ISSN: 1449-2288
Int J Biol Sci 2023; 19(10):2957-2973. doi:10.7150/ijbs.84592 This issue Cite
Research Paper
Ribonuclease 1 Enhances Antitumor Immunity against Breast Cancer by Boosting T cell Activation
1. Department of Molecular and Cellular Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
2. Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
3. Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
4. UTHealth Graduate School of Biomedical Sciences, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
5. Graduate Institute of Biomedical Sciences, Institute of Biochemistry and Molecular Biology, Research Center for Cancer Biology, Cancer Biology and Precision Therapeutics Center, and Center for Molecular Medicine, China Medical University, Taichung 406, Taiwan.
6. Department of Biotechnology, Asia University, Taichung, 413, Taiwan.
*These authors contributed equally to this work
Abstract
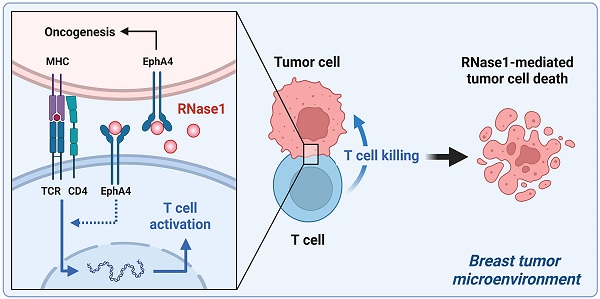
The secretory enzyme human ribonuclease 1 (RNase1) is involved in innate immunity and anti-inflammation, achieving host defense and anti-cancer effects; however, whether RNase1 contributes to adaptive immune response in the tumor microenvironment (TME) remains unclear. Here, we established a syngeneic immunocompetent mouse model in breast cancer and demonstrated that ectopic RNase1 expression significantly inhibited tumor progression. Overall changes in immunological profiles in the mouse tumors were analyzed by mass cytometry and showed that the RNase1-expressing tumor cells significantly induced CD4+ Th1 and Th17 cells and natural killer cells and reduced granulocytic myeloid-derived suppressor cells, supporting that RNase1 favors an antitumor TME. Specifically, RNase1 increased expression of T cell activation marker CD69 in a CD4+ T cell subset. Notably, analysis of cancer-killing potential revealed that T cell-mediated antitumor immunity was enhanced by RNase1, which further collaborated with an EGFR-CD3 bispecific antibody to protect against breast cancer cells across molecular subtypes. Our results uncover a tumor-suppressive role of RNase1 through adaptive immune response in breast cancer in vivo and in vitro, providing a potential treatment strategy of combining RNase1 with cancer immunotherapies for immunocompetent patients.
Keywords: breast cancer, ribonuclease 1, tumor microenvironment, T cell activity, antitumor immunity, tumor-infiltrating immune cells

 Global reach, higher impact
Global reach, higher impact