10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2011; 7(4):476-486. doi:10.7150/ijbs.7.476 This issue Cite
Research Paper
Critical Developmental Stages for the Efficiency of Somatic Cell Nuclear Transfer in Zebrafish
1. State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China
2. School of Basic Medical Science, Wuhan University, Wuhan, China
Received 2010-12-24; Accepted 2011-4-1; Published 2011-4-16
Abstract
Somatic cell nuclear transfer (SCNT) has been performed extensively in fish since the 1960s with a generally low efficiency of approximately 1%. Little is known about somatic nuclear reprogramming in fish. Here, we utilized the zebrafish as a model to study reprogramming events of nuclei from tail, liver and kidney cells by SCNT. We produced a total of 4,796 reconstituted embryos and obtained a high survival rate of 58.9-67.4% initially at the 8-cell stage. The survival rate exhibited two steps of dramatic decrease, leading to 8.7-13.9% at the dome stage and to 1.5-2.96% by the shield stage. Concurrently, we observed that SCNT embryos displayed apparently delayed development also at the two stages, namely the dome stage (1:30 ± 0:40) and the shield stage (2:50 ± 0:50), indicating that the dome and shield stage are critical for the SCNT efficiency. Interestingly, we also revealed that an apparent alteration in klf4 and mycb expression occurred at the dome stage in SCNT embryos from all the three donor cell sources. Taken together, these results suggest that the dome stage is critical for the SCNT efficiency, and that alternated gene expression appears to be common to SCNT embryos independently of the donor cell types, suggesting that balanced mycb and klf4 expression at this stage is important for proper reprogramming of somatic nuclei in zebrafish SCNT embryos. Although the significant alteration in klf4 and mycb expression was not identified at the shield stage between ZD and SCNT embryos, the importance of reprogramming processes at the shield stage should not be underestimated in zebrafish SCNT embryos.
Keywords: SCNT, reprogramming, dome stage, shield stage, klf4 and mycb
Introduction
Somatic cell nuclear transfer (SCNT) has widely been done mostly in aquaculture fish species since the 1960s [1-5]. Recently, SCNT has successfully been applied to laboratory fish models, leading to the production of cloned zebrafish and medaka [6-11]. A generally low efficiency of approximately 1% has been documented for SCNT in fish and other species [12-13]. However, this can be compensated since many eggs are readily available in fish [14]. The ability and convenience for dynamic observation would bring even greater utility to fish in SCNT research, especially the zebrafish, which due to its transparent embryos and suitability for forward-genetics studies is already used extensively as a model vertebrate organism for studying development and disease.
In 2002, Lee et al. obtained first feeding SCNT zebrafish by transplanting long-term cultured cells into enucleated eggs [6], and a similar SCNT procedure was independently established also in our laboratory [11]. Recently, Siripattarapravat et al. developed a method using laser-ablated metaphase II eggs as recipients and an egg activation protocol after nuclear reconstruction in zebrafish, producing only SCNT embryos but not adults [15]. Although apparently healthy SCNT embryos were reported [6, 10-11, 15-16], a major obstacle of zebrafish SCNT experiments is the paucity of knowledge about somatic nuclear reprogramming in reconstituted eggs. The SCNT efficiency may vary considerably with the source and types of somatic donor cells, because they may have different capacities to be reprogrammed by ooplasma. It is therefore intriguing to identify factors that regulate the potential of somatic donor cells for reprogramming. One way is to analyze the gene expression profile [17]. Reprogramming-related alterations of gene expression have been documented in mammalian SCNT embryos [18-20]. Previously, we have observed altered gene expression also in fish SCNT [10, 21], which is most evident at the dome stage [10], when the majority of SCNT embryos derived from kidney cells exhibited incomplete reprogramming processes.
Developmental retardation of various mammalian SCNT embryos during the pre-implantation stages is also a well-documented phenomenon [20]. Concerns also existed as to whether a similar form of retardation occurs in zebrafish. Based on the data of kidney cell derived SCNT embryos, we speculated that these SCNT embryos would finally fail to develop into adult animals, and that the dome stage is a significant developmental stage in these zebrafish SCNT embryos [10]. Although these previous studies have provided a solid basis for understanding the reprogramming process in zebrafish SCNT embryos, it leaves the unresolved issue of how the dome stage retardation and incomplete reprogramming progresses could commonly occur in zebrafish SCNT embryos derived from other cells. It was suggested that the type of donor cells could affect the development of nuclear transferred embryos or the somatic nuclear reprogramming process in the oocyte [12, 22]; thus, this issue should be verified in zebrafish SCNT embryos derived from different cells.
Previously, we have reported that mycb and klf4 have altered expression in the kidney cell-derived zebrafish SCNT embryos [10], which is similar to reprogramming of differentiated cells into a pluripotent state in vitro [23-24]. These observations suggest that a balance between mycb and klf4 expression may be important for the reprogramming process in zebrafish SCNT embryos. We have also revealed that an apparent difference in klf4 and mycb expression occurs at the dome stage [10]. Since the dome and shield stage are critical for the SCNT efficiency, proper mycb and klf4 expression at the dome stage may be important for reprogramming. In this study, we made use of three different sources of donor cells from tail, kidney and liver to produce zebrafish SCNT embryos. We analyzed their development and gene expression profile of SCNT embryos at critical stages. We found that SCNT embryos derived from different donor cell sources were similar in development and gene expression.
Results
Early development of kidney cell derived SCNT embryos in zebrafish
In the present study, eight stages of early development were chosen for monitoring SCNT embryogenesis. These are 2-, 8-, 256-cell stages, high, dome, 30% epiboly, shield and 75% epiboly stages. SCNT embryos were staged on the basis of morphological features, by comparison to the developmental stages of the ZD embryos [25].
Observations of the early development of SCNT embryos obtained by injection of enucleated eggs with nuclei from donor kidney cells (KC SCNT embryos) as recorded in Table 1 showed that in the three batches 69.0% (1044/1511) of the transplanted eggs cleaved. The overwhelming majority, 94.1% (983/1044) of these cleaved embryos easily developed to the 8-cell stage. The great majority, 79.6% (783/983) of 8-cell stage SCNT embryos developed to the 256-cell stage. There were 46.94% to 50.44% of the SCNT embryos that developed to the high stage, although only 13.3% (201/1511) of these transplants developed to the dome stage. The developmental block apparently occurred between the high and dome stage, which seemed to be a critical stage in the development of SCNT embryos [21, 26-27]. Interestingly, 88.57-97.47% of these dome stage embryos easily developed to the 30% epiboly stage. Unfortunately, just 2.60-2.96% of the transplanted eggs could complete blastulae to undergo gastrulation, and 14.28-18.75% of these shield stage embryos could further develop to 75% epiboly. In this study, we focused on the early development of SCNT, so we did not further investigate the SCNT embryos. However, it is worth mentioning that SCNT embryos beyond gastrulation usually develop into adult individuals [27]. Our findings indicated that dome stage and shield stage are the barrier stages that results in low SCNT efficiency [6, 10, 16, 21, 27-30].
Nuclear transplants generated using kidney cells
| No. Of Egg operated | No. Of 2-cell stage | No. Of 8-cell stage | No. Of 256-cell stage | No. Of High stage | No. Of Dome stage | No. Of 30% Epiboly | No. Of Shield stage | No. Of 75% Epiboly |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |
| 405 (Exp.1) | 271 | 257 | 211 | 197 | 55 | 51 | 12 | 2 |
| (66.91) | (63.45) | (52.10) | (48.64) | (13.58) | (12.59) | (2.96) | (0.49) | |
| 539 (Exp.2) | 375 | 344 | 273 | 253 | 70 | 62 | 14 | 2 |
| (69.57) | (63.82) | (50.65) | (46.94) | (12.99) | (11.50) | (2.60) | (0.37) | |
| 567 (Exp.3) | 398 | 382 | 299 | 286 | 79 | 77 | 16 | 3 |
| (70.19) | (67.37) | (52.73) | (50.44) | (13.93) | (13.58) | (2.82) | (0.53) |
Nuclear transplants generated using liver cells
| No. Of Egg operated | No. Of 2-cell stage | No. Of 8-cell stage | No. Of 256-cell stage | No. Of High stage | No. Of Dome stage | No. Of 30% Epiboly | No. Of Shield stage | No. Of 75% Epiboly |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |
| 387 (Exp.1) | 266 | 228 | 201 | 190 | 49 | 46 | 8 | 1 |
| (68.73) | (58.91) | (51.94) | (49.09) | (12.66) | (11.89) | (2.06) | (0.25) | |
| 520 (Exp.2) | 342 | 317 | 263 | 247 | 58 | 58 | 13 | 1 |
| (65.77) | (60.96) | (50.57) | (47.50) | (11.15) | (11.15) | (2.50) | (0.19) | |
| 312 (Exp.3) | 210 | 196 | 164 | 151 | 38 | 36 | 7 | 1 |
| (67.31) | (62.82) | (52.56) | (48.40) | (12.18) | (11.54) | (2.24) | (0.14) |
Early development of liver cell derived SCNT embryos in zebrafish
Table 2 summarizes the early development of liver cell derived SCNT embryos (LC SCNT embryos), where 67.1% (818/1219) of the transplanted eggs cleaved after nuclear transfer; and 90.6% (741/818) of these embryos easily developed to the 8-cell stage. The cleavage rate of LC SCNT embryos was very similar to that of the KC SCNT embryos. However, 84.40% (628/744) of the 8-cell stage embryos developed to the 256-cell stage, which was significantly higher than the 79.6% rate of the KC SCNT embryos. The 8-cell stage and 256-cell stage are subdivided stages of the cleavage period and blastulae period, respectively. The different developmental rates demonstrated that KC and LC SCNT embryos undergo different reprogramming processes between the cleavage and blastulae periods.
Interestingly, there were 47.5-49.1% SCNT embryos that developed to the high stage, and only 11.15%-11.89% of these transplants developed to the dome stage. Therefore, there was no significant difference between the KC and LC SCNT embryos until the blastulae stage, as 96.5% (140/145) of these dome-stage embryos easily developed to the 30% epiboly stage. Notably, only 2.06-2.50% of the transplanted eggs could complete blastulae to undergo gastrulation, and 7.69-14.2% of them developed to 75% epiboly. Compared with that of the KC SCNT embryos, more LC SCNT embryos underwent incomplete reprogramming from blastulae to the gastrula periods.
Early development of tail cell derived SCNT embryos in zebrafish
The early development of tail cell derived SCNT embryos (TC SCNT embryos) are summarized in Table 3, where 67.2% (1389/2066) of the transplanted eggs cleaved after nuclear transfer; and 89.8% (1246/1389) of these embryos, easily developed to the 8-cell stage. Of these 8-cell stage SCNT embryos, 76.2% (950/1246) developed to the 256-cell stage. There were 39.7-44.8% SCNT embryos that developed to the high stage; however, only 8.9% (183/2066) of these transplants developed to the dome stage. From cleavage to the blastulae period, the developmental rate of TC SCNT embryos was the lowest among the three types of SCNT embryos at every subdivided stage. Interestingly, 96.1-98.4% of these dome stage embryos also easily developed to the 30% epiboly stage. Unfortunately, only 1.5-1.8% of the transplanted eggs could complete blastulae to undergo gastrulation, resulting in the lowest efficiency recorded between the blastulae and gastrula stages. However, 9.1-14.2% of them could develop to 75% epiboly in the gastrula period, indicating that the block between the blastulae and gastrula periods had more of an effect on the development of SCNT embryos than that in the gastrula period.
Main features in early development of zebrafish SCNT embryos
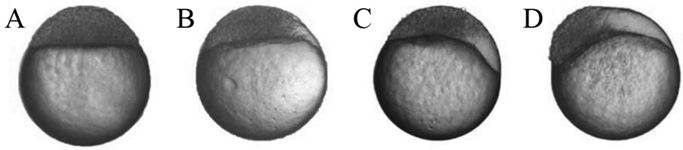
A staging series is a tool that provides accuracy in developmental studies [25]. According to the staging series of zebrafish, we recorded the early development of kidney, liver and tail cell derived SCNT embryos. Fig. 1A shows normal morphology in the blastulae period of zebrafish SCNT embryos. During development, several SCNT embryos displayed abnormal morphology in the blastulae period (Fig. 1 B-D). In following the abnormal SCNT embryos, we observed that they could not complete the normal progression through the blastulae period, and remained at any given subdivision of the blastulae period without any morphological changes for several hours.
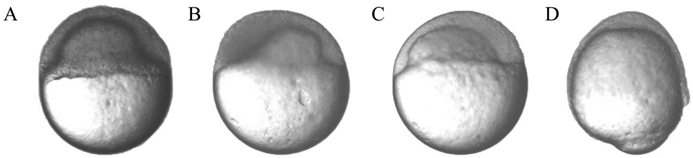
Although there was no significant morphological difference among different types of donor cells in the gastrula period under the microscope, the morphogenetic cell movements of involution, convergence, and extension occurred, producing the primary germ layers and the embryonic axis [25]. Several SCNT embryos also displayed abnormal morphology in the gastrula period (Fig. 2 A-D). The significant abnormal morphology suggested that a number of developmental pathways could have progressed in an aberrant manner, and there were some alterations in the temporal and spatial dynamics of gene expression [31]. Therefore, some of these SCNT embryos underwent incomplete reprogramming processes that prevented them from developing into adults.
Nuclear transplants generated using tail cells
| No. Of Egg operated | No. Of 2-cell stage | No. Of 8-cell stage | No. Of 256-cell stage | No. Of High stage | No. Of Dome stage | No. Of 30% Epiboly | No. Of Shield stage | No. Of 75% Epiboly |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |
| 587 (Exp.1) | 391 | 348 | 261 | 233 | 51 | 49 | 9 | 1 |
| (66.61) | (59.28) | (44.46) | (39.69) | (8.69) | (8.35) | (1.53) | (0.17) | |
| 767 (Exp.2) | 512 | 465 | 346 | 317 | 72 | 70 | 14 | 2 |
| (66.75) | (60.63) | (45.11) | (41.33) | (9.39) | (9.13) | (1.83) | (0.26) | |
| 712 (Exp.3) | 486 | 433 | 343 | 319 | 63 | 62 | 11 | 1 |
| (68.26) | (60.81) | (48.17) | (44.80) | (8.85) | (8.71) | (1.54) | (0.14) |
The morphology of zebrafish SCNT embryos during the blastulae period. (A) The normal morphology of zebrafish SCNT embryos in the blastulae period; (B-D) The abnormal morphology of zebrafish SCNT embryos in blastulae period; part of the animal pole cells of these SCNT embryos did not participate in the development of SCNT embryos.
The abnormal morphology of zebrafish SCNT embryos during the gastrula period. (A-C) The abnormal morphology of zebrafish SCNT embryos at the 40% to 50% epiboly stage; (D) The abnormal morphology of zebrafish SCNT embryos at the 80% epiboly stage.
Developmental timing (hour:min) of nuclear transplantsa.
| 2-cell stage | 8-cell stage | 256-cell stage | High stage | Dome stage | 30% Epiboly | Shield stage | 75% Epiboly | |
|---|---|---|---|---|---|---|---|---|
| ZD embryos | 0:45 | 1:15 | 2:30 | 3:20 | 4:20 | 4:40 | 6:00 | 8:00 |
| KC SCNT embryosb | 0:50±0:05 | 1:25±0:05 | 2:40±0:05 | 3:50±0:10 | 5:30±0:20; ∞ | 5:55±0:30; ∞ | 8:30±0:50; ∞ | 10:45±1:00; ∞ |
| LC SCNT embryosc | 0:50±0:05 | 1:25±0:05 | 2:40±0:05 | 3:50±0:10 | 5:30±0:25; ∞ | 5:55±0:30; ∞ | 8:35±0:50; ∞ | 11:00±1:00; ∞ |
| TC SCNT embryosd | 0:50±0:05 | 1:25±0:05 | 2:50±0:10 | 4:00±0:20 | 5:50±0:40; ∞ | 6:30±0:40; ∞ | 8:50±0:50; ∞ | 11:30±1:00; ∞ |
a Each stage except the shield stage and 75% epiboly includes samples beyond 30 embryos, the timing is the average timing of this stage adjusted by the floating range;
b KC SCNT embryos: kidney cell derived SCNT embryos;
c LC SCNT embryos: liver cell derived SCNT embryos;
d TC SCNT embryos: tail cell derived SCNT embryos;
∞: embryos developed in several hours without any morphological changes until death.
We previously mentioned that there have been differences observed in the speed of development between zebrafish SCNT embryos and ZD embryos; however, there are no detailed and accurate experimental data on these differences. In the present study, a systematic analysis was performed on the developmental timing of the SCNT embryos derived from the different cell types (Table 4). During the cleavage period, the developmental timing of SCNT embryos (0:50 ± 0:05) was a slightly slower than that of the ZD embryos (0:45), while the timing of SCNT embryos had no significant difference among these SCNT embryos derived from the different cell types. From the 256-cell stage, the timing of the TC SCNT embryos was different from that of the KC and LC SCNT embryos. At the dome stage, differences in developmental timing appeared between the KC and LC SCNT embryos, and several SCNT embryos were blocked in this stage. As development proceeds, developmental delay became more obvious in SCNT embryos, with the majority of affected SCNT embryos being at the dome stage (1:30 ± 0:40), shield stage (2:50 ± 0:50) and the 75% epiboly stage (3:30 ± 1:00) Combining results in Tables 1, 2 and 3, the developmental speed of SCNT embryos sharply decreased at these stages, implying that the developmental delay in zebrafish SCNT embryos occurred primarily during early stages of development, and that these stages play an important role in the development of SCNT embryos.
Molecular features of early development in zebrafish SCNT embryos
We have previously showed that mycb (B94) is down-regulated but klf4 (B92) is up-regulated in KC SCNT embryos, leading to a notion that KLF4 and MYCB proteins are of significant importance in the reprogramming process in zebrafish SCNT embryos [10]. In the present study, we examined whether gene klf4 and mycb were similarly expressed in LC and TC SCNT embryos as in KC SCNT embryos.
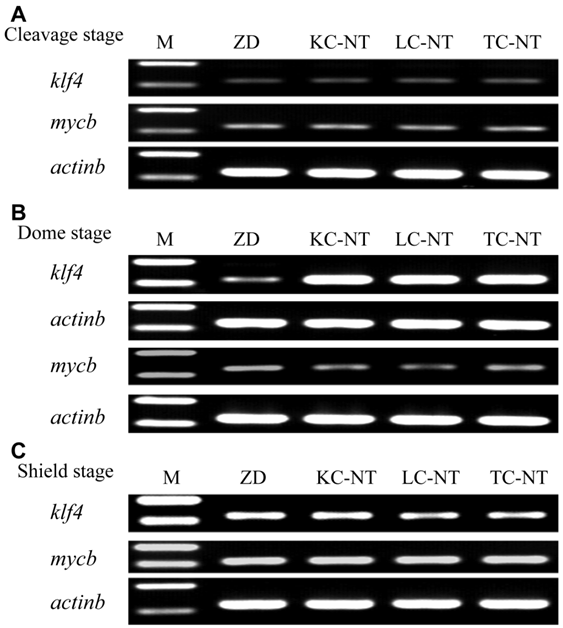
We performed a semi-quantitative RT-PCR analysis of klf4 and mycb expression in ZD and SCNT embryos at the cleavage, dome and shield stages (Fig. 3). At the cleavage stage, there was no difference between ZD and SCNT embryos, as they all exhibited a comparably low level of expression for both klf4 and mycb (Fig. 3A). At the dome stage, there was a significant difference between ZD and SCNT embryos, because the klf4 expression was dramatically higher in SCNT embryos than ZD embryos, whereas no significant difference was detected among the KC, LC and TC SCNT embryos (Fig. 3B). When development proceeded to the shield stage, the difference disappeared between ZD and SCNT embryos on the expression of these genes (Fig. 3C). These results indicate that there is a transient upregulation of klf4 expression at the dome stage in SCNT embryos from donor nuclei of the three different cell types used.
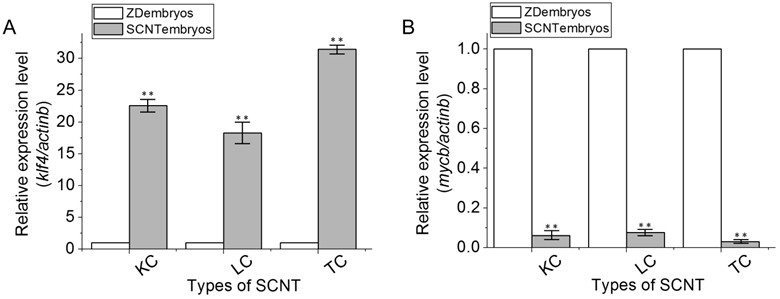
To validate the semi-quantitative RT-PCR data, we performed the real-time RT-PCR analysis by using the comparative Ct method with the formula 2-ΔΔCT with the actb gene as an endogenous control. Using Statistica 6.0 (Statsoft, Krakow, Poland), three independent batches of SCNT embryos and ZD embryos at the dome stage were evaluated and statistically compared by calculating the nonparametric correlation coefficients (Spearman r). The results showed significant correlation among the three independent experiments (P < 0.01). Compared to ZD embryos, SCNT embryos exhibited significant upregulation of klf4 expressions (Fig. 4A), but significant downregulation of mycb expression (Fig. 4B). Again, these changes were found to be specific to SCNT embryos, because no significant differences in the expression of both genes were found among LC, TC and KC SCNT embryos that had received donor nuclei of different cell types.
Gene expression between SCNT embryos and ZD embryos by semi-quantitative RT-PCR analysis. RNA expression was identified using RT-PCR during three developmental stages of zebrafish embryos among ZD embryos, KC SCNT embryos, LC SCNT embryos and TC SCNT embryos. (A) The relative expression of klf4 and mycb gene at the cleavage stage; (B) The relative expression of klf4 and mycb gene at the dome stage; (C) The relative expression of klf4 and mycb gene at the shield stage.
Real-time RT-PCR analyses of klf4 and mycb gene expression. RNA expression was identified using real-time RT-PCR among ZD embryos, KC SCNT embryos, LC SCNT embryos and TC SCNT embryos at the dome stage. (A) Gene klf4. (B) Gene mycb. Data are means ± s.d (bars above columns) of three samples; *, 0.01≤p ≤0.05; **, p ≤0.01.
Discussion
In the present study, we investigated the early developmental fate of different donor cells following their transfer into recipient enucleated eggs in zebrafish SCNT embryos. It is known that failures in the development are usually the main causes of incomplete reprogramming processes [10, 28]. However, few if any studies have focused on the early developmental events or significant reprogramming stage of zebrafish SCNT embryos after nuclear transfer. Here, we provided the first detailed analysis of the early developmental characteristics of SCNT embryos in zebrafish.
Early development of zebrafish SCNT embryos
Following the transfer of nuclei from differentiated cells to enucleated eggs in zebrafish, only a few of the nuclear transplants are able to develop into adult animals. Although apparently healthy zebrafish SCNT embryos were reported [6, 10-11, 15-16], the early development of zebrafish SCNT embryos has remained poorly characterized up until now. Different ZD embryos, even together within a single clutch, develop at slightly different rates [25], so it would not be surprising that SCNT embryos would develop differently as well. Therefore, identifying significant developmental stages for SCNT embryos would be a valuable tool to provide accurate timing in reprogramming studies after nuclear transfer.
Although we demonstrated that zebrafish SCNT embryos undergo significant reprogramming processes during the dome stage after comparative analyses of differentially expressed genes between SCNT embryos and ZD embryos, the evidence that the dome stage is a significant developmental period of zebrafish SCNT embryos remains to be determined. First, there are only five stages to be chosen, which is too broad to provide this evidence. Second, the type of donor cells could affect the development of nuclear transferred embryos [12, 22] and leaves doubt of how the dome stage retardation could occur in zebrafish SCNT embryos derived from other cells. To clarify these issues and to achieve a more comprehensive overview of differentially expressed genes between KC SCNT and ZD embryos, we chose kidney, liver and tail cells to transplant. According to the developmental rate after NT (Table 1, 2, 3), a total of 4,796 reconstructed embryos were produced. Remarkably, there was little difference between the developmental rates of the KC, LC and TC SCNT embryos at the early developmental stages. However, the survival rate, which was initially high (58.9-67.4%) at the 8-cell stage, sharply decreased to 8.7-13.9% at the dome stage and to 1.50-2.96% by the shield stage, regardless of the origin of the donor cells, indicating that there are some important biological or regulatory processes which occurred at these stages. Because the essential difference between the SCNT and ZD embryos is the reprogramming processes after nuclear transfer, the dome stage and shield stage may be the critical periods during which a blockade in development could cause the low nuclear transfer efficiency.
Main features of early development in zebrafish SCNT embryos
During development, several SCNT embryos display abnormal morphology in the blastulae period (Fig. 1 B-D) and gastrula period (Fig. 2 A-D). Although there was no significant morphological characteristic of the cells in the early developmental period, the spatial placement of the cell in the embryo could affect the accuracy of the temporal and spatial gene expression. The significant abnormal morphology indicated that these SCNT embryos had undergone the incomplete reprogramming processes and could not develop into adulthood.
Even if zebrafish SCNT embryos were strictly incubated in standardized conditions as ZD embryos [10, 25], there would be significant differences in the rate of development between these two type of embryos. We had previously mentioned this developmental lag phenomenon between KC SCNT embryos and ZD embryos [10], but a systematic analysis of the developmental timing was only first demonstrated in the present study (Table 4). During the cleavage period, the developmental timing of SCNT embryos (0:50 ± 0:05) was slightly slower than that of ZD embryos (0:45). As time progressed, the developmental lag phenomenon was more and more obvious. Interestingly, from the dome stage, a significant difference of developmental timing appeared between the SCNT embryos and ZD embryos. The large developmental lag occurred at the dome stage (1:30 ± 0:40), shield stage (2:50 ± 0:50) and 75% epiboly (3:30 ± 1:00) stage. Combining results in Tables 1, 2 and 3, the developmental rate of the SCNT embryos showed sharp decreases at these stages. Regardless of the developmental rate and timing in zebrafish SCNT embryos, there were significant events which apparently occurred at the dome and shield stages. The essential difference between the SCNT and ZD embryos is the reprogramming processes after nuclear transfer. It is widely accepted that reprogramming can be divided into two major events that occur just after SCNT: 1) the reversal to pluripotency and 2) the establishment of new differentiation programs [28]. In addition, we have previously provided gene expression evidence of the failure of embryos to reverse pluripotency at the dome stage [10]. Accordingly, we speculate the processes required to reverse pluripotency is critical for the long developmental lag at the dome stage. Thus, the dome stage may play an important role on the development of SCNT embryos after nuclear transfer.
Molecular characterization of early development in zebrafish SCNT embryos
We have previously shown that KC SCNT embryos displayed normal morphology but underwent incomplete reprogramming processes. After comparative analyses of differentially expressed genes between SCNT embryos and ZD embryos, mycb and klf4 were identified [10]. In mammals, the balance between MYC and KLF4 may be important for the generation of induced pluripotent stem cells [23-24]. Surprisingly, mycb and klf4 in zebrafish KC SCNT embryos were differentially expressed in the same manner as those in the reprogrammed mammalian cells in vitro. To date, there is no evidence that mycb and klf4 perform reprogramming functions in zebrafish. However, it is possible that mycb and klf4 may participate in the reprogramming process of the donor nuclei, as it constitutes the essential difference between SCNT and ZD embryos. Wang et al identified seven medaka pluripotency genes (containing klf4), and speculated the homologs/paralogs of klf4 gene is perhaps also the pluripotency gene in other lower vertebrates[32]. Furthermore, klf4 may participate in the reversal of the donor cell to pluripotency in the recipient cell after NT, and the balance between the effects of mycb and klf4 may be important for the reprogramming process in zebrafish SCNT embryos. In the present study, we examined whether the klf4 and mycb genes were correctly expressed as the reprogramming related marker in zebrafish LC and TC SCNT embryos. The semi-quantitative RT-PCR analysis in the expression of klf4 and mycb was performed among the ZD, KC-SCNT, LC-SCNT, TC-SCNT embryos (Fig. 3). Interestingly, there are significant differences between ZD and SCNT embryos just at the dome stage (Fig. 3B). And the expressions of klf4 and mycb in LC and TC SCNT embryos were similar to that in KC SCNT embryos at the dome stage, which are confirmed by the quantitative real-time RT-PCR analysis (Fig. 4). These results provided molecular evidence that the dome stage is a significant developmental stage for SCNT embryos. Thus, klf4 and mycb could be used to evaluate the reprogramming process at the dome stage in zebrafish SCNT embryos.
In summary, we utilized the zebrafish as a model to study reprogramming events of nuclei from tail, liver and kidney cells. The initially high survival rate of zebrafish SCNT embryos at the 8-cell stage (58.9-67.4%) sharply decreased thereafter (8.7-13.9% at the dome stage and 1.50-2.96% by the shield stage). The developmental lag phenomenon was first reported in zebrafish (Table 4), and a large developmental lag was observed at the dome stage (1:30 ± 0:40), shield stage (2:50 ± 0:50) and 75% epiboly (3:30 ± 1:00) stage. These indicating that the dome and shield stage are critical for the SCNT efficiency. However, there was an apparent difference in the klf4 and mycb expression profiles between SCNT embryos and ZD embryos just at the dome stage, suggesting that the klf4 and mycb genes could be the reprogramming related marker in zebrafish SCNT embryos at this stage, and this period is the significant reprogramming stage after NT that results in low nuclear transfer efficiency in zebrafish. It is worth mentioning that it could not be underestimated that the shield stage is a critical stage for the efficiency of SCNT in zebrafish.
Materials and methods
Zebrafish Strain and Maintenance
The AB/Tubingen zebrafish (Danio rerio ) was used for these experiments. Zebrafish were raised and maintained under standard laboratory conditions, and embryos were staged by morphological features [25].
The research animals were provided with the best possible care and treatment and are under the care of a specialized technician. All procedures were approved by the Institute of Hydrobiology, Chinese Academy of Sciences, and were conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals.
Media for nuclear transfer
Eggs were maintained in Hank's saline solution (0.137 M NaCl, 5.4 mM KCl, 0.025 mM Na2HPO4, 0.44 mM KH2PO4, 1.3 mM CaCl2, 1.0 mM MgSO4, 4.2 mM NaHCO3) supplemented with 1.5% BSA (w/v; St. Louis, MO, USA). This working medium was kept at 4oC until nuclear transfer. Prior to nuclear transfer, streptomycin (100 U/ml) and ampicillin (100 U/ml) were added to the working medium and mixed briefly.
Preparation of donor cells
On the day prior to nuclear transfer, primary cells were collected from the tail, liver and kidney tissues of adult male zebrafish (AB strain). Briefly, these tissues were placed into a 0.25% trypsin solution (w/v; Sigma, St. Louis, MO, USA) for 15 min at 20-25oC, dissociated in Holtfreter dissociation solution (Ca2+-free Holtfreter solution containing 0.15 mM EDTA), collected by centrifugation, and washed several times using Holtfreter solution (0.35% NaCl, 0.01% CaCl2, 0.005% KCl [w/v], 100 IU/ml streptomycin, and 100 IU/ml ampicillin). The dissociated cells were maintained at 4oC in JM199 medium until nuclear transfer. Normally, the dissociated cells were used for nuclear transfer within 60 min.
Preparation of recipient eggs
For egg collection, zebrafish were artificially induced to spawn. The quality of eggs plays an important role in SCNT. High quality eggs are slightly granular and yellowish in color, whereas immature eggs are whitish or withered, and the best eggs appear intact and smooth on the yolk surface. Unfertilized embryos were placed into a trypsin solution of 0.25% (w/v; Sigma) for 3 min, and the softened chorion was subsequently removed by microsurgery. Once activated, the egg cytoplasm coalesced, moved toward the animal pole, and formed the blastodisc. The blastodisc of the zebrafish required approximately 12 min to form at 25oC and became a full-sized one-cell egg after 40 min. Therefore, these eggs could act as recipients for up to 40 min following activation at 28oC.
Somatic cell nuclear transfer
To remove the egg pronucleus, we placed recipient eggs in an agar plate filled with Hank's saline solution. The second polar body of the dechorionated egg was visible under a 403 stereomicroscope. The egg nucleus underneath the second polar body was removed by aspiration with a fine glass needle. Enucleated eggs were maintained in a 1.5% agar (w/v; Sigma) plate filled with Hank's saline solution. Nuclear transfer was conducted using either an Eppendorf microinjection system (Model 5171/5246, Hamburg, Germany) with a Nikon TE300 microscope (Nikon, Melville, NY, USA) or a Narishige system (NT-188NE, Leeds Precision Instruments, Minneapolis, MN, USA) with an Axiovert 200 microscope (Carl Zeiss). Donor cells were ruptured by aspiration into the transfer needle, which had an approximately 12-µm inner diameter smaller than the cell, and were transplanted into the cytoplasm of the enucleated eggs at the animal pole. Nuclear transplants were transferred into an agar plate filled with Holtfreter's solution. SCNT was performed three times for each batch of donor cells and recipient eggs. Nuclear transplants were cultured in Holtfreter's solution at 28oC prior to collection.
The SCNT experiments in fish are limited by the inability to directly label the SCNT embryos. To address this issue, we created a negative control as in our previous study [10]. Four hours later, none of the Hanks saline solution-injected embryos survived, demonstrating successful SCNT.
Developmental observation and collection of zebrafish SCNT embryos
The whole processes of embryonic development of zebrafish ZD embryos and zebrafish SCNT embryos cultured in Holtfreter's solution at 28oC were examined under an Olympus SZX-12 microscope. The series of stages for development of the zebrafish embryo were determined as previously described [25]. The serial timings of the early development of embryos were recorded and statistically analyzed. For real-time quantitative RT-PCR analysis, embryos were collected at the dome stage from SCNT embryos derived from tail, liver and kidney cells.
Total RNA extraction and cDNA synthesis
Total RNA was extracted from batches of embryos (n = 100) using the SV Total RNA Isolation System Kit (Promega, CA, USA). We analyzed the integrity of the RNA integrity by its electrophoretic mobility on 1.5% agarose gels in 1× TAE buffer. The UV absorbance of the RNA was also measured at 260 nm (A260) and 280 nm (A280), and the RNA purity was determined using the ratio of A260:A280 (Eppendorf Biometer, Hanburg, Germany).
Gene expression by semi-quantitative RT-PCR and quantitative real-time RT-PCR analysis
Two genes (klf4 and mycb, klf4 forward: 5'-GTT GGG AAG GTT GTG G-3', klf4 reverse: 5'-ATC TGA GCG GGA GAA A-3'; mycb forward: 5'-TGC GAT GAT GCG GAC TA-3', mycb reverse: 5'-TCA GCG TGC AAA GAC G-3') were analyzed in the samples by semi-quantitative RT-PCR which was performed using an Applied Biosystems 9700 (Applied biosystems, Foster City, CA, USA). β-actin was amplified as an endogenous control (actb forward: 5'-GAT GAT GAA ATT GCC GCA CTG-3', actb reverse: 5'-ACC AAC CAT GAC ACC CTG ATG T-3'). Reactions were performed using the following conditions: an initial incubation at 94oC for 5 min, followed by 30-35 cycles (30 cycles for all genes at the cleavage and shield stage, 28 cycles for klf4 and actb gene at the dome stage, 35 cycles for mycb and actb gene at the dome stage) at 94oC for 10 sec, 50-60oC for 30 sec (klf4 at 50 oC, mycb at 55 oC and actb at 60 oC) and 72oC for 30 sec, followed by holding at 72oC for 7 min and ending at 20oC forever.
Two genes (klf4 and mycb) were chosen to be analyzed in the samples by quantitative real-time RT-PCR which was performed using an Applied Biosystems 7000 Real-Time PCR System (Applied biosystems, Foster City, CA, USA) [8]. cDNA samples and a pair of primers were diluted in ddH2O and plated in triplicate in adjacent wells. Three wells without any templates were also included on each plate as negative controls. β-actin was amplified together with the target gene as an endogenous control in each well with a VIC-labeled probe to normalize expression levels among samples. Reactions were performed using the following conditions: an initial incubation at 95oC for 10 min, followed by 40 cycles at 95oC for 10 sec and 60oC for 1 min. Output data generated by the instrument onboard software was transferred to a custom designed Microsoft Excel spreadsheet for analysis. The differential mRNA expression of each candidate gene was calculated by the comparative Ct method using the formula 2-ΔΔCT method [33].
Ethics Committee Approval
The research animals are provided with the best possible care and treatment and are under the care of a specialized technician. All procedures were approved by the Institute of Hydrobiology, Chinese Academy of Sciences, and were conducted in accord with the Guiding Principles for the Care and Use of Laboratory Animals.
Acknowledgements
We thank Ms. Ming Li for technical assistance in nuclear transfer and Ms. Chao Qiu for valuable suggestions. This work was supported by National Natural Science Foundation of China (Grant No. 30900853), the Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20090141120015) and China Postdoctoral Science Foundation funded project (Grant No. 201003505).
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Gasaryan KG, Hung NM, Neyfakh AA. et al. Nuclear transplantation in teleost Misgurnus fossilis L. Nature. 1979;280:585-587
2. Zahnd JP, Porte A. Morphologic signs of nuclear material transfer in the cytoplasm of the ovocytes of certain species of fish. C R Acad Sci Hebd Seances Acad Sci D. 1966;262:1977-1978
3. Yan SY, Lu DY, Du M. et al. Nuclear transplantation in teleosts. Hybrid fish from the nucleus of crucian and the cytoplasm of carp. Sci Sin B. 1984;27(10):1029-1034
4. Chen H, Yi Y, Chen M. et al. Studies on the developmental potentiality of cultured cell nuclei of fish. Int J Biol Sci. 2010;6:192-198
5. Deng C, Liu H. An unknown piece of early work of nuclear reprogramming in fish eggs. Int J Biol Sci. 2010;6:190-191
6. Lee KY, Huang H, Ju B. et al. Cloned zebrafish by nuclear transfer from long-term-cultured cells. Nat Biotechnol. 2002;20:795-799
7. Wakamatsu Y, Ju B, Pristyaznhyuk I. et al. Fertile and diploid nuclear transplants derived from embryonic cells of a small laboratory fish, medaka (Oryzias latipes). Proc Natl Acad Sci U S A. 2001;98:1071-1076
8. Murphey RD, Zon LI. Attack of the fish clones. Nat Biotechnol. 2002;20:785-786
9. Yi M, Hong N, Hong Y. Generation of medaka fish haploid embryonic stem cells. Science. 2009;326:430-433
10. Luo D, Hu W, Chen S. et al. Identification of differentially expressed genes between cloned and zygote-developing zebrafish (Danio rerio) embryos at the dome stage using suppression subtractive hybridization. Biol Reprod. 2009;80:674-684
11. Hu W, Wang YP, Chen SP, Zhu ZY. Nuclear transplantation in different strains of zebrafish. Chin Sci Bull. 2002;47:1277-1280
12. Wakayama T, Yanagimachi R. Cloning of male mice from adult tail-tip cells. Nat Genet. 1999;22:127-128
13. Yan SY, Tu M, Yang HY. et al. Developmental incompatibility between cell nucleus and cytoplasm as revealed by nuclear transplantation experiments in teleost of different families and orders. Int J Dev Biol. 1990;34:255-266
14. Manabu Hattori HH, Ekaterina Bubenshchikova, Yuko Wakamatsu. Nuclear Transfer of Embryonic Cell Nuclei to Non-enucleated and Activated Eggs in Zebrafish, Danio rerio. Int J Biol Sci. 2011
15. Siripattarapravat K, Pinmee B, Venta PJ. et al. Somatic cell nuclear transfer in zebrafish. Nat Methods. 2009;6:733-735
16. Ju B, Huang H, Lee KY. et al. Cloning zebrafish by nuclear transfer. Methods Cell Biol. 2004;77:403-411
17. Zhou W, Sadeghieh S, Abruzzese R. et al. Transcript levels of several epigenome regulatory genes in bovine somatic donor cells are not correlated with their cloning efficiency. Cloning Stem Cells. 2009;11:397-405
18. Vassena R, Han Z, Gao S. et al. Tough beginnings: alterations in the transcriptome of cloned embryos during the first two cell cycles. Developmental biology. 2007;304:75-89
19. Humpherys D, Eggan K, Akutsu H. et al. Abnormal gene expression in cloned mice derived from embryonic stem cell and cumulus cell nuclei. Proc Natl Acad Sci U S A. 2002;99:12889-12894
20. Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Developmental biology. 2004;272:483-496
21. Pei DS, Sun YH, Chen SP. et al. Identification of differentially expressed genes from the cross-subfamily cloned embryos derived from zebrafish nuclei and rare minnow enucleated eggs. Theriogenology. 2007;68:1282-1291
22. Eggan K, Akutsu H, Loring J. et al. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc Natl Acad Sci U S A. 2001;98:6209-6214
23. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676
24. Takahashi K, Tanabe K, Ohnuki M. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872
25. Kimmel CB, Ballard WW, Kimmel SR. et al. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253-310
26. Kane DA, Kimmel CB. The zebrafish midblastula transition. Development (Cambridge, England). 1993;119:447-456
27. Sun YH, Chen SP, Wang YP. et al. Cytoplasmic impact on cross-genus cloned fish derived from transgenic common carp (Cyprinus carpio) nuclei and goldfish (Carassius auratus) enucleated eggs. Biol Reprod. 2005;72:510-515
28. Alberio R, Campbell KH, Johnson AD. Reprogramming somatic cells into stem cells. Reproduction. 2006;132:709-720
29. Ju B, Pristyazhnyuk I, Ladygina T. et al. Development and gene expression of nuclear transplants generated by transplantation of cultured cell nuclei into non-enucleated eggs in the medaka Oryzias latipes. Dev Growth Differ. 2003;45:167-174
30. Zhao HB, Zhu ZY. Nuclear transplantation of somatic cells of transgenic red carp (Cyprinus carpio haematopterus). Yi Chuan Xue Bao. 2002;29:406-412
31. Yin C, Ciruna B, Solnica-Krezel L. Convergence and extension movements during vertebrate gastrulation. Curr Top Dev Biol. 2009;89:163-192
32. Wang D, Dwarakanath MA, Wang T. et al. Identification of Pluripotency Genes in the Fish Medaka. Int J Biol Sci. 2010
33. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method Methods. 2001;25:402-408
Author biography
Dr Wei Hu heads the Fish Gene-Engineering Group, which focuses on understanding the reprogramming mechanisms of zebrafish SCNT embryos, the molecular mechanism in fish reproductive biology, fish genetic engineering and ecological risk evaluation of transgenic fish. The group is a major department of State Key Laboratory of Freshwater Ecology and Biotechnology, in the Institute of Hydrobiology.
Dr Da-Ji Luo is a research scientist with 7 years of research experience in the zebrafish SCNT embryos area. Dr Luo has a special interest in elucidating the molecular mechanisms of reprogramming processes after nuclear transfer in zebrafish.
![]() Corresponding author: Dr Wei Hu, State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China. Tel: 86-27-68780051, Fax: 86-27-68780051; E-mail: huweiac.cn
Corresponding author: Dr Wei Hu, State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China. Tel: 86-27-68780051, Fax: 86-27-68780051; E-mail: huweiac.cn

 Global reach, higher impact
Global reach, higher impact