10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(13):5330-5342. doi:10.7150/ijbs.101438 This issue Cite
Review
Molecular changes in intraocular fluid: implications for myopia
1. Department of Ophthalmology, Shanghai Ninth People's Hospital, China.
2. College of Health Science and Technology, Shanghai Jiao Tong University School of Medicine, China.
3. Department of Laboratory Medicine, the First Affiliated Hospital, Fujian Medical University, China.
Received 2024-7-25; Accepted 2024-9-22; Published 2024-9-30
Abstract
Myopia is the most common eye disease in the world which is caused by a mismatch between the optical power of the eye and its excessive axial length. Scleral remodeling, oxidative stress, inflammation, pathological states of angiogenesis and fibrosis and metabolism are closely associated with the onset and progression of myopia and the pathological changes that may ultimately result. Intraocular fluid is a collective term for the fluid within the eye, and changes in its composition can reflect the physiological and pathological status within the eye, with aqueous humor and vitreous being the commonly tested specimens. Recent studies have revealed potential changes in a variety of molecules in intraocular fluid during myopia progression. Abnormal expression of these molecules may reflect different stages of myopia and provide new perspectives for disease monitoring and treatment. Therefore, in this review, we systematically review the molecular changes in intraocular fluid associated with myopia, as well as the possible mechanisms, with a view to informing basic myopia research and clinical work.
Keywords: Intraocular fluid, Aqueous humor, Vitreous, Myopia, Molecular changes
Introduction
Myopia represents a prevalent ocular disorder characterized by AL elongation [1], with its incidence escalating globally [2]. As of 2010, the worldwide prevalence was recorded at 28.3%, with projections suggesting an increase to 39.9% by 2030 and 49.8% by 2050 [2]. This persistent rise underscores the imperative for a robust public health intervention. Myopia markedly impairs the educational achievements, quality of life, and employment opportunities of adolescents, imposing substantial burdens on individuals, families, and socioeconomic structures. Significantly, the economic impact attributed to uncorrected myopia was estimated at approximately US$244 billion globally in 2015, with East Asia experiencing the most significant economic strain [3]. Moreover, myopia, whether low, moderate or high, is associated with a higher risk of several severe ophthalmic complications including cataracts, glaucoma, myopic macular degeneration, retinal detachment and even blindness [4]. Therefore, in-depth understanding of the pathogenesis of myopia and searching for effective preventive and therapeutic methods have become important topics in ophthalmic research.
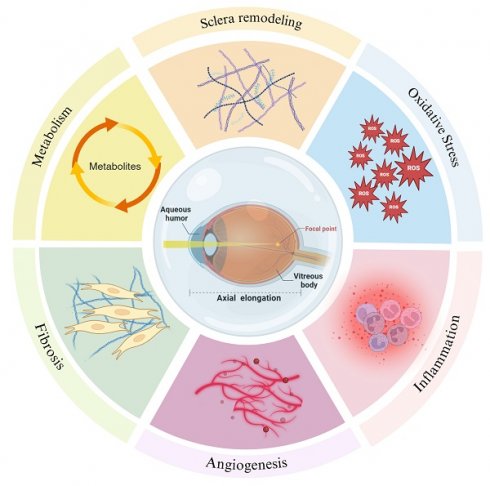
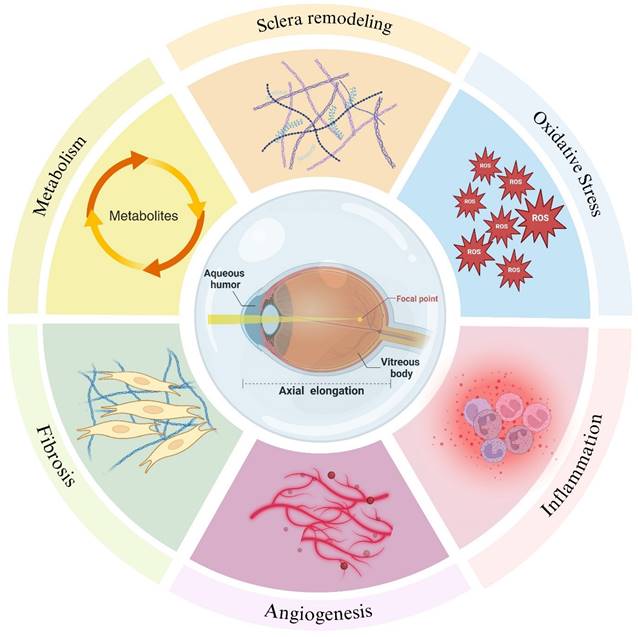
The intraocular fluid, encompassing AH and vitreous body, are integral in sustaining the ocular microenvironment. They provide essential nutrients to intraocular structures, regulate intraocular pressure, and facilitate the removal of metabolic byproducts [5,6]. Extensive research substantiates that a diverse array of biomolecules within intraocular fluid is intricately linked to myopia progression through various biological processes such as scleral remodeling, oxidative stress, inflammation, the pathological states of angiogenesis and fibrosis and metabolism (Figure 1). Fluctuations in the molecular composition of intraocular fluid may signify critical phases in the development of myopia (Table 1). This review endeavors to elucidate the correlations between molecular alterations in intraocular fluid and myopia progression, aiming to furnish novel insights and directions for future investigations.
Scleral remodeling
The sclera is instrumental in the initiation and progression of myopia, with alterations in its biomechanical properties, particularly the imbalance between degradation and synthesis of the scleral ECM, forming the structural foundation for AL hyperextension [7]. As a microenvironment interacting directly with the sclera, shifts in the molecular composition of intraocular fluid can reflect the state of scleral remodeling to some extent. Subsequent research has revealed that variations in the expression of MMPs, TIMPs, and TGF-βs within the intraocular fluid are intricately linked to the process of scleral remodeling.
MMPs, a family of zinc-dependent endopeptidases involved in the degradation of a variety of proteins in the ECM, such as collagen and elastin, while TIMPs serve as specific inhibitors of MMPs [8]. Thus, the balance between MMPs and TIMPs is critical for scleral remodeling [7]. Animal model studies on myopia have demonstrated that increased expression of MMP-2 correlates with reorganization of the scleral structure and ECM remodeling [9,10]. Furthermore, enhanced MMP-2 activity and reduced TIMP-2 activity have been observed to contribute to scleral remodeling in guinea pig [11], mouse [12] and chick [13,14] models. Theoretically, TIMPs, as inhibitors of MMPs, should be reduced in myopic progression, but some studies shown that TIMPs expression decreases with increasing MMP-2 [15,16], which may be related to the in vivo homeostasis hypothesis [17,18]. Interestingly, a recent study have shown that low doses of TIMP-2 promote MMP-2 activation, whereas high doses inhibit its activation, reduce collagen degradation, and shorten the AL, suggesting that TIMP-2 supplementation may be helpful in controlling myopia progression [19]. These findings indicate a nuanced regulatory interplay between MMPs and TIMPs in the remodeling of the sclera.
Molecular changes in intraocular fluid during the development of myopia reflect scleral remodeling, oxidative stress, inflammation, pathological states of angiogenesis and fibrosis and metabolism. Created with Biorender.com.
Myopia-related molecular changes in human intraocular fluid
| Molecules | Sample source | Molecular changes | Ref.1 | AL association | Ref.2 |
|---|---|---|---|---|---|
| MMP-2 | AH, Vitreous | Increased | [20-23] | Positive correlation | [20,23] |
| TIMP-1 | AH | Increased | [20,21] | Positive correlation | [20] |
| TIMP-2 | AH, Vitreous | Increased | [20-22] | Positive correlation | [20] |
| TIMP-3 | AH | Increased | [20,21] | Positive correlation | [20] |
| TGF-β1 | AH | No Significant Change | [29] | Positive correlation | [29] |
| TGF-β2 | Vitreous | No Significant Change | [22] | —— | |
| AH | Increased | [27,29] | Positive correlation | [28] | |
| T-AOC | AH | Decreased | [36,37] | Negative correlation | [36,37] |
| Total nitrate | AH | Increased | [36] | Positive correlation | [36] |
| MCP-1 | AH, Vitreous | Increased | [28,49-51] | Positive correlation | [28] |
| —— | No correlation | [56] | |||
| sICAM-1 | AH | Increased | [28] | Positive correlation | [28] |
| AH | —— | No correlation | [56] | ||
| svCAM-1 | AH | —— | No correlation | [56] | |
| IL-1β | AH | Increased | [37] | No correlation | [37,55] |
| IL-1ra | AH | Decreased | [51] | —— | |
| IL-4 | Vitreous humor | Increased | [49] | —— | |
| IL-5 | Vitreous humor | Increased | [49] | —— | |
| IL-6 | AH, Vitreous | Increased | [37,49,50,52,53] | Positive correlation | [37,52,53] |
| AH | No Significant Change | [23,51] | No correlation | [55] | |
| IL-8 | AH | No Significant Change | [23,51] | No correlation | [55] |
| AH, Vitreous | Increased | [50,52] | Positive correlation | [52] | |
| IL-10 | AH, Vitreous | Increased | [50] | No correlation | [55] |
| IL-12p | AH | —— | No correlation | [55] | |
| TNF-α | AH | No Significant Change | [37] | No correlation | [37,55] |
| IP-10 | Vitreous | Increased | [49,50] | —— | |
| IFN-γ | Vitreous | Increased | [49] | —— | |
| Eotaxin | Vitreous | Increased | [49] | —— | |
| MIP-1α | Vitreous | Increased | [49] | —— | |
| G-CSF | Vitreous | Increased | [49] | —— | |
| VEGF | AH, Vitreous | Decreased | [23,36,52,53,62-64] | Negative correlation | [23,36,52,53,55,56,59,61-63] |
| Vitreous | Increased | [49,50] | Positive correlation | [50] | |
| PEDF | AH | No Significant Change | [23,62] | No correlation | [23,62] |
| AH | Decreased | [67] | —— | ||
| VEGF/PEDF | AH | Decreased | [62] | —— | |
| EGF | AH | Increased | [72] | Positive correlation | [72] |
| IGF-2IGF-2RIGFBP-1IGFBP-2IGFBP-3IGFBP-4IGFBP-6 | Vitreous | Increased | [74] | —— | |
| GDF-15 | AH | Increased | [60] | Positive correlation | [60] |
| HGF | AH | Increased | [36,60] | Positive correlation | [60] |
| PDGF-AA | AH | Increased | [60] | Positive correlation | [60] |
Molecular changes associated with myopia have also been identified in human intraocular fluid as well. In an observational study involving patients with HM or cataract, intraoperatively removed AH was examined and found to contain MMP-2, MMP-3, TIMP-1, TIMP-2, and TIMP-3, but MMP-1 was not detected. Statistical analysis revealed that the levels of MMP-2, TIMP-1, TIMP-2, and TIMP-3 were positively correlated with AL, whereas MMP-3 showed no correlation [20]. Considering the interaction between MMPs and TIMPs, further analysis showed that MMP-2 levels were elevated in AH in patients with HM, while MMP-3 levels remained unchanged. TIMP-1, TIMP-2, and TIMP-3 levels were positively associated with MMP-2 levels, but there was no significant association between MMP-3 and the levels of TIMP-2 or TIMP-3 and the TIMP- 1 levels were only slightly correlated with MMP-3 levels [21]. These results align with the hypothesis that an increase in TIMPs is a compensatory response to maintain homeostasis. Additionally, changes in the vitreous body associated with myopia have drawn research interest. Zhuang et al. obtained vitreous samples from highly myopic patients undergoing vitrectomy, finding significantly elevated MMP-2 activity and levels, as well as an increased MMP-2/TIMP-2 ratio compared to controls [22]. In myopic macular degeneration, MMP-2 levels were significantly elevated and positively correlated with AL [23].
There are three major TGF-β isoforms in mammals, which are crucial in regulating cell growth, differentiation, immune responses, and ECM formation [24]. TGF-βs have a strong association with myopia. For instance, TGF-β2 levels are significantly elevated in both the retina and sclera of chicks with FDM [25]. In tree shrews with experimentally induced myopia, a decline in various TGF-β isoforms correlates with AL elongation [26]. In human studies, Zhu et al. analyzed AH sample from post-cataract surgery using real-time polymerase chain reaction and immunofluorescence staining, and found that TGF-β2 expression in the AH of patients with HM exceeded that of controls [27]. Consistent results were also found in a recent study, which demonstrated that TGF-β2 levels in AH increased by 3.43% for each 1 mm increase in AL [28]. Moreover, a prospective study revealed significantly higher TGF-β2 levels in the AH of patients with highly myopic cataracts compared to those with non-highly myopic cataracts. While TGF-β1 levels were not significantly altered, they were found to correlate with the patient's age and AL [29]. Notably, this study found that TGF-β2 levels in the second eye of patients with HM were higher than those in the first eye, which may be related to the fact that cataract surgery promoted TGF-β2 expression in the other eye. Although the majority of research underscores a significant role for TGF-β, particularly TGF-β2, in myopia development, some studies report no significant differences. Zhuang et al. used enzyme-linked immunosorbent assay to measure TGF-β2 concentrations in the vitreous of HM patients and found them comparable to controls [22]. This disparity might be attributed to differences in the environments of the AH and vitreous or variations in control group selection. Given the importance of TGF-β in the development of myopia, a Meta-analysis explored the relationship between TGF-β gene polymorphisms and myopia, and found that the rs4803455 and rs1800469 loci of TGF-β1 were associated with myopia, suggesting that further research and treatment of myopia based on these findings may be important. Yet rs1982073 and rs2241716 for TGF-β1 and rs7550232 for TGF-β2 were not significantly associated with myopia [30], indicating that further studies are still needed to explain these inconsistent results.
In summary, altered biomechanical properties of the sclera, particularly an imbalance between the degradation and synthesis of the ECM, form the basis of myopic AL hyperextension. Changes in MMPs, TIMPs, and TGF-βs in intraocular fluid are closely associated with scleral remodeling, and these molecules may serve as potential indicators of myopia progression. Given the complexity of myopia mechanisms and the inconsistency of findings, more in-depth studies are still needed in the future.
Oxidative Stress
Oxidative stress is a state of imbalance between the production of ROS in the intra- and extracellular environment and the antioxidant defense system [31]. An excessive buildup of ROS in the intraocular fluid has been linked to the onset of various ophthalmic conditions, including cataracts, glaucoma, and retinal disorders [31]. Studies have pointed to a significant association between oxidative stress and the development of myopia. At the retinal level, proteomic analysis of samples from rabbit models of myopia revealed that oxidative stress may be a key mechanism in the progression of myopia [32]. Another investigation in a chicken model induced by FDM revealed that the expression of neuronal NO synthase was increased, and that antioxidant intervention could mitigate myopia progression [33]. At the scleral level, intense ROS release during oxidative stress may induce apoptosis in scleral cells, thereby fostering the growth of myopic sclera [34]. In patients with HM, the substantial reduction of antioxidant enzymes and other protective agents can lead to cellular damage, apoptosis, and potentially impair visual functions [35].
In recent studies, the relationship between oxidative stress levels in the intraocular fluid and myopia development has garnered attention. T-AOC, an indicator of all antioxidant substances, and total nitrite/nitrate levels, an important indicator for assessing the state of oxidative stress, were measured in the eyes of middle-aged and elderly HM patients with AH. It was found that T-AOC values were significantly lower and total nitrite/nitrate levels were significantly higher in patients with HM, reflecting higher levels of oxidative stress in the intraocular environment. Further analysis revealed that T-AOC was negatively correlated with refraction and AL, indicating that the lower the T-AOC value, the greater the degree of myopia. Whereas, total nitrite/nitrate levels were positively correlated with refraction and AL, indicating that higher levels of oxidative stress were associated with more myopia [36].
Similar results were obtained in our study, where T-AOC levels were significantly lower in patients with AL ≥ 28 mm compared to patients with AL ≤ 26 mm in the testing of AH samples collected from 40 young patients (70 eyes) undergoing implantable collamer lens surgery. Additional correlation analysis confirmed a negative association between T-AOC and AL [37]. The above results were consistent in both young and elderly patients with HM, emphasizing the potential role of oxidative stress in the development of myopia. Regarding the vitreous, examination of samples from animal models of myopia combined with bioinformatics analysis suggests that oxidative stress and lipid metabolism are pathways involved in compensatory eye elongation [38].
Overall, oxidative stress appears to play a significant role in myopia development within the intraocular fluid environment, particularly in HM patients. The reduced antioxidant capabilities and increased oxidative markers highlight a pathogenic process that potentially accelerates myopia by altering the biological functions of the retina and sclera.
Inflammation
Inflammation is the body's natural response to harmful stimuli and typically benefits the organism when controlled [39]. It serves as a key factor in the development of myopia, as evidenced by studies indicating that the expression of ocular inflammatory cytokines escalates with the progression of myopia in various animal models including tree shrews [40], guinea pigs [41], hamsters [42,43], and chicks [44]. These studies suggest that an increased state of ocular inflammation accelerates the development of myopia. Additionally, patients with inflammatory diseases have been reported to exhibit a higher prevalence of myopia [43,45]. In comparison, various markers of inflammation such as elevated circulating neutrophils, reduced monocytes, eosinophils, and lymphocytes [46], along with increased neutrophil-to-lymphocyte ratios and platelet-to-lymphocyte ratios, demonstrate an imbalance in patients with high and pathological myopia. These findings suggest that individuals with myopia may experience a systemic inflammatory state[47,48].
Intraocular fluid is one of the main sites of inflammatory response in myopia development. Research shows that myopic eyes exhibit elevated levels of inflammatory cytokines in the vitreous or AH compared to non-myopic eyes. For instance, significant increases in cytokines such as MCP-1, IL-5, IL-6, IFN-γ, IP-10, eotaxin, macrophage inflammatory protein-1α, IL-4, and granulocyte colony-stimulating factor have been documented in the vitreous of patients with HM [49]. Similarly, increased levels of IL-6, IL-8, IL-10, IP-10, and MCP-1 have been observed in HM and its complications, further indicating a pro-inflammatory state in highly myopic eyes [50]. In the AH of patients with highly myopic cataracts, the expression of IL-1ra was decreased, whereas the expression of MCP-1 was elevated, suggesting that the anterior chamber of the eye may be in a pro-inflammatory state [51]. Moreover, the positive correlation of MCP-1 with AL reinforces its association with myopia progression [28]. IL-6 and IL-8, as typical inflammatory factors, are similarly significantly altered in the atrium in addition to the vitreous. It has been found that IL-6 and IL-8 are significantly higher in highly myopic eyes than in non-highly myopic eyes and have a significant positive correlation with the AL [52,53]. Notably, the tight junctions of the cornea are diminished in eyes suffering from allergic conjunctivitis, leading to increased levels of intraocular inflammatory cytokine expression, which may be related to cytokine penetration, with high levels of MCP-1, IL-6, IL-8, and TNF-α being recognized as a potential contributor to the promotion of retinal inflammation and myopic progression [54] , indicating a more profound effect of inflammation on myopic progression. The development of mCNV is closely associated with the upregulation of inflammatory cytokines. It has been shown that the inflammatory cytokines IL-6, IL-8, IL-10, IP-10 and MCP-1 were significantly increased in AH in the eyes of highly myopic patients with and without mCNV compared to controls [50]. However, the evidence for an association between myopia and inflammation is not consistent. One study found no significant correlation between AL and concentrations of inflammatory cytokines such as IL-1β, IL-6, IL-8, IL-10, IL-12p, and TNF-α in AH of cataract patients with AL ranging from 22.6 to 31.5 mm [55]. Similarly, our research detected no significant changes in TNF-α [37]. Additionally, other investigations reported that IL-6 and IL-8 levels were not significantly elevated compared to controls [23,51], and that log-transformed concentrations of MCP-1, sICAM-1, and svCAM-1 showed no correlation with refractive error or AL [56]. Such discrepancies could stem from variations in sample size, testing methodologies, control group selection, and inclusion criteria. In particular, most of the samples were from cataract patients, which can have an effect on the molecular changes of intraocular fluid, and this may be one of the reasons for the inconsistent results of the study. Therefore, younger patients should be included as controls in future studies. Our recent study took this aspect into account by including non-cataract patients aged 18-45 years and found that IL-1β and IL-6 concentrations were significantly higher in the AH of patients with AL ≥28 mm compared with those with AL ≤26 mm, with IL-6 positively correlating with AL [37], presenting a consistent performance with previous studies [52,53].
Given the role of inflammation in various ocular diseases and the complex mechanisms underlying myopia, further research is essential to clarify the relationship between inflammation and myopia and to uncover the underlying mechanisms.
Angiogenesis
VEGF is a critical bioactive protein integral to angiogenesis [57]. Studies have shown that aberrant VEGF expression is strongly associated with a variety of serious ocular diseases [57]. The involvement of VEGF in myopia development is increasingly acknowledged. A study reported that intravitreal injection of VEGF165 effectively slowed myopia progression in guinea pig models of FDM-induced myopia [58]. Significant changes in VEGF have also been found in the human eye. Most studies noted that in patients with HM and mCNV, VEGF levels in the AH were significantly decreased and showed a negative correlation with AL [23,36,52,53,55,56,59-62]. Notably, there were variations in the experimental procedures of the researchers. The study by Sawada et al. excluded patients with uveitis, myopic atrophy, and diabetes mellitus to minimize confounding by VEGF expression, but did not establish a normal control group and was a cross-sectional study, which limited the inference of causality [61]. Shin et al. enhanced the robustness of their findings through subgroup analysis but included patients with conditions that could compromise the generalizability of the results [62]. Yuan et al. reported a decrease in AH VEGF levels corresponding to increases in AL, but did not account for the impact of intraocular volume on these levels [53]. Conversely, Wong et al. controlled for AL in their analysis, yet faced limitations due to the small scale and cross-sectional nature of their study [23]. In vitreous samples, Zhu et al. and Hu et al. found that VEGF concentrations decreased as AL increased [55,59]. However, one study showed significantly higher levels of VEGF in the vitreous of patients with HM, and the authors hypothesized that this may be related to a low-grade inflammatory state in the eye rather than differences in the type of retinopathy [49].
In mCNV patients, the role of VEGF has also received attention from researchers. It was reported that VEGF levels in AH of mCNV patients were lower compared to controls, but there was no correlation between VEGF and AL. It is hypothesized that VEGF may be mainly localized in the retina and choroid, resulting in low levels in AH. Another theory proposes that due to the extended AL and consequently larger intraocular volume in HM patients, VEGF might be diluted in the anterior chamber and vitreous cavity [63]. Notably, low VEGF levels in the AH of patients with mCNV have also been found to be accompanied by other cytokine imbalances[64]. Moreover, VEGF levels were notably higher in the vitreous of highly myopic patients with mCNV compared to controls, although no disparity was noted in AH levels. This may reflect altered hemodynamics in the dilated retinal pigment epithelium cells and choroid in the posterior pole [50]. In response to the inconsistency of test results in AH as well as vitreous samples, the study introduced a novel approach by considering VEGF mass—calculating the total amount of VEGF (concentration multiplied by volume) rather than concentration alone, accounting for changes in AL and vitreous volume to more accurately assess VEGF's bioactivity within the eye [50].
PEDF is a potent inhibitor of angiogenesis [65]. Typically, in normal ocular tissues, the regulation of angiogenesis is governed by the equilibrium between VEGF and PEDF [66]. Despite expectations of elevated PEDF levels in highly myopic eyes to counterbalance reduced VEGF, it has observed diminished PEDF levels in the AH of patients with HM [67]. Conversely, while one investigation found no significant disparity in PEDF levels between highly myopic individuals and control subjects, the VEGF/PEDF ratio was notably reduced in the highly myopic group [62]. This suggests potential damage to the fundus and may partially clarify the predominant influence on VEGF secretion relative to PEDF in scenarios of fundus alterations triggered by HM, thus accounting for the unanticipated lower PEDF levels [23,62].
Angiogenesis is a prominent feature of PM, and anti-VEGF therapy has been widely used to inhibit this pathological process. Although measuring VEGF concentrations in intraocular fluid can shed light on angiogenic activity, it does not fully encapsulate the complex dynamics of angiogenesis. Emerging research suggests that evaluating the total VEGF content might offer a more holistic view of angiogenic processes. Moreover, maintaining the balance between VEGF and PEDF is crucial for the regulation of angiogenesis, making the VEGF/PEDF ratio a potentially superior metric for assessing the state of angiogenesis compared to the levels of individual factors.
Changes in growth factors
Experimental animal studies have demonstrated that both topical and intraocular administration of multiple growth factors can influence externally induced AL elongation [68-70]. These growth factors undergo specific changes in the intraocular fluid during the development of myopia. EGF is a biologically active polypeptide whose primary role is to promote cell growth, proliferation, and differentiation [71]. One study identified a negative correlation between the total intraocular EGF and AL, indicating that EGF may be linked to pathological changes in the fundus in cases of HM [72]. This was calculated by multiplying the EGF concentration by the intraocular volume in a method consistent with Zhang et al. [50]. IGFs, crucial for tissue growth, cellular proliferation, and repair, are believed to play a significant role in myopia development [69,73]. Insulin microarray analysis of AH and vitreous samples from patients with PM showed that insulin, IGF-2, IGF-2R, and IGFBPs were significantly increased in patients with PM [74]. GDF-15, part of the TGF-β superfamily, participates in the TGF-β signaling pathway, influencing inflammatory responses and promoting fibrosis [75,76]. HGF, extensively studied in various ocular diseases, including corneal and retinal disorders, has been shown to upregulate MMP-2 expression in scleral fibroblasts of myopic guinea pigs, potentially contributing to myopia development through the degradation of scleral ECM [70]. PDGF was first isolated from platelet extracts in the early 1970s and classified as a mitogen for fibroblasts and mesenchymal-derived cells [77]. Notably, levels of GDF-15, HGF, and PDGF-AA were found to be significantly higher in the AH of patients with HM compared to controls. Given the pro-fibrotic effects of these growth factors, researchers have hypothesized their involvement in the fibrotic processes associated with HM [36,60].
Other potential molecules
With the advancement of omics technologies, researchers have obtained significant insights by analyzing AH or vitreous samples from myopic or highly myopic patients (Figure. 2). Transcriptomics and proteomics, two vital branches of omics research, have offered fresh perspectives in understanding the molecular underpinnings of myopia (Table 2). Transcriptomics have revealed an abundance of miRNAs in AH [78], often encapsulated within exosomes [79]. The altered miRNA expression profiles are closely linked to myopia progression. Chen et al. first analyzed the miRNA expression profiles of exosomes in myopic patients' AH and found that the total RNA content in the myopic group was 2.78 times higher than that in the control group. By Open Array, they identified five myopia-specific miRNAs (has-miR-582-3p, has-miR-17-5p, has-miR-885-3p, has-miR-19b-3p, and has-miR-450b-5p), which may be six common myopia-related genes as potential targets [80]. NGS was used to detect a total of 249 mature miRNAs and 17 novel miRNAs that were differentially expressed in myopia, which may be involved in the regulation of the TNF, MAPK, PI3K- AKT, and HIF-1 signaling pathways, and were verified by quantitative polymerase chain reaction [81]. In another study, also using the NGS combined with immunofluorescence and western blot validation, it was concluded that hsa-miR-142-3p may be involved in the pathologic progression of HM by reducing collagen I in scleral fibroblasts by targeting TGF-β1 [82]. The researchers also noted whether there were corresponding changes in the vitreous of the patients. Detection of miRNAs in the vitreous using Low Density Arrays analysis revealed that let-7c was significantly upregulated in the vitreous of highly myopic eyes, while miR-200a was significantly downregulated. Further analysis showed that the up-regulated miRNA target genes involved MAPK and multiple inflammatory signaling pathways; the down-regulated miRNA target genes involved the PI3K/AKT pathway [83].
Transcriptomics and proteomics applied to the detection of myopia-related intraocular fluid molecules
| Sample source | Detection methods | Changes | Relevant mechanisms involved | Specific miRNA or protein | Ref. | |
|---|---|---|---|---|---|---|
| miRNAs | AH | Open Array | The amount of AH RNA was up 2.78-fold in the highly myopic group | —— | Has-miR-582-3p, has-miR-17-5p, has-miR-885-3p, has-miR-19b-3p and has-miR-450b-5p | [80] |
| miRNAs | AH | NGS | 249 miRNAs and 17 novel miRNAs differentially expressed in myopia | TNF, MAPK, PI3K-Akt and HIF-1 signaling pathways | Has-let-7i-5p, has-miR-127-3p and has-miR-98-5p | [81] |
| miRNA | AH | NGS | miR-142-3p was upregulated and positively correlated with ocular AL | Targeting TGF-β1 to reduce collagen I in scleral fibroblasts | miR-142-3p | [82] |
| miRNAs | Vitreous | Low Density Array | Let-7c miRNA was upregulated and miR-200a was downregulated | Up-regulated: 23 pathways including MAPK pathways; down-regulated: 32 pathways including PI3K/AKT | Let-7c, miR-200a | [83] |
| Proteins | AH | iTRAQ | 123 were up-regulated and 87 were down-regulated proteins | —— | —— | [84] |
| Proteins | AH | iTRAQ | 97 were up-regulated and 49 were down-regulated | —— | —— | [85] |
| Proteins | AH | iTRAQ | 32 up-regulated and 26 down-regulated proteins | PLG affects the composition of collagen fibers and ECM leading to scleral thinning | PLG | [86] |
| Proteins | AH | Conventional Method | Albumin; transthyretin, DBP were significantly elevated. | DBP may be involved in the APOA1 arrest pathway | Albumin, transthyretin, DBP | [89] |
| Proteins | AH | Label-free | 63 up-regulated and 38 down-regulated | APOA1 as a stop signal for myopia progression may be a key protein and therapeutic target for PM | APOA1 | [90] |
| Proteins | AH | Label-free | Glial fibrillary acidic proteins and complement-related proteins were elevated | JAK-STAT signaling pathway | Glial fibrillary acidic proteins and complement-related proteins | [91] |
Overview of transcriptomics, proteomics, and metabolomics in analyzing molecular changes in intraocular fluid related to myopia. Created with Biorender.com.
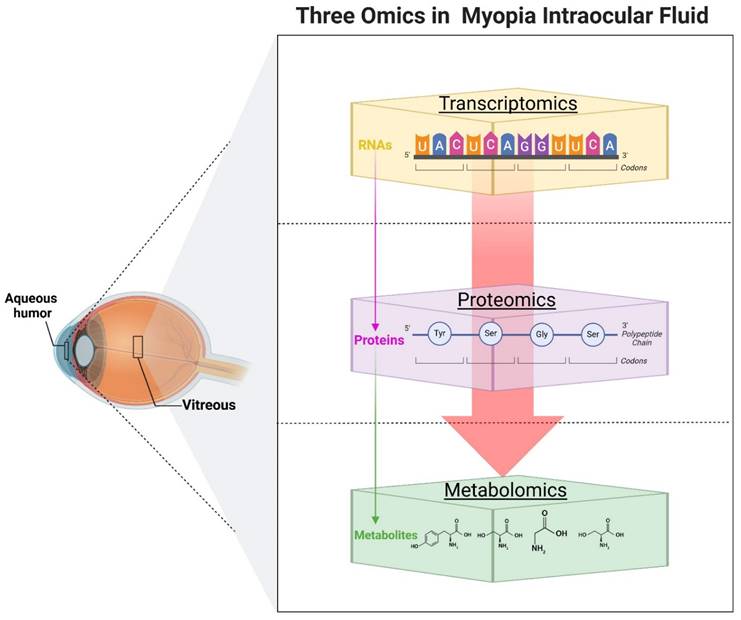
Proteomics serves as the one of the final steps downstream of the omics cascade, focusing on global protein measurement. An iTRAQ-based quantitative proteomics study identified 210 proteins differentially expressed in HM patients, with 123 proteins up-regulated and 87 proteins down-regulated in patients with HM [84]. However, results from other ITRAQ-based studies varied, possibly due to differences in sample size and inclusion criteria. One found that 146 proteins were differentially expressed among proteins associated with the HM group compared with the control group, of which 97 proteins were upregulated and 49 proteins were downregulated in patients with HM [85]. In another study, 58 differentially expressed proteins were identified, of which 32 were up-regulated and 26 were down-regulated. Bioinformatic analysis and confirmation by enzyme-linked immunosorbent assay suggested that PLG may be a candidate biomarker for HM. Its overexpression may lead to scleral thinning by affecting the composition of collagen fibers and ECM of the sclera [86]. APOA1 has emerged as a significant protein in several studies, associated with myopia arrest in animal models [87] and identified in both chick embryo vitreous [38] and AH exosomes of HM patients [88]. APOA1's role is supported by its implication in oxidative stress and lipid metabolism pathways [38]. A proteomics study using old experimental methods indicated that DBP, albumin, and transthyretin were significantly increased in highly myopic eyes. The researchers suggested that DBP may be involved in the APOA1 arrest pathway and thus play a specific role in the development of myopia [89]. The results of a study on PM also ultimately point to APOA1 [90]. Additionally, proteomics has extended to studying mCNV, with elevated levels of glial fibrillary acidic protein and complement-related proteins identified, primarily involving the JAK-STAT pathway [91].
Metabolomics, as another step in the final stage o in the cascade of omics, is increasingly elucidating the association between metabolite alterations in intraocular fluid and the progression of myopia (Table 3). Both AH and vitreous have been central to myopia-related metabolomic studies. Through untargeted approaches using CE-MS and LC-MS, over 40 metabolites have been identified. Higher levels of aminooctanoic acid, arginine, citrulline and sphinganine were observed in patients with HM compared to those with low myopia. These metabolites play pivotal roles in NO synthesis and cellular signaling, suggesting their involvement in myopia development by influencing intraocular pressure regulation and ocular growth. Additionally, low myopia patients exhibited higher levels of aminoundecanoic acid, dihydro-retinoic acid and cysteinylglycine disulfide. These metabolites may be linked to retinal health and antioxidant defense related to retinoid signal transduction, indicating a potential protective role at different myopia stages [92]. Using GC-TOF-MS in an untargeted manner, 29 metabolites showed significant changes between HM patients and controls, with 27 increasing and 2 decreasing. Further metabolic network analysis highlighted the prominence of amino acid metabolism pathways. Specific amino acid metabolites, such as glutamine, N-alpha-acetyl-L-ornithine, nicotinoylglycine, and o-hydroxyhippuric acid, were markedly upregulated. Levels of oxalic acid, linoleic acid methyl ester, and thymidine also significantly increased, with the latter two being associated with enhanced ROS generation and oxidative stress in HM patients. The rise in oxalic acid might relate to calcium binding and subsequent impacts on calcium concentration, contributing to myopia progression. Thymidine levels are thought to correlate strongly with certain features of AL elongation [93]. Shao et al. utilized LC-MS to analyze AH metabolites in patients with AL less than 24 mm (Group A), between 24-26 mm (Group B), and greater than 26 mm (Group C). Comparative and enrichment analyses across these groups revealed that differential metabolites were involved in Taurine and hypotaurine metabolism, vitamin B6 metabolism, pantothenate, and coenzyme A biosynthesis.
Metabolomics of intraocular fluid components
| Detection methods | Sample source | Altered biomarkers | Potential mechanisms | AL association | Ref. |
|---|---|---|---|---|---|
| CE-MS and LC-MS | AH | Aminooctanoic acid, L-arginine, citrulline, aminoundecanoic acid, L-cysteinylglycine disulfide trihydroxyphenyl-gamma-valerolactone, dihydropteroic acid, dodecanedioic acid, aminocyclohexanecarboxylic acid, butyryl-L-carnitine, pantothenic acid, didehydro-retinoic acid, sphinganine, histidinyl-phenylalanine, dimethylnonanoyl carnitine, PC(O-32:2)//PC(P-32:1), PC (42:6), C24 sulfatide, PC(P-42:2)//PC(O-42:3), laccer(d40:0), trihexosylceramide (d36:2), neuacagalcer(d42:2) | Oxidative stress, NO synthesis and retinoic acid signaling | —— | [92] |
| GC-TOF-MS | AH | N-alpha-acetyl-L-ornithine, nicotinoylglycine, oxalacetic acid, o-hydroxyhippuric acid, oxalic acid, ribose, cis-gondoic acid, linoleic acid methyl ester, thymidine, phosphate, indole-3-acetamide, 2-aminophenol, 2-ketoadipate, 3-phenyllactic acid, cis-phytol, conduritol b epoxide, salicin | Amino acid metabolic pathways, oxidative stress and calcium ion binding | Thymidine | [93] |
| UHPLC-MS/MS | AH | SQH, inosine, uridine, hypoxanthine, pseudouridine, 3-nitro-L-tyrosine, phosphopyruvic acid, L-threo-3-phenylserine, DL-0-tyrosine, APK | Bile secretion, insulin secretion, thyroid hormone synthesis and cGMP-PKG signaling pathway | Serine, methionine, proline, creatine, lysine, arginine, tyrosine, threonine, glutamine, asparagine | [74] |
| Vitreous | D-(-)-glutamine, hypoxanthine, prostaglandin G2, decanoylcarnitine, Dl-glutamic acid, L-threonic acid, L-(+)-citrulline, citramalate, L-pyroglutamic acid, KPH | Bile secretion, thyroid hormone synthesis, insulin secretion and cGMP-PKG signaling pathway | |||
| LC-MS | AH | (s)-actinidine, 1,2,3,4,6,7,8-heptachlorodibenzofuran, 2,2'-iminodipropan-l-ol, 2(n)-methyl-norsalsolinol, 3-methoxytyramine, 5-methoxytryptophol, acetaminophen, cerulenin, decanoylcarnitine, hippuric acid, indoleacetaldehyde, I-octanoylcarnitine, levofloxacin, imsp01080031, n-(2,4-dimethylphenyl)formamide, n-acetylarylamine, n-acetylornithine, n,n-dimethyl-safingol, nadolol, netilmicin, orsellinic acid, oxybuprocaine, p-cresol sulfate, pantothenic acid, paracetamol sulfate, pyridoxal, pyrocatechol sulfate, sodium tetradecyl sulfate, spisulosine, theophylline, tryptophol | Taurine and hypotaurine metabolism, vitamin B6 metabolism, pantothenate, and coenzyme A biosynthesis | 5-methoxytryptophol and cerulenin | [94] |
| UHPLC-MS/MS | Vitreous | L-carnitine, propionylcarnitine, theophylline, glycerol tripropanoate, theobromine, gamma glutamyltyrosine, L-kynurenine, pivaloylcarnitine, 1-(beta-D-R-ribofuranosyl)-1,4-dihydronicotinamide, isobutyry-L-carnitine, N-acetylornithine, L-2,3-dihydrodipicolinate,N-acetylhistidine, monoethylglycinexylidide, 5-hydroxyindoleacetic acid, aspartame, L.acetylcarnitine, dehydroneotenone, 4-guanidinobutanoic acid, uric acid, 16-methylheptadecanoic acid, indoxyl sulfate, estradiol | Tryptophan metabolic pathway, uric acid metabolic pathway, oxidative stress and inflammation | —— | [95] |
Further trend analysis and determination of differential metabolites based on FC values, coupled with receiver operating characteristic curve analysis and correlation analysis, identified 5-methoxytryptophol and cerulenin as potential biomarkers significantly associated with abnormal axial myopia [94]. A study on PM, using an untargeted UHPLC-MS/MS approach, analyzed AH and vitreous humor samples from PM and non-myopic patients. The analysis revealed significant differences in 104 AH metabolites and 114 VH metabolites between the groups. Enrichment analysis identified four metabolomic pathways associated with PM: bile secretion, insulin secretion, thyroid hormone synthesis, and the cGMP-PKG signaling pathway. Additionally, ten amino acids were significantly associated with AL [74]. Another study employing similar techniques investigated the metabolomic characteristics of vitreous in PM patients, identifying significant changes in 19 metabolites within the tryptophan metabolism pathway, notably elevated levels of L-kynurenine, indicative of increased oxidative stress and inflammatory response. Furthermore, significantly elevated uric acid levels were correlated with PM complications, suggesting its potential as a PM biomarker. The study also observed changes in amino acid (gamma-glutamyltyrosine and 4-guanidinobutyric acid) and lipid metabolites (L-carnitine, acetyl-L-carnitine and propionylcarnitine), associated with heightened oxidative stress in PM [95].
It is worth noting that multi-omics research has been gradually utilized in the exploration of disease mechanisms. As a multifactorial disease, the combined application of multi-omics can provide a more comprehensive and in-depth understanding of the changes in the development of myopia, especially in the screening of certain meaningful biomarkers. In conclusion, the application of omics technologies to AH and vitreous samples has uncovered crucial molecular alterations and potential biomarkers. These findings are instrumental in deepening our understanding of myopia's mechanisms, aiding in its diagnosis, and fostering the development of targeted treatment strategies.
Conclusion
The etiology of myopia is shaped by an intricate interplay of genetic predispositions and environmental exposures, thereby complicating the elucidation of its underlying mechanisms. Despite considerable advancements in the field, a definitive account of the factors driving myopia progression remains elusive. Current findings on myopia are mostly based on animal and cellular experiments due to the difficulty of obtaining specimens from the human eye. With the popularization of cataract surgery and advances in vitrectomy surgery, we are able to obtain intraocular fluid, including AH and vitreous, in an ethical manner. However, the widespread adoption of cataract extraction and the refinements in vitrectomy techniques have facilitated the ethical collection of intraocular fluid, including AH and vitreous body, thus enriching our understanding of myopia at the molecular level. According to existing studies, there are corresponding changes in the molecules in the intraocular fluid during the progression of myopia as well as in the later stages of the pathology, which point to scleral remodeling, oxidative stress, inflammation, pathological states of angiogenesis and fibrosis and metabolism. Yet researchers face certain challenges in interpreting these changes. On the one hand, this may be due to the fact that the source of AH and vitreous molecules is not limited to the eye, but can partly come from plasma [96,97]. On the other hand, limitations of the studies themselves, such as insufficient sample sizes, varying inclusion criteria, differences in statistical methods, and failure to adequately take into account the dilutional effect of AL elongation, may lead to inconsistent results. Moreover, the majority of human ocular specimens are derived from elderly patients undergoing cataract surgery, which may not offer comprehensive insights comparable to those obtained from younger individuals. Fortunately, researchers are gradually overcoming these challenges with improvements in study design and advances in assay technology. Developments in microscopic specimens and high-throughput assay technologies have led to more precise analysis of molecular changes in intraocular fluid. Researchers are increasingly recognizing the importance of robust study designs and have begun to consider the effect of AL on results, expand sample sizes, and include low myopia and younger patients as controls to improve the scientific validity of their studies. In summary, the study of molecular changes in intraocular fluid has been instrumental in shedding light on the mechanisms of myopia. Despite existing challenges, ongoing technological advancements and methodological improvements provide optimism for a more thorough and direct comprehension of myopia at the human level in the foreseeable future. Such advancements are poised to enhance the strategies for prevention and management of myopia.
Abbreviations
AH: aqueous humor
AKT: serine-threonine kinase
AL: axial length
APOA1: apolipoprotein A1
cGMP: cyclic guanosine monophosphate
DBP: vitamin D-binding protein
ECM: extracellular matrix
EGF: epidermal growth factor
FDM: form-deprivation myopia
GDF: growth differentiation factor
G-CSF: granulocyte colony stimulating factor
HM: high myopia
HGF: hepatocyte growth factor
HIF-1: hypoxia-inducible factor-1
IGF: insulin-like growth factor
IGFBP: IGF-binding protein
IL: interleukin
IL-1ra: IL-1 receptor antagonist
IFN-γ: interferon-γ
IP-10: interferon-γ-induced protein 10
JAK: janus tyrosine kinase
MAPK: mitogen-activated protein kinase
mCNV: myopic choroidal neovascularization
MCP-1: monocyte chemoattractant protein-1
MIP-1α: macrophage inflammatory protein-1α
MMP: matrix metalloproteinase
NGS: next-generation sequencing
NO: nitric oxide
PDGF: platelet-derived growth factor
PEDF: pigment epithelium-derived factor
PI3K: phosphatidyl-inositol 3-kinas
PM: pathologic myopia
PKG: protein kinase G
ROS: reactive oxygen species
sICAM-1: soluble intercellular adhesion molecule-1
svCAM-1: soluble vascular cell adhesion molecule-1
STAT: signal transducer and activator of transcription
T-AOC: total antioxidant capacity
TGF-β: transforming growth factor-β
TIMP: tissue inhibitors of metalloproteinase
TNF: tumor necrosis factor
VEGF: vascular endothelial growth factor
Acknowledgements
Funding
This work was supported by research grants from the Clinical Research Program of 9th People's Hospital, Shanghai Jiao Tong University School of Medicine (JYLJ202117); Shanghai Municipal Health Commission (202140416); the National Natural Science Foundation of China (82203260); and Shanghai Eye Disease Research Center (2022ZZ01003).
Author contributions
All authors have read and agreed to the published version of the manuscript. The figures were created with BioRender.com.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chakraborty R, Landis EG, Mazade R, Yang V, Strickland R, Hattar S. et al. Melanopsin modulates refractive development and myopia. Exp Eye Res. 2022;214:108866
2. Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P. et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-42
3. Naidoo KS, Fricke TR, Frick KD, Jong M, Naduvilath TJ, Resnikoff S. et al. Potential lost productivity resulting from the global burden of myopia: systematic review, meta-analysis, and modeling. Ophthalmology. 2019;126(3):338-46
4. Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, Klaver CCW. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(4):49
5. To CH, Kong CW, Chan CY, Shahidullah M, Do CW. The mechanism of aqueous humour formation. Clin Exp Optom. 2002;85(6):335-49
6. Zong Y, Gao QY, Hui YN. Vitreous function and intervention of it with vitrectomy and other modalities. Int J Ophthalmol. 2022;15(6):857-67
7. Harper AR, Summers JA. The dynamic sclera: extracellular matrix remodeling in normal ocular growth and myopia development. Exp Eye Res. 2015;133:100-11
8. de Almeida LGN, Thode H, Eslambolchi Y, Chopra S, Young D, Gill S. et al. Matrix metalloproteinases: from molecular mechanisms to physiology, pathophysiology, and pharmacology. Pharmacol Rev. 2022;74(3):712-68
9. Guggenheim JA, McBrien NA. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci. 1996;37(7):1380-95
10. Siegwart JT, Norton TT. Steady state mRNA levels in tree shrew sclera with form-deprivation myopia and during recovery. Invest Ophthalmol Vis Sci. 2001;42(6):1153-9
11. Ding X, Fu D, Ge S, Guan Q, Chen M, Yu Z. DNA methylation and mRNA expression of IGF-1 and MMP-2 after form-deprivation myopia in guinea pigs. Ophthalmic Physiol Opt. 2020;40(4):491-501
12. Zhao F, Zhou Q, Reinach PS, Yang J, Ma L, Wang X. et al. Cause and effect relationship between changes in scleral matrix metallopeptidase-2 expression and myopia development in mice. Am J Pathol. 2018;188(8):1754-67
13. Rada JA, Perry CA, Slover ML, Achen VR. Gelatinase a and TIMP-2 expression in the fibrous sclera of myopic and recovering chick eyes. Invest Ophthalmol Vis Sci. 1999;40(13):3091-9
14. Rada JA, Brenza HL. Increased latent gelatinase activity in the sclera of visually deprived chicks. Invest Ophthalmol Vis Sci. 1995;36(8):1555-65
15. McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res. 2003;22(3):307-38
16. McBrien NA, Cornell LM, Gentle A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Invest Ophthalmol Vis Sci. 2001;42(10):2179-87
17. Moore CS, Crocker SJ. An alternate perspective on the roles of TIMPs and MMPs in pathology. Am J Pathol. 2012;180(1):12-6
18. Gardner J, Ghorpade A. Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system. J Neurosci Res. 2003;74(6):801-6
19. Liu HH, Kenning MS, Jobling AI, McBrien NA, Gentle A. Reduced scleral TIMP-2 expression is associated with myopia development: TIMP-2 supplementation stabilizes scleral biomarkers of myopia and limits myopia development. Invest Ophthalmol Vis Sci. 2017;58(4):1971-81
20. Jia Y, Hu DN, Zhu D, Zhang L, Gu P, Fan X. et al. MMP-2, MMP-3, TIMP-1, TIMP-2, and TIMP-3 protein levels in human aqueous humor: relationship with axial length. Invest Ophthalmol Vis Sci. 2014;55(6):3922-8
21. Jia Y, Hu DN, Sun J, Zhou J. Correlations between MMPs and TIMPs levels in aqueous humor from high myopia and cataract patients. Curr Eye Res. 2017;42(4):600-3
22. Zhuang H, Zhang R, Shu Q, Jiang R, Chang Q, Huang X. et al. Changes of TGF-β2, MMP-2, and TIMP-2 levels in the vitreous of patients with high myopia. Graefes Arch Clin Exp Ophthalmol. 2014;252(11):1763-7
23. Wong CW, Yanagi Y, Tsai ASH, Shihabuddeen WA, Cheung N, Lee SY. et al. Correlation of axial length and myopic macular degeneration to levels of molecular factors in the aqueous. Sci Rep. 2019;9:15708
24. Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther. 2003;98(2):257-65
25. Seko Y, Shimokawa H, Tokoro T. Expression of bFGF and TGF-beta 2 in experimental myopia in chicks. Invest Ophthalmol Vis Sci. 1995;36(6):1183-7
26. Jobling AI, Gentle A, Metlapally R, McGowan BJ, McBrien NA. Regulation of scleral cell contraction by transforming growth factor-beta and stress: competing roles in myopic eye growth. J Biol Chem. 2009;284(4):2072-9
27. Zhu XJ, Chen MJ, Zhang KK, Yang J, Lu Y. Elevated TGF-β2 level in aqueous humor of cataract patients with high myopia: potential risk factor for capsule contraction syndrome. J Cataract Refract Surg. 2016;42(2):232-8
28. Pan S, Yuan J, Jin Y, Liu X, Wu S, Wang Y. et al. Innate immune responsive inflammation in development of progressive myopia. Eye. 2024;38(8):1542-8
29. Yan W, Zhang Y, Cao J, Yan H. TGF-β2 levels in the aqueous humor are elevated in the second eye of high myopia within two weeks after sequential cataract surgery. Sci Rep. 2022;12(1):17974
30. Zhu X, Xu B, Dai L, Wang Z, Feng L, Zhao J. Association between TGF-β gene polymorphism and myopia: a systematic review and meta-analysis. Medicine. 2022;101(30):e29961
31. Böhm EW, Buonfiglio F, Voigt AM, Bachmann P, Safi T, Pfeiffer N. et al. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. 2023;68:102967
32. Moon CE, Ji YW, Lee JK, Han K, Kim H, Byeon SH. et al. Retinal proteome analysis reveals a region-specific change in the rabbit myopia model. Int J Mol Sci. 2023;24(2):1286
33. Carr BJ, Stell WK. Nitric oxide (NO) mediates the inhibition of form-deprivation myopia by atropine in chicks. Sci Rep. 2016;6(1):9
34. Liu TX, Wang Z. Biomechanics of sclera crosslinked using genipin in rabbit. Int J Ophthalmol. 2017;10(3):355-60
35. Tsubota K. Anti-aging approach for ocular disorders: from dry eye to retinitis pigmentosa and myopia. Nippon Ganka Gakkai Zasshi. 2017;121(3):232-48
36. Merida S, Villar VM, Navea A, Desco C, Sancho-Tello M, Peris C. et al. Imbalance between oxidative stress and growth factors in human high myopia. Front Physiol. 2020;11:463
37. Yu Q, Wang C, Liu Z, Yue Y, Hsiao Y, Zhou Q. et al. Association between inflammatory cytokines and oxidative stress levels in aqueous humor with axial length in human myopia. Exp Eye Res. 2023;237:109670
38. Yu FJ, Lam TC, Liu LQ, Chun RKM, Cheung JKW, Li KK. et al. Isotope-coded protein label based quantitative proteomic analysis reveals significant upregulation of apolipoprotein A1 and ovotransferrin in the myopic chick vitreous. Sci Rep. 2017;7:12649
39. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428-35
40. Ku H, Chen JJY, Hu M, Tien PT, Lin HJ, Xu G. et al. Myopia development in tree shrew is associated with chronic inflammatory reactions. Curr Issues Mol Biol. 2022;44(9):4303-13
41. Zeng L, Li X, Liu J, Liu H, Xu H, Yang Z. RNA-seq analysis reveals an essential role of the tyrosine metabolic pathway and inflammation in myopia-induced retinal degeneration in guinea pigs. Int J Mol Sci. 2021;22(22):12598
42. Hsu YA, Chen CS, Wang YC, Lin ES, Chang CY, Chen JJY. et al. Anti-inflammatory effects of resveratrol on human retinal pigment cells and a myopia animal model. Curr Issues Mol Biol. 2021;43(2):716-27
43. Lin HJ, Wei CC, Chang CY, Chen TH, Hsu YA, Hsieh YC. et al. Role of chronic inflammation in myopia progression: clinical evidence and experimental validation. EBioMedicine. 2016;10:269-81
44. Riddell N, Crewther SG. Novel evidence for complement system activation in chick myopia and hyperopia models: a meta-analysis of transcriptome datasets. Sci Rep. 2017;7(1):9719
45. Xu R, Zheng J, Liu L, Zhang W. Effects of inflammation on myopia: evidence and potential mechanisms. Front Immunol. 2023;14:1260592
46. Qi J, Pan W, Peng T, Zeng L, Li X, Chen Z. et al. Higher circulating levels of neutrophils and basophils are linked to myopic retinopathy. Int J Mol Sci. 2022;24(1):80
47. Wang X, He Q, Zhao X, Li H, Liu L, Wu D. et al. Assessment of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with high myopia. BMC Ophthalmol. 2022;22(1):464
48. Icel E, Ucak T, Karakurt Y, Yilmaz H, Tasli NG, Turk A. The relation of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio with high axial myopia. Ocul Immunol Inflamm. 2020;28(3):396-401
49. Wei Q, Zhuang X, Fan J, Jiang R, Chang Q, Xu G. et al. Proinflammatory and angiogenesis-related cytokines in vitreous samples of highly myopic patients. Cytokine. 2021;137:155308
50. Zhang S, Mao J, Chen N, Fang Y, Chen Y, Zheng Z. et al. Difference in aqueous concentration and vitreous mass of cytokines in high myopias with and without choroidal neovascularization. Front Med. 2022;9:1029425
51. Zhu X, Zhang K, He W, Yang J, Sun X, Jiang C. et al. Proinflammatory status in the aqueous humor of high myopic cataract eyes. Exp Eye Res. 2016;142:13-8
52. De Piano M, Cacciamani A, Balzamino BO, Scarinci F, Cosimi P, Cafiero C. et al. Biomarker signature in aqueous humor mirrors lens epithelial cell activation: new biomolecular aspects from cataractogenic myopia. Biomolecules. 2023;13(9):1328
53. Yuan J, Wu S, Wang Y, Pan S, Wang P, Cheng L. Inflammatory cytokines in highly myopic eyes. Sci Rep. 2019;9(1):3517
54. Wei CC, Kung YJ, Chen CS, Chang CY, Lin CJ, Tien PT. et al. Allergic conjunctivitis-induced retinal inflammation promotes myopia progression. EBioMedicine. 2018;28:274-86
55. Zhu D, Yang DY, Guo YY, Zheng YF, Li JL, Wang B. et al. Intracameral interleukin 1β, 6, 8, 10, 12p, tumor necrosis factor α and vascular endothelial growth factor and axial length in patients with cataract. PLoS One. 2015;10(2):e0117777
56. Jonas JB, Tao Y, Neumaier M, Findeisen P. VEGF and refractive error. Ophthalmology. 2010;117(11):2234.e1
57. Pozarowska D, Pozarowski P. The era of anti-vascular endothelial growth factor (VEGF) drugs in ophthalmology, VEGF and anti-VEGF therapy. Cent Eur J Immunol. 2016;41(3):311-6
58. Sun R, Peng Q, Zhang F, Gao H, Li T, Wang L. et al. Effect of vascular endothelial growth factor 165 on dopamine level in the retinas of guinea pigs with form-deprivation myopia. PeerJ. 2023;11:e16255
59. Hu Q, Liu G, Deng Q, Wu Q, Tao Y, Jonas JB. Intravitreal vascular endothelial growth factor concentration and axial length. Retina. 2015;35(3):435-9
60. Zhu X, Du Y, Truscott RJW, He W, Zhoug P, Lu Y. Profiling and bioinformatic analysis of differentially expressed cytokines in aqueous humor of high myopic eyes - clues for anti-VEGF injections. Curr Eye Res. 2020;45(1):97-103
61. Sawada O, Miyake T, Kakinoki M, Sawada T, Kawamura H, Ohji M. Negative correlation between aqueous vascular endothelial growth factor levels and axial length. Jpn J Ophthalmol. 2011;55(4):401-4
62. Shin YJ, Nam WH, Park SE, Kim JH, Kim HK. Aqueous humor concentrations of vascular endothelial growth factor and pigment epithelium-derived factor in high myopic patients. Mol Vision. 2012;18(240):2265-70
63. Sawada O, Kawamura H, Kakinoki M, Sawada T, Ohji M. Vascular endothelial growth factor in the aqueous humour in eyes with myopic choroidal neovascularization. Acta Ophthalmol. 2011;89(5):459-62
64. Shchuko AG, Zaitseva NV, Yurieva TN, Chernykh ER, Mikhalevich IM, Shevela EY. et al. Intraocular cytokines and their correlations with clinical parameters in patients with myopic choroidal neovascularization. Ophthalmologica. 2017;237(2):96-104
65. Steele FR, Chader GJ, Johnson LV, Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. PNAS. 1993;90(4):1526-30
66. Gao G, Li Y, Zhang D, Gee S, Crosson C, Ma J. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 2001;489(2-3):270-6
67. Ogata N, Imaizumi M, Miyashiro M, Arichi M, Matsuoka M, Ando A. et al. Low levels of pigment epithelium-derived factor in highly myopic eyes with chorioretinal atrophy. Am J Ophthalmol. 2005;140(5):937-9
68. Dong L, Shi XH, Li YF, Jiang X, Wang YX, Lan YJ. et al. Blockade of epidermal growth factor and its receptor and axial elongation in experimental myopia. FASEB J. 2020;34(10):13654-70
69. Tang RH, Tan J, Deng ZH, Zhao SZ, Miao YB, Zhang WJ. Insulin-like growth factor-2 antisense oligonucleotides inhibits myopia by expression blocking of retinal insulin-like growth factor-2 in guinea pig. Clin Exp Ophthalmol. 2012;40(5):503-11
70. Li XJ, Yang XP, Wan GM, Wang YY, Zhang JS. Effects of hepatocyte growth factor on MMP-2 expression in scleral fibroblasts from a guinea pig myopia model. Int J Ophthalmol. 2014;7(2):239-44
71. Dong F, Call M, Xia Y, Kao WWY. Role of EGF receptor signaling on morphogenesis of eyelid and meibomian glands. Exp Eye Res. 2017;163:58-63
72. Jonas JB, Dong L, Da Chen S, Neumaier M, Findeisen P, Panda-Jonas S. et al. Intraocular epidermal growth factor concentration, axial length, and high axial myopia. Graefes Arch Clin Exp Ophthalmol. 2021;259(11):3229-34
73. Metlapally R, Ki CS, Li YJ, Tran-Viet KN, Abbott D, Malecaze F. et al. Genetic association of insulin-like growth factor-1 polymorphisms with high-grade myopia in an international family cohort. Invest Ophthalmol Vis Sci. 2010;51(9):4476-9
74. Lian P, Zhao X, Song H, Tanumiharjo S, Chen J, Wang T. et al. Metabolic characterization of human intraocular fluid in patients with pathological myopia. Exp Eye Res. 2022;222:109184
75. Zhou YM, Li MJ, Zhou YL, Ma LL, Yi X. Growth differentiation factor-15 (GDF-15), novel biomarker for assessing atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease. Int J Clin Exp Med. 2015;8(11):21201-7
76. Strelau J, Schmeer C, Peterziel H, Sackmann T, Herold-Mende C, Steiner H. et al. Expression and putative functions of GDF-15, a member of the TGF-beta superfamily, in human glioma and glioblastoma cell lines. Cancer Lett. 2008;270(1):30-9
77. Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79(4):1283-316
78. Dunmire JJ, Lagouros E, Bouhenni RA, Jones M, Edward DP. MicroRNA in aqueous humor from patients with cataract. Exp Eye Res. 2013;108:68-71
79. van der Merwe Y, Steketee MB. Extracellular vesicles: biomarkers, therapeutics, and vehicles in the visual system. Curr Ophthalmol Rep. 2017;5(4):276-82
80. Chen CF, Hua K, Woung LC, Lin CH, Chen CT, Hsu CH. et al. Expression profiling of exosomal miRNAs derived from the aqueous humor of myopia patients. Tohoku J Exp Med. 2019;249(3):213-21
81. Zhu Y, Li W, Zhu D, Zhou J. microRNA profiling in the aqueous humor of highly myopic eyes using next generation sequencing. Exp Eye Res. 2020;195:108034
82. Li Q, Zheng Q, He J, Li L, Xie X, Liang H. Hsa-miR-142-3p reduces collagen I in human scleral fibroblasts by targeting TGF-β1 in high myopia. Exp Eye Res. 2022;219:109023
83. Ando Y, Keino H, Inoue M, Hirota K, Takahashi H, Sano K. et al. Circulating vitreous microRNA as possible biomarker in high myopic eyes with macular hole. Int J Mol Sci. 2022;23(7):3647
84. Ji Y, Rong X, Ye H, Zhang K, Lu Y. Proteomic analysis of aqueous humor proteins associated with cataract development. Clin Biochem. 2015;48(18):1304-9
85. Xiang M, Zhang X, Li Q, Wang H, Zhang Z, Han Z. et al. Identification of proteins in the aqueous humor associated with cataract development using iTRAQ methodology. Mol Med Rep. 2017;15(5):3111-20
86. Wen K, Shao X, Li Y, Li Y, Li Y, Wang Q. et al. The plasminogen protein is associated with high myopia as revealed by the iTRAQ-based proteomic analysis of the aqueous humor. Sci Rep. 2021;11(1):8789
87. Bertrand E, Fritsch C, Diether S, Lambrou G, Müller D, Schaeffel F. et al. Identification of apolipoprotein a-I as a “STOP” signal for myopia. Mol Cell Proteomics. 2006;5(11):2158-66
88. Tsai CY, Chen CT, Lin CH, Liao CC, Hua K, Hsu CH. et al. Proteomic analysis of exosomes derived from the aqueous humor of myopia patients. Int J Med Sci. 2021;18(9):2023-9
89. Duan X, Lu Q, Xue P, Zhang H, Dong Z, Yang F. et al. Proteomic analysis of aqueous humor from patients with myopia. Mol Vision. 2008;14:370-7
90. Xue M, Ke Y, Ren X, Zhou L, Liu J, Zhang X. et al. Proteomic analysis of aqueous humor in patients with pathologic myopia. J Proteomics. 2021;234:104088
91. Yu H, Zhong Z, Zhao Y, Luo H, Sun J, Wang R. et al. Insights into myopic choroidal neovascularization based on quantitative proteomics analysis of the aqueous humor. BMC Genomics. 2023;24(1):767
92. Barbas-Bernardos C, Armitage EG, Garcia A, Merida S, Navea A, Bosch-Morell F. et al. Looking into aqueous humor through metabolomics spectacles - exploring its metabolic characteristics in relation to myopia. J Pharm Biomed Anal. 2016;127:18-25
93. Ji Y, Rao J, Rong X, Lou S, Zheng Z, Lu Y. Metabolic characterization of human aqueous humor in relation to high myopia. Exp Eye Res. 2017;159:147-55
94. Shao J, Zhang Z, Cai X, Wu X, Huang B, Shen Y. et al. Correlation analysis of aqueous humor metabolomics with myopic axial length and choroidal parameters. BMC Ophthalmol. 2023;23(1):356
95. Tang YP, Zhang XB, Hu ZX, Lin K, Lin Z, Chen TY. et al. Vitreous metabolomic signatures of pathological myopia with complications. Eye. 2023;37(14):2987-93
96. Wu CW, Sauter JL, Johnson PK, Chen CD, Olsen TW. Identification and localization of major soluble vitreous proteins in human ocular tissue. Am J Ophthalmol. 2004;137(4):655-61
97. Bishop PN, Takanosu M, Le Goff M, Mayne R. The role of the posterior ciliary body in the biosynthesis of vitreous humour. Eye. 2002;16(4):454-60
Author contact
![]() Corresponding authors: Jibo Zhou, MD, PhD, E-mail: zhoujibo1000com; Fang Li, MD, PhD, E-mail: 244377889com.
Corresponding authors: Jibo Zhou, MD, PhD, E-mail: zhoujibo1000com; Fang Li, MD, PhD, E-mail: 244377889com.

 Global reach, higher impact
Global reach, higher impact