ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2015; 11(12):1458-1468. doi:10.7150/ijbs.13444 This issue Cite
Research Paper
Krüppel-Like Factor 5 Promotes Epithelial Proliferation and DNA Damage Repair in the Intestine of Irradiated Mice
1. School of Radiation Medicine and Protection, Medical College of Soochow University, Suzhou, Jiangsu 215123, China
2. Collaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions, Suzhou, Jiangsu 215123, China
3. Institute of Medical Biotechnology, Medical College of Soochow University, Suzhou, Jiangsu 215007, China
4. Jiangsu Stem Cell Key Laboratory, Medical College of Soochow University, Suzhou, Jiangsu 215007, China
5. Key Laboratory of Obstetric, Gynecologic and Pediatric Diseases and Birth Defects of the Ministry of Education, West China Institute of Women and Children's Health, and Department of Pediatrics, Huaxi Second University Hospital, Sichuan University, Chengdu, Sichuan 610041, China.
*These authors contributed equally to this work.
Abstract
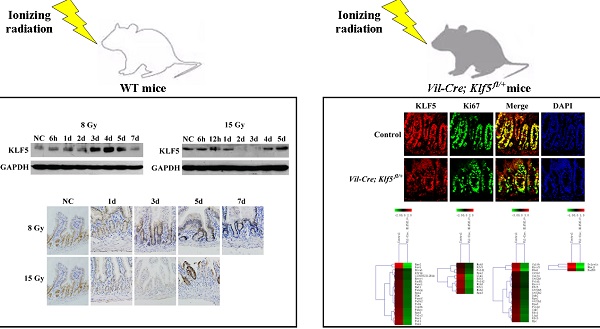
BACKGROUND & AIMS: High doses of radiation induce severe DNA damage in intestinal epithelial cells, especially crypt cells, and cause intestinal injury, but the underlying molecular mechanisms remain unclear. Krüppel-like factor 5 (KLF5), a zinc finger-containing transcription factor, is induced by various stress stimuli and is involved in cell proliferation and survival. The role of KLF5 in radiation-induced intestinal injury was investigated here.
METHODS: Wild type mice were treated with 8 or 15 Gy total body irradiation (TBI). KLF5 content and cellular localization in the small intestines of irradiated mice were detected by Western blot and immunohistochemical analysis. Mice with intestinal-specific knockdown of KLF5 (Vil-Cre; Klf5fl/+ mice) were generated and their response to radiation was compared with controls. Morphological changes were determined by hematoxylin and eosin staining. Proliferation was examined by Ki67 immunostaining. The molecular response of the small intestine after KLF5 knockdown was investigated using microarrays.
RESULTS: KLF5 expression correlated with the progression of intestinal damage. Decreased levels of KLF5 in the gut were associated with increased damage to the intestinal mucosa and reduced epithelial proliferation after TBI. Our microarray data disclosed that KLF5 knockdown down-regulated genes related to DNA damage repair pathways such as nucleotide excision repair, mismatch repair, non-homologous end joining and the Fanconi anemia pathway, which may suggest a novel function of KLF5.
CONCLUSIONS: Our study illustrates that KLF5 may modulate DNA repair pathways to prevent intestinal injury induced by TBI. KLF5 signaling provides a novel field for identification of potential therapeutic targets for the treatment of radiation-induced intestinal damage.
Keywords: Krüppel-like factor 5, ionizing radiation, intestinal injury, mouse

 Global reach, higher impact
Global reach, higher impact