10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2018; 14(6):608-615. doi:10.7150/ijbs.22763 This issue Cite
Research Paper
Screening of multimeric β-xylosidases from the gut microbiome of a higher termite, Globitermes brachycerastes
1. College of Plant Protection, Hunan Agricultural University, Changsha, 410128, China;
2. Institute of Plant Physiology and Ecology, Chinese Academy of Sciences, Shanghai, 200032, China.
3. Department of Entomology, University of Kentucky, Lexington, KY, 40546-0091, USA.
* These authors contributed equally to this work.
Abstract
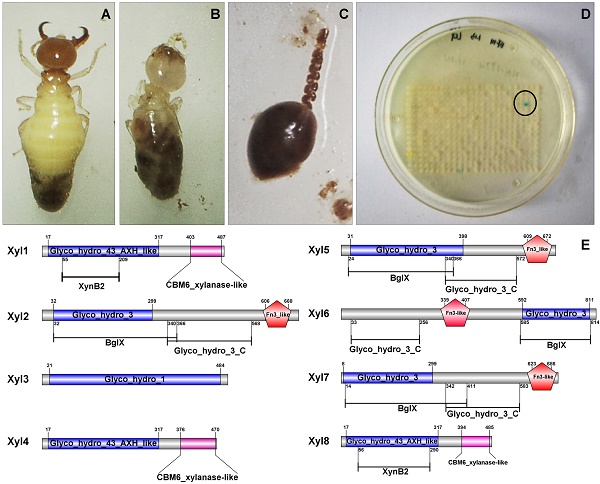
Termite gut microbiome is a rich reservoir for glycoside hydrolases, a suite of enzymes critical for the degradation of lignocellulosic biomass. To search for hemicellulases, we screened 12,000 clones from a fosmid gut library of a higher termite, Globitermes brachycerastes. As a common Southeastern Asian genus, Globitermes distributes predominantly in tropical rain forests and relies on the lignocellulases from themselves and bacterial symbionts to digest wood. In total, 22 positive clones with β-xylosidase activity were isolated, in which 11 representing different restriction fragment length polymorphism (RFLP) patterns were pooled and subjected to 454 pyrosequencing. As a result, eight putative β-xylosidases were cloned and heterologously expressed in Escherichia coli BL21 competent cells. After purification using Ni-NTA affinity chromatography, recombinant G. brachycerastes symbiotic β-xylosidases were characterized enzymatically, including their pH and temperature optimum. In addition to β-xylosidase activity, four of them also exhibited either β-glucosidase or α-arabinosidases activities, suggesting the existence of bifunctional hemicellulases in the gut microbiome of G. brachycerastes. In comparison to multimeric protein engineering, the involvement of naturally occurring multifunctional biocatalysts streamlines the genetic modification procedures and simplifies the overall production processes. Alternatively, these multimeric enzymes could serve as the substitutes for β-glucosidase, β-xylosidase and α-arabinosidase to facilitate a wide range of industrial applications, including food processing, animal feed, environment and waste management, and biomass conversion.
Keywords: Globitermes brachycerastes, gut microbiome, fosmid library, glycoside hydrolases, β-xylosidase, multimeric hemicellulase

 Global reach, higher impact
Global reach, higher impact