10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2013; 9(7):693-701. doi:10.7150/ijbs.6427 This issue Cite
Research Paper
Production of Polyploids and Unreduced Gametes in Lilium auratum × L. henryi Hybrid
1. Department of Agricultural Education, Sunchon National University, Sunchon, 540-742, Korea;
2. Department of Horticultural Science, Kyungpook National University, Daegu 702-701, Korea.
3. BU Biodiversity and Breeding, Plant Research International, P.O. Box 16, 6700 AA, Wageningen, Netherlands.
Received 2013-4-8; Accepted 2013-5-28; Published 2013-7-19
Abstract
Intergenomic F1 hybrids between L. auratum x L. henryi and their BC1 progeny were investigated through genomic in situ hybridization technique (GISH) to determine their potential value in lily breeding. We confirmed that F1 intergenomic hybrids possessed a set of chromosomes (x=12) from both parents and that flowers of the F1 auratum × henryi hybrid showed an intermediate morphological phenotype. Pollen size, viability and germination ability were measured through microscopic observations. F1 intergenomic hybrids produced a relevant frequency of 2n-gametes, which were successfully used to perform crosses with Oriental hybrids, resulting in the triploid Oriental Auratum Henryi (OAuH) hybrid. Twenty BC1 plants were generated by crossing between four different Oriental hybrid cultivars and F1 AuH hybrids using an in vitro embryo rescue technique, after which the genome constitution and chromosome composition were analyzed by GISH. All plants were triploid, showing 12 from female parents (diploid Oriental hybrid) and 24 from male parents (diploid F1 AuH hybrid). Overall, 16 out of 20 BC1 progeny possessed recombinant chromosomes with 1-5 crossover sites per plant. Cytological analysis of 20 BC1 plants by GISH verified that the occurrence of 2n pollen formation in all F1 AuH hybrids was derived from the FDR (first division restitution) mechanism, in which the genome composition of all BC1 plants possess 12 Oriental + 12 L. auratum + 12 L. henryi chromosomes. Allotriploids derived from the AuH hybrid were used as female for crossing with the diploid Oriental hybrid cultivar 'Sorbonne' and considerable numbers of plants (0-6.5 plants per ovary) were only obtained when female OAuH (BC1) triploids were used. Taken together, the results of this study indicate that production and analysis of F1 AuH hybrids and their progeny through sexual polyploidization can be useful for efficient creation of important horticultural traits.
Keywords: Allotriploid, Polyploidization, Homoeologous recombination, Interspecific hybrid, 2n-gamete.
Introduction
There are about 80 species in the genus Lilium, which is taxonomically classified into seven sections [1, 2]. Currently, the most important groups for commercial breeding comprise of the Trumpet lily group, including L. longiflorum of the section Leucolirion, the Asiatic hybrid group of the section Sinomartagon and the Oriental hybrid group of the section Archelirion [3]. All three sections comprise species with distinct, desirable horticultural characteristics. L. henryi belongs to neither Archelirion nor Leucolirion, and shows intermediate phenotypic characteristics of both sections [4]. The interspecific hybridization technique has been applied to introduce some interesting traits such as virus resistance in L. henryi and botrytis resistance in L. auratum. Combination of the desirable characteristics of different species is an important goal in lily breeding [5]. Although interspecific hybridization is laborious and time consuming but it has played an important role in lily breeding [6]. Interspecific hybridization and polyploidization are closely related to F1 sterility and most of the F1 interspecific hybrids between widely related species are sterile. Therefore, many studies have been conducted to restore fertility via mitotic chromosome doubling (mitotic polyploidization). Despite the presence of large genomes in lilies (76 pg/2C) and a fairly large basic chromosome number (x=12), numerous polyploid cultivars have been successful in this crop [5, 7]. Additionally, interspecific hybridization followed by polyploidization has contributed to the development of useful breeding materials as well as cultivars [5]. In many cases, spindle inhibiting or chromosome doubling agents such as colchicine or oryzalin have been successfully applied for the induction of polyploids [8]. However, in some cases numerically unreduced (2n) gametes have been shown to be useful for inducing polyploids using interspecific hybrids that are otherwise sterile [9]. Furthermore, Asano (1984) analyzed the behaviour of meiotic chromosomes and the fertility of pollen and found abnormal chromosome separation at meiosis I and the formation of unreduced gametes [10].
GISH enables distinction of parental chromosomes of interspecific hybrids or intergenomic hybrids [11]. This technique has enabled better insight into various aspects of intergenomic recombination and the modes of origin of 2n-gametes in some lily hybrids [12].
In present study, we analyzed the composition of parental chromosomes F1 AuH hybrid (L. auratum × L. henryi) and their BC1 progeny produced by backcrossing between F1 AuH hybrids and Oriental hybrids through GISH. An important feature of these sexual ployploids was that they possessed homoeologous recombinant chromosomes in their complements. Furthermore, the mechanism of 2n-gametes production in the F1 AuH hybrid was described. Finally, we evaluated BC2 progeny derived from backcrossing between BC1 allotriploids and Oriental hybrids.
Materials and methods
Plant materials
The F1 AuH hybrid materials used in this experiment were developed by crossing between L. auratum (2n=24; hereafter Au) and L. henryi (2n=24; hereafter H) [13]. BC1 progeny used for chromosome analysis by GISH were produced by crossing male F1 AuH hybrids with four different Oriental hybrid cultivars, 'Stargazer', 'Journey's End', 'Dominique', and 'Darlings' [14]. Reciprocal crosses were conducted between female F1 AuH hybrids and male L. auratum and L. henryi, and vice versa.
To produce BC2 progeny, the BC1 progenies were also crossed with female L. henryi Oriental hybrid cultivars 'Sorbonne' and L. auratum as a male. Plants were grown in pots containing a peat based soil mixture in the greenhouse at temperatures varying from 20ºC-25ºC during day time and 14ºC-18ºC at night. Present research was carried out in Wageningen University, Netherland (WUR).
Pollen viability and germination
To measure the pollen size and stainability, pollens were collected from fully open flowers, mounted in a drop of lactophenol acid-fuchsin and viewed under the microscope. Classification of pollen size was determined using a calibrated micrometer. Pollen were collected after flower anthesis and then cultured for 24 hours at 25ºC in artificial agar medium containing 100g/L sucrose, 5g/L bacteriological agar, 20mg/L boric acid and 200mg/L calcium nitrate. The pollen was classified as large (2n) and small (n) depending on size and then counted to determine the germination range.
Pollination and embryo rescue
All crosses were conducted by cut style pollination method (CSM) and encapsulated with aluminum foil on top of the cut stigma for a week. Embryo rescue was then carried out before abortion. The embryo were subsequently dissected under the stereomicroscope and placed on 1/2 MS medium containing 80g/L of sucrose for germination in vitro [15]. Pre and post-fertilization were then investigated by checking ovary enlargement and embryo formation after pollination.
Chromosome preparation
Root tips were harvested in a saturated α-bromonaphtalene solution in the early morning and kept overnight at 4ºC for accumulation of metaphase cells. Then this material was fixed in ethanol - acetic acid solution (3:1) for at least 2 hours, after which they were stored at -20ºC. The root tips were then treated with a pectolytic enzyme mixture (0.3% pectolyase Y23, 0.3% cellulase RS and 0.3% cytohelicase) in 10mM citric acid buffer at 37ºC for 1 hour, after which they were squashed in a drop of 60% acetic acid solution. Slides were then frozen by dipping in liquid nitrogen, after which their cover slips were removed using a razor blade. Before air-drying, the slides were dehydrated in an absolute ethanol for several minutes, after which they were stored at 4ºC for several weeks prior to in situ hybridization.
Genomic in situ hybridization
The GISH protocol was basically the same as that described by Lim et al. (2001) [16]. In this genomic DNA (1 - 10 kb) from L. henryi was used as a probe after labeling with digoxigenin by nick translation according to the manufacturer's instructions (Boehringer Mannheim, Germany). Sheared herring sperm DNA was used to block the non-Henryi-specific DNA sequences. After detection steps, the slides were counter-stained with 5 μg/mL propidium iodide (PI). The images were photographed with a Zeiss Axiophot microscope equipped with epi-fluorescence illumination and single band filters for FITC and Cy3/PI using 400 ISO color negative film. Finally, the film was scanned at 1200 dpi using an HP film scanner and the contrast and color balance were adjusted using Photoshop 5.5 (Adobe Inc. USA).
Results
Analysis of 2n-gamete production of L. auratum × L. henryi hybrid
To obtain progeny of the F1 AuH hybrid, we examined the pollen viability using pollen staining (Fig. 1B; Table 1) and tested in vitro pollen germination to re-confirm Asano's observations [10] (Table 2) and produce their BC1 progeny of the AuH hybrid (Table 3).
Features of the AuH (L. aurutum × L. henryi) interspecific hybrid . (A) Flowers of AuH (L. aurutum x L. henryi) interspecific hybrid; (B) stained pollen grains of AuH interspecific hybrid at the tetrad stage; (C) genomic in situ hybridization (GISH) of the mitoticchromosome of the F1 AuH hybrid.
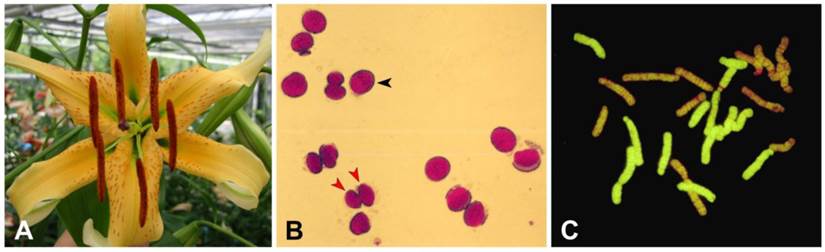
Pollen viability of AuH (L. aurutum × L. henryi) interspecific hybrid, 82111.
| Accession No. | Genotype | Large (2n) | Normal (n) | Total survival (%) | ||
|---|---|---|---|---|---|---|
| Stained (%) | Unstained (%) | Stained (%) | Unstained (%) | |||
| 82111 | AuH | 308 (42.0) | 19 (2.6) | 121 (16.5) | 285 (38.9) | 58.5 |
Pollen germination of AuH (L. aurutum × L. henryi) interspecific hybrid, 82111, on artificial agar medium.
| Accession no. | Genome | Total | Pollen type and germination | Average germination (%) | |||
|---|---|---|---|---|---|---|---|
| No. of 2n pollen (%) | Germination from 2n gametes | No. of n pollen | Germination from n-gametes | ||||
| 82111 | AuH | 640 | 300 | 207 (69%) | 340 | 6 (1.7%) | 33.2 |
BC1 progeny obtained by reciprocal crosses of AuH (L. aurutum × L. henryi) interspecific hybrid, 82111.
| Cross combination | No. of flowers pollinated | Pre-F | Post-F | No. plants derived | Av. plantlets per ovary | |
|---|---|---|---|---|---|---|
| Female | Male | |||||
| 82111 | L. auratum | 11 | 0 | 5 | 3 | 0.27 |
| L. auratum | 82111 | 5 | 0 | 0 | 4 | 0.80 |
| 82111 | L. henryi | 10 | 0 | 8 | 1 | 0.10 |
| L. henryi | 82111 | 5 | 0 | 4 | 0 | 0.00 |
Two types of pollen were primarily observed, aborted small pollen grains (Fig. 1A red arrow) and well-filled large pollen grains (Fig. 1A black arrow). These types of pollen were considered to be a sterile (n) gamete and fertile (2n) gamete, respectively. After pollen testing, 308 (42%) pollen grains were found to be produce viable 2n pollen (Table 1). To investigate the viability and function of pollen for crossing, these pollens were also germinated in vitro. Germination of pollen on artificial agar medium revealed that, of the 640 pollen grains tested, 300 were large pollen grains (2n), among which 207 (69%) germinated (Table 2) which is quite high percentage.
Use of 2n pollen for production of progeny
We confirmed that the F1 AuH hybrid can be utilized to obtain progeny by using the 2n gametes. To investigate the crossability and intergenomic recombination using the F1 AuH hybrid, we conducted reciprocal crosses of AuH hybrids. Generation of progeny by reciprocal crossing was more successful when female F1 AuH hybrids were used (Table 3). In addition, crossing with L. auratum led to more efficient generation of progeny. Finally, progeny were rarely obtained post-fertilization when crossing with L. henryi. These findings indicate that the F1 AuH hybrid has both female and male fertility. During ploidy levels analysis, all 20 BC1 progeny was triploid (Table 4) with equal number of chromosomes. These findings demonstrated that the F1 AuH hybrid contributed balanced diploid chromosome complements.
Chromosome constitution and intergenomic recombination
Two parental genomes of L. henryi and L. auratum in the F1 AuH hybrids (Fig. 1C) were clearly distinguishable after GISH. Probing with DIG-labeled total genomic DNA of L. henryi as a probe resulted in 12 green-labeled chromosomes, indicating that these chromosomes were derived from L. henryi and the others originated from L. auratum. Probe hybridization was uniform throughout the chromosome. These results further confirmed that L. henryi was distinct from L. auratum and belonged to the Archelirion section (Oriental hybrid group) of the genus Lilium.
To investigate chromosome number, frequency and type of recombinant chromosomes, all 20 BC1 progeny were analyzed by GISH and the results are summarized in Table 4. All 20 BC1 progeny had a triploid chromosome composed of 12 L. henryi chromosomes and 24 Oriental chromosomes. These findings were expected because a backcross of the 2n gamete from the F1 AuH hybrid contributes one set each of L. henryi and L. auratum (Oriental hybrid) genomes. These findings clearly confirmed that chromosome sets of the parental genomes of F1 AuH hybrid remain intact in resulting 2n gametes. Moreover, because the chromosome composition of the BC1 progeny comprised non-sister chromatids from a reduction division at anaphase I, all of the progeny were generated from the first division restitution (FDR) 2n pollen produced by the F1 AuH hybrid.
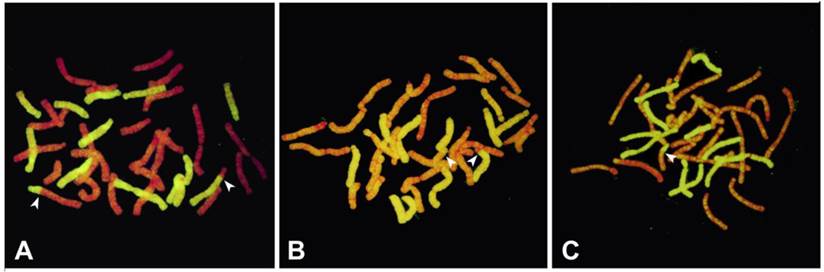
It was observed that among 20 BC1 progeny, four plants (20%) did not have any recombinant chromosomes (Table 4), while the other 16 (80%) contain recombinant chromosomes. The recombination (breakpoint) also occurred on 1-5 points per plant in the long or short arms, showing a variable range of recombinant chromosomes that occurred randomly (Table 4, Fig. 2).
A-C. GISH on mitotic metaphase chromosomes of BC1 progeny derived from interspecific AuH hybrids. Arrowheads indicate the homoeologous recombination sites between L. auratum and L. henryi; (A) Oriental hybrid `Darling` × AuH interspecific hybrid (85864-5); 12 chromosomes originated from L. henryi (yellowish-green fluorescence); 24 chromosomes originated from Oriental hybrid `Darling` and L. auratum (red fluorescence); (B) Oriental hybrid `Stargazer` × AuH interspecific hybrid (83275-15); 12 chromosomes originated from L. henryi (yellowish-green fluorescence); 24 chromosomes originated from Oriental hybrid `Darling` and L. auratum (red fluorescence); (C) Oriental hybrid `Journey`s End` × AuH interspecific hybrid (82936-5); 12 chromosomes originated from L. henryi (yellowish-green fluorescence); 24 chromosomes originated from Oriental hybrid `Darling` and L. auratum (red fluorescence).
Genome constitution and chromosome composition of BC1 progeny in cross combinations between each Oriental hybrid and interspecific AuH hybrid, 82111, as determined by GISH.
| Accession no. | Genotype | Cross combination | Ploidy level | Chromosome number | Chromosome constitution | No. of recombinant chromosomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | O | Au | H | Au/Hz | H/Auy | Total | ||||
| 82111 | AuH | L. auratum | L. henryi | 2× | 2n=24 | 12 | 12 | 0 | 0 | 0 | |
| 82396-1 | O AuH | Journery's End | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 1 | 2 | 3 |
| 82396-2 | O AuH | Journery's End | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 1 | 1 | 2 |
| 82396-3 | O AuH | Journery's End | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 1 | 2 | 3 |
| 82396-4 | O AuH | Journery's End | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 1 | 0 | 1 |
| 82396-5 | O AuH | Journery's End | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 1 | 1 | 2 |
| 82342-3 | O AuH | Stargazer | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 0 | 0 | 0 |
| 82342-6 | O AuH | Stargazer | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 0 | 2 | 2 |
| 83275-1 | O AuH | Stargazer | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 0 | 0 | 0 |
| 83275-3 | O AuH | Stargazer | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 0 | 1 | 1 |
| 83275-5 | O AuH | Stargazer | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 2 | 3 | 5 |
| 83275-7 | O AuH | Stargazer | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 2 | 1 | 3 |
| 83275-8 | O AuH | Stargazer | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 1 | 2 | 3 |
| 83275-12 | O AuH | Stargazer | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 1 | 2 | 3 |
| 83275-15 | O AuH | Stargazer | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 2 | 1 | 3 |
| 85863-1 | O AuH | Dorminique | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 1 | 1 | 2 |
| 85863-2 | O AuH | Dorminique | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 0 | 1 | 1 |
| 85864-1 | O AuH | Darling | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 2 | 2 | 4 |
| 85864-2 | O AuH | Darling | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 0 | 0 | 0 |
| 85864-5 | O AuH | Darling | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 1 | 1 | 2 |
| 85864-6 | O AuH | Darling | 82111 | 3× | 2n=36 | 12 | 12 | 12 | 0 | 0 | 0 |
z chromosome having a L. auratum centromere with L. henryi recombination sites. y chromosome having a L. henryi centromere with L. auratum recombination sites.
Producing BC2 progeny
To assess the possibility of producing BC2 progeny from allotriploid OAuH hybrids, we investigated the pollen viability by pollen staining and germination (Table 5) prior to using them for backcross. The range of pollen staining (0-48%) and germination percentage (0-4.3%) varied depending on the genotypes and plants. However, there was no relationship between germination percentage and pollen viability. Based on these results, we primarily used the female OAuH hybrids because of the low level of germination (0-4.3%).
Extensive reciprocal or backcrossing of OAuH (BC1) triploids followed by in vitro ovule and embryo culture generated several BC2 progeny (Table 6). Backcrosses of BC1 progeny were successful when male L. auratum was used, but no progeny were obtained from backcrosses with L. henryi. These findings were consistent with those observed when reciprocal crosses of F1 AuH hybrids were conducted to produce their BC1 progeny.
Four genotypes of OAuH triploids (primarily female, with some male) were backcrossed with the diploid Oriental hybrid cultivar 'Sorbonne'. Successful results were only obtained when the OAuH triploid was used as the female and the number of plants per ovary varied from 0-6.5 depending on the genotypes and plants. Moreover, no progeny were produced when male OAuH triploids were used. Among the four different BC1 genotypes, BC1 plants '83275-5', '83275-8' and '82342-4' were highly successful at production of BC2 progeny, with a maximum of 13.0 plants per ovary being obtained in plant number 83275-5 (Table 6).
Discussion
The interspecific hybrid was verified to produce the 2n gametes, indicating great potential for meiotic polyploidization in a breeding program. F1 AuH hybrids are valuable to lily breeding due to the desirable horticultural traits of its parents, which include virus resistance from L. henryi and botrytis resistance from L. auratum, therefore, in this study, we re-examined features of the F1 AuH hybrid and produced their subsequent progeny.
Pollen viability and germination of BC1 progeny derived from AuH hybrid, 82111, on artificial agar medium.
| Access no. | Geno-type | Cross combinations | Stained pollen (%) | Germination (%) | |
|---|---|---|---|---|---|
| Female (Oriental hybrids) | Male (AuH) | ||||
| 82396-1 | O AuH | Journey' End | 82111 | 21.9 | 0 |
| 82396-3 | O AuH | Journey' End | 82111 | 6.9 | 0 |
| 82396-4 | O AuH | Journey' End | 82111 | 0.0 | 0 |
| 82396-5 | O AuH | Journey' End | 82111 | 26.2 | 3.9 |
| 82342-3 | O AuH | Stargazer | 82111 | 26.4 | 0 |
| 83275-1 | O AuH | Stargazer | 82111 | 4.1 | 0 |
| 83275-3 | O AuH | Stargazer | 82111 | 44.6 | 2.7 |
| 83275-5 | O AuH | Stargazer | 82111 | 21.1 | 0 |
| 83275-7 | O AuH | Stargazer | 82111 | 48.0 | 0 |
| 83275-8 | O AuH | Stargazer | 82111 | 0.0 | 0 |
| 83275-15 | O AuH | Stargazer | 82111 | 42.6 | 0 |
| 85863-1 | O AuH | Dominique | 82111 | 29.6 | 2.0 |
| 85863-2 | O AuH | Dorminique | 82111 | 38.1 | 2.3 |
| 85864-1 | O AuH | Darling | 82111 | 34.9 | 4.3 |
| 85864-2 | O AuH | Darling | 82111 | 21.2 | 0 |
| 85864-5 | O AuH | Darling | 82111 | 0.0 | 0 |
| 85864-6 | O AuH | Darling | 82111 | 22.0 | 0 |
Numbers of BC2 progeny obtained by backcrosses between OAuH hybrids (BC1) and Oriental hybrid, L. auratum or L. henryi.
| Cross combinations | Geno-type | No. of flowers pollinated | Pre- fertilization barrier (%) | Post- fertilization barrier (%) | No. of plants obtained | No. of plants/ovary | |
|---|---|---|---|---|---|---|---|
| Female | Male | ||||||
| Sorbonne | 82396-1 | O OAuH | 12 | 0(0) | 12(100) | 0 | 0 |
| Sorbonne | 85864-1 | O OAuH | 12 | 0(0) | 12(100) | 0 | 0 |
| 82396-1a | Sorbonne | OAuH O | 5 | 0(0) | 2(40.0) | 3 | 0.6 |
| 82396-3 | Sorbonne | OAuH O | 9 | 0(0) | 8(88.9) | 1 | 0.1 |
| 82396-4 | Sorbonne | OAuH O | 7 | 0(0) | 2(28.6) | 9 | 1.3 |
| 82396-5 | Sorbonne | OAuH O | 7 | 0(0) | 0(0) | 11 | 1.6 |
| 82342-4 | Sorbonne | OAuH O | 7 | 0(0) | 3(42.9) | 40 | 5.7 |
| 83275-1 | Sorbonne | OAuH O | 1 | 1(100) | 0(0) | 0 | 0 |
| 83275-3 | Sorbonne | OAuH O | 3 | 0(0) | 0(0) | 6 | 2.0 |
| 83275-7 | Sorbonne | OAuH O | 6 | 0(0) | 2(33.3) | 3 | 0.5 |
| 83275-8 | Sorbonne | OAuH O | 4 | 0(0) | 0(0) | 26 | 6.5 |
| 85863-1 | Sorbonne | OAuH O | 10 | 0(0) | 7(70.0) | 4 | 0.4 |
| 85863-2 | Sorbonne | OAuH O | 8 | 3(37.5) | 4(50.0) | 9 | 1.1 |
| 85864-1 | Sorbonne | OAuH O | 12 | 0(0) | 5(41.7) | 0 | 0 |
| 85864-2 | Sorbonne | OAuH O | 7 | 7(100) | 0(0) | 0 | 0 |
| 85864-6 | Sorbonne | OAuH O | 8 | 0(0) | 3(37.5) | 8 | 1.0 |
| 83275-5 | L. auratum | OAuH Au | 5 | 0(0) | 0(0) | 65 | 13.0 |
| 83275-15 | L. auratum | OAuH Au | 4 | 2(50.0) | 1(25.0) | 2 | 0.5 |
| 83275-5 | L. henryi | OAuH H | 8 | 8(100) | 0(0) | 0 | 0 |
| 83275-15 | L. henryi | OAuH H | 5 | 5(100) | 0(0) | 0 | 0 |
While Asano (1984) reported that 15% of F1 AuH hybrid pollen grains germinated abnormally while only 1.6% germinated normally [10], Van Tuyl et al. (1989) found that 91.2% of the pollen cells were present as dyads at the tetrad stage in the same hybrid plant [9]. Our findings also showed a high percentage of pollen staining (42%) and germination (69%). The variation between these studies could be explained by the effects of environmental factors on the formation and viability of 2n pollen. It has been reported that high temperatures caused a low frequency of 2n-gamtes [9]. Additionally, Chung et al. (2009) showed that production of 2n pollen by OA hybrids differed depending on the season in which it was measured [17]. Pollen performance is also influenced by the pollen genotype: some genotypes exhibited higher pollen germination %age whereas some genotypes showed very low germination percentage under same environmental conditions [18].
GISH techniques have been applied to determine the origin of 2n gametes in Lilium and offered a new perspective for elucidation of restitution mechanisms, the extent of genetic recombination and composition of 2n-gametes. As expected, all of the BC1 progeny originated from the F1 AuH hybrid were triploid, without any aneuploid progeny. Production of aneuploid BC1 progeny is not common [17, 19, 20, 21, 22], although LA interspecific hybrids showed the potential to produce a large amount aneuploid pollen [23].
Many studies of lilies have shown that F1 interspecific hybrids produced functionally unreduced gametes and were successfully used for production of BC1 progeny [19, 20]. However, success in introgression is related to both the level of homoeologous recombination between parental genomes during meiosis in the F1 hybrids and their fertility [24]. The percentage of recombinant triploid BC1 progeny was 62.5% and 65.8% in ALAs and AOA, respectively [20, 25].
It is well known that intergenomic translocations are more likely to occur in the F1 hybrids of distantly related species because the homoeologous chromosomes are forced to pair and 2n gametes resulting from such meioses are most likely to transmit recombinant chromosomes to progeny with sexual polyploidy [26]. This was observed in the progeny of many F1 interspecific hybrids including Gasteria-Aloe [27], Alstroemeria species [28, 29] and Lilium species [17, 30]. Our data showing a high rate of recombination again confirmed that L. henryi is distinct from L. autraum (Oriental hybrid group) [4]. Moreover, it should be noted that the percentage of recombinant chromosomes in the BC1 progeny was variable depending on the genotypes used as the 2n gamete donor in OA hybrids from 35.7% with 952400-1 to 79.1% with 951502-1 [20]. Therefore, it might be desirable to screen diverse populations of F1 interspecific hybrids producing 2n gametes for frequencies of chromosome pairing and chiasma formation.
Based on the above research, it was assumed that chromosome pairing and crossing over are genetically controlled and thus genotype dependent; accordingly, a high percentage of recombination might be attributed to high genome divergence between L. aurutum and L. henryi when compared with OA hybrid genomes in Lilium. In conclusion, we confirmed that the 2n gametes of the F1 AuH hybrid are highly valuable to polyploid breeding of lilies and the genetic variation of 2n gametes caused by intergenomic recombination dramatically increases the chances of selecting new cultivars from the BC1 population.
In addition to intergenomic recombination, the mechanism of 2n gamete formation is another important aspect for sexual polyploidization. From the cytogenetic point of view, unreduced gametes are known to be formed via three different mechanisms; (i) an incomplete first meiotic division (first division restitution, FDR), (ii) an incomplete second meiotic division (second division restitution, SDR) and (iii) an indeterminate meiotic restitution (IMR) [31, 32, 33]. In the present study, all triploid BC1 progeny of AuH hybrid resulted from FDR 2n pollens because of the presence of 12 centromeres in each of the two sets of homoeologous chromosomes of L. henryi and L. auratum in the AuH hybrids [34], together with a complete set of the genome of the Oriental hybrid as female. The value of FDR gametes is in transferring heterosis and parental gene combinations intact in sexual polyploids.
Previous studies elucidated that the mechanisms of 2n pollen derived from F1 interspecific hybrids in lilies are FDR and IMR [17, 19], and that FDR is the predominant phenomenon involved in production of 2n gametes from F1 OA hybrids [20, 21] and from F1 LA hybrids [35]. Taken together, these data suggest that the chromosomal compositions of FDR gametes are more balanced than those of IMR gametes, resulting in FDR gametes being more viable and having a higher transmission rate than IMR gametes because of being suitable for selection as cultivars in nature. These findings are in accordance with Zhou et al. 2008 studies [36]. Indeed, since Lim et al. (2001) discovered the IMR mechanism in LA interspecific hybrids [17], Barba-Gonzales (2005a) found that some F1 OA hybrids produced 2n gametes via an IMR mechanism [20].
BC1 progeny were produced from a large number of backcrosses of F1 AuH hybrids by crossing with Oriental hybrids through the use of 2n gametes. These BC1 progeny have important features; namely, homoeologous recombinant chromosomes. However, allotriploids generally cannot be easily used as parents in lily breeding because of their high degree of sterility due to unbalanced meiosis. Despite this restriction, there are several instances among crop plants in which allotriploids have been successfully used as parents, such as Arachis hypogea [37], Triticum-Aegilops hybrids [38, 39], Triticum-Hordeum hybrids [40], Alstroemeria species hybrids [41], and Festuca-Lolium hybrids [42].
In lily, allotriploids (ALA) of Longiflorum × Asiatic hybrids have been successfully used for production of BC2 progeny. These triploids can be crossed with both diploid and tetraploid parents to yield aneuploid progeny consisting of near-diploids or near-tetraploid to pentaploid offspring [35]. Furthermore, it has been reported that allotriploids AOA genotypes can be used as parents in crosses with diploid or tetraploid individuals to produce considerable numbers of BC2 progeny [43].
Interestingly, the product of BC2 progeny was only successful in cases in which the BC1 progeny (82396-1) were female (Table 6). These results well support Zhou's hypothesis, “Five same genomes of endosperm are essential for its development in allotriploid x diploid/tetraploid crosses of Lilium [44]. Indeed, Lim et al. (2003) demonstrated that egg cells of triploid BC1 (ALA) produce BC2 progeny with a fairly wide range of chromosomes and this wide range of chromosomes could contribute to the viability of egg cells and chromosome balance of embryos and endosperms [35].
Conclusion
BC2 production derived from F1 AuH interspecific hybrids has potential value because an introgression of segments of the recombinant chromosomes can be transmitted to further generations. However, more studies of the BC2 progeny to identify the recombinant chromosomes transmitted are necessary to establish systematic and meaningful procedures for polyploidy breeding.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Comber HF. A new classification of the Lilium. Lily Yearbook Royal Horti Soc London. 1947;15:86-105
2. De Jong PC. Some notes on the evolution of lilies. The Lily Yearbook of the North Amer Lily Soc. 1974;27:23-28
3. Anderson NO, Younis A, Sun Y. Intersimple sequence repeats distinguish genetic differences in easter lily 'Nellie White' clonal ramets within and among bulb growers over years. J Amer Soc Hort Sci. 2010;135:445-455
4. Van Tuyl JM, Dijken A van, Chi HS. et al. Breakthroughs in interspecific hybridization of Lily. Acta Hort. 2000;508:83-90
5. Van Tuyl JM, Van Diёn MP, Van Creij MGM, Van Kleinwee TCM, Franken J, Bino RJ. Application of in vitro pollination, ovary culture, ovule culture and embryo rescue for overcoming incongruity barriers in interspecific Lilium crosses. Plant Sci. 1991;74:115-126
6. Anderson NO, Plattes A, Opitz E, Younis A. Transgressive Segregant, Interspecific Hybrids between Lilium ×formolongi and L. martagon with Unique Morphology. Acta Hort. 2011;900:181-188
7. Schmitzer E. A survey of named polyploidy lilies of the Asiatic section. Quart Bulletin North Amer Lily Soc. 1991;45:6-12
8. Van TJM, Meijer B, Van Diёn MP. The use of oryzalin as an alternative for colchicine in vitro chromosome doubling of Lilium and Nerine. Acta Horti. 1992;325:625-630
9. Van TJM, De Vries JN, Bino RJ, Kwakkenbos AAM. Identification of 2n-pollen producing interspecific hybrids of Lilium using flow cytometry. Cytologia. 1989;54:737-745
10. Asano Y. Fertility of a hybrid between distantly related species in Lilium. Cytologia. 1984;49:447-456
11. Schwarzacher T, Anamthawat-Jonsson K, Harrison GE, Islam AKMR, Jiak JZ, Leitch AR, Miller TE, Reader SM, Rogers WJ, Shi M, Heslop-Harrison JS. Genomic in situ hybridization to identify alien chromosomes and chromosome segments in wheat. Theor Appl Genet. 1992;84:778-786
12. Van Tuyl JM, Chung MY, Chung JD, Lim KB. Introgression with Lilium hybrids. The Lily Yearbook of the North Amer Lily Soc. 2002;55:17-22
13. Asano Y, Myoda H. Studies on crosses between distantly related species of Lilies. I. For the intrastylar pollination technique. J Japan Soc Hort Sci. 1977;46:59-65
14. Van Tuyl JM. Survey of research on mitotic and meiotic polyploidization at CPRO-DLO. The Lily Yearbook of the North Amer Lily Soc. 1993;43:10-18
15. Van Tuyl JM, Boon E. Variation in DNA-content in the genus Lilium. Acta Hort. 1997;430:829-835
16. Lim KB, Ramanna MS, Jong JH, Jacobsen E, van Tuyl JM. Indeterminate meiotic restitution(IMR): a novel type of meiotic nuclear restitution mechanism detected in interspecific lily hybrids by GISH. Theor Appl Genet. 2001;103:219-230
17. Chung MY, Chung JD, Van Tuyl JM, Lim KB. GISH analysis of subsequent progeny crossed with 2n-gametes of F1 OA interspecific hybrid. Kor J Hort Sci Technol. 2009;27:649- 656
18. Lokker AC, Barba-Gonzalez R, Lim KB, Ramanna MS, van Tuyl JM. Genotypic and environmental variation in production of 2n-gametes of oriental x asiatic lily hybrids. Acta Hort. 2005;673:453-456
19. Barba-Gonzalez R, Lokker AC, Lim KB, Ramanna MS, Van Tuyl JM. Use of 2n gametes for the production of sexual polyploids from sterile Oriental x Asiatic hybrids of lilies (Lilium). Theor Appl Genet. 2004;109:1125-1132
20. Barba GR, Ramanna MS, Visser RGF, Van TJM. Intergenomic recombination in F1 lily hybrids (Lilium) and its significance for genetic variation in the BC1 progenies as revealed by GISH and FISH. Genome. 2005;48:884-894
21. Barba GR, Lim KB, Ramanna MS, Visser RGF, Van TJM. Occurrence of 2n gametes in the F1 hybrids of Oriental × Asiatic lilies (Lilium): Relevance to intergenomic recombination and backcrossing. Euphytica. 2005;143:67-73
22. Zhou S, Ramanna MS, Visser RGF, Van Tuyl JM. Genome composition of triploid lily cultivars derived from sexual polyploidization of Longiflorum × Asiatic hybrids (Lilium). Euphytica. 2008;160:207-215
23. Zhou S. Intergenomic recombination and introgression breeding in longiflorum × Asiatic lilies. PhD thesis, Wageningen University and Research Centre, The Netherlands. 2007:111
24. Van Tuyl JM, De Jeu MJ. Methods for overcoming interspecific crossing barriers. In: Shivanna K-R, Sawhney V-K (eds), pollen biotechnology for crop production and improvement, Cambridge University Press. 1997
25. Lim KB, Chung JD, Van Kronenburg BCE, Ramanna MS, De Jong JH, Van Tuyl JM. Introgression of Lilium rubellum Baker chromosomes into L. longiflorum Thunb.: a genome painting study of the F1 hybrid, BC1 and BC2 progenies. Chromosome Res. 2000;8:119-125
26. Ramanna MS, Jacobsen E. Relevance of sexual polyploidization for crop improvement - A review. Euphytica. 2003;133:3-18
27. Takahashi C, Leitch IJ, Ryan A, Bennett MD, Brandham PE. The use of genomic in situ hybridization (GISH) to show transmission of recombinant chromosomes by a partially fertile bigeneric hybrid, Gasteria lutzii × Aloe aristata (Aloaceae) to its progeny. Chromosoma. 1997;105:342-348
28. Kamstra SA, Kuipers AGJ, De JMJ, Ramanna MS, Jacobsen E. The extent and position of homoeologous recombination in a distant hybrid of Alstroemeria: a molecular cytogenetic assessment of first generation backcross progenies. Chromosoma. 1999;108:52-63
29. Ramanna MS, Kuipers AGJ, Jacobsen E. Occurrence of numerically unreduced (2n) gametes in Alstroemeria interspecific hybrids and their significance for sexual polyploidisation. Euphytica. 2003;133:95-106
30. Anderson NO, Younis A, Optiz E. Development of Colored, Non-Vernalization-Requiring Seed Propagated Lilies. Acta Hort. 2009;836:193-198
31. Mok DWS, Peloquin SJ. The inheritance of three mechanisms of diplandroid(2n pollen) formation in diploid potatoes. Heredity. 1975;35:295-302
32. McCoy TJ. The inheritance of 2n-pollen formation in diploid alfalfa Medicago sativa. Can J Genet Cytol. 1982;24:315-323
33. Hermsen JGT. Mechanisms and genetic implications of 2n gametes formation. Iowa State J Res. 1984;58:421-434
34. Ramanna MS. A re-examination of the mechanism of 2n gamete formation in potato and its implications for breeding. Euphytica. 1979;28:537-561
35. Lim KB, Ramanna MS, Jacobsen E, van Tuyl JM. Evaluation of BC2 progenies derived from 3x-2x and 3x-4x crosses of Lilium: a GISH analysis. Theor Appl Genet. 2003;106:568-574
36. Zhou S, Li K, Zhou G. Analysis of endosperm development of allotriploid × diploid/tetraploid crosses in Lilium. Euphytica. 2012;184:401-412
37. Simpson CE, Davis KS. Meiotic behavior of a male-fertile triploid Arachis L. hybrid. Crop Sci. 1983;23:581-584
38. Vardi A, Zohary D. Introgression in wheat via triploid hybrids. Heredity. 1967;22:541-560
39. Xu S.J, Y.S. Dong. 1992. Fertility and meiotic mechanisms of hybrids between chromosome autoduplication tetraploid wheats and Aegilops species. Genome. 1967;35:379-384
40. Blanco A, Fracchiolla GV, Greco B. Intergeneric wheat × barley hybrid. J of Hered. 1986;77:98-100
41. Kamstra SA, Ramanna MS, de Jeu MJ, Kuipers AGJ, Jacobsen E. Homoeologous chromosome pairing in the distant hybrid Alstroemeria aurea × A inodora and the genome composition of its backcross derivatives determined by fluorescence in situ hybridization with species specific probes. Heredity. 1999;82:69-78
42. Morgan WG, King LP, Koch S, Harper JA, Thomas HM. Introgression of chromosomes of Festuca arundinacea var. glaucescens into Lolium multiflorum revealed by genomic in situ hybridisation (GISH). Theor Appl Genet. 2001;103:696-701
43. Barba GR, Van SAA, Visser RGF, Ramanna MS, Van TJM. Progenies of allotriploids of Oriental × Asiatic lilies (Lilium) examined by GISH analysis. Euphytica. 2006;151:243-250
44. Zhou S, Ramanna MS, Visser RGF, van TJM. Analysis of the meiosis in the F1 hybrids of Longiflorum × Asiatic (LA) of lilies (Lilium) using genomic in situ hybridization J. Genet Genom. 2008;35:687-695
Author contact
![]() Corresponding author: e-mail: kblimac.kr. Tel: +82 53 950 5728 Fax: +82 53 950 6496.
Corresponding author: e-mail: kblimac.kr. Tel: +82 53 950 5728 Fax: +82 53 950 6496.

 Global reach, higher impact
Global reach, higher impact