ISSN: 1449-2288
Int J Biol Sci 2023; 19(6):1846-1860. doi:10.7150/ijbs.79654 This issue Cite
Research Paper
Loss of HRD functional phenotype impedes immunotherapy and can be reversed by HDAC inhibitor in ovarian cancer
1. State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Sun Yat-sen University Cancer Center, Guangzhou, China.
2. Department of Gynecological Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China.
3. Department of Radiation Oncology & Therapy, Jilin Provincial Key Laboratory of Radiation Oncology & Therapy, The First Hospital of Jilin University, Changchun, China.
4. Department of Radiation Oncology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, China
# Dong Yang, Fu-Xue Huang, Wei Wei, Qia-Qia Li contributed equally to this article.
Abstract
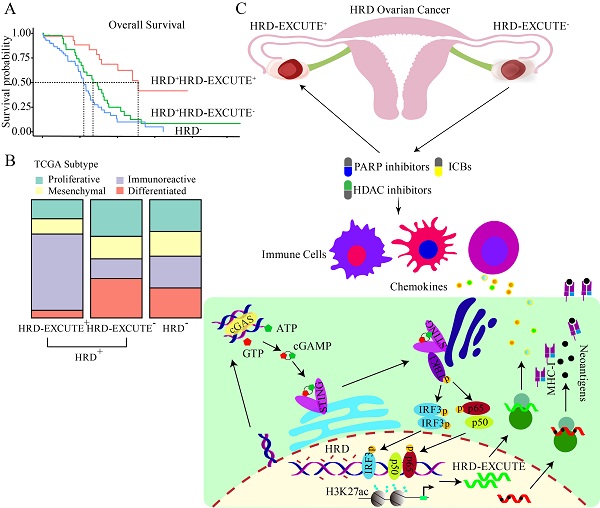
In recent years, homologous recombination deficiency (HRD) has not achieved the expected substantial promotion of immunotherapeutic efficacy in ovarian cancer. This study aims to explore the role of HRD functional phenotype as a powerful biomarker in identifying HRD patients who may benefit from immunotherapy. HRD functional phenotype, namely HRD-EXCUTE, was defined as the average level of the 15 hub genes upregulated in HRD ovarian cancer. A decision tree was plotted to evaluate the critical role of HRD-EXCUTE in HRD patients. Agents inducing HRD-EXCUTE were identified by CMAP web (Connectivity Map). The mechanisms and immunotherapeutic effect of PARPi and HDACi in promoting HRD-EXCUTE was examined in vitro and in vivo. The decision tree plotted on the basis of HRD and HRD-EXCUTE indicated the HRD patients without the HRD functional phenotype were largely unresponsive to immunotherapy, which was validated by the immunotherapeutic cohorts. Furthermore, loss of HRD-EXCUTE in the HRD patients attenuated immunogenicity and inhibited immune cells in tumor microenvironment. Moreover, Niraparib combined with Entinostat induced HRD-EXCUTE by activating the cGAS-STING pathway and increasing the histone acetylation. The combination therapy could enhance the cytotoxicity of immune cells, and promote pro-immune cells infiltrating into ascites, resulting in inhibited ovarian cancer growth. The HRD functional phenotype HRD-EXCUTE was set up as a potent biomarker to identify whether HRD patients can benefit from immunotherapy. Loss of HRD-EXCUTE in HRD patients were largely insensitive to immunotherapy. The combination of PARPi with HDACi could improve the efficacy of the PARPi-based immunotherapy in ovarian cancer by augmenting the HRD functional phenotype.
Keywords: Ovarian cancer, Homologous recombination deficiency, Transcriptomic functional Phenotype, Immunotherapy

 Global reach, higher impact
Global reach, higher impact