10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2011; 7(4):460-468. doi:10.7150/ijbs.7.460 This issue Cite
Research Paper
Nuclear Transfer of Embryonic Cell Nuclei to Non-enucleated Eggs in Zebrafish, Danio rerio
Laboratory of Freshwater Fish Stocks, Bioscience and Biotechnology Center, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8601, Japan.
Received 2011-1-1; Accepted 2011-4-10; Published 2011-4-15
Abstract
We previously established a novel method for nuclear transfer in medaka (Oryzias latipes) using non-enucleated, diploidized eggs as recipients for adult somatic cell nuclei. Here we report the first attempt to apply this method to another fish species. To examine suitability of using non-enucleated eggs as recipients for nuclear transfer in the zebrafish (Danio rerio), we transferred blastula cell nuclei from a wild-type donor strain to non-enucleated, unfertilized eggs from a golden recipient strain. As a result, 31 of 184 (16.8%) operated eggs developed normally and reached the adult stage. Twenty-eight (15.2%) of these transplants showed wild-type phenotype and the remaining three (1.6%) were golden. Except for one individual that exhibited diploid/tetraploid mosaicism, all of the wild-type nuclear transplants were either triploid or diploid. While all of 19 triploid transplants were infertile, a total of six transplants (21.4%) were fertile (five of the eight diploid transplants and one transplant exhibiting ploidy mosaicism). Except for one diploid individual, all of the fertile transplants transferred both the wild-type golden gene allele (slc24a5) as well as the phenotype, the wild-type body color, to their F1 and F2 progeny in a typical Mendelian fashion. PCR analysis of slc24a5 suggested that triploidy originated from a fused nucleus in the diploid donor and haploid recipient nuclei, and that the sole origin of diploidy was the diploid donor nucleus. The results of the present study demonstrated the suitability of using non-enucleated eggs as recipients for nuclear transfer experiments in zebrafish.
Keywords: nuclear transfer, embryonic cells, non-enucleated eggs, zebrafish
Introduction
Nuclear transfer is an important technique in animal cloning and also for demonstrating the germline transmission of genetically engineered genomes. Since the birth of Dolly, the first cloned sheep [1], attempts to produce animals through adult somatic cell nuclear transfer have been successful in a variety of mammalian species [2, 3]. However, despite their importance as an animal resource in fundamental biology, biomedical research, and food production, reports on efficient nuclear transfer in fish are still limited [4]. Indeed, several parameters remain to be determined before nuclear transfer can be widely and efficiently applied to various fish species.
Nuclear transfer in fish has been studied for a half century, starting with the initial work of Tung and colleagues in the 1960s [5, 6]. In the transfer of embryonic cell nuclei to enucleated eggs, transplants have been generated in cyprinid fish [7-10], loach, Misgurnus fossilis [11], medaka, Oryzias latipes [12] and zebrafish, Danio rerio [13, 14]. However, until our recent work using newly established methods in the small freshwater fish model, medaka [15, 16], nuclear transfer using adult somatic cell nuclei had not yet been reported. In those studies, we used non-enucleated and diploidized eggs as recipients for the transfer of adult somatic cell nuclei and consistently obtained diploid and fertile transplants that originated from the donor nuclei. Application of this method to other fish species will facilitate new studies involving genetic modification and cloning in both basic and applied fields of fish research. We therefore sought to apply this method to another fish model, the zebrafish.
Since no studies on nuclear transfer using non-enucleated recipient eggs have been reported to date in zebrafish, we first attempted to determine whether non-enucleated eggs were suitable for use as recipient for nuclear transfer in this species by employing a nuclear transfer approach that we successfully implemented in medaka to transfer embryonic cell nuclei to non-enucleated unfertilized eggs [17-19]. Our results revealed that, as in medaka, non-enucleated recipient eggs could also be used to produce diploid or triploid adult transplants in zebrafish.
Materials and Methods
Fish
A zebrafish strain with the wild-type body color, Riken Wild (Fig. 1B, E), was obtained from the Riken Brain Science Institute (National BioResource Project) for use as the donor fish for nuclear transfer. A commercially available golden variety of zebrafish was used as the recipient fish and for crosses with nuclear transplants (NTs) and their progeny. The golden variety shows hypopigmentation of the melanophores in the skin as well as in the retinal pigment epithelium (RPE) (Fig. 1C, F), both of which are phenotypes resulting from a mutation in the golden gene, slc24a5 [20]. The wild-type phenotype is genetically dominant to the golden phenotype, and in combinations of wild-type donors and golden recipients, NTs originating from donor nuclei can be identified by the wild-type pigmentation in the body, that is, by the appearance of densely melanized melanophores in the skin of embryos.
Zebrafish nuclear transplant and donor and recipient strains. (A) to (C) Larval fish at the hatching stage. (D) to (F) Fish at the adult stage. (A) and (D) Nuclear transplant No. 19 with wild-type body color. (B) and (E) Donor strain with wild-type body color. (C) and (F) Recipient strain with golden body color. Scale bars represent 250 μm in (A) to (C), and 5 mm in (D) to (F).
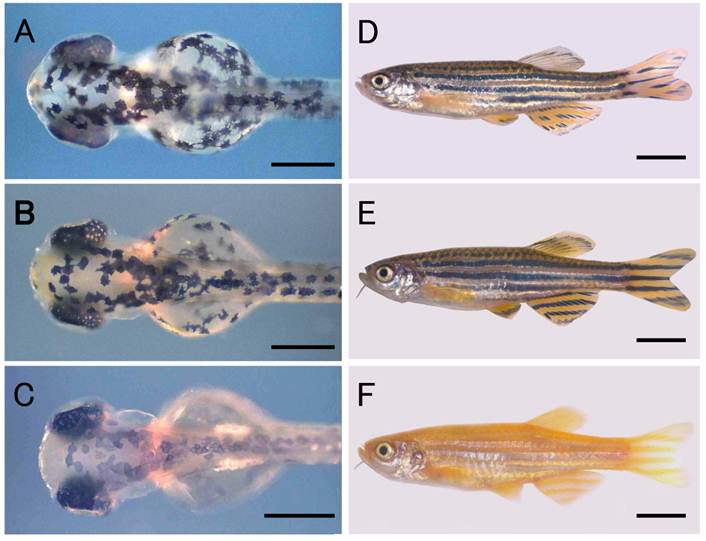
The fish were maintained in 16 L tanks under a 14 h light:10 h dark cycle at 28ºC, and were fed paramecium, Artemia nauplii larvae, and the commercially available artificial feeds, Otohime β1 (Marubeni Nisshin Feed, Japan) and Kyowa N250 (Kyowa Hakko Kogyo, Japan). The developmental stages of the embryos were determined according to the criteria of Westerfield [21].
Preparation of donor cells
Fertilized eggs obtained from natural spawning were incubated at 28.5ºC. Upon reaching the sphere stage, a subsample of the embryos was dechorionated with forceps under a stereomicroscope (MZAPO; Leica, Switzerland). The blastoderms of the embryos were then separated from their yolks with forceps and washed three times with Hank's solution (Gibco Laboratories, MD), rinsed in Ca2+- and Mg2+-free Dulbecco's phosphate-buffered saline (Takara Bio, Japan), and dissociated into single cells by gentle pipetting. The cells were then collected by centrifugation and stored at 20ºC in Leibovitz's L-15 medium (Gibco) containing 0.01% neutral red. Several batches of donor cells were prepared for a single experiment, all of which were used within 1 hour.
Preparation of recipient eggs
Immediately after observing the onset of spawning behavior in the fish tanks, the females were separated from males and placed into a container to prevent spawning. Each female was then anesthetized in 0.1% tricaine solution for several tens of seconds before having her belly gently squeezed using the fingers to obtain unfertilized eggs. The eggs were activated by placing them in a dish containing tap water for 1 min before transferring them to a 6 cm dish filled with Holtfreter's solution at 24ºC; all of the eggs were activated in this way. Activated eggs with a chorion were then used as recipients for nuclear transfer for up to 40 min.
Nuclear transfer
The procedures used for nuclear transfer in zebrafish were the same as those described for medaka by Bubenshchikova et al. [19], with slight modification. Recipient eggs were placed in a V-shaped groove on a 2% agar plate in a 6 cm dish containing Hank's solution with 1.5% bovine serum albumin (Sigma-Aldrich, MO). Donor cells were spread on the same plate. A single donor cell was then slightly ruptured by suction into a glass microcapillary tube (inner diameter: 22-24 μm) and transferred to the center of the blastodisc of a recipient egg. Nuclear transfer was conducted using a hydraulic injector (Cell-Tram Oil; Eppendorf, Germany) connected to a micromanipulator (MO-202; Narishige, Japan) under a stereomicroscope (MZ16; Leica) at approximately 20ºC with the dish placed on a cooling plate (Thermo Plate; Tokai Hit, Japan). Each operated egg was then transferred to a separate well on a 24-well plate (Greiner Bio-one, Germany) containing Holtfreter's solution with penicillin and streptomycin at final concentrations of 100 U/ml and 100 μg/ml, respectively. Plates were incubated at 24ºC for the first 24 hours and then at 26ºC until hatching. Larvae were reared to the adult stage at 28ºC.
Observation of a donor marker; wild-type melanin pigmentation
Melanin pigmentation was initially observed in the RPE of wild-type embryos at 24 hours post fertilization (hpf) after incubation at 28.5ºC; however, pigmentation was not apparent in embryos of the golden variety at the same stage of development. Melanophores appeared in the skin of wild-type embryos at 48 hpf. In the golden variety, pigmentation of the embryonic melanophores was weaker than it was in the wild-type; in the adults of the wild-type fish, dark, melanophore-rich stripes are observed in the skin, but these were absent in the golden variety. We used these melanin pigmentation characteristics as one of the donor markers for detecting the contribution of donor nuclei in the development of the NTs.
Ploidy estimation
Ploidy estimation was performed using methods described elsewhere [16]. Briefly, either part of the caudal fin from an adult fish or a whole recently hatched larva was minced in extraction buffer (CyStain DNA 2step, Partec GmbH, Germany) and incubated for 10 to 20 min at room temperature. This homogenate was then stained with 4-6-diamino-2-phenylindole dihydrochloride (CyStain DNA 2step, Partec) and the ploidy of these samples was determined using a flow cytometer (Ploidy Analyzer, Partec).
Genetic crosses
Each of the adult NTs obtained in this study was crossed with the golden variety to assess their fertility and the transmission of donor markers to their F1 and F2 progeny. Each F1 fish was then backcrossed with the golden variety to obtain the F2 generation.
Molecular genotyping
slc24a5 in the NTs and their progeny was assayed by the polymerase chain reaction (PCR). Genomic DNA was extracted from the caudal fin of adult NTs and F1 progeny, as well as whole bodies of F2 hatchlings, using a commercially available extraction kit (GenElute Mammalian Genomic DNA Miniprep Kit; Sigma-Aldrich). PCR was performed using 0.5 units of Ex-Taq DNA polymerase (Takara Bio), 100 nM of each primer, and 30 to 40 ng of template DNA in a total volume of 20 μl. The primers 5'-TCTGGAACTATTGACTCTTCAC-3' (forward) and 5'-AGGAGACACGCGCCATCATAC-3' (reverse) were used to amplify a 335-bp sequence of the golden gene. The PCR profile consisted of 35 cycles, with denaturation at 94ºC for 30 sec, annealing at 60ºC for 30 sec, and extension at 72ºC for 1 min. The PCR products were then digested using Alu restriction enzyme (Takara Bio) and analyzed on a 12% polyacrylamide gel.
Results
Development of nuclear transplants
The development of NTs is summarized in Table 1. In total, we operated on 184 eggs in six independent experiments. Of the NTs produced, 139 (75.5%) developed to blastulae, 83 (45.1%) survived to the segmentation stage (20-somite stage), and 60 (32.6%) hatched. Fifty-three (28.8%) of the hatchlings exhibited wild-type pigmentation in the skin and RPE (Fig. 1A) and the remainder exhibited the golden phenotype. A total of 31 (16.8%) larvae developed normally and reached the adult stage within two months after hatching. Twenty-eight (15.2%) of these 31 fish had the wild-type phenotype (Fig. 1D) and the remaining three (1.6%) were golden. All of the subsequent analyses were performed using these adult NTs.
Fertility of nuclear transplants and transmission of the wild-type body color to progeny
The characteristics of the NTs are summarized in Table 2. Of the 28 adult NTs showing the wild-type body color, two (Nos. 1 and 2) (7.1%) were female and the others (92.9%) were male. All of the NTs were individually crossed with specimens of the golden variety to examine their fertility and the transmission of the wild-type body color, one of the donor markers, to the resulting progeny. Twenty-one males (Nos. 3 through 23) exhibited active reproductive behavior and their golden partners spawned 76 to 303 eggs per spawning event per week. However, the majority of these eggs were unfertilized. In those cases where fertilization was successful, all of the embryos died during embryonic development; most died at the blastula or gastrula stages, and a small number showing varying degrees of abnormal morphology developed until the hatching stage. One female (No. 1) showed active reproductive behavior with golden male partners, but she did not spawn in ten mating sessions over a two-month period. Thus, of the 28 NTs with the wild-type phenotype, 22 (78.6%) were infertile.
On the other hand, the six remaining NTs (21.4%), which consisted of five males (Nos. 24 through 28) and one female (No. 2), were all fertile. Crossing experiments using these fertile NTs are summarized in Table 3. All of F1 progeny produced by four males (Nos. 24, 25, 27 and 28) and No. 2 female showed the wild-type body color. They developed normally from the embryonic and postembryonic development stages through to the adult stage. In a cross with male No.26, three (1.7%) of the 176 F1 progeny showed the golden phenotype and died at the hatching stage, whereas all of the other F1 progeny (98.3%) showed the wild-type phenotype and grew normally to the adult stage. None of the three NTs with the golden phenotype (Nos. 29 through 31) exhibited active spawning behavior or produced any eggs. In the F2 generation, produced by crossing wild-type F1 progeny with the golden variety, body color segregated into wild-type and golden progeny in a 1:1 ratio. Thus, except for male No. 26 with the wild-type phenotype, all of the fertile NTs transmitted the wild-type phenotype to their progeny in a typical Mendelian fashion.
Development of nuclear transplants produced by transfer of blastula cell nuclei into non-enucleated unfertilized zebrafish eggs.
| No. of operated eggs | No. of developed nuclear transplants | |||||||
|---|---|---|---|---|---|---|---|---|
| Cleavage | Blastula | Gastrula | Segmentation | Hatching | Adults | |||
| Total | 184 | 154 (83.7)a | 139 (75.5) | 109 (59.2) | 83 (45.1) | 60 (32.6) | 31 (16.8) | |
| Body color | Wild type | 53 (28.8) | 28 (15.2) | |||||
| Golden | 07 (03.8) | 03 (01.6) | ||||||
a Numbers shown in parentheses represent the percentages with respect to the total number of operated eggs.
Characters of adult fish generated by transfer of blastula cell nuclei into non-enucleated unfertilized zebrafish eggs.
| Fish No | Body color | Sex | Fertility | Ploidy | allele of slc24a |
|---|---|---|---|---|---|
| 1 | W | F | - | 2N | W |
| 2 | W | F | + | 2N | W |
| 3 | W | M | - | 3N | W+V² |
| 4 | W | M | - | 3N | W+V |
| 5 | W | M | - | 3N | W+V |
| 6 | W | M | - | 3N | W+V |
| 7 | W | M | - | 3N | W+V |
| 8 | W | M | - | 3N | W+V |
| 9 | W | M | - | 3N | W+V |
| 10 | W | M | - | 3N | W+V |
| 11 | W | M | - | 3N | W+V |
| 12 | W | M | - | 3N | W+V |
| 13 | W | M | - | 3N | W+V |
| 14 | W | M | - | 3N | W+V |
| 15 | W | M | - | 3N | W+V |
| 16 | W | M | - | 3N | W+V |
| 17 | W | M | - | 3N | W+V |
| 18 | W | M | - | 3N | W+V |
| 19 | W | M | - | 3N | W+V |
| 20 | W | M | - | 3N | W+V |
| 21 | W | M | - | 3N | W+V |
| 22 | W | M | - | 2N | W |
| 23 | W | M | - | 2N | W |
| 24 | W | M | + | 2N | W |
| 25 | W | M | + | 2N | W |
| 26 | W | M | + | 2N | W |
| 27 | W | M | + | 2N | W |
| 28 | W | M | + | 2N+4N¹ | W+V |
| 29 | G | ND | - | 2N | V |
| 30 | G | ND | - | 2N | V |
| 31 | G | ND | - | 2N | V |
F, female; G, golden; M, male; ND, not determined; V, variant; W, wild type
1 mosaicism of diploidy and tetraploidy, 2 both of wild-type and variant allele
Transmission of a donor marker, body color, to the progeny of six nuclear transplants generated by the transfer of blastula cell nuclei to non-enucleated unfertilized zebrafish eggs.
| Fish No | Sex | F1 | F2 | |||||
|---|---|---|---|---|---|---|---|---|
| Number of embryos | Body color | F1 offspring used for crossing to obtatin F2 | Number of embryos | Body color | ||||
| Wild type n (%) | Golden n (%) | Wild type n (%) | Golden n (%) | |||||
| 2 | F | 594 | 594 (100.0) | 10 (1.5%) | 6 | 714 | 360 (50.4%) | 354 (49.6%) |
| 24 | M | 627 | 627 (100.0) | 10 (1.5%) | 6 | 804 | 397 (49.4%) | 407 (50.6%) |
| 25 | M | 782 | 651 (100.0) | 10 (1.5%) | 6 | 737 | 363 (49.3%) | 374 (50.7%) |
| 26 | M | 176 | 173(198.3) | 3 (1.7%) | 6 | 487 | 242 (49.7%) | 245 (50.3%) |
| 27 | M | 756 | 756 (100.0) | 10 (1.5%) | 6 | 598 | 299 (50.0%) | 299 (50.0%) |
| 28 | M | 560 | 560 (100.0) | 10 (1.5%) | 6 | 771 | 391 (50.7%) | 380 (49.3%) |
F, female; M, male
Ploidy status of nuclear transplants and their progeny
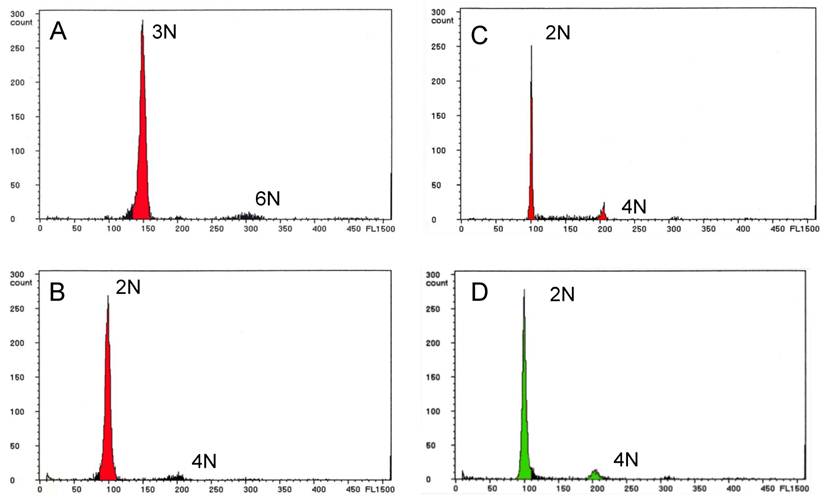
Ploidy status was determined by flow cytometry and the data are summarized in Table 2. Of the 22 infertile NTs with the wild-type phenotype, 19 (67.9%) males (Nos. 3 through 21) were triploid (Fig. 2A), and two males (Nos. 22 and 23) and one female (No. 1) were diploid. The embryos showing abnormal morphology that were obtained from some of the infertile NTs all exhibited aneuploidy (data not shown).
Of the six fertile NTs with the wild-type phenotype, four males (Nos. 24 through 27) and one female (No. 2) were diploid (Fig. 2B), and their F1 progeny were also diploid (Fig. 2C). The last male NT (No. 28) was a mosaic of diploid and tetraploid cells, but produced only diploid F1 progeny. Thus, a total of eight (28.6%) of the NTs with the wild-type phenotype were diploid, and only one (3.5%) was a mosaic of diploid and tetraploid cells. All of the F2 progeny were diploid, as were the three NTs with the golden phenotype and the control fish from the donor strain (Fig. 2D).
Molecular genotyping of slc24a5 in nuclear transplants and their progeny
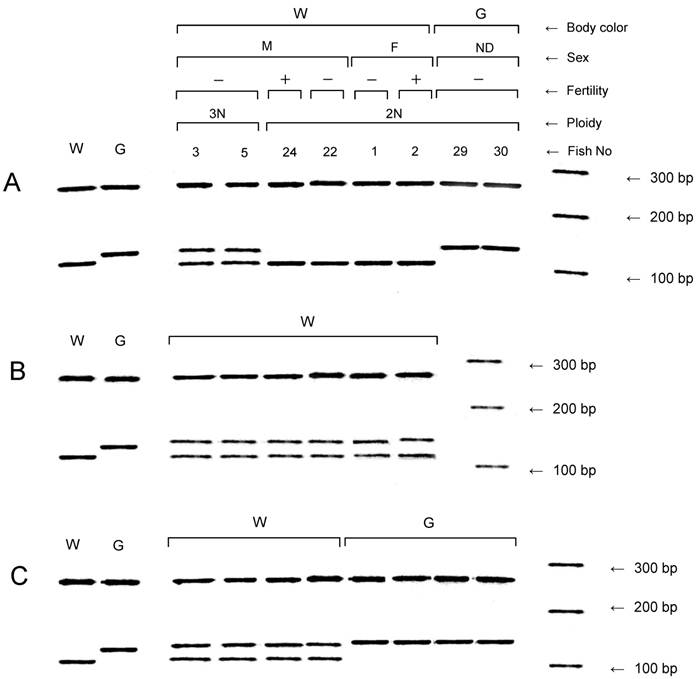
All of the NTs and the six F1 and eight F2 progeny from each of the six fertile NTs were assayed for the other donor marker, slc24a5, by PCR (Fig. 3). The wild-type allele of the gene was confirmed in all of the NTs with the wild-type phenotype, but not in the NTs with the golden phenotype (Table 2 and Fig. 3A). A variant allele of the gene was detected in NTs with the golden phenotype, as well as in all triploid NTs and the mosaic NT which exhibited both diploidy and tetraploidy (No. 28). However, the variant allele was not observed in the diploid NTs with the wild-type phenotype.
In addition to the variant allele, the sequence of the wild-type allele was detected in all 36 of the F1 progeny examined (Fig. 3B). In the F2 generation, the wild-type and variant alleles were detected in individuals with the wild-type phenotype, but the variant allele was only detected in individuals with the golden phenotype (Fig. 3C).
Ploidy analysis of nuclear transplants and F1 progeny by flow cytometry. (A) Nuclear transplant No. 20 showing triploidy. (B) Fertile nuclear transplant No. 27 showing diploidy, and (C) its F1 progeny showing diploidy. (D) Control diploid zebrafish with wild-type body color.
Detection of the golden gene, slc24a5, in nuclear transplants and F1 and F2 progeny. (A) Nuclear transplants. (B) and (C) F1 and F2 progeny, respectively, derived from a fertile diploid nuclear transplant (No. 24) showing the wild-type body color. Right-most lanes in A, B and C, respectively, show a 100 bp ladder. Controls from the wild type strain (W) and the golden variety (G) are shown in the lanes to the left. F, female; G, golden body color; M, male; ND, not determined; W, wild-type body color; +, fertile; -, infertile.
Discussion
In this study, we transferred blastula cell nuclei from a wild-type donor strain to non-enucleated unfertilized eggs of a recipient golden variety of zebrafish to determine whether non-enucleated eggs were suitable for use as nuclear transfer recipients in this species. As a result, 15.2% of the operated eggs reached the adult stage exhibiting the wild-type phenotype, a donor marker, with most being either triploid or diploid. PCR analysis of the slc24a5 gene suggested that the triploid NTs arose due to fusion of the diploid-donor and haploid-recipient genomes, whereas the diploid NTs originated solely from the diploid donor nuclei.
Half of the diploid NTs were fertile and transferred the donor genetic marker to their F1 and F2 progeny in a Mendelian fashion, while one of the diploid NTs was also fertile and produced a small number of F1 progeny with the golden phenotype and numerous progeny with the wild-type phenotype. The origin of these golden F1 progeny and the mechanism by which they are produced is currently unknown and will need to be elucidated in future research. However, the remainder of the diploid NTs and all of the triploid NTs were infertile. One NT with wild-type body coloration was a fertile diploid and tetraploid mosaic. Results of genetic crosses and PCR analysis of the slc24a5 gene in this individual suggest that the diploid cells originated from the diploid donor nucleus, but they did not undergo fusion with the recipient nucleus. Conversely, the tetraploid cells appear to have formed as a result of the fusion of the diploid donor nucleus and a diploidized recipient egg nucleus. Interestingly, the F1 progeny originated from the diploid nuclei but not from the tetraploid nuclei.
Three individuals with the golden phenotype were also obtained in the present study. These fish were diploid and carried only the variant allele of slc24a5, not the wild-type allele. While these findings suggest that the golden individuals originated from recipient nuclei, the diploidization mechanism of the recipient haploid nuclei has not yet been clarified.
The results of the present study corroborate those of our previous studies in medaka, and the characteristics of the NTs described here are considered to be closer to those reported by Bubenshchikova et al. [19] than those of Niwa et al. [17, 18]. For example, as in the report on medaka by Bubenshchikova et al., both diploid and triploid NTs with donor markers were obtained in the zebrafish of the present study, whereas only triploid NTs with donor markers were obtained in the studies of Niwa et al.. Several genetically distinct wild populations of medaka are known to exist [22]. Bubenshchikova et al. used both donor and recipient strains that originated from a population in southern Japan, while Niwa et al. used strains from populations in both northern and southern Japan for the donor and the recipient strains, respectively. In zebrafish, such differences in the genetic characteristics of different populations are not known. It has been reported that the cytoplasmic environment of the recipient affects the development of NTs in cases where donors and recipients are derived from different families or orders [23, 24]. The results of this and previous studies suggest that the likelihood of successfully developing diploid NTs with donor markers increases when the donor and recipient have similar genetic backgrounds.
Although males accounted for most of the wild-type NTs, the reason for this marked sex bias in NTs is not known. Widely varying sex ratios in the offspring of different pairs of parental fish have been reported in zebrafish, but the mechanism underlying sex determination in this species has not yet been clarified [25].
The successful generation of NTs with donor markers was approximately ten times greater in the present study on zebrafish (15.2%) than it was in a previous study on medaka (1.5%) [19]. While the reasons for this disparity are unclear, there were several differences in the experimental systems between these two studies. For example, in the zebrafish experiments, donor nuclei were transferred to activated eggs, whereas in medaka, donor nuclei were transferred to non-activated eggs. In addition, in the medaka experiments, egg activation occurred at the same time as nuclear transfer by stimulating the eggs through pricking with transfer needles.
Over the last 20 years of mammalian cloning studies, enucleated metaphase II oocytes have been used as recipients in nuclear transfer experiments [3]. This is because using zygotes as recipients is not well suited to the development of NT embryos [26]. Similarly, nuclear transfer using recipient eggs arrested at metaphase II by incubation in fish body fluids has also been reported in fish [14, 27]. On the other hand, Egli et al. [28] reported successful cloning in mice using zygotes at the first mitotic phase as recipients. Zygote nuclei have been reported to contain factors that are necessary for the reprogramming of the introduced genome and for embryonic development [29, 30]. The high nuclear transfer success rates achieved in the present study using activated eggs with haploid maternal nuclei as recipients may be indicative of similar conditions in the recipient eggs of fish and mammalian zygotes.
By comparing the similarities of the results obtained in the present study with those obtained previously in medaka, the possibility of successfully applying the methods established in medaka to other fish species is suggested. Nuclear transfer of adult somatic cells to non-enucleated diploidized eggs in zebrafish will be the next step toward achieving that goal.
Acknowledgements
We thank C. Inoue for providing technical assistance. This work was supported by the Program for the Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) and funded by a Research Fellowship from Nagoya University (MH).
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Wilmut I, Schnieke AE, McWhir J. et al. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810-813
2. Meissner A, Jaenisch R. Mammalian nuclear transfer. Dev Dyn. 2006;235:2460-2469
3. Thuan NV, Kishigami S, Wakayama T. How to improve the success rate of mouse cloning technology. J Reprod Dev. 2010;56(1):20-30
4. Wakamatsu Y. Novel method for the nuclear transfer of adult somatic cells in medaka fish (Oryzias latipes): Use of diploidized eggs as recipients. Dev Growth Dif. 2008;50:427-436
5. Di Berardino MA. Genomic potential of differentiated cells. New York, USA: Columbia University Press. 1997
6. Zhu ZY, Sun YH. Embryonic and genetic manipulation in fish. Cell Res. 2000;10:17-27
7. Yan SY. The nucleo-cytoplasmic interactions as revealed by nuclear transplantation in fish. In: (ed.) Malacinski GM. Cytoplasmic Organization Systems: a primer in developmental biology. New York: McGraw-Hill. 1989:61-81
8. Yan SY. Cloning in Fish: Nucleocytoplasmic Hybrids. Hong Kong, China: Educational and Cultural Press. 1998
9. Sun YH, Chen SP, Wang YP. et al. Cytoplasmic impact on cross-genus cloned fish derived from transgenic common carp (Cyprinus carpio) nuclei and goldfish (Carassius auratus) enucleated eggs. Biol Reprod. 2005;72:510-515
10. Pei DS, Sun YH, Chen SP. et al. Identification of differentially expressed genes from the cross-subfamily cloned embryos derived from zebrafish nuclei and rare minnow enucleated eggs. Theriogenology. 2007;68:1282-1291
11. Gasaryan KG, Hung NM, Neyfakh AA. et al. Nuclear transplantation in teleost Misgurnus fossilis. Nature. 1979;280:585-587
12. Wakamatsu Y, Ju B, Pristyaznhyuk I. et al. Fertile and diploid nuclear transplants derived from embryonic cells of a small laboratory fish, medaka (Oryzias latipes). Proc Natl Acad Sci USA. 2001;98:1071-1076
13. Lee KY, Huang H, Ju B. et al. Cloned zebrafish by nuclear transfer from long-term-cultured cells. Nat Biotech. 2002;20:795-799
14. Siripattarapravat K, Pinmee B, Venta PJ. et al. Somatic cell nuclear transfer in zebrafish. Nat Methods. 2009;6:733-735
15. Bubenshchikova E, Kaftanovskaya E, Motosugi N. et al. Reprogramming of adult somatic cell nuclei to pluripotency in fish, medaka (Oryzias latipes), by a novel method of nuclear transfer using diploidized eggs as recipients. Dev Growth Dif. 2007;49:699-709
16. Bubenshchikova E, Kaftanovskaya E, Hattori M. et al. Nuclear transplants from adult somatic cells generated by a novel method using diploidized eggs as recipients in medaka fish (Oryzias latipes). Cloning Stem Cells. 2008;10:443-452
17. Niwa K, Ladygina T, Kinoshita M. et al. Transplantation of blastula nuclei to non-enucleated eggs in the medaka, Oryzias latipes. Dev Growth Dif. 1999;41:163-172
18. Niwa K, Kani K, Kinoshita M. et al. Expression of GFP in nuclear transplants generated by transplantation of embryonic cell nuclei from GFP-transgenic fish into nonenucleated eggs of medaka, Oryzias latipes. Cloning stem Cells. 2000;2:23-34
19. Bubenshchikova E, Ju B, Pristyazhnyuk I. et al. Generation of fertile and diploid fish, medaka (Oryzias latipes), from nuclear transplantation of blastula and four-somite-stage embryonic cells into nonenucleated unfertilized eggs. Cloning Stem Cells. 2005;7:255-264
20. Lamason RL, Mohideen MA, Mest JR. et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782-1786
21. Westerfield M. The Zebrafish Book. 2.1. ed. Eugene, USA: Univ Oregon Press. 1994
22. Sakaizumi M, Moriwaki K, Egami N. Allozymic variation and regional differentiation in wild populations of the fish Oryzias latipes. Copeia. 1983;1983:311-318
23. Yan SY, Tu M, Yang HY. et al. Developmental incompatibility between cell nucleus and cytoplasm as revealed by nuclear transplantation experiments in teleost of different families and orders. Int J Dev Biol. 1990;35:255-265
24. Fujimoto T, Saito T, Sakao S. et al. Developmental potential of embryonic cells in a nucleocytoplasmic hybrid formed using a goldfish haploid nucleus and loach egg cytoplasm. Int J Dev Biol. 2010;54:827-835
25. Orban L, Sreenivasan R, Olsson PE. Long and winding roads: Testis differentiation in zebrafish. Mol Cell Endocrinol. 2009;312:35-41
26. Wakayama T, Tateno H, Mombaerts P. et al. Nuclear transfer into mouse zygotes. Nat Genet. 2000;24:108-109
27. Le Bail P-Y, Depince A, Chenais N. et al. Optimization of somatic cell injection in the perspective of nuclear transfer in goldfish. BMC Dev Biol. 2010;10:64
28. Egli D, Rosains J, Birkhoff G. et al. Developmental reprogramming after chromosome transfer into mitotic mouse zygotes. Nature. 2007;447:679-685
29. Gręda P, Karasiewicz J, Modliński JA. Mouse zygotes as recipients in embryo cloning. Reproduction. 2006;132:741-748
30. Ogushi S, Palmieri C, Fulka H. et al. The maternal nucleus is essential for early embryonic development in mammals. Science. 2008;319:613-616
Author contact
![]() Corresponding author: Email: ywakamateonet.ne.jp
Corresponding author: Email: ywakamateonet.ne.jp

 Global reach, higher impact
Global reach, higher impact