10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2016; 12(11):1363-1371. doi:10.7150/ijbs.16334 This issue Cite
Research Paper
Aloe-emodin suppresses hypoxia-induced retinal angiogenesis via inhibition of HIF-1α/VEGF pathway
1. Laboratory of Chinese Materia Medica, Department of Pharmacology, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan 646000, China;
2. Post-Doctoral Research Station, KangHong Pharmaceutical Group, Chengdu, Sichuan 610036, China;
3. Post-Doctoral Mobile Station, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan 610041, China.
* Jianming Wu and Xiao Ke are co-first authors of this article.
Received 2016-5-31; Accepted 2016-8-22; Published 2016-10-25
Abstract
Background: Aloe-emodin (AE) has been reported to possess the antiangiogenic effect on laser induced choroidal neovascularization. AE inhibits the vessel formation in the zebrafish embryos. However, it is still unclear whether AE can alleviate neovascularization. Here, we investigated the inhibitory effect of AE on the hypoxia-induced retinal neovascularization and the possible mechanisms.
Methods: We established a vascular endothelial growth factor (VEGF) secretion model under chemical induced hypoxia by exposure of 150 µM CoCl2 to the ARPE-19 cells, then treated the cells with different concentrations of AE (0.2, 1.0 and 5.0 µg/mL) or a special hypoxia-inducible factor 1α (HIF-1α) inhibitor [3-(5'-hydroxymethyl-2'-furyl)-1-benzylindazole, YC-1, 1.0 µg/mL]. The cellular supernatants were collected 48 h later to measure the VEGFA concentrations by human VEGFA enzyme-linked immunosorbent assay (ELISA) kits, the mRNA expressions of VEGFA, HIF-1α and prolyl hydroxylase-2 (PHD-2) by quantitative reverse transcription-PCR (qRT-PCR) and the protein expressions of HIF-1α and PHD-2 by Western blots. For in vivo study, the rat pups with oxygen-induced retinopathy were treated with Conbercept ophthalmic injection (1.0 mg/kg) or AE (5.0 and 10.0 mg/kg) for five days, then the retinal avascular areas were assessed via visualization of the retinal vasculature with ADPase and hematoxylin & eosin (H&E) stains.
Results: AE inhibits the VEGFA secretion of ARPE-19 cells under hypoxia condition, decreases the mRNA expressions of VEGFA and PHD-2 and the protein expressions of VEGFA, HIF-1α and PHD-2 in vitro and prevents hypoxia-induced retinal neovascularization in vivo.
Conclusions: AE ameliorates retinal neovascularization throuth inhibition of the HIF-1α/VEGF signaling pathway. AE may be developed as a potential drug for the prevention and treatment of diabetic retinopathy.
Keywords: Aloe-emodin, vascular endothelial growth factor, angiogenesis, hypoxia-inducible factor-1, oxygen-induced retinopathy.
Introduction
Aloe-emodin [AE, 1,8-dihydroxy-3-(hydroxymethyl)anthracene-9,10-dione], an anthraquinone from the famous traditional Chinese medicine Radix et Rhizoma Rhei (RRR, Da-huang in Chinese) and other plants [1], exhibits many beneficial effect including antiangiogenic [2], antineoplastic [3], anti-metastasis [4], strengthening of immune function and other pharmacological effects [5]. AE has been frequently used as the quality control marker for RRR and its preparations. Recently, it was reported that some preparations of Radix et Rhizoma Rhei, such as Da-Huang-Zhe-Chong pill and Keluoxin capsule, could relieve the retinal neovascularization of diabetic retinopathy (DR) [6,7]. Study has proved that AE has the inhibitory effect on vessel formation in the zebrafish embryos [2], but it is still unclear for its effect on hypoxia-induced retinal neovascularization, which is the most important pathophysiologic process in DR.
DR is a serious microvascular complication in the patients with diabetes mellitus, which causes vitreous hemorrhage, tractional retinal detachment and visual deterioration [8, 9]. Furthermore, increased vascular permeability leads to macular edema in the patients with DR [10]. Therefore, DR becomes the main cause of blindness in the adults. Vascular endothelial growth factor (VEGF) plays the key role in the retinal neovascularization of DR during the pathological process, which stimulates the proliferation and migration of the vascular endothelial cells and increases the vascular permeability [11]. Therefore, hypoxia is one of the most potent triggers of VEGFA (the primary and most important member of the VEGF family) expression, involving in the processes of the DNA transcription, mRNA stabilization, translation and release of VEGFA [12], which is mainly controlled by the hypoxia inducible factor-1α (HIF-1α), a transcription factor that regulates hypoxia-inducible genes, including VEGFA for angiogenic response [13]. HIF, a heterodimeric transcriptional factor, is activated and stabilized under the hypoxic condition. Also, HIF promotes the expressions of gene products [14], including angiogenesis factors (eg. VEGFA and TGF-β3) [15, 16], erythropoiesis factor (eg. EPO) [17], cell survival and proliferation factors (eg. IGF-2, ID2 and NOS) and so on [18, 19]. HIF-1 is composed of two subunits of HIF-1α and HIF-1β. HIF-1α is an oxygen sensitive subunit and its expression is induced under hypoxic conditions. HIF-1β is also known as aryl hydrocarbon receptor nuclear translocator (ARNT) and is constitutively expressed. HIF-1β binds with HIF-1α and aryl hydrocarbon receptor (AhR) facilitating its translocation to the nucleus to promote the mRNA expression and the protein generation of VEGF [20]. In consequence, HIF-1α up-regulates the expression of VEGFA under hypoxia, leading to increase vascular permeability and retinal neovascularization. On the contrary, inhibition of HIF-1α decreases retinal neovascularization in hypoxia condition [21-23]. These findings suggest that HIF-1α/VEGF pathway plays the critical role in retinal neovascularization. Here, we studied the effect of AE on retinal neovascularization in the ARPE-19 cells in vitro and in the rat pups in vivo.
Materials and Methods
Reagents
AE (Lot: 110795-201007) was purchased from National Institutes for Food and Drug Control (Beijing, China) and dissolved in dimethyl sulfoxide (DMSO) as stock solution of 10.0 mg/mL. Conbercept Ophthalmic Injection (Lot: 20110610B) was provided by Chengdu Kanghong Pharmaceutical Group Co., Ltd. (Chengdu, Sichuan, China). Human VEGFA enzyme-linked immunosorbent assay (ELISA) kits (Lot: DVE00) were purchased from R&D Systems Inc. (Stillwater, MN, USA). Fetal bovine serum (FBS), DMEM/F12 medium and trypsin-EDTA solution were provided from GIBCO Invitrogen (Carlsbad, CA, USA). DMSO, Tetramethylethylenediamine (TEMED), ammonium sulfide and adenosine diphosphate (ADP) were purchased from Sigma (St. Louis, MO, USA). Anti-HIF-1α antibody, anti-PHD-2 antibody and YC-1 were obtained from Abcam (Cambridge, MA, USA). PrimeScript RT reagent kit with gDNA Eraser and SYBR Premix Ex TaqTM were provided from Takara-Bio (Kusatsu, Shiga, Japan).
Cell culture
The human acute retinal pigment epithelial-19 (ARPE-19) cells (Lot: 60279299) were purchased from the American Type Culture Collection (ATCC), and cultured in DMEM/F12 medium with 10% FBS, 100 μg/mL streptomycin and 100 units/mL penicillin in an incubator at 37 0C with 5% CO2. After 90% confluence, the cells were digested with 0.25% trypsin-0.02% EDTA for passage.
Determination of VEGFA secretion by ARPE-19 cells under chemical induced hypoxia
As previous report [11], the ARPE-19 cells were plated into 96-well plates at a density of 8 × 103 cell/well. After being cultured for 24 h, the culture medium was replaced with fresh FBS free DMEM/F12 medium. 24 h later, 150 µM CoCl2 was exposure to the cells for mimicking hypoxic condition. Then, the cells were treated with 1.0 µg/mL YC-1 (positive control) or 0.2, 1.0 and 5.0 µg/mL AE while the culture medium containing same concentration of DMSO in the AE solution as control. Conditioned medium was collected 48 h later and centrifuged at 800 ×g for 5 min, and the supernatants were transferred to vials for VEGFA analysis. All experiments were performed in triplicate.
The concentrations of VEGFA protein were measured using the human VEGFA Quantikine ELISA kits following the manufacturer's instruction. Absorbance values (450 nm) were recorded in triplicate using M5 Microplate Reader (SpectraMax, Molecular Devices, Sunnyvale, CA, USA). The concentrations of VEGFA were calculated from a standard curve. The sensitivity of the VEGFA kits was 5.0 pg/mL.
RNA isolation and analysis of VEGFA, HIF-1α and PHD-2 mRNA expression
ARPE-19 cells were seeded at a density of 1 × 105 cells/well in 6-well plate and allowed to adhere overnight, then submitted to the same treatments as described above. After being cultured for 24 h, the culture medium was withdrawn, and the cells were washed with cold PBS before harvest. The cell pellets were collected for mRNA extraction after microcentrifuging at 800 ×g for 5 min at 4 0C. The total RNA from ARPE-19 cells was isolated with TRIzol reagent. The cDNA was synthesized using the cDNA synthesis kit according to the manufacturer's protocol. The amounts of each gene mRNA transcripts to β-actin were determined by qRT-PCR using the SYBR pre-mixed system and specific primers. The primer sequences for VEGFA, prolyl hydroxylase-2 (PHD-2), HIF-1α, and β-actin were as follows: Homo VEGFA 5'-CGA AAC CAT GAA CTT TCT GC-3' (forward) and 5'-CCT CAG TGG GCA CAC ACT CC-3' (reverse). Homo PHD-2 5'- AAA CCA TTG GGC TGC TCA T-3' (forward) and 5'-CGT ACA TAA CCC GTT CCA TTG-3' (reverse). Homo HIF-1α 5'-ACA AGT CAC CAC AGG ACA G-3' (forward) and 5'-AGG GAG AAA ATC AAG TCG-3' (reverse). Homo β-actin 5'-AGC GGG AAA TCG TGC GTG AC-3' (forward) and 5'- AGT TTC GTG GAT GCC ACA GGA C-3' (reverse). qRT-PCR was performed using Opticon 3 continuous fluorescence detector (MJ Research Inc., Waltham, MA, USA). The comparative cycle threshold (Ct) method was used and the relative transcript amount of the target gene was normalized to that of β-actin using the 2-ΔΔCt method.
Analysis of HIF-1α and PHD-2 proteins expression by Western blot
The ARPE-19 cells were plated at a density of 2.0 × 105 cells/bottle in culture bottles and submitted to the same treatments as described above. After being cultured for 24 h, the culture media were withdrawn, and the cells were washed with cold PBS for harvest. The cell pellets were disrupted in cell RIPA buffer (50 mM Tris-HCl, 0.5% NP-40, 120 mM NaCl, 0.1mM Na3VO4, 1 mM EDTA, 1 mM PMSF, 1 mM NaF, and 1 μg/mL leupeptin, pH 7.5), then the lysates were centrifuged at 12,000 rpm, 4 0C for 15 min. The protein concentrations were measured using the BCA method, 30 μg protein was electrophoresed on 7.5% density of SDS-acrylamide gels. Following electrophoresis, the proteins were transferred from the gel to a nitrocellulose membrane with an electric transfer system. Non-specific binding was blocked by 5% skim milk in TBST buffer (5 mM Tris-HCl, 136 mM NaCl and 0.1% Tween-20, pH 7.6) for 1 h. The blots were incubated at 4 0C overnight with antibodies against HIF-1α (1:2000), PHD-2 (1:1500) or β-actin (1:800) and were washed three times with 1× TBST. Then, the blots were incubated for 1 h at room temperature with a 1:4,000 dilution of horseradish peroxidase-labeled anti-rabbit or anti-mouse IgG and washed three times with 1× TBST, the membranes were developed and detected by incubation within the ECL Western blot detection reagents.
In vivo study in the rat model of oxygen-induced retinopathy
All animal experiments were conducted strictly in accordance with University's guidelines and were approved by the Committee on Use and Care of Animals of Sichuan University (Chengdu, Sichuan, China). Ten pregnant (16-20-week old and body weight 310-350 g, for OIR model study) Sprague Dawley (SD) rats (SPF Grade, Certificate No. SCXK2013-24) were purchased from the Experimental Animal Centre, Sichuan Provincial Academy of Medical Sciences in China (Chengdu, Sichuan, China). OIR was induced in the SD rat pups following the protocol as described previously [24]. In brief, the normalized litters of 40 SD rat pups with their nursing mothers on postnatal day 7 (PD 7) were placed in an 80% oxygen atmosphere for 5 days. After being returned to normoxia conditions, the rat pups were intraperitoneal (i.p.) administered with Conbercept ophthalmic injection at 1.0 mg/kg or AE at 5.0 and 10.0 mg/kg once a day for 5 days (daily× 5) starting on the PD 12, and used same volume of normal saline as control. Conbercept ophthalmic injection is a potent VEGF blocker for wet age-related macular degeneration as used as positive control. The dose selection of Conbercept ophthalmic injection is based on our previous studies. Five days later (on PD 17), the rat pups were sacrificed (the pups in the control group were bred under normoxia conditions until PD 17), and the eyes were enucleated and fixed in fresh 4% paraformaldehyde for 2 h. The eyecups were dissected and the retinal flatmounts were created and stained with ADPase [25]. The retinal avascular areas were assessed through visualization of the retinal vasculature with infusion of ADPase stain examined under fluorescent microscopy. In each whole mount, the total pre-retinal neovascular areas were measured using Image-pro Plus System and expressed as the percentage of the respective average in relation to total retinal areas. The retinas were also histologically examined. Serial sections of paraffin-embedded small pieces of retina (6 mm) were stained by hematoxylin and eosin (H&E). Images were taken under microscopy, and endothelial nuclei extended beyond the inner limiting membrane into the vitreous were manually counted in a blind manner [26].
Statistical analysis
All the data were reported as mean ± standard deviation (SD). Statistical significance of the data was analyzed by one-way univariate analysis of variance (ANOVA) for comparison of more than two groups. A difference at p < 0.05 was considered to be statistically significant (as marked as *). The higher significance level was set at p < 0.01 (as marked as **).
Results
AE inhibits the secretion of VEGFA in the ARPE-19 cells under hypoxia condition
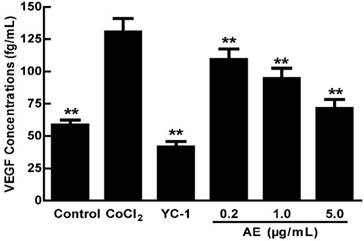
In order to study the effect of AE on VEGFA secretion under hypoxia, the concentrations of VEGFA in the cultured ARPE-19 cells were measured by ELISA after treatment of 150 µM CoCl2 alone or in combination with AE at 0.2, 1.0 or 5.0 µg/mL. As shown in Figure 1, the level of VEGFA in the culture cells was significantly increased after treatment with 150 µM CoCl2 compared to that of non-CoCl2 treated cells (p < 0.01). However, YC-1 at 1.0 µg/mL and AE at 0.2, 1.0 and 5.0 µg/mL markedly decreased the secretion of VEGFA compared to that of the cells treated with CoCl2 alone (p < 0.01). These results indicate that AE inhibits the secretion of VEGFA in the culture ARPE-19 cells.
The effects of AE on VEGFA secretion in the ARPE-19 cells at 48 h of hypoxic condition. The results are representative of at least three independent experiments run in triplicate and expressed as the mean ± SD. ** p < 0.01 vs CoCl2-treated group. The control cells were treated with culture medium with 0.1% DMSO.
The effects of AE on the gene expressions of VEGFA (A), HIF-1α (B) and PHD-2 (C) in the ARPE-19 cells at 24 h of normoxia or hypoxic condition. The results are representative of at least three independent experiments run in triplicate and expressed as the mean ± SD. ** p < 0.01 vs CoCl2-treated group. The control cells were treated with culture medium with 0.1% DMSO.
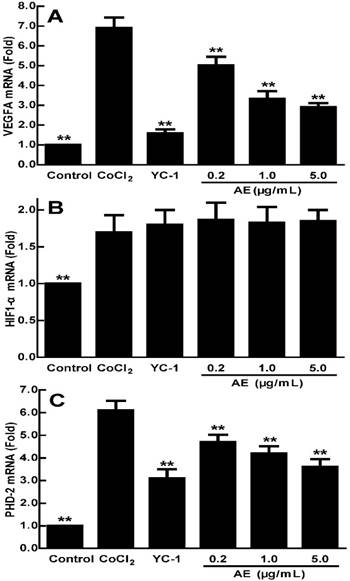
Effects of AE on the mRNA expressions of VEGFA, HIF-1α and PHD-2 in the ARPE-19 cells
VEGFA expression can be induced by post-transcriptional mechanisms directed at the VEGFA mRNA or its relative regulator [27]. AE may affect the mRNA expressions of VEGFA, HIF-1α and PHD-2, so we examined the mRNA expressions of VEGFA, HIF-1α and PHD-2 in the ARPE-19 cells by qRT-PCR analysis. As shown in Figure 2, the expression of VEGFA mRNA increased significantly in the ARPE-19 cells treated with 150 µM CoCl2 for 24 h (p < 0.01) (Figure 2A). AE at 0.2-5.0 µg/mL significantly decreased the high-expression of VEGFA mRNA induced by CoCl2 (p < 0.01) at a dose-dependent manner (Figure 2A), indicating that AE blocked the elevated expression of VEGFA mRNA induced by hypoxia. The data in Figure 2B show that the combination of 150 µM CoCl2 with YC-1 at 1.0 µg/mL or AE at 0.2-5.0 µg/mL did not affect the mRNA expression of HIF-1α compared to that of CoCl2 alone. However, the data in Figure 2C show that the mRNA expression of PHD-2 was increased (p < 0.01) under CoCl2 induced hypoxia conditions, while it is significantly down-regulated by YC-1 (1.0 µg/mL) and AE (0.2-5.0 µg/mL) compared to that of CoCl2 alone (p < 0.01). These results show that AE can decrease the mRNA expressions of VEGFA and PHD-2 in the ARPE-19 cells.
The effects of AE on the protein expressions of HIF-1α (A) and PHD-2 (B) in the ARPE-19 cells at 24 h of normoxia or hypoxic condition. The results are representative of at least three independent experiments run in triplicate and expressed as the mean ± SD. ** p < 0.01 vs CoCl2-treated group. The control cells were treated with culture medium with 0.1% DMSO.
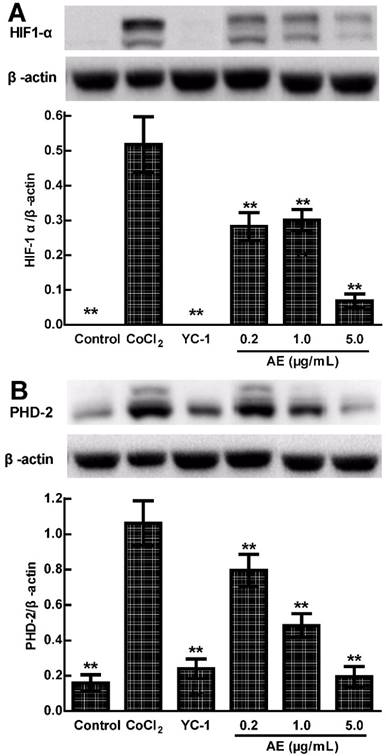
Effects of AE on the levels of HIF-1α and PHD-2 protein in the ARPE-19 cells
VEGFA is induced by hypoxia and regulated by HIF-1α and its degradation regulator PHD-2 [20]. We used the anti-HIF-1α and anti-PHD-2 antibodies to determine the protein expressions of HIF-1α and PHD-2 in the cultured ARPE-19 cells under hypoxia condition. Western blots analysis showed that the protein expressions of HIF-1α and PHD-2 were significantly up-regulated in the ARPE-19 cells treated with 150 µM CoCl2 for 24 h compared to that of control treated with medium (p < 0.01) and significantly down-regulated by YC-1 or AE compared to that of CoCl2 alone (p < 0.01) (Figure 3A & 3B). These results indicate that the inhibition of AE on VEGFA secretion of the ARPE-19 cells under hypoxia condition is associated with the protein expressions of HIF-1α and PHD-2.
AE inhibits hypoxia-induced retinal neovascularization in vivo
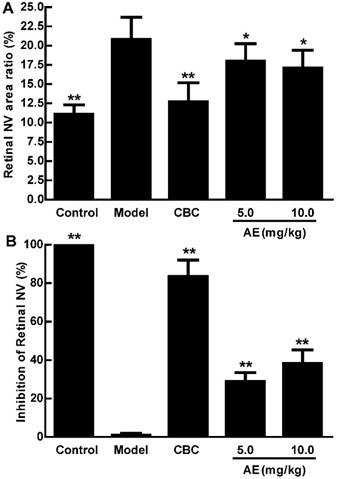
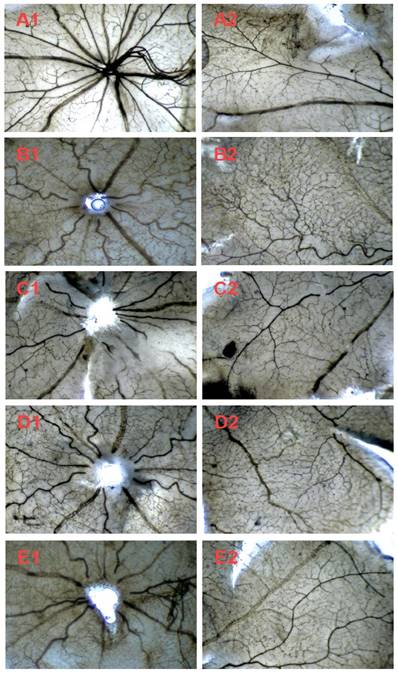
We further investigated the in vivo effect of AE on the hypoxia-induced retinal neovascularization of the superficial vascular plexuses with ADPase stain in a rat model of oxygen-induced retinopathy after we demonstrated the in vitro effect of AE on VEGFA secretion of the ARPE-19 cells. Rat pups were exposed to 80% oxygen from PD 7 to PD 12 to induce retinal vaso-obliteration, and then returned to room air to make the retina relatively hypoxic, resulting in the formation of retinal neovascularization. On the PD 17, the OIR rats treated with vehicle increased the pre-retinal neovascular area ration significantly compared to that of the normal rats treated with the vehicle (p < 0.01). However, AE (5 and 10 mg/kg) and Conbercept (1 mg/kg) markedly reversed the hypoxia-induced neovascular area ration, inhibited retinal neovascularization and alleviated the neovascularization, vessel tortuosity, and dilated vessels of the OIR rats significantly (Figure 4A & 4B and Figure 5).
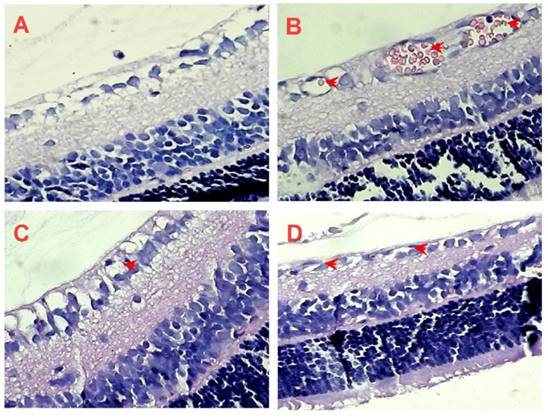
In order to further confirm the inhibitory effect of AE on retinal neovascularization, we counted on the vascular cell nuclei extended beyond the internal limiting membrane in the retinal tissue sections of rats with H&E stain. Nuclei anterior to the internal limiting membrane were not found in the retinal tissue sections of the control rats (Figure 6A), but a lot of neovascular nuclei and vessels were found in the retinal tissue sections of the OIR rats (Figure 6B). Interestingly, the neovascular nuclei and vessels in the retinal tissue sections were remarkably reduced by the Conbercept and AE treatments (Figure 6C & 6D).
The effects of AE on pre-retinal neovascular area ration in the OIR rats. A: Retinal neovascular area ration. B: Inhibition of retinal neovascular area. The results were expressed as means ± SD (n = 10). * p < 0.05, ** p < 0.01, compared to control group (normal saline treatment).
Discussion
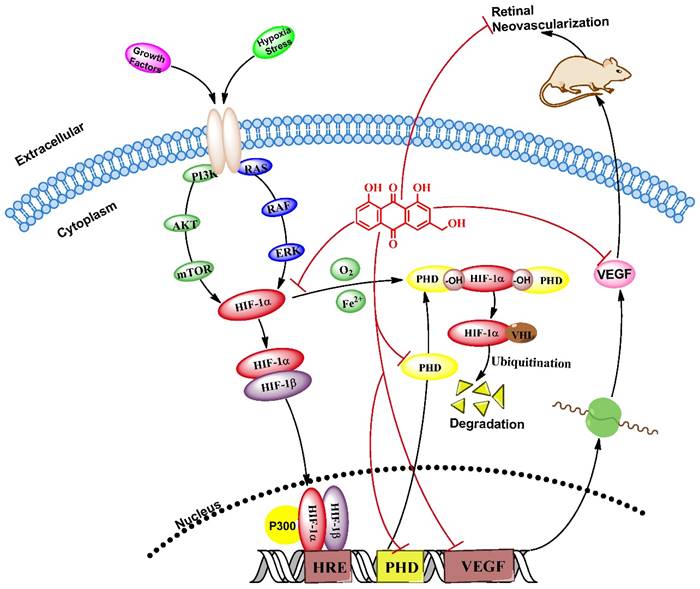
We investigated the role of AE in prevention of hypoxia-induced retinal neovascularization via the HIF-1α/VEGF signaling pathway using VEGFA secretion model of ARPE-19 cells induced by CoCl2 in vitro and neovascularization model of oxygen-induced retinopathy in vivo. Our results reveal that AE can inhibit VEGFA secretion in the ARPE-19 cells under hypoxia condition, down-regulate mRNA expressions of VEGFA and PHD-2, and decrease the protein expressions of VEGFA, HIF-1α and PHD-2 in vitro. Furthermore, AE also can prevent hypoxia-induced retinal neovascularization in vivo. The results show that AE can alleviate hypoxia-induced retinal angiogenesis through suppression of the HIF-1α/VEGF signaling pathway (Figure 7). Therefore, our findings support the major mechanism of Aloe-emodin in the DR-treatment and suggest that AE is an effective agent against DR. More importantly, AE may potentially be developed as a natural drug for DR treatment. However, it needs to be demonstrated the pharmacological activity and pharmacokinetics in different animal models of retinopathy, toxic effects, preparation and stability research, quality control and so on before clinical trials for validation.
The effects of Conbercept and AE on retinal neovascularization of the OIR rats in the retinal sections with ADPase stain (40×). Central (A1-E1) and peripheral (A2-E2) retinal regions of the rat pups fostered under normoxia condition (control) and OIR rats fostered under 80% oxygen. The rats were administered by intraperitoneal (i.p.) injection with normal saline (control), Conbercept 1.0 mg/kg, or AE 5.0 mg/kg and 10 mg/kg, daily x 5. Ten rats were used for each group.
VEGF plays the key role in the retinal neovascularization in the pathological process of DR, which involved in several functions, such as angiogenesis, vasculogenesis, endothelial cell growth and so on.The human VEGF family was composed of five members, including VEGF (or VEGFA), VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF). They can bind to three receptor protein-tyrosine kinases for the VEGF family of ligands (VEGFR1, VEGFR2, and VEGFR3) and two non-enzymatic receptors (neuropilin-1 and -2). VEGFA is the primary and important member of the VEGF family in angiogenesis [28].
The effects of AE on retinal neovascularization in the retinal tissue sections with H&E stain (400×). (A) Control rats, the rat pups were fostered under normoxia condition; (B-D) OIR rats pups were under 80% oxygen and intraperitoneally (i.p.) administered with normal saline (control), Conbercept 1.0 mg/kg, or AE 10 mg/kg, daily x 5. Ten rats were used for each group.
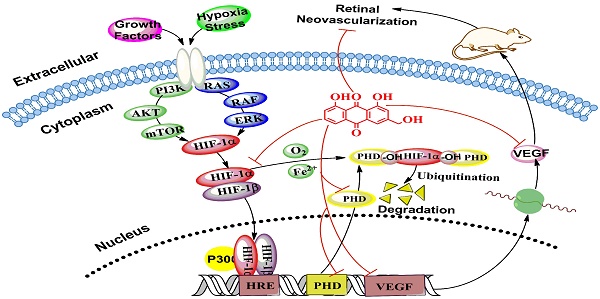
AE alleviates hypoxia-induced retinal angiogenesis through suppression of the HIF-1α/VEGF signaling pathway.
Retinal pigment epithelium cells, located between photoreceptors and the choriocapillaris (capillaries forming the inner vascular layer of the choroid), play an important role in maintaining retinal homeostasis [29-31]. In the present study, we utilized 150 mM CoCl2, a widely used chemical compound to mimic hypoxia condition in the cultured ARPE-19 cells to study the regulation of VEGFA expression. Our data revealed that the mRNA expression was significantly increased at 24 h and the VEGFA secretion was markedly elevated at 48 h in the ARPE-19 cells under hypoxia condition. The time different response with CoCl2 exposure may result from that the mRNA transcription, translation and secretion of VEGFA occurred at different time. In addition, the data also demonstrate that AE can simultaneously inhibit the mRNA transcription and protein expression of VEGFA. However, VEGF expression was also be regulated by PKC, inflammation and so on [23, 33], so AE suppresses the VEGF expression could be through the PKC or inflammation pathway, which needs to be further investigated. HIF plays the critical role in the retinal neovascularization. However, HIF-1α can be hydroxylated by PHD (especially PHD-2), the hydroxylated HIF-1α will be multi-ubiquitinated and degraded in the proteasome. Conversely, the mRNA expression of PHD is upregulated by hypoxia through a HIF-1α dependent signaling pathway [34]. In addition, PHD activity is inhibited under low oxygen tension [35]. This counter action could explain that the expression of PHD-2 is up-regulated by CoCl2 and down-regulated by AE through inhibition of HIF-1α protein expression as previous reported [36]. In the present study, we found the mRNA expressions of VEGFA and PHD-2 and the protein expressions of VEGFA, HIF-1α and PHD-2 can be induced in the CoCl2 hypoxia-mimicking conditions. AE can inhibit the increase induced by CoCl2 in the dose-dependent manner, indicating that AE may inhibit the secretion of VEGFA via the HIF-1α/VEGF signaling pathway. In order to demonstrate the inhibitory effect of AE on retinal neovascularization in vivo, the rat model of oxygen-induced retinopathy was used to investigate the pharmacological effect of AE on ischemic retinopathy induced by hyperoxia and followed by returning to normoxia [24]. Increased retinal neovascularization was found in the OIR rats and AE remarkably alleviated the neovascularization, vessel tortuosity and dilated vessels in the OIR rats, suggesting AE may be a potent inhibitor of retinal neovascularization through the HIF-1α/VEGF signaling pathway.
HIF-1α undergoes quick degradation under normoxic conditions and has a very short half-life (~ 5 min) [37]. However, in hypoxic conditions, several pathways have been shown to control HIF-1α stability and transcriptional activity via post-transnational modifications involving hydroxylation, acetylation, ubiquitination, and phosphorylation reactions, including hypoxic regulation pathway (pVHL-dependent or pVHL-independent) [38], growth factor signaling pathway [39], Mdm2 pathway [40], Hsp90 pathway and so on [41]. Therefore, our further investigation into the molecular mechanisms associated with the effects of AE on anti-retinal neovascularization should study its effect on the upstream regulation, degradation of HIF-1α and HRE transcription.
Conclusions
AE plays an important role in inhibition of VEGFA secretion, through down-regulating the mRNA expressions of VEGFA and PHD-2, decreasing the protein expressions of VEGFA, HIF-1α and PHD-2 in the cultured ARPE-19 cells. AE also inhibits retinal neovascularization in a rat model of oxygen-induced retinopathy in vivo. These results may provide important insights into potential discovery and development of AE as a novel drug for DR treatment clinically. However, more studies are warranted for validating the pharmacological effects on other animal models of retinopathy and associated mechanism(s) against DR.
Abbreviations
AE: Aloe-emodin; DR: diabetic retinopathy; OIR: oxygen-induced retinopathy; VEGF: vascular endothelial growth factor; HIF-1α: hypoxia-inducible factor-1α; PHD-2: prolyl hydroxylase-2; qRT-PCR: quantitative real time PCR; YC-1: 3-(5'-hydroxymethyl-2'-furyl)-1-benzylindazole; FBS: fetal bovine serum; ELISA: enzyme-linked immunosorbent assay; PD: postnatal day.
Acknowledgements
The authors thank Prof. Shousong Cao (Southwest Medical University) for proof-reading and editing of the manuscript. This work was supported by Technology Support Program of Science and Technology Department of Sichuan Province, China (2012SZ0038).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jin W, Wang YF, Ge RL. et al. Simultaneous analysis of multiple bioactive constituents in Rheum tanguticum Maxim. ex Balf. by high-performance liquid chromatography coupled to tandem mass spectrometry. Rapid Commun Mass Sp. 2007;21:2351-60
2. Varinska L, Mirossay L, Mojzisova G. et al. Antiangiogenic effect of selected phytochemicals. Pharmazie. 2010;65:57-63
3. Kong L, Bao Y, Yu Z. et al. Screening of antineoplastic components in Radix et Rhizoma Rhei using chromatographic fingerprints. Se Pu. 2005;23:470-6
4. Lu JJ, Bao JL, Wu GS. et al. Quinones derived from plant secondary metabolites as anti-cancer agents. Anticancer Agents in Med Chem. 2013;13:456-63
5. Chen R, Zhang J, Hu Y. et al. Potential antineoplastic effects of Aloe-emodin: a comprehensive review. Am J Chin Med. 2014;42:275-88
6. Wei L, Li D, Ye R. Effects of Da Huang Zhe Chong Pill on VI factor correlative antigen and fibrinogen in the patient of non-insulin dependent diabetic nephropathy at stage IV. J Tradit Chin Med. 2003;44:30-2
7. Hu JF, Wang W, Wu JM. et al. Inhibitory Effects of Keluoxin Capsule on Hypoxia-induced Angiogenesis in Retina. Chin J Exp Tradit Med Form. 2014;10:156-60
8. Vaziri K, Schwartz SG, Relhan N. et al. New Therapeutic Approaches in Diabetic Retinopathy. Rev Diabet Stud. 2015;12:196-210
9. Jo DH, An H, Chang DJ. et al. Hypoxia-mediated retinal neovascularization and vascular leakage in diabetic retina is suppressed by HIF-1alpha destabilization by SH-1242 and SH-1280, novel hsp90 inhibitors. J Mol Med. 2014;92:1083-1092
10. Sugimoto M, Cutler A, Shen B. et al. Inhibition of EGF signaling protects the diabetic retina from insulin-induced vascular leakage. Am J Pathol. 2013;183:987-95
11. Rosen R, Vagaggini T, Chen Y. et al. Zeaxanthin inhibits hypoxia-induced VEGF secretion by RPE cells through decreased protein levels of hypoxia-inducible factors-1alpha. Biomed Res Int. 2015;2015:687386
12. Pages G, Pouyssegur J. Transcriptional regulation of the Vascular Endothelial Growth Factor gene-a concert of activating factors. Cardiovasc Res. 2005;65:564-73
13. Poon E, Harris AL, Ashcroft M. Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Reviews in Molecular Medicine. 2009;11:e26
14. Zhang W, Petrovic JM, Callaghan D. et al. Evidence that hypoxia-inducible factor-1 (HIF-1) mediates transcriptional activation of interleukin-1beta (IL-1beta) in astrocyte cultures. J Neuroimmunol. 2006;174:63-73
15. Filippi I, Carrarelli P, Luisi S. et al. Different Expression of Hypoxic and Angiogenic Factors in Human Endometriotic Lesions. Reprod Sci. 2016;23:492-7
16. Ahn GO, Seita J, Hong BJ. et al. Transcriptional activation of hypoxia-inducible factor-1 (HIF-1) in myeloid cells promotes angiogenesis through VEGF and S100A8. Proc Natl Acad Sci USA. 2014;111:2698-703
17. Jelkmann W. Regulation of erythropoietin production. J Physiol. 2011;589:1251-8
18. Krotova K, Patel JM, Block ER. et al. Hypoxic upregulation of arginase II in human lung endothelial cells. Am J Physiol-Cell Ph. 2010;299:1541-8
19. Thomas R, Kim MH. HIF-1 alpha: a key survival factor for serum-deprived prostate cancer cells. Prostate. 2008;68:1405-15
20. Masoud GN, Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378-89
21. Kim JH, Kim JH, Yu YS. et al. Deguelin inhibits retinal neovascularization by down-regulation of HIF-1alpha in oxygen-induced retinopathy. J Cell Mol Med. 2008;12:2407-15
22. DeNiro M, Al-Halafi A, Al-Mohanna FH. et al. Pleiotropic effects of YC-1 selectively inhibit pathological retinal neovascularization and promote physiological revascularization in a mouse model of oxygen-induced retinopathy. Mol Pharmacol. 2010;77:348-67
23. Iwase T, Fu J, Yoshida T. et al. Sustained delivery of a HIF-1 antagonist for ocular neovascularization. J Control Relea. 2013;172:625-33
24. Ricci B. Oxygen-induced retinopathy in the rat model. Doc Ophthalmol. 1990;74:171-7
25. Matsubara M, Saito Y, Nakanishi-Ueda T. et al. Influence of the difference of breastfeeding volume on a rat model of oxygen-induced retinopathy. J Clin Biochem Nutr. 2014;55:129-34
26. Winners-Mendizabal OG, Orge FH, Di Fiore JM. et al. Hypoxia-hyperoxia paradigms in the development of oxygen-induced retinopathy in a rat pup model. J Neonatal Perinatal Med. 2014;7:113-7
27. Osera C, Martindale JL, Amadio M. et al. Induction of VEGFA mRNA translation by CoCl2 mediated by HuR. RNA Bio. 2015;12:1121-30
28. Roskoski RJ. Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol. 2007;62:179-213
29. Marneros AG, Fan J, Yokoyama Y. et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167:1451-9
30. Kurihara T, Westenskow PD, Friedlander M. Hypoxia-inducible factor (HIF)/vascular endothelial growth factor (VEGF) signaling in the retina. Adv Exp Med Biol. 2014;801:275-81
31. Le YZ, Bai Y, Zhu M. et al. Temporal requirement of RPE-derived VEGF in the development of choroidal vasculature. J Neurochem. 2010;112:1584-92
32. Jiang Y, Zhang Q, Steinle JJ. Beta-adrenergic receptor agonist decreases VEGF levels through altered eNOS and PKC signaling in diabetic retina. Growth Factors. 2015;33:192-9
33. Wang J, Xu X, Elliott MH. et al. Muller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297-305
34. Berra E, Benizri E, Ginouves A. et al. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082-90
35. Brito LG, Schiavon VF, Andrade JM. et al. Expression of Hypoxia-inducible factor 1-alpha and vascular endothelial growth factor-C in locally advanced breast cancer patients. Clinics. 2011;66:1313-20
36. Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66:6264-70
37. Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642-7
38. Tug S, Delos RB, Fandrey J. et al. Non-hypoxic activation of the negative regulatory feedback loop of prolyl-hydroxylase oxygen sensors. Biochem Bioph Res Commun. 2009;384:519-23
39. Kaidi A, Qualtrough D, Williams AC. et al. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683-91
40. Zhou CH, Zhang XP, Liu F. et al. Modeling the interplay between the HIF-1 and p53 pathways in hypoxia. Sci Rep. 2015;5:13834
41. Eschricht S, Jarr KU, Kuhn C. et al. Heat-shock-protein 90 protects from downregulation of HIF-1alpha in calcineurin-induced myocardial hypertrophy. J Mol Cell Cardiol. 2015;85:117-26
Author contact
![]() Corresponding authors: Xiaofeng Hao (e-mail: hxfcom) Address: Post-Doctoral Research Station, KangHong Pharmaceutical Group, No.36, Shuxi Road, Chengdu 610036, China. Telephone: +86-28-87519670. Zhirong Zhang (e-mail: zrzzlsina.com) Address: West China School of Pharmacy, Sichuan University, No.17, Section 3, South Renmin Road, Chengdu 610041, China. Telephone: +86-28-85501566.
Corresponding authors: Xiaofeng Hao (e-mail: hxfcom) Address: Post-Doctoral Research Station, KangHong Pharmaceutical Group, No.36, Shuxi Road, Chengdu 610036, China. Telephone: +86-28-87519670. Zhirong Zhang (e-mail: zrzzlsina.com) Address: West China School of Pharmacy, Sichuan University, No.17, Section 3, South Renmin Road, Chengdu 610041, China. Telephone: +86-28-85501566.

 Global reach, higher impact
Global reach, higher impact