ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2022; 18(1):426-440. doi:10.7150/ijbs.64640 This issue Cite
Research Paper
Engineering of human mesenchymal stem cells resistant to multiple natural killer subtypes
1. Center of Reproduction, Development & Aging, and Institute of Translational Medicine, Faculty of Health Sciences, University of Macau, Taipa, Macau, China.
2. Ministry of Education Frontiers Science Center for Precision Oncology, University of Macau, Taipa, Macau, China.
3. School of Life Science and Technology, ShanghaiTech University, Shanghai, China.
4. The Shenzhen Key Laboratory of Health Sciences and Technology, International Graduate School at Shenzhen, Tsinghua University, Shenzhen, China.
Abstract
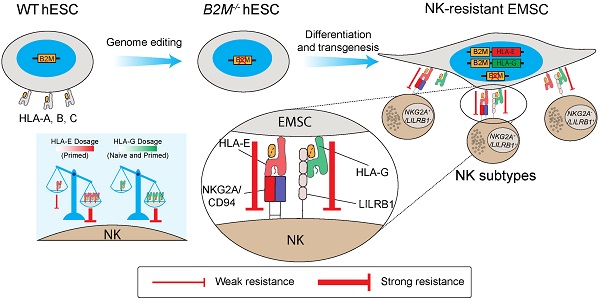
Mesenchymal stem cells (MSCs) as a therapeutic promise are often quickly cleared by innate immune cells of the host including natural killer (NK) cells. Efforts have been made to generate immune-escaping human embryonic stem cells (hESCs) where T cell immunity is evaded by defecting β-2-microglobulin (B2M), a common unit for human leukocyte antigen (HLA) class I, and NK cells are inhibited via ectopic expression of HLA-E or -G. However, NK subtypes vary among recipients and even at different pathologic statuses. It is necessary to dissect and optimize the efficacy of the immune-escaping cells against NK subtypes. Here, we first generated B2M knockout hESCs and differentiated them to MSCs (EMSCs) and found that NK resistance occurred with B2M-/- EMSCs expressing HLA-E and -G only when they were transduced via an inducible lentiviral system in a dose-dependent manner but not when they were inserted into a safe harbor. HLA-E and -G expressed at high levels together in transduced EMSCs inhibited three major NK subtypes, including NKG2A+/LILRB1+, NKG2A+/LILRB1-, and NKG2A-/LILRB1+, which was further potentiated by IFN-γ priming. Thus, this study engineers MSCs with resistance to multiple NK subtypes and underscores that dosage matters when a transgene is used to confer a novel effect to host cells, especially for therapeutic cells to evade immune rejection.
Keywords: Human embryonic stem cells, mesenchymal stem cells, natural killer cells, innate immunity, immune rejection

 Global reach, higher impact
Global reach, higher impact