10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(14):5608-5672. doi:10.7150/ijbs.98107 This issue Cite
Review
Harnessing the Anti-Inflammatory Properties of Polyphenols in the Treatment of Inflammatory Bowel Disease
1. Department of Medicine and Medical Specialities, Faculty of Medicine and Health Sciences, Network Biomedical Research Center for Liver and Digestive Diseases (CIBEREHD), University of Alcalá, Alcalá de Henares, Spain.
2. Ramón y Cajal Institute of Sanitary Research (IRYCIS), Madrid, Spain.
3. Department of Surgery, Medical and Social Sciences, Faculty of Medicine and Health Sciences, University of Alcalá, Alcalá de Henares, Spain.
4. Department of Biomedicine and Biotechnology, University of Alcalá, 28801 Alcalá de Henares, Spain.
5. Department of General and Digestive Surgery, General and Digestive Surgery, Príncipe de Asturias University Hospital, Alcalá de Henares, Spain.
6. Gastroenterology and Hepatology Departament. Ramón y Cajal University Hospital, Madrid, Spain; Ramón y Cajal Institute for Health Research (IRYCIS), Madrid, Spain; Network Research Center for Liver and Digestive Diseases (CIBEREHD), Madrid, Spain; University of Alcalá, Madrid, Spain.
7. Immune System Diseases-Rheumatology, Oncology Departament and Internal Medicine (CIBEREHD), Príncipe de Asturias University Hospital, Alcalá de Henares, Spain.
8. Unit of Biochemistry and Molecular Biology, Department of System Biology (CIBEREHD), University of Alcalá, Alcalá de Henares, Spain.
# Contributed equally.
Received 2024-5-5; Accepted 2024-8-25; Published 2024-10-14
Abstract
Inflammatory bowel disease (IBD) encompasses a spectrum of chronic inflammatory conditions affecting the gastrointestinal tract, notably ulcerative colitis (UC) and Crohn's disease (CD). Both UC and CD result from the interplay between genetic and environmental factors that trigger an exacerbated immune response against gut microorganisms, leading to non-resolving inflammatory damage in the mucosa of specific zones in the intestine. Despite extensive research, current treatments often entail invasive interventions with considerable adverse effects on patient well-being. Consequently, there is a pressing need to find alternative and complementary therapeutic strategies aimed at ameliorating chronic inflammation and restoring intestinal barrier integrity. Polyphenols are plant-based compounds formed naturally or as semi-synthetic/synthetic derivatives with proven health-promoting effects and translational applications in a broad spectrum of chronic diseases. Preclinical models of IBD largely support the efficacy of a broad variety of polyphenols due to their well-documented antioxidant and modulatory properties on the immune system and gut microbiota. Likewise, a growing number of studies using distinct types of polyphenols are being conducted in humans, although more efforts are still warranted. In the present review, the main polyphenols investigated in vitro and in vivo models of IBD will be summarized, as well as the available trials or observational data accessible in humans. Finally, the role of polyphenols in the clinical context of IBDs, along with the main problematics regarding their translational issues and concerns will be discussed, including bioavailability, their inclusion in healthy dietary patterns and foods, interaction with other drugs, and other important points to be addressed by future research.
Keywords: Chron's disease, ulcerative colitis, inflammatory bowel disease, polyphenols, adjunctive therapy, immunomodulatory agents, antioxidants, gut microbiota
1. Introduction
1.1. What are inflammatory bowel diseases?
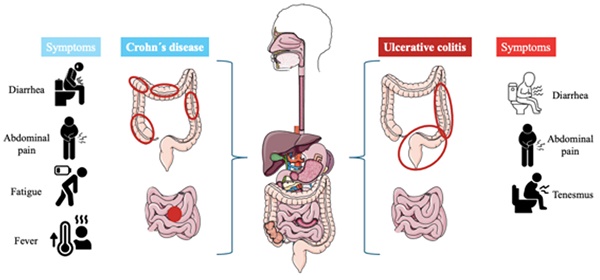
Inflammatory bowel diseases (IBD) are defined as chronic intestinal inflammation resulting from the interplay between genetic and environmental variables that impact immune responses [1]. The two primary categories of inflammatory bowel illnesses are ulcerative colitis (UC) and Crohn´s disease (CD) [2]. Weight loss, diarrhea, stomach discomfort, and rectal bleeding are a few of the symptoms of them, but inflammation is the major characteristic shared by them [3]. Men and women are equally affected by these illnesses, which can strike teenagers and adults. There are several distinctions in the symptoms of UC and CD, despite the similarities between the symptoms of these two illnesses [4].
UC is a chronic disease characterized by generalized inflammation of the rectal and colonic mucosa [5]. In 95% of the cases, UC primarily affects the rectum and may extend continuously and circumferentially to other parts of the large intestine. The clinical course usually includes periods of remission and flare-ups, which may occur spontaneously or in response to treatment [6]. The incidence has increased in several regions of the world, especially in developing countries. Both genetic predisposition and environmental factors play a role in the etiology of the disease. Several studies have identified specific clinical and demographic features associated with distinct UC phenotypes and bad prognoses [7]. On the other hand, Crohn's disease is a chronic inflammatory disorder affecting the gastrointestinal tract, characterized by lesions that can appear anywhere from the mouth to the anus and can lead to extraintestinal complications [8]. The prevalence of Crohn´s disease is increasing in both adults and children. Genetic predispositions to this disease have been discovered, along with the identification of specific environmental factors related to its occurrence [9]. Typical symptoms are diarrhea, abdominal pain, rectal bleeding, fever, weight loss, and fatigue [10]. Figure 1 describes the differences and similarities between UC and CD.
1.2. What are polyphenols and what are their mechanisms of action?
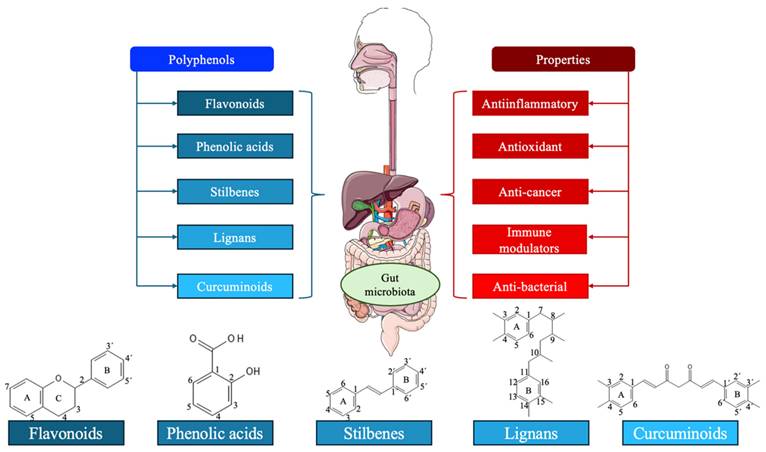
Polyphenols are a class of organic compounds that are predominantly found in fruits, green tea, vegetables, and whole grains [11]. They can also be semi-synthetic or synthetic organic chemicals characterized by one or more hydroxyl moieties on one or more aromatic rings [12]. With 8,000 different structural variations, they represent the largest group of secondary metabolites synthesized through shikimate/ phenyl propanoic or polyketide pathways in plants [13]. Broadly, polyphenols can be classified into five groups: flavonoids, phenolic acids, stilbenes, lignans, and curcuminoids, or they can be divided into different subclasses attending to their number of phenol units, their molecular structures, the linkage types between phenol units, and the substituent groups. Thanks to their aromatic rings, double bonds, and numerous functional groups, polyphenols have effective antioxidant, anti-inflammatory, immune-modulatory, and anti-cancer properties [14-17]. Figure 2 summarizes the main types and properties of polyphenols.
The left describes the progression of Crohn´s disease, the inflammation (red circles) can appear anywhere in the digestive tract and usually appears at the end of the small intestine, also it can occur in patches across the digestive tract. On the other hand, the right is described as ulcerative colitis, the inflammation affects the large intestine and rectum, and it can be extended to the entire colon or only part of its colon.
Polyphenols have a wide range of compounds. This narrative review describes the actions of five of them. First, it explains the different properties that have them, anti-inflammatory, antioxidant, ant-cancer, immune modulators, and anti-bacterial. Secondly, it describes the chemical structure of the five groups, which their derivates have in common, and will be described in the next point.
One of the primary challenges faced by humans pertains to the presence of reactive oxygen species (ROS), which play intricate roles in various biological functions. These functions include combating pathogens, regulating blood pressure, and mediating cellular signaling processes [18]. Their production can be modulated by physiological processes, or it can be introduced through the exogenous via [19]. In normal conditions, there are more antioxidants than free radicals, but at the moment when the accumulation of ROS is higher than the antioxidants in cells or tissues, this process is called oxidative stress. It is caused because ROS has unpaired electrons, providing a higher chemical reactivity, and making it act as a potentially toxic molecule to induce an amount of degenerative disease by damaging the biomolecules [20]. One of the key actions of polyphenols is their antioxidant capacity to scavenge ROS, including both free radicals and non-free radicals like hydrogen peroxide (H2O2), superoxide, and ions (O-2), hydroxyl radical (HO-), ozone (O3). They combat oxidative stress generated by lipids and nucleic acids by donating a single electron (SET) or through hydrogen atom transfer [21]. As a consequence of this, they interrupt the initiation of radical reactions like the oxidation of proteins and sugar, peroxidation of lipids, and oxidative damage to nucleic acids [22]. Also, they can chelate the ions of transition metals inhibiting the formation of free radicals in the Fenton and Haber-Weiss reaction [23]. Another benefit, in this case, they act as co-antioxidants, which are involved in the regeneration of essential vitamins. Therefore, thanks to their antioxidant properties and their ability to propitiate ROS and free radicals, polyphenols are useful in improving human health, also aiding in the prevention of multiple diseases, and reducing the aging process [24].
On the other hand and as has been described before, polyphenols also exert a pivotal anti-inflammatory and immunomodulatory activity. In the last years, in vivo and in vitro models have shown that dietary phenolic compounds are able to modulate the NLPR3 pathway [25], having a protective activity on inflammation. NLRP3 is an important node that links the signaling pathways between inflammation and the redox response, thus influencing cellular responses against ROS [18]. Also, it is suggested that they have a radical scavenging activity, NADPH oxidases (NOX) inhibition, the regulation of enzymes involved in arachidonic acid metabolism, arginine metabolism, MAPK pathway, and the inhibition of pro-inflammatory enzymes such as cyclooxygenase (COX) 2, inducible nitric oxide synthase (iNOS), lipoxygenase (LOX), inhibition of nuclear factor kappa B (NF-κB), and the activation of activator protein-1 (AP-1) DNA binding [26]. On the other hand, some studies suggest that polyphenols have effects on the expression of various inflammatory mediators, including interleukin-1beta, (IL-1β), interleukin-6, (IL-6), and tumor necrotic factor alpha (TNF-α) [27].
Polyphenols can also exert direct effects on the gut microbiota —a diverse community of bacteria, fungi, viruses, and other microorganisms that play crucial roles in the host, including reinforcing intestinal integrity, regulating metabolism, defending against pathogens, and modulating the immune system [28]. Although the precise mechanism remains incompletely understood, it is theorized that polyphenol metabolites may stimulate beneficial gut bacteria [29]. Increasing research suggests that the presence of phenolic compounds may enhance the beneficial actions of probiotics [30]. In parallel, polyphenols show antibacterial activity against a large number of bacteria (including Gram-positive and Gram-negative bacteria) and fungi [31], thus explaining their regulatory role on gut microbiota. However, more research is needed to delineate how polyphenols and related metabolites, either phase II metabolites or those generated by the gut microbiota, might interact with systemic tissues, using in vitro and in vivo models [32].
Finally, polyphenols are also being investigated for their antitumoral activities. Indeed, a broad spectrum of studies supports the multiple anticarcinogenic properties of plant-derived polyphenols, including their inhibitory effects on the proliferation of cancer cells, tumor expansion, angiogenesis, inflammation, and metastasis whereas some studies show potential synergistic effects when polyphenol treatment combined with chemotherapeutic agents [33].
1.3. Polyphenols and Inflammatory Bowel Diseases. Where is the potential?
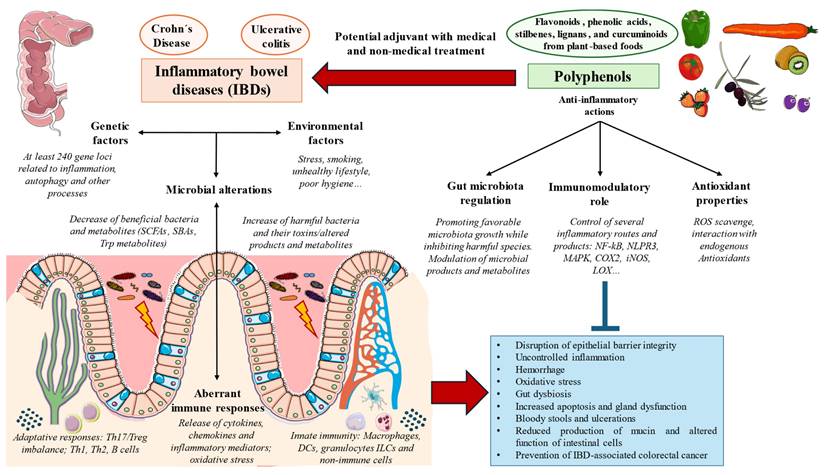
As mentioned above, IBD is a complex and multifactorial disease triggered by the interaction between genetic and environmental factors. The interaction between genetic and environmental factors triggers an impaired immune response against gut microorganisms in IBDs leading to non-resolving inflammatory damage in the mucosa of specific zones in the intestine [34]. Regarding the genetic component, at least 240 gene loci related to inflammatory responses (mainly in the nucleotide oligomerization domain -NOD- receptors, chemokines, cytokines), autophagy, and antimicrobial peptides seem to be associated with the predisposition and occurrence of IBD [35]. Environmental factors associated with IBD pathogenesis include stress, smoking, unhealthy lifestyle, and poor hygiene, whereas the use of non-steroid anti-inflammatory drugs, antibiotics, or appendectomy has also been associated with IBDs [36].
An altered microbiota (gut dysbiosis) is a central mechanism implicated in the pathogenesis of IBD. A set of bacteria seems to be associated with IBD development, including Mycobacterium paratuberculosis, adherent-invasive E. coli (AIEC), Helicobacter pylori and non-pylori species, Campylobacter concisus, Enterococcus faecium, enterotoxigenic Bacteroides fragilis (ETBF), Fusobacterium varium and Ruminococcus gnavus, whereas alterations in the mycome and virome have also been observed influencing immune responses [37,38]. Conversely, some microorganisms are inversely related to IBDs such as Faecalibacterium prausnitzii, Roseburia species, particularly Roseburia hominis and Roseburia intestinalis, Ruminococcaceae, including Clostridium leptum and Clostridium sporogenes, E. coli, Bacteroides fragilis, and Akkermansia muciniphila. These microorganisms are responsible for producing favorable microbial metabolites such as short-chain fatty acids (SCFAs), tryptophan derivatives, and secondary bile acids, among other products, playing crucial roles in regulating immunity, reducing inflammation, and maintaining gut homeostasis [37]. Collectively, the phenomena of gut dysbiosis are directly involved in the exacerbated inflammatory responses related to IBD via direct interactions with the immune system or through the production and release of toxins/microbial metabolites with potential immunomodulatory effects [39].
Regarding the immunological changes occurring in IBDs, a broad spectrum of changes affecting both the innate and adaptative immune systems have been reported. The aberrant innate immunity occurring in the gut of patients affected by IBD encompasses immune and non-immune cells, involved in the sensing and response to the gut microorganisms. These cells include 1) Paneth cells, tuft cells, and other epithelial cells (implicated in the secretion of antimicrobial peptides that contribute to limiting bacterial growth and invasion); 2) globet cells (responsible for producing mucine, serving as prevents the entry and invasion of microorganisms in the different gut layers); and 3) gut epithelial cells (enterocytes) and stromal cells, responsible for detecting invading bacteria through extracellular and intracellular pattern recognition receptors (Toll-like receptors — TLRs and NOD-like receptors-NLRs). Innate immune cells include macrophages, granulocytes, innate lymphoid cells (ILCs), and dendritic cells (DCs), involved in the rapid initiation of inflammatory responses mediated by the secretion of cytokines and chemokines and recruitment of inflammatory adaptative cells [40,41]. Adaptative immune responses associated with IBDs are mainly represented by B cells (implicated in humoral response and T helper cells, particularly Th1, Th2, Th17, and regulatory T cells (Tregs) [42]. Within these cells, compelling evidence seems to defend that Th17 and Treg could have greater relevance in the development of IBDs; however, the dual role that these cells and their released products partly explain the difficulties in the available therapies directed against these and other inflammatory mediators [40,43]. Immune dysfunction is accompanied by aberrant levels of a broad spectrum of cytokines tightly linked to IBD pathogenesis, including IL-1β, IL-18, IL-33, IL-6, IL-10, IL-17 (and their isoforms), TNF-α, tumor growth factor beta (TGF-β), along with chemokines IL-8, chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α and 1β, MCP-3, MIP-3α, CXCL5, CXCL8, CXCL10, and RANTES [40]. Interestingly, inflammatory responses associated with UC are dominated by cytokines such as IL-4, IL-5, IL-9, and IL-13 secreted by Th2 cells, whereas in the case of CD, IL-1, IL-6, IL-8, TNF-α, and IFN-γ secreted by Th1 and Th17 cells are more abundant [44]. Therefore, cytokine and immune response patterns can be important for understanding and discerning both entities.
Finally, other important processes beyond altered immunity and dysbiosis are playing a pivotal role in IBD development. For instance, oxidative/nitrosative stress is also a critical factor implicated in the initiation and progression of IBD. Overproduction of ROS and oxidative stress is triggered during inflammation because of the inflammatory responses that occur in the colonic tissue [45]. All these mechanisms lead to significant changes in the functioning of the gut, from a molecular to a systemic level.
Polyphenols belong to a group of natural compounds contained in foods and plant sources known as nutraceuticals with proven benefits either in health promotion or disease prevention [46]. In the event of IBDs, the relevance of polyphenols has been described in previous literature, exerting their benefits in this condition by many different mechanisms including the reduction of epithelial damage, inflammation, hemorrhage, oxidative stress, gut dysbiosis, apoptosis, gland dysfunction, bloody stools, and ulcerations while promoting an increase in mucin content, number of crypts and reinforcing the integrity of the epithelial barrier [47]. Because of this, a growing number of studies suggested that these actions make polyphenols a potential therapeutic approach as an available adjuvant to medical and non-medical treatment to aid in the clinical management of IBD, although the available evidence to support their clinical use is still limited [48]. Besides, previous works have demonstrated that patients with IBDs show an increased risk of developing colorectal cancer (CRC) [49], making the use of polyphenols a promising strategy to prevent and also aid in the management of this concern [50,51]. Figure 3 summarizes the pathophysiological basis of IBDs and the main actions of polyphenols.
In the present narrative review, a search for the principal polyphenols currently investigated in the context of IBDs (including flavonoids, phenolic acids, stilbenes, lignans, curcuminoids, and other polyphenols from plant-based sources) will be conducted using the bibliographic databases PubMed, Scopus, and ScienceDirect. For each polyphenol explored, the search terms will be ("inflammatory bowel disease" OR "Crohn's disease" OR "ulcerative colitis"). Subsequently, the available studies about these terms will be evaluated, highlighting the main findings/conclusions obtained and discerning their origin (preclinical or clinical). As most studies have been conducted in vitro or in vivo, we will highlight those polyphenols explored in humans with a specific subsection, differentiating into observational or intervention studies. Finally, a critical perspective will be provided on the main limitations and issues surrounding the use of polyphenols in general and in the context of IBDs in particular, based on the most updated scientific evidence.
2. Polyphenols in inflammatory bowel disease
In this section, the use of the main types of polyphenols (flavonoids, phenolic acids, stilbenes, lignans, curcuminoids, and others) in IBDs will be summarized. As aforementioned, most studies have been conducted in vitro or in vivo. In vitro models to study IBDs commonly include Caco-2, HT29, or RAW264.7 cells, as well as organoids [52]. Animal models of IBD are commonly mice or rats. IBD can be induced chemically by agents like dextran sulfate sodium (DSS), 2,4,6-trinitrobenzene sulfonic acid (TNBS), acetic acid (AA), and oxalazone, genetically (knock-out models), or being induced by specific bacteria [53,54]. Thus, in vitro and animal models used in the different studies will also be remarked.
2.1. Flavonoids
Plants produce their synthesis and are extensively distributed in the tissue plant as a glycoside form. The flavonoid structure generally comprises aglycones (the non-sugar fragment part of the appropriate glycoside) or glycosides. They all have a basic structure made of diphenyl propane (C6-C3-C6), their phenolic rings (ring A and ring B) are connected by a heterocyclic ring, and their ring C is usually a closed pyran [55].
Pathogenesis of IBDs and the mechanism of action of polyphenols in this condition.
The diversity of the structures of the flavonoid molecules rises from different modifications in the oxidation situation of the central pyran ring, and the hydroxylation sequence. As a result of these combinations, there are described a wide range of compounds such as flavones, flavonols, flavanones, isoflavones, flavanols, and anthocyanidins/anthocyanins. These modifications are determined by whether a double bond exists between C2 and C3, and the formation of a carbonyl group by C4 [56].
Besides, flavonoids are not considered a stated nutrient yet due to their physiological functions in plants, they are important in the human diet as healthy ingredients. According to epidemiological studies, diets based on an abundance of flavonoids are relational with an increase in longevity and the reduction of cardiovascular disease incidence, and cancer risk [57]. Their biological function is determined by their bioavailability, their anti-inflammatory, and antioxidant properties, and other activities such as vasodilatory, anticancer effects, and anti-ischemia [58].
2.1.1. Flavones
This subgroup is an important part of flavonoids. Their skeleton comprises 2-phenyl-γ-pyrone, which is involved in the heterocyclic pyrone ring connected with two phenyl rings [59]. Another characteristic of the flavones is the position of the O-glycoside joint, it appears in the C3 and C7 positions. This linkage units the sugar group with the aglycone [60]. Their C-glycosides joints do have not much research about their function. In nature, they are found in herbs like parsley and celery and grains such as oats, rye, barley, and sorghum.
As part of the flavone subgroup, it is formed by many components, here it is described the action of apigenin, baicalein, baicalin, wogonoside, wogonin, luteolin, tangeretin, galangin, nobiletin, and chrysin.
A) Apigenin
Apigenin is a natural compound present in parsley, chamomile, celery, vine spinach, artichokes, and oregano [61]. It suggests having anti-inflammatory and antioxidant properties that are beneficial in IBD therapy. Also, it can inhibit the transactivation promoted by tumor necrosis factor (TNF)-α [62]. There is one study that shows the efficacy of apigenin in the murine DSS colitis model through blocking the inflammasome vias by the production of IL-1β and downregulation of iNOS and COX-2 and decreasing serum levels of matrix metalloproteinase-3 [63]. Apigenin can also significantly relieve the intestinal pathological injury in these animal models, increasing goblet cell quantity and mucin secretion, promoting anti-inflammatory cytokines IL-10 expression, and inhibiting the expression of proinflammatory cytokines, TNF-α, IL-1β, IL-6, and MPO activity of colon tissue [64]. It is shown that in combination with epigallocatechin-3-gallate (EGCG) in the Flavo-Natin has protective functions in the oxazolone-induced colitis model, another of the benefits is the maintenance of the intestinal epithelial barrier, shaping microbiota, and their proinflammatory and antioxidant properties [65]. Also, apigenin is found to inhibit the inflammation promoted by carcinogenesis in general, by repressing the signal transducer and activator of transcription (STAT)-3- NF-κB signaling [66]. Apigenin lead to increased zonulin 1 (ZO-1), claudin-1 and occludin expressions to restore the integrity of the intestinal barrier, regulating the microbial populations of Akkermansia, Turicibacter, Klebsiella, Romboutsia, etc., and its metabolites (SCFAs), thus attenuating DSS-induced colon injury [64]. Apigenin can also inhibit both canonical and non-canonical NLRP3 inflammasome pathways by decreasing proinflammatory IL-1β and IL-18 cytokine levels and regulating cleaved caspase-1 and caspase-11 enzymes [67].
B) Baicalein and baicalin
Baicalein and its aglycone baicalein possess multi-fold therapeutic properties and are mainly found in the roots of Oroxylum indicum (L.) Kurz and Scutellaria baicalensis Georgiis (SBG) [68]. Both baicalein and baicalin have shown significant and potential benefits for IBDs. In vitro and in vivo models have shown that baicalein reduces the impact of IBD by inhibiting the COX-2 activity and decreases the phosphorylation of Ikappa kinase (IKK)-β degrading the Ikappa-beta-alfa (IκΒα) [69], also ameliorating UC by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s [70] and through the inhibition of TLR4/MyD88 signaling cascade as well as inactivation of NLRP3 inflammasome [71]. Moreover, the modulatory effects of baicalein in Ahr receptors can restore the balance of Th17/Treg cells and diminish proinflammatory cytokines such as IL-17, IL-6, and TNF-α while increasing anti-inflammatory cytokines such as IL-10 and TGF-β; and epithelial protective cytokine IL-22 in UC mice [72]. Also, baicalein is involved in the reduction of the phosphorylation of p65 and the nuclear translocation and downregulation of the DNA-binding activity of the NF-κB in the STAT3 and cyclin D1 expression [73]. It seems that engineered formulas like baicalein-decorated zinc phosphates could exert greater improvements in animal models of colitis when compared to baicalein alone [74]. In parallel, baicalein can also make synergy with other compounds such as betaine, alleviating colonic inflammation and preventing associated tumorigenesis [75].
Baicalin exerts similar actions to baicalein on autoimmune diseases by regulating cell proliferation and STAT gene expression [76]. Liang et al. [77] explored in vivo the use alone or in combination of baicalin and baicalein from using Scutellaria baicalensis herb (SB). They observed that Baicalin and baicalein had significantly different effects on UC, as well as when both compounds were combined. They show that the combination of the two drugs provides a more comprehensive treatment; and also that compared with baicalein, baicalin was more potent for the treatment of large intestine disease. Baicalin has also been shown to act as an anti-inflammatory, anticarcinogenic antioxidant, and immunomodulatory drug, also aiding in the maintenance of intestinal barrier and flora balance [78]. The studies highlight its effects in the regulation of Th17/Treg populations [79], promoting the proliferation of CD4(+)CD29(+) cells and modulating immunosuppressive pathways [80], enhancing the polarization of the anti-inflammatory phenotype in macrophages (M2) [81]. Also, it is implicated in the down-regulation of the expression of MIF, the number of macrophages, and the amount of macrophage-related cytokines, including MCRP1 MIP-3α [82], inhibition of IL-33 expression and subsequent NF-κB activation [83], blockage of the TLR4/NF-κB-p65/IL-6 signaling pathway [84], regulation of the autophagic flux [85], modulation of gut microbiota and SCFAs [86], amelioration of inflammation, oxidative stress and apoptosis in intestinal cells [87,88], and control of sphingolipid metabolism and sphingolipid signaling pathway [89]. The combination of baicalin with berberin or emodin has also demonstrated significant synergic benefits in the treatment of animal models of UC [90,91].
Two studies conducted by Yu et al. [80,92] assessed the efficacy and effects of baicalin in human cells from patients with UC (N=33 divided into active and inactive groups) and compare it with irritable bowel syndrome and healthy subjects. In these studies, peripheral mononuclear immune cells were extracted from these groups and cultured them in vitro. Baicalin was added at different concentrations (5, 10, 20, or 40 µmol). In one study, they observed that the percentages of CD4+CD29+ T cells were lower with 40 and 20 μmol/L baicalin treatments compared to the no baicalin treatment, driving a significant increase in the expression of IL-4, TGF-β1, and IL-10, and the p-STAT6/STAT6 ratio. In parallel, these treatments decreased the expression of IFN-γ, IL-5, IL-6, RORC, Foxp3, and T-bet, as well as the ratios of T-bet/GATA-3, p-STAT4/STAT4, and p-NF-κB/NF-κB [80]. Likewise, 40 µmol baicalin significantly decreased IL23R gene expression in UC patients, whereas the 20 µmol and 40 µmol baicalin treatments significantly decreased p-STAT4/STAT4 ratios IFN-γ and IL-4 and increased p-STAT6/STAT6 ratios and IL-10 levels [92].
C) Luteolin
Luteolin is a natural compound found in carrots, parsley, broccoli, peppers, celery, olive oil, onion leaves, cabbages, apple skins, chrysanthemum flowers, peppermint, thyme, rosemary, and oregano [93]. Its expression is involved in inhibiting pro-inflammatory mediators such as COX-2, TNF-α, and interleukin (IL)-6, and appears in regulating multiple vias like NF-κB [94]. Recent studies suggest that this natural recurse decreases inflammation in rats with ulcerative colitis by regulating the gut microbiota [94]. In the cell line HT-29 colon epithelial cells, luteolin negatively affects the regulation of inflammatory signaling cascades due to their anti-inflammatory action, inhibiting the JAK/STAT pathway [95]. Likewise, luteolin seems to inhibit TNF-α-induced IL-8 production in this cell line through blockade in the phosphorylation of MAPKs, following IkappaB degradation and NF-kappaB activation [96] Also, in RAW264.7 cells, luteolin acts as an antagonist of the IKKα/β by blocking its phosphorylation and the action of NF-κB [97]. Regarding DSS-induced UC in rats, luteolin seems to reduce colonic inflammation and intestinal barrier damage through the modulation of various pathways including the suppression of the STAT3 signaling pathway by SHP-1 [98] or restoring the balance between NCR-ILC3/NCR+ILC3 [99]. Likewise, the administration of luteolin seems to drive favorable changes in the gut microbiota, enhancing the levels of lactobacillus, Bacteroides, Roseburia, and Butyricicoccus while reducing DSS-induced enhanced ratios of Lactobacillus and Prevotella_9 [100]. Intraperitoneal administration of luteolin was also shown to improve the relative abundance of anti-inflammatory microorganisms (i.e. Clostridia UCG-014, Enterorhabdus, Blautia and Lachnospiraceae NK4A136 group) while attenuating pro-inflammatory species (From the genera Turicibacter, Streptococcus, Staphylococcus, Clostridium sensu stricto 1, Romboutsia, Parasutterella, and Escherichia-Shigella) [101]. Interestingly, luteolin strongly demonstrated utility in alleviating associated physical UC symptoms compared to apigenin or Xanthohumol administration. On the other hand, luteolin (20 and 50mg/kg) significantly attenuated the disease activity index (DAI), colon shortening, and histological damage while decreasing the expression of inflammatory mediators, such as iNOS, TNF-α, and IL-6 [102]. Luteolin can also stimulate total antioxidant defenses (promoting the activity of the superoxide dismutase (SOD) or the catalase (CAT) and alleviating oxidative stress, mainly through the Nrf2 signaling pathway and the decrease of malondialdehyde (MDA) [103]. Finally, luteolin led to metabolomic changes in UC rats, leading to reductions in l-malic acid, creatinine, l-glutamine, and l-lactic acid levels accompanied by elevations in dimethyl sulfone, 5-methylcytosine, cysteine-S-sulfate, and jasmonic acid levels [103]. Furthermore, differential metabolites primarily participated in d-glutamine and d-glutamate metabolism, glutathione metabolism, and citrate cycle pathways, thus demonstrating the multiple roles of this polyphenol.
D) Wogonoside and wogonin
Together with baicalein, baicalin, wogonoside, and wogonin are polyphenols representative of the flavone group, extracted from plants of the genus Sculletaria such as SBG [104]. Wogonoside seems to alleviate colitis by protecting against intestinal barrier dysfunction through the reinforcement of tight junctions via regulation of the MLCK/pMLC2 signaling pathway in Caco2 cells [105], whereas in DSS-induced UC mice this compound seemed to lead to dual inhibition of NF-κB and NLRP3 inflammasome [106]. Wogonin treatment effectively prevented colonic ulceration, neutrophil infiltration, oxidative stress, pro-inflammatory cytokines, and histological changes in DSS mice models. In more detail, Zhou et al. [107] showed that this compound promoted apoptosis by inhibiting Bcl-2 and enhancing the expressions of Bax, caspase-3, and caspase-9. Likewise, it led to a marked downregulation of COX-2 and iNOS, which led to the suppression of NF-κB. Moreover, wogonin also regulated the Nrf2 signaling pathway and decreased the activation of TLR-4/NF-κB. Recently, Ye et al. [108] showed that the effects of this polyphenol were also partly mediated by regulating the plasticity of ILC3/ILC1. They hypothesized that its specific mechanism is to bind to AhR directly or activate the AhR pathway indirectly by altering the tryptophan metabolisms of gut microbiota.
E) Other relevant flavones
Other less studied but relevant flavones investigated include tangeretin, nobiletin, and chrysin. Tangeretin is another flavone member and a main compound of the Citrus Spp. pericarp. It is studied that in DSS-induced colitis mice, it improves the reduction of colonic tissue damage, and increases the activity of the gut microbiota [109]. Through oral administration, it also can inhibit the IL-12 and TNF-α expression, as other flavones can interact in the NF-κB pathway in UC attenuated [110]. Nobiletin, a flavone found in citrus peels, exerted anti-inflammatory effects in TNBS-induced colitis through the downregulation of iNOS and COX-2 expression, restoring barrier function through the inhibition of the Akt-NF-κB-MLCK pathway [111]. Lastly, chrysin is a flavone extracted from honey, propolis, and various plants, fruits, and even fungi [112,113]. This polyphenol was able to prevent chemically induced colitis in vivo through the regulation of the PXR/NF-κB pathway [114].
Overall, these results support the relevance of flavones in the clinical management of IBDs, particularly demonstrated in vitro and in vivo. More studies in humans are still required.
2.1.2. Flavonols
This group has the typical structure within the plane of the 3-hydroxyflavone base. They are different from the rest of the flavonoids because they only have one hydroxyl group at the C-3 position, also their O-glycoside is in the C-7 position [115]. Their main representatives are quercetin and kaempferol; however other flavonols like galangin, myricetin, or isorhamnetin should also be highlighted.
A) Quercetin
Quercetin is one of the bioflavonoids with a wide range of uses in treating metabolic and inflammatory diseases. It is abundantly present in citrus, and green leafy vegetables like broccoli, flowers, and nuts [116]. It is known to act in the intestinal by integrating the mucosa barrier, improving the increase of the colonic microbiota, moderating the oxidative stress response, and resettling the local immune homeostasis [117]. Through a systematic review and meta-analysis, Hu et al. [118] concluded that preclinical evidence suggests that quercetin is a potential agent to consider in IBD treatment. In more detail, they observed that quercetine could reduce histological score, DAI, IL-1β, TNF-α, nitric oxide (NO), MDA, myeloperoxidase (MPO) activity and increase colon length, weight change degree, interleukin-10 (IL-10), glutathione (GSH), SOD and CAT. However, they observed that due to the low methodological quality and the small number of studies included some cautions must be considered with these results. Other preclinical works have also found significant anti-inflammatory effects of polyphenols in the management of IBD. For instance, quercetin seems to act in vivo through the modulation of intestinal microbiota, leading to the re-establishment of healthy microbiomes that favor mucosal healing, and the inhibition of PI3K/AKT signaling [119]. Likewise, it was shown to balance the proportion between anti-inflammatory M2 and proinflammatory M1 macrophages, facilitating intestinal repair [120]. In one study where quercetin is administrated orally in a water-soluble inclusion form, accompanied by hydroxypropyl-b-cyclodextrin (Que-HP-β-CD) in an experimental model of UC in mice, they show a therapeutic/prophylactic potential of this combination to treat the UC [121]. On the other hand, in rat intestinal microvascular endothelial cells (RIMVECs), quercetin downregulates pyroptosis, the levels of inflammatory factors, and the elimination of the intestinal barrier produced by the lipopolysaccharide (LPS) by reducing the activation of the NLRP3 inflammasome [122]. Quercetin was also able to repair intestinal barrier dysfunction in vitro by activating AhR-mediated enhancement of TJs to alleviate UC [123]. Also, some studies have remarked on the relevance of quercetin as a non-toxic and safe bioactive compound with a marked antiviral activity, being suggested as a potential tool against viral-associated IBD [124]. Similarly, quercetin appears to exert therapeutic effects on C. rodentium-induced colitis [125], suggesting the relevance of this polyphenol against viral and bacterial infections.
Observational and intervention studies in humans
Two observational studies have specifically focused on the relationship between quercetin intake and IBDs in humans. Lu et al. [126] analyzed a prospective cohort of 187,709 IBD-free participants from the UK Biobank collecting dietary information to estimate the daily quercetin intake. After almost 10 years of follow-up, they reported 863 incident IBD, finding that participants in the highest quintiles were associated with a lower risk of IBD and UC but not CD when compared to those in the lowest quintile [126]. Similarly, Wang et al. [127] included 2,293 participants with IBD (764 with CD and 1529 with UC) from the UK Biobank and follow them for almost 10 years. During this period, they observed that patients with higher dietary intake of quercetin presented a lower risk of enterotomy and all-cause mortality in IBD when compared to patients located in the lowest quartile intake of quercetin.
One intervention study [128] evaluated the relevance of quercetin in patients with IBD. The study compared two groups of patients receiving different flavonoid mixtures for hemorrhoidal disease (diosmin, troxerutin, rutin, hesperidin, and quercetin as the study group versus hesperidin, diosmetin, isoroifolin, and linarin in purified micronized fraction as the control group. They report that both groups presented bleeding improvement with no significant difference between the groups after 1 and 6 months. However, patient satisfaction after 6 months was significantly higher in the study group receiving the mixture of diosmin, troxerutin, rutin, hesperidin, and quercetin. Therefore, these studies support a potential association between dietary quercetin intake with prevention and improved outcomes related to IBDs, although further intervention studies are required to evaluate the therapeutic effects of quercetin in humans. In a pilot study, Ryan et al. [129] aimed to identify the effects of a nutrition support formula on blood nutrient parameters in adults with IBD. The formula contained a mixture of micronutrients (including methylated forms of folate and vitamin B12), macronutrients, and phytonutrients (including curcumin, XN, ginger compounds, and quercetin). 10 participants with Crohn's disease or ulcerative colitis consumed a micronutrient and phytonutrient-rich beverage twice daily for 12 weeks. Significant increases in serum folate and decreases in red cell distribution width were observed. Modulation of leukocyte subtypes was noted, with a decrease in neutrophils and an increase in lymphocytes. Other parameters, including RBC count, hemoglobin, hematocrit, electrolytes, albumin, and inflammatory markers, did not change significantly.
B) Kaempferol
Kaempferol also known as 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, forms part of the flavonols group due to its properties as an anti-inflammatory, and anti-ulcerative properties, having multiple activities in different molecular pathways [130]. This flavonoid is mainly found in many edible plants like tea, broccoli, cabbage, kale, beans, endive, leek, tomato, strawberries and grapes, and herbal medicinal plants (e.g. Ginkgo biloba, Tilia spp, Equisetum spp, Moringa oleifera, Sophora japonica and propolis) [131]. Similar to quercetin, kaempferol seems to be effective at protecting colonic mucosa from DSS-induced UC [132]. Qu et al. [133] showed that kaempferol may be relevant for treating IBD by regulating the gut microbiota and TLR4-related signaling pathways. In more detail, kaempferol seems to act by elevating the levels of ZO-1, occludin, and claudin-1, reducing the levels of IL-1β, IL-6, and TNF-α and the transcription of an array of inflammatory signaling molecules, accompanied by an increase in IL-10 mRNA expression. In RIMVECs is demonstrated that kaempferol reduces LPS-induced inflammatory mediators, such as TNF-α, IL-1β, IL-6, and vascular cell adhesion molecule-1 (VCAM-1), acting at the toll-like receptor 4 (TLR4), NF-κB, and STAT [134]. Also, kaempferol reduces the IL-8 secretion and barrier dysfunction of the cell line Caco-2 monolayer by the LPS-induced epithelial-endothelial model through the blocking of the NF-κB signaling pathway [135]. Apart from these effects, Kaempferol can balance the intestinal microbiome in mice by elevating the Firmicutes to Bacteroidetes ratio; increasing the linear discriminant analysis scores of beneficial bacteria, such as Prevotellaceae and Ruminococcaceae; and reducing the richness of Proteobacteria in DSS-challenged mice [133].
C) Other relevant flavanols
As previously mentioned, other important types of flavonols studied in IBD include galangin, myricetin, fisetin, or isorhamnetin. Galangin is a natural flavonoid isolated from ginger, gangal, honey, and propolis. This compound seems to act as an HSP90β inhibitor, a component significantly increased in the mucosal biopsies of UC patients and the colons of colitis mice, which show a direct correlation with disease severity [136]. Indeed, galangin was shown to potentially alleviate colitis by inhibiting HSP90β and perturbing fatty acid synthesis-mediated NLRP3 inflammasome activation. Galangin demonstrated dose-dependent regulatory effects in vitro, reducing nitrites, IL-6, and TNF-α levels. In vivo, oral administration of galangin alleviated colitis, reduced proinflammatory cytokines (TNF-α and IL-6), increased anti-inflammatory IL-10, and decreased MPO, nitrites, and TBARS levels while increasing SOD [137]. Other works have also found that galganin downregulates Toll-like receptor 4 (TLR4) expression, and suppresses NF-κB p65 activation [138], also leading to significant increases in autophagy proteins and recovery of beneficial bacteria like Lactobacillus spp., and increased Butyricimonas spp. [139].
Myricetin is another flavonol mainly found in fruits, vegetables, berries, teas, and wine, including cranberry, dock, sweet potato leaves, chard, broad beans, and immature seeds the foods with the richest content in this polyphenol [140]. Qu et al. [141] have found that myricetin can reduce the severity of inflammation in acute UC and significantly improve the condition. The administration of myricetin (80 mg/kg) increased the levels of IL-10 and TGFβ while augmenting the proportion of Treg cells. In a similar line, Zhao et al. found that myricetin administered orally at 200, 100, or 50 mg/kg to DSS-induced UC mice alleviated body weight loss in a dose-dependent manner and significantly reduced histology scores. Besides, myricetin decreased the production of NO, MPO, MDA, IL-1β, and IL-6 while increasing the activity of SOD and GSH [142]. Likewise, other studies have found that myricetin and M10, a myricetin-3-O-β-d-lactose sodium salt can normalize Firmicutes and Actinobacteria populations, leading to a marked increase in Akkermansia muciniphila and decrease in pathogenic microorganisms, such as Ruminococcus and Parabacteroides [143]. Some studies have found that M10 exerts higher activities in preventing UC through inhibiting necroptosis [143] however, it has also been demonstrated that the majority of M10 is metabolized to myricetin via fecal microbiota and that both compounds are mostly located in inflamed tissues, exerting their immunomodulatory actions [144].
Fisetin is abundantly found as a dietary flavonoid found in various fruits (strawberries, apples, mangoes, persimmons, kiwis, and grapes), vegetables (tomatoes, onions, and cucumbers), nuts, and wine [145]. Past works have found that fisetin can inhibit senescence markers (p53, Bcl2, Cxcl1, and Mcp1)in DSS-induced UC in mice, upregulating the expression of micro RNAs (miRNAs) miR-149-5p, miR-96-5p, miR-34a-5p, and miR-30e-5p and the abundance of Akkermansia muciniphila, which is negatively correlated with senescence and inflammation [146]. Likewise, fisetin may exert an important anti-inflammatory activity via inhibition of Akt, p38 MAPK, and NF-κB signaling in the colon tissues of DSS-exposed mice, also enhancing GSH and reducing MDA levels [147].
Isorhamnetin glycosides are primarily extracted from various plant-based foods or medicinal plants such as Opuntia ficus-indica, Hippophae rhamnoides, and Ginkgo biloba [148]. Animal models have shown that isorhamnetin can alleviate IBD via PXR-mediated up-regulation of xenobiotic metabolism and down-regulation of NF-κB signaling [149]. Isorhamnetin can also inhibit ferroptosis, a special type of programmed cell death mediated by iron independent of its previously reported targets MEK1 and PI3K, but alleviated oxidative stress by targeting and activating NRF2.
2.1.3. Flavanones
Their structure is based on the generic structure of flavonoids, a flavan nucleus formed of two aromatic rings linked through a dihydropyrone ring (2,3-dihydro-2-phenylchromen-4-one). They are particularly abundant in fresh fruits and citrus [150]. The most relevant flavanones studied in IBDs include naringin, naringenin, hesperidin, hesperitin, eriodictol, and to a lesser extent, eriocitrin and poncirin.
A) Naringin and naringenin
Naringin (4′,5,7-trihydroxyflavanone-7-rhamnoglucoside) and its aglycone form naringenin are two flavanones found mainly in citrus fruits, including lemon, orange, mandarin, and grapefruit [151]. Preclinical models have shown that naringin can ameliorate the pathogenic symptoms of UC by inhibiting inflammatory response and regulating intestinal microbiota in vivo Among other mechanisms, naringin can improve DAI, colon length shortening, and pathological damage, decrease tissue and serum secretion of inflammatory cytokines, as well as the oxidative stress markers [152]. Similarly, treatment with naringin significantly increased rat body weight and various hematological parameters including hemoglobin, red blood cells, and platelet count, while decreasing spleen weight, colon weight, colon weight to length ratio, macroscopic score, adhesion score, diarrhea score, stool consistency, rectal bleeding score, and white blood cell count [153]. Naringin also significantly increased colonic levels of SOD, GSH, and CAT, while decreasing MDA, xanthine oxidase (XO), colonic NO, and MPO levels [153,154]. Some of the underlying mechanisms associated with the beneficial effects of naringin include the stimulation of PPARγ and the inhibition of the NF-κB and the NLRP3 inflammasome [155,156]. Likewise, naringin also increases the expression of TJ proteins and the relative abundance of Firmicutes/Bacteroides while reducing the content of Proteobacteria to improve the intestinal flora disorder caused by DSS [157]. Because of the pleiotropic effects of naringin, some studies have also evidenced that this compound can prevent important medical complications associated with IBDs. For instance, Liu et al. [158] found that this polyphenol can attenuate intestinal fibrosis, a common complication associated with CD. Similarly, naringin was able to prevent colorectal carcinogenesis by suppressing robust ER stress-induced autophagy in colorectal mucosal cells [159]. Li et al. [160] have also found that naringin may have great potential for the treatment of bone loss in glucocorticoid-treated IBD rats via blocking oxidative stress and promoting bone formation, whereas combined effects of poncirin and naringin from Poncirus trifoliata extracts can alleviate depressive behavior in DSS-induced models of colitis [161].
On the other hand, naringenin is suggested to act as an important immunomodulator against T cell-mediated autoimmune diseases like IBDs [162]. Naringenin was also able to alleviate acetic acid-induced UC in rats in a dose-dependent manner, increasing colonic mucus content and reducing the expression of various inflammatory and oxidative stress markers [163,164].
B) Hesperidin and hesperitin
Hesperidin and its aglycone form, hesperetin can be richly found in citrus fruits such as lemon, sweet oranges, bitter oranges, citron, clementines, and mandarins as well as in Menthae piperitae, Hypericum perforatum, and Salvie officinalis [165]. Treatment with hesperidin significantly reduced neutrophil infiltration, edema, colon shortening, and macro and microscopic damages induced by intracolonic administration of acetic acid in mice [166]. The improvement of colitis after hesperidin treatment is related to the inhibition of pro-inflammatory cytokines TNF-α, IL-6, IL-1β, and IL-33 as well as NF-κB activation in the colon. Likewise, hesperidin can alleviate colonic sphingosine phosphate phosphatase 2 messenger RNA expression and sphingosine kinase-1 levels, thus suppressing the subsequent downstream inflammatory and apoptotic cascades represented by decreased macrophage inflammatory protein-1α and enhancement of B-cell lymphoma 2 immunohistochemistry expression. While improving mitochondrial biogenesis by increasing the peroxisome proliferator-activated receptor-gamma-coactivator 1-α level [167]. Similarly, this marker can act as a potent antioxidant evidenced by marked alleviations of the NO and peroxynitrite levels, increasing total antioxidant capacity, and activating the SOD enzyme [166,167] also improving DAI, MPO activity and MDA content [168].
Regarding hesperitin, past works have evidenced that this compound may ameliorate DSS-induced colitis by maintaining an epithelial barrier via blocking the intestinal epithelial necroptosis [169]. Hesperetin was also shown to alleviate TNBS-induced ulcerative colitis through antioxidant (increasing GSH and SOD while decreasing NO content), anti-inflammatory properties (reducing IL-6, TNF-α, CD45 and NF-kB), antiapoptotic (diminishing caspase 3 and Bax expression) and through modulating JAk2/STAT3/SOCS3 [170,171].
C) Eriodictyol, eriocitrin and poncirin
Eriodictyol is another polyphenol abundantly found in citrus fruits, vegetables, and most of the medicinal plants [172]. Eriocitrin is prominently found in lemons [173] and poncirin in hardy oranges and mandarins [174]. Previous studies have demonstrated that erodicytol is able to decrease MPO expression and regulate the cytokine parameters and oxidative stress in TNBS-induced intestinal tissues of rats. Specifically, the levels of TNF-α, IL-1β, IL-6, IL-10, IL-2, and IL-12 SOD, CAT, GSH-Px, and MDA were modulated in rats with colitis, also inhibiting TLR4/NF-κB pathway activation [175]. Likewise, it seems to upregulate the Sonic hedgehog (Shh) pathway, reducing DAI, colon shortening, histological score, and apoptosis in the colon while augmenting the expression of the tight junction proteins ZO-1 and occluding [176]. One study shows that eriocitrin (30 mg/kg) demonstrated significant attenuation activity against the DSS-stimulated severe colitis in experimental animals, counteracting body-weight loss, colon shortening, histopathological injury, inflammatory cells infiltration, and the secretion of inflammatory cytokines [177]. Together with naringin, the use of poncirin from Poncirus trifoliata extract seems to exert antidepressant effects in mice by restoring vascular endothelial cell integrity in the hippocampus and controlling the neuroinflammatory responses of microglia at the Cornu Ammonis 1 (CA1) and dentate gyrus (DG) regions of the hippocampus [178].
2.1.4. Isoflavones
They are mostly defined as phytoestrogens because they present a chemical structure similar to human estrogen, acting as a physical mimic of natural estrogens by binding to their receptors [179]. They are also considered polyphenols because of their chemical structure, which is formed by two benzene rings (A and B rings) linked with a heterocyclic pyran ring (C ring) [180]. The most important dietary sources of isoflavones are soybeans and soy derivatives, although they can also be found in other legumes such as green beans, and mung beans and in various medicinal plants [181]. Daidzein and Genistein are the most relevant isoflavones explored in the context of IBD, although other isoflavones like glycitein, formononetin, biochanin A, equol, and irilone should also be mentioned herein.
A) Daidzein
Daidzein is a critical isoflavone with pleiotropic effects in intestinal cells. Apart from soy and legumes, currants and raisins are another important source of both daidzein and genistein [182]. In vitro, studies have shown that this compound is able to upregulate metallothionein gene expression and induce CAT activity while decreasing SOD activity in unstimulated Caco-2 cells, but not when the cells were challenged with lipid hydroperoxides [183]. Likewise, other works have also found that daidzein can also improve TJ integrity in Caco-2 cells [184], also being able to attenuate LPS-induced inflammatory responses from intestinal cells, interfering with NF-kB-dependent molecular mechanisms [185].
One of the derivates of Daidzein the 8-Hydroxydaidzein can also play a role as an anti-inflammatory compound in activated macrophages such as RAW 264.7 cells by controlling the proinflammatory cytokines and NF-κB pathway, suggesting that Daidzein could block DSS-induced UC and reducing inflammatory factor expression [186]. Another study found that Daidzein interacted with soybean meal diet-induced intestinal inflammatory responses, in the anti-inflammatory response of the action of Daidzein involved p38, JNK, and NF-κB pathways, leading Daidzein to act as an antioxidant to resist the oxidative damage produced [187]. Daidzein-rich isoflavone aglycones administered to mice for 1 week before inducing UC by DSS leaded to decreased inflammation and tissue damage in the colon than the control mice [188]. More specifically, a decrease in various cytokines such as interferon-gamma, IL-6, and IL-12p40 secretion, and an increase in IL-10 secretion was observed, along with low cell-activation status of antigen-presenting cells (APC) and an inhibition of IL-6 and IL-8 production by TLR2 and TLR4-stimulated monocytes in a dose-dependent manner. Similarly, supplementation with daidzein was shown to reduce the level of myeloperoxidase MPO and inhibit the expression of TNF-α, IL-1β, and IL-6, in the colonic tissues, inhibiting the production of NO and prostaglandin E2 in LPS-stimulated RAW 264.7 macrophages [189].
Observational studies in humans
Observational studies conducted in humans have obtained interesting results regarding the association of daidzein with IBDs. Skolmowska et al. found that high intake of daidzein and tota [190]l isoflavones seemed to reduce the mucus in the feces of UC patients; whereas, a high intake of daidzein alone might drive to an increased fecal pus. In a similar line, Ohfuji et al. [191] found that dietary isoflavone consumption seemed to be associated with an increased risk of UC, particularly in females. It should be highlighted that whereas the study of Skolmowska et al. was conducted in European subjects, the work made by Ohfuji et al. was developed in Japan, existing significant differences in the consumption of isoflavones per day across these regions (25,000-50,000 µg of isoflavones/day in Japan and <1000 µg in Europe) [192]. In agreement with this, Głąbska et al. [193] also found in European patients with UC in remission a direct association between lack of gastrointestinal pain with higher intakes of daidzein, daidzein per 1000 kcal of diet and total isoflavone consumption when compared to those reporting abdominal pain. Therefore, the dose is a relevant factor to consider regarding the role of daidzein and polyphenols in general in human health and IBDs.
B) Genistein
Genistein is shown to be involved in reducing DSS-induced colitis, and also slants M1 macrophages to an M2 phenotype, suggesting that genistein works to be part of the treatment of IBD [194]. Chen et al. [195] also found in DSS-induced colitis mice that genistein was able to inhibit NLRP3 inflammasome via TGR5-cAMP signaling in macrophages. Genistein increased LPS-induced COX2 expression and decreased LPS-induced phosphorylation of IκBα in IEC18 cells [196]. In the colon, expression of COX-2 mRNA and protein was reduced after geinistein treatment together with a decrease in MPO activity [197]. Both genistein and daidzein seemed to inhibit signal translation and activator of transcription 1 (STAT-1) leading to decreased expression of iNOS [198]. Genistein at high concentrations (300µM) can also prevent oxidative stress-induced tyrosine kinase-mediated phosphorylation of the TJ proteins occludin and ZO-1, preventing the breakdown of the intestinal epithelial barrier in Caco-2 cells [199]. In these cells, genistein reduced (4- to 8-fold) IL-1beta-induced IL-8 secretion but promoted NF-kB activity [200]. Genistein can also exert anti-inflammatory effects through the modulation of the gut microbiota. For instance, past works have found that genistein can reduce the growth rate of Lactococcus lactis subsp. lactis, Slackia equolifaciens, and Bacteroides fragilis, while augmenting the growth rate of F. prausnitzii and Lactobacillus rhamnosus, being these changes associated with increased SCFA production [201]. In line with this, Jia et al. claimed that the benefits of genistein in macrophage polarization balance can also be attributed to their associated improvements in intestinal microbiota and its metabolites like SCFAs in DSS-induced UC in mice [202]. UC rats treated with 25-mg/kg genistein showed improved inflammatory cell infiltration, hemorrhage, and destruction of intestinal glands by enhancing the expression of peroxisome proliferator-activated receptor-gamma coactivator (PGC-1), mitochondrial transcription factor A (TFAM), nuclear factor erythroid 2-related factor-2 (Nrf2), heme oxygenase-1 (HO-1), and BCL2 and reduced the expression of BAX, caspase-3, caspase-8, and caspase-9 [203]. Likewise, genisteine can also reduce the activation of the INF-γ/JAK1/STAT1 and INF-γ /TLR-4/ NF-κB signaling pathways and modulate the IRF-1/iNOS/NO and IL-6/JAK2/STAT3/COX-2 pathways and consequently, reduced the levels of TNF-α and IL-1β [204]. Furthermore, it seems that when combined with EVOO, genistein showed more beneficial effects in decreasing inflammation in comparison with pure oils or genistein alone [205]
On the other hand, some negative effects related to genistein have also been observed. For instance, high prenatal and postnatal exposure to genistein and daidzein (genistein: 240 μg/g feed; daidzein: 232 μg/g feed) seemed to enhance acute inflammation markers, influencing the expression of MPO and COX-2 when compared to those having a very low intake of both genistein and daidzein (<10 μg/g feed) [206]. In another study employing human colonic organoids (hCOs), genistein exerts its detrimental effects on the intestinal mucosa via negative regulation of stem/progenitor cell function [207]. More studies are warranted to find proper doses before considering the administration of both genistein and daidzein in IBD patients.
C) Other isoflavones: Glycitein, formononetin, biochanin A, and irilone
Glycitein, formononetin, biochanin A, and irilone are also important isoflavones mainly found in soybeans in the case of the former and red clover in the case of the latter [208]. The relevance of these isoflavones in IBDs has been less studied than the previously mentioned.
Regarding glycitein, molecular docking demonstrated that this compound together with other polyphenols plays a critical role in treating UC with Fuzi-Lizhong Pill (FLP) and Huangqin Decoction (HQT) [209]. Similarly, this compound was also identified as a key modulator of the activity of Codonopsis pilosula, responsible for alleviating UC through the inhibition of the PI3K/Akt signaling pathway [210]. Finally, Głąbska et al. [211] observed that patients with UC in remission reported a lack of constipation and lower intakes of glycitein and glycitein per 1000 kcal of diet.
Formononetin has also been recognized as a critical bioactive compound explaining the success of UC therapy of FLP and HQT [209], Sijunzi Decotion [212], Radix Astragali [213], Sophora flavescens [214,215], Hedysarum multijugum [216], Lizhong Decotion [217] and hydroalcoholic extract of Brazilian red propolis (HERP), also rich in daidzein and biochanin A [218]. Biochanin A inhibited the elevation of ROS, IL-1β, IL-18, and TNF-α release, nitrite production, and the expression of iNOS and COX-2 in RAW 264.7 cells under LPS stimulation [219]. In Muc2-/- mice, biochanin A alleviated UC by restoring the intestinal barrier and promoting autophagy (upregulating TJ proteins, AMPK/mTOR/ULK1 pathway), inhibiting apoptosis and favoring proliferation through reducing caspase 3 expression, and increasing PCNA and Ki67 levels [220]. Biochanin A has also been shown to ameliorate UC in DSS mice thanks to its anti-inflammatory activity by inhibiting the MAPK/NF-κB (p65) axis [221].
On the other hand, equol is a bacterial metabolite of isoflavones with multiple health benefits associated [222]. The role of equol in IBDs however is still controversial. For instance, Sakai et al. [223] described that equol promoted DSS-induced UC in mice by downregulating the production of IL-10 by T cells. Conversely, Li et al. observed that indole 3 acetic acid, an indole derivative, alleviates DSS-induced colitis by promoting the production of Equol from Bifidobacterium pseudolongum [224]. Also, it is known that equol can lead to an increased growth rate of Lactobacillus rhamnosus, a bacteria with anti-inflammatory effects and a protective effect on the intestinal barrier [225].
2.1.5. Flavanols
They form part of the flavonoids group thanks to their C2 and C3 rings, they do not present a double bond between them, and the absence of a carbonyl group on the C4 ring. In nature, flavanols are divided into four groups: flavan-3-ols, flavan-4-ols, isoflavan-3,4-ols and flavan-3,4-ols. The most used of them is flavan-3-ols, followed by flavan-4-ols. They have a wide range starting with simple monomers and going through oligomers and also divided into aglycones or glycosides [226]. Flavanols are found in many foods, including cocoa, tea, cereals, legumes, fruits, vegetables, forages, hops, beers, red wine, grapes, and apples. The main flavanols explored in IBDs include catechins and proanthocyanidins/procyanidins.
A) Catechins
Catechins are natural polyphenols that are present in green tea, cocoa beans, and grapes [227]. Catechins are formed by epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and epigallocatechin-3-gallate (EGCG) [228,229]. Their natural properties control the infiltration and proliferation of immune cells like colonic epithelial cells, macrophages, T lymphocytes, and neutrophils [230]. As the other flavonoids, they exert an anti-inflammatory activity increasing or decreasing the inflammation induced by oxidative stress, through different vias such as MAPK NF-κB, Nrf2, and STAT 1/3 pathways. Also, it is demonstrated that catechins have important modulatory effects on the gut microbiota, which could be of great relevance in the management of IBD. Among other changes, catechins can kill certain pathogenic bacteria, like Clostridium perfringens, Erwinia, Pseudomonas, Clavibacter, Xanthomonas, Agrobacterium spp. Staphylococcus spp., Vibrio parahaemolyticus, Bacillus cereus, and Plesiomonas shigelloides, promoting the growth of beneficial bacteria, like Bifidobacterium spp. in human studies while modulating the proportion of Firmicutes, Bacteroidetes, and other bacterial phyla in vitro [231].
Specifically, EC was shown to ameliorate inflammation in animal models of UC through the inhibition of NF-κB activation along with a decrease in TNF-α, IL-6, NO, MPO, and MDA and an increase in antioxidant enzymes [232]. However, most studies have focused on EGCG as a potential treatment for IBDs. EGCG forms part of the catechins and their biological activity in IBD is related to its anti-inflammatory properties [233]. Yu et al. [234] showed that both high- and low-dose EGCG treatment (50 mg/kg/day and 20 mg/kg/day, respectively) alleviated body weight loss and DAI of DSS-induced colitis, preventing colon shortening and improving intestinal permeability and histopathological changes. Moreover, EGCG treatment attenuated colon inflammation by reducing the levels of pro-inflammatory cytokines IL-6, MCP-1, and TNF-α, and inhibiting CD3+ T cell and CD68+ macrophage infiltration. These results were supported by a preclinical meta-analysis conducted by Wei et al. [235] that included 19 studies involving 309 animals, showing that EGCG was associated with a decrease in IL-1β, IL-6, and interferon-γ; together with alterations in MDA, SOD, GSH, and CAT levels. Besides, they claimed that oral administration exhibited superior efficacy over other forms of administration, whereas the optimal dosage range was 32-62 mg/kg/day, with an intervention duration of 4.8-13.6 days in animals. However, more studies are still warranted in humans. Other works have found synergic effects of EGCG with peracetylated-epigallocatechin-3-gallate (AcEGCG) [236] and quercetin [237].
B) Proanthocyanidins and procyanidins
Proanthocyanidins (also known as condensed tannins) are compounds formed by the polymerization of flavan-3-ol (catechin, epicatechin, epigallocatechin, and epiafzelechin) commonly found in fruits, nuts, bark, chocolate, wine, and some plant seeds and flowers [238]. Depending on the type of monomers, PACs can be classified into procyanidins, prodelphinidins, and propelargonidins. Procyanidins are the most common types of proanthocyanidins and are exclusively formed by catechin and epicatechin molecules [239]. Prodelphinidins and propelargonidins are composed of (-)-gallocatechin/(-)-epigallocatechin and (+)-afzelechin/(-)-epiafzelechin monomers, respectively, although they are notably less distributed in nature [240]. Procyanidins can be categorized into A-type and B-type depending on the stereo configuration and linkage between monomers. Consequently, there are different types of A and B-type procyanidins, being procyanidin A1 and A2, B1, B2, B3, and B4 the most common members [241].
The use of grape seed proanthocyanidin extract (GSPE) has demonstrated multiple benefits in animal models of UC. Wang et al. [242] demonstrated in DSS-induced UC in mice that after administration of GSPE the serum and colonic tissue levels of IL-1β, IL-6, TNF-α, NF-κB, Keap-1 NO, and MDA decreased, whereas SOD, Nrf2 and HO-1 proteins content increased. They reported that catechin, epicatechin, and procyanidins B1, B2, and B4 were mostly responsible for the beneficial effects observed. In a similar line, Sheng et al. [243] also found that GSPE improved DAI, pathological scores, and oxidative stress in these animals and promoted upregulation of ZO-1 occludin, and claudin-1 mRNA levels of colon tissue. Likewise, the expression level of proinflammatory cytokines and the NLRP3 inflammasome mRNA levels of colon tissue were also reduced, whereas 16S rRNA analysis showed that GSPE rebalanced the gut microbiota, by reducing Bacteroidetes, Dubosiella, and Veillonella, increasing Verrucomicrobia and Akkermansia, and elevating the Firmicutes to Bacteroidetes ratio. Chu et al. also reported that GSPE elevated the expression of anti-inflammatory cytokine IL-10 in the colon tissues and serum of DSS-induced colitis mice by suppressing the NF-κB signaling pathway, being able to ameliorate LPS-induced inflammation in RAW264.7 cells [243]. In canins with IBDs, favorable effects from GSPE on the gut microbiota were also accompanied by improvements in bile acid metabolism [244]. Persimmon-derived proanthocyanidins can also ameliorate colon inflammation, DAI, and macrophage activation in DSS-induced UC mice, while influencing gut microbiota composition, leading to an increase of alpha diversity and Bacteroidetes along with a decrease in Enterobacteriaceae and Enterococcus [245]. Importantly, it should be noted that the microbiota and polyphenols have a bidirectional interaction that should be considered. For instance, the metabolism of proanthocyanidin dimers from cranberries can also be affected by an altered microbiota associated with UC, as shown by Diaz et al. [246], thus affecting the response to this compound.
Regarding procyanidins, various studies have shown promising results from their use in in vivo and in vitro models. Previous works have found that procyanidins can prevent the polarization of macrophages to the M1 type and downregulate the levels of proinflammatory factors in cells, whereas in vitro studies found that this effect was attributed to the regulation of the STAT3 and NF-κB pathways [247]. Other in vitro models have also shown that procyanidins can have a significant effect on ROS clearance, also suppressing the expression of MMP9, NF-κB, and the NLRP3 inflammasome in colonic tissue of DSS-induced UC mice [248]. Procyanidins from the peanut skin (PSP) can also attenuate DSS-induced UC inflammation in mice by increasing the relative abundance of Clostridium XlVb and Anaerotruncus, along with SCFA production and reducing the relative abundance of Alistipes at the genus level [249]. PSP is particularly rich in procyanidins A, which are shown to modulate inflammatory (TNF-α, IL-β, IL-6, and IL-10) and oxidative stress markers (MDA, T-SOD, NO, and iNOS) in DSS-induced UC mice and an increase in the relative abundance of Lachnospiraceae_NK4A136_group, Oscillibacter, and Roseburia and a decrease of Bacteroides, Helicobacter, Parabacteroides, Escherichia-Shigella, and Enterobacter after PSP treatment [250]. Procyanidin A can also influence DSS-induced colitis in mice, procyanidins exert their activity in the AMPK/mTOR/p70S6K pathway by decreasing levels in p-p70S6K and p-mTOR in this pathway [251]. Procyanidin B2 seems to suppress oxidative stress by modulating Nrf2/ARE signaling [252], whereas it can also alleviate intestinal inflammation and protect intestinal mucosal functions and structural integrity by inhibiting intestinal PI3K/AKT signaling pathway [253].
2.1.6. Anthocyanins and anthocyanidins
Anthocyanins are found as a red pigment in a variety of berries, currants, and grapes, and consist of an anthocyanidin bound to one to three sugar molecules like arabinose, galactose, glucose, rhamnose, and xylose [254]. Therefore, anthocyanidins are the aglycone form of anthocyanins, and the main types are cyanidin, delphinidin, pelargonidin, peonidin, petunidin, and malvidin [255]. In general, anthocyanins are only partially absorbed and have shown limited biological activity in enterocytes [256]. Numerous studies have focused on the antioxidant properties of anthocyanins, but their anti-inflammatory effects have also been extensively studied in non-intestinal tissues [257]. Research has demonstrated radical scavenging and modulatory activities in the gut microbiota from natural compounds containing anthocyanins [258,259]. Anthocyanin-rich fruits can be divided into three groups based on the types of aglycones of their anthocyanins: pelargonidin group, cyanidin/peonidin group, and multiple anthocyanidins group. This fact can have important implications as compelling evidence suggests a trend for fruits rich in cyanidin, peonidin, or pelargonidin glycosides to exhibit more reproducible-anti-inflammatory effects than blueberries, which contain mostly delphinidin, malvidin, and petunidin glycosides [260]. Delphinidin, malvidin, and petunidin glycosides are unstable and can be further fragmented into smaller molecules, being this fact possibly associated with these observations.
However, a broad spectrum of studies have evaluated the role of anthocyanins in cellular and animal models of IBD. Anthocyanins are generally used from berry extracts, including barberry, bilberry, blueberry, mulberry, raspberry, or strawberry, although purple (sweet) potato or dark-purple rice extracts or red cabbage have also been used.
A) Barberry, bilberry, bulberry, and cranberry anthocyanins
Barberry anthocyanins belong to the Berberidaceae family and consist of full anthocyanins, their main property is their ability to reduce macroscopic ulcer index and ulcer area, colon wet weight/length ratio, and inflammation-inducing cell infiltration [261].
Bilberry and blueberry anthocyanins are members of the Ericaceae family, and as aforementioned, the anthocyanins are mainly malvidin, delphinidin, and petudin, although they also have an important content of cyanidin [262,263]. In Drosophila melanogaster, anthocyanins from bilberries seem to ameliorate DSS-induced UC and improve the antioxidant capacity by modulating NRF2 signaling pathways [264]. Oral administration of bilberries reduced disease severity and inflammation in both acute and chronic DSS-induced colitis mice, decreasing IFN-γ and TNFα secretion. Both bilberries and their anthocyanins prevented apoptosis in colonic epithelial cells caused by inflammation [265]. On the other hand, treatment with blueberry extract promoted a significant decrease in vitro in nuclear and cytoplasmic generated ROS compared to controls, also increasing cell viability following treatment with the pro-inflammatory cytokines [266]. Pereira et al. compared the efficacy of anthocyanin-rich fraction from Portuguese blueberries and 5-aminosalicylic acid in the TNBS-induced UC rat model. They observed that despite both agents exerting anti-inflammatory and antioxidant properties, the greater actions of anthocyanins to downregulate iNOS to decrease leukocyte infiltration and to increase antioxidant defenses in the colon may account for the much higher anti-inflammatory action of anthocyanins [267].
Cranberry is also a member of the Ericaceae family plant. Differentially from bilberries and blueberries, the main anthocyanins in cranberry samples are cyanidin and peonidin glycosides [268]. However, their use in animal models of IBD has also been evaluated in the past. Xiao et al. [269] reported that both cranberry extract and dried cranberries significantly reduced DAI in a murine colitis model, with dried cranberries demonstrating superior efficacy in preventing colitis and mitigating inflammatory markers compared to cranberry extract, suggesting the potential utility of cranberries in IBD prevention and symptom reduction. Most of the benefits associated with the anthocyanins and other polyphenols of cranberries are attributable to their regulatory role on gut microbiota. Dietary cranberry supplementation was shown to mitigate DSS-induced alterations in fecal microbiota, increasing the abundance of beneficial bacteria such as Lactobacillus and Bifidobacterium while decreasing potentially harmful bacteria such as Sutterella and Bilophila [270]. Zhang et al. [271] investigated the effects of cranberry concentrate Type M (CTM) on adherent-invasive Escherichia coli (AIEC) LF82, associated with CD at different infection stages, revealing significant reductions in AIEC LF82 levels in a simulated mucus layer with 0.5 and 1 mM CTM concentrations. Moreover, both fermented and unfermented CTM at 1 mM demonstrated decreased adhesion and invasion of AIEC LF82 in human-derived Caco-2 epithelial cells, indicating potential antipathogenic effects mediated by gut microbiome modulation. It should also be noted that the gut microbiota can also exert a significant influence on the effects of cranberries. Sirven et al. [272], compared the metabolism of cranberry polyphenols between healthy individuals and those with UC, revealing that healthy microbiomes generated higher concentrations of specific metabolites, possibly due to differences in microbiota composition.
Intervention studies in humans
Biedermann et al. [273] tested the effect of a daily standardized anthocyanin-rich bilberry preparation in a pilot study on 13 patients with mild to moderate UC after a follow-up of 9 weeks. They reported that at the end of the 6-week treatment interval, 63.4% of patients achieved remission, while 90.9% of patients showed a response. Equally, they observed a decrease in total Mayo score in all patients, whereas fecal calprotectin levels significantly decreased during the treatment phase. Despite no serious adverse outcomes observed, an increase in calprotectin levels and DAI was noticed after cessation of bilberry intake. Roth et al. [274] also studied the molecular mechanisms and effects of anthocyanins extracted from bilberries in vitro by analyzing colonic tissue and serum samples of 13 mild to moderate UC patients treated with an oral anthocyanin-rich bilberry preparation during an open-label clinical trial. The histopathological analysis determined that reduced amounts of the pro-inflammatory cytokines IFN-γ, TNF-α, and phosphorylated (activated) p65-NF-κB were reduced in these patients. Likewise, responsive patients to the received treatment showed enhanced levels of Th17-cell specific cytokine IL-22 and immunoregulatory cytokine IL-10 as well as reduced serum levels of TNF-α and MCP-1, but enhanced levels of IL-17A, in contrast to patients that did not reach remission after the use of this compound.
B) Mulberry, raspberry, and strawberry anthocyanins
Mulberry (Morus alba L.) is a moraceous plant rich in many anthocyanins, highlighting cyanidin-3-O-glucoside, followed by cyanidin-3-O-rutinoside [275]. Mulberry anthocyanins have also proven significant effects on IBD. Mo et al. [276] demonstrated that these compounds can inhibit DSS-induced clinical symptoms and colonic damage, reduce intestinal inflammation and oxidative stress, restore intestinal barrier integrity, and maintain immune homeostasis. Likewise, these compounds were able to regulate the structure of intestinal microbiota by reducing the level of potentially harmful bacteria (Escherichia-Shigella) and enriching the relative abundance of beneficial bacteria (Allobaculum, Akkermansia, and Muribaculaceae) [276].
Black and red raspberries are two Rubus species with differential content in anthocyanins. Black raspberries seem to have a higher content of anthocyanins, being cyanidin-3-xylosyl-rutinoside as the most common member [277]. Red raspberries contain ∼92.1 ± 19.7 mg anthocyanins/100 g of fresh fruit in a ratio of 32:1 cyanidin- and pelargonidin-based anthocyanins [278]. The relevance of the anthocyanins of both raspberries has been supported in past works. Montrose et al. [279] found that dietary intervention of freeze-dried black raspberries (BRB) improved body mass maintenance and reduced colonic shortening and ulceration in DSS-induced UC mice, but did not affect plasma NO or colon MDA levels. It suppressed several pro-inflammatory cytokines and significantly reduced colonic phosphor-IκBα and cyclooxygenase 2 levels, along with plasma prostaglandin E₂. Similarly, Huang et al. [279] also reported that BRB reduced colonic inflammation in IL-10 knockout mice by correcting dysregulated TLR-4 signaling, which downregulated PGE2. BRBs also decreased spleen macrophage percentages and altered plasma levels of inflammatory mediators, reducing PGE2 and PGI2 while increasing 15-lipoxygenase and its product, 13-S-hydroxyoctadecadienoic acid. Wang et al. also determined whether black raspberries (BRBs) affect promoter methylation of Wnt pathway suppressors in DSS-induced UC. BRB-fed mice showed reduced ulceration, decreased macrophage and neutrophil staining, and decreased NF-κB p65 nuclear localization. By day 7, BRBs demethylated the dkk3 promoter, increasing its mRNA expression, and decreasing levels of β-catenin, c-Myc, DNMT3B, HDAC1, HDAC2, and MBD2 in the colon and bone marrow [280]. Moreover, another study [281] also found that BRB inhibited colonic ulceration and, ultimately, colon cancer partly through inhibiting aberrant epigenetic events that dysregulate Wnt signaling. Regarding red raspberry (RB), one work [282] found that RB supplementation reduced body weight loss, DAI, and colon shortening in DSS-treated mice, and protected colonic structure by suppressing NF-κB signaling and reducing inflammatory markers. It also decreased neutrophil infiltration, MCP-1 mRNA expression, and xanthine oxidase content, while enhancing catalase, claudin-3, ZO-1 protein, MUC-2 mRNA, and AMPK activation. In more detail, Bibi et al. [283] observed in the same animal models that RB supplementation reduced the DAI score and histologic damage by 38.9%, decreased inflammatory mediator expression by 20-70%, CD4 T cell infiltration by 50%, and α4β7 integrin and related adhesion molecules by 33.3%. RB also promoted epithelial repair, goblet cell density, and expression of Klf4, Hes1, Muc2, and intestinal alkaline phosphatase by 20-200%, while reducing proliferating cell nuclear antigen by 70% and β-catenin and STAT3 signaling by 19-33%, enhancing p53 stability and reducing oncogenic gene expression by 50-60%. Overall, these studies suggest that BRBs and RBs are promising agents to consider for the clinical management of IBDs, although clinical studies in humans are still warranted.
Strawberries belong Rosaceae family and their anthocyanins are mainly derived from pelargonidin and cyanidin aglycones [284]. Some animal studies have found that the supplementation of anthocyanins from strawberries, through oral and rectal administration, reduced significantly the inflammation focus and mitigated epithelial necrosis and lesions [47,285,286]. Ghattamaneni et al. [287] evaluated the possible role of pelargonidin 3-glucoside (P3G)-enriched strawberry added to the diet for the final 6 weeks in IBD rats to provide a dose of 8 mg P3G/kg/day. They observed that P3G consumption reversed DSS-induced UC with healthy stools and mucosal lining of the ileum and colon including increased villi, crypts, and goblet cells and reduced inflammation. Thus, despite clinical evidence being needed, anthocyanins from strawberries also seem an interesting option to treat IBDs.
C) Other sources of anthocyanins: Purple (sweet) potato, black/purple rice extracts, and red cabbage
Anthocyanins can also be found in other foods rather than berries, including purple (sweet) potato and dark/purple rice. Pigmented potato (Solanum tuberosum L.) and purple sweet potato (Ipomoea batatas L.) are two major sources of anthocyanins. The former has abundant acylated derivatives of anthocyanins [288], whereas the latter is one of the major sources of anthocyanins with a content ranging from 3.31 to 13.90 mg/g of fresh weight depending on the variety, climate, soil, and harvest conditions [289]. Animal models have shown that purple-fleshed potato supplementation prevented DSS-induced weight loss, colon shortening, and increases in spleen and liver weights in mice [290]. It reduced intestinal permeability, colonic MPO activity, pro-inflammatory interleukins (IL-6 and IL-17), pathogenic bacteria, and flagellin levels, with P25 also decreasing systemic MPO and increasing Akkermansia muciniphila. In a similar line, Li et al. also found that supplementation with purple- or red-fleshed potatoes at 20% w/w mitigated the DSS-induced reduction in colon length and mucin 2 expression levels, and the increase in permeability, spleen weight, myeloperoxidase (MPO) activity, and expression levels of inflammatory cytokines (IL-6, IL-17, and IL1-β) in non-antibiotic mice, but not in gut microbiota ablated mice [291] In a recent study, Sun et al. [292] divided six-week-old C57BL/6J male mice into two groups, one receiving a standard diet and the other with 10% purple potato powder, for 7 weeks. At week 5, each group was subdivided into two, with or without 2.5% DSS induction for 7 days, followed by 7 days of recovery. Purple potato supplementation improved DAI, reversed colonic damage induced by DSS, restored tight junction proteins and homeobox 2 levels, and enhanced mitochondrial function, suggesting its potential for IBD intervention. Chen et al. [293] evaluated the role of pelargonidin-3-galactoside (Pg3gal) from purple sweet potatoes on DSS-induced colonic inflammation in a murine model of UC. They observed that Pg3gal significantly attenuated DSS-induced UC, improved colon size, tissue condition, and inhibited proinflammatory cytokine production. It also modulated gut microbiota, reducing Proteobacteria and Deferribacteres while increasing Firmicutes, Bacteroidetes, and Verrucomicrobia.
On the other hand, purple rice is also a major source of anthocyanins, in particular C3G (Cyanidin-3-O-glucoside) and P3G (Peonidin-3-O-glucoside) [294]. Black rice is also a major source of anthocyanins, although contains a lesser amount of polyphenols than purple rice [295,296]. Thipart et al. [297] evaluated the effects of the anthocyanins from purple rice in acetic acid-induced UC and indomethacin-induced CD rats, showing that the microbiota was modulated in both cases. The relative abundances of beneficial bacteria, especially the Lachnospiraceae NK4A136 group and Lactobacillus, were decreased in the AA-induced UC model, whereas some opportunistic pathogens (Bacteroides, Escherichia/Shigella, Fusobacterium, and Veillonella) seemed to be raised by indomethacin-induced CD, suggesting that beneficial effects from this extract could be reported in UC models. Regarding black rice anthocyanins, one study [298] demonstrates that dietary black rice anthocyanin-rich extracts and rosmarinic acid could alleviate the symptoms and inflammation of DSS-induced colitis in mice by modulating MPO, NO, IL-6, IL-1β, TNF-α, iNOS and COX-2 levels, exerting more notable effects when used in combination.
Lastly, Red cabbage also represent a major source of anthocyanins, which are mainly derivatives of cyanidin-3-diglucoside-5-glucoside [299]. In a study using C57BL/6J mice, the use of red cabbage juice (RCJ) significantly improved body weight, survival, and reduced DAI scores in DSS-induced colitis. RCJ enhanced colonic barrier integrity, increased SCFA-producing bacteria, activated PPAR-γ, and suppressed NFκB signaling, leading to reduced inflammatory cytokine production, indicating potential for IBD prevention and treatment [300]. Overall, compelling evidence support the use of anthocyanins in the management of IBDs, although most studies have been performed in vivo and in vitro.
2.1.7. Chalcones
Chalcones are phenolic compounds categorized as 'open-chain flavonoids' synthesized through the shikimate pathway, serving as precursors to flavonoids. Structurally, chalcones consist of two aromatic rings connected by an α,β-unsaturated ketone unit, with some variants known as dihydrochalcones featuring a saturated ketone instead [301]. Natural chalcones commonly include phenolic hydroxyl groups and are frequently substituted with prenyl or geranyl moieties on the aromatic rings, with numerous examples documented in the literature. In the field of IBDs, some compounds like xanthohumol, isoliquiritigenin, cardamon in, or phloretin must be highlighted, with others like licochacone A, butein, flavokawain B or artepillin C showing some promising implications.
A) Xanthohumol
Xanthohumol (XN) is a prominent prenylated flavonoid present in the hop plant (Humulus lupulus L.) with many favorable effects in health and translational applications, acting as an anti-inflammatory, antimicrobial, antioxidant, immunomodulatory, antigenotoxic and antiangiogenic agent [302]. XN can exert its benefits through different mechanisms, including the inhibition of the TLR4/MD-2 complex or the suppression of macrophage iNOS expression, NO, and IFN-γ production [303]. This compound has also been shown to inhibit the TNF-α-activated NF-κB pathway in vitro and in vivo [304]. Cho et al. [305] evaluated the use of xanthohumol in DSS-induced colitis mice. They observed that XN alleviated colitis symptoms, prevented colonic lesions, and inhibited pro-inflammatory cytokines, oxidative stress, and COX-2 expression. Besides, they show that this compound also suppressed the IKKβ/NF-κB signaling pathway, highlighting XN's potential as a therapeutic agent for colitis. In parallel, Yung et al. [306] examined the anti-fibrotic mechanism of XN on TGF-β1-induced intestinal fibrosis in human intestinal fibroblasts (HIFs). XN reduced fibrosis-related gene expression, restored altered cell shape, and inhibited both NF-κB and Smad signaling pathways. It interfered with TGF-Receptor I and Smad3 binding, highlighting the potential of XN in mitigating TGF-β1-induced fibrosis through TGF-β/Smad signaling inhibition. Restivo et al. [307] assessed the anti-inflammatory effects of a polyethoxylated flavone fraction (PMFF) from Citrus sinensis (particularly rich in nobiletin) and a prenylflavonoid fraction (PFF) from Humulus lupulus (with high levels of XN) both individually and combined (MIX), using an IL-1β-stimulated Caco-2 cell model of IBD. PMFF, PFF, and MIX reduced NO production, with MIX also inhibiting prostaglandin E2 release, NF-κB activation, and enhancing Nrf2 activation and antioxidant responses. Notably, the effects of MIX surpassed those of the individual fractions, highlighting the synergistic anti-inflammatory and anti-oxidative potential of nobiletin and XN. Magadán-Corpas et al. [308] assessed the effects of intraperitoneal injection of the flavonoids apigenin, luteolin, and XN in reducing inflammation and modulating gut microbiota in a murine model of ulcerative colitis. They observed that both luteolin and XN notably increased anti-inflammatory microorganisms and decreased pro-inflammatory species. All flavonoids reduced pro-inflammatory cytokines, with luteolin significantly alleviating physical UC symptoms. Apigenin showed limited microbiota impact due to solubility issues and accumulation in the mesentery.
Intervention studies in humans
To date, no studies have specifically evaluated the role of XN in humans with IBD. However, after a successful phase I trial confirming the safety and tolerability of 24 mg XN daily for 8 weeks his phase II clinical trial protocol aims to evaluate the safety and tolerability of 24 mg of xanthohumol (XN) daily, Langley et al. [309] are testing the safety and tolerability of the same dose in adults with active CD. This triple-masked, randomized, placebo-controlled study will involve up to 32 participants over 8 weeks. Outcomes will include adverse events, inflammatory biomarkers, platelet function, CD clinical activity, stool microbial composition, and XN metabolism. The study will compare these results with those from healthy adults in the phase I trial, showing the therapeutic potential of XN in CD and informing its broader clinical applications. Likewise, in a pilot study, Ryan et al. [310] aimed to identify the effects of a nutrition support formula on blood nutrient parameters in adults with IBD. The formula contained a mixture of micronutrients (including methylated forms of folate and vitamin B12), macronutrients, and phytonutrients (including curcumin, XN, ginger compounds, and quercetin). 10 participants with Crohn's disease or ulcerative colitis consumed a micronutrient and phytonutrient-rich beverage twice daily for 12 weeks. Significant increases in serum folate and decreases in red cell distribution width were observed. Modulation of leukocyte subtypes was noted, with a decrease in neutrophils and an increase in lymphocytes. Other parameters, including RBC count, hemoglobin, hematocrit, electrolytes, albumin, and inflammatory markers, did not change significantly. Therefore, XN can represent an interesting and potential flavonoid with some preliminary evidence in humans. However, more studies are warranted before drawing any relevant conclusion.
B) Isoliquiritigenin
Isoliquiritigenin (ISL) is a major bioactive chalcone compound isolated from the roots of plants belonging to licorice, including Glycyrrhiza uralensis, Mongolian glycyrrhiza, and Glycyrrhiza glabra [311]. Among the many effects of ISL, the literature has described its ability to inhibit upstream of the NF-κB, NLRP3, and MAPK pathway, as well as to activate the Nrf2 pathway, thus exerting important anti-inflammatory and antioxidant effects [312]. In DSS-induced UC mice, ISL ameliorated the reduction of body weight while improving colon length and structural integrity, diarrhea, bloody stool, DAI scores, and MPO activity [313]. These effects were related to the suppression of the phosphorylation of ERK1/2 and p38 and the inactivation of NK-κB. Pretreatment with ISL significantly reduced indomethacin-induced intestinal damage and inhibited increases in cleaved caspase-1 and mature IL-1β protein levels [314]. The protective effects of ISL were linked to its inhibition of NLRP3 inflammasome activation, as demonstrated by the lack of protective effect in NLRP3-/- and caspase-1-/- mice. ISL was shown to reduce the expression of the inflammatory markers IL-8, IL-1β, and COX-2, and inhibits NF-κB activation in TNF-α-stimulated HT-29 cells. Besides, it activates Nrf2 and its target genes and prevents the secretion of HMGB1 by reducing its acetylation through histone deacetylase (HDAC) activation [315]. Collectively, these preclinical studies show that ISL is a potential agent for being explored in IBD. Also, meta-analysis and systematic reviews support the potential of licorice extract and its active compounds in UC preclinical studies, with potent anti-inflammatory, antioxidative, immunomodulatory, and microbiota-regulating effects as primary mechanisms of licorice extract and its compounds in treating UC [316]. However, they acknowledges limitations including study quality variations, publication bias, and unexplored negative outcomes, warranting cautious interpretation and further clinical investigation.
C) Cardamonin
Cardamonin is a natural chalcone first discovered found in cardamom spice but it can also be found in various plants, exerting anti-inflammatory, antioxidant, antineoplastic, metabolic, and antimicrobial effects [317]. Cardamonin demonstrated anti-inflammatory properties by reducing nitrous oxide production and downregulating iNOS, TNF-α, and IL-6 expression in RAW 264.7 cells without affecting cell viability while inhibiting NF-kB signaling. In a DSS-induced colitis mouse model, cardamonin protected against colitis symptoms, also showing therapeutic potential in colitis-associated cancer models [318]. Likewise, cardamomin effectively inhibited necroptosis in vitro by blocking RIPK1/3 phosphorylation and disrupting necrosome formation in HT29, L929, and RAW264.7 cell lines, whereas in vivo, oral administration of cardamonin attenuates DSS-induced colitis in mice by mitigating intestinal barrier damage, suppressing necroinflammation, and reducing MLKL phosphorylation [319]. Cardamonin effectively mitigated DSS-induced symptoms in mice, including body weight loss, diarrhea, colon shortening, and histological damage [320]. This was associated with reduced inflammatory markers (MPO, NO, TNF-α, IL-6) in the colon, and inhibition of TLR-4 expression. Mechanistically, cardamonin blocked NF-κB and MAPK signaling pathways, including inhibition of NF-κB p65 nuclear translocation and downstream target gene expression, demonstrating its potential as a therapeutic agent for IBD. Oral and rectal administration of cardamonin significantly improved symptoms and histopathological changes in DSS- and TNBS-induced colitis in mice, as evidenced by reduced DAI scores, MPO activity, and colon length shortening [321]. Cardamonin downregulated inflammatory markers (IL-1β, TNF-α, IL-6, NLRP3, cleaved caspase-1, ASC, cleaved IL-1β) in colonic tissues and inhibited NLRP3 inflammasome activation in THP-1 and bone marrow-derived macrophages. Mechanistically, cardamonin activated AhR, leading to enhanced AhR/ARNT complex formation, nuclear translocation, and XRE reporter gene activity. It also upregulated Nrf2 and its target genes, particularly NQO1, and these effects were attenuated by AhR antagonist CH223191 [321]. Rats treated orally with 10 or 30 mg/kg/day of cardamonin for 14 days before induction of UC with 3% AA showed decreased disease activity and macroscopic damage indices, along with significant histopathological improvement [322]. Cardamonin also reduced levels of MPO, iNOS, NF-κB, TNFα, and MDA. Immunohistochemical analysis indicated decreased expression of COX-2 and caspase-3 in cardamonin-treated groups, suggesting its protective effects against AA-induced colitis by mitigating inflammation, oxidative stress, and apoptosis. Overall, these preclinical studies support the potential use of cardamonin in IBDs, although broader efforts in humans are still warranted.
D) Phloretin
Phloretin is a chalcone present abundantly in apples, pears, and strawberries acting as a potent antioxidant and modulating several signaling pathways and molecular mechanisms [323]. Kapoor and Padwad [324] studied the many effects of this compound in vitro models of gut inflammation, developed by co-culture of Caco2 (intestinal epithelial) cells and RAW264.7 macrophages. They reported that phloretin reduced LPS-induced inflammation by lowering NO levels, oxidative stress, and mitochondrial membrane depolarization in Caco-2 cells, as indicated by decreased ROS and enhanced MMP. It also attenuated inflammatory cytokines (IL-8, TNF-α, IL-1β, IL-6) and inhibited NF-κB, iNOS, and Cox-2 expression. Phloretin maintained epithelial integrity by regulating tight junction proteins (ZO-1, occludin, Claudin-1, JAM) and reducing LPS-induced Cox-2 levels through modulation of Src expression. Additionally, phloretin combined with sodium pyruvate showed enhanced anti-inflammatory activity by targeting NF-κB signaling pathways. In a rat model of AA-induced colitis, phloretin, administered orally either before or after induction of colitis (50 mg/kg), effectively reduced plasma ALP and LDH levels, inflammatory markers (MPO, NO, eosinophil peroxidase), and colon ICAM-1 gene expression [325]. It also restored tissue GSH levels, and prevented mucosal damage as confirmed by histopathological analysis, suggesting its potential as a natural therapeutic agent for UC management and future clinical applications. Phloretin (60 mg/kg) administered daily attenuated UC symptoms in mice induced by DSS, by reducing inflammation markers, preserving intestinal barrier integrity, and modulating systemic immune responses [326]. This effect was attributed to the ability of phroletin to increase beneficial bacteria like Bacteroidetes, Alistipes, and Lactobacillus, while decreasing harmful Firmicutes, Oscillibacter, and Ruminiclostridium_6. Another study also found that this compound restored the disturbed faecal microbiota in DSS-induced mice and improved metabolic pathways by balancing fecal metabolites like norepinephrine, mesalazine, tyrosine, 5-acetyl-2,4-dimethyloxazole, and 6-acetyl-2,3-dihydro-2-(hydroxymethyl)-4(1H)-pyridinone [326]. Correlation analysis revealed that various microorganisms were positively or inversely associated with these metabolites, evidencing the relevance of phloretin in the gut microbiota. Finally, phloretin administration reduced DSS-induced colitis by modulating NLRP3, TLR4, and PPARγ pathways, enhancing the expression of ZO-1 and occluding, and reducing serum LPS levels while restoring the balance of Escherichia coli and Lactobacillus in the gut [327].
E) Other chalcones
Other chalcones have also shown promising but less established therapeutic effects on IBD, as is the case of licochacone A, butein, flavokawain B, or artepillin C. Licochalcone A (LA), the predominant chalcone in Glycyrrhiza inflata, demonstrated significant protective effects against DSS-induced colitis in mice, reducing weight loss, DAI, histological damage, and gut inflammation [328]. LA preserved intestinal barrier integrity by inhibiting apoptosis and maintaining tight junction protein expression. Dose-dependent effects were observed, with lower doses of LA primarily enhancing gut barrier integrity and higher doses focusing on anti-inflammatory actions. Additionally, LA modulated gut microbiota composition, particularly at lower doses, and exerted anti-UC effects partly through MAPK pathway inhibition, highlighting its potential therapeutic role in UC management [328]. Likewise, LA seems to ameliorate DSS-induced UC by inhibiting NF-κB-regulated pro-inflammatory signaling and activating Nrf2-regulated cytoprotective protein expression [329]. Butein, a major constituent of Toxicodendron vernicifluum ameliorated colitis in IL-10(-/-) mice by reducing the colonic inflammatory score by > 50%, reducing the expression levels of IL-6, IL-1β, IFN-γ pSTAT3 and MMP-9, also inhibiting IL-6-induced activation of STAT3 in Colo 205 cells [330]. Flavokawain B (FKB), found in plants from the Zingiberaceae and Kava family, has equally demonstrated significant therapeutic effects in a DSS-induced IBD mouse model by reducing weight loss, restoring colon length, and mitigating inflammation [331]. In this study, FKB targeted TLR2 to inhibit the formation of the TLR2-MyD88 complex, thereby suppressing the NF-κB signaling pathway both in vivo and in vitro. These findings suggest that FKB's anti-inflammatory properties involve direct modulation of TLR2, highlighting its potential as a therapeutic agent for IBD treatment. Finally, artipilin C (ARC) contained in Brazilian Green Propolis ameliorates UC and colitis-associated colorectal cancer by targeting p21-activated kinase 1 (PAK1) [332]. The inhibition of PAK1 activation by ARC reduces NF-κB-mediated inflammation and enhances PPAR-γ activity, potentially maintaining intestinal integrity under inflammatory and neoplastic conditions.
Despite these studies, future research should focus on validating the effects through in vitro and in vivo studies in murine and human models of UC, emphasizing the transition towards clinical trials. Table 1 summarizes the effects of the main flavonoids explored in this section, highlighting the main dietary sources, mechanisms of action described in preclinical studies and clinical trials/studies in humans with doses used, if pertinent.
Flavonoids explored in the context of inflammatory bowel diseases (IBDs)
| Subgroups | Compounds | Dietary/Exogenous sources | Mechanisms of action (Preclinical models) | Studies in humans (including observational and intervention studies | References |
|---|---|---|---|---|---|
Flavones | Apigenin | Parsley, chamomile, celery, vine spinach, artichokes and oregano | Shows anti-inflammatory and antioxidant properties by inhibiting TNF-α transactivation and blocking inflammasome pathways. It alleviates intestinal injury in animal models, promotes anti-inflammatory cytokine expression, and maintains the intestinal epithelial barrier. Also, it inhibits inflammation related to carcinogenesis by repressing STA3-NF-κB signaling and regulating gut microbiota and SCFA production | - | [63,65,66,333] |
| Baicalein | Oroxylum indicum | Reduces IBD impact by inhibiting COX-2 activity, modulating AhR/IL-22 pathways, and inactivating the NLRP3 inflammasome, among other mechanisms | - | [70-75] | |
| Baicalin | Scutellaria baicalensis | Act as an anti-inflammatory, anticarcinogenic, and immunomodulatory agent, improving intestinal health by regulating pathways such as TLR4/NF-κB, AhR/IL-22 and autophagic flux. And also, it is efficacy in human UC cells, reducing pro-inflammatory markers | - | [76,78-80,82,84,88,90,92] | |
| Luteolin | Carrots, parsley, broccoli, peppers, celery, olive oil, onion leaves, cabbages, apple skins, chrysanthemum flowers, peppermint, thyme, rosemary, and oregano | Inhibits pro-inflammatory mediators such as COX-2, TNF-α, and IL_6, and regulates pathways like NF-κB, JAK/STAT, and MAPKs, reducing inflammation in ulcerative colitis | - | [93,95-97,100,102,103] | |
| Wogonoside | Schulletaria | Alleviate colitis by protecting against intestinal barrier dysfunction through the reinforcement of tight junctions via the MLCK/pMLC2 signaling pathway in Caco2 cells. It also leads to the dual inhibition of NF-κB and the NLPR3 inflammasome in DSS-induced UC mice | - | [105,106] | |
| Wogonin | Schulletaria | Prevents colonic ulceration, neutrophil infiltration, oxidative stress, proinflammatory cytokines, and histological changes in DSS-induced colitis models. It promotes apoptosis by inhibiting Bcl-2 and iNOS to suppress NF-κB. Also, it regulates the Nrf2 and TLR-4/ NF-κB pathways and modulates ILC3/ILC1 plasticity | - | [107,108] | |
| Tangeretin | Citrus spp pericarp | Improves colonic tissue damage reduction and enhances gut microbiota activity in DSS-induced colitis mice. Oral administration inhibits UL-12 ND TNF-α expression through interaction with the NF-κB pathway | - | [109,110] | |
| Nobiletin | Citrus peels | Exhibits anti-inflammatory effects in TNBS-induced colitis by downregulating iNOS and COX-2 expression. It restores barrier functions by inhibiting the Akt/ NF-κB MLCK pathway | - | [111] | |
| Chrysin | Honey, propolis, and various plants, fruits, and fungi | Downregulates the PXR/ NF-κB pathway in induced colitis in vivo | - | [112-114] | |
Flavonols | Quercetin | Citrus, and green leafy vegetables like broccoli, flowers, and nuts | Enhances intestinal integrity, modulates microbiota, and reduces inflammation in IBD. It acts through pathways including inhibition of NF-κB, modulation of PI3K/AKT signaling, activation of AhR for TJ enhancement and suppression of NLPR3 inflammasome | Two observational studies linked higher quercetin intake to lower IBD risk and improved outcomes in UK biobank participants. An intervention study found higher patient satisfaction with quercetin containing flavonoid mixtures for hemorrhoidal disease in IBD patients. A pilot study showed potential benefits of quercetin-rich beverages on blood nutrient parameters in IBD patients, indicating the need for further research | [119,120,122,124,126,128-130] |
| Kaempferol | Edible plants such as tea, broccoli, cabbage, kale, beans, endive, leek, tomato, strawberries and grapes, and herbal medical plants | It is protective against DSS-induced UC by enhancing colonic mucosal integrity and regulating gut microbiota. It reduces inflammatory markers like IL-1β, IL-6, and TNF-α, while increasing IL-10 expression through pathways involving TLR4, NF-κB, and STAT | - | [131,132,134,135] | |
| Galangin | Ginger, gangal, honey, and propolis | Inhibits HSP90β, elevated in UC mucosal biopsies and colitis, correlating with disease severity. It alleviates colitis by blocking HSP90β and suppressing NLRP3 inflammasome activation, reducing inflammatory markers in vitro and in vivo. Also downregulates TLR4, inhibits NF-κB p65, and promotes autophagy proteins | - | [136-139] | |
| Myricetin | Cranberry, dock, sweet potato leaves, chard, broad beans, and immature seeds | Reduces inflammation in acute UC by increasing IL-10 and TGFβ levels, enhancing Treg cell proportions | - | [140-144,334] | |
| Fisetin | Strawberries, apples, mangoes, persimmons, kiwis, grapes, tomatoes, onions, cucumbers, nuts and wine | Inhibits senescence markers and upregulates miRNAs and Akkermansia muciniphila in DSS-induced UC mice, correlating with reduced senescence and inflammation. It also exerts anti-inflammatory effects by inhibiting akt, p38, MAPK, and NF-κB signaling in colonic tissues, fomenting GSH levels, and reducing MDA | - | [145-147] | |
| Isorhamnetin | Opuntia ficus-indica, Hippophae rhamnoides, and Ginkgo biloba | It can alleviate IBD through PXR-mediated up-regulation of xenobiotic metabolism and down-regulation of NF-κB signaling. Also, it can inhibits ferroptosis | - | [148,149] | |
Flavanones | Naringin | Lemon, orange, mandarin, and grapefruit | Reduces inflammation, oxidative stress, and improves intestinal microbiota in UC models. It enhances antioxidant enzymes, decreases inflammatory cytokines, and modulates PPARγ, NF-κB, and NLPR3 inflammasome pathways. Also exhibits potential in preventing intestinal fibrosis, colorectal carcinogenesis, bone loss in IBD, and alleviating depressive behaviour in colitis models | - | [152-156,158,160] |
| Naringingenin | Lemon, orange, mandarin, and grapefruit | Act as an important immunomodulator against T cell-dilated autoimmune diseases like IBDs | - | [162,163] | |
| Hesperidin | Lemon, sweet oranges, bitter oranges, citron, clementines, and mandarins, as well as in Menthae piperitae, Hypericum perforatum, and Salvie officinalis | Reduces neutrophil infiltration, colon damage, and inflammation by inhibiting pro-inflammatory cytokines (TNF-α, IL-6, IL-1β and IL-33, and NF-κB activation | - | [128,165-168] | |
| Hesperetin | Lemon, sweet oranges, bitter oranges, citron, clementines, and mandarins, as well as in Menthae piperitae, Hypericum perforatum, and Salvie officinalis | Amilorates DSS-induced colitis by maintaining an epithelial barrier through blocking the intestinal epithelial necroptosis. Also, alleviates TNBS-induced ulcerative colitis through antioxidant, anti-inflammatory properties, and by modulating JAK2/STAT3/SOCS3 | - | [169,170] | |
| Eriodictyol | Citrus fruits, vegetables, and most medical plants | Decreases MPO expression and modulates cytokines and oxidative stress in TNBS-induced colitis in rats, inhibiting the TLR4/NF-κB pathway. It also upregulates the Hh pathway, reducing DAI, colon shortening, histological scores, and apoptosis while increasing tight junction proteins ZO-1 and occluding | - | ||
| Eriocitrin | Lemons | Significantly alleviated DSS-stimulated severe colitis in experimental animals, reducing body-weight loss, colon shortening, histopathological injury, inflammatory cell infiltration, and inflammatory cyrokine secretion | - | [173,177] | |
| Poncirin | Hardy oranges and mandarins. Poncirus trifoliata | Seems to extert antidepressant effects in mice by restoring vascular endothelial cell integrity in the hippocampus and controlling the neuroinflammatory responses of CA1 and DG regions of the hippocampus | - | [178] | |
| Isoflavones | Daidzein | Soy, legumes, currants and raisins | In vitro studies show daidzein upregulates metallothionein gene expression, induces CAT activity, and improves tight junction integrity in Caco-2 cells. Also, attenuates LPS-induced inflammatory responses via NF-κB dependent mechanism and reduces DSS-induced UC inflammation by modulating p38, JNK, and NF-κB pathways | Observational studies suggest daidzein may reduce mucus in UC patients' feces but increase fecal pus with high intake, while isoflavone consumption may raise UC risk, especially in females. European studies linked higher daidzein intake to reduced gastrointestinal plain in UC patients. These findings highlight the importance of dosage in the impact of daidzein and polyphenols on IBD | [182-186,189-191,193] |
| Genistein | Soy, legumes, currants and raisins | It has reduced DSS-induced colitis by shifting macrophages to an M2 phenotype, inhibiting the NLPR3 inflammasome, and modulating gut microbiota and inflammatory pathways. It enhances the expression of protective proteins and reduces pro-inflammatory markers. | - | [194-202] | |
| Glycitein | Soybeans | Is identified as a critical modulator in UC treatments like Fuzi-Lizhong Pill and Huangquin Decotion, alleviates UC by inhibiting the PI3K7Akt signaling pathway. Also, UC patients in remission reported less constipation and lower glycitein intake | - | [193,209,210] | |
| Formononetin | Soybeans | Bioactive compound that explains the success of UC therapy of FLP and HQT, and others. | - | [212-215,217,218] | |
| Biochanin A | Soybeans | Inhibits the elevation of ROS, IL-1β, IL-18, and TNF-α release, nitrite production, and the expression of iNOS and COX-2 in RAW 264.7 cells under LPS stimulation. Also promotes the restore of the intestinal barrier and promotes autophagy. | - | [219,220] | |
| Equol | Soybeans | A bacterial metabolite of isoflavones, has controversial roles I IBD. One study shows that equol promotes DSS-induced UC by downregulating IL-10 production, while in other study observed that equol production alleviated colitis by promoting beneficial gut microbiota | - | [222,223] | |
Flavanols | Catechins (Focus on EC and EGCG) | Epicatechin (EC), epicatheni gallate (ECG), epigallocatechin (EGC), and epigallocatechin-3-gallate (EGCG) | Catechins exter anti-inflammatory effects in IBD by modulating immune cell infiltration, oxidative stress pathways (MAPK, NF-κB , Nrf2, STAT 1/3)m and gut microbiota. EC specially reduces inflammation markers and increases antioxidant enzymes in UC models, while EGCG improves colitis symptoms by lowering pro-inflammatory cytokines and enhancing intestinal health | - | [224-237] |
| Proanthocyanidins (condensed tanins) | Fruits, nuts, bark, chocolate, wine, and some plant seeds and flowers | GSPE benefits animal models of UC by reducing inflammation markers and oxidative stress while increasing antioxidant proteins like SOD and Nrf2. Also improves gut health by rebalancing microbiota, promoting beneficial bacteria, and enhancing tight junction protein expression | - | [242,244-246] | |
| Procyanidins | Catechin and epicatechin molecules | Show promise in treating UC by preventing M1 macrophage polarization and downregulating pro-inflammatory factors via STAT3 and NF-κB pathways. They also enhance ROS clearance, suppress MMP9 and NLPR3 inflammasome expression, and modulate gut microbiota and SCFA production | - | [247-257] | |
| Anthocyanins | Anthocyanidins (Cyanidin, delphidin, malvidin, peonidin, pelargodin, petudin) + 1-3 sugar molecules (arabinose, galactose, glucose, rhamnose, and xylose) | Barberry, bilberry, blueberry, cranberry, currants, grapes, mulberry black/red raspberry, grapes, strawberry, purple (sweet) potatoes, dark/purple rice and red cabbage. | Barberry anthocyanins reduce inflammation and ulcer induces UC models. Bilberry, blueberry, and cranberry anthocyanins mitigate colitis symptoms, enhance antioxidant capacity, and regulate gut microbiota, with cranberries showing superior efficacy in reducing colitis severity. Mulberry, raspberry, strawberry, and anthocyanins from purple potato, rice, and red cabbage also demonstrate significant anti-inflammatory and gut microbiota modulation effects, suggesting their potential in managing IBDs | In a pilot study, 63.4% of UC patients achieved remission and 90.9% showed a response to a daily anthocyanin-rich bilberry preparation, with decreased total Mayo scores and fecal calprotectin levels during the treatment phase. Molecular analysis revealed reduced pro-inflammatory cytokines and increased immunoregulatory cytokines in responsive patients, with no serious adverse effects reported. However, calprotectin levels and DAI increased after stopping bilberry intake | [261-300] |
Chalcones | Xanthohumol | Humulus lupulus L. | Demonstrates anti-inflammatory and anti-fibrotic effects in colitis by inhibiting pathways such as TLR4/MD-2, NF-κB, and TGF-β/Smad, ad reducing oxidative stress and pro-inflammatory cytokines. Studies show XN alleviates colitis symptoms, prevents colonic lesions, and modulates gut microbiota, increasing anti-inflammatory microorganism | No studies have specially evaluated xanthohumol (XN) in humans with IBD, but a phase II clinical trial is testing its safety and tolerability in adults with active Chron´s disease. A pilot study with a micronutrient and phytonutrient-rich formula, including XN, showed significant increases in serum folate and changes in leukocyte subtypes in IBD patients. Although preliminary evidence is promising, further studies are needed to confirm XN´s potential in IBD treatment | [101,301-307,309,310] |
| Isoliquiritigenin | Glycyrrhiza uralensis, Mongolian glycyrrhiza, and Glycyrrhiza glabra | Exhibits anti-inflammatory and antioxidants effects by inhibiting NF-κB, NLRP3, and MAPK pathways, and activating Nrf2. In DSS-induced UC mice, ISL improved symptoms and colon integrity by suppressing ERK1/2 and p38 phosphorylation, and reducing inflammation markers. Preclinical studies suggest ISL and licorice extract as potential IBD treatments | - | [311-316] | |
| Cardamonin | Various plants | Exhibits anti-inflammatory properties by reducing NO production and downregulating iNOS, TNF-α, and IL-6 expression, while inhibiting NF-κB signaling. In DSS-induced colitis mice, it mitigates symptoms, reduces inflammatory markers, and blocks NF-κB and MAPK pathways | - | [317-322] | |
| Phloretin | Apples, pears, and strawberries | Acts as a potent antioxidant and anti-inflammatory agent by modulating signaling pathways and maintaining epithelial integrity. It reduces inflammation markers, oxidative stress, and mitochondrial membrane depolarization in vitro and in a rat and mouse colitis models, while also preserving gut microbiota balance | - | [323-327] | |
| Liochalcone A | Glycyrrhiza inflata | Significantly protected mice against DSS-induced colitis, reducing weight loss, disease activity index, histological damage, and inflammation while preserving intestinal barrier integrity and modulating gut microbiota | - | [328,329] | |
| Butein | Toxicodendron verniciflumm | Significantly ameliorated colitis in Il-10 (-/-) mice by reducing the colonic inflammatory score by over 50%. It lowered the expression of Il-6, IL-1β, IFN-γ, pSTAT3, and MMP-9, and inhibiting IL-6 induced STAT3 activation in colon 205 cells | - | [330] | |
| Flavokawain B | Zingiberaceae and Kawa family | Demonstrated significant therapeutic effects in a DSS-induced IBD mouse models by reducing weight loss, restoring colon length, and mitigating inflammation. It targeted TLR2 to inhibit the TLR2-MyD88 complex formation, suppressing the NF-κB signaling pathway in vivo and in vitro | - | [331] | |
| Artipilin C | Brazilian green apples propolis | It ameliorates UC and colts-associated colorectal cancer by targeting p21-activated kinase 1 (PAK1). | - | [332] |
2.2. Phenolic acids
Their basic structure is based on having one carboxylic acid group. They are presented in amides, esters, or glycosides and in occasional situations are in free form [335]. Their production is through the shikimic acid by the phenylpropanoid via, they can have chemistry structure possesses a C6-C3 structure (phenylpropanoid type), or a C6-C1 (phenylmethyl type), having an ancestor in their synthesis of lignins and other phenolics [336]. Also, they are divided into three sub-groups: hydroxybenzoic and hydroxycinnamic acids [337]. They are found to have an antioxidant effect, according to some studies, they have a range of activities that promote their antioxidant activity, it is suggested that the main pathway is radical scavenging through hydrogen atom donation.
2.2.1. Hydroxybenzoic acids
Hydroxybenzoic acids have a common structure with benzoic acid in the structure C6-C1 [336]. Flavonoids contribute to the action to promote IBD, by reducing inflammation and promoting mucosal barrier function, in DSS-induced colitis [338]. The most important compounds are gallic acid, ellagic acid, protocatechuic acid, and vanillic acid.
A) Gallic and ellagic acid
On the one hand, gallic acid (GA) also known as 3,4,5-trihydroxy benzoic acid is found in plants, and fruits such as grapes, blackberries, strawberries, and raspberries [339]. Ellagic acid (EA) is a condensed dimmer of gallic acid found in fruits, nuts, and seeds including pomegranates, raspberries, strawberries, walnuts, and almonds [340]. According to past works [47] both GA and EA are able to ameliorate intestinal inflammation through several mechanisms, consequently ameliorating macroscopic colonic damage by mitigating edema, deep ulcerations, and hemorrhage.
In the case of GA, past works have shown that this compound downregulates the NLPR3 inflammasome protein and RNA expression in DSS-induced colitis in mice, improving the anti-inflammatory properties of this compound [341]. It also is suggested to improve colitis by decreasing the deleterious metabolite ammonia while ameliorating gut microbiota dysbiosis [342]. More specifically, GA can raise the proportion of probiotic bacteria like Lactobacillaceae and Prevotellaceae, while reducing some pathogenic species, mainly in the Firmicutes and Proteobacteria phyla [343]. These microbial variations are also related to metabolic changes including augmented carbohydrate and bile acid metabolism, decreasing amino acid metabolism. In parallel, Leng et al. [344] also reported that the use of GA altered the diversity of the gut microbiota and activated the bile acid metabolic pathway, being this fact associated with enhanced ILC3 cells in mesenteric lymph nodes and lamina propria accompanied by reductions in TNF-α, IFN-γ, IL-6, IL-17A, and IL-23, and elevations in IL-10, TGF-β and IL-22. GA also stimulates the expressions of TJ proteins, ameliorates cell apoptosis, and oxidative stress, and suppresses the activation of the NF-κB/MAPK pathway to alleviate LPS-induced intestinal inflammation in Caco-2 cells [345]. Similar results and conclusions were obtained in TNBS-induced UC in mice [346]. In DSS-mice, GA also exerted anti-inflammatory mechanisms by suppressing the IL-6/p-STAT3 (Y705) and p65-NF-κB activation [347].
Regarding EA, in vitro studies in caco 2 cells show that it acts through NF-κB and ERK1/2 inhibition, breaking the cycle of inflammation, oxidative stress, redox-sensitive pathway activation, and intestinal permeabilization [348]. Moreover, a diet supplemented with EA mitigates oxidative stress during colitis by bolstering Nrf2 signaling pathways in piglets treated with paraquat, protecting against intestinal injury by facilitating the maintenance of tight junction structure and intestinal barrier integrity, while also preserving jejunal and ileal morphology, including villus height, goblet cell number, and the ratio of villus height to crypt depth [349]. Similar observations were made in TNBS-induced colitis mice, as EA acted by maintaining mucin secretion and intestinal barrier function [350]. The anti-inflammatory effects of this compound have also been observed in acute and chronic models of DSS-induced UC mice. In acute models, a dietary supplement of EA (2%) led to reductions in IL-6, TNF-α, and IFN-γ, whereas in the chronic UC model, EA significantly inhibited the progression of the disease, reducing intestinal inflammation and decreasing histological scores, mainly through the downregulation of COX-2 and iNOS and the blockage of signaling pathways like p38 MAPK, NF-κB, and STAT3 [351]. Similar conclusions were drawn for animal models of CD [352,353]. Like GA, EA also seems to have important modulatory effects on gut microbiota. Co-treatment with a relevant dose (60 mg/kg/day) of EA for 7 days significantly reduced DSS-induced gut barrier dysfunction, endotoxemia, and inflammatory injuries in the gut, liver, and brain in mice. This was achieved by modulating gut microbiota composition (reducing Bacteroides and E. coli and increasing the abundance of Lactobacillus) and inhibiting elevated oxidative and nitrative stress markers [354]. Likewise, EA also shows a favorable effect on gut microbiota and immune response in piglets treated with paraquat [355], showing the multiple potential benefits associated with this molecule.
B) Protocatechuic acid and vanillic acid
Protocatechuic acid (PCA) is a member of the hydroxybenzoic acids, it is also known as 3,4-dyhyrdroxybenzoic acid found in medical herbs, vegetables, and various plants such as olives, roselle, du-zhong, calamondin, and white wine grapes [356]. Yang et al. [357] showed that PCA reduced the levels of the disease activity index, inflammatory factors, and histological damage in UC mice, mainly through the modulation of Bacteroidetes. Moreover, PCA seemed to downregulate the level of ferroptosis in the colon tissue, evidenced by a reduced iron overload, decreased GSH depletion, and a lower level of MDA production, whereas similar results were observed in Erastin-treated Caco-2 cells. PCA can also lead to reduced levels of IL-1β, IL-6, and TNF-α, also ameliorating DSS-induced ZO-1 and claudin-2/4 redistribution [358]. Crespo et al. suggested that PCA could modulate the sphingosine kinase (SphK)/S1P system and related signaling pathways to exert its anti-inflammatory effects in TNBS models of IBD [359]. They observed that PCA administration effectively prevented colonic damage, weight loss, and MPO activity increase induced by TNBS, while also modulating antioxidant enzyme expression, proinflammatory cytokines, and signaling molecules such as AKT, ERK, pSTAT3, and NF-κB p65. In another study, PCA administration significantly prevented colitis symptoms, reduced pro-inflammatory cytokines and liver toxicity markers, and protected against oxidative damage in both the colon and liver, highlighting its chemoprotective role [360].
Vanillic acid (VA) is a natural benzoic acid derivative commonly found in herbs, rice, maize, and edible plants and fruits [361]. Ni et al. demonstrated the relevance of the use of VA in treating DSS-induced colitis by restoring intestinal epithelium homeostasis through the inhibition of ferroptosis. They reported that VA acted by direct targeting of carbonic anhydrase IX (CAIX, CA9), leading to the activation of insulin-induced gene-2 (INSIG2) and subsequent interaction with stromal interaction molecule 1 (STIM1), resulting in the translocation of SCAP-SREBP1 and the upregulation of stearoyl-CoA desaturase 1 (SCD1) transcription. This cascade effectively inhibits ferroptosis-mediated excessive death of intestinal epithelial cells, preserving intestinal barrier integrity and mitigating unresolved inflammation. On the other hand, Kim et al. [362] showed that VA reduced clinical manifestations of colitis such as weight loss, colon length shortening, and disease activity index. Moreover, VA suppressed the expression of COX-2 and the activation of transcription nuclear factor-κB p65 in DSS-treated colon tissue, also decreasing plasma levels of IL-6 suggesting its efficacy in regulating chronic intestinal inflammation.
2.2.2. Hydroxycinnamic acids
These form part of this group due to their chemical structure in this case has a cinnamic acid structure with one or more hydroxyl groups linked with the phenyl ring, in different from hydroxybenzoic acids, that this last one does not have a cinnamic structure. They are found in fruits, vegetables, grains, coffee, and tea [363]. Some common hydroxycinnamic acids are chlorogenic acid, caffeic acid, ferulic acid, sinapic acid, rosmarinic acid, coumarinic acid, and quinic acid.
A) Chlorogenic and caffeic acid
Chlorogenic acid and caffeic acid are the two critical phenolic acids found in coffee, representing along with caffeine some of the major bioactive compounds in this drink [364]. Caffeic acid is also found in various plant-based foods, including fruits and vegetables, and drinks like tea or wine. It consists of a cinnamic acid moiety with a hydroxyl group substituted at the 3-position on the phenyl ring. In RAW 264.7 cells and colon epithelial cells, HT-29 is found that caffeic acid acts as an anti-inflammatory modulator by suppressing the production of nitric oxide (NO), IL-1β, IL-6, IL-8, and TNF-α, suggesting their potential therapeutic in IBD [365]. Xiang et al. [366] demonstrated that caffeic acid could attenuate DSS-induced murine UC by interfering with the activation of macrophages, thus representing an alternative therapeutic option for this condition. Other works have also demonstrated that caffeic acid could exert its benefits in these animals by increasing the Akkermansia population [367]. One derivated of caffeic acid is FA-97 and it is synthetic and is found to reduce DSS-induced colitis against oxidative stress by the activation of the Nrf2/HO-1 pathway [368]. In parallel, caffeic acid phenyl ester is a bioactive compound of propolis extract with proven anti-inflammatory actions. Among their protective and therapeutic effects against IBDs demonstrated in vitro and in DSS-induced UC mice models, the studies have shown that this compound is able to ameliorate NLRP3 inflammasome activity [369], by reducing myeloperoxidases and proinflammatory cytokines [370] and also through modulating NF-kB activation while reducing intercellular adhesion molecules (ICAM)-1 and vascular cell adhesion molecules (VCAM) [371].
On the other hand, chlorogenic acid is also found in a plethora of plant sources like apples, artichoke, betel, burdock, carrots, eggplants, grapes, kiwi, potatoes, tea or tomatoes, among others [372]. Chemically, it comprises a caffeic acid moiety and a quinic acid moiety; hence, it is also referred to as 5-O-caffeoylquinic acid. Gao et al. [373] claimed that chlorogenic acid is able to reduce DSS-induced colonic mucosal damage, by alleviating DSS-induced inflammation, oxidative stress, and apoptosis in the colon, while reducing ERK1/2, p -ERK, p38, p-p38, JNK, and p-JNK protein expression. Similar findings were made by Vukelić et al. [374], who also observed that chlorogenic acid suppressed the activation of pro-inflammatory and apoptotic signaling pathways in these animals. Zeng et al. [375] showed in these animal models and in RAW264.7 cells that chlorogenic acid prevented colitis by downregulating miR-155 expression and inactivating the NF-κB/NLRP3 inflammasome pathway in macrophages. Maslin et al. [376] observed that despite the addition of quercetin and chlorogenic acid to a DSS-induced UC mice did not protect against indicators of injury and inflammation, or fecal SCFA concentrations compared to the control diet, these compounds influenced the expression of various injuries repair molecules, pro-inflammatory cytokines, SCFA transport proteins, and NF-κB inhibitory molecules. Thus, they suggested that these compounds exert their maximum benefits in healthy individuals or during periods of remission, whereas they may suppress some of the signaling involved in inflammation promotion during active disease stages [376]. Among the main benefits of chlorogenic acid in the gut microbiota in DSS-mice, is a marked decrease in the proportion of Firmicutes and Bacteroidetes, and microbial diversity, along with a marked increase in Akkermansia has been reported by Zhang et al. [377]. Similar observations were made for IL-10 knockout mice models of IBD, as chlorogenic acid was able to reduce the expression levels of iNOS, IL-1β, and TNF-α [378], whereas chlorogenic acid was able to inhibit the growth of Bacteroides and the accumulation of Bacteroides-derived LPS, in indomethacin-induced colitis [379].
B) Ferulic and sinapic acid
Ferulic acid has a similar structure as caffeic acid, but it contains an additional methoxy group linked to the phenyl ring. It is presented in oranges, apples, and tomatoes [380]. It is suggested to have a protective action in the intestinal tight junctions, and also suppresses the endoplasmic reticulum (ER) stress, NO generation, and inflammation in Caco-2 and T84 cells, demonstrating its anti-inflammatory and anti-oxidant activities [381]. In vivo, the relevance of ferulic acid has been demonstrated in multiple animal models using 2,4,6-trinitrobenzene sulfonic acid, intrarectal acetic acid, DSS and TNBS-induced UC [382-385]. In this sense, Yu et al. [385] show that ferulic acid can alleviate intestinal injury in UC rats and inhibit inflammatory factor levels (IL-6, IL-12, and IL-1β), apoptosis-related protein expression (caspase-1 and caspase-3) and the TXNIP/NLRP3 signaling pathway. Besides, greater effects were observed for those animals identified in the middle and high intervention group (20 mg/kg of ferulic acid per day during 14 days and 250 mg/kg, respectively) when compared to the low intervention group (10 mg/kg per day). Simultaneously, Ghasemi-Dehnoo et al. [383] showed that ferulic acid administered orally one hour after the UC induction and during five days at 20, 40, and 60 mg/kg doses ameliorated UC through the inhibition of the LPS-TLR4-NF-κB and NF-κB-INOS-NO signaling pathways. Importantly it seems that higher doses were also associated with broader improvements. Sadar et al. [382] also reported that ferulic acid could ameliorate TNBS-induced colitis by inhibiting IFN-γ induced inflammatory cascade, driving a concomitant decrease in the release of pro-inflammatory cytokines. Likewise, a greater concentration of the compound (40mg/kg once per day during 14 days) drove to greater improvements when compared to middle doses (20mg/kg day) and low doses (10 mg/day) A ferulic acid derivate designed as C1a (ferulic acid conjugated with octopamine) was shown to effectively alleviates clinical signs and inflammatory mediators in vitro and in vivo models of IBD, showing even greater benefits that ferulic acid alone at the same doses (1 or 5 mg/kg per day during 14 days) [384].
Sinapic acid (SA) forms part of the hydroxycinnamic acids by their similarity in their structure, which has in common with ferulic acid, but in this case, it also has a vinyl group attached to the phenyl ring. It is found in various edible foods like berries, citric fruits, oil seeds, wheat, rice, spices, and plants like Salvia officinalis and Myristica fragrans [386]. Jang et al. [387] observed that SA was able to mitigate stimulus-induced delocalization of tight junction proteins, reduce intestinal permeability, and suppress inflammatory cytokine expression both in vitro and in vivo. SA is directly bound to transforming growth factor beta-activated kinase 1 (TAK1), inhibiting NF-κB and MAPK/ATF-2 pathways, thereby regulating mitogen-activated protein kinase (MLCK) expression. Dietary sinapic acid also improved gut microbiota imbalance and IBD symptoms, suggesting its potential as a nutraceutical and pharmaceutical agent for IBD treatment by targeting TAK1 and inhibiting subsequent NF-κB and ATF-2 signaling. Pretreatment with SA (40 mg/kg/day) significantly mitigated colonic injuries and acetic acid-induced UC symptoms in rats, reducing oxidative stress (MDA, NO levels), restoring antioxidant/oxidant balance (catalase, glutathione levels), suppressing inflammation (TNF-α, IL-6, MPO, PGE2, COX-2, NF-κB), and inhibiting apoptosis (Bax, caspase-3), while increasing anti-apoptotic protein Bcl-2 expression [388]. Oral administration of SA (10, 30, and 100 mg/kg) to female Balb/c mice post-TNBS instillation significantly improved colonic weight and length, reduced macroscopic and microscopic colonic changes, and lowered myeloperoxidase activity, malondialdehyde levels, and tumor necrosis factor-alpha expression compared to TNBS control [389]. Li et al. [390] reported that SA supplementation could significantly improve clinical symptoms, reduce pathological changes, repair intestinal mucosal barrier function, and maintain epithelial homeostasis by inhibiting NLRP3 inflammasome activation and decreasing pro-inflammatory cytokine expression. SA also increased antioxidant enzyme expression via the Nrf2/keap1 pathway, enhanced autophagy through the AMPK-Akt/mTOR signaling pathway, and reduced intestinal fibrosis-associated proteins Collagen-I and α-SMA. Similarly, Qian et al. [391] observed that SA administration attenuated oxidative damage by enhancing SOD, GSH, and CAT activity while decreasing serum and colonic mRNA levels of proinflammatory cytokines. Mechanistically, SA reduced NLRP3 inflammasome activation and enhanced intestinal barrier integrity by upregulating ZO-1, occludin, and claudin-1 expression. In vitro, SA also influenced cell viability, decreasing epithelial permeability and restoring the protein and mRNA expression of claudin 1, and ZO-1 in LPS-treated Caco-2 cells [392].
C) Rosmarinic, coumaric and quinic acid
Rosmarinic acid (RA) is a type of polyphenol belonging to the class of phenolic acids. It is specifically an ester of hydroxycinnamic acid, found in various plants, including rosemary, basil, and mint, among others. RA has been studied for its potential antioxidant, anti-inflammatory, and antimicrobial properties, making it an important component of many herbs and plant-derived foods [393]. In the event of IBDs, RA has proven its efficacy in alleviating intestinal inflammation, ameliorating tight junction damage, gut dysbiosis, endoplasmic reticulum stress, cell death, and smooth muscle contractile dysregulation [394]. Formiga et al. [395] observed that RA had an important anti-inflammatory activity in the gut through cytoprotection, mucosal barrier maintenance, and modulation of antioxidant and immunomodulatory systems. Indeed, they observed that oral administration of RA (25-200 mg/kg) reduced macroscopic lesions, ulcerative area, intestinal weight/length ratio, and diarrheal index. At higher doses (200 mg/kg), RA decreased MDA and MPO, restored GSH levels, enhanced SOD activity, reduced IL-1β and TNF-α levels, and also modulated T cell populations, reducing mRNA transcription of inflammatory markers, and enhanced gene expression and positive cell staining for MUC-2 and ZO-1. In a mouse model of DSS-induced colitis, RA significantly reduced colitis severity, as evidenced by decreased disease activity index scores, colonic damage, and colon length. RA also lowered levels of inflammatory cytokines (IL-6, IL-1β, IL-22) and protein expression of COX-2 and iNOS, inhibiting NF-kappa B and STAT3 activation and subsequently reducing pro-survival gene activity dependent on these transcription factors [396]. Similarly, the use of chitosan/nutriose-coated niosomes to increase RA local bioavailability led to significant improvements in acute colitis through the downregulation of the protein expression of inflammasome components such as NLR family pyrin domain-containing 3 (NLRP3), adaptor protein (ASC) and caspase-1, and the consequent reduction of IL-1β levels [397]. Also, this nanovesicle drove a significant increase in the expression of Nrf2 and HO-1.
Coumaric acid is a phenolic acid from the hydroxycinnamic acid family synthesized through the shikimate pathway with phenylalanine and tyrosine as precursors [398]. It is abundantly found in fruits (apples, pears, grapes, oranges, tomatoes, and berries), vegetables (e.g. beans, potatoes, and onions), cereals (e.g. maize, oats, and wheat), mushrooms, and medicinal herbs [399]. Quinic acids are polyphenol esters formed of hydroxycinnamic acids and quinic acid mainly found in plants (Yerba mate, white, green teas, and coffee), microalgae, and cyanobacteria [400]. One study conducted in 64 male Wistar rats evaluated the use of quinic acid in treating UC induced by acetic acid in rats. This acid, especially at higher doses, demonstrated promising therapeutic potential for UC by significantly increasing the expression of HO-1, Nrf2, and NQO1 mRNA, while decreasing tissue levels of TNF-α and IL-1β protein in colon tissue [401]. Regarding quinic acid, one study [402] showed that this compound attenuated UC by inhibiting the TLR4-NF-κB and NF-κB-iNOS-NO signaling pathways, thereby reducing colitis-related complications such as oxidative stress, inflammation, apoptosis, and histopathological damage in the same animal models. Table 2 summarizes the effects of the main phenolic acids explored in this section.
2.3. Stilbenes
They are considered phytochemicals, some of them, also are included as phytoalexins, and their chemical composition is formed by a 1,2-diphenylethylene backbone. They are found in berries, grapes, peanuts, and red wine [403]. The three most important stilbenes are, resveratrol, piceatannol and pterostilbene.
A) Resveratrol
Resveratrol (RES) also known as 3,4,5-trihydroxystilbene is presented mainly in wine and grapes [404]. RES is a potent antioxidant and anti-inflammatory molecule widely explored in the management of immunoinflammatory diseases like IBD [405]. The mechanism of action of RES involves multiple immune responses and signaling pathways. RES is absorbed quickly and metabolized into various derivatives. However, the poor water solubility of this molecule and its low bioavailability limit its clinical applications [406]. RES also presents a bidirectional relationship with gut microbiota, showing modulatory effects on the microbial community, which in turn influence its metabolism and action [407]. More specifically, in vitro studies have shown that RES reduces inflammation in UC by decreasing IL-1β levels and increasing IL-11 production, primarily through the modulation of the Nrf2 pathway in both TNF-α challenged Caco-2 cells and patient tissue samples [408]. RES and 3-(4-hydroxyphenyl)-propionic acid (4HPP), a microbial metabolite of RES, significantly reduced paracellular permeability and proinflammatory cytokine secretion in LPS-treated Caco-2 cells, correlating with increased TJ protein expression [409]. Both compounds ameliorated intestinal barrier dysfunction and colonic inflammation in colitis mice, mediated through AMPK activation and regulation of the SIRT1/NF-κB pathway, whereas dihydro resveratrol (DHR), another microbial metabolite of RES, showed no significant effects either in vivo or in vitro. RES was equally able to reverse the LPS-induced downregulation of occludin, ZO-1, and claudin-1 in HT-29 cells as well as attenuating the Notch1 pathway and subsequently reducing IL-6 and TNF-α levels [410].
Phenolic acids explored in the context of inflammatory bowel diseases (IBDs)
| Subgroups | Compounds | Dietary/Exogenous sources | Mechanisms of action (Preclinical models) | Studies in humans (including observational and intervention studies | References |
|---|---|---|---|---|---|
| Hydroxybenzoic | Gallic acid | Grapes, blackberries, strawberries, and raspberries | Downregulates the NLPR3 inflammasome and improves colitis in DSS-induced mice by reducing ammonia levels and gut microbiota dysbiosis, increasing beneficial bacteria like Lactobacillaceae and Prevotellacea. It modulates metabolic pathways, enhancing carbohydrate and bile acid metabolism while decreasing amino acid metabolism, and reduces pro-inflammatory cytokines while increasing anti-inflammatory cytokines | - | [341-347] |
| Ellagic acid | Fruits, nuts, and seeds, such as pomegranates, raspberries, strawberries, walnuts, and almonds | Inhibits NF-κB and ERK1/2 in Caco-2 cells, reducing inflammation, oxidative stress, and intestinal permeability. Dietary EA supplementation bolsters Nrf2 pathways, protecting against oxidative stress and maintaining gut barrier integrity and morphology in piglets and TNBS-induced colitis mice. Also, reduces inflammation and histological scores in the acute and chronic DSS-induced UC models by downregulating COX-2, iNOS, and blocking p38, MAPK, NF-κB, and STAT3 | - | [348-353] | |
| Protocatechuic acid | Olives, roselle, du-zhong, calamondin, and white wine grapes | One study demonstrated that PCA reduced disease activity, inflammation, and histological damage in UC mice by modulating Bacteroidetes and downregulating ferroptosis, with similar effects in Erastin-treated Caco-2 cells. Also, it reduced IL-1β, IL-6, and TNF-α levels, and improved tight junction protein distribution in DSS-induced colitis | - | [356-360] | |
| Vanillic acid | Herbs, rice, maize, and edible plants and fruits | It is demonstrated that VA treats DSS-induced mice colitis by inhibiting ferroptosis and restoring intestinal epithelium homeostasis through a CAIX-INSIG2-STIM1-SCAP-SREBP1-SCD1 pathway, preserving barrier integrity and reducing inflammation. In another study, it is found that VA alleviated colitis symptoms, reduced COX-2 expression, NF-κB p65 activation, and plasma IL-6 levels | - | [361,362] | |
| Hydroxycinnamic | Caffeic acid | Plant-based foods, including fruits and vegetables, and drinks like tea or wine | Act as an anti-inflammatory modulator by suppressing NO. IL-1β, IL-6, IL-8, and TNF-α in RAAW 264.7 and HT-29 cells. It attenuates DSS-induced murine UC by interfering with macrophage activation and increasing Akkermansia populations | - | [364-371] |
| Chlorogenic acid | Apples, artichoke, betel, burdock, carrots, eggplants, grapes, kiwi, potatoes, tea or tomatoes, among others | Alleviates DSS-induced colonic damage by reducing inflammation, oxidative stress, and apoptosis while modulating ERK1/2. P38, and JNK pathways. It downregulates miR-155 and inactivates the NF-κB/NLRP3 pathway in macrophages, offering protective effects against colitis | - | [373-379] | |
| Ferulic acid | Orange, apples, and tomatoes | It demonstrates effects on intestinal tight junctions, suppressing ER stress, NO generation, and inflammation in vitro. In vivo, it alleviates colitis in various animal models by inhibiting inflammatory pathways, and reducing cytokine levels | - | [380-385] | |
| Sinapic acid | Berries, citric fruits, oil seeds, wheat, rice, spices, and plants like Salvia officinalis and Myristica fragans | It mitigates intestinal permeability, reduces inflammatory cytokine expression, and restores tight junction protein localization both in vitro and in vivo. It inhibits the NF-κB and MAPK/ATF-2 pathways by binding to TAK1, improves gut microbiota imbalance, and reduces IBD symptoms, showing potential as a nutraceutical and pharmaceutical agent for IBD treatments | - | [386-392] | |
| Rosmarinic acid | Rosemary, basil, and mint, among others | Demonstrates significant antioxidant, anti-inflammatory, and antimicrobial properties, proving effective in alleviating intestinal inflammation, ut dysbiosis, and tight junction damage in IBD | - | [393-397] | |
| Coumaric acid | Apples, pears, grapes, oranges, tomatoes, berries, beans, potatoes, onions, maize, oats, wheat, mushrooms and medicinal herbs | - | - | [399,401] | |
| Quinic acid | Yerba mate, white, green teas, coffee, microalgae, and cyanobacteria | One study demonstrated the therapeutic potential for UC by significantly increasing HO-1, Nrf2, and NQO1 mRNA expression while reducing TNF-α and IL-1β protein levels in colon tissue. Also attenuates UC by inhibiting the TLR4-NF-κB and NF-κB-iNOS-No signaling pathway, reducing oxidative stress, inflammation, apoptosis, and histopathological damage | - | [400,402] |
Serra et al. [411] compared the role of cyanidin-3-glucoside and RES with 5-aminosalicylic acid (5-ASA) in HT-29 intestinal cells. The results showed that both polyphenols, at lower concentrations than 5-ASA, activated Nrf2, increased HO1 and glutamate cysteine ligase mRNA expression, enhanced the reduced-to-oxidized glutathione ratio, and inhibited reactive species production, with resveratrol and 5-ASA also increasing nuclear PPAR-γ levels. They concluded that these compounds could act as complementary nutraceuticals in managing intestinal inflammation in inflammatory bowel disease. The stimulation of the Nrf2/HO‑1 pathway by RES reduced clinical symptoms, inflammatory responses, and intestinal mucosal barrier damage in experimental UC mice as well [412]. On the other hand, Garcia et al. [413] demonstrated the antifibrotic effects of RES on intestinal smooth muscle cells from a CD rat model, finding that this compound decreased cell numbers through cell cycle arrest and apoptosis, and reduced collagen synthesis and procollagen mRNA expression. Similar conclusions were obtained in the PG-PS rat model of CD, as the use of RES significantly reduced inflammatory cytokines and TGF-β1 and demonstrated a promising trend in decreasing tissue fibrosis [414]. Resveratrol also enhances xenophagy, promoting autophagy-dependent clearance of intracellular bacteria in vitro (in intestinal epithelial cells and macrophages) and in vivo (transgenic GFP-LC3 zebrafish), suggesting potential for developing pro-autophagic nutrients to maintain intestinal homeostasis and combat infections [415].
Multiple animal models have also validated the relevance of RES in the management of IBD through several additional mechanisms. These actions included down-regulation of Wnt signaling pathway [416], regulation of gut microbiota diversity [417], regulation of arginine metabolism in macrophages [418], modulation of SUMO1 through the Wnt/β-catenin pathway [419], inhibition of STAT3 O-GlcNAcylation and reduction JAK2/STAT3 pathway activity [420], suppression of the intestinal inflammatory cascade reaction, and regulation of autophagy and SIRT1/mTOR signaling [421,422], modulation of the PI3K/Akt/VEGFA pathway [423], reduction of neutrophil infiltration, inhibition of adhesive molecules, restoration of the NO and redox status [424,425], recovery of the Treg/Th17 balance and the HIF-1α/mTOR signaling pathway [426], induction of immunosuppressive CD11b(+) Gr-1(+) cells [427] promotion of MUC2 synthesis via the ANRIL-miR-34a axis [427], NLRP-3 inflammasome repression [428], AMPK-mediated activation of CDX2 and the regulation of the SIRT1/NF-κB pathway [429], downregulation of miR-31 [430,431], inhibition of SphK1 , and downregulation of the p38 MAPK [432]. Overall, in vivo and in vitro studies show that RES can have important effects in alleviating IBD, modulating a plethora of mechanisms associated with this chronic condition.
Intervention studies in humans
Human studies have demonstrated significant benefits from resveratrol supplementation in people with UC. One work [433] analyzed the effects of the Mediterranean diet (MD) combined with curcumin and resveratrol supplementation on disease activity, serum inflammatory markers, and quality of life in patients with mild to moderate UC. In this multicenter three-arm randomized controlled trial, participants were randomized into the MD, MD + curcumin, and MD + resveratrol groups. All groups followed the MD for 8 weeks, with the addition of either curcumin (1600 mg/day) or resveratrol (500 mg/day) supplementation for the respective groups. Results demonstrated that all interventions effectively reduced disease activity and inflammation while improving the quality of life in UC patients. Similar results were obtained in a randomized, double-blind, placebo-controlled study [434], in which 50 patients with active mild to moderate UC were given either a 500-mg resveratrol or placebo capsule for 6 weeks. The resveratrol group showed significant reductions in serum inflammatory markers, including TNF-α, c reactive protein (CRP) and NF-κB activity in PBMCs with no significant changes in the placebo group. Quality of life scores (IBDQ-9) increased and clinical colitis activity index scores decreased significantly in the resveratrol group compared to the placebo group. Likewise, 500 mg/day resveratrol capsules or the same amount of placebo for 6 weeks given to 56 patients with mild to moderate UC also reported that the intervention group show an increase in total antioxidant capacity, serum levels of SOD, and the quality of life, along with a decrease in MDA levels and disease activity [435].
Overall, these studies support that 500 mg resveratrol supplementation can improve quality of life and reduce disease activity in UC patients, especially in combination with a healthy diet such as MD. However, the long-term effects of resveratrol in these patients require further investigation.
B) Piceatannol
Piceatannol (PIC) is a natural compound found in various plants, including grapes, peanuts, and blueberries. It is structurally similar to resveratrol and has gained attention due to its potential health benefits. For instance, past works have demonstrated that PIC is able to reduce several proinflammatory mediators in activated immune cells and induce regulatory T cells in vitro while modulating adipocyte function [436]. PIC was shown to repress inflammation, inhibit cell apoptosis, and regulate microbiota composition, with some synergic effects when combined with 3'-hydroxy pterostilbene [437]. Both compounds seemed to increased representative probiotic species, including Akkermansiaceae and Lactobacillus intestinalis, while exerting inhibitory effects on several bacterial species (Spiroplasmataceae and Acholeplasmataceae). Another study showed that oral administration of resveratrol or PIC (10 mg/kg body weight each) for 7 constitutive days attenuated the DSS-induced inflammatory injury, upregulation of iNOS expression, and activation of NF-kappaB, STAT3, and ERK [438]. Likewise, PIC ameliorated the disruption of the colonic architecture, along with a significant reduction in colonic myeloperoxidase (MPO) activity, and a decrease in the production of inflammatory mediators such as nitric oxide (NO), prostaglandin (PGE2, as well as various pro-inflammatory cytokines [439].
C) Pterostillbene
Pterostilbene (PTS) (3,5-dimethoxy-4-hydroxystilbene), is an analogue of resveratrol that has the same health-promoting properties as resveratrol [440]. This compound is mainly found in blueberries and Pterocarpus marsupium heartwood [441]. In DSS-induced mouse colitis models, PTS is shown to inhibit the activity of NLRP3 inflammasome, demonstrating its anti-inflammatory properties [442]. In UC mice induced by high-fat diet (HFD) and DSS, the use of PTS significantly reduced inflammation, aberrant crypt foci formation, and colon weight-to-length ratio, by ameliorating IL-1β, C/EBP homologous protein (CHOP), COX-2, and TGF-β1 while maintaining mucin2 and E-cadherin expressions [443]. PTS can also inhibit DC-mediated T cell proliferation, reducing Th1 and Th17 populations, and increasing Treg populations. Likewise, this compound suppressed DC-induced inflammatory cytokine production by attenuating the transcription factor PU.1 and promoting Foxp3+ Treg differentiation, effectively alleviating symptoms of DSS-induced colitis and decreasing TNF-α expression in mice [444]. Table 3 summarizes the effects of the main stilbenes explored in this section.
2.4. Lignans
This group is formed by two phenylpropane motives united by a C6-C3 bond. They are found in plants such as the latter, flaxseed, and seams seed, and also can be found in fish, meat, oilseed, and beverages [446].
Stilbenes explored in the context of inflammatory bowel diseases (IBDs)
| Subgroups | Compounds | Dietary/Exogenous sources | Mechanisms of action (Preclinical models) | Studies in humans (including observational and intervention studies | References |
|---|---|---|---|---|---|
| Stilbenes | Resveratrol | Wine and grapes | Is a potent antioxidant and anti-inflammatory molecule explored for managing IBD. It modulates responses and signaling pathways, but its poor water solubility and low bioavailability limit clinical applications. It influences gut microbiota, reducing inflammation, enhancing tight junction protein expression, and modulating several pathways | Human studies show that resveratrol supplementation benefits people with UC. In a trial with the Mediterranean diet (MD) plus resveratrol or curcumin, all groups showed reduced disease activity and inflammation, and improved quality of life. Another study with 500 mg/day resveratrol for 6 weeks reported significant reductions in inflammatory markers and improved quality of life compared to placebo. Overall, 500 mg resveratrol supplementation enhances life quality and reduces disease activity in UC patients, but long-term effects need further study | [405-415,417-429,431,432,434,435] |
| Piceatannol | Grapes, peanuts, and blueberries | Is similar to resveratrol, has shown promise in reducing inflammation and modulating immune response. It decreases proinflammatory mediators, inhibits cell apoptosis, and improves microbiota composition, particularly beneficial probiotics. Oral administration of PIC attenuates DSS-induced colonic inflammation, decreases inflammatory mediators, and improves colonic architecture | - | [436-439] | |
| Pterostilbene | Blueberries and Pterocarpus marsupium heartwood | Exhibits anti-inflammatory effects in DSS-induced models. It reduces inflammation, aberrant crypt foci, and maintains mucin2 and E-cadherin expression, while also inhibiting Th1 and Th17 cell proliferation and promoting Treg differentiation. Also decreases, TNF-α expression and alleviates colitis symptoms | - | [440-444] |
Firstly, it should be highlighted the multiple studies conducted with flaxseed and flaxseed oils, particularly rich in lignans. Indeed, flaxseeds represent the first plant and food with the highest content of lignans, with approximately 301,000 μg of lignans /100 g [447], at present the maximal known content of any foodstuff. The major lignan in flaxseed is called secoisolariciresinol diglucoside (SDG) [448]. Because of this, a significant number of preclinical studies support the relevance of lignans in the treatment of IBDs, whereas some clinical trials have also been conducted in this sense. However, additional lignans present in sesame and other sources should also be highlighted. In this section, these groups of lignans will be summarized.
A) Flaxseed and secoisolariciresinol diglucoside
Some in vivo models have found that flaxseed oil supplementation had a beneficial health effect in a physically active mouse model of CD susceptibility, leading to favorable changes in the gut microbiota [449]. Aqueous-methanolic crude extracts of Flaxseed (Fs.Cr) and Flaxseed oil were evaluated for their therapeutic effects on AA-induced colitis in mice, with microscopic analysis of colon tissue and assessments of antispasmodic and antibacterial activities [450].
Flaxseed oil reduced mortality and colonic ulcers more effectively, while Fs.Cr increased mucin content, exhibited stronger anti-inflammatory effects, and demonstrated significant antispasmodic and antibacterial activities in these animals. Fs.Cr was found to regulate several additional key molecules involved in IBD, reducing the levels of TNF-α, IFN-γ, MDA and MPO, and increasing IL-17 SOD, GPX, CAT, and total GSH, thus demonstrating their role in decreasing UC severity by reducing oxidative damage, inflammation, and promoting mucosal repair [451]. Silva et al. [452] explored the fractions containing phenolic compound isolate (Phi) and phenolic reduced-flaxseed protein hydrolysate (phr-FPH) from flaxseed in TNBS-induced UC mice. They observed that both Phi and phr-FPH reduced levels of TNF-α and NO in stimulated macrophages. Moreover, Phi and phr-FPH treatments prevented weight loss and colon inflammation in colitic BALB/c mice, also decreasing T cell proliferation, Th1 and Th17 cells, and pro-inflammatory cytokines, while increasing Treg cells in spleen cell cultures from Phi-treated mice. SDG also showed promising effects in the DSS-induced colitis model and LPS-stimulated RAW264.7 macrophages. In more detail, SDG supplementation attenuated colitis severity, reduced macrophage infiltration, and lowered inflammatory cytokine levels while inhibiting NLRP1 inflammasome activation, partly through NF-κB disruption, suggesting its potential as an IBD treatment [453]. Lignans and flaxseed oligosaccharides and proteins seem to benefit significantly in animal models of IBD [454,455]. Conversely, another work [456] found that neither the administration of extra virgin olive oil (EVOO) nor flaxseed oil alone or in combination had significant benefits for preventing or ameliorating any symptoms in DSS-induced acute UC mice. Indeed, their use was associated with slight adverse effects such as increased spleen weight-to-body weight ratio and inflammatory markers. In a similar line, ground flaxseed supplementation seemed to ameliorate the benefits from a reduced-fat diet in a Citrobacter rodentium-induced model of colitis [457].
Intervention studies in humans
Interestingly some studies in humans evaluating the role of flaxseed supplementation have been conducted by Morshedzadeh et al. [458-461]. In a first open-labeled randomized controlled trial with 75 UC patients, Morshedzadeh et al. [459] randomly assigned subjects into one of the following groups for 12 weeks: group 1) receiving 30 g/day of grounded flaxseed (GF); group 2) receiving 10 g/day of flaxseed oil (FO) and group 3) receiving placebo. They observed that both flaxseed and flaxseed oil, attenuate inflammatory markers, disease severity, blood pressure, and waist circumference when compared to the placebo. In more detail, a significant decrease was observed in fecal calprotectin, erythrocyte sedimentation rate, INF-γ, IL-6, waist circumference, diastolic blood pressure, and systolic blood pressure, and a significant increase was noted in TGF-β and Inflammatory Bowel Disease Questionnaire-Short form (IBDQ-9) score in the GF and FO groups compared to the control. No significant difference was observed between the FO and GF groups except for TGF-β. Similarly, they showed significant reductions in hs-CRP and Mayo score, alongside increased quality of life in both GF and FO groups compared to the control. FO showed a significant increase in IL-10 concentration but no significant changes were observed in TLR4 between intervention and control groups [458]. Besides, the severity of the disease positively correlated with the decrease in Mayo score. When they compared the effects on metabolic syndrome parameters of Flaxseed supplementation (30 g/day GF) with placebo in 70 patients with mild to moderate UC, reduced insulin, HOMA-IR, triglyceride, and total cholesterol levels were observed along with increasing HDL levels [460]. Improvements were also noted in SCCAI score and TNF-α although no significant differences were observed in body weight, BMI, waist circumferences, or blood pressure between the intervention and control groups. In another trial, 64 with mild and moderate Flaxseed supplementation (30g/day of GF) resulted in a significant reduction in resistin and visfatin concentration and a significant increase in adiponectin levels [461]. Therefore, flaxseed supplementation (30 g/day GF or 10g/day FO) during 12 weeks seemed to have positive effects on patients with mild to moderate UC, improving a broad spectrum of inflammatory, metabolic, and clinical markers. However, additional long-term studies are required before drawing any definitive conclusion.
B) Sesame seeds, sesamin and sesamol
Sesame seeds exhibit the second-highest lignan concentration, with a total of 39,348μg/100g [462]. Major lignans of sesame are sesamin and sesamolin, although other important members include sesamol, sesaminol, its epimers, and episesamin [463]. The use of sesame oil has demonstrated significant benefits in the treatment of IBD in preclinical models. For instance, sesame oil can decrease the levels of MPO [464], whereas other studies have found that 4 mL/kg for 7 days of this product can ameliorate TNBS-induced UC in rats by reducing the degree of inflammation, fibrosis, and acidic mucin while increasing neutral mucin [465]. On the other hand, sesame cake, a by-product of sesame oil production, improved symptoms of DSS-induced colitis, ameliorated histopathological damage of the mucus layer in colon tissues, and decreased pro-inflammatory cytokines apoptosis and oxidative stress in colitis-induced colon tissues [466]. Likewise, the use of Kanjangs (Fermented Soy Sauce and Sesame Sauce) exerted an anticolitic effect partially by reducing the serum levels of proinflammatory cytokines and inhibiting the mRNA expression of these factors in the colon tissue of DSS-induced C57BL/6 mice of UC [467]. Interestingly, low-dose treatment (4 mL/kg) with the fermented sauces resulted in broader anticolitic effects than consumption of a higher quantity (8 mL/kg).
Regarding specific lignans from sesame, sesamin (SES) exerts a protective action against UC by activating AKT/ERK and the increasing signaling of Nrf2 [468]. Likewise, SES treatment reduced the DAI values and improved the histopathology of the colon in the DSS-treated mice, diminishing TNF-α, IL-1β, and IL-6 production caused by DSS through the modulation of the NF-κB and MAPK signaling pathways [469]. Sesamol has also obtained some interesting results in this field. Zhao et al. [470] demonstrated that sesamol treatment (100 mg/kg per day) for 6 weeks inhibited the DSS-induced bodyweight loss of mice, along with the DSS-induced histopathological changes and inflammatory responses by targeting the NF-κB signaling pathway in mice colon. Moreover, they showed that sesamol prevented gut barrier damage by enhancing the expression of tight junction proteins (occludin, claudin-1, and ZO-1), recovering the loss of gut mucus layer while increasing SCFAs through changes in the gut microbiome structure by enhancing the relative abundance of Coprococcuscus, Butyricicoccus, Odoribacter, and AF12 [470]. In a similar experimental design, Xia et al. [471] observed that sesamol treatment (100 mg/kg per day) for 6 weeks prevented inflammatory response, epithelial barrier dysfunction, and depression-like and anxiety-like behaviors in DSS-induced UC mice via gu the-brain axis. In more detail, sesamol alleviated neuroinflammatory reactions by suppressing the TLR-4/NF-κB pathway, protected against oxidative stress, and upregulated the Nrf2 antioxidant signaling pathway, improving brain-derived neurotrophic factor (BDNF) by upregulating the BDNF/TrkB/CREB signaling pathway, restored synaptic impairments and enhanced norepinephrine and serotonin levels. Their correlation analysis also showed that the gut barrier and lipopolysaccharide (LPS) content in the serum were strongly associated with behavioral performance and the biochemical indexes of the brain. Similarly, sesamol also can reduce the histological damage, MPO, and nitrite content in albino rats after 1 week of treatment [472].
C) Other lignans
Apart from the lignans from flaxseed or sesame, we must emphasize the potential applications of other lignans like schisandrin, magnolol, magnolin, honokiol, arctigenin, enterolactone, enterodiol, koreanaside A and fargesin.
Schisandrin (SCH) is a lignan and one of the primary active compounds from the widely used traditional medicinal plant Schisandra chinensis with sedative, hypnotic, anti-aging, antioxidant, and immunomodulatory properties [473]. There are various types of SCH including SCH-A, B, or C. In a study [474] where mice were divided into six groups (control, model, 5-ASA, and SCH at varying doses), SCH-treated mice showed substantial weight gain, alleviated colitis severity, decreased inflammatory factors, and improved gut microbiota (GM) composition and bile acid conversion, suggesting SCH's potential as a UC treatment through the regulation of the SGK1/NLRP3 pathway and GM balance. Another study [475] demonstrated that SCH-B reduced IL-17A production in CD4+ T cells by targeting STAT3 in vitro. Notably, Sch B showed therapeutic effects on DSS-induced acute and chronic colitis and CD4+CD45RBhigh T cell-induced colitis, identifying TH17 cells as the direct target mediating its anti-inflammatory effects. The preventative effect of SCHB on UC and colitis-associated cancer was evaluated, showing that SCH-B enhanced intestinal epithelial barrier protection through FAK activation and regulates gut microbiota, both of which are crucial for its protective effects [476]. SCH-B significantly reduced TNF-α, IL-1β, INF-γ, and IL-6 concentrations and mRNA expression levels in colon tissue and inhibited phosphorylation of IκBα, NF-κB p65, p38 MAPK, c-Jun NH2-terminal kinase, and extracellular signal-regulated kinase in DSS-induced UC mice [477]. Schisandrin B demonstrated anti-inflammatory effects in both in vivo and in vitro colitis models by suppressing the NLRP3 inflammasome and inducing the AMPK/Nrf2 signaling pathway, protecting against ROS-induced mitochondrial damage and alleviating epithelial cell damage via regulation of pyroptosis [478]. SCH-C also improved intestinal permeability dysfunction across three IBD model systems by enhancing epithelial barrier integrity through upregulation of ZO-1 and occluding [479]. In Caco-2 cells, Schisandrin C reversed IL-1β-induced increases in MLCK and p-MLC expression, preventing cytoskeletal contraction and subsequent intestinal permeabilization. Additionally, Schisandrin C inhibited NF-ĸB and p38 MAPK signaling pathways, which regulate MLCK expression and tight junction complex reorganization. On the other hand, deoxyschisandrin, another lignan found in this Schisandra could ameliorate symptoms of UC, reducing the levels of inflammatory cytokines, suppressing CD4 T cell infiltration, and effectively inhibiting apoptosis in the colon of DSS-induced UC mice [480]. Another study shows that this compound demonstrated a cytoprotective effect against H2O2-induced apoptotic cell death in human intestinal epithelial cells (HCT116). Deoxyschisandrin inhibited H2O2-induced apoptosis as evidenced by Annexin V and propidium iodide flow cytometry assays, and it blocked caspase-3 activation by preventing pro-caspase-3 cleavage. Additionally, deoxyschisandrin suppressed H2O2-induced NF-kappaB activation by inhibiting IkappaBalpha degradation and NF-kappaB translocation to the nucleus, suggesting its potential as a protective agent against oxidative stress-induced intestinal cell apoptosis [481].
Magnolol and honokiol are two lignans abundantly found in Magnolia officinalis plant with pleiotropic and anti-inflammatory actions [482]. The use of Magnolia officinalis bark extract (MBE) formed by both magnolol and honokiol prevented weight loss and suppressed the activation of the proinflammatory cytokine IL6 in DSS-induced colitis. Besides, it restored the length of the damaged colon and decreased the expression of necroptosis markers in mice with DSS-induced colitis [483]. Considering their properties separately, magnolol is a lignan that decreases the activity of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α in DSS-treated mice [484]. Magnolol demonstrated dose-dependent enhancement of phagocytosis and inhibition of NO production at the concentration range of 10-40 μM, whereas, in a DSS-induced colitis model, magnolol improved colitis symptoms, including body weight loss and colon length, attenuating pro-inflammatory cytokine levels and histopathological manifestations via modulation of MAPK and NF-κB signaling pathways [485]. In rats with TNBS-induced colitis, magnolol at various doses significantly reduced colonic MPO activity and serum levels of IL-17. Histological analysis showed medium and high doses of magnolol improved DAI and thymus index, and downregulated NF-κB p65 mRNA and TLR-4 protein expression. It can also suppress IL-12 expression through modulation of NF-κB and PPAR-γ pathways and enhanced ZO-1 and occludin expression in colonic tissue [486]. Similarly, honokiol can significantly improve DAI, colon length, and histopathological scores in vivo, and reduce inflammatory mediators while enhancing TJ proteins in vitro [487]. In a mouse model of UC induced by DSS, honokiol administration suppressed proinflammatory cytokines TNF-α, IL-6, IL-1β, and IFN-γ, upregulated PPAR-γ expression, and inhibited the TLR4-NF-κB signaling pathway, attenuating gasdermin-D-mediated macrophage pyroptosis [488]. Through bioactivity-guided chromatography and molecular experiments, HON was identified as a Transient receptor potential vanilloid 4 (TRPV4) antagonist, targeting the Q239 residue to inhibit channel opening and improve endothelial permeability [489]. This mechanism enhanced VE-Cadherin expression and localization, protecting the endothelial barrier and suggesting potential therapeutic applications for alleviating IBD. Honokiol also inhibited caspase-1 activation, and apoptosis speck-like protein oligomerization, suggesting dual inhibition of NLRP3 inflammasome priming and activation processes through the modulation of the SLC3A2/L-leucine/mTORC1/NLRP3 pathways [490]. Finally, Chen et al. [490] also demonstrated that honokiol could partially protect against colitis by regulating Th17 differentiation through activating SIRT3, leading to inhibition of the STAT3/RORγt signaling pathway.
Magnolin (MGL) is another lignan produced by plants of the Magnolia genus [491]. Animal experiments demonstrated that MGL treatment mitigated weight loss, colon shortening, DA, colitis histological scores, and inflammatory factor expression [492]. MGL also improved intestinal barrier function by preserving tight junction proteins (ZO-1 and Claudin-1) and inhibiting intestinal epithelial cell apoptosis induced by TNBS and TNF-α in mice and colon organoids. Furthermore, MGL exerted its protective effects by inhibiting the PI3K/AKT signaling pathway [492] and the arachidonate 5-lipoxygenase (ALOX5)-mediated ferroptosis, inhibiting M1 while promoting M2 macrophages [493].
Arctigenin is a natural lignan extracted from Arctium lappa with potential actions in IBD.
Arctigenin significantly mitigates colitis by reducing body weight loss, DAI, and histological damage in the colon. It enhances intestinal epithelial cell recovery, reduces neutrophil and macrophage infiltration, and suppresses pro-inflammatory cytokines and oxidative stress markers while inhibiting MAPK and NF-κB pathways [494]. Similarly, this compound inhibits Th17 and Th1 cell differentiation by reducing STAT3 and STAT4 phosphorylation, respectively, and suppresses mTORC1 pathway activity independently of PI3K/AKT and ERK signaling in DSS-induced colitis mice [495]. Orally administered arctigenin alleviated colitis in mice by enhancing mucosal healing, primarily through accelerating colonic epithelial cell migration without affecting proliferation through the activation of the focal adhesion kinase (FAK) [496].
Enterolactone and enterodiol are two lignans resulting from the metabolism of other lignans by the gut microbiota [497]. Enterolactone was useful to mitigate inflammation-induced loss of intestinal epithelial barrier integrity and oxidative stress in vitro [498]. In more detail, enterolactone at 200 nM maintained or improved Trans-Epithelial Electrical Resistance (TEER) values in HCT-8 and Caco-2/RAW-264.7 coculture models, enhancing ZO-1 protein expression in HCT-8 cells under inflammatory conditions. Likewise, Koreanaside A, a lignan isolated from the flower of Forsythia koreana alleviated inflammatory responses by downregulating AP-1, NF-κB, and JAK/STAT signaling in LPS-induced macrophages and DSS-induced colitis mice [499]. Fargesin, another bioactive lignan isolated from Flos Magnoliae, has demonstrated anti-inflammatory effects on chemically induced IBD through NF-κB signaling suppression [499]. Collectively, lignans seem to be interesting compounds to be used in patients with IBDs, although further studies are still required. Table 4 summarizes the effects of the main lignans explored in this section.
2.5. Other types of polyphenols
2.5.1. Curcuminoids (Curcumina, demetoxicurcumina, bisdemetoxicurcumina)
Curcuminoids are phenolic compounds commonly used as a spice, pigment, and additive also utilized as a therapeutic agent in several foods derived from the plant Turmeric (Curcuma longa Linn) [500]. The main type of curcuminoid is the curcumin. Curcuminoids in general and curcumin, in particular, have proven to act as a potent anti-inflammatory and antioxidant agent [501]. Likewise, they can work as anti-tumor, anti-apoptotic, anti-fibrosis, or immunomodulatory agents, having been investigated in a plethora of human diseases [502]. In the context of IBDs, curcumin has presented a consistent body of evidence, acting through several mechanisms. The principal anti-inflammatory activity of this agent is through the inhibition of IBD inflammation by modulating the NF-κB pathway [503]. Furthermore, it inhibits inflammatory cascades by suppressing the COX enzyme and prostaglandin E-2 (PGE2), also influencing various molecular signaling pathways related to inflammation, apoptosis, and oxidative stress, like PPARγ, PI3K, TLR-4, Akt, mTOR, ERK5, AP-1, TGF-β, PAK1, Wnt, β-catenin, Shh, Rac1, p38MAPK, EBPα, NLRP3 inflammasome, Nrf2, Notch-1, AMPK, STAT3, and MyD-88, crucial in IBD development [504]. It also interacts with IL-1, IL-6, and IL-12, and decreases the levels of ROS having a good result in UC and CD patients [505]. Also, curcumin reduced the expression of TNF-α, IL-6, and IL-17 while increasing the anti-inflammatory cytokine IL-10 [506]. In parallel, curcumin also modulates iron metabolism proteins and reduces iron stores in a DSS-induced colitis mouse model, which should be considered in IBD management by monitoring erythroid parameters [507]. It may also interact with transient receptor potential vanilloid receptor 1 (TRPV1), attenuating visceral hyperalgesia and colitis [508]. Finally, curcumin has also the ability to regulate autophagy, reducing the expression of key autophagy-related genes such as Beclin-1, ATG5, and LC3II, while increasing B cell lymphoma 2 (bcl-2), thereby improving colitis [509]. Interestingly, preclinical models have shown that curcumin can decrease the expression of Th1 cytokines (IL-12, IFN-gamma, TNF-alpha, IL-1) and increase the expression of Th2 cytokines (IL-4 and IL-10) in colon mucosa, promoting a shift from Th1 to Th2 [510]. It should also be noted that curcumin use has some important challenges that need to be addressed. For instance, it is known that curcumin is unstable under physiological conditions and the bioavailability after oral administration of this compound is very low [511]. Following oral administration, curcumin is quickly metabolized through reduction, sulfation, and glucuronidation in the liver, kidneys, and intestinal mucosa, leading to low intestinal absorption. The most common strategy to improve curcumin's poor pharmacokinetic profile involves combining it with piperine, a natural alkaloid found in black pepper (Piper nigrum), which effectively inhibits the glucuronidation process [512]. To enhance its properties, researchers have also explored various approaches, including the development of compounds, the use of liposomes, and the synthesis of nanoparticles or curcumin analogs [513]. Therefore, curcumin has the potential to modulate IBDs through several mechanisms described in preclinical models. However, distinctively compared to other polyphenols, curcumin stands out as the most extensively studied component in clinical trials and human studies. In the following subsection, the principal studies conducted in this field and their most relevant conclusions will be summarized.
Intervention studies in humans
Curcuminoids represent one of the most broadly explored polyphenols in the management of multiple diseases in humans, including in IBD. Indeed, various doses of curcumin have been tested and explored in patients with IBDs (i.e. 550 mg /three times daily-1 per month, and 1 g /twice times a daily-6 month) showing promising results that remain to be fully explored [514]. In this subsection, the design and results obtained from the main clinical trials will be presented.
Lignans explored in the context of inflammatory bowel diseases (IBDs)
| Subgroups | Compounds | Dietary/Exogenous sources | Mechanisms of action (Preclinical models) | Studies in humans (including observational and intervention studies | References |
|---|---|---|---|---|---|
| Lignans | Flaxseed and Secoisolariciresinol diglucoside | Flaxseed and flaxseed oil | Reduces mortality and colonic ulcers, while aqueous-methanolic crude extracts (Fs.Cr) enhance mucin content and exhibit anti-inflammatory, antispasmodic, and antibacterial effecs. Phenolic compounds from flaxseed and SGD also reduce inflammation and improve mucosal repair in colitis models | Human studies on flaxseed oil found that flaxseed supplementation benefits UC patients. In a 12-week trial with 75 UC patients, both 30 g/day of grounded flaxseed (GF) and 10 g/day of flaxseed oil (FO) significantly reduced inflammatory markers, disease severity, blood pressure, and waist circumference compared to placebo. Additional trials showed improvements in metabolic syndrome parameters, inflammatory markers, and quality of life. However, long-term studies are needed for definitive conclusions | [449-461] |
| Sesame seeds, sesamin, and sesamol | Sesame seeds, sesamin, and sesamol | Have shown notables benefits in treating IBD in preclinical models. Sesame oil and sesame cake reduce inflammation, fibrosis, and oxidative stress, while sesamin and sesamol specially mitigate UC symptoms by modulating inflammatory and oxidative pathways, enhancing gut barrier function, and improving both physical and mental health in affected animals | - | [462-472] | |
| Schisandrin | Schisandra chinesis | Show promise in treating UC. SCH treatments alleviate colitis severity, improve gut microbiota, and modulate inflammatory pathways, including SGK1/NLRP3 and NLRP3 inflammasome. Specifically, Schinsandrin B reduces pro-inflammatory cytokines and enhances epithelial barrier protection, while Schisandrin c improves intestinal permeability and Deoxyschinsandrin offers cytoprotection against oxidative stress-induced cell apoptosis | - | [474-480] | |
| Magnolol and Honokiol | Magnolia officinalis | Magnolol reduces pro-inflammatory cytokines and improves colitis symptoms by modulating MAPK and NF-κB pathways, while honokiol enhances epithelial barrier integrity, reduces cytokines, and inhibits NLRP3 inflammasome activation | - | [482-492] | |
| Magnolin | Magnolia genus | Animal studies showed that MGL treatment alleviates weight loss, colon shortening, disease activity, and inflammation in colitis models. MGL also enhances intestinal barrier functions by preserving tight junction proteins and preventing epithelial cells apoptosis | - | [491-493] | |
| Arctigenin | Arctium lappa | Alleviates colitis by reducing body weight loss, disease activity index, and colon histological damage. It promotes intestinal epithelial cell recovery, decreases immune cell infiltration and suppresses inflammation and oxidative stress by inhibiting MAPK and NF-κB pathways | - | [494-496] | |
| Enterolactone and enterodiol | Resulting from the metabolism of other lignans by the gut microbiota | Mitigates inflammation-induced loss of intestinal epithelial barrier integrity and oxidative stress in vitro | - | [497,498] | |
| Koreanaside A | Forsythia koreana | Alleviates inflammatory response by downregulating AP-1, NF-κB, and JAK/STAT signaling in LPS-induced macrophages and DSS-induced colitis mice | - | [499] | |
| Fargesin | Flos magnoliae | Anti-inflammatory effects on chemically induced IBD through NF-κB, signaling suppression | - | [499] |
Most studies evaluating curcuminoids in humans have been conducted in UC patients. In a randomized, double-blind, multicenter trial [515], 89 patients with quiescent UC were given either curcumin (2g/day) plus sulfasalazine or mesalamine, or a placebo plus sulfasalazine or mesalamine for 6 months. Only 4.65% of the curcumin group relapsed compared to 20.51% in the placebo group, with significant improvements in both clinical activity index (CAI) and endoscopic index (EI) for the curcumin group. During a 6-month follow-up, 8 additional patients in the curcumin group and 6 in the placebo group relapsed, suggesting curcumin is a promising and safe maintenance therapy for UC remission. In another randomized, double-blind pilot trial involving 45 patients with mild-to-moderate distal UC participants received either NCB-02 (standardized curcumin preparation) enema plus oral 5-ASA or placebo enema plus oral 5-ASA. After 8 weeks, the NCB-02 group showed a 56.5% treatment response compared to 36.4% in the placebo group, with clinical remission in 43.4% versus 22.7% and endoscopic improvement in 52.2% versus 36.4%. Per protocol analysis indicated significantly better outcomes for the NCB-02 group in clinical response, clinical remission, and endoscopic improvement, suggesting that NCB-02 may offer greater improvements in disease activity for patients with mild-to-moderate distal UC [516]. Lang et al. [517] developed a multicenter randomized, placebo-controlled, double-blind study of 50 mesalamine-treated patients with active mild-to-moderate UC. Curcumin (3 g/day) was given to 26 patients while 24 received a placebo for one month. Clinical remission (measured by Simple Clinical Colitis Activity Index- SCCAI ≤2) was achieved by 53.8% of the curcumin group compared to none in the placebo group, and clinical response (≥3 point reduction in SCCAI) occurred in 65.3% of the curcumin group versus 12.5% in the placebo group. Additionally, endoscopic remission (partial Mayo score ≤1) was seen in 38% of curcumin-treated patients compared to none in the placebo group, with adverse events being rare and similar between the groups. Similarly, Salomon et al. [518] conducted a placebo-controlled double-blind study to assess the efficacy of curcumin in inducing remission in 5-ASA treatment-resistant patients with mild-to-moderate UC. 50 patients with SCCAI scores between 5-12 were randomized to receive either 3 g of curcumin or placebo daily for 30 days alongside maximal 5-ASA therapy. Clinical remission, defined as SCCAI ≤2, was achieved in 54% (14/26) of curcumin-treated patients compared to none in the placebo group. A significant clinical response (≥3-point decrease in SCCAI) was observed in 65.3% of curcumin-treated patients versus 12.5% in the placebo group (P < 0.001, OR 13.2). Endoscopic remission (partial Mayo score ≤1) was achieved by 36% (8/22) of curcumin-treated patients and none in the placebo group (P = 0.035, OR 23.5). These findings highlight curcumin as a promising adjunct therapy for mild-to-moderate active UC, demonstrating efficacy in both clinical and endoscopic outcomes without notable side effects.
Simultaneously, in a randomized, double-blinded controlled trial involving 56 adults diagnosed with mild to moderate UC based on the SCCAI, participants were randomly assigned to receive either curcuminoids nanomicelles (80 mg, three times daily, orally) plus mesalamine (3 g/day, orally) or placebo plus mesalamine for four weeks [519]. Assessments at baseline and weeks 2 and 4 showed a significant reduction in the urgency of defecation and improved general condition in the curcuminoid nanomicelles group compared to the placebo group. Additionally, the mean SCCAI score was significantly lower in the curcuminoid nanomicelles group at week 4 (1.71 ± 1.84) compared to the placebo group (2.68 ± 2.09, p = 0.050). This study suggests that adding curcuminoid nanomicelles to standard mesalamine therapy improves symptoms and reduces clinical activity in patients with ulcerative colitis In another clinical trial, Banerjee et al. [520] randomized mild to moderately active UC patients on standard mesalamine to receive either 50 mg twice daily bio-enhanced curcumin (BEC) or a placebo. At 6 weeks, clinical and endoscopic remission rates were 44.1% and 35.3% in the BEC group, respectively, compared to none in the placebo group, with significantly higher clinical response in the BEC group (52.9% vs. 14.3%). At 3 months, the BEC group had clinical remission, clinical response, and endoscopic remission rates of 55.9%, 58.8%, and 44%, respectively, compared to 5.7%, 28.6%, and 5.7% in the placebo group, with 95% and 84% of BEC responders maintaining remission at 6 and 12 months, and no significant side effects observed. In a randomized double-blind clinical trial conducted by Sadeghi et al. [521], 70 patients with mild-to-moderate UC were assigned to receive either curcumin (1,500 mg/day) or a placebo for 8 weeks. Curcumin supplementation significantly improved the SCCAI and scores on the Inflammatory Bowel Disease Questionnaire-9, enhancing quality of life Additionally, curcumin reduced serum hs-CRP levels and erythrocyte sedimentation rate (ESR) compared to placebo, highlighting its potential benefit as an adjunct therapy for mild-to-moderate UC alongside conventional treatments. Very recently, Ben-Horin et al. [522] evaluated the efficacy of the herbal combination of curcumin-QingDai (CurQD) in patients with active UC. The study consisted of two parts: In Part I of the study, an open-label trial evaluated the initial efficacy of CurQD in patients with active UC identified by specific clinical and endoscopic criteria. Part II involved a placebo-controlled trial where active UC patients were randomized in a 2:1 ratio to receive enteric-coated CurQD 3 g/d or placebo for 8 weeks, with continuation of maintenance therapy for responders. Then they focused on clinical response and endoscopic improvement. Part I demonstrated promising initial responses, with 70% achieving clinical improvement. Part II confirmed the efficacy of CurQD, showing significantly higher rates of clinical response (85.7% vs. 30.7%) and remission (50% vs. 8%) compared to placebo, alongside increased mucosal expression of CYP1A1, suggesting potential therapeutic benefits via the aryl-hydrocarbon receptor pathway. Also and as aforementioned, one study [445] analyzed the effects of the MD combined with curcumin and resveratrol supplementation on disease activity, serum inflammatory markers, and quality of life in patients with mild to moderate UC, showing that these interventions effectively reduced disease activity and inflammation while improving quality of life in UC patients.
Other studies have also found positive results regarding the role of curcumin in the management of CD. In a pilot study conducted by Holt et al. [523] five patients with ulcerative proctitis and five with CD were enrolled. Patients with ulcerative proctitis were treated with 550 mg of curcumin twice daily for one month, followed by 550 mg three times daily for another month, while CD patients received 360 mg of curcumin three times daily for one month and then four times daily for the remaining two months. Assessments included blood tests, inflammation markers (sedimentation rate and C-reactive protein), sigmoidoscopies, and biopsies at baseline and the end of the study. Results showed improvements in symptoms and reduced medication use in ulcerative proctitis patients, while CD patients exhibited lowered DAI scores and sedimentation rates following curcumin therapy. Theracurmin®, a highly bioavailable curcumin derivative with potent anti-inflammatory properties, was evaluated in a randomized, double-blinded study involving patients with active mild-to-moderate CD [523]. Administered at 360 mg/day for 12 weeks, Theracurmin® demonstrated significant reductions in clinical disease activity by week 12 (p = 0.005) and achieved clinical remission rates of 35%, 40%, and 40% at weeks 4, 8, and 12, respectively. Endoscopic remission rates and healing of anal lesions were also notable in the Theracurmin® group compared to placebo, with no serious adverse events reported. In a clinical trial conducted in a pediatric population [524], children with CD or UC in remission or with mild disease (Pediatric Crohn's Disease Activity Index [PCDAI] <30 or Pediatric Ulcerative Colitis Activity Index [PUCAI] score <34) received curcumin alongside standard therapy. Starting at 500 mg twice daily for 3 weeks, doses were escalated to 1 g twice daily at week 3 and to 2 g twice daily at week 6 using a forced-dose titration design. Measures of disease activity (PUCAI/PCDAI) and side effects were assessed at weeks 3, 6, and 9. Curcumin was well tolerated overall, with increased gassiness reported by only 2 patients consistently across visits. This study suggested that curcumin could be considered as adjunctive therapy alongside conventional treatments for patients seeking combined conventional and alternative medicine approaches.
Despite these positive results, some studies have failed to find significant benefits from curcumin in the management of IBD. A double-blind randomized controlled trial across 8 centers in France included 62 patients with CD post-bowel resection who received azathioprine and were randomly assigned to either oral curcumin (3 g/day) or placebo for 6 months [525]. The evaluation included a colonoscopy, CD activity index, laboratory tests, and quality of life questionnaires. At month 6, postoperative recurrence (Rutgeerts' index score ≥i2) was observed in 58% of the curcumin group versus 68% of the placebo group, with a higher incidence of severe recurrence (Rutgeerts' index score ≥i3) in the curcumin group. Clinical recurrence rates and quality of life scores did not significantly differ between groups. Severe adverse events were reported in 6% of the placebo group and 16% of the curcumin group. The study concluded curcumin showed no superior efficacy over placebo in preventing CD recurrence post-surgery with thiopurine treatment. Likewise, Kedia et al. [526] conducted an 8-week randomized clinical trial to investigate the effectiveness of oral curcumin in achieving clinical remission among patients with mild-to-moderate ulcerative colitis. Participants were assigned to receive either mesalamine combined with curcumin (450 mg/day) or mesalamine with a placebo. The study outcomes showed no significant differences between the curcumin and placebo groups in terms of rates of treatment failure, mucosal healing, clinical response, or clinical remission by the end of the trial. These findings suggest that a daily dose of 450 mg of curcumin did not successfully induce remission in patients with mild-to-moderate ulcerative colitis.
Based on the collective findings from various clinical trials exploring the efficacy of curcuminoids in IBD, including UC and CD, several conclusions can be drawn. Curcuminoids, investigated at varying doses and formulations, demonstrate promising potential in managing IBD, particularly in inducing and maintaining remission in patients with mild-to-moderate disease activity. Studies have consistently shown significant improvements in clinical response, endoscopic remission, and reduction of inflammatory markers with curcumin supplementation, suggesting its role as an effective adjunct therapy alongside conventional treatments. Systematic reviews and meta-analysis agree that the use of curcumin and curcuminoids in the management of both UC and CD show promising results, with a greater level of evidence in the case of the former [527]. However, they also sustain that there is a need to standardize the dose and the formulations of curcumin, the time of treatment, and the route of administration before drawing definitive conclusions [528]. Moreover, conflicting results from certain trials underscore the variability in outcomes and highlight the need for further large-scale, multicenter studies to validate these findings.
2.5.2. Polyphenols from olives and extra virgin olive oil
Olives and EVOO stand out as one of the most representative foods of MedDiet. EVOO contains over 30 phenolic compounds, including the most represented oleuropein (OLE), both in the glycated and in the aglycone form, hydroxytyrosol (HT), and others like verbascoside, oleocanthal or oleachin [529]. Olives and EVOO act as potent immunomodulatory, antioxidant, antiapoptotic, anti-inflammatory, and antimutagenic agents, with significant benefits in the different organs and systems of the body, including the digestive system and the gut [530,531]. Numerous preclinical studies have exhibited solid evidence of the mechanisms by which polyphenol-rich EVOO or specific polyphenols isolated from these compounds provide their anti-inflammatory, antioxidant, antitumor, and microbiota-modulation effects whereas some human studies that explored the effects of EVOO on patients with IBD further support its relevance [532]. Herein we will summarize the main evidence around EVOO, HT, and oleuropein conducted in the field of IBDs.
A) Extra virgin olive oil
Preclinical models conducted in vitro and in vivo have shown multiple roles from EVOO in the management of IBD. The impact of olive oil phenolic extract in the inflammatory response was evaluated by Muto et al. [533] in Caco-2 cells treated with LPS or IL-1β. This compound was found to prevent IL-8 expression and secretion in LPS-treated cells, while in IL-1β-treated cells, it was able to inhibit IL-8 promoter activity but enhanced IL-8 mRNA stability, leading to increased protein expression, with involvement of the p38 and ERK signaling pathways. Also, different variants of EVOO from Spain were shown to exert protective effects on cell integrity and a reduction in ROS production in Caco-2 cells, highlighting the health benefits of the Picual variety [534]. Likewise, antioxidant polyphenols from EVOO can decrease cytotoxicity and ROS production in Caco-2 cells exposed to alternariol, a mycotoxin that can contaminate olives [535]. In animal models, EVOO diets exerted a noteworthy beneficial effect in chronic DSS-induced colitis by cytokine modulation and COX-2 and iNOS reduction via downregulation of p38 MAPK, with greater results if the diet is supplemented with HT [536]. In another study [537], mice on an EVOO+polyphenol extract (PE) diet showed significantly reduced DAI, cell proliferation, and levels of MCP-1, TNF-α, COX-2, and iNOS compared to the control group, as well as down-regulated JNK phosphorylation, prevented IκBα degradation, and PPARγ deactivation. They concluded that EVOO-PE supplementation effectively mitigates experimental colitis through PPARγ up-regulation and inhibition of nuclear transcription factor-kappa B and MAPK signaling pathways. Similarly, Takashima et al. [538] showed the effect of chronic intake of 5% EVOO on inflammation, cell proliferation, and signal transducers and activators of transcription (STAT) in a DSS-induced colitis rat model. EVOO significantly attenuated inflammation as assessed by disease activity index, body weight loss, and histological scores. It also reduced the expressions of STAT3, phosphorylated STAT3 (pSTAT3), COX-2, and iNOS induced by DSS, while mitigating increases in cell proliferation (PCNA) and restoring apoptosis (cleaved caspase-3).
Despite these favorable results, other studies have failed to find any significant benefits from EVOO in the management of IBD. HLA-B27 transgenic rats fed diets containing corn oil (CO), EVOO with high phenolic content, or olive oil with low phenolic content (ROO) for 3 months showed that CO-induced intestinal inflammation characterized by diarrhea, increased myeloperoxidase activity, and mucosal injury, which was not mitigated by EVOO [539]. Nonetheless, EVOO significantly reduced TNFα gene expression in the colon mucosa and lowered total cholesterol levels compared to CO-fed rats, suggesting potential benefits in managing hypercholesterolemia and minimizing statin-associated myotoxicity. Another work [540] investigated the effects of dietary interventions with EVOO and fish oil (FO) on a DSS-induced colitis mouse model. Despite dietary supplementation with EVOO, FO, or a combination of both, none of the interventions ameliorated symptoms or inflammatory markers associated with colitis. Additionally, mice supplemented with FO showed an increased spleen weight-to-body weight ratio, while the combination of EVOO and FO led to elevated TNF-α levels compared to controls.
Intervention studies in humans
To date, few clinical trials have been conducted evaluating the effectiveness of EVOO and their polyphenols in the clinical management of IBD. In a crossover clinical trial involving forty patients with UC, Morvaridi et al. [541] showed that consumption of EVOO significantly decreased erythrocyte sedimentation rate and hs-CRP. Gastrointestinal symptoms including bloating, constipation, fecal urgency, incomplete defecation, and overall gastrointestinal symptom severity (GSRS) were also notably reduced after EVOO consumption. In a randomized, controlled, double-blind, crossover trial involving 12 participants [542], ingestion of three raw virgin olive oils differing in phenolic compound (PC) content was assessed over 3-week periods with intervening washout periods. PC-enriched virgin olive oil containing a mixture of olive oil and thyme (FVOOT) significantly decreased serum ox-LDL levels and increased bifidobacteria populations and phenolic metabolite protocatechuic acid compared to baseline and to virgin olive oil naturally containing PC (VOO) (P < 0.05). These findings suggest that PC-enriched olive oils, particularly FVOOT, may confer cardioprotective benefits through modulation of microbial populations and antioxidant metabolites. It must be highlighted that despite additional studies in humans are still required, the inclusion of EVOO and other food sources contained in MedDiet has proven significant benefits in patients with IBDs. Following 6 months of MedDiet resulted in improved BMI, reduced waist circumference, and decreased prevalence of liver steatosis in both UC and CD patients [543]. Furthermore, fewer patients experienced active disease and elevated inflammatory biomarkers after adhering to the MedDiet, indicating potential benefits for managing IBD-related symptoms and metabolic health. In a prospective, randomized controlled trial from 2017 to 2021 involving adults with quiescent ulcerative colitis (UC), participants were assigned to either a Mediterranean diet pattern (MDP) or a control high-fiber diet (CHD) for 12 weeks [544]. The MDP group showed higher tolerance and significantly lower levels of fecal calprotectin (>100 μg/g) compared to the CHD group (75% vs. 20%). Additionally, the MDP group exhibited increased levels of total fecal short-chain fatty acids (SCFAs), specifically acetic acid and butyric acid, and favorable changes in gut microbiota associated with protective roles in colitis, such as Alistipes finegoldii and Flavonifractor plautii, as well as SCFA-producing Ruminococcus bromii. These findings suggest that the MDP may serve as a beneficial and sustainable dietary approach for maintaining clinical remission and managing UC. Importantly, MedDiet shows similar benefits to other well-established diets like Specific Carbohydrate Diet (SCD) to achieve symptomatic remission and regulate different clinical variables, defining the adherence to a diet as the most important factor to maximize the benefits from these approaches [545].
Overall, despite more clinical trials and human studies specifically focusing on EVOO and its polyphenols are warranted, the inclusion of this food and nutrients in MedDiet is a promising translational strategy for patients with IBDs.
B) Hydroxytyrosol
HT (3,4-dihydroxyphenyl-ethanol) is one of the most important compounds found in EVOO and is broadly studied in preclinical models of IBD. Elmaksoud et al. [546] investigated the therapeutic potential of olive leaf extract standardized with 25% hydroxytyrosol (OLES-25%HYT) in treating induced ulcerative colitis in albino rats. Compared to untreated ulcerative colitis rats, OLES-25%HYT significantly reduced mortality rate and DAI while also decreasing oxidative stress markers such as MDA, MPO, and NO and increasing antioxidant enzymes including SOD, CAT, and GPX in colon tissue. Additionally, OLES-25%HYT downregulated pro-inflammatory cytokines and the apoptotic gene Bax, while upregulating the anti-apoptotic gene Bcl2, demonstrating its intestinal anti-inflammatory, antioxidant, and anti-apoptotic effects in experimental ulcerative colitis models. Mao [547] also showed that HT supplementation ameliorated colon pathology and apoptosis, increased antioxidant capacity, and reduced expression of NLRP3 inflammasome components and pro-inflammatory cytokines in the DSS-induced colitis model. Additionally, HT promoted a favorable shift in gut microbiota composition towards probiotics and increased levels of short-chain fatty acids, highlighting its potential therapeutic benefits in ulcerative colitis through multiple mechanisms. HT is also able to counteract the effects of Benzo[a]pyrene (B[a]P) in human colonic epithelial cells (HCoEpC), decreasing the production of pro-inflammatory cytokines and ERK1/2 and mTOR activation while promoting autophagy and mitophagy [548]. HT alone or combined with pectin/alginate and olive oil seems to be effective against inflammation in TNBS-induced colitis, alleviating inflammatory infiltration [549]. In vivo and in vitro models show that medium-chain triglycerides led to greater damage and colitis symptoms, while the use of HT with eicosapentaenoic acid and docosahexaenoic acid significantly reduced damage and inflammation, as well as the combined use of fish and olive oil [550]. Sanchez-Fidalgo et al. [551] divided six-week-old mice into three dietary groups: standard, EVOO, and HT-enriched EVOO, and then induced with colitis using 3% DSS. Diets enriched with EVOO significantly reduced clinical and histological damage, decreased mortality by about 50%, and improved cytokine profiles, including maintaining TNF-α near control levels and increasing IL-10, while downregulating COX-2 and iNOS. HT supplementation further enhanced these benefits, especially in reducing iNOS levels and adding antioxidant effects, suggesting improved outcomes for chronic colitis management.
C) Oleuropein
OLE is a secoiridoid, an ester of elenolic acid and HT with a oleosidic skeleton. Past works have remarked on the potential translational role of OLE in the context of IBDs. An experimental study using rat models of UC compared the effects of this compound with normal controls and untreated colitis-induced [552]. Colonic tissue analysis showed OLE significantly reduced oxidative stress markers (MDA, MPO, NO) and increased antioxidant enzyme levels (SOD, CAT, GPX) while downregulating pro-inflammatory cytokines and the pro-apoptotic gene Bax and upregulating the anti-apoptotic gene Bcl2. OLE demonstrated significant intestinal anti-inflammatory, antioxidant, and anti-apoptotic effects, reducing both the mortality rate and disease activity index in ulcerative colitis. In another work, the anti-inflammatory effects of OLE in a mouse model of chronic colitis induced by DSS were evaluated [553]. Mice receiving a diet supplemented with 0.25% OLE for 56 days showed reduced inflammatory symptoms, improved disease activity index, and histopathological changes, alongside decreased inflammatory cell recruitment and lower levels of IL-1β and IL-6, with increased IL-10 in colon tissue. OLE also reduced COX-2 and iNOS expression, suppressed p38 MAPK phosphorylation, potentially upregulated annexin A1, and improved intestinal wound healing, indicating its promise as a treatment for UC. Biopsies from 14 patients with active UC were cultured with Escherichia coli lipopolysaccharide (EC-LPS) and OLE OLE treatment significantly reduced the expression of cyclooxygenase-2 (COX-2) and interleukin-17 (IL-17), as well as IL-17 levels in culture supernatants, compared to EC-LPS treatment alone. Histological analysis showed that OLE-treated samples had reduced inflammatory cell infiltration and improved inflammatory damage, demonstrating OLE's anti-inflammatory activity in UC colonic biopsies [554].
2.5.3. Coumarins
Coumarins are a class of benzopyrones, consisting of a benzene ring fused to an alpha-pyrone ring, also entailing important effects on human health and promising translational implications [555]. Coumarins can be found in vegetables, spices, fruits, and medicinal plants including all parts of the plants-fruits, roots, stems and leaves; however cinnamon stands out as one of the edible foods with the highest concentration of coumarins [556]. The main types of coumarins include esculetin, aesculin, umbelliferone, fraxetin, daphnetin, coumarin, and paepalentin.
Esculetin is a coumarin compound derived from the bark of Fraxinus chinensis Roxb and its glycoside form is called aesculin. Both compounds are characterized by exerting potent antioxidant and anti-inflammatory properties [557]. Esculetin, administered at 5 mg/kg in a rat model of TNBS-induced IBD, demonstrated potent intestinal anti-inflammatory effects, preventing an increase in MDA levels, countering GSH depletion, reducing epithelial cell apoptosis, and inhibiting the secretion of pro-inflammatory cytokines (IL-1β, IL-2, IFN-γ) in vitro. In vivo, esculetin reduced colonic TNF-α and IL-1β levels and inhibited MPO and alkaline phosphatase activities, being effective at a 10-fold lower dose than sulphasalazine and showed comparable efficacy to prednisolone [558]. Using network pharmacology and molecular docking, one study [559] identified 50 potential gene targets of esculetin against UC, including core genes such as AKT1, STAT1, CCND1, and PTGS2. Pathway enrichment analysis revealed the prolactin (PRL) signaling pathway as crucial in esculetin's action against UC, supported by strong molecular docking affinity to PRL and its receptor. Another research explored the role of esculetin and 4-methyl-esculetin in a rat model of colitis induced by TNBS [560]. Esculetin reduced lesion extension, and diarrhea incidence, and restored GSH levels in acute colitis, whereas 4-methyl-esculetin seemed to exert a superior efficacy, also inhibiting MPO and alkaline phosphatase activities, beneficial in acute and relapse colitis models likely due to its methyl group at C-4. Indeed, more studies have proven the efficacy of 4-methyl-esculetin in IBD models.
In a TNBS-induced rat colitis model, 4-methyl-esculetin exhibited efficacy comparable to prednisolone and sulfasalazine, reducing macroscopic damage scores, restoring intestinal architecture, and preventing GSH depletion and alkaline phosphatase activity [561]. During colitis relapse, it improved histological inflammation and biochemically inhibited MPO, alkaline phosphatase, and metalloproteinase 9 activities, while also decreasing MDA and IL-1β levels. In vitro, 4-methyl-esculetin inhibited IL-1β, IL-8, IL-2, and IFN-γ production, indicating its potential as a potent intestinal anti-inflammatory agent. Similar results were obtained in DSS-induced colitis mice, as 4-methyl-esculetin administered orally at 25 mg/kg daily, improved microscopic parameters, reduced MPO activity, lowered colonic IL-6 levels, and prevented GSH depletion compared to the DSS-control group [562].
On the other hand aesculin demonstrated minimal cytotoxicity in vivo and in RAW264.7 macrophages, significantly alleviated DSS-induced colitis symptoms, and reduced inflammatory factors such as iNOS, IL-1β, and TNF-α in both peritoneal macrophages and colonic tissues [563]. Importantly, aesculin attenuated NF-κB signaling activity and enhanced PPAR-γ nuclear localization in DSS-induced mice and LPS-stimulated macrophages, suggesting its therapeutic potential in UC through modulation of these pathways.
Other types of coumarins have also shown significant benefits in preclinical models of IBD. Witaicenis et al. [564] evaluated six coumarin derivatives of plant origin (scopoletin, scoparone, fraxetin, 4-methyl-umbelliferone, esculin, and daphnetin) in TNBS-induced colitis in rats. They reported that treatment with aesculin, scoparone, and daphnetin produced the best protective effects. In their experiments, all coumarin derivatives showed antioxidant activity in the DPPH assay, while daphnetin and fraxetin also showed antioxidant activity by inhibiting lipid peroxidation. Coumarins, except for 4-methyl-umbelliferone, also showed antioxidant activity through the counteraction of GSH levels or the inhibition of MPO activity. He et al. observed that daphnetin effectively alleviated colitis severity in mice by improving intestinal structure and increasing levels of ZO-1, occludin, and BCL-2, while reducing Bax and cleaved caspase 3 expression [565]. Daphnetin also suppressed MDA and SOD activities, inflammatory cytokine levels, and apoptosis in both in vivo and in vitro models. Mechanistically, daphnetin inhibited JAK2/STAT signaling via REG3A activation, highlighting its potential therapeutic role in UC treatment by targeting these pathways [565]. Fraxetin mitigated DSS-induced symptoms including body weight loss, colon shortening, tissue damage, and DAI. Mechanistically, this compound can inhibit the NF-κB pathway and NLRP3 inflammasome activation, reducing inflammatory responses, restoring gut barrier function by enhancing goblet cells and tight junction proteins (ZO-1 and occludin) and modulating intestinal microbiota diversity [566].
Umbelliferone (UMB), a potent coumarin derivative with antioxidant and anti-inflammatory properties, was investigated in an acetic acid-induced UC rat model [567]. UMB (30 mg/kg, oral) significantly improved macroscopic and histological tissue damage, reduced colonic TNF-α, IL-6, MPO, and VCAM-1 levels, and downregulated TLR4, NF-κB, and iNOS gene and protein expression, indicating strong anti-inflammatory effects. Additionally, UMB upregulated SIRT1 and PPARγ signaling pathways, mitigating oxidative injury and inflammation [568].
Paepalantine (9,10-dihydroxy-5,7-dimethoxy-1H-naptho(2,3c)pyran-1-one) is an isocoumarin previously isolated from the capitula of the Brazilian endemic Paepalanthus bromelioides. Administered at doses of 5 and 10 mg/kg, paepalantine significantly mitigated TNBS-induced colonic damage in both intact mucosa and mucosal recovery settings, as demonstrated histologically and biochemically [569]. This protective effect was attributed to paepalantine's ability to enhance colonic oxidative status by preventing glutathione depletion and inhibiting colonic nitric oxide activity.
Lastly, Coumarin and its derivate, 4-hydroxycoumarin were evaluated in TNBS-induced colitis models of rats [570]. Both compounds, administered at doses of 5 and 25 mg/kg, significantly reduced colonic damage, observed macroscopically, microscopically, and biochemically. This protective effect was associated with the prevention of GSH depletion due to colonic inflammation. Overall, the literature supports the relevance of coumarins and derivates in preclinical models of IBD, although studies in humans to confirm their applications are warranted.
2.5.4. Flavonolignanes (Silimarin)
Flavonolignans are the major bioactive components presented in the Milk thistle (Silybum marianum) mostly known for their hepatoprotective properties, but also standing out for their immunomodulatory, antioxidant, and antitumoral role [571]. Flavonolignans in S. marianum (collectively known as silymarin) are structurally diverse, with 23 constituents being isolated from purple- and white-flowering variants. Flavonolignans, compounds found in silymarin, are often mischaracterized by their name, as they are not composed of separate flavonoid and lignan units. Instead, they are biogenetically related to lignans and neolignans, sharing similar biosynthetic pathways, and are derived from two phenylpropanoid units with an additional structure that classifies them under flavonoids [572]. These compounds exhibit a wide structural diversity due to various linkages of the C6C3 unit to the flavonoid nucleus, often forming dioxane, furan, cyclohexane rings, or simple ether side chains, and they frequently exist as stereoisomers in nature. The 3 more important isomers are silybin (or silibinin), silydianin, and silychristin, being the former the most active of these compounds [573].
Silymarin (SM) is a potent anti-inflammatory molecule capable of inhibiting NF-κB pathways and optimizing the redox balance in the cell through activating antioxidant enzymes and non-enzymatic antioxidants via Nrf2 activation [574]. Also, some studies have remarked on the relevance of SM and quercetin as non-toxic and safe bioactive compounds with a marked antiviral activity, being suggested as a potential tool against viral-associated IBD [575]. Mechanistically, the protection and antiviral effects of these bioactive compounds include a decrease in oxidative damage, inhibition of viral binding and replication, RNA synthesis, caspase enzymes, viral proteases, and viral assembly. Another study [576] investigated the effects of SM and L-arginine (L-Arg) on IBD progression in a TNBS-induced colitis rat model, finding that both treatments ameliorated colitis symptoms, decreased serum TNF-α levels, inhibited colonic iNOS, NF-κB, and cytochrome c expression, and increased HSP70 expression. The oral bioavailability of SM is poor due to rapid metabolism, low intestinal permeability, and poor water solubility; and varies significantly between species and is influenced by the methods of preparation [574]. Because of this, some studies have developed different systems to improve silymarin's bioavailability, with promising results obtained when compared to conventional approaches [577,578].
Silibin has also proven significant benefits in preclinical models of IBD. A rat model of colitis induced by TNBS to evaluate the anti-inflammatory effects of silibinin and ursodeoxycholic acid (UDCA), alone and in combination, with dexamethasone as a control. The treatments significantly reduced NF-κB activity, IL-1β, TNF-α, TBARS, protein carbonyl, and MPO levels, while enhancing antioxidant power, with combination therapy showing the most notable improvements [579]. Chemopreventive effects of silibin were evaluated on colitis-associated cancer (CAC) mouse model, finding that this component significantly inhibited intestinal tumor cell viability, reduced tumor size and number, decreased colitis and tumor scores, and promoted apoptosis while inhibiting proliferation [580]. Additionally, silibinin reduced inflammatory cytokine production, improved the colonic mucosal barrier, and suppressed STAT3 phosphorylation, indicating its potential as a chemopreventive agent against CAC.
Intervention studies in humans
A randomized, double-blinded, placebo-controlled clinical trial involving 80 UC patients in remission was conducted from September 2009 to October 2010 [581]. Patients were randomly assigned to receive either SM (140 mg daily) or placebo (composed of lactose monohydrate, corn starch, and magnesium stearate) alongside their standard therapy for 6 months. Ten patients discontinued the study due to adverse effects (4 in the SM group) or disease flare-ups (6 in the placebo group). The SM group showed significant improvements in hemoglobin levels and erythrocyte sedimentation rate, as well as a decrease in DAI. After 6 months, 35 out of 38 patients in the SM group remained in complete remission without flare-ups, compared to 21 out of 32 patients in the placebo group. These findings suggest that SM supplementation may effectively help maintain remission in UC patients. Overall, table 5 summarizes the effects of the rest of the polyphenols explored in this section.
3. Understanding the precise role of polyphenols as part of an integrative therapy in IBD
As explained before IBDs, represented by UC and CD, are two major inflammatory conditions potentially characterized by an abnormal mucosal immunological response associated with a heterogeneous phenotypic spectrum, triggered by gastrointestinal and extraintestinal affectations, or with atypical or non-specific symptoms [582]. Although conventional treatments have been shown to be effective in controlling symptoms, many patients experience significant side effects derived from their use, and a percentage of them do not respond adequately to these therapies [583]. In this context, the use of natural compounds, such as dietary polyphenols could be of great aid to avoid the negative effects of normal therapies, acting as complementary therapies from the available armamentarium [584]. Throughout the present manuscript, the relevance of the main types of polyphenols and their mechanisms of action in IBDs have been reviewed. However, to fully understand the role of polyphenols in the medical management of these conditions, it is necessary to focus on the critical points and potential limitations derived from their use. These issues mainly revolve around the bioavailability of polyphenols, potential interactions with other treatments, and security considerations.
Curcuminoids, olive-associated polyphenols (including secoiridoids and 5-HT), coumarins, and flavonolignans
| Subgroups | Compounds | Dietary/Exogenous sources | Mechanisms of action (Preclinical models) | Studies in humans (including observational and intervention studies | References |
|---|---|---|---|---|---|
| Curcuminoids | Curcumin | Turmeric | Is a potent anti-inflammatory and antioxidant with broad therapeutic potential, including anti-tumor, anti-apoptotic, and immunomodulatory effects. It modulates multiple inflammatory pathways, including NF-κB, COX, and various cytokines, and influences iron metabolism and autophagy in IBDs | Human studies show curcumin´s promise managing IBD, particularly UC. Clinical trials report significant improvements in disease activity, inflammatory markers, and quality of life with curcumin supplementation. However, some studies yielded mixed results, underscoring the need for standardized dosages, formulations, and further large-scale trials to validate curcumin´s efficacy as an adjunct therapy in IBd management | [501-528] |
| Secoiridoids and polyphenols from olives/EVOO | Extra virgin olive oil | Olives/EVOO | Preclinical studies demonstrated the potential of EVOO in managing IBD, with findings showing that EVOO can reduce inflammation, oxidative stress, and cell damage in various models. It has been found to modulates cytokines, COX-2, and iNOS while influencing signaling pathways like MAPk and PPARγ | Clinical trials on EVOO and its poluphenols for IBd are limited. Studies have shown that EVOO can reduce inflammation markers and gastrointestinal symptoms in Uc patients and that phenolic-rich EVOO can enhance cardioprotective benefits and gut microbiota. Also, Mediterranean diet, including EVOO, has demonstrated over all benefits for IBD management, such as improved BMI, reduced inflammation, and better disease management compared to other diets | [533-535,537-545] |
| Hydroxytyrosol | Has shown promising results in preclinical IBD models. Studies demonstrate that HT reduces inflammation, oxidative stress, and apoptosis while enhancing antioxidant defenses and gut microbiota composition | - | [546-551] | ||
| Oleuropein | Studies reveal that Ole reduces oxidative stress, inflammation, and apoptosis, and improves disease activity in UC models. It decreases pro-inflammatory cytokines and COX-2 expression while promoting healing and enhancing antioxidant defenses | - | [552-554] | ||
| Coumarins | Esculetin and 4- methylesculetin | Cinnamon; Vegetables, spices, fruits, and medicinal plants including all parts of the plants-fruits, roots, stems and leaves | Esculetin at 5 mg/kg in a rat TNBS-induced IBD model, exhibited strong anti-inflammatory effects, reducing MDA levels, preventing GSH depletion, and decreasing pro-inflammatory cytokines. It showed efficacy comparable to prednisolone and was 10 tomes more effective than sulfasalazine. Similarly, 4-methyl-esculetin demonstrated superior efficacy in reducing colitis symptoms, preventing GSH depletion, and inhibiting inflammation, with effects comparable to established treatments | - | [557-562] |
| Aesculin | Showing minimal cytotoxicity, significantly alleviated DSS-induced colitis symptoms by reducing inflammatory factors like iNOS, IL-1β, and TNF-α. It also attenuated NF-κB signaling and enhanced PPARγ nuclear localization | - | [563] | ||
| Daphnetin | He et al. found that daphnetin alleviated colitis severity in mice by improving intestinal structure, increasing ZO-1, occludin, and BCL-2 levels, and reducing Bax and cleaved caspase 3 expression. It also suppressed MAD, SOD activities, inflammatory cytokines, and apoptosis, while inhibiting JAK/STAT signaling via REG3A activation | - | [565] | ||
| Fraxetin | Demonstrated notable efficacy in mitigating DSS-induced colitis symptoms, including body weight loss, colon shortening, tissue damage, and disease activity index. It also inhibits NF-κB pathway, suppression of NLRP3 inflammasome, restoration of Gut barrier function, and modulation of gut microbiota | - | [566] | ||
| Umbelliferone | Improves macroscopic and histological damage in an acetic acid-induced UC rat model. It reduced inflammatory markers, downregulated TLR4, NF-κB, and iNOS, and upregulated SIRT1 and PPARγ signaling pathways | - | [567] | ||
| Paepalantine | Alleviates TNBS-induced colonic damage in mice at 5 and 10 mg/kg. it improved mucosal recovery and reduced oxidative stress by preventing glutathione depletion and inhibiting nitric oxide activity | - | [569] | ||
| Coumarin | Reduces colonic damage in TNBS-induced colitis models. Their protective effect was linked to preventing glutathione depletion caused by inflammation | - | [570] | ||
| Flavonolignanes | Silymarin | Milk thistle (Sylybum marianum) | Is a potent anti-inflammatory that inhibits NF-κB pathways and enhances antioxidant defenses via Nrf2 activation. It shows potential as a tool against viral-associated IBD due to its properties and effectiveness in reducing colitis symptoms | In a randomized, double-blinded trial with 80 UC patients, silibin supplementation improved hemoglobin levels, erythrocyte sedimentation rate, and disease activity index, helping maintain remission better than placebo | [573,575-578,581] |
| Silibin | Sylybum marianum | Has ant-inflammatory and chemopreventine benefits I preclinical IBD models. In a TNBS-induced colitis rat model, silybin reduced NF-κB activity and inflammatory markers, enhancing antioxidant defenses | - | [579,580] |
3.1. Bioavailability of polyphenols
Regarding the formulations and dosages of polyphenols, it is crucial to consider the bioavailability and stability of these compounds, as well as the most appropriate route of administration.
The poor bioavailability of polyphenols represents one of the major concerns to be addressed. The literature [585] highlights that polyphenols show a low bioavailability due to their interaction with other nutrients along with metabolic processes mediated by the liver (phase I and II metabolism), intestine, and microbiota. Oral administration is the most usual pathway for polyphenols given pharmacologically, leading to these problems of bioavailability [586]. In the case of humans, it is difficult to imagine other pathways of administration, due to the confortability of using oral pills. Various formulations, such as liposomes, pro-liposomes, micro- and nanocapsules, polymeric capsules, phospholipid-polyphenol complex, micro- and nanoemulsions, micro- and nanoparticles, or even more complex systems are being explored to improve the delivery and therapeutic efficacy of polyphenols in patients with IBD [587,588]. However it should be noted that the biological activities and therapeutic effects of polyphenols are also potentially mediated by their metabolites formed in the gut, liver, or gut microbiota, therefore demonstrating the complexity of polyphenols in this context [589,590]. In general, gallic acid and isoflavones are the most well-absorbed polyphenols, followed by catechins, flavanones, and quercetin glucosides, although with different kinetics. Proanthocyanidins, the galloylated tea catechins, and the anthocyanins are the least well-absorbed polyphenols [590]. These properties are directly related to other important points such as finding adequate doses of polyphenols. In the case of clinical applications of polyphenols in IBD, the optimal dosage may vary depending on the type of polyphenol, and also on the severity of the disease. Thus, in the case of polyphenols with poor bioavailability like quercetin or RES, a long period of administration together with high daily doses of these compounds are generally required for observing their therapeutic effects, as previously described [591]. Therefore, issues regarding the optimal dosage, vehicles of administration, or specific considerations related to the bioavailability of each polyphenol a pivotal points of research to be explored by further studies.
The inclusion of polyphenols from diets and foods rich in these compounds is also a critical point of study. The literature has shown the promising adjuvant effects of different dietary patterns characterized by high content of polyphenols on the clinical management of IBDs including the anti-inflammatory [592], Mediterranean [593], and FODMAP diets [594]. With specific differences and punctualizations, these dietary patterns include plant-based sources rich in polyphenols, including vegetables, legumes fruits and spices. However, to understand the role of polyphenols contained in different food sources, a critical concept should be introduced herein: the food matrix. The literature defines a food matrix as the “physical domain that contains and/or interacts with specific constituents of a food (e.g., a nutrient) providing functionalities and behaviors which are different from those exhibited by the components in isolation or a free state” [595]. This means that for example, isolated quercetin exerts different effects than quercetin contained in food sources like broccoli, as quercetin would be included in a broad and complex food matrix, interacting with other nutrients that may affect its activity. The relevance of the food matrix in the bioavailability of polyphenols is perhaps one of the most interesting topics of nutrition research. Indeed, the literature recognizes that the food matrix can either enhance or diminish the bioavailability of polyphenols, although the mechanism by which it works remains to be fully elucidated [596]. Bohn [597] conducted an extensive review detailing how dietary factors and food matrix affected polyphenol bioavailability. In this review, it is highlighted that dietary fiber (such as hemicellulose), divalent minerals, and viscous and protein-rich meals are likely to cause detrimental effects on polyphenol bioaccessibility; whereas digestible carbohydrates, dietary lipids (especially for hydrophobic polyphenols, e.g., curcumin), and additional antioxidants may enhance polyphenol availability Likewise, after epithelial uptake, polyphenols such as flavonoids may diminish phase II metabolism and excretion to enhance polyphenol bioavailability, whereas various polyphenols may act synergistically due to their influence on efflux transporters such as p-glycoprotein [597]. Other examples more specific include the effects of pectin with quercetin, improving its bioavailability through gut flora and function changes; the sugar content in matrices affecting RES bioavailability, with lower absorption in grape juice compared to pure forms and also there are mixed results on milk's impact on polyphenol bioavailability [598]. The fat content of cocoa enhances the digestibility of some phenolic compounds (especially procyanidins) [599], whereas EVOO polyphenols appear to be highly bioavailable with greater absorption in a dose-dependent way and when administered as an olive oil solution compared to an aqueous solution [598]. Likewise, as occurring with oral supplementation, the bioavailability of polyphenols present in foods is in general low, and in vitro, studies observed clear differences in the mechanisms of polyphenols when used in their pure form and at high concentrations versus when ingested through foods rich in these compounds [600]. This, however, does not mean that they do not have favorable or preventive effects against different pathologies, but it demonstrates that the food matrix is an important element to be considered in the therapeutic effects of polyphenols. Therefore, the consideration of the food matrix and specific combinations of polyphenols with other nutrients can be used to maximize the benefits of these compounds.
3.2. Safety considerations and interaction of polyphenols with available therapies
Notwithstanding we have strongly supported the benefits of polyphenols in the clinical management of IBDs, it is important to remark that polyphenols can also have certain harmful effects that are worth mentioning. Among other effects, polyphenols can block iron uptake, inhibit digestive enzymes, alter intestinal microbiota, and hormonal balance, and interact with different drugs [601], which may lead to undesired adverse effects in these patients.
Regarding the point, it is well-established that one of the most common complications seen in IBD patients is iron deficiency anemia (IDA) [601]. Despite iron can be supplemented intravenously, oral supplementation of this mineral is the most common therapeutic option. Polyphenols are able to chelate the ions of transition metals (e.g., Fe and Cu), inhibiting the formation of free radicals in the Fenton and Haber-Weiss reactions [601]. Catechins are one of the most important inhibitors of iron absorption [602]. Despite in general this action of polyphenols is considered positive as part of their antioxidant properties; this effect could be particularly detrimental for IBD patients with IDA. For instance, past works have demonstrated that 20-50 mg total polyphenols/serving reduced Fe absorption from the meal by 50-70%, whereas beverages containing 100-400 mg total polyphenols/serving reduced Fe absorption by 60-90% [603]. Therefore, the studies discouraged the use of black and herb teas, coffee, and coca for patients with low levels of iron, with the greatest detrimental effects for black tea.
On the other hand, flavonoids can form complexes with proteins through nonspecific forces such as hydrogen bonding and hydrophobic effects, as well as by covalent bond formation [604]. This effect can affect the utilization of food proteins and digestive enzymes, which may lead to impaired function and disturbances during biochemical reactions or processes that a given enzyme catalyzes. Digestive enzymes have been shown to present significant benefits in the clinical management of gastrointestinal diseases like IBD [605,606]. Therefore, if digestive enzymes are used in IBD patients, the effect of flavonoids in regulating these proteins should also be considered.
The role of polyphenols on gut microbiota and hormonal balance are perhaps two concerns more complex and less studied. In the case of the former, and as it has been remarked throughout the whole manuscript, polyphenols partly exert their therapeutic action by modulating the gut microbiota. In general, these effects are considered positive; however, the precise role of gut microbiota in IBDs is not fully understood yet, and some specific individual factors and variations should be considered to understand if the changes that occurred in these microbial are favorable, neutral or harmful [607]. Therefore, more efforts are needed in this sense. Secondly, the available literature recognizes a possible role of isoflavones in the alteration of the hormonal balance, affecting both estrogen and thyroid hormone regulation. However, there is a great heterogeneity in the available studies regarding these effects. As IBD might be associated with estrogen and thyroid hormone dysregulation [608,609], further studies should be conducted to evaluate potential side effects derived from the use of isoflavones in IBD.
Finally, the interaction of polyphenols with different treatments should also be explored. The main pharmacological therapies for IBDs include aminosalicylates, corticosteroids, immunomodulators, antibiotics, and biologics [610]. While a certain percentage of patients may benefit from several of these therapies, many others either do not respond to treatment at all or lose response over time. Furthermore, the negative side effects of conventional therapies frequently place restrictions on them. Polyphenols might interact with drug components and enzymes like cytochrome P450, which can alter drug metabolism and affect their therapeutic effects [611]. Likewise, dietary polyphenols are potential substrates and/or inhibitors of Phase II enzymes like UDP-glucuronosyltransferases due to the presence of hydroxylic groups (-OH) that give them structural similarity with drugs' metabolites. Known polyphenols that inhibit drug-metabolizing enzymes include quercetin, resveratrol, chrysin, anthocyanins, naringenin, apigenin, coumarins, kaempferol, acacetin, luteolin, diosmetin, caffeic acid, and gallic acid [601]. Additionally, polyphenols can affect drug transport through interactions with drug transporters [612]. In parallel, certain plants and herb-based products rich in polyphenols when consumed with medications, require careful monitoring due to potential interactions. Healthcare providers need to educate patients about these risks, emphasizing that natural products are not always safe, and patients should consult pharmacists before using herbal supplements alongside medications. Effective communication about potential side effects is crucial, especially for drugs with a narrow therapeutic index like warfarin, cyclosporine A, and digoxin [601]. Besides, the effects of long-term use of polyphenols remain to be better understood. In this sense, it is important to consider the possible synergy or antagonism between polyphenols and conventional medications used in the treatment of IBD. More research is needed to evaluate how polyphenols may interact with drugs such as corticosteroids, immunomodulators, and biological drugs and whether these interactions may influence the effectiveness or safety of the treatment. Figure 4 explains the context of polyphenols and future issues to address in IBD patients.
4. Conclusions
Polyphenols are a class of natural compounds that have garnered significant interest due to their extensive biological effects, particularly their potent antioxidant and modulatory properties on the immune system and gut microbiota. To date, most research has demonstrated the benefits of polyphenols in preclinical models, acting through several well-reported mechanisms. Proportionally, few studies have been conducted in humans, with curcumin standing out as the most studied polyphenol, with some observational or translational studies performed on quercetin, isoflavones, resveratrol, silymarin xanthohumol, along with EVOO, flaxseed and anthocyanins from berries. Throughout the tables, important data relative to the studies conducted in humans are summarized. Also and as a limitation of our study, polyphenols are part of the bioactive compounds of different medicinal plants that could have been potentially investigated in humans not collected here. Finally, clinicians and patients must understand the precise role of polyphenols as an adjunctive therapy for IBDs. There are still many issues to be investigated before drawing clear recommendations related to their use, including concerns related to their bioavailability, dosage, formulation, administration, possible interactions with treatments, and potential adverse effects for some patients in certain contexts. To date, the most feasible recommendation would be to incorporate plant-based sources rich in polyphenols, including fruits, berries, cocoa and derivates, vegetables, legumes, nuts or spices, and to some extent coffee or tea, always included as part of a healthy dietary pattern such as MedDiet, considering the food matrix and dietary advice to maximize the bioavailability and effects of polyphenols. Further clinical trials should be conducted evaluating the use of isolated polyphenols in IBD subjects, whereas the use of curcumin, resveratrol, or biphenol-rich nutraceuticals could also be considered or tested by clinicians due to their promising benefits [613,614], although many cautions should be taken, as to date it is difficult to find standardized protocols, formulas or recommendations regarding their use. As interest in integrative approaches to healthcare continues to grow, polyphenols may emerge as valuable adjuncts to conventional treatments, offering patients additional avenues for managing their condition and enhancing their quality of life.
Understanding the precise role of polyphenols as part of integrative therapy in IBD. By their anti-inflammatory properties, polyphenols have been shown to reduce inflammation in patients with Chron´s disease, while also affording protection to intestinal tissues and decreasing ulceration in those with ulcerative colitis. As a result, there is a growing utilization of polyphenols, as their administration offers improved outcomes and increases comfort for patients, especially when compared to conventional therapies. However, more investigations are needed to improve the effects of polyphenols such as their interaction with other drugs, or synergies with other biological compounds, and also the dosage and bioavailability of polyphenols.
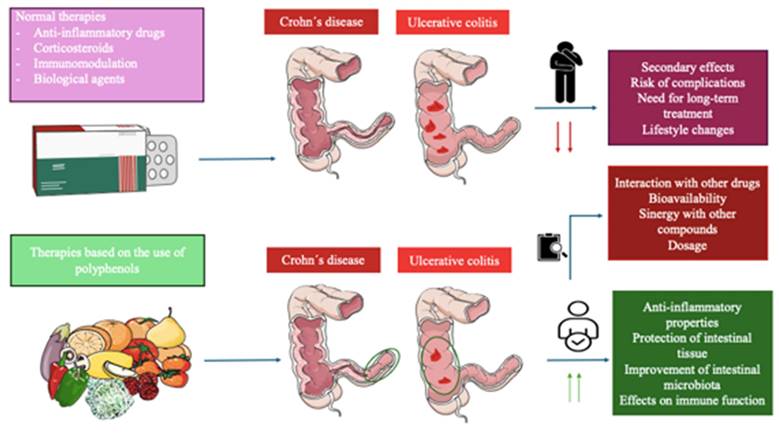
Acknowledgements
Funding
This work was partially supported by grants from the Mutua Madrileña, Programa de Actividades de I+D de la Comunidad de Madrid en Biomedicina (grant no. P2022/BMD‑7321), Halekulani S.L., ProACapital and MJR.
Author contributions
All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Flynn S, Eisenstein S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg Clin North Am [Internet]. 2019Dec1;99(6):1051-62 Available from: https://pubmed.ncbi.nlm.nih.gov/31676047/
2. Seyedian SS, Nokhostin F, Dargahi Malamir M. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019;12:113-22
3. Rosen MJ, Dhawan A, Saeed SA. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr [Internet]. 2015Nov1;169(11):1053-60 Available from: https://pubmed.ncbi.nlm.nih.gov/26414706/
4. Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature [Internet]. 2011Jun16;474(7351):307-17 Available from: https://pubmed.ncbi.nlm.nih.gov/21677747/
5. Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol [Internet]. 2010;105(3):501-23 Available from: https://pubmed.ncbi.nlm.nih.gov/20068560/
6. César Da Silva B, Lyra AC, Rocha R, Oliveira G, Bruno César Da Silva S, Santana GO. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. 2014; Available from: http://dx.doi.org/10.3748/wjg.v20.i28.9458.
7. Segal JP, Jean-Frédéric LeBlanc A, Hart AL. Ulcerative colitis: an update. Clin Med (Lond) [Internet]. 2021Mar1;21(2):135-9 Available from: https://pubmed.ncbi.nlm.nih.gov/33762374/
8. Guijarro LG, Cano-Martínez D, Toledo-Lobo MV, Ruiz-Llorente L, Chaparro M, Guerra I. et al. Evaluation of AIF-1 (Allograft Inflammatory Factor-1) as a Biomarker of Crohn's Disease Severity. Biomedicines [Internet]. 2022 Mar 1;10(3). Available from: https://pubmed.ncbi.nlm.nih.gov/35327530/
9. Guijarro LG, Cano-Martínez D, Toledo-Lobo MV, Salinas PS, Chaparro M, Gómez-Lahoz AM. et al. Relationship between IGF-1 and body weight in inflammatory bowel diseases: Cellular and molecular mechanisms involved. Biomed Pharmacother [Internet]. 2021 Dec 1;144. Available from: https://pubmed.ncbi.nlm.nih.gov/34601192/
10. Goens D, Micic D. Role of Diet in the Development and Management of Crohn's Disease. Curr Gastroenterol Rep [Internet]. 2020Apr1;22(4):1-9 Available from: https://link.springer.com/article/10.1007/s11894-020-0755-9
11. Rudrapal M, Khairnar SJ, Khan J, Dukhyil A Bin, Ansari MA, Alomary MN. et al. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front Pharmacol [Internet]. 2022Feb14;13:806470 Available from: www.frontiersin.org
12. Tresserra-Rimbau A, Lamuela-Raventos RM, Moreno JJ. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem Pharmacol. 2018Oct1;156:186-95
13. Gasmi A, Mujawdiya PK, Noor S, Lysiuk R, Darmohray R, Piscopo S. et al. Polyphenols in Metabolic Diseases. Molecules [Internet]. 2022 Oct 127(19). Available from: /pmc/articles/PMC9570923/
14. Alov P, Tsakovska I, Pajeva I. Computational studies of free radical-scavenging properties of phenolic compounds. Curr Top Med Chem [Internet]. 2015Dec15;15(2):85-104 Available from: https://pubmed.ncbi.nlm.nih.gov/25547098/
15. Makarewicz M, Drożdż I, Tarko T, Duda-Chodak A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants (Basel) [Internet]. 2021Feb1;10(2):1-70 Available from: https://pubmed.ncbi.nlm.nih.gov/33525629/
16. MC R, I A, JL R. Anti-inflammatory agents from plants: progress and potential. Curr Med Chem [Internet]. 2012Jun19;19(14):2088-103 Available from: https://pubmed.ncbi.nlm.nih.gov/22414101/
17. Spagnuolo C, Russo M, Bilotto S, Tedesco I, Laratta B, Russo GL. Dietary polyphenols in cancer prevention: the example of the flavonoid quercetin in leukemia. Ann N Y Acad Sci [Internet]. 2012;1259(1):95-103 Available from: https://pubmed.ncbi.nlm.nih.gov/22758641/
18. Hussain T, Tan B, Yin Y, Blachier F, Tossou MCB, Rahu N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid Med Cell Longev [Internet]. 2016. 2016 Available from: /pmc/articles/PMC5055983/
19. Duda-Chodak A, Tarko T. molecules Possible Side Effects of Polyphenols and Their Interactions with Medicines. 2023; Available from: https://doi.org/10.3390/molecules28062536.
20. Ballard JWO, Towarnicki SG. Mitochondria, the gut microbiome and ROS. Cell Signal [Internet]. 2020 Nov 1;75. Available from: https://pubmed.ncbi.nlm.nih.gov/32810578/
21. Martin JR, Gupta MK, Page JM, Yu F, Davidson JM, Guelcher SA. et al. A porous tissue engineering scaffold selectively degraded by cell-generated reactive oxygen species. Biomaterials. 2014Apr1;35(12):3766-76
22. Shavandi A, Bekhit AEDA, Saeedi P, Izadifar Z, Bekhit AA, Khademhosseini A. Polyphenol uses in biomaterials engineering. Biomaterials. 2018Jun1;167:91-106
23. Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nature Reviews Immunology 2013 13:5 [Internet]. 2013Apr25;13(5):349-61 Available from: https://www.nature.com/articles/nri3423
24. Gao X, Xu Z, Liu G, Wu J. Polyphenols as a versatile component in tissue engineering. Acta Biomater. 2021Jan1;119:57-74
25. Ortega MA, De Leon-Oliva D, García-Montero C, Fraile-Martinez O, Boaru DL, de Castro AV. et al. Reframing the link between metabolism and NLRP3 inflammasome: therapeutic opportunities. Front Immunol [Internet]. 2023 14. Available from: https://pubmed.ncbi.nlm.nih.gov/37545507/
26. Wang X, Qi Y, Zheng H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants [Internet]. 2022 Jun 1;11(6). Available from: /pmc/articles/PMC9220293/
27. Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. 2016Apr1;8:33-42
28. Natividad JMM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res [Internet]. 2013Mar;69(1):42-51 Available from: https://pubmed.ncbi.nlm.nih.gov/23089410/
29. Marín L, Miguélez EM, Villar CJ, Lombó F. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. Biomed Res Int [Internet]. 2015 Feb 23. 2015 Available from: https://pubmed.ncbi.nlm.nih.gov/25802870/
30. Pacheco-Ordaz R, Wall-Medrano A, Goñi MG, Ramos-Clamont-Montfort G, Ayala-Zavala JF, González-Aguilar GA. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett Appl Microbiol [Internet]. 2018Jan1;66(1):25-31 Available from: https://pubmed.ncbi.nlm.nih.gov/29063625/
31. Manso T, Lores M, de Miguel T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics [Internet]. 2022 Jan 1;11(1). Available from: /pmc/articles/PMC8773215/
32. Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front Nutr. 2018Sep21;5:370438
33. Bhosale PB, Ha SE, Vetrivel P, Kim HH, Kim SM, Kim GS. Functions of polyphenols and its anticancer properties in biomedical research: a narrative review. Transl Cancer Res [Internet]. 2020Dec1;9(12):7619 Available from: /pmc/articles/PMC8798728/
34. Saez A, Herrero-Fernandez B, Gomez-Bris R, Sánchez-Martinez H, Gonzalez-Granado JM. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. International Journal of Molecular Sciences 2023, Vol 24, Page 1526 [Internet]. 2023Jan12;24(2):1526 Available from: https://www.mdpi.com/1422-0067/24/2/1526/htm
35. Jarmakiewicz-Czaja S, Zielińska M, Sokal A, Filip R. Genetic and Epigenetic Etiology of Inflammatory Bowel Disease: An Update. Genes (Basel) [Internet]. 2022 Dec 1 [cited. 2024 Jul 10];13(12). Available from: /pmc/articles/PMC9778199/
36. Qelliny MR, Elgarhy OH, Khaled KA, Aly UF. Colon Drug Delivery Systems for the Treatment of Inflammatory Bowel Disease. Journal of advanced Biomedical and Pharmaceutical Sciences [Internet]. 2019Oct1;2(4):164-84 Available from: https://jabps.journals.ekb.eg/article_50287.html
37. Liu S, Zhao W, Lan P, Mou X. The microbiome in inflammatory bowel diseases: from pathogenesis to therapy. Protein Cell [Internet]. 2021May1;12(5):331 Available from: /pmc/articles/PMC8106558/
38. Kumar S, Kumar A. Microbial pathogenesis in inflammatory bowel diseases. Microb Pathog. 2022Feb1;163:105383
39. Ortega M, García-Montero C, Fraile-Martínez O, Monserrat J, Álvarez-Mon MA. La microbiota intestinal en la salud y en la enfermedad. Medicine - Programa de Formación Médica Continuada Acreditado. 2022Dec1;13(69):4054-63
40. Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014Jan1;13(1):3-10
41. Briseño CG, Murphy TL, Murphy KM. Complementary Diversification of Dendritic Cells and Innate Lymphoid Cells. Curr Opin Immunol. 2014 29: 69-78
42. Huang Y, Chen Z. Inflammatory bowel disease related innate immunity and adaptive immunity. Am J Transl Res [Internet]. 2016;8(6):2490 Available from: /pmc/articles/PMC4931145/
43. Lee SH, Kwon J eun, Cho M La. Immunological pathogenesis of inflammatory bowel disease. Intest Res [Internet]. 2018 [cited. 2024 Jul 10];16(1):26. Available from: /pmc/articles/PMC5797268/
44. Lin Y, Liu H, Bu L, Chen C, Ye X. Review of the Effects and Mechanism of Curcumin in the Treatment of Inflammatory Bowel Disease. Front Pharmacol [Internet]. 2022 Jun 20;13. Available from: /pmc/articles/PMC9250976/
45. Sahoo DK, Heilmann RM, Paital B, Patel A, Yadav VK, Wong D. et al. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front Endocrinol (Lausanne) [Internet]. 2023Aug28;14:1217165 Available from: www.biorender.com
46. Alvarez-Mon MA, Ortega MA, García-Montero C, Fraile-Martinez O, Monserrat J, Lahera G. et al. Exploring the Role of Nutraceuticals in Major Depressive Disorder (MDD): Rationale, State of the Art and Future Prospects. Pharmaceuticals (Basel) [Internet]. 2021 Aug 1;14(8). Available from: https://pubmed.ncbi.nlm.nih.gov/34451918/
47. Caban M, Lewandowska U. Polyphenols and the potential mechanisms of their therapeutic benefits against inflammatory bowel diseases. J Funct Foods. 2022Aug1;95:105181
48. Hagan M, Hayee H, Rodriguez-Mateos A, Santos-Buelga C. molecules (Poly)phenols in Inflammatory Bowel Disease and Irritable Bowel Syndrome: A Review. Molecules [Internet]. 2021;26:1843 Available from: https://doi.org/10.3390/molecules
49. Sato Y, Tsujinaka S, Miura T, Kitamura Y, Suzuki H, Shibata C. Inflammatory Bowel Disease and Colorectal Cancer: Epidemiology, Etiology, Surveillance, and Management. Cancers (Basel) [Internet]. 2023 Aug 1;15(16). Available from: /pmc/articles/PMC10452690/
50. Zhao Y, Jiang Q. Roles of the Polyphenol-Gut Microbiota Interaction in Alleviating Colitis and Preventing Colitis-Associated Colorectal Cancer. Adv Nutr [Internet]. 2021Mar1;12(2):546-65 Available from: https://pubmed.ncbi.nlm.nih.gov/32905583/
51. Long J, Guan P, Hu X, Yang L, He L, Lin Q. et al. Natural Polyphenols as Targeted Modulators in Colon Cancer: Molecular Mechanisms and Applications. Front Immunol [Internet]. 2021 Feb 16 [cited. 2024 Jul 10];12:635484. Available from: www.frontiersin.org
52. Joshi A, Soni A, Acharya S. In vitro models and ex vivo systems used in inflammatory bowel disease. In vitro models [Internet]. 2022 Apr 25 [cited. 2024 Jul 10];1(3):213. Available from: /pmc/articles/PMC9036838/
53. Kiesler P, Fuss IJ, Strober W. Experimental Models of Inflammatory Bowel Diseases. Cell Mol Gastroenterol Hepatol. 2015Mar1;1(2):154-70
54. Lee CH, Koh SJ, Radi ZA, Habtezion A. Animal models of inflammatory bowel disease: novel experiments for revealing pathogenesis of colitis, fibrosis, and colitis-associated colon cancer. Intest Res. 2023;21(3):295-305
55. Vezza T, Rodríguez-Nogales A, Algieri F, Utrilla MP, Rodriguez-Cabezas ME, Galvez J. Flavonoids in Inflammatory Bowel Disease: A Review. Nutrients [Internet]. 2016 Apr 9 [cited. 2024 Mar 17];8(4). Available from: /pmc/articles/PMC4848680/
56. Liu RH. Dietary bioactive compounds and their health implications. J Food Sci. 2013 Jun;78(SUPPL.1)
57. Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci [Internet]. 2016 Jan 8 [cited. 2024 Mar 17];5:1-15. Available from: /pmc/articles/PMC5465813/
58. Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG. et al. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules [Internet]. 2020 Nov 1 [cited. 2024 Mar 17];25(22). Available from: https://pubmed.ncbi.nlm.nih.gov/33187049/
59. Hostetler GL, Ralston RA, Schwartz SJ. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv Nutr [Internet]. 2017 May 1 [cited. 2024 Mar 17];8(3):423-35. Available from: https://pubmed.ncbi.nlm.nih.gov/28507008/
60. Wang X, Cao Y, Chen S, Lin J, Bian J, Huang D. Anti-Inflammation Activity of Flavones and Their Structure-Activity Relationship. J Agric Food Chem [Internet]. 2021 Jul 7 [cited. 2024 Mar 17];69(26):7285-302. Available from: https://pubmed.ncbi.nlm.nih.gov/34160206/
61. Shankar E, Goel A, Gupta K, Gupta S. Plant flavone apigenin: An emerging anticancer agent. Curr Pharmacol Rep [Internet]. 2017 Dec 1 [cited. 2024 Jul 10];3(6):423. Available from: /pmc/articles/PMC5791748/
62. Márquez-Flores YK, Villegas I, Cárdeno A, Rosillo MÁ, Alarcón-de-la-Lastra C. Apigenin supplementation protects the development of dextran sulfate sodium-induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways. J Nutr Biochem [Internet]. 2016 Apr 1 [cited. 2024 Mar 18];30:143-52. Available from: https://pubmed.ncbi.nlm.nih.gov/27012631/
63. Lin TJ, Yin SY, Hsiao PW, Yang NS, Wang IJ. Transcriptomic analysis reveals a controlling mechanism for NLRP3 and IL-17A in dextran sulfate sodium (DSS)-induced colitis OPEN. SCIENTIFIC RepoRTs | [Internet]. 2018 [cited. 2024 Mar 18];8:14927. Available from: www.nature.com/scientificreports
64. Fu R, Wang L, Meng Y, Xue W, Liang J, Peng Z. et al. Apigenin remodels the gut microbiota to ameliorate ulcerative colitis. Front Nutr [Internet]. 2022 Dec 16 [cited. 2024 Jul 10];9. Available from: https://pubmed.ncbi.nlm.nih.gov/36590200/
65. Li M, Weigmann B. Effect of a Flavonoid Combination of Apigenin and Epigallocatechin-3-Gallate on Alleviating Intestinal Inflammation in Experimental Colitis Models. J Mol Sci [Internet]. 2023 [cited. 2024 Mar 18];24. Available from: https://doi.org/10.3390/ijms242216031
66. Ai XY, Qin Y, Liu HJ, Cui ZH, Li M, Yang JH. et al. Apigenin inhibits colonic inflammation and tumorigenesis by suppressing STAT3-NF-κB signaling. 2017 [cited. 2024 Mar 18]; Available from: www.impactjournals.com/oncotarget
67. Márquez-Flores YK, Villegas I, Cárdeno A, Rosillo MÁ, Alarcón-de-la-Lastra C. Apigenin supplementation protects the development of dextran sulfate sodium-induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways. J Nutr Biochem [Internet]. 2016 Apr 1 [cited. 2024 Jul 10];30:143-52. Available from: https://pubmed.ncbi.nlm.nih.gov/27012631/
68. Hu Z, Guan Y, Hu W, Xu Z, Ishfaq M. An overview of pharmacological activities of baicalin and its aglycone baicalein: New insights into molecular mechanisms and signaling pathways. Iran J Basic Med Sci [Internet]. 2022 Jan 1 [cited. 2024 Jul 10];25(1):14. Available from: /pmc/articles/PMC9118284/
69. Zhong X, Surh YJ, Do SG, Shin E, Shim KS, Lee CK. et al. Baicalein Inhibits Dextran Sulfate Sodium-induced Mouse Colitis. J Cancer Prev [Internet]. 2019 Jun 30 [cited. 2024 Mar 18];24(2):129-38. Available from: https://pubmed.ncbi.nlm.nih.gov/31360692/
70. Li Y yang, Wang X jing, Su Y lin, Wang Q, Huang S wei, Pan Z feng. et al. Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol Sin [Internet]. 2022 Jun 1 [cited. 2024 Jul 10];43(6):1495-507. Available from: https://pubmed.ncbi.nlm.nih.gov/34671110/
71. Luo X, Yu Z, Deng C, Zhang J, Ren G, Sun A. et al. Baicalein ameliorates TNBS-induced colitis by suppressing TLR4/MyD88 signaling cascade and NLRP3 inflammasome activation in mice. Sci Rep [Internet]. 2017 Dec 1 [cited. 2024 Jul 10];7(1). Available from: https://pubmed.ncbi.nlm.nih.gov/29180692/
72. Liu C, Li Y, Chen Y, Huang S, Wang X, Luo S. et al. Baicalein Restores the Balance of Th17/Treg Cells via Aryl Hydrocarbon Receptor to Attenuate Colitis. Mediators Inflamm [Internet]. 2020 [cited 2024 Jul 10]. 2020 Available from: https://pubmed.ncbi.nlm.nih.gov/33082710/
73. Jang JY;, Im E;, Kim ND. Therapeutic Potential of Bioactive Components from Scutellaria baicalensis Georgi in Inflammatory Bowel Disease and Colorectal Cancer: A Review. 2023 [cited. 2024 Mar 18]; Available from: https://doi.org/10.3390/ijms24031954
74. Liu L, Wu W, Li S, Ma L, Liu Y, Wang X. et al. Engineered baicalein-decorated zinc phosphates for synergistic alleviation of inflammatory bowel disease by repairing the mucosal barrier and relieving oxidative stress. Biomater Sci [Internet]. 2023 Oct 11 [cited. 2024 Jul 10];11(23):7678-91. Available from: https://pubmed.ncbi.nlm.nih.gov/37870399/
75. Kim DH, Sung B, Chung HY, Kim ND. Modulation of Colitis-associated Colon Tumorigenesis by Baicalein and Betaine. J Cancer Prev [Internet]. 2014 Sep 30 [cited. 2024 Jul 10];19(3):152-60. Available from: https://pubmed.ncbi.nlm.nih.gov/25337584/
76. Xu J, Liu J, Yue G, Sun M, Li J, Xiu X. et al. Therapeutic effect of the natural compounds baicalein and baicalin on autoimmune diseases. Mol Med Rep [Internet]. 2018 Jul 1 [cited. 2024 Jul 10];18(1):1149-54. Available from: https://pubmed.ncbi.nlm.nih.gov/29845272/
77. Liang S, Deng X, Lei L, Zheng Y, Ai J, Chen L. et al. The comparative study of the therapeutic effects and mechanism of baicalin, baicalein, and their combination on ulcerative colitis rat. Front Pharmacol [Internet]. 2019 [cited. 2024 Jul 10];10:1466. Available from: /pmc/articles/PMC6923254/
78. Wang X, Xie L, Long J, Liu K, Lu J, Liang Y. et al. Therapeutic effect of baicalin on inflammatory bowel disease: A review. J Ethnopharmacol [Internet]. 2022 Jan 30 [cited. 2024 Jul 10];283. Available from: https://pubmed.ncbi.nlm.nih.gov/34666140/
79. Zhu L, Xu LZ, Zhao S, Shen ZF, Shen H, Zhan L Bin. Protective effect of baicalin on the regulation of Treg/Th17 balance, gut microbiota and short-chain fatty acids in rats with ulcerative colitis. Appl Microbiol Biotechnol [Internet]. 2020 Jun 1 [cited. 2024 Jul 10];104(12):5449-60. Available from: https://pubmed.ncbi.nlm.nih.gov/32322944/
80. Yu FY, Huang SG, Zhang HY, Ye H, Chi HG, Zou Y. et al. Effects of baicalin in CD4 + CD29 + T cell subsets of ulcerative colitis patients. World J Gastroenterol [Internet]. 2014 Nov 7 [cited. 2024 Jul 10];20(41):15299-309. Available from: https://pubmed.ncbi.nlm.nih.gov/25386078/
81. Zhu W, Jin Z, Yu J, Liang J, Yang Q, Li F. et al. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int Immunopharmacol [Internet]. 2016 Jun 1 [cited. 2024 Jul 10];35:119-26. Available from: https://pubmed.ncbi.nlm.nih.gov/27039210/
82. Dai SX, Zou Y, Feng YL, Liu HB, Zheng XB. Baicalin down-regulates the expression of macrophage migration inhibitory factor (MIF) effectively for rats with ulcerative colitis. Phytother Res [Internet]. 2012 Apr [cited. 2024 Jul 10];26(4):498-504. Available from: https://pubmed.ncbi.nlm.nih.gov/21887805/
83. Zhang CL, Zhang S, He WX, Lu JL, Xu YJ, Yang JY. et al. Baicalin may alleviate inflammatory infiltration in dextran sodium sulfate-induced chronic ulcerative colitis via inhibiting IL-33 expression. Life Sci [Internet]. 2017 Oct 1 [cited. 2024 Jul 10];186:125-32. Available from: https://pubmed.ncbi.nlm.nih.gov/28802904/
84. Feng J, Guo C, Zhu Y, Pang L, Yang Z, Zou Y. et al. Baicalin down regulates the expression of TLR4 and NFkB-p65 in colon tissue in mice with colitis induced by dextran sulfate sodium. Int J Clin Exp Med [Internet]. 2014 Nov 30 [cited. 2024 Jul 10];7(11):4063. Available from: /pmc/articles/PMC4276173/
85. Rizzo V, Ferlazzo N, Currò M, Isola G, Matarese M, Bertuccio MP. et al. Baicalin-Induced Autophagy Preserved LPS-Stimulated Intestinal Cells from Inflammation and Alterations of Paracellular Permeability. Int J Mol Sci [Internet]. 2021 Mar 1 [cited. 2024 Jul 10];22(5):1-11. Available from: https://pubmed.ncbi.nlm.nih.gov/33652555/
86. Wang J, Zhu N, Su X, Gao Y, Yang R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells. 2023;12:793
87. Li J, Bai J, Tuerdi N, Liu K. Long non-coding RNA MEG3 promotes tumor necrosis factor-alpha induced oxidative stress and apoptosis in interstitial cells of cajal via targeting the microRNA-21 /I-kappa-B-kinase beta axis. Bioengineered [Internet]. 2022 [cited. 2024 Jul 10];13(4):8676-88. Available from: https://www.tandfonline.com/action/journalInformation?journalCode=kbie20
88. Yao J, Cao X, Zhang R, Li YX, Xu ZL, Zhang DG. et al. Protective Effect of Baicalin Against Experimental Colitis via Suppression of Oxidant Stress and Apoptosis. Pharmacogn Mag [Internet]. 2016 Jul 1 [cited. 2024 Jul 10];12(47):225-34. Available from: https://pubmed.ncbi.nlm.nih.gov/27601854/
89. Wu Q, Wu X, Wang M, Liu K, Li Y, Ruan X. et al. Therapeutic Mechanism of Baicalin in Experimental Colitis Analyzed Using Network Pharmacology and Metabolomics. Drug Des Devel Ther [Internet]. 2023 [cited. 2024 Jul 10];17:1007-24. Available from: https://pubmed.ncbi.nlm.nih.gov/37025160/
90. Yan Y, Li L, Wu K, Zhang G, Peng L, Liang Y. et al. A Combination of Baicalin and Berberine Hydrochloride Ameliorates Dextran Sulfate Sodium-Induced Colitis by Modulating Colon Gut Microbiota. J Med Food [Internet]. 2022 Aug 1 [cited. 2024 Jul 10];25(8):853-62. Available from: https://pubmed.ncbi.nlm.nih.gov/35980327/
91. Xu B, Huang S, Chen Y, Wang Q, Luo S, Li Y. et al. Synergistic effect of combined treatment with baicalin and emodin on DSS-induced colitis in mouse. Phytother Res [Internet]. 2021 Oct 1 [cited. 2024 Jul 10];35(10):5708-19. Available from: https://pubmed.ncbi.nlm.nih.gov/34379340/
92. CAO SY, YE SJ, WANG WW, WANG B, ZHANG T, PU YQ. Progress in active compounds effective on ulcerative colitis from Chinese medicines. Chinese Journal of Natural Medicines. 2019Feb1;17(2):81-102
93. Lopez-Lazaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem [Internet]. 2009 Dec 31 [cited. 2024 Jul 13];9(1):31-59. Available from: https://pubmed.ncbi.nlm.nih.gov/19149659/
94. Gendrisch F, Esser PR, Schempp CM, Wölfle U. Luteolin as a modulator of skin aging and inflammation. BioFactors [Internet]. 2021 Mar 1 [cited. 2024 Mar 18];47(2):170-80. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/biof.1699
95. Nunes C, Almeida L, Barbosa RM, Laranjinha J. Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food Funct [Internet]. 2017 Jan 1 [cited. 2024 Mar 18];8(1):387-96. Available from: https://pubmed.ncbi.nlm.nih.gov/28067377/
96. Kim JA, Kim DK, Kang OH, Choi YA, Park HJ, Choi SC. et al. Inhibitory effect of luteolin on TNF-alpha-induced IL-8 production in human colon epithelial cells. Int Immunopharmacol [Internet]. 2005 Jan [cited. 2024 Jul 13];5(1):209-17. Available from: https://pubmed.ncbi.nlm.nih.gov/15589482/
97. Xue L, Jin X, Ji T, Li R, Zhuge X, Xu F. et al. Luteolin ameliorates DSS-induced colitis in mice via suppressing macrophage activation and chemotaxis. Int Immunopharmacol [Internet]. 2023 Nov 1 [cited. 2024 Mar 18];124(Pt B). Available from: https://pubmed.ncbi.nlm.nih.gov/37776768/
98. Li BL, Zhao DY, Du PL, Wang XT, Yang Q, Cai YR. Luteolin alleviates ulcerative colitis through SHP-1/STAT3 pathway. Inflamm Res [Internet]. 2021 Jun 1 [cited. 2024 Jul 13];70(6):705-17. Available from: https://pubmed.ncbi.nlm.nih.gov/34014331/
99. Xie X, Zhao M, Huang S, Li P, Chen P, Luo X. et al. Luteolin alleviates ulcerative colitis by restoring the balance of NCR-ILC3/NCR+ILC3 to repairing impaired intestinal barrier. Int Immunopharmacol [Internet]. 2022 Nov 1 [cited. 2024 Jul 13];112. Available from: https://pubmed.ncbi.nlm.nih.gov/36182875/
100. Li B, Du P, Du Y, Zhao D, Cai Y, Yang Q. et al. Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci [Internet]. 2021 Mar 15 [cited. 2024 Jul 13];269. Available from: https://pubmed.ncbi.nlm.nih.gov/33434535/
101. Magadán-Corpas P, Pérez-Valero Á, Ye S, Sordon S, Huszcza E, Popłoński J. et al. Gut Microbiota and Inflammation Modulation in a Rat Model for Ulcerative Colitis after the Intraperitoneal Administration of Apigenin, Luteolin, and Xanthohumol. Int J Mol Sci [Internet]. 2024 Mar 1 [cited. 2024 Jul 13];25(6). Available from: https://pubmed.ncbi.nlm.nih.gov/38542210/
102. Li Y, Shen L, Luo H. Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway. Int Immunopharmacol [Internet]. 2016 Nov 1 [cited. 2024 Jul 13];40:24-31. Available from: https://pubmed.ncbi.nlm.nih.gov/27569028/
103. Li B, Guo Y, Jia X, Cai Y, Zhang Y, Yang Q. Luteolin alleviates ulcerative colitis in rats via regulating immune response, oxidative stress, and metabolic profiling. Open Med (Wars) [Internet]. 2023 Jan 1 [cited. 2024 Jul 13];18(1). Available from: https://pubmed.ncbi.nlm.nih.gov/37693835/
104. Zhao T, Tang H, Xie L, Zheng Y, Ma Z, Sun Q. et al. Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Pharm Pharmacol [Internet]. 2019 [cited. 2024 Mar 18];71(9):1353-69. Available from: https://pubmed.ncbi.nlm.nih.gov/31236960/
105. Huang S, Fu Y, Xu B, Liu C, Wang Q, Luo S. et al. Wogonoside alleviates colitis by improving intestinal epithelial barrier function via the MLCK/pMLC2 pathway. Phytomedicine [Internet]. 2020 Mar 1 [cited. 2024 Jul 13];68. Available from: https://pubmed.ncbi.nlm.nih.gov/32062328/
106. Sun Y, Zhao Y, Yao J, Zhao L, Wu Z, Wang Y. et al. Wogonoside protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-κB and NLRP3 inflammasome activation. Biochem Pharmacol [Internet]. 2015 Mar 15 [cited. 2024 Jul 13];94(2):142-54. Available from: https://pubmed.ncbi.nlm.nih.gov/25677765/
107. Zhou Y, Dou F, Song H, Liu T. Anti-ulcerative effects of wogonin on ulcerative colitis induced by dextran sulfate sodium via Nrf2/TLR4/NF-κB signaling pathway in BALB/c mice. Environ Toxicol [Internet]. 2022 Apr 1 [cited. 2024 Jul 13];37(4):954-63. Available from: https://pubmed.ncbi.nlm.nih.gov/35044701/
108. Ye Q, Huang S, Wang Y, Chen S, Yang H, Tan W. et al. Wogonin improves colitis by activating the AhR pathway to regulate the plasticity of ILC3/ILC1. Phytomedicine [Internet]. 2024 Jun 1 [cited. 2024 Jul 13];128. Available from: https://pubmed.ncbi.nlm.nih.gov/38518634/
109. Chen B, Luo J, Han Y, Du H, Liu J, He W. et al. Dietary Tangeretin Alleviated Dextran Sulfate Sodium-Induced Colitis in Mice via Inhibiting Inflammatory Response, Restoring Intestinal Barrier Function, and Modulating Gut Microbiota. J Agric Food Chem [Internet]. 2021 Jul 14 [cited. 2024 Mar 18];69(27):7663-74. Available from: https://pubmed.ncbi.nlm.nih.gov/34182753/
110. Eun SH, Woo J Te, Kim DH. Tangeretin Inhibits IL-12 Expression and NF-κB Activation in Dendritic Cells and Attenuates Colitis in Mice. Planta Med [Internet]. 2017 Apr 1 [cited. 2024 Mar 18];83(6):527-33. Available from: https://pubmed.ncbi.nlm.nih.gov/27806407/
111. Xiong Y, Chen D, Yu C, Lv B, Peng J, Wang J. et al. Citrus nobiletin ameliorates experimental colitis by reducing inflammation and restoring impaired intestinal barrier function. Mol Nutr Food Res [Internet]. 2015 May 1 [cited. 2024 Jul 13];59(5):829-42. Available from: https://pubmed.ncbi.nlm.nih.gov/25655748/
112. Talebi M, Talebi M, Farkhondeh T, Kopustinskiene DM, Simal-Gandara J, Bernatoniene J. et al. An updated review on the versatile role of chrysin in neurological diseases: Chemistry, pharmacology, and drug delivery approaches. Biomed Pharmacother [Internet]. 2021 Sep 1 [cited. 2024 Jul 13];141. Available from: https://pubmed.ncbi.nlm.nih.gov/34328092/
113. Stompor-gorący M, Bajek-bil A, Machaczka M. Chrysin: Perspectives on Contemporary Status and Future Possibilities as Pro-Health Agent. Nutrients [Internet]. 2021 Jun 1 [cited. 2024 Jul 13];13(6). Available from: /pmc/articles/PMC8232110/
114. Dou W, Zhang J, Zhang E, Sun A, Ding L, Chou G. et al. Chrysin ameliorates chemically induced colitis in the mouse through modulation of a PXR/NF-κB signaling pathway. J Pharmacol Exp Ther [Internet]. 2013 Jun [cited. 2024 Jul 13];345(3):473-82. Available from: https://pubmed.ncbi.nlm.nih.gov/23536316/
115. Do Socorro Chagas MS, Behrens MD, Moragas-Tellis CJ, Penedo GXM, Silva AR, Gonçalves-De-Albuquerque CF. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid Med Cell Longev [Internet]. 2022 [cited 2024 Mar 18]. 2022 Available from: https://pubmed.ncbi.nlm.nih.gov/36111166/
116. Anand David AV, Arulmoli R, Parasuraman S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn Rev [Internet]. 2016 Jul 1 [cited. 2024 Mar 18];10(20):84. Available from: /pmc/articles/PMC5214562/
117. Lyu YL, Zhou HF, Yang J, Wang FX, Sun F, Li JY. Biological Activities Underlying the Therapeutic Effect of Quercetin on Inflammatory Bowel Disease. Mediators Inflamm [Internet]. 2022 [cited 2024 Mar 18]. 2022 Available from: /pmc/articles/PMC9338876/
118. Hu S, Zhao M, Li W, Wei P, Liu Q, chen S. et al. Preclinical evidence for quercetin against inflammatory bowel disease: a meta-analysis and systematic review. Inflammopharmacology [Internet]. 2022 Dec 1 [cited. 2024 Jul 13];30(6):2035-50. Available from: https://pubmed.ncbi.nlm.nih.gov/36227442/
119. Zhang Q, Wen F, Sun F, Xu Z, Liu Y, Tao C. et al. Efficacy and Mechanism of Quercetin in the Treatment of Experimental Colitis Using Network Pharmacology Analysis. Molecules [Internet]. 2022 Jan 1 [cited. 2024 Jul 13];28(1). Available from: https://pubmed.ncbi.nlm.nih.gov/36615338/
120. Gao F, Zhu F, Shuai B, Wu M, Wei C, Yuan Y. et al. Quercetin ameliorates ulcerative colitis by restoring the balance of M2/M1 and repairing the intestinal barrier via downregulating cGAS-STING pathway. Front Pharmacol [Internet]. 2024 [cited. 2024 Jul 13];15. Available from: https://pubmed.ncbi.nlm.nih.gov/38774206/
121. Kottakis G, Kambouri K, Giatromanolaki A, Valsami G, Kostomitsopoulos N, Tsaroucha A. et al. medicina Effects of the Antioxidant Quercetin in an Experimental Model of Ulcerative Colitis in Mice. 2022 [cited. 2024 Mar 18]; Available from: https://doi.org/10.3390/medicina59010087
122. Zhang HX, Li YY, Liu ZJ, Wang JF. Quercetin effectively improves LPS-induced intestinal inflammation, pyroptosis, and disruption of the barrier function through the TLR4/NF-κB/NLRP3 signaling pathway in vivo and in vitro. Citation: Food & Nutrition Research. 2022;2022:66
123. Wang X, Xie X, Li Y, Xie X, Huang S, Pan S. et al. Quercetin ameliorates ulcerative colitis by activating aryl hydrocarbon receptor to improve intestinal barrier integrity. Phytother Res [Internet]. 2024 Jan 1 [cited. 2024 Jul 13];38(1):253-64. Available from: https://pubmed.ncbi.nlm.nih.gov/37873559/
124. Zarenezhad E, Abdulabbas HT, Kareem AS, Kouhpayeh SA, Barbaresi S, Najafipour S. et al. Protective role of flavonoids quercetin and silymarin in the viral-associated inflammatory bowel disease: an updated review. Arch Microbiol [Internet]. 2023 Jun 1 [cited. 2024 Jul 13];205(6). Available from: https://pubmed.ncbi.nlm.nih.gov/37249707/
125. Lin R, Piao M, Song Y. Dietary Quercetin Increases Colonic Microbial Diversity and Attenuates Colitis Severity in Citrobacter rodentium-Infected Mice. Front Microbiol [Internet]. 2019 [cited. 2024 Jul 13];10(MAY). Available from: https://pubmed.ncbi.nlm.nih.gov/31156598/
126. Lu SY, Dan L, Sun S, Fu T, Chen J. Dietary quercetin intake is associated with lower ulcerative colitis risk but not Crohn's disease in a prospective cohort study and in vivo experiments. Food Funct [Internet]. 2024 May 11 [cited. 2024 Jul 13];15(12). Available from: https://pubmed.ncbi.nlm.nih.gov/38807501/
127. Wang T, Lu S yuan, Dan L, Sun Y, Fu T, Tian L. et al. Higher Dietary Quercetin Intake Is Associated with Lower Risk of Adverse Outcomes among Individuals with Inflammatory Bowel Disease in a Prospective Cohort Study. J Nutr [Internet]. 2024 Jun 1 [cited. 2024 Jul 13];154(6):1861-8. Available from: https://pubmed.ncbi.nlm.nih.gov/38677479/
128. Corsale I, Carrieri P, Martellucci J, Piccolomini A, Verre L, Rigutini M. et al. Flavonoid mixture (diosmin, troxerutin, rutin, hesperidin, quercetin) in the treatment of I-III degree hemorroidal disease: a double-blind multicenter prospective comparative study. Int J Colorectal Dis [Internet]. 2018 Nov 1 [cited. 2024 Jul 13];33(11):1595-600. Available from: https://pubmed.ncbi.nlm.nih.gov/29934701/
129. Ryan JJ, Hanes DA, Bradley RD, Contractor N. Effect of a Nutrition Support Formula in Adults With Inflammatory Bowel Disease: A Pilot Study. Glob Adv Health Med [Internet]. 2019 Jul 1 [cited. 2024 Jul 13];8. Available from: https://pubmed.ncbi.nlm.nih.gov/31384513/
130. Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S. et al. Quercetin, Inflammation and Immunity. Nutrients [Internet]. 2016 Mar 15 [cited. 2024 Mar 18];8(3). Available from: /pmc/articles/PMC4808895/
131. M. Calderon-Montano J, Burgos-Moron E, Perez-Guerrero C, Lopez-Lazaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem [Internet]. 2011 Apr 12 [cited. 2024 Jul 13];11(4):298-344. Available from: https://pubmed.ncbi.nlm.nih.gov/21428901/
132. Park MY, Ji GE, Sung MK. Dietary kaempferol suppresses inflammation of dextran sulfate sodium-induced colitis in mice. Dig Dis Sci [Internet]. 2012 Feb [cited. 2024 Jul 13];57(2):355-63. Available from: https://pubmed.ncbi.nlm.nih.gov/21901258/
133. Qu Y, Li X, Xu F, Zhao S, Wu X, Wang Y. et al. Kaempferol Alleviates Murine Experimental Colitis by Restoring Gut Microbiota and Inhibiting the LPS-TLR4-NF-κB Axis. Front Immunol [Internet]. 2021 Jul 22 [cited. 2024 Jul 13];12. Available from: https://pubmed.ncbi.nlm.nih.gov/34367139/
134. Bian Y, Liu P, Zhong J, Hu Y, Fan Y, Zhuang S. et al. Kaempferol inhibits multiple pathways involved in the secretion of inflammatory mediators from LPS-induced rat intestinal microvascular endothelial cells. Mol Med Rep [Internet]. 2019 Mar 1 [cited. 2024 Mar 18];19(3):1958-64. Available from: https://pubmed.ncbi.nlm.nih.gov/30569099/
135. Bian Y, Dong Y, Sun J, Sun M, Hou Q, Lai Y. et al. Protective Effect of Kaempferol on LPS-Induced Inflammation and Barrier Dysfunction in a Coculture Model of Intestinal Epithelial Cells and Intestinal Microvascular Endothelial Cells. J Agric Food Chem [Internet]. 2020 Jan 8 [cited. 2024 Mar 18];68(1):160-7. Available from: https://pubmed.ncbi.nlm.nih.gov/31825618/
136. Yang L, Ma X yu, Mu K xin, Dai Y, Xia Y feng, Wei Z feng. Galangin Targets HSP90β to Alleviate Ulcerative Colitis by Controlling Fatty Acid Synthesis and Subsequent NLRP3 Inflammasome Activation. Mol Nutr Food Res [Internet]. 2023 Jun 1 [cited. 2024 Jul 13];67(11). Available from: https://pubmed.ncbi.nlm.nih.gov/37002873/
137. Sangaraju R, Nalban N, Alavala S, Rajendran V, Jerald MK, Sistla R. Protective effect of galangin against dextran sulfate sodium (DSS)-induced ulcerative colitis in Balb/c mice. Inflamm Res [Internet]. 2019 Aug 2 [cited. 2024 Jul 13];68(8):691-704. Available from: https://pubmed.ncbi.nlm.nih.gov/31147743/
138. Gerges SH, Tolba MF, Elsherbiny DA, El-Demerdash E. The natural flavonoid galangin ameliorates dextran sulphate sodium-induced ulcerative colitis in mice: Effect on Toll-like receptor 4, inflammation and oxidative stress. Basic Clin Pharmacol Toxicol [Internet]. 2020 Jul 1 [cited. 2024 Jul 13];127(1):10-20. Available from: https://pubmed.ncbi.nlm.nih.gov/31943791/
139. Xuan H, Ou A, Hao S, Shi J, Jin X. Galangin Protects against Symptoms of Dextran Sodium Sulfate-induced Acute Colitis by Activating Autophagy and Modulating the Gut Microbiota. Nutrients [Internet]. 2020 Feb 1 [cited. 2024 Jul 13];12(2). Available from: https://pubmed.ncbi.nlm.nih.gov/32013062/
140. Taheri Y, Suleria HAR, Martins N, Sytar O, Beyatli A, Yeskaliyeva B. et al. Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications. BMC Complement Med Ther [Internet]. 2020 Aug 1 [cited. 2024 Jul 13];20(1). Available from: /pmc/articles/PMC7395214/
141. Qu X, Li Q, Song Y, Xue A, Liu Y, Qi D. et al. Potential of myricetin to restore the immune balance in dextran sulfate sodium-induced acute murine ulcerative colitis. J Pharm Pharmacol [Internet]. 2020 Jan 1 [cited. 2024 Jul 13];72(1):92-100. Available from: https://pubmed.ncbi.nlm.nih.gov/31724745/
142. Zhao J, Hong T, Dong M, Meng Y, Mu J. Protective effect of myricetin in dextran sulphate sodium-induced murine ulcerative colitis. Mol Med Rep [Internet]. 2013 Feb [cited. 2024 Jul 13];7(2):565-70. Available from: https://pubmed.ncbi.nlm.nih.gov/23232835/
143. Miao RR, Zhan S, Hu XT, Yuan WM, Wu LJ, Cui SX. et al. Myricetin and M10, a myricetin-3-O-β-d-lactose sodium salt, modify composition of gut microbiota in mice with ulcerative colitis. Toxicol Lett [Internet]. 2021 Aug 1 [cited. 2024 Jul 13];346:7-15. Available from: https://pubmed.ncbi.nlm.nih.gov/33811973/
144. Zhao J, Yuan W, Wang S, Zhang H, Chen D, Niu X. et al. Comparative Pharmacokinetics and Tissue Distribution of M10 and Its Metabolite Myricetin in Normal and Dextran-Sodium-Sulfate-Induced Colitis Mice. Molecules [Internet]. 2022 Dec 1 [cited. 2024 Jul 13];27(23). Available from: https://pubmed.ncbi.nlm.nih.gov/36500233/
145. Pal HC, Pearlman RL, Afaq F. Fisetin and Its Role in Chronic Diseases. Adv Exp Med Biol [Internet]. 2016 Sep 1 [cited. 2024 Jul 13];928:213-44. Available from: https://pubmed.ncbi.nlm.nih.gov/27671819/
146. Ashiqueali SA, Chaudhari D, Zhu X, Noureddine S, Siddiqi S, Garcia DN. et al. Fisetin modulates the gut microbiota alongside biomarkers of senescence and inflammation in a DSS-induced murine model of colitis. Geroscience [Internet]. 2024 Jun 1 [cited. 2024 Jul 13];46(3):3085-103. Available from: https://pubmed.ncbi.nlm.nih.gov/38191834/
147. Sahu BD, Kumar JM, Sistla R. Fisetin, a dietary flavonoid, ameliorates experimental colitis in mice: Relevance of NF-κB signaling. J Nutr Biochem [Internet]. 2016 Feb 1 [cited. 2024 Jul 13];28:171-82. Available from: https://pubmed.ncbi.nlm.nih.gov/26878795/
148. Wang H, Chen L, Yang B, Du J, Chen L, Li Y. et al. Structures, Sources, Identification/Quantification Methods, Health Benefits, Bioaccessibility, and Products of Isorhamnetin Glycosides as Phytonutrients. Nutrients [Internet]. 2023 Apr 1 [cited. 2024 Jul 13];15(8). Available from: /pmc/articles/PMC10143801/
149. Dou W, Zhang J, Li H, Kortagere S, Sun K, Ding L. et al. Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway. J Nutr Biochem [Internet]. 2014 [cited. 2024 Jul 13];25(9):923-33. Available from: https://pubmed.ncbi.nlm.nih.gov/24913217/
150. Barreca D, Gattuso G, Bellocco E, Calderaro A, Trombetta D, Smeriglio A. et al. Flavanones: Citrus phytochemical with health-promoting properties. BioFactors [Internet]. 2017 Jul 8 [cited. 2024 Jul 13];43(4):495-506. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/biof.1363
151. Stabrauskiene J, Kopustinskiene DM, Lazauskas R, Bernatoniene J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines [Internet]. 2022 Jul 1 [cited. 2024 Jul 13];10(7). Available from: /pmc/articles/PMC9313440/
152. Cao R, Wu X, Guo H, Pan X, Huang R, Wang G. et al. Naringin Exhibited Therapeutic Effects against DSS-Induced Mice Ulcerative Colitis in Intestinal Barrier-Dependent Manner. Molecules [Internet]. 2021 Nov 1 [cited. 2024 Jul 13];26(21). Available from: https://pubmed.ncbi.nlm.nih.gov/34771012/
153. Hambardikar VR, Mandlik DS. Protective effect of naringin ameliorates TNBS-induced colitis in rats via improving antioxidant status and pro-inflammatory cytokines. Immunopharmacol Immunotoxicol [Internet]. 2022 [cited. 2024 Jul 13];44(3):373-86. Available from: https://pubmed.ncbi.nlm.nih.gov/35254187/
154. Kumar VS, Rajmane AR, Adil M, Kandhare AD, Ghosh P, Bodhankar SL. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J Biomed Res [Internet]. 2014 [cited. 2024 Jul 13];28(2):132-45. Available from: https://pubmed.ncbi.nlm.nih.gov/24683411/
155. Cao H, Liu J, Shen P, Cai J, Han Y, Zhu K. et al. Protective Effect of Naringin on DSS-Induced Ulcerative Colitis in Mice. J Agric Food Chem [Internet]. 2018 Dec 19 [cited. 2024 Jul 13];66(50):13133-40. Available from: https://pubmed.ncbi.nlm.nih.gov/30472831/
156. Dong J, Chen Y, Yang F, Zhang W, Wei K, Xiong Y. et al. Naringin Exerts Therapeutic Effects on Mice Colitis: A Study Based on Transcriptomics Combined With Functional Experiments. Front Pharmacol [Internet]. 2021 Aug 24 [cited. 2024 Jul 13];12. Available from: https://pubmed.ncbi.nlm.nih.gov/34504431/
157. Yang Q, Liang Q, Balakrishnan B, Belobrajdic DP, Feng QJ, Zhang W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients. 2020;12:381
158. Liu J, Xu L, Wang L, Wang Q, Yu L, Zhang S. Naringin Alleviates Intestinal Fibrosis by Inhibiting ER Stress-Induced PAR2 Activation. Inflamm Bowel Dis [Internet]. 2024 Apr 1 [cited. 2024 Jul 13]; Available from: https://pubmed.ncbi.nlm.nih.gov/38557865/
159. Zhang YS, Wang F, Cui SX, Qu XJ. Natural dietary compound naringin prevents azoxymethane/dextran sodium sulfate-induced chronic colorectal inflammation and carcinogenesis in mice. Cancer Biol Ther [Internet]. 2018 [cited. 2024 Jul 13];19(8):735-44. Available from: https://pubmed.ncbi.nlm.nih.gov/29580144/
160. Li C, Zhang J, Lv F, Ge X, Li G. Naringin protects against bone loss in steroid-treated inflammatory bowel disease in a rat model. Arch Biochem Biophys [Internet]. 2018 Jul 15 [cited. 2024 Jul 13];650:22-9. Available from: https://pubmed.ncbi.nlm.nih.gov/29753722/
161. Lee KI, Kim MS, Yuk HJ, Jo Y, Kim HJ, Kim J. et al. Alleviating depressive-like behavior in DSS-induced colitis mice: Exploring naringin and poncirin from Poncirus trifoliata extracts. Biomed Pharmacother [Internet]. 2024 Jun 1 [cited. 2024 Jul 13];175. Available from: https://pubmed.ncbi.nlm.nih.gov/38772154/
162. Liu Z, Niu X, Wang J. Naringenin as a natural immunomodulator against T cell-mediated autoimmune diseases: literature review and network-based pharmacology study. Crit Rev Food Sci Nutr [Internet]. 2023 [cited. 2024 Jul 13];63(32):11026-43. Available from: https://pubmed.ncbi.nlm.nih.gov/35776085/
163. Al-Rejaie SS, Abuohashish HM, Al-Enazi MM, Al-Assaf AH, Parmar MY, Ahmed MM. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J Gastroenterol [Internet]. 2013 Sep 14 [cited. 2024 Jul 13];19(34):5633-44. Available from: https://pubmed.ncbi.nlm.nih.gov/24039355/
164. Azuma T, Shigeshiro M, Kodama M, Tanabe S, Suzuki T. Supplemental naringenin prevents intestinal barrier defects and inflammation in colitic mice. J Nutr [Internet]. 2013 Jun [cited. 2024 Jul 13];143(6):827-34. Available from: https://pubmed.ncbi.nlm.nih.gov/23596159/
165. Wdowiak K, Walkowiak J, Pietrzak R, Bazan-Woźniak A, Cielecka-Piontek J. Bioavailability of Hesperidin and Its Aglycone Hesperetin—Compounds Found in Citrus Fruits as a Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)—Mini-Review. Nutrients [Internet]. 2022 Jul 1 [cited. 2024 Jul 13];14(13). Available from: /pmc/articles/PMC9268531/
166. Guazelli CFS, Fattori V, Ferraz CR, Borghi SM, Casagrande R, Baracat MM. et al. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem Biol Interact [Internet]. 2021 Jan 5 [cited. 2024 Jul 13];333. Available from: https://pubmed.ncbi.nlm.nih.gov/33171134/
167. Shafik NM, Gaber RA, Mohamed DA, Ebeid AM. Hesperidin modulates dextran sulfate sodium-induced ulcerative colitis in rats: Targeting sphingosine kinase-1- sphingosine 1 phosphate signaling pathway, mitochondrial biogenesis, inflammation, and apoptosis. J Biochem Mol Toxicol [Internet]. 2019 Jun 1 [cited. 2024 Jul 13];33(6). Available from: https://pubmed.ncbi.nlm.nih.gov/30811821/
168. Xu L, Yang Z lin, Li P, Zhou Y qiang. Modulating effect of Hesperidin on experimental murine colitis induced by dextran sulfate sodium. Phytomedicine [Internet]. 2009 Oct [cited. 2024 Jul 13];16(10):989-95. Available from: https://pubmed.ncbi.nlm.nih.gov/19386481/
169. Zhang J, Lei H, Hu X, Dong W. Hesperetin ameliorates DSS-induced colitis by maintaining the epithelial barrier via blocking RIPK3/MLKL necroptosis signaling. Eur J Pharmacol [Internet]. 2020 Apr 15 [cited. 2024 Jul 13];873. Available from: https://pubmed.ncbi.nlm.nih.gov/32035144/
170. Elhennawy MG, Abdelaleem EA, Zaki AA, Mohamed WR. Cinnamaldehyde and hesperetin attenuate TNBS-induced ulcerative colitis in rats through modulation of the JAk2/STAT3/SOCS3 pathway. J Biochem Mol Toxicol [Internet]. 2021 May 1 [cited. 2024 Jul 13];35(5):e22730. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/jbt.22730
171. Polat FR, Karaboğa L. Immunohistochemical examination of anti-inflammatory and anti-apoptotic effects of hesperetin on trinitrobenzene sulfonic acid induced colitis in rats. Biotech Histochem [Internet]. 2019 Apr 3 [cited. 2024 Jul 13];94(3):151-8. Available from: https://pubmed.ncbi.nlm.nih.gov/30383440/
172. Deng Z, Hassan S, Rafiq M, Li H, He Y, Cai Y. et al. Pharmacological Activity of Eriodictyol: The Major Natural Polyphenolic Flavanone. Evid Based Complement Alternat Med [Internet]. 2020 [cited 2024 Jul 13]. 2020 Available from: /pmc/articles/PMC7752289/
173. Yao L, Liu W, Bashir M, Nisar MF, Wan C (Craig). Eriocitrin: A review of pharmacological effects. Biomedicine & Pharmacotherapy. 2022Oct1;154:113563
174. Patel DK. Therapeutic Potential of Poncirin Against Numerous Human Health Complications: Medicinal Uses and Therapeutic Benefit of an Active Principle of Citrus Species. Endocr Metab Immune Disord Drug Targets [Internet]. 2021 Jan 11 [cited. 2024 Jul 13];21(11):1974-81. Available from: https://pubmed.ncbi.nlm.nih.gov/33423654/
175. Hu LH, Liu JY, Yin J Bin. Eriodictyol attenuates TNBS-induced ulcerative colitis through repressing TLR4/NF-kB signaling pathway in rats. Kaohsiung J Med Sci [Internet]. 2021 Sep 1 [cited. 2024 Jul 13];37(9):812-8. Available from: https://pubmed.ncbi.nlm.nih.gov/34042266/
176. Wang R, Shen L, Li H, Peng H. Eriodictyol attenuates dextran sodium sulphate-induced colitis in mice by regulating the sonic hedgehog signalling pathway. Pharm Biol [Internet]. 2021 [cited. 2024 Jul 13];59(1):974-85. Available from: https://pubmed.ncbi.nlm.nih.gov/34348563/
177. Guo G, Shi W, Shi F, Gong W, Li F, Zhou G. et al. Anti-inflammatory effects of eriocitrin against the dextran sulfate sodium-induced experimental colitis in murine model. J Biochem Mol Toxicol [Internet]. 2019 Nov 1 [cited. 2024 Jul 13];33(11). Available from: https://pubmed.ncbi.nlm.nih.gov/31593355/
178. Hong I, Kaang BK. The Complexity of Ventral CA1 and Its Multiple Functionalities. Genes Brain Behav. 2022;21:e12826
179. Park H, Jin UH, Orr AA, Echegaray SP, Davidson LA, Allred CD. et al. Isoflavones as Ah Receptor Agonists in Colon-Derived Cell Lines: Structure-Activity Relationships. Chem Res Toxicol [Internet]. 2019 Nov 18 [cited. 2024 Mar 18];32(11):2353-64. Available from: https://pubmed.ncbi.nlm.nih.gov/31621310/
180. Jung YS, Rha CS, Baik MY, Baek NI, Kim DO. A brief history and spectroscopic analysis of soy isoflavones. Food Sci Biotechnol [Internet]. 2020 Dec 1 [cited. 2024 Mar 18];29(12):1605-17. Available from: https://link.springer.com/article/10.1007/s10068-020-00815-6
181. Zaheer K, Humayoun Akhtar M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit Rev Food Sci Nutr [Internet]. 2017 Apr 13 [cited. 2024 Jul 13];57(6):1280-93. Available from: https://pubmed.ncbi.nlm.nih.gov/26565435/
182. Liggins J, Bluck LJC, Runswick S, Atkinson C, Coward WA, Bingham SA. Daidzein and genistein content of fruits and nuts. J Nutr Biochem [Internet]. 2000 [cited. 2024 Jul 13];11(6):326-31. Available from: https://pubmed.ncbi.nlm.nih.gov/11002128/
183. Wijeratne SSK, Cuppett SL. Soy isoflavones protect the intestine from lipid hydroperoxide mediated oxidative damage. J Agric Food Chem [Internet]. 2007 Nov 28 [cited. 2024 Jul 13];55(24):9811-6. Available from: https://pubmed.ncbi.nlm.nih.gov/17960878/
184. Noda S, Tanabe S, Suzuki T. Differential effects of flavonoids on barrier integrity in human intestinal Caco-2 cells. J Agric Food Chem [Internet]. 2012 May 9 [cited. 2024 Jul 13];60(18):4628-33. Available from: https://pubmed.ncbi.nlm.nih.gov/22506771/
185. Calvello R, Aresta A, Trapani A, Zambonin C, Cianciulli A, Salvatore R. et al. Bovine and soybean milk bioactive compounds: Effects on inflammatory response of human intestinal Caco-2 cells. Food Chem [Internet]. 2016 Nov 1 [cited. 2024 Jul 13];210:276-85. Available from: https://pubmed.ncbi.nlm.nih.gov/27211648/
186. Shen J, Li N, Zhang X. Daidzein Ameliorates Dextran Sulfate Sodium-Induced Experimental Colitis in Mice by Regulating NF-κB Signaling. Journal of Environmental Pathology, Toxicology and Oncology [Internet]. 2019 [cited. 2024 Mar 18];38(1):29-39. Available from: https://www.dl.begellhouse.com/journals/0ff459a57a4c08d0,4b309b253eb144f8,09ff16a8457417f2.html
187. Yu G, Liu Y, Ou W, Dai J, Ai Q, Zhang W. et al. The protective role of daidzein in intestinal health of turbot (Scophthalmus maximus L.) fed soybean meal-based diets. Scientific Reports. 2021;11:1 [Internet]. 2021 Feb 8 [cited 2024 Mar 18];11(1):1-13. Available from: https://www.nature.com/articles/s41598-021-82866-1
188. Morimoto M, Watanabe T, Yamori M, Takebe M, Wakatsuki Y. Isoflavones regulate innate immunity and inhibit experimental colitis. J Gastroenterol Hepatol [Internet]. 2009 [cited. 2024 Jul 13];24(6):1123-9. Available from: https://pubmed.ncbi.nlm.nih.gov/19220665/
189. Shen J, Li N, Zhang X. Daidzein Ameliorates Dextran Sulfate Sodium-Induced Experimental Colitis in Mice by Regulating NF-κB Signaling. J Environ Pathol Toxicol Oncol [Internet]. 2019 [cited. 2024 Jul 13];38(1):29-39. Available from: https://pubmed.ncbi.nlm.nih.gov/30806288/
190. Skolmowska D, Głabska D, Guzek D, Lech G. Association between Dietary Isoflavone Intake and Ulcerative Colitis Symptoms in Polish Caucasian Individuals. Nutrients [Internet]. 2019 Aug 1 [cited. 2024 Jul 13];11(8):1936. Available from: /pmc/articles/PMC6722525/
191. Ohfuji S, Fukushima W, Watanabe K, Sasaki S, Yamagami H, Nagahori M. et al. Pre-Illness Isoflavone Consumption and Disease Risk of Ulcerative Colitis: A Multicenter Case-Control Study in Japan. PLoS One [Internet]. 2014 Oct 14 [cited. 2024 Jul 13];9(10). Available from: /pmc/articles/PMC4196952/
192. Messina M, Ho S, Alekel DL. Skeletal Benefits of Soy Isoflavones: A Review of the Clinical Trial and Epidemiologic Data. Curr Opin Clin Nutr Metab Care. 2004;7:649-658
193. Głąbska D, Guzek D, Grudzińska D, Lech G. Influence of dietary isoflavone intake on gastrointestinal symptoms in ulcerative colitis individuals in remission. World J Gastroenterol [Internet]. 2017 Aug 8 [cited. 2024 Jul 13];23(29):5356. Available from: /pmc/articles/PMC5550784/
194. Abron JD, Singh NP, Price RL, Nagarkatti M, Nagarkatti PS, Singh UP. Genistein induces macrophage polarization and systemic cytokine to ameliorate experimental colitis. PLoS One [Internet]. 2018 Jul 1 [cited. 2024 Mar 18];13(7). Available from: https://pubmed.ncbi.nlm.nih.gov/30024891/
195. Chen Y, Le TH, Du Q, Zhao Z, Liu Y, Zou J. et al. Genistein protects against DSS-induced colitis by inhibiting NLRP3 inflammasome via TGR5-cAMP signaling. Int Immunopharmacol [Internet]. 2019 Jun 1 [cited. 2024 Jul 13];71:144-54. Available from: https://pubmed.ncbi.nlm.nih.gov/30901677/
196. López-Posadas R, Ballester I, Mascaraque C, Suárez MD, Zarzuelo A, Martínez-Augustin O. et al. Flavonoids exert distinct modulatory actions on cyclooxygenase 2 and NF-κB in an intestinal epithelial cell line (IEC18). Br J Pharmacol [Internet]. 2010 Aug [cited. 2024 Jul 13];160(7):1714. Available from: /pmc/articles/PMC2936843/
197. Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Diel P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur J Nutr [Internet]. 2009 Jun [cited. 2024 Jul 13];48(4):213-20. Available from: https://pubmed.ncbi.nlm.nih.gov/19234664/
198. Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-Inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-kappaB Activations, Whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit only NF-kappaB Activation along with Their Inhibitory Effect on iNOS Expression and NO Production in Activated Macrophages. Mediators Inflamm [Internet]. 2007 [cited. 2024 Jul 13];2007:45673. Available from: /pmc/articles/PMC2220047/
199. Rao RK, Basuroy S, Rao VU, Karnaky KJ, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-β-catenin complexes from the cytoskeleton by oxidative stress. Biochemical Journal [Internet]. 2002 Dec 1 [cited. 2024 Jul 13];368(2):471-81. Available from: /biochemj/article/368/2/471/40075/Tyrosine-phosphorylation-and-dissociation-of
200. Romier B, Van De Walle J, During A, Larondelle Y, Schneider YJ. Modulation of signalling nuclear factor-kappaB activation pathway by polyphenols in human intestinal Caco-2 cells. Br J Nutr [Internet]. 2008 [cited. 2024 Jul 13];100(3):542-51. Available from: https://pubmed.ncbi.nlm.nih.gov/18377686/
201. Wu ZY, Sang LX, Chang B. Isoflavones and inflammatory bowel disease. World J Clin Cases [Internet]. 2020 Jun 6 [cited. 2024 Jul 13];8(11):2081-2091. Available from: /pmc/articles/PMC7281056/
202. Jia Q, Fang S, Yang R, Ling Y, Mehmood S, Ni H. et al. Genistein alleviates dextran sulfate sodium-induced colitis in mice through modulation of intestinal microbiota and macrophage polarization. Eur J Nutr [Internet]. 2024 [cited. 2024 Jul 13]; Available from: https://pubmed.ncbi.nlm.nih.gov/38592519/
203. Alharbi TS, Alshammari ZS, Alanzi ZN, Althobaiti F, Elewa MAF, Hashem KS. et al. Therapeutic effects of genistein in experimentally induced ulcerative colitis in rats via affecting mitochondrial biogenesis. Mol Cell Biochem [Internet]. 2024 Feb 1 [cited. 2024 Jul 13];479(2):431-44. Available from: https://pubmed.ncbi.nlm.nih.gov/37084167/
204. Elhefnawy EA, Zaki HF, El Maraghy NN, Ahmed KA, Abd El-Haleim EA. Genistein and/or sulfasalazine ameliorate acetic acid-induced ulcerative colitis in rats via modulating INF-γ/JAK1/STAT1/IRF-1, TLR-4/NF-κB/IL-6, and JAK2/STAT3/COX-2 crosstalk. Biochem Pharmacol [Internet]. 2023 Aug 1 [cited. 2024 Jul 13];214. Available from: https://pubmed.ncbi.nlm.nih.gov/37414101/
205. Tanideh N, Sadeghi F, Amanat S, Firoozi D, Noorafshan A, Iraji A. et al. Protection by pure and genistein fortified extra virgin olive oil, canola oil, and rice bran oil against acetic acid-induced ulcerative colitis in rats. Food Funct [Internet]. 2020 Jan 1 [cited. 2024 Jul 13];11(1):860-70. Available from: https://pubmed.ncbi.nlm.nih.gov/31942583/
206. Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Degen GH, Diel P. In utero and postnatal exposure to a phytoestrogen-enriched diet increases parameters of acute inflammation in a rat model of TNBS-induced colitis. Arch Toxicol [Internet]. 2008 May 27 [cited. 2024 Jul 13];82(12):941-50. Available from: https://link.springer.com/article/10.1007/s00204-008-0309-7
207. Tsuchiya M, Ito G, Hama M, Nagata S, Kawamoto A, Suzuki K. et al. Functional analysis of isoflavones using patient-derived human colonic organoids. Biochem Biophys Res Commun [Internet]. 2021 Feb 26 [cited. 2024 Jul 13];542:40-7. Available from: https://pubmed.ncbi.nlm.nih.gov/33486190/
208. Ollberding NJ, Lim U, Wilkens LR. et al. Legume, Soy, Tofu, and Isoflavone Intake and Endometrial Cancer Risk in Postmenopausal Women in the Multiethnic Cohort Study. J Natl Cancer Inst. 2012;104:67-76
209. Hu X, Liu W, He M, Qiu Q, Zhou B, Liu R. et al. Comparison of the molecular mechanisms of Fuzi Lizhong Pill and Huangqin decoction in the treatment of the cold and heat syndromes of ulcerative colitis based on network pharmacology. Comput Biol Med [Internet]. 2023 Jun 1 [cited. 2024 Jul 13];159. Available from: https://pubmed.ncbi.nlm.nih.gov/37084637/
210. Li F, Yang Y, Ge J, Wang C, Chen Z, Li Q. et al. Multi-omics revealed the mechanisms of Codonopsis pilosula aqueous extract in improving UC through blocking abnormal activation of PI3K/Akt signaling pathway. J Ethnopharmacol [Internet]. 2024 Jan 30 [cited. 2024 Jul 13];319(Pt 2). Available from: https://pubmed.ncbi.nlm.nih.gov/37820998/
211. Ollberding NJ, Lim U, Wilkens LR. et al. Legume, Soy, Tofu, and Isoflavone Intake and Endometrial Cancer Risk in Postmenopausal Women in the Multiethnic Cohort Study. J Natl Cancer Inst. 2012;104:67-76
212. Zhang D, Duan S, He Z, Zhu Z, Li Z, Yi Q. et al. Sijunzi Decoction Targets IL1B and TNF to Reduce Neutrophil Extracellular Traps (NETs) in Ulcerative Colitis: Evidence from Silicon Prediction and Experiment Validation. Drug Des Devel Ther [Internet]. 2023 [cited. 2024 Jul 13];17:3103-28. Available from: https://pubmed.ncbi.nlm.nih.gov/37868820/
213. Ren J, Ding Y, Li S, Lei M. Predicting the anti-inflammatory mechanism of Radix Astragali using network pharmacology and molecular docking. Medicine [Internet]. 2023 Sep 1 [cited. 2024 Jul 13];102(35):E34945. Available from: https://pubmed.ncbi.nlm.nih.gov/37657026/
214. Chen M, Ding Y, Tong Z. Efficacy and Safety of Sophora flavescens (Kushen) Based Traditional Chinese Medicine in the Treatment of Ulcerative Colitis: Clinical Evidence and Potential Mechanisms. Front Pharmacol [Internet]. 2020 Dec 10 [cited. 2024 Jul 13];11. Available from: https://pubmed.ncbi.nlm.nih.gov/33362558/
215. Chen L, Shao J, Luo Y, Zhao L, Zhao K, Gao Y. et al. An integrated metabolism in vivo analysis and network pharmacology in UC rats reveal anti-ulcerative colitis effects from Sophora flavescens EtOAc extract. J Pharm Biomed Anal [Internet]. 2020 Jul 15 [cited. 2024 Jul 13];186. Available from: https://pubmed.ncbi.nlm.nih.gov/32371325/
216. Zhang Z, Chong W, Xie X, Liu Y, Shang L, Li L. Hedysarum multijugum Maxim treats ulcerative colitis through the PI3K-AKT and TNF signaling pathway according to network pharmacology and molecular docking. Ann Transl Med [Internet]. 2022 Oct [cited. 2024 Jul 13];10(20):1132-1132. Available from: https://pubmed.ncbi.nlm.nih.gov/36388782/
217. Zhang Y, Li W wen, Wang Y, Fan Y wen, Wang Q yi, Liu C. et al. Investigation of the material basis and mechanism of Lizhong decoction in ameliorating ulcerative colitis based on spectrum-effect relationship and network pharmacology. J Ethnopharmacol [Internet]. 2024 Apr 6 [cited. 2024 Jul 13];323. Available from: https://pubmed.ncbi.nlm.nih.gov/38159822/
218. Barbosa Bezerra G, de Menezes de Souza L, dos Santos AS, de Almeida GKM, Souza MTS, Santos SL. et al. Hydroalcoholic extract of Brazilian red propolis exerts protective effects on acetic acid-induced ulcerative colitis in a rodent model. Biomed Pharmacother [Internet]. 2017 Jan 1 [cited. 2024 Jul 13];85:687-96. Available from: https://pubmed.ncbi.nlm.nih.gov/27955827/
219. Kulhari U, Rajanan A, Ambujakshan A, Verma S, Mugale MN, Sahu BD. Biochanin A mitigates ulcerative colitis and intestinal inflammation in mice by inhibiting MAPK/NF-kB (p65) axis. J Biochem Mol Toxicol [Internet]. 2024 Jun 1 [cited. 2024 Jul 13];38(6). Available from: https://pubmed.ncbi.nlm.nih.gov/38764152/
220. Zhang H, Wang X, Zhao L, Zhang K, Cui J, Xu G. Biochanin a ameliorates DSS-induced ulcerative colitis by improving colonic barrier function and protects against the development of spontaneous colitis in the Muc2 deficient mice. Chem Biol Interact [Internet]. 2024 May 25 [cited. 2024 Jul 13];395. Available from: https://pubmed.ncbi.nlm.nih.gov/38648921/
221. Zhang H, Wang X, Zhao L. et al. Biochanin a Ameliorates DSS-Induced Ulcerative Colitis by Improving Colonic Barrier Function and Protects against the Development of Spontaneous Colitis in the Muc2 Deficient Mice. Chem Biol Interact. 2024;395:111014
222. Mayo B, Vázquez L, Flórez AB. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients [Internet]. 2019 Sep 1 [cited. 2024 Jul 13];11(9). Available from: /pmc/articles/PMC6770660/
223. Sakai T, Furoku S, Nakamoto M, Shuto E, Hosaka T, Nishioka Y. et al. Soy isoflavone equol perpetuates dextran sulfate sodium-induced acute colitis in mice. Biosci Biotechnol Biochem [Internet]. 2011 [cited. 2024 Jul 13];75(3):593-5. Available from: https://pubmed.ncbi.nlm.nih.gov/21389602/
224. Li M, Han X, Sun L, Liu X, Zhang W, Hao J. Indole-3-acetic acid alleviates DSS-induced colitis by promoting the production of R-equol from Bifidobacterium pseudolongum. Gut Microbes [Internet]. 2024 [cited. 2024 Jul 13];16(1). Available from: https://pubmed.ncbi.nlm.nih.gov/38528729/
225. Hur H-G, Rafii F. Biotransformation of the Isoflavonoids Biochanin A, Formononetin, and Glycitein by Eubacterium Limosum. FEMS Microbiol Lett. 2000;192:21-25
226. Luo Y, Jian Y, Liu Y, Jiang S, Muhammad D, Wang W. Citation: molecules Flavanols from Nature: A Phytochemistry and Biological Activity Review. 2022 [cited. 2024 Mar 18]; Available from: https://doi.org/10.3390/molecules27030719
227. Fathima A, Rao JR. Selective toxicity of Catechin-a natural flavonoid towards bacteria. Appl Microbiol Biotechnol [Internet]. 2016 Jul 1 [cited. 2024 Mar 18];100(14):6395-402. Available from: https://pubmed.ncbi.nlm.nih.gov/27052380/
228. Braicu C, Ladomery MR, Chedea VS, Irimie A, Berindan-Neagoe I. The relationship between the structure and biological actions of green tea catechins. Food Chem [Internet]. 2013 [cited. 2024 Mar 18];141(3):3282-9. Available from: https://pubmed.ncbi.nlm.nih.gov/23871088/
229. Meltzer SM, Monk BJ, Tewari KS. Green tea catechins for treatment of external genital warts. Am J Obstet Gynecol. 2009;200(3):233.e1-233.e7
230. Hold GL, Smith M, Grange C, Watt ER, El-Omar EM, Mukhopadhya I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol [Internet]. 2014 Feb 7 [cited. 2024 Mar 18];20(5):1192-210. Available from: https://pubmed.ncbi.nlm.nih.gov/24574795/
231. Fan FY, Sang LX, Jiang M, Mcphee DJ. molecules Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. 2017 [cited. 2024 Mar 18]; Available from: www.mdpi.com/journal/molecules
232. Zhang HJ, Deng AJ, Zhang ZH, Yu ZH, Liu Y, Peng SY. et al. The protective effect of epicatechin on experimental ulcerative colitis in mice is mediated by increasing antioxidation and by the inhibition of NF-κB pathway. Pharmacol Rep [Internet]. 2016 Jun 1 [cited. 2024 Jul 13];68(3):514-20. Available from: https://pubmed.ncbi.nlm.nih.gov/26878122/
233. Wei R, Liu X, Wang Y, Dong J, Wu F, Mackenzie GG. et al. (-)-Epigallocatechin-3-gallate mitigates cyclophosphamide-induced intestinal injury by modulating the tight junctions, inflammation and dysbiosis in mice. Food Funct [Internet]. 2021 Nov 21 [cited. 2024 Mar 18];12(22):11671-85. Available from: https://pubmed.ncbi.nlm.nih.gov/34730149/
234. Du Y, Ding H, Vanarsa K, Soomro S, Baig S, Hicks J. et al. Low dose Epigallocatechin Gallate Alleviates Experimental Colitis by Subduing Inflammatory Cells and Cytokines, and Improving Intestinal Permeability. Nutrients [Internet]. 2019 Aug 1 [cited. 2024 Jul 13];11(8). Available from: https://pubmed.ncbi.nlm.nih.gov/31362373/
235. Wei F, Li D, Chen X, Li Y, Zeng Y, Cai Y. et al. Therapeutic effects of epigallocatechin-3-gallate for inflammatory bowel disease: A preclinical meta-analysis. Phytomedicine [Internet]. 2024 Jun 1 [cited. 2024 Jul 13];128. Available from: https://pubmed.ncbi.nlm.nih.gov/38503153/
236. Chiou YS, Ma NJL, Sang S, Ho CT, Wang YJ, Pan MH. Peracetylated (-)-epigallocatechin-3-gallate (AcEGCG) potently suppresses dextran sulfate sodium-induced colitis and colon tumorigenesis in mice. J Agric Food Chem [Internet]. 2012 Apr 4 [cited. 2024 Mar 18];60(13):3441-51. Available from: https://pubmed.ncbi.nlm.nih.gov/22409325/
237. Hu M, Huang Y, Du X, Liu G, Qi B, Li Y. The synergistic effect of epigallocatechin-3-gallate and quercetin co-loaded hydrogel beads on inflammatory bowel disease. Food Funct [Internet]. 2023 Mar 28 [cited. 2024 Jul 13];14(10):4539-51. Available from: https://pubmed.ncbi.nlm.nih.gov/37067270/
238. Qi Q, Chu M, Yu X, Xie Y, Li Y, Du Y. et al. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Reviews International [Internet]. 2023 Aug 25 [cited 2024 Jul 13];39(7):4581-609. Available from: https://www.tandfonline.com/doi/abs/10.1080/87559129. 2022 2029479
239. Zhao S, Zhang L, Yang C, Li Z, Rong S. Procyanidins and Alzheimer's Disease. Molecular Neurobiology. 2019;56:8 [Internet]. 2019 Jan 16 [cited 2024 Mar 18];56(8):5556-67. Available from: https://link.springer.com/article/10.1007/s12035-019-1469-6
240. González-Quilen C, Rodríguez-Gallego E, Beltrán-Debón R, Pinent M, Ardévol A, Blay MT. et al. Health-Promoting Properties of Proanthocyanidins for Intestinal Dysfunction. Nutrients 2020, Vol 12, Page 130 [Internet]. 2020 Jan 2 [cited. 2024 Jul 13];12(1):130. Available from: https://www.mdpi.com/2072-6643/12/1/130/htm
241. Rue EA, Rush MD, van Breemen RB. Procyanidins: a comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem Rev [Internet]. 2018 Feb 1 [cited. 2024 Jul 13];17(1):1. Available from: /pmc/articles/PMC5891158/
242. Wang X, Quan S, Li J, Liu Y, Sun H, Zhang J. et al. Protective Effects of Grape Seed Proanthocyanidin Extract in Preventing DSS Induced Ulcerative Colitis Based on Pharmacodynamic, Pharmacokinetic and Tissue Distribution. Curr Drug Metab [Internet]. 2022 Jun 13 [cited. 2024 Jul 13];23(6):496-505. Available from: https://pubmed.ncbi.nlm.nih.gov/35692132/
243. Sheng K, Zhang G, Sun M, He S, Kong X, Wang J. et al. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct [Internet]. 2020 Sep 1 [cited. 2024 Jul 13];11(9):7817-29. Available from: https://pubmed.ncbi.nlm.nih.gov/32808642/
244. Zhang M, Mo R, Wang H, Liu T, Zhang G, Wu Y. Grape seed proanthocyanidin improves intestinal inflammation in canine through regulating gut microbiota and bile acid compositions. FASEB J [Internet]. 2023 Dec 1 [cited. 2024 Jul 13];37(12). Available from: https://pubmed.ncbi.nlm.nih.gov/37933950/
245. Kitabatake M, Matsumura Y, Ouji-Sageshima N, Nishioka T, Hara A, Kayano S ichi. et al. Persimmon-derived tannin ameliorates the pathogenesis of ulcerative colitis in a murine model through inhibition of the inflammatory response and alteration of microbiota. Sci Rep [Internet]. 2021 Dec 1 [cited. 2024 Jul 13];11(1). Available from: https://pubmed.ncbi.nlm.nih.gov/33790314/
246. Diaz MS, Mertens-Talcott SU, Talcott ST. Intestinal Microbiome Metabolism of Cranberry (Vaccinium macrocarpon) Proanthocyanidin Dimers, but Not Trimers, Is Altered by Dysbiosis in Ulcerative Colitis Ex Vivo. J Agric Food Chem [Internet]. 2024 Feb 28 [cited. 2024 Jul 13];72(8):4184-94. Available from: https://pubmed.ncbi.nlm.nih.gov/38350030/
247. Shi Y, Zhang H, Li S, Xin D, Li S, Yan B. et al. Procyanidin improves experimental colitis by regulating macrophage polarization. Biomedicine & Pharmacotherapy. 2023Sep1;165:115076
248. Chen L, You Q, Hu L, Gao J, Meng Q, Liu W. et al. The Antioxidant Procyanidin Reduces Reactive Oxygen Species Signaling in Macrophages and Ameliorates Experimental Colitis in Mice. Front Immunol [Internet]. 2018 Jan 5 [cited. 2024 Jul 13];8(JAN). Available from: https://pubmed.ncbi.nlm.nih.gov/29354126/
249. Wang N, Chen W, Cui C, Zheng Y, Yu Q, Ren H. et al. The Peanut Skin Procyanidins Attenuate DSS-Induced Ulcerative Colitis in C57BL/6 Mice. Antioxidants (Basel) [Internet]. 2022 Nov 1 [cited. 2024 Jul 13];11(11). Available from: https://pubmed.ncbi.nlm.nih.gov/36358470/
250. Huang B, Wang L, Liu M, Wu X, Lu Q, Liu R. The underlying mechanism of A-type procyanidins from peanut skin on DSS-induced ulcerative colitis mice by regulating gut microbiota and metabolism. J Food Biochem [Internet]. 2022 Jul 1 [cited. 2024 Jul 13];46(7). Available from: https://pubmed.ncbi.nlm.nih.gov/35218055/
251. Zhang H, Lang W, Liu X, Bai J, Jia Q, Shi Q. Procyanidin A1 alleviates DSS-induced ulcerative colitis via regulating AMPK/mTOR/p70S6K-mediated autophagy. J Physiol Biochem [Internet]. 2022 Feb 1 [cited. 2024 Mar 18];78(1):213-27. Available from: https://pubmed.ncbi.nlm.nih.gov/35001346/
252. Zhu X, Tian X, Yang M, Yu Y, Zhou Y, Gao Y. et al. Procyanidin B2 Promotes Intestinal Injury Repair and Attenuates Colitis-Associated Tumorigenesis via Suppression of Oxidative Stress in Mice. Antioxid Redox Signal [Internet]. 2021 Jul 10 [cited. 2024 Jul 13];35(2):75-92. Available from: https://pubmed.ncbi.nlm.nih.gov/32940048/
253. Jiang C, Zhu P, Shi Y, Xiang W, Ge S, Zhang Z. et al. [Protective effect of procyanidin B2 on intestinal barrier and against enteritis in a mouse model of trinitrobenzene sulphonic acid-induced colitis]. Nan Fang Yi Ke Da Xue Xue Bao [Internet]. 2019 Jul 20 [cited. 2024 Jul 13];39(7):778-83. Available from: https://pubmed.ncbi.nlm.nih.gov/31340909/
254. Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res [Internet]. 2017 [cited. 2024 Mar 18];61(1). Available from: /pmc/articles/PMC5613902/
255. Mattioli R, Francioso A, Mosca L, Silva P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules [Internet]. 2020 Sep 1 [cited. 2024 Jul 13];25(17). Available from: /pmc/articles/PMC7504512/
256. Crozier A, Del Rio D, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Aspects Med [Internet]. 2010 Dec [cited. 2024 Mar 18];31(6):446-67. Available from: https://pubmed.ncbi.nlm.nih.gov/20854839/
257. Farzaei MH, El-Senduny FF, Momtaz S, Parvizi F, Iranpanah A, Tewari D. et al. An update on dietary consideration in inflammatory bowel disease: anthocyanins and more. Expert Rev Gastroenterol Hepatol [Internet]. 2018 Oct 3 [cited. 2024 Mar 18];12(10):1007-24. Available from: https://pubmed.ncbi.nlm.nih.gov/30136591/
258. Kaspar KL, Park JS, Brown CR, Mathison BD, Navarre DA, Chew BP. Pigmented potato consumption alters oxidative stress and inflammatory damage in men. J Nutr [Internet]. 2011 Jan 1 [cited. 2024 Mar 18];141(1):108-11. Available from: https://pubmed.ncbi.nlm.nih.gov/21106930/
259. Verediano TA, Stampini Duarte Martino H, Dias Paes MC, Tako E. Effects of Anthocyanin on Intestinal Health: A Systematic Review. Nutrients [Internet]. 2021 Apr 1 [cited. 2024 Jul 13];13(4). Available from: /pmc/articles/PMC8074038/
260. Fang J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition [Internet]. 2015 Nov 1 [cited. 2024 Jul 13];31(11-12):1301-6. Available from: https://pubmed.ncbi.nlm.nih.gov/26250485/
261. Minaiyan M, Ghannadi A, Mahzouni P, Jaffari-Shirazi E. Comparative Study of Berberis vulgaris Fruit Extract and Berberine Chloride Effects on Acetic Acid-Induced Colitis in Rats. Iran J Pharm Res [Internet]. 2011 [cited. 2024 Mar 18];10(1):97. Available from: /pmc/articles/PMC3869597/
262. Paturi G, Mandimika T, Butts CA, Zhu S, Roy NC, McNabb WC. et al. Influence of dietary blueberry and broccoli on cecal microbiota activity and colon morphology in mdr1a(-/-) mice, a model of inflammatory bowel diseases. Nutrition [Internet]. 2012 Mar [cited. 2024 Mar 18];28(3):324-30. Available from: https://pubmed.ncbi.nlm.nih.gov/22113065/
263. Chu WK, Cheung SCM, Lau RAW. Bilberry (Vaccinium myrtillus L.). Herbal Medicine: Biomolecular and Clinical Aspects: Second Edition [Internet]. 2011 Mar 28 [cited 2024 Jul 13];55-71. Available from: https://www.ncbi.nlm.nih.gov/books/NBK92770/
264. Zhang G, Gu Y, Dai X. Protective Effect of Bilberry Anthocyanin Extracts on Dextran Sulfate Sodium-Induced Intestinal Damage in Drosophila melanogaster. Nutrients [Internet]. 2022 Jul 1 [cited. 2024 Jul 13];14(14). Available from: https://pubmed.ncbi.nlm.nih.gov/35889832/
265. Piberger H, Oehme A, Hofmann C, Dreiseitel A, Sand PG, Obermeier F. et al. Bilberries and their anthocyanins ameliorate experimental colitis. Mol Nutr Food Res [Internet]. 2011 Nov [cited. 2024 Jul 13];55(11):1724-9. Available from: https://pubmed.ncbi.nlm.nih.gov/21957076/
266. Driscoll K, Deshpande A, Datta R, Ramakrishna W. Anti-inflammatory Effects of Northern Highbush Blueberry Extract on an In Vitro Inflammatory Bowel Disease Model. Nutr Cancer [Internet]. 2020 Oct 2 [cited. 2024 Jul 13];72(7):1178-90. Available from: https://pubmed.ncbi.nlm.nih.gov/31588794/
267. Pereira SR, Pereira R, Figueiredo I, Freitas V, Dinis TCP, Almeida LM. Comparison of anti-inflammatory activities of an anthocyanin-rich fraction from Portuguese blueberries (Vaccinium corymbosum L.) and 5-aminosalicylic acid in a TNBS-induced colitis rat model. PLoS One [Internet]. 2017 Mar 1 [cited 2024 Jul 13];12(3):e0174116. Available from: https://pubmed.ncbi.nlm.nih.gov/28329021/
268. Urbstaite R, Raudone L, Janulis V. Phytogenotypic Anthocyanin Profiles and Antioxidant Activity Variation in Fruit Samples of the American Cranberry (Vaccinium macrocarpon Aiton). Antioxidants [Internet]. 2022 Feb 1 [cited. 2024 Jul 13];11(2). Available from: /pmc/articles/PMC8868480/
269. Xiao X, Kim J, Sun Q, Kim D, Park CS, Lu TS. et al. Preventive effects of cranberry products on experimental colitis induced by dextran sulphate sodium in mice. Food Chem [Internet]. 2015 Jan 15 [cited. 2024 Jul 13];167:438-46. Available from: https://pubmed.ncbi.nlm.nih.gov/25149009/
270. Cai X, Han Y, Gu M, Song M, Wu X, Li Z. et al. Dietary cranberry suppressed colonic inflammation and alleviated gut microbiota dysbiosis in dextran sodium sulfate-treated mice. Food Funct [Internet]. 2019 Oct 1 [cited. 2024 Jul 13];10(10):6331-41. Available from: https://pubmed.ncbi.nlm.nih.gov/31524900/
271. Zhang D, Verstrepen L, De Medts J, Duysburgh C, Van den Abbeele P, Marzorati M. et al. A Cranberry Concentrate Decreases Adhesion and Invasion of Escherichia coli (AIEC) LF82 In Vitro. Pathogens [Internet]. 2021 Sep 1 [cited. 2024 Jul 13];10(9). Available from: https://pubmed.ncbi.nlm.nih.gov/34578249/
272. Sirven MA, Venancio VP, Shankar S, Klemashevich C, Castellón-Chicas MJ, Fang C. et al. Ulcerative colitis results in differential metabolism of cranberry polyphenols by the colon microbiome in vitro. Food Funct [Internet]. 2021 Dec 21 [cited. 2024 Jul 13];12(24):12751-64. Available from: https://pubmed.ncbi.nlm.nih.gov/34847216/
273. Biedermann L, Mwinyi J, Scharl M, Frei P, Zeitz J, Kullak-Ublick GA. et al. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis - an open pilot study. J Crohns Colitis [Internet]. 2013 May 1 [cited. 2024 Jul 13];7(4):271-9. Available from: https://pubmed.ncbi.nlm.nih.gov/22883440/
274. Roth S, Spalinger MR, Gottier C, Biedermann L, Zeitz J, Lang S. et al. Bilberry-Derived Anthocyanins Modulate Cytokine Expression in the Intestine of Patients with Ulcerative Colitis. PLoS One [Internet]. 2016 May 1 [cited. 2024 Jul 13];11(5). Available from: https://pubmed.ncbi.nlm.nih.gov/27152519/
275. Kim I, Lee J. Variations in Anthocyanin Profiles and Antioxidant Activity of 12 Genotypes of Mulberry (Morus spp.) Fruits and Their Changes during Processing. Antioxidants [Internet]. 2020 Mar 1 [cited 2024 Jul 13];9(3):242. Available from: /pmc/articles/PMC7139643/
276. Mo J, Ni J, Zhang M, Xu Y, Li Y, Karim N. et al. Mulberry Anthocyanins Ameliorate DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Antioxidants [Internet]. 2022 Sep 1 [cited. 2024 Jul 13];11(9). Available from: /pmc/articles/PMC9496020/
277. Toshima S, Hirano T, Kunitake H. Comparison of anthocyanins, polyphenols, and antioxidant capacities among raspberry, blackberry, and Japanese wild Rubus species. Sci Hortic. 2021Jul27;285:110204
278. Burton-Freeman BM, Sandhu AK, Edirisinghe I. Red Raspberries and Their Bioactive Polyphenols: Cardiometabolic and Neuronal Health Links. Advances in Nutrition [Internet]. 2016 [cited. 2024 Jul 13];7(1):44. Available from: /pmc/articles/PMC4717884/
279. Montrose DC, Horelik NA, Madigan JP, Stoner GD, Wang LS, Bruno RS. et al. Anti-inflammatory effects of freeze-dried black raspberry powder in ulcerative colitis. Carcinogenesis [Internet]. 2011 Mar 1 [cited. 2024 Jul 13];32(3):343-50. Available from: https://pubmed.ncbi.nlm.nih.gov/21098643/
280. Wang LS, Kuo CT, Stoner K, Yearsley M, Oshima K, Yu J. et al. Dietary black raspberries modulate DNA methylation in dextran sodium sulfate (DSS)-induced ulcerative colitis. Carcinogenesis [Internet]. 2013 Dec [cited. 2024 Jul 13];34(12):2842-50. Available from: https://pubmed.ncbi.nlm.nih.gov/24067901/
281. Wang LS, Kuo CT, Huang THM, Yearsley M, Oshima K, Stoner GD. et al. Black raspberries protectively regulate methylation of Wnt pathway genes in precancerous colon tissue. Cancer Prev Res (Phila) [Internet]. 2013 Dec [cited. 2024 Jul 13];6(12):1317-27. Available from: https://pubmed.ncbi.nlm.nih.gov/24129635/
282. Bibi S, Kang Y, Du M, Zhu MJ. Dietary red raspberries attenuate dextran sulfate sodium-induced acute colitis. J Nutr Biochem [Internet]. 2018 Jan 1 [cited. 2024 Jul 13];51:40-6. Available from: https://pubmed.ncbi.nlm.nih.gov/29091813/
283. Bibi S, Du M, Zhu MJ. Dietary Red Raspberry Reduces Colorectal Inflammation and Carcinogenic Risk in Mice with Dextran Sulfate Sodium-Induced Colitis. J Nutr [Internet]. 2018 May 1 [cited. 2024 Jul 13];148(5):667-74. Available from: https://pubmed.ncbi.nlm.nih.gov/29897487/
284. Giampieri F, Forbes-Hernandez TY, Gasparrini M, Alvarez-Suarez JM, Afrin S, Bompadre S. et al. Strawberry as a health promoter: an evidence based review. Food Funct [Internet]. 2015 May 1 [cited. 2024 Mar 18];6(5):1386-98. Available from: https://pubmed.ncbi.nlm.nih.gov/25803191/
285. Chen T, Shi N, Afzali A. Chemopreventive Effects of Strawberry and Black Raspberry on Colorectal Cancer in Inflammatory Bowel Disease. Nutrients [Internet]. 2019 Jun 1 [cited. 2024 Mar 18];11(6). Available from: /pmc/articles/PMC6627270/
286. Han D, Wu Y, Lu D, Pang J, Hu J, Zhang X. et al. Polyphenol-rich diet mediates interplay between macrophage-neutrophil and gut microbiota to alleviate intestinal inflammation. Cell Death & Disease. 2023;14:10 [Internet]. 2023 Oct 9 [cited 2024 Apr 14];14(10):1-14. Available from: https://www.nature.com/articles/s41419-023-06190-4
287. Ghattamaneni NK, Sharma A, Panchal SK, Brown L. Pelargonidin 3-glucoside-enriched strawberry attenuates symptoms of DSS-induced inflammatory bowel disease and diet-induced metabolic syndrome in rats. Eur J Nutr [Internet]. 2020 Oct 1 [cited. 2024 Jul 13];59(7):2905-18. Available from: https://pubmed.ncbi.nlm.nih.gov/31696323/
288. Eichhorn S, Winterhalter P. Anthocyanins from pigmented potato (Solanum tuberosum L.) varieties. Food Research International. 2005Oct1;38(8-9):943-8
289. Rodríguez-Mena A, Ochoa-Martínez LA, González-Herrera SM, Rutiaga-Quiñones OM, González-Laredo RF, Olmedilla-Alonso B. Natural pigments of plant origin: Classification, extraction and application in foods. Food Chem. 2023Jan1;398:133908
290. Li S, Wang T, Wu B, Fu W, Xu B, Pamuru RR. et al. Anthocyanin-containing purple potatoes ameliorate DSS-induced colitis in mice. J Nutr Biochem [Internet]. 2021 Jul 1 [cited. 2024 Jul 13];93. Available from: https://pubmed.ncbi.nlm.nih.gov/33705951/
291. Li S, Wang T, Fu W, Kennett M, Cox AD, Lee D. et al. Role of Gut Microbiota in the Anti-Colitic Effects of Anthocyanin-Containing Potatoes. Mol Nutr Food Res [Internet]. 2021 Dec 1 [cited. 2024 Jul 13];65(24). Available from: https://pubmed.ncbi.nlm.nih.gov/34633750/
292. Sun Q, Bibi S, Xue Y, Du M, Chew B, Zhu MJ. Dietary purple potato supplement attenuates DSS-induced colitis in mice: impact on mitochondrial function. J Nutr Biochem [Internet]. 2024 Apr 1 [cited. 2024 Jul 13];126. Available from: https://pubmed.ncbi.nlm.nih.gov/38253109/
293. Chen J, Jiang F, Xu N, Dong G, Jiang J, Wang M. et al. Anthocyanin Extracted from Purple Sweet Potato Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice by Suppressing Pyroptosis and Altering Intestinal Flora Structure. J Med Food [Internet]. 2024 Feb 1 [cited. 2024 Jul 13];27(2):110-22. Available from: https://pubmed.ncbi.nlm.nih.gov/38181190/
294. Zheng R long, Ren T, Niu C tuo, Zheng F yun, Wang J jing, Liu C feng. et al. Anthocyanins composition and antioxidant activity of purple rice and color degradation under sunlight exposure of purple rice wine. Journal of Food Measurement and Characterization [Internet]. 2022 Jun 1 [cited. 2024 Jul 13];16(3):1889-900. Available from: https://link.springer.com/article/10.1007/s11694-022-01285-6
295. Sholikhah U, Parjanto, Handoyo T, Yunus A. Anthocyanin Content in Some Black Rice Cultivars. IOP Conf Ser Earth Environ Sci [Internet]. 2021 Mar 1 [cited. 2024 Jul 13];709(1):012076. Available from: https://iopscience.iop.org/article/10.1088/1755-1315/709/1/012076
296. Das AB, Goud V V, Das C. Extraction of phenolic compounds and anthocyanin from black and purple rice bran (Oryza sativa L.) using ultrasound: A comparative analysis and phytochemical profiling. Ind Crops Prod. 2017Jan1;95:332-41
297. Thipart K, Gruneck L, Phunikhom K, Sharpton TJ, Sattayasai J, Popluechai S. Dark-purple rice extract modulates gut microbiota composition in acetic acid- and indomethacin-induced inflammatory bowel disease in rats. Int Microbiol [Internet]. 2023 May 1 [cited. 2024 Jul 13];26(2):423-34. Available from: https://pubmed.ncbi.nlm.nih.gov/36484910/
298. Zhao L, Zhang Y, Liu G, Hao S, Wang C, Wang Y. Black rice anthocyanin-rich extract and rosmarinic acid, alone and in combination, protect against DSS-induced colitis in mice. Food Funct [Internet]. 2018 May 1 [cited. 2024 Jul 13];9(5):2796-808. Available from: https://pubmed.ncbi.nlm.nih.gov/29691532/
299. Ghareaghajlou N, Hallaj-Nezhadi S, Ghasempour Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021Dec15;365:130482
300. Jean Wilson E, Sirpu Natesh N, Ghadermazi P, Pothuraju R, Prajapati DR, Pandey S. et al. Red Cabbage Juice-Mediated Gut Microbiota Modulation Improves Intestinal Epithelial Homeostasis and Ameliorates Colitis. Int J Mol Sci [Internet]. 2023 Jan 1 [cited. 2024 Jul 13];25(1). Available from: https://pubmed.ncbi.nlm.nih.gov/38203712/
301. Jasim HA, Nahar L, Jasim MA, Moore SA, Ritchie KJ, Sarker SD. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules [Internet]. 2021 Aug 1 [cited. 2024 Jul 13];11(8). Available from: https://pubmed.ncbi.nlm.nih.gov/34439870/
302. Liu M, Hansen PE, Wang G, Qiu L, Dong J, Yin H. et al. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules. 2015;20(1):754-79
303. Sahoo DK, Heilmann RM, Paital B, Patel A, Yadav VK, Wong D. et al. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front Endocrinol (Lausanne) [Internet]. 2023 [cited. 2024 Jul 13];14:1217165. Available from: /pmc/articles/PMC10493311/
304. Gupta SC, Tyagi AK, Deshmukh-Taskar P, Hinojosa M, Prasad S, Aggarwal BB. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem Biophys. 2014Oct1;559:91-9
305. Cho JM, Yun SM, Choi YH, Heo J, Kim NJ, Kim SH. et al. Xanthohumol prevents dextran sulfate sodium-induced colitis via inhibition of IKKβ/NF-κB signaling in mice. Oncotarget [Internet]. 2018 Jan 1 [cited. 2024 Jul 13];9(1):866. Available from: /pmc/articles/PMC5787519/
306. Yun SM, Han YM, Song MY, Lee DY, Kim HS, Kim SH. et al. Xanthohumol Interferes with the Activation of TGF-β Signaling in the Process Leading to Intestinal Fibrosis. Nutrients [Internet]. 2022 Jan 1 [cited. 2024 Jul 13];15(1). Available from: https://pubmed.ncbi.nlm.nih.gov/36615756/
307. Restivo I, Basilicata MG, Giardina IC, Massaro A, Pepe G, Salviati E. et al. A Combination of Polymethoxyflavones from Citrus sinensis and Prenylflavonoids from Humulus lupulus Counteracts IL-1β-Induced Differentiated Caco-2 Cells Dysfunction via a Modulation of NF-κB/Nrf2 Activation. Antioxidants (Basel) [Internet]. 2023 Aug 1 [cited. 2024 Jul 13];12(8). Available from: https://pubmed.ncbi.nlm.nih.gov/37627616/
308. Tuli HS, Aggarwal V, Parashar G. et al. Xanthohumol: A Metabolite with Promising Anti-Neoplastic Potential. Anticancer Agents Med Chem. 2022;22:418-432
309. Langley BO, Ryan JJ, Phipps J, Buttolph L, Bray B, Aslan JE. et al. Xanthohumol microbiome and signature in adults with Crohn's disease (the XMaS trial): a protocol for a phase II triple-masked, placebo-controlled clinical trial. Trials [Internet]. 2022 Dec 1 [cited. 2024 Jul 13];23(1). Available from: https://pubmed.ncbi.nlm.nih.gov/36273173/
310. Neumann HF, Frank J, Venturelli S, Egert S. Bioavailability and Cardiometabolic Effects of Xanthohumol: Evidence from Animal and Human Studies. Mol Nutr Food Res. 2022;66:e2100831
311. Peng F, Du Q, Peng C, Wang N, Tang H, Xie X. et al. A Review: The Pharmacology of Isoliquiritigenin. Phytother Res [Internet]. 2015 Jul 1 [cited. 2024 Jul 13];29(7):969-77. Available from: https://pubmed.ncbi.nlm.nih.gov/25907962/
312. Chen Z, Ding W, Yang X, Lu T, Liu Y. Isoliquiritigenin, a potential therapeutic agent for treatment of inflammation-associated diseases. J Ethnopharmacol [Internet]. 2024 Jan 10 [cited. 2024 Jul 13];318(Pt B). Available from: https://pubmed.ncbi.nlm.nih.gov/37604329/
313. Choi YH, Bae JK, Chae HS, Choi YO, Nhoek P, Choi JS. et al. Isoliquiritigenin ameliorates dextran sulfate sodium-induced colitis through the inhibition of MAPK pathway. Int Immunopharmacol [Internet]. 2016 Feb 1 [cited. 2024 Jul 13];31:223-32. Available from: https://pubmed.ncbi.nlm.nih.gov/26771170/
314. Nakamura S, Watanabe T, Tanigawa T, Shimada S, Nadatani Y, Miyazaki T. et al. Isoliquiritigenin Ameliorates Indomethacin-Induced Small Intestinal Damage by Inhibiting NOD-Like Receptor Family, Pyrin Domain-Containing 3 Inflammasome Activation. Pharmacology [Internet]. 2018 Apr 1 [cited. 2024 Jul 13];101(5-6):236-45. Available from: https://pubmed.ncbi.nlm.nih.gov/29393276/
315. Chi JH, Seo GS, Cheon JH, Lee SH. Isoliquiritigenin inhibits TNF-α-induced release of high-mobility group box 1 through activation of HDAC in human intestinal epithelial HT-29 cells. Eur J Pharmacol [Internet]. 2017 [cited. 2024 Jul 13];796:101-9. Available from: https://pubmed.ncbi.nlm.nih.gov/28012970/
316. Lu P De, Yuan MC, Quan XP, Chen JF, Zhao YH. Preclinical studies of licorice in ulcerative colitis: A systematic review with meta-analysis and network pharmacology. J Ethnopharmacol [Internet]. 2022 Oct 5 [cited. 2024 Jul 13];296. Available from: https://pubmed.ncbi.nlm.nih.gov/35671864/
317. Gonçalves LM, Valente IM, Rodrigues JA. An Overview on Cardamonin. J Med Food [Internet]. 2014 Jun 6 [cited. 2024 Jul 13];17(6):633. Available from: /pmc/articles/PMC4060836/
318. James S, Aparna JS, Babu A, Paul AM, Lankadasari MB, Athira SR. et al. Cardamonin Attenuates Experimental Colitis and Associated Colorectal Cancer. Biomolecules [Internet]. 2021 May 1 [cited. 2024 Jul 13];11(5). Available from: https://pubmed.ncbi.nlm.nih.gov/33947113/
319. Shen X, Chen H, Wen T, Liu L, Yang Y, Xie F. et al. A natural chalcone cardamonin inhibits necroptosis and ameliorates dextran sulfate sodium (DSS)-induced colitis by targeting RIPK1/3 kinases. Eur J Pharmacol [Internet]. 2023 Sep 5 [cited. 2024 Jul 13];954. Available from: https://pubmed.ncbi.nlm.nih.gov/37302524/
320. Ren G, Sun A, Deng C, Zhang J, Wu X, Wei X. et al. The anti-inflammatory effect and potential mechanism of cardamonin in DSS-induced colitis. Am J Physiol Gastrointest Liver Physiol [Internet]. 2015 Sep 1 [cited. 2024 Jul 13];309(7):G517-27. Available from: https://pubmed.ncbi.nlm.nih.gov/26251468/
321. Wang K, Lv Q, Miao Y meng, Qiao S miao, Dai Y, Wei Z feng. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem Pharmacol [Internet]. 2018 Sep 1 [cited. 2024 Jul 13];155:494-509. Available from: https://pubmed.ncbi.nlm.nih.gov/30071202/
322. Ali AA, Abd Al Haleem EN, Khaleel SAH, Sallam AS. Protective effect of cardamonin against acetic acid-induced ulcerative colitis in rats. Pharmacol Rep [Internet]. 2017 Apr 1 [cited. 2024 Jul 13];69(2):268-75. Available from: https://pubmed.ncbi.nlm.nih.gov/28129600/
323. Nakhate KT, Badwaik H, Choudhary R, Sakure K, Agrawal YO, Sharma C. et al. Therapeutic Potential and Pharmaceutical Development of a Multitargeted Flavonoid Phloretin. Nutrients [Internet]. 2022 Sep 1 [cited. 2024 Jul 13];14(17). Available from: https://pubmed.ncbi.nlm.nih.gov/36079895/
324. Kapoor S, Padwad YS. Phloretin suppresses intestinal inflammation and maintained epithelial tight junction integrity by modulating cytokines secretion in in vitro model of gut inflammation. Cell Immunol [Internet]. 2023 Sep 1 [cited. 2024 Jul 13];391-392. Available from: https://pubmed.ncbi.nlm.nih.gov/37506521/
325. Arya V S, Kanthlal S K. Phloretin Ameliorates Acetic Acid Induced Colitis Through Modulation of Immune and Inflammatory Reactions in Rats. Endocr Metab Immune Disord Drug Targets [Internet]. 2021 Jun 24 [cited. 2024 Jul 13];21(1):163-72. Available from: https://pubmed.ncbi.nlm.nih.gov/32579511/
326. Wu M, Li P, An Y, Ren J, Yan D, Cui J. et al. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol Res [Internet]. 2019 Dec 1 [cited. 2024 Jul 13];150. Available from: https://pubmed.ncbi.nlm.nih.gov/31689519/
327. Zhang Z, Li S, Cao H, Shen P, Liu J, Fu Y. et al. The protective role of phloretin against dextran sulfate sodium-induced ulcerative colitis in mice. Food Funct [Internet]. 2019 Jan 1 [cited. 2024 Jul 13];10(1):422-31. Available from: https://pubmed.ncbi.nlm.nih.gov/30604787/
328. Zhang J, Li N, Cao L, Sun Y, Qing DG, Xu XQ. et al. The Regulatory Effects of Licochalcone A on the Intestinal Epithelium and Gut Microbiota in Murine Colitis. Molecules [Internet]. 2021 Jul 2 [cited. 2024 Jul 13];26(14). Available from: https://pubmed.ncbi.nlm.nih.gov/34299424/
329. Liu D, Huo X, Gao L, Zhang J, Ni H, Cao L. NF-κB and Nrf2 pathways contribute to the protective effect of Licochalcone A on dextran sulphate sodium-induced ulcerative colitis in mice. Biomed Pharmacother [Internet]. 2018 Jun 1 [cited. 2024 Jul 13];102:922-9. Available from: https://pubmed.ncbi.nlm.nih.gov/29710547/
330. Lee SD, Choe JW, Lee BJ, Kang MH, Joo MK, Kim JH. et al. Butein effects in colitis and interleukin-6/signal transducer and activator of transcription 3 expression. World J Gastroenterol [Internet]. 2015 Jan 14 [cited. 2024 Jul 13];21(2):465-74. Available from: https://pubmed.ncbi.nlm.nih.gov/25593461/
331. Chen Y, Jin T, Zhang M, Hong B, Jin B, Hu C. et al. Flavokawain B inhibits NF-κB inflammatory signaling pathway activation in inflammatory bowel disease by targeting TLR2. Toxicol Appl Pharmacol [Internet]. 2024 May 1 [cited. 2024 Jul 13];486. Available from: https://pubmed.ncbi.nlm.nih.gov/38583725/
332. da Silva LM. Perspectives on the Role of P21-Activated Kinase 1 (PAK1) in the Intestinal Anti-inflammatory and Antitumor Potential of Artepillin C. Curr Mol Pharmacol [Internet]. 2024 Apr 27 [cited. 2024 Jul 13];17(1). Available from: https://pubmed.ncbi.nlm.nih.gov/37185322/
333. Hirata Y, Motoyama M, Kimura S. et al. Artepillin C, a Major Component of Brazilian Green Propolis, Inhibits Endoplasmic Reticulum Stress and Protein Aggregation. Eur J Pharmacol. 2021;912:174572
334. Zhou XL, Yang J, Qu XJ, Meng J, Miao RR, Cui SX. M10, a Myricetin-3-O-b-D-Lactose Sodium Salt, Prevents Ulcerative Colitis Through Inhibiting Necroptosis in Mice. Front Pharmacol [Internet]. 2020 Sep 11 [cited. 2024 Jul 13];11. Available from: https://pubmed.ncbi.nlm.nih.gov/33041798/
335. Mandal SM, Chakraborty D, Dey S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav [Internet]. 2010 [cited. 2024 Mar 18];5(4):359. Available from: /pmc/articles/PMC2958585/
336. Kumar N, Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. 2019 [cited 2024 Mar 18]; Available from: https://doi.org/10.1016/j.btre. 2019 e00370
337. Simón J, Casado-Andrés M, Goikoetxea-Usandizaga N, Serrano-Maciá M, Luz Martínez-Chantar M. Nutraceutical Properties of Polyphenols against Liver Diseases. [cited. 2024 Mar 18]; Available from: www.mdpi.com/journal/nutrients
338. Han X, Li M, Sun L, Liu X, Yin Y, Hao J. et al. p-Hydroxybenzoic Acid Ameliorates Colitis by Improving the Mucosal Barrier in a Gut Microbiota-Dependent Manner. Nutrients [Internet]. 2022 Dec 1 [cited. 2024 Mar 18];14(24). Available from: /pmc/articles/PMC9784546/
339. Wianowska D, Olszowy-Tomczyk M. A Concise Profile of Gallic Acid—From Its Natural Sources through Biological Properties and Chemical Methods of Determination. Molecules [Internet]. 2023 Feb 1 [cited. 2024 Jul 13];28(3). Available from: /pmc/articles/PMC9919014/
340. Harper P. A Review of the Dietary Intake, Bioavailability and Health Benefits of Ellagic Acid (EA) with a Primary Focus on Its Anti-Cancer Properties. Cureus [Internet]. 2023 Aug 8 [cited. 2024 Jul 13];15(8). Available from: /pmc/articles/PMC10484468/
341. Yu TY, Feng YM, Kong WS, Li SN, Sun XJ, Zhou G. et al. Gallic acid ameliorates dextran sulfate sodium-induced ulcerative colitis in mice via inhibiting NLRP3 inflammasome. Front Pharmacol [Internet]. 2023 Jan 25 [cited. 2024 Mar 18];14. Available from: https://pubmed.ncbi.nlm.nih.gov/36762118/
342. Peng J, Liu T, Meng P, Luo Y, Zhu S, Wang Y. et al. Gallic acid ameliorates colitis by trapping deleterious metabolite ammonia and improving gut microbiota dysbiosis. mBio [Internet]. 2024 Feb 1 [cited. 2024 Mar 18];15(2):e0275223. Available from: https://pubmed.ncbi.nlm.nih.gov/38126747/
343. Li Y, Xie Z, Gao T, Li L, Chen Y, Xiao D. et al. A holistic view of gallic acid-induced attenuation in colitis based on microbiome-metabolomics analysis. Food Funct [Internet]. 2019 Jul 1 [cited. 2024 Jul 13];10(7):4046-61. Available from: https://pubmed.ncbi.nlm.nih.gov/31225554/
344. Leng Y, Zhang X, Zhang Q, Xia J, Zhang Y, Ma C. et al. Gallic acid attenuates murine ulcerative colitis by promoting group 3 innate lymphocytes, affecting gut microbiota, and bile acid metabolism. J Nutr Biochem [Internet]. 2024 Jun [cited. 2024 Jul 13];131:109677. Available from: https://pubmed.ncbi.nlm.nih.gov/38844081/
345. Chu C, Ru H, Chen Y, Xu J, Wang C, Jin Y. Gallic acid attenuates LPS-induced inflammation in Caco-2 cells by suppressing the activation of the NF-κB/MAPK signaling pathway. Acta Biochim Biophys Sin (Shanghai) [Internet]. 2024 Mar 22 [cited. 2024 Jul 13];56(6):905-15. Available from: https://pubmed.ncbi.nlm.nih.gov/38516705/
346. Zhu L, Gu PQ, Shen H. Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis. Int Immunopharmacol [Internet]. 2019 Feb 1 [cited. 2024 Jul 13];67:129-37. Available from: https://pubmed.ncbi.nlm.nih.gov/30544066/
347. Pandurangan AK, Mohebali N, Esa NM, Looi CY, Ismail S, Saadatdoust Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int Immunopharmacol [Internet]. 2015 Oct 1 [cited. 2024 Mar 18];28(2):1034-43. Available from: https://pubmed.ncbi.nlm.nih.gov/26319951/
348. Iglesias DE, Cremonini E, Fraga CG, Oteiza PI. Ellagic acid protects Caco-2 cell monolayers against inflammation-induced permeabilization. Free Radic Biol Med [Internet]. 2020 May 20 [cited. 2024 Jul 13];152:776-86. Available from: https://pubmed.ncbi.nlm.nih.gov/31981623/
349. Xiao Y, Huang R, Wang N, Deng Y, Tan B, Yin Y. et al. Ellagic Acid Alleviates Oxidative Stress by Mediating Nrf2 Signaling Pathways and Protects against Paraquat-Induced Intestinal Injury in Piglets. Antioxidants [Internet]. 2022 Feb 1 [cited. 2024 Apr 14];11(2):252. Available from: https://www.mdpi.com/2076-3921/11/2/252/htm
350. Peng B, Xue L, Yu Q, Zhong T. Ellagic acid alleviates TNBS-induced intestinal barrier dysfunction by regulating mucin secretion and maintaining tight junction integrity in rats. Int J Food Sci Nutr [Internet]. 2023 [cited. 2024 Jul 13];74(4):476-86. Available from: https://pubmed.ncbi.nlm.nih.gov/37455358/
351. Marín M, María Giner R, Ríos JL, Carmen Recio M. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J Ethnopharmacol [Internet]. 2013 Dec 12 [cited. 2024 Jul 13];150(3):925-34. Available from: https://pubmed.ncbi.nlm.nih.gov/24140585/
352. Rosillo MA, Sanchez-Hidalgo M, Cárdeno A, Alarcón De La Lastra C. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn's disease. Biochem Pharmacol [Internet]. 2011 Oct 1 [cited. 2024 Jul 13];82(7):737-45. Available from: https://pubmed.ncbi.nlm.nih.gov/21763290/
353. Rosillo MA, Sánchez-Hidalgo M, Cárdeno A, Aparicio-Soto M, Sánchez-Fidalgo S, Villegas I. et al. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol Res [Internet]. 2012 Sep [cited. 2024 Jul 13];66(3):235-42. Available from: https://pubmed.ncbi.nlm.nih.gov/22677088/
354. Kim DH, Kim JS, Kwon JH, Kwun IS, Baek MC, Kwon GS. et al. Ellagic Acid Prevented Dextran-Sodium-Sulfate-Induced Colitis, Liver, and Brain Injury through Gut Microbiome Changes. Antioxidants [Internet]. 2023 Oct 1 [cited. 2024 Jul 13];12(10):1886. Available from: /pmc/articles/PMC10604018/
355. Jin H, Che S, Wu K, Wu M. Ellagic acid prevents gut damage via ameliorating microbe-associated intestinal lymphocyte imbalance. Food Funct [Internet]. 2022 Aug 22 [cited. 2024 Jul 13];13(19):9822-31. Available from: https://pubmed.ncbi.nlm.nih.gov/36040795/
356. Semaming Y, Pannengpetch P, Chattipakorn SC, Chattipakorn N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Evid Based Complement Alternat Med [Internet]. 2015 [cited 2024 Jul 13]. 2015 Available from: /pmc/articles/PMC4337037/
357. Yang X, Sun X, Zhou F, Xiao S, Zhong L, Hu S. et al. Protocatechuic Acid Alleviates Dextran-Sulfate-Sodium-Induced Ulcerative Colitis in Mice via the Regulation of Intestinal Flora and Ferroptosis. Molecules [Internet]. 2023 May 1 [cited. 2024 Jul 13];28(9). Available from: https://pubmed.ncbi.nlm.nih.gov/37175184/
358. So BR, Kim S, Jang SH, Kim MJ, Lee JJ, Kim SR. et al. Dietary protocatechuic acid redistributes tight junction proteins by targeting Rho-associated protein kinase to improve intestinal barrier function. Food Funct [Internet]. 2023 Apr 25 [cited. 2024 Mar 18];14(10):4777-91. Available from: https://pubmed.ncbi.nlm.nih.gov/37128780/
359. Crespo I, San-Miguel B, Mauriz JL, de Urbina JJO, Almar M, Tuñón MJ. et al. Protective Effect of Protocatechuic Acid on TNBS-Induced Colitis in Mice Is Associated with Modulation of the SphK/S1P Signaling Pathway. Nutrients [Internet]. 2017 Mar 16 [cited. 2024 Jul 13];9(3). Available from: https://pubmed.ncbi.nlm.nih.gov/28300788/
360. Farombi EO, Adedara IA, Awoyemi O V, Njoku CR, Micah GO, Esogwa CU. et al. Dietary protocatechuic acid ameliorates dextran sulphate sodium-induced ulcerative colitis and hepatotoxicity in rats. Food Funct [Internet]. 2016 Feb 1 [cited. 2024 Jul 13];7(2):913-21. Available from: https://pubmed.ncbi.nlm.nih.gov/26691887/
361. Matejczyk M, Ofman P, Juszczuk-Kubiak E, Świsłocka R, Shing WL, Kesari KK. et al. Biological effects of vanillic acid, iso-vanillic acid, and orto-vanillic acid as environmental pollutants. Ecotoxicol Environ Saf [Internet]. 2024 Jun 1 [cited. 2024 Jul 13];277. Available from: https://pubmed.ncbi.nlm.nih.gov/38663192/
362. Kim SJ, Kim MC, Um JY, Hong SH. The beneficial effect of vanillic acid on ulcerative colitis. Molecules [Internet]. 2010 Oct [cited. 2024 Jul 13];15(10):7208-17. Available from: https://pubmed.ncbi.nlm.nih.gov/20959795/
363. Yang L, Nao J, Dong X. The Therapeutic Potential of Hydroxycinnamic Acid Derivatives in Parkinson's Disease: Focus on In Vivo Research Advancements. J Agric Food Chem [Internet]. 2023 Jul 26 [cited. 2024 Mar 18];71(29):10932-51. Available from: https://pubmed.ncbi.nlm.nih.gov/37432913/
364. Erskine E, Gültekin Subaşl B, Vahapoglu B, Capanoglu E. Coffee Phenolics and Their Interaction with Other Food Phenolics: Antagonistic and Synergistic Effects. ACS Omega [Internet]. 2022 Jan 1 [cited. 2024 Jul 13];7(2):1595. Available from: /pmc/articles/PMC8772327/
365. Kim CK, Yu J, Le DD, Han S, Yu S, Lee M. Anti-inflammatory activity of caffeic acid derivatives from Ilex rotunda. Int Immunopharmacol [Internet]. 2023 Feb 1 [cited. 2024 Mar 18];115. Available from: https://pubmed.ncbi.nlm.nih.gov/36571918/
366. Xiang C, Liu M, Lu Q, Fan C, Lu H, Feng C. et al. Blockade of TLRs-triggered macrophage activation by caffeic acid exerted protective effects on experimental ulcerative colitis. Cell Immunol [Internet]. 2021 Jul 1 [cited. 2024 Jul 13];365. Available from: https://pubmed.ncbi.nlm.nih.gov/33932876/
367. Zhang Z, Wu X, Cao S, Wang L, Wang D, Yang H. et al. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice. Oncotarget [Internet]. 2016 May 31 [cited. 2024 Jul 13];7(22):31790-9. Available from: https://pubmed.ncbi.nlm.nih.gov/27177331/
368. Mei Y, Wang Z, Zhang Y, Wan T, Xue J, He W. et al. FA-97, a New Synthetic Caffeic Acid Phenethyl Ester Derivative, Ameliorates DSS-Induced Colitis Against Oxidative Stress by Activating Nrf2/HO-1 Pathway. Front Immunol [Internet]. 2020 Jan 8 [cited. 2024 Mar 18];10. Available from: https://pubmed.ncbi.nlm.nih.gov/31969881/
369. Dai G, Jiang Z, Sun B, Liu C, Meng Q, Ding K. et al. Caffeic Acid Phenethyl Ester Prevents Colitis-Associated Cancer by Inhibiting NLRP3 Inflammasome. Front Oncol [Internet]. 2020 May 6 [cited. 2024 Jul 13];10. Available from: https://pubmed.ncbi.nlm.nih.gov/32435622/
370. Khan MN, Lane ME, McCarron PA, Tambuwala MM. Caffeic acid phenethyl ester is protective in experimental ulcerative colitis via reduction in levels of pro-inflammatory mediators and enhancement of epithelial barrier function. Inflammopharmacology [Internet]. 2018 Apr 1 [cited. 2024 Jul 13];26(2):561-9. Available from: https://pubmed.ncbi.nlm.nih.gov/28528363/
371. Pandurangan AK, Mohebali N, Hasanpourghadi M, Esa NM. Caffeic Acid Phenethyl Ester Attenuates Dextran Sulfate Sodium-Induced Ulcerative Colitis Through Modulation of NF-κB and Cell Adhesion Molecules. Appl Biochem Biotechnol [Internet]. 2022 Mar 1 [cited. 2024 Jul 13];194(3):1091-104. Available from: https://pubmed.ncbi.nlm.nih.gov/35040047/
372. Santana-Gálvez J, Cisneros-Zevallos L, Jacobo-Velázquez DA. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules : A Journal of Synthetic Chemistry and Natural Product Chemistry [Internet]. 2017 Mar 1 [cited. 2024 Jul 13];22(3). Available from: /pmc/articles/PMC6155416/
373. Gao W, Wang C, Yu L, Sheng T, Wu Z, Wang X. et al. Chlorogenic Acid Attenuates Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice through MAPK/ERK/JNK Pathway. Biomed Res Int [Internet]. 2019 [cited 2024 Jul 13]. 2019 Available from: https://pubmed.ncbi.nlm.nih.gov/31139644/
374. Vukelić I, Detel D, Pučar LB, Potočnjak I, Buljević S, Domitrović R. Chlorogenic acid ameliorates experimental colitis in mice by suppressing signaling pathways involved in inflammatory response and apoptosis. Food Chem Toxicol [Internet]. 2018 Nov 1 [cited. 2024 Jul 13];121:140-50. Available from: https://pubmed.ncbi.nlm.nih.gov/30165128/
375. Zeng J, Zhang D, Wan X, Bai Y, Yuan C, Wang T. et al. Chlorogenic Acid Suppresses miR-155 and Ameliorates Ulcerative Colitis through the NF-κB/NLRP3 Inflammasome Pathway. Mol Nutr Food Res [Internet]. 2020 Dec 1 [cited. 2024 Jul 13];64(23). Available from: https://pubmed.ncbi.nlm.nih.gov/33078870/
376. Maslin LA, Weeks BR, Carroll RJ, Byrne DH, Turner ND. Chlorogenic Acid and Quercetin in a Diet with Fermentable Fiber Influence Multiple Processes Involved in DSS-Induced Ulcerative Colitis but Do Not Reduce Injury. Nutrients [Internet]. 2022 Sep 1 [cited. 2024 Jul 13];14(18). Available from: https://pubmed.ncbi.nlm.nih.gov/36145086/
377. Zhang Z, Wu X, Cao S, Cromie M, Shen Y, Feng Y. et al. Chlorogenic Acid Ameliorates Experimental Colitis by Promoting Growth of Akkermansia in Mice. Nutrients [Internet]. 2017 Jul 1 [cited. 2024 Jul 13];9(7). Available from: https://pubmed.ncbi.nlm.nih.gov/28661454/
378. Lee YM, Shin DW, Lim BO. Chlorogenic Acid Improves Symptoms of Inflammatory Bowel Disease in Interleukin-10 Knockout Mice. J Med Food [Internet]. 2020 Oct 1 [cited. 2024 Jul 13];23(10):1043-53. Available from: https://pubmed.ncbi.nlm.nih.gov/33054539/
379. Yan Y, Zhou X, Guo K, Zhou F, Yang H. Chlorogenic Acid Protects Against Indomethacin-Induced Inflammation and Mucosa Damage by Decreasing Bacteroides-Derived LPS. Front Immunol [Internet]. 2020 Jun 3 [cited. 2024 Jul 13];11. Available from: https://pubmed.ncbi.nlm.nih.gov/32582202/
380. Li D, Rui Y xin, Guo S duo, Luan F, Liu R, Zeng N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci [Internet]. 2021 Nov 1 [cited. 2024 Mar 18];284. Available from: https://pubmed.ncbi.nlm.nih.gov/34481866/
381. Hwang HJ, Lee SR, Yoon JG, Moon HR, Zhang J, Park E. et al. Ferulic Acid as a Protective Antioxidant of Human Intestinal Epithelial Cells. Antioxidants (Basel) [Internet]. 2022 Aug 1 [cited. 2024 Mar 18];11(8). Available from: https://pubmed.ncbi.nlm.nih.gov/35892649/
382. Sadar SS, Vyawahare NS, Bodhankar SL. Ferulic acid ameliorates TNBS-induced ulcerative colitis through modulation of cytokines, oxidative stress, iNOs, COX-2, and apoptosis in laboratory rats. EXCLI J [Internet]. 2016 Aug 9 [cited. 2024 Jul 13];15:482-99. Available from: https://pubmed.ncbi.nlm.nih.gov/27822176/
383. Ghasemi-Dehnoo M, Amini-Khoei H, Lorigooini Z, AnjomShoa M, Rafieian-Kopaei M. Ferulic acid ameliorates ulcerative colitis in a rat model via the inhibition of two LPS-TLR4-NF-κB and NF-κB-INOS-NO signaling pathways and thus alleviating the inflammatory, oxidative and apoptotic conditions in the colon tissue. Inflammopharmacology [Internet]. 2023 Oct 1 [cited. 2024 Jul 13];31(5):2587-97. Available from: https://pubmed.ncbi.nlm.nih.gov/37432553/
384. Kim YY, Hur G, Jang HJ, Jeong S, Lee SW, Lee SJ. et al. Ferulic Acid Derivatives Ameliorate Intestine Barrier Destruction by Alleviating Inflammatory Responses in Dextran Sulfate Sodium-Induced Inflammatory Bowel Disease. Toxics [Internet]. 2024 Apr 1 [cited. 2024 Jul 13];12(4). Available from: https://pubmed.ncbi.nlm.nih.gov/38668491/
385. Yu S, Qian H, Zhang D, Jiang Z. Ferulic acid relieved ulcerative colitis by inhibiting the TXNIP/NLRP3 pathway in rats. Cell Biol Int [Internet]. 2023 Feb 1 [cited. 2024 Jul 13];47(2):417-27. Available from: https://pubmed.ncbi.nlm.nih.gov/36251276/
386. Pandi A, Kalappan VM. Pharmacological and therapeutic applications of Sinapic acid-an updated review. Mol Biol Rep [Internet]. 2021 Apr 1 [cited. 2024 Mar 18];48(4):3733-45. Available from: https://pubmed.ncbi.nlm.nih.gov/33988797/
387. Jang S, Kim S, So BR, Kim Y, Kim CK, Lee JJ. et al. Sinapic acid alleviates inflammatory bowel disease (IBD) through localization of tight junction proteins by direct binding to TAK1 and improves intestinal microbiota. Front Pharmacol [Internet]. 2023 [cited. 2024 Mar 18];14. Available from: https://pubmed.ncbi.nlm.nih.gov/37649894/
388. Shahid M, Raish M, Ahmad A, Bin Jardan YA, Ansari MA, Ahad A. et al. Sinapic Acid Ameliorates Acetic Acid-Induced Ulcerative Colitis in Rats by Suppressing Inflammation, Oxidative Stress, and Apoptosis. Molecules [Internet]. 2022 Jul 1 [cited. 2024 Jul 13];27(13). Available from: https://pubmed.ncbi.nlm.nih.gov/35807383/
389. Lee JY. Anti-inflammatory effects of sinapic acid on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice. Arch Pharm Res [Internet]. 2018 Feb 1 [cited. 2024 Jul 13];41(2):243-50. Available from: https://pubmed.ncbi.nlm.nih.gov/29392509/
390. Li WY, Liu JY, Wang ZX, Wang KY, Huang CX, He W. et al. Sinapic Acid Attenuates Chronic DSS-Induced Intestinal Fibrosis in C57BL/6J Mice by Modulating NLRP3 Inflammasome Activation and the Autophagy Pathway. ACS Omega [Internet]. 2023 Jan 9 [cited. 2024 Jul 13];9(1):1230-41. Available from: https://pubmed.ncbi.nlm.nih.gov/38222654/
391. Qian B, Wang C, Zeng Z, Ren Y, Li D, Song J Le. Ameliorative Effect of Sinapic Acid on Dextran Sodium Sulfate- (DSS-) Induced Ulcerative Colitis in Kunming (KM) Mice. Oxid Med Cell Longev [Internet]. 2020 [cited 2024 Jul 13]. 2020 Available from: https://pubmed.ncbi.nlm.nih.gov/33312339/
392. Lan H, Zhang LY, He W, Li WY, Zeng Z, Qian B. et al. Sinapic Acid Alleviated Inflammation-Induced Intestinal Epithelial Barrier Dysfunction in Lipopolysaccharide- (LPS-) Treated Caco-2 Cells. Mediators Inflamm [Internet]. 2021 [cited 2024 Mar 18]. 2021 Available from: https://pubmed.ncbi.nlm.nih.gov/34539242/
393. Ijaz S, Iqbal J, Abbasi BA, Ullah Z, Yaseen T, Kanwal S. et al. Rosmarinic acid and its derivatives: Current insights on anticancer potential and other biomedical applications. Biomed Pharmacother [Internet]. 2023 Jun 1 [cited. 2024 Apr 14];162. Available from: https://pubmed.ncbi.nlm.nih.gov/37062215/
394. Li K, Wu J, Xu S, Li X, Zhang Y, Gao X jiao. Rosmarinic acid alleviates intestinal inflammatory damage and inhibits endoplasmic reticulum stress and smooth muscle contraction abnormalities in intestinal tissues by regulating gut microbiota. Microbiol Spectr [Internet]. 2023 Oct 17 [cited. 2024 Apr 14];11(5). Available from: https://pubmed.ncbi.nlm.nih.gov/37594285/
395. Formiga R de O, Alves Júnior EB, Vasconcelos RC, Guerra GCB, de Araújo AA, de Carvalho TG. et al. p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: Role of Antioxidant System and Immunomodulation. Int J Mol Sci [Internet]. 2020 Aug 2 [cited. 2024 Jul 13];21(16):1-23. Available from: https://pubmed.ncbi.nlm.nih.gov/32824269/
396. Jin BR, Chung KS, Cheon SY, Lee M, Hwang S, Noh Hwang S. et al. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci Rep [Internet]. 2017 Apr 6 [cited. 2024 Jul 13];7. Available from: https://pubmed.ncbi.nlm.nih.gov/28383063/
397. Marinho S, Illanes M, Ávila-Román J, Motilva V, Talero E. Anti-Inflammatory Effects of Rosmarinic Acid-Loaded Nanovesicles in Acute Colitis through Modulation of NLRP3 Inflammasome. Biomolecules [Internet]. 2021 Feb 1 [cited. 2024 Apr 14];11(2):1-17. Available from: https://pubmed.ncbi.nlm.nih.gov/33530569/
398. El-Seedi HR, El-Said AMA, Khalifa SAM, Göransson U, Bohlin L, Borg-Karlson AK. et al. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J Agric Food Chem [Internet]. 2012 Nov 7 [cited. 2024 Jul 13];60(44):10877-95. Available from: https://pubmed.ncbi.nlm.nih.gov/22931195/
399. Pei K, Ou J, Huang J, Ou S. p-Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J Sci Food Agric [Internet]. 2016 Jul 1 [cited. 2024 Jul 13];96(9):2952-62. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/jsfa.7578
400. Valanciene E, Malys N. Advances in Production of Hydroxycinnamoyl-Quinic Acids: From Natural Sources to Biotechnology. Antioxidants [Internet]. 2022 Dec 1 [cited. 2024 Jul 13];11(12). Available from: /pmc/articles/PMC9774772/
401. Ekhtiar M, Ghasemi-Dehnoo M, Mirzaei Y, Azadegan-Dehkordi F, Amini-Khoei H, Lorigooini Z. et al. The coumaric acid and syringic acid ameliorate acetic acid-induced ulcerative colitis in rats via modulator of Nrf2/HO-1 and pro-inflammatory cytokines. Int Immunopharmacol [Internet]. 2023 Jul 1 [cited. 2024 Jul 13];120. Available from: https://pubmed.ncbi.nlm.nih.gov/37182450/
402. Ghasemi-Dehnoo M, Lorigooini Z, Amini-Khoei H, Sabzevary-Ghahfarokhi M, Rafieian-Kopaei M. Quinic acid ameliorates ulcerative colitis in rats, through the inhibition of two TLR4-NF-κB and NF-κB-INOS-NO signaling pathways. Immun Inflamm Dis [Internet]. 2023 Aug 1 [cited. 2024 Jul 13];11(8). Available from: https://pubmed.ncbi.nlm.nih.gov/37647443/
403. Li S, Tan HY, Wang N, Cheung F, Hong M, Feng Y. The Potential and Action Mechanism of Polyphenols in the Treatment of Liver Diseases. Oxid Med Cell Longev [Internet]. 2018 [cited 2024 Mar 18]. 2018 Available from: https://pubmed.ncbi.nlm.nih.gov/29507653/
404. Breuss JM, Atanasov AG, Uhrin P. Molecular Sciences Resveratrol and Its Effects on the Vascular System. 2019 [cited. 2024 Mar 18]; Available from: www.mdpi.com/journal/ijms
405. Nunes S, Danesi F, Del Rio D, Silva P. Resveratrol and inflammatory bowel disease: the evidence so far. Nutr Res Rev [Internet]. 2018 Jun 1 [cited. 2024 Jul 13];31(1):85-97. Available from: https://pubmed.ncbi.nlm.nih.gov/29191255/
406. Shi Y, Zhou J, Jiang B, Miao M. Resveratrol and inflammatory bowel disease. Ann N Y Acad Sci [Internet]. 2017 [cited. 2024 Jul 13];1403(1):38-47. Available from: https://pubmed.ncbi.nlm.nih.gov/28945937/
407. Hu Y, Chen D, Zheng P, Yu J, He J, Mao X. et al. The Bidirectional Interactions between Resveratrol and Gut Microbiota: An Insight into Oxidative Stress and Inflammatory Bowel Disease Therapy. Biomed Res Int [Internet]. 2019 [cited 2024 Jul 13]. 2019 Available from: https://pubmed.ncbi.nlm.nih.gov/31179328/
408. Sabzevary-Ghahfarokhi M, Soltani A, Luzza F, Larussa T, Rahimian G, Shirzad H. et al. The protective effects of resveratrol on ulcerative colitis via changing the profile of Nrf2 and IL-1β protein. Mol Biol Rep [Internet]. 2020 Sep 1 [cited. 2024 Jul 13];47(9):6941-7. Available from: https://pubmed.ncbi.nlm.nih.gov/32888128/
409. Zhang B, Zhang Y, Liu X, Yin J, Li X, Zhang X. et al. Differential Protective Effect of Resveratrol and Its Microbial Metabolites on Intestinal Barrier Dysfunction is Mediated by the AMPK Pathway. J Agric Food Chem [Internet]. 2022 Sep 14 [cited. 2024 Jul 13];70(36):11301-13. Available from: https://pubmed.ncbi.nlm.nih.gov/36066018/
410. Luo Y, Yu X, Zhao P, Huang J, Huang X. Effects of Resveratrol on Tight Junction Proteins and the Notch1 Pathway in an HT-29 Cell Model of Inflammation Induced by Lipopolysaccharide. Inflammation [Internet]. 2022 Dec 1 [cited. 2024 Jul 13];45(6):2449-64. Available from: https://pubmed.ncbi.nlm.nih.gov/35705831/
411. Serra D, Almeida LM, Dinis TCP. Anti-inflammatory protection afforded by cyanidin-3-glucoside and resveratrol in human intestinal cells via Nrf2 and PPAR-γ: Comparison with 5-aminosalicylic acid. Chem Biol Interact [Internet]. 2016 Dec 25 [cited. 2024 Jul 13];260:102-9. Available from: https://pubmed.ncbi.nlm.nih.gov/27818126/
412. Xinya YU, Xiaoxi LI, Yunchun XU, Yuwei LI, Zhou Y, Zhang J. et al. Resveratrol ameliorates ulcerative colitis by upregulating Nrf2/HO-1 pathway activity: Integrating animal experiments and network pharmacology. Mol Med Rep [Internet]. 2024 May 1 [cited. 2024 Jul 13];29(5). Available from: https://pubmed.ncbi.nlm.nih.gov/38488031/
413. Garcia P, Schmiedlin-Ren P, Mathias JS, Tang H, Christman GM, Zimmermann EM. Resveratrol causes cell cycle arrest, decreased collagen synthesis, and apoptosis in rat intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol [Internet]. 2012 Feb [cited. 2024 Jul 13];302(3). Available from: https://pubmed.ncbi.nlm.nih.gov/22052016/
414. Rahal K, Schmiedlin-Ren P, Adler J, Dhanani M, Sultani V, Rittershaus AC. et al. Resveratrol has antiinflammatory and antifibrotic effects in the peptidoglycan-polysaccharide rat model of Crohn's disease. Inflamm Bowel Dis [Internet]. 2012 Apr [cited. 2024 Jul 13];18(4):613-23. Available from: https://pubmed.ncbi.nlm.nih.gov/22431488/
415. Al Azzaz J, Rieu A, Aires V, Delmas D, Chluba J, Winckler P. et al. Resveratrol-Induced Xenophagy Promotes Intracellular Bacteria Clearance in Intestinal Epithelial Cells and Macrophages. Front Immunol [Internet]. 2019 [cited. 2024 Jul 13];9:3149. Available from: https://pubmed.ncbi.nlm.nih.gov/30693000/
416. Liu X, Wu YL, Liu KL, Cui XL, DU XX, Zhang WQ. [Effects of resveratrol on ulcerative colitis in mice and its mechanism]. Zhongguo Ying Yong Sheng Li Xue Za Zhi [Internet]. 2019 Sep 1 [cited. 2024 Jul 13];35(5):447-53. Available from: https://pubmed.ncbi.nlm.nih.gov/31894679/
417. Yu B, Wang Y, Tan Z, Hong Z, Yao L, Huang S. et al. Resveratrol ameliorates DSS-induced ulcerative colitis by acting on mouse gut microbiota. Inflammopharmacology [Internet]. 2024 Jun 1 [cited. 2024 Jul 13];32(3):2023-33. Available from: https://pubmed.ncbi.nlm.nih.gov/38492181/
418. Xu X, Ocansey DKW, Pei B, Zhang Y, Wang N, Wang Z. et al. Resveratrol alleviates DSS-induced IBD in mice by regulating the intestinal microbiota-macrophage-arginine metabolism axis. Eur J Med Res [Internet]. 2023 Dec 1 [cited. 2024 Mar 18];28(1). Available from: https://pubmed.ncbi.nlm.nih.gov/37660064/
419. Wang J, Zhang Z, Fang A, Wu K, Chen X, Wang G. et al. Resveratrol Attenuates Inflammatory Bowel Disease in Mice by Regulating SUMO1. Biol Pharm Bull [Internet]. 2020 Mar 1 [cited. 2024 Jul 13];43(3):450-7. Available from: https://pubmed.ncbi.nlm.nih.gov/32115503/
420. Yaqin Z, Kehan W, Yi Z, Naijian W, Wei Q, Fei M. Resveratrol alleviates inflammatory bowel disease by inhibiting JAK2/STAT3 pathway activity via the reduction of O-GlcNAcylation of STAT3 in intestinal epithelial cells. Toxicol Appl Pharmacol [Internet]. 2024 Mar 1 [cited. 2024 Jul 13];484. Available from: https://pubmed.ncbi.nlm.nih.gov/38437956/
421. Zhang L, Hui XUE, Zhao G, Qiao C, Xiaomei SUN, Pang C. et al. Curcumin and resveratrol suppress dextran sulfate sodium-induced colitis in mice. Mol Med Rep [Internet]. 2019 Apr 1 [cited. 2024 Jul 13];19(4):3053-60. Available from: https://pubmed.ncbi.nlm.nih.gov/30816479/
422. Pan HH, Zhou XX, Ma YY, Pan WS, Zhao F, Yu MS. et al. Resveratrol alleviates intestinal mucosal barrier dysfunction in dextran sulfate sodium-induced colitis mice by enhancing autophagy. World J Gastroenterol [Internet]. 2020 Sep 7 [cited. 2024 Jul 13];26(33):4945-59. Available from: https://pubmed.ncbi.nlm.nih.gov/32952341/
423. Zhu F, Zheng J, Xu F, Xi Y, Chen J, Xu X. Resveratrol Alleviates Dextran Sulfate Sodium-Induced Acute Ulcerative Colitis in Mice by Mediating PI3K/Akt/VEGFA Pathway. Front Pharmacol [Internet]. 2021 Aug 23 [cited. 2024 Jul 13];12. Available from: https://pubmed.ncbi.nlm.nih.gov/34497510/
424. Abdallah DM, Ismael NR. Resveratrol abrogates adhesion molecules and protects against TNBS-induced ulcerative colitis in rats. Can J Physiol Pharmacol [Internet]. 2011 Nov [cited. 2024 Jul 13];89(11):811-8. Available from: https://pubmed.ncbi.nlm.nih.gov/22029500/
425. Yao J, Wang JY, Liu L, Li YX, Xun AY, Zeng W Sen. et al. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch Med Res [Internet]. 2010 May [cited. 2024 Jul 13];41(4):288-94. Available from: https://pubmed.ncbi.nlm.nih.gov/20637373/
426. Yao J, Wei C, Wang JY, Zhang R, Li YX, Wang LS. Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World J Gastroenterol [Internet]. 2015 Jun 7 [cited. 2024 Jul 13];21(21):6572-81. Available from: https://pubmed.ncbi.nlm.nih.gov/26074695/
427. Singh UP, Singh NP, Singh B, Hofseth LJ, Taub DD, Price RL. et al. Role of resveratrol-induced CD11b(+) Gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(-/-) mice. Brain Behav Immun [Internet]. 2012 Jan [cited. 2024 Jul 13];26(1):72-82. Available from: https://pubmed.ncbi.nlm.nih.gov/21807089/
428. Sun H, Cai H, Fu Y, Wang Q, Ji K, Du L. et al. The Protection Effect of Resveratrol Against Radiation-Induced Inflammatory Bowel Disease via NLRP-3 Inflammasome Repression in Mice. Dose Response [Internet]. 2020 Apr 1 [cited. 2024 Jul 13];18(2). Available from: https://pubmed.ncbi.nlm.nih.gov/32636719/
429. Prakash V, Bose C, Sunilkumar D. et al. Resveratrol as a Promising Nutraceutical: Implications in Gut Microbiota Modulation, Inflammatory Disorders, and Colorectal Cancer. Int J Mol Sci. 2024;25:3370
430. Alattar A, Alshaman R, Al-Gayyar MMH. Therapeutic Effects of Sulforaphane in Ulcerative Colitis: Effect on Antioxidant Activity, Mitochondrial Biogenesis and DNA Polymerization. Redox Rep. 2022;27:128-138
431. Alrafas HR, Busbee PB, Nagarkatti M, Nagarkatti PS. Resveratrol Downregulates miR-31 to Promote T Regulatory Cells during Prevention of TNBS-Induced Colitis. Mol Nutr Food Res [Internet]. 2020 Jan 1 [cited. 2024 Jul 14];64(1). Available from: https://pubmed.ncbi.nlm.nih.gov/31730734/
432. Abdin AA. Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur J Pharmacol [Internet]. 2013 [cited. 2024 Jul 14];718(1-3):145-53. Available from: https://pubmed.ncbi.nlm.nih.gov/24055189/
433. Erol Doğan Ö, Karaca Çelik KE, Baş M, Alan EH, Çağın YF. Effects of Mediterranean Diet, Curcumin, and Resveratrol on Mild-to-Moderate Active Ulcerative Colitis: A Multicenter Randomized Clinical Trial. Nutrients [Internet]. 2024 May 16 [cited. 2024 Jul 14];16(10). Available from: https://pubmed.ncbi.nlm.nih.gov/38794742/
434. Samsami-kor M, Daryani NE, Asl PR, Hekmatdoost A. Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch Med Res [Internet]. 2015 May 1 [cited. 2024 Jul 14];46(4):280-5. Available from: https://pubmed.ncbi.nlm.nih.gov/26002728/
435. Samsamikor M, Daryani NE, Asl PR, Hekmatdoost A. Resveratrol Supplementation and Oxidative/Anti-Oxidative Status in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch Med Res [Internet]. 2016 May 1 [cited. 2024 Jul 14];47(4):304-9. Available from: https://pubmed.ncbi.nlm.nih.gov/27664491/
436. Rakib A, Mandal M, Showkat A, Kiran S, Mazumdar S, Singla B. et al. Piceatannol induces regulatory T cells and modulates the inflammatory response and adipogenesis. Biomedicine & Pharmacotherapy. 2023May1;161:114514
437. Lin WS, Chueh TL, Nagabhushanam K, Ho CT, Pan MH. Piceatannol and 3'-Hydroxypterostilbene Alleviate Inflammatory Bowel Disease by Maintaining Intestinal Epithelial Integrity and Regulating Gut Microbiota in Mice. J Agric Food Chem [Internet]. 2023 Feb 1 [cited. 2024 Apr 14];71(4):1994-2005. Available from: https://pubmed.ncbi.nlm.nih.gov/36688924/
438. Youn J, Lee JS, Na HK, Kundu JK, Surh YJ. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr Cancer [Internet]. 2009 [cited. 2024 Apr 14];61(6):847-54. Available from: https://pubmed.ncbi.nlm.nih.gov/20155626/
439. Kim YH, Kwon HS, Kim DH, Cho HJ, Lee HS, Jun JG. et al. Piceatannol, a stilbene present in grapes, attenuates dextran sulfate sodium-induced colitis. Int Immunopharmacol. 2008Dec10;8(12):1695-702
440. Kim H, Seo KH, Yokoyama W. Chemistry of Pterostilbene and Its Metabolic Effects. J Agric Food Chem [Internet]. 2020 Nov 18 [cited. 2024 Mar 18];68(46):12836-41. Available from: https://pubmed.ncbi.nlm.nih.gov/32125846/
441. McCormack D, McFadden D. A Review of Pterostilbene Antioxidant Activity and Disease Modification. Oxid Med Cell Longev [Internet]. 2013 [cited. 2024 Jul 14];2013:15. Available from: /pmc/articles/PMC3649683/
442. Ruan B, Rong M, Ming Z, Wang K, Liu X, Deng L. et al. Discovery of pterostilbene analogs as novel NLRP3 inflammasome inhibitors for potential treatment of DSS-induced colitis in mice. Bioorg Chem [Internet]. 2023 Apr 1 [cited. 2024 Mar 18];133. Available from: https://pubmed.ncbi.nlm.nih.gov/36841048/
443. Fan-Jiang PY, Lee PS, Nagabhushanam K, Ho CT, Pan MH. Pterostilbene Attenuates High-Fat Diet and Dextran Sulfate Sodium-Induced Colitis via Suppressing Inflammation and Intestinal Fibrosis in Mice. J Agric Food Chem [Internet]. 2021 Jun 30 [cited. 2024 Jul 14];69(25):7093-103. Available from: https://pubmed.ncbi.nlm.nih.gov/34152136/
444. Yashiro T, Yura S, Tobita A, Toyoda Y, Kasakura K, Nishiyama C. Pterostilbene reduces colonic inflammation by suppressing dendritic cell activation and promoting regulatory T cell development. FASEB J [Internet]. 2020 Nov 1 [cited. 2024 Jul 14];34(11):14810-9. Available from: https://pubmed.ncbi.nlm.nih.gov/32964554/
445. Erol Doğan Ö, Karaca Çelik KE, Baş M, Alan EH, Çağın YF. Effects of Mediterranean Diet, Curcumin, and Resveratrol on Mild-to-Moderate Active Ulcerative Colitis: A Multicenter Randomized Clinical Trial. Nutrients [Internet]. 2024 May 16 [cited. 2024 Jul 15];16(10). Available from: https://pubmed.ncbi.nlm.nih.gov/38794742/
446. Durazzo A, Lucarini M, Camilli E, Marconi S, Gabrielli P, Lisciani S. et al. Dietary Lignans: Definition, Description and Research Trends in Databases Development. Molecules 2018, Vol 23, Page 3251 [Internet]. 2018 Dec 8 [cited. 2024 Mar 18];23(12):3251. Available from: https://www.mdpi.com/1420-3049/23/12/3251/htm
447. Rodríguez-García C, Sánchez-Quesada C, Toledo E, Delgado-Rodríguez M, Gaforio JJ. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules [Internet]. 2019 [cited. 2024 Jul 14];24(5). Available from: /pmc/articles/PMC6429205/
448. Touré A, Xueming X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr Rev Food Sci Food Saf [Internet]. 2010 May 1 [cited 2024 Jul 14];9(3):261-9. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1541-4337. 2009 00105.x
449. Plissonneau C, Sivignon A, Chassaing B, Capel F, Martin V, Etienne M. et al. Beneficial Effects of Linseed Supplementation on Gut Mucosa-Associated Microbiota in a Physically Active Mouse Model of Crohn's Disease. Int J Mol Sci [Internet]. 2022 Jun 1 [cited. 2024 Jul 14];23(11). Available from: https://pubmed.ncbi.nlm.nih.gov/35682570/
450. Palla AH, Gilani AUH, Bashir S, Ur Rehman N. Multiple Mechanisms of Flaxseed: Effectiveness in Inflammatory Bowel Disease. Evid Based Complement Alternat Med [Internet]. 2020 [cited 2024 Jul 14]. 2020 Available from: https://pubmed.ncbi.nlm.nih.gov/32765633/
451. Palla AH, Iqbal NT, Minhas K, Gilani AH. Flaxseed extract exhibits mucosal protective effect in acetic acid induced colitis in mice by modulating cytokines, antioxidant and antiinflammatory mechanisms. Int Immunopharmacol [Internet]. 2016 Sep 1 [cited. 2024 Jul 14];38:153-66. Available from: https://pubmed.ncbi.nlm.nih.gov/27280586/
452. e Silva FGD, Paiatto LN, Yamada AT, Netto FM, Simioni PU, Tamashiro WMSC. Intake of Protein Hydrolysates and Phenolic Fractions Isolated from Flaxseed Ameliorates TNBS-Induced Colitis. Mol Nutr Food Res [Internet]. 2018 Sep 1 [cited. 2024 Jul 14];62(17). Available from: https://pubmed.ncbi.nlm.nih.gov/29932491/
453. Wang Z, Chen T, Yang C, Bao T, Yang X, He F. et al. Secoisolariciresinol diglucoside suppresses Dextran sulfate sodium salt-induced colitis through inhibiting NLRP1 inflammasome. Int Immunopharmacol [Internet]. 2020 Jan 1 [cited. 2024 Jul 14];78. Available from: https://pubmed.ncbi.nlm.nih.gov/31812068/
454. Xu Z, Chen W, Deng Q, Huang Q, Wang X, Yang C. et al. Flaxseed oligosaccharides alleviate DSS-induced colitis through modulation of gut microbiota and repair of the intestinal barrier in mice. Food Funct [Internet]. 2020 Sep 1 [cited. 2024 Jul 14];11(9):8077-88. Available from: https://pubmed.ncbi.nlm.nih.gov/32856645/
455. Caetano-Silva ME, Rund LA, Vailati-Riboni M, Pacheco MTB, Johnson R. Copper-Binding Peptides Attenuate Microglia Inflammation through Suppression of NF-KB Pathway. Mol Nutr Food Res. 2021;65:e2100153
456. de Paula do Nascimento R, Lima AV, Oyama LM, Paiotti APR, Cardili L, Martinez CAR. et al. Extra virgin olive oil and flaxseed oil have no preventive effects on DSS-induced acute ulcerative colitis. Nutrition [Internet]. 2020 Jun 1 [cited. 2024 Jul 14];74. Available from: https://pubmed.ncbi.nlm.nih.gov/32179382/
457. Määttänen P, Lurz E, Botts SR, Wu RY, Yeung CW, Li B. et al. Ground flaxseed reverses protection of a reduced-fat diet against Citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol [Internet]. 2018 Nov 1 [cited. 2024 Jul 14];315(5):G788-98. Available from: https://pubmed.ncbi.nlm.nih.gov/30095298/
458. Morshedzadeh N, Shahrokh S, Chaleshi V, Karimi S, Mirmiran P, Zali MR. The effects of flaxseed supplementation on gene expression and inflammation in ulcerative colitis patients: An open-labelled randomised controlled trial. Int J Clin Pract [Internet]. 2021 May 1 [cited. 2024 Jul 14];75(5). Available from: https://pubmed.ncbi.nlm.nih.gov/33482045/
459. Morshedzadeh N, Shahrokh S, Aghdaei HA, Amin Pourhoseingholi M, Chaleshi V, Hekmatdoost A. et al. Effects of flaxseed and flaxseed oil supplement on serum levels of inflammatory markers, metabolic parameters and severity of disease in patients with ulcerative colitis. Complement Ther Med [Internet]. 2019 Oct 1 [cited. 2024 Jul 14];46:36-43. Available from: https://pubmed.ncbi.nlm.nih.gov/31519285/
460. Morshedzadeh N, Rahimlou M, Shahrokh S, Karimi S, Mirmiran P, Zali MR. The effects of flaxseed supplementation on metabolic syndrome parameters, insulin resistance and inflammation in ulcerative colitis patients: An open-labeled randomized controlled trial. Phytotherapy Research [Internet]. 2021 Jul 1 [cited. 2024 Jul 14];35(7):3781-91. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ptr.7081
461. Morshedzadeh N, Rahimlou M, Shahrokh S, Chaleshi V, Mirmiran P, Zali MR. The effects of flaxseed supplementation on concentration of circulating adipokines in patients with ulcerative colitis. Gastroenterol Hepatol Bed Bench [Internet]. 2023 [cited. 2024 Jul 14];16(1):24-33. Available from: https://pubmed.ncbi.nlm.nih.gov/37070105/
462. Wei P, Zhao F, Wang Z. et al. Sesame (Sesamum Indicum L.): A Comprehensive Review of Nutritional Value, Phytochemical Composition, Health Benefits, Development of Food, and Industrial Applications. Nutrients. 2022;14:4079
463. Andargie M, Vinas M, Rathgeb A, Möller E, Karlovsky P. Lignans of Sesame (Sesamum indicum L.): A Comprehensive Review. Molecules [Internet]. 2021 Feb 2 [cited 2024 Jul 14];26(4):883. Available from: https://pubmed.ncbi.nlm.nih.gov/33562414/
464. Ostovan M, Fazljou SMB, Khazraei H, Khodaei MA, Torbati M. The Anti-Inflammatory Effect of Pistacia Lentiscus in a Rat Model of Colitis. J Inflamm Res [Internet]. 2020 [cited. 2024 Jul 14];13:369-76. Available from: https://pubmed.ncbi.nlm.nih.gov/32801830/
465. Periasamy S, Hsu DZ, Chandrasekaran VRM, Liu MY. Sesame oil accelerates healing of 2,4,6-trinitrobenzenesulfonic acid-induced acute colitis by attenuating inflammation and fibrosis. JPEN J Parenter Enteral Nutr [Internet]. 2013 Sep [cited. 2024 Jul 14];37(5):674-82. Available from: https://pubmed.ncbi.nlm.nih.gov/23243149/
466. Tungalag T, Park JY, Park KW, Yang DK. Sesame cake extract attenuates dextran sulfate sodium-induced colitis through inhibition of oxidative stress in mice. Food Sci Biotechnol [Internet]. 2023 Feb 1 [cited. 2024 Jul 14];33(3):699-709. Available from: https://pubmed.ncbi.nlm.nih.gov/38274181/
467. Song J Le, Choi JH, Seo JH, Lim YI, Park KY. Anti-Colitic Effects of Kanjangs (Fermented Soy Sauce and Sesame Sauce) in Dextran Sulfate Sodium-Induced Colitis in Mice. J Med Food [Internet]. 2014 Sep 9 [cited. 2024 Jul 14];17(9):1027. Available from: /pmc/articles/PMC4152797/
468. Bai X, Gou X, Cai P, Xu C, Cao L, Zhao Z. et al. Sesamin Enhances Nrf2-Mediated Protective Defense against Oxidative Stress and Inflammation in Colitis via AKT and ERK Activation. Oxid Med Cell Longev [Internet]. 2019 [cited 2024 Mar 18]. 2019 Available from: https://pubmed.ncbi.nlm.nih.gov/31534619/
469. Chen S, Zhang CL, Shen HQ, Zhou XF, Li JH, Yu JL. et al. Sesamin protects against DSS-induced colitis in mice by inhibiting NF-κB and MAPK signaling pathways. Food Funct [Internet]. 2021 Feb 21 [cited. 2024 Apr 14];12(4):1688-94. Available from: https://pubmed.ncbi.nlm.nih.gov/33496702/
470. Zhao B, Xia B, Li X, Zhang L, Liu X, Shi R. et al. Sesamol Supplementation Attenuates DSS-Induced Colitis via Mediating Gut Barrier Integrity, Inflammatory Responses, and Reshaping Gut Microbiome. J Agric Food Chem [Internet]. 2020 Sep 30 [cited. 2024 Jul 15];68(39):10697-708. Available from: https://pubmed.ncbi.nlm.nih.gov/32893621/
471. Xia B, Liu X, Li X, Wang Y, Wang D, Kou R. et al. Sesamol ameliorates dextran sulfate sodium-induced depression-like and anxiety-like behaviors in colitis mice: the potential involvement of the gut-brain axis. Food Funct [Internet]. 2022 Mar 7 [cited. 2024 Jul 15];13(5):2865-83. Available from: https://pubmed.ncbi.nlm.nih.gov/35179534/
472. Kondamudi PK, Kovelamudi H, Mathew G, Nayak PG, Rao MC, Shenoy RR. Investigation of sesamol on myeloperoxidase and colon morphology in acetic acid-induced inflammatory bowel disorder in albino rats. ScientificWorldJournal [Internet]. 2014 [cited 2024 Jul 15]. 2014 Available from: https://pubmed.ncbi.nlm.nih.gov/24616646/
473. Wang X, Wang X, Yao H, Shen C, Geng K, Xie H. A comprehensive review on Schisandrin and its pharmacological features. Naunyn Schmiedebergs Arch Pharmacol [Internet]. 2024 Feb 1 [cited. 2024 Jul 15];397(2):783-94. Available from: https://pubmed.ncbi.nlm.nih.gov/37658213/
474. Wang X, Shen C, Wang X, Tang J, Wu Z, Huang Y. et al. Schisandrin protects against ulcerative colitis by inhibiting the SGK1/NLRP3 signaling pathway and reshaping gut microbiota in mice. Chin Med [Internet]. 2023 Dec 1 [cited. 2024 Jul 15];18(1). Available from: https://pubmed.ncbi.nlm.nih.gov/37674245/
475. Ma Z, Xu G, Shen Y, Hu S, Lin X, Zhou J. et al. Schisandrin B-mediated TH17 cell differentiation attenuates bowel inflammation. Pharmacol Res [Internet]. 2021 Apr 1 [cited. 2024 Jul 15];166. Available from: https://pubmed.ncbi.nlm.nih.gov/33545313/
476. Li J, Lu Y, Wang D, Quan F, Chen X, Sun R. et al. Schisandrin B prevents ulcerative colitis and colitis-associated-cancer by activating focal adhesion kinase and influence on gut microbiota in an in vivo and in vitro model. Eur J Pharmacol [Internet]. 2019 Jul 5 [cited. 2024 Jul 15];854:9-21. Available from: https://pubmed.ncbi.nlm.nih.gov/30951716/
477. Nasser MI, Zhu S, Chen C, Zhao M, Huang H, Zhu P. A Comprehensive Review on Schisandrin B and Its Biological Properties. 2020 [cited. 2024 Jul 15]; Available from: https://doi.org/10.1155/2020/2172740
478. Zhang W, Wang W, Shen C, Wang X, Pu Z, Yin Q. Network pharmacology for systematic understanding of Schisandrin B reduces the epithelial cells injury of colitis through regulating pyroptosis by AMPK/Nrf2/NLRP3 inflammasome. Aging [Internet]. 2021 Oct 15 [cited. 2024 Jul 15];13(19):22193-209. Available from: https://pubmed.ncbi.nlm.nih.gov/34628369/
479. Kim MR, Cho SY, Lee HJ, Kim JY, Nguyen UTT, Ha NM. et al. Schisandrin C improves leaky gut conditions in intestinal cell monolayer, organoid, and nematode models by increasing tight junction protein expression. Phytomedicine [Internet]. 2022 Aug 1 [cited. 2024 Jul 15];103. Available from: https://pubmed.ncbi.nlm.nih.gov/35689901/
480. Zhang WF, Yang Y, Su X, Xu DY, Yan YL, Gao Q. et al. Deoxyschizandrin suppresses dss-induced ulcerative colitis in mice. Saudi J Gastroenterol [Internet]. 2016 Nov 1 [cited. 2024 Jul 15];22(6):448-55. Available from: https://pubmed.ncbi.nlm.nih.gov/27976641/
481. Hou Y, An J, Hu XR, Sun BB, Lin J, Xu D. et al. Ghrelin inhibits interleukin-8 production induced by hydrogen peroxide in A549 cells via NF-κB pathway. Int Immunopharmacol. 2009Jan;9(1):120-6
482. CHEN C, ZHANG QW, YE Y, LIN LG. Honokiol: A naturally occurring lignan with pleiotropic bioactivities. Chin J Nat Med [Internet]. 2021 Jul 1 [cited. 2024 Apr 14];19(7):481-90. Available from: https://pubmed.ncbi.nlm.nih.gov/34247771/
483. Lee KI, Kim HJ, Kim H, Kim MS, Kim JI, Park KS. Magnolia officinalis Bark Extract Prevents Enterocyte Death in a Colitis Mouse Model by Inhibiting ROS-Mediated Necroptosis. Antioxidants (Basel) [Internet]. 2022 Dec 1 [cited. 2024 Apr 14];11(12). Available from: https://pubmed.ncbi.nlm.nih.gov/36552643/
484. Zhao L, Xiao HT, Mu HX, Huang T, Lin ZS, Zhong LLD. et al. Magnolol, a Natural Polyphenol, Attenuates Dextran Sulfate Sodium-Induced Colitis in Mice. Molecules [Internet]. 2017 Jul 20 [cited. 2024 Mar 18];22(7). Available from: https://pubmed.ncbi.nlm.nih.gov/28726741/
485. Chen H, Fu W, Chen H, You S, Liu X, Yang Y. et al. Magnolol attenuates the inflammation and enhances phagocytosis through the activation of MAPK, NF-κB signal pathways in vitro and in vivo. Mol Immunol [Internet]. 2019 Jan 1 [cited. 2024 Jul 15];105:96-106. Available from: https://pubmed.ncbi.nlm.nih.gov/30500626/
486. Shen P, Zhang Z, He Y, Gu C, Zhu K, Li S. et al. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci [Internet]. 2018 Mar 1 [cited. 2024 Jul 15];196:69-76. Available from: https://pubmed.ncbi.nlm.nih.gov/29355546/
487. Wang L, Wang J. Honokiol Ameliorates DSS-Induced Mouse Colitis by Inhibiting Inflammation and Oxidative Stress and Improving the Intestinal Barrier. Oxid Med Cell Longev [Internet]. 2022 [cited 2024 Jul 15]. 2022 Available from: https://pubmed.ncbi.nlm.nih.gov/36578522/
488. Wang N, Kong R, Han W, Bao W, Shi Y, Ye LP. et al. Honokiol alleviates ulcerative colitis by targeting PPAR-γ-TLR4-NF-κB signaling and suppressing gasdermin-D-mediated pyroptosis in vivo and in vitro. Int Immunopharmacol [Internet]. 2022 Oct 1 [cited. 2024 Jul 15];111. Available from: https://pubmed.ncbi.nlm.nih.gov/35901530/
489. Niu L, Wang S, Xu Y, Zu X, You X, Zhang Q. et al. Honokiol targeting ankyrin repeat domain of TRPV4 ameliorates endothelial permeability in mice inflammatory bowel disease induced by DSS. J Ethnopharmacol [Internet]. 2024 May 10 [cited. 2024 Jul 15];325. Available from: https://pubmed.ncbi.nlm.nih.gov/38296175/
490. Cai X, Jiang X, Zhao M, Su K, Tang M, Hong F. et al. Identification of the target protein and molecular mechanism of honokiol in anti-inflammatory action. Phytomedicine [Internet]. 2023 Jan 1 [cited. 2024 Jul 15];109. Available from: https://pubmed.ncbi.nlm.nih.gov/36610140/
491. Bhuia MS, Wilairatana P, Chowdhury R, Rakib AI, Kamli H, Shaikh A. et al. Anticancer Potentials of the Lignan Magnolin: A Systematic Review. Molecules [Internet]. 2023 May 1 [cited. 2024 Jul 15];28(9). Available from: /pmc/articles/PMC10180476/
492. Zhang M, Song X, Liu S, Zhang N, Yang M, Gao P. et al. Magnolin inhibits intestinal epithelial cell apoptosis alleviating Crohn's disease-like colitis by suppressing the PI3K/AKT signalling pathway. Int Immunopharmacol [Internet]. 2024 Jun 15 [cited. 2024 Jul 15];134. Available from: https://pubmed.ncbi.nlm.nih.gov/38733829/
493. Yao T, Yao YY, Wang JZ, Jiang SM, Li LJ. Magnolin alleviated DSS-induced colitis by inhibiting ALOX5-mediated ferroptosis. Kaohsiung J Med Sci [Internet]. 2024 Apr 1 [cited. 2024 Jul 15];40(4):360-73. Available from: https://pubmed.ncbi.nlm.nih.gov/38340032/
494. Wu X, Yang Y, Dou Y, Ye J, Bian D, Wei Z. et al. Arctigenin but not arctiin acts as the major effective constituent of Arctium lappa L. fruit for attenuating colonic inflammatory response induced by dextran sulfate sodium in mice. Int Immunopharmacol [Internet]. 2014 [cited. 2024 Jul 15];23(2):505-15. Available from: https://pubmed.ncbi.nlm.nih.gov/25284342/
495. Wu X, Dou Y, Yang Y, Bian D, Luo J, Tong B. et al. Arctigenin exerts anti-colitis efficacy through inhibiting the differentiation of Th1 and Th17 cells via an mTORC1-dependent pathway. Biochem Pharmacol [Internet]. 2015 Aug 3 [cited. 2024 Jul 15];96(4):323-36. Available from: https://pubmed.ncbi.nlm.nih.gov/26074264/
496. Guo Y, Liu X, Tao Y, Zhu Y, Zhang J, Yu X. et al. Arctigenin promotes mucosal healing in ulcerative colitis through facilitating focal adhesion assembly and colonic epithelial cell migration via targeting focal adhesion kinase. Int Immunopharmacol [Internet]. 2024 Feb 15 [cited. 2024 Jul 15];128. Available from: https://pubmed.ncbi.nlm.nih.gov/38280335/
497. Wang LQ. Mammalian phytoestrogens: Enterodiol and enterolactone. J Chromatogr B Analyt Technol Biomed Life Sci [Internet]. 2002 Sep 25 [cited. 2024 Jul 15];777(1-2):289-309. Available from: https://pubmed.ncbi.nlm.nih.gov/12270221/
498. Almousa AA, Meurens F, Krol ES, Alcorn J. Linoorbitides and enterolactone mitigate inflammation-induced oxidative stress and loss of intestinal epithelial barrier integrity. Int Immunopharmacol [Internet]. 2018 Nov 1 [cited. 2024 Jul 15];64:42-51. Available from: https://pubmed.ncbi.nlm.nih.gov/30145469/
499. Kim TW, Shin JS, Chung KS, Lee YG, Baek NI, Lee KT. Anti-Inflammatory Mechanisms of Koreanaside A, a Lignan Isolated from the Flower of Forsythia koreana, against LPS-Induced Macrophage Activation and DSS-Induced Colitis Mice: The Crucial Role of AP-1, NF-κB, and JAK/STAT Signaling. Cells [Internet]. 2019 Oct 1 [cited. 2024 Apr 14];8(10). Available from: https://pubmed.ncbi.nlm.nih.gov/31569788/
500. Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives - A review. J Tradit Complement Med [Internet]. 2017 Apr 1 [cited. 2024 Jul 15];7(2):205. Available from: /pmc/articles/PMC5388087/
501. Silvestro S, Sindona C, Bramanti P, Mazzon E, Prata C, Maraldi T. et al. Molecular Sciences A State of the Art of Antioxidant Properties of Curcuminoids in Neurodegenerative Diseases. J Mol Sci [Internet]. 2021 [cited. 2024 Mar 18];22. Available from: https://doi.org/10.3390/ijms22063168
502. Salehi B, Stojanović-Radić Z, Matejić J, Sharifi-Rad M, Anil Kumar N V, Martins N. et al. The therapeutic potential of curcumin: A review of clinical trials. Eur J Med Chem. 2019Feb1;163:527-45
503. Brumatti LV, Marcuzzi A, Tricarico PM, Zanin V, Girardelli M, Bianco AM. Curcumin and inflammatory bowel disease: potential and limits of innovative treatments. Molecules [Internet]. 2014 Dec 1 [cited. 2024 Mar 18];19(12):21127-53. Available from: https://pubmed.ncbi.nlm.nih.gov/25521115/
504. Fallahi F, Borran S, Ashrafizadeh M, Zarrabi A, Pourhanifeh MH, Khaksary Mahabady M. et al. Curcumin and inflammatory bowel diseases: From in vitro studies to clinical trials. Mol Immunol. 2021Feb1;130:20-30
505. Goulart RDA, Barbalho SM, Lima VM, Souza GA De, Matias JN, Araújo AC. et al. Effects of the Use of Curcumin on Ulcerative Colitis and Crohn's Disease: A Systematic Review. J Med Food [Internet]. 2021 Jul 1 [cited. 2024 Mar 18];24(7):675-85. Available from: https://pubmed.ncbi.nlm.nih.gov/33155879/
506. Yue W, Liu Y, Li X, Lv L, Huang J, Liu J. Curcumin ameliorates dextran sulfate sodium-induced colitis in mice via regulation of autophagy and intestinal immunity. Turk J Gastroenterol [Internet]. 2019 Mar 1 [cited. 2024 Apr 14];30(3):290-8. Available from: https://pubmed.ncbi.nlm.nih.gov/30923033/
507. Samba-Mondonga M, Constante M, Fragoso G, Calvé A, Santos MM. Curcumin induces mild anemia in a DSS-induced colitis mouse model maintained on an iron-sufficient diet. PLoS One [Internet]. 2019 Apr 1 [cited. 2024 Apr 14];14(4). Available from: https://pubmed.ncbi.nlm.nih.gov/31026259/
508. Martelli L, Ragazzi E, Di Mario F, Martelli M, Castagliuolo I, Dal Maschio M. et al. A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterology and motility [Internet]. 2007 Aug [cited. 2024 Apr 14];19(8):668-74. Available from: https://pubmed.ncbi.nlm.nih.gov/17640182/
509. Younes OA, Elsherbiny DM, Hanna DMF, Gad AM, Azab SS. Tocilizumab Unfolds Colo-Protective and Immunomodulatory Effect in Experimentally Induced Ulcerative Colitis via Mitigating Autophagy and ER Stress Signaling. Inflammopharmacology. 2024
510. Zhang M, Deng CS, Zheng JJ, Xia J. Curcumin regulated shift from Th1 to Th2 in trinitrobenzene sulphonic acid-induced chronic colitis. Acta Pharmacol Sin [Internet]. 2006 Aug [cited. 2024 Jul 15];27(8):1071-7. Available from: https://pubmed.ncbi.nlm.nih.gov/16867261/
511. Lin Y, Liu H, Bu L, Chen C, Ye X. Review of the Effects and Mechanism of Curcumin in the Treatment of Inflammatory Bowel Disease. Front Pharmacol [Internet]. 2022 Jun 20 [cited. 2024 Jul 15];13. Available from: /pmc/articles/PMC9250976/
512. Pituch-Zdanowska A, Dembiński Ł, Banaszkiewicz A. Old but Fancy: Curcumin in Ulcerative Colitis—Current Overview. Nutrients [Internet]. 2022 Dec 1 [cited. 2024 Jul 15];14(24). Available from: /pmc/articles/PMC9781182/
513. Ji J, Ma Z, Wang Y. Advancing Gastrointestinal Health: Curcumin's Efficacy and Nanopreparations. Molecules [Internet]. 2024 Apr 1 [cited. 2024 Jul 15];29(7). Available from: https://pubmed.ncbi.nlm.nih.gov/38611938/
514. Yang H, Zhang H, Tian L. et al. Curcumin Attenuates Lupus Nephritis by Inhibiting Neutrophil Migration via PI3K/AKT/NF-ΚB Signalling Pathway. Lupus Sci Med. 2024;11:e001220
515. Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A. et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol [Internet]. 2006 Dec [cited. 2024 Jul 15];4(12):1502-6. Available from: https://pubmed.ncbi.nlm.nih.gov/17101300/
516. Singla V, Pratap Mouli V, Garg SK, Rai T, Choudhury BN, Verma P. et al. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis - a randomized, placebo-controlled, pilot study. J Crohns Colitis [Internet]. 2014 Mar 1 [cited. 2024 Jul 15];8(3):208-14. Available from: https://pubmed.ncbi.nlm.nih.gov/24011514/
517. Lang A, Salomon N, Wu JCY, Kopylov U, Lahat A, Har-Noy O. et al. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin Gastroenterol Hepatol [Internet]. 2015 Aug 1 [cited. 2024 Jul 15];13(8):1444-1449.e1. Available from: https://pubmed.ncbi.nlm.nih.gov/25724700/
518. Salomon N, Lang A, Kopylov U, Lahat A, Har-Noy O, Wu J. et al. Curcumin Add-on Therapy for Remission Induction in Mild-moderate Active Ulcerative Colitis: A Multi-center, Randomized, Placebo-Controlled Trial. Clinical Gastroenterology and Hepatology. 2015Jul1;13(7):1381-2
519. Masoodi M, Mahdiabadi MA, Mokhtare M, Agah S, Kashani AHF, Rezadoost AM. et al. The efficacy of curcuminoids in improvement of ulcerative colitis symptoms and patients' self-reported well-being: A randomized double-blind controlled trial. J Cell Biochem [Internet]. 2018 Nov 1 [cited. 2024 Jul 15];119(11):9552-9. Available from: https://pubmed.ncbi.nlm.nih.gov/30132960/
520. Banerjee R, Pal P, Penmetsa A, Kathi P, Girish G, Goren I. et al. Novel Bioenhanced Curcumin With Mesalamine for Induction of Clinical and Endoscopic Remission in Mild-to-Moderate Ulcerative Colitis: A Randomized Double-Blind Placebo-controlled Pilot Study. J Clin Gastroenterol [Internet]. 2021 Sep 1 [cited. 2024 Jul 15];55(8):702-8. Available from: https://pubmed.ncbi.nlm.nih.gov/32889959/
521. Sadeghi N, Mansoori A, Shayesteh A, Hashemi SJ. The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis. Phytother Res [Internet]. 2020 May 1 [cited. 2024 Jul 15];34(5):1123-33. Available from: https://pubmed.ncbi.nlm.nih.gov/31802559/
522. Ben-Horin S, Salomon N, Karampekos G, Viazis N, Lahat A, Ungar B. et al. Curcumin-QingDai Combination for Patients With Active Ulcerative Colitis: A Randomized, Double-Blinded, Placebo-Controlled Trial. Clin Gastroenterol Hepatol [Internet]. 2024 Feb 1 [cited. 2024 Jul 15];22(2):347-356.e6. Available from: https://pubmed.ncbi.nlm.nih.gov/37302449/
523. Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: A pilot study. Dig Dis Sci [Internet]. 2005 Nov [cited. 2024 Jul 15];50(11):2191-3. Available from: https://link.springer.com/article/10.1007/s10620-005-3032-8
524. Suskind DL, Wahbeh G, Burpee T, Cohen M, Christie D, Weber W. Tolerability of curcumin in pediatric inflammatory bowel disease: a forced-dose titration study. J Pediatr Gastroenterol Nutr [Internet]. 2013 Mar [cited. 2024 Jul 15];56(3):277-9. Available from: https://pubmed.ncbi.nlm.nih.gov/23059643/
525. Bommelaer G, Laharie D, Nancey S, Hebuterne X, Roblin X, Nachury M. et al. Oral Curcumin No More Effective Than Placebo in Preventing Recurrence of Crohn's Disease After Surgery in a Randomized Controlled Trial. Clin Gastroenterol Hepatol [Internet]. 2020 Jun 1 [cited. 2024 Jul 15];18(7):1553-1560.e1. Available from: https://pubmed.ncbi.nlm.nih.gov/31470175/
526. Kedia S, Bhatia V, Thareja S, Garg S, Mouli VP, Bopanna S. et al. Low dose oral curcumin is not effective in induction of remission in mild to moderate ulcerative colitis: Results from a randomized double blind placebo controlled trial. World J Gastrointest Pharmacol Ther [Internet]. 2017 May 6 [cited. 2024 Jul 15];8(2):147-54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28533925
527. Yin J, Wei L, Wang N, Li X, Miao M. Efficacy and safety of adjuvant curcumin therapy in ulcerative colitis: A systematic review and meta-analysis. J Ethnopharmacol [Internet]. 2022 May 10 [cited. 2024 Jul 15];289. Available from: https://pubmed.ncbi.nlm.nih.gov/35091013/
528. Goulart RDA, Barbalho SM, Lima VM, Souza GA De, Matias JN, Araújo AC. et al. Effects of the Use of Curcumin on Ulcerative Colitis and Crohn's Disease: A Systematic Review. J Med Food [Internet]. 2021 Jul 1 [cited. 2024 Jul 15];24(7):675-85. Available from: https://pubmed.ncbi.nlm.nih.gov/33155879/
529. Bucciantini M, Leri M, Nardiello P, Casamenti F, Stefani M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants [Internet]. 2021 Jul 1 [cited. 2024 Jul 15];10(7). Available from: /pmc/articles/PMC8300823/
530. Bilal RM, Liu C, Zhao H, Wang Y, Farag MR, Alagawany M. et al. Olive Oil: Nutritional Applications, Beneficial Health Aspects and its Prospective Application in Poultry Production. Front Pharmacol [Internet]. 2021 Aug 25 [cited. 2024 Jul 15];12. Available from: /pmc/articles/PMC8424077/
531. Jiménez-Sánchez A, Martínez-Ortega AJ, Remón-Ruiz PJ, Piñar-Gutiérrez A, Pereira-Cunill JL, García-Luna PP. Therapeutic Properties and Use of Extra Virgin Olive Oil in Clinical Nutrition: A Narrative Review and Literature Update. Nutrients [Internet]. 2022 Apr 1 [cited. 2024 Jul 15];14(7). Available from: /pmc/articles/PMC9003415/
532. Vrdoljak J, Kumric M, Vilovic M, Martinovic D, Tomic IJ, Krnic M. et al. Effects of Olive Oil and Its Components on Intestinal Inflammation and Inflammatory Bowel Disease. Nutrients [Internet]. 2022 Feb 1 [cited. 2024 Jul 15];14(4). Available from: /pmc/articles/PMC8875923/
533. Muto E, Dell'Agli M, Sangiovanni E, Mitro N, Fumagalli M, Crestani M. et al. Olive oil phenolic extract regulates interleukin-8 expression by transcriptional and posttranscriptional mechanisms in Caco-2 cells. Mol Nutr Food Res [Internet]. 2015 Jun 1 [cited. 2024 Jul 15];59(6):1217-21. Available from: https://pubmed.ncbi.nlm.nih.gov/25708117/
534. Borges TH, Cabrera-Vique C, Seiquer I. Antioxidant properties of chemical extracts and bioaccessible fractions obtained from six Spanish monovarietal extra virgin olive oils: assays in Caco-2 cells. Food Funct [Internet]. 2015 Jul 1 [cited. 2024 Jul 15];6(7):2375-83. Available from: https://pubmed.ncbi.nlm.nih.gov/26087367/
535. Chiesi C, Fernandez-Blanco C, Cossignani L, Font G, Ruiz MJ. Alternariol-induced cytotoxicity in Caco-2 cells. Protective effect of the phenolic fraction from virgin olive oil. Toxicon [Internet]. 2015 [cited. 2024 Jul 15];93:103-11. Available from: https://pubmed.ncbi.nlm.nih.gov/25451539/
536. Sánchez-Fidalgo S, Sánchez De Ibargüen L, Cárdeno A, Alarcón De La Lastra C. Influence of extra virgin olive oil diet enriched with hydroxytyrosol in a chronic DSS colitis model. Eur J Nutr [Internet]. 2012 Jun [cited. 2024 Jul 15];51(4):497-506. Available from: https://pubmed.ncbi.nlm.nih.gov/21874330/
537. Sánchez-Fidalgo S, Cárdeno A, Sánchez-Hidalgo M, Aparicio-Soto M, De la Lastra CA. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J Nutr Biochem [Internet]. 2013 Jul [cited. 2024 Jul 15];24(7):1401-13. Available from: https://pubmed.ncbi.nlm.nih.gov/23337347/
538. Takashima T, Sakata Y, Iwakiri R, Shiraishi R, Oda Y, Inoue N. et al. Feeding with olive oil attenuates inflammation in dextran sulfate sodium-induced colitis in rat. J Nutr Biochem [Internet]. 2014 Feb [cited. 2024 Jul 15];25(2):186-92. Available from: https://pubmed.ncbi.nlm.nih.gov/24445043/
539. Bigagli E, Toti S, Lodovici M, Giovannelli L, Cinci L, D'Ambrosio M. et al. Dietary Extra-Virgin Olive Oil Polyphenols Do Not Attenuate Colon Inflammation in Transgenic HLAB-27 Rats but Exert Hypocholesterolemic Effects through the Modulation of HMGCR and PPAR-α Gene Expression in the Liver. Lifestyle Genom [Internet]. 2018 Feb 1 [cited. 2024 Jul 15];11(2):99-108. Available from: https://pubmed.ncbi.nlm.nih.gov/30630166/
540. de Paula do Nascimento R, Lima AV, Oyama LM, Paiotti APR, Cardili L, Martinez CAR. et al. Extra virgin olive oil and flaxseed oil have no preventive effects on DSS-induced acute ulcerative colitis. Nutrition [Internet]. 2020 Jun 1 [cited. 2024 Jul 15];74. Available from: https://pubmed.ncbi.nlm.nih.gov/32179382/
541. Morvaridi M, Jafarirad S, Seyedian SS, Alavinejad P, Cheraghian B. The effects of extra virgin olive oil and canola oil on inflammatory markers and gastrointestinal symptoms in patients with ulcerative colitis. Eur J Clin Nutr [Internet]. 2020 Jun 1 [cited. 2024 Jul 15];74(6):891-9. Available from: https://pubmed.ncbi.nlm.nih.gov/31901082/
542. Martín-Peláez S, Mosele JI, Pizarro N, Farràs M, de la Torre R, Subirana I. et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: implications of human gut microbiota. Eur J Nutr [Internet]. 2017 Feb 1 [cited. 2024 Jul 15];56(1):119-31. Available from: https://pubmed.ncbi.nlm.nih.gov/26541328/
543. Chicco F, Magrì S, Cingolani A, Paduano D, Pesenti M, Zara F. et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm Bowel Dis [Internet]. 2021 Jan 1 [cited. 2024 Jul 15];27(1):1. Available from: /pmc/articles/PMC7737160/
544. Haskey N, Estaki M, Ye J, Shim RK, Singh S, Dieleman LA. et al. A Mediterranean Diet Pattern Improves Intestinal Inflammation Concomitant with Reshaping of the Bacteriome in Ulcerative Colitis: A Randomised Controlled Trial. J Crohns Colitis [Internet]. 2023 Oct 1 [cited. 2024 Jul 15];17(10):1569-78. Available from: https://pubmed.ncbi.nlm.nih.gov/37095601/
545. Lewis JD, Sandler RS, Brotherton C, Brensinger C, Li H, Kappelman MD. et al. A Randomized Trial Comparing the Specific Carbohydrate Diet to a Mediterranean Diet in Adults With Crohn's Disease. Gastroenterology [Internet]. 2021 Sep 1 [cited. 2024 Jul 15];161(3):837-852.e9. Available from: https://pubmed.ncbi.nlm.nih.gov/34052278/
546. Elmaksoud HAA, Motawea MH, Desoky AA, Elharrif MG, Ibrahimi A. Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed Pharmacother [Internet]. 2021 Oct 1 [cited. 2024 Jul 15];142. Available from: https://pubmed.ncbi.nlm.nih.gov/34463261/
547. Miao F. Hydroxytyrosol alleviates dextran sodium sulfate-induced colitis by inhibiting NLRP3 inflammasome activation and modulating gut microbiota in vivo. Nutrition [Internet]. 2022 May 1 [cited. 2024 Jul 15];97. Available from: https://pubmed.ncbi.nlm.nih.gov/35248848/
548. Santarelli R, Pompili C, Gilardini Montani MS, Evangelista L, Gonnella R, Cirone M. 3,4-Dihydroxyphenylethanol (DPE or Hydroxytyrosol) Counteracts ERK1/2 and mTOR Activation, Pro-Inflammatory Cytokine Release, Autophagy and Mitophagy Reduction Mediated by Benzo[a]pyrene in Primary Human Colonic Epithelial Cells. Pharmaceutics [Internet]. 2022 Mar 1 [cited. 2024 Jul 15];14(3). Available from: https://pubmed.ncbi.nlm.nih.gov/35336037/
549. Voltes A, Bermúdez A, Rodríguez-Gutiérrez G, Reyes ML, Olano C, Fernández-Bolaños J. et al. Anti-Inflammatory Local Effect of Hydroxytyrosol Combined with Pectin-Alginate and Olive Oil on Trinitrobenzene Sulfonic Acid-Induced Colitis in Wistar Rats. J Invest Surg [Internet]. 2020 Jan 2 [cited. 2024 Jul 15];33(1):8-14. Available from: https://pubmed.ncbi.nlm.nih.gov/29764253/
550. Reddy KVK, Naidu KA. Oleic acid, hydroxytyrosol and n-3 fatty acids collectively modulate colitis through reduction of oxidative stress and IL-8 synthesis; in vitro and in vivo studies. Int Immunopharmacol [Internet]. 2016 Jun 1 [cited. 2024 Jul 15];35:29-42. Available from: https://pubmed.ncbi.nlm.nih.gov/27016717/
551. Zhou Y, Wang D, Duan H. et al. The Potential of Natural Oils to Improve Inflammatory Bowel Disease. Nutrients. 2023;15:2606
552. Motawea MH, Elmaksoud HAA, Elharrif MG, Desoky AAE, Ibrahimi A. Evaluation of Anti-inflammatory and Antioxidant Profile of Oleuropein in Experimentally Induced Ulcerative Colitis. Int J Mol Cell Med [Internet]. 2020 [cited. 2024 Jul 16];9(3):224-33. Available from: https://pubmed.ncbi.nlm.nih.gov/33274185/
553. Giner E, Recio MC, Ríos JL, Giner RM. Oleuropein protects against dextran sodium sulfate-induced chronic colitis in mice. J Nat Prod [Internet]. 2013 Jun 28 [cited. 2024 Jul 16];76(6):1113-20. Available from: https://pubmed.ncbi.nlm.nih.gov/23758110/
554. Larussa T, Oliverio M, Suraci E, Greco M, Placida R, Gervasi S. et al. Oleuropein Decreases Cyclooxygenase-2 and Interleukin-17 Expression and Attenuates Inflammatory Damage in Colonic Samples from Ulcerative Colitis Patients. Nutrients [Internet]. 2017 Apr 15 [cited. 2024 Jul 16];9(4). Available from: https://pubmed.ncbi.nlm.nih.gov/28420140/
555. Hano C. Antioxidant and Anti-aging Action of Plant Polyphenols. [cited. 2024 Jul 16]; Available from: https://www.researchgate.net/publication/342924827_Antioxidant_and_Anti-Aging_Action_of_Plant_Polyphenols
556. Lončar M, Jakovljević M, Šubarić D, Pavlić M, Služek VB, Cindrić I. et al. Coumarins in Food and Methods of Their Determination. Foods [Internet]. 2020 May 1 [cited. 2024 Jul 16];9(5). Available from: https://pubmed.ncbi.nlm.nih.gov/32443406/
557. Ju S, Tan Y, Wang Q, Zhou L, Wang K, Wen C. et al. Antioxidant and anti-inflammatory effects of esculin and esculetin (Review). Exp Ther Med [Internet]. 2024 Apr 12 [cited. 2024 Jul 16];27(6). Available from: /pmc/articles/PMC11046185/
558. Witaicenis A, Luchini AC, Hiruma-Lima CA, Felisbino SL, Justulin LA, Garrido-Mesa N. et al. Mechanism and Effect of Esculetin in an Experimental Animal Model of Inflammatory Bowel Disease. http://dx.doi.org/101177/1721727X1301100213 [Internet]. 2013 May 1 [cited. 2024 Jul 16];11(2):433-46. Available from: https://journals.sagepub.com/doi/10.1177/1721727X1301100213?icid=int.sj-abstract.similar-articles.4
559. Cai T, Cai B. Network pharmacology and molecular docking reveal potential mechanism of esculetin in the treatment of ulcerative colitis. Medicine [Internet]. 2023 Nov 10 [cited. 2024 Jul 16];102(45):E35852. Available from: https://pubmed.ncbi.nlm.nih.gov/37960728/
560. Witaicenis A, Seito LN, Di Stasi LC. Intestinal anti-inflammatory activity of esculetin and 4-methylesculetin in the trinitrobenzenesulphonic acid model of rat colitis. Chem Biol Interact [Internet]. 2010 Jul [cited. 2024 Jul 16];186(2):211-8. Available from: https://pubmed.ncbi.nlm.nih.gov/20380826/
561. Witaicenis A, Luchini AC, Hiruma-Lima CA, Felisbino SL, Garrido-Mesa N, Utrilla P. et al. Suppression of TNBS-induced colitis in rats by 4-methylesculetin, a natural coumarin: comparison with prednisolone and sulphasalazine. Chem Biol Interact [Internet]. 2012 Jan 5 [cited. 2024 Jul 16];195(1):76-85. Available from: https://pubmed.ncbi.nlm.nih.gov/22119283/
562. Witaicenis A, de Oliveira ECS, Tanimoto A, Zorzella-Pezavento SFG, de Oliveira SL, Sartori A. et al. 4-methylesculetin, a coumarin derivative, ameliorates dextran sulfate sodium-induced intestinal inflammation. Chem Biol Interact [Internet]. 2018 Jan 25 [cited. 2024 Jul 16];280:59-63. Available from: https://pubmed.ncbi.nlm.nih.gov/29217385/
563. Tian X, Peng Z, Luo S, Zhang S, Li B, Zhou C. et al. Aesculin protects against DSS-Induced colitis though activating PPARγ and inhibiting NF-кB pathway. Eur J Pharmacol [Internet]. 2019 Aug 15 [cited. 2024 Jul 16];857. Available from: https://pubmed.ncbi.nlm.nih.gov/31202807/
564. Witaicenis A, Seito LN, Da Silveira Chagas A, De Almeida LD, Luchini AC, Rodrigues-Orsi P. et al. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine [Internet]. 2014 Feb 15 [cited. 2024 Jul 16];21(3):240-6. Available from: https://pubmed.ncbi.nlm.nih.gov/24176844/
565. He Z, Liu J, Liu Y. Daphnetin attenuates intestinal inflammation, oxidative stress, and apoptosis in ulcerative colitis via inhibiting REG3A-dependent JAK2/STAT3 signaling pathway. Environ Toxicol [Internet]. 2023 Sep 1 [cited. 2024 Jul 16];38(9):2132-42. Available from: https://pubmed.ncbi.nlm.nih.gov/37209277/
566. Sun X, Jin X, Wang L, Lin Z, Feng H, Zhan C. et al. Fraxetin ameliorates symptoms of dextran sulphate sodium-induced colitis in mice. Heliyon [Internet]. 2023 Jan 15 [cited. 2024 Jul 16];10(1). Available from: https://pubmed.ncbi.nlm.nih.gov/38163213/
567. Abdel-Wahab BA, Alkahtani SA, Alqahtani AA, Hassanein EHM. Umbelliferone ameliorates ulcerative colitis induced by acetic acid via modulation of TLR4/NF-κB-p65/iNOS and SIRT1/PPARγ signaling pathways in rats. Environ Sci Pollut Res Int [Internet]. 2022 May 1 [cited. 2024 Jul 16];29(25):37644-59. Available from: https://pubmed.ncbi.nlm.nih.gov/35066822/
568. Tao Z, Jin Z, Wu J. et al. Sirtuin Family in Autoimmune Diseases. Front Immunol. 2023;14:1186231
569. Di Stasi LC, Camuesco D, Nieto A, Vilegas W, Zarzuelo A, Galvez J. Intestinal anti-inflammatory activity of paepalantine, an isocoumarin isolated from the capitula of Paepalanthus bromelioides, in the trinitrobenzenesulphonic acid model of rat colitis. Planta Med [Internet]. 2004 Apr [cited. 2024 Jul 16];70(4):315-20. Available from: https://pubmed.ncbi.nlm.nih.gov/15095146/
570. Luchini AC, Rodrigues-Orsi P, Cestari SH, Seito LN, Witaicenis A, Pellizzon CH. et al. Intestinal anti-inflammatory activity of coumarin and 4-hydroxycoumarin in the trinitrobenzenesulphonic acid model of rat colitis. Biol Pharm Bull [Internet]. 2008 Jul [cited. 2024 Jul 16];31(7):1343-50. Available from: https://pubmed.ncbi.nlm.nih.gov/18591772/
571. Bijak M. molecules Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism. 2017 [cited. 2024 Jul 16]; Available from: www.mdpi.com/journal/molecules
572. Csupor D, Csorba A, Hohmann J. Recent advances in the analysis of flavonolignans of Silybum marianum. J Pharm Biomed Anal [Internet]. 2016 Oct 25 [cited. 2024 Jul 16];130:301-17. Available from: https://pubmed.ncbi.nlm.nih.gov/27321822/
573. Koltai T, Fliegel L. Role of Silymarin in Cancer Treatment: Facts, Hypotheses, and Questions. J Evid Based Integr Med [Internet]. 2022 Jan 1 [cited. 2024 Jul 16];27. Available from: https://pubmed.ncbi.nlm.nih.gov/35018864/
574. Sahoo DK, Heilmann RM, Paital B, Patel A, Yadav VK, Wong D. et al. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front Endocrinol (Lausanne) [Internet]. 2023 [cited. 2024 Jul 16];14:1217165. Available from: /pmc/articles/PMC10493311/
575. Zarenezhad E, Abdulabbas HT, Kareem AS, Kouhpayeh SA, Barbaresi S, Najafipour S. et al. Protective role of flavonoids quercetin and silymarin in the viral-associated inflammatory bowel disease: an updated review. Arch Microbiol [Internet]. 2023 Jun 1 [cited. 2024 Jul 16];205(6). Available from: https://pubmed.ncbi.nlm.nih.gov/37249707/
576. Al-Drees AM, Khalil MS. Histological and immunohistochemical effects of L-arginine and silymarin on TNBS-induced inflammatory bowel disease in rats. Histol Histopathol [Internet]. 2016 Nov 1 [cited. 2024 Jul 16];31(11):1259-70. Available from: https://pubmed.ncbi.nlm.nih.gov/26979994/
577. Miroliaee AE, Esmaily H, Vaziri-Bami A, Baeeri M, Shahverdi AR, Abdollahi M. Amelioration of experimental colitis by a novel nanoselenium-silymarin mixture. Toxicol Mech Methods [Internet]. 2011 Mar [cited. 2024 Jul 16];21(3):200-8. Available from: https://pubmed.ncbi.nlm.nih.gov/21247366/
578. Nguyen THT, Trinh NT, Tran HN, Tran HT, Le PQ, Ngo DN. et al. Improving silymarin oral bioavailability using silica-installed redox nanoparticle to suppress inflammatory bowel disease. J Control Release [Internet]. 2021 Mar 10 [cited. 2024 Jul 16];331:515-24. Available from: https://pubmed.ncbi.nlm.nih.gov/33616078/
579. Esmaily H, Vaziri-Bami A, Miroliaee AE, Baeeri M, Abdollahi M. The correlation between NF-κB inhibition and disease activity by coadministration of silibinin and ursodeoxycholic acid in experimental colitis. Fundam Clin Pharmacol [Internet]. 2011 Dec [cited. 2024 Jul 16];25(6):723-33. Available from: https://pubmed.ncbi.nlm.nih.gov/21077947/
580. Zheng R, Ma J, Wang D, Dong W, Wang S, Liu T. et al. Chemopreventive Effects of Silibinin on Colitis-Associated Tumorigenesis by Inhibiting IL-6/STAT3 Signaling Pathway. Mediators Inflamm [Internet]. 2018 [cited 2024 Jul 16]. 2018 Available from: https://pubmed.ncbi.nlm.nih.gov/30498394/
581. Rastegarpanah M, Malekzadeh R, Vahedi H, Mohammadi M, Elahi E, Chaharmahali M. et al. A randomized, double blinded, placebo-controlled clinical trial of silymarin in ulcerative colitis. Chin J Integr Med [Internet]. 2015 Dec 1 [cited. 2024 Jul 16];21(12):902-6. Available from: https://pubmed.ncbi.nlm.nih.gov/22528757/
582. De Souza HSP, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol [Internet]. 2016 Jan 24 [cited. 2024 Mar 18];13(1):13-27. Available from: https://pubmed.ncbi.nlm.nih.gov/26627550/
583. Giudici F, Fiorindi C, Gubatan J, Kulkarni C V, Talamantes SM, Temby M. et al. Dietary Exposures and Interventions in Inflammatory Bowel Disease: Current Evidence and Emerging Concepts. 2023 [cited. 2024 Mar 18]; Available from: https://doi.org/10.3390/nu15030579
584. Srour B, Kordahi MC, Bonazzi E, Deschasaux-Tanguy M, Touvier M, Chassaing B. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol Hepatol [Internet]. 2022 Dec 1 [cited. 2024 Mar 18];7(12):1128-40. Available from: http://www.thelancet.com/article/S2468125322001698/fulltext
585. Di Lorenzo C, Colombo F, Biella S, Stockley C, Restani P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients [Internet]. 2021 Jan 1 [cited. 2024 Apr 14];13(1):1-30. Available from: /pmc/articles/PMC7833401/
586. Renaud J, Martinoli MG. Considerations for the Use of Polyphenols as Therapies in Neurodegenerative Diseases. Int J Mol Sci [Internet]. 2019 Apr 2 [cited. 2024 Jul 16];20(8). Available from: /pmc/articles/PMC6514961/
587. Anand S, Sowbhagya R, Ansari MA, Alzohairy MA, Alomary MN, Almalik AI. et al. Polyphenols and Their Nanoformulations: Protective Effects against Human Diseases. Life [Internet]. 2022 Oct 1 [cited. 2024 Apr 14];12(10). Available from: /pmc/articles/PMC9604961/
588. Pawłowski W, Caban M, Lewandowska U. Cancer Prevention and Treatment with Polyphenols: Type IV Collagenase-Mediated Mechanisms. Cancers (Basel). 2024;16:3193
589. Xie L, Vance T, Kim B, Lee SG, Caceres C, Wang Y. et al. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: a randomized controlled trial. Nutr Res [Internet]. 2017 Jan 1 [cited. 2024 Apr 14];37:67-77. Available from: https://pubmed.ncbi.nlm.nih.gov/28215316/
590. Zhao Y, Sun J, Liu Y. et al. Metabolic Basis for Superior Antioxidant Capacity of Red-Fleshed Peaches. Food Chem X. 2024;23:101698
591. Bertelli A, Biagi M, Corsini M, Baini G, Cappellucci G, Miraldi E. Polyphenols: From Theory to Practice. Foods [Internet]. 2021 Nov 1 [cited. 2024 Jul 16];10(11). Available from: /pmc/articles/PMC8621732/
592. Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: A case series report. Nutr J [Internet]. 2014 Jan 16 [cited. 2024 Mar 18];13(1):1-7. Available from: https://nutritionj.biomedcentral.com/articles/10.1186/1475-2891-13-5
593. Martínez-González MA, Gea A, Ruiz-Canela M. The Mediterranean Diet and Cardiovascular Health. Circ Res [Internet]. 2019 Mar 1 [cited. 2024 Mar 18];124(5):779-98. Available from: https://www.ahajournals.org/doi/abs/10.1161/CIRCRESAHA.118.313348
594. Weber AT, Shah ND, Sauk J, Limketkai BN. Popular Diet Trends for Inflammatory Bowel Diseases: Claims and Evidence. Current Treatment Options in Gastroenterology. 2019;17:4 [Internet]. 2019 Nov 8 [cited 2024 Mar 18];17(4):564-76. Available from: https://link.springer.com/article/10.1007/s11938-019-00248-z
595. Aguilera JM. The food matrix: implications in processing, nutrition and health. Crit Rev Food Sci Nutr [Internet]. 2019 Dec 16 [cited. 2024 Jul 16];59(22):3612-29. Available from: https://pubmed.ncbi.nlm.nih.gov/30040431/
596. Čepo DV, Radić K, Šalov M, Turčić P, Anić D, Komar B. Food (Matrix) Effects on Bioaccessibility and Intestinal Permeability of Major Olive Antioxidants. Foods 2020, Vol 9, Page 1831 [Internet]. 2020 Dec 9 [cited. 2024 Apr 14];9(12):1831. Available from: https://www.mdpi.com/2304-8158/9/12/1831/htm
597. Bohn T. Dietary factors affecting polyphenol bioavailability. Nutr Rev [Internet]. 2014 [cited. 2024 Jul 16];72(7):429-52. Available from: https://pubmed.ncbi.nlm.nih.gov/24828476/
598. D'Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R. Bioavailability of the Polyphenols: Status and Controversies. Int J Mol Sci [Internet]. 2010 Apr [cited. 2024 Jul 16];11(4):1321. Available from: /pmc/articles/PMC2871118/
599. Ortega N, Reguant J, Romero MP, Macià A, Motilva MJ. Effect of fat content on the digestibility and bioaccessibility of cocoa polyphenol by an in vitro digestion model. J Agric Food Chem [Internet]. 2009 Jul 8 [cited. 2024 Jul 16];57(13):5743-9. Available from: https://pubmed.ncbi.nlm.nih.gov/19492841/
600. Stromsnes K, Lagzdina R, Olaso-gonzalez G, Gimeno-mallench L, Gambini J. Pharmacological properties of polyphenols: Bioavailability, mechanisms of action and biological effects in in vitro studies, animal models and humans. Biomedicines [Internet]. 2021 Aug 1 [cited. 2024 Apr 14];9(8). Available from: /pmc/articles/PMC8392236/
601. Duda-Chodak A, Tarko T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules [Internet]. 2023 Mar 1 [cited. 2024 Apr 14];28(6). Available from: /pmc/articles/PMC10058246/
602. Chen B, Zhang W, Lin C, Zhang L. A Comprehensive Review on Beneficial Effects of Catechins on Secondary Mitochondrial Diseases. Int J Mol Sci [Internet]. 2022 Oct 1 [cited. 2024 Apr 14];23(19):11569. Available from: /pmc/articles/PMC9569714/
603. Fukushima Y, Ohie T, Yonekawa Y, Yonemoto K, Aizawa H, Mori Y. et al. Coffee and green tea as a large source of antioxidant polyphenols in the Japanese population. J Agric Food Chem [Internet]. 2009 Feb 25 [cited. 2024 Apr 14];57(4):1253-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19187022
604. Kumar S, Pandey AK. Chemistry and Biological Activities of Flavonoids: An Overview. The Scientific World Journal [Internet]. 2013 [cited. 2024 Apr 14];2013:16. Available from: /pmc/articles/PMC3891543/
605. Ianiro G, Pecere S, Giorgio V, Gasbarrini A, Cammarota G. Digestive Enzyme Supplementation in Gastrointestinal Diseases. Curr Drug Metab [Internet]. 2016 Jan 26 [cited. 2024 Apr 14];17(2):187. Available from: /pmc/articles/PMC4923703/
606. Leighton MP, Lam C, Mehta S, Spiller RC. Efficacy and mode of action of mesalazine in the treatment of diarrhoea-predominant irritable bowel syndrome (IBS-D): Study protocol for a randomised controlled trial. Trials. 2013 Jan 9;14(1)
607. Ortega M, García-Montero C, Fraile-Martínez O, Monserrat J, Álvarez-Mon MA. The gut microbiota in sickness and in health. Medicine (Spain). 2022Dec1;13(69):4054-63
608. Jacenik D, Cygankiewicz AI, Mokrowiecka A, Małecka-Panas E, Fichna J, Krajewska WM. Sex- and Age-Related Estrogen Signaling Alteration in Inflammatory Bowel Diseases: Modulatory Role of Estrogen Receptors. Int J Mol Sci [Internet]. 2019 Jul 1 [cited. 2024 Apr 14];20(13). Available from: /pmc/articles/PMC6651503/
609. Shizuma T. Concomitant Thyroid Disorders and Inflammatory Bowel Disease: A Literature Review. Biomed Res Int [Internet]. 2016 [cited 2024 Apr 14]. 2016 Available from: /pmc/articles/PMC4794572/
610. Cai Z, Wang S, Li J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front Med (Lausanne) [Internet]. 2021 Dec 20 [cited. 2024 Apr 14];8:765474. Available from: /pmc/articles/PMC8720971/
611. Kampschulte N, Alasmer A, Empl MT, Krohn M, Steinberg P, Schebb NH. Dietary Polyphenols Inhibit the Cytochrome P450 Monooxygenase Branch of the Arachidonic Acid Cascade with Remarkable Structure-Dependent Selectivity and Potency. J Agric Food Chem [Internet]. 2020 Aug 26 [cited. 2024 Apr 14];68(34):9235-44. Available from: https://pubs.acs.org/doi/abs/10.1021/acs.jafc.0c04690
612. Hussain SA, Sulaiman AA, Alhaddad H, Alhadidi Q. Natural polyphenols: Influence on membrane transporters. J Intercult Ethnopharmacol [Internet]. 2016 [cited. 2024 Apr 14];5(1):97. Available from: /pmc/articles/PMC4805155/
613. Liu F, Li D, Wang X, Cui Y, Li X. Polyphenols intervention is an effective strategy to ameliorate inflammatory bowel disease: a systematic review and meta-analysis. Int J Food Sci Nutr [Internet]. 2021 [cited. 2024 Jul 16];72(1):14-25. Available from: https://pubmed.ncbi.nlm.nih.gov/32369394/
614. Giang J, Lan X, Crichton M, Marx W, Marshall S. Efficacy and safety of biophenol-rich nutraceuticals in adults with inflammatory gastrointestinal diseases or irritable bowel syndrome: A systematic literature review and meta-analysis. Nutr Diet [Internet]. 2022 Feb 1 [cited. 2024 Jul 16];79(1):76-93. Available from: https://pubmed.ncbi.nlm.nih.gov/33960587/
Author contact
![]() Corresponding author: miguel.angel.ortega92com.
Corresponding author: miguel.angel.ortega92com.

 Global reach, higher impact
Global reach, higher impact