10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2011; 7(4):469-475. doi:10.7150/ijbs.7.469 This issue Cite
Research Paper
Sperm Nuclear Transfer and Transgenic Production in the Fish Medaka
1. Department of Biological Sciences, National University of Singapore, 10 Kent Ridge Crescent, Singapore 119260;
2. Key Laboratory of Freshwater Ecology, Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences, No. 8, 1st Wudayuan Road, Donghu Hi-Tech Development Zone, Wuhan, Hubei 430223, China.
#Present address: Department of Orthopaedic Surgery, National University of Singapore; DSO Kent Ridge 27, Medical Drive Singapore 119074
Received 2010-12-29; Accepted 2011-4-10; Published 2011-4-16
Abstract
Sperm nuclear transfer or intracytoplasmic sperm injection (ICSI) is a powerful assisted reproductive technology (ART) for treating human male infertility. Controversial reports of increased birth defects have raised concerns about the ART's safety. The cause for birth defects, however, has remained elusive for analysis in human because of the sample size, male infertility genetics, physiological heterogeneity and associated procedures such as embryo manipulations. Animal models are required to evaluate factors leading to the increased birth defects. Here we report the establishment of medakafish model for ICSI and transgenic production. This small laboratory fish has high fecundity and easy embryology. We show that ICSI produced a 5% high percentage of fertile animals that exhibited both paternal and maternal contribution as evidenced by the pigmentation marker. Furthermore, when sperm were pre-incubated with a plasmid ubiquitously expressing RFP and subjected to ICSI, 50% of sperm nuclear transplants showed germline transmission. We conclude that medaka is an excellent model for ICSI to evaluate birth defects and that sperm nuclear transfer can mediate stable gene transfer at high efficiency. Although more demanding for experimentation, sperm-mediated transgenesis should be particularly applicable for aquaculture species with a lengthy generation time and/or a large adult body size.
Keywords: medaka, nuclear transfer, sperm, gene transfer, transgenesis
Introduction
New assisted reproductive technology (ART) greatly enhances our ability to treat human infertility. For example, the development of sperm nuclear transfer or intracytoplasmic sperm injection (ICSI) has revolutionized the use of ART for treating human male infertility [1]. Since the birth of baby Louise Brown in 1978, who was conceived by in vitro fertilization (IVF) [2], ICSI in combination with IVF has increasingly been used to achieve pregnancies in couples with male infertility, resulting in more than 1 million babies worldwide conceived in this manner. In 2003 nearly 3% of children born in Scandinavia and 1.7% of children born in France were conceived using ICSI [3]. ART overcomes natural barriers that prevent fertilization. In contrast to many other therapeutic procedures, ART has, however, never been rigorously tested for safety before clinical use. The safety of ART has been questioned for 33 years since the first IVF-mediated birth and 19 years after the first use of ICSI. There is an incomplete and controversial picture of the risks associated with ART, which suggests that ICSI-conceived children are at increased risk of congenital defects and epigenetic syndromes [4].
In human, it is challenging to establish whether ART-associated risks are related to the technology itself or the genetic and physiological defects of the parents [3]. In this regard, studies in model organisms will provide experimental data on genetic and technical parameters of ART-mediated reproduction. In particular, lower vertebrates such as fish offer excellent systems to evaluate ICSI-associated risks. Fish produce a large number of gametes and have external embryology. Nuclear transfer in fish has a long history of research [5]. Cell culture-derived nuclear transplant was obtained in 1986 [6], even 10 years before the cloned sheep Dolly [7]. Recently, procedures for somatic cell nuclear transfer have been well established in several species including zebrafish [8; 9] and medaka [10]. Medaka is an excellent model for stem cell biology [11-14], germ cell biology [15; 16] and reproductive biology [15; 17; 18]. Most importantly, we have shown in medaka that haploid embryonic stem cell nuclear transfer can generate a fertile semicloned fish called Holly [19]. In addition, ICSI has also been used to mediate stable gene transfer in zebrafish [20]. This study was aimed at the use of medaka as fish model to develop procedures for ICSI and sperm-mediated gene transfer.
2. Materials and Methods
2.1. Fish
Work with fish followed the guidelines on the care and use of animals for scientific purposes of the National Advisory Committee for laboratory animal research in Singapore. Medaka was maintained under an artificial photoperiod of 14-h light to 10-h [16] darkness.
2.2. Plasmids
Plasmid DNA was prepared according to the Qiagen midi-preps procedure (Diagen). Two plasmids used were pHygGFP (Invitrogen) expressing a fusion between hygromycin resistance and green fluorescent protein (GFP) and pDsRED-N1 expressing red fluorescent protein (RFP; Invitrogen). For linearization, plasmids were cut with NotI and purified with the Qiagen column.
2.3. Sperm nuclei preparation
Fish were anaesthetized in the Arowana stabilizer (Qian Hu Corp Ltd, Singapore). Testes were dissected from two adult males of i1 albino medaka [19]. Sperm were prepared and subjected to demembranation by using digitonin [21], incorporating the modifications of the Browder laboratory (http://www.ucalgary.ca/UofC/eduweb/virtualembryo/frogs2.html), e.g., omission of protease inhibitors. The density of nuclei was counted on a hemocytometer following staining with propidium iodide (final 10 μg/ml) for 10 min. Aliquots of 10-μl suspension at approximately 100 nuclei/nl were made in 0.2-ml tubes, quickly frozen in liquid nitrogen and stored at -80°C until use.
2.4. Oocyte preparation
Spawning females were selected one day before the experiment. On the day of experiment, fish were subjected sequentially to anesthetic treatment in the Arowana stabilizer, 70% ethanol spray and surface-drying by a paper towel to remove any residual water. Ovaries were dissected in medaka oocyte medium (MOM; 9.8 g/L medium M199 and antibiotics, pH 8.0) in a 60-mm Petri dish, and mature oocytes were released and transferred into 5 ml of MOM in a 35-mm-dish at room temperature for use within 6 h.
2.5. Sperm nuclear transfer
Mature oocytes were arranged in rows on 1.5%-agarose ramps in Ringer´s solution in 6-cm dishes covered with MOM, so that the animal pole demarcated by the micropyle facing up with an angle of approximately 40 degree pointing to the microinjection needle tip. Nuclear suspension was diluted to 10 nuclei/nl in nucleus dilution buffer (250 mM sucrose, 75 mM KCl, 0.5 mM spermidine trihydrochloride, 0.2 mM spermine tetrahydrochloride, 30% glycerol, pH 7.4), and loaded onto a flat surface. Single nuclei were sucked into the microinjection needle. Approximately 100 pl of suspension containing one nucleus on average was injected through the micropyle into non-enucleated oocytes. Injection needles were made by pulling thin-walled capillaries (Clarks GC100T) on a Flaming-Brown puller (Sutter P87 with box filament), and the tips were broken with forceps to make an opening with an outer diameter of approximately 10-μm, ~3 times the diameter of approximately 4 μm for medaka sperm nuclei[15]. Loading of nuclear suspension by sucking a negative pressure into the injection needle was monitored under a Leica MZ12 dissecting microscope, as sperm nuclei are visible with dark field illumination. Nuclear injection proceeded in two steps. Under bright field illumination, the microinjection needle was allowed to penetrate the tough chorion through the micropyle by a quick forward movement in the first step, so that the tip reached the cell membrane outsides the cytoplasm that is visible as a crescent-like transparent area of approximately 20 μm in diameter and 10 μm in depth. On the oocyte surface, the micropyle appears as an approximately 5-μm milky spot residing in the center of an attachment-free area of approximately 20-μm directly atop of the cytoplasm. In the second step, the needle penetrated quickly the cell membrane into the cytoplasm in the presence of a positive pressure to form an outflow to deliver nuclei and simultaneously prevent cytoplasm inward flow that causes damage. Injection was monitored by the formation of a “bulb-like” droplet of injected suspension and a cortical reaction-like movement - rapid retraction of cytoplasmic and cell membrane in response to needle penetration across the cell membrane. At the end of injection, the needle was quickly withdrawn from the oocyte again in the continuous presence of a positive pressure. After injection of all oocytes completed, MOM was discarded and embryo rearing medium (ERM; [13]) was added. In ERM, well-injected oocytes became transparent within 15 min, while non-injected oocytes and badly treated oocytes became opaque. After 1.5-h incubation in ERM, cleavage occurred as in artificially inseminated embryos, and non-cleaving embryos were removed. The operated oocytes were incubated in ERM in Petri dishes until hatching and fry were grown in glass tanks.
2.6. Sperm-mediated gene transfer
Plasmid DNA was diluted at final 100 ng/μl in water. A 10-μl aliquot of sperm nuclear suspension was thawed on ice, to which 2 μl of circular pHygGFP or pDsRED-N1 was added. After incubation at room temperature for 10 min, the nuclei were injected into non-enucleated oocytes as described above.
2.7. Microscopy
Whole embryos were visualized on a Leica MZFIII stereo microscope and photographed using a Nikon E4500 digital camera (Nikon Corp). Observation and documentation of sperm and embryos at higher magnifications were under a Zeiss Axiovert invert microscope by using a Zeiss AxioCam MRc digital camera [11]. Certain embryos were fixed in 2% formaldehyde in PBS for 15 min at room temperature, then rinsed in PBS and stored at 4°C. This light fixation diminishes autofluorescence.
2.8. Statistics
Statistical analyses were calculated by using Graphad Prism v4.0. Data consolidated were presented as mean ± s. d. and p values were calculated by using non-parametric student's t-test. Genetic data were evaluated by using chi-square test [19].
3. Results
3.1. Sperm nuclear transfer
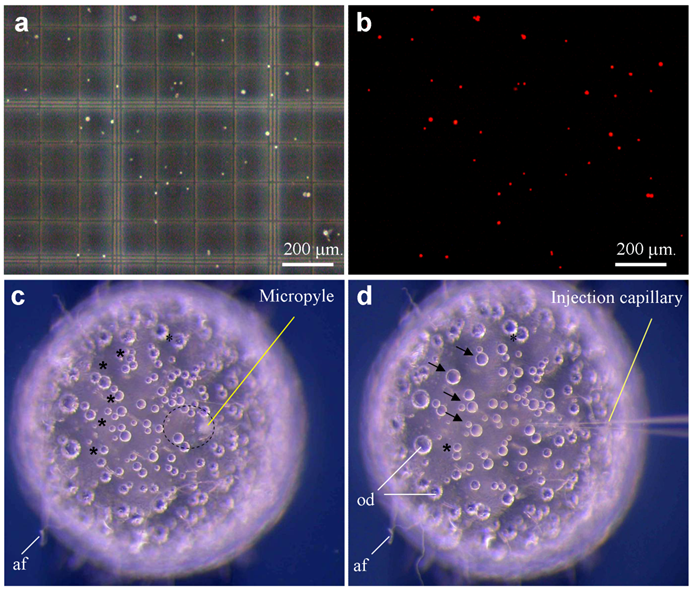
Medaka sperm nuclei were treated with digitonin, and damage to the plasma membrane was monitored by staining with propidium iodide (PI) as a vital fluorescent dye. Control sperm nuclei were rarely labeled when incubated in 1 μg/ml PI prior to digitonin treatment, whereas all digitonin-treated nuclei were fluorescent (Fig. 1a-b). To establish the conditions that prevent nuclei from settling down during the injection procedure, we made use of two approaches. First, nuclei in sperm dilution buffer that contains 30% glycerol remained to be suspended individually. Second, individual nuclei were loaded by sucking at regular intervals from the tip under a dissecting microscope. Nuclei loaded in this way remained floating in the needle, providing a reliable rate of nuclear injection for up to 1 h.
Sperm nuclei were then injected into the cytoplasm at the animal pole region of eggs. On the egg surface of the animal pole, there is an area of ~20 μm in diameter, which is free of attachment filaments. Roughly at the center of this smooth area, a milky spot called micropyle is visible, extending from the surface to the cytoplasm (Fig. 1c), which is the canal that the sperm makes use for penetration during natural fertilization. Medaka eggs have tough chorion and are thus robust for orientation in an agarose groove and for precise penetration by an injection needle (Fig. 1d). However, the tough chorion prevents easy needle penetration. After testing several regimes, we established a 2-step procedure for nuclear injection through the micropyle. The first step aims at chorion penetration and orientation of a proper angle and position relative to the cytoplasm. The second step penetrates the cell membrane and simultaneously injects the nuclear suspension, which are monitored by a cortical reaction-like retraction and a tiny “bulb-like” volume resulting from injected suspension. The formation of a bulb is indicative of proper injection and delivery. During each step of penetration and withdrawal, the application of a quick movement and positive pressure was found to be essential to prevent damage due to a change in the egg pressure.
Out of 223 eggs that received sperm nuclear injection in four independent experiments, 19 reached the gastrula stage, 15 hatched out to swimming fry and 11 developed into adult fish, producing efficiencies of 8.5%, 6.7% and 4.9% for the respective stages (Table 1). Sperm nuclear transplants (SNTs) that survived to advanced stages beyond day 3 post fertilization exhibited a normal development or nearly so, and more importantly, biparental contribution from both sperm nucleus and oocyte nucleus as evidenced by wildtype pigmentation (see below). Therefore, sperm nuclear transfer allows a relatively high efficiency of 5% for normal development in medaka.
Sperm nuclei and ICSI. (a-b) Sperm after demembranation and propidium iodide staining (red) were detected under phase-contrast (a) and fluorescent optics (b). A sperm nucleus is 4 μm. (c) Mature oocyte showing micropyle and the cytoplasmic region (circle) free of attachment filaments (af). (d) The same oocyte shown in (c) after sperm nuclear transfer, showing the injection capillary and oil droplets (od) that are fused (arrow) and fusing (asterisks). The oocyte is 1 mm in diameter.
Sperm nuclear transplantation
| Method of injection | Number of experiments | Eggs injected | Gastrula, n (%)1) | Fry, n (%)1) | Adult, n (%)1) | Germline transmission n (%)2) |
|---|---|---|---|---|---|---|
| ICSI | 4 | 223 | 19 (8.5) | 13 (5.8) | 11 (4.9) | Nd |
| ICSI + linear pDsRed-N1 | 3 | 112 | 8 (7.1) | 7 (6.3) | 6 (5.5) | 3 (50) |
1) Derived by comparison to the total number of eggs injected.
2) Derived by comparison to the number of adults.
3.2. Sperm mediated gene transfer
We then tested the SNT-mediated gene transfer. For this, demembranated sperm were incubated with DNA of plasmid pDsRed-N1 and similarly injected through the micropyle into the ooplasm. In total, 112 oocytes were operated in two batches of experiments, leading to 8 embryos at the gastrula stage, 7 fry that ultimately developed into 6 adults, thus an efficiency of 5%. This indicates that preincubation with plasmid DNA does not compromise the ICSI efficiency.
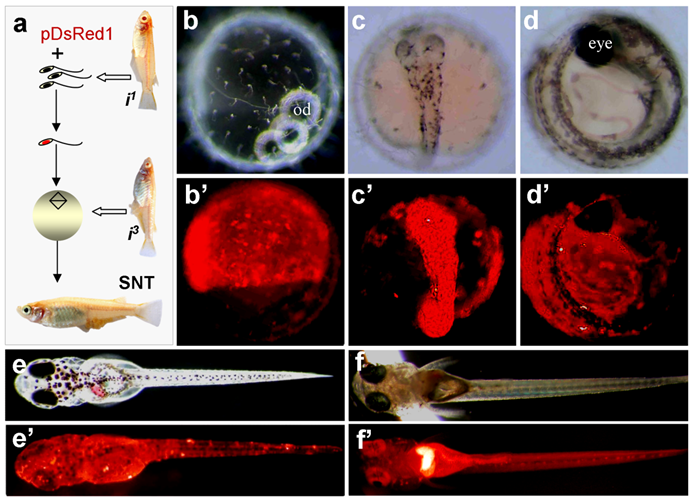
In our experiments, sperm nuclei from albino strain i1 were transplanted into non-enucleated mature oocytes of albino strain i3. The combination of the nuclei between these two albino strains by fertilization or semicloning via haploid embryonic stem cell nuclear transfer produces wild-type pigmented F1 [19]. All SNTs were found to be of biparental constitution, as they displayed wild-type pigmentation at advanced embryonic stages and adulthood (Fig. 2a-e). Notably, 5 out of the 8 embryos possessed RFP expression at a high level. One of them is illustrated in Fig. 2a-e.
One embryo died before hatching. Six out of the 7 fry survived to adulthood, leading to 2 male and 4 female adults. Germline transmission was investigated by monitoring RFP expression in F1 hybrids from test-crossing between individual SNTs and non-transgenic i1 medaka. Of the 6 SNT adults, 4 showed germline transmission, producing an efficiency of 67%. Approximately 50% of F1 progeny displayed the 1:1 ratio of Mendelian segregation of albino and wildtype pigmentation (Table 2), conforming to biparental contribution from both the egg and sperm, as has been described for Holly production by direct transfer of i1-derived haploid embryonic stem cell nuclei into the i3 eggs [19]. The proportion of RFP-positive progeny ranged from 17% to 87% (Table 2). Transgenic RFP expression in these F1 progeny was found to be in the entire body (Fig. 2f and f').
Production of transgenic sperm nuclear transplant. (a) Flow chat of the procedure. Sperm nuclei after demembration treatments are incubated with pDsRed1 DNA and individually injected into normal mature eggs, resulting in the restoration of diploidy in the eggs that develop into sperm nuclear transplants containing sperm-mediated transgene pDsRed1. Notably, the combination of nuclei between two albino strains produces wildtype pigmentation, most evident in the eye. (b-e') A representative sperm nuclear transplant at different stages of development, showing RFP expression and wildtype pigmentation. (f and f') Transgenic F1 progeny from a cross between the sperm nuclear transplant male (shown in Table 1) and albino strains i1, showing pigmentation and ubiquitous RFP expression.
Germline transmission in F1 progeny1)
| SNT (sex) | Total of Progeny | RPF-positive progeny, n (%) | RPF-negative progeny, n (%) | ||||
|---|---|---|---|---|---|---|---|
| Subtotal2) | Albino3) | Pigmented3) | Subtotal2) | Albino3) | Pigmented3) | ||
| 1 (♀) | 53 | 9 (17) | 4 | 5 | 44 | 21 | 23 |
| 2 (♀) | 64 | 13 (22) | 5 | 8 | 51 | 23 | 28 |
| 3 (♀) | 73 | 41 (56) | 23 | 18 | 32 | 19 | 13 |
| 4 (♂) | 200 | 174 (87) | 91 | 87 | 22 | 11 | 11 |
| Sum | 390 | 237 (61) | 123 (52) | 118 (48) | 149 (39) | 74 (50) | 75 (50) |
1) SNTs were crossed to the opposite sex of non-transgenic i1 medaka. RFP expression was monitored throughout embryonic development. Pigmentation was monitored from day 3 post fertilization onwards.
2) Derived by comparison to the total number of progeny observed.
3) Derived by comparison to the subtotal of progeny.
4. Discussion
In this study, we report sperm nuclear transfer and gene transfer in the fish medaka. We show that sperm nuclear transfer with or without a step of preincubation with DNA reproducibly gives rise to a 5% efficiency for the production of adult nuclear transplants. This efficiency is comparable to that for semicloning of Holly by using the mitotic nuclei of haploid embryonic stem cells [19], but higher than that of first somatic cell nuclear transfer experiments in the crucian carp [6]. The high success rate of sperm nuclear transfer and semicloning may largely be ascribed to the omission of enucleation, which represents a major step responsible for a reduction in survival rate due to mechanical damages in host eggs.
Our observations that both sperm and haploid embryonic stem cells generate a comparably high efficiency of nuclear transplant production indicate that haploid meiotic and mitotic nuclei are not different in animal production. In human, round spermatid injection (ROSI) has been proposed as a treatment for men in whom mature sperm forms (elongating spermatids or spermatozoa) cannot be identified for ICSI [22]. ROSI has not been widely performed or as successful as ICSI. Because ROSI involves the use of immature meiotic products, the procedure presents new technical challenges and raises new unresolved genetic concerns on the safety. In this regard, medaka may provide a testing organism.
We demonstrate that sperm nuclear transfer can mediate stable gene transfer at high efficiency of up to 67%. Although this procedure is more demanding than cytoplasmic microinjection, sperm-mediated transgenesis should be particularly applicable for aquaculture species with a lengthy generation time and/or a large adult body size. In fact, sperm-mediated transgenesis has recently applied to flounder [23] and catfish [24] by artificial insemination with sperm that have been pre-incubated with DNA.
Acknowledgements
We thank J. Deng for collecting fish samples. This work was supported by the Biomedical Research Council of Singapore (R-08-1-21-19-585 & SBIC-SSCC C-002-2007).
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17-18
2. Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2:366
3. Matzuk MM, Lamb DJ. The biology of infertility: Research advances and clinical challenges. Nat Med. 2008;14:1197-1213
4. Alukal JP, Lamb DJ. Intracytoplasmic sperm injection (ICSI)--what are the risks? Urol Clin North Am. 2008;35:277-288
5. Yan SY, Tu M, Yang HY, Mao ZG. et al. Developmental incompatibility between cell nucleus and cytoplasm as revealed by nuclear transplantation experiments in teleost of different families and orders. Int J Dev Biol. 1990;34:255-266
6. Chen H, Yi Y, Chen M, Yang X. Studies on the developmental potentiality of cultured cell nuclei of fish. Int J Biol Sci. 2010;6:192-198
7. Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64-66
8. Lee KY, Huang H, Ju B, Yang Z, Lin S. Cloned zebrafish by nuclear transfer from long-term-cultured cells. Nat Biotechnol. 2002;20:795-799
9. Siripattarapravat K, Pinmee B, Venta PJ, Chang CC, Cibelli JB. Somatic cell nuclear transfer in zebrafish. Nat Methods. 2009;6:733-735
10. Wakamatsu Y, Ju B, Pristyaznhyuk I, Niwa K. et al. Fertile and diploid nuclear transplants derived from embryonic cells of a small laboratory fish, medaka (oryzias latipes). Proc Natl Acad Sci U S A. 2001;98:1071-1076
11. Hong N, Li M, Zeng Z, Yi M. et al. Accessibility of host cell lineages to medaka stem cells depends on genetic background and irradiation of recipient embryos. Cell Mol Life Sci. 2010Apr;67(7):1189-1202
12. Hong Y, Winkler C, Schartl M. Pluripotency and differentiation of embryonic stem cell lines from the medakafish (Oryzias latipes). Mech Dev. 1996;60:33-44
13. Hong Y, Winkler C, Schartl M. Production of medakafish chimeras from a stable embryonic stem cell line. Proc Natl Acad Sci U S A. 1998;95:3679-3684
14. Yan Y, Du J, Chen T, Yi M. et al. Establishment of medakafish as a model for stem cell-based gene therapy: Efficient gene delivery and potential chromosomal integration by baculoviral vectors. Exp Cell Res. 2009;315:2322-2331
15. Hong Y, Liu T, Zhao H, Xu H. et al. Establishment of a normal medakafish spermatogonial cell line capable of sperm production in vitro. Proc Natl Acad Sci U S A. 2004;101:8011-8016
16. Li M, Hong N, Xu H, Yi M. et al. Medaka vasa is required for migration but not survival of primordial germ cells. Mech Dev. 2009;126:366-381
17. Xu H, Li M, Gui J, Hong Y. Fish germ cells. Sci China Life Sci. 2010;53:435-446
18. Xu H, Li Z, Li M, Wang L, Hong Y. Boule is present in fish and bisexually expressed in adult and embryonic germ cells of medaka. PLoS One. 2009;4:e6097
19. Yi M, Hong N, Hong Y. Generation of medaka fish haploid embryonic stem cells. Science. 2009;326:430-433
20. Jesuthasan S, Subburaju S. Gene transfer into zebrafish by sperm nuclear transplantation. Dev Biol. 2002;242:88-95
21. Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal fgf signaling requirements during gastrulation. Development. 1996;122:3173-3183
22. Miki H, Hirose M, Ogonuki N, Inoue K. et al. Efficient production of androgenetic embryos by round spermatid injection. Genesis. 2009;47:155-160
23. Lanes CF, Sampaio LA, Marins LF. Evaluation of dnase activity in seminal plasma and uptake of exogenous DNA by spermatozoa of the brazilian flounder Paralichthys orbignyanus. Theriogenology. 2009;71:525-533
24. Collares T, Campos VF, Seixas FK, Cavalcanti PV. et al. Transgene transmission in south american catfish (Rhamdia quelen) larvae by sperm-mediated gene transfer. J Biosci. 2010;35:39-47
Author contact
![]() Corresponding author: Prof. Yunhan Hong, Department of Biological Sciences, National University of Singapore, 10 Kent Ridge Crescent, Singapore 119260. Fax: +65 6779 2486; Tel: +65 6516 2915; Email: dbshyhedu.sg
Corresponding author: Prof. Yunhan Hong, Department of Biological Sciences, National University of Singapore, 10 Kent Ridge Crescent, Singapore 119260. Fax: +65 6779 2486; Tel: +65 6516 2915; Email: dbshyhedu.sg

 Global reach, higher impact
Global reach, higher impact