Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2017; 13(10):1309-1319. doi:10.7150/ijbs.20254 This issue Cite
Research Paper
Adenovirus-Mediated Gene Transfer of microRNA-21 Sponge Inhibits Neointimal Hyperplasia in Rat Vein Grafts
1. Department of Cardiothoracic Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, 400016, China;
2. Department of Cardiothoracic Surgery, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, 200233, China;
3. Centre for Clinical Pharmacology, William Harvey Research Institute, Queen Mary University of London, London EC1M 6BQ, United Kingdom;
4. Key Laboratory for Regenerative Medicine, Ministry of Education, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China.
* contributed equally to this work
Received 2017-3-24; Accepted 2017-7-20; Published 2017-10-17
Abstract
Background: Vein graft failure due to neointimal hyperplasia remains an important and unresolved complication of cardiovascular surgery. microRNA-21 (miR-21) plays a major role in regulating vascular smooth muscle cell (VSMC) proliferation and phenotype transformation. Thus, the purpose of this study was to determine whether adenovirus-mediated miR-21 sponge gene therapy was able to inhibit neointimal hyperplasia in rat vein grafts.
Methods: Adenovirus-mediated miR-21 sponge was used to inhibit VSMC proliferation in vitro and neointimal formation in vivo. To improve efficiency of delivery gene transfer to the vein grafts, 20% poloxamer F-127 gel was used to increase virus contact time and 0.25% trypsin to increase virus penetration. Morphometric analyses and cellular proliferation were assessed for neointimal hyperplasia and VSMC proliferation.
Results: miR-21 sponge can significantly decrease the expression of miR-21 and proliferation in cultured VSMCs. Cellular proliferation rates were significantly reduced in miR-21 sponge-treated grafts compared with controls at 28 days after bypass surgery (14.6±9.4 vs 34.9±10.8%, P=0.0032). miR-21 sponge gene transfer therapy reduced the intimal/media area ratio in vein grafts compared with the controls (1.38±0.08 vs. 0.6±0.10, P<0.0001). miR-21 sponge treatment also improved vein graft hemodynamics. We further identified that phosphatase and tensin homolog (PTEN) is a potential target gene that was involved in the miR-21-mediated effect on neointimal hyperplasia in vein grafts.
Conclusions: Adenovirus-mediated miR-21 sponge gene therapy effectively reduced neointimal formation in vein grafts. These results suggest that there is potential for miR-21 sponge to be used to prevent vein graft failure.
Keywords: MicroRNA, Neointimal formation, Gene therapy, Vein graft disease.
Introduction
Vein graft failure of coronary artery bypass grafting occurs as a result of neointimal hyperplasia, which is characterized by the proliferation and migration of vascular smooth muscle cells (VSMC) [1]. Gene therapy has generated interest as a potential therapeutic alternative for the treatment and prevention of neointimal hyperplasia. Vein grafts are ideally suited to gene therapy due to the short time window (approximately 40-60 min) prior to graft, during which the vein grafts are maintained ex vivo to minimize the risk of systemic exposure. Numerous preclinical studies and clinical trials have documented the potential to prevent neointimal formation by inhibiting VSMC proliferation and migration [2-5].
Increasing evidence indicates that microRNAs (miRNAs) regulate key genetic programs in cardiovascular biology, physiology and disease. Several experimental studies have evaluated the role and translational therapeutic potential of miRNAs in the regulation of VSMC phenotype and neointimal formation [6-10]. Among the miRNAs dysregulated in the vascular wall with neointimal formation, miR-21 has been implicated in VSMC proliferation via the target genes phosphatase and tensin homolog (PTEN) [11]. miR-21 is up-regulated in the vascular walls with neointimal formation following carotid artery balloon injury, and its down-regulation decreases neointima formation. miR-21 up-regulation by repetitive ischemia correlates with excessive cell proliferation in metabolic syndrome and miR-21 down-regulation blocks cell proliferation [12]. Inhibition of miR-21 reduces in-stent restenosis in animals [13]. However, whether miR-21 is involved in regulation of neointimal lesion formation in vein grafts has not been investigated.
The previous studies prompted us to determine whether miR-21 may be used as a novel gene therapeutic approach for the prevention of vein graft failure by inhibiting neointimal formation.
Recent studies have reported that miRNA sponges are valuable tools to induce miRNA loss-of-function in in vitro and in vivo studies in cell lines and transgenic organisms over extended durations [14, 15]. miRNA sponges contain multiple tandem complementary miRNA antisense binding sites (MBS) for an miRNA of interest, and are an innovative approach used to sequester miRNAs from their endogenous targets [15]. Viral vectors may be used for miRNA sponge-based therapy by producing a high level of prolonged expression of the sponge, and by providing an efficient method for in vivo delivery [16].
Therefore, the aim of the present study was to determine whether miR-21 sponge therapy is a useful novel gene therapeutic approach for the prevention of vein graft failure by inhibiting neointimal hyperplasia, using adenovirus (Ad)-mediated gene transfer. In addition, to enhance the efficiency of Ad-mediated gene transfer to the vein graft surface, we used poloxamer F-127 gel to increase viral contact time, and a low concentration of trypsin to increase viral penetration.
Methods
miRNA sponge design
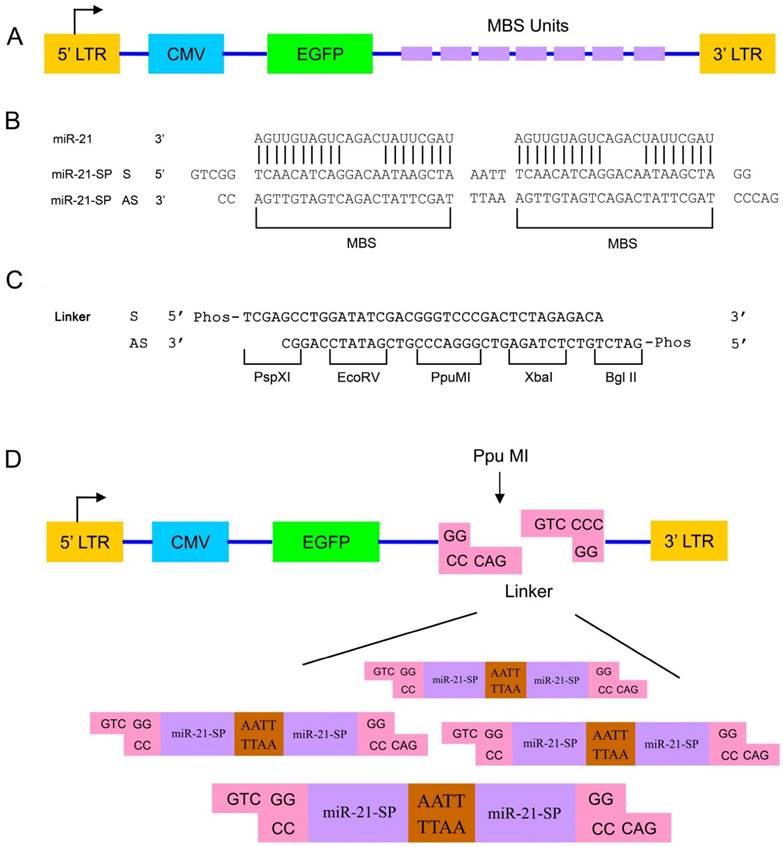
The basic approach for miRNA sponge design was performed as described in our previous studies [17] (Fig. 1A). Briefly, oligonucleotides were designed with two identical MBS, but each MBS mismatches in the middle portion to create a bulge at positions 9-12 of the miR-21 sequence (Fig. 1B). The two MBS were separated by a short 4 nucleotide sequence (AATT). The 5' and 3' ends of the oligonucleotide duplex consist of overhangs that are compatible with the PpuMI restriction endonuclease. Furthermore, the PpuMI restriction endonuclease on the left of the MBS was modified (Fig. 1B) to allow for flexibility in potential further directional subcloning.
To enable directional cloning of the oligonucleotide duplexes, the linker was inserted in the adenovirus vector. The linker design was used to introduce a PpuMI restriction enzyme recognition site in the Ad. A 5' PspXI site and a 3' BglII site were added to the ends of the linker for efficient directional subcloning (Fig. 1C). By ligation of oligonucleotide duplexes with PpuMI compatible ends with a PpuMI-digested adenovirus vector, sponge constructs with a variable number of MBS were generated in a single ligation reaction (Fig. 1D).
Gene transfer vectors
The miR-21 sponge oligonucleotide and the reverse complementary sequence were synthesized by the Beijing Genomics Institute (Shenzhen, China). The sequences were as follows: sense, 5'-GTCGG TCAACATCAGGACAATAAGCTAAATTTCAACATCAGGACAATAAGCTA GG -3'; and antisense, 5'-GACCCTAGCTTATCAGACTGATGTTGAAATTTAGCTTATCAGACTGATGTTGACC-3'. The two oligonucleotides were annealed to make a double-stranded DNA fragment with PpuMI overhangs. The sponge linker oligonucleotide and the reverse complementary sequence were synthesized by the Beijing Genomics Institute. The sequences were as follows: i) 5'- TCGAGCCTGGATATCGACGGGTCCCGACTCTAGAGACA-3'; and ii) 5'- GATCTGTCTCTAGAGTCGGGACCCGTCGATATCCAGGC-3'. The control sponge was as described previously [18]. The adenovirus vector was constructed and propagated in 293T cells according to standard protocols [19]. The virus transduction titer was 1.5x1010 plaque forming units (pfu)/ml, as determined by a plaque titration assay on the 293T cells.
VSMC isolation, culture and gene transfer
Primary rat VSMCs were isolated from the aortas of male Sprague-Dawley (SD) rats (150 -180g) by the explant method, as previously described [20]. For the infection of VSMC, cells were incubated with Ad-miR-21-SP for 48 h. The Ad-GFP control sponge (Ad-Control-SP) was used as a negative control.
Proliferation assays
The methods of the VSMC proliferation assay were as described previously [21]. VSMCs were plated in 24-well plates and grown to 60% confluence. VSMCs were serum starved for 24 h and stimulated with platelet-derived growth factor (20 ng/ml) for 48 h, following which cell proliferation was assessed by cell counting and BrdU assays. BrdU incorporation assays were performed according to the manufacturer's instructions (Roche Diagnostics, Basel, Switzerland). The experiment was repeated three times independently.
Design of miR-21 sponges and directional cloning strategy. (A) Schematic overview of construction of adenoviru (Ad) mediated miRNA sponges. (B) The sense and antisense sequences design used to generate the miR-21 sponge with bulged binding sites. (C) The linker design used to introduce a PpuMI restriction enzyme recognition site in the Ad. A 5' PspXI site and a 3' BglII site were added to the ends of the linker for efficient directional subcloning. (D) The method to ligate miR-21 sponge oligonucleotide duplexes into the Ad. Each oligonucleotide duplex contains two MBS and phosphorylated PpuMI restriction enzyme compatible overhangs to generate miR-21 sponge with varying sizes using a single ligation reaction.
Surgical procedure and therapeutic interventions
Male SD rats (200-250g) underwent interposition bypass grafting of the autologous jugular vein to the carotid artery (n = 6 for each group at each time point). The procedure used for vein grafting was similar to that described previously [22]. To locally deliver Ad to the vein graft walls and avoid any potential systemic side effects, we applied an established local delivery model via the F-127 pluronic gel, as previously described [6, 23]. Briefly, Vein segments were gently flushed to remove residual blood, and approximately 100 µl Ad solution was infused under gentle distending pressure for 30 minutes at room temperature. The vein wall remained well-distended for the duration of the virus incubation period. Then after end-to-end anastomoses of the vein segment to common carotid artery, 100 µl Ad solution was preloaded into 100 µl 20% F-127 pluronicgel containing 0.25% trypsin at 4°C, and was gently painted around the grafted vein segments including the anastomosis sites. All the protocols involving experimental animals were approved by the Institutional Animal Care and Use Committee of the Chinese University of Hong Kong, and all the experimental procedures complied with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA). All experimental methods were performed in accordance with the approved guidelines. All efforts were made to minimize animal suffering.
Statistical analysis
Values are presented as the mean ± standard deviation. Comparisons were made using the Student's t-test or analysis of variance, and P <0.05 was considered to indicate a statistically significant difference. SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA), was used for all statistical analyses.
Results
miR-21 sponge inhibits miR-21 expression and VSMC proliferation
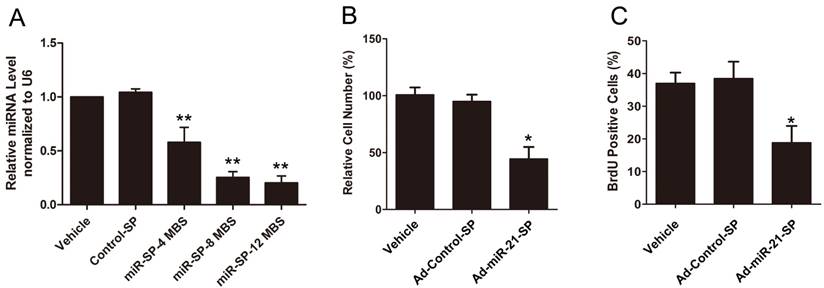
With the 1:1000 vector/duplex ratio ligation, the mean number of MBS was 8.5 (range 4-20) in a total of 40 colonies, and 34% of all analyzed clones had 8 or more MBS. Sequencing (Beijing Genomics Institute) of 16 clones containing different inserts and insert lengths confirmed the expected number of MBS in the correct orientation for all the clones.
To knockdown miR-21, VSMCs were infected with Ad-miR-21-SP containing 4-12 of miR-21 MBS. Compared with the empty vector, Ad-miR-21-SP inhibited the expression of miR-21, with increasing numbers of MBS producing a stronger effect following 48 h of infection (Fig. 2A). The miR-21 sponge with 4 MBS showed a mild inhibitory effect, whilst sponges with 8 and 12 MBS showed almost identical significantly strong inhibitory effects. By contrast, no miR-21 inhibition was observed in the vehicle and Ad-Control-SP groups. We therefore used Ad-miR-21-SP with 12 MBS in the subsequent experiments. As shown in Fig. 2B and C, Ad-miR-21-SP significantly decreased the number of proliferating cells compared with the vehicle and Ad-Control-SP groups, measured using cell counting and BrdU incorporation assay.
(A) The effect of miR-21 sponge with MBS number of four, eight, and twelve on the expression of miR-21 in cultured rat VSMC. (B) The effect of miR-21 sponge inhibited VSMC proliferation in vitro determined by cell counting. (C) The effect of miR-21 sponge on VSMC proliferation as determined by BrdU analysis. Data presented are the mean of three independent experiments. **P<0.001 compared with control sponge. *P<0.05 compared with vehicle control.
Animals and systemic measurements
All rats successfully received a vein graft. A single rat in the Ad-miR-21-SP group died during the induction of anesthesia. All animals that recovered from the first operation survived the entire period of observation. No significant differences were noted in the behavior or feeding habits between the treated groups. There was no significant difference in body weight between the Ad-Control-SP (n = 6 per time point) and Ad-miR-21-SP group (n = 6 per time point) following the operation (228.9±8.6 g vs 223.4±6.9 g at 7 days, P =0.126; 243.6±4.7 vs 240.3±6.3, P = 0.187 at 14 days; 256.6±3.4 vs 253.3±4.1, P =0.764 at 28 days). Body temperatures were also similar between the control and the Ad-treated groups in the 7 days following surgery (Fig. 3). The vein grafts from the Ad-miR-21-SP group were all patent at 3 weeks; however, one graft in the Ad-Control-SP group was completely occluded due to thrombosis at 3 weeks.
miR-21 sponge decreases miR-21 expression in vivo
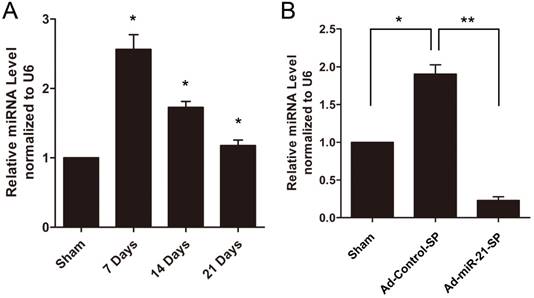
In the present study, the vein grafts were isolated at 7, 14 and 21 days after surgery, and the uninjured veins in the sham group served as controls. As shown in Fig. 4A, miR-21 expression was significantly upregulated in the vein grafts compared with the sham group. In subsequent experiments, we applied sponges to knockdown the expression of miR-21 using Ad-miR-21-SP. As shown in Fig. 4B, treatment with the miR-21 sponge significantly decreased miR-21 expression in the vein graft wall at 14 days compared with Ad-Control-SP.
miR-21 sponge improves hemodynamics of vein grafts
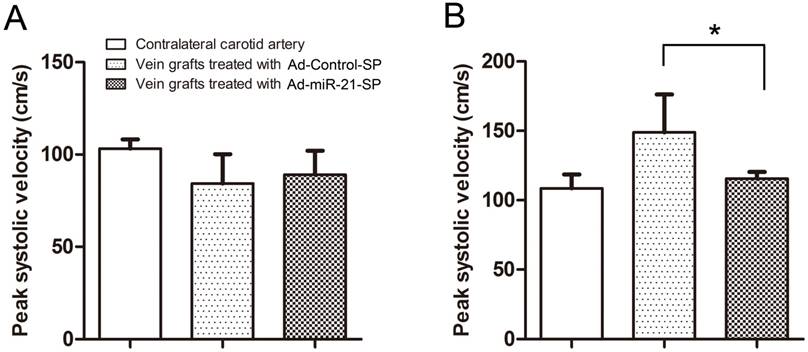
The size differences between the native artery and the vein graft were noted, in particular when relatively large diameter vein grafts were anastomosed to relatively small diameter arteries. In such a case, the blood flow velocity of the vein grafts either in the Ad-Control-SP treated group or Ad-miR-21-SP treated group was lower than in the native carotid arteries, however the difference was not statistically significant (Fig. 5A). When measured again at 28 days after surgery, the peak-systolic velocity (PSV) in the anastomosis of the vein grafts was significantly lower in the Ad-miR-21-SP compared with the Ad-Control-SP treated grafts (115.5±4.9 cm/s and 148.8±27.3, respectively; P = 0.005; Fig. 5B). There was no significant difference in the velocity in the contralateral carotid arteries (Sham group) compared with the Ad-miR-21-SP treated grafts (108.6±9.8 vs. 115.5±4.9 cm/s, respectively; P = 0.069). Consistent with the velocity shown in Fig. 5B, the PSV ratio was significantly lower in the Ad-miR-21-SP treated grafts compared with the Ad-Control-SP treated grafts at 28 days after surgery (1.86±0.42 vs 1.32±0.26; P = 0.004).
There was no significant difference in body temperatures of rats from different groups during 7 days after vein grafts surgery.
Expression of miR-21 in rat vein grafts. (A) The time course changes of miR-21 expression determined by qRT-PCR. (B) miR-21 was downregulated by Ad-miR-21-sponge in vein grafts (n = 6 for each group per time point). Data presented are the mean of three independent experiments. *P<0.05 compared with uninjured veins, **P<0.001 compared with control sponge.
The peak systolic velocity in the anastomosis of vein grafts (n = 6 for each group). (A) The Doppler peak-systolic velocity in vein grafts and contralateral untreated carotid arteries after completion of surgery. (B) The Doppler peak-systolic velocity in vein grafts and contralateral untreated carotid arteries 28 days after surgery. Data presented are the mean of three independent experiments. * P<0.05 compared with control sponge.
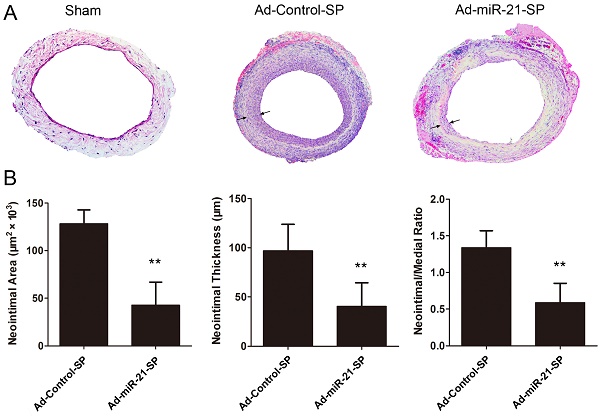
miR-21 sponge inhibits vein graft neointima formation
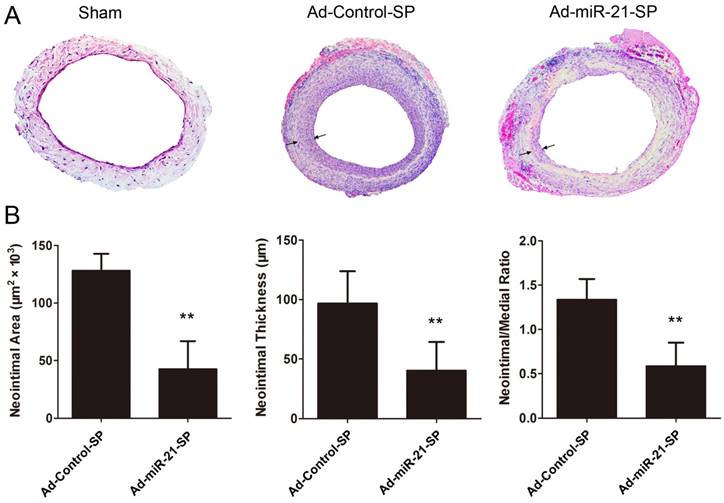
Fig. 6 presents the quantitative morphometry of hematoxylin and eosin (HE) sections at 28 days, indicating that the neointimal area was significantly decreased in the Ad-miR-21-SP treated grafts compared with the Ad-Control-SP treated grafts (128.3±5.2 vs 43.6±8.6, respectively, P < 0.0001; Fig. 6A). Similarly, the neointimal thickness of the Ad-miR-21-SP treated group was reduced by 56% compared with the Ad-Control-SP (96.9±9.5 vs 40.8±8.4, respectively, P = 0.0006; Fig. 6B). In addition, the neointima to media ratio, which is a more sensitive parameter for assessing relative changes in intima and media thickness, is shown in Fig. 6A. The neointima/media thickness ratio was significantly decreased in the Ad-miR-21-SP treated grafts compared with the Ad-Control-SP treated grafts (1.38±0.08 vs 0.6±0.10, P < 0.0001; Fig. 5B).
miR-21 sponge suppresses VSMC proliferation in vivo
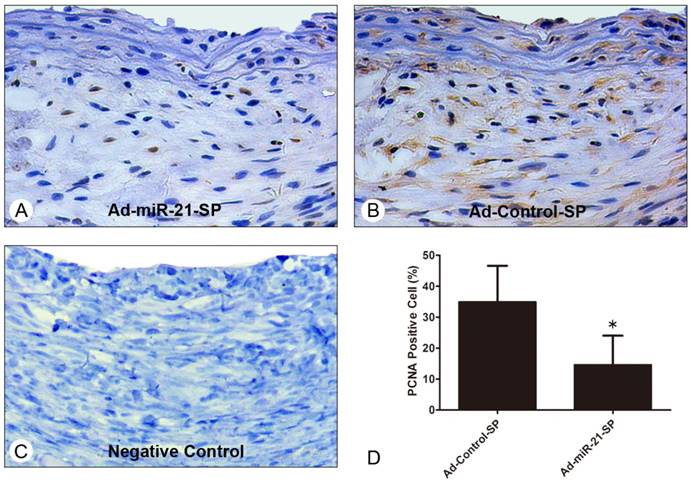
It is known that VSMC proliferation and neointimal migration are the major causes of neointimal hyperplasia. In order to obtain further insight into whether Ad-miR-21-SP affected VSMC proliferation in vein graft remodeling, we examined the expression index of PCNA in the vein graft sections at 28 days after surgery. The number of proliferating cell nuclear antigen (PCNA)-positive VSMCs was calculated, and was compared with the total number of VSMCs in that field to produce the PCNA index as determined by the HE-stained image. Representative immunofluorescence of PCNA and its negative control is shown in Fig. 7, and the vein grafts treated with Ad-miR-21-SP were observed to substantially reduce the upregulation of the cell proliferative marker PCNA in the vein grafts (34.9±10.8 vs. 14.6±9.4%, P = 0.0032) at 28 days after surgery, compared with those infected with Ad-GFP.
A strategy which would prevent intimal hyperplasia and restenosis while preserving the endothelium is the aim for gene therapeutic intervention [1]. Therefore, we evaluated whether Ad-miR-21-SP simultaneously accelerated endothelial cell proliferation and the regeneration of the injured endothelium. At 28 days following surgery, the vein graft sections were stained with lectin Dolichos biflorus agglutinin to detect vascular endothelial cells, however, no significant differences in the luminal coverage of endothelial cells were observed between the groups (data not shown).
PTEN is a target gene of miR-21 in vein grafts
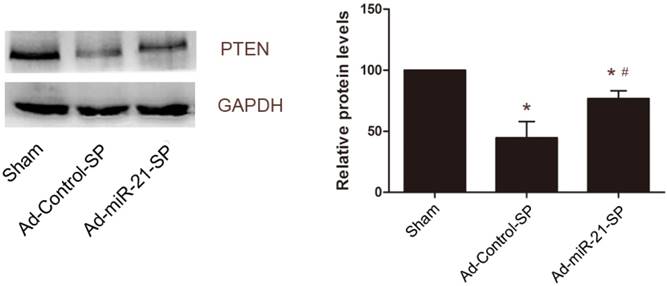
miR-21 has previously been shown to target PTEN mRNAs, leading to reduced expression of the gene products in VSMCs [11]. Therefore, we then determined whether PTEN was a potential target gene of miR-21 that may contribute to vein graft neointimal formation. As shown in Fig. 8, PTEN was downregulated in vein grafts compared with the sham group at 28 days after surgery. In the Ad-miR-21-SP-treated vein grafts, the expression levels of PTEN were upregulated (Fig. 8). These results are similar to a previous study.
miR-21 sponge inhibits neointimal formation (arrows) in vein grafts 28 days after surgery (n = 6 for each group). (A) Representative H-E stained photomicrographs of vein grafts from different groups. (B) Quantification of neointimal area, neointimal thickness, neointima-to-media thickness ratio in vein grafts from different groups. Data presented are the mean of three independent experiments. **P<0.001 compared with control sponge.
Immunohistochemical PCNA staining in vein grafts 28 days after surgery (n = 6 for each group). (A) Representative immunohistochemical staining images of PCNA in vein grafts treated with control sponge. (B) Representative immunohistochemical staining images of PCNA in vein grafts treated with Ad-miR-21-sponge (Ad-miR-21-SP). (C) Negative controls of sections stained with same protocol without primary antibody. (D) The percentage of PCNA positive cells in vein grafts treated with Ad-miR-21-sponge (Ad-miR-21-SP) was significantly lower than that with control sponge. Data presented are the mean of three independent experiments. *P<0.05 compared with control sponge.
The effect of miR-21 sponge on expression levels of PTEN in vein grafts. Representative Western blot of PTEN protein in the vein grafts treated with control sponge or miR-21 sponge. Data presented are the mean of three independent experiments. *P<0.05 compared with sham group; # P<0.05 compared with control sponge.
Discussion
Vascular anastomosis performed with sutures is the cornerstone of cardiovascular surgery. However, the long-term benefit of bypass surgery is limited by the development of neointimal formation within the vein graft. To date, no optimal treatments are available for vein graft failure [24]. Therefore, further studies on the development of effective therapies that inhibit the formation of neointimal hyperplasia to improve the long-term patency of vein grafts are required.
It is well established that the phenotypic modulation of VSMCs plays a critical role in the pathogenesis of a variety of proliferative cardiovascular diseases, including postangioplasty restenosis, bypass vein graft failure, arteriole hypertension and transplantation arteriopathy [25]. The phenotypic modulation in VSMCs is accompanied by significantly accelerated proliferation and migration, and the production of extracellular matrix components. Ultimately, these cellular events result in the formation of vascular neointima, which is the main pathological lesion in long-term vein graft failure. As previously mentioned, treatment of vein graft failure should aim to reduce VSMC activation, migration and proliferation.
The selection of the correct target for gene therapy applications is one of the biggest challenges faced by gene therapists. Currently, multiple lines of evidence suggest that miRNAs play pivotal roles in the control of VSMC function and the response to vascular injury through targeting transcription factors or key signaling molecules involved in VSMC proliferation and migration. miR-21 has been identified as an important regulator of VSMC proliferation and neointimal lesion formation via its target genes PTEN and Bcl-2. In cultured cells, upregulation of miR-21 resulted in increased VSMC proliferation and decreased VSMC apoptosis. By contrast, inhibition of miR-21 decreases the proliferation of VSMCs, and increases apoptosis. In vivo, inhibition of miR-21 decreases neointima formation in the rat carotid artery after balloon injury [11]. On the basis of the same mechanism, overexpression of miR-21 induces the proliferation of VSMCs in the aortic wall predominantly through the inhibition of PTEN, and thus protects against accelerated murine aortic aneurysm progression and rupture [26]. In the present study, we found that miR-21 expression was significantly increased in the vein graft walls with neointimal proliferation. Moreover, treatment with Ad-miR-21-SP reduced the proliferation of cultured VSMCs. Our recent data supports that miR-21 is associated with the proliferation of VSMCs, and suggests that local delivery of miR-21 inhibitors is an effective therapeutic means for the prevention of neointimal hyperplasia in vein grafts.
To achieve prolonged inhibition of miR-21 in vivo, we designed a directional cloning method to rapidly and efficiently generate miRNA-21 sponge constructs with varying sizes using a single ligation reaction (Fig. 1). We believe that miRNA sponges with more than 10 MBS can be easily generated by this directional cloning strategy. Furthermore, we successfully used miR-21 sponges against miR-21 to determine the effect on VSMC growth. miRNA sponge technology is an innovative method used to generate RNAs that contain multiple tandem complementary MBS against an miRNA of interest. When vectors encoding the miRNA sponges are transiently transfected into cells, miRNA sponges can be expressed to reduce the miRNA targets as strongly as the conventional anti-miRNA oligonucleotides fragments. In addition, synthetic inhibitor oligonucleotides are effective for short-term experiments but are not suitable for long-term experiments due to degradation and dilution caused by cell proliferation [27]. By contrast, the experimental application of miRNA sponges is particularly useful in long-term experiments. These competitive inhibitors are transcripts expressed from strong promoters (i.e. cytomegalovirus), containing multiple tandem MBS against miRNA targets.
We applied Ad-encoding miR-21 sponges by directly painting them onto vein grafts using a poloxamer gel to prolong viral contact with the vein grafts, and used low concentrations of trypsin to increase viral penetration. The current study observed that 20% poloxamer hydrogel containing a low concentration of trypsin enhanced miR-21 sponge delivery to the vein graft wall. Our data suggests that luminal gene delivery using infusion under gentle distending pressure combined with the painting delivery method of the Ad/poloxamer/trypsin complexes is a safe and highly efficient method of ex vivo gene transfer to vein grafts.
Poloxamer F127 is a synthetic polymer that is a liquid at colder temperatures (4 to 5°C) and gelatinous at body temperature, and has a good solubilizing capacity and low toxicity, and therefore is considered a good medium for drug delivery systems [23]. This unique property allows solutions containing poloxamers and other compounds to be mixed well at colder temperatures and then solidified into a gel to prolong contact time and induce a slow release of the transgene into target organs at body temperature [28]. March et al. were the first to report that the combination of adenoviruses with poloxamers significantly increased the gene transfer efficiency in a culture of VMSCs up to 10-fold [29]. Kikuchi et al. reported for the first time that complexing the virus to the gelatinous poloxamer with mild trypsin and applying it directly to the atria enabled specific delivery, and resulted in 100% transmural gene transfer using the epicardial painting method [28]. Handa et al. demonstrated that the incorporation of trypsin into the poloxamer hydrogel significantly increased vessel wall gene transfer. In addition, trypsin at 0.25 and 0.5% resulted in greater gene transfer at the same level without affecting intimal hyperplasia and inflammation [23]. Furthermore, we suggest that removing as much of the perivascular fat from the vein grafts as possible has the potential to extend this gene transfer method. In addition, the animal temperature and hemodynamic parameters were assessed after Ad exposure.
miRNAs are involved in the pathogenesis of various cardiovascular diseases and have become an intriguing target for therapeutic intervention, and investigating the role of miRNAs in cardiovascular disease is a new dimension for cardiovascular research. Considering the vast number of miRNAs and the profound effects of those currently identified on the cardiovascular system, it is certain that many new and unanticipated roles of miRNAs in the control of cardiovascular function are waiting to be discovered. Further information regarding the functions of these aberrantly expressed miRNAs in cardiovascular disease is required. More importantly, identifying their gene targets and the signaling pathways responsible for their cardiovascular effects is critical for future studies. Progress during further studies of gene expression inhibition techniques, such as miRNA sponges, is likely to result in the development of miRNA or miRNA cluster-based therapy, and a combination of miRNAs and/or miRNA cluster treatment strategies for the prevention of neointimal formation and atherosclerosis in vein grafts. In addition, increased safety and efficiency of gene delivery systems will be required in additional studies. In addition to the use of miRNAs as therapeutic targets, they may serve a valuable functional assessment method for vein graft pathologies. Furthermore, we should be aware that research into the roles of miRNAs in cardiovascular disease is at an early stage, and there remains a long way to go before miRNA-related therapies can be widely applied to clinical practice.
Recent studies have demonstrated that endothelial-derived cells contribute to neointimal formation through endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling [30]. Similar to epithelial-to-mesenchymal transition (EMT), EndMT is the process by which endothelial cells lose their cell-specific markers and morphology and acquire a mesenchymal cell-like phenotype. Furthermore, it has been reported that miR-21 is involved in cardiac TGF-β-mediated EndMT via the PTEN/Akt pathway [31]. These previous studies established EndMT is an important mechanism underlying neointimal formation in vein grafts, and miRNAs are critical endogenous regulators for EndMT in vascular pathology. Therefore, the identification of additional miRNAs and analysis of the functions in EndMT may provide new insights into the mechanisms of neointimal formation in vein grafts. Future studies will move research on the function of miRNA in this field to the next level. The ultimate goal, of course, is the development of new safe and efficient therapeutic strategies for vein graft failure.
Rat model is one of the most common animal models for studying vein graft failure and therapeutic interventions. By 3 days after graft, the VSMC in the vein graft have changed to a synthetic phenotype. By 7 days there is an increase in graft thickening, and adventitial vascularisation gradually increases for the next 3 weeks [32, 33]. The endothelial repair was almost completed on the suture line as well as on the stitches by 14 days. There is a robust increase in vein graft thickening at 7 days and neointimal hyperplasia was generated at 14 days, which continues to develop during 12 weeks after vein grafting [33-35]. Vein grafts generate a thick neointima after 4 weeks, which significantly reduced the lumen of the vessel. Therefore, we usually choose to follow up at 4 to 6 weeks considering the cost of study. We regret that we are unable to have a longer follow-up. Therefore, short term follow up is a limitation of this study.
Conclusions
The present study identified that miR-21 is a key regulator of vein graft failure by targeting, at least in part, the PTEN gene. miR-21 expression was substantially upregulated in proliferating VSMCs and vein grafts with neointimal hyperplasia after bypass surgery. Knockdown of miR-21 by miR-21 sponges suppressed VSMC proliferation and neointima formation in vivo. Although miR-21sponge inhibited neointimal lesion formation significantly compared to control group (Ad-Control-SP group), local delivery of miR-21 sponge partial prevents neointima formation. These results suggest that there is potential for the use of miR-21 sponges in preventing vein graft failure.
Acknowledgements
This work was supported by grants from the Science and Technical Commission of Shanghai Municipality, China [Key Technologies R&D Program, No. 15411952400], the Key Program of Health Bureau of Chongqing, China [ID: 2013-1-015] and Research Grants of The First Affiliated Hospital of Chongqing Medical University [ID:PYJJ2017-11].
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wang XW, Zhao XJ, Xiang XY. Gene therapy for vein graft failure. J Card Surg. 2013;28:144-147
2. West NE, Qian H, Guzik TJ. et al. Nitric oxide synthase (nNOS) gene transfer modifies venous bypass graft remodeling: effects on vascular smooth muscle cell differentiation and superoxide production. Circulation. 2001;104:1526-1532
3. Mayr U, Mayr M, Li C. et al. Loss of p53 accelerates neointimal lesions of vein bypass grafts in mice. Circ Res. 2002;90:197-204
4. Petrofski JA, Hata JA, Gehrig TR. et al. Gene delivery to aortocoronary saphenous vein grafts in a large animal model of intimal hyperplasia. J Thorac Cardiovasc Surg. 2004;127:27-33
5. Alexander JH, Hafley G, Harrington RA. et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446-2454
6. Liu X, Cheng Y, Zhang S. et al. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476-487
7. Cheng Y, Liu X, Yang J. et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158-166
8. Merlet E, Atassi F, Motiani RK. et al. miR-424/322 regulates vascular smooth muscle cell phenotype and neointimal formation in the rat. Cardiovasc Res. 2013;98:458-468
9. Sun Y, Chen D, Cao L. et al. MiR-490-3p modulates the proliferation of vascular smooth muscle cells induced by ox-LDL through targeting PAPP-A. Cardiovasc Res. 2013;100:272-279
10. Cordes KR, Sheehy NT, White MP. et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705-710
11. Ji R, Cheng Y, Yue J. et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579-1588
12. Hutcheson R, Chaplin J, Hutcheson B. et al. miR-21 normalizes vascular smooth muscle proliferation and improves coronary collateral growth in metabolic syndrome. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:4088-4099
13. McDonald RA, Halliday CA, Miller AM. et al. Reducing In-Stent Restenosis: Therapeutic Manipulation of miRNA in Vascular Remodeling and Inflammation. J Am Coll Cardiol. 2015;65:2314-2327
14. Kluiver J, Gibcus JH, Hettinga C. et al. Rapid generation of microRNA sponges for microRNA inhibition. PLoS One. 2012;7:e29275
15. Ebert MS, Sharp PA. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043-2050
16. Gentner B, Schira G, Giustacchini A. et al. Stable knockdown of microRNA in vivo by lentiviral vectors. Nat Methods. 2009;6:63-66
17. Wang XW, He XJ, Lee KC. et al. MicroRNA-221 sponge therapy attenuates neointimal hyperplasia and improves blood flows in vein grafts. Int J Cardiol. 2016;208:79-86
18. Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721-726
19. Bett AJ, Haddara W, Prevec L. et al. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci U S A. 1994;91:8802-8806
20. Brown C, Pan X, Hassid A. Nitric oxide and C-type atrial natriuretic peptide stimulate primary aortic smooth muscle cell migration via a cGMP-dependent mechanism: relationship to microfilament dissociation and altered cell morphology. Circ Res. 1999;84:655-667
21. Miyake T, Aoki M, Morishita R. Inhibition of anastomotic intimal hyperplasia using a chimeric decoy strategy against NFkappaB and E2F in a rabbit model. Cardiovasc Res. 2008;79:706-714
22. Shi C, Patel A, Zhang D. et al. Plasminogen is not required for neointima formation in a mouse model of vein graft stenosis. Circ Res. 1999;84:883-890
23. Handa M, Li W, Morioka K. et al. Adventitial delivery of platelet-derived endothelial cell growth factor gene prevented intimal hyperplasia of vein graft. J Vasc Surg. 2008;48:1566-1574
24. Bhardwaj S, Roy H, Yla-Herttuala S. Gene therapy to prevent occlusion of venous bypass grafts. Expert Rev Cardiovasc Ther. 2008;6:641-652
25. Muto A, Model L, Ziegler K. et al. Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Circ J. 2010;74:1501-1512
26. Maegdefessel L, Azuma J, Toh R. et al. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci Transl Med. 2012;4:122ra122
27. Kluiver J, Slezak-Prochazka I, Smigielska-Czepiel K. et al. Generation of miRNA sponge constructs. Methods. 2012;58:113-117
28. Kikuchi K, McDonald AD, Sasano T. et al. Targeted modification of atrial electrophysiology by homogeneous transmural atrial gene transfer. Circulation. 2005;111:264-270
29. March KL, Madison JE, Trapnell BC. Pharmacokinetics of adenoviral vector-mediated gene delivery to vascular smooth muscle cells: modulation by poloxamer 407 and implications for cardiovascular gene therapy. Hum Gene Ther. 1995;6:41-53
30. Cooley BC, Nevado J, Mellad J. et al. TGF-beta signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med. 2014;6:227ra234
31. Kumarswamy R, Volkmann I, Jazbutyte V. et al. Transforming growth factor-beta-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol. 2012;32:361-369
32. McGeachie J, Campbell P, Prendergast F. Vein to artery grafts. A quantitative study of revascularization by vasa vasorum and its relationship to intimal hyperplasia. Ann Surg. 1981:100-107
33. Thomas AC. Animal models for studying vein graft failure and therapeutic interventions. Curr Opin Pharmacol. 2012;12:121-126
34. Sterpetti AV, Cucina A, Lepidi S. et al. Formation of myointimal hyperplasia and cytokine production in experimental vein grafts. Surgery. 1998;123:461-469
35. Schachner T, Laufer G, Bonatti J. In vivo (animal) models of vein graft disease. Eur J Cardiothorac Surg. 2006;30:451-463
Author contact
![]() Corresponding authors: Chun Huang, M.D, PhD, Associate Professor, Department of Cardiothoracic Surgery, The First Affiliated Hospital of Chongqing Medical University, NO. 001 Youyi Road, Chongqing, 400016, China Phone: +86-23-89011132; Fax number: +86-23-68811487 Email addresses: hcsurgerycom Qing-Chen Wu, MD, Professor, Department of Cardiothoracic Surgery, The First Affiliated Hospital of Chongqing Medical University, NO. 001 Youyi Road, Chongqing, 400016, China Email addresses: cqmusurgerycom
Corresponding authors: Chun Huang, M.D, PhD, Associate Professor, Department of Cardiothoracic Surgery, The First Affiliated Hospital of Chongqing Medical University, NO. 001 Youyi Road, Chongqing, 400016, China Phone: +86-23-89011132; Fax number: +86-23-68811487 Email addresses: hcsurgerycom Qing-Chen Wu, MD, Professor, Department of Cardiothoracic Surgery, The First Affiliated Hospital of Chongqing Medical University, NO. 001 Youyi Road, Chongqing, 400016, China Email addresses: cqmusurgerycom

 Global reach, higher impact
Global reach, higher impact