10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2020; 16(8):1324-1334. doi:10.7150/ijbs.40551 This issue Cite
Research Paper
MSTN Mutant Promotes Myogenic Differentiation by Increasing Demethylase TET1 Expression via the SMAD2/SMAD3 Pathway
1. State Key Laboratory of Reproductive Regulation & Breeding of Grassland Livestock, Inner Mongolia University, Hohhot, 010070, China
2. School of Life Science, Inner Mongolia University, Hohhot, 010070, China
Abstract
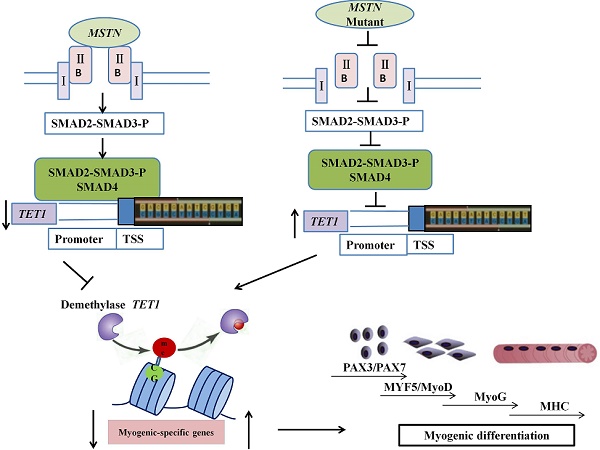
Myostatin (MSTN) is mostly expressed in skeletal muscle and plays crucial roles in the negative regulation of muscle mass development. The methylation and demethylation of myogenesis-specific genes are major regulatory factors in muscle satellite cell differentiation. The present study was designed to investigate the mechanism of myogenic differentiation regulated by MSTN mutation (MT) and the methylation/demethylation state of downstream genes. The results showed that, in the MSTN-/+ satellite cells, a higher myotube fusion index and a larger myotube length were observed compared to the wild type controls; the genes associated with myogenesis were all up-regulated compared to the WT controls. The methylation of the promoters and gene bodies of PAX3, PAX7, MyoD, and MyoG were all down-regulated, while the expression of the key demethylase TET1 was significantly promoted. ChIP-qPCR was used to demonstrate that the SMAD2/SMAD3 complex combined with the promoter of TET1 to inhibit the activity of TET1 promoter, indicating that MSTN may regulate TET1 via SMAD2/SMAD3. The overexpression of TET1 in wild type cells promoted myogenic differentiation, increased the myotube index, and reduced the methylation of the associated genes. On the contrary, the knockdown of TET1 in the MSTN mutant cells resulted in the opposite phenomena as in the overexpressed cells. In conclusion, the myostatin mutant showed an increased transcriptional activity of TET1, inducing higher levels of demethylation and improving the transcriptional activity levels of myogenic differentiation-associated genes. The binding of SMAD2/SMAD3 directly to the TET1 promoter region indicated that the MSTN mutant demethylated the myogenesis-specific genes by up-regulating TET1, which is directly controlled by SMAD2/SMAD3.
Keywords: MSTN mutant, myogenic differentiation, ten-eleven translocation methylcytosine dioxygenase 1 (TET1), SMAD2/SMAD3, DNA methylation

 Global reach, higher impact
Global reach, higher impact