10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(11):4114-4127. doi:10.7150/ijbs.95463 This issue Cite
Research Paper
Nerve growth factor promote VCAM-1-dependent monocyte adhesion and M2 polarization in osteosarcoma microenvironment: Implications for larotrectinib therapy
1. Translational Medicine Research Center, China Medical University Hospital, Taichung, Taiwan
2. Department of Medical Laboratory Science and Biotechnology, Asia University, Taichung, Taiwan
3. Department of Sports Medicine, College of Health Care, China Medical University, Taichung, Taiwan
4. Department of Orthopedic Surgery, China Medical University Hospital, Taichung, Taiwan
5. Department of Orthopedic Surgery, China Medical University Beigang Hospital, Yunlin, Taiwan
6. Translational Medicine Center, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan
7. School of Oral Hygiene, College of Oral Medicine, Taipei Medical University, Taipei City, Taiwan.
8. Department of Pharmacology, School of Medicine, China Medical University, Taichung, Taiwan
9. Chinese Medicine Research Center, China Medical University, Taichung, Taiwan
10. Department of Medical Research, China Medical University Hsinchu Hospital, Hsinchu, Taiwan
Received 2024-2-19; Accepted 2024-7-9; Published 2024-8-1
Abstract
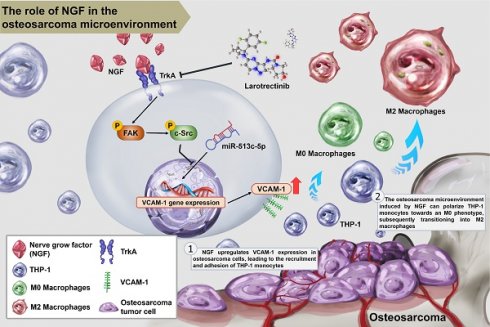
Osteosarcoma is the most prevalent form of primary malignant bone tumor, primarily affecting children and adolescents. The nerve growth factors (NGF) referred to as neurotrophins have been associated with cancer-induced bone pain; however, the role of NGF in osteosarcoma has yet to be elucidated. In osteosarcoma samples from the Genomic Data Commons data portal, we detected higher levels of NGF and M2 macrophage markers, but not M1 macrophage markers. In cellular experiments, NGF-stimulated osteosarcoma conditional medium was shown to facilitate macrophage polarization from the M0 to the M2 phenotype. NGF also enhanced VCAM-1-dependent monocyte adhesion within the osteosarcoma microenvironment by down-regulating miR-513c-5p levels through the FAK and c-Src cascades. In in vivo xenograft models, the overexpression of NGF was shown to enhance tumor growth, while the oral administration of the TrK inhibitor larotrectinib markedly antagonized NGF-promoted M2 macrophage expression and tumor progression. These results suggest that larotrectinib could potentially be used as a therapeutic agent aimed at mitigating NGF-mediated osteosarcoma progression.
Keywords: osteosarcoma, NGF, larotrectinib, M2 macrophage, miR-513c-5p
Introduction
Osteosarcoma is the most common form of primary malignant bone cancer, affecting mainly children and teenagers. Men are more susceptible to the disease than women, as indicated by a male-to-female ratio of 1.5-2:1 [1]. Common sites for osteosarcoma include the proximal tibia (19%), proximal humerus (10%), and distal femur (42%) [2, 3]. Recent advances in chemotherapy for non-metastatic osteosarcoma have boosted the five-year survival rate from 15% to 60% [4]. Note however that roughly 25% of osteosarcoma patients have metastases at the time of diagnosis [5], and the prognosis in these cases remains poor. Thus, the overall five-year survival rate for patients with metastatic osteosarcoma is below 30% [6]. This has prompted extensive research into the development of novel pre-treatment prognostic indicators and novel targeted medications as adjuvants to chemotherapy.
Researchers have established that malignant growth depends on interactions between tumor cells and cells within the tumor microenvironment [7, 8], which is a complex system involving a variety of cell types, including endothelial, fibroblast, and inflammatory cells (e.g., dendritic and macrophage cells) [7]. Most of these cells are macrophages, specific subpopulations of which are drawn in by cytokines released by the tumor [9, 10]. These tumor-associated macrophages (TAMs) can have a profound effect on tumor growth; however, the effect varies with phenotype. M1 macrophages are typically anti-tumoral, whereas M2 macrophages are typically pro-tumoral [11]. TAMs have been linked to the progression and spread of cancer within the tumor microenvironment [12, 13].
Vascular cell adhesion molecule 1 (VCAM-1) plays a role in controlling the growth, migration, and death of cancer cells [14, 15]. In osteosarcoma, VCAM-1 expression is correlated with tumor formation and disease stage [16]. Various growth agents have been shown to promote VCAM-1 expression over the course of osteosarcoma progression and metastasis [17, 18]. We hypothesize that diminishing VCAM-1 synthesis is a logical therapeutic strategy aimed at limiting osteosarcoma growth.
Tumor cells have the ability to stimulate the formation of sympathetic and sensory nerves, which can lead to cancer-induced bone pain (CIBP) [19], the treatment of which becomes increasingly difficult as the tumor grows [20]. The mechanisms underlying CIBP include a variety of neuropathological events, including inflammation, ischemia, compression, or damage [21]. Researchers have recently reported a strong correlation between CIBP and elevated nerve growth factor (NGF) expression levels [21]. NGF is an important neurotrophic factor, which promotes the development of sympathetic and sensory nerves. It is abundantly expressed in the tumor microenvironment, where it interacts with various cell types to facilitate cancer progression [22]. NGF is catalyzed by the high-affinity receptor tropomyosin receptor kinase A (TrkA), which is a kinase shown to regulate tumor growth, neuronal differentiation, neural proliferation, and nociceptor responsiveness as well as prevent programmed cell death [22, 23]. The NGF/TrkA axis has been implicated in the metastasis of various cancers, including breast cancer [24], chondrosarcoma [25], colon cancer [26], pancreatic cancer [27], and prostate cancer [28]. Studies have shown that blocking NGF or TrkA expression can significantly slow the growth of tumors, reduce pain-related activities, and ameliorate bone loss in sarcoma patients [25, 29].
Researchers in the field of medicinal chemistry have made considerable strides in elucidating TrK inhibitors. Larotrectinib (LOXO-101) is a highly specific small molecule inhibitor of the entire TrK family, including TrkA, TrkB, and TrkC. The FDA has approved its usage in the treatment of malignancies that are TRK fusion-positive, including lung [30] and breast [31] cancer. In the current study, we determined that NGF facilitates VCAM-1-dependent monocyte adhesion and M2 polarization in the osteosarcoma microenvironment. We also determined that larotrectinib inhibits NGF-mediated effects in vitro and in vivo, which suggests the potential of using larotrectinib to develop novel therapeutic agents for osteosarcoma.
Materials and Methods
NGF recombinant protein was obtained from PeproTech (Rocky Hill, NJ, USA). p-FAK (Tyr397) (Catalog No: #3283) and p-c-Src (Tyr416) (Catalog No: #2101) antibodies were obtained from Cell Signaling (Danvers, MA, USA). FAK (Tyr397) (GTX129840) antibodies were purchased from GeneTex (Hsinchu, Taiwan). c-Src (Catalog No: 25978-1-AP), CD163 (Catalog No: 16646-1-AP), and CD206 (Catalog No: 18704-1-AP) antibodies were purchased from Proteintech (Rosemont, IL, USA). VCAM-1 (EPR5047) (Catalog No: ab134047) and NGF (EP1320Y) antibodies (Catalog No: ab52918) were obtained from Abcam (Cambridge, MA, USA). β-actin antibodies (Catalog No: a5441) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Cell culture supplements were obtained from Invitrogen (Carlsbad, CA, USA). siRNAs against VCAM-1, FAK, c-Src, and controls were purchased from Dharmacon (Lafayette, CO, USA). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Analysis of mRNA expression profiles from the Genomic Data Commons (GDC)
Transcriptome profiles of osteosarcoma were accessed using the TCGA database via the GDC database. RNA-Seq analysis was performed on osteosarcoma samples to derive gene expression profiles of macrophage markers and the neurotrophin family [32].
Cell cultures
Human osteosarcoma cell lines 143B and MG63 and the human monocyte cell line THP-1 were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 10% FBS, penicillin, and streptomycin at 37°C under a humidified atmosphere of 5% CO2 [33].
THP-1 cells were differentiated into M0 macrophages via stimulation with 100 ng/ml phorbol 12-myristate 13-acetate (PMA) for 24 h followed by incubation in DMEM medium for 24 h.
Analysis monocyte adhesion to osteosarcoma
Osteosarcoma cells (1 × 105) were seeded in 12-well plates and allowed to reach a confluency of roughly 90%. The osteosarcoma cells were then exposed to NGF for 24 h. Monocytes were stimulated via exposure to BCECF-AM (10 μM) for 1 h, followed by co-culturing with osteosarcoma cells for 1 h. Non-adherent monocytes were then washed away, and adherent monocytes were quantified using a fluorescence microscope [34].
MicroRNA (miRNA) database searches
We employed the miRNA database miRWalk: Home (http://mirwalk.umm.uni-heidelberg.de/) to predict miRNAs that could potentially target the VCAM-1 gene. Incorporating the miRDB database with a filter set to a minimum threshold of 0.8 resulted in the identification of 14 miRNAs with potential binding affinity to the VCAM-1 gene.
Real-time quantitative PCR analysis of mRNA and miRNA
Total RNA was extracted from osteosarcomas using TRIzol reagent (MDBio; Taipei, Taiwan). Subsequently, 1 μg of RNA was reverse-transcribed into cDNA using an oligo-DT primer, in accordance with the protocol outlined by the manufacturer (Invitrogen; Carlsbad, CA, USA). Quantitative PCR (qPCR) was performed using SYBR Green with sequence-specific primers (Invitrogen; Carlsbad, CA, USA). qPCR assays were performed using the StepOnePlus system (Applied Biosystems; Foster City, CA, USA) [35, 36]. The sequences of all primers are listed in Table 1.
List of PCR and primer used for the experiments
| Target mRNA | Forward primer (5'→3') | Reverse primer (5'→3') |
|---|---|---|
| CD14 | GACCTAAAGATAACCGGCACC | GCAATGCTCAGTACCTTGAGG |
| CD68 | GGAAATGCCACGGTTCATCCA | TGGGGTTCAGTACAGAGATGC |
| CD80 | AAACTCGCATCTACTGGCAAA | GGTTCTTGTACTCGGGCCATA |
| IL-1b | TTCGACACATGGGATAACGAGG | TTTTTGCTGTGAGTCCCGGAG |
| CD163 | GCGGGAGAGTGGAAGTGAAAG | GTTACAAATCACAGAGACCGCT |
| CD206 | GGGTTGCTATCACTCTCTAGC | TTTCTTGTCTGTTGCCGTAGTT |
| VCAM-1 | TTTGACAGGCTGGAGATAGACT | TCAATGTGTAATTTAGCTCGGCA |
| GAPDH | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
Western blot analysis
Protein samples extracted from osteosarcoma cells using RIPA buffer underwent electrophoretic separation using SDS-PAGE gels (7.5-12%) prior to transfer onto PVDF membranes (Merck; Darmstadt, Germany). After blocking with 5% non-fat milk, the membranes were exposed to primary antibodies at 4°C overnight and then exposed to specific secondary antibodies at room temperature for 1 h. Target protein expression was detected using an ECL kit (Millipore, USA) and visualized using the ImageQuant™ LAS 4000 biomolecular imager [37-39]. Bands were subsequently digitized using UN-SCAN-IT gel 6.1 software. Graphs were generated using GraphPad Prism after normalizing the data using Microsoft Excel.
Mouse xenograft models
Male nude mice were obtained from BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan). All procedures involving animal studies were granted approval by the Institutional Animal Care and Use Committee and conducted in accordance with the Guidelines of Animal Experimentation set forth by Shin Kong Wu Ho-Su Memorial Hospital (Protocol No. 113SKH007). Under 1.5-2.5% isoflurane anesthesia, 5 × 106 143B or 143B/NGF cells were subcutaneously injected into the right flank of each animal in a solution comprising 50% serum-free DMEM and 50% Matrigel (total volume of 100 μL). The 143B/NGF+Larotrectinib group was also administered larotrectinib (50 mg/kg) orally three times a week. After a four-week tumor growth period, the mice were euthanized via CO2 inhalation. The harvested tumors were fixed in 10% formalin in preparation for analysis [25, 40].
Immunohistochemistry (IHC)
Immunohistochemistry assays were conducted on mouse tissues. The primary antibodies employed in immunohistochemical analysis were CD163, CD206, and VCAM-1 diluted at a ratio of 1:250. Quantification was performed in accordance with the protocol detailed in our prior publications [41, 42]. Following the application of biotin-labeled secondary antibodies, the antibody binding signal was visualized using the NovoLink Polymer Detection System (Leica Biosystems Inc, IL, US) using 3,3'-diaminobenzidine with hematoxylin for counterstaining. Positive expression levels were quantified by IHC staining results, based on scores ranging from 1 (weak) to 5 (strong) [43].
Statistical analysis
The data are presented as mean ± standard deviation (SD). Statistical analysis was performed using the two-tailed Student's t-test to determine the significance of between-group differences, with the level of significance set at 0.05.
Results
NGF facilitated the polarization of macrophages to the M2 phenotype in the osteosarcoma microenvironment
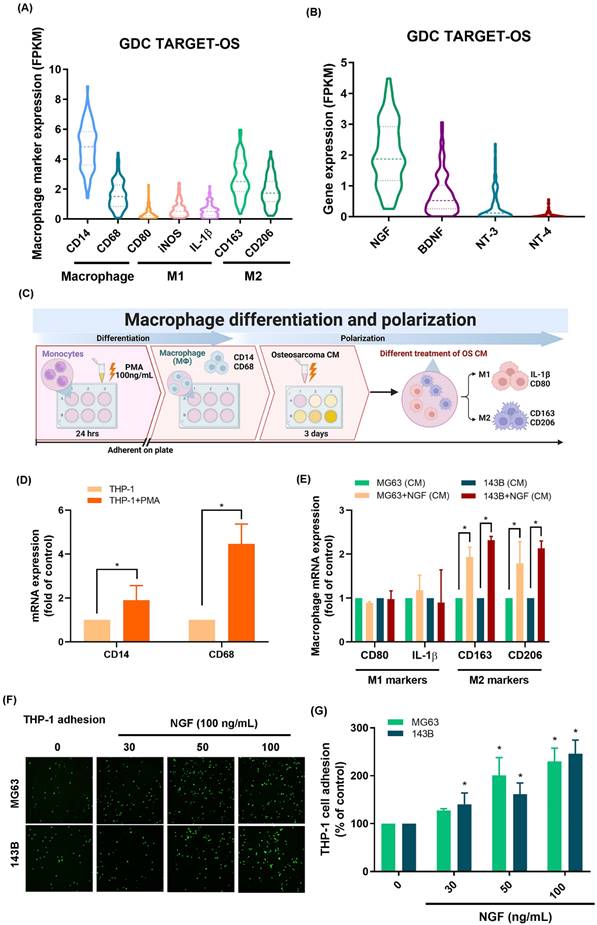
Researchers have previously demonstrated that TAMs exhibiting the M2 phenotype can accelerate tumor growth [7]. Analysis of 88 osteosarcoma tissue samples from the GDC data portal revealed elevated CD14 and CD68 levels (macrophage markers) as well as the presence of CD163 and CD206 (M2 macrophage markers); however, M1 macrophage markers (CD80, iNOS and IL-1β) were not detected (Fig. 1A).
As for neurotrophin factors, it was found that the expression level of NGF mRNA exceeded those of BDNF, NT-3, and NT-4 (Fig. 1B). We then determined whether NGF promoted the polarization of M2 macrophages (Fig. 1C). Stimulating THP-1 cells with PMA resulted in the differentiation of THP-1 cells into M0 macrophages, as evidenced by the mRNA expression of M0 markers CD14 and CD68 (Fig. 1D). Exposing M0 macrophages to NGF-treated osteosarcoma conditioned medium was also shown to enhance polarization to the M2 phenotype, but not to the M1 phenotype (Fig. 1E). These findings suggest that in the osteosarcoma microenvironment, NGF enhances the polarization of naïve macrophages into the M2 phenotype.
NGF facilitated VCAM-1-dependent monocyte adhesion to osteosarcoma
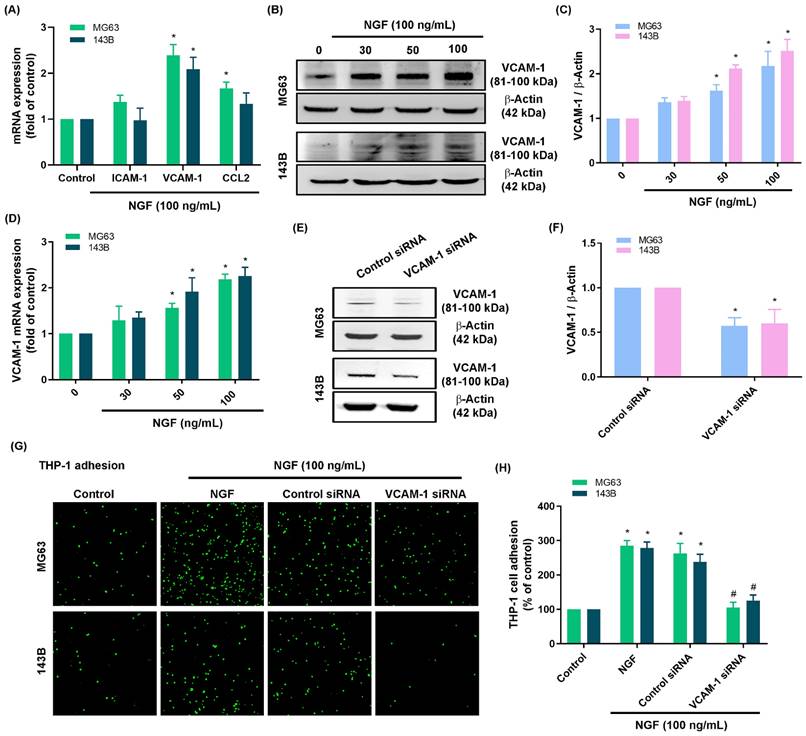
The infiltration of macrophages into the tumor microenvironment is a critical step in tumor proliferation and growth. Treating osteosarcoma cell lines (143B and MG63 cells) with NGF was shown to increase monocyte adhesion in a concentration-dependent manner (Fig. 1F&G). The screening of candidate targets in NGF-treated osteosarcomas revealed that the NGF-induced upregulation of VCAM-1 was more pronounced than that of ICAM-1 or CCL2 (Fig. 2A). NGF also enhanced VCAM-1 protein and mRNA expression levels in a concentration-dependent manner (Fig. 2B-D). Osteosarcoma cells transfected with VCAM-1 siRNA were shown to antagonize VCAM-1 expression and moderate NGF-induced monocyte adhesion (Fig. 2E-H), which indicates that NGF augments VCAM-1-dependent monocyte adhesion to osteosarcoma.
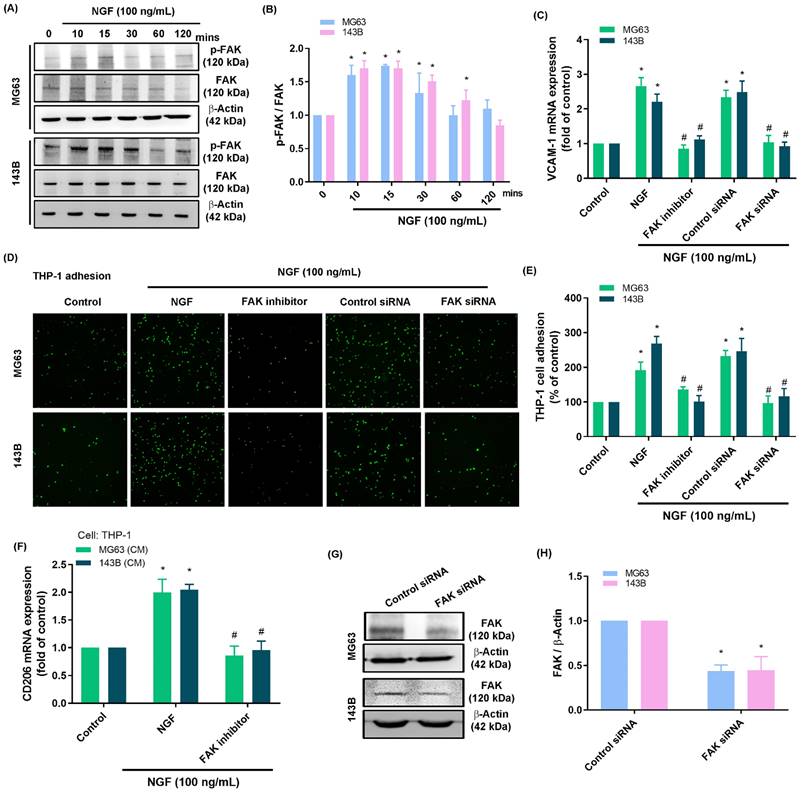
NGF induced VCAM-1 production and monocyte adhesion by inhibiting miR-513c-5p expression via the FAK and c-Src pathways
Activation of the FAK and c-Src pathways is an essential step in the progression of osteosarcoma [44]. Stimulating osteosarcomas with NGF was shown to enhance FAK and c-Src phosphorylation in a time-dependent manner (Fig. 3A&B and 4A&B). Stimulating osteosarcoma cells with an FAK inhibitor or c-Src inhibitor (PP2) eliminated VCAM-1 synthesis, monocyte adhesion, and CD206 expression (Fig. 3C-E and 4C-E). Administration of the supernatant collected from these cells to THP-1 cells affected the expression of M2 macrophage marker CD206 mRNA expression (Fig. 3F and 4F). Transfecting osteosarcoma cells with FAK or c-Src siRNA was also shown to reduce VCAM-1 synthesis and monocyte adhesion (Fig. 3C-H and 4C-H). Stimulating osteosarcoma cells with an FAK inhibitor suppressed the NGF-facilitated phosphorylation of c-Src (Fig. 4I&J). These results suggest that FAK and c-Src pathway activation is involved in the NGF-mediated synthesis of VCAM-1 and monocyte adhesion.
Researchers have compiled considerable evidence that miRNAs play crucial roles in the progression and apoptosis of cancer cells [45, 46]. In the current study, we searched the miRWalk and miRDB online databases to identify miRNA targets within the 3'-UTR region of VCAM-1 mRNA (Fig. 5A). Among the 14 candidate miRNAs, miR-513c-5p presented the most pronounced down-regulation after NGF stimulation (Fig. 5B&C). Exposure to NGF markedly suppressed miR-513c-5p generation in two osteosarcoma cell lines in a concentration-dependent manner (Fig. 5D). The schematic diagram in Fig. 5E shows that the VCAM-1 3'-UTR contains a miR-513c-5p binding site. We investigated the impact of miR-513c-5p binding to the VCAM-1 3'-UTR, the results show that NGF was shown to enhance the luciferase activity of the wild-type VCAM-1 3'-UTR, whereas the mutant VCAM-1 3'-UTR generated no such response (Fig. 5F). Transfecting osteosarcoma cells with an miR-513c-5p mimic significantly reduced NGF-induced monocyte adhesion, VCAM-1, and CD206 expression (Fig. 5G-I). Administration of the supernatant collected from these cells to THP-1 cells affected the expression of M2 macrophage marker CD206 mRNA expression (Fig. 5J). Pre-treating osteosarcoma cells with FAK and c-Src inhibitors or their respective siRNAs blocked the effects of NGF in repressing miR-513c-5p expression (Fig. 5K&L). These results suggest that NGF augments VCAM-1-dependent monocyte adhesion by suppressing miR-513c-5p generation via the FAK and c-Src pathways.
Larotrectinib blocked NGF-induced tumor growth of osteosarcoma cells
The effects of NGF were examined in vivo by generating an osteosarcoma cell line that overexpressed NGF (143B/NGF). It was found that 143B/NGF cells promoted NGF and VCAM-1 expression and monocyte adhesion (Fig. 6A-E). Larotrectinib is an orally administered ATP-competitive inhibitor of the TrK family, which blocks the expression of NGF [47]. Cell viability assays demonstrated that larotrectinib (10 - 100 μM) did not have cytotoxic effects on 143B/NGF cells (Fig. 6F). Larotrectinib was also shown to suppress monocyte adhesion to 143B/NGF cells in a concentration-dependent manner (Fig. 6G&H).
NGF facilitates the polarization of macrophages to the M2 phenotype in the osteosarcoma microenvironment. (A&B) mRNA expression of macrophage and neurotrophin factors in osteosarcoma tissue based on analysis of samples from the GDC data portal; (C) Schematic of Macrophage Differentiation and Polarization; (D) qPCR analysis of THP-1 cells, following incubation with PMA for 24 h; (E) qPCR analysis showing mRNA expression after treating osteosarcoma cells with NGF for 24 h and then applying conditioned medium to M0 macrophage; (F&G) Fluorescence microscope images of THP-1 cells adhered to osteosarcoma cells, following incubation with NGF for 24 h. All experiments were repeated at least three times. * p < 0.05 compared with the control group.
NGF enhances VCAM-1 generation and monocyte adhesion to osteosarcoma. (A) qPCR results indicating mRNA expression after treating osteosarcoma cells with NGF for 24 h; (B-D) Western blot and qPCR results indicating VCAM-1 expression after treating osteosarcoma cells with NGF for 24 h; (E&F) Western blot analysis indicating VCAM-1 expression following transfection of osteosarcoma cells with VCAM-1 siRNA; (G&H) THP-1 adhesion in osteosarcomas transfected with VCAM-1 siRNA and then stimulated with NGF for 24 h. All experiments were repeated at least three times. * p < 0.05 compared with the control group; # p < 0.05 compared with the NGF-treated group.
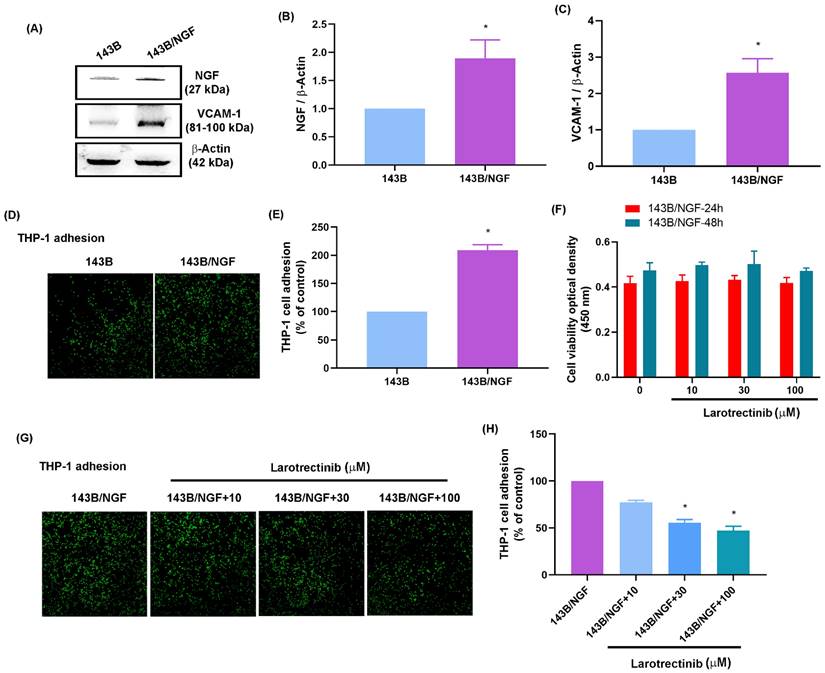
In an in vivo xenograft model involving the subcutaneous injection of osteosarcoma cells into the right flank of mice, it was found that the resulting tumor volume and weight of the overexpressing NGF cell line (143B/NGF) were markedly greater than those of the 143B cell line (Fig. 7A-C). The oral administration of larotrectinib significantly inhibited NGF-mediated tumor growth (Fig. 7A-C). IHC staining revealed that NGF overexpression enhanced CD163, CD206, and VCAM-1 expression (Fig. 7D-G). We also observed that CD163 and CD206 were positively correlated with VCAM-1 expression levels (Fig. 7H&I). Moreover, larotrectinib inhibited the expression of CD163, CD206, and VCAM-1 (Fig. 7D-G). Taken together, these findings suggest that the NGF-promoted growth of osteosarcoma involves macrophage infiltration and M2 macrophages expression. Our findings also indicate that larotrectinib suppresses these effects.
Discussion
Researchers are amassing considerable evidence of a correlation between cancer prognosis and tumor innervation, particularly in cancers with pronounced innervation, such as pancreatic, head and neck, prostate, and colorectal cancers [48]. Analysis of patient tumor samples has revealed a positive correlation between tumor innervation density and the rates of metastasis, incidence, and mortality. Note that the extensive distribution of sensory nerves in bones often leads to CIBP in individuals with osteosarcoma [49]. Researchers have also demonstrated that NGF secreted by tumors stimulates nerve regeneration and plays a key role in promoting the survival, motility, and invasion of tumor cells [50]. Moreover, the knockout of Circ_0000006 potentiates the effects of doxorubicin on osteosarcoma-repressed cell proliferation, migration, and invasion via the miR-646/BDNF pathway [51]. Exosomes have been implicated in the secretion of cellular hormones (including NGF) fostering an inflammatory and immunosuppressive microenvironment [48]. Macrophages are highly adaptive cells capable of migration into and adaption to the cancer microenvironment. Several researchers have posited that tumors be viewed as complex microenvironments, rather than individual tumor cells [52]. In the current study, we obtained in vitro and in vivo evidence of NGF facilitating VCAM-1-dependent monocyte adhesion and M2 macrophage polarization in the osteosarcoma microenvironment.
NGF increases VCAM-1 production and monocyte adhesion to osteosarcomas via FAK signaling. (A&B) Western blot analysis showing FAK phosphorylation in osteosarcomas stimulated with NGF; (C-E) THP-1 adhesion and VCAM-1 expression in osteosarcomas incubated with FAK inhibitor or transfected with FAK siRNA and then stimulated with NGF for 24 h; (F) The supernatant collected from osteosarcoma cells to THP-1 cells affected the expression of M2 macrophage marker CD206 mRNA expression; (G&H) Western blot analysis showing FAK expression following transfection of osteosarcoma cells with FAK siRNA. All experiments were repeated at least three times. * p < 0.05 compared with the control group; # p < 0.05 compared with the NGF-treated group.
NGF increases VCAM-1 production and monocyte adhesion to osteosarcomas via c-Src signaling. (A&B) Western blot analysis indicating c-Src phosphorylation after stimulating osteosarcoma cells with NGF; (C-E) THP-1 adhesion and VCAM-1 expression in osteosarcoma cells incubated with PP2 or transfected with c-Src siRNA and then stimulated with NGF for 24 h; (F) The supernatant collected from osteosarcoma cells to THP-1 cells affected the expression of M2 macrophage marker CD206 mRNA expression; (G&H) Western blot analysis showing c-Src expression following transfection of osteosarcoma cells with c-Src siRNA; (I&J) Western blot analysis showing c-Src phosphorylation after incubating osteosarcoma cells with FAK inhibitor followed by stimulation with NGF. All experiments were repeated at least three times. * p < 0.05 compared with the control group; # p < 0.05 compared with the NGF-treated group.
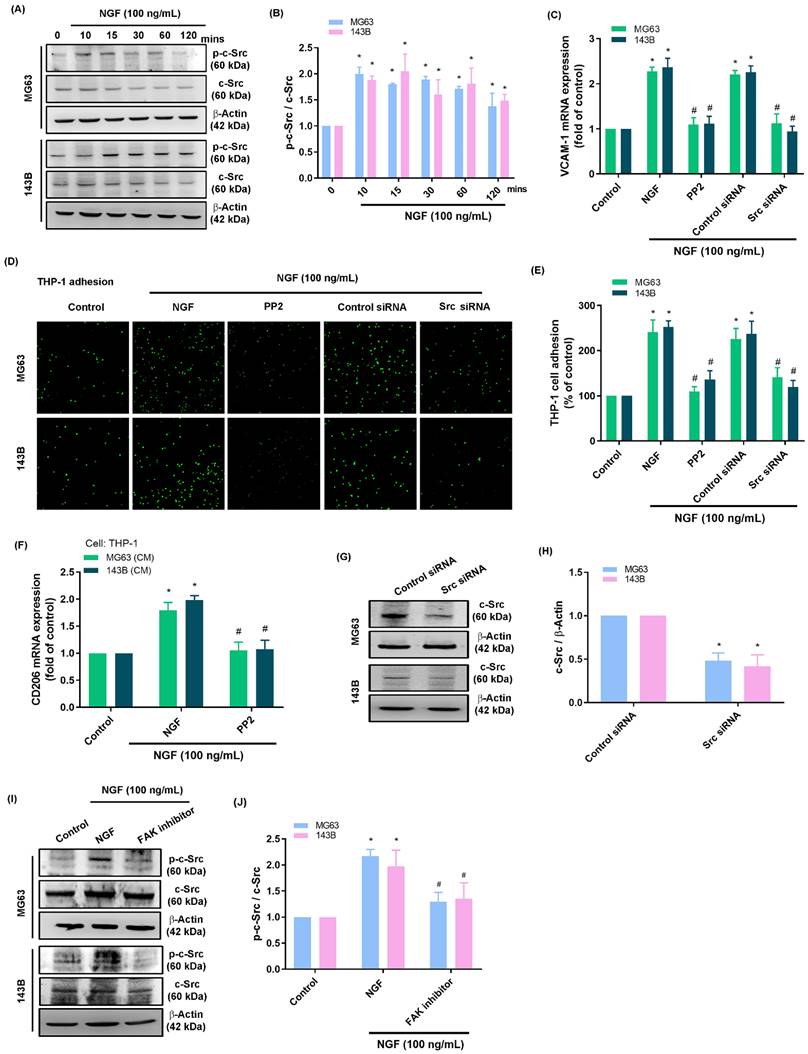
NGF promotes VCAM-1 production and monocyte adhesion through the down-regulation of miR-513c-5p expression. (A) miRNAs predicted to bind with VCAM-1 3'-UTR based on analysis of two miRNA prediction databases; (B-D) qPCR results showing miRNA expression after treating osteosarcoma cells with NGF; (E) miR-513c-5p binding site in VCAM-1 3'-UTR; (F) Osteosarcoma cells were transfected with the VCAM-1 3'-UTR wild-type or mutant plasmid for 24 h, then stimulated with NGF for 24 h, and relative luciferase activity was measured; (G-I) THP-1 adhesion and VCAM-1 expression in osteosarcomas transfected with miR-513c-5p mimic and then stimulated with NGF for 24 h; (J) The supernatant collected from osteosarcoma cells to THP-1 cells affected the expression of M2 macrophage marker CD206 mRNA expression; (K&L) miRNA expression in osteosarcoma cells incubated with FAK inhibitor and PP2 or transfected with FAK and c-Src siRNA and then stimulated with NGF for 24 h. All experiments were repeated at least three times. * p < 0.05 compared with the control group; # p < 0.05 compared with the NGF-treated group.
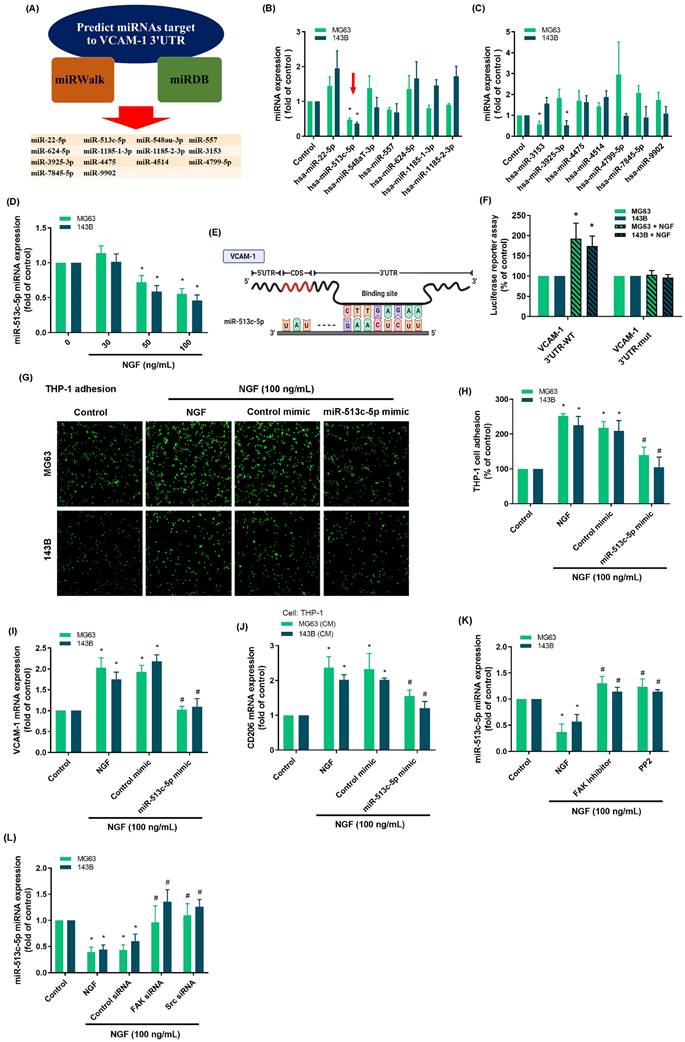
Larotrectinib blocks NGF-induced monocyte adhesion to osteosarcomas. (A-C) Western blot and qPCR results showing NGF and VCAM-1 expression in 143B and NGF-overexpressing (143B/NGF) cells; (D&E) Adhesion of THP-1 cells to 143B and 143B/NGF cells; (F) MTT assay results indicating viability of 143B/NGF cells incubated with larotrectinib for 24 or 48 h; (G&H) THP-1 adhesion in osteosarcoma cells incubated with larotrectinib and then stimulated with NGF for 24 h. All experiments were repeated at least three times. * p < 0.05 compared with the control group; # p < 0.05 compared with the NGF-treated group.
Receptor tyrosine kinases are important signaling molecules that regulate the proliferation, survival, differentiation, apoptosis, and migration of cells [53]. The aberrant activation of tyrosine kinases has been linked to a variety of cancers. The activation of non-mutated kinases can lead to genomic alterations and contribute to the development and metastasis of various cancers, particularly those with a low mutation rate (e.g., prostate cancer).
Researchers have identified several tyrosine kinase inhibitors (TKIs) with varying degrees of activity against TRKA, TRKB, and/or TRKC. These TKIs can be broadly classified as multi-kinase inhibitors with activity against a range of targets, including TRK or more selective TRK inhibitors [53]. Multi-kinase inhibitors include entrectinib, crizotinib, cabozantinib, lestaurtinib, altiratinib, foretinib, ponatinib, nintedanib, merestinib, MGCD516, PLX7486, DS-6051b, and TSR-011 [54]. First-generation tropomyosin receptor kinase (TrK) inhibitors (larotrectinib and entrectinib) act as ATP-competitive inhibitors specifically binding to the ATP binding site in the active conformation of their target enzyme (DFG-in) [53]. Currently, larotrectinib is the most specific TrK family inhibitor undergoing assessment for the treatment of cancer. It is also a novel candidate for targeting the NGF-mediated progression of osteosarcoma.
The tumor microenvironment plays an important role in the development and spread of tumors. Recent research has revealed that the infiltration of inflammatory cells into the stroma surrounding the tumor is crucial to its growth [55, 56]. Macrophages are the most prevalent inflammatory cells in the tumor environment. Previous research has demonstrated that macrophages can easily penetrate the borders of tumors [57, 58]. In the current study, our aim was to elucidate the means by which NGF enhances monocyte adhesion in the osteosarcoma microenvironment. The GDC data portal revealed elevated levels of M1 macrophage markers (CD163 and CD206) without any M2 macrophage markers in osteosarcoma samples. The overexpression of NGF has been shown to promote tumor growth and the expression of M2 macrophage markers in vivo. Our data suggest that the effects of NGF in facilitating the growth of osteosarcoma involve the expression of M2 macrophage markers.
Larotrectinib antagonizes NGF-induced osteosarcoma growth in vivo. (A-C) Osteosarcoma cells were subcutaneously injected into the right flanks of mice. The 143B/NGF+Larotrectinib group was administered larotrectinib (50 mg/kg) orally three times a week. The tumor size and weight were measured after sacrificing the mice at four weeks; (D-G) IHC analysis indicating protein expression levels of CD163, CD206, and VCAM-1 in tumors; (H&I) Correlation between CD163 or CD206 versus VCAM-1 expression levels. All experiments were repeated at least three times. * p < 0.05 compared with the 143B group; # p < 0.05 compared with the 143B/NGF group.
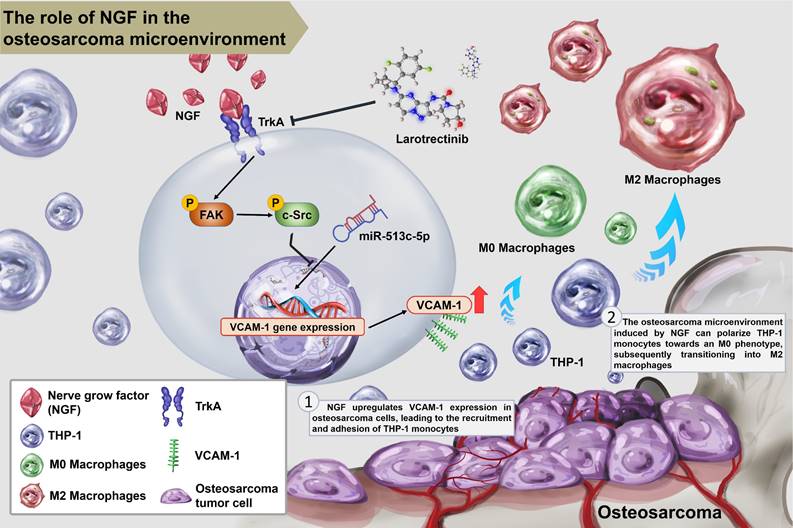
Schematic diagram illustrating the mechanism underlying the effects of NGF in an osteosarcoma microenvironment. NGF facilitates VCAM-1-dependent monocyte invasion into the osteosarcoma microenvironment and then enhances M2 macrophage polarization by inhibiting miR-513c-5p levels through the FAK and c-Src signaling cascades. Larotrectinib effectively suppresses NGF-induced cell growth.
FAK-induced c-Src pathway activation has been shown to play a crucial role in mediating various cellular activities [59, 60]. In the current study, we discovered that FAK and c-Src inhibitors moderated the effects of NGF in promoting VCAM-1 production as well as monocyte adhesion. Genetic inhibition induced by FAK and c-Src siRNAs yielded similar effects. Incubating osteosarcoma cells with NGF was shown to augment FAK and c-Src phosphorylation. The fact that FAK inhibitors reversed NGF-enhanced c-Src phosphorylation suggests that in human osteosarcoma, NGF activates the FAK and c-Src signaling pathways and in so doing promotes VCAM-1-regulated monocyte adhesion. Multiple lines of evidence highlight the significance of the FAK and c-Src pathways in tumor functions. For instance, amphiregulin induces VEGF-A synthesis and angiogenesis in human chondrosarcoma via the FAK/c-Src pathway [61]. Moreover, D-pinitol interferes with the motility of prostate cancer cells by suppressing FAK and c-Src cascades [62]. Finally, NGF has been shown to facilitate chondrosarcoma metastasis through the activation of FAK/c-Src signaling [29].
miRNAs have been implicated in a number of diseases, such as cancer, obesity, cardiovascular diseases, and diabetes [63, 64], and recent research has demonstrated that miRNAs play key roles in the cancer microenvironment. For example, miR-30a antagonists inhibit LOX expression and affect thyroid cancer differentiation [65]. Moreover, the expression of miR-141-3p and miR-145-5p has been shown to suppress the migration and invasion of renal cell carcinoma [66]. In chondrosarcoma, miR-27b and miR-519d are regulated by adipocytokines [15, 16, 51]. Bioinformatics analysis in this study identified VCAM-1 as a direct target of miR-513c-5p. This was the first study to demonstrate a correlation between the miR-513c-5p/VCAM-1 axis and macrophages in osteosarcoma. We demonstrated that miR-513c-5p expression was profoundly down-regulated after NGF treatment and that an miR-513c-5p mimic eliminated the NGF-mediated synthesis of VCAM-1 and cell monocyte adhesion. Moreover, the inhibition of FAK and c-Src countered the decrease in miR-513c-5p synthesis induced by NGF, which suggests that NGF modulates VCAM-1-dependent monocyte adhesion to osteosarcoma cells by inhibiting miR-513c-5p levels via the FAK and c-Src pathways.
Experiments in the current study confirmed that the oral administration of the TrK inhibitor larotrectinib significantly inhibited the effects of NGF on M2 macrophage levels and osteosarcoma progression; however, it is important to consider the complexity of the in vivo tumor microenvironment. Further research will be required to elucidate the effects of larotrectinib on other cells associated with osteosarcoma progression. Moreover, researchers will have to overcome various challenges in the use of molecular therapy to target TrK inhibitors. It will be necessary to identify selective inhibitors and assess their efficacy in preclinical and clinical studies. It will also be necessary to develop effective delivery methods for the transport of these compounds to target cells. Nonetheless, the targeting of TrK inhibitors remains a promising therapeutic strategy for various diseases, including osteosarcoma.
In summary, this study demonstrated that NGF augments VCAM-1-dependent monocyte adhesion within the osteosarcoma microenvironment and facilitates M2 macrophage polarization by inhibiting miR-513c-5p expression via the FAK and c-Src signaling cascades. Larotrectinib presented anti-tumor effects through the inhibition of NGF-promoted tumor growth (Fig. 8).
Acknowledgements
This study was supported by grants from Taiwan's Ministry of Science and Technology (MOST 111-2314-B-039-048-MY3), National Science and Technology Council (NSTC 112-2314-B-341-005 and NSTC 112-2320-B-039-035-MY3), China Medical University (CMU112-ASIA-05; CMU112-ASIA-10), China Medical University Hospital (DMR-113-200; DMR-113-070; DMR-113-071; DMR-113-201), and Shin Kong Wu Ho-Su Memorial Hospital (2024SKHBND002).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Li J, Zhang Y, Sun F, Zhang G, Pan XA, Zhou Q. Long Noncoding RNA PCGEM1 Facilitates Tumor Growth and Metastasis of Osteosarcoma by Sponging miR-433-3p and Targeting OMA1. Orthop Surg. 2023;15:1060-71
2. Wu X, Ma S, Wu Z, Zhao Q. Global scientific trends on matrix metalloproteinase and osteosarcoma: A bibliometric and visualized analysis. Frontiers in oncology. 2023;13:1064815
3. Hou CH, Lin FL, Tong KB, Hou SM, Liu JF. Transforming growth factor alpha promotes osteosarcoma metastasis by ICAM-1 and PI3K/Akt signaling pathway. Biochemical pharmacology. 2014;89:453-63
4. Wu C, Gong S, Duan Y, Deng C, Kallendrusch S, Berninghausen L. et al. A tumor microenvironment-based prognostic index for osteosarcoma. J Biomed Sci. 2023;30:23
5. Gong G, Ganesan K, Xiong Q, Zheng Y. Anti-Invasive and Anti-Migratory Effects of Ononin on Human Osteosarcoma Cells by Limiting the MMP2/9 and EGFR-Erk1/2 Pathway. Cancers. 2023;15:758
6. Liu JF, Chen PC, Chang TM, Hou CH. Thrombospondin-2 stimulates MMP-9 production and promotes osteosarcoma metastasis via the PLC, PKC, c-Src and NF-kappaB activation. Journal of cellular and molecular medicine. 2020;24:12826-39
7. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904-12
8. Lin J, Rao D, Zhang M, Gao Q. Metabolic reprogramming in the tumor microenvironment of liver cancer. J Hematol Oncol. 2024;17:6
9. Zhang X, Yu C, Zhao S, Wang M, Shang L, Zhou J. et al. The role of tumor-associated macrophages in hepatocellular carcinoma progression: A narrative review. Cancer Med. 2023;12:22109-29
10. Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315-22
11. Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605-12
12. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F. et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425-40
13. Krstic J, Trivanovic D, Jaukovic A, Santibanez JF, Bugarski D. Metabolic Plasticity of Stem Cells and Macrophages in Cancer. Front Immunol. 2017;8:939
14. Schlesinger M, Bendas G. Vascular cell adhesion molecule-1 (VCAM-1)-an increasing insight into its role in tumorigenicity and metastasis. Int J Cancer. 2015;136:2504-14
15. Chang AC, Chen PC, Lin YF, Su CM, Liu JF, Lin TH. et al. Osteoblast-secreted WISP-1 promotes adherence of prostate cancer cells to bone via the VCAM-1/integrin alpha4beta1 system. Cancer Lett. 2018;426:47-56
16. Chao CC, Lee CW, Chang TM, Chen PC, Liu JF. CXCL1/CXCR2 Paracrine Axis Contributes to Lung Metastasis in Osteosarcoma. Cancers. 2020;12:459
17. Wang LH, Tsai HC, Cheng YC, Lin CY, Huang YL, Tsai CH. et al. CTGF promotes osteosarcoma angiogenesis by regulating miR-543/angiopoietin 2 signaling. Cancer Lett. 2017;391:28-37
18. Chao CC, Lee WF, Yang WH, Lin CY, Han CK, Huang YL. et al. IGFBP-3 stimulates human osteosarcoma cell migration by upregulating VCAM-1 expression. Life sciences. 2021;265:118758
19. Yoneda T, Hiasa M, Okui T, Hata K. Cancer-nerve interplay in cancer progression and cancer-induced bone pain. J Bone Miner Metab. 2023;41:415-427
20. Yang H, Wang Y, Zhen S, Wang B, Jiao M, Liu L. et al. AMPK activation attenuates cancer-induced bone pain by reducing mitochondrial dysfunction-mediated neuroinflammation. Acta Biochim Biophys Sin (Shanghai). 2023;55:460-471
21. Jing D, Zhao Q, Zhao Y, Lu X, Feng Y, Zhao B. et al. Management of pain in patients with bone metastases. Frontiers in oncology. 2023;13:1156618
22. Di Donato M, Giovannelli P, Migliaccio A, Castoria G. The nerve growth factor-delivered signals in prostate cancer and its associated microenvironment: when the dialogue replaces the monologue. Cell Biosci. 2023;13:60
23. Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature. 1986;319:743-8
24. Bruno F, Arcuri D, Vozzo F, Malvaso A, Montesanto A, Maletta R. Expression and Signaling Pathways of Nerve Growth Factor (NGF) and Pro-NGF in Breast Cancer: A Systematic Review. Curr Oncol. 2022;29:8103-20
25. Tzeng HE, Lin SL, Thadevoos LA, Lien MY, Yang WH, Ko CY. et al. Nerve growth factor promotes lysyl oxidase-dependent chondrosarcoma cell metastasis by suppressing miR-149-5p synthesis. Cell death & disease. 2021;12:1101
26. Han S, Wang D, Huang Y, Zeng Z, Xu P, Xiong H. et al. A reciprocal feedback between colon cancer cells and Schwann cells promotes the proliferation and metastasis of colon cancer. Journal of experimental & clinical cancer research: CR. 2022;41:348
27. Zhang W, He R, Yang W, Zhang Y, Yuan Q, Wang J. et al. Autophagic Schwann cells promote perineural invasion mediated by the NGF/ATG7 paracrine pathway in pancreatic cancer. Journal of experimental & clinical cancer research: CR. 2022;41:48
28. Chen WY, Wen YC, Lin SR, Yeh HL, Jiang KC, Chen WH. et al. Nerve growth factor interacts with CHRM4 and promotes neuroendocrine differentiation of prostate cancer and castration resistance. Commun Biol. 2021;4:22
29. Tzeng HE, Lin SL, Thadevoos LA, Ko CY, Liu JF, Huang YW. et al. The mir-423-5p/MMP-2 Axis Regulates the Nerve Growth Factor-Induced Promotion of Chondrosarcoma Metastasis. Cancers. 2021;13:3347
30. Boulanger MC, Temel JS, Mino-Kenudson M, Ritterhouse LL, Dagogo-Jack I. Primary Resistance to Larotrectinib in a Patient With Squamous NSCLC With Subclonal NTRK1 Fusion: Case Report. JTO Clin Res Rep. 2023;4:100501
31. Liu J, Zhang Y, Zhu Y, Tian L, Tang M, Shen J. et al. Research Progress on Small Molecules Inhibitors Targeting TRK Kinases. Curr Med Chem. 2023;30:1175-92
32. Trang NTN, Lai CY, Tsai HC, Huang YL, Liu SC, Tsai CH. et al. Apelin promotes osteosarcoma metastasis by upregulating PLOD2 expression via the Hippo signaling pathway and hsa_circ_0000004/miR-1303 axis. Int J Biol Sci. 2023;19:412-25
33. Hou CH, Lin FL, Hou SM, Liu JF. Hyperthermia induces apoptosis through endoplasmic reticulum and reactive oxygen species in human osteosarcoma cells. International journal of molecular sciences. 2014;15:17380-95
34. Liu JF, Chen PC, Chang TM, Hou CH. Monocyte Chemoattractant Protein-1 promotes cancer cell migration via c-Raf/MAPK/AP-1 pathway and MMP-9 production in osteosarcoma. Journal of experimental & clinical cancer research: CR. 2020;39:254
35. Lee H-P, Wu Y-C, Chen B-C, Liu S-C, Li T-M, Huang W-C. et al. Soya-cerebroside reduces interleukin production in human rheumatoid arthritis synovial fibroblasts by inhibiting the ERK, NF-κB and AP-1 signalling pathways. Food Agr Immunol. 2020;31:740-50
36. Lee H-P, Liu S-C, Wang Y-H, Chen B-C, Chen H-T, Li T-M. et al. Cordycerebroside A suppresses VCAM-dependent monocyte adhesion in osteoarthritis synovial fibroblasts by inhibiting MEK/ERK/AP-1 signaling. Journal of Functional Foods. 2021;86:104712
37. Lee HP, Wang SW, Wu YC, Tsai CH, Tsai FJ, Chung JG. et al. Glucocerebroside reduces endothelial progenitor cell-induced angiogenesis. Food Agr Immunol. 2019;30:1033-45
38. Su CM, Tang CH, Chi MJ, Lin CY, Fong YC, Liu YC. et al. Resistin facilitates VEGF-C-associated lymphangiogenesis by inhibiting miR-186 in human chondrosarcoma cells. Biochemical pharmacology. 2018;154:234-42
39. Wu KM, Hsu YM, Ying MC, Tsai FJ, Tsai CH, Chung JG. et al. High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in H9c2 cardiomyoblast cells via ROS suppression. Nutrition & metabolism. 2019;16:36
40. Hou CH, Lin FL, Hou SM, Liu JF. Cyr61 promotes epithelial-mesenchymal transition and tumor metastasis of osteosarcoma by Raf-1/MEK/ERK/Elk-1/TWIST-1 signaling pathway. Molecular cancer. 2014;13:236
41. Su C-H, Lin C-Y, Tsai C-H, Lee H-P, Lo L-C, Huang W-C. et al. Betulin suppresses TNF-α and IL-1β production in osteoarthritis synovial fibroblasts by inhibiting the MEK/ERK/NF-κB pathway. Journal of Functional Foods. 2021;86:104729
42. Lee KT, Su CH, Liu SC, Chen BC, Chang JW, Tsai CH. et al. Cordycerebroside A inhibits ICAM-1-dependent M1 monocyte adhesion to osteoarthritis synovial fibroblasts. Journal of food biochemistry. 2022;46:e14108
43. Achudhan D, Liu SC, Lin YY, Lee HP, Wang SW, Huang WC. et al. Antcin K inhibits VEGF-dependent angiogenesis in human rheumatoid arthritis synovial fibroblasts. Journal of food biochemistry. 2022;46:e14022
44. Shao S, Piao L, Guo L, Wang J, Wang L, Wang J. et al. Tetraspanin 7 promotes osteosarcoma cell invasion and metastasis by inducing EMT and activating the FAK-Src-Ras-ERK1/2 signaling pathway. Cancer Cell Int. 2022;22:183
45. Zhang P, Zhang J, Quan H, Wang J, Liang Y. MicroRNA-143 expression inhibits the growth and the invasion of osteosarcoma. J Orthop Surg Res. 2022;17:236
46. Doghish AS, Elballal MS, Elazazy O, Elesawy AE, Shahin RK, Midan HM. et al. miRNAs as potential game-changers in bone diseases: Future medicinal and clinical uses. Pathol Res Pract. 2023;245:154440
47. Kong W, Zhu H, Zheng S, Yin G, Yu P, Shan Y. et al. Larotrectinib induces autophagic cell death through AMPK/mTOR signalling in colon cancer. Journal of cellular and molecular medicine. 2022;26:5539-50
48. Silverman DA, Martinez VK, Dougherty PM, Myers JN, Calin GA, Amit M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer research. 2021;81:1431-40
49. Zarghooni K, Bratke G, Landgraf P, Simon T, Maintz D, Eysel P. The Diagnosis and Treatment of Osteosarcoma and Ewing's Sarcoma in Children and Adolescents. Dtsch Arztebl Int. 2023;120:405-412
50. Peng T, Guo Y, Gan Z, Ling Y, Xiong J, Liang X. et al. Nerve Growth Factor (NGF) Encourages the Neuroinvasive Potential of Pancreatic Cancer Cells by Activating the Warburg Effect and Promoting Tumor Derived Exosomal miRNA-21 Expression. Oxid Med Cell Longev. 2022;2022:8445093
51. Amuti A, Liu D, Maimaiti A, Yu Y, Yasen Y, Ma H. et al. Doxorubicin inhibits osteosarcoma progression by regulating circ_0000006/miR-646/ BDNF axis. J Orthop Surg Res. 2021;16:645
52. Squadrito ML, De Palma M. Macrophage regulation of tumor angiogenesis: implications for cancer therapy. Mol Aspects Med. 2011;32:123-45
53. Yan W, Lakkaniga NR, Carlomagno F, Santoro M, McDonald NQ, Lv F. et al. Insights into Current Tropomyosin Receptor Kinase (TRK) Inhibitors: Development and Clinical Application. J Med Chem. 2019;62:1731-60
54. Wang H, Qi L, Zhong C, Fang X, Yuan Y. The Genomic and Proteomic Profiles of NTRK Genes and Trk Receptors in Liver Hepatocellular Carcinoma. Clin Med Insights Oncol. 2023;17:11795549231180840
55. Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332-7
56. Bissell MJ, Kenny PA, Radisky DC. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harb Symp Quant Biol. 2005;70:343-56
57. Halin S, Rudolfsson SH, Van Rooijen N, Bergh A. Extratumoral macrophages promote tumor and vascular growth in an orthotopic rat prostate tumor model. Neoplasia. 2009;11:177-86
58. Kusuma I, Yapson GYA, Nolan J, Wiratnaya IGE, Supadmanaba IGP. Clinicopathological and prognostic significance of CXCR4 expression in osteosarcoma: a meta-analysis. BioMedicine. 2022;12:34-43
59. Lin LW, Wang SW, Huang WC, Huynh TK, Lai CY, Ko CY. et al. Melatonin Inhibits VEGF-Induced Endothelial Progenitor Cell Angiogenesis in Neovascular Age-Related Macular Degeneration. Cells. 2023;12:799
60. Tai HC, Wang SW, Swain S, Lin LW, Tsai HC, Liu SC. et al. Melatonin suppresses the metastatic potential of osteoblastic prostate cancers by inhibiting integrin alpha(2) beta(1) expression. J Pineal Res. 2022;72:e12793
61. Wang CQ, Huang YW, Wang SW, Huang YL, Tsai CH, Zhao YM. et al. Amphiregulin enhances VEGF-A production in human chondrosarcoma cells and promotes angiogenesis by inhibiting miR-206 via FAK/c-Src/PKCdelta pathway. Cancer Lett. 2017;385:261-70
62. Lin TH, Tan TW, Tsai TH, Chen CC, Hsieh TF, Lee SS. et al. D-pinitol inhibits prostate cancer metastasis through inhibition of alphaVbeta3 integrin by modulating FAK, c-Src and NF-kappaB pathways. International journal of molecular sciences. 2013;14:9790-802
63. Yang N, Zhu S, Lv X, Qiao Y, Liu YJ, Chen J. MicroRNAs: Pleiotropic Regulators in the Tumor Microenvironment. Frontiers in immunology. 2018;9:2491
64. Cardoso AL, Fernandes A, Aguilar-Pimentel JA, de Angelis MH, Guedes JR, Brito MA. et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. 2018;47:214-77
65. Boufraqech M, Nilubol N, Zhang L, Gara SK, Sadowski SM, Mehta A. et al. miR30a inhibits LOX expression and anaplastic thyroid cancer progression. Cancer research. 2015;75:367-77
66. Liep J, Kilic E, Meyer HA, Busch J, Jung K, Rabien A. Cooperative Effect of miR-141-3p and miR-145-5p in the Regulation of Targets in Clear Cell Renal Cell Carcinoma. PloS one. 2016;11:e0157801
Author contact
![]() Corresponding author: Chih-Yang, Lin PhD; E‑mail: T016406skh.org.tw, Chih-Hsin Tang, PhD; E-mail: chtangcmu.edu.tw
Corresponding author: Chih-Yang, Lin PhD; E‑mail: T016406skh.org.tw, Chih-Hsin Tang, PhD; E-mail: chtangcmu.edu.tw

 Global reach, higher impact
Global reach, higher impact