10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2013; 9(2):142-148. doi:10.7150/ijbs.5453 This issue Cite
Short Research Communication
Enhanced Cytotoxicity for Colon 26 Cells Using Doxorubicin-Loaded Sorbitan Monooleate (Span 80) Vesicles
Division of Chemical Engineering, Graduate School of Engineering Science, Osaka University, 1-3 Machikaneyama-cho, Toyonaka, Osaka 560-8531, Japan.
Received 2012-10-26; Accepted 2012-12-19; Published 2013-1-17
Abstract
Span 80 (sorbitan monooleate) vesicles behaved differently from conventional phospholipid vesicles (liposomes) because the former had a more fluid interface. After doxorubicin hydrochloride (DOX) was encapsulated into the Span 80 vesicle (loading efficiency: 63 %), DOX-loaded Span 80 vesicles (DVs) were thereafter added to Colon 26 cells. It was suggested, from the flow cytometric analysis and confocal laser microscopic observation, that DVs directly deliver DOX into the cytoplasm of Colon 26 cells. DVs showed the different delivery manner from the DOX-loaded liposomes (DLs). It is considered that the difference of delivery manner between DVs and DLs resulted in the difference of cytotoxicity (IC50); i.e. IC50 values for DVs and DLs were 5 and > 30 μM, respectively. The results obtained herein would give the fundamental findings which can contribute to the improvement of formulation of conventional liposome-based carrier and its cytotoxicity.
Keywords: Span 80 vesicles, Colon 26, Cytotoxicity
Introduction
In the cancer treatment, various anti-cancer drugs have been investigated: RNA interference- or small interference RNAs-based drugs[1]; paclitaxel[2]; curcumin[3]; or doxorubicin[4-6]. For the efficient delivery of drugs to cancer cells, various carriers, such as nanoparticle[7], lipoplex[8], liposome which is a closed-phospholipid bilayer membrane[4, 9], vesicles composed of detergents [10], have been proposed as promising delivery strategies. Of those carriers, the liposome has an advantage for both an encapsulation of anti-cancer drug[2], and an easy modification of liposomal surface[4]. For examples, Doxil® is a doxorubicin (DOX) incorporated into long-circulating PEGylated liposomes, which is a well-known commercial drug using the liposome. It has been demonstrated that Doxil® could decrease cardiotoxicity and improve tumor accumulation via the enhanced permeability and retention (EPR) mechanism[4]. Meanwhile, Doxil® has drawbacks on its own low efficiency of drug delivery, which is attributed at least in part to its uptake by the endocytosis pathway[11, 12]. The incorporation of cationic lipids[9], and the modification of liposomes by monoclonal anticancer antibody have improved the efficiency of drug delivery and cytotoxicity[4].
DOX contained in Doxil® is the anti-cancer drug studied well from the variety of aspects. It has been reported that DOX showed the similar self-quenching property to calcein[13]. DOX can be easily up-taken by cells via permeation across cell membranes[5]. Therefore, DOX preferably induced the strong side-effect, although DOX disappears by a rapid opsonization and an up-take by the reticuloendothelial system (RES) of liver and spleen[14, 15]. To suppress the side-effect of DOX, a chemical conjugate of DOX was then investigated by using poly(ethylene glycol) to produce the prodrug[16], or anti-body directed against the cancer cells[17]. Alternatively, the enhanced cytotoxicity of DOX could be achieved by a chemical conjugate with DNA[18]. The utilization of DOX without any chemical conjugation or complicated pretreatment would demand the carrier that can directly delivers DOX into cell inside.
The direct delivery of drug requires the strong interaction of carrier with cell membranes. Conventionally, liposomes composed of phospholipids induced no membrane fusion with cell membranes, except for the liposomes incorporating positively charged lipids. As stated before, liposomes shows low delivery efficiency due to their internalization by the endocytosis pathway[11, 12]. Alternatively, vesicles composed of nonionic detergent, namely niosome, have recently been studied[19]. One of typical noisomes is vesicles prepared by Span 80 which is a sorbitan monooleate composed of a sorbitol group and acyl chain[20, 21]. Our previous study has revealed that Span 80 vesicles have very fluid, flexible and wet surface by contrasting with conventional liposomes[21]. It is therefore expected that Span 80 vesicles can be used as a promising vesicle formulation to overcome the problems of conventional liposome-based carrier such as Doxil®.
In this study, we prepared the novel vesicle formulation including anti-cancer drug by using Span 80 vesicles. First, DOX was loaded into Span 80 vesicles by a remote loading method[22, 23]. The obtained vesicles were called as DVs. We investigated the binding of DVs to cell membranes and their cytotoxicity by contrasting with the conventional liposomal carrier containing DOX (DLs). Colon 26 cells were herein used as a case study because it has been reported, from the previous study [10], that Span 80 vesicle-based delivery system could show a certain anti-cancer activity to Colo 201 cells (human colon cancer cell line).
Material and Method
Materials
Sorbitan monooleate (Span 80), polyoxyethylene sorbitan monooleate (Tween 80) and Doxorubicin hydrochloride were purchased from Wako Pure Chemical Industries (Osaka, Japan). Cholesterol was obtained from Sigma-Aldrich (St. Louis, MO, USA). A zwitterionic phospholipid, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), was purchased from the NOF Corporation (Tokyo, Japan).
Preparation of Span 80 vesicles
Span 80 vesicles were prepared by the two-step emulsification method as previously described in detail[20, 24]. 6 ml of n-hexane containing 252 mg Span 80 and48 mg cholesterol was added to 0.6 ml of 155 mM ammonium sulfate solution, followed by the first emulsification for 6 min at 17,500 rpm using a micro-homogenizer NS-310E 2 (Microtec Co.Ltd., Funabashi, Japan). The solvent obtained from the water-in-oil emulsion was evaporated in a rotary evaporator at 28 oC under reduced pressure, yielding a water-lipid emulsion. After that, 6 ml of PBS (pH 7.4) containing 96 mg of Tween 80 was added, followed by mixing with the homogenizer for 2 min at 3500 rpm to obtain the heterogeneous Span 80 vesicles suspension. The heterogeneous vesicles suspension was stirred with a magnetic stirrer for 3 h at room temperature, followed by storage overnight at 4 oC. The vesicles were then purified by an ultracentrifugation (50,000 rpm at 4oC for 120 min) in a Himac centrifuge CR15B (Hitachi Koki Co.Ltd., Tokyo, Japan). The purified Span 80 suspension was passed through 100-nm nucleopore track-etch polycarbonate membranes and purified twice by the ultracentrifugation.
Preparation of liposome
Liposomes were prepared according to the previous literatures[21, 25]. Phospholipids were dissolved in a chloroform solution. The organic solvent was removed by evaporation in a rotary evaporator. The residual lipid film, after drying under a vacuum overnight, was hydrated with the ammonium sulfate. The suspension was subjected to five cycles of freezing and thawing and was then extruded through polycarbonate filter with 100 nm in pore size, followed by ultracentrifugation, as described above. The lipid concentrations were determined by phosphorous analysis.
Loading of DOX into Span 80 vesicles
DOX was loaded into Span 80 vesicles by a remote loading method[22, 23]. In short, an aliquot of ammonium sulphatesolution (155 mM) was added into the Span 80 vesicles. This Span 80 vesicle suspension (50 mM) was then mixed with a DOX saline solution (1 mM). To accelerate a loading of DOX, the sample was then heated to 60oC for 10 min. Afterwards, the unloaded material was removed by using a gel permeation chromatography(SepharoseTM4B).
TEM observation of vesicles
Vesicles prepared here were observed by transmission electron microscopy (TEM). TEM images were obtained by the following procedure. In brief, a 5 μl aliquot of diluted solution was placed on a copper grid (400-mesh) covered with a carbon-coated collodion film for 1 min and the excess sample solution was removed by blotting with filter paper. After the residual solution had dried up, the grid was negatively stained with a 2% (wt/vol) uranyl acetate solution. Again, the liquid on the grid was removed with filter paper and dried. EM images were acquired using a JEOL 100CX transmission microscope (JEOL, Tokyo, Japan) with an acceleration voltage of 80 kV.
Cell cultivation
Mice colon adenocarcinoma cell line (Colon 26 cells) was obtained from Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University (Sendai, Japan). Colon 26 cells were cultured with Eagle's minimum essential medium (E-MEM) containing 10% fetal bovine serum (FBS) in a humidified atmosphere of 5% CO2 at 37 oC.
Observation by confocal laser scanning microscopy
Cells were seeded at 2 × 105 cells/ml in a cell culture dish in complete medium, E-MEM with 10% FBS. The cells were then incubated for 24 h in a humidified atmosphere of 5% CO2 and at a temperature of 37oC. Then, the test samples were added to cells and left them for another 1 h under 5% CO2 atmosphere and 37oC for incubation. Afterwards these cells were observed by a confocal laser scanning microscope (Fluoview, Olympus).
Flowcytometry
Cells were seeded at 2 × 105 cells/ml in a cell culture dish in complete medium, E-MEM, with 10% FBS for Colon 26 cells. Cells were then incubated for 24 h in a humidified atmosphere of 5% CO2 and at a temperature of 37oC. Then, test samples were added to the cells and were further incubated for another 1 h under 5% CO2 atmosphere and 37°C for incubation. After incubation cells were removed by EDTA-Trypsin and washed by PBS buffer (pH 7.4) twice. Cells were analyzed by flowcytometry (Life Technologies, Gaithersburg, MD, USA). Fluorescence intensity excited at 488 nm was monitored with BP filter (575/24 nm).
MTT assay
The cytotoxicity of DV on Colon 26 cells was evaluated by an MTT assay (Promega, USA). Cells were seeded at 2 × 105 cells/ml, 100 μl in 96-well microtiter plates in complete medium, E-MEM, with 10% FBS. Cells were then incubated for 24 h in a humidified atmosphere of 5% CO2 and at a temperature of 37°C. Then, DOX-loaded Span 80 vesicles (DVs) were added to wells for 24 h-incubation under 5% CO2 atmosphere and 37°C. The supernatant including DVs were thereafter left. The sample in the presence of neither DOX nor vesicles was a positive control. The cell viability was assayed by adding 15μL of MTT solution (5 mg/ml). After incubation at 37°C for another 4 h, 100 μL of Stop mix solution (20% sodium dodecyl sulfate in 50% dimethyl stateamide) was added to the plate and left it for another 1 h to dissolve the obtained crystal. The absorbance of each well was measured by using a microplate reader (Bio-Rad CA, USA) at a test wave length of 550 nm and 655 nm. The ratio of cell viability was calculated by the following:(Ratio of cell viability) = (A1-A3) / (A2-A3), where A1, A2, and A3 mean the absorbance vesicle (10 ml) + cell suspension (90 ml), that of cell suspension (100 ml), and that of culture medium (100 ml) as a negative control, respectively.
Results and Discussion
Loading of DOX into Span 80 vesicles
In the first series of experiments, the loading of DOX into Span 80 vesicles was investigated with a remote loading method. 1 mM of DOX was, by a remote loading method[22, 23], loaded into Span 80 vesicles (composed of 252 mg Span 80 and 48 mg cholesterol) with 100 nm in diameter. Span 80 vesicles including DOX (DVs) were separated by a size exclusion chromatography. The concentration of DOX for the fraction of Span 80 vesicles was measured, by disrupting DVs with sodium dodecyl sulfate (SDS). The molar ratio of DOX, included in the vesicle fraction, to initial DOX (1 mM) was estimated to be 63 %.The vesicles by TEM were observed to investigate the influence of loading of DOX on the vesicle structure. Span 80 vesicles have the similar vesicular structure to the conventional liposome (Figure 1A). The same was true for DVs although DVs appear more aggregated (Figure 1B) in comparison with Span 80 vesicles. This aggregation might be a transient one resulting from the pretreatment for TEM observation, although DVs are likely to fuse due to their fluid and flexible surface [20]. In actual, the DVs were confirmed to be stably dispersed in the solution because their mean diameter (131.5 nm) was constant for several weeks, from the dynamic light scattering method.
Loading of DOX into Span 80 vesicles. TEM images of A) Span 80 vesicles and B) DVs. Scale bar represents 200 nm. C) Fluorescence spectra of free DOX, DVs and DVs + SDS. Inset: concentration dependency of DOX fluorescence intensity at 590 nm. D) Relationship between λmax of DOX in binary water/ethanol system and the corresponding dielectric constant. E) Schematic illustration of DV. For Span 80 vesicles, see Experimental section.
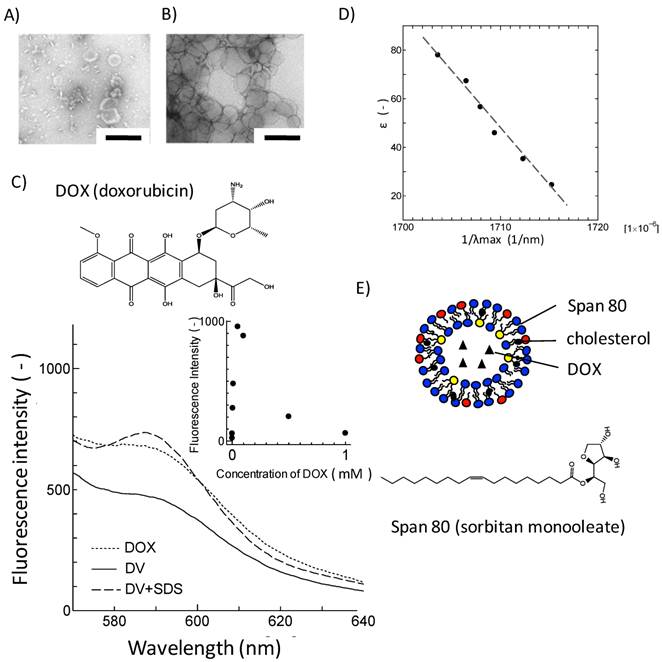
It is unclear, from the observation with TEM, how DOX was loaded in Span 80 vesicles. The loading nature of DOX in Span 80 vesicles was investigated from the aspect of fluorescence property of DOX. Figure 1C shows the fluorescence spectra for free DOX, DVs, and SDS-treated DVs excited at λex= 480 nm. Free DOX has an emission peak at around 590 nm, corresponding to the fluorophore. The concentration dependency of DOX fluorescence was shown in the inset of Figure 1C. DOX was self-quenched above 100 μM, which is consistent with the previous report[13]. Furthermore, DVs that contain DOX at the same concentration as free DOX indicated the reduction of DOX fluorescence intensity (Figure 1C), suggesting the incorporation of DOX into Span 80 vesicles at higher concentration range. Besides, the SDS treatment led to the increase in the fluorescence of DOX, which was probably because of a dequenching of DOX. This also strongly suggested the incorporation of DOX in Span 80 vesicles at high concentration.
Furthermore, the solubilization of DOX in Span 80 vesicles was investigated in terms of the solvatochromic property. The emission property of fluorescence molecules in solvent depends on the dielectic constant of solvent[26]. The fluorescence spectra for DOX were then measured with varying the dielectric constant of the binary water/ethanol system as a solvent. The reciprocal of λmax giving the maximal fluorescence intensity of DOX was proportional to the dielectric constant of solvent (Figure 1D).The fluorescence spectrum for DVs showed the emission peak at 590 nm in λmax (Figure 1C), corresponding to the bulk aqueous environment (ε ~ 75) (estimated by Figure 1D). Taking into consideration that the dielectric constantmonotonously changes along the vesicle membrane[27], it is considered that DOX would be present at the same dielectric environment as the bulk aqueous phase, although some of DOX might be solubilized at membrane region of Span 80 vesicle.
From the above results, it is considered that DOX was contained in the Span 80 vesicles as schematically shown in Figure 1E. It has been reported that the membrane interface of Span 80 vesicles was considerably fluid due to the intense mobility of headgroup for sorbitol ester[21]. Therefore, DVs might interact with cell membranes enough to deliver DOX into cell inside.
Interaction of DVs with cell membranes
Span 80 vesicles have the dynamic interface advantageous for their interaction with cell membranes[21]. DVs would preferably bind to the cell membrane by contrasting them with the DOX-loaded liposomes (DLs). In this section, the binding of DVs to the cell membranes and the delivery of DOX to cells was examined.
Delivery of DOX by Span 80 vesicles and liposomes. A) Confocal laser microscopic images (left) DVs and (right) DLs. Green fluorescence: NBD-PE; red fluorescence: DOX. Flowcytogram of Colon 26 cells at B) 37 and C) 4 oC. a: control (PBS), b:DLs, c: Free DOX, and d: DVs. Other detailed experimental conditions were referred to experimental section. Fluorescence intensity was monitored with the condition of ex: 488 nm/ em. 575 nm (575/24 nm BP filter).
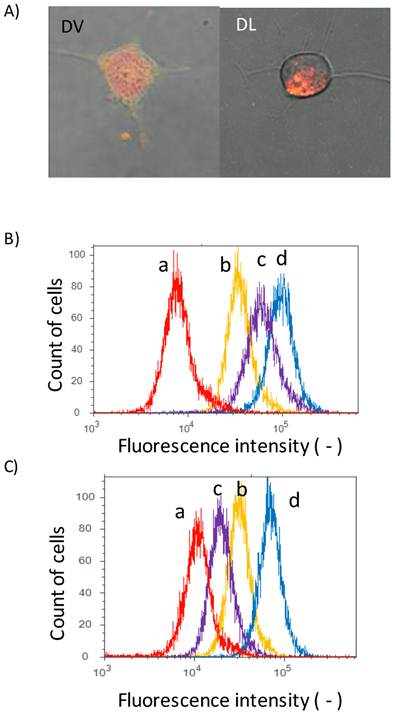
The interaction of DVs with cell membranes was investigated by a confocal laser microscope. Colon 26 cells were herein used as model cells. Fluorescence lipid NBD-PE was herein incorporated into DVs because such NBD-stained DVs should show both red fluorescence from DOX and green fluorescence from NBD-PE. Colon 26 cells were incubated for 12 h with NBD-stained DVs. Green fluorescence derived from NBD-PE was observed at cellular membranes, as shown in Figure 2A. This observation implies that the lipid mixing between the Span 80 vesicles and cell membranes occurred. Red fluorescence derived from DOX was also observed inside Colon 26 cells, implying the delivery of DOX into the cytoplasm of cells. From this double-staining experiment, it is considered that the Span 80 vesicle delivered DOX into the cytoplasm of cells by the interaction of Span 80 vesicles with cellular membranes, although we could not rule out the possibility that DOX was involved in endosomes or lysosomes. Actually, Span 80 vesicles have the dynamic interface advantageous for the strong interaction between vesicles[21]. It has been reported, from the composition analysis of Span 80 vesicles, that Span 80 vesicles involved the tri-ester body with three acyl chains prior to the generation of negative curvature[20]. The generation of negative curvature is an indispensable process of the membrane fusion[28]. Besides, from the fluorescence resonance energy transfer experiment, Span 80 vesicles induced the activity of membrane fusion at 43 oC, whereas no fusion activity at 25 oC [20]. These previous findings are compatible with the observations shown in Figure 2A. In contrast, in the case of DLs, the dotted red fluorescence in cytosol of Colon 26 cells could be observed. This observation was the definite evidence that the DLs were internalized by the endocytosis pathway. From the observation using the confocal laser microscopy, the delivery manner of DVs was found to be different from that of DLs.
In addition, we measured the fluorescence of DOX with the flow cytometry to investigate the binding of DVs to cells. Peaks for free DOX (peak c), DVs (peak d) and DLs (peak b) were shifted to the higher fluorescence region relative to the control (peak a) (Figure 2B), suggesting that DVs and DLs evidently bound to Colon 26 cells. In order to clarify the internalization mechanism of DVs and DLs, their binding to cells was investigated under 4 oC-inclubation that could inhibit the endocytosis pathway. Both peaks for DLs (peak b) and free DOX (peak c) was shifted to the control (peak a) (Figure 2C). The observed shift of DLs probably resulted from the binding of DLs under 4 oC-inclubation, as reported in the previous literature [4]. It was therefore considered that DLs used herein was internalized via an endocytosis pathway. In contrast, the peak for free DOX (peak c) was obviously shifted to the control peak. Free DOX permeates across the cell membranes[5]. It is considered that the 4 oC-incubation reduced the permeability of cell membranes to DOX. From these results, DVs was thus found to be up-taken by the different pathway from the endocytosis observed in DLs.
Cytotoxicity of DV System
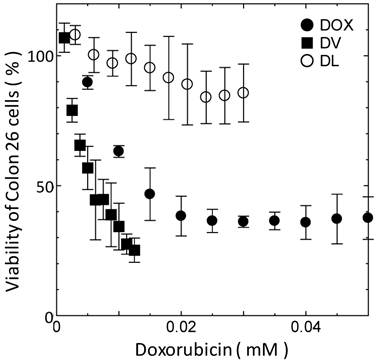
The difference in delivery manner between DVs and DLs would affect their cytotoxicity. The cytotoxicity of DVs was investigated with the MTT assay. Span 80 vesicle alone was found to show no toxicity below 2 mM (data not shown). The same was true for liposomes under the same concentration. The cell viability of DVs was therein investigated below 2 mM of Span 80. Figure 3 shows the cell viability of Colon 26 cells as a function of DOX concentration. DVs induced the larger decremental change of cell viability as compared with free DOX, whereas DLs induced the smaller decremental change of viability. From Figure 3, IC50 values for DVs, free DOX, and DLs were 5, 15, and >30 μM, respectively. It is considered that the highest IC50 value for DLs resulted from their internalization through an endocytosis pathway as shown in Figure 2B and C. Reduction of IC50 value of free DOX relative to DLs might be a result that free DOX was able to directly permeate across the cell membranes[5]. Likewise, a remarkable reduction of IC50 value was observed in the case of DVs. DVs appeared to directly deliver DOX into the cytoplasm of cells, probably due to the interaction between Span 80 vesicle membranes and cellular membranes (Figure 2). Although further investigation is needed, it is likely that the drug delivery using DVs is substantially efficient in contrast to the conventional systems using liposome (Doxil®) based on the above results.
Cell viability of Colon 26 cells as function of DOX concentration. All the experiments were performed at least three times.
In conclusion, we prepared Span 80 vesicles containing DOX (DVs) with a 63 % in loading efficiency. It was demonstrated that DVs could successfully deliver DOX into the cytoplasm of tumor cells via the direct interaction of vesicle membranes with cellular membranes, which could improve the cytotoxicity. The above advantage could successfully be induced under the condition that Span 80 vesicles themselves showed low toxicity (below 2 mM of Span 80). Taking into consideration that both Eucheuma serra agglutinin (ESA) with high targeting activity to tumor cells and PEGylated lipid with a stealth function could be incorporated into Span 80 vesicles[10], a combination of DVs with ESA/PEGylated lipid might improve their cytotoxicity and the targeting activity of conventional liposome-based carrier (i.e. Doxil®). The present study would give the fundamental findings with respect to the alternative strategy to deliver DOX into the cytoplasm of tumor cells. A further in vitro and in vivo research would achieve the construction of novel drug carrier using DV system.
Acknowledgements
The fundamental concept of this study was conducted by the Research Group of “Membrane Stress Biotechnology” and the Sigma Multidisciplinary Research Laboratory Group (Grad. Sc. of Engineering Science, Osaka University) “Membranomics”. It was partly supported by the Cabinet Office, Government of Japan through its “Funding Program for Next Generation World-Leading Researchers” (GR066) and Grants-in-Aid for Scientific Research (Nos. 23656525 and 24686086 ) from the Ministry of Education, Science, Sports, and Culture of Japan (MEXT). The TEM images were taken at the Research Center for Ultrahigh Voltage Electron Microscopy, Osaka University, Japan. The authors report no declarations of interest.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Oh YK, Park TG. siRNA delivery systems for cancer treatment. Advanced drug delivery reviews. 2009;61:850-62
2. Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. Journal of controlled release: official journal of the Controlled Release Society. 2007;117:68-79
3. Yallapu MM, Gupta BK, Jaggi M, Chauhan SC. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. Journal of colloid and interface science. 2010;351:19-29
4. Elbayoumi TA, Torchilin VP. Enhanced cytotoxicity of monoclonal anticancer antibody 2C5-modified doxorubicin-loaded PEGylated liposomes against various tumor cell lines. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2007;32:159-68
5. Veronese FM, Schiavon O, Pasut G, Mendichi R, Andersson L, Tsirk A. et al. PEG-doxorubicin conjugates: influence of polymer structure on drug release, in vitro cytotoxicity, biodistribution, and antitumor activity. Bioconjugate chemistry. 2005;16:775-84
6. Park H, Yang J, Lee J, Haam S, Choi IH, Yoo KH. Multifunctional nanoparticles for combined doxorubicin and photothermal treatments. ACS nano. 2009;3:2919-26
7. Sami H, Maparu AK, Kumar A, Sivakumar S. Generic Delivery of Payload of Nanoparticles Intracellularly via Hybrid Polymer Capsules forBioimaging Applications. PLoS ONE. 2012;7:e36195. doi:10.1371/journal.pone.0036195
8. Ma B, Zhang S, Jiang H, Zhao B, Lv H. Lipoplex morphologies and their influences on transfection efficiency in gene delivery. Journal of controlled release: official journal of the Controlled Release Society. 2007;123:184-94
9. Wu J, Lee A, Lu Y, Lee RJ. Vascular targeting of doxorubicin using cationic liposomes. International journal of pharmaceutics. 2007;337:329-35
10. Omokawa Y, Miyazaki T, Walde P, Akiyama K, Sugahara T, Masuda S. et al. In vitro and in vivo anti-tumor effects of novel Span 80 vesicles containing immobilized Eucheuma serra agglutinin. International journal of pharmaceutics. 2010;389:157-67
11. Gupta B, Torchilin VP. Monoclonal antibody 2C5-modified doxorubicin-loaded liposomes with significantly enhanced therapeutic activity against intracranial human brain U-87 MG tumor xenografts in nude mice. Cancer immunology, immunotherapy: CII. 2007;56:1215-23
12. O'Donnell RT, Martin SM, Ma Y, Zamboni WC, Tuscano JM. Development and characterization of CD22-targeted pegylated-liposomal doxorubicin (IL-PLD). Investigational new drugs. 2010;28:260-7
13. Mohan P, Rapoport N. Doxorubicin as a molecular nanotheranostic agent: effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Molecular pharmaceutics. 2010;7:1959-73
14. Han HD, Lee A, Song CK, Hwang T, Seong H, Lee CO. et al. In vivo distribution and antitumor activity of heparin-stabilized doxorubicin-loaded liposomes. International journal of pharmaceutics. 2006;313:181-8
15. Han HD, Lee A, Hwang T, Song CK, Seong H, Hyun J. et al. Enhanced circulation time and antitumor activity of doxorubicin by comblike polymer-incorporated liposomes. Journal of controlled release: official journal of the Controlled Release Society. 2007;120:161-8
16. Pawar SK, Badhwar AJ, Kharas F, Khandare JJ, Vavia PR. Design, synthesis and evaluation of N-acetyl glucosamine (NAG)-PEG-doxorubicin targeted conjugates for anticancer delivery. International journal of pharmaceutics. 2012;436:183-93
17. Yu DS, Chu TM, Yeh MY, Chang SY, Ma CP, Han SH. Antitumor activity of doxorubicin-monoclonal antibody conjugate on human bladder cancer. The Journal of urology. 1988;140:415-21
18. Swift LP, Cutts SM, Nudelman A, Levovich I, Rephaeli A, Phillips DR. The cardio-protecting agent and topoisomerase II catalytic inhibitor sobuzoxane enhances doxorubicin-DNA adduct mediated cytotoxicity. Cancer chemotherapy and pharmacology. 2008;61:739-49
19. Kaur IP, Garg A, Singla AK, Aggarwal D. Vesicular systems in ocular drug delivery: an overview. International journal of pharmaceutics. 2004;269:1-14
20. Kato K, Walde P, Koine N, Ichikawa S, Ishikawa T, Nagahama R. et al. Temperature-sensitive nonionic vesicles prepared from Span 80 (sorbitan monooleate). Langmuir: the ACS journal of surfaces and colloids. 2008;24:10762-70
21. Hayashi K, Shimanouchi T, Kato K, Miyazaki T, Nakamura A, Umakoshi H. Span 80 vesicles have a more fluid, flexible and "wet" surface than phospholipid liposomes. Colloids and surfaces B, Biointerfaces. 2011;87:28-35
22. Madden TD, Harrigan PR, Tai LC, Bally MB, Mayer LD, Redelmeier TE. et al. The accumulation of drugs within large unilamellar vesicles exhibiting a proton gradient: a survey. Chemistry and physics of lipids. 1990;53:37-46
23. Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochimica et biophysica acta. 1993;1151:201-15
24. Kato K, Walde P, Koine N, Imai Y, Akiyama K, Sugahara T. Molecular composition of nonionic vesicles prepared from Span 80 or Span 85 by a two-step emulsification method. J Disper Sci Technol. 2006;27:1217-22
25. Shimanouchi T, Sasaki M, Hiroiwa A, Yoshimoto N, Miyagawa K, Umakoshi H. et al. Relationship between the mobility of phosphocholine headgroups of liposomes and the hydrophobicity at the membrane interface: A characterization with spectrophotometric measurements. Colloid Surface B. 2011;88:221-30
26. Muller M, Zschornig O, Ohki S, Arnold K. Fusion, leakage and surface hydrophobicity of vesicles containing phosphoinositides: influence of steric and electrostatic effects. The Journal of membrane biology. 2003;192:33-43
27. Konopasek I, Kvasnicka P, Amler E, Kotyk A, Curatola G. The transmembrane gradient of the dielectric constant influences the DPH lifetime distribution. FEBS letters. 1995;374:338-40
28. Kao PH, Chiou YL, Chen YJ, Lin SR, Chang LS. Guanidination of notexin promotes its phospholipase A(2) activity-independent fusogenicity on vesicles with lipid-supplied negative curvature. Toxicon: official journal of the International Society on Toxinology. 2012;59:47-58
Author contact
![]() Corresponding author: Tel: +81-(0)6-6850-6287, Fax: +81-(0)6-6850-6286 E-MAIL: umakoshies.osaka-u.ac.jp.
Corresponding author: Tel: +81-(0)6-6850-6287, Fax: +81-(0)6-6850-6286 E-MAIL: umakoshies.osaka-u.ac.jp.

 Global reach, higher impact
Global reach, higher impact