10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(7):2132-2149. doi:10.7150/ijbs.75890 This issue Cite
Research Paper
Plasminogen Activator Inhibitor-1 Potentiates Neutrophil Infiltration and Tissue Injury in Colitis
1. Department of Pediatrics, Ruijin Hospital, Shanghai Jiao Tong University, School of Medicine; Shanghai, 200025, China.
2. Division of Rheumatology, Department of Internal Medicine, School of Medicine, University of Utah; Salt Lake City, Utah, 84132, USA.
3. Molecular Medicine Program, University of Utah; Salt Lake City, Utah, 84132, USA.
4. Department of Emergency, Shanghai Jiahui International Hospital; Shanghai, 200233, China.
5. Institute of tropical medicine, Hainan Medical University; Haikou, 570228, China.
Abstract
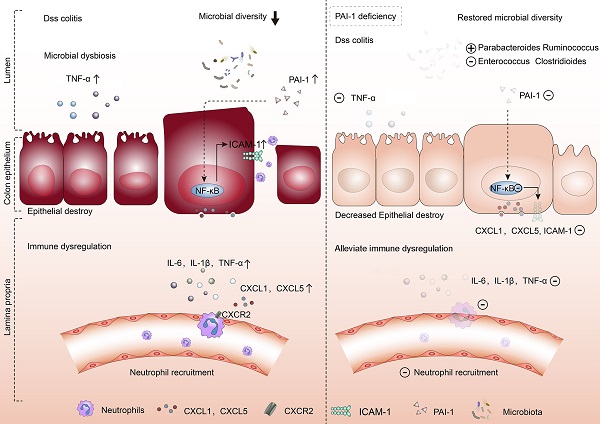
The mechanism underlying inflammatory bowel disease (IBD) remains unclear. We aimed to identify early diagnostic biomarkers and understand their roles in the pathogenesis of IBD.
Methods: We identified plasminogen activator inhibitor-1 (PAI-1) as a potential key gene that is upregulated in IBD based on published transcriptomic datasets. To further determine the role of PAI-1 in disease pathogenesis, we induced colitis in wild-type (WT) and PAI-1 knockout (KO) mice by administering dextran sulfate sodium (DSS). We used an RNA array of genes and 16S rRNA sequencing of the microbiome to analyze PAI-1 function. The colon and serum PAI-1 levels in humans were further evaluated for their diagnostic value.
Results: PAI-1 expression was significantly increased in patients and DSS-induced WT mice but reduced in PAI-1 KO mice. These changes were associated with significantly decreased neutrophil infiltration in colonic tissues. The RNA array revealed that the CXC chemokines CXCL1 and CXCL5 and their common receptor CXCR2 were among the most significantly different genes between the PAI-1 KO mice with DSS-induced colitis and the WT mice. Mechanistically, PAI-1 deficiency led to blunted activation of the NF-κB pathway in the colon epithelium. The gut microbiome was altered in the PAI-1 KO mice, which showed enriched abundances of short-chain fatty acid-producing genera and diminished abundances of pathogenic genera. Receiver operating characteristic (ROC) curve analysis revealed the diagnostic value of PAI-1.
Conclusions: Our data suggest a previously unknown function of PAI-1 inducing neutrophil-mediated chemokine expression by activating the NF-κB pathway and affecting the function of the gut microbiome. PAI-1 could be a potential diagnostic biomarker and a therapeutic target in IBD.
Keywords: chemokines, inflammatory bowel disease, intestinal epithelium, microbiota, PAI-1.

 Global reach, higher impact
Global reach, higher impact