10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2016; 12(11):1298-1308. doi:10.7150/ijbs.16569 This issue Cite
Research Paper
Histone Deacetylase Inhibitor Trichostatin a Promotes the Apoptosis of Osteosarcoma Cells through p53 Signaling Pathway Activation
1. Department of Orthopedics, Jinling Hospital, School of Medicine, Nanjing University, Nanjing, Jiangsu, China.
2. Center for Translational Medicine, Nanjing University Medical School, Nanjing, Jiangsu, PR China.
3. Jiangsu Key Laboratory for Molecular Medicine, Nanjing University Medical School, Nanjing, PR China.
* These authors contributed equally to this work.
Abstract
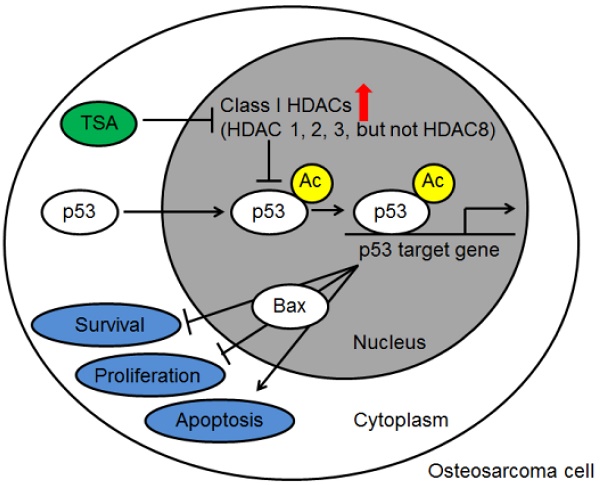
Purpose: The purpose of this study was to investigate the profile of histone deacetylase (HDAC) activity and expression in osteosarcoma cells and tissues from osteosarcoma patients and to examine the mechanism by which a histone deacetylase (HDAC) inhibitor, Trichostatin A (TSA), promotes the apoptosis of osteosarcoma cells.
Methods: HDAC activity and histone acetyltransferase (HAT) activity were determined in nuclear extracts of MG63 cells, hFOB 1.19 cells and tissues from 6 patients with primary osteosarcoma. The protein expression of Class I HDACs (1, 2, 3 and 8) and the activation of the p53 signaling pathway were examined by Western blot. Cell growth and apoptosis were determined by 3-(4, 5-dimethyl-2-thiazolyl)-2H-tetrazolium bromide (MTT) assay and flow cytometry, respectively.
Results: Nuclear HDAC activity and class I HDAC expression were significantly higher in MG63 cells than in hFOB 1.19 cells, and a similar trend was observed in the human osteosarcoma tissues compared with the paired adjacent non-cancerous tissues. TSA significantly inhibited the growth of MG63 cells and promoted apoptosis in a dose-dependent manner through p53 signaling pathway activation.
Conclusion: Class I HDACs play a central role in the pathogenesis of osteosarcoma, and HDAC inhibitors may thus have promise as new therapeutic agents against osteosarcoma.
Keywords: osteosarcoma, HDAC, Trichostatin A, apoptosis, p53

 Global reach, higher impact
Global reach, higher impact