10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2011; 7(7):968-977. doi:10.7150/ijbs.7.968 This issue Cite
Research Paper
In vitro Evaluation of Natural Marine Sponge Collagen as a Scaffold for Bone Tissue Engineering
1. Division of Orthopaedic Surgery, Guangdong Academy of Medical Sciences, Guangdong General Hospital, Guangzhou, Guangdong, 510080, P.R. China
2. Centre for Orthopaedic Research, School of Surgery, University of Western Australia, Western Australia, 6009, Australia
3. Laboratory of Orthopaedic Cellular and Molecular Biology, Institute of Health Sciences, Shanghai Institutes of Biological Sciences, Chinese Academy of Sciences and Shanghai JiaoTong University, Shanghai 200025, P.R. China
# These authors contributed equally to this paper.
Received 2011-4-18; Accepted 2011-7-17; Published 2011-8-7
Abstract
The selection of a suitable scaffold matrix is critical for cell-based bone tissue engineering. This study aimed to identify and characterize natural marine sponges as potential bioscaffolds for osteogenesis. Callyspongiidae marine sponge samples were collected from the Fremantle coast of Western Australia. The sponge structure was assessed using scanning electron microscopy (SEM) and Hematoxylin and eosin. Mouse primary osteoblasts were seeded onto the sponge scaffold and immunostained with F-actin to assess cell attachment and aggregation. Alkaline phosphatase expression, von Kossa staining and real-time PCR were performed to examine the osteogenic potential of sponge samples. SEM revealed that the sponge skeleton possessed a collagenous fibrous network consisting of interconnecting channels and a porous structure that support cellular adhesion, aggregation and growth. The average pore size of the sponge skeleton was measured 100 to 300 μm in diameter. F-actin staining demonstrated that osteoblasts were able to anchor onto the surface of collagen fibres. Alkaline phosphatase expression, a marker of early osteoblast differentiation, was evident at 7 days although expression decreased steadily with long term culture. Using von Kossa staining, mineralisation nodules were evident after 21 days. Gene expression of osteoblast markers, osteocalcin and osteopontin, was also observed at 7, 14 and 21 days of culture. Together, these results suggest that the natural marine sponge is promising as a new scaffold for use in bone tissue engineering.
Keywords: natural marine sponge, bone tissue engineering
Introduction
Bone tissue engineering aims to mimic the natural process of bone formation by delivering a source of cells and/or growth factors in a scaffold matrix which can induce cellular attachment, migration, proliferation and osteoblastic differentiation 1, 2. Despite the significant regenerative capacity of bone tissue, promoting bone regeneration is a focal point in tissue engineering due to its need in treating serious clinical conditions, such as spinal arthrodesis, fixation of prosthetic implants, restoration of maxillofacial structures, pathological bone fractures and non union fracture 1, 3.
One of the key aspects of bone tissue engineering is the use of scaffold matrices. A wide variety of scaffold materials are currently available and their selection depends on whether the properties of the scaffold material closely match the properties of the tissue it seeks to replace 4. An ideal scaffold for bone tissue engineering must possess suitable biocompatibility, osteoconductive and osteoinductive capacities together with a structure which mimics the trabecular network of bone tissue 1. Both natural and synthetic scaffolds have been investigated as biomaterials in bone tissue engineering and each have their benefits. The physiochemical properties of synthetic polymers can easily be altered to adjust porosity, microstructure, degradation rate, and mechanics. However, natural scaffolds are often more biocompatible and offer a better biointeractive surface for cell attachment and growth 5. In particular, natural scaffolds that are derived from biological tissues display highly optimized structures and comprise extracellular matrix components that offer a foundation for cell attachment, migration and proliferation 6. It has been well documented that naturally derived materials such as purified collagen and extracellular matrix are promising as bioscaffolds in tissue engineering 7, 8.
Marine sponges or poriferans, belonging to the phylum Porifera, are aquatic, sessile, filter-feeding metazoans of collagen origin 9. The Western Australia coast houses a large and diverse range of marine sponges including rare species unique to the area 10. In addition to being an important part of the ecosystem in which they live, marine sponges are also economically and scientifically important. For example, marine sponges have been used as commercial bath sponges since early Greek civilization and more recently as potential sources of therapeutic drugs and antibiotic substances 11, 12. Marine sponges display a structure which mimics the cancellous architecture of bone tissue. The complex canal system within sponges creates a porous environment which is ideal for cellular integration when combined with cells for tissue engineering. In this study, we aimed to identify the potential of marine sponges as a bioscaffold to promote osteogenesis.
MATERIALS AND METHODS
Preparation of marine sponge
The marine sponge samples were collected from the Fremantle coast (south mole region) of Western Australia. All sponge samples were processed to free the sponge skeleton of cellular debris (Zheng, MH. Australia Provision Patent No 2008901451). To facilitate microstructural examination and seeding of osteoblasts, samples were cut down in size to a 5 mm2 area, 3 mm in thickness. To ensure sterility, all sponge samples were γ-irradiated, and stored in sterile conditions at -4oC prior to experimental use.
Histological examinations
Scanning electron microscopy (SEM) was performed to characterize the micro-architecture of marine sponges and cell growth on the scaffold. Sponge-cell constructs were fixed in 2.5 % glutaraldehyde for 60 min at room temperature. Samples were rinsed twice in 0.05 M cacodylate buffer for 5 min, then post-fixed in 1% osmium tetroxide (w/v) in cacodylate buffer for 60 min. Samples were washed 3 times in 0.5 M cacodylate buffer for 5 min each, and post-fixed in 1% tannic acid (w/v) in 0.05 M cacodylate buffer for 60 min to prevent shrinkage of cells for SEM analysis. Samples were stained for 60 min in 0.5% uranyl acetate (w/v) and rinsed well in saline solution. Samples were then dehydrated stepwise in 25%, 50%, 70% (twice), 95%, and absolute ethanol for 30 min RT each, and stored in super dry ethanol until critical point dried in an Emitech K850 critical point drying apparatus. Marine sponge without cells were fixed in 2.5 % glutaraldehyde and directly subjected to dehydration. The dried samples were then mounted on aluminum studs and sputter coated with 20 nm gold/palladium. Samples were viewed in a Phillips XL30 scanning electron microscope at 15 kV. Marine sponge skeleton without cells were fixed, paraffin embedded, and sectioned into 5µm sections for hematoxylin and eosin (H&E) staining.
Isolation and cultivation of primary osteoblasts
Osteoblasts were extracted from calvariae of neonatal C57BL/6J mice and cultivated in vitro. The execution of the animals was approved by the Animal Ethic Committee of the University of Western Australia. Briefly, the calvariae was removed from the skull, cleaned from other connective tissue and digested with collagenase type II (400units/ml; SIGMA-ALDRICH, St. Louis, MO, USA) at 37°C for 1 hr to release cells from the tissue. Digestion was terminated by addition of fetal bovine serum (FBS, TRACE, Sydney, Australia). Cells were pelleted by centrifugation at 1,500rpm for 5 min. The medium was then removed from the tube and the cell pellet was resuspended in 5 ml growth medium consisting of α-minimum essential medium (α-MEM, Gibco, New York, USA), 10% FBS, 1% penicillin/streptomycin solution (Invitrogen, Carlsbad, CA, USA), and 10mM L-Glutamine (Gibco, New York, USA). The osteoblast suspension was transferred to a 25 cm2 tissue culture flask for monolayer culture and incubated at 37°C in a humid environment with 5% carbon dioxide. Culture medium was change every two days until cell confluency was reached. To increase cell numbers, cells were then transferred into 75 cm2 flasks using 0.05% trypsin (Gibco, New York, USA).
Seeding of osteoblast cells onto the marine sponge
Primary osteoblasts were resuspended in osteoblast differentiation medium containing a cocktail of 100nM dexamethasone, 10mM β-glycerophosphate, and 50μg/ml ascorbic acid-2-phosphate (SIGMA-ALDRICH, St. Louis, MO, USA) to a final concentration of 5 x 105 cells/ml. Pre-soaked pieces of sponge were placed separately in the wells of 24-well plates, and 1ml of cell suspension was slowly added to each well. Cells were cultured at 37oC, 5% CO2 for10-12 hr (to allow cellular adherence). Seeded constructs were then given a further 1ml of differentiation medium. Medium was changed every 2-3 days. Sponge-cell constructs were cultured for 4, 7, 14 and 21 days. At 4 and 14 days, samples were fixed and subjected to confocal microscopy examination. At 7, 14 and 21 days RNA was extracted from samples and cDNA used for real-time PCR analysis to measure expression of osteoblastic gene markers.
Confocal Microscopy examination
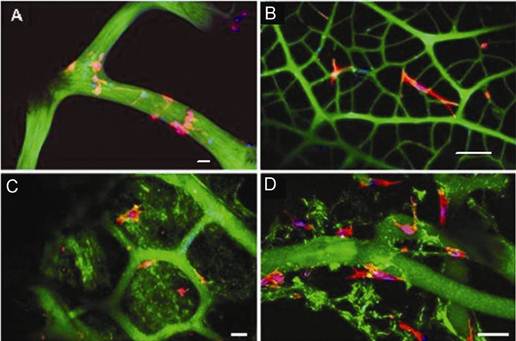
F-actin immunostaining was performed on osteoblast-sponge constructs to visualise attachment of seeded cells onto the matrix of the sponge. Staining was conducted according to the method previously described by Helfrich & Ralston (2003) 13. Hoechst bis-benzimide was used as a nuclear stain. Marine sponges were placed into Coverglass® chamber slides (NUNC) and immersed in aqueous solution. Detection of fluorochromes (Rhodamine-phalloidin, Hoechst 33258) was carried out using a confocal laser-scanning microscope (CLSM, MRC-1000, Bio-Rad) equipped with a krypton-argon laser using a 20X water immersion objective lens (NIKON, NA= 1.0) or 10X dry lens (NIKON, NA=0.7). Twenty serial optical sections (z= 1µm) were acquired satisfying the Nyquist criteria for sampling. The serial optical sections (z-stacks) from each sponge were collected for the construction of three-dimensional images. Auto-fluorescence was used to image sponge skeletons. Confocal images were collected as Bio-Rad PIC files and analysed using Confocal Assistant 4.02. The fibrous skeleton appeared green, cells red and nucleus purple.
Alkaline phosphatase staining
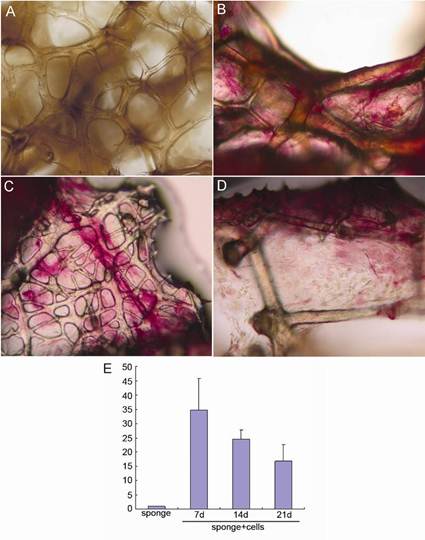
Osteoblast differentiation at days 7, 14 and 21 of culture was quantified using the Sigma Leukocyte Alkaline Phosphatse kit (SIGMA-ALDRICH, St. Louis, MO, USA) according to manufacturer's instructions with minor modifications. The sponge-cell constructs were washed three times in 1хPBS, fixed in 4% paraformaldehyde for 15 min at room temperature, then washed three more times with 1хPBS. Each fixed sponge sample was then added to the substrate solution containing naphthol AS-BI phosphate, fast red violet LB and sodium nitrite. The resulting insoluble, diffuse pink dye deposit indicated the sites of alkaline phosphatise activity. The alkaline levels were quantitatively assessed by the measurement of positively stained areas, and the values were expressed as the mean ± standard deviation. Statistical significances were determined using Students' t test. The experiments were performed at least 3 times and the results of the representative experiments were presented.
Von Kossa staining
Von Kossa staining was performed to assess the level of calcium deposition by the osteoblasts seeded on the marine sponge. The sponges were incubated with 5% silver nitrate solution under ultraviolet light for 60 min. Un-reacted silver was removed with 5% sodium thiosulfate for 5 min. The sponges were then imaged using the epifluorescence Nikon Diaphot 300 inverted microscope.
RNA extraction and Real-time RT-PCR
Osteoblastic gene expression was analyzed in sponge-cell constructs after 7, 14 and 21 days cultivation. The sponge-cell constructs were freeze pulverized in liquid nitrogen and subjected to RNA extraction using the Qiagen RNAeasy Mini kit (Qiagen Pty Ltd, Hilden, Germany) according to the manufacturer's instruction. RNA was quantified by spectrophotometric measurement. RNA was stored at -80°C immediately after extraction until required. Complementary DNA was synthesized from 4 µg of RNA using 200 units of M-MLV Reverse Transcriptase (New England Biolabs, USA), 40 units of RNase Inhibitor (New England Biolabs, USA), 1.25mM dNTP (Promega Corp, Madison, WI), and 5 µm Oligo dT in a total volume of 20 µl. The samples were incubated at 42°C for 1 hr and heated for a further 10 min at 92ºC in a thermal cycler (Perkins-Elmer GeneAmp 2400, USA). Upon completion, samples were stored at -20ºC for additional experimental use.
Relative quantitative real-time PCR was performed on a 384-well/plate ABI Prism 7000 Sequence Detection machine (Applied Biosystems, Foster City, CA) using Sybr Green PCR Master Mix (Applied Biosystems, Foster City, CA). Each PCR reaction (total volume 10 µl) contained 5 µl SybrGreen PCR Master Mix, 2.5 µl ddH2O, 1.5 µl cDNA, and 5 mM each of the forward and reverse primers. The real-time PCR cycling profile comprised an initial denaturation of 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec, an annealing temperature of 60°C for 20 sec, and extension of 72℃ for 20 sec followed by a final extension at 72°C for 1 min. The housekeeping gene 18S was included as an endogenous control. The expression of osteoblastic markers osteopontin, osteocalcin, and alkaline phosphatase, were studied using the primers listed in table 1. A cycle threshold (Ct) value was obtained for each sample and analyzed by the Sequence Detection System software (Applied Biosystems, Foster City, USA). The comparative 2− ΔΔCT method was used to calculate the relative expression of each target gene as previously described 14. Briefly, the mean Ct value of target gene was normalized to its averaged Ct values of the housekeeping gene 18S to give a ΔCt value, which was further normalized to control samples to obtain a ΔΔCt value.
RESULTS
Characterization of the sponge skeleton by SEM and H&E staining
The sponges collected from the Fremantle coast of Western Australian are a member of the sponge family Callyspongiidae (belonging to the order Haplosleridia). The sponge is irregular in shape and cushion-like and scattered with oscules at regular intervals over its surface (Figure 1A). The microstructure of the sponge, as examined by SEM, is composed of regularly interconnected spongin fibres forming the choanosomal (mesophylic) skeleton (Figure 1B). The primary, secondary and occasional tertiary fibres form rectangular meshed networks. Primary fibres are either ramified (branched) to form secondary fibres and tertiary fibres (Figure 1C). The spongin fibres range from 30 to 50 µm in diameter, and create pores ranging 100 to 300 µm in diameter. The fibres of the scaffold contain occasional siliceous spicules which are attached or embedded within the fibrous network in a random orientation. These spicules are 20 to 40 μm in length and align longitudinally along the fibre axis. No cellular debris was observed on the skeleton of the marine sponge. H&E staining shows the collagen fibres and interconnection of the skeleton (Figure 1D).
Osteoblast attachment and growth within the sponge scaffold
Confocal examination of cell-sponge constructs cultured in osteoblast differentiation medium for 4 and 14 days was carried out to assess cellular attachment, cell density and growth within and around the spongin fibres. Attachment between the cell membrane of osteoblasts and the substrate surface was evident by F-actin staining. Figure 2 shows that after 4 days culture, cells were incorporated into the skeleton of the sponge scaffold and spread along fibres of the scaffold. Extensive cellular proliferation was observed on the surface of fibrous sponge skeleton after 14 days cultivation (Figure 3).
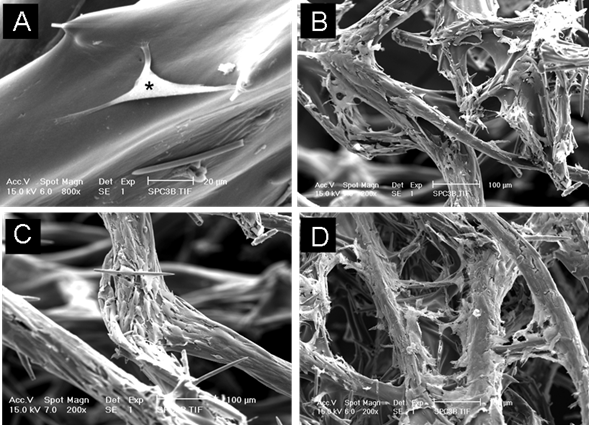
The cells formed a thin layer over the ectosomal skeleton of the sponge and infiltrated the pores of the spongin fibres. Collectively, these results indicate that osteoblasts were able to anchor on the surface of spongin fibres, bridge interconnecting collagen spaces, proliferate and extend growth into the porous structure. SEM examination of the sponge-cell constructs confirmed the cell attachment and growth on maring sponge, as evidenced by the single cell attachment after 4 days culture to a layer of cell mass covering the collagen skeleton after 21 days (Figure 4).
Sequences of primers used for Real-time PCR
| Refseq No. | Gene | Sequence |
|---|---|---|
| NM_199173 | Osteocalcin | Forward:5' AGG GCA GCG AGG TAG TGA AGA 3' Reverse: 5' AAG GGC AAG GGG AAG AGG AAA GAA 3' |
| NM_000478 | alkaline phosphatase | Forward: 5' TGC TCC CAC GCG CTT GTG CCT GGA 3' Reverser: 5' CTG GCA CTA AGG AGT TAG TAA G 3' |
| NM_001040060 | Osteopontin | Forward: 5'TGAGGAAAAGCAGAATGCTGTGTCC 3' Reverse: 5'GTTGCTGGCAGGTCCGTGGG 3' |
| HQ387008 | 18S | Forward: 5' CCT GCG GCT TAA TTT GAC TC 3' Reverse: 5' AAC TAA GAA CGG CCA TGC AC 3' |
(A) Photograph of sponge samples that were collected, scale bar = 5cm; (B) SEM micrographs of sponge skeletons at low magnification showing the regular fibre network and irregularly arranged fibres of the sponge at low magnification; (C) high magnification displaying the ectosomal skeleton including branches of the choanosomal fibre network, and occasional spicules; and (D) the collagen skeleton of marine sponge stained with Hematoxylin and Eosin.

Fluorescent confocal micrographs of sponge-cell constructs after 4 days culture. Cells were stained with F-actin (red) together with nuclear stain Hoechst bis-benzimide (purple). Autofluorescence (green) was used to image spongin fibres. (A) Attachment of cells to the fibres of the marine sponge. (B) Cells bridging pores of ectosomal skeletal fibres. (C) Low magnification of cells within the fibre skeleton, debris is visible. (D) High magnification of cellular attachment onto fibres of the skeleton. Tissue debris is also visible. Scale bar = 20 µm.
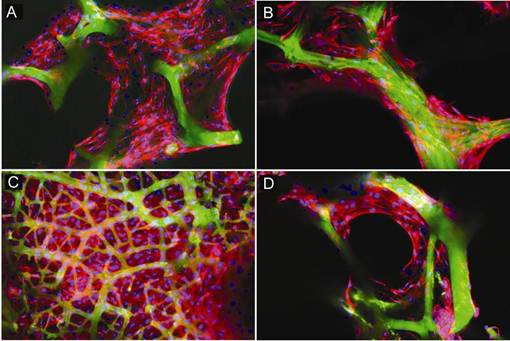
Confocal microscopy showing F-actin fluorescent staining of cells (red) and nucleus (purple) on sponge skeletons (green autofluorescense) after 14 days culture. (A) Low magnification, cells form a mat over the ectosomal skeleton. (B) High magnification, cells form a thin layer over the fibres of the scaffold. (C) Low magnification, cells infiltrate pores of the ectosomal skeletal fibres. (D) High magnification showing cellular infiltration of a pore of the sponge skeleton. Scale bar = 20 µm.
Scanning electron microscopy examination of osteoblasts cultured on marine sponges for 4 days (A), 7days (B), 14 days (C) and 21 days (D). (* indicates single cell anchoring on the skeleton)
Osteoconductivity of sponge-cell constructs
Both alkaline phosphatase and von Kossa staining were used to examine osteoblast differentiation and mineralization within the sponge scaffolds. Alkaline phosphatise activity was observed from day 7 to day 21 of culture, although the expression levels steadily decreased over time (Figure 5). After 21 days of culture, von Kossa staining demonstrated a dark staining pattern within the sponge matrix identifying the presence of calcium nodules within the sponge and therefore the mineralisation of the extracellular matrix. In contrast, no staining was observed in sponge contructs without cells (Figure 6).
Light micrographs showing alkaline phosphatase staining. (A) Sponge skeletons without cells; (B) Sponge-cell constructs after 7days in vitro culture; (C) Sponge-cell constructs after 14 days in vitro culture; (D) Sponge-cell constructs after 21 days in vitro culture; (E) Quantification of alkaline phosphatase staining of different groups, error bars represent mean±standard deviation, n=3.
Light micrographs showing von Kossa staining. (A) Sponge skeletons; (B) Sponge-cell constructs after 21 days in vitro culture; (C) higher magnifications of sample A, 200x; (D) higher magnification of sample B, 200x.

Gene expression of osteoblastic markers in sponge-cell constructs
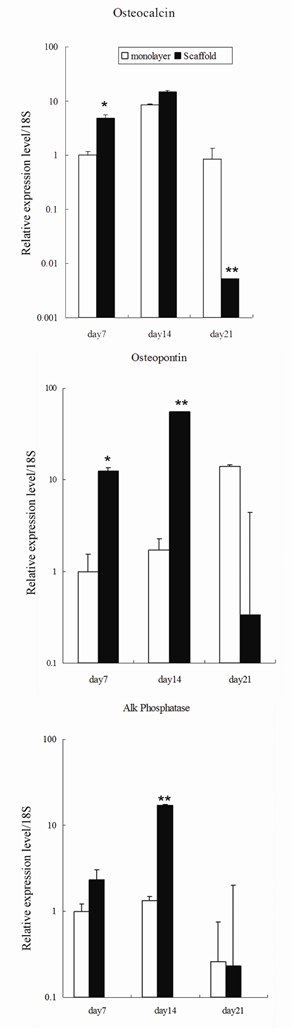
The osteoconductive potential of the marine sponge in vitro was assessed using real-time PCR to quantify gene expression of osteogenic genes in the cell-sponge constructs. Primary calvarial osteoblasts were seeded on the sea sponge and cultivated in osteoblast differentiation medium for 7, 14 and 21 days. Cells were also cultivated in a monolayer (without sponge) as a control. Osteocalcin, osteopontin, and alkaline phosphatase gene expression was detected in cell-sponge constructs at all time points using real time PCR. The mRNA expression levels of osteocalcin and osteopontin in cell-sponge constructs after 7 days cultivation were significantly higher than the expression in cells cultured on monolayer (P<0.05), although the levels did decrease at 21 days of culture. At day 14, there was a significant increase in the level of alkaline phosphatase gene expression in the cells seeded in the sponge compared to those cultured in monolayer (Figure 7).
Gene expression of osteoblastic markers in sponge-cell constructs after 7 days, 14 days and 21 days culture. Mouse osteoblasts were seeded on the scaffold or cultured in monolayer and cultivated in osteoblastic differentiation medium. The expression of osteocalcin, osteopontin, and alkaline phosphatase of the cells in the spongin scaffold were compared to expression by cells cultured on monolayer by real-time PCR. Mean Ct value of target genes was normalized to housekeeping gene 18S. Results are shown as mean ± standard error. Student T-test determined statistical significance where * p<0.05 and ** p<0.01.
DISCUSSION
The skeletons of Poriferans appear to have unique properties that make them appealing as a potential bioscaffolds for cell-based bone tissue engineering. These properties include the fibrous skeleton, the collagenous composition, the ability to hydrate to a high degree, and the possession of open interconnected channels created by a porous structured network 15. This study demonstrates that Poriferans are able to induce osteoblast attachment, proliferation, migration and differentiation in vitro, suggesting its potential as a bioscaffold for bone tissue engineering. The sponge-cell constructs support osteogenesis as evidenced by alkaline phosphatase expression, an early marker of osteoblast differentiation, mineral deposition, and the expression of osteogenic markers such as osteocalcin and osteopontin.
Green et al. (2003) has firstly reported the fiber skeleton of natural marine sponge and suggested its application for tissue engineered bone 6. The Spongia was shown to support the attachment, growth and differentiation of osteoprogenitor cells in the skeleton with void dimensions ranging from 500 to 1250μm 6. As known, osteoblasts respond directly to the pore dimensions of scaffold framework 6. Formation of new tissue is greatly influenced by the composition, porosity and three-dimensional structure of the scaffold onto which cells are cultured 16, 17. Porosity within the marine sponge skeleton is created by a system of pores (ostia), channels and chambers throughout the sponge body that provide the living sponge with nutrition and gas exchange from the surrounding water. The open porosity of this scaffold enables maximal invasion of cells and bone tissue that is necessary for bone reconstruction, whilst minimizing the volume of space taken up by the biomaterial 18. The pore dimension of the scaffold is also critical for osteoblast integration into bone tissue. It has been shown that the optimum pore dimensions for bone tissue regeneration in synthetic scaffolds is between 100 and 250 μm 20. Vogel and Baneyx (2003) demonstrated that these pore dimensions correlate directly to mineralized bone formation, and are therefore suitable for most bone engineering purposes 21. In this study, the pore dimension of the sponge, ranging from 100~300μm was comparable to the pore size in human compact bone 19, suggesting it may be used as an ideal scaffold for tissue engineered bone.
Collagen is the dominant component of the skeletal matrix of the sea sponge and is the major protein constituent of the extracellular matrix of bone. The use of collagen-based sponges as scaffolds in tissue engineering has a number of advantages. The collagenous composition functions as a cell-matrix adhesion molecule to support cell adhesion 22. Since it is a 'native' constituent it is well recognized by cells within the scaffold 23, 24. Additionally, the mesh-like orientation of collagen fibres within the sponge skeleton is microstructurally similar to the lattice-work of fibres in human trabecular bone 19, 25. This study demonstrated that the collagen fibres of the marine sponge skeleton indeed provide a suitable framework for the attachment, migration and proliferation of osteoblasts. The aggregation of osteoblastic cells on spongin fibres may be attributed by the collagenous composition of the sponge fibres together with the presentation of matrix moieties at the skeleton surface. Extracellular adhesion molecules, such as aggregation factor, interact with adhesion receptors composed of scavenger receptor cysteine-rich domains 26-28. Although the specificity of marine sponge to osteoblasts is unknown, these molecules, as well as the collagens and responding integrins, constitute a system that are crucial for specific cell adhesion 29, 30.
The chemotactic properties of collagen are also advantageous to its use in tissue engineering scaffolds 31. The collagenous poriferan skeleton is comparable to the connective tissue of more complex organisms; and is analogous to collagen type XIII 15, therefore providing a natural environment for cellular attachment and aggregation. Previous studies using infrared spectrosopy have demonstrated that the amino acid composition of spongin and collagenous fibrils is similar to that of vertebrate collagen 9. In addition, even though the amino acid composition and carbohydrate contents of selected collagenous sponge skeletons has been identified 9, further characterization of the exact chemical composition of the marine sponge would provide further understanding of the mechanisms involved in the cellular patterning observed in this study.
Identification of suitable scaffolds onto which osteogenic cells can be seeded to generate functional three-dimensional tissues is a major research goal. It is evident that the skeleton of Poriferans is dynamic, and consists of a complex composition of molecules which regulate cellular behaviour and phenotype 28, 32, 33. In support of this, the osteogenic potential of the cell-sponge construct was reinforced by gene expression of osteoblast markers. Similarly, human osteoprogenitor cells were found to attach to the spongin skeleton of Spongia sea sponges in serum-free medium within 16 hours of in vitro culture 15. Due to their ability to hydrate to a high degree, marine sponge skeletons have also shown to adsorb and release recombinant human BMP-2 and induce expression of alkaline phosphatase in a myoblast cell line 15. This indicates that sponge skeletons offer huge potential as engineering structures that promote bone cell differentiation and osteogenesis. The limitation of using marine sponge for tissue engineered bone have been suggested by Green et al. 6. Firstly, the biodegradability of nature sponge is not desirable as the sponge skeleton could resistant to varies chemical dissolutions and enzymes. No degradation of the collagen skeleton has been observed when it was implanted in nude rat for 21-week period (data not shown). Secondly, althought there is no evidence of marine sponge carrying unknown pathogens, the possibility of immunogenicity could not be ruled out. Thirdly, there is lack of specificity of marine sponge to bone formation. Marine sponge supports osteoblastic cell attachment and growth as well as bacteria and other cell type. Therefore, further research direction points to further clarification of the biodegradability, immunogenicity and the bone forming specificity of marine sponge by animal studies. In vivo bone formation by marine sponge shall be tested in small and large animal models before promising to be a potential scaffold for tissue engineered bone regeneration. Furthermore, surface modification, such as Hydroxyapatite coating, may facilitate osteoblastic cell homing and bone formation.
Taken together, this study indicates that the natural marine sponge skeleton is favourable as a new source of bioscaffold for the repair of bone defects. This project supports the study by Green et al. (2003), advocating sponge collagen as a bioscaffold material in bone tissue engineering. The pore size, microstructure, nanostructure, permeability, and composition of the sea sponge skeleton appear to facilitate successful cellular attachment and proliferation. Their potential as osteoinductive and conductive frameworks together with the abundance and structural diversity of natural marine sponge skeletons indicates promise as a new source of scaffold for tissue regeneration.
Acknowledgements
This work was supported by an Australian Research Council Linkage grant (LP110100581) and Natural Science Foundation and Medical Scientific Research Foundation of Guangdong Province, China.
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Bruder SP, Fox BS. Tissue engineering of bone. Cell based strategies. Clin Orthop Relat Res. 1999:S68-83
2. Goldstein SA, Patil PV, Moalli MR. Perspectives on tissue engineering of bone. Clin Orthop Relat Res. 1999:S419-423
3. Gittens SA, Uludag H. Growth factor delivery for bone tissue engineering. J Drug Target. 2001;9:407-429
4. Vats A, Tolley NS, Polak JM, Gough JE. Scaffolds and biomaterials for tissue engineering: a review of clinical applications. Clin Otolaryngol Allied Sci. 2003;28:165-172
5. Sharma B, Elisseeff JH. Engineering structurally organized cartilage and bone tissues. Ann Biomed Eng. 2004;32:148-159
6. Green D, Walsh D, Mann S, Oreffo RO. The potential of biomimesis in bone tissue engineering: lessons from the design and synthesis of invertebrate skeletons. Bone. 2002;30:810-815
7. Badylak S, Liang A, Record R, Tullius R, Hodde J. Endothelial cell adherence to small intestinal submucosa: an acellular bioscaffold. Biomaterials. 1999;20:2257-2263
8. Burg KJ, Culberson CR, Greene KG, Loebsack AB, Roland WR, Holder WDJr. et al. Absorbable mesh aids in development of discrete, tissue-engineered constructs. Crit Rev Biomed Eng. 2000;28:383-387
9. Simpson TL. The cell biology of sponges. New York: Springer-Verlag. 1984
10. Fromont J, Vanderklift M, Kendrick G. Marine sponges of the Dampier Archipelago, Western Australia: patterns of species distributions, abundance and diversity. Biodiversity and Conservation. 2006;15:3731-3750
11. Hooper JNA, Van Soest RW. Systema Porifera: a guide to the classification of sponges; Vol 1. New York: Kluwer Academic Plenum. 2002
12. Shim JS, Lee H-S, Shin J, Kwon HJ. Psammaplin A, a marine natural product, inhibits aminopeptidase N and suppresses angiogenesis in vitro. Cancer Letter. 2003;203:163-169
13. Helfrich MH, Ralstron SH. Methods in molecular medicine: Bone research protocols; Vol 80. New Jersey: Humana Press. 2003
14. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408
15. Green D, Howard D, Yang X, Kelly M, Oreffo RO. Natural marine sponge fiber skeleton: a biomimetic scaffold for human osteoprogenitor cell attachment, growth, and differentiation. Tissue Eng. 2003;9:1159-1166
16. Lu Q, Ganesan K, Simionescu DT, Vyavahare NR. Novel porous aortic elastin and collagen scaffolds for tissue engineering. Biomaterials. 2004;25:5227-5237
17. Pineda LM, Busing M, Meinig RP, Gogolewski S. Bone regeneration with resorbable polymeric membranes. III. Effect of poly(L-lactide) membrane pore size on the bone healing process in large defects. J Biomed Mater Res. 1996;31:385-394
18. Gibson LJ, Ashby MF. Celluar solids: structures and properties. London: Pergamon Press. 1988
19. Bilezikian JP, Rodan GA, Raisz LG. Principles of bone biology; second ed. San Diego: Academic Press. 2002
20. Karande TS, Ong JL, Agrawal CM. Diffusion in musculoskeletal tissue engineering scaffolds: design issues related to porosity, permeability, architecture, and nutrient mixing. Ann Biomed Eng. 2004;32:1728-1743
21. Vogel V, Baneyx G. The tissue engineeting puzzle: a molecular perspective. Annu Rev Biomed Eng. 2003;5:441-463
22. Diehl-Seifert B, Kurelec B, Zahn RK, Dorn A, Jericevic B, Uhlenbruck G. et al. Attachment of sponge cells to collagen substrata: effect of a collagen assembly factor. J Cell Sci. 1985;79:271-285
23. Exposito JY, Cluzel C, Garrone R, Lethias C. Evolution of collagens. Anat Rec. 2002;268:302-316
24. Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cell Mater. 2003;5:29-39
25. Datta N, Holtorf HL, Sikavitsas VI, Jansen JA, Mikos AG. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26:971-977
26. Blumbach B, Pancer Z, Diehl-Seifert B, Steffen R, Munkner J, Muller I. et al. The putative sponge aggregation receptor. Isolation and characterization of a molecule composed of scavenger receptor cysteine-rich domains and short consensus repeats. J Cell Sci. 1998;111( Pt 17):2635-2644
27. Pfeifer K, Frank W, Schroder HC, Gamulin V, Rinkevich B, Batel R. et al. Cloning of the polyubiquitin cDNA from the marine sponge Geodia cydonium and its preferential expression during reaggregation of cells. J Cell Sci. 1993;106( Pt 2):545-553
28. Schutze J, Krasko A, Diehl-Seifert B, Muller WE. Cloning and expression of the putative aggregation factor from the marine sponge Geodia cydonium. J Cell Sci. 2001;114:3189-3198
29. Muller WE, Krasko A, Skorokhod A, Bunz C, Grebenjuk VA, Steffen R. et al. Histocompatibility reaction in tissue and cells of the marine sponge Suberites domuncula in vitro and in vivo: central role of the allograft inflammatory factor 1. Immunogenetics. 2002;54:48-58
30. Wimmer W, Blumbach B, Diehl-Seifert B, Koziol C, Batel R, Steffen R. et al. Increased expression of integrin and receptor tyrosine kinase genes during autograft fusion in the sponge Geodia cydonium. Cell Adhes Commun. 1999;7:111-124
31. Postlethwaite AE, Seyer JM, Kang AH. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc Natl Acad Sci U S A. 1978;75:871-875
32. Muller WE. Origin of metazoan adhesion molecules and adhesion receptors as deduced from cDNA analyses in the marine sponge Geodia cydonium: a review. Cell Tissue Res. 1997;289:383-395
33. Muller WE, Wiens M, Muller IM, Schroder HC. The chemokine networks in sponges: potential roles in morphogenesis, immunity and stem cell formation. Prog Mol Subcell Biol. 2004;34:103-143
Author contact
![]() Corresponding author: W/Prof. Minghao. Zheng, Centre for Orthopaedic Research, University of Western Australia, M Block, QEII Medical Centre, Monash Avenue, Nedlands, Western Australia 6009, Australia. Tel: 61-8-9346-4050; E-mail: minghao.zhengedu.au. Prof. Qiujian Zheng, Division of Orthopaedic Surgery, Guangdong Academy of Medical Sciences, Guangdong General Hospital, 10th floor, No. 106, Zhongshan er Road, Guangzhou, Guangdong, 510080, P.R. China. Tel: 86-20-83827812; Email: zqj650com
Corresponding author: W/Prof. Minghao. Zheng, Centre for Orthopaedic Research, University of Western Australia, M Block, QEII Medical Centre, Monash Avenue, Nedlands, Western Australia 6009, Australia. Tel: 61-8-9346-4050; E-mail: minghao.zhengedu.au. Prof. Qiujian Zheng, Division of Orthopaedic Surgery, Guangdong Academy of Medical Sciences, Guangdong General Hospital, 10th floor, No. 106, Zhongshan er Road, Guangzhou, Guangdong, 510080, P.R. China. Tel: 86-20-83827812; Email: zqj650com

 Global reach, higher impact
Global reach, higher impact