 ± 0.05 | 0.14 ± 0.03† |
| Diabetic + Glibenclamide | 0.30 ± 0.08† | 1.07 ± 0.07*,†† | 0.64 ± 0.09 | 0.29 ± 0.03 | 0.14 ± 0.04† |
| Diabetic + Glibenclamide + Honey | 0.21 ± 0.04**,† | 1.14 ± 0.08† | 0.82 ± 0.12 | 0.41 ± 0.03 | 0.10 ± 0.02**,† |
| Diabetic + Metformin | 0.38 ± 0.19*,† | 1.38 ± 0.16 | 0.87 ± 0.09 | 0.34 ± 0.03 | 0.17 ± 0.08*,† |
| Diabetic + Metformin + Honey | 0.17 ± 0.02**,†† | 0.89 ± 0.15*,† | 0.67 ± 0.11 | 0.24 ± 0.04† | 0.07 ± 0.01**,† |
Data are expressed as mean ± SEM. Each group consisted of five to seven animals. Values are statistically significant at * p < 0.05, ** p < 0.01 compared to non-diabetic control; † p < 0.05, †† p < 0.01 compared to diabetic control.
Discussion
In STZ-induced diabetic rats, insulin deficiency or hypoinsulinemia develops as a consequence of irreversible destruction of the β-cells of the pancreas resulting in hyperglycemia [20]. Glibenclamide, metformin or honey significantly reduced blood glucose concentrations in our study which is similar to findings from previous studies [12; 21-23]. However, the hypoglycemic effect of tualang honey was not observed in the non-diabetic rats similar to our previous finding [12-13]. This indicates that similar to what has been previously reported for metformin [24], the hypoglycemic effect of honey might also be glucose dependent and thus possessing euglycemic rather than hypoglycemic effect in non-diabetic rats.
The mechanisms of action of glibenclamide and metformin are well documented [25-26]. On the contrary, it is still unknown how honey mediates its hypoglycemic effect in diabetes. However, based on current literature, one may suggest that tualang honey might possibly exert its hypoglycemic effect through fructose which is the predominant constituent [27]. Fructose neither increases plasma glucose nor its metabolism requires insulin secretion [28]. Dietary fructose is known to activate glucokinase which is a key enzyme involved in the intracellular metabolism of glucose. It catalyzes the conversion of glucose to glucose-6-phosphate thereby decreasing blood glucose [29]. A previous study also reported that fructose stimulated insulin secretion from an isolated pancreas [30]. However, stronger evidence in support of the role of fructose in mediating hypoglycemic effect of honey is provided by Curry and his colleagues [31]. They found that in rat pancreas preparations, there was no insulin response to fructose when very low or no concentrations of glucose were present in the medium. In contrast, it was observed that with higher glucose concentrations, insulin response to fructose was elicited [31]. These findings seem to corroborate ours where we found that tualang honey showed hypoglycemic effect in diabetic rats but did not produce such an effect in non-diabetic rats.
Furthermore, honey is reported to have a lower glycemic index compared to many other carbohydrates [32]. With regards to the compositions of tualang honey [fructose (29.6 %), glucose (30.0 %), fructose/glucose ratio (0.99)], the nearly 1:1 ratio of fructose to glucose might indicate its uniqueness in comparison to some types of honey which have 1:2 ratio of fructose to glucose [33]. Besides, unlike other common kinds of honey which are produced by Apis mellifera [27], tualang honey is produced by Apis dorsata [13]. Moreover, tualang honey is of polyfloral origin compared to others which are from monofloral sources. So, these and other properties may distinguish tualang honey from other types of honey. In addition to fructose and glucose, honey is known to contain fructooligosaccharides which provide prebiotic benefits to the intestinal microflora such as bifidobacteria and lactobacilli, which are probiotics [34-36]. Since the gastrointestinal microflora is now identified to play a key role in health and disease, honey may help improve gut health in diabetes and perhaps impact on glucose management by additional mechanisms which need to be explored.
Honey also contains elements such as zinc, selenium, copper, calcium, potassium, chromium, manganese, etc [27]. Some of these minerals are reported to play vital roles in the maintenance of normal glucose tolerance and insulin secretion from the pancreatic β-cells [37-38]. Other ions such as copper and zinc are also known to be involved in glucose and insulin metabolism [39-40]. Even though the amounts of these minerals in honey may be small, it is worthwhile to note that these trace elements are not actually required in large quantities. Daily supplementation of the diabetic rats with tualang honey for four weeks might attain sufficient concentrations of these minerals to elicit pharmacological responses which synergistically contribute to the hypoglycemic effect. Furthermore, improved secretion of insulin from the pancreas (possibly due to protective effect on beta cells) might contribute to the hypoglycemic effect of tualang honey.
Tight glycemic control is now recognized as one of the main therapeutic goals to prevent diabetic complications [1, 2]. HbA1c is commonly used as an indicator of glycemic control [41]. Findings from the DCCT showed that there is a relationship between HbA1c and glycemic control [1]. Since HbA1c measures blood glucose control over a period of eight to twelve weeks, it will not be a valid test in this study which lasted for four weeks. Therefore, we measured fructosamine which reflects a two to three week period of glycemic control prior to blood testing. Fructosamine is a stable product formed as result of protein modification by glucose and its serum level is reported to increase in diabetes [42]. Fructosamine can undergo oxidative cleavage leading to the formation of advanced glycation end products (AGEs), which are implicated in long-term complications of diabetes mellitus [42]. We observed that fructosamine was significantly elevated in the serum of the diabetic rats similar to what was previously reported [43]. Our results further showed that unlike honey which significantly decreased fructosamine levels, neither glibenclamide nor metformin reduced the elevated levels of fructosamine. A previous study also found that glibenclamide did not reduce fructosamine in diabetic rats [43].
Surprisingly, significantly reduced fructosamine levels were observed when glibenclamide or metformin was combined with tualang honey. Therefore, this suggests that these hypoglycemic drugs in combination with tualang honey produced better glycemic control. It is still unclear how tualang honey decreased fructosamine concentration. However, this may be linked to the previously reported antioxidant effect of tualang honey [12-13]. Previous studies have reported that antioxidants such as vitamins C and E reduce HbA1c values by inhibiting protein glycation [44-45]. Besides, Gly-13-C, an antioxidant was also reported to reduce fructosamine in diabetic rats [43]. The same groups of diabetic animals that showed significantly reduced fructosamine levels also exhibited much lower glucose concentrations. Therefore, based on these results, one may suggest that the combination of hypoglycemic drugs with honey or possibly other antioxidants may reduce the formation and/or the deleterious effects of advanced glycation endproducts (AGEs) which may perhaps delay the development and progression of diabetic complications.
Induction of diabetes by STZ resulted in loss of body weight in the diabetic control rats. Our results showed that neither glibenclamide nor metformin prevented weight loss. On the other hand, these drugs in combination with honey reduced weight loss in diabetic rats. The mechanisms by which honey improves body weight are unclear. Insulin is reported to have anabolic effect on protein metabolism by stimulating protein synthesis and retarding protein degradation [46]. Although tualang honey increased serum insulin, considering the levels of insulin in the diabetic rats that received glibenclamide or metformin in combination with honey, the additional effect of tualang honey on body weight in these rats could not be attributed to insulin. Perhaps, the fructose constituent of tualang honey [47] or improved glycemic control due to tualang honey might contribute to weight gain.
Increased serum levels of urea and creatinine, indicators of impaired renal function [48], were observed in the diabetic control rats. Their levels remained elevated in the diabetic rats treated with tualang honey, glibenclamide or metformin. However, glibenclamide or metformin in combination with honey significantly decreased serum creatinine. This suggests that combination of hypoglycemic drugs with tualang honey may have beneficial effect in preventing renal damage, perhaps through improved glycemic control and amelioration of oxidative stress as previously reported [12-13]. Microalbuminuria is one of the clinical manifestations of diabetic nephropathy. Albuminuria, which may be due to leakage of albumin by damaged glomerular membrane, is reported to develop in untreated diabetes mellitus [48]. Reduced levels of serum albumin and albumin/globulin in the diabetic rats might indicate renal leakage. Treatment with tualang honey or metformin increased serum albumin and albumin/globulin ratio. These data suggest that tualang honey may help in the repair of renal damage.
Diabetes is characterized by insulin deficiency which causes increased lipolysis in adipose tissues and increased entry of free fatty acids (FFA) to the liver [49]. Increased FFA and insulin independent glucose uptake in the liver is reported to stimulate triglyceride synthesis [50]. This increased synthesis of triglycerides tends to reduce protein content of lipoproteins, especially in VLDL and LDL while the triglyceride content increases [51]. This alteration in the protein and triglyceride compositions results in reduced uptake of these lipoproteins by their receptors [51]. As a result, dyslipidemia related to abnormalities in serum lipoproteins is an impending atherogenic risk factor in diabetes [52]. In this study, the diabetic control rats showed insignificant increases in TG and VLDL levels while no significant change was observed in total cholesterol, HDL cholesterol and LDL cholesterol levels. Our findings are similar to those of Bin and his colleagues who also found insignificant lipid abnormalities in diabetic rats [53]. However, other researchers did report significant changes in lipid abnormalities [49]. The decreased levels of TG and VLDL cholesterol in diabetic rats that received glibenclamide or metformin seem to corroborate previous findings that these drugs do ameliorate dyslipidemia [21, 22, 25-26]. Besides, significantly lower levels of VLDL cholesterol and TG observed in diabetic rats that received a combination of glibenclamide or metformin with tualang honey further demonstrate that tualang honey combined with these hypoglycemic agents produces synergistic effect in reducing VLDL cholesterol and TG in diabetic rats.
In conclusion, these results indicate that addition of tualang honey to glibenclamide or metformin in STZ-induced diabetic rats helps to achieve serum glucose and fructosamine levels much lower than those achieved with glibenclamide or metformin alone. Honey as an adjunct to glibenclamide or metformin leads to an improved glycemic control and some additional metabolic benefits not achieved with either glibenclamide or metformin alone. This study seems to suggest that the combination of honey with oral hypoglycemic agents may be a valuable adjuvant therapy to achieve and/or maintain glycemic control and possibly reduce or delay the onset of diabetic complications. A similar study in human subjects is desirable to determine if these results can be appropriately extrapolated to human diabetes.
Acknowledgements
This work was supported by a grant (1001/PPSP/81202020) from Universiti Sains Malaysia (USM). We would like to acknowledge USM Fellowship awarded to the first author. We also express our appreciation to Federal Agricultural Marketing Authority (FAMA), Kedah, Malaysia for supplying tualang honey.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. The Diabetes Control and Complications Trial (DCCT) Research Group. The effect of intensive treatment of diabetes on the development and progression of long term complications in the diabetes control in insulin dependent diabetes mellitus. N Engl J Med. 1993;329:977-986
2. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837-853
3. Turner RC, Cull CA, Frighi V. et al. UK Prospective Diabetes Study Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281:2005-2012
4. UK Prospective Diabetes Study Group. UK Prospective Diabetes Study 16: Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249-1258
5. Yale JF, Valiquett TR, Ghazzi MN. et al. The effect of a thiazolidinedione drug, troglitazone, on glycemia in patients with type 2 diabetes mellitus poorly controlled with sulfonylurea and metformin; a multicenter, randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;134:737-745
6. Cook MN, Girman CJ, Stein PP. et al. UK Prospective Diabetes Study (UKPDS) Group. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care. 2005;28:995-1000
7. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351-42354
8. Robertson RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: a case of double jeopardy for the pancreatic islet beta cell. Free Radic Biol Med. 2006;41:177-184
9. Grankvist K, Marklund SL, Taljedal IB. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981;199:393-398
10. Kajimoto Y, Kaneto H. Role of oxidative stress in pancreatic beta-cell dysfunction. Ann N Y Acad Sci. 2004;11:168-176
11. Kaneto H, Kajimoto Y, Miyagawa J. et al. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic β-cells against glucose toxicity. Diabetes. 1999;48:2398-2406
12. Erejuwa OO, Sulaiman SA, Ab Wahab MS. et al. Effects of Malaysian tualang honey supplementation on glycemia, free radical scavenging enzymes and markers of oxidative stress in kidneys of normal and streptozotocin-induced diabetic rats. Int J Cardiol. 2009;137:S45
13. Erejuwa OO, Sulaiman SA, Ab Wahab MS. et al. Antioxidant protection of Malaysian tualang honey in pancreas of normal and streptozotocin-induced diabetic rats. Ann Endocrinol. 2010;71(4):291-296
14. Barham D, Trinder P. An improved color reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97:142-145
15. Warnick GR, Nguyen T, Alberts AA. Comparison of improved precipitation methods for quantification of high-density lipoprotein cholesterol. Clin Chem. 1985;31:217
16. Siedel J, Hagele EO, Ziegenhorn J. et al. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29:1075-1080
17. Foster LB, Dunn RT. Stable reagents for determination of serum triglycerides by colorimetric hantzsch condensation method. Clin Chem. 1973;19:338-340
18. Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of LDL-C in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:449-502
19. Tomas L. Clinical laboratory diagnostics, 1st ed. Frankfurt: Verlagsgesellschaft. 1998:208-214
20. Rakieten N, Rakieten ML, Nadkarni MV. Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother Rep. 1963;29:91-98
21. Panneerselvam K, Kuppuswamy K, Kodukkur VP. Hypolipidemic activity of 18β-glycyrrhetinic acid on streptozotocin-induced diabetic rats. Eur J Pharmacol. 2009;612(1-3):93-97
22. Zheng L, Jianchun L, Zhaojun Z. et al. The antidiabetic effects of cysteinyl metformin, a newly synthesized agent, in alloxan- and streptozocin-induced diabetic rats. Chem Biol Interact. 2008;173(1):68-75
23. Al-Khalidi A, Jawad FH, Tawfiq NH. Effects of bees honey, zahdi dates and its syrup on blood glucose and serum insulin of diabetics. Nutr Rep Int. 1980;21(5):631-643
24. Jayesh BM, Balaraman R. Metformin reduces blood pressure and restores endothelial function in aorta of streptozotocin-induced diabetic rats. Life Sci. 2006;78(22):2615-2624
25. Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65(3):385-411
26. Wiernsperger NF, Bailey CJ. The antihyperglycaemic effect of metformin: therapeutic and cellular mechanisms. Drugs. 1999;58:31-39
27. Bogdanov S, Jurendic T, Sieber R. et al. Honey for nutrition and health: a review. J Am Coll Nutr. 2008;27(6):677-689
28. Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58(suppl):754S-765S
29. Watford M. Small amounts of dietary fructose dramatically increase hepatic glucose uptake through a novel mechanism of glucokinase activation. Nutr Rev. 2002;60:253-257
30. Grodsky GM, Rafts AA, Bennett LL. Effects of carbohydrates on secretion of insulin from isolated rat pancreas. Am J Physiol. 1963;205:638-644
31. Curry DL, Curry KP, Gomez M. Fructose potentiation of insulin secretion. Endocrinology. 1972;91:1493-1498
32. Abdulrhman M, El-Hefnawy M, Hussein R. et al. The glycemic and peak incremental indices of honey, sucrose and glucose in patients with type 1 diabetes mellitus: effects on C-peptide level—a pilot study. Acta Diabetol. 2009 [Epub ahead of print]
33. Bahrami M, Ataie-Jafari A, Hosseini S. et al. Effects of natural honey consumption in diabetic patients: an 8-week randomized clinical trial. Int J Food Sci Nutr. 2009;60(7):618-626
34. Chow J. Probiotics and prebiotics: a brief overview. J Ren Nutr. 2002;12:76-86
35. Sanz ML, Polemis N, Morales V. et al. In vitro investigation into the potential prebiotic activity of honey oligosaccharides. J Agric Food Chem. 2005;53:2914-2921
36. Kajiwara S, Gandhi H, Ustunol Z. Effect of honey on the growth of and acid production by humann intestinal Bifidobacterium spp.: An in vitro comparison with commercial oligosaccharides and inulin. J Food Protect. 2002;65:214-218
37. Anderson RA, Cheng N, Bryden NA. et al. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786-1791
38. Kar A, Choudhary BK, Bandyopadhyay NG. Preliminary studies on the inorganic constituents of some indigenous hyperglycaemic herbs on oral glucose tolerance test. J Ethnopharmacol. 1999;64:179-184
39. Keil HL, Victor EN. The role of copper in carbohydrate metabolism. J Biol Chem. 1934;106:343-349
40. Arquilla ER, Packer S, Tarmas W. et al. The effect of zinc on insulin metabolism. Endocrinology. 1978;103:1330-1449
41. Rohlfing CL, Wiedmeyer HS, Little RR. et al. Defining the relationship between plasma glucose and HbA1c. Diabetes Care. 2002;25:275-278
42. Kennedy L, Baynes JW. Non-enzymatic glycosylation and the chronic complications of diabetes: an overview. Diabetologia. 1984;26(2):93-8
43. Ashok P, Kyada A, Subbarao P. et al. Antioxidant status of a polyherbomineral formulation (Gly-13-C) in STZ-diabetic rats. Int J Pharmacol. 2010;6:157-172
44. Vinson JA, Howard TB. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J Nutr Biochem. 1996;7(12):659-663
45. Ceriello A, Giugliano D, Quatraro A. et al. Vitamin E reduction of protein glycosylation in diabetes: new prospect for prevention of diabetic complications? Diabetes Care. 1991;14:68-72
46. Levine R. Insulin: the effects and mode of action of the hormone. Vitamins and Hormones. 1982;39:145-173
47. Bocarsly ME, Powell ES, Avena NM. et al. High-fructose corn syrup causes characteristics of obesity in rats: Increased body weight, body fat and triglyceride levels. Pharmacol Biochem Behav. 2010 [Epub ahead of print]
48. Mogensen CE, Steffes MW, Deckert T. et al. Functional and morphological renal manifestations in diabetes mellitus. Diabetologia. 1981;21:89-93
49. Ohno T, Horio F, Tanaka S. et al. Fatty liver and hyperlipidemia in IDDM (insulin-dependent diabetes mellitus) of Streptozotocin-treated shrews. Life Sci. 2000;66:125-131
50. Coppack SW, Jensen MD, Miles JM. In vivo regulation of lipolysis in humans. J Lipid Res. 1994;35:177-193
51. Winocour PH, Durrington PN, Bhatnagar D. et al. Abnormalities of VLDL, IDL, and LDL characterize insulin-dependent diabetes mellitus. Arterioscler Thromb. 1992;12:920-928
52. Verges BL. Dyslipidemia in diabetes mellitus. Review of the main lipoprotein abnormalities and their consequences on the development of atherogenesis. Diabetes Metab. 1999;25:32-40
53. Bin Z, Hanchao S, Junfu Z. et al. Effects of Simvastatin on Oxidative Stress in Streptozotocin-Induced Diabetic Rats: A Role for Glomeruli Protection. Nephron Exp Nephrol. 2005;101:e1-e8
Author contact
![]() Corresponding author: Omotayo O. Erejuwa (erejuwacom) or Siti A. Sulaiman (sbsamrahusm.my); Tel.: +6097666124; Fax: +6097653370.
Corresponding author: Omotayo O. Erejuwa (erejuwacom) or Siti A. Sulaiman (sbsamrahusm.my); Tel.: +6097666124; Fax: +6097653370.

 Global reach, higher impact
Global reach, higher impact