10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(8):2333-2348. doi:10.7150/ijbs.81017 This issue Cite
Review
A Lipid Perspective on Regulated Pyroptosis
1. Laboratory of Stem Cell Regulation with Chinese Medicine and Its Application, School of Pharmacy, Hunan University of Chinese Medicine, Changsha 410208, China.
2. Science and Technology Innovation Center, Hunan University of Chinese Medicine, 410208, Changsha, Hunan, China.
3. Department of Urology, The First Hospital of Hunan University of Chinese Medicine, Changsha 410208, Hunan, China.
4. Hunan Provincial Key Laboratory of Vascular Biology and Translational Medicine, Changsha 410208, Hunan, China.
5. Department of Respiratory Medicine, First Affiliated Hospital, Hunan University of Chinese Medicine, Changsha 410021, Hunan, China.
* Contributed equally to this work.
Received 2022-11-19; Accepted 2023-4-3; Published 2023-4-25
Abstract
Pyroptosis is a novel pro-inflammatory cell programmed death dependent on Gasdermin (GSMD) family-mediated membrane pore formation and subsequent cell lysis, accompanied by the release of inflammatory factors and expanding inflammation in multiple tissues. All of these processes have impacts on a variety of metabolic disorders. Dysregulation of lipid metabolism is one of the most prominent metabolic alterations in many diseases, including the liver, cardiovascular system, and autoimmune diseases. Lipid metabolism produces many bioactive lipid molecules, which are important triggers and endogenous regulators of pyroptosis. Bioactive lipid molecules promote pyroptosis through intrinsic pathways involving reactive oxygen species (ROS) production, endoplasmic reticulum (ER) stress, mitochondrial dysfunction, lysosomal disruption, and the expression of related molecules. Pyroptosis can also be regulated during the processes of lipid metabolism, including lipid uptake and transport, de novo synthesis, lipid storage, and lipid peroxidation. Taken together, understanding the correlation between lipid molecules such as cholesterol and fatty acids and pyroptosis during metabolic processes can help to gain insight into the pathogenesis of many diseases and develop effective strategies from the perspective of pyroptosis.
Keywords: pyroptosis, lipids, lipid metabolism, NLRP3, inflammasome.
Introduction
Pyroptosis is involved in essential innate immunomodulatory mechanism and originally defined as caspase-1-dependent programmed cell death (PCD) by Cookson BT and Brennan MA in 2001 [1]. Pyroptosis occurs primarily in monocytes, macrophages, and dendritic cells, but can also be detected in other cell types, such as endothelial cells, cardiomyocytes, and hepatocytes [2-4]. Pyroptosis is driven by inflammatory caspase‐1, caspase‐4, caspase‐5 and caspase‐11, and executed by the gasdermin (GSDM) family. GSDMs induce membrane rupture and the release of intracellular contents, such as interleukin-1β (IL-1β), IL-18, high mobility group box 1 (HMGB1) and Adenosine triphosphate (ATP), which trigger local or systemic inflammatory responses [5]. Studies demonstrate that pyroptosis has a dual effect on organisms. Mild-to-moderate pyroptosis can accelerate the immune response by recruiting more immune cells to clear endogenous or exogenous danger signals (e.g., oxidative stress, hyperglycemia, inflammation), effectively prevent excessive proliferation of cells, and maintain the normal development and homeostasis of organisms [6, 7]. However, exaggerated pyroptosis leads to an overwhelming inflammatory response [8], to some extent resulting in tissue damage [7], thereby exacerbating disease progression and outcomes, such as cardiovascular diseases, liver diseases, and nervous system diseases [9-11].
Lipids are one of the major nutrients in humans and are pivotal for maintaining cellular structure as well as providing energy and mediating signaling transduction. Lipid biosynthesis and catabolism produce a variety of biological mediators, which are biologically active lipid molecules (also known as signaling molecules or second messengers) that regulate versatile signaling pathways. Previous studies by our group have identified an association between inflammation and lipid accumulation, as well as lipid-triggered inflammatory reactions [12, 13]. In addition, pyroptosis is a form of inflammatory cell death. Recent studies have revealed that lipid metabolism may induce pyroptosis during the process of diseases, suggesting a close relationship between lipid metabolism and pyroptosis. However, the molecular mechanism between lipid and pyroptosis remains largely unclear.
The Mechanism and Features of Pyroptosis
Generally, gasdermin family members are core among the pyroptosis pathway, which can be cleaved and activated by inflammatory caspase. There are six members of gasdermin family in human, including GSDMA, GSDMB, GSDMC, GSDMD, GSDME and DFNB59. Except for DFNB59, other gasdermins consist of a pore-forming N-terminal (NT) domain and a C-terminal (CT) regulatory domain, and the NT of gasdermins induce pyroptosis. Among these proteins, GSDMD is extensively studied in pyroptosis and has been identified as the executioner of pyroptosis [14-16].
Pyroptosis can be divided into the canonical inflammasome pathway and the noncanonical inflammasome pathway. Canonical pyroptotic death is mediated by inflammasome assembly [5]. When the host is resistant to microbial infection, multimolecular complexes called inflammasomes will be activated. In addition, inflammasomes are also associated with non-microbial diseases. Accumulating evidence shows that intracellular risks and environmental stimuli, such as cholesterol crystals (CHCs), oxidized LDL (ox-LDL), uric acid crystals, saturated fatty acids (SFA), DNA, mitochondrial ROS and lysophosphatidylcholine (LPC), can lead to the assembly of inflammasome [17]. Most inflammasomes are composed of three components: cytosolic pattern recognition receptors (PRRs, also known as inflammasome sensors), the adaptor ASC (apoptosis-associated spec-like protein containing a CARD), and the cysteine protease caspase-1. So far, five PRRs (NLRP1, NLRP3, NLRC4, Pyrin, and AIM2) have been identified as inflammasome-formation [18, 19]. Given its potential role in several human diseases, the NLRP3 inflammasome has been the object of extensive research among these inflammasomes, which converts pro-caspase-1 into caspase-1. Activated caspase-1 cleaves the full-length GSDMD into GSDMD-N and GSDMD-C. GSDMD-N oligomerizes and boosts cell membrane pore formation by binding to membranes and destroying the stability of the membranes [15]. Moreover, caspase-1 processes the pro-inflammatory cytokines, including pro-IL-1β and pro-IL-18 to be IL-1β and IL-18, accompanied with the release of other cell contents (such as lactate de-hydrogenase (LDH) and HMGB1) through the cell membrane pore, resulting in cell swelling and pyroptosis [6, 20].
In the non-canonical pyroptosis pathway, Shao et al. found that bacterial lipopolysaccharide (LPS) (or host-derived oxidized phospholipids) entered the cytoplasm through Toll-like receptors 4(TLR4) /MD2/CD14 signal pathway and bacterial outer membrane vesicles, and then directly activated caspase-4/5/11 [21, 22]. The activation of caspase-11 or caspase-4/5 does not require the PRR-mediated inflammasome. Activated caspase-4/5/11 can cleave GSDMD to GSDMD-N directly, but they are not accompanied by the cleavage of inflammatory factors pro-IL-1β and pro-IL-18 [15, 23]. However, they are able to mediate the maturation and secretion of IL-1β/ IL-18 through the NLRP3/caspase-1 pathway in some cells such as macrophages, leading to the inflammatory response [24]. This also suggests that other members of caspase are involved in the inflammatory process by promoting caspase-1 activation [25]. Therefore, the canonical and non-canonical pathways of pyroptosis are different in their induced signaling and initiation process, but eventually form pores on the plasma membrane (PM) by GSDMD and trigger pyroptosis through the inflammatory caspase.
In addition to the above two pathways, a caspase-3 dependent pathway has been revealed recently. In the case of administration of chemotherapeutic agents (e.g., DNA binding / modified compounds such as doxorubicin, cisplatin or topoisomerase inhibitors: etoposide, CPT-11, etc.), caspase-3 is activated to induce cleavage of GSDME, resulting in significant pyroptosis.
The morphology of cells changes obviously during pyroptosis. Swelling of cell membrane before rupture can be observed under scanning electron microscopy, and many bubble-like protrusions appear on the surface of cell membrane. Pyroptosis can be identified with dyes of lower molecular weights, such as 7-amino actinomycin (7-AAD), propidine iodide (PI), and ethidium bromide (EtBr) [26]. In addition, there is a very specific type of DNA damage at the early stage of pyroptosis, which is positive for dUTP nick end labeling (TUNEL) staining. Recently, it has been found that in some cases, activated GSDMD does not always occur membrane rupture and pyroptosis. Macrophages, dendritic cells, and neutrophils are able to survive in the cleavage of GSDMD cleavage activated by inflammasomes, which are defined as cell hyperactivation [27], and it can be distinguished by detecting lactate dehydrogenase in cell culture supernatant. The release of cytokines can locally induce large-scale inflammatory response at the site of infection, causing cell death. Dead cells no longer participate in any immunomodulatory activities. If pyroptosis is balanced by the excessive activation of the cells described above, then the scorched cells will continue to secrete inflammatory factors and constantly affect immunomodulatory events. However, the detailed mechanism determining when GSDMD cleavage triggers scorching or hyperactivation is still unclear.
Multiple Lipid Classes: Different Effects in Pyroptosis
Lipids such as cholesterol, together with Glycolipids and phospholipids, not only function as a major component of biological membranes, but also serve as the supply and storage of energy [28]. Fatty acids (FAs) are the fundamental components of complex lipids such as phospholipids and glycolipids, which can be esterified with glycerol to form triglycerides and stored in lipid droplets. Under energy stress conditions, they can be oxidized and hydrolyzed by FA to produce ATP. In addition, lipids also play significant roles as signaling molecules. Cholesterol and oxysterol can activate downstream gene expression through sterol regulatory element-binding protein (SREBP). Phospholipases (PLC, PLD, and PLA) can catalyze the formation of bioactive second messenger molecules including diacylglycerol, lysophosphatidic acid, and arachidonic acid (AA) [29, 30]. These different species of lipid molecules play key roles in regulating the fundamental processes of life, from energy storage to cell membranes formation to signal transduction Herein, we discuss these molecules and their derivatives that are produced in lipid metabolism and are associated with pyroptosis.
Cholesterol Derivatives and Pyroptosis
Intracellular cholesterol is maintained through de novo synthesis and exogenous uptake. Exogenous cholesterol follows an intricate pathway and then converts into free cholesterol in cells. However, excess free cholesterol may be toxic, which can cause pyroptosis both in vivo and in vitro. Specifically, overloading free cholesterol induces the expression of sphingomyelin synthase 1 (SMS1) in hepatocytes, and the diacylglycerol (DAG) produced by SMS1 activates protein kinase Cδ (PKCδ) and NLR family CARD domain-containing protein 4 (NLRC4) inflammasome to induce pyroptosis, thus triggering the development of non-alcoholic steatohepatitis (NASH) [30]. Elevated free cholesterol can also activate endoplasmic reticulum (ER) stress by stimulating mSREBP1 in intervertebral disc degeneration, thereby inducing pyroptosis in nucleus pulposus cells [31].
Free cholesterol is transported from late lysosomes to other subcellular organelles, such as PM and ER [32]. When cellular cholesterol trafficking is blocked, the cholesterol in PM decreases, resulting in the inhibition of NLRP3 assembly and the reduction of caspase-1, IL-1β and IL-18, eventually suppressing pyroptosis [33]. However, another study has shown that when the cholesterol content in PM reached 30%, the binding, oligomeric assembly, and pore formation of GSDMD-N could be reduced [34]. Generally, pyroptosis requires the N-terminal of the GSDMs to form pores in PM. These studies suggest that the content of PM cholesterol may directly affect pyroptosis through inflammasomes or combination with GSDMD. The relationship between PM cholesterol and pyroptosis is worth further exploration in the future. In the ER, cholesterol can be esterified. But the supersaturation of unesterified cholesterol in cells may cause precipitation of cholesterol crystals (CHCs) in the vascular wall, which is coincide with the earliest recruitment of inflammatory cells [35, 36]. Hypercholesterolemia promotes the formation of CHCs and causes coronary endothelial cell damage in vivo and in vitro through NLRP3 activation. However, pretreatment with caspase-1 or HMGB1 inhibitors could significantly reverse endothelial-dependent vasodilation injury [37]. A study has shown that the optimum dose of CHCs to induce pyroptosis was 0.5 mg/ml in human umbilical vein endothelial cells (HUVECs) [38]. One of the critical mechanisms of CHCs-stimulated pyroptosis is lysosomal rupture. CHCs cause lysosomal damage, allowing lysosomal contents such as cathepsin B (CTSB) to penetrate into the cytoplasm and activate the NLRP3 inflammasome [39]. On the other hand, CHCs in areas of plaque necrosis can stimulate the overproduction of mitochondrial reactive oxygen species (mtROS), thereby inducing the activation of NLRP3 inflammasome and caspase-1 [2, 36, 38]. ROS have been considered to play an important role in NLRP3 inflammasome activation and pyroptosis [40]. Colchicine can suppress the uptake of CHCs by endothelial cells, and attenuate the occurrence of NLRP3 inflammasome and pyroptosis by inhibiting mtROS production and oxidative stress through the AMP-dependent kinase (AMPK) / Sirtuin1 pathway [38]. The detailed mechanism of CHCs-induced pyroptosis remains unclear. In sum, these findings suggest a critical role for CHCs in the activation of pyroptosis.
Cholesterol can also be converted to oxysterols by chemical oxidation, such as 24(S)-hydroxycholesterol (24(S)-OHC), 27-OHC, 7β-OHC, and 25-OHC [41]. Oxysterols can cause many types of cell death, such as apoptosis, autophagy, and necrosis [42]. Recent studies have shown that oxysterol can also induce pyroptosis [43]. 25-OHC promotes P2X7 receptor-dependent pyroptosis in skin cells, leading to the emergence of skin degenerative diseases [43]. P2X7 receptor (P2X7) is a ligand-gated ion channel activated by extracellular ATP, which can assemble NLRP3 inflammasome [44]. Another study also showed that ATP released by Pannexin-1, a non-selective and large-pore channels, activated P2X7 to allow small cations including K+ and Na+ to cross the PM and mediate pyroptosis [45]. However, It has been found that in murine macrophages, 25-OHC can reduce the cellular content of sterols, IL-1β transcription and extensive inhibition of the inflammasome activation by antagonizing SREBP processing, thereby achieving an anti-inflammatory effect [46], which may trigger a potential inhibition of pyroptosis. In addition, excessive intracellular accumulation of 27-OHC induces lysosome dysfunction in co-cultured SH-SY5Y cells and C6 cells, and changes the levels of lysosome protein. CTSB is leaked through lysosomal membrane permeabilization (LMP) into the cytosol and induces NLRP3-dependent neuronal pyroptosis, causing neurodegenerative diseases such as Alzheimer's disease [47].
Fatty Acids and Pyroptosis
Fatty acids (FAs) are the hydrocarbon components of most lipids, usually with an even number of carbon atoms, ranging from 2-26. According to whether the hydrocarbon chain is saturated, fatty acids can be divided into saturated (SFA), monounsaturated (MUFA), and polyunsaturated fatty acids (PUFA). PUFAs are divided into two categories: omega-3 (ω-3) PUFAs and ω-6 PUFAs. FAs can also be divided into three types according to their amino acid chain lengths: short-, medium-, and long-chain FAs (SCFAs, MCFAs, and LCFAs, respectively [48].
Different FA types have distinct effects, even opposite influences. For obesity-related osteoarthritis, diet rich in SFA, MUFA, and n-6 PUFA can activate the TLR4/NF-κB signaling pathway in articular cartilage, which in turn upregulates the NLRP3 inflammasome, thereby inducing pyroptosis [49]. Excessive free fatty acids (FFAs) induce tissue injury by activating pyroptosis, which is usually used to establish the cell model of NASH [50-53]. Palmitic acid (PA) is the most dominant SFA in diet and is the main FFAs in plasma [54]. Pyroptosis is the primary type of PA-induced cell death, which is related to activation of various protein kinases, ER stress, and recruitment of macrophages [55, 56]. The combination of these factors leads to inflammasome-related cell death. PA induces NLRP3 mediated pyroptosis in HepG2 cells via ER stress [54]. PA-induced lipotoxicity also causes mitochondrial damage and the release of mtDNA into the cytosol, thus triggering the cyclic GMP-AMP synthase (cGAS) - stimulator of interferon genes (STING) signaling pathway, which switches on the initiation of NLRP3 inflammasome-dependent pyroptosis in cardiomyocytes, promoting myocardial hypertrophy [57]. In addition, higher levels of PA promote the polarization of macrophage M1, and upregulate the expression of cathepsin S (CTSS) through the transcription factor interferon regulatory factor 5 (IRF5). CTSS is transported in the exosomes, which can upregulate caspase-1 and trypsinogen in acinar cells, thus promoting pyroptosis and pancreatic tissue damage [58, 59]. Besides, PA can enhance the endocytosis of LPS produced by gram-negative bacteria in the intestines of mice into intestinal neurons in a lipid raft-dependent manner, thus promoting LPS cleaving caspase-11 to regulate pyroptosis through non-classical pathway, eventually leading to the loss of enteric neuronal and enteric motility disorder [60]. In sum, SFAs, especially PAs, can activate inflammasome-dependent pyroptosis in different ways.
In addition, many FAs can inhibit pyroptosis, thereby alleviating tissue damage. The diet rich in n-3 PUFA has anti-inflammatory and anti-pyroptosis effects. Docosahexaenoic acid (DHA), as a representative of ω-3 PUFA, is a potent inhibitor of both caspase-1 activation and IL-1β secretion [61, 62]. DHA at a physiologically relevant concentration (less than 50 µM) is capable of attenuating pyroptosis [63, 64]. DHA suppresses TLR4/NF-κB and NLRP3/caspase-1/GSDMD signaling pathways, thereby attenuating osteoarthritis [49]. Further studies have shown that the anti-pyroptosis effect of DHA is to inhibit the assembly of NLRP3 inflammasome by promoting the interaction between G protein-coupled receptor (GPR) 120 and NLRP3. GPR120 is a PUFA receptor that mediates anti-inflammatory effects via PM internalization into the cytoplasm [29]. The activation of GPRs causes the binding of β-arrestin2 to GPRs, and subsequent internalization of β-arrestin2, which then attaches to NLRP3 and prevents the assembly and activation of NLRP3 inflammasome [65-67]. Meanwhile, DHA inhibits hypoxia/restoration (H/R)-induced injury by suppressing pyroptosis of hepatocytes induced by liver I/R injury in vivo and in vitro through the phosphatidylinositol-3-kinase /protein kinase B (PI3K/Akt) pathway [68]. DHA can also exert an anti-inflammatory activity in the treatment of acute keratitis by alleviating the non-canonical pyroptosis [69]. However, more than 50 μM of DHA can play a pro-inflammatory role. DHA (200 µM) at a higher concentration (within the physiological dose range) induces Bv-2 cell pyroptosis through 12-lipoxygenase (12-LOX). In detail, 12-LOX can produce one or more metabolism and activate a pro-inflammatory cell death program [63, 70]. Similarly, all-trans retinoic acid (ATRA) secreted by hepatic stellate cells (HSC) can bind to retinoic acid receptors in KCs and activate the transcription activity of NLRP3. In addition, ATRA can also block autophagy, lead to excessive accumulation of ROS, and then activate NLRP3 inflammasome, thus inducing the pyroptosis of macrophages [71]. In addition, a study has shown that DHA can cause pyroptosis of triple-negative breast cancer cells MDA-MB-231 [72]. Treatment with 200μM of DHA in breast cancer cells can lead to NF-κB translocation, caspase-1 and GSDMD activation, IL-1β secretion, HMGB1 translocation, pore membrane formation, and loss of membrane [72]. It is noteworthy that DHA has no significant effect on human non-cancerous mammary epithelial cells MCF-10A or PBMCs, indicating that this fatty acid has cytotoxicity only on cancer cells [72]. Therefore, ω-3 supplementation during therapy of breast cancer patients can be used as a new therapeutic strategy.
In addition to DHA, the metabolite short chain fatty acids (SCFAs) of gut microbiota including propionate (C3) and butyrate (C4) have been reported to be beneficial for pyroptosis and treatment of wear particle-induced osteolysis, in which C4 instead of C3 requires the GRP109a receptor in this process [73]. 10-hydroxy-2-decenoic acid (10-HAD), the major fatty acid in royal jelly, can inhibit pyroptosis and treat ulcerative colitis [74]. NLRP3 mediated pyroptosis induced by PA can be antagonized by oleic acid (OA), one of MUFAs [54]. Chondrocytes treated with MUFAs showed the down-regulation of TLR4/NF-κB and NLRP3/caspase-1/GSDMD signaling pathways, while a MUFA-enriched high-fat diet stimulates the expression of TLR4/NF-κB and NLRP3 inflammasome proteins. The contradictory results of MUFAs in vitro and in vivo may be due to the fact that the high-fat diet rich in MUFA contains not only MUFAs, but also other pro-inflammatory FAs, such as SFA and n-6 PUFA. These FAs can weaken or even reverse the effects of MUFAs, resulting in the contradiction between the experimental results in vitro and in vivo [49].
Phospholipids and its Oxidative Derivatives and Pyroptosis
Phospholipids (PLs) are amphiphilic lipids that exist in the cell membranes of all plants and animals. They are arranged in lipid bilayers and include two major categories, glycerophospholipids, and sphingolipids. The PLs in most cell membranes are glycerophospholipids, which are composed of FA esterified into the glycerol back bone, phosphate groups, and hydrophilic residues (such as choline). Glycerophospholipids can be further divided into phosphatidylinositol (PI), cardiolipin (CL), phosphatidylserine (PS), phosphatidylcholine (PC), and phosphatidylethanolamine (PE) according to the substituents. The GSDMD-N possess lipid-binding and regulatory activities. GSDMD-N has the strongest binding with CL (a kind of mitochondrial and bacterial lipid) and phosphatidylinositol phosphates (PIPs), the phosphorylated products of PI [75, 76]. This powerful combination enables GSDMD-N to locate the inner leaflet of the PM, form membrane-disrupting pores, and execute pyroptosis. And the same combination also exists on regulatorof cell death-1(RCD-1), a remote homolog of the N-terminal domain of gasdermin [77]. phosphatidylinositol (4,5) bisphosphate (PIP2) and its synthetic precursor phosphatidylinositol-4-phosphate (PI4P) are the two main PIPs in PM. Decreasing their concentration can reduce the binding and oligomerization of GSDMD-N [16, 78]. The accumulation of PI4P can potentiate the activation of NLRP3 inflammasome. In detail, NLRP3 is recruited into the dispersed trans-Golgi network (dTGN) through ionic bonding between its conserved polybasic region and negatively charged PI4P, and then interacts with ASC to activate the downstream signal cascade [79, 80]. It has been shown that the dTGN is of endosomal origin. The endosomal accumulation of PI4P further impairs the trafficking of endosome to TGN [81].
Although the combination of GSDMD-N and PS is weak [75], GSDMD-N can also form oligomeric pores by binding with PS [82]. Another study has shown that oligomeric pores mediated calcium influx, which induced PS transfer from inner leaflets to outer leaflets through transmembrane protein 16F, a calcium-dependent phospholipid scramblase [83]. The asymmetric distribution of glycerophospholipids across the inner and outer leaflets of the PM is crucial for cellular integrity and signal transduction [84]. Actually, GSDMD-N does not bind directly with PE or PC (the major lipids on both plasma membrane leaflets) [75]. However, polyene PC, a clinical practice commonly used in the treatment of fatty liver, can reduce the expressions of GSDMD-N and other pyroptosis-related proteins [85]. PC can be decomposed to lysophosphatidylcholine (LPC) under the catalysis of phospholipase A2 (PLA2). It is reported that LPC, can induce pyroptosis in human monocytes and human endothelial cells. LPC can also induce foam cell formation through LD biogenesis, which depends on the activation of NLRP3/caspase-1 [86]. Taken together, alteration of the phospholipid composition or distribution in the membrane directly affects the combination of GSDMD-N and lipids, which may be a potential strategy for inhibiting pyroptosis.
1-palmitoyl-2-arachidonoyl-snglycero-3-phosphorylcholine (PAPC) is a class of natural phospholipids. Under the condition of inflammation or oxidative stress, the side chains of polyunsaturated fatty acids in phospholipids are oxidized and modified to produce oxidized PAPC (oxPAPC). OxPAPC is usually present in dead cells, with concentrations of 10-100 μM at the site of injury [87]. Similar to CHCs, OxPAPC is found in the early stage of atherosclerosis in mice and accumulated in the blood vessels of foam cells [36, 86], indicating that they contribute to inflammation in the early stage. However, unlike CHCs, oxPAPC exhibits anti-inflammatory effects and inhibits pyroptosis during gram-negative bacterial sepsis [88]. This is due to the competitive combination of oxPAPC and LPS. LPS activates TLR4, triggers transcription on the cell surface, and activates caspase-4/11 in the cytosolic, resulting in pyroptosis [60]. OxPAPC not only antagonizes TLR4, but also directly binds to caspase-4 and caspase-11, and competes with LPS, thereby inhibiting LPS-induced pyroptosis [88]. Interestingly, compared with LPS, the combination of the above oxPAPC will not promote pyroptosis due to the unique positively charged amino acid residues [87]. Moreover, oxPAPC induces an enhanced activation state of dendritic cells, called “hyperactive”, and then induces potent adaptive immune responses [87]. Therefore, oxPAPC and its derivatives may provide a basis for therapies that target pyroptosis in gram-negative bacterial sepsis.
Low-Density Lipoprotein and its Oxidative Derivatives and Pyroptosis
Low-density lipoprotein (LDL) is a complex particle containing protein and lipids, and its outermost layer is surrounded by a lipid core and monomeric protein ApoB-100. LDL can induce inflammasome activation [89], but there are no reports related to pyroptosis LDL contains polyunsaturated fatty acids. When the antioxidant activity of LDL is impaired, ROS and reactive nitrogen species result in lipid oxidation to produce oxidized LDL (ox-LDL) [90]. Ox-LDL is the main factor to promote foam cell formation and atherosclerosis, which may be one of the reasons why ox-LDL is used as an inducer in almost all studies on pyroptosis in atherosclerosis [91].
Ox-LDL can trigger pyroptosis directly or indirectly. On the one hand, ox-LDL can be directly recognized by TLR4, which can activate a series of downstream signals, such as NF-κB p65 phosphorylation, to promote transcription of pro-IL-1β and pro-caspase-1 [92, 93]. In addition, ox-LDL activated pyroptosis in primary human aortic EC through non-canonical NF-κB pathway that upregulated the transcription factor IRF-1 through RelB/p52. IRF-1 interacted with the GSDMD promoter at -526/-515 and the caspase-1 promoter at -11/10 to promote the expression and caspase-1-mediated activation of GSDMD [94]. In addition, ox-LDL can also assist NLRP3 inflammasome assembly in various indirect ways. Ox-LDL can induce mitochondrial dysfunction and ROS release through multiple pathways, resulting in subsequent pyroptosis. Specifically, ox-LDL can down-regulate tet methylcytosine dioxygenase 2 (TET2) [95], thus reducing mitochondrial dysfunction [96]. Zhaolin et al. showed that ox-LDL might induce EC pyroptosis and promote the development of atherosclerosis by regulating miR-125a-5p/ TET2 pathway [95]. TET2 can inhibit the methylation of ubiquinol-cytochrome c reductase core protein 1 (UQCRC1), a subunit of mitochondrial complex III. Deletion of UQCRC1 can lead to excessive ROS production [97]. Similarly, fibroblast growth factor 21 also inhibits ox-LDL-induced pyroptosis through the TET2-UQCRC1-ROS pathway [98]. The proprotein convertase subtilisin/kexin type 9 (PCSK9) is an important protein involved in lipid metabolism and AS. Ox-LDL induces the pyroptosis of HUVECs in a concentration-dependent manner through PCSK9/UQCRC1/ROS pathway. PCSK9 can downregulate UQCRC1 expression and mediate ox-LDL-induced pyroptosis of HUVECs [99]. Furthermore, UQCRC1 can be directly down-regulated by ox-LDL, and the silence of UQCRC1 aggravates HUVEC pyroptosis and the damage of mitochondrial function. TET2 is also an inhibitor of succinate dehydrogenase B (SDHB) and its deletion leads to the up-regulation of SDHB expression and activity by reducing the recruitment of histone deacetylase 2 [100]. Overexpression of SDHB in HUVECs impairs mitochondrial function, increases ROS level, and enhances pyroptosis, while knockout of SDHB can resist HUVEC pyroptosis [101]. These studies indicate that ox-LDL can induce oxidative stress and mitochondrial disorder through a variety of ways, thus leading to the production of ROS [95]. Undoubtedly, the elevated ROS also disrupts mitochondrial functions [99]. Through this interaction and regulation, the participation of ROS is considered as the driving force of pyroptosis [102]. ROS not only serves as an efficient trigger of the NLRP3 inflammasome, but also directly promotes GSDMD cleavage in pyroptosis, and up-regulates the oxidative modification of cysteine in GSDMD [102]. Therefore, ROS is a triggering factor that activates NLRP3 inflammasomes agent and "effector" molecule [103].
Ca2+ influx is the upstream regulator of NLRP3 inflammasome activation. Studies have shown that ox-LDL induces the closure of macrophage K+ channels to open Ca2+ channels [104], and upregulates the expression of calcium-sensing receptor (CaSR) in rat aortic VSMCs in a time and dose-dependence manners to promote Ca2+ influx [105]. Sebastian Rühl et al. found that the influx of Ca2+ through GSDMD pores was served as a signal for cells to initiate membrane repair by recruiting the endosomal sorting complexes required by transport (ESCRT) machinery to damaged membrane areas, such as the PM. Inhibition of the ESCRT-III machinery strongly exacerbates the activation of canonical or noncanonical inflammasome-dependent pyroptosis as well as the release of IL-1β in both human and murine cells [106]. These studies suggest that ox-LDL may induce pyroptosis by promoting Ca2+ influx, and the rupture of membrane pores after pyroptosis is due to the Ca2+ influx activating ESCRT to repair cell membranes, and prevent the amplification of inflammation followed pyroptosis.
Ox-LDL can also induce K+ efflux via the activation of big conductance Ca2+-activated K+ channels (BKCa). In addition, P2X7 generates channels to promote K+ efflux [107]. Definitely, K+ efflux is an essential upstream factor of caspase-1 activation. High levels of extracellular K+ can block the inflammatory responses induced by ox-LDL in HUVECs [108], while low concentrations of intracellular K+ are sufficient to trigger NLRP3 inflammasome [18]. Ox-LDL up-regulates mixed lineage kinase domain-like (MLKL) protein, which activates NLRP3-induced pyroptosis by stimulating intracellular K+ efflux. MLKL-induced caspase-1 activation and IL-1β maturation can be abolished by NLRP3 specific inhibitor MCC950 [108].
Pyroptosis is a form of pro-inflammatory cell death. Conversely, autophagy is a cell survival mechanism, which allows cells to survive by adapting to stress. There two cell processes are important components of immune regulation. In particular, the activity of autophagosome plays a pivot role in regulating cell deaths, and blocking autophagy promotes the pyroptosis of ox-LDL-treated macrophages via the p62/Nrf2/ARE axis [109]. Mitochondrial receptor NIX inhibits ox-LDL-induced pyroptosis of human macrophage through autophagy and inhibition of caspase-1 activation [110]. Ox-LDL-treated ECs exhibit increased pyroptosis mediated by myeloid cells trigger receptors1 (TREM-1) and decreased autophagy induced by Sirt6 [111].
Synthetic Lipids and their Potential Application and Pyroptosis
Lipid nanoparticles (LNP) are multicomponent lipid systems that typically contain phospholipids, cationic lipids, cholesterol, and polyethylene glycolated lipids. Cationic lipids are key components of LNP, which can be permanently charged or acquire their charge at an acid pH and are also known as ionizable lipids. Cationic lipids possess a significant ability to stimulate the innate immune system, and are generally considered a safe alternative to viral vectors [112]. The traditional type of lipid nanoparticles refers to liposomes. Several studies have demonstrated that the structure of lipids can affect these immune responses. Lipids with lysine head groups, ditetradecyl hydrophobic chains, and propyl or pentyl spacers, respectively, such as L3C14 and L5C14 liposomes, are most effective in activating the NLRP3 inflammasome [113]. A previous study has shown that the arginine-based liposomes, such as A3C14 liposomes, can induce the most effective lysosomal disruption and NLRP3 inflammasome activation [114]. Varying the concentrations of different lipid components in lipid nanoparticle formulations, the most notable of which are ionizable, cationic lipids, and cholesterol, can change their impact on the activation of NLRP3 inflammasomes, mainly due to the delay of endosomal rupture/fusing [115]. In sum, the structural effect of cationic liposomes on the activation of NLRP3 inflammasome has provided insights into the application of lipid nanoparticles in improving immune response. The ability of cationic lipid nanoparticles can be exploited in gene therapy, anticancer or antiviral immunotherapies.
In vitro and in vivo evidence of lipid and its metabolites regulate pyroptosis.
| Category of lipid | In vivo | In vitro | Effect on pyroptosis | mechanism | Ref |
|---|---|---|---|---|---|
| CHCs | NLRP3-/-mice | Mouse primary coronary arterial endothelial cells | Promote | mtROS↑/NLRP3↑ | [40] |
| Cholesterol | HFHCD-fed mice | hepatocyte | Promote | DAG↑/PKCδ↑/NLRC4↑ | [30] |
| HCD-fed rats | NP cells | Promote | mSREBP1↑/ER stress↑ | [31] | |
| 27-OHC | NA | SH-SY5Y cells, C6 cells | Promote | LMP↑/CTSB↑/NLRP3↑/caspase-1↑ | [47] |
| 25-OHC | NA | NHEK,HaCaT cells, | Promote | P2X7R↑/NLRP3↑/caspase-1↑ | [43] |
| PA | obesity-associated osteoarthritis mice | SW1353 chondrocytes | promote | TLR4↑/NF-κB↑ NLRP3↑/caspase-1↑/GSDMD↑ | [49] |
| NAFLD rat | HepG2 cells | Promote | ER stress↑/NLRP3↑ | [54] | |
| hyperlipidemic pancreatitis mice | Rat pancreatic acinar cells | Promote | IRF5↑/CTSS↑/caspase1↑/GSDMD↑ | [59] | |
| caspase-11-knockout mice, Western diet-fed mice | enteric neuronal cells | Promote | caspase-11↑/GSDMD↑ | [60] | |
| DCM mice | Myocardial cells | Promote | cGAS↑/STING↑ | [57] | |
| DHA | Buffalo rat | Kupffer cells | Inhibit | PI3K↑/Akt↑/caspase-1↑ | [68] |
| Acute keratitis rats | Human corneal epithelial cells | Inhibit | Caspase-11↓/p30↓ | [69] | |
| LPS-exposed mice | Kuppfer cells | Inhibit | GPR120↑/β-arrestin2↑/ NLRP3↓ | [29] | |
| obesity-associated osteoarthritis mice | SW1353 chondrocytes | Inhibit | TLR4↓/NF-κB↓ NLRP3↓/caspase-1↓/GSDMD↓ | [49] | |
| NA | murine microglia cells | Promote DHA (200µM) | 12-LOX↑ | [63] | |
| NA | MDA-MB-231 cells,4T1 cells | Promote DHA (200µM) | NF-κB translocation↑, caspase-1↑/GSDMD↑ | [72] | |
| OA | NAFLD rat | HepG2 cells | Inhibit | ER stress↓/NLRP3↓ | [54] |
| Propionate、butyrate | C57BL/6J male mice | BMDMs、THP-1、Osteoclast differentiation | Inhibit | NLRP3↓/Caspase-1↓/GSDMD-N↓ | [73] |
| 10-HDA | ulcerative colitis mice | THP-1 | Inhibit | NLRP3↓/Caspase-1↓/GSDMD-N↓ | [74] |
| PI(4,5)P2 | Gsdmd-/- mice | HEK293T、Hela、iBMDM | Promote | GSDMD-N↑ | [75] |
| PI4P | Gsdmd-/- mice | HEK293T、HeLa | Promote | GSDMD-N↑ | [78] |
| cardiolipin | NA | Hela cells、293T | Promote | GSDMD-N↑ | [16] |
| phosphatidylserine | NA | Asymmetric plasma membrane composition | Promote | GSDMD-N↑ | [82] |
| LPC | NA | THP-1、HUVEC | Promote | NLRP3↑/Caspase-1↑/GSDMD-N↑ | [86] |
| oxPAPC | Casp1-/-, Casp11-/-, Tlr4-/-, Tlr3-/-, and Cd36-/- mice | BM cells were flushed from femurs and tibias | Inhibit | TLR4↓/caspase4,11↑ | [88] |
The timeline of pyroptosis
Lipid Metabolism: A New Indicator of Pyroptosis
Key Enzymes in Lipid Synthesis: A Potential Aspects of Regulate Pyroptosis
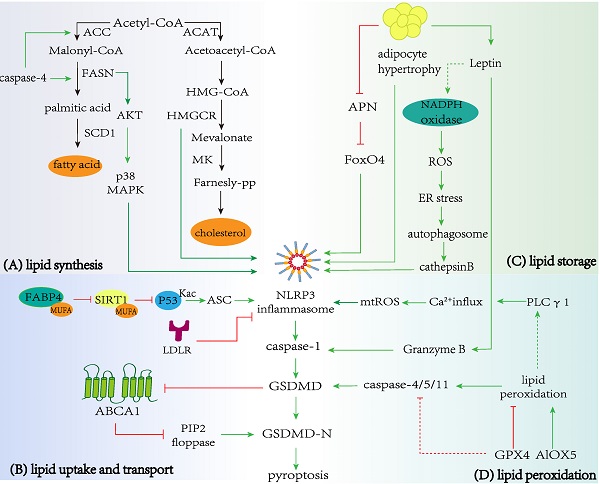
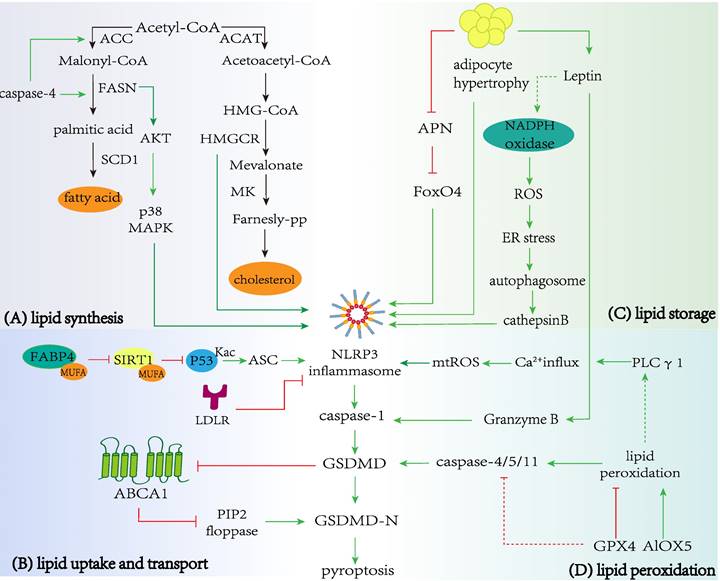
Nearly 30 enzymatic processes convert acetyl CoA into cholesterol, such as mevalonate pathway [116]. Inhibition of the mevalonate pathway leads to pyroptosis in Raw 264.7 monocyte cells [117]. 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR), as the key rate-limiting enzyme of the mevalonate pathway in cholesterol synthesis [118], is positively correlated with inflammation [119, 120] and interacts with NLRP3 [121]. HMGCR knockdown can reduce pyroptosis [121]. Notably, the use of statins (a class of HMGCR inhibitors) including simvastatin and atorvastatin can attenuate pyroptosis [31]. Mevalonate kinase (MK) is another important kinase in the mevalonate pathway, and its deficiency is associated with an auto-immune disease known as Mevalonate Kinase Deficiency (MKD). It has been shown that pyroptosis in MKD is a fundamental step to induce the inflammatory phenotype of MKD patients [122].
Fatty acid synthase (FASN) is the key enzyme to govern the de novo synthesis of fatty acids, which can convert acetyl-CoA, malonyl-CoA, and NADPH into SFA [123, 124]. One study showed that the increase of FASN-mediated lipid synthesis of macrophages could enhance the caspase-1 activation mediated by NLRP3 and IL-1β expression through Akt and P38 MAPK pathways [125]. Uncoupling protein-2 (UCP2) is a mitochondrial transport protein family located in the inner membrane of mitochondrial. It acts as a critical regulator of glucose-dependent de novo lipid synthesis in vivo and in vitro, and can up-regulate the expression of FASN in response to LPS and other stimuli [126]. Another study revealed that knockdown or overexpression of UCP2 in hepatocytes could suppress or up-regulate FFAs-mediated pyroptosis respectively, which is manifested by the expressions of pyroptotic gene and accelerated cell death [127]. This may be due to the activation of FASN mediates the increase of lipid synthesis, and ultimately promotes pyroptosis. SMS1 is an enzyme that generates sphingomyelin (SM) and DAG from de novo-synthesized ceramide [128]. A recent study has shown that the overexpression of SMS1 in hepatocytes induces hepatocyte pyroptosis through the DAG-PKCδ-NLRC4 axis [30].
Conversely, pyroptosis also seems to affect lipid metabolism. GSDMD plays a key role in the pathogenesis of steatohepatitis by regulating lipogenesis. It has been found that knockout of GSDMD can reduce the expression of the lipogenic gene (such as FAS, PPARγ, SCD-1, and SREBP-1), while increasing the expression of lipolytic genes such as PPARα, CPT-1, ACO, LCAD, Cyp4a10 and Cyp4a14, to alleviate steatosis [129-131]. In addition, caspase-4 can promote the synthesis and accumulation of fatty acids by up-regulating the expression of acetyl coenzyme A carboxylase, FASN, SREBP-1 and SREBP-2, and increasing the number of lipid droplets, and ultimately accelerate the progress of pancreatic cancer [132].
Lipid uptake is involved in Pyroptosis
Cholesterol can be transported through LDL binding to LDL receptor (LDLR) and internalization [133]. A recent study has demonstrated that the expression of LDLR is down-regulated following acute cerebral ischemia, which exacerbated neuronal pyroptosis and inflammatory response by provoking the activation and recruitment of NLRP3 inflammasome, leading to the enlargement of cerebral infarct volume and the aggravation of neurological function defect [134].
The known FA protein transporters in PM include cluster of differentiation 36 (CD36) and FA-binding proteins (FABPs). CD36 is a scavenger receptor that can induce macrophage pyroptosis by regulating ox-LDL uptake [135, 136]. In addition, the activation of inflammasome by Porphyromonas gingivalis (Pg) LPS in the oral cavity is mediated by CD36/scavenger receptor-B2 (SR-B2) and TLR2, and leads to systemic release of pro-atherosclerotic IL-1β, as well as induces macrophage pyroptosis. However, pyroptosis is reduced in the absence of CD36/SR-B2 [137].
Lipid Storage: Hypertrophic Adipocytes induce Obese Adipocyte Pyroptosis
In a state of caloric excess, white adipose tissue mainly stores the surplus energy in the form of triglycerides. The volume of adipose tissue can be increased, in one of two main ways: hypertrophy or hyperplasia [138]. The activation of NLRP3-dependent caspase-1 in hypertrophic adipocytes induces the pyroptosis of obese adipocytes, causing macrophage recruitment with metabolic consequences. These may be directly related to the large number of CHCs observed in transmission electron microscopy [139, 140]. It has been reported that Nrf1 plays a key role in energy homeostasis by regulating lipid metabolism. Adipocyte-specific knockout of Nrf1 [Nrf1(f)-KO] in mice disturbs the expressions of lipolytic genes in adipocytes, resulting in white adipocyte hypertrophy, followed by severe adipose inflammation and pyroptosis [141, 142]. Furthermore, overexpression of Nrf1 has been shown to inhibit tubular epithelial cell pyroptosis [143]. Knockout of BMPR2 in adipocytes disrupts the phosphorylation of the lipid-droplet-coating protein (perilipin), and downregulates the lipolysis of white adipocytes, leading to subsequent caspase-1- dependent pyroptosis and inflammation [144]. In addition to energy storage, adipose tissue is an active endocrine organ that regulates lipid metabolism by secreting adipokines like adiponectin (APN) [145]. The secretion of APN in hypertrophic adipocytes is decreased [146, 147]. APN can suppress lipopolysaccharide-induced pyroptosis by inhibiting forkhead transcription factor O 4 (FoxO4) in human aortic epithelial cells [148]. In addition, leptin is also a hormone primarily derived from adipose tissue, and its plasma levels are correlated with fat storage [149]. Leptin can modulate lipid metabolism in hepatocytes, resulting in hepatic steatosis [150]. A study has shown that leptin exerts direct hepatocyte pyroptosis via ROS production/ER stress/autophagy induction/cathepsin B maturation/NLRP3 inflammasomes axis, leading to potential liver injury [151]. Moreover, leptin can also trigger hepatocyte pyroptosis through CD8+ T lymphocytes. This effect relies on the Granzyme B released by CD8+ T lymphocytes [152]. Altogether, these results suggest that failing lipid metabolism renders adipocytes vulnerable to pyroptosis, and prevention of adipocyte hypertrophy may improve the disease associated with pyroptosis.
The Interaction between Lipid Transport and Pyroptosis
Excess cholesterol in the ER drives the activation of the LXR transcription factors, which mediate cholesterol efflux by controlling the expression of the cholesterol export molecules ATP-binding cassette transporter 1 (ABCA1) and ATP-binding cassette subfamily G member 1 (ABCG1). ABCA1/G1-mediated cholesterol efflux is the initial step of reverse cholesterol transport (RCT). It is worth noting that ABCA1 also has certain anti-inflammatory effects. The lack of ABCA1/ABCG1 in cells leads to cholesterol accumulation and activation of NLRP3 inflammasomes [153, 154]. A study has shown that ABCG1-knockout mice disturbed cholesterol metabolism and exacerbated pyroptosis after traumatic brain injury [155]. ABCA1 expression can reduce the binding of GSDMD-N to PM, thus preventing cell lysis. Furthermore, ABCA1 is the floppase of PIP2 that transfers PIP2 from the inner to the outer leaflet of the PM, thereby reducing the content of PIP2 on the leaflets inside the PM [156]. As the ligand of GSDMD-N fragments, PIP2 promotes cell rupture by transfection of GSDMD-N [75, 157], indicating that ABCA1 may indirectly inhibit GSDMD-induced membrane pore disruption and scorch death through PIP2. It is noteworthy that the pore formation caused by GSDMD can decrease the ability of cells to effectively transport cholesterol via the ABCA1-apoA1 pathway [157], which may increase cholesterol accumulation. Therefore, GSDMD-/- macrophages and mice can resist the reduction of RCT induced by pyroptosis.
Fatty acid binding protein 4 (FABP4) is a carrier protein of fatty acids, which is mainly expressed in macrophages and adipocytes and can regulate lipid metabolism. The increase of intracellular MUFA level in macrophages with FABP4 deletion leads to up-regulation of UCP2 expression, inhibition of ROS production, reduction of ER stress, and NF-κB activation and cytokine release attenuated, eventually leading to the anti-inflammatory phenotype in both animal and cell models [158, 159]. The latest study further provides a mechanism, that is, the combination of FABP4 and MUFA can reduce the activation of silent mating type information regulation 2 homolog 1 (SIRT1) and the acetylation of p53. Pharmacological inhibition or genetic deletion of FABP4 in macrophages can deacetylate and inactivate p53 through SIRT1, which is the result of the loss of ASC expression. Lack of ASC prevents assembly of the NLRP3 inflammasome, GSDMD processing, and functional activation of pyroptosis [160]. Together, these studies support a possible role for adipose tissue in promoting pyroptosis. Further studies are warranted to reveal molecular links between lipid droplet formation and pyroptosis inhibition.
Lipid Peroxidation: not the only Feature of Ferroptosis
Lipid peroxidation refers to the process of lipid peroxide generation mediated by free radicals on the cell membranes and organelle membranes [161]. Glutathione peroxidase 4 (GPX4) is an antioxidant defense enzyme that can repair oxidative damage [162]. Kang et al. showed that the inactivation of GPX4 gene increased lipid peroxidation-dependent caspase-11 activation, and aggravated GSDMD-mediated pyroptosis in macrophages as well as septic lethality in mice [163]. And other studies have also demonstrated the ability of GPX4 to inhibit pyroptosis [164, 165], which may be related to lipid peroxidation. 5-Lipoxygenase (ALOX5) is an iron-containing and nonheme dioxygenase that catalyzes the peroxidation of PUFAs such as AA. Inhibition of lipid peroxidation by ALOX5 limits the activation of caspase-11 inflammasome and pyroptosis in macrophages, which provides a potential strategy for the treatment of sepsis [166]. These results indicate that lipid peroxidation is closely related to pyroptosis. Interestingly, only lipid peroxides produced by the oxidation of phospholipids can induce GSDMD-N-mediated pyroptosis in BMDMs, instead of lipid peroxides formed by cholesterol and glycolipids in PM [163]. Furthermore, lipid peroxidation of PM leads to phospholipase C (PLC) γ1 activation. PLCγ1, as an important second messenger, converts PIP2 to inositol 1,4,5-trisphosphate (IP3), resulting in the mobilization of ER calcium pool [167]. On the one hand, PLCγ1-regulated calcium signal controls GSDMD-mediated pyroptosis by promoting GSDMD-N translocation to the PM [163, 168]. On the other hand, the mobilization of intracellular calcium stores can also induce calcium flux and mtROS production, which triggers the activation of NLRP3 inflammasome and caspase-1, leading to IL-1β release as well as GSDMD-mediated pyroptosis [169].
Fatty Acid Oxidation
The main pathway of fatty acid catabolism is through fatty acid beta-oxidation (FAO), which is up-regulated under long-term fasting, exercise, or metabolic stress. Moon et al. have demonstrated that FAO promotes the activation of NLRP3 inflammasome through NADPH oxidase 4 (NOX4). Mechanistically, NOX4 acts as a source of cellular superoxide anion, which enhances the expression of carnitine palmitoyltransferase 1A (CPT1A) to promote the activation of NLRP3 inflammasome, and CPT1A is a key enzyme in the FAO pathway [170]. Another study showed that NOX promoted the production of mtROS or self-derived ROS, and activated NLRP3 inflammasome. It was further found that NOX4 was positively correlated with the expressions of caspase-1 and GSDMD-N, and inhibition of NOX4 could prevent cardiomyocyte pyroptosis [171]. Since NOX4-derived mtROS is important for regulating the expression of the CPT1A [170], it is believed that NOX4-derived mtROS may stimulate NLRP3 by activating CPT1A-modulated FAO, thus leading to pyroptosis.
Regulation of lipid metabolism in pyroptosis. A Lipid synthesis and pyroptosis. Enzymes in cholesterol and fatty acid biosynthesis, including FASN and HMGCR can promote the occurrence and development of pyroptosis. In addition, the pyroptosis-related protein caspase-4 promote critical enzymes in fatty acid synthesis, including ACC and FASN. B Lipid uptake and transport with pyroptosis. LDLR and ABCA1 restrain NLRP3 inflammasome activation and prevent caspase-1 cleaving GSDMD to promote the release of the N-terminal domain, which executes pores formation and pyroptosis. The combination of FABP4 and MUFA can reduce activation of SIRT1 and acetylation of p53, promoting NLRP3-dependent pyroptosis. C Lipid storage and pyroptosis. Hypertrophic adipocytes can induce NLRP3-dependent caspase-1 activation and pyroptosis, and obese adipocytes also regulate the secretion of APN and leptin. APN and leptin promote pyroptosis via FoxO4 or ROS production/ER stress/autophagy induction/cathepsin B maturation axis respectively. D Lipid peroxidation and pyroptosis. GPX4 and ALOX5 inhibit or promote lipid peroxidation respectively, leading to caspase-11-dependent pyroptosis. Lipid peroxidation triggers NLRP3 inflammasome and caspase-1 activation by inducing PLC γ1 activation.
Conclusion
Pyroptosis is an inflammatory programmed death pathway. Numerous types of lipids, such as CHCs, ox-LDL, and SFAs, act as signal molecules to activate pyroptosis. Conversely, lipids such as UFAs can inhibit pyroptosis. In general, most of these lipids activate NLRP3-dependent pyroptosis by inducing mitochondrial dysfunction, ER stress, and lysosomal disruption, while other types of lipids directly activate GSDMD without relying on NLRP3. Pyroptosis is usually induced by LPS and ATP (or Nigericin) [172]. But accumulating studies used lipids (such as ox-LDL and PA) to establish pyroptosis model, especially in the studies of cardiovascular diseases and NAFLD. Lipid metabolism is regulated by complex intracellular signal network and constitutes an essential part of cell fate regulation. Meanwhile, enzymes or proteins related to lipid metabolism also affect pyroptosis. For example, the GSDMD-N fragments cleaved by caspase-1 trigger pyroptotic macrophages in a PLC1-dependent manner.
Cholesterol in the endoplasmic reticulum is involved in the activation of NLRP3. Silencing NLRP3 and ASC in macrophages, CHCs are unable to release IL-1β demonstrated its ability to activate NLRP3 inflammasome. Cardiolipin is beneficial to the formation of NLRP3 inflammasome. SFAs, such as PA, stimulate TLRs and induce K+-dependent NLRP3 inflammasomes. By contrast, UFAs prevent SFAs-induced NLRP3 activation. In addition to these endogenous host-derived lipids, synthetic lipids (cationic and ionizable lipids) also have been described to regulate the activation of inflammasome. All of these have been discussed in detail in the review by Pizzuto M et al [173]. It is easy to see the important role of lipids in the activation of NLRP3 inflammasome. To date, there are still some issues to be solved. The extent of pyroptosis caused by activation of NLRP3 inflammasome is still unclear, and how inflammasomes develop into pyroptosis requires further exploration. The relationship between synthetic lipids and NLRP3 may have potential roles in synthetic lipids and pyroptosis. Lipids can activate the occurrence of pyroptosis, but there are few studies on whether the large-scale inflammatory response caused by pyroptosis will affect lipid metabolism in turn, and thus the level of blood lipids. It is well known that the inflammatory response can aggravate the imbalance of lipid metabolism, promotes the accumulation and uptake of lipids, as well as inhibit efflux. Therefore, the alterations and status of lipid metabolism-related proteins, such as ABCA1/G1, ABCG5/8, PCSK9, etc. in the process of cytokinesis, deserve in-depth investigations. In addition, as a form of programmed death, pyroptosis plays a protective role, especially in immune defense, but the inflammatory factors released by excessive pyroptosis can lead to a certain degree of tissue damage.
It is exciting to note that inhibitors of the inflammasome-pyroptosis pathway have been identified. Several drugs targeting pyroptosis such as MCC950, VX-765, z-VAD-fmk, have been developed and validated in vitro cell culture studies and animal models of inflammation-related diseases in vivo, but prospective clinical trials are also required to potentially translate them into clinical practice. This is because the activation and action mechanism of pyroptosis is extremely intricate, and a consensus model has not been formed yet. Therefore, a large number of experiments, especially clinical trials, are still urgently required. Other potential drug candidates, such as autophagy inducers, antioxidants, and miRNA reagents, need further development. These studies will help to deepen the understanding of the pathogenesis of many diseases, and develop effective treatment strategies from the perspective of pyroptosis.
Abbreviations
AA: Arachidonic acid; ABCA1: ATP-binding cassette transporter 1; ABCG1: ATP-binding cassette subfamily G member 1; ALOX5: 5-Lipoxygenase; AMPK: AMP-dependent kinase; APN: adipokines like adiponectin; ASC: apoptosis-associated spec-like protein containing a CARD; ATP: Adenosine triphosphate; BMPR2: bone morphogenetic protein receptor type 2; CD36: cluster of differentiation 36; CHCs: cholesterol crystals; COPII: coat protein complex II; CPT1A: carnitine palmitoyltransferase 1A; CTSB: cathepsin B; CTSD: cathepsin D; DHA: Docosahexaenoic acid; ER: endoplasmic reticulum; EtBr: ethidium bromide; FABP4: Fatty acid binding protein 4; FABPs: FA-binding proteins; FAO: fatty acid beta-oxidation; FAs: Fatty acids; FASN: Fatty acid synthase; FoxO4: forkhead transcription factor O 4; GPR: G protein-coupled receptor; GPX4: Glutathione peroxidase 4; GSDMA: gasdemin A; GSDMB: gasdemin B; GSDMC: gasdemin C; GSDMD: gasdemin D; GSDME: gasdemin E; GSMD: Gasdermin; H/R: hypoxia/restoration; HFHCD: high-fat and high-cholesterol diet; HMGB1: high mobility group box 1; HMGCR: 3-hydroxy-3-methylglutaryl coenzyme A reductase; IL-1β: interleukin-1β; IP3: inositol 1,4,5-trisphosphate; LDH: lactate de-hydrogenase; LDL: Low-density lipoprotein; LPC: lyso-PC; LPS: lipopolysaccharide; LXR: Liver X receptors; MAPK: mitogen activated protein kinase; MK: Mevalonate Kinase; MKD: Mevalonate Kinase Deficiency; mtROS: mitochondrial reactive oxygen species; MUFA: monounsaturated; NASH: non-alcoholic steatohepatitis; NF-κB: nuclear factor kappa B; NOX4: NADPH oxidase 4; OA: oleic acid; ox-LDL: oxidized LDL; oxPAPC: oxidized PAPC; PA: palmitic acid; PAPC: 1-palmitoyl-2-arachidonoyl-snglycero-3-phosphorylcholine; PCD: programmed cell death; PCSK9: proprotein convertase subtilisin/kexin type 9; Perilipin: the lipid-droplet-coating protein; Pg: Porphyromonas gingivalis; PI: propidine iodide; PI3K/Akt: phosphatidylinositol-3-kinase/protein kinase B; PIP2: phosphatidylinositol(4,5)bisphosphate; PKCδ: protein kinaseCδ; PLC: phospholipase C; PLs: Phospholipids; PRRs: pattern recognition receptors; PUFA: polyunsaturated fatty acids; RCT: reverse cholesterol transport; ROS: reactive oxygen species; SDHB: succinate dehydrogenase B; SFA: saturated fatty acids; SIRT1: silent mating type information regulation 2 homolog 1; SR-B2: scavenger receptor-B2; SREBP: Sensor response element binding protein; TET2: Tet methylcytosine dioxygenase 2; TLR4: Toll-like receptor 4; TUNEL: dUTP nick end labeling; UCP2: Uncoupling proteins 2; UQCRC1: ubiquinol-cytochrome c reductase core protein 1; 7-AAD: 7-amino actinomycin; 12-LOX: 12-lipoxygenase.
Acknowledgements
Funding
This work was supported by the National Natural Sciences Foundation of China (No.82274159, 81973668 and 81774130), the National Science Fundation of Hunan Province (No. 2022JJ80088), the Key Project of the Educational Department of Hunan Province (No. 20A375, 21A0226), Open Fund of the State Key Laboratory Cultivation Base Co-constructed by the Ministry of Traditional Chinese Medicine Powder and Innovative Drug Research in Hunan Province (No. 21PTKF1004), the Scientific Research Project of Changsha Science and Technology Bureau (No. kq2004060), Key Project of Hunan Provincial Health Commission (202213055529), Pharmaceutical Open Fund of Domestic First-class Disciplines (cultivation) of Hunan Province (2021YX07), the Outstanding Youth Project of Educational Department of Hunan Province (22B0355) and the First-Class Discipline of Pharmaceutical Science of Hunan.
Author contributions
All authors contributed to the development of this review article and approved the submitted version. Yun Qiu and Ya-Ning Shi searched the literature and drafted the manuscript. Li Qin and Ai Guo Dai conceived and designed the review. Neng Zhu, Shuo Zhang, Chan-Juan Zhang, Jia Gu, Peng He examined the literature and made the figures. Li Qin made a critical revision of the review.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113-4
2. Zhaolin Z, Guohua L, Shiyuan W, Zuo W. Role of pyroptosis in cardiovascular disease. Cell Prolif. 2019;52:e12563
3. Shi H, Gao Y, Dong Z, Yang J, Gao R, Li X. et al. GSDMD-Mediated Cardiomyocyte Pyroptosis Promotes Myocardial I/R Injury. Circ Res. 2021;129:383-96
4. Liu Z, Wang M, Wang X, Bu Q, Wang Q, Su W. et al. XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol. 2022;52:102305
5. Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128
6. Aachoui Y, Sagulenko V, Miao EA, Stacey KJ. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr Opin Microbiol. 2013;16:319-26
7. Orning P, Lien E, Fitzgerald KA. Gasdermins and their role in immunity and inflammation. J Exp Med. 2019;216:2453-65
8. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009 7
9. Al Mamun A, Wu Y, Jia C, Munir F, Sathy KJ, Sarker T. et al. Role of pyroptosis in liver diseases. Int Immunopharmacol. 2020;84:106489
10. He X, Fan X, Bai B, Lu N, Zhang S, Zhang L. Pyroptosis is a critical immune-inflammatory response involved in atherosclerosis. Pharmacol Res. 2021;165:105447
11. Tan Y, Chen Q, Li X, Zeng Z, Xiong W, Li G. et al. Pyroptosis: a new paradigm of cell death for fighting against cancer. J Exp Clin Cancer Res. 2021;40:153
12. Tuñón J, Badimón L, Bochaton-Piallat ML, Cariou B, Daemen MJ, Egido J. et al. Identifying the anti-inflammatory response to lipid lowering therapy: a position paper from the working group on atherosclerosis and vascular biology of the European Society of Cardiology. Cardiovasc Res. 2019;115:10-9
13. Qin L, Zhu N, Ao BX, Liu C, Shi YN, Du K. et al. Caveolae and Caveolin-1 Integrate Reverse Cholesterol Transport and Inflammation in Atherosclerosis. Int J Mol Sci. 2016;17:429
14. Katoh M, Katoh M. Identification and characterization of human DFNA5L, mouse Dfna5l, and rat Dfna5l genes in silico. Int J Oncol. 2004;25:765-70
15. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660-5
16. Ding J, Wang K, Liu W, She Y, Sun Q, Shi J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111-6
17. He Y, Hara H, Núñez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends in biochemical sciences. 2016;41:1012-21
18. He Y, Hara H, Núñez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci. 2016;41:1012-21
19. Zhaolin Z, Guohua L, Shiyuan W, Zuo W. Role of pyroptosis in cardiovascular disease. Cell proliferation. 2019;52:e12563
20. Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature immunology. 2009;10:241-7
21. Yang J, Zhao Y, Shao F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr Opin Immunol. 2015;32:78-83
22. Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561-74
23. Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666-71
24. Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends In Biochemical Sciences. 2017;42:245-54
25. Yin Y, Pastrana JL, Li X, Huang X, Mallilankaraman K, Choi ET. et al. Inflammasomes: sensors of metabolic stresses for vascular inflammation. Front Biosci (Landmark Ed). 2013;18:638-49
26. Zhang W, Liang Z. [Comparison between annexin V-FITC/PI and Hoechst33342/PI double stainings in the detection of apoptosis by flow cytometry]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2014;30:1209-12
27. Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity. 2018;48:35-44.e6
28. Kapourchali FR, Surendiran G, Goulet A, Moghadasian MH. The Role of Dietary Cholesterol in Lipoprotein Metabolism and Related Metabolic Abnormalities: A Mini-review. Crit Rev Food Sci Nutr. 2016;56:2408-15
29. Fan G, Li Y, Chen J, Zong Y, Yang X. DHA/AA alleviates LPS-induced Kupffer cells pyroptosis via GPR120 interaction with NLRP3 to inhibit inflammasome complexes assembly. Cell death & disease. 2021;12:73
30. Koh EH, Yoon JE, Ko MS, Leem J, Yun JY, Hong CH. et al. Sphingomyelin synthase 1 mediates hepatocyte pyroptosis to trigger non-alcoholic steatohepatitis. Gut. 2021;70:1954-64
31. Yan J, Li S, Zhang Y, Deng Z, Wu J, Huang Z. et al. Cholesterol Induces Pyroptosis and Matrix Degradation via mSREBP1-Driven Endoplasmic Reticulum Stress in Intervertebral Disc Degeneration. Front Cell Dev Biol. 2021;9:803132
32. Varsano N, Beghi F, Elad N, Pereiro E, Dadosh T, Pinkas I. et al. Two polymorphic cholesterol monohydrate crystal structures form in macrophage culture models of atherosclerosis. Proc Natl Acad Sci U S A. 2018;115:7662-9
33. de la Roche M, Hamilton C, Mortensen R, Jeyaprakash AA, Ghosh S, Anand PK. Trafficking of cholesterol to the ER is required for NLRP3 inflammasome activation. J Cell Biol. 2018;217:3560-76
34. Mulvihill E, Sborgi L, Mari SA, Pfreundschuh M, Hiller S, Müller DJ. Mechanism of membrane pore formation by human gasdermin-D. Embo j. 2018 37
35. Kellner-Weibel G, Yancey PG, Jerome WG, Walser T, Mason RP, Phillips MC. et al. Crystallization of free cholesterol in model macrophage foam cells. Arterioscler Thromb Vasc Biol. 1999;19:1891-8
36. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357-61
37. Zhang Y, Li X, Pitzer AL, Chen Y, Wang L, Li P-L. Coronary endothelial dysfunction induced by nucleotide oligomerization domain-like receptor protein with pyrin domain containing 3 inflammasome activation during hypercholesterolemia: beyond inflammation. Antioxid Redox Signal. 2015;22:1084-96
38. Yang M, Lv H, Liu Q, Zhang L, Zhang R, Huang X. et al. Colchicine Alleviates Cholesterol Crystal-Induced Endothelial Cell Pyroptosis through Activating AMPK/SIRT1 Pathway. Oxid Med Cell Longev. 2020;2020:9173530
39. Rajamäki K, Lappalainen J, Oörni K, Välimäki E, Matikainen S, Kovanen PT. et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765
40. Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210-5
41. Dias IHK, Milic I, Lip GYH, Devitt A, Polidori MC, Griffiths HR. Simvastatin reduces circulating oxysterol levels in men with hypercholesterolaemia. Redox Biol. 2018;16:139-45
42. Schroepfer GJ Jr. Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev. 2000;80:361-554
43. Olivier E, Dutot M, Regazzetti A, Laprévote O, Rat P. 25-Hydroxycholesterol induces both P2X7-dependent pyroptosis and caspase-dependent apoptosis in human skin model: New insights into degenerative pathways. Chem Phys Lipids. 2017;207:171-8
44. Zeng C, Wang R, Tan H. Role of Pyroptosis in Cardiovascular Diseases and its Therapeutic Implications. Int J Biol Sci. 2019;15:1345-57
45. Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity. 2015;43:923-32
46. Reboldi A, Dang EV, McDonald JG, Liang G, Russell DW, Cyster JG. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science. 2014;345:679-84
47. Chen S, Zhou C, Yu H, Tao L, An Y, Zhang X. et al. 27-Hydroxycholesterol Contributes to Lysosomal Membrane Permeabilization-Mediated Pyroptosis in Co-cultured SH-SY5Y Cells and C6 Cells. Front Mol Neurosci. 2019;12:14
48. Ghosh A, Gao L, Thakur A, Siu PM, Lai CWK. Role of free fatty acids in endothelial dysfunction. Journal of Biomedical Science. 2017;24:50
49. Jin X, Dong X, Sun Y, Liu Z, Liu L, Gu H. Dietary Fatty Acid Regulation of the NLRP3 Inflammasome via the TLR4/NF-B Signaling Pathway Affects Chondrocyte Pyroptosis. Oxidative Medicine and Cellular Longevity. 2022;2022:3711371
50. Shen T, Lei T, Chen L, Zhu B-B, Xu B-L, Zhang C-P. et al. Gardenoside Hinders Caspase-1-Mediated Hepatocyte Pyroptosis Through the CTCF/DPP4 Signaling Pathway. Frontiers In Physiology. 2021;12:669202
51. Meng Z, Zhu B, Gao M, Wang G, Zhou H, Lu J. et al. Apigenin alleviated PA-induced pyroptosis by activating autophagy in hepatocytes. Food & Function. 2022;13:5559-70
52. Wang Y, Li C, Ali I, Li L, Wang G. N-acetylcysteine modulates non-esterified fatty acid-induced pyroptosis and inflammation in granulosa cells. Mol Immunol. 2020;127:157-63
53. Shi H, Zhang Y, Xing J, Liu L, Qiao F, Li J. et al. Baicalin attenuates hepatic injury in non-alcoholic steatohepatitis cell model by suppressing inflammasome-dependent GSDMD-mediated cell pyroptosis. International Immunopharmacology. 2020;81:106195
54. Zeng X, Zhu M, Liu X, Chen X, Yuan Y, Li L. et al. Oleic acid ameliorates palmitic acid induced hepatocellular lipotoxicity by inhibition of ER stress and pyroptosis. Nutr Metab (Lond). 2020;17:11
55. Nemecz M, Constantin A, Dumitrescu M, Alexandru N, Filippi A, Tanko G. et al. The Distinct Effects of Palmitic and Oleic Acid on Pancreatic Beta Cell Function: The Elucidation of Associated Mechanisms and Effector Molecules. Frontiers In Pharmacology. 2018;9:1554
56. Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflammation Research: Official Journal of the European Histamine Research Society [et Al]. 2019;68:915-32
57. Yan M, Li Y, Luo Q, Zeng W, Shao X, Li L. et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS-STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discovery. 2022;8:258
58. Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N. et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nature Immunology. 2011;12:231-8
59. Xia W, Lu Z, Chen W, Zhou J, Zhao Y. Excess fatty acids induce pancreatic acinar cell pyroptosis through macrophage M1 polarization. BMC Gastroenterol. 2022;22:72
60. Ye L, Li G, Goebel A, Raju AV, Kong F, Lv Y. et al. Caspase-11-mediated enteric neuronal pyroptosis underlies Western diet-induced colonic dysmotility. J Clin Invest. 2020;130:3621-36
61. Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C. et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154-63
62. Wierenga KA, Wee J, Gilley KN, Rajasinghe LD, Bates MA, Gavrilin MA. et al. Docosahexaenoic Acid Suppresses Silica-Induced Inflammasome Activation and IL-1 Cytokine Release by Interfering With Priming Signal. Frontiers in immunology. 2019;10:2130
63. Srikanth M, Chandrasaharan K, Zhao X, Chayaburakul K, Ong W-Y, Herr DR. Metabolism of Docosahexaenoic Acid (DHA) Induces Pyroptosis in BV-2 Microglial Cells. Neuromolecular Med. 2018;20:504-14
64. Rajasinghe LD, Chauhan PS, Wierenga KA, Evered AO, Harris SN, Bates MA. et al. Omega-3 Docosahexaenoic Acid (DHA) Impedes Silica-Induced Macrophage Corpse Accumulation by Attenuating Cell Death and Potentiating Efferocytosis. Frontiers In Immunology. 2020;11:2179
65. Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32:203-27
66. Hong L, Zahradka P, Cordero-Monroy L, Wright B, Taylor CG. Dietary Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) Operate by Different Mechanisms to Modulate Hepatic Steatosis and Hyperinsulemia in Zucker Rats. Nutrients. 2019 11
67. Meyers AK, Zhu X. The NLRP3 Inflammasome: Metabolic Regulation and Contribution to Inflammaging. Cells. 2020 9
68. Li Z, Zhao F, Cao Y, Zhang J, Shi P, Sun X. et al. DHA attenuates hepatic ischemia reperfusion injury by inhibiting pyroptosis and activating PI3K/Akt pathway. Eur J Pharmacol. 2018 835
69. Wang P, Zhu C, Liu M, Yuan Y, Ke B. The inhibiting effect of Aspirin Triggered-Resolvin D1 in non-canonical pyroptosis in rats with acute keratitis. Exp Eye Res. 2022;218:108938
70. Herr DR, Yam TYA, Tan WSD, Koh SS, Wong WSF, Ong WY. et al. Ultrastructural Characteristics of DHA-Induced Pyroptosis. Neuromolecular Med. 2020;22:293-303
71. Yu Z, Xie X, Su X, Lv H, Song S, Liu C. et al. ATRA-mediated-crosstalk between stellate cells and Kupffer cells inhibits autophagy and promotes NLRP3 activation in acute liver injury. Cellular Signalling. 2022;93:110304
72. Pizato N, Luzete BC, Kiffer LFMV, Corrêa LH, de Oliveira Santos I, Assumpção JAF. et al. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci Rep. 2018;8:1952
73. Wu Y-L, Zhang C-H, Teng Y, Pan Y, Liu N-C, Liu P-X. et al. Propionate and butyrate attenuate macrophage pyroptosis and osteoclastogenesis induced by CoCrMo alloy particles. Mil Med Res. 2022;9:46
74. Huang S, Tao R, Zhou J, Qian L, Wu J. Trans-10-Hydroxy-2-Decenoic Acid Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice via Regulating the Inflammasome-Mediated Pyroptotic Pathway and Enhancing Colonic Barrier Function. Molecular Nutrition & Food Research. 2022;66:e2100821
75. Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153-8
76. Cian MB, Mettlach JA, Zahn AE, Giordano NP, Minor KE, McClelland M. et al. Cardiolipin Biosynthesis Genes Are Not Required for Salmonella enterica Serovar Typhimurium Pathogenesis in C57BL/6J Mice. Microbiol Spectr. 2022;10:e0261721
77. Daskalov A, Mitchell PS, Sandstrom A, Vance RE, Glass NL. Molecular characterization of a fungal gasdermin-like protein. Proc Natl Acad Sci U S A. 2020;117:18600-7
78. Chai Q, Yu S, Zhong Y, Lu Z, Qiu C, Yu Y. et al. A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin. Science (New York, NY). 2022;378:eabq0132
79. He S, Li L, Chen H, Hu X, Wang W, Zhang H. et al. PRRSV Infection Induces Gasdermin D-Driven Pyroptosis of Porcine Alveolar Macrophages through NLRP3 Inflammasome Activation. J Virol. 2022;96:e0212721
80. Chen J, Chen ZJ. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. 2018;564:71-6
81. Zhang Z, Venditti R, Ran L, Liu Z, Vivot K, Schürmann A. et al. Distinct changes in endosomal composition promote NLRP3 inflammasome activation. Nature Immunology. 2023;24:30-41
82. Schaefer SL, Hummer G. Sublytic gasdermin-D pores captured in atomistic molecular simulations. Elife. 2022 11
83. Yang X, Cheng X, Tang Y, Qiu X, Wang Y, Kang H. et al. Bacterial Endotoxin Activates the Coagulation Cascade through Gasdermin D-Dependent Phosphatidylserine Exposure. Immunity. 2019 51
84. Young MM, Bui V, Chen C, Wang H-G. FTY720 induces non-canonical phosphatidylserine externalization and cell death in acute myeloid leukemia. Cell Death & Disease. 2019;10:847
85. Biao Y, Chen J, Liu C, Wang R, Han X, Li L. et al. Protective Effect of Decoction Against Non-Alcoholic Fatty Liver Disease Through Inhibition of ROS/NLRP3/IL-1β Pathway by Nrf2 Signaling Activation. Frontiers In Pharmacology. 2022;13:877924
86. Corrêa R, Silva LFF, Ribeiro DJS, Almeida RDN, Santos IO, Corrêa LH. et al. Lysophosphatidylcholine Induces NLRP3 Inflammasome-Mediated Foam Cell Formation and Pyroptosis in Human Monocytes and Endothelial Cells. Front Immunol. 2019;10:2927
87. Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J. et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 2016;352:1232-6
88. Chu LH, Indramohan M, Ratsimandresy RA, Gangopadhyay A, Morris EP, Monack DM. et al. The oxidized phospholipid oxPAPC protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nat Commun. 2018;9:996
89. Wang P, Zhang W, Feng Z, Zhang J, Sun Y, Zhang W. LDL-induced NLRC3 inflammasome activation in cardiac fibroblasts contributes to cardiomyocytic dysfunction. Mol Med Rep. 2021 24
90. Pérez-Torres I, Guarner-Lans V, Soria-Castro E, Manzano-Pech L, Palacios-Chavarría A, Valdez-Vázquez RR. et al. Alteration in the Lipid Profile and the Desaturases Activity in Patients With Severe Pneumonia by SARS-CoV-2. Front Physiol. 2021;12:667024
91. Dunér P, To F, Alm R, Gonçalves I, Fredrikson GN, Hedblad B. et al. Immune responses against fibronectin modified by lipoprotein oxidation and their association with cardiovascular disease. J Intern Med. 2009;265:593-603
92. Zhang M, Xue Y, Chen H, Meng L, Chen B, Gong H. et al. Resveratrol Inhibits MMP3 and MMP9 Expression and Secretion by Suppressing TLR4/NF-κB/STAT3 Activation in Ox-LDL-Treated HUVECs. Oxid Med Cell Longev. 2019;2019:9013169
93. Song Y, Hou M, Li Z, Luo C, Ou J-S, Yu H. et al. TLR4/NF-κB/Ceramide signaling contributes to Ox-LDL-induced calcification of human vascular smooth muscle cells. Eur J Pharmacol. 2017;794:45-51
94. Fan X, Li Q, Wang Y, Zhang D-M, Zhou J, Chen Q. et al. Non-canonical NF-κB contributes to endothelial pyroptosis and atherogenesis dependent on IRF-1. Transl Res. 2022
95. Zhaolin Z, Jiaojiao C, Peng W, Yami L, Tingting Z, Jun T. et al. OxLDL induces vascular endothelial cell pyroptosis through miR-125a-5p/TET2 pathway. J Cell Physiol. 2019;234:7475-91
96. Guo X, Zhong W, Chen Y, Zhang W, Ren J, Gao A. Benzene metabolites trigger pyroptosis and contribute to haematotoxicity via TET2 directly regulating the Aim2/Casp1 pathway. EBioMedicine. 2019;47:578-89
97. Zeng J, Tao J, Xia L, Zeng Z, Chen J, Wang Z. et al. Melatonin inhibits vascular endothelial cell pyroptosis by improving mitochondrial function via up-regulation and demethylation of UQCRC1. Biochem Cell Biol. 2021;99:339-47
98. Chen JJ, Tao J, Zhang XL, Xia LZ, Zeng JF, Zhang H. et al. Inhibition of the ox-LDL-Induced Pyroptosis by FGF21 of Human Umbilical Vein Endothelial Cells Through the TET2-UQCRC1-ROS Pathway. DNA Cell Biol. 2020;39:661-70
99. Zeng J, Tao J, Xi L, Wang Z, Liu L. PCSK9 mediates the oxidative low-density lipoprotein-induced pyroptosis of vascular endothelial cells via the UQCRC1/ROS pathway. Int J Mol Med. 2021;47:1
100. Chen J, Zhang J, Wu J, Zhang S, Liang Y, Zhou B. et al. Low shear stress induced vascular endothelial cell pyroptosis by TET2/SDHB/ROS pathway. Free Radic Biol Med. 2021;162:582-91
101. Wu P, Chen J, Chen J, Tao J, Wu S, Xu G. et al. Trimethylamine N-oxide promotes apoE(-/-) mice atherosclerosis by inducing vascular endothelial cell pyroptosis via the SDHB/ROS pathway. J Cell Physiol. 2020;235:6582-91
102. Wang Y, Shi P, Chen Q, Huang Z, Zou D, Zhang J. et al. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J Mol Cell Biol. 2019;11:1069-82
103. Abais JM, Xia M, Zhang Y, Boini KM, Li P-L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22:1111-29
104. Su J, Zhou H, Liu X, Nilsson J, Fredrikson GN, Zhao M. oxLDL antibody inhibits MCP-1 release in monocytes/macrophages by regulating Ca(2+) /K(+) channel flow. J Cell Mol Med. 2017;21:929-40
105. Li H-X, Kong F-J, Bai S-Z, He W, Xing W-J, Xi Y-H. et al. Involvement of calcium-sensing receptor in oxLDL-induced MMP-2 production in vascular smooth muscle cells via PI3K/Akt pathway. Mol Cell Biochem. 2012;362:115-22
106. Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science (New York, NY). 2018;362:956-60
107. Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142-53
108. Wu Q, He X, Wu LM, Zhang RY, Li LM, Wu CM. et al. MLKL Aggravates Ox-LDL-Induced Cell Pyroptosis via Activation of NLRP3 Inflammasome in Human Umbilical Vein Endothelial Cells. Inflammation. 2020;43:2222-31
109. Liu J, Wang C, Li J, Yu Y, Liu Y, Liu H. et al. Autophagy blockage promotes the pyroptosis of ox-LDL-treated macrophages by modulating the p62/Nrf2/ARE axis. J Physiol Biochem. 2021:1-11
110. Peng X, Chen H, Li Y, Huang D, Huang B, Sun D. Effects of NIX-mediated mitophagy on ox-LDL-induced macrophage pyroptosis in atherosclerosis. Cell Biol Int. 2020;44:1481-90
111. Zi Y, Yi-An Y, Bing J, Yan L, Jing T, Chun-Yu G. et al. Sirt6-induced autophagy restricted TREM-1-mediated pyroptosis in ox-LDL-treated endothelial cells: relevance to prognostication of patients with acute myocardial infarction. Cell Death Discov. 2019;5:88
112. Lonez C, Bessodes M, Scherman D, Vandenbranden M, Escriou V, Ruysschaert J-M. Cationic lipid nanocarriers activate Toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomedicine. 2014;10:775-82
113. Li T, He J, Horvath G, Próchnicki T, Latz E, Takeoka S. Lysine-containing cationic liposomes activate the NLRP3 inflammasome: Effect of a spacer between the head group and the hydrophobic moieties of the lipids. Nanomedicine. 2018;14:279-88
114. Li T, Zehner M, He J, Próchnicki T, Horvath G, Latz E. et al. NLRP3 inflammasome-activating arginine-based liposomes promote antigen presentations in dendritic cells. Int J Nanomedicine. 2019;14:3503-16
115. Forster Iii J, Nandi D, Kulkarni A. mRNA-carrying lipid nanoparticles that induce lysosomal rupture activate NLRP3 inflammasome and reduce mRNA transfection efficiency. Biomater Sci. 2022;10:5566-82
116. Zhang C, Zhu N, Li H, Gong Y, Gu J, Shi Y. et al. New dawn for cancer cell death: Emerging role of lipid metabolism. Mol Metab. 2022;63:101529
117. Marcuzzi A, Piscianz E, Girardelli M, Crovella S, Pontillo A. Defect in mevalonate pathway induces pyroptosis in Raw 264.7 murine monocytes. Apoptosis. 2011;16:882-8
118. Long J, Zhang CJ, Zhu N, Du K, Yin YF, Tan X. et al. Lipid metabolism and carcinogenesis, cancer development. Am J Cancer Res. 2018;8:778-91
119. Varghese JF, Patel R, Yadav UCS. Sterol regulatory element binding protein (SREBP) -1 mediates oxidized low-density lipoprotein (oxLDL) induced macrophage foam cell formation through NLRP3 inflammasome activation. Cell Signal. 2019;53:316-26
120. Takei A, Nagashima S, Takei S, Yamamuro D, Murakami A, Wakabayashi T. et al. Myeloid HMG-CoA Reductase Determines Adipose Tissue Inflammation, Insulin Resistance, and Hepatic Steatosis in Diet-Induced Obese Mice. Diabetes. 2020;69:158-64
121. Zha S, Yu X, Wang X, Gu Y, Tan Y, Lu Y. et al. Topical Simvastatin Improves Lesions of Diffuse Normolipemic Plane Xanthoma by Inhibiting Foam Cell Pyroptosis. Front Immunol. 2022;13:865704
122. Tricarico PM, Marcuzzi A, Piscianz E, Monasta L, Crovella S, Kleiner G. Mevalonate kinase deficiency and neuroinflammation: balance between apoptosis and pyroptosis. Int J Mol Sci. 2013;14:23274-88
123. Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202-8
124. Song Z, Xiaoli AM, Yang F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients. 2018 10
125. Moon J-S, Lee S, Park M-A, Siempos II, Haslip M, Lee PJ. et al. UCP2-induced fatty acid synthase promotes NLRP3 inflammasome activation during sepsis. J Clin Invest. 2015;125:665-80
126. Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F. et al. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53(Suppl 1):S130-5
127. Zhong H, Liu M, Ji Y, Ma M, Chen K, Liang T. et al. Genipin Reverses HFD-Induced Liver Damage and Inhibits UCP2-Mediated Pyroptosis in Mice. Cell Physiol Biochem. 2018;49:1885-97
128. Bienias K, Fiedorowicz A, Sadowska A, Prokopiuk S, Car H. Regulation of sphingomyelin metabolism. Pharmacol Rep. 2016;68:570-81
129. Xu B, Jiang M, Chu Y, Wang W, Chen D, Li X. et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2018;68:773-82
130. Gao X, Liu S, Tan L, Ding C, Fan W, Gao Z. et al. Estrogen Receptor α Regulates Metabolic-Associated Fatty Liver Disease by Targeting NLRP3-GSDMD Axis-Mediated Hepatocyte Pyroptosis. J Agric Food Chem. 2021;69:14544-56
131. Tang Q, Liu W, Yang X, Tian Y, Chen J, Hu Y. et al. ATG5-Mediated Autophagy May Inhibit Pyroptosis to Ameliorate Oleic Acid-Induced Hepatocyte Steatosis. DNA and Cell Biology. 2022;41:1038-52
132. Song W, Liu Z, Wang K, Tan K, Zhao A, Li X. et al. Pyroptosis-related genes regulate proliferation and invasion of pancreatic cancer and serve as the prognostic signature for modeling patient survival. Discov Oncol. 2022;13:39
133. Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21:225-45
134. Sun R, Peng M, Xu P, Huang F, Xie Y, Li J. et al. Low-density lipoprotein receptor (LDLR) regulates NLRP3-mediated neuronal pyroptosis following cerebral ischemia/reperfusion injury. J Neuroinflammation. 2020;17:330
135. Lin J, Shou X, Mao X, Dong J, Mohabeer N, Kushwaha KK. et al. Oxidized low density lipoprotein induced caspase-1 mediated pyroptotic cell death in macrophages: implication in lesion instability? PLoS One. 2013;8:e62148
136. Wang Y, Fang C, Xu L, Yang B, Song E, Song Y. Polybrominated Diphenyl Ether Quinone Exposure Induces Atherosclerosis Progression via CD36-Mediated Lipid Accumulation, NLRP3 Inflammasome Activation, and Pyroptosis. Chem Res Toxicol. 2021;34:2125-34
137. Brown PM, Kennedy DJ, Morton RE, Febbraio M. CD36/SR-B2-TLR2 Dependent Pathways Enhance Porphyromonas gingivalis Mediated Atherosclerosis in the Ldlr KO Mouse Model. PLoS One. 2015;10:e0125126
138. Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nature Reviews Molecular Cell Biology. 2019;20:242-58
139. Cinti S. UCP1 protein: The molecular hub of adipose organ plasticity. Biochimie. 2017;134:71-6
140. Giordano A, Murano I, Mondini E, Perugini J, Smorlesi A, Severi I. et al. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. Journal of lipid research. 2013;54:2423-36
141. Hou Y, Liu Z, Zuo Z, Gao T, Fu J, Wang H. et al. Adipocyte-specific deficiency of Nfe2l1 disrupts plasticity of white adipose tissues and metabolic homeostasis in mice. Biochemical and Biophysical Research Communications. 2018;503:264-70
142. Ren S, Hou Y, Zuo Z, Liu Z, Wang H, Xu Y. et al. Protracted rosiglitazone treatment exacerbates inflammation in white adipose tissues of adipocyte-specific Nfe2l1 knockout mice. Food Chem Toxicol. 2020;146:111836
143. Wang R, Zhao H, Zhang Y, Zhu H, Su Q, Qi H. et al. Identification of MicroRNA-92a-3p as an Essential Regulator of Tubular Epithelial Cell Pyroptosis by Targeting Nrf1 via HO-1. Front Genet. 2020;11:616947
144. Qian S, Pan J, Su Y, Tang Y, Wang Y, Zou Y. et al. BMPR2 promotes fatty acid oxidation and protects white adipocytes from cell death in mice. Commun Biol. 2020;3:200
145. Booth A, Magnuson A, Fouts J, Foster M. Adipose tissue, obesity and adipokines: role in cancer promotion. Horm Mol Biol Clin Investig. 2015;21:57-74
146. Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023-33
147. Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439-51
148. Zhang L, Yuan M, Zhang L, Wu B, Sun X. Adiponectin alleviates NLRP3-inflammasome-mediated pyroptosis of aortic endothelial cells by inhibiting FoxO4 in arteriosclerosis. Biochemical and Biophysical Research Communications. 2019;514:266-72
149. Ahima RS, Flier JS. Leptin. Annual Review of Physiology. 2000;62:413-37
150. Rotundo L, Persaud A, Feurdean M, Ahlawat S, Kim H-S. The Association of leptin with severity of non-alcoholic fatty liver disease: A population-based study. Clin Mol Hepatol. 2018;24:392-401
151. Baral A, Park P-H. Leptin Induces Apoptotic and Pyroptotic Cell Death via NLRP3 Inflammasome Activation in Rat Hepatocytes. International Journal of Molecular Sciences. 2021 22
152. Zhang Q, Wang J, Huang F, Yao Y, Xu L. Leptin induces NAFLD progression through infiltrated CD8+ T lymphocytes mediating pyroptotic-like cell death of hepatocytes and macrophages. Dig Liver Dis. 2021;53:598-605
153. Xiao H, Lu M, Lin TY, Chen Z, Chen G, Wang W-C. et al. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128:632-42
154. Westerterp M, Fotakis P, Ouimet M, Bochem AE, Zhang H, Molusky MM. et al. Cholesterol Efflux Pathways Suppress Inflammasome Activation, NETosis, and Atherogenesis. Circulation. 2018;138:898-912
155. Xu H, Zheng L, Chen X, Pang Q, Yan Y, Liu R. et al. Brain-specific loss of Abcg1 disturbs cholesterol metabolism and aggravates pyroptosis and neurological deficits after traumatic brain injury. Brain pathology (Zurich, Switzerland). 2022: e13126.
156. Vivas O, Tiscione SA, Dixon RE, Ory DS, Dickson EJ. Niemann-Pick Type C Disease Reveals a Link between Lysosomal Cholesterol and PtdIns(4,5)P(2) That Regulates Neuronal Excitability. Cell Rep. 2019;27:2636-48.e4
157. Opoku E, Traughber CA, Zhang D, Iacano AJ, Khan M, Han J. et al. Gasdermin D Mediates Inflammation-Induced Defects in Reverse Cholesterol Transport and Promotes Atherosclerosis. Front Cell Dev Biol. 2021;9:715211
158. Hui X, Li H, Zhou Z, Lam KS, Xiao Y, Wu D. et al. Adipocyte fatty acid-binding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH2-terminal kinases and activator protein-1. J Biol Chem. 2010;285:10273-80
159. Harjes U, Bridges E, McIntyre A, Fielding BA, Harris AL. Fatty acid-binding protein 4, a point of convergence for angiogenic and metabolic signaling pathways in endothelial cells. J Biol Chem. 2014;289:23168-76
160. Huang Y, Yong P, Dickey D, Vora SM, Wu H, Bernlohr DA. Inflammasome Activation and Pyroptosis via a Lipid-regulated SIRT1-p53-ASC Axis in Macrophages From Male Mice and Humans. Endocrinology. 2022 163
161. Anthonymuthu TS, Kenny EM, Bayır H. Therapies targeting lipid peroxidation in traumatic brain injury. Brain Res. 2016;1640:57-76
162. Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317-31
163. Kang R, Zeng L, Zhu S, Xie Y, Liu J, Wen Q. et al. Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis. Cell Host Microbe. 2018;24:97-108 e4
164. Gu X, Wang Y, He Y, Zhao B, Zhang Q, Li S. MiR-1656 targets GPX4 to trigger pyroptosis in broilers kidney tissues by activating NLRP3 inflammasome under Se deficiency. J Nutr Biochem. 2022;105:109001
165. Fan R, Sui J, Dong X, Jing B, Gao Z. Wedelolactone alleviates acute pancreatitis and associated lung injury via GPX4 mediated suppression of pyroptosis and ferroptosis. Free Radic Biol Med. 2021;173:29-40
166. Chen R, Zhu S, Zeng L, Wang Q, Sheng Y, Zhou B. et al. AGER-Mediated Lipid Peroxidation Drives Caspase-11 Inflammasome Activation in Sepsis. Front Immunol. 2019;10:1904
167. Kadamur G, Ross EM. Mammalian phospholipase C. Annu Rev Physiol. 2013;75:127-54
168. Liu H, Tang D, Zhou X, Yang X, Chen AF. PhospholipaseCγ1/calcium-dependent membranous localization of Gsdmd-N drives endothelial pyroptosis, contributing to lipopolysaccharide-induced fatal outcome. Am J Physiol Heart Circ Physiol. 2020;319:H1482-h95
169. Li J, Wang X, Mei KC, Chang CH, Jiang J, Liu X. et al. Lateral size of graphene oxide determines differential cellular uptake and cell death pathways in Kupffer cells, LSECs, and hepatocytes. Nano Today. 2021 37
170. Moon JS, Nakahira K, Chung KP, DeNicola GM, Koo MJ, Pabón MA. et al. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat Med. 2016;22:1002-12
171. Zeng C, Duan F, Hu J, Luo B, Huang B, Lou X. et al. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. 2020;34:101523
172. Hsu CG, Chávez CL, Zhang C, Sowden M, Yan C, Berk BC. The lipid peroxidation product 4-hydroxynonenal inhibits NLRP3 inflammasome activation and macrophage pyroptosis. Cell Death and Differentiation. 2022;29:1790-803
173. Pizzuto M, Pelegrin P, Ruysschaert J-M. Lipid-protein interactions regulating the canonical and the non-canonical NLRP3 inflammasome. Prog Lipid Res. 2022;87:101182
Author contact
![]() Corresponding author: Prof. Li Qin, E-mail: lqinedu.cn or Prof. Ai-Guo Dai, E-mail: daiaiguocom, School of Pharmacy, Hunan University of Chinese Medicine, 300 Xueshi Road, Hanpu Science and Education District, Changsha 410208, Hunan, China.
Corresponding author: Prof. Li Qin, E-mail: lqinedu.cn or Prof. Ai-Guo Dai, E-mail: daiaiguocom, School of Pharmacy, Hunan University of Chinese Medicine, 300 Xueshi Road, Hanpu Science and Education District, Changsha 410208, Hunan, China.

 Global reach, higher impact
Global reach, higher impact