10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(9):3845-3858. doi:10.7150/ijbs.70958 This issue Cite
Review
VEGF/VEGFR-Targeted Therapy and Immunotherapy in Non-small Cell Lung Cancer: Targeting the Tumor Microenvironment
1. Laboratory of Molecular Pharmacology, Department of Pharmacology, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan 646000, China
2. Department of Oncology, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan 646000, China
3. Cell Therapy & Cell Drugs of Luzhou Key Laboratory, Luzhou, Sichuan 646000, China
4. South Sichuan Institute of Translational Medicine, Luzhou, Sichuan 646000, China
5. Department of Oncology and Hematology, The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Luzhou, Sichuan 646000, China
6. Center of Excellence for Molecular Imaging (CEMI), Department of Radiologic Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand
# These authors contributed equally to this work.
Received 2022-1-12; Accepted 2022-4-23; Published 2022-5-29
Abstract
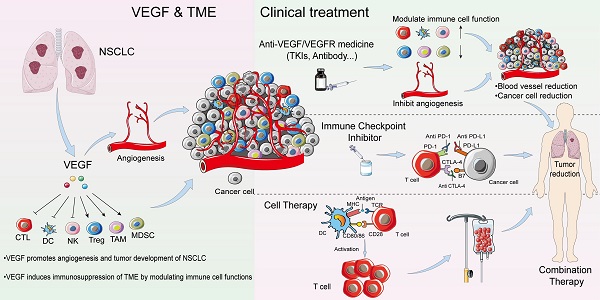
Non-small cell lung cancer (NSCLC) is the leading cause of death by cancer worldwide. Despite developments in therapeutic approaches for the past few decades, the 5-year survival rate of patients with NSCLC remains low. NSCLC tumor is a complex, heterogeneous microenvironment, comprising blood vessels, cancer cells, immune cells, and stroma cells. Vascular endothelial growth factors (VEGFs) are a major mediator to induce tumor microvasculature and are associated with the progression, recurrence, and metastasis of NSCLC. Current treatment medicines targeting VEGF/VEGF receptor (VEGFR) pathway, including neutralizing antibodies to VEGF or VEGFR and receptor tyrosine kinase inhibitors, have shown good treatment efficacy in patients with NSCLC. VEGF is not only an important angiogenic factor but also an immunomodulator of tumor microenvironment (TME). VEGFs can suppress antigen presentation, stimulate activity of regulatory T (Treg) cells, and tumor-associated macrophages, which in turn promote an immune suppressive microenvironment in NSCLC. The present review focuses on the angiogenic and non-angiogenic functions of VEGF in NSCLC, especially the interaction between VEGF and the cellular components of the TME. Additionally, we discuss recent preclinical and clinical studies to explore VEGF/VEGFR-targeted compounds and immunotherapy as novel approaches targeting the TME for the treatment of NSCLC.
Keywords: NSCLC, tumor microenvironment, VEGF/VEGFR pathway, immunotherapy, clinical trials
1. Introduction
Lung cancer is a leading cause of cancer-related death, and non-small cell lung cancer (NSCLC) is the most common type of lung cancer [1]. The prognosis of NSCLC remains poor, and the overall recovery and survival rates of patients remain low, as most patients with NSCLC have advanced cancer or extensive metastases before diagnosis [2-4]. The tumor microenvironment (TME) of NSCLC is complex, comprising various types of immune cells and vasculature. Vascular endothelial growth factor (VEGF) is mainly secreted by the endothelial cells of blood vessels; however, it can also be generated by the immune cells of the TME, such as tumor-associated macrophages (TAM) [5], tumor-associated neutrophils (TAN) [6, 7], mast cells (MC) [8-10], myeloid-derived suppressor cells (MDSC) [11-13], and natural killer cells (NK) [14, 15]. The VEGF is not only linked with multiple functions in angiogenesis but also suppresses immune cells and promotes local and systemic immunosuppression in cancer. In recent years, owing to the successful development of immune checkpoint inhibitors, vaccines, VEGF/VEGF receptor (VEGFR)-targeting compounds, and other immunotherapeutic agents for cancer treatment, a new approach to treat various tumors, which combines anti-angiogenic drugs with immunotherapy, has emerged [16]. Emerging evidence demonstrates potential synergistic efficiency between VEGF/VEGFR-targeting compounds and immunotherapy for NSCLC treatment. In the present review, we focus on the angiogenic and non-angiogenic functions of VEGF in NSCLC, in particular, on the interaction between VEGF and the cellular components of the TME. In addition, we discuss recent preclinical and clinical studies to explore VEGF/VEGFR-targeted compounds and immunotherapy as novel approaches targeting the TME for NSCLC treatment.
2. Functions of VEGF/VEGFR in NSCLC
2.1 Angiogenic function: tumor vasculature
VEGF is mainly secreted by tumor cells, some stromal cells, and endothelial cells in the TME [17]. The VEGF family comprises several members, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, placental growth factor (PLGF), and endocrine gland-derived VEGF (EG-VEGF) [17, 18].
The members of the VEGF family perform their functions by binding with their receptors. VEGF receptors are categorized into two types: tyrosine kinase receptors (VEGF receptors, VEGFR), which include VEGFR-1, VEGFR-2, and VEGFR-3, and neuropilin receptors (NRPs), which include NRP-1 and NRP-2 [19, 20]. NRPs are the co-receptors of VEGF; the binding of VEGF and NRPs enhances the stability of the receptor complex [19].
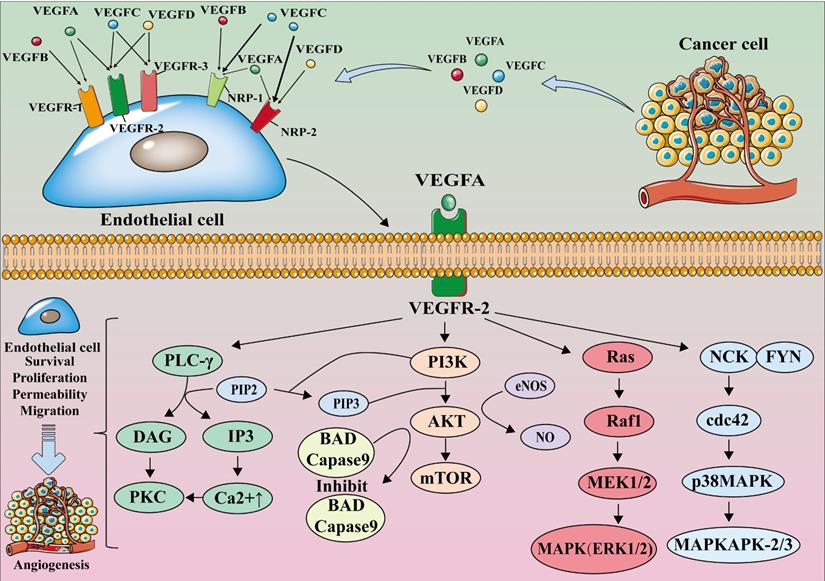
The VEGF family members selectively bind to VEGFR. VEGF-A is the major member of the VEGF family for angiogenesis; it is expressed in all vascular tissues, macrophages, tumor cells, and other cells [21, 22]. Moreover, it can bind to both VEGFR-1 and VEGFR-2, but it primarily binds to the latter for dimerization, autophosphorylation, and activation, thereby becoming crucial in downstream signaling, causing the proliferation and migration of endothelial cell, and performing angiogenic functions. [23-25].
VEGF-A binds to VEGFR-2 and activates phospholipase C γ (PLC-γ). Activated PLC-γ can hydrolyze the membrane component phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) to produce inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 induces intracellular Ca2+ release and improves vascular permeability. Ca2+ causes protein kinase C (PKC) to bind and polymerize to the plasma membrane, which is activated by DAG; PKC can also act as an upstream activator of the Raf1-MEK1/2-ERK1/2 pathway, which is central to endothelial cell proliferation [26]. The binding of VEGF-A to VEGFR-2 also phosphorylates phosphoinositide 3-Kinase (PI3K), and the activated PI3K binds to the substrate PIP2 converting it to phosphatidylinositol 3-phosphate (PIP3). PIP3 induces serine/threonine-specific protein kinase (AKT) phosphorylation, which activates endothelial nitric oxide synthase (eNOS) to produce nitric oxide (NO) and induces endothelial cell proliferation and migration [27, 28] (Figure 1).
VEGF-B mainly binds to VEGFR-1 and NRP-1, and plays an important role in tumor angiogenesis and in the improvement of ischemic conditions [29, 30]. VEGF-C and VEGF-D mainly bind to VEGFR-3 and participate in lymphangiogenesis [30, 31]. VEGF-D is associated with tumor metastasis to regional lymph nodes [32-34]. Furthermore, PIGF mainly binds to VEGFR-1 and regulates the growth and maturation of blood vessels by regulating endothelial cell and parietal cell proliferation [35] (Figure 1).
2.2 Non-angiogenic function: role of VEGF in the functions of TME cell components in NSCLC
2.2.1 VEGF and cancer cells
Evidence suggests that VEGF acts in tumors not only by promoting angiogenesis but also by directly working on cancer cells [36]. VEGF can promote tumor development and progression by interacting with receptors expressed on tumor cells through autocrine and/or paracrine mechanisms [37]. In addition to tyrosine kinases, NRPs can regulate the function and transportation of growth factor receptors and integrins, thus playing a crucial role in mediating VEGF action on tumor cells [37]. Autocrine VEGF in lung cancer has been shown to activate mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) and PI3K/AKT signaling pathways to promote cell proliferation in NSCLC; additionally, NRP1 plays a central role in regulating VEGF-driven NSCLC cell proliferation [38]. However, studies showed that blocking endogenous VEGF with bevacizumab, the VEGF antibody, did not inhibit NSCLC cell line growth, suggesting that VEGF alone does not maintain lung cancer cell proliferation in vitro [39, 40]. We believe that the development of NSCLC is caused by a combination of factors in the TME. Therefore, in a single cell line without tumor angiogenesis or TME, blocking VEGF alone does not effectively inhibit tumor cell growth. it is likely that VEGF-VEGFR-targeted therapy also acts on the TME to reverse the immunosuppression therein, thus inhibiting tumor growth. Therefore, we infer that combining immunotherapy with VEGF-VEGFR-targeted therapy may exert better therapeutic effects on NSCLC.
The VEGF-VEGFR signaling pathway. VEGF-A binds to VEGFR-2 for dimerization, autophosphorylation, and activation leading to endothelial cell survival, proliferation, permeability, and migration. mTOR, mammalian target of rapamycin; MAPK, mitogen-activated protein kinase; MEK, MAP kinase kinase.
2.2.2 VEGF and T cells
Tumor cells evade immune defence by suppressing T cell function, for example, by upregulating the expression levels of T cell checkpoints [41, 42]. VEGF-A enhances the expression of PD-1 and other suppressive checkpoints, such as CTLA-4, on the surface of T cells, and suppresses the activity of CD8+ T cell, manifesting as a progressive state of dysfunction leading to the blocking of the effector function of T cells [43-45]. The inhibitory functions of checkpoints could be reversed by anti-angiogenic agents, as when VEGF is blocked, tumor blood vessels decrease and tumor tissue hypoxia is induced, which in turn activates hypoxia-inducible factor (HIF-1α), and promotes cytokine production to activate CD8+ T cells [43-45]. However, recently, studies have reported that tumor hypoxia, angiogenesis, and immunosuppression can modulate each other, thus promoting tumor progression and compromising the clinical effectiveness of antitumor therapy [46].
Furthermore, VEGF can directly inhibit T cell function. Continuous infusion of recombinant VEGF in non-tumor patients resulted in a decrease in the number of T cells and the ratio of T to B cells in lymph nodes and spleens [47]. Additionally, recombinant VEGF-infused mice showed smaller thymus compared with control group mice infused with phosphate-buffered saline, indicating the immune suppressing quality of VEGF [48]. In another study, inhibiting VEGF activity by endostatin, a VEGF specific antibody, enhanced infiltration of mature CD8+ T cells and reduced the number of immunosuppressive cells in tumors [49]. VEGF-A directly inhibits T cell proliferation and cytotoxic activity through VEGFR-2 in advanced ovarian cancer [50]. In addition, VEGF-A has been shown to induce the expression of the transcription factor thymocyte selection-associated high mobility group box (TOX) in T cells, which drives a depletion-specific transcriptional program in T cells. In the case of PD-1 blockade resistance, combined blockade of PD-1 and VEGF-A activities restores the anti-tumor function of T cells, resulting in better tumor control [51] (Figure 2A).
In addition to directly regulating T cell function, VEGF also can suppress T cell function by regulating Fas ligand (FasL) levels, which is upregulated by VEGF-A in the TME [52, 53]. FasL is expressed on the surface of T cells and in tumor endothelium; however, it is not seen in a normal vascular system [48, 54]. In human tumors, the expression of FasL in endothelial cells leads to the loss of CD8+ T cells [48, 54]. In mice, endothelium-secreted FasL suppresses the infiltration of T cells in tumors, thus promoting tumor growth [48, 54] (Figure 2A).
2.2.3 VEGF and Treg
Treg cells are a major subset of CD4+ T cells. The characteristic phenotype of Treg cells is CD25+ CD4+ FoxP3+ T cell [55, 56]. Some preclinical and clinical studies have shown that Treg cells are one of the most common immunosuppressive cell types in tumors [57, 58]. They hinder immune surveillance against cancer in healthy individuals, prevent patients with tumors from producing effective anti-tumor immunity, and cause the occurrence and development of various malignant tumors, including NSCLC [59]. Treg cells negatively regulate immune response through two mechanisms: (1) by direct contact to inhibit the activation of target cells, such as directly up-regulating the expression of CTLA-4 through FoxP3+, thus inhibiting the expression of IL-2, lymphocyte activating protein 3 (LAG-3), CD39/73, and NRP-1 to induce immunosuppression [60, 61], and (2) through a humoral and cytokine secretion mechanism, which involves inducing the production of soluble immunosuppressive molecules, such as IL-10, IL-35, transforming growth factor-β (TGF-β), adenosine, prostaglandin E-2 (PGE-2), or galactose lectin-1 (Gl-1) [60, 61].
The effect of VEGF on CTL, Treg, and TAM. A) VEGF-A enhances the expression of PD-1 and FasL on CTL, thereby promoting CTL cell failure and leading to immunosuppression. B) VEGF-A can make Treg recruited in the tumor microenvironment and increase the number of Treg. VEGF-A can induce Treg differentiation by producing immature DC. Treg cells inhibit the activation of target cells through FoxP3+ direct contact; up-regulate the expression of CTLA-4; inhibit the expression of IL-2, LAG-3, CD39/73, and NRP-1; or cause immunosuppression by producing soluble immunosuppressive molecules. C) Under the action of VEGF-A, monocytes/macrophages in the blood are gathered around tumor cells and differentiate into TAM. The M2 cells in TAM can promote tumorigenesis and development.
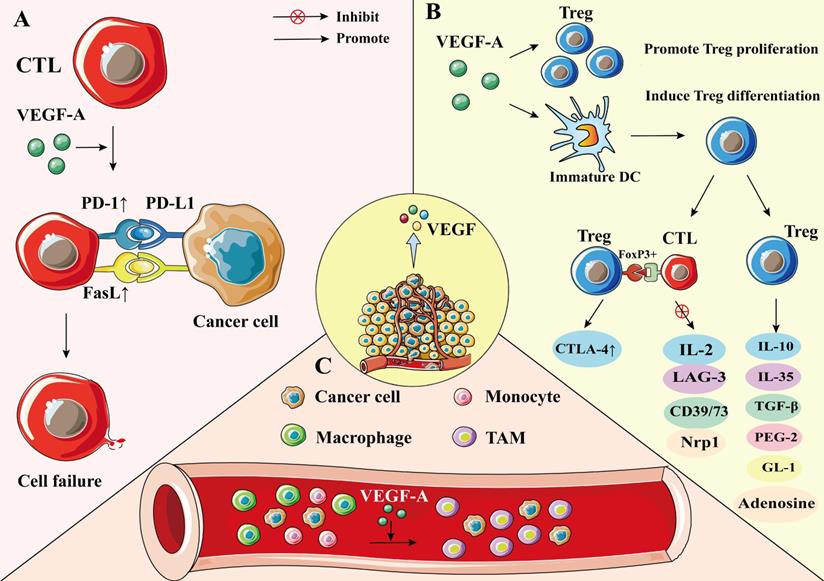
In cancer patients, VEGF-A expression was positively correlated with levels of intratumoral Tregs [62]. VEGF-A can promote Treg differentiation by inducing immature dendritic cell (DC) numbers [50]. Additionally, VEGF-A can directly regulate Treg recruitment in the TME by binding with VEGFR2, which in turn stimulates the proliferation of Treg cells, and enhances the immunosuppressive function [50, 63, 64]. Terme et al. reported that tumor-bearing mice show higher expression levels of VEGFR-2 on Treg cells than healthy mice, and that neutralizing VEGFR-2 by specific antibodies can reduce the proportion of Tregs in the spleen of nude mice, indicating that VEGFR-2 signaling plays an important role in regulating Treg functions [65]. Furthermore, VEGF-A promotes the infiltration of Treg cells in the TME by binding to NRP-1 [66]. When the NRP-1 gene was knocked down in T cells, the number of Treg cells decreased, whereas the number of CD8+ T cells increased (Figure 2B) [66].
2.2.4 VEGF and TAM
TAMs are dynamic cells with multiple polarization states; they are important mediators of cancer development and progression [67]. TAMs are present in all stages of tumor development, making them the most abundant immune cells in the TEM [68]. There are two types of TAMs, M1 and M2 phenotypes. The M1 phenotype exhibits antitumor effects, whereas M2 is involved in promoting tumor progression [69]. TAMs cause immunosuppression by producing cytokines, chemokines, growth factors, and triggering the release of suppressive immune checkpoint proteins in T cells [70]. Hwang et al. showed that M2 TAM significantly enhanced the expression levels of VEGF-A and VEGF-C of NSCLC cells, whereas M1 TAM only upregulated the expression levels of VEGF-A in NSCLC cells, indicating that TAMs are significantly associated with vascular and lymph angiogenesis, which in turn promotes the progression of NSCLC [5]. Additionally, TAM receptors, including Tyro3, Axl, and Mark, bias macrophage polarization toward a pro-tumor M2-like phenotype. These receptors are promising therapeutic targets for tumor-associated macrophages [71].
TAM of the TME is mainly differentiated from bone marrow-derived blood monocytes and monocytic MDSC under the stimulation of tumor cell-secreted cytokines, including VEGF-A, IL-4, and IL-10 [68, 72]. VEGF-A recruits TAM mainly by binding to VEGFR-1 on the TAM surface [50]. TAM expresses PD-L1, which inhibits T cell receptor signaling upon binding to PD-1, leading to T cell inactivation [50]. Moreover, M2 macrophages can also release VEGFA, VEGFC, and other growth factors, which may in turn promote cancer progression [70, 73] (Figure 2C).
2.2.5 VEGF and dendritic cells
DCs are antigen-presenting cells and have the strongest antigen presentation ability in vivo [74]. DCs can produce cytokines and stimulate the differentiation of effector T and NK cells [75, 76]. Therefore, DC dysfunction is one of the mechanisms leading to anti-tumor immunodeficiency.
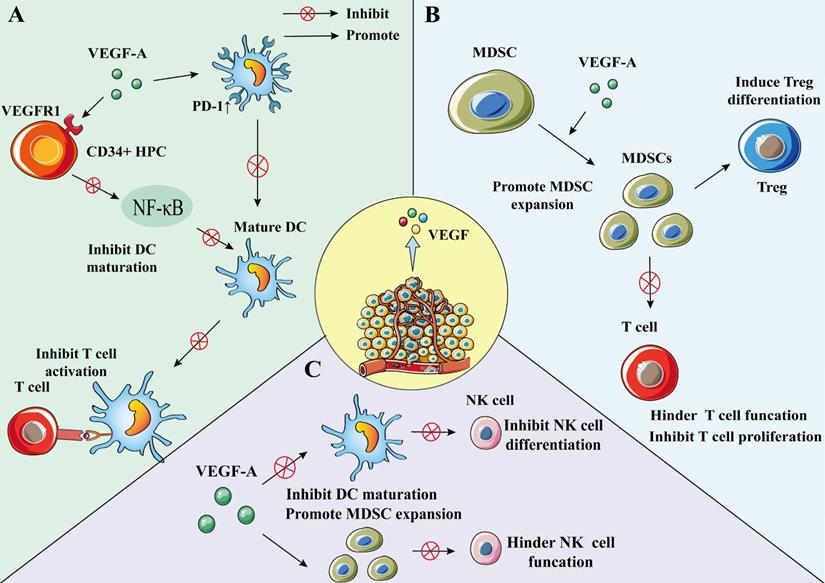
DC can be differentiated from the early stage of hematopoietic progenitor cell (HPC) [77]; however, this process may be regulated by VEGF-A [78]. Through VEGFR-1, VEGF-A can bind to HPC CD34+ cells and inhibit nuclear factor-κB (NF-κB), which is the activator of transcription factors in these cells, thereby inhibiting the differentiation and maturation of DC [78, 79]. VEGF can also inhibit DC function by up-regulating PD-1 [80]. The inhibiton of DC maturation reduces T cell tumor infiltration and exerts immunosuppressive effect [80]. Recent findings suggest that VEGF can impair mature DCs' migration ability and immune function through VEGFR-2-mediated RhoA-cofilin1 pathway [81].
Increased immature DCs in cancer patients are associated with increased VEGF levels, which are involved in mediating DC dysfunction [78]. Bevacizumab can affect the maturation and function of DCs in vivo by slightly increasing DC numbers and significantly reducing immature myeloid cell numbers [82]. Additionally, bevacizumab can reverse the inhibition of VEGF-induced monocyte differentiation into DC in vitro [83].
An investigation of the relationship between VEGF and DC cells using a mouse model found that recombinant VEGF significantly altered DC growth and development at relevant concentrations, with a reduction in the proportion of mature DCs in lymph nodes and spleens of mice [47].
Furthermore, the results of a clinical study evaluating the relationship between DC infiltration and VEGF expression in NSCLC (132 primary NSCLC treated with surgery) showed that the mean number of infiltrating DCs in the VEGF high expression group was lower than that in the low expression group [84], indicating that VEGF may regulate the infiltration of DC into NSCLC tumor (Figure 3A).
The effect of VEGF on DC, MDSC, and NK cell. A) VEGF-A can bind to VEGFR1 on CD34+ HPC, inhibit NF-κB, an activator of transcription factors in these cells, and inhibit the differentiation and maturation of DC. VEGF-A can also increase the expression of PD-1 in DC, resulting in a decrease in the number of DCs. To inhibit the activation of T cells by mature DCs. Lead to immunosuppression. B) VEGF-A can promote the expansion of MDSC, and MDSC can inhibit the proliferation of tumor-specific T cells and promote the development of Treg. C) VEGF-A can inhibit the differentiation of NK cells by inhibiting DC maturation. VEGF can also increase the number of MDSCs and inhibit the function of NK cells.
2.2.6 VEGF and MDSC
MDSC is a heterogeneous population comprising immature myeloid cells, which are precursor cells of macrophages, DC, or granulocytes features [85]. MDSCs are characterized by bone marrow origin, immaturity, and suppression of immune response [85]. They can promote tumor cell survival, angiogenesis, tumor cell invading, and metastases [11, 86]. Additionally, MDSC induces immune tolerance and can suppress effector T cells and NK cells to induce immune responses [85, 87]. Moreover, MDSC could inhibit the growth of tumor-specific T cells and promote the development of Treg cells, which plays a pivotal role in immunosuppression and immune escape [86, 88, 89]. MDSCs are also found to be involved in the differentiation of Treg cells. In cancer patients, an increase in MDSC in peripheral blood causes a decrease in mature DCs [90]. Many studies have reported that MDSCs play an important role in mediating a variety of tumor-related immunosuppressive functions and tumor immune escape, including NSCLC [89, 91].
VEGF-A is a factor that can promote the amplification of MDSCs; the use of bevacizumab can inhibit VEGF function and MDSC proliferation [50, 92]. Research on the effect of lactones on immune cells in the TME showed that MDSC proportion reduced in both the low- and high-dose endostatin groups compared to the control group [49] (Figure 3B).
2.2.7 VEGF and natural killer cells
NK cells are a subgroup of cytotoxic innate lymphoid cells in the innate immune system and have a unique killing effect on tumor cells [93]. VEGF can inhibit the differentiation of NK cells by inhibiting DC maturation [78, 94]. Furthermore, VEGF can increase the number of MDSCs and inhibit NK cell function, in turn leading to the phenomenon of immune escape [89, 95, 96].
Studies have shown that NK cells can secrete VEGF-A under hypoxic conditions, which is a characteristic of the TME [97]. Under such hypoxic conditions, VEGF secretion is a transient phenomenon because when NK cells return to peripheral blood, this phenomenon can be reversed [97, 98] (Figure 3C).
In summary, VEGF not only promotes tumor growth by promoting angiogenesis but also acts on various immune cells in the TME, which leads to immunosuppression. Therefore, in treating NSCLC, the selection of VEGF-VEGFR-targeted drugs can inhibit tumors from two aspects.
3. VEGF-targeted therapy and immunotherapy for NSCLC
3.1 VEGF/VEGFR inhibitors and antibodies
VEGF is overexpressed in NSCLC; the expression levels are higher in the tumorous than the surrounding normal lung tissue [99]. The high expression of VEGF is related to tumor recurrence, low survival rate, metastasis, and death [99, 100]. VEGF is essential for tumor growth and immunosuppression. Therefore, targeted drugs that inhibit the VEGF pathway, such as anti-VEGF monoclonal antibodies and tyrosine kinase inhibitors (TKIs), are used for NSCLC treatment.
Over the past few decades, several VEGF inhibitors have been approved for NSCLC treatment. Among them, bevacizumab, a humanized monoclonal immunoglobulin G 1 (IgG1) antibody that can bind to VEGF-A, has shown good efficacy in treating NSCLC [101]. Studies show that chemotherapy combined with bevacizumab prolongs progression-free survival (PFS) and overall survival (OS) in patients with NSCLC compared to chemotherapy alone [23]. In addition to combination therapy with chemotherapeutics, bevacizumab has also shown encouraging effects in combination with immunotherapy [80]. Data from several clinical trials involving the combination of several immune checkpoint inhibitors and bevacizumab show that when combined with immunotherapy, bevacizumab can prolong survival rate [80]. Bevacizumab is also approved by the European Medicines Agency and the United States Food and Drug Administration as first-line treatment for advanced, metastatic, or recurrent NSCLC.
Another VEGF targeting antibody, ramucirumab, is a complete human IgG1 antibody, which can block the interaction between VEGFR-2 and VEGF ligand [102, 103]. Ramucirumab functions by inhibiting its signaling pathway by selectively binding to VEGFR-2 [104]. Additionally, it blocks the activation of VEGFR-2 by ligands other than VEGF-A, including VEGF-C and VEGF-D [105].
Nintedanib is a small molecule TKI [106]. In a phase III clinical study, the differences between patients with NSCLC treated with docetaxel with and without nintedanib were evaluated. PFS was significantly longer in patients treated with nintedanib than those in the placebo group [107]. Moreover, the OS of docetaxel plus nintedanib was significantly longer than that of docetaxel plus placebo [107].
Furthermore, some other TKIs targeting the VEGF receptor pathway have been tested in clinical trials. For example, lorlatinib has shown clinical activity in patients with advanced NSCLC [108]. Another phase II trial showed significantly longer PFS in some patients with NSCLC treated with fruquintinib [109].
3.2 Immune checkpoint inhibitor
The immune checkpoint is an immunomodulatory protein that can cause immunosuppression. Antibodies that block the receptors of cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death protein (PD-1) or its ligand PD-L1 have been approved for clinical use [110]. PD-1 and PD-L1 are mainly expressed in immune cells, such as NK cells, DC, CD4+, and CD8+ T cells [111-113]. PD-1 interacts with its ligand PD-L1 to inhibit T cell activation and proliferation, leading to immune escape [114]. Significant correlations were observed between the expression levels of PD-L1 and the expression levels of angiogenic factors, such as VEGFA and HIF-1α [115]. CTLA-4 is an extracellular surface protein that can control immune suppression, mainly by stimulating T cell receptors [116].
The immune checkpoint inhibitors currently approved as first-line therapy fall into three main categories: Anti-PD-1, Anti-PD-L1, and Anti-CTLA-4. The main inhibitors used for first-line treatment of NSCLC include pembrolizumab [117-119], nivolumab [120], toripalimab [121], sintilimab (Anti-PD-1) [122], atezolizumab [26, 123], durvalumab (Anti-PD-L1) [124, 125], and lpilimumab (Anti-CTLA-4) [120, 126].
VEGF-A inhibits immune activation and induces immunosuppression by affecting various immune cells in the TME. Therefore, immune escape can be suppressed by inhibiting the effect of VEGF, and then combining immune checkpoint inhibitors to treat NSCLC. First, anti-angiogenic drugs can normalize tumor blood vessels and cause tumor immune cells (such as tumor-infiltrating lymphocytes) to increase in NSCLC. Immune checkpoint inhibitor can relieve the inhibition of PD-1 and PD-L1 on T cells, and the synergistic effect of the two shows better therapeutic effects on solid tumors [127]. A clinical trial of anti-VEGF drugs combined with immune checkpoint inhibitors is currently underway (NCT04340882).
3.3 Vaccines and cell therapy
Cancer vaccines are an active immunotherapeutic intervention against malignancies. Their intended function is to bypass immune tolerance in the TME, thereby suppressing tumor survival. A new antigenic vaccine reported for the treatment of NSCLC combines NEO-PV-01 with PD-1 blockade; it induces T cells with cell-killing effects and has the ability to translocate to tumors [128]. A Phase II trial showed the benefit of TG4010 (MVA-MUC1-IL2) vaccine in combination with first-line chemotherapy for advanced NSCLC [129].
Additionally, a clinical trial on the safety and feasibility of CRISPR-edited T cells in patients with refractory NSCLC has been completed [130]. Another type of CAR-T cell therapy involves reprogramming the patient's T cells and infusing the modified T cells into the patient's body to attack the cancer cells. However, compared to malignant blood diseases, CAR-T cells have shown limited success in treating solid tumors, including NSCLC [131]. Floris Dammeijer et al. found that tumor vaccines and cellular immunotherapy improved OS and PFS in patients with NSCLC, and that cellular immunotherapy was more effective than tumor vaccines [132]. Combining VEGF-VEGFR-targeted therapy with a cancer vaccine to bypass immune tolerance in the TME may surpass the expectations of its effectiveness. Nevertheless, more preclinical and clinical studies will be needed to demonstrate this in the future.
3.4 Combining VEGF/VEGFR inhibitors and immunotherapy for NSCLC treatment
3.4.1 Preclinical studies
A preclinical study in a human lung adenocarcinoma xenograft model showed that bevacizumab improves the antitumor effect of cytokine-induced killer (CIK) cell transfer therapy [133]. The CIK cells in the single treatment group showed no significant antitumor activity compared to the control group, while bevacizumab inhibited tumor growth and reached statistical significance [133]. CIK cell therapy combined with VEGF inhibitor therapy showed significant antitumor activity compared to other groups [133]. Similar studies in mouse models have also demonstrated that recombinant human endonuclease improves the therapeutic effect of employing CIK cells in lung cancer, revealing the mechanism of the antitumor effect of VEGF inhibitors in combination with immune cell therapy [134].
A study evaluating the synergistic effect of small-molecule tyrosine kinase inhibitor (bosutinib) and tumor vaccine showed that cabozantinib treatment combined with therapeutic tumor vaccine improved the proliferation and function of T cells [135]. Tregs from mice treated with cabozantinib alone had a significantly lower ability to regulate CD4+ T cell proliferation than Tregs from control mice [135]. Moreover, the regulatory ability of Tregs in mice treated with tumor vaccine alone reduced significantly [135]. Surprisingly, the combination of cabozantinib and tumor vaccine application eliminated Treg function, as the CD4+ T cells of the mice with Treg were not significantly different from those of mice without Treg [135]. Additionally, mice treated with cabozantinib combined with tumor vaccines showed a significant increase in both CD3+ lymphocyte and CD8+ T cell infiltration, indicating an increase in tumor killing effects [135]. Other investigators have reported that a low-dose of anti-VEGFR2 antibodies can reprogram the immunosuppressive TME in a manner that enhances anti-cancer vaccine therapy [136].
In 2017, a Japanese research team reported a study using a mouse model, contributing new data on anti-tumor immunotherapy combining VEGF inhibitor (sunitinib) with agonist antibody of death receptor-5. Compared with a single therapy, combined therapy reduced tumor growth rate and increased the number of CD4+ Foxp3- and CD8+ T cells in patients with tumor [137].
Furthermore, VEGFR combined with T-lymphocyte adoptive transfer also showed potential clinical significance in cancer treatment. A preclinical study showed that adoptive transfer of human VEGFR-1 specificity with chimeric antigen receptor (CAR)-modified T lymphocytes (V-1CAR) can delay tumor growth and formation in mice [138]. The results showed that in a mouse model of NSCLC cell A549 xenograft in NOD-SCID BALB/c mice, the growth of A549 xenografts was inhibited by V-1CAR modified T lymphocytes [138].
Moreover, a recent study found that anti-PD-L1 antibody combined with anti-VEGF antibody treatment inhibited tumor growth and increased CD8+ T cell infiltration compared with anti-VEGF monotherapy [139]. These preclinical results support the idea that treatment efficacy can be improved by combining VEGF inhibitor with immunotherapy.
3.4.2 Clinical studies
Some clinical trials of anti-angiogenic drugs combined with immunotherapy for the treatment of NSCLC are ongoing (Table 1).
In a phase III study (NCT02366143), to examine whether VEGF blockade enhances the efficacy of immunotherapy, atezolizumab was added to the combination of bevacizumab and chemotherapy treatment. At the time of data truncation, in the intent-to-treat wild-type (ITT-WT) population, the median PFS of the group with bevacizumab and atezolizumab was significantly longer than that of the group without atezolizumab [140]. Furthermore, after 6 months of treatment, the PFS rate of the group with bevacizumab and atezolizumab was higher than that of the group without atezolizumab [140]. In the Teff-high WT population, the median PFS was significantly longer in the group with atezolizumab than in the group without atezolizumab [140]. Moreover, similar results were observed for OS in the ITT-WT population. However, the rate of objective response was not confirmed, and there were more data for the group with atezolizumab than for that without atezolizumab; the results were similar in the Teff-high WT population [140].
Data from clinical trials of anti-angiogenic drugs combined with immunotherapy for the treatment of NSCLC
| NCT ID | Relevant Compound(s) | Phase | Outcome Measures | Status | Study Title |
|---|---|---|---|---|---|
| NCT02366143 | Atezolizumab + Bevacizumab + Paclitaxel + Carboplatin | Ⅲ | PFS, 8.3 (95% CI, 7.7-9.8) OS, 19.2 (95% CI, 17.0-23.8) | Completed | A Study of Atezolizumab in Combination with Carboplatin Plus (+) Paclitaxel With or Without Bevacizumab Compared with Carboplatin+Paclitaxel+Bevacizumab in Participants with Stage IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC) |
| NCT02039674 | Pembrolizumab + Paclitaxel + Carboplatin + Bevacizumab | I/II | Objective Response Rate, DLT | Completed | A Study of Pembrolizumab (MK-3475) in Combination with Chemotherapy or Immunotherapy in Participants with Non-small Cell Lung Cancer (MK-3475-021/KEYNOTE-021) |
| NCT01454102 | Bevacizumab + Nivolumab | I | ORR, 16.7% (95% CI, 2.1-48.4%) PFSR, 58.3% (95% CI, 27.0-80.1%) | Completed | Study of Nivolumab (BMS-936558) in Combination with Gemcitabine/Cisplatin, Pemetrexed/Cisplatin, Carboplatin/Paclitaxel, Bevacizumab Maintenance, Erlotinib, Ipilimumab or as Monotherapy in Subjects with Stage IIIB/IV Non-small Cell Lung Cancer (NSCLC) (CheckMate 012) |
| NCT01633970 | Atezolizmab + Bevacizumab | I | DLT | Completed | A Study of Atezolizumab Administered in Combination with Bevacizumab and/or with Chemotherapy in Participants with Locally Advanced or Metastatic Solid Tumors |
| NCT02443324 | Ramucirumab + Pembrolizumab | I | DLT, DCR, Objective Response Rate | Active, not recruiting | A Study of Ramucirumab Plus Pembrolizumab in Participants with Gastric or GEJ Adenocarcinoma, NSCLC, Transitional Cell Carcinoma of the Urothelium, or Biliary Tract Cancer |
| NCT02856425 | Pembrolizumab + Nintedanib | I | MTD | Recruiting | Trial of Pembrolizumab and Nintedanib |
| NCT04046614 | Nintedanib + Nivolumab | I/II | PFS, ORR | Recruiting | Feasibility and Safety of Nintedanib in Combination with Nivolumab in Pretreated Patients with Advanced or Metastatic NSCLC of Adenocarcinoma Histology |
| NCT03377023 | Nivolumab + Ipilimumab + Nintedanib | I/II | MTD, DCR, Objective Response Rate | Active, not recruiting | Phase I/II Study of Nivolumab and Ipilimumab Combined with Nintedanib in Non-Small Cell Lung Cancer |
| NCT03689855 | Ramucirumab + Atezolizumab | II | ORR OS PFS | Active, not recruiting | Ramucirumab and Atezolizumab After Progression on Any Immune Checkpoint Blocker in NSCLC |
| NCT00828009 | Bevacizumab + Tecemotide | II | PFS, 14.9 (95% CI, 11.0-20.9) OS, 42.7 (95% CI, 21.7-63.3) | Completed; results published | BLP25 Liposome Vaccine and Bevacizumab After Chemotherapy and Radiation Therapy in Treating Patients with Newly Diagnosed Stage IIIA or Stage IIIB Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery |
| NCT02574078 | Bevacizumab + Nivolumab | I/II | PFS, 6.7 (4.1-NA) OS, 30.8 (8.8-NA) | Completed; results published | A Study of Nivolumab in Advanced Non-Small Cell Lung Cancer (NSCLC) |
| NCT02681549 | Pembrolizumab + Bevacizumab | II | BMRR, ORR | Recruiting | Pembrolizumab Plus Bevacizumab for Treatment of Brain Metastases in Metastatic Melanoma or Non-small Cell Lung Cancer |
| NCT03527108 | Nivolumab + Ramucirumab | II | DCR, ORR, PFS | Recruiting | Nivolumab Plus Ramucirumab in Patients With Recurrent, Advanced, Metastatic NSCLC |
| NCT02572687 | Ramucirumab + MEDI4736 | I | DLT, ORR, DCR | Completed | A Study of Ramucirumab (LY3009806) Plus MEDI4736 in Participants With Advanced Gastrointestinal or Thoracic Malignancies |
| NCT03786692 | Carboplatin + Pemetrexed + Bevacizumab + Atezolizumab | II | PFS, ORR | Recruiting | Phase II Randomized Trial of Carboplatin+Pemetrexed+Bevacizumab+/- Atezolizumab in Stage IV NSCLC |
| NCT02174172 | Atezolizumab + PEG-interferon alfa-2a + Bevacizumab | I | RP2D, RECIST | Completed | A Study to Assess the Safety and Tolerability of Atezolizumab in Combination With Other immune-modulating Therapies in Participants With Locally Advanced or Metastatic Solid Tumors |
| NCT03616691 | Atezolizumab+ Bevacizumab | II | ORR, PFS | Unknown | Atezolizumab Monotherapy and Consequent Therapy With Atezolizumab Plus Bevacizumab for NSCLC |
| NCT03647956 | Atezolizumab + Bevacizumab | II | Objective response rate, PFS, TTP, DoR | Unknown | Atezolizumab in Combination With Bevacizumab, Carboplatin and Pemetrexed for EGFR-mutant Metastatic NSCLC Patients After Failure of EGFR Tyrosine Kinase Inhibitors |
| NCT03713944 | Carboplatin + Pemetrexed + Atezolizumab + Bevacizumab | II | PFS, ORR, DCR | Active, not recruiting | Carboplatin Plus Pemetrexed Plus Atezolizumab Plus Bevacizumab in Chemotherapy and Immunotherapy-naïve Patients With Stage IV Non-squamous Non-small Cell Lung Cancer |
| NCT03836066 | Atezolizumab + Bevacizumab | II | Efficacy of atezolizumab in combination with bevacizumab | Active, not recruiting | Atezolizumab Plus Bevacizumab in First-Line NSCLC Patients |
PFS: progression-free survival; OS: overall survival; CI: confidence interval; ORR: overall response rate; PFSR: progression-free survival rate; DLT: Dose-limiting toxicity; DCR: disease control rate; MTD: maximum tolerated dose; BMRR: brain metastasis response rate; RP2D: recommended phase II dose; RECIST: response evaluation criteria in solid Tumors; TTP: time to progression; DoR: duration of response.
In a phase I study (NCT01454102), the efficacy of nivolumab in combination with bevacizumab was compared to the efficacy of nivolumab alone in patients with advanced NSCLC. The results showed that a higher proportion of subjects were progression-free and survived at 24 weeks in the nivolumab plus bevacizumab group than in the nivolumab monotherapy group. Moreover, the objective response rate (ORR) of nivolumab plus bevacizumab and nivolumab alone arms was 16.7% and 23.1%, respectively.
In another phase I study (NCT02443324), the effectiveness and safety of ramucirumab and pembrolizumab were evaluated in the treatment of different tumor entities, including NSCLC. The ORR of the treated population was 42.3%, and the median PFS was 9.3 months. However, the treated population did not reach the median OS [141].
A study on pembrolizumab plus paclitaxel plus carboplatin plus with/without bevacizumab in stage IIIB/IV NSCLC is also under evaluation (NCT02039674). The results of pembrolizumab plus bevacizumab showed an ORR of 56%, a PFS of 7.1 months, and a median OS of 16.7 months [142].
Moreover, an open phase IB study evaluating the safety, pharmacology, and initial efficacy of the combination of atezolizumab and bevacizumab in the treatment of advanced solid tumors including NSCLC has been completed. However, the results of the study have not yet been published (NCT01633970).
Many studies are currently ongoing. A phase IB trial has been launched with the aim to evaluate the efficacy of pembrolizumab and nintedanib in the treatment of solid tumors, including advanced NSCLC (NCT02856425), and participants are being recruited. A phase I/II study of the efficacy of nivolumab and ipilimumab combined with nintedanib in NSCLC has been initiated, but no results have been published so far (NCT03377023). Additionally, a Phase I/II trial evaluating the feasibility and safety of nintedanib in combination with nivolumab in pretreated patients with advanced or metastatic adenocarcinoma histologic NSCLC has been initiated and is currently recruiting participants (NCT04046614).
Overall, the combination of immunotherapy and VEGF-VEGFR-targeted therapy for NSCLC has yielded encouraging results for such treatment. However, for best therapeutic results, the effects of different anti-angiogenic medications, drug doses, and timing of the combination of the two drugs need to be addressed in future clinical trials.
4. Conclusion and Perspectives
This review evaluated the latest knowledge on two therapies, VEGF-VEGFR-targeted therapy and immunotherapy, for the treatment of NSCLC. In addition to promoting tumor angiogenesis, VEGF also promotes immunosuppression in NSCLC. The application of a combination of VEGF/VEGFR-targeted therapy and immunotherapy in preclinical and clinical studies has shown good efficacy in the treatment of NSCLC by regulating the immunosuppressive TME. Results from the currently enrolling clinical studies also support that the combination therapy is a promising approach for the treatment of NSCLC. Future preclinical and clinical studies need to address some key questions, such as what are the specific biomarkers in response to the combination therapy? This therapeutic approach targeting the TME certainly has complex biological effects. Combination therapy may further increase this complexity, posing an increased risk of toxicity and the possibility of immune-related adverse events. For example, immunotherapy can sometimes lead to serious immune-related adverse events, and these toxic reactions can usually be resolved by stopping treatment or reducing the dose. Therefore, it is important to optimize the dose, timing of administration, and sequence of administration of VEGF-VEGFR-targeted drug therapy and immunotherapy in future clinical studies. Since the use of VEGF-VEGFR-targeted drugs allows the drugs or immune cells in immunotherapy to reach the tumor site more easily through normalized blood vessels, the dose of immunotherapy drugs can be reduced appropriately in combination therapy.
While the employment of combination therapy offers a promising future for NSCLC treatment, further studies must investigate how these therapeutic strategies interact with each other to regulate the immunosuppressive TME and kill cancer cells. New technologies, such as single-cell sequencing and spatial transcriptomics, may improve our knowledge. These technologies can help us understand the interactions among angiogenesis, cancer cells, and immune cells of the immunosuppressive TME of NSCLC. Meanwhile, we can design new approaches to target angiogenic and immunosuppressive environment for NSCLC treatment.
Acknowledgements
This work was supported by Science and Technology Research Fund of Sichuan Administration of Traditional Chinese Medicine (No. 2021MS528), and the Science and Technology Strategic Cooperation Programs of Luzhou Municipal People's Government and Southwest Medical University (Grant nos. 2019LZXNYDJ25, and 2019LZXNYDJ45).
Author Contributions
YZ, SG, JD, JL, and ZX wrote the first draft of manuscript and first revision; all authors revised the manuscript for second times before submission. All authors contributed to manuscript revision, read and approved the submitted version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398:535-54
2. Hao C, Liu G, Tian G. Autophagy inhibition of cancer stem cells promotes the efficacy of cisplatin against non-small cell lung carcinoma. Ther Adv Respir Dis. 2019;13:1753466619866097
3. Toyokawa G, Yamada Y, Tagawa T, Oda Y. Significance of spread through air spaces in early-stage lung adenocarcinomas undergoing limited resection. Thorac Cancer. 2018;9:1255-61
4. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446-54
5. Hwang I, Kim JW, Ylaya K, Chung EJ, Kitano H, Perry C. et al. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J Transl Med. 2020;18:443
6. De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457-74
7. Guimarães-Bastos D, Frony AC, Barja-Fidalgo C, Moraes JA. Melanoma-derived extracellular vesicles skew neutrophils into a pro-tumor phenotype. J Leukoc Biol. 2021
8. Komi DEA, Khomtchouk K, Santa Maria PL. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clinical reviews in allergy & immunology. 2020;58:298-312
9. McHale C, Mohammed Z, Gomez G. Human Skin-Derived Mast Cells Spontaneously Secrete Several Angiogenesis-Related Factors. Frontiers in immunology. 2019;10:1445
10. Sammarco G, Varricchi G, Ferraro V, Ammendola M, De Fazio M, Altomare DF. et al. Mast Cells, Angiogenesis and Lymphangiogenesis in Human Gastric Cancer. International journal of molecular sciences. 2019 20
11. Rahma OE, Hodi FS. The Intersection between Tumor Angiogenesis and Immune Suppression. Clinical cancer research: an official journal of the American Association for Cancer Research. 2019;25:5449-57
12. Vetsika E-K, Koukos A, Kotsakis A. Myeloid-Derived Suppressor Cells: Major Figures that Shape the Immunosuppressive and Angiogenic Network in Cancer. Cells. 2019 8
13. Mortezaee K. Myeloid-derived suppressor cells in cancer immunotherapy-clinical perspectives. Life Sci. 2021;277:119627
14. Albini A, Bruno A, Noonan DM, Mortara L. Contribution to Tumor Angiogenesis From Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front Immunol. 2018;9:527
15. Guan Y, Chambers CB, Tabatabai T, Hatley H, Delfino KR, Robinson K. et al. Renal cell tumors convert natural killer cells to a proangiogenic phenotype. Oncotarget. 2020;11:2571-85
16. Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27:1345-56
17. Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176:1248-64
18. Uemura A, Fruttiger M, D'Amore PA, De Falco S, Joussen AM, Sennlaub F. et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog Retin Eye Res. 2021;84:100954
19. Dallinga MG, Habani YI, Schimmel AWM, Dallinga-Thie GM, van Noorden CJF, Klaassen I. et al. The Role of Heparan Sulfate and Neuropilin 2 in VEGFA Signaling in Human Endothelial Tip Cells and Non-Tip Cells during Angiogenesis In Vitro. Cells. 2021 10
20. Sarabipour S, Mac Gabhann F. VEGF-A121a binding to Neuropilins - A concept revisited. Cell Adh Migr. 2018;12:204-14
21. McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Müller KH, Sharkey AM. et al. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996;98:482-9
22. Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273:114-27
23. Chu BF, Otterson GA. Incorporation of Antiangiogenic Therapy Into the Non-Small-Cell Lung Cancer Paradigm. Clin Lung Cancer. 2016;17:493-506
24. Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871-82
25. Das M, Wakelee H. Targeting VEGF in lung cancer. Expert Opin Ther Targets. 2012;16:395-406
26. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N. et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. The New England journal of medicine. 2018;378:2288-301
27. Olsson A-K, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359-71
28. Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19:2003-12
29. Korpela H, Hätinen O-P, Nieminen T, Mallick R, Toivanen P, Airaksinen J. et al. Adenoviral VEGF-B186R127S gene transfer induces angiogenesis and improves perfusion in ischemic heart. iScience. 2021;24:103533
30. Zhou Y, Zhu X, Cui H, Shi J, Yuan G, Shi S. et al. The Role of the VEGF Family in Coronary Heart Disease. Front Cardiovasc Med. 2021;8:738325
31. Chakraborty A, Upadhya R, Usman TA, Shetty AK, Rutkowski JM. Chronic VEGFR-3 signaling preserves dendritic arborization and sensitization under stress. Brain Behav Immun. 2021;98:219-33
32. Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: Review. Blood Cells, Molecules, and Diseases. 2007;38:258-68
33. Al-Shareef H, Hiraoka S-I, Tanaka N, Shogen Y, Lee A-D, Bakhshishayan S. et al. Use of NRP1, a novel biomarker, along with VEGF-C, VEGFR-3, CCR7 and SEMA3E, to predict lymph node metastasis in squamous cell carcinoma of the tongue. Oncology Reports. 2016;36:2444-54
34. Sopo M, Anttila M, Hämäläinen K, Kivelä A, Ylä-Herttuala S, Kosma V-M. et al. Expression profiles of VEGF-A, VEGF-D and VEGFR1 are higher in distant metastases than in matched primary high grade epithelial ovarian cancer. BMC Cancer. 2019;19:584
35. Kong X, Bu J, Chen J, Ni B, Fu B, Zhou F. et al. PIGF and Flt-1 on the surface of macrophages induces the production of TGF-β1 by polarized tumor-associated macrophages to promote lung cancer angiogenesis. Eur J Pharmacol. 2021;912:174550
36. Frezzetti D, Gallo M, Maiello MR, D'Alessio A, Esposito C, Chicchinelli N. et al. VEGF as a potential target in lung cancer. Expert Opin Ther Targets. 2017;21:959-66
37. Goel HL, Mercurio AM. VEGF targets the tumour cell. Nature reviews Cancer. 2013;13:871-82
38. Barr MP, Gray SG, Gately K, Hams E, Fallon PG, Davies AM. et al. Vascular endothelial growth factor is an autocrine growth factor, signaling through neuropilin-1 in non-small cell lung cancer. Molecular cancer. 2015;14:45
39. Li H, Takayama K, Wang S, Shiraishi Y, Gotanda K, Harada T. et al. Addition of bevacizumab enhances antitumor activity of erlotinib against non-small cell lung cancer xenografts depending on VEGF expression. Cancer Chemother Pharmacol. 2014;74:1297-305
40. Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA. et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:3484-94
41. Lei X, Lei Y, Li J-K, Du W-X, Li R-G, Yang J. et al. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126-33
42. Galassi C, Musella M, Manduca N, Maccafeo E, Sistigu A. The Immune Privilege of Cancer Stem Cells: A Key to Understanding Tumor Immune Escape and Therapy Failure. Cells. 2021 10
43. Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139-48
44. Martino EC, Misso G, Pastina P, Costantini S, Vanni F, Gandolfo C. et al. Immune-modulating effects of bevacizumab in metastatic non-small-cell lung cancer patients. Cell Death Discov. 2016;2:16025
45. de Almeida PE, Mak J, Hernandez G, Jesudason R, Herault A, Javinal V. et al. Anti-VEGF Treatment Enhances CD8 T-cell Antitumor Activity by Amplifying Hypoxia. Cancer immunology research. 2020;8:806-18
46. Bannoud N, Dalotto-Moreno T, Kindgard L, García PA, Blidner AG, Mariño KV. et al. Hypoxia Supports Differentiation of Terminally Exhausted CD8 T Cells. Front Immunol. 2021;12:660944
47. Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S. et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150-66
48. Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S. et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878-86
49. Liu X, Nie W, Xie Q, Chen G, Li X, Jia Y. et al. Endostatin reverses immunosuppression of the tumor microenvironment in lung carcinoma. Oncol Lett. 2018;15:1874-80
50. Bourhis M, Palle J, Galy-Fauroux I, Terme M. Direct and Indirect Modulation of T Cells by VEGF-A Counteracted by Anti-Angiogenic Treatment. Frontiers in immunology. 2021;12:616837
51. Kim CG, Jang M, Kim Y, Leem G, Kim KH, Lee H. et al. VEGF-A drives TOX-dependent T cell exhaustion in anti-PD-1-resistant microsatellite stable colorectal cancers. Sci Immunol. 2019 4
52. Tang R, Xu J, Zhang B, Liu J, Liang C, Hua J. et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13:110
53. Flores-Mendoza G, Rodríguez-Rodríguez N, Rubio RM, Madera-Salcedo IK, Rosetti F, Crispín JC. Fas/FasL Signaling Regulates CD8 Expression During Exposure to Self-Antigens. Frontiers in immunology. 2021;12:635862
54. Dorothee G, Vergnon I, Menez J, Echchakir H, Grunenwald D, Kubin M. et al. Tumor-infiltrating CD4+ T lymphocytes express APO2 ligand (APO2L)/TRAIL upon specific stimulation with autologous lung carcinoma cells: role of IFN-alpha on APO2L/TRAIL expression and -mediated cytotoxicity. J Immunol. 2002;169:809-17
55. Ohkura N, Sakaguchi S. Transcriptional and epigenetic basis of Treg cell development and function: its genetic anomalies or variations in autoimmune diseases. Cell research. 2020;30:465-74
56. Mikami N, Kawakami R, Sakaguchi S. New Treg cell-based therapies of autoimmune diseases: towards antigen-specific immune suppression. Curr Opin Immunol. 2020;67:36-41
57. Zappasodi R, Sirard C, Li Y, Budhu S, Abu-Akeel M, Liu C. et al. Rational design of anti-GITR-based combination immunotherapy. Nat Med. 2019;25:759-66
58. Han P, Hou Y, Zhao Y, Liu Y, Yu T, Sun Y. et al. Low-dose decitabine modulates T-cell homeostasis and restores immune tolerance in immune thrombocytopenia. Blood. 2021;138:674-88
59. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109-18
60. Deng G, Song X, Fujimoto S, Piccirillo CA, Nagai Y, Greene MI. Foxp3 Post-translational Modifications and Treg Suppressive Activity. Frontiers in immunology. 2019;10:2486
61. Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019;110:2080-9
62. Sun L, Xu G, Liao W, Yang H, Xu H, Du S. et al. Clinicopathologic and prognostic significance of regulatory T cells in patients with hepatocellular carcinoma: a meta-analysis. Oncotarget. 2017;8:39658-72
63. Shitara K, Nishikawa H. Regulatory T cells: a potential target in cancer immunotherapy. Ann N Y Acad Sci. 2018;1417:104-15
64. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325-40
65. Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O. et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73:539-49
66. Hansen W, Hutzler M, Abel S, Alter C, Stockmann C, Kliche S. et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med. 2012;209:2001-16
67. Zhou K, Cheng T, Zhan J, Peng X, Zhang Y, Wen J. et al. Targeting tumor-associated macrophages in the tumor microenvironment. Oncology letters. 2020;20:234
68. Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019;30:36-50
69. Myers KV, Amend SR, Pienta KJ. Targeting Tyro3, Axl and MerTK (TAM receptors): implications for macrophages in the tumor microenvironment. Molecular cancer. 2019;18:94
70. Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76
71. Park JE, Dutta B, Tse SW, Gupta N, Tan CF, Low JK. et al. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift. Oncogene. 2019;38:5158-73
72. Lapeyre-Prost A, Terme M, Pernot S, Pointet AL, Voron T, Tartour E. et al. Immunomodulatory Activity of VEGF in Cancer. Int Rev Cell Mol Biol. 2017;330:295-342
73. Lia A, Annese T, Fornaro M, Giannini M, D'Abbicco D, Errede M. et al. Perivascular and endomysial macrophages expressing VEGF and CXCL12 promote angiogenesis in anti-HMGCR immune-mediated necrotizing myopathy. Rheumatology (Oxford). 2021
74. Morante-Palacios O, Fondelli F, Ballestar E, Martínez-Cáceres EM. Tolerogenic Dendritic Cells in Autoimmunity and Inflammatory Diseases. Trends in immunology. 2021;42:59-75
75. Bosch NC, Voll RE, Voskens CJ, Gross S, Seliger B, Schuler G. et al. NF-κB activation triggers NK-cell stimulation by monocyte-derived dendritic cells. Ther Adv Med Oncol. 2019;11:1758835919891622
76. Lucarini V, Melaiu O, Tempora P, D'Amico S, Locatelli F, Fruci D. Dendritic Cells: Behind the Scenes of T-Cell Infiltration into the Tumor Microenvironment. Cancers. 2021 13
77. Anirudh S, Rosenberger A, Schwarzenberger E, Schaefer C, Strobl H, Zebisch A. et al. TNFα Rescues Dendritic Cell Development in Hematopoietic Stem and Progenitor Cells Lacking C/EBPα. Cells. 2020 9
78. Han Z, Dong Y, Lu J, Yang F, Zheng Y, Yang H. Role of hypoxia in inhibiting dendritic cells by VEGF signaling in tumor microenvironments: mechanism and application. Am J Cancer Res. 2021;11:3777-93
79. Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 2018;15:310-24
80. Chen DS, Hurwitz H. Combinations of Bevacizumab With Cancer Immunotherapy. Cancer J. 2018;24:193-204
81. Long J, Hu Z, Xue H, Wang Y, Chen J, Tang F. et al. Vascular endothelial growth factor (VEGF) impairs the motility and immune function of human mature dendritic cells through the VEGF receptor 2-RhoA-cofilin1 pathway. Cancer Sci. 2019;110:2357-67
82. Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R. et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115-24
83. Strauss L, Volland D, Kunkel M, Reichert TE. Dual role of VEGF family members in the pathogenesis of head and neck cancer (HNSCC): possible link between angiogenesis and immune tolerance. Med Sci Monit. 2005;11:BR280-BR92
84. Inoshima N, Nakanishi Y, Minami T, Izumi M, Takayama K, Yoshino I. et al. The influence of dendritic cell infiltration and vascular endothelial growth factor expression on the prognosis of non-small cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2002;8:3480-6
85. Hegde S, Leader AM, Merad M. MDSC: Markers, development, states, and unaddressed complexity. Immunity. 2021;54:875-84
86. Dysthe M, Parihar R. Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1224:117-40
87. Weber R, Groth C, Lasser S, Arkhypov I, Petrova V, Altevogt P. et al. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cellular immunology. 2021;359:104254
88. Tesi RJ. MDSC; the Most Important Cell You Have Never Heard Of. Trends Pharmacol Sci. 2019;40:4-7
89. Ma J, Xu H, Wang S. Immunosuppressive Role of Myeloid-Derived Suppressor Cells and Therapeutic Targeting in Lung Cancer. J Immunol Res. 2018;2018:6319649
90. Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED. et al. Clinical significance of defective dendritic cell differentiation in cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000;6:1755-66
91. Fleming V, Hu X, Weber R, Nagibin V, Groth C, Altevogt P. et al. Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression. Front Immunol. 2018;9:398
92. Koinis F, Vetsika EK, Aggouraki D, Skalidaki E, Koutoulaki A, Gkioulmpasani M. et al. Effect of First-Line Treatment on Myeloid-Derived Suppressor Cells' Subpopulations in the Peripheral Blood of Patients with Non-Small Cell Lung Cancer. J Thorac Oncol. 2016;11:1263-72
93. Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18:85-100
94. Wang L, Dai Y, Zhu F, Qiu Z, Wang Y, Hu Y. Efficacy of DC-CIK-based immunotherapy combined with chemotherapy in the treatment of intermediate to advanced non-small cell lung cancer. Am J Transl Res. 2021;13:13076-83
95. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology. 2009;9:162-74
96. Kolahian S, Oz HH, Zhou B, Griessinger CM, Rieber N, Hartl D. The emerging role of myeloid-derived suppressor cells in lung diseases. Eur Respir J. 2016;47:967-77
97. Hawke LG, Whitford MKM, Ormiston ML. The Production of Pro-angiogenic VEGF-A Isoforms by Hypoxic Human NK Cells Is Independent of Their TGF-beta-Mediated Conversion to an ILC1-Like Phenotype. Front Immunol. 2020;11:1903
98. Krzywinska E, Kantari-Mimoun C, Kerdiles Y, Sobecki M, Isagawa T, Gotthardt D. et al. Loss of HIF-1α in natural killer cells inhibits tumour growth by stimulating non-productive angiogenesis. Nature communications. 2017;8:1597
99. Eguchi R, Wakabayashi I. HDGF enhances VEGF-dependent angiogenesis and FGF-2 is a VEGF-independent angiogenic factor in non-small cell lung cancer. Oncology reports. 2020;44:14-28
100. Jung WY, Min K-W, Oh YH. Increased VEGF-A in solid type of lung adenocarcinoma reduces the patients' survival. Sci Rep. 2021;11:1321
101. Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R. et al. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017
102. Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L. et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655-69
103. Syed YY. Ramucirumab: A Review in Hepatocellular Carcinoma. Drugs. 2020;80:315-22
104. Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S. et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780-7
105. Janning M, Loges S. Anti-Angiogenics: Their Value in Lung Cancer Therapy. Oncol Res Treat. 2018;41:172-80
106. Kovalchuk B, Berghoff AS, Karreman MA, Frey K, Piechutta M, Fischer M. et al. Nintedanib and a bi-specific anti-VEGF/Ang2 nanobody selectively prevent brain metastases of lung adenocarcinoma cells. Clin Exp Metastasis. 2020;37:637-48
107. Reck M, Kaiser R, Mellemgaard A, Douillard J-Y, Orlov S, Krzakowski M. et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143-55
108. Shaw AT, Solomon BJ, Chiari R, Riely GJ, Besse B, Soo RA. et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2019;20:1691-701
109. Lu S, Chen G, Sun Y, Sun S, Chang J, Yao Y. et al. A Phase III, randomized, double-blind, placebo-controlled, multicenter study of fruquintinib in Chinese patients with advanced nonsquamous non-small-cell lung cancer - The FALUCA study. Lung cancer (Amsterdam, Netherlands). 2020;146:252-62
110. Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. 2021;16:223-49
111. Cha J-H, Chan L-C, Li C-W, Hsu JL, Hung M-C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol Cell. 2019;76:359-70
112. Sivori S, Pende D, Quatrini L, Pietra G, Della Chiesa M, Vacca P. et al. NK cells and ILCs in tumor immunotherapy. Mol Aspects Med. 2021;80:100870
113. Dammeijer F, van Gulijk M, Mulder EE, Lukkes M, Klaase L, van den Bosch T. et al. The PD-1/PD-L1-Checkpoint Restrains T cell Immunity in Tumor-Draining Lymph Nodes. Cancer cell. 2020 38
114. Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B. et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Molecular cancer. 2019;18:10
115. Koh YW, Lee SJ, Han J-H, Haam S, Jung J, Lee HW. PD-L1 protein expression in non-small-cell lung cancer and its relationship with the hypoxia-related signaling pathways: A study based on immunohistochemistry and RNA sequencing data. Lung Cancer. 2019;129:41-7
116. Chen R, Ganesan A, Okoye I, Arutyunova E, Elahi S, Lemieux MJ. et al. Targeting B7-1 in immunotherapy. Med Res Rev. 2020;40:654-82
117. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F. et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. The New England journal of medicine. 2018;378:2078-92
118. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J. et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. The New England journal of medicine. 2018;379:2040-51
119. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England journal of medicine. 2015;372:2018-28
120. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E. et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. The New England journal of medicine. 2019;381:2020-31
121. Wang Z, Ying J, Xu J, Yuan P, Duan J, Bai H. et al. Safety, Antitumor Activity, and Pharmacokinetics of Toripalimab, a Programmed Cell Death 1 Inhibitor, in Patients With Advanced Non-Small Cell Lung Cancer: A Phase 1 Trial. JAMA Netw Open. 2020;3:e2013770
122. Gao S, Li N, Gao S, Xue Q, Ying J, Wang S. et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2020;15:816-26
123. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH. et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. The New England journal of medicine. 2020;383:1328-39
124. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. The New England journal of medicine. 2017;377:1919-29
125. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. The New England journal of medicine. 2018;379:2342-50
126. Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS. et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375-86
127. Kudo M. Scientific Rationale for Combined Immunotherapy with PD-1/PD-L1 Antibodies and VEGF Inhibitors in Advanced Hepatocellular Carcinoma. Cancers (Basel). 2020 12
128. Faucher JL, Lacronique-Gazaille C, Frebet E, Trimoreau F, Donnard M, Bordessoule D. et al. "6 markers/5 colors" extended white blood cell differential by flow cytometry. Cytometry A. 2007;71:934-44
129. Ramlau R, Quoix E, Rolski J, Pless M, Lena H, Lévy E. et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2008;3:735-44
130. Lu Y, Xue J, Deng T, Zhou X, Yu K, Deng L. et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med. 2020;26:732-40
131. Qu J, Mei Q, Chen L, Zhou J. Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): current status and future perspectives. Cancer Immunol Immunother. 2021;70:619-31
132. Dammeijer F, Lievense LA, Veerman GDM, Hoogsteden HC, Hegmans JP, Arends LR. et al. Efficacy of Tumor Vaccines and Cellular Immunotherapies in Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34:3204-12
133. Tao L, Huang G, Shi S, Chen L. Bevacizumab improves the antitumor efficacy of adoptive cytokine-induced killer cells therapy in non-small cell lung cancer models. Med Oncol. 2014;31:777
134. Shi S, Wang R, Chen Y, Song H, Chen L, Huang G. Combining antiangiogenic therapy with adoptive cell immunotherapy exerts better antitumor effects in non-small cell lung cancer models. PLoS One. 2013;8:e65757
135. Kwilas AR, Ardiani A, Donahue RN, Aftab DT, Hodge JW. Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J Transl Med. 2014;12:294
136. Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J. et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561-6
137. Pircher A, Wolf D, Heidenreich A, Hilbe W, Pichler R, Heidegger I. Synergies of Targeting Tumor Angiogenesis and Immune Checkpoints in Non-Small Cell Lung Cancer and Renal Cell Cancer: From Basic Concepts to Clinical Reality. Int J Mol Sci. 2017 18
138. Wang W, Ma Y, Li J, Shi HS, Wang LQ, Guo FC. et al. Specificity redirection by CAR with human VEGFR-1 affinity endows T lymphocytes with tumor-killing ability and anti-angiogenic potency. Gene Ther. 2013;20:970-8
139. Ishikura N, Sugimoto M, Yorozu K, Kurasawa M, Kondoh O. Anti-VEGF antibody triggers the effect of anti-PD-L1 antibody in PD-L1 and immune desert-like mouse tumors. Oncology reports. 2022 47
140. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N. et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378:2288-301
141. Herbst RS, Arkenau HT, Bendell J, Arrowsmith E, Wermke M, Soriano A. et al. Phase 1 Expansion Cohort of Ramucirumab Plus Pembrolizumab in Advanced Treatment-Naive NSCLC. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2021;16:289-98
142. Gadgeel SM, Stevenson JP, Langer CJ, Gandhi L, Borghaei H, Patnaik A. et al. Pembrolizumab and platinum-based chemotherapy as first-line therapy for advanced non-small-cell lung cancer: Phase 1 cohorts from the KEYNOTE-021 study. Lung Cancer. 2018;125:273-81
Author contact
![]() Corresponding author: E-mail addresses: yueshuizhaoedu.cn (Yueshui Zhao), Jing.li9com (Jing Li), xzg555898com (Zhangang Xiao)
Corresponding author: E-mail addresses: yueshuizhaoedu.cn (Yueshui Zhao), Jing.li9com (Jing Li), xzg555898com (Zhangang Xiao)

 Global reach, higher impact
Global reach, higher impact