10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(15):5849-5857. doi:10.7150/ijbs.77030 This issue Cite
Research Paper
Humoral Immunogenicity to SARS-CoV-2 Vaccination in Liver Transplant Recipients: A Systematic Review and Meta-Analysis
1. Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Soonchunhyaung University College of Medicine, Bucheon, Republic of Korea.
2. Department of Pediatrics, Kyung Hee University Hospital, Kyung Hee University College of Medicine, Seoul, Republic of Korea.
3. Department of Data Science, Sejong University College of Software Convergence, Seoul, Republic of Korea.
4. Sungkyunkwan University School of Medicine, Suwon, Republic of Korea.
5. Department of Pediatrics, Yonsei University College of Medicine, Seoul, Republic of Korea.
6. Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea.
7. Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Republic of Korea.
8. Yonsei Liver Center, Severance Hospital, Yonsei University Health System, Seoul, Republic of Korea.
Received 2022-7-11; Accepted 2022-8-20; Published 2022-9-21
Abstract
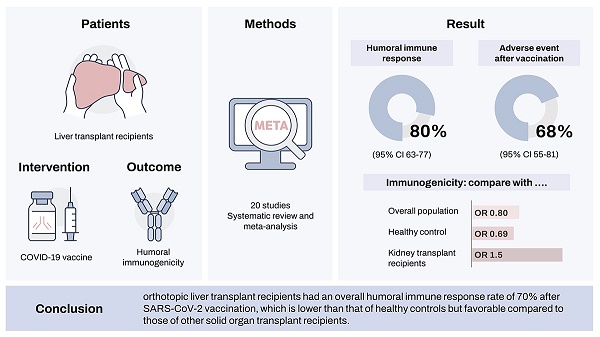
Solid organ transplant recipients generally show reduced immunogenicity to various vaccines. We aimed to assess the immunogenicity of the immune response among orthotopic liver transplant (OLT) recipients after the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination. A systematic search was performed to evaluate immunogenicity or adverse events reported after SARS-CoV-2 vaccination. The pooled analysis of 20 studies showed a humoral immune response rate of 0.70 (95% confidence interval [CI], 0.63-0.77) after SARS-CoV-2 vaccination among OLT recipients. The immunogenicity among OLT recipients was significantly lower compared to the overall population and healthy controls, with odds ratios (ORs) of 0.80 and 0.69. However, it was significantly higher than that of patients receiving other organ transplants, especially kidneys, with an OR of 1.50. Male sex, old age, chronic kidney disease, obesity, and multiple or high immunosuppressant doses significantly increased the risk of unresponsiveness in patients with OLT. The overall incidence of any adverse event after vaccination was 0.68 (95% CI, 0.55-0.81), similar to that of control. OLT recipients had an overall humoral immune response rate of 70% after SARS-CoV-2 vaccination, which is lower than that of healthy controls but favourable compared to those of other solid organ transplant recipients.
Keywords: Meta-analysis, liver transplant, vaccine, SARS-CoV-2, immunogenicity
Introduction
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to more than 6 million deaths, dramatically increasing the burden on healthcare systems worldwide [1-5]. Since vaccination against SARS-CoV-2 is the most appropriate way to achieve herd immunity, more than 5 billion people globally have received at least one dose as of April 2022. Considering that patients who had organ (or liver) transplant have worse COVID-19 outcomes than general population, vaccination against SARS-CoV-2 is generally recommended for this population [6-10].
In contrast, solid organ transplant recipients generally show reduced immunogenicity to a number of vaccines. For example, extensive studies on influenza vaccines have shown that antibody- and cell-mediated immune responses are lower in solid organ transplant recipients than in the general population [11,12]. This is primarily due to inhibited lymphocyte activation, interaction with antigen-presenting cells, and decreased B-cell memory responses led by the lifelong administration of immunosuppressive agents [13]. Nevertheless, vaccination against influenza has been associated with a reduction in influenza-associated complications in patients receiving solid organ transplants. Likewise, although the overall response rate of the first dose of mRNA vaccine against SARS-CoV-2 among solid organ transplant recipients was disappointingly low, it seems prudent to vaccinate immunocompromised patients since the benefits outweigh the risks [14]. Only 17% of these vaccinated individuals achieved detectable antibodies to the SARS-CoV-2 spike protein, although the response rate might vary depending on the type of transplanted organ and/or immunosuppressive agents [13]. However, pooled analyses of serial vaccine pharmacodynamics, effectiveness, and response duration in terms of humoral or cellular immunity against SARS-CoV-2 specified for populations receiving orthotopic liver transplants (OLT) are currently scarce.
Therefore, herein we aimed to explore the trends of humoral immune response immunogenicity among patients receiving OLT after completion of the SARS-CoV-2 vaccine series and assess the identified risk factors for vaccine nonresponse.
Materials and Methods
The protocol for this review was registered with PROSPERO (International Prospective Register of Systematic Reviews, CRD42022324652) in advance. This systematic review and meta-analysis were performed according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Inclusion criteria, exclusion criteria, and study outcomes
Studies were included if they were randomised controlled trials, cross-sectional studies, or cohort studies, including those of prospective and retrospective designs that reported on the immunogenicity of COVID-19 vaccination in adult OLT recipients (>19 years old) of deceased or living donor liver transplants. For the COVID-19 vaccine, both mRNA and viral vector vaccines were investigated. The exclusion criteria were as follows: i) case reports, ii) case series of fewer than five patients, and iii) reviews. The primary outcome of interest was the proportion of liver transplant recipients with a serological antibody response to a full or partial COVID-19 vaccination dose.
Search strategy
We searched for synonymous terms and used them to develop the search strategy. The keywords used in the Patient/Problem, Intervention, Comparison, and Outcome (PICO) model are shown in the Supplementary material and method section. We searched the PubMed (Medline), EMBASE, and Cochrane Library databases using terms Medical Subject Headings (MeSH) terms to identify studies published in English between 1 January 2019 and 31 March 2022. The search strategies and results of each database search are shown in the Supplementary material and method section. The search terms included liver transplantation-related index words and COVID-19 vaccine-related index words.
Study selection and data extraction
Two authors independently screened the titles and abstracts. Two reviewers (BKK and JJY) independently screened the full-text articles for study relevance. Any discrepancy between the two reviewers was resolved by JIS or JYK after discussion. The two researchers also independently performed the risk of bias assessment and extracted the study data, including the characteristics and results, and recorded them in a standard form.
Methodological quality and risk of bias assessment
We used the Risk of Bias Assessment tool for Nonrandomised Studies (RoBANS) [15] to assess the risk of bias; the overall results are shown in the Supplementary material risk of bias section. Any discrepancy was resolved by two authors (BKK and JJY) after discussion. Publication bias was assessed using funnel plots (Figure S1, S2).
Statistical analysis
The pooled prevalence was derived using a random-effects model. Characteristics were compared between the OLT and control groups using a random-effects model as the risk ratio for continuous variables and the Freeman-Tukey variant of the arcsine square root transformed proportion for binary variables. [16] The risk factors were recorded as odds ratios (ORs) with 95% confidence intervals. We evaluated inter-study heterogeneity using the I2 metric of inconsistency and the P value of the Cochran Q test. I2, the ratio of inter-study variance to the sum of intra-study and inter-study variance, ranges from 0% to 100%. To explain inter-study heterogeneity, a meta-regression was conducted to examine the influence of other factors on clinical outcomes. Statistical analyses were performed using RevMan 5 (Cochrane Library) or the meta package in R (version 4.1.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of included studies
Based on the title and abstract screening, we identified 26 potentially relevant studies. Among them, six were excluded for the following reasons: wrong patient population (n=2), overlapping populations (n=2), case reports (n=2), and reviews (n=1). As a result, 20 studies were included in the meta-analysis (Figure S3). Information regarding the enrolled patients is presented in Table 1.
Demographics and characteristics of studies included in the systematic review and meta-analysis
| Study | Country | Study design | Inclusion | Exclusion | Vaccine type | Dose | Response evaluation | Objective indicator of humoral immunogenicity | Antibodies | LT duration (yrs) | Age (median) | Male (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Herrera (2021) [46] | Spain | Prospective | heart and liver transplant recipients | prior COVID | Moderna | 2 | 4 weeks after 2nd vaccine | Presence of anti-S IgG spike | Anti-S IgG | 4.6 | 61.5 | 73 |
| Cholankeril (2021) [47] | USA | Prospective | LT recipients | prior COVID | Pfizer | 2 | 30 to 75 days after 2nd vaccine | Semi-quantitative anti-S IgG value > 1 | Anti-S IgGAnti-nucleocapsid IgG | 3.3 | 63 | 70 |
| Huang (2002) [48] | USA | Retrospective | adult organ transplantation | prior COVID | mRNA vaccine (Pfizer or Moderna) | 2 | day 30-90 after 2nd vaccine | anti-S IgG titer > 1:50 | Anti-S IgGAnti-nucleocapsid IgG | 3.2 | 62 | 60.7 |
| Davidov (2022) [17] | Israel | Prospective | LT recipients | prior COVID | Pfizer | 2 | 36 days after 2nd vaccine | anti-RBD IgG titers > 1.1 sample-to-cutoff ratio /neutralizing antibodies | Anti-RBD IgGNeutralizing antibodies | 6 | 64 | 56.6 |
| D'Offizi (2021) [49] | Italy | Retrospective | LT recipients | prior COVID | mRNA vaccine (Pfizer or Moderna) | 2 | 2 weeks after 2nd vaccine | anti-S IgG > 7.2 BAU/mL | Anti-S IgG | 6 | 59 | 70 |
| Fernández-Ruiz (2021) [50] | Spain | Prospective | KT or LT recipients | prior COVID | Moderna | 2 | 2 weeks after 2nd vaccine | Presence of anti-S IgG spike | Anti-S IgG | |||
| Guarino (2022) [51] | Italy | Prospective | LT recipients | prior COVID | Pfizer | 2 | 1 month and 3 months after 2nd vaccine | anti-S IgG >25 AU/mL | Anti-S IgG | 14.08 | 64.8 | 75.4 |
| Mulder (2002) [52] | Netherlands | Retrospective | LT recipients | prior COVID | mRNA vaccine or ChAdOx1 nCoV-19 | 2 | 4 weeks after 2nd vaccine | Presence of anti-S IgG spike | Anti-S IgG | 5.5 | 59 | 60 |
| Marion (2022) [22] | France | Retrospective | sold organ transplantation | mRNA vaccine (Pfizer and Moderna) | 2 | 4 weeks after 2nd vaccine | Presence of anti-S IgG spike | Anti-S IgG | ||||
| Nazaruk (2021) [53] | Poland | Retrospective | KT or LT recipients | prior COVID | Pfizer | 2 | 4-8 weeks after the 2nd vaccine | anti-S IgG > 50 AU/mL /neutralizing antibodies | Anti-S IgGNeutralizing antibodies | 14.8 | 58.4 | 80 |
| Erol (2021) [54] | Turkey | Prospective | KT or LT recipients | prior COVID | Sinovac or Pfizer | 2 | 4-6 weeks after the 2nd vaccine | anti-S IgG > 50 AU/mL | Anti-S IgG | |||
| Rabinowich (2021) [32] | Israel | Prospective | LT recipients | Pfizer | 2 | 10-20 days after the 2nd vaccine | anti-S IgG > 15 AU/mL /anti-nucleocapsid IgG | Anti-S IgGAnti-nucleocapsid IgG | 5 | 60.1 | 30 | |
| Rahav (2021) [55] | Israel | Prospective | immunocompromised | prior COVID | Pfizer | 2 | 2-4 weeks after the 2nd vaccine | anti-RBD IgG titers > 1.1 /neutralizing antibodies | Anti-RBD IgGNeutralizing antibodies | 68 | 52.8 | |
| Rashidi-Alavijeh (2021) [56] | Germany | Prospective | LT recipients | Pfizer | 2 | 15 days after the 2nd vaccine | anti-S IgG > 13 AU/mL | Anti-S IgG | 8 | 57 | 60.5 | |
| Ruether (2022) [18] | Germany | Prospective | LT recipients or liver cirrhosis | mRNA vaccine or vector-based vaccine(AZD1222; AstraZeneca) | 2 | 4 weeks after the 2nd vaccine | anti-S IgG > 33.8 BAU/mL /anti-RBD IgG | Anti-S IgGAnti-RBD IgG | 17 | 55 | 57.2 | |
| Sakai (2022) [57] | Japan | Retrospective | LC or LT recipients | prior COVID | Pfizer | 2 | 2 weeks after the 2nd vaccine | anti-RBD IgG titers > 1.0 AU/mL | Anti-RBD IgG | 15.5 | 65 | 76.8 |
| Strauss (2021) [58] | USA | Retrospective | LT recipients | mRNA vaccine (Pfizer or Moderna) | 2 | 4 weeks after the 2nd vaccine | anti-S1 IgG > 1.1 AU/mL /anti-RBD IgG > 0.8 U/mL | Anti-S1 IgGAnti-RBD IgG | 6.9 | 64 | 43 | |
| Thuluvath (2021) [59] | USA | Prospective | LT recipients and those with chronic liver disease (CLD) with andwithout cirrhosis | prior COVID | mRNAvaccines or after the single dose of Johnson & Johnson vaccine | 2 | 4 weeks after the 2nd vaccine | anti-S IgG > 0.4 U/mL | Anti-S IgG | 65.7 | 66 | |
| Timmermann (2021) [60] | Germany | Retrospective | LT recipients | mRNAvaccines or after the single dose of Johnson & Johnson vaccine | 2 | 3 weeks after the 2nd vaccine | Presence of anti-S IgG spike/anti-nucleocapsid IgG | Anti-S IgGAnti-nucleocapsid IgG | 14.4 | 66.1 | 63.6 | |
| Toniutto (2022) [61] | Italy | Retrospective | LT recipients | prior COVID | Pfizer | 2 | 1, 4, 6 month after the 2nd vaccine | anti-RBD IgG > 0.8 U/mL /anti-nucleocapsid IgG > 0.8 > 10 kAU/L | Anti-RBD IgGAnti-nucleocapsid IgG | 7.8 | 57.9 | 70.2 |
Abbreviations: LT, liver transplantation; KT, kidney transplantation; RBD, receptor binding protein.
Summary of the immunogenicity rates of COVID-vaccination in patients with liver transplantation recipients
| Subgroup/Subset | No. of studies | No. of patients responder/total | Pooled event rate (M-H, Random) | 95% CI | I2 | P for heterogeneity |
|---|---|---|---|---|---|---|
| Overall | ||||||
| Immunogenicity rate, overall | 20 | 1,680/2,416 | 0.70 | 0.63 to 0.77 | 91% | <0.01 |
| Country | ||||||
| Europe | 11 | 1,181/1,592 | 0.74 | 0.66 to 0.81 | 89% | <0.01 |
| America | 4 | 327/566 | 0.56 | 0.36 to 0.74 | 95% | <0.01 |
| Middle east | 3 | 118/192 | 0.63 | 0.46 to 0.79 | 82% | <0.01 |
| East | 2 | 54/66 | 0.90 | 0.62 to 1.00 | 75% | 0.05 |
| Types of vaccine | ||||||
| Moderna only | 2 | 64/71 | 0.88 | 0.69 to 1.00 | 61% | 0.11 |
| Pfizer only | 9 | 708/980 | 0.71 | 0.63 to 0.79 | 85% | <0.01 |
| mRNA vaccine (Moderna, Pfizer or Sinovac) | 5 | 350/571 | 0.66 | 0.47 to 0.82 | 94% | <0.01 |
| mRNA vaccines + vector vaccines | 4 | 568/794 | 0.64 | 0.46 to 0.80 | 95% | <0.01 |
| Study design | ||||||
| Prospective | 11 | 608/1029 | 0.69 | 0.58 to 0.79 | 91% | <0.01 |
| Retrospective | 9 | 985/1387 | 0.71 | 0.61 to 0.80 | 93% | <0.01 |
| Publish year | ||||||
| 2021 | 12 | 519/756 | 0.72 | 0.60 to 0.83 | 91% | <0.01 |
| 2022 | 8 | 1161/1660 | 0.68 | 0.58 to 0.77 | 93% | <0.01 |
| Diagnosis tool | ||||||
| Anti-Spike immunoglobulin | 16 | 1453/2117 | 0.69 | 0.60 to 0.77 | 93% | <0.01 |
| Anti-RBD immunoglobulin | 4 | 227/299 | 0.76 | 0.71 to 0.81 | 0% | 0.55 |
* CI: confidence interval; M-H: Mantel-Haenszel; No.: number; RR: risk ratio.
As shown in Table 1, all 20 studies were conducted in various countries, mostly within Europe (n=11), followed by North America (n=4), the Middle East (n=3), and East Asia (n=2). In most studies, patients with a prior history of COVID-19 infection were excluded from the analysis. The Pfizer vaccine alone was the most common vaccine used in the study (n=9), followed by the mRNA vaccine (Pfizer or Moderna, n=5), mRNA vaccine or vector vaccine (n=4), and Moderna vaccine alone (n=2). Anti-S immunoglobulin G (IgG) was most commonly used as an evaluation tool for immunogenicity, and anti-RBD IgG or anti-nucleocapsid IgG was used.
Immunogenicity rates of COVID-19 vaccination among OLT recipients
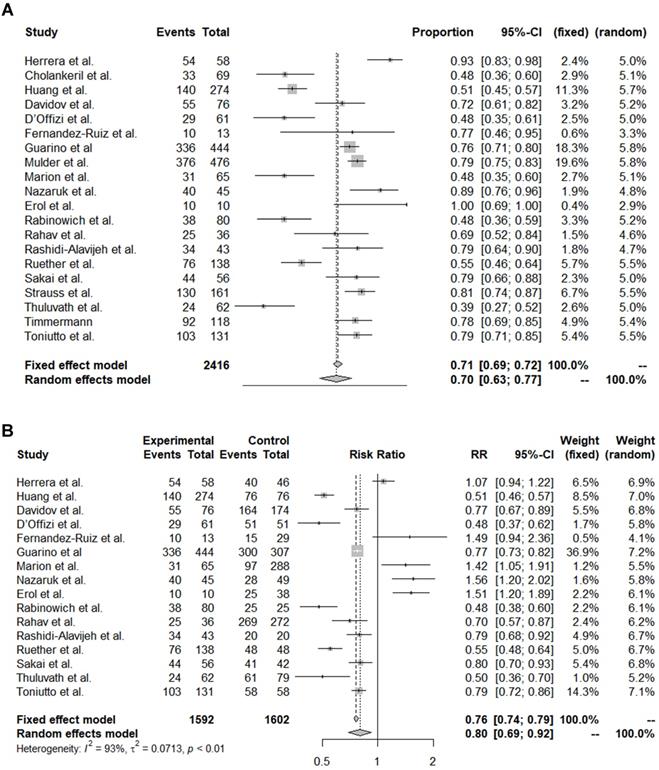
The pooled immunogenicity rate of OLT recipients against the COVID-19 vaccine was 0.70 (95% CI, 0.63-0.77) (Table 2, Figure 1A). A stratified analysis of the countries of the study subjects revealed that the immunogenicity of European (event rate, 0.74) and Asian (event rate, 0.56) patients was higher than that of American (event rate, 0.56) and the Middle Eastern (event rate, 0.63) patients. By vaccine type, the immunogenicity rate of Moderna (event rate, 0.88) and Pfizer alone (event rate, 0.71) was higher than that of the mRNA mixed group (event rate, 0.66) and the mRNA + vector vaccine group (event rate, 0.64). On the other hand, there was no significant difference in the immunogenicity rate by study design, year of publication, or diagnostic tool.
Next, we analysed how the immunogenicity of the OLT patient group differed from that of the control groups (Table S1, Figure 1B). Among the 20 papers, 16 reported differences in immunogenicity between OLT and control groups. The immunogenicity of the OLT recipients was significantly lower than that of the overall control group, with an OR of 0.80 (95% CI, 0.69-0.92). In particular, the immunogenicity of OLT recipients was significantly lower than that of healthy controls (pooled OR from 10 studies, 0.69; 95% CI, 0.63-0.77) or liver cirrhosis patients (pooled OR from 2 studies, 0.54; 95% CI, 0.47-0.62). On the other hand, the immunogenicity rate of OLT recipients was significantly higher than that of recipients of other organ transplants, such as kidneys (pooled OR from 6 studies, 1.50; 95% CI, 1.35-1.67) or hearts (pooled OR from 3 studies, 1.44; 95% CI, 0.89-2.32).
Finally, we performed a meta-regression analysis because of the high inter-study heterogeneity (Table S2). The analysis showed no association of the pooled adjusted OR with age, sex, body mass index, comorbidities, and immunosuppressant type or number.
Forest plots of immunogenicity rates. (A) Pooled immunogenicity rate. (B) Comparison of liver transplantation recipient and control groups.
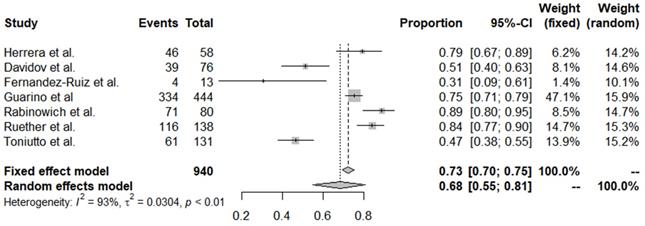
Forest plots of all adverse events reported after coronavirus disease 2019 vaccine administration.
Adverse event of COVID-vaccination in patients with liver transplantation recipients
| Subgroup/Subset | No. of studies | No. of patients AE/total | Pooled event rate (M-H, Random) | 95% CI | I2 | P for heterogeneity |
|---|---|---|---|---|---|---|
| Overall | ||||||
| Adverse events, overall | 7 | 671/940 | 0.68 | 0.55 to 0.81 | 93% | <0.01 |
| Country | ||||||
| Europe | 5 | 561/784 | 0.67 | 0.51 to 0.81 | 93% | <0.01 |
| Middle east | 2 | 110/156 | 0.72 | 0.31 to 0.98 | 96% | <0.01 |
| Types of vaccine | ||||||
| Moderna only | 2 | 50/71 | 0.58 | 0.12 to 0.97 | 91% | <0.01 |
| Pfizer only | 4 | 505/731 | 0.67 | 0.48 to 0.83 | 95% | <0.01 |
| mRNA vaccines + vector vaccines | 1 | 116/138 | 0.84 | 0.77 to 0.90 | NA | NA |
| Publish year | ||||||
| 2021 | 3 | 121/151 | 0.72 | 0.45 to 0.92 | 89% | <0.01 |
| 2022 | 4 | 550/789 | 0.65 | 0.48 to 0.81 | 95% | <0.01 |
* CI: confidence interval; M-H: Mantel-Haenszel; No.: number; RR: risk ratio.
Risk factors for the unresponsiveness to vaccination among OLT recipients
We identified all risk factors described in the studies. In our meta-analysis, a total of 10 risk factors were accessible for calculation: sex, age, chronic kidney disease (CKD), obesity, multiple immunosuppressant use, high steroid dose, mycophenolate mofetil, tacrolimus, time since OLT, and vaccination in 1st year after transplantation (Table S3). All risk factors but high-dose tacrolimus use were significantly associated with vaccine unresponsiveness among OLT recipients. In particular, CKD (OR, 27.56; 95% CI, 10.06-87.54), vaccination in 1st year after transplantation (OR, 18.53; 95% CI, 7.67-44.79), and the use of multiple immunosuppressants (OR, 10.40; 95% CI, 6.12-17.68) significantly increased the risk of unresponsiveness.
Adverse events after COVID-19 vaccination among OLT recipients
Of the 20 studies, 7 reported adverse events after vaccination. The overall incidence of adverse events was 0.68 (95% CI, 0.55-0.81) (Table 3, Figure 2). The incidence of these adverse events did not differ significantly by country, vaccine type, or publication year. Serious adverse events (SAEs) were reported in 9 papers; 1 patient for Bell's palsy [17] and 6 patients for joint pain/fever, fatigue/headache/muscle pain requiring hospitalization [11, 18] were reported in 2 papers, but there was no occurrence of SAE in the remaining 7 papers. Also, no deaths have been reported in these vaccinated patients and there were no studies reporting the rate of COVID-19 infection after vaccination.
Discussion
In the present systematic review and meta-analysis, we observed that OLT recipients had a 70% (95% CI, 0.68-0.77) overall humoral immune response after vaccination against SARS-CoV-2, which was significantly lower than that of the controls, with a risk ratio (RR, 0.80; 95% CI, 0.69-0.92). In particular, compared to that of healthy controls, the OR of 0.69 (95% CI, 0.63-0.77) of the vaccine response among OLT recipients was relatively lower. However, compared to patients receiving transplants of other solid organs such as kidneys or hearts, OLT recipients showed a trend toward relatively favourable responses with an OR of 1.50 (95% CI, 1.35-1.67) and 1.44 (95% CI, 0.89-2.32), respectively. There are several possible explanations for this finding. First, it might be related to the fact that most thoracic or kidney transplant patients receive induction therapy [19,20], whereas only less than 30% of OLT recipients receive induction therapy [21]. In a similar context, we found that OLT patients receiving calcineurin inhibitor-based immunosuppressive regimens were more likely to be responders than those receiving a mycophenolate mofetil-based regimen [22]. Lastly, liver is the major immune-modulating organ, thus, restoring liver function after OLT could be more beneficial in achieving immune response after the vaccination [23].
Notably, among various risk factors, the presence of CKD showed the highest impact on non-response to vaccine, with a pooled OR of 27.56 (95% CI 10.06-87.54). Even in patients with CKD who are not receiving immunosuppressive agents, impaired adaptive immunity is often observed. Antibody production by B lymphocytes decreases with the dysfunction of antigen-presenting cells and memory T cell apoptosis under uremic conditions [24]. Furthermore, both insufficient erythropoietin (EPO) and vitamin D can, in part, contribute to the dysregulation of immunomodulation [25]. Considering that OLT recipients are subject to CKD development during their life owing to nephrotoxic calcineurin inhibitors as well as peri-operative medical condition [26-29], the presence of CKD itself among OLT recipients might have an additional impact compared with other risk factors. However, further studies are required to determine whether therapeutic intervention with EPO and vitamin D supplementation in OLT patients with CKD might positively affect the efficacy of the SARS-CoV-2 vaccine. In addition, the depth of immunosuppression, which was also closely associated with a higher non-response when the vaccine was received within the 1st year after transplantation, had a strong impact on the humoral response. In our meta-regression analysis, the use of one immunosuppressive agent increased the humoral immune response compared with the use of two or more immunosuppressive agents (p=0.002). In addition, the presence of CKD negatively impacted the immune response with marginal significance (p=0.052). Nevertheless, solid organ transplantation experts [30,31] recommend the maintenance of immunosuppressive agents, including mycophenolate mofetil) when recipients of solid organs receive the SARS-CoV-2 vaccination owing to the potential concern of graft rejection. Further clinical trials are needed to elucidate this issue.
In terms of adverse events, about 68% of OLT recipients experienced any kinds of adverse events. Although the currently approved SARS-CoV-2 vaccines include novel mRNA types, there are no theoretical concerns that they should not be safe for immunosuppressed individuals, as they contain no live viruses capable of replication within the vaccinated host. In the first studies assessing the safety of SARS-CoV-2 mRNA vaccines among patients who received solid organ transplants, the type and rate of adverse events have been similar to those of non-immunosuppressed controls (70-85% of pain at the injection site and 15-20% of fatigue) [32-34].
In general, more than 20% of patients with cirrhosis show unresponsiveness to the SARS-CoV-2 vaccination [35] because they are also considered immunocompromised. Hepatic fibrosis impairs the synthesis of innate immunity proteins and pattern recognition receptors, and the absolute counts and functions of B and T lymphocytes are affected by diverse mechanisms, including co-stimulation marker downregulation, memory cell loss, and T cell exhaustion [35,36]. Nevertheless, notably, OLT patients showed significantly lower immunogenicity than patients with cirrhosis (OR, 0.54; 95% CI, 0.47-0.62). This indicates that the use of immunosuppressive agents in the presence of other associated comorbidities including renal insufficiency (either primary or secondary to underlying liver disease) [37-41], should offset the benefit of OLT, that is, restored hepatic functional reserve and normalised portal pressure in achieving a humoral response to the SARS-CoV-2 vaccination.
This study had several limitations. First, we could not assess the cellular immune response by T cells among the OLT recipients. There were only 4 articles reporting the cellular immunogenicity in these patients' group. However, it was impossible to derive an integrated result appropriately, since the evaluation tool for cellular response was heterogeneous among studies. Although a serological response to a virus-specific antibody is generally the major endpoint in the evaluation of vaccine efficacy [42], coordinated and lasting CD4+ and CD8+ T cell responses with the proper specificity, phenotype, and function are also likely critical components of antiviral immunity against SARS-CoV-2 [43] since circulating antibodies to SARS-CoV-2 may be short-lived or of low magnitude and/or potency [44,45]. Further studies measuring the secretion of interferon-gamma by peripheral blood lymphocytes upon SARS-CoV-2 glycoprotein stimulation are required to resolve this issue. In a similar context, further studies are required to determine whether booster doses of vaccines based on mRNA or viral vectors can affect the overall humoral and cellular immune responses among OLT recipients. Similarly, whether there is a correlation between diminishing antibody levels after vaccination and SARS-CoV-2 infection susceptibility or severity should be assessed through further research. In addition, although the rate of achieving immune response could be the only available indicator at present, data regarding other clinical outcomes such as rate of SARS-CoV-2 infection after vaccination, complication or mortality have been still insufficient so far. Further long-term follow-up should be required to address this issue.
In conclusion, because OLT recipients are at risk of developing a higher rate of COVID-19-related complications, they are at priority for receiving immunisations against SARS-CoV-2. The humoral response to vaccines against SARS-CoV-2 among OLT recipients was relatively lower than that of healthy controls, with a similar level of adverse events compared to the historical controls. However, OLT recipients had a more favourable response than patients who received kidney or heart transplants. Interventions to achieve higher response rates, such as booster vaccinations, higher vaccine dosages, and intradermal administration, require assessment in well-designed clinical trials.
Abbreviations
OLT: orthotopic liver transplant; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ORs: odds ratios; COVID-19: Coronavirus disease 2019; PROSPERO: International Prospective Register of Systematic Reviews; MOOSE: Meta-analysis of Observational Studies in Epidemiology; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PICO: Patient/Problem, Intervention, Comparison, and Outcome; MeSH: Medical Subject Headings; RoBANS: Risk of Bias Assessment tool for Nonrandomised Studies; CKD: chronic kidney disease.
Supplementary Material
Supplementary materials and methods, figures and tables.
Acknowledgements
We would like to Jae-Young Kim in Research Factory Inc. (www.rfactory.co.kr) for consulting the statistical analysis. We thank Eun-Ae Jung for performing the search strategy.
Ethics approval and consent to participate
Ethics approval was waived from the Institutional Review Board and our study conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Financial support
This research was supported by the Soonchunhyang University Research Fund and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HV22C023300). This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HV22C023300).
Author Contributions
1) Study concept and design: Jae Il Shin and Beom Kyung Kim;
2) Provision of study materials or patients: Jeong-Ju Yoo, Jae Il Shin, and Beom Kyung Kim;
3) Collection and assembly of data: Jeong-Ju Yoo and Beom Kyung Kim;
4) Data analysis and interpretation: Jeong-Ju Yoo, Dong Keon Yon, and Seung Won Lee;
5) Manuscript writing: Jeong-Ju Yoo, and Beom Kyung Kim;
6) Final approval of manuscript: All authors.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Amin M. COVID-19 and the liver: overview. Eur J Gastroenterol Hepatol. 2021;33(3):309-11
2. Metawea MI, Yousif WI, Moheb I. COVID 19 and liver: An A-Z literature review. Dig Liver Dis. 2021;53(2):146-52
3. Spearman CW, Aghemo A, Valenti L, Sonderup MW. COVID-19 and the liver: A 2021 update. Liver Int. 2021;41(9):1988-98
4. Ekpanyapong S, Bunchorntavakul C, Reddy KR. COVID-19 and the Liver: Lessons Learnt from the EAST and the WEST, A Year Later. J Viral Hepat. 2022;29(1):4-20
5. Buchy P, Buisson Y, Cintra O. et al. COVID-19 pandemic: lessons learned from more than a century of pandemics and current vaccine development for pandemic control. Int J Infect Dis. 2021;112:300-17
6. Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14(5):612-20
7. Webb GJ, Marjot T, Cook JA. et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5(11):1008-16
8. Cho JY, Kim SS, Lee YS, Song DS, Lee JH, Kim JH. Management of liver diseases during the pandemic of coronavirus disease-19. Clin Mol Hepatol. 2020;26(3):243-50
9. Lee YR, Kang MK, Song JE. et al. Clinical outcomes of coronavirus disease 2019 in patients with pre-existing liver diseases: A multicenter study in South Korea. Clin Mol Hepatol. 2020;26(4):562-76
10. Di Maira T, Berenguer M. COVID-19 and liver transplantation. Nat Rev Gastroenterol Hepatol. 2020;17(9):526-8
11. Mombelli M, Rettby N, Perreau M, Pascual M, Pantaleo G, Manuel O. Immunogenicity and safety of double versus standard dose of the seasonal influenza vaccine in solid-organ transplant recipients: A randomized controlled trial. Vaccine. 2018;36(41):6163-9
12. Héquet D, Pascual M, Lartey S. et al. Humoral, T-cell and B-cell immune responses to seasonal influenza vaccine in solid organ transplant recipients receiving anti-T cell therapies. Vaccine. 2016;34(31):3576-83
13. Boyarsky BJ, Werbel WA, Avery RK. et al. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. Jama. 2021;325(17):1784-6
14. Sonani B, Aslam F, Goyal A, Patel J, Bansal P. COVID-19 vaccination in immunocompromised patients. Clin Rheumatol. 2021;40(2):797-8
15. Kim SY, Park JE, Lee YJ. et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66(4):408-14
16. Bender R, Friede T, Koch A. et al. Methods for evidence synthesis in the case of very few studies. Res Synth Methods. 2018;9(3):382-92
17. Davidov Y, Tsaraf K, Cohen-Ezra O. et al. Immunogenicity and Adverse Effects of the 2-Dose BNT162b2 Messenger RNA Vaccine Among Liver Transplantation Recipients. Liver Transpl. 2022;28(2):215-23
18. Ruether DF, Schaub GM, Duengelhoef PM. et al. SARS-CoV2-specific Humoral and T-cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin Gastroenterol Hepatol. 2022;20(1):162-72.e9
19. Penninga L, Møller CH, Gustafsson F, Gluud C, Steinbrüchel DA. Immunosuppressive T-cell antibody induction for heart transplant recipients. Cochrane Database Syst Rev. 2013(12): Cd008842.
20. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9 Suppl 3: S1-155.
21. Bittermann T, Hubbard RA, Lewis JD, Goldberg DS. The use of induction therapy in liver transplantation is highly variable and is associated with posttransplant outcomes. Am J Transplant. 2019;19(12):3319-27
22. Marion O, Del Bello A, Abravanel F. et al. Predictive Factors for Humoral Response After 2-dose SARS-CoV-2 Vaccine in Solid Organ Transplant Patients. Transplant Direct. 2022;8(1):e1248
23. Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(2 Suppl 1):S54-62
24. Ando M, Shibuya A, Tsuchiya K, Akiba T, Nitta K. Reduced expression of Toll-like receptor 4 contributes to impaired cytokine response of monocytes in uremic patients. Kidney Int. 2006;70(2):358-62
25. Hou YC, Lu KC, Kuo KL. The Efficacy of COVID-19 Vaccines in Chronic Kidney Disease and Kidney Transplantation Patients: A Narrative Review. Vaccines (Basel). 2021 9(8)
26. Kim JM, Kim DG, Kim J. et al. Outcomes after liver transplantation in Korea: Incidence and risk factors from Korean transplantation registry. Clin Mol Hepatol. 2021;27(3):451-62
27. Oh J, Kim JM. Immunologic strategies and outcomes in ABO-incompatible living donor liver transplantation. Clin Mol Hepatol. 2020;26(1):1-6
28. Kim KD, Lee JE, Kim JM. et al. Cost-effectiveness and long-term outcomes of liver transplantation using hepatitis B core antibody-positive grafts with hepatitis B immunoglobulin prophylaxis in Korea. Clin Mol Hepatol. 2021;27(4):603-15
29. Kang I, Lee JG, Choi SH. et al. Impact of everolimus on survival after liver transplantation for hepatocellular carcinoma. Clin Mol Hepatol. 2021;27(4):589-602
30. Available from https://www.myast.org/covid-19-vaccine-faq-sheet
31. Available from https://ishlt.org/ishlt/media/Documents/COVID19_Vaccine-Recommendations_3-15-2021.pdf
32. Rabinowich L, Grupper A, Baruch R. et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435-8
33. Grupper A, Rabinowich L, Schwartz D. et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719-26
34. Ou MT, Boyarsky BJ, Motter JD. et al. Safety and Reactogenicity of 2 Doses of SARS-CoV-2 Vaccination in Solid Organ Transplant Recipients. Transplantation. 2021;105(10):2170-4
35. Ai J, Wang J, Liu D. et al. Safety and Immunogenicity of SARS-CoV-2 Vaccines in Patients With Chronic Liver Diseases (CHESS-NMCID 2101): A Multicenter Study. Clin Gastroenterol Hepatol. 2021
36. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61(6):1385-96
37. Shin J, Yu JH, Jin YJ. et al. Acute-on-chronic liver failure as a major predictive factor for mortality in patients with variceal bleeding. Clin Mol Hepatol. 2020;26(4):540-53
38. Lesmana CRA, Raharjo M, Gani RA. Managing liver cirrhotic complications: Overview of esophageal and gastric varices. Clin Mol Hepatol. 2020;26(4):444-60
39. Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for liver cirrhosis: Varices, hepatic encephalopathy, and related complications. Clin Mol Hepatol. 2020;26(2):83-127
40. Piano S, Tonon M, Angeli P. Changes in the epidemiology and management of bacterial infections in cirrhosis. Clin Mol Hepatol. 2021;27(3):437-45
41. Yoon KT, Liu H, Lee SS. β-blockers in advanced cirrhosis: More friend than enemy. Clin Mol Hepatol. 2021;27(3):425-36
42. Zimmermann P, Ritz N, Perrett KP, Messina NL, van der Klis FRM, Curtis N. Correlation of Vaccine Responses. Front Immunol. 2021;12:646677
43. DiPiazza AT, Graham BS, Ruckwardt TJ. T cell immunity to SARS-CoV-2 following natural infection and vaccination. Biochem Biophys Res Commun. 2021;538:211-7
44. Long QX, Tang XJ, Shi QL. et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200-4
45. Robbiani DF, Gaebler C, Muecksch F. et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437-42
46. Herrera S, Colmenero J, Pascal M. et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant. 2021;21(12):3971-9
47. Cholankeril G, Al-Hillan A, Tarlow B. et al. Clinical Factors Associated With Lack of Serological Response to SARS-CoV-2 Messenger RNA Vaccine in Liver Transplantation Recipients. Liver Transpl. 2022;28(1):123-6
48. Huang HJ, Yi SG, Mobley CM. et al. Early humoral immune response to two doses of severe acute respiratory syndrome coronavirus 2 vaccine in a diverse group of solid organ transplant candidates and recipients. Clin Transplant. 2022;36(5):e14600
49. D'Offizi G, Agrati C, Visco-Comandini U. et al. Coordinated cellular and humoral immune responses after two-dose SARS-CoV2 mRNA vaccination in liver transplant recipients. Liver Int. 2022;42(1):180-6
50. Fernández-Ruiz M, Almendro-Vázquez P, Carretero O. et al. Discordance Between SARS-CoV-2-specific Cell-mediated and Antibody Responses Elicited by mRNA-1273 Vaccine in Kidney and Liver Transplant Recipients. Transplant Direct. 2021;7(12):e794
51. Guarino M, Esposito I, Portella G. et al. Humoral Response to 2-dose BNT162b2 mRNA COVID-19 Vaccination in Liver Transplant Recipients. Clin Gastroenterol Hepatol. 2022
52. Mulder MB, van der Eijk AA, GeurtsvanKessel CH. et al. High antibody response in relation to immunosuppressive blood levels in liver transplant recipients after SARS-CoV-2 vaccination: an observational, cohort study. Gut. 2022
53. Nazaruk P, Monticolo M, Jędrzejczak AM. et al. Unexpectedly High Efficacy of SARS-CoV-2 BNT162b2 Vaccine in Liver versus Kidney Transplant Recipients-Is It Related to Immunosuppression Only? Vaccines (Basel). 2021 9(12)
54. Erol C, Yanik Yalcin T, Sari N. et al. Differences in Antibody Responses Between an Inactivated SARS-CoV-2 Vaccine and the BNT162b2 mRNA Vaccine in Solid-Organ Transplant Recipients. Exp Clin Transplant. 2021;19(12):1334-40
55. Rahav G, Lustig Y, Lavee J. et al. BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: A prospective cohort study. EClinicalMedicine. 2021;41:101158
56. Rashidi-Alavijeh J, Frey A, Passenberg M. et al. Humoral Response to SARS-Cov-2 Vaccination in Liver Transplant Recipients-A Single-Center Experience. Vaccines (Basel). 2021 9(7)
57. Sakai A, Morishita T, Matsunami H. Antibody Response After a Second Dose of the BNT162b2 mRNA COVID-19 Vaccine in Liver Transplant Recipients. Transpl Int. 2022;35:10321
58. Strauss AT, Hallett AM, Boyarsky BJ. et al. Antibody Response to Severe Acute Respiratory Syndrome-Coronavirus-2 Messenger RNA Vaccines in Liver Transplant Recipients. Liver Transpl. 2021;27(12):1852-6
59. Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75(6):1434-9
60. Timmermann L, Globke B, Lurje G. et al. Humoral Immune Response following SARS-CoV-2 Vaccination in Liver Transplant Recipients. Vaccines (Basel). 2021 9(12)
61. Toniutto P, Falleti E, Cmet S. et al. Past COVID-19 and immunosuppressive regimens affect the long-term response to anti-SARS-CoV-2 vaccination in liver transplant recipients. J Hepatol. 2022
Author contact
![]() Corresponding authors: contributed equally to this work. Jae Il Shin, M.D., Ph.D. Department of Pediatrics, Yonsei University College of Medicine, Seoul, Republic of Korea. 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Republic of Korea. E-mail: shinjiac; Beom Kyung Kim, M.D., Ph.D. Department of Internal Medicine, Yonsei University College of Medicine. 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Republic of Korea. Tel.: +82-(0)2-2228-1930, Fax: +82-(0) 2-393-6884, E-mail: beomkkimac.
Corresponding authors: contributed equally to this work. Jae Il Shin, M.D., Ph.D. Department of Pediatrics, Yonsei University College of Medicine, Seoul, Republic of Korea. 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Republic of Korea. E-mail: shinjiac; Beom Kyung Kim, M.D., Ph.D. Department of Internal Medicine, Yonsei University College of Medicine. 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Republic of Korea. Tel.: +82-(0)2-2228-1930, Fax: +82-(0) 2-393-6884, E-mail: beomkkimac.

 Global reach, higher impact
Global reach, higher impact