10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(15):5667-5680. doi:10.7150/ijbs.77126 This issue Cite
Research Paper
Identification of Gαi3 as a novel molecular therapeutic target of cervical cancer
1. Obstetrics and Gynecology Department, The Affiliated Zhangjiagang Hospital of Soochow University, Institute of Neuroscience, Soochow University, Suzhou, China.
2. Department of Otorhinolaryngology Head and Neck Surgery, Children's Hospital of Soochow University, Suzhou, China.
3. Department of Radiotherapy and Oncology, Affiliated Kunshan Hospital of Jiangsu University, Suzhou, China.
4. Center of Translational Medicine, The Affiliated Zhangjiagang Hospital of Soochow University, Suzhou, China.
#Equal contribution
Received 2022-7-17; Accepted 2022-8-25; Published 2022-9-6
Abstract
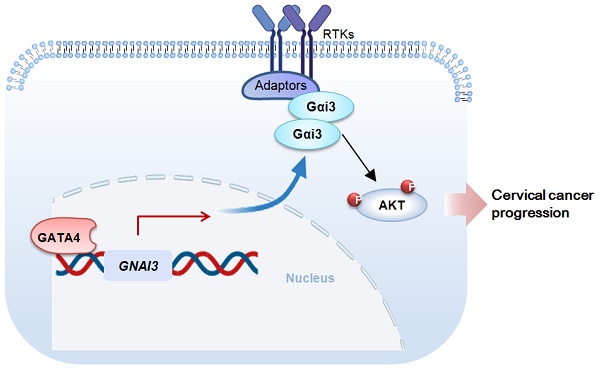
Here we studied expression and potential functions of Gαi3 in cervical cancer. The bioinformatics analysis together with the results from local patients' tissues revealed that Gαi3 expression was remarkably elevated in human cervical cancer tissues and different cervical cancer cells, and was associated with poor overall survival and poor disease-specific survival of patients. Gαi3 depletion resulted in profound anti-cervical cancer activity. In primary or immortalized cervical cancer cells, Gαi3 shRNA or CRISPR/Cas9-caused Gαi3 knockout/KO largely hindered cell proliferation and migration, and provoked apoptosis. On the contrast, ectopic Gαi3 overexpression further enhanced cervical cancer proliferation and migration. Akt-mTOR activation in primary cervical cancer cells was significantly reduced after Gαi3 silencing or KO, but was augmented following Gαi3 overexpression. Further studies revealed that the transcription factor GATA4 binding to Gαi3 promoter region was significantly enhanced in cervical cancer tissues and cells. Gαi3 expression was decreased by GATA4 shRNA, but upregulated following GATA4 overexpression. In vivo, the growth of cervical cancer xenografts was robustly suppressed after Gαi3 silencing or KO. Gαi3 depletion and Akt-mTOR inactivation were detected in Gαi3-silenced/-KO cervical cancer xenograft tissues. Together, upregulated Gαi3 is a valuable oncotarget of cervical cancer.
Keywords: Cervical cancer, Targeted therapy, Gαi3, Akt-mTOR
Introduction
Cervical cancer seriously threatens women's health globally [1, 2]. The number of cases in developing countries accounts for over 85% of the world. Although the screening (mainly HPV screening) of cervical cancer has been relatively complete [1, 2], and the surgical techniques, radiotherapy equipment, and chemotherapy have been gradually improved, the clinical treatment of advanced and recurrent cervical cancer is still unsatisfactory, and the prognosis is still poor [3-5]. The chemotherapy response for advanced cervical cancer is between 20% and 36%, and the survival time is less than one year [3-5]. Although significant achievements have been made in cervical cancer therapy, the overall survival/prognosis for recurrent and metastatic patients is extremely poor [3, 4].
Molecularly-targeted agents, including the anti-angiogenic drugs, immuno-suppressants and EGFR blockers, are being tested for advanced cervical cancer [6, 7]. Bevacizumab combined with chemotherapy were shown to improve overall survival in certain advanced cervical cancer patients [6, 8-10]. However, for many advanced cancers, the novel targeted therapies are still in urgent need [11-14].
Gαi proteins contain three primary subunits, Gαi1/2/3 [15]. Studies from our group have shown that Gαi proteins are essential novel proteins in transducing signals by receptor tyrosine kinases (RTKs) [16-22] and non-RTK receptors [17, 23]. Gαi proteins mediate activation oncogenic signalings (PI3K-Akt-mTOR and Erk-MAPK) by associating with ligand-activated receptors (RTKs and others) [16-22]. We have recently identified that Gαi proteins are elevated in different human cancers, essential for tumorigenesis and cancer progression [16, 20, 24-26]. Gαi3's expression and potential functions cervical cancer are explored here.
Materials and methods
Reagents
The antibodies were reported early [20]. LY294002 together other chemicals/reagents were provided by Sigma (St. Louis, Mo).
Cells
The fresh cervical cancer tissues or the paracancerous epithelial tissues were first digested. The digested human cells were washed, centrifuged, and incubated in complete medium with penicillin/streptomycin and DNase (500 U). Cell suspensions were thereafter filtered, centrifuged, and resuspended. The primary cancer cells or cervical epithelial cells were cultivated in described medium [27] with minor modifications. Here, the primary human cervical cancer cells (“priCC-1” and “priCC-2”) and the primary human cervical epithelial cells (“priCEpi-1” and “priCEpi-2”), derived from same two primary patients, were obtained. The immortalized cervical cancer cell lines, Caski and HeLa229, were provided by the Cell Bank of Institute of Biological Science of CAS (Shanghai, China). The protocols of this study were approved from the Ethics Committee of Soochow University and were according to the principles of Helsinki declaration.
Human tissues
The human tissues, including cervical cancer tissues and matched paracancerous normal cervical tissues, were obtained from a total of twenty patients who were administrated at the Affiliated Hospitals of Soochow University. Each single patient provided the written-informed consent. The patients' information was listed in Table 1. All cancers are squamous cell carcinomas. Tissue slides were subject to immunohistochemistry (IHC) staining (using the described protocols [16]).
Genetic modification of Gαi3
Gαi3 shRNA, Gαi3 knockout (KO) by using the established CRISPR/Cas9 strategy, as well as ectopic Gαi3 overexpression using a lentiviral construct were reported previously [16, 26].
Genetic modification of GATA4
The lentiviral constructs encoding the GATA4 shRNA or the GATA4-expressing sequence, as well as their relative control constructs, were reported in our previous study [28]. The constructs were individually and stably transduced to the primary human cervical cancer cells. Expression of GATA4 was always tested.
Constitutively-active mutant Akt1 (caAkt1)
In brief, the caAkt1 (S473D)-expressing lentivirus (see our previous studies [29, 30]) was added to cultured primary human cervical cancer cells. caAkt1-expressing stable cells were then formed by using puromycin.
The information of the cervical cancer patients
| No. | Age | Stage | Differentiation | Left lymph node | Right lymph node | Metastasis | P16 | Ki67 |
|---|---|---|---|---|---|---|---|---|
| 1 | 29 | IB2 | High | 0/6 | 0/8 | no | + | +, 67% |
| 2 | 42 | IIIC1p | Mid to Low | 0/7 | 1/9 | no | + | +, 85% |
| 3 | 52 | IB1 | High | 0/10 | 0/10 | no | + | +, 50% |
| 4 | 69 | IB1 | High | 0/9 | 0/10 | no | + | +, 75% |
| 5 | 40 | IIA1 | Mid | 0/6 | 0/10 | no | + | +, 90% |
| 6 | 49 | IB2 | Mid to Low | 0/12 | 0/11 | no | - | +, 70% |
| 7 | 62 | IA1 | Low | NA | NA | no | NA | NA |
| 8 | 40 | IIA1 | Mid | 0/6 | 0/10 | no | + | +, 90% |
| 9 | 35 | IB1 | High | O/11 | 2/19 | no | + | +, 20% |
| 10 | 57 | IIA2 | Mid | 0/8 | 0/17 | no | + | +, 70% |
| 11 | 48 | IB2 | Low | NA | NA | no | NA | NA |
| 12 | 44 | IIA2 | Mid | 0/11 | 0/10 | no | NA | NA |
| 13 | 34 | IIA | Low | 2/5 | 0/14 | no | NA | NA |
| 14 | 42 | IIA1 | Low | 0/11 | 0/16 | no | NA | NA |
| 15 | 64 | IIA | Low | 0/6 | 0/6 | no | NA | NA |
| 16 | 48 | IIA2 | Low | 2/5 | 2/7 | no | NA | NA |
| 17 | 57 | IA1 | High | NA | NA | no | NA | NA |
| 18 | 55 | IIA1 | Low | 0/10 | 0/16 | no | NA | NA |
| 19 | 44 | IA2 | Low | NA | NA | no | NA | NA |
| 20 | 27 | IB2 | High | 0/8 | 0/14 | no | NA | NA |
“NA” stands for not available.
Other assays
Cellular function assays, including CCK-8 (testing cell viability), nuclear EdU/DAPI staining (testing cell proliferation) and “Transwell” migration, as well as the nuclear TUNEL/DAPI staining, Annexin V-PI flow cytometry, Caspase-3 activity assay were reported early [16, 18, 26, 31]. Gene and protein detections by quantitative real-time PCR (qRT-PCR) and Western blotting were reported early [19, 22, 26]. The detailed protocols of GATA4 chromosome immunoprecipitation (ChIP) were reported previously [28]. mRNA primers were reported previously [16, 26]. The uncropped blotting images were listed in Figure S1.
Animal studies
The nude mice were reported previously [24]. priCC-1 cells, at seven million cells of each xenograft, were subcutaneously (s.c.) injected mice's flanks. Within three weeks cervical cancer xenografts were formed (~ 80 mm3). The intratumoral injection of Gαi3 shRNA AAV (adeno-associated viruses) or scramble control shRNA AAV was reported previously [26]. The IHC staining of xenograft slides were reported early [16, 20]. The measurement of tumors was reported early [26]. Soochow University's Ethics Committee and IACUC reviewed the protocols.
Statistical analyses
In vitro experiments here were repeated five times with similar results observed each time. Data were normally distributed and were presented as mean ± standard deviation (SD). Statistical comparison and P values calculation were described early [26, 28].
Results
Gαi3 overexpression in cervical cancer is correlated with poor overall survival
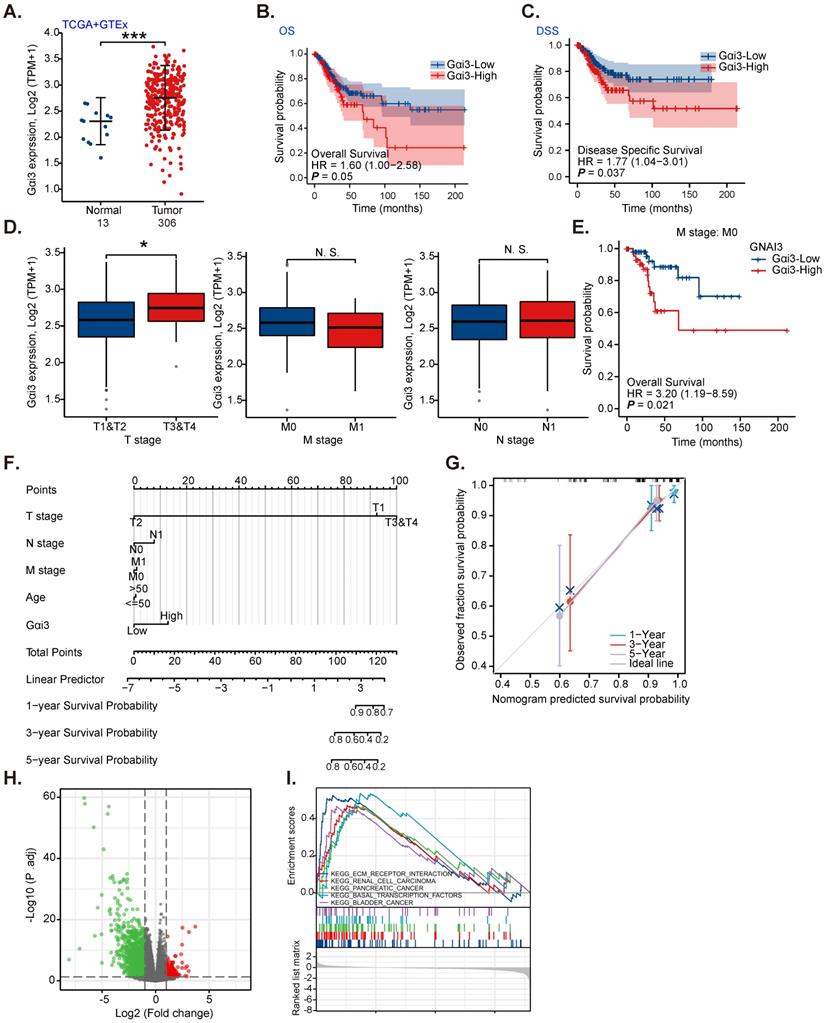
TCGA and the Genotype-Tissue Expression (GTEx) databases reveal that the number of Gαi3 (GNAI3) mRNA transcripts in cervical cancer tissues (“Tumor”, n= 306) was remarkably higher than it in normal cervical tissues (“Normal”, n = 13) (Figure 1A). High Gαi3 expression was correlated with the low overall survival (OS, HR=1.60, P = 0.05, Figure 1B) and low disease-specific survival (DSS, HR=1.77, P =0.037, Figure 1C). High Gαi3 expression was significantly correlated with poor prognosis in advanced T-stage cervical cancers (Figure 1D). Gαi3 overexpression in cervical cancer was however not associated with M-stage and N-stage status (Figure 1D). In addition, subgroup analysis of different clinical characteristics showed that Gαi3 overexpression in M0 cervical cancer patients was significantly associated with poor prognosis (HR=3.20, P = 0.021, Figure 1E).
Alignment Diagram (Nomogram) prediction map based on clinical parameters and Gαi3 expression could effectively predict the occurrence probability of 1-, 3-, and 5-year survival response (Figure 1F and G). The high Gαi3 expression predicting poor 1-, 3-, and 5-year survival response is highly consistent with the actual clinical results (Figure 1F and G).
Next, TCGA results were analyzed and the differentially expressed gene (DEGs) were retrieved to examine co-expression genes with Gαi3 in cervical cancer tissues. The volcanic map of Gαi3-assocaited DEGs is shown in Figure 1H (|LogFC|>1, Adjust P-value < 0.05). KEGG pathway analysis (Figure 1I) found that Gαi3-associated DEGs were enriched in different oncogenic cascades including extracellular matrix (ECM) receptor interaction, renal cell cancer (RCC), pancreatic cancer, basal transcription factors and bladders (Figure 1I). These results implied that Gαi3-associated DEGs could be involved in carcinogenesis and cancer progression. Together, these bioinformatics results show that Gαi3 overexpression in cervical cancer is correlated with poor overall survival.
Gαi3 upregulation in cervical cancer tissues of local patients
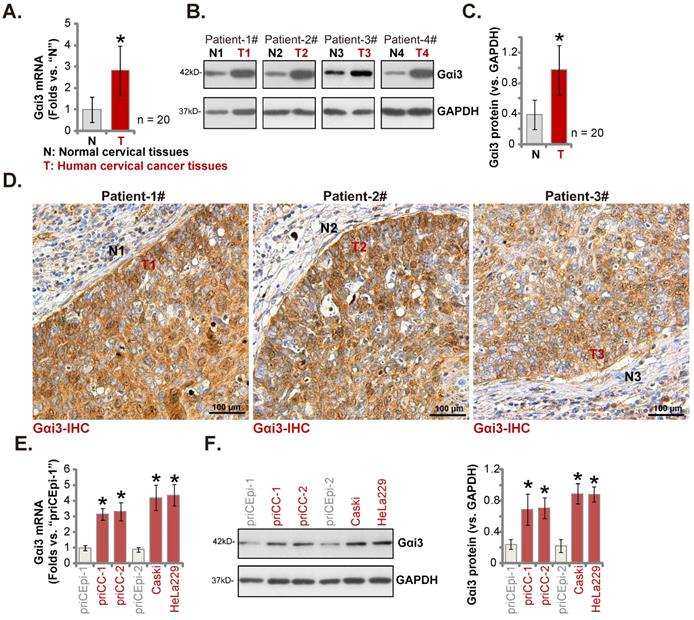
Next we examined Gαi3 expression in local cervical cancer tissues. The cervical cancer tissues (“T”) and matched paracancerous cervical epithelial tissues (“N”) of twenty (n = 20) primary cervical cancer patients were obtained. Gαi3 mRNA levels in the cervical cancer tissues were s higher (Figure 2A). Moreover, Gαi3 protein expression was remarkably elevated in four patients (Patient 1# to 4#)'s cervical cancer tissues (Figure 2B). When combining all twenty sets patient tissues' blotting data, we discovered that Gαi3 protein upregulation in cervical cancer tissues was significant (Figure 2C). In addition, the immunohistochemistry (IHC) staining results of Patient 1# to 3# further supported robust Gαi3 protein upregulation in cervical cancer (Figure 2D). Gαi3 expression in human cervical cancer cells was tested as well. As shown expression levels of both Gαi3 mRNA and protein were elevated in the primary human cervical cancer cells (“priCC-1” and “priCC-2”) and immortalized lines (Caski and HeLa229) (Figure 2E and F). The relative low Gαi3 expression was obsereved in primary cervical epithelial cells (“priCEpi-1” and “priCEpi-2”) (Figure 2E and F). These results clearly supported Gαi3 overexpression in cervical cancer.
Gαi3 overexpression in cervical cancer is correlated with poor overall survival. TCGA cohorts plus GTEx database revealed Gαi3 transcripts in 306 cases of cervical cancer tissues (“Tumor”) and 13 cases of normal epithelial tissues (“Normal”) (A). TCGA cervical cancer cohorts (CESC) showed the Kaplan Meier Survival curve of Gαi3-low (in blue) and Gαi3-high (in red) cervical cancer patients (B and C). The subgroup analyses of Gαi3 mRNA expression and clinical characteristics of cervical patients in TCGA cervical cancer cohorts (CESC) were shown (D and E). Nomogram for high Gαi3 expression in predicting 1-, 3- and 5-year overall survival probability of cervical cancer patients was shown (F and G). The volcano map of differentially expressed gene (DEGs) based on Gαi3 expression in TCGA cervical cancer cohorts (CESC) was shown (H); KEGG pathway analysis of Gαi3-associated DEGs and enriched pathways were presented (I). ***P < 0.001; *P < 0.05; “N. S.” means P > 0.05.
Gαi3 upregulation in cervical cancer tissues of local patients. Listed genes and proteins in cervical cancer tissues (“T”) and matched normal cervical epithelial tissues (“N”) from a total of twenty (n = 20) primary cervical cancer patients were measured, and results were quantified (A-C). IHC images confirmed Gαi3 protein upregulation in cervical cancer tissue slides of three representative patients (D). Gαi3 mRNA and protein expression in listed cervical cancer cells and cervical epithelial cells was shown (E and F). *P < 0.05 versus “N” tissues/priCEpi-1 cells. Scale bar = 100 µm.
shRNA-induced silencing of Gαi3 inhibits cervical cancer cell growth and migration
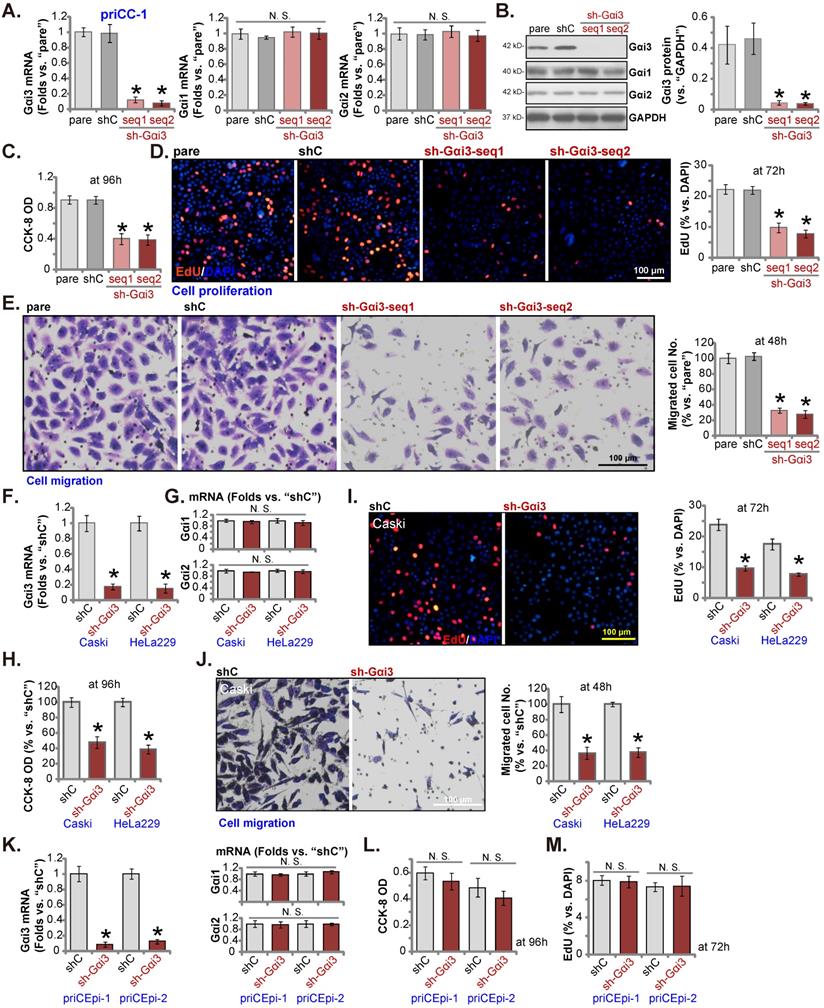
In order to test whether Gαi3 could exert pro-cancerous activity, the shRNA strategy was utilized. Two lentiviral Gαi3 shRNAs, “sh-Gαi3-seq1” and “sh-Gαi3-seq2” [26], were transduced to priCC-1 primary cancer cells. Following selection, stable priCC-1 cells bearing Gαi3 shRNA were formed. Gαi3 mRNA was silenced in sh-Gαi3-bearing stable priCC-1 cells (Figure 3A). Gαi1 /2 mRNA expression was however unchanged (Figure 3A). Gαi3 shRNAs in priCC-1 cells also resulted in remarkable Gαi3 protein downregulation (Figure 3B), leaving Gαi1/2 protein expression unaffected (Figure 3B). CCK-8 OD, or cell viability, was decreased in Gαi3-silenced priCC-1 cells (Figure 3C). In addition, Gαi3 silencing robustly hindered EdU incorporation and decreased EdU-positive nuclei percentage in priCC-1 cells, causing significant proliferation inhibition (Figure 3D). Silencing Gαi3 by the targeted shRNAs slowed priCC-1 cell in vitro migration (Figure 3E) assays. These results showed that Gαi3 shRNA provoked significant anti-cancer activity in primary cervical cancer cells.
The established Caski and HeLa229 cells were transduced with sh-Gαi3-seq1, and stable cells formed, namely “sh-Gαi3” cells. The applied Gαi3 shRNA led to significant Gαi3 mRNA silencing in the immortalized cervical cancer cells (Figure 3G), and Gαi1/2 mRNA expression was unchanged (Figure 3H). Similar to the results in priCC-1 primary cells, Gαi3 silencing inhibited viability (Figure 3H), EdU incorporation/proliferation (Figure 3I) and migration (Figure 3J) in the immortalized cells. The sh-Gαi3-seq1-containing lentivirus was transfected to the primary human cervical epithelial cells, priCEpi-1 and priCEpi-2 (see Figure 2). The stable cells with the shRNA were established and they were named as “sh-Gαi3” epithelial cells, where Gαi3 mRNA levels were significantly downregulated (Figure 3K). Gαi1/2 mRNA expression was unaffected (Figure 3K). Interestedly, Gαi3 silencing failed to significantly decrease viability (Figure 3L) and nuclear EdU incorporation (Figure 3M) in primary cervical epithelial cells.
shRNA-induced silencing of Gαi3 inhibits cervical cancer cell growth and migration. The primary priCC-1 cells, the established Caski and HeLa229 cells, priCEpi-1 and priCEpi-2 epithelial cells were stably transduced with the applied lentiviral Gαi3 shRNA (“sh-Gαi3”, seq1/seq2 standing for two different sequences) or the scramble control shRNA (“shC”), listed genes and proteins were tested (A, B, F, G and K). Cells were further cultivated for another 48-96h, cell viability (C, H, and L), proliferation (D, I and M), migration (E and J) were tested. “pare” were the parental control cells (same for all Figures). *P < 0.05 versus “pare”/“shC” group. “N. S.” means P > 0.05. Scale bar = 100 µm.
Gαi3 silencing provokes apoptosis in cervical cancer cells. priCC-1 cells, the established Caski and HeLa229 cells, priCEpi-1 and priCEpi-2 epithelial cells were stably transduced with the applied lentiviral Gαi3 shRNA (“sh-Gαi3”, seq1/seq2) or the scramble control shRNA (“shC”), cells were further cultured for 72-96h, the Caspase-3 activity was tested (A, D and F); Cell apoptosis was tested by nuclear TUNEL/DAPI staining (B, E and G) and Annexin V flow cytometry (C) assays. *P < 0.05 versus “shC” group. “N. S.” means P > 0.05. Scale bar = 100 µm.
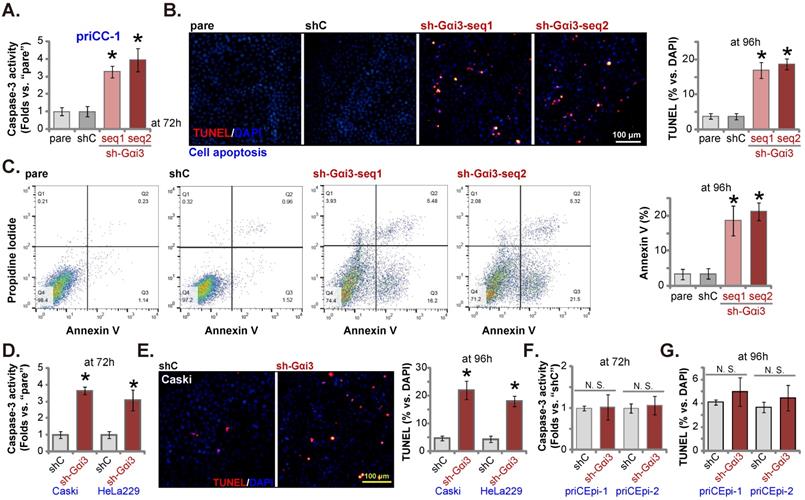
Gαi3 silencing provokes apoptosis in cervical cancer cells
Next we examined the potential effect of Gαi3 silencing on cell apoptosis. As shown, in priCC-1 cells expressing Gαi3 shRNA (“sh-Gαi3-seq1” or “sh-Gαi3-seq2”), increased Caspase-3 activity was detected (Figure 4A). TUNEL-positively stained nuclei (Figure 4B) and Annexin V-positive cells (Figure 4C) were significantly boosted after Gαi3 silencing, supporting apoptosis activation. In Caski and HeLa229 cells, shRNA-induced stable knockdown of Gαi3 enhanced the Caspase-3 activity (Figure 4D) and TUNEL nuclei percentage (Figure 4E). In priCEpi-1 and priCEpi-2 normal cell silencing of Gαi3, using sh-Gαi3-seq1, failed to augment the Caspase-3 activity (Figure 4F) and TUNEL-positively stained nuclei number (Figure 4G).
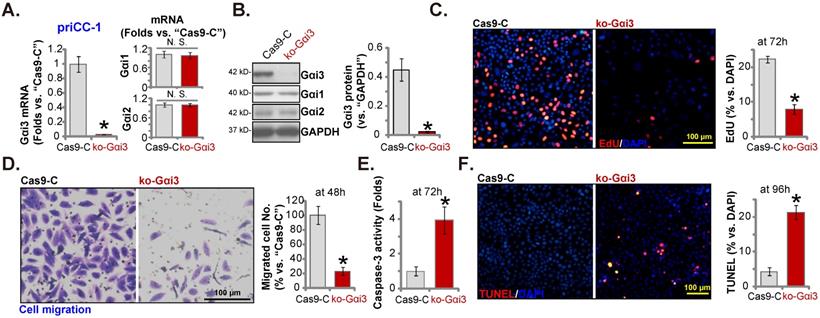
Gαi3 KO results in robust anti-cervical cancer cell activity
To further support the pro-cancerous activity of Gαi3, the CRISPR/Cas9 gene editing strategy, as described [26], was employed to knockout (KO) Gαi3 in cervical cancer cells (“ko-Gαi3” priCC-1 cells). As compared to the control Cas9-expressing priCC-1 cells with the lenti-CRISPR/Cas9 empty vector (“Cas9-C”), Gαi3 was depleted in the ko-Gαi3 priCC-1 cells (Figure 5A and B), where Gαi1/2 expression was unchanged (Figure 5A and B). Gαi3 KO inhibited priCC-1 cell proliferation and reduced EdU nuclei percentage (Figure 5C). In addition, priCC-1 in vitro cell migration (Figure 5D) was largely hindered following Gαi3 KO. In the ko-Gαi3 priCC-1 cells, the Caspase-3 activity (Figure 5F) and the TUNEL percentage (Figure 5G) were both increased. Therefore, Gαi3 KO exerted robust anti-cancer activity in primary cervical cancer cells.
Gαi3 overexpression exerts pro-cervical cancer activity
Gαi3 silencing or KO resulted in robust anti-cervical cancer cell activity. Ectopic overexpression Gαi3 could therefore possibly induce opposite activity. A lentiviral Gαi3-expressing construct, as reported in our previous studies [16, 18, 26], was transduced to priCC-1 cells. These cells were named as “OE-Gαi3” cells where Gαi3 expression was robustly increased (Figure 6A and B). Gαi1/2 expression was unchanged (Figure 6A and B). In priCC-1 cells, ectopic Gαi3 overexpression promoted cell proliferation and EdU incorporation (Figure 6C). Moreover, Gαi3 overexpression accelerated priCC-1 cell in vitro migration (Figure 6D).
Gαi3 KO results in robust anti-cervical cancer cell activity. The primary priCC-1 cells, bearing a lenti-CRISPR/Cas9-Gαi3-KO construct (“ko-Gαi3”) or the empty vector (“Cas9-C”), were established, and listed genes and proteins were measured (A and B); Cells were further cultured for another 48-96h, cell proliferation and migration (C and D), as well as the Caspase-3 activity (E) and apoptosis (F) were tested. *P < 0.05 versus “Cas9-C” group. “N. S.” means P > 0.05. Scale bar = 100 µm.
Gαi3 overexpression exerts pro-cervical cancer activity. The primary priCC-1 cells, the established Caski and HeLa229 cells, priCEpi-1 and priCEpi-2 epithelial cells, bearing the lentiviral Gαi3-expressing construct (“OE-Gαi3”) or the empty vector (GV369, “Vec”), were established, and expression of listed genes and proteins were tested (A, B, E and H); Cells were further cultured for 48-96h, EdU incorporation/proliferation (C, F and J), migration (D and G), and viability (I) were measured. *P < 0.05 versus “Vec” group. “N. S.” means P > 0.05. Scale bar = 100 µm.
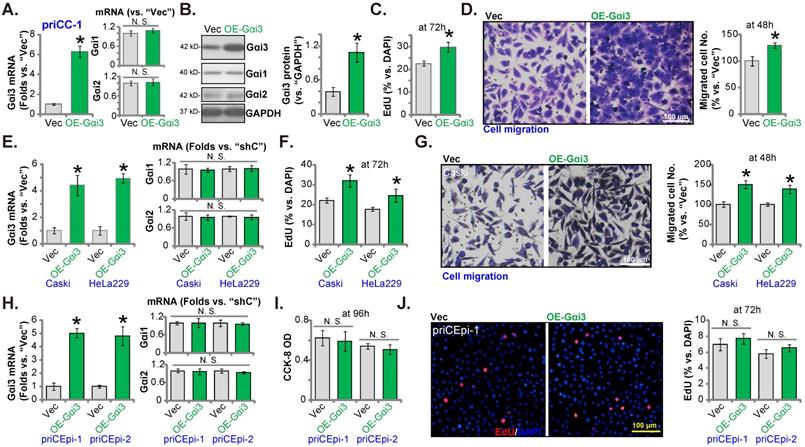
The same lentiviral Gαi3-expressing construct (“OE-Gαi3”) were stably transduced to Caski cells and HeLa229 cells, causin Gαi3 mRNA overexpression (Figure 6E). Gαi1/2 mRNA expression was unchanged (Figure 6E). OE-Gαi3 enhanced EdU incorporation (Figure 6F) and migration (Figure 6G) in Caski and HeLa229 cells. The Gαi3-expressing construct was stably transduced to priCEpi-1 and priCEpi-2 epithelial cells. Stable cells, or OE-Gαi3 cells, were formed. Gαi3 mRNA (but not Gαi1/2 mRNA) upregulation expression was detected in the OE-Gαi3 epithelial cells (Figure 6H). However, Gαi3 overexpression exerted no significant effects on CCK-8 viability (Figure 6I) and EdU incorporation/proliferation (Figure 6J) in priCEpi-1 and priCEpi-2 cells.
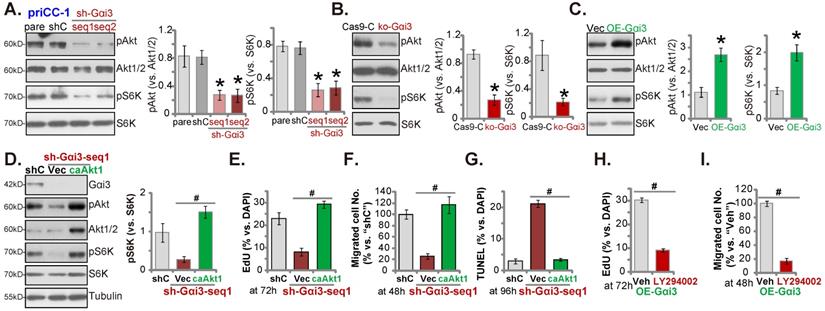
Gαi3 is important for Akt-mTOR activation in cervical cancer cells
Gαi proteins association with multiple RTKs (EGFR, VEGFR, TrkB and others [16, 18, 19, 22, 26]) is required for mediating downstream Akt-mTOR activation. In priCC-1 cells, shRNA-induced silencing of Gαi3 largely inhibited phosphorylation of Akt (at Ser-473) and S6K (at Thr-389) (Figure 7A). Moreover, KO of Gαi3 (see Figure 5) remarkably decreased Akt-S6K phosphorylation in priCC-1 cells. Contrarily, Gαi3 overexpression (OE-Gαi3) in priCC-1 cells enhanced Akt-S6K phosphorylation (Figure 7C). Akt1/2 and S6K expression levels were unchanged in the Gαi3-altered priCC-1 cells (Figure 7A-C). Gαi3 is therefore important for Akt-mTOR activation in priCC-1 cells.
To test that Gαi3 silencing-provoked anti-cancer cell activity was due to inactivating Akt-mTOR signaling cascade, the lentivirus encoding caAkt1 [26]) was transduced to sh-Gαi3-seq1-expressing priCC-1 primary cells. caAkt1 completely restored Akt-S6K phosphorylation in the Gαi3-silenced priCC-1 cells (Figure 7D) without affecting Gαi3 protein expression (Figure 7D). Importantly, Gαi3 shRNA-induced proliferation inhibition (Figure 7E), migration reduction (Figure 7F) and apoptosis (Figure 7G) were almost completely abolished by caAkt1. Thus Gαi3 silencing-induced anti-cervical cancer cell activity was possibly due to inactivating Akt-mTOR activation. Next, we found that the Akt-mTOR blocker LY294002 [32] largely inhibited proliferation (Figure 7H) and migration (Figure 7I) of OE-Gαi3 priCC-1 cells.
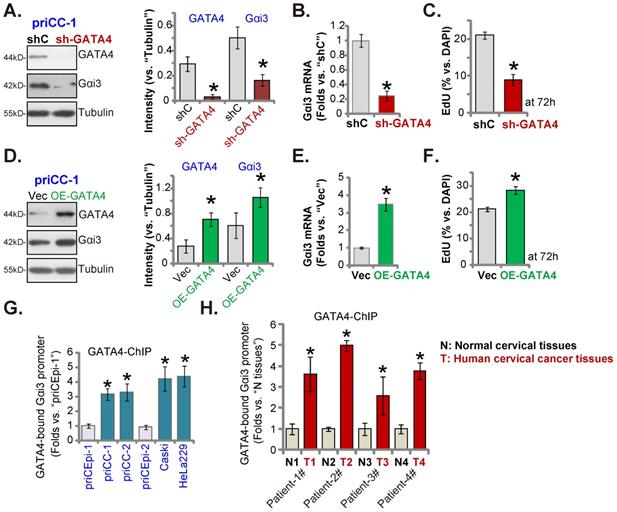
GATA4 is important for Gαi3 expression in cervical cancer cells
Our group [28] and others have supported that GATA4 is one important transcription factor for Gαi3 [33]. We therefore analyzed whether GATA4 was the primary mechanism of Gαi3 overexpression in cervical cancer. GATA4 shRNA-expressing lentivirus [28] was stably transfected to primary human cervical cancer priCC-1 cells, resulting in robust GATA4 silencing (Figure 8A). GATA4 shRNA robustly decreased Gαi3 mRNA and protein (Figure 8A and B) expression in priCC-1 cells. Moreover, GATA4 shRNA inhibited priCC-1 cell proliferation and decreased EdU-incorporated nuclei ratio (Figure 8C).
On the contrary, the GATA4-overexpressing lentiviral construct was stably transduced to priCC-1 cells to establish OE-GATA4 cells, where GATA4 protein level was remarkably increased (Figure 8D). OE-GATA4 resulted in Gαi3 mRNA (Figure 8E) and protein (Figure 8D) upregulation in priCC-1 cells. Cell proliferation, tested by EdU incorporation, was enhanced by GATA4 overexpression (Figure 8F). Therefore, GATA4 is indeed essential for Gαi3 expression in cervical cancer cells.
Remarkably, GATA4 chromosome immunoprecipitation (ChIP) results revealed that GATA4-Gαi3 promoter DNA binding [33] in cervical cancer cells was robustly higher than that in priCEpi-1 and priCEpi-2 epithelial cells (Figure 8G). Moreover, in cervical cancer tissues of four representative patients, GATA4 binding to the Gαi3 promoter DNA was significantly higher than that in the matched surrounding normal cervical tissues (Figure 8H). These results implied that increased GATA4-Gαi3 promoter binding could be one primary mechanism of Gαi3 overexpression in cervical cancer.
Gαi3 is important for Akt-mTOR activation in cervical cancer cells. priCC-1 cells bearing the described genetic modifications were established and culture, and listed proteins in total cell lysates were measured (A-C). Akt-S6K phosphorylation was quantified (A-C). The sh-Gαi3-seq1-expressing priCC-1 cells were further stably transduced with a lentiviral constitutively-active Akt1 (caAkt1, S473D) construct or the empty vector (“Vec”), and expression of listed proteins was shown (D); Cells were further cultivated for 48-96h, cell proliferation, migration and apoptosis were examined by the described assays, and results were quantified (E-G). OE-Gαi3 priCC-1 cells were treated with LY294002 (5 µM) or 0.1% DMSO (“Veh”) for the designated hours, cell proliferation and migration were tested similarly and results were quantified (H and I). “pare” stands for the parental control cells. *P < 0.05 versus “pare”/“Cas9-C”/“Vec” group. # P < 0.05. “N. S.” means P > 0.05.
GATA4 is important for Gαi3 expression in cervical cancer cells. Expression of listed genes and proteins in primary priCC-1 cells with described GATA4 genetic modification was shown (A, B, D and E). Cells were further cultivated for 72h and cell proliferation was tested by measuring EdU-positive nuclei percentage (C and F). Chromosome IP (ChIP) revealed the relative amount of Gαi3 promoter DNA binding to GATA4 protein in the listed cervical cancer cells and epithelial cells (G) as well as in the listed human tissues of four representative patients (H). *P < 0.05 versus “shC”/“Vec”/“priCEpi-1 cells”/“N” tissues.
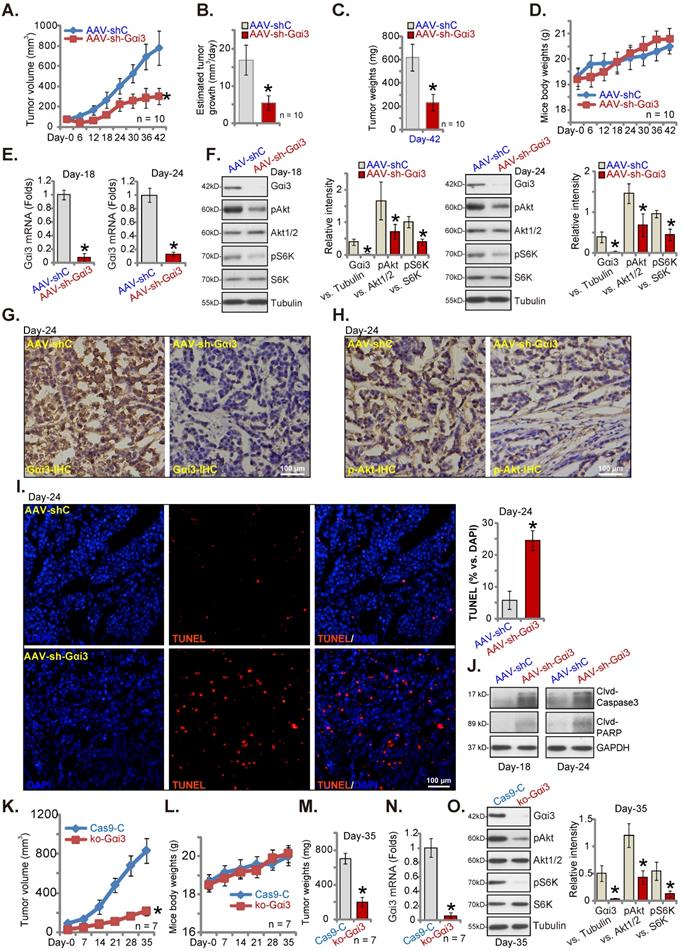
Gαi3 depletion suppresses cervical cancer xenograft growth in nude mice
Gαi3's role on cervical cancer cell growth in vivo was explored. priCC-1 cells, at seven million cells per mouse, were s.c. injected to nude mice right flanks. After three weeks, the xenografts were formed (~ 80 mm3). Mice were then randomly separated into two groups, receiving daily (for ten days) intratumoral injection of AAV-packed Gαi3 shRNA (AAV-sh-Gαi3 [26]) or control AAV shRNA (AAV-shC [26]). Figure 9A demonstrated that AAV-sh-Gαi3 injection remarkably hindered priCC-1 xenograft growth in nude mice. The estimated daily tumor growth, in mm3 per day [26], was remarkably inhibited after AAV-sh-Gαi3 treatment (Figure 9B). At Day-42, priCC-1 xenografts were isolated and the AAV-sh-Gαi3 group priCC-1 xenografts were lighter than the AAV-shC group xenografts (Figure 9C). The mice body weights between the two groups were indifferent (Figure 9D). Thus, intratumoral injection of Gαi3 shRNA-AAV remarkably suppressed priCC-1 xenograft growth.
At Day-18 and 24, one priCC-1 xenograft in the treatment and control group mice was carefully isolated (total four xenografts). Signaling proteins were tested. Western blotting and qRT-PCR assaying of fresh tissue lysates found that Gαi3 mRNA (Figure 9E) and protein (Figure 9F) were silenced in AAV-sh-Gαi3-injected xenografts. Moreover, Akt-S6K phosphorylation was significantly decreased (Figure 9F). The representative IHC images further confirmed Gαi3 protein silencing in AAV-sh-Gαi3-injected priCC-1 xenografts (at Day-24, Figure 9G). In addition, IHC images verified Akt inhibition in Gαi3-silenced priCC-1 xenografts (at Day-24, Figure 9H). The fluorescence staining of priCC-1 xenograft slides demonstrated increased TUNEL staining in AAV-sh-Gαi3-injected tumors (at Day-24, Figure 9I), indicating apoptosis activation. Moreover, Caspase-3 and PARP cleavages were increased (Figure 9J). Thus, Gαi3 silencing by AAV-sh-Gαi3 injection robustly suppressed Akt-mTOR activation and provoked apoptosis in priCC-1 xenografts.
To further support the important role of Gαi3 in cervical cancer cell growth in vivo, ko-Gαi3 priCC-1 cells or “Cas9-C” cells (see Figure 5) were s.c. injected to nude mice's flanks. The tumor recordings were started three weeks after (labeled as “Day-0”). The growth of ko-Gαi3 priCC-1 xenografts was largely inhibited when compared to the Cas9-C priCC-1 xenografts (Figure 9K). Animal body weights were again indifferent (Figure 9L). At Day-35 we found that Gαi3 KO priCC-1 xenografts were much lighter than Cas9-C xenografts (Figure 9M). Gαi3 mRNA and protein were depleted in ko-Gαi3 priCC-1 xenografts (Figure 9N and O), where Akt-S6K phosphorylation was remarkably decreased (Figure 9O).
Gαi3 depletions suppresses cervical cancer xenograft growth in nude mice. The priCC-1 xenograft-bearing nude mice were intratumorally injected daily with AAV-packed Gαi3 shRNA (AAV-sh-Gαi3) or control AAV shRNA (AAV-shC). Tumor volumes (A), the estimated daily tumor growth (B), priCC-1 xenograft weights (at Day-42, C) and animal body weights (D) were shown. At Day-18/Day-24, one priCC-1 xenograft in AAV-sh-Gαi3 and AAV-shC groups was carefully isolated, listed genes and proteins in the xenograft tissue lysates were tested (E, F and J). The representative IHC images of Gαi3 and p-Akt (Ser-473) were presented (G and H). The representative fluorescence images showing nuclear TUNEL and DAPI staining in xenograft slides were presented as well (I). The ko-Gαi3 priCC-1 cells or the Ca9-C control priCC-1 cells were s.c. injected to nude mice's right flanks. After three weeks, recordings were started (“Day-0”). Tumor volumes (K) and the mice body weights (L) were presented. At Day-35, priCC-1 xenografts were isolated and weighted (M). The listed genes and proteins in the xenograft lysates were tested (N and O). *P < 0.05 versus “AAV-shC”/“Ca9-C” group. Scale bar = 100 µm.
Discussion
The mortality rate of cervical cancer has obvious regional differences [3, 4, 34]. Traditional treatment methods, including surgery, radiotherapy and chemotherapy, have their limitations. With the latest development of targeted therapy, especially the use of targeted drugs such as bevacizumab, the survival time of certain cervical cancer patients could be prolonged [7, 9, 12]. However for most cervical cancer patients, the novel targeted therapies are in urgent need [7, 9, 12].
Our recent studies have shown that Gαi3 could be a novel therapeutic oncotarget of human cancer [16, 26]. We previously found that Gαi3 expression is elevated in osteosarcoma and correlates with poor overall survival [26]. Gαi3 is important for osteosarcoma cell growth Conversely, Gαi3 shRNA or KO robustly inhibited osteosarcoma cell growth [26]. Moreover, Gαi3 overexpression was detected in human glioma tissues and is significantly correlated with poor overall survival [16]. Gαi3 silencing potently inhibited patient-derived glioma xenograft orthotopic growth [16]. Overexpression of Gαi3, on the other hand, significantly enhanced glioma growth [16].
Our study supports that Gαi3 is a valuable oncotarget of cervical cancer. The bioinformatics analysis revealed that the number of Gαi3 mRNA transcripts is elevated in human cervical cancer tissues, and Gαi3 upregulation was correlated with patients' poor overall survival and DSS. Gαi3 mRNA and protein levels in local cervical cancer tissues were upregulated. Remarkably, Gαi3 depletion resulted in robust anti-cervical cancer cell activity. In different cervical cancer cells, Gαi3 silencing or KO resulted in robust anti-cancer activity. Conversely, ectopic overexpression of Gαi3 further promoted cervical cancer proliferation. In vivo, the growth of cervical cancer xenografts was remarkably hindered after Gαi3 silencing or KO. These results clearly supported that targeting Gαi3 could be a promising therapeutic strategy against cervical cancer.
Our group has identified Gαi proteins, Gαi1 and Gαi3, as key signaling molecules mediating downstream signalings by a number RTKs [16, 18-22, 25, 26, 28] and non-RTK receptors [17]. Gαi1 and Gαi3 can associate with ligand-activated RTKs to transduce downstream mitogenic/oncogenic signaling cascades [16, 18-22, 25, 26, 28]. For example, Gαi1/3 located in the VEGFR2 endocytosis complex, essential for VEGF (vascular endothelial growth factor)-induced endocytosis of VEGFR2 and downstream signaling transduction [18]. Moreover, Gαi1/3 proteins are required for brain-derived neurotrophic factor (BDNF)-induced signaling activation [19]. In addition, Gαi1/3 proteins can associate with EGF-stimulated EGFR and the adaptor protein Gab1, mediating downstream Akt-mTOR cascade activation [22].
The Akt-mTOR signaling is an important target for the development of cervical cancer therapeutics [35, 36]. Here we found that Gαi3 is vital for Akt-mTOR cascade activation in cervical cancer cells. Akt-mTOR activation was significantly inhibited after Gαi3 silencing or KO, but augmented following Gαi3 overexpression. Reduced Akt-S6K phosphorylation was also detected in cervical cancer xenograft tissues with Gαi3 silencing or KO. Notably, Gαi3 silencing-induced anti-cervical cancer activity, including proliferation inhibition, migration reduction and apoptosis induction, were almost reversed following Akt-S6K re-activation by caAkt1. Moreover, Gαi3 overexpression-induced proliferation and migration acceleration was largely inhibited by LY294002, the PI3K-Akt-mTOR inhibitor, in cervical cancer cells.
An early study using luciferase reporter assay and chromatin Immunoprecipitation (ChIP) demonstrated that the transcription factor GATA4 can directly bind to the promoter region of Gαi3 and regulate its transcriptional activity and expression [33]. The very recent study of our group has shown that GATA4 is an important transcription factor of Gαi3 in endothelial cells [28]. We further discovered that phosphoenolpyruvate carboxykinase 1 (PCK1) associated with phosphorylated GATA4, promoting Gαi3 transcription and expression in endothelial cells [28]. It will then lead to increased Akt-mTOR activation and pro-angiogenesis response [28]. Here in cervical cancer cells, Gαi3 was decreased following GATA4 shRNA, but was upregulated following GATA4 overexpression. Significantly, an increased binding between GATA4 and Gαi3 promoter region in both cervical cancer tissues and various cervical cancer cells was detected. These results implied that GATA4-mediated increased Gαi3 transcription could be a primary mechanism of Gαi3 upregulation in cervical cancer.
Conclusion
These data suggest that targeting Gαi3 would be a promising therapeutic strategy against cervical cancer. Specific pharmacological inhibitors or pipeline drugs blocking Gαi3 association with RTKs should then inhibit downstream oncogenic cascade activation and cervical cancer progression.
Supplementary Material
Supplementary figures.
Acknowledgements
Funding
This work was generously supported by Key Research and Development Program of Jiangsu Province (No. BE2019652) and National Natural Science Foundation of China (81922025, 81802511, 81974388, 82171461, 81771457). A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. The authors declare that they have no competing interests. This study was approved by Ethics Committee of Soochow University. All institutional and national guidelines for the care were carefully followed.
Ethical Approval and Consent to participate
This study was approved by the Ethics Committee of Soochow University.
Availability of data and material
All data generated during this study are included in this published article. Data will be made available upon request.
Author contributions
All authors conceived the idea and designed the work, contributed to acquisition of data.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30
3. Arbyn M, Weiderpass E, Bruni L, de Sanjose S, Saraiya M, Ferlay J. et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191-e203
4. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169-82
5. Vu M, Yu J, Awolude OA, Chuang L. Cervical cancer worldwide. Curr Probl Cancer. 2018;42:457-65
6. Ferrall L, Lin KY, Roden RBS, Hung CF, Wu TC. Cervical Cancer Immunotherapy: Facts and Hopes. Clin Cancer Res. 2021;27:4953-73
7. Crafton SM, Salani R. Beyond Chemotherapy: An Overview and Review of Targeted Therapy in Cervical Cancer. Clin Ther. 2016;38:449-58
8. de Azevedo CR, Thuler LC, de Mello MJ, Ferreira CG. Neoadjuvant Chemotherapy Followed by Chemoradiation in Cervical Carcinoma: A Review. Int J Gynecol Cancer. 2016;26:729-36
9. Duenas-Gonzalez A, Serrano-Olvera A, Cetina L, Coronel J. New molecular targets against cervical cancer. Int J Womens Health. 2014;6:1023-31
10. Zagouri F, Sergentanis TN, Chrysikos D, Filipits M, Bartsch R. Molecularly targeted therapies in cervical cancer. A systematic review. Gynecol Oncol. 2012;126:291-303
11. Gheorghe AS, Dumitrescu EA, Komporaly IA, Mihaila RI, Lungulescu CV, Stanculeanu DL. New Targeted Therapies and Combinations of Treatments for Cervical, Endometrial, and Ovarian Cancers: A Year in Review. Curr Oncol. 2022;29:2835-47
12. Vora C, Gupta S. Targeted therapy in cervical cancer. ESMO Open. 2018;3:e000462
13. Zhang C, Liao Y, Liu P, Du Q, Liang Y, Ooi S. et al. FABP5 promotes lymph node metastasis in cervical cancer by reprogramming fatty acid metabolism. Theranostics. 2020;10:6561-80
14. Fang J, Zhang B, Wang S, Jin Y, Wang F, Ding Y. et al. Association of MRI-derived radiomic biomarker with disease-free survival in patients with early-stage cervical cancer. Theranostics. 2020;10:2284-92
15. Fan H, Li P, Zingarelli B, Borg K, Halushka PV, Birnbaumer L. et al. Heterotrimeric Galpha(i) proteins are regulated by lipopolysaccharide and are anti-inflammatory in endotoxemia and polymicrobial sepsis. Biochim Biophys Acta. 2011;1813:466-72
16. Wang Y, Liu YY, Chen MB, Cheng KW, Qi LN, Zhang ZQ. et al. Neuronal-driven glioma growth requires Galphai1 and Galphai3. Theranostics. 2021;11:8535-49
17. Bai JY, Li Y, Xue GH, Li KR, Zheng YF, Zhang ZQ. et al. Requirement of Galphai1 and Galphai3 in interleukin-4-induced signaling, macrophage M2 polarization and allergic asthma response. Theranostics. 2021;11:4894-909
18. Sun J, Huang W, Yang SF, Zhang XP, Yu Q, Zhang ZQ. et al. Galphai1 and Galphai3mediate VEGF-induced VEGFR2 endocytosis, signaling and angiogenesis. Theranostics. 2018;8:4695-709
19. Marshall J, Zhou XZ, Chen G, Yang SQ, Li Y, Wang Y. et al. Antidepression action of BDNF requires and is mimicked by Galphai1/3 expression in the hippocampus. Proc Natl Acad Sci U S A. 2018;115:E3549-E58
20. Liu YY, Chen MB, Cheng L, Zhang ZQ, Yu ZQ, Jiang Q. et al. microRNA-200a downregulation in human glioma leads to Galphai1 over-expression, Akt activation, and cell proliferation. Oncogene. 2018;37:2890-902
21. Zhang YM, Zhang ZQ, Liu YY, Zhou X, Shi XH, Jiang Q. et al. Requirement of Galphai1/3-Gab1 signaling complex for keratinocyte growth factor-induced PI3K-AKT-mTORC1 activation. J Invest Dermatol. 2015;135:181-91
22. Cao C, Huang X, Han Y, Wan Y, Birnbaumer L, Feng GS. et al. Galpha(i1) and Galpha(i3) are required for epidermal growth factor-mediated activation of the Akt-mTORC1 pathway. Sci Signal. 2009;2:ra17
23. Li X, Wang D, Chen Z, Lu E, Wang Z, Duan J. et al. Galphai1 and Galphai3 regulate macrophage polarization by forming a complex containing CD14 and Gab1. Proc Natl Acad Sci U S A. 2015;112:4731-6
24. Lv Y, Wang Y, Song Y, Wang SS, Cheng KW, Zhang ZQ. et al. LncRNA PINK1-AS promotes G alpha i1-driven gastric cancer tumorigenesis by sponging microRNA-200a. Oncogene. 2021;40:3826-44
25. Liu F, Chen G, Zhou L, Wang Y, Zhang Z, Qin X. et al. YME1L overexpression exerts pro-tumorigenic activity in glioma by promoting Gαi1 expression and Akt activation. Protein Cell. 2022
26. Bian ZJ, Shan HJ, Zhu YR, Shi C, Chen MB, Huang YM. et al. Identification of Galphai3 as a promising target for osteosarcoma treatment. Int J Biol Sci. 2022;18:1508-20
27. Li G, Zhou LN, Yang H, He X, Duan Y, Wu F. Ninjurin 2 overexpression promotes human colorectal cancer cell growth in vitro and in vivo. Aging (Albany NY). 2019;11:8526-41
28. Yao J, Wu XY, Yu Q, Yang SF, Yuan J, Zhang ZQ. et al. The requirement of phosphoenolpyruvate carboxykinase 1 for angiogenesis in vitro and in vivo. Sci Adv. 2022;8:eabn6928
29. Zhang D, Xia H, Zhang W, Fang B. The anti-ovarian cancer activity by WYE-132, a mTORC1/2 dual inhibitor. Tumour Biol. 2016;37:1327-36
30. Yang H, Zhao J, Zhao M, Zhao L, Zhou LN, Duan Y. et al. GDC-0349 inhibits non-small cell lung cancer cell growth. Cell Death Dis. 2020;11:951
31. Gao YY, Ling ZY, Zhu YR, Shi C, Wang Y, Zhang XY. et al. The histone acetyltransferase HBO1 functions as a novel oncogenic gene in osteosarcoma. Theranostics. 2021;11:4599-615
32. Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC Jr, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256-67
33. Guo S, Zhang Y, Zhou T, Wang D, Weng Y, Wang L. et al. Role of GATA binding protein 4 (GATA4) in the regulation of tooth development via GNAI3. Sci Rep. 2017;7:1534
34. Canfell K, Kim JJ, Brisson M, Keane A, Simms KT, Caruana M. et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:591-603
35. Bossler F, Hoppe-Seyler K, Hoppe-Seyler F. PI3K/AKT/mTOR Signaling Regulates the Virus/Host Cell Crosstalk in HPV-Positive Cervical Cancer Cells. Int J Mol Sci. 2019 20
36. Bahrami A, Hasanzadeh M, Hassanian SM, ShahidSales S, Ghayour-Mobarhan M, Ferns GA. et al. The Potential Value of the PI3K/Akt/mTOR Signaling Pathway for Assessing Prognosis in Cervical Cancer and as a Target for Therapy. J Cell Biochem. 2017;118:4163-9
Author contact
![]() Corresponding authors: Dr. Yan Lv, E-mail: lvyanlabcom; Prof. Hai-wei Huang, E-mail: huanghaiwei760401com; Prof. Cong Cao, E-mail: caocongedu.cn.
Corresponding authors: Dr. Yan Lv, E-mail: lvyanlabcom; Prof. Hai-wei Huang, E-mail: huanghaiwei760401com; Prof. Cong Cao, E-mail: caocongedu.cn.

 Global reach, higher impact
Global reach, higher impact