10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(5):1471-1489. doi:10.7150/ijbs.77979 This issue Cite
Research Paper
Timosaponin AIII promotes non-small-cell lung cancer ferroptosis through targeting and facilitating HSP90 mediated GPX4 ubiquitination and degradation
1. Department of Integrated Traditional Chinese and Western Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
2. Health Management Center, Hubei Provincial Hospital of Integrated Chinese & Western Medicine, Wuhan, China.
3. College of International Education, Tianjin University of Traditional Chinese Medicine, Tianjin, China.
4. School of Traditional Chinese Medicine, Xiamen University Malaysia, Selangor. 43900. Malaysia.
5. Russian-Chinese Education and Research Center of System Pathology, South Ural State University, 454080 Chelyabinsk, Russia.
6. Institute of Immunology and Physiology, Ural Branch of the Russian Academy of Science, 620049 Ekaterinburg, Russia.
7. Shum Yiu Foon Sum Bik Chuen Memorial Centre for Cancer and Inflammation Research, School of Chinese Medicine, Hong Kong Baptist University, 999077, Hong Kong SAR, China.
8. Institute of Integrated Bioinfomedicine and Translational Science, School of Chinese Medicine, Hong Kong Baptist University, Hong Kong SAR, China.
9. Institute of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
10. Key Laboratory of Biological Targeted Therapy, the Ministry of Education, Wuhan, China.
11. Clinical Research Center of Cancer Immunotherapy, Wuhan, China.
† These authors contributed equally to this work
Abstract
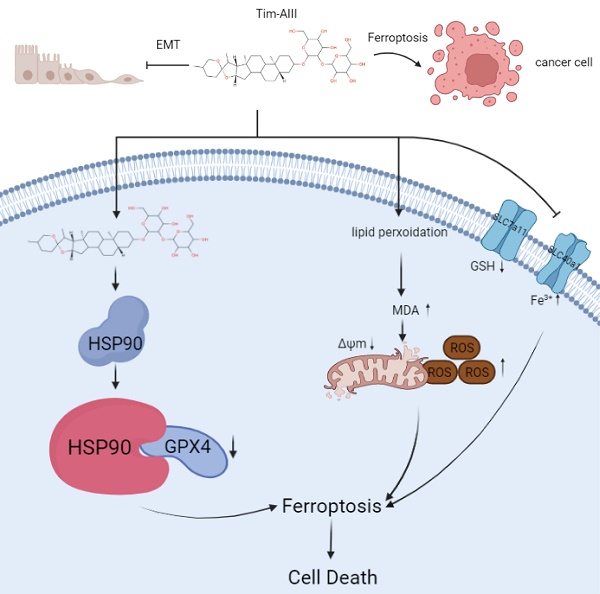
Timosaponin AIII (Tim-AIII), a steroid saponin, exhibits strong anticancer activity in a variety of cancers, especially breast cancer and liver cancer. However, the underlying mechanism of the effects of Tim-AIII-mediated anti-lung cancer effects remain obscure. In this study, we showed that Tim-AIII suppressed cell proliferation and migration, induced G2/M phase arrest and ultimately triggered cell death of non-small cell lung cancer (NSCLC) cell lines accompanied by the release of reactive oxygen species (ROS) and iron accumulation, malondialdehyde (MDA) production, and glutathione (GSH) depletion. Interestingly, we found that Tim-AIII-mediated cell death was reversed by ferroptosis inhibitor ferrostatin-1 (Fer-1). Meanwhile, the heat shock protein 90 (HSP90) was predicted and verified as the direct binding target of Tim-AIII by SwissTargetPrediction (STP) and surface plasmon resonance (SPR) assay. Further study showed that Tim-AIII promoted HSP90 expression and Tim-AIII induced cell death was blocked by the HSP90 inhibitor tanespimycin, indicating that HSP90 was the main target of Tim-AIII to further trigger intracellular events. Mechanical analysis revealed that the Tim-AIII-HSP90 complex further targeted and degraded glutathione peroxidase 4 (GPX4), and promoted the ubiquitination of GPX4, as shown by an immunoprecipitation, degradation and in vitro ubiquitination assay. In addition, Tim-AIII inhibited cell proliferation, induced cell death, led to ROS and iron accumulation, MDA production, GSH depletion, as well as GPX4 ubiquitination and degradation, were markedly abrogated when HSP90 was knockdown by HSP90-shRNA transfection. Importantly, Tim-AIII also showed a strong capacity of preventing tumor growth by promoting ferroptosis in a subcutaneous xenograft tumor model, whether C57BL/6J or BALB/c-nu/nu nude mice. Together, HSP90 was identified as a new target of Tim-AIII. Tim-AIII, by binding and forming a complex with HSP90, further targeted and degraded GPX4, ultimately induced ferroptosis in NSCLC. These findings provided solid evidence that Tim-AIII can serve as a potential candidate for NSCLC treatment.
Keywords: Timosaponin AIII, heat shock protein 90, glutathione peroxidase 4, ferroptosis, non-small cell lung cancer

 Global reach, higher impact
Global reach, higher impact