10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(12):3804-3815. doi:10.7150/ijbs.85765 This issue Cite
Review
Zonula Occludens Proteins Signaling in Inflammation and Tumorigenesis
1. Center for Pancreatic Cancer Research, The South China University of Technology School of Medicine, Guangzhou, China.
2. The Second Affiliated Hospital and Guangzhou First People's Hospital, South China University of Technology School of Medicine, Guangdong, China.
Received 2023-5-1; Accepted 2023-7-10; Published 2023-7-24
Abstract
Tight junction (TJ) is the barrier of epithelial and endothelial cells to maintain paracellular substrate transport and cell polarity. As one of the TJ cytoplasmic adaptor proteins adjacent to cell membrane, zonula occludens (ZO) proteins are responsible for connecting transmembrane TJ proteins and cytoplasmic cytoskeleton, providing a binding platform for transmembrane TJ proteins to maintain the barrier function. In addition to the basic structural function, ZO proteins play important roles in signal regulation such as cell proliferation and motility, the latter including cell migration, invasion and metastasis, to influence embryonic development, tissue homeostasis, damage repair, inflammation, tumorigenesis, and cancer progression. In this review, we will focus on the signal regulating function of ZO proteins in inflammation and tumorigenesis, and discuss the limitations of previous research and future challenges in ZO protein research.
Keywords: ZO protein, tight junction, inflammation, tumorigenesis, signaling
Introduction
In the course of biological evolution from single cell to multicellular organism, intercellular junctions appear and play crucial roles in various complicated and ordered biological processes. There are three categories of intercellular junctions: occluding junction, anchoring junction and gap junction. Each cell junction serves its function individually or communicate mutually to work together.
Occluding junction consists of tight junction and septate junction, two structure and function conserved junctions with some location and interactive partner variations in different species [1]. Tight junction (TJ) exists in vertebrates including humans and mice and locates at cellular apical side. However, septate junction of invertebrates such as Drosophila melanogaster and Caenorhabditis elegans locates at subapical side below adherens junction [2, 3], one category of anchoring junction, indicating that biological evolution is diverse but conservative.
TJ proteins are divided into two sorts according to their cellular location. One is transmembrane proteins comprising claundins, MARVEL domain proteins, junctional adhesion molecules (JAMs) and blood vessel epicardial substance (BVES), and the other is cytoplasmic adaptor proteins including ZO proteins, cingulin and membrane-associated guanylate kinase inverted (MAGI) proteins. Barrier function, the basic structural function of TJ, is accomplished by the cooperation of transmembrane proteins and adaptor proteins. Besides, TJ proteins participate in signal transduction to influence cell biological behaviors. Here we introduce ZO proteins, one of TJ adaptor proteins, from the respect of their structure and function, especially signal regulating roles, in physiological and pathological processes.
ZO protein structure, basic function and functional redundancy
As cytoplasmic adaptor proteins, ZOs play a basic role in connecting transmembrane TJ proteins and cytoplasmic cytoskeleton to maintain TJ barrier function. However, functional redundancy exists among ZO proteins as a result of their structural similarity. In this part, we briefly introduce ZO protein structure and basic function, and discuss their redundant and non-redundant roles under different environments.
Structure and basic function
ZO proteins, comprising ZO-1, ZO-2 and ZO-3, belong to TJ protein families located in cytoplasm adjacent to cell membrane. They and other adaptor proteins constitute protein plaques to provide stable scaffolds for numerous proteins including transmembrane TJ proteins, maintaining the barrier function: One is the “gate function”, which controls the paracellular transport of ions and solutes; and the other is the “fence function”, which prevents substrate mixture of the apical side and the basolateral side of one cell to keep cell polarity [4]. ZO proteins are also a member of membrane-associated guanylate kinase-like (MAGUK-like) protein families, mainly containing three PDZ (postsynaptic density protein 95, discs large, ZO-1) domains, one SRC homology 3 (SH3) domain and one inactivated Guanylate kinase (GUK) domain from the amino terminus to the carboxyl terminus [1], which are crucial for the structural and the signal regulating functions of ZO proteins.
ZO proteins are indispensable for cell membrane localization and function of transmembrane TJ proteins. Although the ZO-1 knockout mouse mammary epithelial cell line EpH4 can also form integral TJ, the formation speed slows down [5]. A similar phenomenon of TJ formation delay has been observed in the ZO-1 knockdown canine kidney epithelial cell line MDCK [6]. Further research found that a large number of TJ proteins lost their membrane localization after simultaneously inhibiting ZO-1 and ZO-2 expressions [7, 8], which elicited enhanced paracellular permeability and therefore deregulated gate function [6, 8, 9]. In the ZO-1 knockout MDCK II cell line, a monoclonal cell line of MDCK cells, subtle ZO-1 overexpression rescued deregulated TJ membrane localization, indicating that low level of ZO-1 is enough to maintain TJ formation and function [10]. Although previous studies have proved that TJ is closely correlated with cell polarity [11, 12] and deficiency of all three ZO proteins triggered abnormal gate function in epithelial cells, however, cell polarity was not changed as expected [7, 8]. As a consequence, which molecules are essential for cell polarity formation and maintenance remains to be further studied.
Functional redundancy and non-redundancy
The function among ZO proteins exists redundancy due to their structural similarity. For example, in ZO-1 and ZO-2 simultaneously inhibited EpH4 cells, overexpression of either ZO-1 or ZO-2 rescued deregulated TJ membrane localization [7]. In addition, Beutel et al. explained the functional redundancy of ZO-1 and ZO-2 from the aspect of phase separation [9]. They found that most reported ZO binding proteins such as TJ proteins, cytoskeleton proteins and transcription factors enriched with ZO proteins through phase separation, and subsequent composition analysis of binding proteins suggested high similarity between ZO-1 and ZO-2, reminding their functional redundancy.
In contrast, lots of evidence proves the existence of non-redundant role of ZO proteins. In vitro assays reported that overexpression of ZO-2 could not rescue the TJ formation speed of the ZO-1 knockout EpH4 cells [5]. Moreover, inhibition of ZO-1 rather than ZO-2 in MDCK cells promoted the paracellular permeability of larger molecules and changed cell morphology [6]. In vivo assays provide more powerful support of the non-redundant roles of ZO proteins: First of all, the tissue distribution of ZO proteins is specific. Even if ZO-1 and ZO-2 are comprehensively distributed on the apical side of lateral membrane of epithelial and endothelial cells, ZO-3 does not exist in endothelial cells [13-15]. In addition, the special structure of heart “intercalated disc” expresses only ZO-1 rather than ZO-2 or ZO-3. ZO-1 conditional knockout in mouse heart induced loss of gap junction protein connexin 40 (Cx40), leading to atrioventricular block [16]. In testis, liver and inner ear ZO-2 gene mutation alone causes severe organ dysfunction [17]. Moreover, either ZO-1 or ZO-2 individually knockout resulted in mouse abnormal embryonic development and embryonic lethality [18, 19]. Though the embryonic development and tissue morphology were not affected in ZO-3 knockout mouse, however, ZO-3 knockout zebrafish induced improved embryonic sensitivity to osmotic pressure and epithelial permeability, contributing to abnormal embryonic development and embryonic lethality [19-21].
It should be noted that the research conclusions above are all under physiological conditions without external stimulus. Recent research has shown that ZO proteins exert extra non-redundant roles under stress, which cannot be detected in ordinary environment. For instance, the arrangement of cytoskeleton actin and microvilli of ZO-1 knockout mouse intestine only changed slightly [22]. However, under chemical or immune injuries, the mucosa of ZO-1 knockout intestine was disrupted severely. Both in vitro and in vivo assays demonstrated that loss of ZO-1 restrained intestinal cell proliferation and promoted cell apoptosis. Mechanistically, ZO-1 bound centrosomes and induced correct spindle split direction in proliferating cells. Another study found ZO-2 knockout mouse liver appeared mild dysfunction and the barrier function of bile duct was not influenced [23]. But under the stimulus of bile acid, the ZO-2 knockout liver was injured severely, which is possibly caused by the bile acid transport and detoxification inability of the liver. In addition, the research mentioned above that ZO-3 had a crucial role in zebrafish rather than mouse embryonic development can be explained by different stresses the two species face: Mammals living on land have only around 20 claudin genes, while teleost fish living in water with more paracellular barrier stress has around 60 claudin genes [24]. In summary, ZO proteins have common redundant roles and distinct specific non-redundant roles.
Signal regulating function of ZO proteins
In addition to the basic function of ZO proteins, they also bind various signal regulating proteins including transcription factors and protein kinases to participate in cellular signal transduction. Exploration of ZO protein signal regulating function will complement their roles in physiological and pathological processes.
Phenomenon of nucleus-membrane shuttling
Parts of MAGUK-like proteins including all three ZO proteins have several both nuclear localization signals and nuclear export signals, hence theoretically they have the ability of moving into and out of nucleus [25]. The cellular localization alteration of ZO proteins probably affects the localization of their binding partners and subsequently influences cell signal transduction. To study the nucleus-membrane shuttling of ZO proteins facilitates us to understand the mechanisms of their signal regulating function (Table 1). ZO-1 is the first ZO protein to be reported owning the ability of nucleus-membrane shuttling [26]. Gottardi et al. found that cell density or confluency was crucial for ZO-1 cellular localization in epithelial cells. ZO-1 located in the nucleus of subconfluent cells, while it was transported out of nucleus to membrane with the rise of cell density. Afterwards, Islas et al. found similar cellular localization behavior of ZO-2 [25]. One difference between ZO-1 and ZO-2 was that ZO-2 nuclear localization could also be observed in some confluent cells, illustrating possible functional difference between them. Nevertheless, to our knowledge, any imaging evidence of ZO-3 nucleus-membrane shuttling has not been observed, even ZO-3 was detected in nuclear proteins [27]. Moreover, Balda et al. indicated that they could not repeat the ZO-1 nuclear localization phenomenon though applying the same cell line and protein detection methods of previous studies [28]. Actually, some studies have demonstrated that in confluent cells, the nuclear ZO protein levels are far lower than those of total ZO proteins [25, 26], which may cause the false negativity of ZO protein nuclear signal because it may be covered by membrane signal. As a result, more advanced technological approaches are necessary for the studying of ZO protein nucleus-membrane shuttling.
Regulation of cell proliferation and motility by ZO proteins
| 1. Cell proliferation regulation | |||||||
|---|---|---|---|---|---|---|---|
| ZO proteins | Protein source | Cell localization | Binding proteins | Effector proteins | Effect | Cell identity | Reference |
| ZO-1 | Exogenous | Membrane | ZONAB | cyclin D1, PCNA | Inhibition | Epithelial | [28-30] |
| ZO-2 | Exogenous | Nucleus | C-Myc | cyclin D1, PCNA | Inhibition | Epithelial | [31] |
| Exogenous | Nucleus | GSK3β | cyclin D1 | Inhibition | Epithelial | [32, 33] | |
| Exogenous | Membrane, nucleus | AP-1 | - | Inhibition | Epithelial | [34, 35] | |
| ZO-1 or/and ZO-2 | Endogenous | Membrane | - | - | None | Epithelial | [5, 7, 42] |
| ZO-3 | Endogenous | Membrane | - | cyclin D1 | Promotion | Epithelial | [36] |
| 2. Cell motility regulation | |||||||
| ZO proteins | Protein source | Cell localization | Binding proteins | Effector proteins | Effect | Cell identity | Reference |
| ZO-1 | Endogenous | Membrane | α, β, γ-catenins | - | Inhibition | Epithelial | [44, 45] |
| Endogenous | Membrane | N-cadherin | - | Promotion | Mesenchymal | [46] | |
| Endogenous | Membrane | Integrin α5β1 | - | Promotion | Mesenchymal | [47] | |
| Endogenous | Membrane | PLP2 | RAC1 | Promotion | Mesenchymal | [48] | |
| Exogenous | Cytoplasm | - | β-catenin signaling | Promotion | Epithelial | [51] | |
| Endogenous/Exogenous | Cytoplasm | - | β-catenin signaling, MMP14 | Promotion | Mesenchymal | [52] | |
| Endogenous/Exogenous | Cytoplasm | - | IL-8 | Promotion | Mesenchymal/ Epithelial | [54] | |
| ZO-2 | Endogenous | Cytoplasm | - | MMP14 | Inhibition | Mesenchymal | [53] |
Cell proliferation regulation
ZO proteins influence cell proliferation through nucleus-membrane shuttling and regulating cell cycle related proteins such as cyclin D1 and proliferating cell nuclear antigen (PCNA). ZO-1 mainly inhibited cell proliferation by binding transcription factor ZO-1-associated nucleic acid-binding protein (ZONAB) with its SH3 domain [28]. ZONAB promoted the transcriptions of cell cycle related proteins cyclin D1 and PCNA by binding directly their promoters [28, 29]. In addition, ZONAB accelerated cell cycle progression by combining with cyclin dependent kinase 4 (CDK4) and cyclin D1 facilitating them to get into nucleus [30]. Consequently, when cell density is high, membranous ZO-1 sequester ZONAB in cytoplasm suppressing its nuclear localization, which inhibits cell proliferation. In contrast, when cell density is low, little membranous ZO-1 cannot sequester ZONAB effectively and cell cycle progresses normally. Similar to ZO-1, ZO-2 also mainly inhibits cell proliferation. González-Mariscal indicated that exogenous ZO-2 inhibited cell proliferation through collaborating with C-Myc and histone deacetylase 1 (HDAC1) in nucleus to form transcription regulatory complex, which bound the cyclin D1 promoter to restrain its transcription [31]. Moreover, they found that ZO-2 regulated cell cycle from the posttranslational level. They observed the phenomenon that ZO-2 got into nucleus in the late G1 phase and got out of nucleus in the M phase, while cyclin D1 got into nucleus in the early G1 phase and got out of nucleus in the late S phase. Exogenous ZO-2 caused the G1/S phase delay by stabilizing the protein kinase glycogen synthase kinase 3 beta (GSK3β), which phosphorylated cyclin D1 on threonine-286 (Thr-286) to promote its degradation [32, 33]. They also discovered that ZO-2 combined with transcription factor activator protein 1 (AP-1), which has been reported to own extensive cell signal regulating functions on cell proliferation, transformation and death, both on membrane and in nucleus [34, 35]. Therefore, membranous ZO-2 may regulate cell proliferation through sequestering AP-1 in cytoplasm. There is little evidence and controversies suggesting the role of ZO-3 in cell proliferation. Surprisingly, cyclin D1 was observed to locate near cell membrane in dividing cells and bound with three ZO proteins in human colon epithelial cells [36]. Inhibition of ZO-3, rather than ZO-2, prevented the cyclin D1 membrane localization leading to cell cycle arrest, and accelerated cyclin D1 degradation in cells treated with protein synthesis inhibitor cycloheximide, which reflected the ZO-3 functional specificity. However, the total cyclin D1 protein level kept constant in cells not treated with cycloheximide, indicating that ZO-3 may influence only a fraction of cyclin D1 near cell membrane in diving cells rather than the entire cyclin D1 protein. Besides, the protein levels and the cell localization of ZO-1 and ZO-2 did not change after ZO-3 inhibition, excluding the influence of ZO-1 and ZO-2 on cyclin D1. On the contrary, inhibition of ZO-3 promoted cell proliferation in MDCK cells [37], which may be caused by the different characteristics of the two cell lines and different experimental methods. In this study, only one stable ZO-3 knockdown monoclonal cell line was selected to be utilized for experiments. Whereas, different monoclonal cell lines are heterogeneous and possibly change their features in the process of long-term culture. Hence it is hard to say that the phenotypes observed from one monoclonal cell line are actually induced by ZO-3.
Except for cell density, the expressions and the cell localization of ZO proteins also change under external stimulus. For example, the nuclear ZO-2 protein increased without increase of the total ZO-2 protein in the condition of chemical stimulus or heat shock, proving ZO-2 nuclear import [38]. Besides, in the cell wound scratch assay, the expressions of ZO-1, ZO-2 and ZO-3 decreased accompanied with ZO-1 and ZO-2 nuclear import, and the expressions of cyclin D1 and PCNA improved in the cells near the wound [25-27, 36]. Because the ZO protein nuclear localization is always accompanied with stronger cell proliferation capacity, some researchers argue that in subconfluent cells nuclear ZO proteins induce cell proliferation, while in confluent cells membranous ZO proteins suppress cell proliferation [25, 36]. However, the theory cannot explain all phenomena. For instance, in some highly differentiated cells which have no or weak proliferation capacity, such as intestinal epithelial cells of the top side of villi and renal tubular cells, large amounts of nuclear staining of ZO proteins can be seen [26, 39]. Moreover, the studies mentioned above proved that nuclear ZO-2 negatively regulated the cyclin D1 expression suppressing cell proliferation [31, 32]. Another study indicated that actually the nuclear ZO proteins increased with increased cell density [27], although their nuclear imaging was hard to observe. All above evidence opposes the theory that nuclear ZO proteins induce cell proliferation. In summary, it seems that all cells with ZO protein nuclear localization in different conditions, including low cell density, external stimulus or highly differentiated stage, undergo more or less survival stress. Therefore, it is supposed that the nuclear localization behavior of ZO proteins is possibly correlated with cell survival.
Notably, the studies of the cell proliferation regulation of ZO proteins above are most based on exogenous ZO proteins, and some exogenous ZO proteins contain only partial domains but not full-length proteins. There may be big differences between supraphysiological level of proteins or protein fragments and physiological level of integral proteins. A study has shown that ZONAB could only bind the ZO-1 protein fragment containing SH3 domain rather than full-length ZO-1 in vitro, and all three ZO proteins could not be found in the complexes that ZONAB bound [37]. Nevertheless, another study indicated that expect ZO-3, both full-length ZO-1 and ZO-2 could bind ZONAB in vitro [9]. The difference might be caused by the internal interaction of ZO proteins, which formed closed conformation to prevent the combination with other proteins [40, 41] and specific protein modifications or something else are possibly needed to expose their crucial binding domains. Furthermore, the cell proliferation regulation function of endogenous ZO proteins is not obvious as expected in physiological condition. In many epithelial cell lines, inhibition of neither ZO-1 nor ZO-2 or the combination of ZO-1 and ZO-2 with distinct inhibition approaches did not influence cell proliferation [5, 7, 42]. However, in another study the combinational inhibition of ZO-1 and ZO-2 suppressed cell proliferation, though it did not work to inhibit ZO-1 or ZO-2 individually [37]. The study indicated that ZONAB was not transported into nucleus after ZO-1 and ZO-2 inhibition but diffused or got lost in cytoplasm. These contradictory phenomena induced by endogenous or exogenous ZO proteins bring challenges to study the cell proliferation regulating function of ZO proteins. It should be considered that endogenous proteins of one family exist function redundancy because of their structural similarity. Besides, we should be cautious to face the conclusions from different research in consideration of the advantages and limitations of different experimental techniques.
ZO protein function on epithelial-mesenchymal transition and cell motility regulation
Epithelial-mesenchymal transition (EMT) is essential for the progression of embryonic development. EMT is reactivated and plays crucial roles in damage repair, tissue fibrosis and cancer progression. In the course of EMT, epithelial cells lose cell junctions and cell polarity and rearrange their cytoskeleton leading to cell morphology alteration [43]. Meanwhile the cells gain better motile ability through reprogrammed gene expression and signal transduction. Epithelium derived cancer cells with epithelial cell features can be transformed to cells with mesenchymal cell features, including enhanced abilities of migration, invasion and metastasis. Cancer cell lines derived from tumors of the same tissue, such as primary tumors or metastatic tumors, are usually various and heterogeneous with different motile abilities. Therefore, as compared with cell lines derived from normal tissues, cancer cell lines may be more suitable for studying cell motility and its regulating mechanisms.
Regulation of epithelial cells
In normal epithelial cells or cancer cells, adherens junction is essential for TJ formation, subsequently influencing cell motility. Rajasekaran et al. found that membrane localization of ZO-1 depended on E-cadherin-mediated adherens junction formation [44]. In virus transfected MDCK cells, the E-cadherin expression was inhibited and membranous ZO-1 localization was lost, while the subsequent overexpression of E-cadherin rescued membranous ZO-1 localization. Mechanistically, catenins of adherens junction, including α-catenin, β-catenin and γ-catenin, combined with ZO-1 and facilitated its transport to membrane. Therefore, loss of adherens junction proteins caused by EMT may be one of the main reasons for abnormal membranous localization of tight junction proteins. The similar result was also observed in cancer cells: In the E-cadherin positive primary breast cancer cell line MCF-7 rather than the E-cadherin negative invasive breast cancer cell line MDA-MB-231, overexpression of insulin-like growth factor I receptor induced ZO-1 expression and increased cell aggregation, and the following ZO-1 inhibition prevented the course, demonstrating that ZO-1 is crucial for cell aggregation which is dependent on E-cadherin-mediated adherens junction formation [45].
Regulation of mesenchymal cells
In addition to motility of epithelial cells, ZO proteins also regulate motility of mesenchymal cells. Because E-cadherin gets lost in mesenchymal cells, signal regulating function of ZO-1 is independent of E-cadherin but depends on other junctional proteins, such as mesenchymal cells specific N-cadherin [46] or rearranged integrins [47]. In melanoma cells ZO-1 bound N-cadherin to enhance not only adherence and invasive ability of cancer cells, but also adherence ability between cancer cells and fibroblasts [46]. In subconfluent or scratched wounded cells of invasive lung cancer or breast cancer, ZO-1 was observed to co-localize with integrin α5β1. After ZO-1 phosphorylation by protein kinase C epsilon (PKCε), ZO-1 combining with integrin α5β1 was transported to the leading edge of cell membrane to guide cell migration, contributing to stronger cell invasive ability [47]. Notably, the ZO-1 leading edge localization was just found in confluent cells, suggesting localization conservation of ZO-1 according to cell density. The similar leading edge localization of ZO-1 occurred in invasive colorectal cancer cells undergoing collective cell migration [48], a feature of invasive or metastatic cancer cells, which means that a group of mutually connected cells migrates to a direction as one unit without losing their cell junction [49]. The study found that in invasive colorectal cancer cells, a colonic epithelium-enriched transmembrane protein PLP2 recruited ZO-1 to the leading edge of cell membrane and induced cytoskeleton actin remodeling to initiate collective cell migration.
Cell motility regulation through the β-catenin signaling pathway
The β-catenin signaling pathway plays indispensable roles in various biological processes, including EMT and cell motility [43, 50]. Some research has shown that ZO proteins influence cell motility through regulating the β-catenin signaling pathway. Reichert et al. illustrated that overexpression of the ZO-1 protein fragment containing only PDZ domains diffused in cytoplasm but not be transported to membrane [51]. Mechanistically, the ZO-1 protein fragment promoted EMT and carcinogenesis in vivo by activating the β-catenin signaling pathway. Because the β-catenin signaling pathway is always dysregulated in cancer cells, ZO-1 protein mutants may induce cancer initiation and progression. Similar cytoplasmic localization of ZO-1 could be also found in invasive breast cancer cells, while ZO-1 located on cell membrane in primary breast cancer cells [52]. The researchers found that cytoplasmic ZO-1 improved cancer cell invasive ability through enhancing the expression of matrix metalloproteinase 14 (MMP14/MT1-MMP), which was associated with activated the β-catenin signaling pathway. They also indicated that both the ZO-1 protein fragment containing only PDZ domains and integral full-length ZO-1 protein could activate the β-catenin signaling pathway in invasive breast cancer cells, suggesting the functional differences of ZO-1 between normal epithelial cells and cancer cells. Afterwards they also found that ZO-2 had the same cytoplasmic localization in invasive lung cancer cells [53]. However, ZO-2 inhibited the MMP14 expression and suppressed ability of cell invasion, suggesting the non-redundant functions of ZO-1 and ZO-2 in cancer cells. In addition to MMP14, IL-8 was also highly expressed in invasive breast cancer cells as compared with primary breast cancer cells [54]. In primary breast cancer cells, overexpression of ZO-1 could enhance the transcription of IL-8 and ability of cell invasion, however, the course was independent of β-catenin signaling pathway, manifesting different signal regulating mechanisms between primary and invasive cancer cells with different ability of invasion.
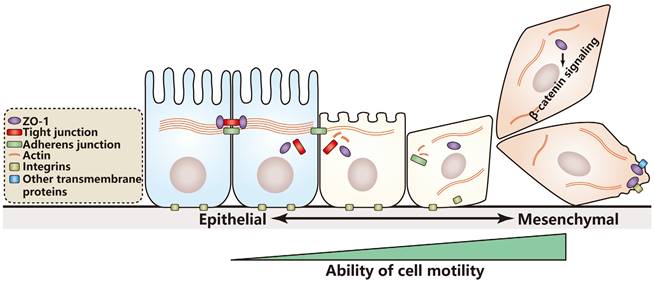
According to the studies above, a hypothesis is proposed: The influence of ZO-1 on cell motility depends on cell identity. In cells with epithelial characteristics, ZO-1 prefers to inhibit cell motility; in contrast, in cells with mesenchymal characteristics, ZO-1 prefers to promote cell motility through binding transmembrane proteins or affecting cell signaling (Figure 1). If the hypothesis is true, it will provide reference value for clinical therapy and prognosis estimation of patients with different cancer stages. However, nearly all studies on the relationship of cell motility and ZO proteins just focus on ZO-1, whether ZO-2 or ZO-3 regulates cell motility remains further research.
Model for cell motility regulation by ZO-1 during EMT. In the course of EMT, epithelial cells lose cell junctions and cell polarity and rearrange their cytoskeleton to gain better motile ability. A hypothesis is proposed that in cells with epithelial characteristics ZO-1 prefers to inhibit cell motility, while in cells with mesenchymal characteristics, ZO-1 prefers to promote cell motility through binding transmembrane proteins or activating the β-catenin signaling.
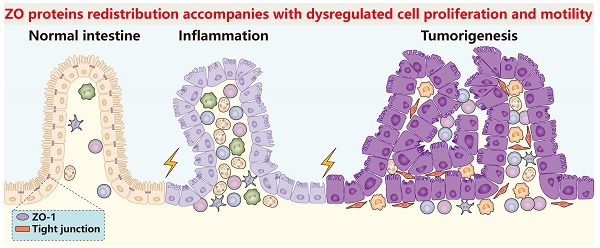
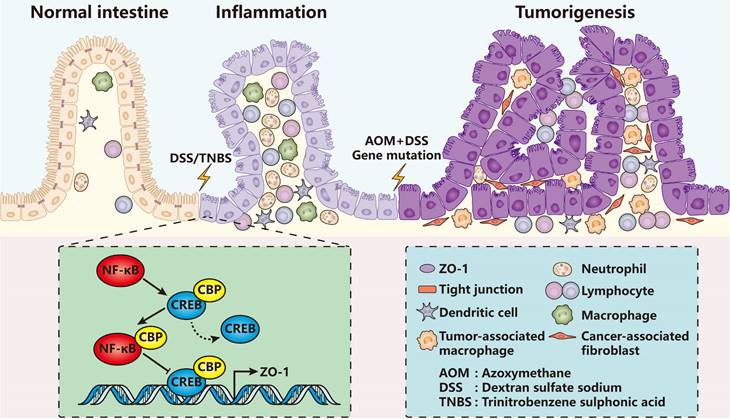
ZO-1 signaling in intestinal inflammation and tumorigenesis. In DSS/TNBS-induced intestinal inflammation or AOM/DSS-induced intestinal tumorigenesis, ZO-1 gets lost accompanied with severe infiltration of various immune cells. Mechanistically, inflammatory activated NF-κB replaces CREB of the CREB-CBP protein complex, which has been proved to promote ZO-1 transcription, forming the NF-κB-CBP complex to repress ZO-1 expression. In turn, loss of ZO-1 aggravates inflammatory damage and accelerates the pathological processes.
ZO proteins in pathological processes
In various pathological processes such as inflammation, tumorigenesis, cancer progression and other diseases, cell identities or states change under external of internal stimuli, usually accompanied with alterations of quantity, category or binding partners of cell junction proteins. Understanding the change rules of ZO proteins in these processes possibly helps us better diagnose and treat patients.
ZO proteins in inflammation
Up to now, it has been repeatedly proved that ZO proteins diffuse or get lost in nearly all types of inflammations comprising colitis [55-62], pancreatitis [63, 64], acute radiation cystitis [65], atopic asthma [66], pleurisy [67], allergic conjunctivitis [68] and so on. For example, in dextran sulfate sodium (DSS) or trinitrobenzene sulphonic acid (TNBS) induced mammary colitis models (Figure 2), the ZO-1 expression level of colon epithelia decreased quickly even on the first day [55, 57, 58]. Similar results were also observed in pancreatic ductal cells in caerulein-induced mammary pancreatitis [63, 64].
ZO proteins are mainly regulated by nuclear factor kappa B (NF-κB) signaling pathway [59, 62, 69] and cytokines including tumor necrosis factor-alpha (TNF-α) [60, 67], interleukin 1 beta (IL-1β) [60], IL-6 [61], IL-8 [70], IL-9 [68], IL-22 [71] and IL-33 [68]. Zhang et al. found that in patients with inflammatory bowel disease (IBD), the expression level of monocarboxylate transporter 4 (MCT4) increased significantly [61]. Overexpression of MCT4 in vitro disrupted intestinal barrier function via inhibiting ZO-1 expression while promoting IL-6 expression. Mechanistically, MCT4 facilitated the formation of NF-κB-CBP (cAMP-response element binding protein (CREB) binding protein) transcription factor complex, therefore preventing the binding of CREB and CBP, the combination of which has been demonstrated to play a central role in transactivation of ZO-1 [72-74]. Another research showed that the NF-κB activity was influenced by a transcription factor forkhead box o4 (Foxo4) in vivo, which reduced in the model of TNBS-induced colitis or in IBD patients [59]. Foxo4 bound with NF-κB and inhibited its transcriptional activity for lots of cytokines such as TNF-α, interferon-gamma (IFN-γ), IL-1β and IL-6. Foxo4 knockout mice resulted in increased intestinal epithelial permeability and sensitivity to TNBS-induced inflammation, and down-regulation of ZO-1 and claudin-1.
Some other factors such as microbes and neurotrophic factors can also regulate the ZO protein expression. Prebiotic modulation in obese and diabetic mice under the stimulus of carbohydrate promoted intestinal epithelial secretion of glucagon like peptide-2 (GLP-2), a proglucagon-derived peptide, which protected the intestinal barrier and prevented the disruption of TJ proteins including ZO-1 and occluding [75]. In addition, glial-derived neurotrophic factor (GDNF) secreted by intestinal glial cells could ameliorate DSS-induced colitis through increasing the ZO-1 expression and inhibiting cell apoptosis and cytokines such as TNF-α and IL-1β [60]. Therefore, we can conclude that ZO proteins are closely associated with inflammation. ZO proteins and proinflammatory factors probably influence reciprocally.
ZO proteins in tumorigenesis and cancer progression
It has been shown that oncogenic viruses including adenovirus and human papillomavirus can directly bind the PDZ domains of ZO proteins, therefore inducing protein degradation [76, 77]. Besides, ZO proteins have highly homologous structures with another MAGUK-like protein Dlg, which has been shown acting as tumor suppressor [78], so ZO proteins are also believed to act as tumor suppressors in cancer.
Although the research on the relationship of ZO proteins and tumorigenesis is very limited, the conclusion is surprisingly consistent that ZO proteins get lost in the course of tumorigenesis [58, 79-84]. The mostly used model suggesting their relationship is colitis-associated colorectal cancer, which can be induced by the combination of DSS and azoxymethane (AOM), and the expression level of intestinal epithelial ZO-1 reduces as compared with that of normal colon epithelium [58, 82-84]. Liu et al. observed that in the mice with adenomatous polyposis coli (Apc) gene mutation which can develop intestinal tumors spontaneously, intestinal mucosa was damaged severely accompanied with increased tumor numbers and the loss of ZO-1 under the stimulation by deoxycholic acid [81]. Furthermore, similar results were found in the course of bladder epithelial tumorigenesis of the phosphatase and tensin homolog (Pten) and serine/threonine kinase 11 (Stk11) double gene knockout mice [80]. Another study showed that ZO-1 located at cell membrane in normal oral mucosa epithelial cells. However, in the cells of dysplasia or tumorigenesis, the ZO-1 expression increased and ZO-1 was transported into nucleus [85]. The immune staining indicated that the proportion of ZO-1 positive cells was far more than the Ki67 positive proliferating cells, suggesting that the ZO-1 nuclear localization is not completely correlated with cell proliferation regulation again. However, the staining pattern specificity of ZO-1 remains to be testified because to our knowledge the extensive ZO-1 nuclear staining in pathological tissues has not been observed in any previous studies. The author saw the similar ZO-1 shuttling as previous studies in oral squamous carcinoma cell lines, but the difference was that ZO-1 diffused in cytoplasm rather than was transported to membrane in confluent cells [85].
ZO proteins influence cancer progression under complicated and disordered background of cancer cells. Many studies indicated loss of ZO proteins during cancer progression was always correlated with poorer prognosis [53, 86-91]. However, on the contrary, in some cases high ZO protein levels accelerated cancer progression [46-48], suggesting that ZO proteins probably play different roles in different cancers or different stages of cancer progression. For instance, ZO-1 inhibition restrained oral squamous carcinoma cell proliferation and invasion [85], while improved cell proliferation and invasion capacity of endometrial cancer [92], liver cancer [93] and pancreatic cancer [94]. Mechanistically, in pancreatic cancer, zinc transporter protein 4 (ZIP4) was highly expressed and inhibited ZO-1 and claudin-1 expressions by regulating a mesenchymal cell marker zinc finger E-box binding homeobox 1 (ZEB1), which bound directly to the promoters of ZO-1 and claudin-1 and repressed their transcriptions. Inhibition of ZIP4 elevated the expression levels of ZO-1 and claudin-1 and the phosphorylation levels of focal adhesion kinase (FAK) and Paxillin, two molecules related with cell adhesion and motility, while subsequent silence of ZO-1 or claudin-1 rescued the phosphorylation levels and the phenomena of attenuated cell proliferation and invasion. The controversies of cancer cell biological regulation by ZO proteins maybe explained through complicated and heterogeneous signal regulating backgrounds of different cancer cells.
ZO proteins in other diseases
Until now, it has been ZO-2 rather than ZO-1 or ZO-3 that is closely related with other human diseases, especially for familial genetic diseases [17]. For instance, familial intrahepatic cholestasis is often observed in children whose ZO-2 gene get lost due to truncating mutations [95-98]. Bile of the patients does not flow through the intrahepatic ducts of the liver and some of them will develop chronic cholestatic hepatitis with cirrhosis and hepatocellular carcinoma in the late stage of the disease. Another familial disease, familial hypercholanemia, is a rare disease characterized by elevated serum bile acid concentration, pruritus, and fat malabsorption. In several Amish patients, the missense mutation V48A in the PDZ1 domain of ZO-2 reduced its stability and binding ability to claudins. In some individuals, the mutation of both ZO-2 and bile acid-CoA:amino acid N-acyltransferase (BAAT) could result in more severe structural damage and affected bile acid transport and circulation [99]. In addition, the missense mutation of ZO-2 can also lead to autosomal dominant non-syndromic hearing loss [100, 101]. Xu et al. found that the adult male ZO-2 knockout chimera mice showed reduced fertility and pathological changes in the testis whose blood-testis barrier was disrupted [99], while whether ZO-2 plays a similar role in humans remains unclear.
Perspectives and challenges
ZO proteins play crucial roles in embryonic development, tissue homeostasis, damage repair, inflammation, tumorigenesis, cancer progression and other diseases. The structural function provides guarantee for barrier function of TJ, and the signal regulating function influences cell proliferation and motility through taking part in signal transduction. Nevertheless, what we have mastered is mainly the structural function and studies on the signal regulating function are limited. Therefore, comprehensively studying the signal regulating function of ZO proteins will help us better understand various biological processes. But before this, we have to overcome the following challenges.
The first challenge is that the functional redundancy of ZO proteins brings us difficulty to study one ZO protein individually. Previous research has reminded us that generally there are no obvious phenotypes by interfering only one ZO protein in physiological condition. In addition, the function of exogenous ZO proteins cannot represent the actual function of endogenous ZO proteins due to their differences of protein structures, expression levels and cell localization, severely obstructing our studies on the function of ZO proteins. Fortunately, the functional redundancy seems not obvious under stress, perhaps because each ZO protein participates in more complicated signal regulating processes and they cannot substitute each other. Generally speaking, more advanced research techniques and more reasonable experimental conditions are necessary.
The second challenge is that it is hard to study the function in specific place of ZO proteins because of their highly dynamic subcellular localization. From in vitro models we can see that not only cell density but also external stimulus can influence the localization of ZO proteins. Because the localization alteration makes it different of binding partners and protein function, it is possible to get various results in different experimental conditions. Therefore, in vitro model is a double-edged sword: As compared with in vivo model, in vitro model can easily reflect the localization alteration of ZO proteins in different conditions, providing more opportunities for us to study the position specific function. Whereas, the dynamic localization makes it difficult to get stable and true results. Only establishing more reasonable in vitro models is able to give play to the unique advantages.
The last challenge is that comprehensive distribution of ZO proteins makes them difficult to be ideal clinical drug targets. Therefore, it is imperative to figure out their functions in different tissues and their upstream and downstream regulatory modes. Otherwise, development of drug delivery approaches specific to pathological areas is requisite in the future to attenuate the side effects of drugs targeting ZO proteins. However, the amount of drug side effects targeting ZO proteins may be less than that as expected because of their functional redundancy. For example, ZO-3 is not expressed in endothelial cells and its protein function in physiological condition is very limited in mammals, it may be a more ideal therapeutic target as compared with ZO-1 and ZO-2 if ZO-3 plays crucial roles in diseases. In addition, just because of comprehensive distribution of ZO proteins and previous definite evidence of their loss under inflammation or in the course of tumorigenesis, they are expected to be ideal early clinical diagnosis markers of numerous pathogenetic processes of different organs.
Abbreviations
TJ: tight junction; ZO: zonula occludens; JAMs: junctional adhesion molecules; MAGI: membrane-associated guanylate kinase inverted; MAGUK-like: membrane-associated guanylate kinase-like; PDZ: postsynaptic density protein 95, discs large, ZO-1; SH3: SRC homology 3; GUK: guanylate kinase; Cx40: connexin 40; PCNA: proliferating cell nuclear antigen; ZONAB: ZO-1-associated nucleic acid-binding protein; CDK4: cyclin dependent kinase 4; HDAC1: histone deacetylase 1; GSK3β: glycogen synthase kinase 3 beta; Thr-286: threonine-286; AP-1: activator protein 1; EMT: epithelial-mesenchymal transition; PKCε: protein kinase C epsilon; MMP14/MT1-MMP: matrix metalloproteinase 14; DSS: dextran sulfate sodium; TNBS: trinitrobenzene sulphonic acid; NF-κB: nuclear factor kappa B; TNF-α: tumor necrosis factor-alpha; IL: interleukin; IBD: inflammatory bowel disease; MCT4: monocarboxylate transporter 4; CREB: cAMP-response element binding protein; CBP: cAMP-response element binding protein binding protein; Foxo4: forkhead box o4; IFN-γ: interferon-gamma; GLP-2: glucagon like peptide-2; GDNF: glial-derived neurotrophic factor; AOM: azoxymethane; Apc: adenomatous polyposis coli; Pten: phosphatase and tensin homolog; Skt11: serine/threonine kinase 11; ZIP4: zinc transporter protein 4; ZEB1: zinc finger E-box binding homeobox 1; FAK: focal adhesion kinase; BAAT: bile acid-CoA:amino acid N-acyltransferase.
Acknowledgements
We are grateful to the members of our laboratory for their critical reading of the manuscript and constructive thoughts. The work is partly supported by National Natural Science Foundation of China (#82072632), Guangzhou Municipality Bureau of Science and Technology, Guangzhou, China (#202102010033) and Natural Science Foundation of Guangdong Province, China (#2022A1515012585).
Author contributions
S.Y. conceptualized, designed, and researched the data for the review article, and wrote the first draft. J.H. and K.X. contributed to manuscript preparation, editing, revision before its submission. All authors approved the submitted final version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564-80
2. Izumi Y, Furuse M. Molecular organization and function of invertebrate occluding junctions. Semin Cell Dev Biol. 2014;36:186-93
3. Simske JS, Hardin J. Claudin family proteins in Caenorhabditis elegans. Methods Mol Biol. 2011;762:147-69
4. Otani T, Furuse M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020;30:805-17
5. Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M. et al. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem. 2004;279:44785-94
6. Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930-40
7. Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M. et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741-54
8. Fanning AS, Van Itallie CM, Anderson JM. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol Biol Cell. 2012;23:577-90
9. Beutel O, Maraspini R, Pombo-Garcia K, Martin-Lemaitre C, Honigmann A. Phase Separation of Zonula Occludens Proteins Drives Formation of Tight Junctions. Cell. 2019;179:923-36 e11
10. Tokuda S, Higashi T, Furuse M. ZO-1 knockout by TALEN-mediated gene targeting in MDCK cells: involvement of ZO-1 in the regulation of cytoskeleton and cell shape. PLoS One. 2014;9:e104994
11. Hoi Sang U, Saier MH Jr, Ellisman MH. Tight junction formation is closely linked to the polar redistribution of intramembranous particles in aggregating MDCK epithelia. Exp Cell Res. 1979;122:384-91
12. Dragsten PR, Blumenthal R, Handler JS. Membrane asymmetry in epithelia: is the tight junction a barrier to diffusion in the plasma membrane? Nature. 1981;294:718-22
13. Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491-502
14. Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 1994;124:949-61
15. Inoko A, Itoh M, Tamura A, Matsuda M, Furuse M, Tsukita S. Expression and distribution of ZO-3, a tight junction MAGUK protein, in mouse tissues. Genes Cells. 2003;8:837-45
16. Dai W, Nadadur RD, Brennan JA, Smith HL, Shen KM, Gadek M. et al. ZO-1 Regulates Intercalated Disc Composition and Atrioventricular Node Conduction. Circ Res. 2020;127:e28-e43
17. Gonzalez-Mariscal L, Gallego-Gutierrez H, Gonzalez-Gonzalez L, Hernandez-Guzman C. ZO-2 Is a Master Regulator of Gene Expression, Cell Proliferation, Cytoarchitecture, and Cell Size. Int J Mol Sci. 2019 20
18. Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M. et al. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell. 2008;19:2465-75
19. Xu J, Kausalya PJ, Phua DC, Ali SM, Hossain Z, Hunziker W. Early embryonic lethality of mice lacking ZO-2, but Not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol Cell Biol. 2008;28:1669-78
20. Adachi M, Inoko A, Hata M, Furuse K, Umeda K, Itoh M. et al. Normal establishment of epithelial tight junctions in mice and cultured cells lacking expression of ZO-3, a tight-junction MAGUK protein. Mol Cell Biol. 2006;26:9003-15
21. Kiener TK, Selptsova-Friedrich I, Hunziker W. Tjp3/zo-3 is critical for epidermal barrier function in zebrafish embryos. Dev Biol. 2008;316:36-49
22. Kuo WT, Zuo L, Odenwald MA, Madha S, Singh G, Gurniak CB. et al. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology. 2021;161:1924-39
23. Xu J, Kausalya PJ, Van Hul N, Caldez MJ, Xu S, Ong AGM. et al. Protective Functions of ZO-2/Tjp2 Expressed in Hepatocytes and Cholangiocytes Against Liver Injury and Cholestasis. Gastroenterology. 2021;160:2103-18
24. Loh YH, Christoffels A, Brenner S, Hunziker W, Venkatesh B. Extensive expansion of the claudin gene family in the teleost fish, Fugu rubripes. Genome Res. 2004;14:1248-57
25. Islas S, Vega J, Ponce L, Gonzalez-Mariscal L. Nuclear localization of the tight junction protein ZO-2 in epithelial cells. Exp Cell Res. 2002;274:138-48
26. Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc Natl Acad Sci U S A. 1996;93:10779-84
27. Qiao X, Roth I, Feraille E, Hasler U. Different effects of ZO-1, ZO-2 and ZO-3 silencing on kidney collecting duct principal cell proliferation and adhesion. Cell Cycle. 2014;13:3059-75
28. Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 2000;19:2024-33
29. Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, Matter K. et al. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol. 2006;26:2387-98
30. Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423-32
31. Huerta M, Munoz R, Tapia R, Soto-Reyes E, Ramirez L, Recillas-Targa F. et al. Cyclin D1 is transcriptionally down-regulated by ZO-2 via an E box and the transcription factor c-Myc. Mol Biol Cell. 2007;18:4826-36
32. Tapia R, Huerta M, Islas S, Avila-Flores A, Lopez-Bayghen E, Weiske J. et al. Zona occludens-2 inhibits cyclin D1 expression and cell proliferation and exhibits changes in localization along the cell cycle. Mol Biol Cell. 2009;20:1102-17
33. Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499-511
34. Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, Gonzalez-Mariscal L. The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Exp Cell Res. 2004;292:51-66
35. Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131-6
36. Capaldo CT, Koch S, Kwon M, Laur O, Parkos CA, Nusrat A. Tight function zonula occludens-3 regulates cyclin D1-dependent cell proliferation. Mol Biol Cell. 2011;22:1677-85
37. Spadaro D, Tapia R, Jond L, Sudol M, Fanning AS, Citi S. ZO proteins redundantly regulate the transcription factor DbpA/ZONAB. J Biol Chem. 2014;289:22500-11
38. Traweger A, Fuchs R, Krizbai IA, Weiger TM, Bauer HC, Bauer H. The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B. J Biol Chem. 2003;278:2692-700
39. Gonzalez-Mariscal L, Namorado MC, Martin D, Luna J, Alarcon L, Islas S. et al. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int. 2000;57:2386-402
40. Fanning AS, Little BP, Rahner C, Utepbergenov D, Walther Z, Anderson JM. The unique-5 and -6 motifs of ZO-1 regulate tight junction strand localization and scaffolding properties. Mol Biol Cell. 2007;18:721-31
41. Lye MF, Fanning AS, Su Y, Anderson JM, Lavie A. Insights into regulated ligand binding sites from the structure of ZO-1 Src homology 3-guanylate kinase module. J Biol Chem. 2010;285:13907-17
42. Hernandez S, Chavez Munguia B, Gonzalez-Mariscal L. ZO-2 silencing in epithelial cells perturbs the gate and fence function of tight junctions and leads to an atypical monolayer architecture. Exp Cell Res. 2007;313:1533-47
43. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178-96
44. Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996;132:451-63
45. Mauro L, Bartucci M, Morelli C, Ando S, Surmacz E. IGF-I receptor-induced cell-cell adhesion of MCF-7 breast cancer cells requires the expression of junction protein ZO-1. J Biol Chem. 2001;276:39892-7
46. Smalley KS, Brafford P, Haass NK, Brandner JM, Brown E, Herlyn M. Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion. Am J Pathol. 2005;166:1541-54
47. Tuomi S, Mai A, Nevo J, Laine JO, Vilkki V, Ohman TJ. et al. PKCepsilon regulation of an alpha5 integrin-ZO-1 complex controls lamellae formation in migrating cancer cells. Sci Signal. 2009;2:ra32
48. Ghosh D, Dutta A, Kashyap A, Upmanyu N, Datta S. PLP2 drives collective cell migration via ZO-1-mediated cytoskeletal remodeling at the leading edge in human colorectal cancer cells. J Cell Sci. 2021 134
49. Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci. 2009;122:3203-8
50. Nusse R, Clevers H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985-99
51. Reichert M, Muller T, Hunziker W. The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells. Evidence for a role of beta-catenin/Tcf/Lef signaling. J Biol Chem. 2000;275:9492-500
52. Polette M, Gilles C, Nawrocki-Raby B, Lohi J, Hunziker W, Foidart JM. et al. Membrane-type 1 matrix metalloproteinase expression is regulated by zonula occludens-1 in human breast cancer cells. Cancer Res. 2005;65:7691-8
53. Luczka E, Syne L, Nawrocki-Raby B, Kileztky C, Hunziker W, Birembaut P. et al. Regulation of membrane-type 1 matrix metalloproteinase expression by zonula occludens-2 in human lung cancer cells. Clin Exp Metastasis. 2013;30:833-43
54. Brysse A, Mestdagt M, Polette M, Luczka E, Hunziker W, Noel A. et al. Regulation of CXCL8/IL-8 expression by zonula occludens-1 in human breast cancer cells. Mol Cancer Res. 2012;10:121-32
55. Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12-9
56. Martinez C, Vicario M, Ramos L, Lobo B, Mosquera JL, Alonso C. et al. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol. 2012;107:736-46
57. Formiga RO, Alves Junior EB, Vasconcelos RC, Guerra GCB, Antunes de Araujo A, Carvalho TG. et al. p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: Role of Antioxidant System and Immunomodulation. Int J Mol Sci. 2020 21
58. Chang ZY, Liu HM, Leu YL, Hsu CH, Lee TY. Modulation of Gut Microbiota Combined with Upregulation of Intestinal Tight Junction Explains Anti-Inflammatory Effect of Corylin on Colitis-Associated Cancer in Mice. Int J Mol Sci. 2022 23
59. Zhou W, Cao Q, Peng Y, Zhang QJ, Castrillon DH, DePinho RA. et al. FoxO4 inhibits NF-kappaB and protects mice against colonic injury and inflammation. Gastroenterology. 2009;137:1403-14
60. Zhang DK, He FQ, Li TK, Pang XH, Cui DJ, Xie Q. et al. Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J Pathol. 2010;222:213-22
61. Zhang S, Xu W, Wang H, Cao M, Li M, Zhao J. et al. Inhibition of CREB-mediated ZO-1 and activation of NF-kappaB-induced IL-6 by colonic epithelial MCT4 destroys intestinal barrier function. Cell Prolif. 2019;52:e12673
62. Ibrahim S, Zhu X, Luo X, Feng Y, Wang J. PIK3R3 regulates ZO-1 expression through the NF-kB pathway in inflammatory bowel disease. Int Immunopharmacol. 2020;85:106610
63. Fallon MB, Gorelick FS, Anderson JM, Mennone A, Saluja A, Steer ML. Effect of cerulein hyperstimulation on the paracellular barrier of rat exocrine pancreas. Gastroenterology. 1995;108:1863-72
64. Schmitt M, Klonowski-Stumpe H, Eckert M, Luthen R, Haussinger D. Disruption of paracellular sealing is an early event in acute caerulein-pancreatitis. Pancreas. 2004;28:181-90
65. Zwaans BMM, Carabulea AL, Bartolone SN, Ward EP, Chancellor MB, Lamb LE. Voiding defects in acute radiation cystitis driven by urothelial barrier defect through loss of E-cadherin, ZO-1 and Uroplakin III. Sci Rep. 2021;11:19277
66. de Boer WI, Sharma HS, Baelemans SM, Hoogsteden HC, Lambrecht BN, Braunstahl GJ. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol. 2008;86:105-12
67. Mazzon E, Cuzzocrea S. Role of TNF-alpha in lung tight junction alteration in mouse model of acute lung inflammation. Respir Res. 2007;8:75
68. Hu J, Gao N, Zhang Y, Chen X, Li J, Bian F. et al. IL-33/ST2/IL-9/IL-9R signaling disrupts ocular surface barrier in allergic inflammation. Mucosal Immunol. 2020;13:919-30
69. Wang H, Segaran RC, Chan LY, Aladresi AAM, Chinnathambi A, Alharbi SA. et al. Gamma Radiation-Induced Disruption of Cellular Junctions in HUVECs Is Mediated through Affecting MAPK/NF-kappaB Inflammatory Pathways. Oxid Med Cell Longev. 2019;2019:1486232
70. Yu H, Huang X, Ma Y, Gao M, Wang O, Gao T. et al. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int J Biol Sci. 2013;9:966-79
71. Hebert KD, McLaughlin N, Galeas-Pena M, Zhang Z, Eddens T, Govero A. et al. Targeting the IL-22/IL-22BP axis enhances tight junctions and reduces inflammation during influenza infection. Mucosal Immunol. 2020;13:64-74
72. Chen J, Xiao L, Rao JN, Zou T, Liu L, Bellavance E. et al. JunD represses transcription and translation of the tight junction protein zona occludens-1 modulating intestinal epithelial barrier function. Mol Biol Cell. 2008;19:3701-12
73. Zhong Y, Zhang B, Eum SY, Toborek M. HIV-1 Tat triggers nuclear localization of ZO-1 via Rho signaling and cAMP response element-binding protein activation. J Neurosci. 2012;32:143-50
74. Lui WY, Wong EW, Guan Y, Lee WM. Dual transcriptional control of claudin-11 via an overlapping GATA/NF-Y motif: positive regulation through the interaction of GATA, NF-YA, and CREB and negative regulation through the interaction of Smad, HDAC1, and mSin3A. J Cell Physiol. 2007;211:638-48
75. Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O. et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091-103
76. Javier RT. Cell polarity proteins: common targets for tumorigenic human viruses. Oncogene. 2008;27:7031-46
77. Hernandez-Monge J, Garay E, Raya-Sandino A, Vargas-Sierra O, Diaz-Chavez J, Popoca-Cuaya M. et al. Papillomavirus E6 oncoprotein up-regulates occludin and ZO-2 expression in ovariectomized mice epidermis. Exp Cell Res. 2013;319:2588-603
78. Hough CD, Woods DF, Park S, Bryant PJ. Organizing a functional junctional complex requires specific domains of the Drosophila MAGUK Discs large. Genes Dev. 1997;11:3242-53
79. Moussalli MJ, Wu Y, Zuo X, Yang XL, Wistuba, II, Raso MG. et al. Mechanistic contribution of ubiquitous 15-lipoxygenase-1 expression loss in cancer cells to terminal cell differentiation evasion. Cancer Prev Res (Phila). 2011;4:1961-72
80. Shorning BY, Griffiths D, Clarke AR. Lkb1 and Pten synergise to suppress mTOR-mediated tumorigenesis and epithelial-mesenchymal transition in the mouse bladder. PLoS One. 2011;6:e16209
81. Liu L, Dong W, Wang S, Zhang Y, Liu T, Xie R. et al. Deoxycholic acid disrupts the intestinal mucosal barrier and promotes intestinal tumorigenesis. Food Funct. 2018;9:5588-97
82. Liang J, Li H, Chen J, He L, Du X, Zhou L. et al. Dendrobium officinale polysaccharides alleviate colon tumorigenesis via restoring intestinal barrier function and enhancing anti-tumor immune response. Pharmacol Res. 2019;148:104417
83. Yan S, Chang J, Hao X, Liu J, Tan X, Geng Z. et al. Berberine regulates short-chain fatty acid metabolism and alleviates the colitis-associated colorectal tumorigenesis through remodeling intestinal flora. Phytomedicine. 2022;102:154217
84. Deng J, Zhao L, Yuan X, Li Y, Shi J, Zhang H. et al. Pre-Administration of Berberine Exerts Chemopreventive Effects in AOM/DSS-Induced Colitis-Associated Carcinogenesis Mice via Modulating Inflammation and Intestinal Microbiota. Nutrients. 2022 14
85. Babkair H, Yamazaki M, Uddin MS, Maruyama S, Abe T, Essa A. et al. Aberrant expression of the tight junction molecules claudin-1 and zonula occludens-1 mediates cell growth and invasion in oral squamous cell carcinoma. Hum Pathol. 2016;57:51-60
86. Tokes AM, Szasz AM, Juhasz E, Schaff Z, Harsanyi L, Molnar IA. et al. Expression of tight junction molecules in breast carcinomas analysed by array PCR and immunohistochemistry. Pathol Oncol Res. 2012;18:593-606
87. Hoover KB, Liao SY, Bryant PJ. Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am J Pathol. 1998;153:1767-73
88. Orban E, Szabo E, Lotz G, Kupcsulik P, Paska C, Schaff Z. et al. Different expression of occludin and ZO-1 in primary and metastatic liver tumors. Pathol Oncol Res. 2008;14:299-306
89. Zhu H, Lu J, Wang X, Zhang H, Tang X, Zhu J. et al. Upregulated ZO-1 correlates with favorable survival of gastrointestinal stromal tumor. Med Oncol. 2013;30:631
90. Chlenski A, Ketels KV, Korovaitseva GI, Talamonti MS, Oyasu R, Scarpelli DG. Organization and expression of the human zo-2 gene (tjp-2) in normal and neoplastic tissues. Biochim Biophys Acta. 2000;1493:319-24
91. Bonnomet A, Syne L, Brysse A, Feyereisen E, Thompson EW, Noel A. et al. A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene. 2012;31:3741-53
92. Du J, Zhang F, Zhang L, Jia Y, Chen H. MicroRNA-103 regulates the progression in endometrial carcinoma through ZO-1. Int J Immunopathol Pharmacol. 2019;33:2058738419872621
93. Zhang X, Wang L, Zhang H, Tu F, Qiang Y, Nie C. Decreased expression of ZO-1 is associated with tumor metastases in liver cancer. Oncol Lett. 2019;17:1859-64
94. Liu M, Yang J, Zhang Y, Zhou Z, Cui X, Zhang L. et al. ZIP4 Promotes Pancreatic Cancer Progression by Repressing ZO-1 and Claudin-1 through a ZEB1-Dependent Transcriptional Mechanism. Clin Cancer Res. 2018;24:3186-96
95. Ge T, Zhang X, Xiao Y, Wang Y, Zhang T. Novel compound heterozygote mutations of TJP2 in a Chinese child with progressive cholestatic liver disease. BMC Med Genet. 2019;20:18
96. Zhou S, Hertel PM, Finegold MJ, Wang L, Kerkar N, Wang J. et al. Hepatocellular carcinoma associated with tight-junction protein 2 deficiency. Hepatology. 2015;62:1914-6
97. Parsons DW, Roy A, Yang Y, Wang T, Scollon S, Bergstrom K. et al. Diagnostic Yield of Clinical Tumor and Germline Whole-Exome Sequencing for Children With Solid Tumors. JAMA Oncol. 2016;2:616-24
98. Vij M, Shanmugam NP, Reddy MS, Sankaranarayanan S, Rela M. Paediatric hepatocellular carcinoma in tight junction protein 2 (TJP2) deficiency. Virchows Arch. 2017;471:679-83
99. Xu J, Anuar F, Ali SM, Ng MY, Phua DC, Hunziker W. Zona occludens-2 is critical for blood-testis barrier integrity and male fertility. Mol Biol Cell. 2009;20:4268-77
100. Kim MA, Kim YR, Sagong B, Cho HJ, Bae JW, Kim J. et al. Genetic analysis of genes related to tight junction function in the Korean population with non-syndromic hearing loss. PLoS One. 2014;9:e95646
101. Wang HY, Zhao YL, Liu Q, Yuan H, Gao Y, Lan L. et al. Identification of Two Disease-causing Genes TJP2 and GJB2 in a Chinese Family with Unconditional Autosomal Dominant Nonsyndromic Hereditary Hearing Impairment. Chin Med J (Engl). 2015;128:3345-51
Author contact
![]() Corresponding author: Center for Pancreatic Cancer Research, South China University of Technology School of Medicine, Guangzhou 510006; Phone: 020-3938-0579; E-mail: scutmedicineedu.cn.
Corresponding author: Center for Pancreatic Cancer Research, South China University of Technology School of Medicine, Guangzhou 510006; Phone: 020-3938-0579; E-mail: scutmedicineedu.cn.

 Global reach, higher impact
Global reach, higher impact