10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(3):981-993. doi:10.7150/ijbs.79852 This issue Cite
Research Paper
Galectin-9 blockade synergizes with ATM inhibition to induce potent anti-tumor immunity
1. Tianjin Key Laboratory of Lung Cancer Metastasis and Tumor Microenvironment, Lung Cancer Institute, Tianjin Medical University General Hospital, Tianjin 300052, P. R. China.
2. Department of Medical Oncology, Tianjin Medical University General Hospital, Tianjin 300052, P. R. China.
3. Department of Lung Cancer Surgery, Tianjin Medical University General Hospital, Tianjin 300052, P.R. China.
4. Antibody Therapeutics, Inc., Hayward, CA, USA.
5. Department of Molecular and Cellular Oncology, The University of Texas M. D. Anderson Cancer Center, Houston, Texas 77030, USA.
6. Graduate Institute of Biomedical Sciences and Center for Molecular Medicine, China Medical University, Taichung 40402, Taiwan.
#These authors contributed equally to this work.
Abstract
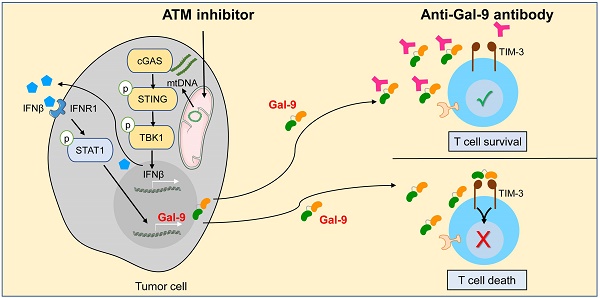
Although current cancer immunotherapies that target PD-1/PD-L1 immune checkpoint to reinvigorate exhausted T cells have achieved impressive clinical outcomes, only a small proportion of patients respond. New therapeutic targets are therefore needed to be identified to further unleash the anti-tumor potential of T cells and benefit more patients. Galectin-9 (Gal-9), initially identified as a ligand for TIM-3 to induce T cell death, acts as an immunosuppressive regulator in the tumor microenvironment (TME) but its potential as a therapeutic target remains largely elusive. Here we show that antibody neutralization of Gal-9, in combination with inhibition of Ataxia telangiectasia mutated (ATM), a kinase essential for DNA damage response (DDR), is a promising modality for cancer immunotherapy. Genetic depletion of ATM in tumors markedly potentiated anti-Gal-9 therapy in a syngeneic mouse model. Mechanistically, ATM inhibition greatly upregulated Gal-9 expression and secretion in a variety of human and murine tumor cells via the cGAS-STING-interferon β (IFNβ) innate immune pathway. Combination of Gal-9 inhibition with AZD1390, a selective ATM inhibitor currently evaluated in clinical trials, significantly suppressed tumor growth and prolonged survival in multiple syngeneic mouse models, including the poorly-immunogenic LLC lung tumors that do not respond to PD-1/PD-L1 blockade, concomitant with increased T cell infiltration. These results reveal Gal-9 induction via STING/IFNβ signaling as an important mechanism mediating tumor immune escape that could be targeted for cancer immunotherapies, and unveil a novel anti-Gal-9-based combination strategy for cancer immunotherapies in a wide variety of malignancies, including those resistant to PD-1/PD-L1 blockade.
Keywords: Cancer immunotherapy, DNA damage response, cGAS-STING, galectin

 Global reach, higher impact
Global reach, higher impact