10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(12):3762-3780. doi:10.7150/ijbs.85813 This issue Cite
Review
B7-H3 in Brain Malignancies: Immunology and Immunotherapy
1. Department of Neurosurgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China
2. Medical Research Center, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Received 2023-5-3; Accepted 2023-7-13; Published 2023-7-24
Abstract
The immune checkpoint B7-H3 (CD276), a member of the B7 family with immunoregulatory properties, has been identified recently as a novel target for immunotherapy for refractory blood cancers and solid malignant tumors. While research on B7-H3 in brain malignancies is limited, there is growing interest in exploring its therapeutic potential in this context. B7-H3 plays a crucial role in regulating the functions of immune cells, cancer-associated fibroblasts, and endothelial cells within the tumor microenvironment, contributing to the creation of a pro-tumorigenic milieu. This microenvironment promotes uncontrolled cancer cell proliferation, enhanced metabolism, increased cancer stemness, and resistance to standard treatments. Blocking B7-H3 and terminating its immunosuppressive function is expected to improve anti-tumor immune responses and, in turn, ameliorate the progression of tumors. Results from preclinical or observative studies and early-phase trials targeting B7-H3 have revealed promising anti-tumor efficacy and acceptable toxicity in glioblastoma (GBM), diffuse intrinsic pontine glioma (DIPG), medulloblastoma, neuroblastoma, craniopharyngioma, atypical teratoid/rhabdoid tumor, and brain metastases. Ongoing clinical trials are now investigating the use of CAR-T cell therapy and antibody-drug conjugate therapy, either alone or in combination with standard treatments or other therapeutic approaches, targeting B7-H3 in refractory or recurrent GBMs, DIPGs, neuroblastomas, medulloblastomas, ependymomas, and metastatic brain tumors. These trials hold promise for providing effective treatment options for these challenging intracranial malignancies in both adult and pediatric populations.
Keywords: B7-H3, brain tumor, tumor microenvironment, cancer immunotherapy, chimeric antigen receptor T cell therapy, antibody-drug conjugate therapy
Introduction
Immune checkpoints (ICs) are a group of antigens that deliver signals that either enhance or suppress immune responses to regulate the activation, direction, and magnitude of host immune responses. Well-known inhibitory ICs, such as programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated protein 4 (CTLA-4), can induce T cell exhaustion and death, contributing to the suppression of anti-tumor immunity and subsequent cancer progression, making them novel therapeutic targets for anti-tumor immunotherapy [1]. B7 family are a group of widely studied immunomodulatory ligands that activate or inhibit the function and proliferation of effector immune cells [2]. These molecules are usually exerting an immune-suppressing effect and are expressed on the surface of both immune cells and cancer cells. They contribute directly to tumor progression or indirectly by influencing the anti-tumor immune system. A total of 10 members of the B7 family have been identified, including B7-1 (CD80), B7-2 (CD86), B7-DC (CD273/PD-L2), B7-H1 (CD274 or PD-L1), B7-H2 (CD275), B7-H3 (CD276), B7-H4 (B7x/B7S1/VTCN1), B7-H5 (GI24/VISTA/PD-1H), B7-H6 (NCR3LG1), and B7-H7 (HHLA2) [2]. Since its discovery in 2001, B7-H3 has garnered significant attention [3]. However, its biological function in the immune system is complex and still being investigated. While some studies have reported its ability to enhance immune function, most studies have found that B7-H3 contributes to tumorigenesis, angiogenesis, invasion, and metastasis through complex mechanisms of action [4]. Blocking or silencing B7-H3 has been shown to eliminate tumors and prolong survival in hematological malignancies and some solid tumors [5].
Patients with brain metastasis, glioblastoma (GBM), or diffuse intrinsic pontine glioma (DIPG) has the most dismal prognosis. Brain metastases make up the majority of intracranial neoplasms and develop when cancer cells from other organs spread through the bloodstream and establish themselves in the brain. Among primary malignant brain tumors in adults, GBM is the most common [6], while DIPG, a high-grade glioma originating from the brainstem, accounts for 10% of all pediatric tumors [7]. Despite remarkable progress made in conventional treatments such as surgery, radiotherapy, and chemotherapy for these brain malignancies, prognosis remains poor compared with other cancers [8]. Although postoperative radiochemotherapy and adjuvant chemotherapy with temozolomide, tumor treating fields, and DCVax-L have shown significant survival benefits in GBMs, research on targeted immunotherapies has unfortunately yielded limited benefits on patient survival over the past two decades [9-11]. However, with the rapid advancement and valuable experience of research on B7-H3 immunology and successful application of immunotherapy targeting B7-H3 in blood cancers and certain advanced solid cancers such as gastrointestinal cancers, ovarian cancers, renal cancers, and liver cancers [12-18], studies investigating the potential of B7-H3 as a treatment for brain tumors, though limited, deserve greater attention and recognition.
A comprehensive and up-to-date review is needed to enhance our understanding on the immunology and immunotherapy of B7-H3 in brain malignancies. Thus, in this review, we made a detailed summary by comprehensively reviewing the latest research progress on the structure and biological functions of B7-H3, its interactions with the tumor microenvironment (TME) components, its pro-tumorigenesis roles and underlying mechanisms of action, current research on B7-H3-related immunology and immunotherapy in different brain tumor types, and promising ongoing clinical trials exploring the anti-tumor efficacy and safety of B7-H3 targeted immunotherapies for brain malignancies.
Structure and Ligands of B7-H3
Structure, expression, and existing forms of B7-H3
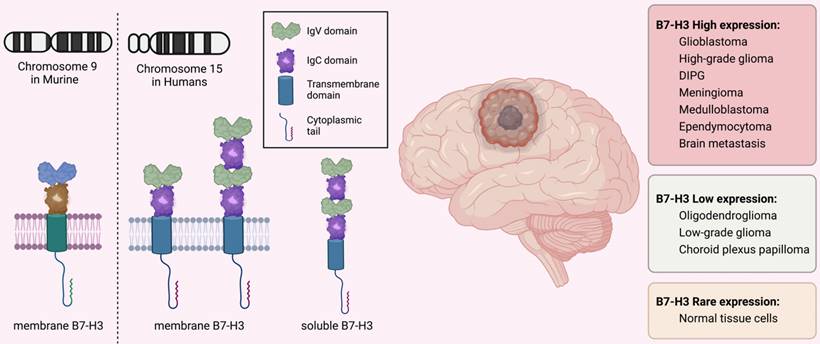
The immune checkpoint B7-H3 is a type I transmembrane glycoprotein consisting of 316 amino acids encoded by 12 exons located in chromosome 9 in murine while in 15q24.1 in humans [19]. It consists of a cytoplasmic tail of 45 amino acids, a single transmembrane segment, and extracellular immunoglobulin domains. In mice, the extracellular domain is made up of an N-terminal IgV and a C-terminal IgC domain, while in humans, there are two isoforms with differing pairs of Ig ectodomains: 2IgB7-H3 (B7-H3 VC) with 93% amino acid similarity to murine 2IgB7-H3 and 4IgB7-H3 (B7-H3 VCVC or B7-H3b), which contains two tandemly duplicated IgV-IgC domains [20-22]. The 4IgB7-H3 isoform is the predominant one in humans and is extensively expressed on the surface of cancerous cells and immune cells (Figure 1).
B7-H3 is expressed in various types of brain tumors including GBM [23, 24], high-grade gliomas [25-27], DIPG [28], pleomorphic xanthoastrocytoma [29], meningioma [30, 31], craniopharyngioma [32, 33], atypical teratoid/rhabdoid tumors (ATRT) [34], medulloblastoma [35, 36], and neuroblastoma [37]. However, the level of its expression varies among different brain tumor types. The expression of B7-H3 is moderate-to-high in GBM, high-grade glioma, DIPG, meningioma, medulloblastoma, and ependymocytoma, while is lower in choroid plexus papilloma, oligodendroglioma, and low-grade glioma [26-28, 35, 38, 39]. The expression of different B7-H3 isoforms varies at different disease stages in GBMs. Specifically, 4IgB7-H3 is detected only in newly-diagnosed GBM cells, while 2IgB7-H3 is predominantly detected in recurrent GBM cells. This provides clinical implications for targeting 4IgB7-H3 in newly-diagnosed GBMs while using 2IgB7-H3 to track recurrence and treatment responses [23]. B7-H3 mRNA is present in most normal cells, but its expression levels are typically low. This is in stark contrast to its high expression in cancer cells [40-42].
The crystal structure of B7-H3 has been reported and the FG loop of IgV domains is the structural basis for its function [41]. Aside from the most extensively studied type of B7-H3 found on the cell membrane (mB7-H3), a "soluble" form of B7-H3 (sB7-H3) has also been detected in plasma [43]. This form of B7-H3 is produced through an alternative splicing process from the fourth intron of the B7-H3 gene, which is mediated by matrix metalloproteinase, and is commonly released from monocytes, monocyte-derived dendritic cells (DCs), activated T lymphocytes, and mB7-H3-positive cancerous cells [43, 44]. Nevertheless, the existence of sB7-H3 remains uncertain due to the lack of evidence regarding its solubility and expression in exosomes [19, 45].
Structure and Expression of B7-H3 (CD276). B7-H3 is encoded by the exons located on chromosome 9 in mice and on chromosome 15 in humans. The molecule consists of a short cytoplasmic tail, a single transmembrane sequence, and extracellular immunoglobulin domains. In mice, the extracellular domain is composed of an N-terminal IgV and a C-terminal IgC, while in humans, the predominant isoform is 4IgB7-H3, which contains two duplicated IgV-IgC domains. B7-H3 expression varies among different brain tumors but is rarely expressed on normal cells. In addition to the membrane-bound form, the soluble form of B7-H3 has also been detected in human plasma.
Ligands of B7-H3
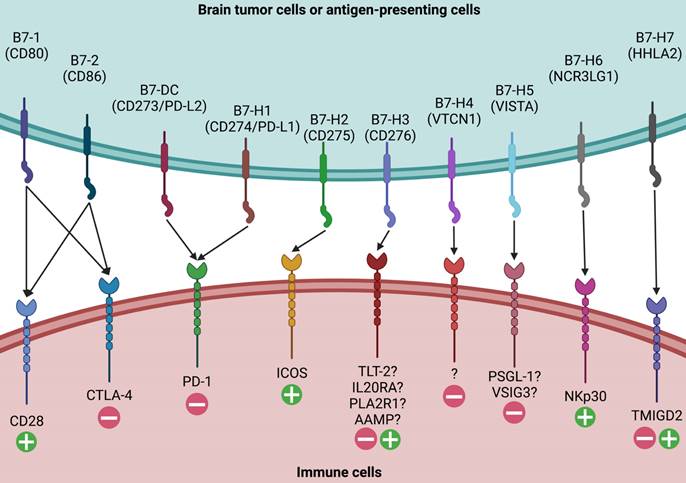
Given the complex and context-specific functions of B7-H3 as both a co-stimulatory and co-inhibitory molecule, it is crucial to identify its binding receptors and understand their interactions. Unlike its superfamily members such as B7-1, B7-2, PD-L1, PD-L2, and B7-H2, which have known ligands like CD28, CTLA-4, PD-1, and inducible T cell costimulatory, the ligands for B7-H3 have yet to be clearly identified (Figure 2).
Four candidates have been proposed as possible binding receptors for B7-H3: triggering receptor expressed on myeloid cells (TREM)-like transcript 2 (TLT-2), interleukin-20 receptor subunit α (IL20RA), phospholipase A2 receptor 1 (PLA2R1), and angio-associated migratory cell protein (AAMP) [46-49]. TLT-2, a single transmembrane protein, was the first identified B7-H3 ligand candidate expressed in macrophages, microglia, neutrophils, and T/B lymphocytes [46, 50, 51]. It contains a putative SH3 binding motif and has multifaceted functions in modulating both the innate and adaptive immune responses [52-54]. However, the binding of B7-H3 and TLT-2 has been disputed since subsequent studies have failed to provide solid evidence to support their interactions in both murine and human cells [55, 56]. IL20RA, a transmembrane glycoprotein with two tandem β-sandwich extracellular domains, has been recently reported as another putative binding target of B7-H3 using high-throughput interactome platforms [47, 48]. It is expressed in the skin, lung, and testis, with low expression levels in circulating immune cells [57, 58]. Studies have reported that the B7-H3-IL20RA binding may have indirect functions in modulating immune responses, potentially via stromal substances or cancer cells [57-59]. PLA2R1, a transmembrane protein with a short cytoplasmic tail, a transmembrane sequence, a tandem C-type lectin domain, a fibronectin type II domain, and a cysteine-rich terminal domain, is another high-affinity B7-H3 binding receptor [48]. However, the mechanism by which the B7-H3-PLA2R1 partner regulates immune responses remains to be elucidated. Recently, AAMP was discovered as another novel binding partner [49]. Researchers have demonstrated that AAMP expressed on glioma cells and immune cells is the binding receptor and has a synergistic effect with B7-H3. The immunosuppressive function of B7-H3 can be down-regulated by targeting AAMP.
Immunological and Non-immunological Functions of B7-H3
Functions through the innate and adaptive immunity
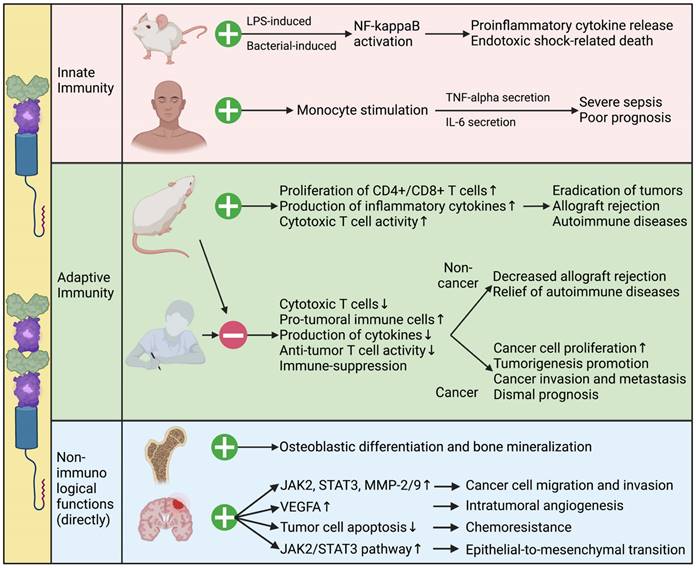
The functions of B7-H3 in modulating innate immunity are multifaceted and intricate. It has been found to enhance both lipopolysaccharide- and bacterial lipoprotein-induced NF-kappaB activation, and promote the release of pro-inflammatory cytokines in a toll-like receptor (TLR)-2- and TLR-4-dependent manner. However, it fails to stimulate inflammatory responses in murine macrophages and inhibits natural-killer (NK) cell activation [60]. In humans, high levels of sB7-H3 in the plasma are correlated with the levels of plasma TNF-alpha and IL-6, and predict poor outcomes in patients with severe sepsis [60].
In adaptive immunity, B7-H3 acts as both a co-stimulator and a co-inhibitor of T cell-mediated immune responses. Initial research has shown that B7-H3 has co-stimulatory effects on the immune system [3]. It increases the proliferation of both CD4+ and CD8+ T cells, stimulates the production of IFN-γ, and enhances cytotoxic T cell activity. Following studies have revealed that B7-H3 leads to the eradication of tumors and the reduction of metastasis through the rapid expansion of tumor-specific CD8+ cytotoxic T cells and NK cells, and increased production of IL-12 [61-63]. B7-H3 also mediates the development of acute and chronic allograft rejection by stimulating T-cell immune responses and promoting the production of cytokines and chemokines [64]. In addition, B7-H3 promotes collagen-induced arthritis and autoimmune encephalomyelitis in murine models by stimulating the activity of T helper type 1 (Th1)/Th17 cells and activating the production of IFN-γ and IL-17 [65]. However, these results have rarely been validated in humans. The lack of B7-H3 binding to TLT-2 and the expression of 4IgB7-H3 isoforms in humans may explain this difference between mice and men.
Contrary to its co-stimulatory immune functions, more recent studies suggest that B7-H3 inhibits T-cell activation and proliferation, thereby negatively regulating adaptive immune responses in both cancerous and non-cancerous conditions. Studies have demonstrated that B7-H3 inhibits cytokine production through signaling pathways involving NFTA, NF-kappaB, and AP-1 factors, which regulate gene transcription and immune cell proliferation [66-68]. In vivo studies have further validated the inhibitory function of B7-H3. B7-H3-knockout mice develop severe asthma with high concentrations of IL-5 and IL-13, eosinophil infiltrations in the lungs, and elevated anti-ovalbumin antibodies in the plasma, indicating the co-inhibitory function of B7-H3 on Th2-cell responses [65]. In allograft models, blocking B7-H3 using monoclonal antibodies results in enhanced IFN-γ production and accelerated rejection, further suggesting the co-inhibitory role of B7-H3 [69]. In cancerous models, B7-H3 has been reported to promote tumorigenesis by inhibiting T-cell mediated anti-tumor immune functions. The expression level of B7-H3 in tumors correlates with a low concentration of infiltrating T lymphocytes, elevated regulatory T (Treg) cell infiltration, and suppression of NK-cell-mediated tumor cell lysis process, leading to accelerated cancer cell proliferation and mobility [70-72].
Ligands for B7 Family Members. Ten members of the B7 family have been identified, including B7-1, B7-2, B7-DC, B7-H1, B7-H2, B7-H3, B7-H4, B7-H5, B7-H6, and B7-H7. While major receptors for the majority of B7 family molecules have been identified, the putative ligands for B7-H3 have not been clearly elucidated. Four candidates, including TLT-2, IL20RA, PLA2R1, and AAMP, have been proposed as the possible binding receptors for B7-H3 and need further validation.
Although numerous investigations have provided evidence for the multifaceted biological functions of B7-H3 in both innate and adaptive immunity in cancerous and non-cancerous settings as discussed above, discrepancies and controversies still exist [2, 73, 74]. One point is that B7-H3 interact with both stimulatory and inhibitory ligands with varied binding affinity, and its final effects are determined by the comprehensive summation of multiple downstream signaling pathways activated by different ligand-receptor partners. Another factor is that the expression level of B7-H3 varies substantially among different tissues and disease models, which may reshape the immune-modulatory functions of B7-H3 and result in tissue- and disease-specific outcomes. Intratumoral heterogeneity, complex tumor microenvironmental context composites, and imbalanced signaling intensity are also potential factors responsible for the dual functions of B7-H3 [4, 19, 73].
Functions through non-immunological mechanisms
Apart from its immunological functions, B7-H3 also plays important non-immunological roles in both normal and cancerous tissues (Figure 3). During embryogenesis, B7-H3 is highly expressed on developing bones and plays a critical role in osteoblastic differentiation and bone mineralization. Inhibition of B7-H3 using B7-H3-Fc suppresses this process [75]. In cancer, B7-H3 has been implicated in metastasis, angiogenesis, and tumorigenesis through various non-immunological mechanisms [12, 76, 77]. Studies have shown that B7-H3 promotes cancer cell migration and invasion by regulating metastasis-associated proteins such as Janus kinase 2 (JAK2), signal transducer and activator of transcription 3 (STAT3), matrix metalloproteinase (MMP)-2, and MMP-9. It also promotes intratumoral angiogenesis by upregulating the expression of vascular endothelial growth factor A (VEGFA) [12, 78, 79]. Moreover, B7-H3 down-regulates tumor cell apoptosis, reduces G2/M-phase arrest, and thus decreases the sensitivity to anti-tumor drugs, leading to increased chemoresistance [80-83]. Recent studies have also demonstrated that B7-H3 promotes epithelial-to-mesenchymal transition (EMT) via the JAK2/STAT3 pathway and stimulates cancer cell glycolytic metabolism [17, 84, 85].
Immunological and Non-immunological Functions of B7-H3. Functions of B7-H3 in modulating tumorigenesis, sepsis, allograft rejection, autoimmune diseases, and osteogenesis via immunological and non-immunological mechanisms are complex and multifaceted, as investigated in murine models and patients. Further exploration is under way.
Interactions of B7-H3 with the Tumor Microenvironment (TME) and Its Effects on Tumor Cells. Although the functions of B7-H3 in regulating tumor behaviors are complex, the majority of studies suggest that it plays pro-tumorigenic roles in assisting the development and progression of cancers. B7-H3 exerts its effects mainly in two ways: 1) indirectly, via communications with components of the TME, including infiltrated immune cells, aberrant vascular networks, and cancer-associated fibroblasts, and 2) directly, via multiple signaling pathways that regulate cancer cell proliferation, apoptosis, stemness, invasion, migration, metabolism, and treatment responses.
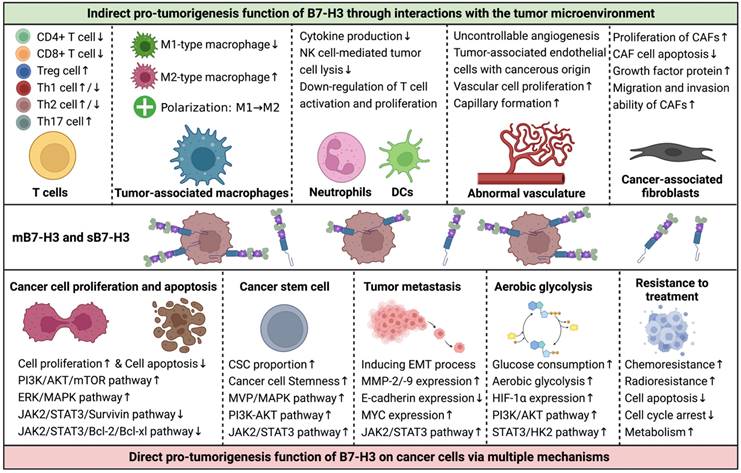
Interactions of B7-H3 with the TME components
The TME is a complex and dynamic structure that includes infiltrating immune cells, stromal components, vascular networks, extracellular matrix, exosomes, cytokines, and chemokines, in addition to malignant cells [86]. The interactions between tumor cells and the TME play a critical role in tumor initiation, progression, metastasis, and therapeutic responses. B7-H3 plays a significant role in tumor biology by modulating the functions of infiltrating immune cells, angiogenesis, and cancer-associated fibroblasts (CAFs) in the TME (Figure 4) [17, 70, 87-93].
B7-H3 and infiltrating immune cells
The major infiltrating immune cells include T cells, TAMs, DCs, NK cells, and neutrophils, which exert both pro- and anti-tumor functions. For instance, CD4+ Th cells, CD8+ cytotoxic T cells, and M1-type TAMs promote cancer-killing functions, while Treg cells and M2-type TAMs suppress anti-tumor immunity and enhance tumorigenesis [86, 89, 94-96].
T lymphocytes are a significant group of adaptive anti-tumor immune cells that infiltrate the TME. Different T cell types perform specific immune functions. Studies have shown that B7-H3 inhibits the proliferation and activity of CD4+ and CD8+ effector T cells, leading to immunity inhibition and tumor progression. Blocking B7-H3 reverses the state of immunosuppression, restores anti-tumor immunity, and creates an anti-tumorigenesis TME [2, 19, 20, 41, 55, 66, 70, 72, 73]. Treg cells represent approximately 7% of CD4+ T lymphocytes and express CD25 and forkhead box P3 (FOXP3), which promotes immune tolerance and helps in cancer immune evasion [94]. CD4+CD25+ Treg cells up-regulate B7-H3 expression and down-regulate MHC-peptide complexes on antigen-presenting DCs, leading to suppressive function in stimulating T-cell-mediated immune responses [95]. Another study indicated a positive correlation between FOXP3+ Treg cells and B7-H3 expression on tumor cells, suggesting a possible cooperative relationship between B7-H3 and Treg cells in cancer immune evasion [71]. Th cells, which differentiate from naïve CD4+ T cells, produce lineage-specific cytokines and play crucial roles in coordinating immune responses to infections and autoimmune diseases [96]. Th cell differentiation is determined by T-cell receptor signaling and other triggering signals, such as those from B7-H3. In a murine model of autoimmune encephalomyelitis, B7-H3-deficient mice experienced more severe inflammation, and Th cells differentiated preferentially toward Th1 rather than Th2 cells, indicating the stimulatory function of B7-H3 for Th2 cells [67]. However, in another model of allergic conjunctivitis, B7-H3 negatively regulated both Th2 and Th1 immune responses [97]. Further conflicting results have shown that B7-H3 in murine models with autoimmune diseases has co-stimulatory functions for Th1 and Th17 cells but co-inhibitory functions for Th2 cells [65]. The contradictory immune effects of B7-H3 on Th cells may arise from the analysis of varied disease settings, highlighting the need for further investigation and validation.
TAMs are crucial immune cells infiltrating the TME and exist in two polarization states: M1-type macrophages that enhance inflammation and promote anti-tumor immune functions, and M2-type macrophages that play a critical role in immune down-regulation and tumor evasion [89]. The polarization of TAMs is constantly in flux and determined by the prevailing signals in the TME. TAMs express high levels of B7-H3, which exhibits pro-metastatic and immunosuppressive functions by promoting tumor angiogenesis and extracellular matrix reconstruction, dampening T-cell infiltration, and suppressing immune responses [92]. Additionally, B7-H3 on cancerous cells and antigen-presenting cells promotes the polarization of TAMs from M1- to M2-macrophages, impairing anti-tumor immune functions and mediating tumor progression [98-101].
Neutrophils and DCs are also prominent components of the TME that express high levels of B7-H3. B7-H3 on DCs and neutrophils correlates with reduced IL-12 production and down-regulated T cell activation and proliferation, resulting in an immune-inhibitory effect and promoting a pro-tumorigenesis TME [74, 102]. Both the cell-binding m4IgB7H3 and the supernatant s4IgB7H3 of glioma samples are functional and significantly suppress NK cell-mediated tumor cell lysis; Silencing B7-H3 in cells makes them more susceptible to NK cell-induced cytotoxicity than unsilenced cells [72].
B7-H3 plays a pivotal role in communicating with multiple immune infiltrates, regulating the activation of immune cells and production of cytokines and chemokines, dictating the features of the TME, and influencing the biology of cancers. In addition, B7-H3 affects immune cells differently and has varied influences on the same immune cells in different cancers, contributing to the complex relationships between B7-H3 and infiltrating immune cells in the TME.
B7-H3 with tumor vasculature and CAFs
Vascular networks in tumors are abnormal and uncontrollable, and angiogenesis is a hallmark of cancer that facilitates improved delivery of oxygen and nutrients, as well as providing a path for tumor metastasis [103]. Research has shown that over half of the endothelial cells in GBMs carry the same genomic alterations as GBM cells, indicating that a large portion of vascular networks in the TME have a cancerous origin [93]. Moreover, CD133+ stem-like cells can differentiate into endothelial progenitor cells. Inhibition of γ-secretase or silencing NOTCH1 blocks the transition of CD133+ cells into endothelial progenitor cells, while blocking VEGF inhibits the subsequent maturation of endothelial progenitors into tumorous endothelium [104]. B7-H3 is overexpressed in tumor-associated endothelial cells and is related to cancer angiogenesis, whereas it is not expressed in normal angiogenic tissues [105]. High-grade gliomas are characterized by more aggressive biological behaviors and have higher expression levels of B7-H3 on both the tumor cells and endothelial cells than low-grade gliomas [27, 72, 84]. Through the NF-kappaB signaling pathway, B7-H3 accelerates vascular cell proliferation and capillary formation by enhancing the production of VEGF [12]. Furthermore, B7-H3 can promote cancer progression and metastasis by accelerating the formation of tumor-associated vessels mediated by CD14+ monocytes [18]. MMP-9 and MMP-2 are also involved in B7-H3-mediated cancerous angiogenesis through the PI3K/AKT/MMP pathway [14, 106, 107].
CAFs that comprise a predominant part of the TME also prompt tumor progression and immune escape, in addition to infiltrating immune cells and aberrant vascular networks [91]. B7-H3 has a co-stimulatory effect on CAFs, and knockdown of B7-H3 inhibits the proliferation of CAFs, increases CAF cell apoptosis, decreases the expression of stromal cell-derived factor-1 and hepatocyte growth factor protein, and thus promotes tumor progression [90]. Moreover, a study has found that B7-H3 is expressed on both the tumor cells and CAFs, and this expression correlates with the migration and invasion ability of CAFs, the secretion of IL-6 and VEGF, and a poor overall prognosis [108].
Mechanisms of B7-H3 in Promoting Tumorigenesis
Cancer cell proliferation and apoptosis
B7-H3 is known to promote cancer cell proliferation and inhibit cancer cell apoptosis, leading to sustained tumor growth and progression [70, 81, 83, 84, 107]. In addition to impairing anti-tumor immune functions, B7-H3 promotes cancer cell proliferation through the activation of several key signaling pathways via the PI3K or MVP/MEK axes [109, 110]. B7-H3 also exerts its anti-apoptotic effects through the JAK2/STAT3 and CDC25A signaling pathways [16, 81, 82, 84, 107]. Confirmatory studies have shown that silencing B7-H3 reduces cancer cell proliferation and enhances cancer cell apoptosis by decreasing the expression of several anti-apoptotic and cell cycle-related proteins [16, 80, 111, 112].
Cancer stem cells
Cancer stem cells (CSCs) or tumor-initiating cells with pluripotent and self-renewing properties are considered the key drivers of tumorigenesis and major sources of treatment resistance and tumor relapse [87]. The regulation of CSCs in gliomas is influenced by genetic alterations, epigenetic factors, TME interactions, niche factors, metabolism, and the immune system [87]. B7-H3 is significantly overexpressed in CSCs compared to non-stem cancer cells [87, 109, 110], suggesting that targeting B7-H3 may be a promising therapeutic strategy for cancers enriched with B7-H3+ CSCs. Studies have revealed that B7-H3 overexpression increases the proportion of CSC populations through MVP/MAPK, PI3K-AKT, and JAK2/STAT3 signaling pathways and also enhances cancer cell stemness, whereas down-regulation of B7-H3 inhibits CSC functions and stemness [84, 110, 113].
Tumor metastasis
Metastasis is another essential hallmark of cancer. B7-H3 has been shown to promote glioma cell invasion and induce the EMT process by upregulating MMP-2/-9 expression and downregulating E-cadherin expression [84]. In glioma cell and cancer stem cell models, both membrane-bound and soluble forms of B7-H3 promote tumor invasiveness into surrounding brain tissues [72]. The JAK2/STAT3/Slug signaling pathway is involved in the EMT process and subsequent tumor invasion and metastasis in gliomas and liver cancers [17, 84], while the PI3K/AKT and MAPK signaling pathways are responsible for EMT in lung cancer and renal cell carcinoma [114, 115]. B7-H3 also upregulates MYC expression, leading to GBM cell differentiation and induction of tumor migration and invasion [116].
Tumor metabolism
Abnormal cancer metabolism, characterized by a shift toward aerobic glycolysis instead of oxidative phosphorylation, fuels tumor evasion, metastasis, and chemotherapy resistance [88]. Studies have revealed that B7-H3 promotes glucose consumption, accelerates aerobic glycolysis, and increases lactate production via stabilizing and upregulating hypoxia inducible factor-1 alpha (HIF-1α) expression and its downstream targets that are mediators of the glycolytic pathway through the PI3K/AKT/mTOR pathway and via activating STAT3 and the subsequent HK2, providing new perspectives of immunomodulation function of B7-H3 on tumor progression [117-119]. In GBMs, expression of B7-H3 is upregulated in areas with hypoxia [120].
Immunotherapeutic Strategies Targeting B7-H3
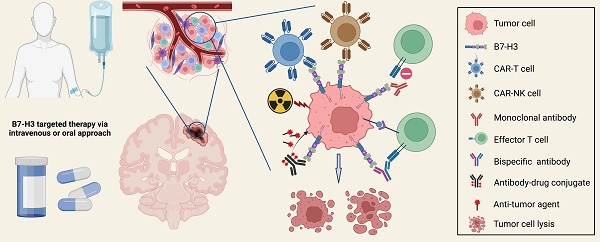
B7-H3 targeted therapies have been increasingly conducted for malignant brain tumors during the past decade. Recent advances in molecular biology and engineering have paved the way for various therapeutic strategies targeting B7-H3, including chimeric antigen receptor T cell (CAR-T) therapy, CAR-NK cell therapy, antibodies blocking, and antibody-drug conjugates therapy (Figure 5). Combining anti-B7-H3 therapies with other treatments is also a promising option for refractory cancers. Most of the aforementioned strategies have undergone verification in preclinical in vitro and in vivo studies, generating promising safety and anti-tumor efficacy data, and anti-B7-H3 clinical trials are now in progress.
CAR-based therapy
A CAR is a protein consisting of an extracellular antigen-binding domain, a transmembrane domain that anchors the CAR structure to the effector cell membrane, and an intracellular signaling-activation domain. CAR-T therapy employs autologous or allogeneic T lymphocytes that have been genetically modified with CARs targeting a specific tumor antigen that plays a crucial role in tumor progression, enabling them to effectively recognize and eliminate targeted cancer cells independently of the major histocompatibility complex (MHC) antigen [121].
CAR-T therapy has shown remarkable success in the treatment of hematological malignancies such as refractory acute lymphoblastic leukemia. B7-H3 targeted CAR-T therapy has demonstrated acceptable tolerance and promising effectiveness in GBMs and several other brain cancers [24, 25, 34, 42, 122-124], with many phase-I/II trials still underway. Since B7-H3 is highly expressed on tumor cells, aberrant vessels, and CAFs while rarely on normal cells, B7-H3-CAR-T therapy can directly target cancer cells and disrupt stroma cells and angiogenesis simultaneously without damaging surrounding normal brain tissues.
Studies have shown that B7-H3-CAR-T therapy exerts potent anti-tumor efficacy in pediatric medulloblastoma [122], pediatric glioma [25], cerebral ATRTs [34], DIPG [124], non-small cell lung cancer (NSCLC) brain metastases [125], craniopharyngioma [32], and GBMs [24, 126, 127]. In a medulloblastoma xenograft model, B7-H3-CAR-T cells effectively crossed the blood-brain barrier (BBB), mediated anti-tumor immune effects, and induced tumor regression [122]. In a study using orthotopic xenografts of pediatric glioma of different grades, both local and systemic B7-H3-CAR-T therapy induced tumor regression and a significant survival advantage [25]. Incurable pediatric ATRTs were treated with intracerebroventricular or intratumoral B7-H3-CAR-T cell administration in mouse models, which exhibited potent anti-tumor effects and reduced systemic inflammatory cytokine production [34]. The first-in-human phase I trial administering locoregional B7-H3-CAR-T cells to pediatric recurrent/refractory CNS tumors and DIPGs was published in 2023. Three patients with DIPGs were evaluated and preliminary tolerability was witnessed. Intracranial immune activation was correlated with the persistent existence of B7-H3-CAR-T cells in the cerebrospinal fluid [124]. Metastatic NSCLC to the brain has an extremely poor prognosis. B7-H3-CAR-T therapy exhibits anti-tumor activity in vitro against NSCLC cells and organoids, and in xenotransplant models against orthotopic and metastatic NSCLC [125]. Researchers revealed that co-expression of CCR2b in B7-H3-CAR-T cells significantly improved the capability of passing the BBB and enhanced anti-tumor immune effects against brain metastases. In craniopharyngioma with high expression of B7-H3, studies have shown that B7-H3-CAR-T cells exerts tumor-killing effects in the 2D model [32]. In GBMs, B7-H3-CAR-T therapy enhances anti-tumor activities, releases effector cytokines, and effectively controls the growth of tumor cell lines and neurospheres [24, 126]. Although CD28-co-stimulated B7-H3-CAR-T cells released more inflammatory cytokines, no additional anti-tumor efficacy was observed. Although B7-H3-CAR-T therapy effectively attenuates tumor growth in various xenografts cancer models, it is not able to completely eradicate tumors [5]. Spatio-temporal tumor heterogeneity, limited transmission through the BBB, and the immunosuppressive TME are challenges that must be overcome to make B7-H3-CAR-T therapy effective in treating brain tumors. One potential solution is the development of next-generation CARs and combination treatments with chemoradiation and biological agents [128].
Therapeutic Strategies Targeting B7-H3. Multiple preclinical studies and early-phase clinical trials have been investigating novel strategies targeting B7-H3, including CAR-T therapy, CAR-NK therapy, monoclonal antibody blocking, bispecific antibody therapy, antibody-drug conjugate (ADC) therapy, and combination treatments with anti-B7-H3 therapies. These strategies have demonstrated remarkable anti-tumor efficacy, improved clinical outcomes, and acceptable drug-related toxicity.
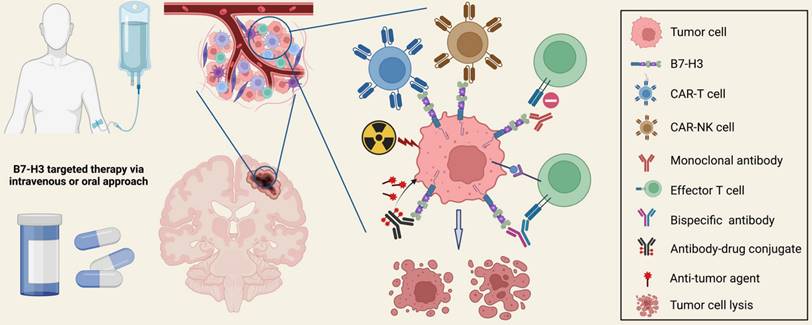
NK cells are innate lymphocytes that lack antigen-specific receptors on T cells while expressing substantial neural cell adhesion molecule CD56, identifying and killing tumor cells, infected cells, and other abnormal cells. Studies have shown that B7-H3 has immunosuppressive effects on NK cells within the TME and in turn de-regulates NK cell-mediated anti-tumor effects [72]. Recently, CAR-NK cells have been generated using the NK-92 cell line, a human non-Hodgkin's lymphoma NK cell line, for cancer immunotherapy to reverse the immunosuppressive TME and enhance the anti-tumor functions of NK cells. In GBMs and brain metastases, CAR-NK therapy targeting EGFRvIII or HER-2 has been explored in preclinical trials showing that CAR-NK therapy enhances activation of NK cells in the TME, induces anti-tumor effects by killing cancer cells and CSCs, and improves survival in rodent brain tumor models [129-131].
Monoclonal and bispecific antibody
Monoclonal antibodies (mAbs) are an important component of anti-cancer therapy and are developed against tumor-expressed antigens. They function through different mechanisms such as direct binding to antigens, blocking antigen-antibody interactions, or mediating antibody-dependent cellular cytotoxicity (ADCC) [132]. The successful application of mAb therapy targeting PD-1, PD-L1, and CTLA-4 for refractory cancers has encouraged the introduction of B7-H3 mAb therapy for tumors that express high levels of B7-H3.
Targeting B7-H3 therapy using mAbs has been shown to activate cytotoxic infiltrating immune cells, reorganize the intratumoral vascular network, enhance the sensitivity and clinical efficacy of chemotherapy, and inhibit tumor progression [85, 92, 133-135]. Targeting B7-H3 with an mAb has demonstrated both safety and efficacy in the stage IV neuroblastoma [28]. The murine IgG1 mAb, 8H9, which is specific for the principal form of B7-H3 (4IgB7-H3), shows promising effects for patients with B7-H3+ tumors and metastases to the central nervous system [136]. In addition to the above-mentioned mouse mAb, a humanized mAb 8H9 targeting the FG loop of B7-H3 has been also designed and exhibits ADCC activity when co-cultured with peripheral blood mononuclear cells [137]. MGA271, a fully humanized IgG1 B7-H3-targeting Fc-engineered mAb with increased activation affinity, enhanced anti-tumor function, and acceptable tolerance, and MJ18, an anti-mouse B7-H3-blocking mAb, have been reported to activate intratumoral anti-cancer immune function and inhibit tumor growth [138, 139].
In contrast to mAbs with only one binding domain, bispecific antibodies (bsAbs) are dual-affinity re-targeting proteins that are artificially generated with two binding specificities [140]. BsAb thus recruits and induces cytotoxic immune cells to approach closely and kill tumor cells, regulate complementary anti-tumor signaling pathways, and mediates cancer cell immune escape. A recent study has shown that bispecific chemically self-assembling nanorings that target CD3 and B7-H3 increase T cell infiltration and facilitate selective cytotoxicity of B7-H3+ medulloblastoma spheroids independent of target MHC class I antigen [141]. Furthermore, B7-H3/CD16 bispecific antibody (BsAb) has been shown to selectively activate NK cell activity, leading to enhanced antitumor efficacy mediated by NK cells [142]. Additionally, B7-H3/4-1BB BsAb has been reported to boost the function of tumor-infiltrating differentiated CD8+ lymphocytes and enhance antitumor immune responses [143].
Antibody-drug conjugates therapy
Antibody-drug conjugates (ADCs) are a novel therapeutic approach for refractory cancers that utilize a humanized mAb and cytotoxic drugs or radionucleotides. Preclinically developed ADC, MGC018, with a duocarmycin payload targeting B7-H3 has shown promising anti-tumor activity in preclinical models of various cancer types and has an acceptable safety profile in cynomolgus monkeys [38, 144]. Another targeting ADC, DS-7300a, containing a B7-H3-targeting mAb and a DNA topoisomerase I inhibitor, exerts potent anti-tumor activities against B7-H3+ tumors and maintained acceptable pharmacokinetic and safety profiles in in vitro and in vivo models [145]. Monomethyl auristatin E- and pyrrolobenzodiazepine-conjugated B7-H3-ADCs have been reported to impair tumor growth, induce tumor regression, and improve survival [40]. In craniopharyngiomas, the B7-H3-targeted DM1-conjugated ADC exhibits tumor-suppression functions in both the traditional 2D cell culture model and the 3D organoid model [32]. Therapy using B7-H3-mAb 8H9 labeled with 131I has shown efficacy and well tolerance in the treatment of recurrent metastatic neuroblastoma [146]. Safety and anti-tumor effects of another ADC 124I-B7-H3 has been verified in a phase I trial for DIPGs [147]. Three patients with embryonal tumor with multilayered rosettes have been treated with 131I-Omburtamab, which has been well-tolerated and shows potential therapeutic benefits following surgery and chemoradiation therapy [148].
Current Understanding of B7-H3-related immunology and immunotherapy in Major Brain Tumor Types
B7-H3 in gliomas
Gliomas are a heterogeneous group of tumors that arise from glia cells or neural stem cells, and are the most common and devastating primary malignant brain tumors in adults, accounting for around 50% of cases. A recent RNA-seq analysis of the TCGA and CGGA databases has revealed that B7-H3 has a higher mRNA level than any other B7 family members in gliomas [149]. Immunostaining of B7-H3 showed that 86% of gliomas were B7-H3-positive, with the majority having moderate to high intensity [39]. Results from a quantitative sandwich ELISA have revealed that sB7-H3 is present in the cerebrospinal fluid and blood serum of patients with gliomas [26]. Clinically, the expression level of B7-H3 is correlated with the malignancy grade, molecular subtype, IDH mutation status, and patient prognosis [26, 27, 84, 126, 149]. B7-H3 is selectively distributed in IDH-wildtype gliomas, mesenchymal gliomas, and high-grade gliomas, making it a potential immunotherapy-targeted biomarker in these refractory subtypes. Mechanistically, B7-H3 enhances proliferation and promotes invasion of glioma cells via activation of the JAK2/STAT3/Slug pathway, and induces EMT processes by downregulating E-cadherin and upregulating MMP-2/-9 protein [84].
Regarding newly-diagnosed GBMs, the most malignant and aggressive form of glioma, despite combined therapy including surgery, radiotherapy, and chemotherapy, the overall survival is only 14.6-16.0 months (20.9 months if with adjuvant tumor treating fields therapy), leading to one of the lowest 5-year survival of 6.8% among 33 human cancers [8]. Almost all GBMs experience recurrence, and there is no established standard of care treatment for these relapsed lesions. Attention has increasingly shifted toward immunotherapeutic treatments targeting novel biomarkers. Among different glioma subtypes, the mRNA expression level of B7-H3 is the highest in GBMs, and the B7-H3 gene promoter is significantly hypomethylated as confirmed in the CGGA and TCGA datasets [149]. B7-H3 is expressed both in the cytoplasm and at the cell membrane and is specifically upregulated in areas with hypoxia [23, 120]. Specifically, 4IgB7-H3 is mainly detected in newly-diagnosed GBMs, while 2IgB7-H3 is specifically expressed in non-cancerous brain tissue and highly expressed in recurrent GBMs [23]. Moreover, B7-H3 and the gene signature including GATA3 and immunosuppressive galactose-specific lectin 3 can help predict the prognosis of GBMs [150].
Several studies have attempted to apply CAR-T cell therapy targeting B7-H3 in GBMs in vitro and in vivo [24, 126, 127]. The anti-tumor functions of B7-H3-CAR-T cells were confirmed in patient-derived GBM cells and GBM cell lines, and the survival rate of B7-H3-CAR-T-cell-treated GBM xenograft murine models and a clinical case was significantly improved [24, 25, 126].
B7-H3 in meningiomas
Meningioma is the most common type of primary brain tumor in adults, with about 80% being benign and treated mainly through gross total surgical resection. However, a small percentage of meningiomas are classified as WHO grade 2 or 3 that are more likely to recur and require additional treatment such as adjuvant radiotherapy. No chemotherapy, targeted therapy, or immunotherapy has been approved for these types of malignancies. Recent studies have identified B7-H3 as the most highly expressed immune checkpoint in meningioma, with expression detected in 75%-100% of samples from both adults and children [30, 39]. In a cohort of 234 B7-H3-positive meningiomas, high B7-H3 expression was associated with male sex, high-grade subtypes, and lack of adjuvant radiotherapy [30]. B7-H3 was found to be expressed in almost all tumor cells in the majority of meningiomas, with elevated expression observed in tumors with gene mutations affecting the PI3K/AKT/mTOR pathway [31].
The protein phosphatase 2A (PP2A) inhibitor LB-100 has been shown to increase the expression of B7-H3 in malignant meningioma cells, making them more sensitive to B7-H3-targeted immunotherapy [151]. Inhibition of nicotinamide phosphoribosyltransferase, which highly expresses in anaplastic meningiomas, suppressed tumor growth and inhibited B7-H3 expression by regulating STAT1, indicating its potential use as a combined treatment for B7-H3-targeted immunotherapy [152]. The first-in-human study of B7-H3-targeted CAR-T cell therapy for treating a recurrent anaplastic meningioma showed a favorable safety profile, but revealed that B7-H3 expression decreased in the tumor part near the region of CAR-T-cell infiltration, requiring further investigation to address antigen loss and enhance CAR-T-cell trafficking [153].
B7-H3 in DIPGs
DIPG is a type of malignant brainstem tumor that affects approximately 300 children in the United States every year. Unfortunately, due to its invasive nature and location, surgical removal is mostly not possible, and radiation therapy is the current standard of care. The median survival for DIPG is less than 12 months, making it a very deadly disease that requires innovative treatment approaches. Recent studies have found that B7-H3 immunoreactivity is present in 100% of DIPG specimens, and its mRNA expression level is significantly higher in DIPGs than in other non-diffuse brainstem gliomas [28].
The open-label, ongoing, first-in-human, BrainChild-03 phase I clinical trial is currently investigating the delivery of locoregional B7-H3-CAR-T cells to children and young adults with DIPGs (NCT04185038), and preliminary data from three DIPG patients revealed that repeated intracranial B7-H3 CAR-T-cell therapy induced local immune activation and persistent evidence of B7-H3-CAR-T cells in the cerebrospinal fluid [124].
B7-H3 in ATRTs
ATRT is a rare but aggressive form of cancer that occurs in children under the age of three and originates in the brain or spinal cord due to genetic mutations in SMARCB1 or SMARCA4. In the United States, approximately 60 children are diagnosed each year. Although multimodal therapies such as surgery, chemotherapy, and radiotherapy are available, the median survival time is only around 17 months. In a multicenter study, 100% of ATRT tumors and cell lines were found to express B7-H3, with 72% being strongly positive and 23% moderately positive [34]. Another study also revealed that all ATRT specimens had B7-H3 immunostaining, with 92.3% exhibiting high and 7.6% showing medium staining intensities [39].
In mice models, CAR-T cells targeting B7-H3 administered via intracerebroventricular or intratumoral approaches showed faster kinetics, greater efficacy, and reduced systemic inflammation compared to systemic delivery of CAR-T cells administered intravenously. Additionally, locoregional CAR-T cell therapy induced antigen-specific protection from tumor relapse in both the brain and periphery regions [34].
B7-H3 in medulloblastomas
Medulloblastoma is the most common malignant embryonal neuroepithelial tumor in children, accounting for 20% of pediatric brain tumors and 40% of posterior fossa tumors. It is classified into 4 molecular subgroups: WNT-activated, SHH-activated, Group-3, and Group-4, and clinical treatment typically involves neurological surgery, radiotherapy, and chemotherapy. The 5-year survival rate is 70%, affected by various factors such as tumor grade and type, patient age, overall health status, and treatment response. B7-H3 is expressed in almost all of pediatric medulloblastomas, and is particularly frequent in Group-4 tumors [35, 39, 154]. In another study, the mRNA level of B7-H3 was found to differ significantly across molecular subgroups, and was significantly higher in tumor tissues than in non-tumor tissues. A higher B7-H3 level was associated with worse survival of patients with pediatric medulloblastomas [36].
Currently, B7-H3-CAR-T cell therapy for medulloblastoma is under investigation, as it has shown significant anti-tumor efficacy in vitro and regression of established medulloblastoma xenografts, particularly in cases where there is a high surface density of B7-H3 protein on the tumor tissues [122]. Additionally, a bispecific chemically self-assembling nanoring that targets both CD3 and B7-H3 has been approved and shown to successfully cross the BBB and stimulate immune activation both intracranially and systemically in an orthotopic medulloblastoma model [141].
B7-H3 in neuroblastoma
Neuroblastoma is the most common extracranial solid tumor of childhood, typically found in the peripheral nervous system and rarely inside the brain. Most tumors in children under one year of age are localized (stages 1-2), while older children often develop highly disseminated tumors with metastases involving the bone marrow, brain, liver, and skin. About half of neuroblastomas are classified as high-risk, with a 5-year survival rate of less than 50%. Despite treatments such as induction chemotherapy, surgery, autologous stem cell rescue, radiation therapy, isotretinoin, and targeted therapy against GD2, nearly 50% of patients with high-risk neuroblastomas relapse. B7-H3, similar to GD2, is highly expressed in both primary and metastatic neuroblastomas, and the immunoreactivity pattern is located on the cytoplasmic membrane and areas of rosette formation or ganglion differentiation [154, 155]. High B7-H3 expression levels in neuroblastoma are associated with decreased survival rates, advanced tumor stages, and increased potential for metastasis [155-157].
In experiments, masking B7-H3 protein with the antibody (5B14) on isolated neuroblastoma cells enhanced NK cell-mediated lysis of tumor cells [37]. Additionally, a combined treatment using artemether and doxorubicin was reported to suppress B7-H3 expression in neuroblastomas at both mRNA and protein levels, with artemether potentially serving as a therapeutic option via regulating B7-H3 for neuroblastomas [156]. The B7-H3-targeting antibody-drug conjugate (ADC) m276-SL-PBD has shown promising results in neuroblastoma patients and cell line-derived mouse models, demonstrating an ultra-high treatment response rate and significant anti-tumor activity [158]. Furthermore, bicistronic-CAR-T cells effectively eliminated neuroblastoma cells expressing GPC2 or B7-H3 and showed resistance to immune exhaustion compared to conventional CAR-T cells targeting a single antigen, indicating that this method may overcome heterogenous expression of targeted antigens in neuroblastomas [159]. A recent phase I trial found that compartmental radioimmunotherapy with anti-B7-H3 monoclonal antibody administered intraventricularly had favorable dosimetry and significant survival benefits for neuroblastomas compared to historical data [160].
B7-H3 in craniopharyngiomas
Craniopharyngiomas are benign neoplasms that develop in the sellar/parasellar region. There are two subtypes: adamantinomatous and papillary craniopharyngiomas. Despite being classified as a grade I benign brain tumor, craniopharyngiomas have a high recurrence rate, even after maximal surgical resection, due to their location near critical skull base structures and their aggressive growth pattern. The relative five-year survival rate is only 85.8%, the lowest among predominantly non-malignant brain tumors [161]. Recent studies have found that B7-H3 is highly expressed in both subtypes of craniopharyngiomas, with more than 90% of tumors showing moderate-to-high-intensity staining, and is correlated with poor prognosis [33, 39, 162].
B7-H3/CD3 bi-specific T cell engagers have shown promising results in vitro, indicating that B7-H3-targeted therapy might be an effective treatment for refractory craniopharyngiomas [33]. B7-H3-targeted CAR-T cell therapy has demonstrated potent tumor-killing effects in 2D cell culture models, but limited efficacy in 3D organoid models. However, the B7-H3 antibody-DM1 conjugate has exhibited tumor-suppressing function in both 2D and 3D models, providing a promising hint for ADC therapy for refractory craniopharyngiomas [32].
B7-H3 in brain metastases
Brain metastases are common intracranial neoplasms that develop when cancer cells from other parts of the body, such as the lungs, breasts, or melanoma, spread to the brain. They affect about 20% of cancer patients and remain a significant cause of cancer-related deaths, despite the use of treatments such as surgery, radiotherapy, chemotherapy, and immunotherapy. Researchers are currently investigating promising treatment options that target novel markers. For instance, a study on metastatic melanoma in vivo and in vitro found that silencing B7-H3 reduced the ability of cancer cells to spread and increased symptom-free survival in mice and rats. The study also showed that B7-H3 regulated several genes associated with metastasis, including MMP-2, STAT3, IL-8, and TIMP-1 and -2 [79]. This finding is unique as it investigated the non-immunological function of B7-H3 in brain metastases. Another study has shown that co-expression of CCR2b in B7-H3-CAR-T cells significantly enhances the permeability of the BBB and increases the effectiveness of CAR-T cell therapy against NSCLC brain metastases both in vivo and in vitro. This study offers a new perspective on using engineered CAR-T cell therapy to control these lethal metastatic lesions [125].
Ongoing Clinical Studies Targeting B7-H3 in Brain Tumors
Several early-phase clinical trials targeting B7-H3 in hematologic and other solid malignancies are ongoing, and some have revealed potent clinical efficacy and favorable tolerance (https://clinicaltrials.gov/). This suggests that novel effective immunotherapies, in addition to conventional treatments, may be available for refractory cancers [2, 5, 19, 98]. In brain cancers, as demonstrated in preclinical studies and limited early-phase trial data, B7-H3 also acts as a promising therapeutic target and is being intensively evaluated in clinical trials. Ongoing clinical trials targeting B7-H3 in brain tumors are listed in Table 1.
B7-H3-CAR-T cell therapy in brain tumors
In the field of CAR-T therapy, clinical trials targeting refractory CNS tumors, such as DIPGs, GBMs, and neuroblastomas, are ongoing to evaluate the safety, feasibility, pharmacokinetics, pharmacodynamics, and therapeutic efficacy of B7-H3-CAR-T therapy. There are seven ongoing trials (NCT04185038, NCT05474378, NCT05366179, NCT05241392, NCT04385173, NCT04077866, NCT04637503) being conducted in children or adult patients. In 2019, Seattle Children's Hospital initiated a non-randomized, parallel-assignment CAR-T-Cell locoregional immunotherapy for children and young adults with DIPGs and recurrent or refractory CNS tumors (NCT04185038). In this trial, eligible patients receive autologous T cells engineered to target B7-H3-expressing cells via an indwelling catheter placed in the tumor resection cavity or the ventricular system. The treatment options vary based on the location and type of tumors. Patients with supratentorial tumors will be assigned to arm A (tumor cavity infusion), patients with metastatic, leptomeningeal, or infratentorial tumors will be assigned to arm B (intraventricular infusion), and patients with DIPGs will be assigned to arm C (ventricular system infusion). Six clinical trials are also ongoing examining the safety and efficacy of B7-H3-CAR-T therapy for adult patients. Stanford University launched a trial last year that schedules B7-H3-CAR-T cells to be administered locoregionally in the ventricular system or in both the ventricular system and the resection cavity (NCT05474378). The primary outcome measures are the number of successful manufacturing products that meet the minimum assigned dose range, the maximum tolerated dose (MTD), and recommended phase II dose (RP2D). In another trial launched last year from the University of North Carolina Lineberger Comprehensive Cancer Center (NCT05366179), autologous B7-H3-CAR-T cells are only infused into the ventricular system of adult patients with relapsed or refractory GBMs. In this trial, a suspension of CAR-T cells (0.5 mL) is given via a Rickham catheter followed by a normal-saline flush (3-5 mL). The other four clinical trials are actively being conducted in China.
Active Clinical Trials Targeting B7-H3 in Patients with Brain Tumors.
| Trial Number | Intervention | Phase | Launch Time | Settings | Sample Size | Patients | Pathology | Primary Endpoints |
|---|---|---|---|---|---|---|---|---|
| NCT04185038 | CAR-T therapy | I | 2019 | Seattle Children's Hospital, US | 90 | Children and young adults | DIPGs and refractory or recurrent CNS tumors | Safety and feasibility |
| NCT05474378 | CAR-T therapy | I | 2022 | Stanford University, US | 39 | Adults | Recurrent GBMs | Number of successful manufacturing products meeting the minimum dose range, the maximum tolerated dose, and the recommended phase II dose |
| NCT05366179 | CAR-T therapy | I | 2022 | University of North Carolina, US | 36 | Adults | Relapsed or refractory GBMs | Safety and tolerability, cytokine release syndrome, and neurotoxicity |
| NCT05241392 | CAR-T therapy | I | 2022 | Beijing Tiantan Hospital, China | 30 | Adults | Recurrent GBMs | Dose limiting toxicity and adverse events |
| NCT04385173 | CAR-T therapy | I | 2020 | Zhejiang University, China | 12 | Adults | Recurrent or refractory GBMs | Adverse events, maximum tolerated dose, overall survival, and progression-free survival |
| NCT04077866 | CAR-T therapy | I/II | 2019 | Zhejiang University, China | 40 | Adults | Recurrent or refractory GBMs | Overall survival |
| NCT04637503 | CAR-T therapy | I/II | 2020 | Shenzhen Geno-Immune Medical Institute, China | 100 | Adults | Relapsed and refractory neuroblastoma | Adverse events |
| NCT05063357 | ADC therapy | I | 2021 | Y-mAbs Therapeutics, US | 36 | Children and young adults | DIPGs that have not progressed following external beam radiation therapy. | Adverse events |
| NCT04743661 | ADC therapy | II | 2021 | Memorial Sloan Kettering Cancer Center and Y-mAbs Therapeutics, US | 62 | Children and young adults | Recurrent medulloblastoma and ependymomas | 2-year event free survival, and percentage of patients who met feasibility criteria in the ependymoma cohort |
| NCT05064306 | ADC therapy | I | 2021 | Memorial Sloan Kettering Cancer Center and Y-mAbs Therapeutics, US | NA | Children and young adults | CNS/leptomeningeal neoplasms | NA |
| NCT03275402 | ADC therapy | II/III | 2017 | Y-mAbs Therapeutics, US | 52 | Children | Neuroblastomas and CNS/leptomeningeal metastases | Overall survival rate at 3 years |
| NCT00089245 | ADC therapy | I | 2004 | Y-mAbs Therapeutics, US | 120 | Children and adults | Refractory, recurrent, or advanced CNS/leptomeningeal cancers | Treatment related toxicities |
In a recently published study from Beijing Tiantan Hospital evaluating MTD and RP2D (NCT05241392), patients are treated with autologous B7-H3-CAR-T cells through an Ommaya device placed into the tumor resection cavity or ventricular system. Two trials are being conducted at the second affiliated hospital of Zhejiang University, with one assessing the pharmacokinetics and pharmacodynamics of CAR-T cell infusion in addition to its safety and clinical effectiveness (NCT04385173), and the other being a randomized, parallel-arm, phase I/II trial expected to enroll 40 patients randomized to receive B7-H3-CAR-T therapy during the adjuvant phase of Temozolomide chemotherapy or to undergo Temozolomide treatment alone (NCT04077866). The primary endpoint is overall survival, while adverse events, MTD, and multiple disease response parameters, including progression-free survival, are the secondary and other outcome measures. Finally, the trial from Shenzhen Geno-Immune Medical Institute utilizes multi-CAR-T cell therapy which targets disialoganglioside, prostate-specific membrane antigen, and B7-H3 in patients with relapsed and refractory neuroblastomas to assess the feasibility, safety, and efficacy of the “multiple targeting” approach and to assess its persistency in patients (NCT04637503).
B7-H3-ADC therapy in brain tumors
ADC therapy is currently under investigation for the treatment of brain cancers. One clinical trial (NCT05063357) is examining the safety and efficacy of convection enhanced delivery of 131I-omburtamab, a murine IgG1 mAb that recognizes B7-H3 and is labeled with Iodine-131. Y-mAbs Therapeutics initiated this trial in 2021 to assess its effectiveness in treating children and young adults with DIPGs that have not progressed following external radiation therapy. Another phase II trial (NCT04743661), led by Memorial Sloan Kettering Cancer Center and Y-mAbs Therapeutics, is investigating the added utility of 131I-omburtamab in combination with irinotecan, temozolomide, and bevacizumab for patients with recurrent medulloblastoma. This trial will involve surgery, induction chemotherapy with irinotecan, temozolomide, and bevacizumab, and continued radioimmunotherapy with 2 doses of 131I-omburtamab. Patients with recurrent B7-H3+ ependymomas who have not responded to surgery, radiation, or other therapies will also be included in the study to assess the therapeutic efficacy of 131I-omburtamab. These two institutes have launched another clinical trial (NCT05064306) enrolling children and young adults who have CNS/leptomeningeal neoplasms to provide them with treatment with 131I-omburtamab. Additionally, a multicenter phase II/III trial (NCT03275402) is enrolling children with neuroblastomas and CNS/leptomeningeal metastases to receive up to 2 cycles of intracerebroventricular infusion with 131I-omburtamab to evaluate its clinical efficacy and safety. In addition to 131I-omburtamab, 131I-8H9 is also being investigated (NCT00089245) to assess its maximum tolerated dose in patients with refractory, recurrent, or advanced CNS/leptomeningeal cancers. 177Lu-DTPA-omburtamab has been tested in two trials (NCT04315246, NCT04167618) involving patients with leptomeningeal metastasis or medulloblastomas to evaluate its safety, pharmacokinetics, and therapeutic responses. However, these two clinical trials were recently withdrawn or terminated due to business priorities.
Current Limitations and Challenges
Due to the low incidence of brain malignancies, enrolling a sufficient number of patients in clinical trials has been challenging. To address this issue, it is crucial for medical centers to collaborate and establish multi-center trials dedicated to the treatment of brain malignancies. By uniting efforts and resources, it will be possible to gather a larger patient population, facilitating the advancement of treatment options for these conditions.
While extensive research has been conducted on the potential ligands of B7-H3, no satisfactory results have been achieved thus far. To enhance the effectiveness of B7-H3 targeted therapy, it remains imperative to identify its binding partner and the downstream signaling pathway molecules associated with its function. Such discoveries would provide more specific and effective targets for therapeutic intervention.
Additionally, combination therapy has demonstrated superior efficacy compared to single treatment modalities in various brain malignancies. Incorporating different treatment approaches, such as focused ultrasound or tumor treating fields, alongside B7-H3 targeted therapy, holds promise as potential candidates for combination treatments. These modalities have the potential to enhance BBB penetration, overcome treatment resistance, and ultimately generate synergistic anti-tumor effects. Exploring these combination strategies could significantly improve the outcomes for patients with brain malignancies.
Conclusions and Perspectives
B7-H3 is widely expressed on cancer cells and immune cells, playing a complex role in tumor progression and immune evasion. Although the crystal structure of B7-H3 has been confirmed, its specific binding receptors and downstream signaling pathways are still being investigated. B7-H3 functions as an immune co-inhibitor by suppressing the production of anti-tumor cytokines, inhibiting the activation and function of innate cytotoxic immune cells, and impairing T-cell immune response, thereby undermining adaptive immunity. Through interactions with infiltrating immune cells, tumor vasculature, and CAFs within the TME, B7-H3 establishes a pro-tumoral environment that supports uncontrolled tumor cell growth, accelerated metabolism, enhanced cancer stemness, and increased resistance to treatment. Recent advancements in understanding B7-H3's mechanisms of action and the rapid development of emerging treatment approaches have led to the investigation of various immunotherapies using CAR-T cells, CAR-NK cells, mAbs, bsAbs, and ADCs. Preclinical studies and early-phase clinical trials targeting B7-H3 in many refractory malignant brain tumor types have shown promising anti-tumor efficacy with limited toxicity. Currently, a growing number of trials focusing on B7-H3 targeted immunotherapy in brain malignancies, particularly GBMs, DIPGs, and brain metastases, are underway. These trials hold promise in providing new options and concepts for the treatment of these highly resistant types of brain tumors.
Abbreviations
AAMP: angio-associated migratory cell protein; ADC: antibody-drug conjugate; ADCC: antibody-dependent cellular cytotoxicity; ATRT: atypical teratoid/rhabdoid tumor; bsAbs: bispecific antibodies; CAF: cancer-associated fibroblast; CAR-T cell: chimeric antigen receptor T cell; CTLA-4: cytotoxic T lymphocyte-associated protein 4; DC: dendritic cell; DIPG: diffuse intrinsic pontine glioma; EMT: epithelial-to-mesenchymal transition; FOXP3: forkhead box P3; GBM: glioblastoma; IC: immune checkpoints; IFN-γ: interferon gamma; IL-4: interleukin-4; IL20RA: interleukin-20 receptor subunit α; JAK2: Janus kinase 2; mAbs: monoclonal antibodies; MMP-2: matrix metalloproteinase 2; MHC: major histocompatibility complex; NK cell: natural-killer cell; MTD: maximum tolerated dose; NSCLC: non-small cell lung cancer; PD-1: programmed cell death protein 1; PD-L1: programmed cell death ligand 1; PLA2R1: phospholipase A2 receptor 1; RP2D: recommended phase II dose; Th1 cell: T helper type 1 cell; Th2 cell: T helper type 2 cell; Th17 cell: T helper type 17 cell; TLR: toll-like receptor; TLT-2: TREM-like transcript 2; TME: tumor microenvironment; Treg cell: regulatory T cell; TREM: triggering receptor expressed on myeloid cell; scFv: single chain antibody fragment; STAT3: signal transducer and activator of transcription 3; VEGFA: vascular endothelial growth factor A.
Acknowledgements
Funding
This work was supported by the National Natural Science Foundation of China (82203527 and 82103302), the Fundamental Research Funds for the Central Universities (3332022009), and the China Postdoctoral Science Foundation (2022M710451). The funding sources have no roles in the conduct of the research; in the preparation of the article study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author Contributions
X.G. and B.X. conceived the study and consulted a large number of literatures. X.G. and Y.W. designed and drew the figures in the manuscript. X.G. and M.C. drafted the manuscript. Y.W., B.X., and W.M. reviewed and critically revised this manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Shiravand Y, Khodadadi F, Kashani SMA. et al. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol. 2022;29:3044-60 doi: 10.3390/curroncol29050247
2. Zhao B, Li H, Xia Y. et al. Immune checkpoint of B7-H3 in cancer: from immunology to clinical immunotherapy. J Hematol Oncol. 2022;15:153. doi: 10.1186/s13045-022-01364-7
3. Chapoval AI, Ni J, Lau JS. et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269-74 doi: 10.1038/85339
4. Liu S, Liang J, Liu Z. et al. The Role of CD276 in Cancers. Front Oncol. 2021;11:654684. doi: 10.3389/fonc.2021.654684
5. Kanchan RK, Doss D, Khan P. et al. To kill a cancer: Targeting the immune inhibitory checkpoint molecule, B7-H3. Biochim Biophys Acta Rev Cancer. 2022;1877:188783. doi: 10.1016/j.bbcan.2022.188783
6. Ostrom QT, Price M, Neff C. et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019. Neuro Oncol. 2022;24:v1-v95 doi: 10.1093/neuonc/noac202
7. Perrone MG, Ruggiero A, Centonze A. et al. Diffuse Intrinsic Pontine Glioma (DIPG): Breakthrough and Clinical Perspective. Curr Med Chem. 2021;28:3287-317 doi: 10.2174/0929867327666200806110206
8. Chen F, Wendl MC, Wyczalkowski MA. et al. Moving pan-cancer studies from basic research toward the clinic. Nat Cancer. 2021;2:879-90 doi: 10.1038/s43018-021-00250-4
9. Stupp R, Mason WP, van den Bent MJ. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-96 doi: 10.1056/NEJMoa043330
10. Stupp R, Taillibert S, Kanner A. et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318:2306-16 doi: 10.1001/jama.2017.18718
11. Liau LM, Ashkan K, Brem S. et al. Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination With Extension of Survival Among Patients With Newly Diagnosed and Recurrent Glioblastoma: A Phase 3 Prospective Externally Controlled Cohort Trial. JAMA Oncol. 2023;9:112-21 doi: 10.1001/jamaoncol.2022.5370
12. Wang R, Ma Y, Zhan S. et al. B7-H3 promotes colorectal cancer angiogenesis through activating the NF-kappaB pathway to induce VEGFA expression. Cell Death Dis. 2020;11:55. doi: 10.1038/s41419-020-2252-3
13. Sun X, Yu Y, Ma L. et al. T cell cytotoxicity toward hematologic malignancy via B7-H3 targeting. Invest New Drugs. 2020;38:722-32 doi: 10.1007/s10637-019-00819-y
14. Cheng R, Wang B, Cai XR. et al. CD276 Promotes Vasculogenic Mimicry Formation in Hepatocellular Carcinoma via the PI3K/AKT/MMPs Pathway. Onco Targets Ther. 2020;13:11485-98 doi: 10.2147/OTT.S271891
15. Li Y, Yang X, Yao P. et al. B7-H3 increases the radioresistance of gastric cancer cells through regulating baseline levels of cell autophagy. Am J Transl Res. 2019;11:4438-49
16. Zhang J, Liu L, Han S. et al. B7-H3 is related to tumor progression in ovarian cancer. Oncol Rep. 2017;38:2426-34 doi: 10.3892/or.2017.5858
17. Kang FB, Wang L, Jia HC. et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45. doi: 10.1186/s12935-015-0195-z
18. Li M, Zhang G, Zhang X. et al. Overexpression of B7-H3 in CD14+ monocytes is associated with renal cell carcinoma progression. Med Oncol. 2014;31:349. doi: 10.1007/s12032-014-0349-1
19. Kontos F, Michelakos T, Kurokawa T. et al. B7-H3: An Attractive Target for Antibody-based Immunotherapy. Clin Cancer Res. 2021;27:1227-35 doi: 10.1158/1078-0432.CCR-20-2584
20. Picarda E, Ohaegbulam KC, Zang X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin Cancer Res. 2016;22:3425-31 doi: 10.1158/1078-0432.CCR-15-2428
21. Steinberger P, Majdic O, Derdak SV. et al. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172:2352-9 doi: 10.4049/jimmunol.172.4.2352
22. Sun M, Richards S, Prasad DV. et al. Characterization of mouse and human B7-H3 genes. J Immunol. 2002;168:6294-7 doi: 10.4049/jimmunol.168.12.6294
23. Digregorio M, Coppieters N, Lombard A. et al. The expression of B7-H3 isoforms in newly diagnosed glioblastoma and recurrence and their functional role. Acta Neuropathol Commun. 2021;9:59. doi: 10.1186/s40478-021-01167-w
24. Nehama D, Di Ianni N, Musio S. et al. B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine. 2019;47:33-43 doi: 10.1016/j.ebiom.2019.08.030
25. Haydar D, Houke H, Chiang J. et al. Cell-surface antigen profiling of pediatric brain tumors: B7-H3 is consistently expressed and can be targeted via local or systemic CAR T-cell delivery. Neuro Oncol. 2021;23:999-1011 doi: 10.1093/neuonc/noaa278
26. Baral A, Ye HX, Jiang PC. et al. B7-H3 and B7-H1 expression in cerebral spinal fluid and tumor tissue correlates with the malignancy grade of glioma patients. Oncol Lett. 2014;8:1195-201 doi: 10.3892/ol.2014.2268
27. Wang Z, Wang Z, Zhang C. et al. Genetic and clinical characterization of B7-H3 (CD276) expression and epigenetic regulation in diffuse brain glioma. Cancer Sci. 2018;109:2697-705 doi: 10.1111/cas.13744
28. Zhou Z, Luther N, Ibrahim GM. et al. B7-H3, a potential therapeutic target, is expressed in diffuse intrinsic pontine glioma. J Neurooncol. 2013;111:257-64 doi: 10.1007/s11060-012-1021-2
29. Kumar A, Mohamed E, Tong S. et al. CXCL14 Promotes a Robust Brain Tumor-Associated Immune Response in Glioma. Clin Cancer Res. 2022;28:2898-910 doi: 10.1158/1078-0432.CCR-21-2830
30. Deng J, Ma M, Wang D. et al. Expression and Clinical Significance of Immune Checkpoint Regulator B7-H3 (CD276) in Human Meningioma. World Neurosurg. 2020;135:e12-e8 doi: 10.1016/j.wneu.2019.10.044
31. Proctor DT, Patel Z, Lama S. et al. Identification of PD-L2, B7-H3 and CTLA-4 immune checkpoint proteins in genetic subtypes of meningioma. Oncoimmunology. 2019;8:e1512943. doi: 10.1080/2162402X.2018.1512943
32. Tang M, Chen C, Wang G. et al. Evaluation of B7-H3 Targeted Immunotherapy in a 3D Organoid Model of Craniopharyngioma. Biomolecules. 2022 12. doi: 10.3390/biom12121744
33. Chen C, Wang Y, Zhong K. et al. Frequent B7-H3 overexpression in craniopharyngioma. Biochem Biophys Res Commun. 2019;514:379-85 doi: 10.1016/j.bbrc.2019.04.142
34. Theruvath J, Sotillo E, Mount CW. et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat Med. 2020;26:712-9 doi: 10.1038/s41591-020-0821-8
35. Li S, Poolen GC, van Vliet LC. et al. Pediatric medulloblastoma express immune checkpoint B7-H3. Clin Transl Oncol. 2022;24:1204-8 doi: 10.1007/s12094-021-02762-y
36. Marques RF, Moreno DA, da Silva L. et al. Digital expression profile of immune checkpoint genes in medulloblastomas identifies CD24 and CD276 as putative immunotherapy targets. Front Immunol. 2023;14:1062856. doi: 10.3389/fimmu.2023.1062856
37. Castriconi R, Dondero A, Augugliaro R. et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101:12640-5 doi: 10.1073/pnas.0405025101
38. Zhou WT, Jin WL. B7-H3/CD276: An Emerging Cancer Immunotherapy. Front Immunol. 2021;12:701006. doi: 10.3389/fimmu.2021.701006
39. Maachani UB, Tosi U, Pisapia DJ. et al. B7-H3 as a Prognostic Biomarker and Therapeutic Target in Pediatric central nervous system Tumors. Transl Oncol. 2020;13:365-71 doi: 10.1016/j.tranon.2019.11.006
40. Seaman S, Zhu Z, Saha S. et al. Eradication of Tumors through Simultaneous Ablation of CD276/B7-H3-Positive Tumor Cells and Tumor Vasculature. Cancer Cell. 2017;31:501-15 e8. doi: 10.1016/j.ccell.2017.03.005
41. Vigdorovich V, Ramagopal UA, Lazar-Molnar E. et al. Structure and T cell inhibition properties of B7 family member, B7-H3. Structure. 2013;21:707-17 doi: 10.1016/j.str.2013.03.003
42. Du H, Hirabayashi K, Ahn S. et al. Antitumor Responses in the Absence of Toxicity in Solid Tumors by Targeting B7-H3 via Chimeric Antigen Receptor T Cells. Cancer Cell. 2019;35:221-37 e8. doi: 10.1016/j.ccell.2019.01.002
43. Chen W, Liu P, Wang Y. et al. Characterization of a soluble B7-H3 (sB7-H3) spliced from the intron and analysis of sB7-H3 in the sera of patients with hepatocellular carcinoma. PLoS One. 2013;8:e76965. doi: 10.1371/journal.pone.0076965
44. Zhang G, Hou J, Shi J. et al. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123:538-46 doi: 10.1111/j.1365-2567.2007.02723.x
45. Purvis IJ, Velpula KK, Guda MR. et al. B7-H3 in Medulloblastoma-Derived Exosomes; A Novel Tumorigenic Role. Int J Mol Sci. 2020 21. doi: 10.3390/ijms21197050
46. Hashiguchi M, Kobori H, Ritprajak P. et al. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci U S A. 2008;105:10495-500 doi: 10.1073/pnas.0802423105
47. Husain B, Ramani SR, Chiang E. et al. A Platform for Extracellular Interactome Discovery Identifies Novel Functional Binding Partners for the Immune Receptors B7-H3/CD276 and PVR/CD155. Mol Cell Proteomics. 2019;18:2310-23 doi: 10.1074/mcp.TIR119.001433
48. Cao S, Peterson SM, Muller S. et al. A membrane protein display platform for receptor interactome discovery. Proc Natl Acad Sci U S A. 2021 118. doi: 10.1073/pnas.2025451118
49. Ciprut S, Berberich A, Knoll M. et al. AAMP is a binding partner of costimulatory human B7-H3. Neurooncol Adv. 2022;4:vdac098. doi: 10.1093/noajnl/vdac098
50. Wang SY, Fu XX, Duan R. et al. The Alzheimer's disease-associated gene TREML2 modulates inflammation by regulating microglia polarization and NLRP3 inflammasome activation. Neural Regen Res. 2023;18:434-8 doi: 10.4103/1673-5374.346468
51. King RG, Herrin BR, Justement LB. Trem-like transcript 2 is expressed on cells of the myeloid/granuloid and B lymphoid lineage and is up-regulated in response to inflammation. J Immunol. 2006;176:6012-21 doi: 10.4049/jimmunol.176.10.6012
52. Halpert MM, Thomas KA, King RG. et al. TLT2 potentiates neutrophil antibacterial activity and chemotaxis in response to G protein-coupled receptor-mediated signaling. J Immunol. 2011;187:2346-55 doi: 10.4049/jimmunol.1100534
53. Zheng H, Liu CC, Atagi Y. et al. Opposing roles of the triggering receptor expressed on myeloid cells 2 and triggering receptor expressed on myeloid cells-like transcript 2 in microglia activation. Neurobiol Aging. 2016;42:132-41 doi: 10.1016/j.neurobiolaging.2016.03.004
54. Xu JC, Gao F, Liu YA. et al. Myeloid cell-like transcript 2 is related to liver inflammation and the pathogenesis of hepatitis B via the involvement of CD8(+)T cell activation. Clin Exp Med. 2019;19:93-104 doi: 10.1007/s10238-018-0534-1
55. Leitner J, Klauser C, Pickl WF. et al. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39:1754-64 doi: 10.1002/eji.200839028
56. Yan R, Yang S, Gu A. et al. Murine b7-h3 is a co-stimulatory molecule for T cell activation. Monoclon Antib Immunodiagn Immunother. 2013;32:395-8 doi: 10.1089/mab.2013.0052
57. Wolk K, Kunz S, Asadullah K. et al. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397-402 doi: 10.4049/jimmunol.168.11.5397
58. Blumberg H, Conklin D, Xu WF. et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9-19 doi: 10.1016/s0092-8674(01)00187-8
59. Ungaro F, Garlatti V, Massimino L. et al. mTOR-Dependent Stimulation of IL20RA Orchestrates Immune Cell Trafficking through Lymphatic Endothelium in Patients with Crohn's Disease. Cells. 2019 8. doi: 10.3390/cells8080924
60. Zhang G, Wang J, Kelly J. et al. B7-H3 augments the inflammatory response and is associated with human sepsis. J Immunol. 2010;185:3677-84 doi: 10.4049/jimmunol.0904020
61. Luo L, Chapoval AI, Flies DB. et al. B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J Immunol. 2004;173:5445-50 doi: 10.4049/jimmunol.173.9.5445
62. Sun X, Vale M, Leung E. et al. Mouse B7-H3 induces antitumor immunity. Gene Ther. 2003;10:1728-34 doi: 10.1038/sj.gt.3302070
63. Lupu CM, Eisenbach C, Lupu AD. et al. Adenoviral B7-H3 therapy induces tumor specific immune responses and reduces secondary metastasis in a murine model of colon cancer. Oncol Rep. 2007;18:745-8
64. Wang L, Fraser CC, Kikly K. et al. B7-H3 promotes acute and chronic allograft rejection. Eur J Immunol. 2005;35:428-38 doi: 10.1002/eji.200425518
65. Luo L, Zhu G, Xu H. et al. B7-H3 Promotes Pathogenesis of Autoimmune Disease and Inflammation by Regulating the Activity of Different T Cell Subsets. PLoS One. 2015;10:e0130126. doi: 10.1371/journal.pone.0130126
66. Hofmeyer KA, Ray A, Zang X. The contrasting role of B7-H3. Proc Natl Acad Sci U S A. 2008;105:10277-8 doi: 10.1073/pnas.0805458105
67. Suh WK, Gajewska BU, Okada H. et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899-906 doi: 10.1038/ni967
68. Ling V, Wu PW, Spaulding V. et al. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics. 2003;82:365-77 doi: 10.1016/s0888-7543(03)00126-5
69. Ueno T, Yeung MY, McGrath M. et al. Intact B7-H3 signaling promotes allograft prolongation through preferential suppression of Th1 effector responses. Eur J Immunol. 2012;42:2343-53 doi: 10.1002/eji.201242501
70. Chen L, Chen J, Xu B. et al. B7-H3 expression associates with tumor invasion and patient's poor survival in human esophageal cancer. Am J Transl Res. 2015;7:2646-60
71. Jin Y, Zhang P, Li J. et al. B7-H3 in combination with regulatory T cell is associated with tumor progression in primary human non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8:13987-95
72. Lemke D, Pfenning PN, Sahm F. et al. Costimulatory protein 4IgB7H3 drives the malignant phenotype of glioblastoma by mediating immune escape and invasiveness. Clin Cancer Res. 2012;18:105-17 doi: 10.1158/1078-0432.CCR-11-0880
73. Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: Friend or foe? Int J Cancer. 2014;134:2764-71 doi: 10.1002/ijc.28474
74. Li ZY, Wang JT, Chen G. et al. Expression, regulation and clinical significance of B7-H3 on neutrophils in human gastric cancer. Clin Immunol. 2021;227:108753. doi: 10.1016/j.clim.2021.108753
75. Suh WK, Wang SX, Jheon AH. et al. The immune regulatory protein B7-H3 promotes osteoblast differentiation and bone mineralization. Proc Natl Acad Sci U S A. 2004;101:12969-73 doi: 10.1073/pnas.0405259101
76. Yuan H, Wei X, Zhang G. et al. B7-H3 over expression in prostate cancer promotes tumor cell progression. J Urol. 2011;186:1093-9 doi: 10.1016/j.juro.2011.04.103
77. Chen YW, Tekle C, Fodstad O. The immunoregulatory protein human B7H3 is a tumor-associated antigen that regulates tumor cell migration and invasion. Curr Cancer Drug Targets. 2008;8:404-13 doi: 10.2174/156800908785133141
78. Liu F, Zhang T, Zou S. et al. B7-H3 promotes cell migration and invasion through the Jak2/Stat3/MMP9 signaling pathway in colorectal cancer. Mol Med Rep. 2015;12:5455-60 doi: 10.3892/mmr.2015.4050
79. Tekle C, Nygren MK, Chen YW. et al. B7-H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. Int J Cancer. 2012;130:2282-90 doi: 10.1002/ijc.26238
80. Liu H, Tekle C, Chen YW. et al. B7-H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation. Mol Cancer Ther. 2011;10:960-71 doi: 10.1158/1535-7163.MCT-11-0072
81. Zhang T, Jiang B, Zou ST. et al. Overexpression of B7-H3 augments anti-apoptosis of colorectal cancer cells by Jak2-STAT3. World J Gastroenterol. 2015;21:1804-13 doi: 10.3748/wjg.v21.i6.1804
82. Ma Y, Wang R, Lu H. et al. B7-H3 promotes the cell cycle-mediated chemoresistance of colorectal cancer cells by regulating CDC25A. J Cancer. 2020;11:2158-70 doi: 10.7150/jca.37255
83. Zhao X, Zhang GB, Gan WJ. et al. Silencing of B7-H3 increases gemcitabine sensitivity by promoting apoptosis in pancreatic carcinoma. Oncol Lett. 2013;5:805-12 doi: 10.3892/ol.2013.1118
84. Zhong C, Tao B, Chen Y. et al. B7-H3 Regulates Glioma Growth and Cell Invasion Through a JAK2/STAT3/Slug-Dependent Signaling Pathway. Onco Targets Ther. 2020;13:2215-24 doi: 10.2147/OTT.S237841
85. Nunes-Xavier CE, Karlsen KF, Tekle C. et al. Decreased expression of B7-H3 reduces the glycolytic capacity and sensitizes breast cancer cells to AKT/mTOR inhibitors. Oncotarget. 2016;7:6891-901 doi: 10.18632/oncotarget.6902
86. Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753. doi: 10.1016/j.pharmthera.2020.107753
87. Biserova K, Jakovlevs A, Uljanovs R. et al. Cancer Stem Cells: Significance in Origin, Pathogenesis and Treatment of Glioblastoma. Cells. 2021 10. doi: 10.3390/cells10030621
88. Park JH, Pyun WY, Park HW. Cancer Metabolism: Phenotype, Signaling and Therapeutic Targets. Cells. 2020 9. doi: 10.3390/cells9102308
89. Pan Y, Yu Y, Wang X. et al. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol. 2020;11:583084. doi: 10.3389/fimmu.2020.583084
90. Zhang S, Zhou C, Zhang D. et al. The anti-apoptotic effect on cancer-associated fibroblasts of B7-H3 molecule enhancing the cell invasion and metastasis in renal cancer. Onco Targets Ther. 2019;12:4119-27 doi: 10.2147/OTT.S201121
91. Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18:99-115 doi: 10.1038/s41573-018-0004-1
92. Cheng N, Bei Y, Song Y. et al. B7-H3 augments the pro-angiogenic function of tumor-associated macrophages and acts as a novel adjuvant target for triple-negative breast cancer therapy. Biochem Pharmacol. 2021;183:114298. doi: 10.1016/j.bcp.2020.114298
93. Ricci-Vitiani L, Pallini R, Biffoni M. et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824-8 doi: 10.1038/nature09557
94. Raffin C, Vo LT, Bluestone JA. T(reg) cell-based therapies: challenges and perspectives. Nat Rev Immunol. 2020;20:158-72 doi: 10.1038/s41577-019-0232-6
95. Mahnke K, Ring S, Johnson TS. et al. Induction of immunosuppressive functions of dendritic cells in vivo by CD4+CD25+ regulatory T cells: role of B7-H3 expression and antigen presentation. Eur J Immunol. 2007;37:2117-26 doi: 10.1002/eji.200636841
96. Zhu J. T Helper Cell Differentiation, Heterogeneity, and Plasticity. Cold Spring Harb Perspect Biol. 2018 10. doi: 10.1101/cshperspect.a030338
97. Fukushima A, Sumi T, Fukuda K. et al. B7-H3 regulates the development of experimental allergic conjunctivitis in mice. Immunol Lett. 2007;113:52-7 doi: 10.1016/j.imlet.2007.07.011
98. Feng R, Chen Y, Liu Y. et al. The role of B7-H3 in tumors and its potential in clinical application. Int Immunopharmacol. 2021;101:108153. doi: 10.1016/j.intimp.2021.108153
99. Mao Y, Chen L, Wang F. et al. Cancer cell-expressed B7-H3 regulates the differentiation of tumor-associated macrophages in human colorectal carcinoma. Oncol Lett. 2017;14:6177-83 doi: 10.3892/ol.2017.6935
100. Miyamoto T, Murakami R, Hamanishi J. et al. B7-H3 Suppresses Antitumor Immunity via the CCL2-CCR2-M2 Macrophage Axis and Contributes to Ovarian Cancer Progression. Cancer Immunol Res. 2022;10:56-69 doi: 10.1158/2326-6066.CIR-21-0407
101. Gao Y, Fang P, Li WJ. et al. LncRNA NEAT1 sponges miR-214 to regulate M2 macrophage polarization by regulation of B7-H3 in multiple myeloma. Mol Immunol. 2020;117:20-8 doi: 10.1016/j.molimm.2019.10.026
102. Schneider T, Hoffmann H, Dienemann H. et al. Non-small cell lung cancer induces an immunosuppressive phenotype of dendritic cells in tumor microenvironment by upregulating B7-H3. J Thorac Oncol. 2011;6:1162-8 doi: 10.1097/JTO.0b013e31821c421d
103. Al-Ostoot FH, Salah S, Khamees HA. et al. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat Res Commun. 2021;28:100422. doi: 10.1016/j.ctarc.2021.100422
104. Wang R, Chadalavada K, Wilshire J. et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829-33 doi: 10.1038/nature09624
105. Seaman S, Stevens J, Yang MY. et al. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11:539-54 doi: 10.1016/j.ccr.2007.04.017
106. Purvis IJ, Avilala J, Guda MR. et al. Role of MYC-miR-29-B7-H3 in Medulloblastoma Growth and Angiogenesis. J Clin Med. 2019 8. doi: 10.3390/jcm8081158
107. Zhou X, Ouyang S, Li J. et al. The novel non-immunological role and underlying mechanisms of B7-H3 in tumorigenesis. J Cell Physiol. 2019;234:21785-95 doi: 10.1002/jcp.28936
108. Zhan S, Liu Z, Zhang M. et al. Overexpression of B7-H3 in alpha-SMA-Positive Fibroblasts Is Associated With Cancer Progression and Survival in Gastric Adenocarcinomas. Front Oncol. 2019;9:1466. doi: 10.3389/fonc.2019.01466
109. Wei X, Li K, Zhang G. et al. B7-H3 promoted proliferation of mouse spermatogonial stem cells via the PI3K signaling pathway. Oncotarget. 2018;9:1542-52 doi: 10.18632/oncotarget.23457
110. Liu Z, Zhang W, Phillips JB. et al. Immunoregulatory protein B7-H3 regulates cancer stem cell enrichment and drug resistance through MVP-mediated MEK activation. Oncogene. 2019;38:88-102 doi: 10.1038/s41388-018-0407-9
111. Wang S, Mou J, Cui L. et al. Astragaloside IV inhibits cell proliferation of colorectal cancer cell lines through down-regulation of B7-H3. Biomed Pharmacother. 2018;102:1037-44 doi: 10.1016/j.biopha.2018.03.127
112. Yu TT, Zhang T, Lu X. et al. B7-H3 promotes metastasis, proliferation, and epithelial-mesenchymal transition in lung adenocarcinoma. Onco Targets Ther. 2018;11:4693-700 doi: 10.2147/OTT.S169811
113. Jiang B, Zhang T, Liu F. et al. The co-stimulatory molecule B7-H3 promotes the epithelial-mesenchymal transition in colorectal cancer. Oncotarget. 2016;7:31755-71 doi: 10.18632/oncotarget.9035
114. Liao H, Ding M, Zhou N. et al. B7-H3 promotes the epithelial-mesenchymal transition of NSCLC by targeting SIRT1 through the PI3K/AKT pathway. Mol Med Rep. 2022; 25. doi: 10.3892/mmr. 2022 12595
115. Xie J, Sun M, Zhang D. et al. Fibronectin enhances tumor metastasis through B7-H3 in clear cell renal cell carcinoma. FEBS Open Bio. 2021;11:2977-87 doi: 10.1002/2211-5463.13280
116. Zhang J, Wang J, Marzese DM. et al. B7H3 regulates differentiation and serves as a potential biomarker and theranostic target for human glioblastoma. Lab Invest. 2019;99:1117-29 doi: 10.1038/s41374-019-0238-5
117. Li Z, Liu J, Que L. et al. The immunoregulatory protein B7-H3 promotes aerobic glycolysis in oral squamous carcinoma via PI3K/Akt/mTOR pathway. J Cancer. 2019;10:5770-84 doi: 10.7150/jca.29838
118. Lim S, Liu H, Madeira da Silva L. et al. Immunoregulatory Protein B7-H3 Reprograms Glucose Metabolism in Cancer Cells by ROS-Mediated Stabilization of HIF1alpha. Cancer Res. 2016;76:2231-42 doi: 10.1158/0008-5472.CAN-15-1538
119. Shi T, Ma Y, Cao L. et al. B7-H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis. 2019;10:308. doi: 10.1038/s41419-019-1549-6
120. Petterson SA, Sorensen MD, Burton M. et al. Differential expression of checkpoint markers in the normoxic and hypoxic microenvironment of glioblastomas. Brain Pathol. 2023;33:e13111. doi: 10.1111/bpa.13111
121. Lin H, Cheng J, Mu W. et al. Advances in Universal CAR-T Cell Therapy. Front Immunol. 2021;12:744823. doi: 10.3389/fimmu.2021.744823
122. Majzner RG, Theruvath JL, Nellan A. et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin Cancer Res. 2019;25:2560-74 doi: 10.1158/1078-0432.CCR-18-0432
123. Vitanza NA, Ronsley R, Choe M. et al. Locoregional CAR T cells for children with CNS tumors: Clinical procedure and catheter safety. Neoplasia. 2023;36:100870. doi: 10.1016/j.neo.2022.100870
124. Vitanza NA, Wilson AL, Huang W. et al. Intraventricular B7-H3 CAR T Cells for Diffuse Intrinsic Pontine Glioma: Preliminary First-in-Human Bioactivity and Safety. Cancer Discov. 2023;13:114-31 doi: 10.1158/2159-8290.CD-22-0750
125. Li H, Harrison EB, Li H. et al. Targeting brain lesions of non-small cell lung cancer by enhancing CCL2-mediated CAR-T cell migration. Nat Commun. 2022;13:2154. doi: 10.1038/s41467-022-29647-0
126. Tang X, Zhao S, Zhang Y. et al. B7-H3 as a Novel CAR-T Therapeutic Target for Glioblastoma. Mol Ther Oncolytics. 2019;14:279-87 doi: 10.1016/j.omto.2019.07.002
127. Tang X, Wang Y, Huang J. et al. Administration of B7-H3 targeted chimeric antigen receptor-T cells induce regression of glioblastoma. Signal Transduct Target Ther. 2021;6:125. doi: 10.1038/s41392-021-00505-7
128. Huang J, Zheng M, Zhang Z. et al. Interleukin-7-loaded oncolytic adenovirus improves CAR-T cell therapy for glioblastoma. Cancer Immunol Immunother. 2021;70:2453-65 doi: 10.1007/s00262-021-02856-0
129. Alkins R, Burgess A, Kerbel R. et al. Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro Oncol. 2016;18:974-81 doi: 10.1093/neuonc/nov318
130. Wang J, Toregrosa-Allen S, Elzey BD. et al. Multispecific targeting of glioblastoma with tumor microenvironment-responsive multifunctional engineered NK cells. Proc Natl Acad Sci U S A. 2021 118. doi: 10.1073/pnas.2107507118
131. Pan C, Zhai Y, Li G. et al. NK Cell-Based Immunotherapy and Therapeutic Perspective in Gliomas. Front Oncol. 2021;11:751183. doi: 10.3389/fonc.2021.751183
132. Tsao LC, Force J, Hartman ZC. Mechanisms of Therapeutic Antitumor Monoclonal Antibodies. Cancer Res. 2021;81:4641-51 doi: 10.1158/0008-5472.CAN-21-1109
133. Lu H, Shi T, Wang M. et al. B7-H3 inhibits the IFN-gamma-dependent cytotoxicity of Vgamma9Vdelta2 T cells against colon cancer cells. Oncoimmunology. 2020;9:1748991. doi: 10.1080/2162402X.2020.1748991
134. Lee YH, Martin-Orozco N, Zheng P. et al. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27:1034-45 doi: 10.1038/cr.2017.90
135. Modak S, Kramer K, Gultekin SH. et al. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. 2001;61:4048-54
136. Xu H, Cheung IY, Guo HF. et al. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69:6275-81 doi: 10.1158/0008-5472.CAN-08-4517
137. Ahmed M, Cheng M, Zhao Q. et al. Humanized Affinity-matured Monoclonal Antibody 8H9 Has Potent Antitumor Activity and Binds to FG Loop of Tumor Antigen B7-H3. J Biol Chem. 2015;290:30018-29 doi: 10.1074/jbc.M115.679852
138. Yamato I, Sho M, Nomi T. et al. Clinical importance of B7-H3 expression in human pancreatic cancer. Br J Cancer. 2009;101:1709-16 doi: 10.1038/sj.bjc.6605375
139. Loo D, Alderson RF, Chen FZ. et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18:3834-45 doi: 10.1158/1078-0432.CCR-12-0715
140. Weidle UH, Kontermann RE, Brinkmann U. Tumor-antigen-binding bispecific antibodies for cancer treatment. Semin Oncol. 2014;41:653-60 doi: 10.1053/j.seminoncol.2014.08.004
141. Mews EA, Beckmann P, Patchava M. et al. Multivalent, Bispecific alphaB7-H3-alphaCD3 Chemically Self-Assembled Nanorings Direct Potent T Cell Responses against Medulloblastoma. ACS Nano. 2022;16:12185-201 doi: 10.1021/acsnano.2c02850
142. Liu J, Yang S, Cao B. et al. Targeting B7-H3 via chimeric antigen receptor T cells and bispecific killer cell engagers augments antitumor response of cytotoxic lymphocytes. J Hematol Oncol. 2021;14:21. doi: 10.1186/s13045-020-01024-8
143. You G, Lee Y, Kang YW. et al. B7-H3x4-1BB bispecific antibody augments antitumor immunity by enhancing terminally differentiated CD8(+) tumor-infiltrating lymphocytes. Sci Adv. 2021 7. doi: 10.1126/sciadv.aax3160
144. Scribner JA, Brown JG, Son T. et al. Preclinical Development of MGC018, a Duocarmycin-based Antibody-drug Conjugate Targeting B7-H3 for Solid Cancer. Mol Cancer Ther. 2020;19:2235-44 doi: 10.1158/1535-7163.MCT-20-0116
145. Yamato M, Hasegawa J, Maejima T. et al. DS-7300a, a DNA Topoisomerase I Inhibitor, DXd-Based Antibody-Drug Conjugate Targeting B7-H3, Exerts Potent Antitumor Activities in Preclinical Models. Mol Cancer Ther. 2022;21:635-46 doi: 10.1158/1535-7163.MCT-21-0554
146. Kramer K, Kushner BH, Modak S. et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97:409-18 doi: 10.1007/s11060-009-0038-7
147. Souweidane MM, Kramer K, Pandit-Taskar N. et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018;19:1040-50 doi: 10.1016/S1470-2045(18)30322-X
148. Bailey K, Pandit-Taskar N, Humm JL. et al. Targeted radioimmunotherapy for embryonal tumor with multilayered rosettes. J Neurooncol. 2019;143:101-6 doi: 10.1007/s11060-019-03139-6
149. Zhang C, Zhang Z, Li F. et al. Large-scale analysis reveals the specific clinical and immune features of B7-H3 in glioma. Oncoimmunology. 2018;7:e1461304. doi: 10.1080/2162402X.2018.1461304
150. Takashima Y, Kawaguchi A, Hayano A. et al. CD276 and the gene signature composed of GATA3 and LGALS3 enable prognosis prediction of glioblastoma multiforme. PLoS One. 2019;14:e0216825. doi: 10.1371/journal.pone.0216825
151. Hu B, Hao S, Miao Y. et al. Inhibiting PP2A Upregulates B7-H3 Expression and Potentially Increases the Sensitivity of Malignant Meningiomas to Immunotherapy by Proteomics. Pathol Oncol Res. 2022;28:1610572. doi: 10.3389/pore.2022.1610572
152. Deng Y, Hu B, Miao Y. et al. A Nicotinamide Phosphoribosyltransferase Inhibitor, FK866, Suppresses the Growth of Anaplastic Meningiomas and Inhibits Immune Checkpoint Expression by Regulating STAT1. Front Oncol. 2022;12:836257. doi: 10.3389/fonc.2022.836257
153. Tang X, Liu F, Liu Z. et al. Bioactivity and safety of B7-H3-targeted chimeric antigen receptor T cells against anaplastic meningioma. Clin Transl Immunology. 2020;9:e1137. doi: 10.1002/cti2.1137
154. Gregorio A, Corrias MV, Castriconi R. et al. Small round blue cell tumours: diagnostic and prognostic usefulness of the expression of B7-H3 surface molecule. Histopathology. 2008;53:73-80 doi: 10.1111/j.1365-2559.2008.03070.x
155. Park JA, Cheung NV. Targets and Antibody Formats for Immunotherapy of Neuroblastoma. J Clin Oncol. 2020;38:1836-48 doi: 10.1200/JCO.19.01410
156. Tan WQ, Chen G, Ye M. et al. Artemether Regulates Chemosensitivity to Doxorubicin via Regulation of B7-H3 in Human Neuroblastoma Cells. Med Sci Monit. 2017;23:4252-9 doi: 10.12659/msm.902068
157. Pulido R, Nunes-Xavier CE. Hopes on immunotherapy targeting B7-H3 in neuroblastoma. Transl Oncol. 2023;27:101580. doi: 10.1016/j.tranon.2022.101580
158. Kendsersky NM, Lindsay J, Kolb EA. et al. The B7-H3-Targeting Antibody-Drug Conjugate m276-SL-PBD Is Potently Effective Against Pediatric Cancer Preclinical Solid Tumor Models. Clin Cancer Res. 2021;27:2938-46 doi: 10.1158/1078-0432.CCR-20-4221
159. Tian M, Cheuk AT, Wei JS. et al. An optimized bicistronic chimeric antigen receptor against GPC2 or CD276 overcomes heterogeneous expression in neuroblastoma. J Clin Invest. 2022 132. doi: 10.1172/JCI155621
160. Kramer K, Pandit-Taskar N, Kushner BH. et al. Phase 1 study of intraventricular (131)I-omburtamab targeting B7H3 (CD276)-expressing CNS malignancies. J Hematol Oncol. 2022;15:165. doi: 10.1186/s13045-022-01383-4
161. Ostrom QT, Cioffi G, Waite K. et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro Oncol. 2021;23:iii1-iii105 doi: 10.1093/neuonc/noab200
162. Wang Y, Deng J, Wang L. et al. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and VISTA in craniopharyngioma. J Immunother Cancer. 2020 8. doi: 10.1136/jitc-2019-000406
Author contact
![]() Corresponding authors: Yu Wang, Tel.: +8615311860318; Email: ywangcn. Bing Xing, Tel.: +8613718696377; Email: xingbingemailcom.
Corresponding authors: Yu Wang, Tel.: +8615311860318; Email: ywangcn. Bing Xing, Tel.: +8613718696377; Email: xingbingemailcom.

 Global reach, higher impact
Global reach, higher impact