10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(14):5673-5694. doi:10.7150/ijbs.100317 This issue Cite
Review
Overcoming Barriers in Photodynamic Therapy Harnessing Nanogenerators Strategies
1. Department of Abdominal Oncology, Radiation Oncology Key Laboratory of Sichuan Province, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu 610041, China.
2. Department of Radiation Oncology, Radiation Oncology Key Laboratory of Sichuan Province, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu 610041, China.
3. Department of Pathology, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu 610041, China.
*These authors contributed equally to this work.
Received 2024-6-30; Accepted 2024-10-3; Published 2024-10-14
Abstract
Photodynamic therapy (PDT) represents a targeted approach for cancer treatment that employs light and photosensitizers (PSs) to induce the generation of reactive oxygen species (ROS). However, PDT faces obstacles including insufficient PS localization, limited light penetration, and treatment resistance. A potential solution lies in nanogenerators (NGs), which function as self-powered systems capable of generating electrical energy. Recent progress in piezoelectric and triboelectric NGs showcases promising applications in cancer research and drug delivery. Integration of NGs with PDT holds the promise of enhancing treatment efficacy by ensuring sustained PS illumination, enabling direct electrical control of cancer cells, and facilitating improved drug administration. This comprehensive review aims to augment our comprehension of PDT principles, explore associated challenges, and underscore the transformative capacity of NGs in conjunction with PDT. By harnessing NG technology alongside PDT, significant advancement in cancer treatment can be realized. Herein, we present the principal findings and conclusions of this study, offering valuable insights into the integration of NGs to overcome barriers in PDT.
Keywords: photodynamic therapy, nanogenerators, treatment resistance, cancer treatment, reactive oxygen species
Introduction
Cancer has emerged as a major cause of mortality [1]. Conventional cancer therapies, encompassing chemotherapy, radiotherapy, targeted therapy, and immunotherapy, are hampered by notable drawbacks such as severe side effects and toxicity towards healthy cells [2, 3]. Photodynamic therapy (PDT) emerges as an appealing alternative with enhanced efficacy, reduced side effects, and heightened precision [4, 5]. PDT employs light and photosensitizers (PSs) to selectively harm cancer cells by generating reactive oxygen species (ROS) [6, 7]. Nonetheless, PDT encounters hurdles like inadequate tumor targeting, restricted light penetration, diminished ROS production, and the emergence of cancer cell resistance.
To address challenges, nanogenerators (NGs) have emerged as a potential solution offering several advantages. These self-powered systems convert biomechanical energy into electrical energy [8, 9], characterized by compactness, cost-effectiveness, and high-power density output [10, 11]. NGs, including Triboelectric Nanogenerators (TENGs) and Piezoelectric Nanogenerators (PENGs), have made significant strides in influencing cellular responses [11, 12], particularly in precise cancer treatment [13, 14]. TENGs have demonstrated efficacy in promoting cell growth, differentiating mouse embryonic osteoblast [15, 16], and guiding nerve cell growth [17]. PENGs have emerged as key players in regulating cell behavior through pathways like MAPK/ERK and cyclic adenosine monophosphate(cAMP) [18], promoting cell adhesion [19], activating downstream signaling cascades, enhancing macrophage motility [20], and inducing neurite outgrowth [21]. These advancements highlight the potential of NGs in positively impacting cellular behavior in cancer therapy. By enhancing ROS production within the tumor microenvironment (TME), NGs contribute to improved therapeutic outcomes while minimizing damage to healthy tissues. Notably, an implantable biodegradable TENG nanofiber patch enhances hydroxyl radical production in specific weakly acidic TME conditions [14]. Another NE based gas-therapy system, can realize nitric oxide (NO) releasing over the blood-brain barrier (BBB) to realize the precise intracranial glioblastoma treatment [13].
NGs offer innovative solutions to combat resistance in PDT by addressing key challenges through various mechanisms. They can enhance oxygen supply within the TME [22], facilitate targeted drug delivery of PSs [23, 24], amplify ROS generation [25, 26], enable synergistic combination therapies [27, 28], modulate the TME [29, 30], induce immunogenic cell death [31, 32], disrupt DNA repair mechanisms [33], influence angiogenic effects [34, 35]. For instance, portable wearable NG-based devices can sustainably power PSs to directly impact cancer cells [36]. NG-driven drug delivery systems address resistance mechanisms in cancer treatment, improving targeting [13, 23, 24], light penetration [37], and ROS generation [26, 38] to enhance PDT efficacy.
This review aims to provide a comprehensive overview of the fundamental principles of PDT, impediments to its effectiveness, and mechanisms of resistance. It also explores the benefits of nanotechnology-enabled (NE) approaches in augmenting PDT efficacy. The physicochemical attributes of nanomaterials and their potential applications in managing deep-seated cancers, modulating cellular apoptosis pathways, overcoming resistance to PDT, and monitoring biochemical products are thoroughly examined. By identifying strategies that can enhance the efficacy of PDT, this review contributes to the progression of cancer treatment. Finally, critical considerations for the clinical implementation of the combination of PDT and NGs in cancer treatment are discussed.
1. PDT and the Barriers
PDT represents a promising therapeutic modality involving the introduction of PSs into tumor tissues, succeeded by light exposure at a specific wavelength. PDT initiates the production of ROS within the TME, culminating in cancer cell eradication. Within PDT, PSs convert light energy into chemical energy upon illumination in the presence of oxygen, leading to ROS generation through two photochemical processes involving adjacent molecules. The initial excited state of the PS (singlet state denoted as 1PS•) is excited to a more stable excited triplet state (3PS•), which then generates ROS, effectively eliminating tumor cells [39]. In addition to tumor cell destruction, PDT can target tumor vascular endothelial cells, boost immune responses, and trigger local inflammation, collectively enhancing its therapeutic efficacy. The mechanisms underlying the anti-cancer effects of PDT are illustrated in Figure 1.
1.1. Dual mechanisms of Photodynamic Reactions
PDT operates through two primary mechanisms involving the generation of ROS: type I and type II reactions. Type I reaction involves complex electron transfer steps, leading to the generation of various ROS, including superoxide radicals (O2-), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH). These reactions initiate upon PS photon absorption, involving energy transfer and single electron reduction processes. This process includes the singlet excited state of the PS (1PS•), where energy transfer occurs upon its return to the ground state, forming an excited triplet state (3PS•), interacting with molecular oxygen to generate singlet state PS and the excited state of singlet oxygen (1O2) that interact with cell membrane lipids [40]. Initial single electron transfer steps generate PS radical anions, which react with oxygen to produce O2-. O2-then engages in redox reactions to produce H2O2, subsequently undergoing single electron reduction to form •OH. The Type II reaction transfers energy to the ground state triplet of molecular oxygen, generating the ground state singlet of the PS and the excited state of 1O2. This energy transfer elevates molecular oxygen to a higher energy state, shifting it from the ground state to an excited triplet state (3O2), while the PS reverts to its ground state. The 3O2 undergoes intersystem crossing to form 1O2, a potent cytotoxic agent pivotal in PDT [41, 42].
Oxygen concentration significantly influences PDT efficacy by modulating treatment reactions. Tumor hypoxia, characterized by low oxygen levels, can diminish PDT effectiveness, particularly in type II PDT [42-45]. While type I PDT reactions can proceed independently of oxygen, its presence influences overall PDT efficacy. In oxygen-rich environments, type I reactions can generate ROS beyond 1O2, contributing to cellular damage and amplifying PDT's cytotoxic effects [43]. Type I and type II processes can occur simultaneously, factors like tumor cell type, PS characteristics, oxygen levels, and tumor oxygenation complexity influence the relative occurrence of type I and type II reactions. Moreover, hypoxia impacts tumor metastasis, drug resistance [46], neovascularization [47], and the tumor immune microenvironment [48], impacting treatment effectiveness.
The outcomes of PDT are influenced by various factors, including tumor cell type and PS characteristics such as photophysical and photochemical properties [49], tissue distribution [50], absorption properties [49, 51], and subcellular localization [49, 51, 52]. In various tumors, differing blood oxygen levels are observed. Non-small cell lung cancers [53], liver cancers [54], and primary/metastatic brain tumors [55] are typically well-vascularized with elevated oxygen levels [54-56]. This oxygen-rich environment commonly favors Type-II reactions. Tumor oxygenation complexity is influenced by factors like depth, necrotic regions, and cancer subtypes [57]. Illustrating Type I and Type II photochemical reaction of different PS is also of significant value. PSs like porphyrin, boron dipyrromethene, cyanine dyes, and aggregation-induced emission molecules have been engineered for enhanced accumulation in deep tumors and exploitation of acidic/hypoxic microenvironments in Type-II PDT reactions [58]. Those PSs has shown the ability of enhancing PS accumulation in deep tumor [59], and taking advantage of acidic and hypoxia TME in the Type II PDT reaction [60-62]. To counter resistance, some PSs are designed for oxygen-independent Type I PDT. NanoPcA was structured to prevent aggregation-caused quenching [63]. A PS complex, coupling cyclometallated Ir (III) with coumarin, was engineered for normoxic/hypoxic conditions [64]. Li et al. devised PSs generating superoxide radical anions for enhanced photochemical properties [65]. Additionally, certain PSs are tailored for both O2-dependent and O2-independent PDT, such as Ti-based Nanoscale Metal-Organic Framework (NMOF) [66], Zr-TBB NMOF [67], heterogeneous covalent organic nanosheet [68].
PDT leverages light exposure and PSs to generate ROS within tumor tissues, inducing tumor inhibition. ROS target cancer cells and blood vessels, disrupting cellular functions and blood supply. They also initiate an immune response against tumor metastasis.
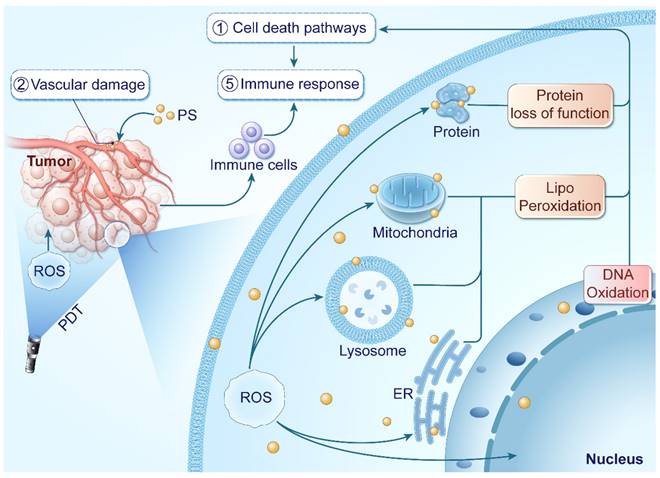
ROS generated during PDT exert cytotoxic effects by attacking biomolecules like lipids, proteins, and DNA [69, 70]. Plasma membrane and organelles such as mitochondria, endoplasmic reticulum (ER), Golgi apparatus, and lysosomes are particularly susceptible due to the high sensitivity of lipids to ROS [71]. Photodynamic reactions induce lipid peroxidation through the production of radicals [72]. 1O2 can directly react with unsaturated lipids, forming lipid peroxides, which disrupt the integrity and function of biomembranes [73]. Furthermore, the generated radicals can initiate free-radical chain reactions, causing secondary modifications to proteins and polynucleotides [72, 74]. DNA is crucial target of ROS in PDT, and damage to DNA can trigger apoptosis in cancer cells [75, 76]. The cumulative effect of these reactions disrupts the structure and functions of organelles, ultimately resulting in cell death. Common cell death modalities in PDT include apoptosis, necrosis, and autophagy. Additionally, non-conventional forms of cell death such as necroptosis, ferroptosis, pyroptosis, parthanatos, and mitotic catastrophe may also occur [77, 78].
1.2. Barriers of PDT
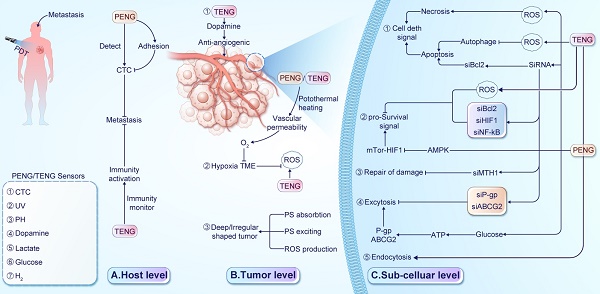
PDT faces several barriers that can limit its effectiveness in treating cancer [79-81]. To enhance the clinical benefits of PDT, it's essential to understand these barriers acting at the host, TME, and sub-cellular levels, which are visually represented in Figure 2.
Barriers of PDT in treating cancer. (A) At the host level, ① PDT affects the cytoskeleton and cell adhesion, potentially enhancing cancer cell migration and invasion. ② Inadequate immune surveillance and regulation can limit the efficacy of PDT against distant lesions. (B) At the TME level, ① the hypoxic TME re-duces oxygen availability and stabilizes hypoxia-inducible factor 1 (HIF-1). ② Damage to blood vessels impairs oxygen supply, exacerbating hypoxia. ③ The location and shape of tumors can affect the absorption, distribution, and excitation of PSs, reducing ROS production. (C) At the subcellular level, ① Up-regulation of transport proteins excrete PSs. ② Enhanced antioxidant systems scavenge ROS in tumor tissues. ③ Activation of pro-survival signaling pathways promotes transcription and tumor progression. ④ Up-regulation of heat shock proteins facilitates the repair of proteins damaged by ROS.
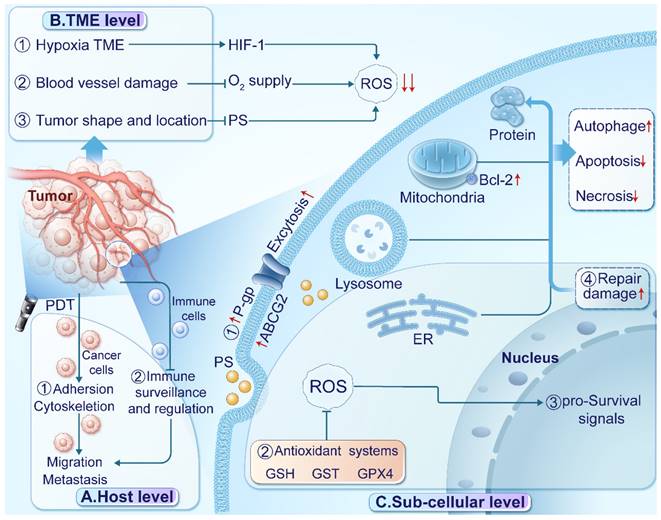
At the host level, limitations in immune response monitoring, cancer cell migration, and treatment interruption impact PDT efficacy. Toxic effects of PSs and PDT on normal tissues also affect patient tolerance. Tumor metastasis poses a significant challenge for PDT efficacy, with limited diffusion range of ROS affecting its effectiveness on distant lesions [82]. Inadequate immune response monitoring, including handling of cell debris, tumor-specific antigens, and immune activation, contributes to suboptimal efficacy against distant lesions [83]. Additionally, PDT's impact on cytoskeletal dynamics, cellular plasticity, and morphology may affect cancer cell migration and invasion [84]. Treatment-induced pain can lead to interruptions, diminishing overall effectiveness. Careful monitoring and managing the side effects are crucial [85, 86].
At the TME level, factors such as hypoxia, acidity, and immunosuppression contribute to resistance mechanisms against PDT. Insufficient oxygen availability and enhanced antioxidant defense mechanisms limit ROS generation, while immunosuppressive microenvironments impede immune activation. Dynamic TME changes during treatment or tumor progression play a significant role in cancer cell adaptation and resistance development [87-89]. Various factors within the TME influence the mechanisms of resistance to PDT [90]. For instance, hypoxia restricts ROS generation [91] by limiting oxygen availability and promotes tumor progression through upregulating hypoxia-inducible factors (HIFs) in PDT-resistant tumors [79]. The acidic TME induces insufficient endogenous H2O2 and boosts cellular antioxidant defense mechanisms, restricting PDT efficacy [92]. Tumor cells establish an immunosuppressive microenvironment by releasing immunosuppressive cytokines, hindering immune activation by PDT [93]. Furthermore, nitric oxide (NO) generated through inducible nitric oxide synthase (iNOS/NOS2) has been associated with PDT resistance, impairing therapeutic efficacy [94]. These microenvironmental factors contribute to resistance development and impact PDT outcomes.
Apart from TME level, tissue penetration depth of light is critical for PDT [95, 96]. Conventional PSs are typically activated by short-wavelength ultraviolet-visible (UV-Vis) light, limiting their effectiveness in treating deep-seated tumors due to insufficient tissue penetration [97]. To address this, various approaches, including near-infrared (NIR) light, X-ray radiation, and self-luminescence have been explored [98]. NIR light (700-1100 nm) penetrates biological tissues more effectively than UV-Vis light (400-700 nm), enhancing treatment depth and reducing absorption by proteins and lipids [99, 100], thereby increasing light availability for tumor-targeted PSs [101, 102]. By utilizing NIR light, researchers can surmount tissue penetration limitations, enhancing PDT outcomes in clinical settings [103-105]. Despite its advantages, the application of NIR light in PDT presents notable challenges. Variability in tissue composition can affect light penetration and absorption, leading to inconsistent therapeutic outcomes. Furthermore, the efficacy of NIR-mediated PDT is contingent upon oxygen availability in the TME, complicating treatment in hypoxic tumors. Thermal effects from NIR exposure can damage surrounding healthy tissues if not carefully managed, and determining the optimal light dosage to mitigate unnecessary damage remains complex [106]. Regulatory and safety concerns regarding NIR use must also be addressed in clinical applications. While NIR light offers deeper penetration than visible light, depth limitations persist that may affect treatment efficacy[106]. Ongoing research aims to overcome these challenges in NIR-induced PDT for Types I and II treatments [107, 108].
The suboptimal tumor-targeting capacity of PSs, leading to inefficient cancer cell uptake, presents a material performance limitation in PDT [33, 109]. In clinical settings, inadequate PS localization hinders treatment efficacy by promoting PS accumulation in healthy tissues rather than specifically within tumors [110, 111]. This nonspecific distribution diminishes cytotoxic effects on cancer cells and elevates the risk of harming adjacent healthy tissues [112, 113]. Furthermore, variable PS concentrations within different tumor regions due to imprecise distribution impede consistent ROS generation upon light activation, thereby compromising treatment efficacy. Resolving the challenge of insufficient tumor-targeting by PSs is pivotal for enhancing PDT precision and effectiveness. Strategies like targeted delivery systems [114], nanocarriers [115], and molecular targeting agents [116] can enhance PS specificity towards cancer cells, optimizing treatment outcomes and reducing off-target effects. Overcoming this limitation can improve PDT's utility as a targeted and minimally invasive therapeutic approach across diverse cancer types.
Sub-cellular factors, including transport proteins, hypoxia-inducible factors, antioxidant systems, and pro-survival signaling pathways, play pivotal roles in PDT resistance. Transport proteins, such as P-glycoprotein (P-gp), multidrug resistance protein (MDR), and ATP-binding cassette transporter G2 (ABCG2) facilitate PS excretion from tumor cells, diminishing their retention [117, 118]. The hypoxic TME influences ROS generation, activating HIF and pro-survival pathways that aid tumor progression [44, 119]. Upregulated antioxidant systems like glutathione (GSH), glutathione S-transferase (GST), and glutathione peroxidase 4 (GPx4) scavenge ROS in tumor tissues [120, 121]. Heat shock proteins assist in ROS-damaged protein repair [122, 123]. Additionally, PDT-treated glioblastoma cells activate the AKT/mTOR signaling pathway via NF-κB signaling, regulating DNA transcription, cytokine production, and cell survival [124]. Autophagy, promoting cell survival at low PDT doses by recycling damaged organelles, contributes to resistance. Negative feedback regulation decreases proapoptotic caspase-9 activity, suppressing cell death [125]. The AMP-activated kinase (AMPK) pathway, activated by 5-aminolevulinic acid (5-ALA) and photoinduced stress in PDT-treated cancers, triggers autophagy, leading to tumor resistance to 5-ALA-PDT [125]. Given these diverse resistance mechanisms, developing novel therapeutic strategies, such as NGs, is crucial for enhancing PDT's clinical utility and overcoming resistance challenges.
2. NGs
The concept of NGs originated from Zhong Lin Wang's theoretical exploration of Maxwell's equations [126]. NGs convert mechanical energy into electrical energy, enabling the analysis of mechanical inputs through electrical output signals [127, 128]. Unlike conventional technologies, NGs are self-sustainability and do not require external power sources.
2.1. Types of NGs
One type of NG is the Piezoelectric Nanogenerator (PENG), which operates based on the piezoelectric effect. Comprising an insulating piezoelectric material with electrodes on its surfaces, the PENG generates electric dipoles when strained, enhancing material polarization. This polarization establishes an electrostatic potential, balanced by electron flow through an external load. Wang's group pioneered the design of PENG in 2006 [8], sparking efforts by researchers to enhance the efficiency, flexibility, and biocompatibility of PENGs for diverse applications [129, 130]. PENGs efficiently convert mechanical energy into electrical energy, providing a reliable power source for PDT devices without external power. Typically made from biocompatible materials, PENGs are suitable for integration into implantable or wearable PDT systems. They can harvest energy from bodily movements, ensuring continuous power generation for extended PDT treatments. Designed at the nanoscale, PENGs enable the development of compact and minimally invasive PDT devices, offering a sustainable energy solution for prolonged use. However, PENGs may have lower power output compared to other energy harvesting technologies, potentially limiting the functionality of high-power PDT devices. They require consistent mechanical vibrations to generate electricity, which can constrain their effectiveness in static environments. The fabrication of PENGs can be complex and may necessitate specialized expertise, increasing production costs. Additionally, PENGs are sensitive to environmental factors such as temperature and humidity, which can affect performance. Integrating PENGs into existing PDT systems may also present challenges related to compatibility and optimization. Extensive research has been conducted to enhance PENG energy through material selection [131-138] and innovative device structures [135, 139, 140].
The Triboelectric Nanogenerator (TENG) is another form of nanogenerator that exploits the triboelectric effect, which arises when two materials contact each other, leading to charge transfer and subsequent electrostatic induction. Comprising two electrodes and at least one pair of triboelectric layers, the TENG generates a potential difference from an electron imbalance when the materials separate, driving electron flow between the top and bottom electrodes on the material surfaces [141]. TENGs achieve high power densities, generating sufficient energy for PDT devices with varying requirements. Their output voltage surpasses that of PENGs, leading to increased research developments in this area [142]. With advancements in nanomaterials, TENGs have reached an area power density of 1200 W/m² and energy conversion efficiencies of 50-85% [143]. They can be scaled to meet the specific power needs of PDT devices, allowing for customization and optimization. TENGs are characterized by low cost, versatility, environmental friendliness, and high conversion efficiency. They can be designed as fibers or integrated into fabrics, making them breathable, comfortable, and suitable for low-cost, large-scale production [144]. However, the size of TENGs may limit their integration into small-scale PDT systems, posing challenges for miniaturization. Furthermore, their design can be complex, necessitating careful optimization to ensure efficient energy generation in PDT applications.
2.2. NG-enhanced PDT
The integration of NGs with PDT holds immense promise in cancer management, warranting further exploration. Previous investigations have shown encouraging results, as illustrated in Figure 3. The efficacy of NGs has been demonstrated in providing precise and sustained light sources to stimulate PSs [36], amplifying ROS generation and incident photo-electron conversion efficiency in wound healing [145], supporting detachable drug/light injector to conforms metronomic PDT at low-dose of light or drug [146], integrating ferroptosis inducer (FIN) and imidazole ketone erastin (IKE) to augment the PDT [147], and delivering therapeutic light doses into deep tissues for PDT [147].
Notably, Liu et al. introduced a groundbreaking self-powered PDT methodology, termed s-PDT, leveraging biomechanical energy for electricity production [36]. This novel technique allows for accurate LED activation and PS stimulation to eradicate cancer cells. The distinctive attributes of the PENG position it as a prime contender for wearable medical equipment in oncology centered around PDT, offering convenient and auspicious treatment avenues.
In a recent review, the utilization of NGs in wound healing technology was explored [149]. For instance, Yu et al. developed a TiO2/BTO/Au multilayered coaxial heterostructured nanorod array, demonstrating enhanced ROS generation and incident photo-electron conversion efficiency under UV-Vis light [145]. This innovation holds promise for boosting ROS and •OH production, potentially enhancing PDT efficacy. The ability of the nanorod array to enhance ROS and •OH production within the UV-Vis light spectrum can be particularly advantageous in targeting and destroying cancer cells during PDT, potentially improving cancer treatment outcomes.
Examples of NGs and PDT combined applications. (A) Human motion driven self-powered photodynamic system for long-term autonomous cancer therapy [36]. (B) A self-powered wireless detachable drug/light injector for metronomic photodynamic therapy in cancer treatment [146]. (C) Implanted, wireless, self-powered photodynamic therapeutic tablet synergizes with ferroptosis inducer for effective cancer treatment [147]. (D) Implantable self-powered therapeutic pellet for wireless photodynamic/sonodynamic hybrid therapy of cancer recurrence inhibition and tumor regression [148]. (E) Self-powered, implantable, and wirelessly controlled NO generation system for intracranial neuroglioma therapy [13]. (A) Reprinted with permission from [36]. Copyright 2020 American Chemical Society. (B) Reprinted with permission, LN: 5882360170575. (D) Reprinted with permission, LN: 5882810859864. (E) Reprinted with permission, LN: 5882811413873.
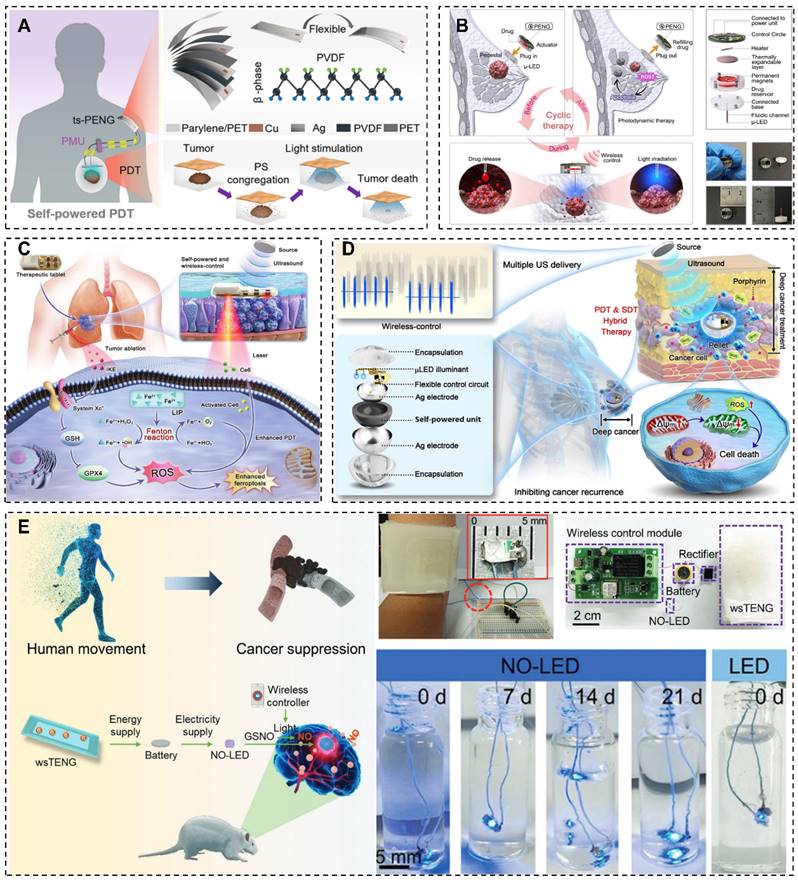
Chen's team presents a self-powered, wireless, and detachable drug/light injector for metronomic PDT in cancer treatment [146]. The device was consistent of a micro light emitting diode (µ-LED) as light source, a syringe needle, a drug reservoir, a thermally-driven pump, and a wireless control circuit. By harnessing body motion energy through connection to various piezoelectric nanogenerators, the device can wirelessly administer precise doses of drugs and light to the subcutaneous tumor. Implementation of this device in metronomic PDT demonstrated notable efficacy, with a 57.4% reduction in average tumor volume compared to the control group. Its compact design, adaptable delivery mechanisms for drugs and light, and minimized adverse effects underscore its advantages.
Furthermore, Zou et al. integrates a ferroptosis inducer and imidazole ketone erastin to develop a self-powered photodynamic therapeutic tablet [147]. In this device, the Fenton reaction was induced to supplement oxygen to enhance reactive oxygen species (ROS) production to augment the sensitivity of photodynamic therapy (PDT). Additionally, PDT facilitates iron ions release from the labile iron pool (LIP), and then, accelerate lipid peroxidation to inducing ferroptosis. This device demonstrated more than 85% tumor inhibition rate in vitro and in vivo experiments, due to overcome inadequate penetration and tumor hypoxia associated with PDT. Moreover, it can reduce the medication dosage to minimize adverse effects.
Additionally, a therapeutic pellet that provides wireless PDT/SDT hybrid therapy was designed by Xue's group, which contains an integrated self-powered unit, light-emitting diode illuminant, and control circuit [148]. The system is powered by ultrasound energy via the piezoelectric effect to support LED, alongside direct SDT treatment, the pellet delivers therapeutic doses of light to support PDT. The implantable system efficiently treats breast cancer in mice, showed great potential to overcome the barrier of PDT in deep tumors.
These investigations spotlight the transformative potential of NGs in PDT. By surmounting the constraints of external power supplies and offering real-time monitoring, NGs position themselves as game-changers in the PDT landscape. Continued research and development are necessary to expedite the clinical adoption of NG-enhanced PDT as a breakthrough approach for various medical challenges.
The intricate design of PENG/TENG brings forth additional benefits, augmenting the effectiveness of PDT in surmounting therapeutic hurdles. Therefore, a thorough exploration of the amalgamation of nanomaterial engineering and PDT is crucial to transform cancer treatment. The fusion of NGs with PDT emerges as a promising tactic to tackle the constraints linked with traditional PDT, as depicted in Figure 4. This fusion confers a distinct edge in countering PDT resistance. Serving as self-sustaining energy reservoirs, NGs expand the scope of PDT to distant or hard-to-reach regions, ensuring treatment availability. Furthermore, they furnish real-time data and responses to mechanical stimuli throughout procedures, enhancing treatment precision and efficacy.
Advantages of NGs in overcoming PDT barriers. Their wearable nature provides sustained electrical power for continuous monitoring and reliable PS excitation. NGs can directly influence cancer cells through modulation of pro-survival pathways, increased adhesion, and gene therapy applications. Moreover, NGs support the development of devices that enhance PDT efficacy, enabling precise and targeted treatment delivery. These advantages highlight the potential of NGs in revolutionizing PDT and improving treatment outcomes for various medical conditions.
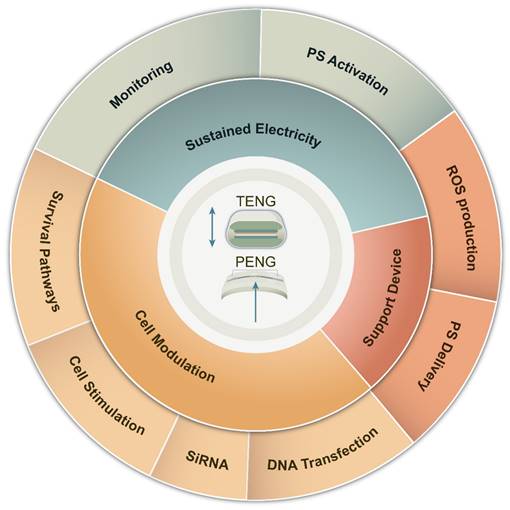
3. Overcoming Tumor Resistance to PDT at the Host Level
3.1. NGs for Biomaterial Sensing
Advancements in biosensor technologies have transformed their power supply, capabilities, and application scenarios [150]. These devices can now detect diverse biomaterial-related signals with heightened precision [151]. Monitoring biomaterials involved in PDT provides valuable insights into the process, status, and efficacy of PDT, leading to enhanced effectiveness and the ability to overcome resistance.
One versatile application of NGs is their use as physical and chemical sensors. Wearable sensors, powered by NGs, gauge biochemical parameters in bodily fluids, illuminating the photodynamic process and alleviating treatment resistance [37, 152, 153]. NGs such as TENGs provide high energy conversion efficiency, resilience in demanding environments, and stable adjustable outputs, rendering them ideal power sources for biomedical detection of analytes like dopamine, glucose, lactate, enzymes, various microbes, as well as chemical substances.
Peng et al. developed a flexible self-powered metal-semiconductor-metal (MSM) photoswitch for UV light detection, featuring a flexible gallium nitride (GaN) membrane on a polyethylene terephthalate (PET) substrate [37]. The asymmetric MSM design effectively separated electron-hole pairs via the intrinsic electric field, eliminating the need for external power sources. This GaN-based photoswitch exhibited superior UV photoresponse, functioning autonomously. The piezoelectric polarization field in GaN membrane has excellent mechanical flexibility that the depletion region can be obtained to further enhance UV on/off ratio up to 154% only under 1% strain. While its attributes suggest promise for enhancing PDT precision in clinical settings, further research is needed to evaluate its applicability in deep-seated tumor monitoring.
Accurate measurement of dopamine levels holds potential as an anti-angiogenic agent in combination treatments for breast and colon cancer, thereby enhancing the efficacy of anticancer drugs [154]. Jie et al. introduced an integrated device that incorporates a sensor leveraging the triboelectric effect for detecting neural signals and neurotransmitters [34]. The device switches from Schottky to ohmic contact when exposed to a voltage pulse from the TENG. In this TENG based integrated device, shows High selectivity and sensitivity (detection limit of 0.5 μM, a linear range from 10 μM to 1 mM). The output voltage and current of the developed TENS can reach 116 V and 33 μA. The Schottky contact biosensor exhibited a tenfold higher response to dopamine compared to the ohmic contact biosensor. The speed of dopamine polymerization in extremely low concentration is the sensing limitation, further low concentration detection can be achieved by microminiaturization of TENG. The Schottky contact biosensor exhibited a tenfold higher response to dopamine compared to the ohmic contact biosensor. The device was tested for simultaneous detection of dopamine and nerve signals pre- and post-treatment. The integration of this device with the TENG shows potential for dopamine determination in cancer therapy, contributing to targeted treatments and enhancing the effectiveness of anticancer drugs.
A TENG-based preconcentrator integrated with a smartphone was developed for immune sensing applications [155]. In this setup, the TENG served as a power source to drive a nanofluidic preconcentrator for electrokinetic trapping of biomolecules. The TENG operated at 3.7 Hz and a rotary TENG was used to investigate higher frequencies (7-37 Hz). The research showcased captured images of immunobeads-filled fluidic channels on a smartphone. During PDT, tumor tissue destruction can release substances like inflammatory cytokines and activate complement [156, 157]. The TENG's role as a power source for the nanofluidic preconcentrator enables the electrokinetic trapping of biomolecules, facilitating immune sensing. This technology offers insights into the immune status of tumors, aiding in decisions regarding the combination of PDT with immune adjuvants. Such a combination has the potential to augment the body's immune system efficacy in combating tumors and potentially induce an "abscopal effect," bolstering the body's anti-tumor capabilities.
Glucose acts as a critical metabolic regulator influencing cancer cell sensitivity to PDT. Modulating glucose metabolism can enhance PDT efficacy by sensitizing tumors or promote resistance mechanisms, highlighting the need to target these pathways for improved outcomes. Combining glucose-modulating agents with PDT offers a promising synergistic cancer treatment strategy. For instance, 2-deoxy-D-glucose (2-DG) can alter glucose utilization pathways, amplifying PDT effectiveness and enhancing therapeutic responses [158]. Glucose oxidase (GOx) significantly reshapes the TME by inducing glucose deprivation [159-161], sensitizing cancer cells to PDT. Utilizing GOx represents an innovative approach to optimizing PDT outcomes. Glucose levels can serve as biomarkers for assessing tumor responses to PDT, facilitating personalized therapeutic interventions and improving patient outcomes. TENGs have been employed for glucose sensors. One approach involves harnessing TENG-generated energy stored in a battery to power a glucose biosensor [162]. Another approach uses a whirligig-inspired TENG to power commercial glucose sensors [163]. This TENG harvest body motion energy from fabricated between a patterned polydimethylsiloxane (PDMS) film and an Al foil was fabricated. The maximum output of voltage and current density are up to 17 V and 0.02 μA/cm2, respectively. The TENG can directly illuminate 30 light-emitting diodes (LEDs) and charge a lithium-ion battery to power a glucose biosensor.
3.2. NGs for Metastasis Detection and Privation
Metastasis and recurrence represent significant hurdles in cancer treatment, often resulting in cancer-related deaths. is an efficacious local anti-cancer treatment, its effectiveness can be compromised by metastatic spread. In response to this challenge, researchers have devised devices incorporating piezoelectric BaTiO3 nanoparticles [19]. These nanoparticles have the capability to convert ultrasonic energy into an electrical signal, facilitating cancer cell adhesion and prompting stem cell differentiation. Moreover, the surface nanotopography of biomaterials has exhibited potential in improving cell adhesion and controlling cell behavior via mechanical transduction pathways. Integrating a PENG device can potentially enhance cancer cell adhesion and mitigate metastasis. By leveraging these advancements in nanotechnology and materials science, researchers aim to address the complex issues of cancer metastasis and recurrence, ultimately advancing the landscape of cancer treatment strategies.
The identification of circulating tumor cells (CTCs) is critical in PDT. While PDT is an effective local anti-cancer treatment, its efficacy may be compromised by metastatic tumors [98]. Immune responses elicited by PDT often lack the potency to eradicate metastatic growths [164], particularly due to the immunosuppressive nature of the TME [93]. Early detection of CTCs is crucial for prognostic assessment and improving survival rates among cancer patients with metastasis. CTCs act as valuable biomarkers for evaluating prognosis across various carcinoma types [165]. However, current ex vivo CTC isolation technologies capture only a small number of CTCs due to the restricted blood volumes obtained from single venipunctures, resulting in statistical variability and an inaccurate representation of tumor cell heterogeneity. To overcome these challenges, Daniel F. Hayes and his team have developed an innovative in vivo intravascular aphaeretic CTC isolation system [166]. This technology enables continuous collection of CTCs directly from a peripheral vein, allowing for the analysis of a greater number of CTCs. Such advancements facilitate precise monitoring and management of tumor metastasis by providing more comprehensive and representative data. Furthermore, NGs have shown potential in enhancing cancer cell adhesion, which may improve CTC analysis [19, 165]. This enhanced understanding of CTC dynamics can inform strategies to combine PDT with immunotherapy or other modalities, thereby strengthening anti-tumor immune responses and preventing metastasis.
4. Overcoming Resistance to PDT at the TME Level
4.1. NGs for PS Excitation
The excitation of PSs in PDT is crucial for its clinical application. Several factors such as light sources, photodynamic penetration, PS absorption, distribution, and activation influence this excitation process. Achieving effective PS activation is essential for successful PDT in clinical settings. However, limited light penetration in deep-seated tumors presents a challenge.
To address this challenge, various strategies have been explored, including the use of light with better tissue penetration and sustained light illumination. Long-wavelength light, particularly within the first (650-950 nm) and second (1000-1350 nm) NIR bio-windows, has been shown to enhance tissue penetration [167]. involving photon upconversion, two-photon excitation, X-ray excitation, or internal self-luminescence of PSs have been developed to treat tumors in deep tissues [105, 168-170]. One such approach is the use of a wearable twinning structure piezoelectric nanogenerator (ts-PENG) in a system called s-PDT [36]. This system converts biomechanical energy into electrical energy to power a miniaturized LED (m-LED) via a power management unit (PMU), delivering light stimulation over tumor tissues. In vivo experiments have demonstrated that the s-PDT system effectively inhibits tumors with intermittent or continuous light stimulation.
Additionally, a TENG-based approach incorporates a nanoprodrug that combines piezoelectric nanomaterials and amphiphilic prodrug molecules [171]. Ultrasound-triggered activation releases chemotherapy drugs and nitric oxide, inhibiting chemoresistance and enhancing antitumor efficiency through controlled drug and NO release.
Efficient and targeted intracellular delivery of PS biomacromolecules remains a challenge in PDT. TENGs offer a promising solution to improve PS delivery. A device combining microneedles and a TENG has been developed, showing increased drug delivery rates [172]. A self-powered microfluidic transport system combining electrowetting and a freestanding mode TENG allows for precise positioning and fast response speeds in PS delivery [173].
Tumor morphology can significantly influence the efficacy of PDT. Some tumor regions may be challenging to access directly with light irradiation, leading to reduced treatment effectiveness. Conversely, flat tumors with regular shapes can receive more comprehensive light exposure, resulting in better treatment outcomes. An innovative drug delivery system based on an integrated triboelectric nanogenerator (iTENG) has been developed to address these challenges [23]. This system can adapt to various implantation sites and enhance drug release during electrical field (EF) stimulation. In both in vitro and in vivo studies, the iTENG has demonstrated remarkable cytotoxicity against cancer cells.
Furthermore, a TENG-powered electrochemotherapy system featuring microneedle electrodes has shown promising results for three dimensional (3D) cells or tissues, improving therapeutic effects in irregularly shaped tumors [24]. These devices are particularly well-suited for 3D tissues, aiding in the endocytosis of PSs and enhancing their penetration, absorption, and distribution within tumors. Consequently, lower PS dosages can be utilized, reducing toxicity to normal tissues. The diverse shapes of iTENGs allow for implantation in deep-seated or irregularly shaped tumors, enhancing their potential for effective treatment across various clinical scenarios. These advancements in nanogenerator technology and drug delivery systems hold promise for improving PDT outcomes in tumors with varied morphologies and complexities.
4.2. NGs for Monitoring the TME
Tumor tissues often exhibit an acidic extracellular environment (pH 6.5-6.9) attributed to the Warburg effect, which is linked to increased metabolic activity, rapid cell proliferation, and inadequate tissue perfusion [174]. The acidic conditions in the TME can significantly influence drug transport and behavior, leading to alterations in drug characteristics. Research indicates that enhancing the pH gradient between tumor and normal tissues can improve the concentration gradient of photosensitizing drugs, thereby enhancing their lipophilicity and cellular uptake [175, 176]. Alluri and colleagues have developed a self-powered biosensor for theranostics that specifically measures blood pH [177]. They utilized the ionotropic gelation technique to create multifunctional biopolymer-piezoelectric composite worm structures featuring wavy and linear patterns. By adjusting the length and weight ratio of piezoelectric nanoparticles, the peak-peak voltage and current decreases around 87% and 71% for composite wavy pattern worm(CWPW) devices when the CWPWs length decreased to 56.4% (L = 1.95 to 0.85 cm) respectively. The pH-dependent conductivity of the composite linear worms (CLWs) was evaluated for potential clinical monitoring applications. The self-powered biosensor generated piezoelectric potential, serving as an independent power source to operate the CLW sensor effectively under varying pH conditions. This innovative technology demonstrates significant promise for monitoring pH fluctuations within the TME and optimizing drug delivery strategies in theranostics. By enabling real-time pH monitoring, this biosensor could potentially enhance our understanding of the TME dynamics and improve the precision of drug delivery in cancer theranostics.
Reflecting the hypoxic TME, lactate serves as a metabolic marker that helps predict treatment success and tumor behavior [178, 179]. Hypoxia-induced lactate accumulation creates a resistant environment, limiting treatment effectiveness and potentially inducing lactic acidosis [180-183]. Detecting lactate levels can predict PDT success, guiding treatment strategies and patient responses. TENGs have demonstrated potential for lactate detection, as well as electrochemical synthesis of nanoparticles. For instance, a self-powered system was developed to detect lactate in sweat, achieving high selectivity for lactate over interfering species like creatinine, uric acid, and glucose [184]. The maximum output voltage and current density reaching 500 V and 14 mA/m2, respectively. Continued advancements in TENG technology could lead to the development of more efficient and convenient lactate monitoring devices, further enhancing the ability to predict PDT success, guide treatment strategies, and improve patient outcomes.
4.3. NGs for Modulating the TME
The hypoxic microenvironment commonly observed in tumors undergoing Photodynamic Therapy (PDT) arises from various factors such as imbalances in oxygen consumption, inefficient oxygen supply due to abnormal tumor vasculature, and the effects of conventional type II PDT [89]. Rapid oxygen depletion and limited blood supply during PDT treatment can promote the development of aggressive malignant cells and treatment-resistant cell populations. Mitochondrial oxidative phosphorylation (OXPHOS) contributes to both tumor hypoxia and mitochondrial ROS production [185-187]. The superoxide dismutase (SOD) catalytic cascade converts mitochondrial superoxide anions (O2•-) into hydroxyl radicals (OH•), enhancing Type I and Type II PDT efficacy and generating O2 to support oxygen-dependent Type II PDT [188-190]. The interplay between OXPHOS-mediated O2 consumption and PDT-induced O2 depletion exacerbates tumor hypoxia.
Besides oxygen exhausting, ROS generated during PDT can directly harm endothelial cells, leading to a reduction in blood supply, which further aggravates hypoxia within the TME [42]. Hypoxia influences the tumor immune microenvironment post-PDT, promoting angiogenic signals, cell survival, proliferation, invasiveness, and metastasis [79, 191]. It also induces the transformation of macrophages into the M2 type, recruiting myeloid-derived suppressor cells (MDSCs) and inhibiting T cell activation [192, 193]. To address the challenges, various strategies have been proposed. These include increasing oxygen supply, utilizing oxygen-independent PDT reactions, stimulating ROS production, and combining PDT with hypoxia-activated or oxygen-independent therapies [98, 118]. By implementing these strategies, PDT can be optimized for effectiveness in hypoxic microenvironments, potentially leading to improved treatment outcomes for cancer patients.
PENG/TENG technologies can power implantable devices that actively release oxygen in hypoxic regions. For instance, a PENG-powered oxygen release system could respond to local oxygen levels, ensuring a steady supply in areas with limited oxygen availability [38]. TENG/PENG technologies were used to power drug delivery systems that can trigger the release of oxygen-sensitizing agents in hypoxic tumor regions [194]. This approach can enhance the efficacy of treatments like PDT by improving oxygen levels within the tumor. PENG/TENG devices were incorporated within tissue-engineered constructs to promote oxygenation in hypoxic environments [195]. These sensors could trigger responses such as oxygen release or drug delivery based on the detected oxygen concentrations, enabling precise and targeted interventions. Designing self-powered oxygen generation systems based on PENG/TENG technologies that can extract oxygen from ambient sources and deliver it to hypoxic tissues [196]. These systems can operate independently of external power sources, making them suitable for sustained oxygen delivery in challenging environments. The use of PENG/TENG-powered systems has also been explored in combination with other therapies to address hypoxia, for example, coupling TENG-powered oxygen release with traditional chemotherapeutic agents to improve drug efficacy in regions with low oxygen levels [25].
NGs boost ROS production in PDT through external stimulation. For example, Yao et al. devised a self-driven catalysis-promoting system called TENG-CatSystem [38]. It comprises a self-driven TENG for electric impulses, a nanozyme featuring a 1D ferriporphyrin covalent organic framework on a carbon nanotube (COF-CNT) to generate ROS, and a conductive hydrogel embedded with COF-CNT for injection into tumor tissues. TENG-CatSystem shows promise in boosting PDT efficacy by addressing key limitations. The COF-CNT-embedded conductive hydrogel, when introduced at the tumor site, elevates local ROS levels, reduces tissue impedance, and enhances PDT precision while minimizing off-target effects. The TENG support amplifies the COF-CNT's peroxidase-like function by up to fourfold, shifting cellular responses from autophagy to apoptosis to counter PDT resistance. Additionally, the TENG-CatSystem enhances peroxidase catalytic conductivity, enabling oxygen-independent ROS generation, a potent strategy against hypoxia in PDT. The self-driven TENG ensures sustained therapeutic action, suitable for at-home use. Similarly, TENGs can boost ROS production in hypoxic TME by converting mechanical energy from body movements or blood flow [26]. By leveraging NGs, researchers aim to surpass oxygen constraints and treatment resistance in hypoxic tumors, ultimately improving therapeutic outcomes.
The acidic TME with a pH of 5.6 to 6.8 originates from cancer cell hypoxia and increased glycolysis [197, 198]. This acidity significantly impacts PDT efficacy through mechanisms such as PS activation, ROS generation, and treatment resistance, with implications for cancer invasion and metastasis [199, 200]. Research indicates that a self-powered TENG system enhances •OH production under acidic conditions, showing promise for boosting PDT effectiveness [14]. Implantable patch incorporating catalytic g-C3N4 nanosheets and the drug doxorubicin (DOX) provides electrical stimulation, significantly increasing •OH production and enhancing cancer treatment outcomes. This approach demonstrates superior tumor suppression in a breast cancer model and highlights the potential of targeting the acidic microenvironment to enhance PDT efficacy and explore combination therapies.
Improving oxygen supply in hypoxic tumors through blood flow can enhance PDT efficacy. Photothermal-mediated heating is a method that can elevate tumor blood flow and oxygen levels, thereby enhancing the outcomes of PDT [201]. Integrating NGs with photothermal capabilities holds promise for synergistic effects with PDT. Another strategy involves utilizing a tumor-specific nanogenerator that generates peroxynitrite to enhance vascular permeability and oxygen supply within the TME [22]. The availability of blood supply to organs not only impacts the absorption and distribution of PSs but also plays a role in influencing the overall effectiveness of PDT. The proportions of PSs binding to tumor tissue after transport through the bloodstream contribute to these variations. In a related development, Jie et al. developed an integrated device based on the triboelectric effect for detecting dopamine in alkaline conditions[34]. Dopamine has been shown to enhance the efficacy of anticancer drugs and may act as an antiangiogenic agent in the treatment of breast and colon cancer [154]. Combining this innovative device with TENG holds significant potential for accurately determining dopamine levels in cancer treatment settings.
PDT exhibits a dual nature in its potential to address tumor metastasis. Firstly, PDT exhibits the ability to trigger immune responses that can impede metastasis [202]. These responses hold promise for sustained protection against metastatic dissemination [31, 32, 203]. However, translating this potential into clinical effectiveness encounters obstacles due to the intricacies of metastatic pathways, tumor diversity, and the necessity for refined treatment regimens [93, 204]. Murillo et al. demonstrated a ZnO nanosheets based PENG to stimulate macrophage motility [20], suggests a potential strategy to enhance PDT's ability to activate immune responses. The enhanced mobility of macrophages could potentially enhance immune cell recruitment and activation at the tumor site post-PDT. Further research is imperative to explore the synergistic effects of combining PENG-induced macrophage stimulation with PDT to enhance immune response, presenting a novel avenue to bolster PDT efficacy against tumor metastasis. Moreover, strategies are essential to reduce CTCs and monitor immune status promptly. Nanotechnology enhances cancer cell adhesion, CTC analysis, and immune status detection [19, 165]. These benefits facilitate accurate tracking of tumor metastasis and immune status, guiding the integration of PDT with other therapies to effectively reduce tumor recurrence and metastasis.
5. Overcoming Tumor Resistance to PDT at the Subcellular Level
5.1. NGs for Modulating PDT-induced Cell Death
Apoptosis, primarily triggered by ROS-mediated oxidative stress during PDT, represents the main mechanism of cell death induced by PDT [205]. Excessive ROS production during photodynamic reactions leads to structural and functional changes in cancer cells [206], activating caspase proteins that initiate signal transduction leading to apoptosis [207]. In contrast, necrosis refers to cell injury that causes premature cell death through autolysis [208]. Shifting the mode of cell death induced by PDT from apoptosis to necrosis can intensify the damage inflicted on cancer cells [205]. Necrosis can occur through various mechanisms, such as the direct targeting of the cell membrane by PSs, ROS-induced activation of necrotic pathways, ATP depletion leading to cell death, or inhibition of the autophagy pathway. Several factors influence necrotic cell death in cancer cells exposed to PDT, including the subcellular localization of PSs, ATP depletion in the cytosol [209], and the specific type of ROS generated during the process [210].
High doses of PDT have the potential to shift the mode of cell death from apoptosis to necrosis by increasing levels of ROS [211]. NGs can enhance ROS production, while ATP depletion can further promote necrosis. In a study conducted by Yang and colleagues [26], a TENG-supported device generated higher levels of ROS, including superoxide anion (O2-•), hydroperoxide radical (HOO•), peroxides (H2O2, ROOH), and hydroxyl radical (HO•), initiating free radical chain reactions that induce various cell death pathways, including apoptosis, necrosis, and other cell death mechanisms.
Additionally, Xue et al. proposed a high-performance TENG-based glucose sensor capable of detecting glucose concentrations without the need for glucose enzymes [153]. This device has the potential to monitor glucose levels, which can be instrumental in assessing the status of cellular ATP levels. Considering the differential immune responses elicited by necrosis and apoptosis, combining these strategies with PDT-induced anti-tumor immunity shows promise and merits further investigation.
In advanced tumors, serves as a mechanism activated to support cell survival and provide energy acquisition [212]. Cancer cells that are resistant to PDT often exhibit elevated levels of the anti-apoptotic protein Bcl-2, which functions to shield them from PDT-induced phototoxicity [212]. Studies have shown that the efficacy of PDT can be improved in cells deficient in the autophagy-related protein5 (ATG5), such as in HeLa and MCF-7 cells, indicating the involvement of autophagy in the adaptive survival response of cells undergoing PDT treatment [213]. To address the challenge of cell survival in PDT-resistant cancer cells, a self-powered TENG has been developed to deliver siRNA targeting genes such as Bcl-2, ATG5 and others to overcome cell survival and proliferation in PDT-resistant cancer cells [33].
The impact of autophagy on cell fate largely depends on the dosage of PDT and ROS generated. At lower doses, autophagy can promote cell survival by recycling damaged organelles. However, at higher doses, autophagy may contribute to organelle damage and ultimately lead to cell death. Research has demonstrated that the TENG-Cat System can enhance ROS production, [33], preventing damaged cells from recycling their impaired organelles and cytoplasmic components [214]. This disruption in the autophagic process may render cells less resistant to PDT-induced damage, potentially improving the effectiveness of PDT in treating resistant cancer cells.
5.2. NGs for Pro-survival Signaling Pathways
PDT can induce various forms of cell death, including apoptosis, necrosis, or autophagic cell death in tumors. However, cancer cells often activate pro-survival signaling pathways such as Bcl-2, HIF-1, AMPK, NRF2, NF-kB, and AKT-TOR to adapt and survive the treatment, which can lead to PDT resistance [215]. Inhibiting these pathways has the potential to enhance the efficacy of PDT.
An integrated TENG system has been developed to efficiently delivers siRNA targeting Bcl-2, HIF1-α, or NF-κB to overcome resistance to PDT [207]. The AMPK signaling pathway, which is involved in regulating autophagy, plays a role in contributing to PDT resistance [213, 216]. For example, the activation of autophagy through the AMPK pathway induced by 5-aminolevulinic acid (5-ALA) photoactivation can lead to tumor resistance and reduced apoptosis [125].
NGs have shown effectiveness in modulating cell activities [217-224]. PENGs have been utilized to modulate signaling pathways like MAPK/ERK and cAMP to promote differentiation in specific cell types, such as PC12 cells [18]. Furthermore, electrical stimulation has been shown to activate AMPK, thereby suppressing autophagy and mTOR-HIF-1 signaling pathways. This suppression can enhance apoptosis, potentially overcoming drug resistance in cancer cells. By targeting these signaling pathways and utilizing innovative nanogenerator systems, researchers aim to improve the effectiveness of PDT and combat resistance mechanisms in cancer treatment.
5.3. NGs for the PSs Excretion and Endocytosis
Intracellular accumulation of PSs is crucial for PDT's cytotoxic effects on tumor cells. However, resistance to PDT can arise due to the excretion of PSs by multidrug resistance transporters such as P-glycoprotein (P-GP) and Breast cancer resistance protein (BCRP/ABCG-2) [117]. Blocking P-GP with Verapamil or ABCG-2 with tyrosine kinase inhibitors like Imatinib mesylate can increase the intracellular content of PSs, thereby enhancing the effectiveness of PDT [225] [226]. Overexpression of P-GP and ABCG-2 can reduce the intracellular concentration of PSs, impacting the photosensitivity of cells [226]. NGs have been shown to down-regulate P-GP and ABCG-2, offering a potential strategy to overcome PDT resistance mediated by these transporters [33].
Mitochondrial dysfunction and impaired ATP synthesis can reduce the drug transport capacity of P-GP in cancer cells. A high-performance MXene/GO-based TENG serves as a noninvasive glucose sensor capable of detecting glucose levels without the need for a glucose enzyme [153]. Monitoring glucose levels is crucial as glucose is metabolized to ATP, which supports the function of the P-GP efflux pump. By detecting glucose concentration, the MXene/GO-based TENG for noninvasive glucose detection may provide insights into monitoring the function of the P-GP efflux pump to some extent. This innovative approach could potentially aid in understanding and optimizing the intracellular accumulation of PSs in tumor cells, thereby overcoming resistance mechanisms and enhancing the efficacy of PDT in cancer treatment.
In the realm of PDT, the selective accumulation of PSs in diseased targets is critical for enhancing treatment outcomes and minimizing non-specific damage to healthy tissues [227]. Endocytosis is the initial step in PS absorption, facilitating their accumulation within cells [228]. However, the selective endocytosis of PSs in cancer cells is often hindered by the hydrophobicity of many PS compounds [118]. Even for hydrophilic PSs, the selective accumulation in diseased targets may not be sufficient for clinical use [229]. To address these challenges, various nanoparticle-based delivery systems, such as liposomes, polymeric micelles, and silica-based nanoparticles, have been developed to improve the selective accumulation of PSs [230-234]. While these methods have shown some benefits, they do not provide comprehensive solutions, as issues remain in terms of sustained delivery, the delivery of large molecular weight PSs, and the targeting of deep-seated or irregularly shaped tumors.
Recent studies have explored alternative strategies to enhance PS delivery and endocytosis. For instance, Yang et al. demonstrated efficient delivery of exogenous materials, including large molecules, into hard-to-transfect primary cells with high cell viability [33]. Additionally, a TENG-powered electrochemotherapy system with microneedle electrodes showed promising results for 3D cell or tissue models, enhancing drug delivery in irregularly shaped tumors [24]. Furthermore, a self-powered drug delivery system comprising a current source derived from a disk TENG (D-TENG) enabled sustained drug delivery and significant enhancement of cancer cell uptake over long-term conditions [235]. Incorporating PSs into such self-powered electrical systems utilizing TENGs has demonstrated the potential to promote endocytosis and increase PS accumulation within tumor cells[23]. By capitalizing on these innovative delivery strategies and self-powered electrical systems, the selective accumulation of PSs in diseased targets can be further enhanced, ultimately improving the efficacy and clinical applicability of PDT.
5.4. NGs for Gene Therapy
NGs have been shown to increase plasma membrane potential and permeability, thereby enabling efficient delivery of siRNA and transfection of DNA plasmids into cells. This approach offers several advantages over conventional methods like electroporation, including high delivery efficiency and improved cell viability.
The self-regulated electro system driven by TENG/PENG has proven effective for gene transfection and siRNA delivery in cancer treatment [33]. An integrated system developed by Zhao et al. utilizes a self-powered TENG to provide a stable voltage pulse source, triggering an increase in plasma membrane potential and permeability [24]. This system achieves efficient delivery of various molecules into different cell types, with delivery efficiency reaching up to 90% and cell viability exceeding 94%. This device can facilitate siRNA delivery for PDT, enabling gene therapy to overcome drug resistance by silencing genes involved in PDT resistance.
Additionally, a fully integrated electrical stimulation cell culture dish (SESD) has been designed to provide self-powered electrical stimulation to adherent cells [236]. Electrical stimulation safely increases intracellular calcium concentration by opening calcium-ion channels, significantly enhancing plasmid transfection efficiency in mammalian cells and promoting cell survival.
Another self-powered electroporation system, utilizing a polypyrrole microfoam electrode, has been developed to deliver biomolecules with high efficiency and cell viability [237]. By leveraging these technologies, the integrated system based on NGs can efficiently deliver siRNA and enhance DNA plasmid transfection, enabling the modulation of tumor resistance in PDT. This innovative approach holds promise for improving gene delivery and therapeutic outcomes in cancer treatment.
Figure 5 presents a comprehensive survey of strategies employing NGs to combat barriers in PDT. NGs exhibit beneficial characteristics, including precise and effective delivery of PSs, ROS generation under hypoxic conditions, and facilitation of biomedical sensors for detecting PDT byproducts and materials that influence treatment outcomes. Additionally, NGs promote the permeability and intracellular penetration of therapeutic agents like PSs, small interfering RNA (siRNA), and DNA plasmids. The programmable nature of NGs allows for the fine-tuning of photodynamic and physiological processes within tumors and the human body, ultimately optimizing PDT efficacy.
6. Conclusion
In this study, we have investigated the capacity of Nanogenerators (NGs) to surmount resistance in PDT, presenting novel approaches to bolster treatment effectiveness. PDT resistance poses a notable obstacle, resulting in subpar results in specific scenarios. Nevertheless, the amalgamation of NGs with PDT exhibits substantial potential in tackling this hurdle. NGs present distinctive benefits by furnishing a localized and sustainable energy reservoir for PDT, thereby circumventing drawbacks linked to conventional light sources. NGs can convert mechanical, frictional, or thermal energy into electrical energy to power the light source for PS activation and ROS generation.
We have explored various types of NGs, encompassing nanowires, nanofibers, and 2D material-based NGs, emphasizing their potential roles in PDT. Integration of these NGs with photosensitizers holds promise for enhancing delivery efficiency, augmenting cellular uptake, and enabling controlled release, thereby heightening the therapeutic efficacy of PDT. Furthermore, NGs offer a means to counter resistance mechanisms commonly encountered in PDT, including hypoxia, limited tissue penetration, and multidrug resistance. By furnishing an oxygen-independent energy source, NGs effectively tackle challenges posed by hypoxic tumor microenvironments. Their unique attributes, such as small size and flexibility, facilitate deep tissue penetration and targeted delivery, enabling effective treatment of tumors in anatomically intricate locations. Moreover, NGs possess the potential to surmount multidrug resistance by triggering cell death pathways that operate independently of drug efflux pumps or cellular resistance mechanisms. This breakthrough opens new avenues for combating drug-resistant tumors, broadening the therapeutic repertoire accessible to medical practitioners.
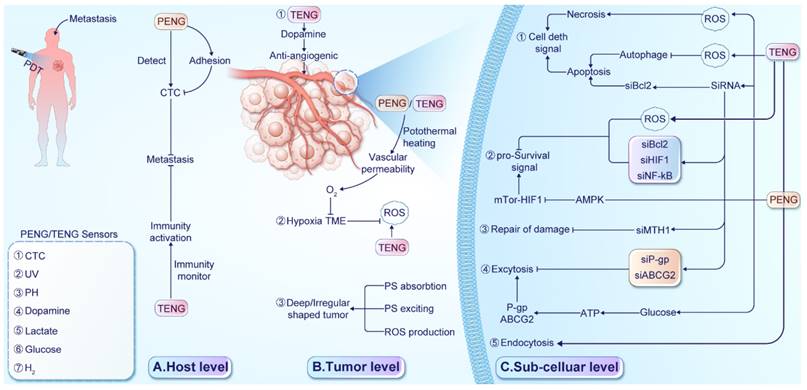
Strategies employed by NGs to overcome tumor resistance to PDT. (A) At the host level, NG-supported devices detect various biomarkers for early identification of metastasis and enhance immune activity to improve survival. (B) At the tumor level, ①A TENG-based sensor detect dopamine levels with anti-angiogenic properties. ② PENG/TENG systems facilitate thermal therapy, increasing O2 supply, relieving hypoxia, and enhancing ROS production. RR-TENG or TENG-Cat directly stimulate ROS production. ③ PENG/TENG-supported drug delivery systems(DDSs) enhance PS absorption, excitation, and ROS production for deep-seated or irregularly shaped tumors. (C) At the subcellular level, ① NG devices influence cell death signals, with TENG enhancing ROS production and inducing necrosis or enhancing apoptosis through Bcl-2 gene silencing or autophagy suppression. ② NG devices suppress pro-survival signals by increasing ROS production and silencing pro-survival genes or activating the AMPK signal to regulate mTOR-HIF1 signaling. ③Silencing the Mutt homolog 1(MTH1) gene reduces protein repair damaged by ROS. ④ TENG devices silence P-gp and ABCG2 to reduce photosensitizer exocytosis. TENG-based glucose sensors detect ATP production. ⑤ A designed D-TENG enhances cancer cell endocytosis and PS absorption.
While NG-PDT technology shows great promise, critical challenges must be tackled to enhance effectiveness and practicality. These include ensuring biocompatibility to prevent adverse immune reactions, developing scalable manufacturing processes for mass production, improving targeting precision to deliver PSs accurately, obtaining regulatory approval for clinical integration, ensuring long-term stability, addressing emerging resistance mechanisms, exploring combination therapies, optimizing cost-effectiveness, and integrating imaging and monitoring for personalized treatment planning. Through collaborative research and innovative approaches, overcoming these challenges can propel the progress of NG-PDT technology, enhancing its efficacy and clinical relevance in cancer therapy.
The development of NG-based PDT technology presents several promising opportunities for advancement. Leveraging NGs, researchers can enable personalized treatment approaches by tailoring PDT regimens to individual patient and cancer characteristics. Multifunctional NG platforms integrating PDT with imaging, drug delivery, or other therapeutic modalities can enhance treatment efficacy and precision. Smart NG-based drug delivery systems allow for on-demand photosensitizer release in response to specific tumor microenvironment stimuli. Theranostic NG applications integrate therapeutic and diagnostic capabilities, enabling real-time monitoring of treatment efficacy. Improved NG targeting can selectively deliver PSs to cancer cells, minimizing off-target effects. Synergistic combinations of NG-PDT with immunotherapy or targeted therapy show potential to overcome resistance mechanisms and achieve enhanced therapeutic outcomes. Remote monitoring and control of PDT parameters through NG systems can offer greater flexibility and precision in treatment administration. Integrating bioinformatics and AI algorithms can optimize NG-PDT protocols based on individual patient profiles. NG-based strategies to mitigate therapeutic resistance in cancer cells can improve long-term treatment responses. Promoting global accessibility of NG-PDT technology through optimized manufacturing and cost-reduction measures can expand its clinical applicability. By capitalizing on these opportunities through collaborative research efforts, the development of NG-PDT technology can be advanced, leading to improved cancer treatment outcomes.
In conclusion, NGs present a promising avenue for overcoming resistance in PDT through the provision of localized and sustainable energy sources, ultimately enhancing treatment effectiveness. The fusion of NGs with PDT stands to transform cancer therapy and elevate patient outcomes. Ongoing exploration and advancement in this realm are poised to drive the clinical implementation of NG-PDT methodologies, offering benefits to patients globally and propelling advancements in the domain of cancer therapeutics.
Abbreviations
2-DG: 2-deoxy-D-glucose
3D: three dimensional
5-ALA: 5-aminolevulinic acid
ABCG2: ATP-binding cassette transporter G2
AKT: Protein kinase B
AMPK: AMP-activated kinase
ATG5: autophogy-related 5
ATP: Adenosine Triphosphate
BBB: blood-brain barrier
BCRP: Breast Cancer Resistance Protein
cAMP: cyclic adenosine monophosphate
CLW: composite linear worm
COF-CNT: covalent organic framework on a carbon nanotube
CTCs: circulating tumor cells
CWPW: composite wavy pattern worm
DDS: drug delivery system
DNA: deoxyribonucleic acid
EF: electrical field
ER: endoplasmic reticulum
ERK: extracellular signal-regulated kinases
FIN: ferroptosis inducer
GaN: gallium nitride
GOx: Glucose oxidase
GSH: glutathione
GST: glutathione S-transferase
GPx4: glutathione peroxidase 4
HIF-1: hypoxia-inducible factor 1
HO•: hydroxyl radical
HOO•: hydroperoxide radical
IKE: imidazole ketone erastin
iNOS/NOS2: inducible nitric oxide synthase
LED: light emitting diode
LIP: labile iron pool
MAPK: mitogen-activated protein kinases
MDR: multidrug resistance
MDSCs: myeloid-derived suppressor cells
MSM: metal-semiconductor-metal
MTH1: Mutt homolog 1
mTOR: Mammalian target of rapamycin
NE: nanotechnology-enabled
NF-κB: Nuclear factor-kappaB
NGs: nanogenerators
NIR: near-infrared
NMOF: Titanium-Based Nanoscale Metal-Organic Framework
NO: nitric oxide
NOS: nitric oxide synthase
O2•-: superoxide anions
O2-: superoxide radicals
OH•: hydroxyl radicals
OXPHOS: oxidative phosphorylation
PDMS: polydimethylsiloxane
PDT: photodynamic therapy
PENGs: Piezoelectric Nanogenerators
PET: polyethylene terephthalate
P-gp: P-glycoprotein
MDR: multidrug resistance protein
PMU: power management unit
PSs: photosensitizers
ROS: reactive oxygen species
SDT: sonodynamic hybrid therapy
siRNA: small interfering RNA
SOD: superoxide dismutase
TENGs: Triboelectric Nanogenerators
TME: tumor microenvironment
UV-Vis: ultraviolet-visible
Acknowledgements
This work was supported by Health Commission of Sichuan Province (2024-803); Chengdu Technology Bureau (2024-YF05-02230-SN); Youth Innovation Project of Sichuan Medical Association (Q19010).
Author contributions
Conceptualization, M. C.; writing—original draft preparation, Y. Z., and P. Z.; writing—review and editing, P. Z., X. C., and M. C.; visualization, Y. Z., P.C. and M. C.; supervision, P. C., M. S., and M. C. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-49
2. Mohile SG, Mohamed MR, Xu H, Culakova E, Loh KP, Magnuson A. et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021;398:1894-904
3. Goldberg RM, Sargent DJ, Morton RF, Mahoney MR, Krook JE, O'Connell MJ. Early detection of toxicity and adjustment of ongoing clinical trials: the history and performance of the North Central Cancer Treatment Group's real-time toxicity monitoring program. J Clin Oncol. 2002;20:4591-6
4. Teh DBL, Bansal A, Chai C, Toh TB, Tucker RAJ, Gammad GGL. et al. A Flexi-PEGDA Upconversion Implant for Wireless Brain Photodynamic Therapy. Adv Mater. 2020;32:e2001459
5. Sando Y, Matsuoka KI, Sumii Y, Kondo T, Ikegawa S, Sugiura H. et al. 5-aminolevulinic acid-mediated photodynamic therapy can target aggressive adult T cell leukemia/lymphoma resistant to conventional chemotherapy. Sci Rep. 2020;10:17237
6. Viswanath D, Won Y-Y. Combining Radiotherapy (RT) and Photodynamic Therapy (PDT): Clinical Studies on Conventional RT-PDT Approaches and Novel Nanoparticle-Based RT-PDT Approaches under Preclinical Evaluation. ACS Biomater Sci Eng. 2022;8:3644-58
7. Agwa MM, Elmotasem H, Elsayed H, Abdelsattar AS, Omer AM, Gebreel DT. et al. Carbohydrate ligands-directed active tumor targeting of combinatorial chemotherapy/phototherapy-based nanomedicine: A review. Int J Biol Macromol. 2023;239:124294
8. Wang ZL, Song J. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science. 2006;312:242-6
9. Fan FR, Tian ZQ, Wang ZL. Flexible triboelectric generator!. Nano Energy. 2012;1:328-34
10. Alagumalai A, Mahian O, Aghbashlo M, Tabatabaei M, Wongwises S, Wang ZL. Towards smart cities powered by nanogenerators: Bibliometric and machine learning-based analysis. Nano Energy. 2021 83
11. Wang H, Cheng J, Wang Z, Ji L, Wang ZL. Triboelectric nanogenerators for human-health care. Science Bulletin. 2021;66:490-511
12. Deng W, Zhou Y, Libanori A, Chen G, Yang W, Chen J. Piezoelectric nanogenerators for personalized healthcare. Chemical Society Reviews. 2022;51:3380-435
13. Yao S, Zheng M, Wang Z, Zhao Y, Wang S, Liu Z. et al. Self-Powered, Implantable, and Wirelessly Controlled NO Generation System for Intracranial Neuroglioma Therapy. Adv Mater. 2022;34:e2205881
14. Zheng M, Yao S, Zhao Y, Wan X, Hu Q, Tang C. et al. Self-Driven Electrical Stimulation-Promoted Cancer Catalytic Therapy and Chemotherapy Based on an Implantable Nanofibrous Patch. ACS Appl Mater Interfaces. 2023;15:7855-66
15. Tang W, Tian J, Zheng Q, Yan L, Wang J, Li Z. et al. Implantable Self-Powered Low-Level Laser Cure System for Mouse Embryonic Osteoblasts' Proliferation and Differentiation. ACS Nano. 2015;9:7867-73
16. Tian JJ, Shi R, Liu Z, Ouyang H, Yu M, Zhao CC. et al. Self-powered implantable electrical stimulator for osteoblasts' proliferation and differentiation. Nano Energy. 2019;59:705-14
17. Jiang W, Li H, Liu Z, Li Z, Tian J, Shi B. et al. Fully Bioabsorbable Natural-Materials-Based Triboelectric Nanogenerators. Adv Mater. 2018;30:e1801895
18. Hoop M, Chen XZ, Ferrari A, Mushtaq F, Ghazaryan G, Tervoort T. et al. Ultrasound-mediated piezoelectric differentiation of neuron-like PC12 cells on PVDF membranes. Sci Rep. 2017;7:4028
19. Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol. 2014;15:825-33
20. Murillo G, Blanquer A, Vargas-Estevez C, Barrios L, Ibanez E, Nogues C. et al. Electromechanical Nanogenerator-Cell Interaction Modulates Cell Activity. Adv Mater. 2017 29
21. Kim JI, Hwang TI, Lee JC, Park CH, Kim CS. Regulating Electrical Cue and Mechanotransduction in Topological Gradient Structure Modulated Piezoelectric Scaffolds to Predict Neural Cell Response. Adv Funct Mater. 2020 30
22. Chen Y, Li ZH, Pan P, Zeng RY, Zhang XZ. Tumor-Specific ONOO(-) Nanogenerator for Improved Drug Delivery and Enhanced Chemotherapy of Tumor. ACS Nano. 2021;15:11514-25
23. Zhao C, Shi Q, Li H, Cui X, Xi Y, Cao Y. et al. Shape Designed Implanted Drug Delivery System for In situ Hepatocellular Carcinoma Therapy. ACS nano. 2022;16:8493-503
24. Zhao C, Yang Y, Cui X, Shan Y, Xue J, Jiang D. et al. Self-Powered Electrical Impulse Chemotherapy for Oral Squamous Cell Carcinoma. Materials (Basel). 2022;15:2060
25. Yao S, Zhao X, Wang X, Huang T, Ding Y, Zhang J. et al. Bioinspired Electron Polarization of Nanozymes with a Human Self-Generated Electric Field for Cancer Catalytic Therapy. Adv Mater. 2022;34:e2109568
26. Yang D, Liu Z, Yang P, Huang L, Huang F, Tao X. et al. A curtain purification system based on a rabbit fur-based rotating triboelectric nanogenerator for efficient photocatalytic degradation of volatile organic compounds. Nanoscale. 2023;15:6709-21
27. Hao Z, Guo S, Tu W, Wang Q, Wang J, Zhang X. et al. Piezoelectric Catalysis Induces Tumor Cell Senescence to Boost Chemo-Immunotherapy. Small. 2024;20:e2309487
28. Truong Hoang Q, Huynh KA, Nguyen Cao TG, Kang JH, Dang XN, Ravichandran V. et al. Piezocatalytic 2D WS(2) Nanosheets for Ultrasound-Triggered and Mitochondria-Targeted Piezodynamic Cancer Therapy Synergized with Energy Metabolism-Targeted Chemotherapy. Adv Mater. 2023;35:e2300437
29. Zhao Y, Wang S, Yao S, Wan X, Hu Q, Zheng M. et al. Piezoelectric Ba(0.85) Sr(0.15) TiO(3) Nanosonosensitizer with Nitric Oxide Delivery for Boosting Cancer Therapy. Small Methods. 2024;8:e2301134
30. Zhong SJ, Xiong C, Zhao YC, Yao SC, Hu QH, Wang SB. et al. Self-Driven Electricity Modulates d-Band Electrons of Copper Single-Atom Nanozyme for Boosting Cancer Therapy. Adv Funct Mater. 2023 33
31. Jalili A, Makowski M, Switaj T, Nowis D, Wilczynski GM, Wilczek E. et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin Cancer Res. 2004;10:4498-508
32. Preise D, Oren R, Glinert I, Kalchenko V, Jung S, Scherz A. et al. Systemic antitumor protection by vascular-targeted photodynamic therapy involves cellular and humoral immunity. Cancer Immunol Immunother. 2009;58:71-84
33. Liu Z, Nie J, Miao B, Li J, Cui Y, Wang S. et al. Self-Powered Intracellular Drug Delivery by a Biomechanical Energy-Driven Triboelectric Nanogenerator. Adv Mater. 2019;31:e1807795
34. Jie Y, Wang N, Cao X, Xu Y, Li T, Zhang X. et al. Self-powered triboelectric nanosensor with poly (tetrafluoroethylene) nanoparticle arrays for dopamine detection. ACS nano. 2015;9:8376-83
35. Sen O, Marino A, Pucci C, Ciofani G. Modulation of anti-angiogenic activity using ultrasound-activated nutlin-loaded piezoelectric nanovectors. Mater Today Bio. 2022;13:100196
36. Liu Z, Xu L, Zheng Q, Kang Y, Shi B, Jiang D. et al. Human motion driven self-powered photodynamic system for long-term autonomous cancer therapy. ACS nano. 2020;14:8074-83
37. Peng M, Liu Y, Yu A, Zhang Y, Liu C, Liu J. et al. Flexible self-powered GaN ultraviolet photoswitch with piezo-phototronic effect enhanced on/off ratio. ACS nano. 2016;10:1572-9
38. Yao SC, Zheng MJ, Wang SB, Huang T, Wang Z, Zhao YC. et al. Self-driven Electrical Stimulation Promotes Cancer Catalytic Therapy Based on Fully Conjugated Covalent Organic Framework Nanocages. Adv Funct Mater. 2022 32
39. Kessel D, Oleinick NL. Cell death pathways associated with photodynamic therapy: an update. Photochem Photobiol. 2018;94:213-8
40. Zhao X, Liu J, Fan J, Chao H, Peng X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: from molecular design to application. Chem Soc Rev. 2021;50:4185-219
41. Chilakamarthi U, Giribabu L. Photodynamic Therapy: Past, Present and Future. Chem Rec. 2017;17:775-802
42. Li X, Kwon N, Guo T, Liu Z, Yoon J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angew Chem Int Ed Engl. 2018;57:11522-31
43. Zhou Z, Song J, Nie L, Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem Soc Rev. 2016;45:6597-626
44. Dang J, He H, Chen D, Yin L. Manipulating tumor hypoxia toward enhanced photodynamic therapy (PDT). Biomater Sci. 2017;5:1500-11
45. Shen ZJ, Ma QM, Zhou XY, Zhang GM, Hao GZ, Sun Y. et al. Strategies to improve photodynamic therapy efficacy by relieving the tumor hypoxia environment. Npg Asia Mater. 2021 13
46. Kumari R, Sunil D, Ningthoujam RS. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: An up-to-date review. J Control Release. 2020;319:135-56
47. Sharma A, Sinha S, Shrivastava N. Therapeutic Targeting Hypoxia-Inducible Factor (HIF-1) in Cancer: Cutting Gordian Knot of Cancer Cell Metabolism. Front Genet. 2022;13:849040
48. Ni Y, Zhou X, Yang J, Shi H, Li H, Zhao X. et al. The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment. Front Cell Dev Biol. 2021;9:637675
49. Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization. Photodiagnosis and photodynamic therapy. 2004;1:279-93
50. Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kędzierska E, Knap-Czop K. et al. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomedicine & pharmacotherapy. 2018;106:1098-107
51. Rocha LGB. Development of a novel photosensitizer for Photodynamic Therapy of cancer. Universidade de Coimbra (Portugal). 2016
52. Rodrigues J, Amorim R, Silva M, Baltazar F, Wolffenbuttel R, Correia J. Photodynamic therapy at low-light fluence rate: In vitro assays on colon cancer cells. IEEE Journal of Selected Topics in Quantum Electronics. 2018;25:1-6
53. Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J. et al. Metabolic Heterogeneity in Human Lung Tumors. Cell. 2016;164:681-94
54. Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T. et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15:157-70
55. Maher EA, Marin-Valencia I, Bachoo RM, Mashimo T, Raisanen J, Hatanpaa KJ. et al. Metabolism of [U-13 C]glucose in human brain tumors in vivo. NMR Biomed. 2012;25:1234-44
56. Molina JR, Sun Y, Protopopova M, Gera S, Bandi M, Bristow C. et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat Med. 2018;24:1036-46
57. Rodrigues JA, Correia JH. Enhanced Photodynamic Therapy: A Review of Combined Energy Sources. Cells. 2022;11:3995
58. Zheng X, Jin Y, Liu X, Liu T, Wang W, Yu H. Photoactivatable nanogenerators of reactive species for cancer therapy. Bioact Mater. 2021;6:4301-18
59. Sun B, Chang R, Cao S, Yuan C, Zhao L, Yang H. et al. Acid-Activatable Transmorphic Peptide-Based Nanomaterials for Photodynamic Therapy. Angew Chem Int Ed Engl. 2020;59:20582-8
60. Cai Y, Ni D, Cheng W, Ji C, Wang Y, Mullen K. et al. Enzyme-Triggered Disassembly of Perylene Monoimide-based Nanoclusters for Activatable and Deep Photodynamic Therapy. Angew Chem Int Ed Engl. 2020;59:14014-8
61. He L, Ni Q, Mu J, Fan W, Liu L, Wang Z. et al. Solvent-Assisted Self-Assembly of a Metal-Organic Framework Based Biocatalyst for Cascade Reaction Driven Photodynamic Therapy. J Am Chem Soc. 2020;142:6822-32
62. Li S, Gu K, Wang H, Xu B, Li H, Shi X. et al. Degradable Holey Palladium Nanosheets with Highly Active 1D Nanoholes for Synergetic Phototherapy of Hypoxic Tumors. J Am Chem Soc. 2020;142:5649-56
63. Li X, Lee D, Huang JD, Yoon J. Phthalocyanine-Assembled Nanodots as Photosensitizers for Highly Efficient Type I Photoreactions in Photodynamic Therapy. Angew Chem Int Ed Engl. 2018;57:9885-90
64. Novohradsky V, Rovira A, Hally C, Galindo A, Vigueras G, Gandioso A. et al. Towards Novel Photodynamic Anticancer Agents Generating Superoxide Anion Radicals: A Cyclometalated Ir(III) Complex Conjugated to a Far-Red Emitting Coumarin. Angew Chem Int Ed Engl. 2019;58:6311-5
65. Li M, Xiong T, Du J, Tian R, Xiao M, Guo L. et al. Superoxide Radical Photogenerator with Amplification Effect: Surmounting the Achilles' Heels of Photodynamic Oncotherapy. J Am Chem Soc. 2019;141:2695-702
66. Lan G, Ni K, Veroneau SS, Feng X, Nash GT, Luo T. et al. Titanium-Based Nanoscale Metal-Organic Framework for Type I Photodynamic Therapy. J Am Chem Soc. 2019;141:4204-8
67. Luo T, Ni K, Culbert A, Lan G, Li Z, Jiang X. et al. Nanoscale Metal-Organic Frameworks Stabilize Bacteriochlorins for Type I and Type II Photodynamic Therapy. J Am Chem Soc. 2020;142:7334-9
68. Wang K, Zhang Z, Lin L, Chen J, Hao K, Tian HY. et al. Covalent Organic Nanosheets Integrated Heterojunction with Two Strategies To Overcome Hypoxic-Tumor Photodynamic Therapy. Chem Mater. 2019;31:3313-23
69. Wang X-Q, Wang W, Peng M, Zhang X-Z. Free radicals for cancer theranostics. Biomaterials. 2021;266:120474
70. Benov L. Photodynamic therapy: current status and future directions. Medical principles and practice. 2014;24:14-28
71. Guéraud F, Atalay M, Bresgen N, Cipak A, Eckl P, Huc L. et al. Chemistry and biochemistry of lipid peroxidation products. Free radical research. 2010;44:1098-124
72. Girotti AW, Kriska T. Role of lipid hydroperoxides in photo-oxidative stress signaling. Antioxidants and Redox Signaling. 2004;6:301-10
73. Girotti AW. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. Journal of Photochemistry and Photobiology B: Biology. 2001;63:103-13
74. Epe B. DNA damage spectra induced by photosensitization. Photochemical & Photobiological Sciences. 2012;11:98-106
75. Rangasamy S, Ju H, Um S, Oh D-C, Song JM. Mitochondria and DNA targeting of 5, 10, 15, 20-tetrakis (7-sulfonatobenzo [b] thiophene) porphyrin-induced photodynamic therapy via intrinsic and extrinsic apoptotic cell death. Journal of medicinal chemistry. 2015;58:6864-74
76. Sparrow JR, Zhou J, Cai B. DNA is a target of the photodynamic effects elicited in A2E-laden RPE by blue-light illumination. Investigative ophthalmology & visual science. 2003;44:2245-51
77. Mishchenko T, Balalaeva I, Gorokhova A, Vedunova M, Krysko DV. Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death Dis. 2022;13:455
78. Yuan H, Han Z, Chen Y, Qi F, Fang H, Guo Z. et al. Ferroptosis Photoinduced by New Cyclometalated Iridium(III) Complexes and Its Synergism with Apoptosis in Tumor Cell Inhibition. Angew Chem Int Ed Engl. 2021;60:8174-81
79. Casas A, Di Venosa G, Hasan T, Batlle A. Mechanisms of resistance to photodynamic therapy. Current medicinal chemistry. 2011;18:2486-515
80. Gilaberte Y, Milla L, Salazar N, Vera-Alvarez J, Kourani O, Damian A. et al. Cellular intrinsic factors involved in the resistance of squamous cell carcinoma to photodynamic therapy. Journal of Investigative Dermatology. 2014;134:2428-37
81. Wei M-F, Chen M-W, Chen K-C, Lou P-J, Lin SY-F, Hung S-C. et al. Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells. Autophagy. 2014;10:1179-92
82. Vankayala R, Hwang KC. Near-infrared-light-activatable nanomaterial-mediated phototheranostic nanomedicines: an emerging paradigm for cancer treatment. Advanced Materials. 2018;30:1706320
83. Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nature Reviews Cancer. 2006;6:535-45
84. Di Venosa G, Perotti C, Batlle A, Casas A. The role of cytoskeleton and adhesion proteins in the resistance to photodynamic therapy. Possible therapeutic interventions. Photochemical & Photobiological Sciences. 2015;14:1451-64
85. Jiang X, Du B, Zheng J. Glutathione-mediated biotransformation in the liver modulates nanoparticle transport. Nature nanotechnology. 2019;14:874-82
86. Zhang X, Chen X, Guo Y, Jia H-R, Jiang Y-W, Wu F-G. Endosome/lysosome-detained supramolecular nanogels as an efflux retarder and autophagy inhibitor for repeated photodynamic therapy of multidrug-resistant cancer. Nanoscale horizons. 2020;5:481-7
87. Shi L, Hu F, Duan Y, Wu W, Dong J, Meng X. et al. Hybrid nanospheres to overcome hypoxia and intrinsic oxidative resistance for enhanced photodynamic therapy. Acs Nano. 2020;14:2183-90
88. Li W, Guo X, Kong F, Zhang H, Luo L, Li Q. et al. Overcoming photodynamic resistance and tumor targeting dual-therapy mediated by indocyanine green conjugated gold nanospheres. Journal of Controlled Release. 2017;258:171-81
89. Chen D, Yu Q, Huang X, Dai H, Luo T, Shao J. et al. A highly-efficient type i photosensitizer with robust vascular-disruption activity for hypoxic-and-metastatic tumor specific photodynamic therapy. Small. 2020;16:2001059
90. Zamarrón A, Lucena SR, Salazar N, Sanz-Rodríguez F, Jaén P, Gilaberte Y. et al. Isolation and characterization of PDT-resistant cancer cells. Photochemical & Photobiological Sciences. 2015;14:1378-89
91. Wang X, Wang Z, Ma W, Wu X, Fang W, Guo C. et al. Construction of a nanotheranostic system Zr-MOF@ PPa/AF@ PEG for improved photodynamic therapy effects based on the PDT-oxygen consumption and hypoxia sensitive chemotherapeutic drug. Journal of Photochemistry and Photobiology B: Biology. 2021;222:112274
92. Wang Z, Shen X, Gao X. Density functional theory mechanistic insight into the peroxidase-and oxidase-like activities of nanoceria. The Journal of Physical Chemistry C. 2021;125:23098-104
93. Wang H, Li J, Wang Z, Wang Y, Xu X, Gong X. et al. Tumor-permeated bioinspired theranostic nanovehicle remodels tumor immunosuppression for cancer therapy. Biomaterials. 2021;269:120609
94. Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochemical journal. 2001;357:593-615
95. Brancaleon L, Moseley H. Laser and non-laser light sources for photodynamic therapy. Lasers Med Sci. 2002;17:173-86
96. Mang TS. Lasers and light sources for PDT: past, present and future. Photodiagnosis Photodyn Ther. 2004;1:43-8
97. Barolet D. Light-emitting diodes (LEDs) in dermatology. Semin Cutan Med Surg. 2008;27:227-38
98. Fan W, Huang P, Chen X. Overcoming the Achilles' heel of photodynamic therapy. Chem Soc Rev. 2016;45:6488-519
99. Wilson BC, Jeeves WP, Lowe DM. In vivo and post mortem measurements of the attenuation spectra of light in mammalian tissues. Photochem Photobiol. 1985;42:153-62
100. Bashkatov AN, Genina EA, Kochubey VI, Tuchin VV. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J Phys D Appl Phys. 2005;38:2543-55
101. Chen G, Qiu H, Prasad PN, Chen X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem Rev. 2014;114:5161-214
102. Simpson CR, Kohl M, Essenpreis M, Cope M. Near-infrared optical properties of ex vivo human skin and subcutaneous tissues measured using the Monte Carlo inversion technique. Phys Med Biol. 1998;43:2465-78
103. Tian G, Gu Z, Zhou L, Yin W, Liu X, Yan L. et al. Mn2+ dopant-controlled synthesis of NaYF4:Yb/Er upconversion nanoparticles for in vivo imaging and drug delivery. Adv Mater. 2012;24:1226-31
104. Wang C, Tao H, Cheng L, Liu Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials. 2011;32:6145-54
105. Cui S, Yin D, Chen Y, Di Y, Chen H, Ma Y. et al. In vivo targeted deep-tissue photodynamic therapy based on near-infrared light triggered upconversion nanoconstruct. ACS Nano. 2013;7:676-88
106. Deng K, Li C, Huang S, Xing B, Jin D, Zeng Q. et al. Recent Progress in Near Infrared Light Triggered Photodynamic Therapy. Small. 2017 13
107. Li Q, Huang C, Liu L, Hu R, Qu J. Enhancing Type I Photochemistry in Photodynamic Therapy Under Near Infrared Light by Using Antennae-Fullerene Complexes. Cytometry A. 2018;93:997-1003
108. Hemmer E, Acosta-Mora P, Mendez-Ramos J, Fischer S. Optical nanoprobes for biomedical applications: shining a light on upconverting and near-infrared emitting nanoparticles for imaging, thermal sensing, and photodynamic therapy. J Mater Chem B. 2017;5:4365-92
109. Wu Y, Li F, Zhang X, Li Z, Zhang Q, Wang W. et al. Tumor microenvironment-responsive PEGylated heparin-pyropheophorbide-a nanoconjugates for photodynamic therapy. Carbohydr Polym. 2021;255:117490
110. Lim CM, Grillo M, Harrison P, Nguyen AM, Gordon L. Photodynamic therapy: A real-world analysis of outcomes of photodynamic therapy in an Australian tertiary dermatology clinic. Australas J Dermatol. 2019;60:339-40
111. Yoshida T, Tokashiki R, Ito H, Shimizu A, Nakamura K, Hiramatsu H. et al. Therapeutic effects of a new photosensitizer for photodynamic therapy of early head and neck cancer in relation to tissue concentration. Auris Nasus Larynx. 2008;35:545-51
112. Sheng C, Pogue BW, Wang E, Hutchins JE, Hoopes PJ. Assessment of photosensitizer dosimetry and tissue damage assay for photodynamic therapy in advanced-stage tumors. Photochem Photobiol. 2004;79:520-5
113. Wang ZJ, He YY, Huang CG, Huang JS, Huang YC, An JY. et al. Pharmacokinetics, tissue distribution and photodynamic therapy efficacy of liposomal-delivered hypocrellin A, a potential photosensitizer for tumor therapy. Photochem Photobiol. 1999;70:773-80
114. Moghassemi S, Dadashzadeh A, Azevedo RB, Feron O, Amorim CA. Photodynamic cancer therapy using liposomes as an advanced vesicular photosensitizer delivery system. J Control Release. 2021;339:75-90
115. Yu XT, Sui SY, He YX, Yu CH, Peng Q. Nanomaterials-based photosensitizers and delivery systems for photodynamic cancer therapy. Biomater Adv. 2022;135:212725
116. Li X, Lee S, Yoon J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem Soc Rev. 2018;47:1174-88
117. Huang Z, Hsu Y-C, Li L-B, Wang L-W, Song X-D, Yow CM. et al. Photodynamic therapy of cancer—Challenges of multidrug resistance. Journal of Innovative Optical Health Sciences. 2015;8:1530002
118. Xie J, Wang Y, Choi W, Jangili P, Ge Y, Xu Y. et al. Overcoming barriers in photodynamic therapy harnessing nano-formulation strategies. Chem Soc Rev. 2021;50:9152-201
119. Wang H, Guo Y, Wang C, Jiang X, Liu H, Yuan A. et al. Light-controlled oxygen production and collection for sustainable photodynamic therapy in tumor hypoxia. Biomaterials. 2021;269:120621
120. Kimáková P, Solár P, Fecková B, Sačková V, Solárová Z, Ilkovičová L. et al. Photoactivated hypericin increases the expression of SOD-2 and makes MCF-7 cells resistant to photodynamic therapy. Biomedicine & Pharmacotherapy. 2017;85:749-55
121. Soares HT, Campos JR, Gomes-da-Silva LC, Schaberle FA, Dabrowski JM, Arnaut LG. Pro-oxidant and antioxidant effects in photodynamic therapy: cells recognise that not all exogenous ROS are alike. ChemBioChem. 2016;17:836-42
122. Rodriguez ME, Cogno IS, Sanabria LSM, Moran YS, Rivarola VA. Heat shock proteins in the context of photodynamic therapy: autophagy, apoptosis and immunogenic cell death. Photochemical & Photobiological Sciences. 2016;15:1090-102
123. Helbig D, Simon JC, Paasch U. Photodynamic therapy and the role of heat shock protein 70. International Journal of Hyperthermia. 2011;27:802-10
124. Coupienne I, Bontems S, Dewaele M, Rubio N, Habraken Y, Fulda S. et al. NF-kappaB inhibition improves the sensitivity of human glioblastoma cells to 5-aminolevulinic acid-based photodynamic therapy. Biochemical Pharmacology. 2011;81:606-16
125. Ji H-T, Chien L-T, Lin Y-H, Chien H-F, Chen C-T. 5-ALA mediated photodynamic therapy induces autophagic cell death via AMP-activated protein kinase. Molecular cancer. 2010;9:1-11
126. Wang ZL. On Maxwell's displacement current for energy and sensors: the origin of nanogenerators. Materials today. 2017;20:74-82
127. Li Z, Chen J, Zhou J, Zheng L, Pradel KC, Fan X. et al. High-efficiency ramie fiber degumming and self-powered degumming wastewater treatment using triboelectric nanogenerator. Nano Energy. 2016;22:548-57
128. Pu X, Zhang C, Wang ZL. Triboelectric nanogenerators as wearable power sources and self-powered sensors. National Science Review. 2023;10:nwac170
129. Shin S-H, Kwon YH, Lee MH, Jung J-Y, Seol JH, Nah J. A vanadium-doped ZnO nanosheets-polymer composite for flexible piezoelectric nanogenerators. Nanoscale. 2016;8:1314-21
130. Zhu G, Yang R, Wang S, Wang ZL. Flexible high-output nanogenerator based on lateral ZnO nanowire array. Nano letters. 2010;10:3151-5
131. Hu Y, Lin L, Zhang Y, Wang ZL. Replacing a battery by a nanogenerator with 20 V output. Adv Mater. 2012;24:110-4
132. Wang X, Song WZ, You MH, Zhang J, Yu M, Fan Z. et al. Bionic Single-Electrode Electronic Skin Unit Based on Piezoelectric Nanogenerator. ACS Nano. 2018;12:8588-96
133. Shin SH, Kim YH, Lee MH, Jung JY, Nah J. Hemispherically aggregated BaTiO3 nanoparticle composite thin film for high-performance flexible piezoelectric nanogenerator. ACS Nano. 2014;8:2766-73
134. Guo W, Tan C, Shi K, Li J, Wang XX, Sun B. et al. Wireless piezoelectric devices based on electrospun PVDF/BaTiO(3) NW nanocomposite fibers for human motion monitoring. Nanoscale. 2018;10:17751-60
135. Xu S, Qin Y, Xu C, Wei Y, Yang R, Wang ZL. Self-powered nanowire devices. Nat Nanotechnol. 2010;5:366-73
136. Johar MA, Kang JH, Ha JS, Lee JK, Ryu SW. Controlled conductivity of p-type CuO/GaN piezoelectric generator to harvest very high piezoelectric potential. J Alloy Compd. 2017;726:765-71
137. Johar MA, Waseem A, Hassan MA, Kang JH, Ha JS, Lee JK. et al. Facile growth of high aspect ratio c-axis GaN nanowires and their application as flexible p-n NiO/GaN piezoelectric nanogenerators. Acta Mater. 2018;161:237-45
138. Jin L, Ma SY, Deng WL, Yan C, Yang T, Chu X. et al. Polarization-free high-crystallization ß-PVDF piezoelectric nanogenerator toward self-powered 3D acceleration sensor. Nano Energy. 2018;50:632-8
139. Qin Y, Wang X, Wang ZL. Microfibre-nanowire hybrid structure for energy scavenging. Nature. 2008;451:809-13
140. Gu L, Cui N, Cheng L, Xu Q, Bai S, Yuan M. et al. Flexible fiber nanogenerator with 209 V output voltage directly powers a light-emitting diode. Nano Lett. 2013;13:91-4
141. Wang ZL, Chen J, Lin L. Progress in triboelectric nanogenerators as a new energy technology and self-powered sensors. Energy & Environmental Science. 2015;8:2250-82
142. Sripadmanabhan Indira S, Aravind Vaithilingam C, Oruganti KSP, Mohd F, Rahman S. Nanogenerators as a Sustainable Power Source: State of Art, Applications, and Challenges. Nanomaterials (Basel). 2019;9:773
143. Yu A, Chen X, Wang R, Liu J, Luo J, Chen L. et al. Triboelectric Nanogenerator as a Self-Powered Communication Unit for Processing and Transmitting Information. ACS Nano. 2016;10:3944-50
144. Dong K, Peng X, An J, Wang AC, Luo J, Sun B. et al. Shape adaptable and highly resilient 3D braided triboelectric nanogenerators as e-textiles for power and sensing. Nat Commun. 2020;11:2868
145. Yu X, Wang S, Zhang XD, Qi AH, Qiao XR, Liu ZR. et al. Heterostructured nanorod array with piezophototronic and plasmonic effect for photodynamic bacteria killing and wound healing. Nano Energy. 2018;46:29-38
146. Guan HY, Zou PJ, Lin R, Xiao L, Fang ZY, Chen JY. et al. Implantable self-powered therapeutic pellet for wireless photodynamic/sonodynamic hybrid therapy of cancer recurrence inhibition and tumor regression. Nano Energy. 2023 105
147. Zou P, Lin R, Fang Z, Chen J, Guan H, Yin J. et al. Implanted, Wireless, Self-Powered Photodynamic Therapeutic Tablet Synergizes with Ferroptosis Inducer for Effective Cancer Treatment. Adv Sci (Weinh). 2023;10:e2302731
148. Lin T, Zou PJ, Lin R, Guan HY, Fang ZY, Chen JY. et al. A self-powered wireless detachable drug/light injector for metronomic photodynamic therapy in cancer treatment. Nano Energy. 2023 116
149. Kao FC, Ho HH, Chiu PY, Hsieh MK, Liao JC, Lai PL. et al. Self-assisted wound healing using piezoelectric and triboelectric nanogenerators. Sci Technol Adv Mater. 2022;23:1-16
150. Kim J, Campbell AS, de Ávila BE-F, Wang J. Wearable biosensors for healthcare monitoring. Nature Biotechnology. 2019;37:389-406
151. Liu X, Cai Z, Gao N, Ye S, Tao T, He H. et al. Controllable preparation of (200) facets preferential oriented silver nanowires for non-invasive detection of glucose in human sweat. Smart Materials in Medicine. 2021;2:150-7
152. Petruk G, Petrlova J, Samsudin F, Giudice RD, Bond PJ, Schmidtchen A. Concentration- and pH-Dependent Oligomerization of the Thrombin-Derived C-Terminal Peptide TCP-25. Biomolecules. 2020;10:1572
153. Xue X, Qu Z, Fu Y, Yu B, Xing L, Zhang Y. Self-powered electronic-skin for detecting glucose level in body fluid basing on piezo-enzymatic-reaction coupling process. Nano Energy. 2016;26:148-56
154. Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clinical Cancer Research. 2008;14:2502-10
155. Fan Y-J, Huang M-Z, Hsiao Y-C, Huang Y-W, Deng C-Z, Yeh C. et al. Enhancing the sensitivity of portable biosensors based on self-powered ion concentration polarization and electrical kinetic trapping. Nano Energy. 2020;69:104407
156. Theodoraki MN, Lorenz K, Lotfi R, Furst D, Tsamadou C, Jaekle S. et al. Influence of photodynamic therapy on peripheral immune cell populations and cytokine concentrations in head and neck cancer. Photodiagnosis Photodyn Ther. 2017;19:194-201
157. Xiang Q, Yang C, Luo Y, Liu F, Zheng J, Liu W. et al. Near-Infrared II Nanoadjuvant-Mediated Chemodynamic, Photodynamic, and Photothermal Therapy Combines Immunogenic Cell Death with PD-L1 Blockade to Enhance Antitumor Immunity. Small. 2022;18:e2107809
158. Li W, Yang Y, Wang J, Ge T, Wan S, Gui L. et al. Establishment of bone-targeted nano-platform and the study of its combination with 2-deoxy-d-glucose enhanced photodynamic therapy to inhibit bone metastasis. J Mech Behav Biomed Mater. 2024;150:106306
159. Zhou J, Liu C, Wang Y, Ding M, Yu N, Liu D. et al. Prodrug and Glucose Oxidase Coloaded Photodynamic Hydrogels for Combinational Therapy of Melanoma. ACS Biomater Sci Eng. 2022;8:4886-95
160. Zhang J, Yang J, Qin X, Zhuang J, Jing D, Ding Y. et al. Glucose Oxidase Integrated Porphyrinic Covalent Organic Polymers for Combined Photodynamic/Chemodynamic/Starvation Therapy in Cancer Treatment. ACS Biomater Sci Eng. 2022;8:1956-63
161. Xia Y, Wu Y, Cao J, Wang J, Chen Z, Li C. et al. Liposomal Glucose Oxidase for Enhanced Photothermal Therapy and Photodynamic Therapy against Breast Tumors. ACS Biomater Sci Eng. 2022;8:1892-906
162. Khandelwal G, Deswal S, Dahiya R. Triboelectric Nanogenerators as Power Sources for Chemical Sensors and Biosensors. ACS Omega. 2022;7:44573-90
163. Zhang H, Yang Y, Hou T-C, Su Y, Hu C, Wang ZL. Triboelectric nanogenerator built inside clothes for self-powered glucose biosensors. Nano Energy. 2013;2:1019-24
164. Liu Z, Xie Z, Li W, Wu X, Jiang X, Li G. et al. Photodynamic immunotherapy of cancers based on nanotechnology: recent advances and future challenges. J Nanobiotechnology. 2021;19:160
165. Fathallah-Shaykh HM, DeAtkine A, Coffee E, Khayat E, Bag AK, Han X. et al. Diagnosing growth in low-grade gliomas with and without longitudinal volume measurements: A retrospective observational study. Plos Med. 2019;16:e1002810
166. Kim TH, Wang Y, Oliver CR, Thamm DH, Cooling L, Paoletti C. et al. A temporary indwelling intravascular aphaeretic system for in vivo enrichment of circulating tumor cells. Nature Communications. 2019;10:1478
167. Smith AM, Mancini MC, Nie S. Second window for in vivo imaging. Nature nanotechnology. 2009;4:710-1
168. Pawlicki M, Collins HA, Denning RG, Anderson HL. Two-photon absorption and the design of two-photon dyes. Angewandte Chemie International Edition. 2009;48:3244-66
169. Scaffidi JP, Gregas MK, Lauly B, Zhang Y, Vo-Dinh T. Activity of psoralen-functionalized nanoscintillators against cancer cells upon X-ray excitation. ACS nano. 2011;5:4679-87
170. Kotagiri N, Sudlow GP, Akers WJ, Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nature nanotechnology. 2015;10:370-9
171. Wang Y, Tang Q, Wu R, Sun S, Zhang J, Chen J. et al. Ultrasound-Triggered Piezocatalysis for Selectively Controlled NO Gas and Chemodrug Release to Enhance Drug Penetration in Pancreatic Cancer. ACS nano. 2023;17:3557-73
172. Bok M, Lee Y, Park D, Shin S, Zhao Z-J, Hwang B. et al. Microneedles integrated with a triboelectric nanogenerator: an electrically active drug delivery system. Nanoscale. 2018;10:13502-10
173. Nie J, Ren Z, Shao J, Deng C, Xu L, Chen X. et al. Self-powered microfluidic transport system based on triboelectric nanogenerator and electrowetting technique. Acs Nano. 2018;12:1491-9
174. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-33
175. Moan J, Peng Q, Evensen JF, Berg K, Western A, Rimington C. Photosensitizing efficiencies, tumor- and cellular uptake of different photosensitizing drugs relevant for photodynamic therapy of cancer. Photochem Photobiol. 1987;46:713-21
176. Friberg EG, Čunderlı́ková B, Pettersen EO, Moan J. pH effects on the cellular uptake of four photosensitizing drugs evaluated for use in photodynamic therapy of cancer. Cancer Letters. 2003;195:73-80
177. Alluri NR, Selvarajan S, Chandrasekhar A, Balasubramaniam S, Jeong JH, Kim S-J. Self powered pH sensor using piezoelectric composite worm structures derived by ionotropic gelation approach. Sensors and Actuators B: Chemical. 2016;237:534-44
178. Yu J, Li Q, Wei Z, Fan G, Wan F, Tian L. Ultra-stable MOF@MOF nanoplatform for photodynamic therapy sensitized by relieved hypoxia due to mitochondrial respiration inhibition. Acta Biomater. 2023;170:330-43
179. Wen J, Luo Y, Gao H, Zhang L, Wang X, Huang J. et al. Mitochondria-targeted nanoplatforms for enhanced photodynamic therapy against hypoxia tumor. J Nanobiotechnology. 2021;19:440
180. Manzoor AA, Schroeder T, Dewhirst MW. One-stop-shop tumor imaging: buy hypoxia, get lactate free. J Clin Invest. 2008;118:1616-9
181. Wigfield SM, Winter SC, Giatromanolaki A, Taylor J, Koukourakis ML, Harris AL. PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br J Cancer. 2008;98:1975-84
182. Wan Y, Fu LH, Li C, Lin J, Huang P. Conquering the Hypoxia Limitation for Photodynamic Therapy. Adv Mater. 2021;33:e2103978
183. Lee DC, Sohn HA, Park ZY, Oh S, Kang YK, Lee KM. et al. A lactate-induced response to hypoxia. Cell. 2015;161:595-609
184. Chen C-H, Lee P-W, Tsao Y-H, Lin Z-H. Utilization of self-powered electrochemical systems: Metallic nanoparticle synthesis and lactate detection. Nano Energy. 2017;42:241-8
185. Viale A, Corti D, Draetta GF. Tumors and mitochondrial respiration: a neglected connection. Cancer Res. 2015;75:3685-6
186. Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705-13
187. Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560-9
188. Wang Y, Cai R, Chen C. The Nano-Bio Interactions of Nanomedicines: Understanding the Biochemical Driving Forces and Redox Reactions. Acc Chem Res. 2019;52:1507-18
189. Yang B, Chen Y, Shi J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem Rev. 2019;119:4881-985
190. Samuel EL, Marcano DC, Berka V, Bitner BR, Wu G, Potter A. et al. Highly efficient conversion of superoxide to oxygen using hydrophilic carbon clusters. Proc Natl Acad Sci U S A. 2015;112:2343-8
191. Sanabria LM, Rodríguez ME, Cogno IS, Vittar NBR, Pansa MF, Lamberti MJ. et al. Direct and indirect photodynamic therapy effects on the cellular and molecular components of the tumor microenvironment. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2013;1835:36-45
192. Wang H, Han X, Dong Z, Xu J, Wang J, Liu Z. Hyaluronidase with pH-responsive dextran modification as an adjuvant nanomedicine for enhanced photodynamic-immunotherapy of cancer. Advanced Functional Materials. 2019;29:1902440
193. Liu H, Hu Y, Sun Y, Wan C, Zhang Z, Dai X. et al. Co-delivery of bee venom melittin and a photosensitizer with an organic-inorganic hybrid nanocarrier for photodynamic therapy and immunotherapy. ACS nano. 2019;13:12638-52
194. Zhao CC, Feng HQ, Zhang LJ, Li Z, Zou Y, Tan PC. et al. Highly Efficient In vivo Cancer Therapy by an Implantable Magnet Triboelectric Nanogenerator. Adv Funct Mater. 2019 29
195. Han Q, Fang ZY, Lin R, Chen JY, Wei XH, Gong CC. et al. Piezo-photodynamic therapy of Au@PEG-ZnO nanostructures enabled with a battery-free wireless cancer therapeutic dot. Nano Energy. 2024 125
196. Ghosh K, Iffelsberger C, Konecny M, Vyskocil J, Michalicka J, Pumera M. Nanoarchitectonics of Triboelectric Nanogenerator for Conversion of Abundant Mechanical Energy to Green Hydrogen. Adv Energy Mater. 2023 13
197. Justus CR, Dong L, Yang LV. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front Physiol. 2013;4:354
198. Shan L. (64)Cu-1,4,7,10-Tetraazacyclododecane-1,4,7-Tris-acetic acid-10-maleimidoethylacetamide-ACEQNPIYWARYADWLFTTPLLLLDLALLVDADEGTG. Molecular Imaging and Contrast Agent Database (MICAD). Bethesda (MD). 2004
199. Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373-84
200. Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A. et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013;73:1524-35
201. Feng L, Tao D, Dong Z, Chen Q, Chao Y, Liu Z. et al. Near-infrared light activation of quenched liposomal Ce6 for synergistic cancer phototherapy with effective skin protection. Biomaterials. 2017;127:13-24
202. Song W, Kuang J, Li CX, Zhang MK, Zheng DW, Zeng X. et al. Enhanced Immunotherapy Based on Photodynamic Therapy for Both Primary and Lung Metastasis Tumor Eradication. Acs Nano. 2018;12:1978-89
203. Korbelik M. Cancer vaccines generated by photodynamic therapy. Photoch Photobio Sci. 2011;10:664-9
204. Chen Z, Liu L, Liang R, Luo Z, He H, Wu Z. et al. Bioinspired Hybrid Protein Oxygen Nanocarrier Amplified Photodynamic Therapy for Eliciting Anti-tumor Immunity and Abscopal Effect. ACS Nano. 2018;12:8633-45
205. Agostinis P, Breyssens H, Buytaert E, Hendrickx N. Regulatory pathways in photodynamic therapy induced apoptosis. Photochemical & Photobiological Sciences. 2004;3:721-9
206. Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell research. 2019;29:347-64
207. Aniogo EC, George BPA, Abrahamse H. Role of Bcl-2 family proteins in photodynamic therapy mediated cell survival and regulation. Molecules. 2020;25:5308
208. Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends in biochemical sciences. 2007;32:37-43
209. Hsieh YJ, Wu CC, Chang CJ, Yu JS. Subcellular localization of Photofrin determines the death phenotype of human epidermoid carcinoma A431 cells triggered by photodynamic therapy: when plasma membranes are the main targets. J Cell Physiol. 2003;194:363-75
210. Al-Mutairi DA, Al-Mutairi DA, Craik JD, Batinic-Haberle I, Benov LT. Induction of oxidative cell damage by photo-treatment with zinc meta N-methylpyridylporphyrin. Free radical research. 2007;41:89-96
211. Mroz P, Yaroslavsky A, Kharkwal GB, Hamblin MR. Cell death pathways in photodynamic therapy of cancer. Cancers. 2011;3:2516-39
212. Maiuri MC, Criollo A, Kroemer G. Crosstalk between apoptosis and autophagy within the Beclin 1 interactome. The EMBO journal. 2010;29:515-6
213. Domagala A, Stachura J, Gabrysiak M, Muchowicz A, Zagozdzon R, Golab J. et al. Inhibition of autophagy sensitizes cancer cells to Photofrin-based photodynamic therapy. BMC cancer. 2018;18:1-10
214. Mohammad-Hadi L, MacRobert AJ, Loizidou M, Yaghini E. Photodynamic therapy in 3D cancer models and the utilisation of nanodelivery systems. Nanoscale. 2018;10:1570-81
215. Broekgaarden M, Weijer R, van Gulik TM, Hamblin MR, Heger M. Tumor cell survival pathways activated by photodynamic therapy: a molecular basis for pharmacological inhibition strategies. Cancer and Metastasis Reviews. 2015;34:643-90
216. Dewaele M, Martinet W, Rubio N, Verfaillie T, de Witte PA, Piette J. et al. Autophagy pathways activated in response to PDT contribute to cell resistance against ROS damage. Journal of cellular and molecular medicine. 2011;15:1402-14
217. Yoon JK, Misra M, Yu SJ, Kim HY, Bhang SH, Song SY. et al. Thermosensitive, stretchable, and piezoelectric substrate for generation of myogenic cell sheet fragments from human mesenchymal stem cells for skeletal muscle regeneration. Advanced Functional Materials. 2017;27:1703853
218. Yin Z, Chen X, Chen JL, Shen WL, Nguyen TMH, Gao L. et al. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31:2163-75
219. Damaraju SM, Shen Y, Elele E, Khusid B, Eshghinejad A, Li J. et al. Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials. 2017;149:51-62
220. Timin AS, Muslimov AR, Zyuzin MV, Peltek OO, Karpov TE, Sergeev IS. et al. Multifunctional scaffolds with improved antimicrobial properties and osteogenicity based on piezoelectric electrospun fibers decorated with bioactive composite microcapsules. ACS applied materials & interfaces. 2018;10:34849-68
221. Zhang Z, Wang Y, Zhang H, Tang Z, Liu W, Lu Y. et al. Hypersonic poration: A new versatile cell poration method to enhance cellular uptake using a piezoelectric nano-electromechanical device. Small. 2017;13:1602962
222. Adadi N, Yadid M, Gal I, Asulin M, Feiner R, Edri R. et al. Electrospun fibrous PVDF-TrFe scaffolds for cardiac tissue engineering, differentiation, and maturation. Advanced Materials Technologies. 2020;5:1900820
223. Wang A, Hu M, Zhou L, Qiang X. Self-powered well-aligned P (VDF-TrFE) piezoelectric nanofiber nanogenerator for modulating an exact electrical stimulation and enhancing the proliferation of preosteoblasts. Nanomaterials. 2019;9:349
224. Kong Y, Liu F, Ma B, Duan J, Yuan W, Sang Y. et al. Wireless localized electrical stimulation generated by an ultrasound-driven piezoelectric discharge regulates proinflammatory macrophage polarization. Advanced Science. 2021;8:2100962
225. Chu ES, Yow CM, Shi M, Ho RJ. Effects of photoactivated 5-aminolevulinic acid hexyl ester on MDR1 over-expressing human uterine sarcoma cells. Toxicology letters. 2008;181:7-12
226. Robey RW, Steadman K, Polgar O, Bates SE. ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy. Cancer biology & therapy. 2005;4:195-202
227. van Hell AJ, Fretz MM, Crommelin DJ, Hennink WE, Mastrobattista E. Peptide nanocarriers for intracellular delivery of photosensitizers. J Control Release. 2010;141:347-53
228. Ackroyd R, Kelty C, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001;74:656-69
229. Wang LY, Shi XY, Yang CS, Huang DM. Versatile RBC-derived vesicles as nanoparticle vector of photosensitizers for photodynamic therapy. Nanoscale. 2013;5:416-21
230. Derycke AS, de Witte PA. Liposomes for photodynamic therapy. Adv Drug Deliv Rev. 2004;56:17-30
231. van Nostrum CF. Polymeric micelles to deliver photosensitizers for photodynamic therapy. Adv Drug Deliv Rev. 2004;56:9-16
232. Kim S, Ohulchanskyy TY, Pudavar HE, Pandey RK, Prasad PN. Organically modified silica nanoparticles co-encapsulating photosensitizing drug and aggregation-enhanced two-photon absorbing fluorescent dye aggregates for two-photon photodynamic therapy. J Am Chem Soc. 2007;129:2669-75
233. Qian J, Gharibi A, He S. Colloidal mesoporous silica nanoparticles with protoporphyrin IX encapsulated for photodynamic therapy. J Biomed Opt. 2009;14:014012
234. Li B, Moriyama EH, Li F, Jarvi MT, Allen C, Wilson BC. Diblock copolymer micelles deliver hydrophobic protoporphyrin IX for photodynamic therapy. Photochem Photobiol. 2007;83:1505-12
235. Chen Q, Deng W, He J, Cheng L, Ren PG, Xu Y. Enhancing Drug Utilization Efficiency via Dish-Structured Triboelectric Nanogenerator. Front Bioeng Biotechnol. 2022;10:950146
236. Yuan M, Li X, Liu J, Zheng Y, Cheng L, Tang N. et al. Fully Integrated Self-Powered Electrical Stimulation Cell Culture Dish for Noncontact High-Efficiency Plasmid Transfection. ACS Applied Materials & Interfaces. 2021;13:54762-9
237. Liu Z, Liang X, Liu H, Wang Z, Jiang T, Cheng Y. et al. High-throughput and self-powered electroporation system for drug delivery assisted by microfoam electrode. ACS nano. 2020;14:15458-67
Author contact
![]() Corresponding author: Prof. Meihua Chen, PhD, Radiation Oncology, Radiation Oncology Key Laboratory of Sichuan Province, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu 610041, China, Phone: +86-28-85420852, email: chenmeihuaorg.cn.
Corresponding author: Prof. Meihua Chen, PhD, Radiation Oncology, Radiation Oncology Key Laboratory of Sichuan Province, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu 610041, China, Phone: +86-28-85420852, email: chenmeihuaorg.cn.

 Global reach, higher impact
Global reach, higher impact