10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(7):2779-2789. doi:10.7150/ijbs.95484 This issue Cite
Review
Seleno-amino Acid Metabolism Reshapes the Tumor Microenvironment: from Cytotoxicity to Immunotherapy
1. Shanghai Frontiers Science Center of TCM Chemical Biology, Institute of Interdisciplinary Integrative Medicine Research and Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, China.
2. School of Pharmacy, Second Military Medical University, Shanghai 200433, China.
3. State Key Laboratory for Quality Ensurance and Sustainable Use of Dao-di Herbs, Institute of Medicinal Plant Development, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, 100700, China.
# These authors contributed equally to this work.
Received 2024-2-19; Accepted 2024-4-26; Published 2024-5-5
Abstract
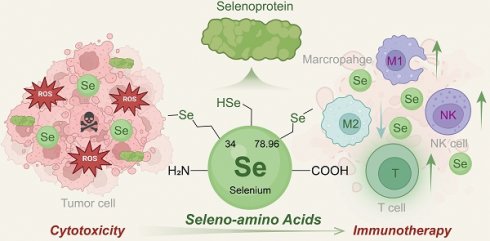
Selenium (Se) is an essential trace element for biological processes. Seleno-amino acids (Se-AAs), known as the organic forms of Se, and their metabolic reprogramming have been increasingly recognized to regulate antioxidant defense, enzyme activity, and tumorigenesis. Therefore, there is emerging interest in exploring the potential application of Se-AAs in antitumor therapy. In addition to playing a vital role in inhibiting tumor growth, accumulating evidence has revealed that Se-AA metabolism could reshape the tumor microenvironment (TME) and enhance immunotherapy responses. This review presents a comprehensive overview of the current progress in multifunctional Se-AAs for antitumor treatment, with a particular emphasis on elucidating the crosstalk between Se-AA metabolism and various cell types in the TME, including tumor cells, T cells, macrophages, and natural killer cells. Furthermore, novel applications integrating Se-AAs are also discussed alongside prospects to provide new insights into this emerging field.
Keywords: Cancer, Selenium, Seleno-amino acid, Metabolic reprogramming, Immunotherapy
Introduction
Cancer continues to be a prominent global cause of mortality, but conventional treatments are frequently limited by issues such as toxicity, resistance, and adverse effects [1]. In recent years, tumor immunotherapy, including immune checkpoint blockade (ICB), adoptive cell transfer, and cancer vaccination, has emerged as a promising alternative for harnessing the power of the immune system to recognize and eliminate cancer cells [2]. However, despite its potential benefits, many cancer patients exhibit a low response to this treatment modality, highlighting the need for innovative strategies that can augment its efficacy [3]. Many studies have shown that abnormal amino acid metabolism can affect both tumor and immune cells in the tumor microenvironment (TME), leading to tumor immune evasion [4]. For example, tryptophan facilitates the survival and activity of CD8+ T cells [5]. However, kynurenine, an amino acid metabolite of tryptophan, can increase the expression level of programmed cell death protein 1 (PD-1) on CD8+ T cells and mediate immunosuppression by activating regulatory T cells (Tregs) [6]. Hence, inspired by these findings, strategies targeting amino acid metabolism to improve the response to tumor immunotherapy have been proposed and entered clinical trials [7].
Selenium (Se) is an essential trace element that plays a crucial role in regulating biological processes [8]. Se-AAs, the organic forms of Se, are considered valuable forms for Se supplementation owing to their high safety and bioavailability compared to those of inorganic Se, such as selenite and selenate [9]. Increasing evidence has indicated that Se plays an important role in cancer progression, drug resistance, and immune evasion [10]. Se-AA deficiency can impair the antitumor effects of chemotherapy and radiotherapy, increasing tumor resistance [11, 12]. In addition to their impact on traditional cancer treatments, Se-AAs and their metabolism have been proven to show excellent potential for tumor immunotherapy, as they significantly improve the immune response by regulating the crosstalk between tumor cells and immune cells and reshaping the TME [13].
Given the diverse functions and implications of Se-AA metabolism relevant to the TME, we summarize recent advancements in the development of Se-AAs for tumor treatment to enhance our understanding of their pharmacological mechanisms, with an emphasis on their immunomodulatory effects on different kinds of cells in the TME. Additionally, we discuss the current applications and perspectives of Se-AAs for more effective tumor treatment as a novel adjunctive therapeutic strategy, which will contribute to tumor immunotherapy developments in the future.
Biological forms of Se
Se exists in nature in three main forms: monomeric Se, inorganic Se, and organic Se. The absorption and utilization of monomeric Se are limited. Inorganic Se exists in the valence states of +4 and +6 and is found predominantly as inorganic selenate (SeO42-) and selenite (SeO32-) in living organisms. However, the estimated toxic effects of inorganic Se intake were found to occur at a level of 16 µg/day, whereas the corresponding threshold for organic Se was determined to be 260 μg/day [14]. Organic Se found within living organisms can be classified into two main types: Se-AAs and Se-containing proteins. Mammals and microorganisms have been observed to contain two major types of Se-AAs: selenocysteine (SeCys) and selenomethionine (SeMet) [15]. The primary means by which humans acquire Se are through Se-enriched plant and animal products [16], typically in the form of SeMet from grains, yeast, and meat proteins, as well as L-Se-methyl selenocysteine (MeSeCys) found in certain plant foods such as garlic and cauliflower [12, 17]. It has been reported that Se deficiency can lead to various diseases, including myocardial infarction, neurological damage, and low immunity. Therefore, understanding the metabolic transformation mechanism of Se within biological systems is essential for investigating its functional role [18, 19].
Physicochemical properties of Se in amino acids
SeCys is formed by substituting the oxygen atom in serine (Ser) at precisely the same position as Cys in its homologous protein. The enhanced biological activity exhibited by SeCys may be attributed to the distinctive physicochemical properties of Se. A thorough comparison of the chemical structures of Cys and SeCys revealed that this disparity arises from the inherent dissimilarities between sulfur (S) and Se atoms [20].
Compared to S, molecules containing Se exhibit lower redox potentials and higher reactivity, and are susceptible to oxidation or reduction. Moreover, when comparing the thiol group to the selenol group, it is evident that the pKa value of the latter is significantly lower. This observation suggested that a larger proportion of the selenol group in SeCys undergoes deprotonation and exists in its more electrophilic state as -Se. This higher reactivity can be attributed to such a transformation. Notably, the electrostatic interaction between Se and other molecules should be emphasized [21].
Based on the above physicochemical comparison, the primary merit of SeCys, which features Se as its active center, lies in its enduring catalytic efficacy during redox reactions [20, 22, 23]. Significantly, Se exhibits a distinctive and readily reversible reaction with oxygen and ROS, which is not observed in sulfur [24]. Therefore, Se-AAs are more versatile than common amino acids.
Absorption and metabolic pathways of Se-AAs
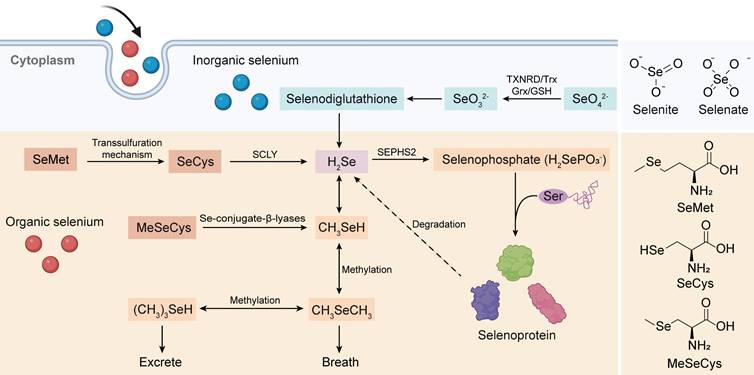
To comprehensively investigate the antitumor mechanism of Se-AAs, we conducted a thorough analysis of the complete Se metabolism pathway, which encompasses both inorganic and organic forms (Figure 1). Overall, hydrogen selenide(H2Se) plays a pivotal role in Se metabolism, as it serves as the nexus between two crucial metabolic pathways. Initially, Se is present in an oxidized state (selenite, Se4+ and selenate, Se6+) within inorganic substances; however, this high-valent form of Se undergoes reduction to its low-valent counterpart through the involvement of reduced glutathione and reduced nicotinamide adenine dinucleotide phosphate (NADPH) within living organisms [25]. The resultant metabolite from this process, H2Se, actively participates in the synthesis of selenoproteins [26] while also demonstrating significant potential as an antitumor agent through the production of methylselenol (CH3SeH) [27]. Although the various forms of Se-AAs undergo distinct metabolic pathways, they can exert antitumor effects either directly or by converting them into selenoproteins [28-30]. The dietary absorption of SeCys does not directly contribute to selenoprotein synthesis; rather, SeCys is metabolized to H2Se by a β-cleaving enzyme (Figure 1) [26], and the substance undergoes conversion from selenide to selenophosphate [31]. Simultaneously, Ser binds to a specialized tRNA, forming a serine-tRNA complex catalyzed by serine-tRNA synthetase. Subsequently, SeCys synthetase facilitates a uniquely specialized process wherein the -OH group of serine is replaced by -SeH derived from selenophosphate. This results in the formation of selenocysteine-RNA (SeCys-tRNA), which ultimately undergoes translation into selenoproteins [32].
SeMet is a popular form of dietary Se due to its exceptional bioavailability and minimal toxicity. As a Se analog of Met, SeMet can actively participate in the process of protein synthesis by substituting Met or converting it to SeCys [25]. In addition, the conversion of SeMet to SeCys occurs through the transsulfuration pathway, which is also responsible for the synthesis of Cys from Met [33]. The final step in the multistep process of synthesizing SeCys from SeMet involves the synthesis of selenohomocysteine and Ser followed by the elimination of SeMet via cystathionine-γ-cleaving enzymes [34]. Studies have demonstrated that rats produce more SeCys when additional dietary SeMet is provided, indirectly suggesting that the storage of SeMet in proteins hinders its efficient metabolism [35].
MeSeCys, which is exclusively consumed as a dietary source and not endogenously present in the human body, is directly cleaved to CH3SeH by β-lyase upon ingestion. As previously mentioned, CH3SeH serves as a pivotal antitumor metabolite of Se; hence, MeSeCys exhibits superior biological activity in vivo compared to other forms of Se compounds [36]. Furthermore, under the influence of β-lyase, MeSeCys can generate selenophosphoric acid, which acts as a precursor for synthesizing diverse selenoproteins and remains active when it is incorporated as a SeCys within various proteins [37].
The crosstalk between Se-AA metabolism and various cells in the TME
The absorption and metabolism of Se have garnered significant attention within the field of oncology [38]. A higher incidence of cancer, including breast cancer, lung cancer, gastric carcinoma, bladder cancer, oophoroma, pancreatic carcinoma, and melanoma, has been observed among individuals with inadequate dietary Se intake or lower plasma Se levels [12, 39, 40]. Furthermore, mutations in the SeCys insertion sequence are associated with impaired lymphocyte proliferation, abnormal cytokine secretion, and telomere shortening. This finding further highlights the importance of Se in cancer treatment [41]. Among the various Se compounds, the clinical application of inorganic Se compounds is limited due to their low lipid solubility, high mutagenicity, and high genotoxicity. Conversely, organic Se compounds, such as SeCys, SeMet, and MeSeCys, demonstrate enhanced cell membrane permeability and reduced side effects and systemic toxicity. Thus, organic Se holds great potential for cancer therapy [42, 43].
Absorption and metabolic pathways of inorganic Se and Se-AAs. Inorganic Se (selenate and selenite) could be stepwise reduced to produce H2Se, which is the bridge connecting inorganic Se metabolism and organic Se metabolism, via the intermediate selenodiglutathione. Meanwhile, Se-AAs (SeMet, SeCys, and MeCys) could also be converted to H2Se, followed by its transformation into selenophosphate for the synthesis of selenoproteins. Furthermore, H2Se is excreted through methylation reactions.
Se-AAs are a class of essential amino acids, and their metabolism has been implicated in numerous tumors. Together with their direct involvement in regulating various signaling pathways crucial for tumor growth and survival, several studies have highlighted the active roles of Se-AAs in remodeling the TME by modulating the crosstalk between immune cells and tumor cells. Se-AAs can activate multiple enzyme systems in lymphocytes and enhance the activity of immune cells, such as NK and T cells. Se-AAs further stimulate the secretion of lymphocyte factors with immunomodulatory effects [44, 45]. Moreover, Se-AAs exhibit the potential for improving the functionality of immunoglobulin M (IgM), immunoglobulin G (IgG), and other antibodies that are crucial factors in humoral immunity [46].
The multiple mechanisms of Se-AAs on tumor cells
The effects of Se-AAs on normal tissues and cells are primarily related to the various biological functions of selenoproteins, ranging from cellular redox regulation to the biosynthesis of hormones [30]. The involvement of selenoproteins in tumor cells is multifaceted, with oxidoreductase being identified as the pivotal selenoprotein [47]. These oxidoreductases include glutathione peroxidase (GPx), thioredoxin reductase (TrxR), and iodothyronine deiodinase (DIO) [43]. The GPx family primarily comprises GPx1, GPx2, GPx3, and GPx4. The enzymatic activity of these selenoproteins effectively counteracts oxidative stress and inhibits cell death processes induced by inflammation [48, 49]. Among them, GPx1 and GPx4 exert their protective effects against lipid peroxidation by effectively neutralizing phosphatases through the action of H2O2, thereby impeding the phosphorylation cascade [50]. GPx2 acts as a negative regulator of the oncogene p53, not only inhibiting its transcriptional activity and promoting the degradation of p53 proteins but also downregulating the downstream target genes of p53. Thus, it plays a vital role in regulating the cell cycle [51]. GPx3 is considered a tumor suppressor due to its role in maintaining the regulation of the thromboxane biosynthesis pathway, thereby inhibiting platelet aggregation [52]. In addition, SeCys-containing TrxRs are present in both the cytoplasm (TrxR1) and mitochondria (TrxR2) and play crucial roles in reducing oxidized thioredoxin, catalyzing NADPH, regulating ascorbic acid levels, and modulating metabolism. These activities ultimately contribute to tumor growth promotion and impact patient prognosis [53].
In addition to selenoproteins, Se-AAs themselves can also exert antitumor effects. SeCys, the active center of selenoproteins, can exert its effects on tumors through the regulation of selenoproteins [54]. For instance, selenophosphate synthetase 2 (SEPHS2), an enzyme regulating SeCys biosynthesis, is crucial for the survival of tumor cells to detoxify Se. The depletion of SEPHS2 in tumor cells led to the accumulation of selenide, a toxic intermediate produced during SeCys biosynthesis, resulting in the inhibition of cell proliferation, loss of colony-forming ability, and cell death [11]. In addition, SeCys plays a role in blocking the tumor cell cycle [55] and promoting the activation of the p38 MAPK, JNK, and ERK signaling pathways while inhibiting AKT activity, inducing DNA damage in tumor cells [56, 57]. SeCys can also induce mitochondrial dysfunction and activate ROS-mediated p53 phosphorylation to facilitate apoptosis in tumor cells [58-60]. At the same time, SeCys has demonstrated targeted inhibition of TrxR1 expression [61] and radiosensitizing effects [62].
Numerous studies have demonstrated the potent inhibitory effects of SeMet on the proliferation of various tumor cells, including breast cancer, prostate cancer, and melanoma cells [63, 64]. SeMet exhibited remarkable selectivity toward tumor cells in comparison to normal diploid fibroblasts or primary cells of the human prostate [65, 66]. Mechanistically, SeMet selectively led to an increase in G2-M cell cycle arrest in tumor cells through the phosphorylation of P-Tyr15-p34/cell division cycle 2 kinase (cdc 2) [66], and induced apoptosis by promoting poly-ADP ribose polymerase (PARP) cleavage and the generation of ROS [67] (Table 1).
MeSeCys has been shown to inhibit the proliferation of certain tumor cell lines, such as A549 [68], LNCap [69], and HOP-62 cells [70]. MeSeCys can also trigger apoptosis in tumor cells by promoting lipid peroxidation and ROS generation [68], as well as inhibiting the PI3K-Akt signaling pathway [70]. In particular, MeSeCys exhibited the potential to normalize angiogenesis and downregulate tumor-related proteins (such as androgen receptor and estrogen receptor α), thus overcoming tumor resistance to various therapeutic approaches, including chemotherapy [70], targeted therapy [71], and androgen deprivation therapy [69] (Table 1).
Se-AAs reprogram macrophage immune responses
Tumor-associated macrophages (TAMs) are prone to polarize to immunosuppressive M2 macrophages in the TME, thereby accelerating cancer progression and metastasis, while proinflammatory M1 macrophages exhibit preferential antitumor immune activation [72]. The majority of therapeutic approaches targeting macrophages have focused on reprogramming TAMs, terminating macrophage recruitment, and interfering with TAM survival [73]. However, existing approaches that depend on the blockade of the colony-stimulating factor 1/colony-stimulating factor 1 receptor (CSF1/CSF1R) axis will unavoidably compromise tissue-resident macrophages, resulting in imprecise therapeutic effectiveness [74]. Chen et al. [75] designed Se nanoparticles coated with mushroom polysaccharides to restore immunity in the malignant pleural effusion of lung cancer. Their study revealed that Se nanoparticles can be gradually metabolized into selenocystine (SeCys2) within macrophages and educate M2 TAMs into an M1 phenotype. These results suggest that SeCys2 plays a key regulatory role in macrophage immune responses (Figure 2B).
Se-AAs regulate T-cell functions
The intake of Se has been shown to impact T-cell-based adaptive immunity. Se deficiency causes atrophy of the thymus, spleen, and lymph nodes in mice, thereby inhibiting the activation and proliferation of T cells. These findings strongly indicate a close relationship between Se levels and compromised T-cell immune function [81]. Se intake also promoted the proliferation and differentiation of activated CD4+ T cells into Th1 cells (Figure 2C), which are known to play a crucial role in antitumor or bacterial infection responses [81].
γδ T cells are a distinct subset of T cells that possess a unique T-cell receptor (TCR) composed of a γ and a δ chain that serves as a bridge between innate and adaptive immunity, making them crucial players in the maintenance of overall immune function [82]. γδ T cells have demonstrated robust therapeutic efficacy against tumors by secreting proapoptotic molecules and inflammatory cytokines without the presence of dendritic cells. To enhance the cytotoxicity of γδ T cells, Hu et al. [83] selected Se nanoparticles to tune the antitumor ability of γδ T cells. The authors found that Se nanoparticles could increase the cancer cell-killing efficacy of γδ T cells and significantly upregulate the expression of natural killer group 2, member D (NKG2D), and interferon γ (IFN-γ) on the surface of γδ T cells while downregulating the expression of PD-1 to reduce their immunosuppressive effects (Figure 2C).
The mechanism diagram illustrates the crosstalk between Se-AA metabolism and various cell types in the TME. A) The multiple action of Se-AAs on tumor cell-related pathways. B) The functions of Se-AAs in programming macrophage immune responses. C) Se-AAs promote T-cell proliferation, differentiation, and cytotoxicity. D) Se-AAs boost the antitumor activities of NK cells.
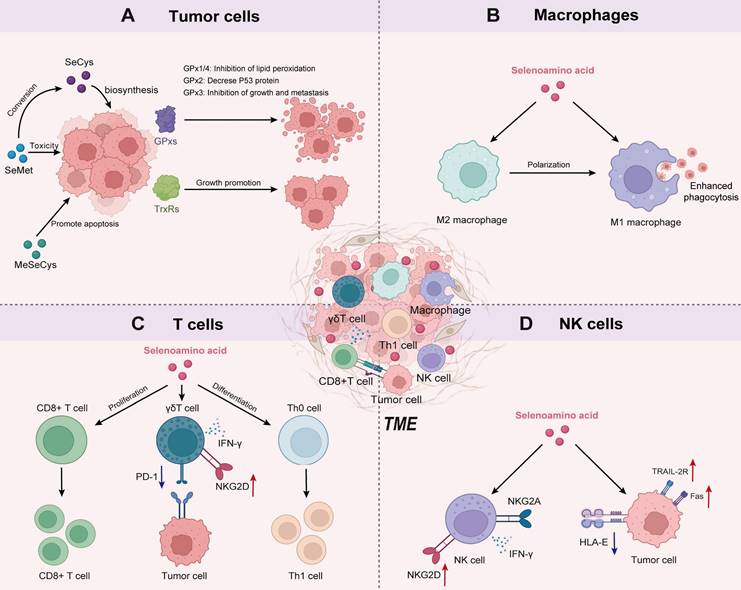
Antitumor mechanism of Se-AAs.
| Se-AAs | Target cells | Effects | Mechanism | Ref. |
|---|---|---|---|---|
| SeCys | MDA-MB-231 cells | Selenoprotein production via the SLC7A11-SEPHS2 axis | Cancer cells can detoxify selenide produced in the SeCys biosynthesis pathway via the SEPHS2 protein, and overexpression of the SEPHS2 protein protects cancer cells against selenite. | [11] |
| SeCys | U251/U87/MG-63 cells | DNA damage and MAPK and AKT pathways modulation | SeCys promotes DNA damage through inducing ROS generation. SeCys causes p38MAPK, JNK and ERK activation and AKT inactivation, thereby inducing DNA damage in tumor cells. Mitochondrial dysfunction and imbalanced Bcl-2 family expression. Activation of TrxR1-targeted inhibition | [55-57], [61] |
| SeCys | A375/MCF-7/MDA-MB-231/HepG2 cells | Cell cycle arrest and apoptosis | Cell cycle arrest with reduced expression of associated proteins, including cyclins A and CDK-2. Activation of caspase-independent apoptosis. Activation of ROS-mediated mitochondrial pathway. Promotion of p53 phosphorylation | [58-60] |
| SeCys | HeLa/Caski/SiHa cells | Radiosensitization | SeCys can act as a radiosensitizer and significantly enhance ROS production in cancer cells following X-ray treatment. | [62] |
| SeCys2 | A375/HeLa/HepG2/MCF-7 cells | Pro-apoptosis | SeCys2 could promote the overproduction of ROS in tumor cells, leading to DNA damage and affecting the p53, AKT, and MAPK pathways to induce apoptosis of tumor cells. SeCys2 enhances 5-FU-induced loss of mitochondrial membrane potential by regulating the expression of Bcl2 family proteins | [76-78] |
| SeCys2 | NK cells and MDA-MB-231 cells | Upregulation of recognition ligands | SeCys2 rebalances the Smad 2/3/Smad 7 signaling pathways to increase the expression of NKG2D on NK cells and NKG2DL on tumor cells, respectively. | [79] |
| SeCys2 | CIK cells and HepG2 cells | Enhanced persistence of CIK cells and regulation of recognition ligands | SeCys2 prolongs the persistence of CIK cells in vivo and effectively enhances the cytotoxicity of CIK cells by regulating the expressions of NKG2D/NKG2DLs and PD-1/PD-L1. | [80] |
| SeMet | LNCaP/PC-3/DU145 cells | Cell cycle arrest | SeMet results in the G2-M cell cycle by phosphorylating cdc2. | [66] |
| SeMet | A549/HepG2 cells | Pro-apoptosis | SeMet promotes glutathione depletion and induces high levels of ROS in tumor cells, further leading to apoptosis. | [67] |
| MeSeCys | A549 cells | Inhibition of tumor cell proliferation | MeSeCys induces lipid peroxidation and increases ROS generation in A549 cells. | [68] |
| MeSeCys | LNCaP cells | Inhibition of castration-resistant progression of LNCaP tumors | MeSeCys downregulates the expression of androgen receptor and prostate-specific antigen, inhibits proliferation and angiogenesis, and induces apoptosis in LNCaP tumors. | [69] |
Overall, the biological effects of Se on T cells largely rely on selenoproteins synthesized from SeCys. Selenoproteins actively participate in various T-cell functions, including regulating TRC-induced calcium flux, modulating the redox activity of T cells, and linking TCR-induced activation to the metabolic reprogramming required for T-cell proliferation and differentiation [84].
Se-AAs enhance NK-cell functions
NK cells are key effector cells in tumor immunotherapy because they can recognize specific cell surface receptors on tumor cells and pathogen-infected cells. This recognition subsequently triggers receptor-mediated cytotoxicity and cytokine production [85]. NK cell cytotoxicity is attributed to the inhibitory effect of the NKG2A receptor on signaling pathways. However, within the immunosuppressive TME, NK cells encounter challenges in recognizing ligands expressed by tumor cells, which significantly impedes the therapeutic efficacy of NK cells [86]. Therefore, it is important to overcome the inhibitory effects of the TME to enhance NK cell activity and tumor cell recognition. Several studies have used organic Se to effectively enhance NK cell recognition by upregulating the expression of NKG2D and NKG2DL, which is dependent on the DNA damage response pathway [87]. Wei et al. [88] introduced the Sec derivative into a peptide consisting of a tumor-targeting sequence and an enzyme cleavage motif to form selenopeptide nanoparticles by self-assembly. The authors found that the combination of chemotherapy and selenopeptide-induced immunotherapy promoted reprogramming of the human leukocyte antigen E/natural killer group 2 member A (HLA-E/NKG2A) axis, activated NK cell recognition of tumors, and ultimately achieved synergistic antitumor therapy (Figure 2D) [88, 89]. In addition, Se-containing complexes can also enhance therapeutic efficacy against prostate cancer by activating the death receptor (TRAIL/FasL) signaling pathway (Figure 2D) [89]. These studies confirmed that Se can significantly enhance the activity of NK cells and effectively kill tumor cells via NK cells.
Antitumor application of Se-AAs
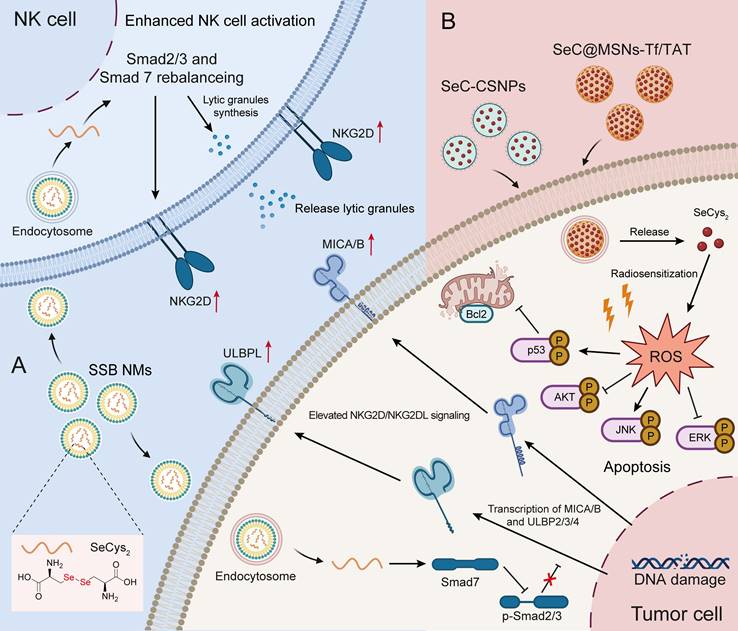
To date, inorganic Se has several limitations due to its immeasurable toxicity, and research on Se-containing drugs has favored organic Se and Se nanoparticles. As a homolog of S, Se not only has antitumor effects but also has stronger chemical reactivity than S. Several studies have explored the utilization of diselenide bonds (Se-Se) as an alternative to disulfide bonds (S-S) in the development of chemotherapeutics, photochemotherapy and photodynamic therapy [90-92]. Similar cystine, which is formed by the disulfide bond between two cysteines, SeCys2 readily forms a dimer due to the decreased redox potential of its selenyl group. Liu C et al. [79] constructed a nanoemulsion system (named SSB NMs) by using SeCys2 in combination with a TGF-β inhibitor, which resulted in a significant enhancement of NK cell-mediated antitumor efficacy against TNBC. Their findings revealed that the potentiation effect of NK cells relies on the upregulation of NKG2D signaling and NKG2D ligands (NKG2DLs). Moreover, SSB NMs effectively rebalanced the TGF-β/TGF-β RI/Smad2/3/Smad 7 signaling pathways to increase the expression of NKG2D on NK cells and NKG2DL on tumor cells (Figure 3A) [79].
Antitumor application of Se-AAs. A) SSB NMs effectively suppressed the TGF-β/TGF-β RI/Smad2/3/Smad 7 signaling pathway, thereby promoting the expression of NKG2D on NK cells and NKG2DL on tumor cells, ultimately enhancing the NK cell-mediated immune response. B) The SeCys2 released by SeC-CSNPs and SeC@MSNs-Tf/TAT synergized with chemotherapy and radiotherapy to promote the overproduction of intracellular ROS, thereby affecting apoptosis-related signaling pathways and ultimately inducing tumor cell apoptosis.
Cytokine-induced killer (CIK) cell-based adoptive cell transfer has great potential in clinical cancer immunotherapy in an MHC-unrestricted manner. However, the limited in vivo persistence and suboptimal therapeutic efficacy of CIK cells significantly constrain their further application [93]. To address these challenges, Liu et al. [80] developed an effective strategy by combining Se nanoparticles with CIK cells for synergistic immunotherapy. These authors found that SeCys2 was the main functional metabolite of Se nanoparticles and not only significantly prolonged the persistence of CIK cells in vivo but also effectively enhanced the cytotoxicity of CIK cells through upregulating the expression of NKG2D/NKG2DLs and PD-1/PD-L1 and reshaping the TME in multiple mouse tumor models (hepatic, breast and prostate tumors) [80].
In addition, numerous studies have demonstrated that SeCys2 can promote the overproduction of intracellular ROS. This phenomenon subsequently leads to DNA damage and influences crucial signaling pathways, such as the p53, AKT, and MAPK pathways, ultimately leading to tumor cell apoptosis (Figure 3B) [76, 77]. Notably, SeCys2-induced ROS can also be combined with traditional chemotherapy [78] and radiotherapy to effectively overcome tumor resistance [62]. For example, SeCys2 effectively promoted 5-FU-induced apoptosis through its modulation of Bcl2 family protein expression and the augmentation of mitochondrial membrane potential (Figure 3B). Moreover, SeCys not only exhibited radiosensitizing effects on tumors [62] but also increased radioresistance in healthy tissue by promoting cytokine secretion and augmenting the population of white blood cells while concurrently reducing bone marrow DNA inhibition [94].
Conclusion and future perspectives
Abnormal tumor metabolism is considered a hallmark of cancer [95] and one of the main underlying factors impeding the efficacy of cancer immunotherapies [96]. In addition to the well-studied glucose-dependent metabolic landscape (termed the “Warburg effect”), amino acid metabolism reprogramming in the TME is also extensively involved in the manipulation of tumor immune escape [97]. Thus, targeting amino acid metabolism in tumors opens up new avenues for cancer immunotherapies by orchestrating the uptake, transport, and metabolism of amino acids, such as glutamine [98], tryptophan [99], and arginine [100]. Among them, Se-AAs are crucial for maintaining the cellular oxidation-reduction balance and the immune system in mammals, and remarkable progress has been made in Se-AA metabolism reprogramming within the TME [101]. With an increasing understanding of the various underlying mechanisms, targeting Se-AA metabolism has emerged as a promising therapeutic strategy with potential clinical implications.
Recently, owing to the unique physiochemical properties and pharmacological activities of Se, numerous Se-containing small molecules have exhibited decreased toxicity and improved antitumor bioactivities by incorporating Se into structural scaffolds, making them promising compounds [102]. Additionally, Se-AAs have also been used as chemical handles for the synthesis and functionalization of peptides and proteins, such as metal-free/metal-catalyzed transformations, traceless chemical modifications, and protein folding [103]. However, current research related to Se-AA-containing drugs, especially peptide drugs, is very scarce. Therefore, it is imperative and highly important to study the pharmacological effects of Se-AA-containing compounds in the future. Considering the potential advantages of organoselenium in medicinal chemistry, we hypothesize that the development of Se-AA-based conjugates will be a powerful strategy for generating novel tumor immunotherapy agents or adjuvants to enhance current regimens used in clinical immunology.
Abbreviations
cdc 2: P-Tyr15-p34/cell division cycle 2 kinase; CH3SeH: Methylselenol; CIK: Cytokine-induced killer; CSF1/CSF1R: Colony-stimulating factor 1/colony-stimulating factor 1 receptor; DIO: iodinated thyrotropine; GPx: Glutathione peroxidase; HLA-E: human leukocyte antigen E; H2Se: Hydrogen selenide; ICB: Immune checkpoint blockade; IFN-γ: Interferon gamma; IgG: Immunoglobulin G; IgM: Immunoglobulin M; MSeA: Methylselenic acid; MeSeCys: L-Se-methyl selenocysteine; MTB: Mycobacterium tuberculosis; NADPH: Nicotinamide adenine dinucleotide phosphate; NKG2A: Killer Cell Lectin Like Receptor C1; NKG2D: natural killer group 2 member D; PARP: poly-ADP ribose polymerase; PD-1: Programmed cell death protein 1; ROS: Reactive oxygen species; S: Sulfur; Se: selenium; Se-AAs: Seleno-amino acids; SeCys: Selenocysteine; SeCys-tRNA: Selenocysteine-RNA; SeCys2: Selenocystine; SeMet: Selenomethionine; SEPHS2: Selenophosphate synthetase 2; Ser: Serine; SIRPα: Signal-regulated protein alpha; TAM: Tumor-associated macrophage; TCR: T-cell receptor; TME: Tumor microenvironment; Tregs: Regulatory T cells; TrxR: Thioredoxin reductase.
Acknowledgements
Funding
This work was supported by the National Natural Science Foundation of China (No. 82322073, 82173846, 82304533), CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2023-I2M-3-009), Key Project at Central Government Level: The Ability Establishment of Sustainable Use for Valuable Chinese Medicine Resources (2060302), Oriental Scholars of Shanghai Universities (TP2022081), Jiangxi Province Thousand Talents Program (jxsq2023102168), Young Talent Lifting Project of China Association of Chinese Medicine [No. CACM-(2021-QNRC2-A08)], Shanghai Rising-Star Program (No.22QA1409100), Shanghai Sailing Program (No. 22YF1445000), 2021 Shanghai Science and Technology Innovation Action Plan (No. 21S11902800), Three-year Action Plan for Shanghai TCM Development and Inheritance Program [No. ZY(2021-2023)-0208, ZY(2021-2023)-0401], High-level Key Discipline of National Administration of Traditional Chinese Medicine (No. zyyzdxk-2023071), Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-D-202004), and Innovation team of high-level local universities in Shanghai: Strategic Innovation Team of TCM Chemical Biology. All figures were created with BioRender.com.
Author contributions
Rui Liang, Aoyu Cheng, and Shengxin Lu conceived the concept and were mainly responsible for writing, painting, and revision this review. Xiaokun Zhang, Maomao Ren, and Jiayi Lin participated in reviewing literature and provided feedback on this review. Ye Wu, Weidong Zhang, and Xin Luan provided guidance and valuable feedback. All authors read, revised, and approved the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
2. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86-104
3. Yang K, Halima A, Chan TA. Antigen presentation in cancer - mechanisms and clinical implications for immunotherapy. Nat Rev Clin Oncol. 2023;20:604-23
4. Zheng Y, Yao Y, Ge T, Ge S, Jia R, Song X. et al. Amino acid metabolism reprogramming: shedding new light on T cell anti-tumor immunity. J Exp Clin Cancer Res. 2023;42:291
5. Huang X, Sun T, Wang J, Hong X, Chen H, Yan T. et al. Metformin reprograms tryptophan metabolism to stimulate CD8+ T-cell function in colorectal cancer. Cancer Res. 2023;83:2358-71
6. Zhang X, Wang C, Wang J, Hu Q, Langworthy B, Ye Y. et al. PD-1 blockade cellular vesicles for cancer immunotherapy. Adv Mater. 2018;30:e1707112
7. Kelly CM, Qin L-X, Whiting KA, Richards AL, Avutu V, Chan JE. et al. A Phase II study of Epacadostat and Pembrolizumab in patients with advanced sarcoma. Clin Cancer Res. 2023;29:2043-51
8. Rayman MP. Selenium and human health. Lancet. 2012;379:1256-68
9. Zhang X, He H, Xiang J, Yin H, Hou T. Selenium-containing proteins/peptides from plants: a review on the structures and functions. J Agric Food Chem. 2020;68:15061-73
10. Vinceti M, Filippini T, Del Giovane C, Dennert G, Zwahlen M, Brinkman M. et al. Selenium for preventing cancer. Cochrane Database Syst Rev. 2018;1:CD005195
11. Carlisle AE, Lee N, Matthew-Onabanjo AN, Spears ME, Park SJ, Youkana D. et al. Selenium detoxification is required for cancer-cell survival. Nat Metab. 2020;2:603-11
12. Fairweather-Tait SJ, Collings R, Hurst R. Selenium bioavailability: current knowledge and future research requirements. Am J Clin Nutr. 2010;91:1484S-91S
13. Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN. Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem Sci. 2014;39:112-20
14. Hu J, Wang Z, Zhang L, Peng J, Huang T, Yang X. et al. Seleno-amino acids in vegetables: a review of their forms and metabolism. Front Plant Sci. 2022;13:804368
15. Mangiapane E, Pessione A, Pessione E. Selenium and selenoproteins: an overview on different biological systems. Curr Protein Pept Sci. 2014;15:598-607
16. Hossain A, Skalicky M, Brestic M, Maitra S, Sarkar S, Ahmad Z. et al. Selenium biofortification: roles, mechanisms, responses and prospects. Molecules. 2021;26:881
17. Kim E, Bisson WH, Löhr CV, Williams DE, Ho E, Dashwood RH. et al. Histone and non-histone targets of dietary deacetylase inhibitors. Curr Top Med Chem. 2016;16:714-31
18. Natasha Shahid M, Niazi NK Khalid S, Murtaza B Bibi I. et al. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ Pollut. 2018;234:915-34
19. Steinbrenner H, Sies H. Selenium homeostasis and antioxidant selenoproteins in brain: implications for disorders in the central nervous system. Arch Biochem Biophys. 2013;536:152-7
20. Maroney MJ, Hondal RJ. Selenium versus sulfur: Reversibility of chemical reactions and resistance to permanent oxidation in proteins and nucleic acids. Free Radic Biol Med. 2018;127:228-37
21. Wang J, Chen M, Zhang Z, Ma L, Chen T. Selenium: From fluorescent probes to biomedical application. Coord Chem Rev. 2023;493:215278
22. Shimodaira S, Asano Y, Arai K, Iwaoka M. Selenoglutathione diselenide: unique redox reactions in the GPx-like catalytic cycle and repairing of disulfide bonds in scrambled protein. Biochemistry. 2017;56:5644-53
23. Wessjohann LA, Schneider A, Abbas M, Brandt W. Selenium in chemistry and biochemistry in comparison to sulfur. Biol Chem. 2007;388:997-1006
24. DeAngelo SL, Győrffy B, Koutmos M, Shah YM. Selenoproteins and tRNA-Sec: regulators of cancer redox homeostasis. Trends Cancer. 2023;9:1006-18
25. Burk RF, Hill KE. Regulation of selenium metabolism and transport. Annu Rev Nutr. 2015;35:109-34
26. Kim SJ, Choi MC, Park JM, Chung AS. Antitumor effects of selenium. Int. J. Mol. Sci. 2021;22:874
27. Morán-Serradilla C, Angulo-Elizari E, Henriquez-Figuereo A, Sanmartín C, Sharma AK, Plano D. Seleno-metabolites and their precursors: a new dawn for several illnesses? Metabolites. 2022;12:874
28. Ye R, Huang J, Wang Z, Chen Y, Dong Y. The role and mechanism of essential selenoproteins for homeostasis. Antioxidants. 2022;11:973
29. Steinbrenner H, Duntas LH, Rayman MP. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. 2022;50:102236
30. Barchielli G, Capperucci A, Tanini D. The role of selenium in pathologies: an updated review. Antioxidants. 2022;11:973
31. Rooseboom M, Commandeur JNM, Vermeulen NPE. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol Rev. 2004;56:53-102
32. Kang D, Lee J, Wu C, Guo X, Lee BJ, Chun J-S. et al. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp Mol Med. 2020;52:1198-208
33. Beilstein MA, Whanger PD. Selenium metabolism and glutathione peroxidase activity in cultured human lymphoblasts. Effects of transsulfuration defects and pyridoxal phosphate. Biol Trace Elem Res. 1992;35:105-18
34. Suzuki KT, Kurasaki K, Suzuki N. Selenocysteine beta-lyase and methylselenol demethylase in the metabolism of Se-methylated selenocompounds into selenide. Biochim Biophys Acta. 2007;1770:1053-61
35. Andreadou I, Menge WM, Commandeur JN, Worthington EA, Vermeulen NP. Synthesis of novel Se-substituted selenocysteine derivatives as potential kidney selective prodrugs of biologically active selenol compounds: evaluation of kinetics of beta-elimination reactions in rat renal cytosol. J Med Chem. 1996;39:2040-6
36. Weekley CM, Harris HH. Which form is that?. The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem Soc Rev. 2013;42:8870-94
37. Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R. et al. Characterization of mammalian selenoproteomes. Science. 2003;300:1439-43
38. Harris HR, Bergkvist L, Wolk A. Selenium intake and breast cancer mortality in a cohort of Swedish women. Breast Cancer Res Treat. 2012;134:1269-77
39. Cai X, Wang C, Yu W, Fan W, Wang S, Shen N. et al. Selenium Exposure and Cancer Risk: an Updated Meta-analysis and Meta-regression. Sci Rep. 2016;6:19213
40. Chen Y-C, Prabhu KS, Das A, Mastro AM. Dietary selenium supplementation modifies breast tumor growth and metastasis. Int J Cancer. 2013;133:2054-64
41. Lu J, Holmgren A. Selenoproteins. J Biol Chem. 2009;284:723-7
42. Valdiglesias V, Pásaro E, Méndez J, Laffon B. In vitro evaluation of selenium genotoxic, cytotoxic, and protective effects: a review. Arch Toxicol. 2010;84:337-51
43. Labunskyy VM, Hatfield DL, Gladyshev VN. Selenoproteins: molecular pathways and physiological roles. Physiol Rev. 2014;94:739-77
44. Liu S, Wei W, Wang J, Chen T. Theranostic applications of selenium nanomedicines against lung cancer. J Nanobiotechnology. 2023;21:96
45. Pan S, Guan J, Xianyu B, Tan Y, Li T, Xu H. A nanotherapeutic strategy to reverse NK cell exhaustion. Adv Mater. 2023;35:e2211370
46. Avery JC, Hoffmann PR. Selenium, selenoproteins, and immunity. Nutrients. 2018;10:1203
47. Davis CD, Tsuji PA, Milner JA. Selenoproteins and cancer prevention. Annu Rev Nutr. 2012;32:73-95
48. Hatfield D. Selenium: its molecular biology and role in human health. Free Radical Res. 2002;36:235
49. Brigelius-Flohé R, Flohé L. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid Redox Signal. 2020;33:498-516
50. Lee S, Lee EK, Kang DH, Lee J, Hong SH, Jeong W. et al. Glutathione peroxidase-1 regulates ASK1-dependent apoptosis via interaction with TRAF2 in RIPK3-negative cancer cells. Exp Mol Med. 2021;53:1080-91
51. Ren Z, Liang H, Galbo PM, Dharmaratne M, Kulkarni AS, Fard AT. et al. Redox signaling by glutathione peroxidase 2 links vascular modulation to metabolic plasticity of breast cancer. Proc Natl Acad Sci U S A. 2022;119:e2107266119
52. Demircan K, Bengtsson Y, Sun Q, Brange A, Vallon-Christersson J, Rijntjes E. et al. Serum selenium, selenoprotein P and glutathione peroxidase 3 as predictors of mortality and recurrence following breast cancer diagnosis: A multicentre cohort study. Redox Biol. 2021;47:102145
53. Ren X, Zou L, Zhang X, Branco V, Wang J, Carvalho C. et al. Redox signaling mediated by thioredoxin and glutathione systems in the central nervous system. Antioxid Redox Signal. 2017;27:989-1010
54. Fan Z, Song J, Guan T, Lv X, Wei J. Efficient expression of glutathione peroxidase with chimeric tRNA in amber-less escherichia coli. ACS Synth Biol. 2018;7:249-57
55. Long M, Wu J, Hao J, Liu W, Tang Y, Li X. et al. Selenocystine-induced cell apoptosis and S-phase arrest inhibit human triple-negative breast cancer cell proliferation. In Vitro Cell Dev Biol Anim. 2015;51:1077-84
56. Wang K, Fu X-T, Li Y, Hou Y-J, Yang M-F, Sun J-Y. et al. Induction of S-phase arrest in human glioma cells by selenocysteine, a natural selenium-containing agent via triggering reactive oxygen species-mediated DNA damage and modulating MAPKs and AKT pathways. Neurochem Res. 2016;41:1439-47
57. Chen T, Wong Y-S. Selenocystine induces S-phase arrest and apoptosis in human breast adenocarcinoma MCF-7 cells by modulating ERK and Akt phosphorylation. J Agric Food Chem. 2008;56:10574-81
58. Wang W, Meng F-B, Wang Z-X, Li X, Zhou D-S. Selenocysteine inhibits human osteosarcoma cells growth through triggering mitochondrial dysfunction and ROS-mediated p53 phosphorylation. Cell Biol Int. 2018;42:580-8
59. Chen T, Wong YS. Selenocystine induces apoptosis of A375 human melanoma cells by activating ROS-mediated mitochondrial pathway and p53 phosphorylation. Cell Mol Life Sci. 2008;65:2763-75
60. Chen T, Wong Y-S. Selenocystine induces caspase-independent apoptosis in MCF-7 human breast carcinoma cells with involvement of p53 phosphorylation and reactive oxygen species generation. Int J Biochem Cell Biol. 2009;41:666-76
61. Fan C-D, Fu X-Y, Zhang Z-Y, Cao M-Z, Sun J-Y, Yang M-F. et al. Selenocysteine induces apoptosis in human glioma cells: evidence for TrxR1-targeted inhibition and signaling crosstalk. Sci Rep. 2017;7:6465
62. Lin H, Yin L, Chen B, Ji Y. Design of functionalized magnetic silica multi-core composite nanoparticles for synergistic magnetic hyperthermia/radiotherapy in cancer cells. Colloids Surf B Biointerfaces. 2022;219:112814
63. Suzuki M, Endo M, Shinohara F, Echigo S, Rikiishi H. Differential apoptotic response of human cancer cells to organoselenium compounds. Cancer Chemother Pharmacol. 2010;66:475-84
64. Sinha R, Pinto JT, Facompre N, Kilheffer J, Baatz JE, El-Bayoumy K. Effects of naturally occurring and synthetic organoselenium compounds on protein profiling in androgen responsive and androgen independent human prostate cancer cells. Nutr Cancer. 2008;60:267-75
65. Redman C, Scott JA, Baines AT, Basye JL, Clark LC, Calley C. et al. Inhibitory effect of selenomethionine on the growth of three selected human tumor cell lines. Cancer Lett. 1998;125:103-10
66. Menter DG, Sabichi AL, Lippman SM. Selenium effects on prostate cell growth. Cancer Epidemiol Biomarkers Prev. 2000;9:1171-82
67. Li T, Xiang W, Li F, Xu H. Self-assembly regulated anticancer activity of platinum coordinated selenomethionine. Biomaterials. 2018;157:17-25
68. Ma J, Huang J, Sun J, Zhou Y, Ji X, Guo D. et al. L-Se-methylselenocysteine sensitizes lung carcinoma to chemotherapy. Cell Prolif. 2021;54:e13038
69. Liu Y, Liu X, Guo Y, Liang Z, Tian Y, Lu L. et al. Methylselenocysteine preventing castration-resistant progression of prostate cancer. Prostate. 2015;75:1001-8
70. Behera C, Sandha KK, Banjare N, Malik SB, Tabassum M, Kumar R. et al. Implication of methylselenocysteine in combination chemotherapy with gemcitabine for improved anticancer efficacy. Eur J Pharm Sci. 2022;176:106238
71. Li Z, Carrier L, Belame A, Thiyagarajah A, Salvo VA, Burow ME. et al. Combination of methylselenocysteine with tamoxifen inhibits MCF-7 breast cancer xenografts in nude mice through elevated apoptosis and reduced angiogenesis. Breast Cancer Res Treat. 2009;118:33-43
72. Rao L, Zhao S-K, Wen C, Tian R, Lin L, Cai B. et al. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv Mater. 2020;32:e2004853
73. Kumari N, Choi SH. Tumor-associated macrophages in cancer: recent advancements in cancer nanoimmunotherapies. J Exp Clin Cancer Res. 2022;41:68
74. Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res. 2021;81:1201-8
75. Song Z, Luo W, Zheng H, Zeng Y, Wang J, Chen T. Translational nanotherapeutics reprograms immune microenvironment in malignant pleural effusion of lung adenocarcinoma. Adv Healthc Mater. 2021;10:e2100149
76. He L, Lai H, Chen T. Dual-function nanosystem for synergetic cancer chemo-/radiotherapy through ROS-mediated signaling pathways. Biomaterials. 2015;51:30-42
77. Yu B, Li H, Zhang J, Zheng W, Chen T. Rational design and fabrication of a cancer-targeted chitosan nanocarrier to enhance selective cellular uptake and anticancer efficacy of selenocystine. J Mater Chem B. 2015;3:2497-504
78. Fan C, Chen J, Wang Y, Wong Y-S, Zhang Y, Zheng W. et al. Selenocystine potentiates cancer cell apoptosis induced by 5-fluorouracil by triggering reactive oxygen species-mediated DNA damage and inactivation of the ERK pathway. Free Radic Biol Med. 2013;65:305-16
79. Liu C, Lai H, Chen T. Boosting natural killer cell-based cancer immunotherapy with selenocystine/transforming growth factor-beta inhibitor-encapsulated nanoemulsion. ACS Nano. 2020;14:11067-82
80. Liu T, Xu L, He L, Zhao J, Zhang Z, Chen Q. et al. Selenium nanoparticles regulates selenoprotein to boost cytokine-induced killer cells-based cancer immunotherapy. Nano Today. 2020;35:100975
81. Steinbrenner H, Al-Quraishy S, Dkhil MA, Wunderlich F, Sies H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv Nutr. 2015;6:73-82
82. Ribot JC, Lopes N, Silva-Santos B. γδ T cells in tissue physiology and surveillance. Nat Rev Immunol. 2021;21:221-32
83. Hu Y, Liu T, Li J, Mai F, Li J, Chen Y. et al. Selenium nanoparticles as new strategy to potentiate γδ T cell anti-tumor cytotoxicity through upregulation of tubulin-α acetylation. Biomaterials. 2019;222:119397
84. Ma C, Hoffmann PR. Selenoproteins as regulators of T cell proliferation, differentiation, and metabolism. Semin Cell Dev Biol. 2021;115:54-61
85. Han X, Shen S, Fan Q, Chen G, Archibong E, Dotti G. et al. Red blood cell-derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci Adv. 2019;5:eaaw6870
86. Borst L, van der Burg SH, van Hall T. The NKG2A-HLA-E axis as a novel checkpoint in the tumor microenvironment. Clin Cancer Res. 2020;26:5549-56
87. Adjei IM, Jordan J, Tu N, Trinh TL, Kandell WM, Wei S. et al. Functional recovery of natural killer cell activity by nanoparticle-mediated delivery of transforming growth factor beta 2 small interfering RNA. J Interdiscip Nanomed. 2019;4:112-98
88. Wei Z, Yi Y, Luo Z, Gong X, Jiang Y, Hou D. et al. Selenopeptide nanomedicine activates natural killer cells for enhanced tumor chemoimmunotherapy. Adv Mater. 2022;34:e2108167
89. Lai H, Zeng D, Liu C, Zhang Q, Wang X, Chen T. Selenium-containing ruthenium complex synergizes with natural killer cells to enhance immunotherapy against prostate cancer via activating TRAIL/FasL signaling. Biomaterials. 2019;219:119377
90. Shao D, Zhang F, Chen F, Zheng X, Hu H, Yang C. et al. Biomimetic diselenide-bridged mesoporous organosilica nanoparticles as an X-ray-responsive biodegradable carrier for chemo-immunotherapy. Adv Mater. 2020;32:e2004385
91. Weekley CM, Aitken JB, Vogt S, Finney LA, Paterson DJ, de Jonge MD. et al. Metabolism of selenite in human lung cancer cells: X-ray absorption and fluorescence studies. J Am Chem Soc. 2011;133:18272-9
92. Deepagan VG, Kwon S, You DG, Nguyen VQ, Um W, Ko H. et al. In situ diselenide-crosslinked polymeric micelles for ROS-mediated anticancer drug delivery. Biomaterials. 2016;103:56-66
93. Gao X, Mi Y, Guo N, Xu H, Xu L, Gou X. et al. Cytokine-induced killer cells as pharmacological tools for cancer immunotherapy. Front Immunol. 2017;8:774
94. Du J, Gu Z, Yan L, Yong Y, Yi X, Zhang X. et al. Poly(vinylpyrollidone)- and selenocysteine-modified Bi2Se3 nanoparticles enhance radiotherapy efficacy in tumors and promote radioprotection in normal tissues. Adv Mater. 2017;29:1701268
95. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74
96. Somarribas Patterson LF, Vardhana SA. Metabolic regulation of the cancer-immunity cycle. Trends Immunol. 2021;42:975-93
97. Bader JE, Voss K, Rathmell JC. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell. 2020;78:1019-33
98. Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619-34
99. Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18:379-401
100. Yang Y, Bedford MT. Protein arginine methyltransferases and cancer. Nat Rev Cancer. 2013;13:37-50
101. Jacob C, Giles GI, Giles NM, Sies H. Sulfur and selenium: the role of oxidation state in protein structure and function. Angew Chem Int Ed. 2003;42:4742-58
102. Hou W, Xu H. Incorporating selenium into heterocycles and natural products─from chemical properties to pharmacological activities. J Med Chem. 2022;65:4436-56
103. Zhao Z, Laps S, Gichtin JS, Metanis N. Selenium chemistry for spatio-selective peptide and protein functionalization. Nat Rev Chem. 2024;8:211-29
Author contact
![]() Corresponding authors: wuyeedu.cn (Y. W), wdzhangycom (W.-D. Zhang), luanxinedu.cn (X. Luan).
Corresponding authors: wuyeedu.cn (Y. W), wdzhangycom (W.-D. Zhang), luanxinedu.cn (X. Luan).

 Global reach, higher impact
Global reach, higher impact