10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(14):5779-5792. doi:10.7150/ijbs.99556 This issue Cite
Review
Micro(nano)plastics: an Emerging Burden for Human Health
Department of Precision Medicine, University of Campania Luigi Vanvitelli, Via L. De Crecchio 7, 80138 Naples, Italy.
Received 2024-6-12; Accepted 2024-9-25; Published 2024-10-21
Abstract
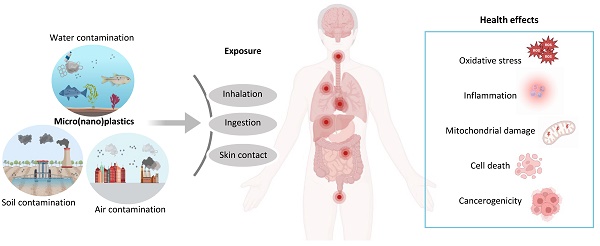
The escalation of plastic pollution represents a global environmental and health problem. Important toxic effects have been attributed to the increasing diffusion of microplastics (MPs) and nanoplastics (NPs) derived from the degradation of plastics. These particles have been ubiquitously observed in the environment, with humans being continuously exposed via ingestion, inhalation and skin contact. Nonetheless, the cellular homeostasis imbalance induced by micro- and nano- plastics (MNPs) in human health has been only recently shown, while most evidence and molecular mechanisms derived from studies in vitro and in vivo models. To date, the majority of available results testified the accumulation of MNPs in the cardiovascular, nervous, reproductive and digestive systems, and recently clear evidence about cardiovascular toxic effects of MNPs has been provided in humans. In this context, this review aims to provide a comprehensive update about the most recent studies reporting the effects of MNPs in different models, focusing on the available evidence in the main areas of study related to human health. Hopefully, this review will contribute to raise awareness about the toxicity and oxidative alteration exerted by MNPs, supporting the elaboration of new strategies to counteract plastic pandemics.
Keywords: microplastics, nanoplastics, oxidative stress, toxic effects, human health threat
Introduction
Plastics represent heterogeneous materials defined as “synthetic or heavily modified natural polymers” in a solid state and insoluble in water at 20°C [1]. Their chemical composition is responsible of their environmental behavior, so as denser materials tend to deposit in the ocean bottom while lighter ones tend to float on the ocean surface [1]. It is possible to distinguish three main phases in plastic life cycle: (i) production, resulting from energy-intensive and catalytic transformations, (ii) use, as single-use plastics, synthetic fibers and construction, and (iii) disposal. To date, plastic sustainable disposal, such as through recycling, is still below 10% globally, resulting in huge amounts of plastic wastes dispersed into the environment, accumulating in landfills, disrupting ecosystems and harming human's health [2].
Microplastics (MPs), fragments with a particle size of ≤ 5 mm, and nanoplastics (NPs), with a particle size of ≤ 1 μm, are hazardous forms of plastics ubiquitously found from the atmosphere to the hydrosphere [3]. The global threat associated to the continuous release of MPs and NPs is promptly emerging, as of the accumulating evidence regarding the toxic effects on environmental and human health [4-6]. Indeed, the occurrence of micro- and nanoplastics (MNPs) has been widely demonstrated in different ecosystems, representing an important source of harm due to their high biological incompatibility [7]. However, given their ubiquitous presence, humans continuously come into contact with MNPs through inhalation, skin contact and ingestion [8], thus accumulating in blood, lungs, gastrointestinal and genitourinary tract, possibly causing multiple toxic effects [9]. Moreover, also everyday kitchen activities, such as using plastic utensils and non-stick cookware, are a significant source of MPs ingestion, highlighting a pervasive daily source of exposure [10]. Several studies assessed the toxic effects exerted by these small particles in vitro and in vivo models. MNPs exposure determined reactive oxygen species (ROS) formation as their main cellular effect, resulting in cell growth suppression, mitochondrial dysfunction and endoplasmic reticulum stress [11,12]. The alterations of the redox state induced by MNPs has been attributed to extracellular and intracellular processes. Particularly, different factors, namely mechanical forces, temperature, light, chemicals and other variables can alter MPs surface molecule's structure leading to the formation of free radicals [13]. Indeed, after MNPs are taken up by cells these particles are transported to lysosomes and then to mitochondria where they can alter the membrane potential, leading to increased ROS formation [13]. Moreover, MNPs surface could adsorb harmful ligands as well as live microbial pathogens, functioning as a delivery system and amplifying their toxicity [14]. Recent reports demonstrated a particle size-dependent absorption of endocrine disruptors pollutants, altering hormone receptors signaling and resulting in endocrine dysfunctions [6]. Different studies are reporting toxic effects of MNPs at several levels, supporting their biological toxicity in different body districts. Interestingly, metagenomic analysis on gut microbiota evidenced that polystyrene MPs with a diameter ranging between 0.05-0.1 µm can affect fungal and bacterial composition and interaction, altering microbial metabolic pathways and inducing antibiotic resistance phenomena [15]. In vitro human placental cells can internalize MNPs, possibly affecting pregnancy progression [16]. MNPs exerted toxic effect on mice intestinal cells causing ROS-mediated apoptotic cell death and are responsible of cardiovascular performances impairment, hemolysis and increased thrombotic risk at macro- and micro- vascular level [17,18]. NPs can be endocytated and induce autophagy activation and autophagosome formation, particularly in neurons, which are more susceptible to NPs exposure related stress and degeneration [19]. Furthermore, human exposure to MNPs has been also identified as a cancer risk factors, exerting genotoxic and cytotoxic effects, according to the Agency for Toxic Substances and Disease Registry (ATSDR) reports [20]. Even though more and more studies are evidencing toxic effects related to MNPs in various organs and tissues, most evidence available focuses on nervous, gastrointestinal, cardiovascular and reproductive system. Nonetheless, these systems have also been preliminarily investigated in human subjects, above all in the cardiovascular system. In this scenario, it is compelling to produce and spread evidence about the interplay between plastics consumption and health and environmental risk, as the base to restore the human right to live in a healthy environment, recognized by the United Nations [21]. This review aims to provide the reader with the most recent and significant evidence about the effects of MPs and NPs in the nervous, reproductive, cardiovascular and digestive system, also highlighting the presence of studies in human models and to raise awareness about the emerging health and safety hazards to be faced by humanity.
MNPs: what we know
Evidence indicate MNPs as widespread toxic pollutants, with environmental long-standing permanence. MNPs can present with different aspects, such as beads, fibers and films, and have different sizes and surfaces, determining their toxicity [22]. Most of the available studies focused on the effects of MNPs on the nervous, reproductive, cardiovascular and digestive systems. As it follows, some of the most recent and relevant in vitro and in vivo evidence on the toxic effects of MNPs will be discussed.
Effects of MNPs on the nervous system
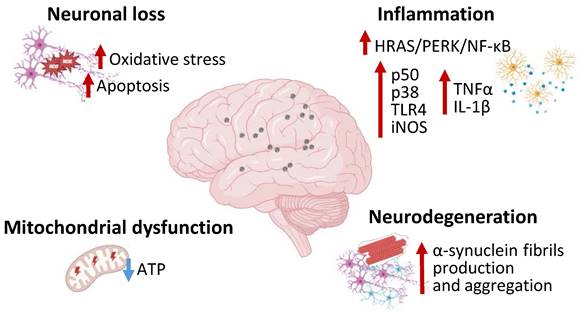
Different studies investigated the effects of environmentally relevant MNPs on central nervous system, reporting their ability in causing excessive ROS and apoptosis in human nervous cell lines, with stronger effects detected in cortical neurons models [23,24] (Figure 1). Recently, evidence on the bioaccumulation of MNPs in the central nervous system has been provided via autoptic investigations on human brains and assessed via Pyrolysis Gas Chromatography-Mass Spectrometry [25]. However, there is still no clear evidence reporting the consequences of MNPs accumulation on the human central nervous system. A pivotal role of MNPs-related neuronal cells degeneration has been attributed to oxidative stress. Specifically, MNPs exposure led to cortical neurons oxidative cell death and microbial pathogens can produce biofilms in MPs surface and exacerbate the neurotoxic effect [24].
Polystyrene MPs can accumulate in the brain and lead to anxiety-like behavior related to microglial activation and inflammatory response. In BV2 microglial cells polystyrene MPs treatment determined the activation of the HRAS/protein kinase R-like endoplasmic reticulum kinase (PERK)/nuclear factor (NF)-κB mediated inflammation and tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β expression [26]. Microglial N9 cells treated with NPs demonstrated p50, p38 and toll-like receptor (TLR)4 upregulation along with a notable increase in inducible nitric oxide synthase (iNOS) mRNA levels, evidencing the pro-inflammatory property of NPs [27] (Figure 1). Polystyrene NPs can induce ferroptosis and increased oxidative stress, related to altered glutathione metabolism, in brain microvascular cells, thus impairing blood brain barrier permeability and reducing zonula occludens 1 (ZO-1) protein expression [28]. Moreover, polystyrene NPs determine downregulation of myelin basic protein (MBP) and myelin oligodendrocyte glycoprotein (MOG), thus damaging myelin structure, reducing oligodendrocytes number and leading to motor defects in vivo [29]. Mice treated with polystyrene NPs exhibited impaired excitatory neurons energy metabolism, demonstrated by decreased adenosine triphosphate (ATP) cellular content and downregulated ATP-associated genes expression, as well as astrocytes and microglia inflammatory response, ultimately leading to a Parkinson's Disease-like phenotype [30]. In this context, anionic NPs have been observed to promote α-synuclein protein fibrils formation and propagation via direct interaction with specific α-synuclein domains [31]. NPs can be endocytated in a clathrine-dependent mode by neurons, causing lysosomal function impairment, reducing α-synuclein clearance [31]. Furtherly, Jeong et al. described the capacity of NPs to exacerbate the extrapyramidal symptoms in C. Elegans and human cell models, testified by dopaminergic pauperization, locomotor dysfunction, and buildup of α-Synuclein aggregates [32] (Figure 1). In SH-SY5Y neuroblastoma cells NPs exposure resulted in cellular accumulation, with significant mitochondrial damages, altered morphology, reduced membrane potential and ATP production. Further investigations indicated that NPs mitochondrial disruption was associated to complex I interference and excessive mitophagy activation via AMPK/ULK1 pathway induction, thus leading to cell death [33]. Overall, MNPs displayed multiple toxic effects on the nervous system, being correlated to impaired energy metabolism and neuronal dysfunction and degradation. The effects of MNPs on α-synuclein metabolism shed a new light on the correlation between the environment and neurodegenerative diseases, such as Parkinson's disease and other forms of dementia, suggesting their involvement in chronic degenerative diseases. Most studies concerning the effects of plastics on the nervous system have focused on the role of NPs more than MPs, which would suggest a major susceptibility of neurons and other neural cells to NPs. It could be hypothesized that in vivo systems NPs can penetrate brain barrier more efficiently than MPs, in virtue of their smaller size, and exert toxic effects. In addition, NPs, compared to MPs, could enter neuronal cells with peculiar, efficient but still unknown mechanisms and alter cellular specific signaling pathways [19]. The plausible mechanism of action in the nervous system could be related to excessive ROS production induced by MNPs in neurons and other neural cells determining endoplasmic reticulum stress, mitochondrial damages, activation of NF-κB inflammatory pathway, damage in myelin structure, leading to ferroptosis or mitophagy cell death process. Nonetheless, further studies are required to clarify the mechanisms of MNPs toxicity on the nervous system.
MNPs effects on the nervous system. Image representing the molecular pathways including inflammation, neurodegeneration, mitochondrial dysfunction, neuronal loss, activated in the nervous system during MNPs exposure. MNPs causes neural HRAS/PERK/NF-kB pathway activation and increased p50, p38, TLR4, iNOS, TNF-α, IL-1β expression. MNPs are also associated to neurodegeneration with increased α-synuclein production and aggregation along with mitochondrial dysfunction and neuronal loss. PERK—Protein kinase RNA-like endoplasmic reticulum kinase; NF-κB—Nuclear factor κB; TLR4—Toll-like receptor 4; iNOS—inducible nitric oxide synthase; TNF-α—tumor necrosis factor-α; IL-1 β—interleukin-1β.
MNPs on the reproductive system
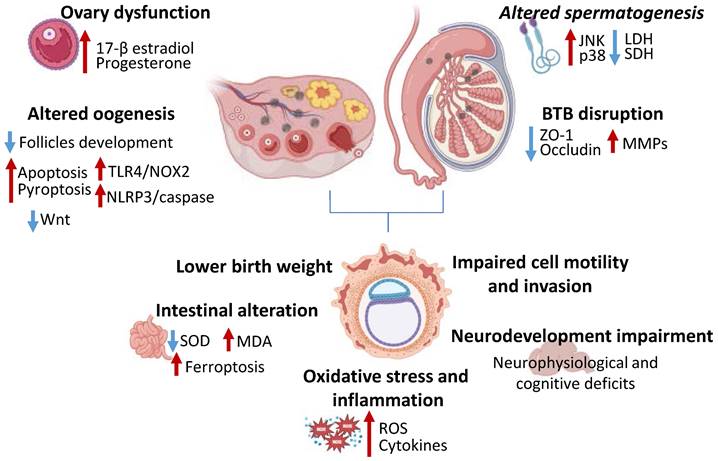
Among the toxic effects exerted by MNPs, the impact on reproduction, including male and female reproductive system as well as embryonal development, is alarming [34,35]. Montano et al. detected MPs in the ovarian follicular fluid of women undergoing assisted reproductive treatment. Their study revealed a significant correlation between MPs concentration and levels of follicle-stimulating hormone (FSH), as well as weaker associations with body mass index (BMI), age, and estradiol [36]. Moreover, a recent study conducted on 22 female patients evidenced the accumulation of MPs, including polyurethane, polypropylene, polystyrene and polyethylene, in human endometrium. Even though this result suggests the existence of toxicity on female's reproductive system, the effects are still to be assessed [37]. In addition, MNPs have also been identified in the vagina, which has been characterized with a thinner and more permeable stratum corneum, making it more susceptible to their effects. For instance, Pontecorvi et al. utilized a human vaginal keratinocyte cell model to reveal that internalized MNPs impaired the expression of junctional and adherence proteins, disturbed the organization of the actin cortex and influenced genes involved in oxidative stress signaling pathways and miRNAs crucial for vaginal epithelial barrier function [38]. In males, the investigation of 6 testis and 30 semen human specimens assessed the accumulation of plastic particles in the reproductive system [39]. A similar study evaluated the concentration of MPs in human testis, with polyethylene being the most abundant, and the presence of an inverse correlation between testis weight and MPs concentration [40]. The accumulation of MPs has also been reported in prostate samples of 6 out of 12 patients examined [41]. Additionally, the study carried out by Chen et al. revealed high MNPs concentration and significant impairment of human semen function. Indeed, polystyrene NPs were capable of penetrating and damaging sperm cells [42]. Finally, a recent study assessed the accumulation of MPs in human placental samples, with polyethylene being the most prevalent [43]. Indeed, in the study carried out by Amereh et al., increased exposure to MPs was associated to lower birth weight, reduced length, smaller head circumference, in intrauterine growth restriction (IUGR) pregnancies compared to those classified normal pregnancies [44]. Current evidence demonstrated MNPs accumulation via the blood-placental barrier during pregnancy and trough the breast milk during nursing in different organs, leading to a dysregulated metabolism and impaired immune and reproductive function [45,46]. Given the bioaccumulation of MNPs in human reproductive system, several studies are being conducted to evaluate their toxicity in vitro and in vivo models.
Treatment with polystyrene MPs in HTR-8/sVneo human placenta cell lines led to increased ROS and inflammatory cytokines production and reduced cell survival [47] (Figure 2). Moreover, MPs treatment impaired cell motility and invasion ability, negatively affecting the placentation process [47]. Polystyrene NPs exposure altered cell membrane integrity and induced autophagy initiation and autophagosome production, along with mitochondrial damage, in human umbilical vein endothelial cells (HUVECs) [48,49]. It has been observed that maternal administration of polystyrene NPs in mice impaired progeny nervous system development and led to neurophysiological and cognitive deficits [50]. Tang et al. demonstrated that mice exposed to polystyrene NPs during gestation displayed lower birth weight and higher adult weight compared to non-NPs exposed mice [51]. Moreover, it has been shown that the intake of MPs during the prenatal and early post-natal period can provoke neurodevelopmental disorders in mice offspring caused by a diminished dopamine transporter protein in the brain, or dysregulated gene expression [52]. Relevant findings indicate that NPs accumulation in the blood-brain barrier is significantly higher during the early embryonic period due to the underdeveloped fetal blood-brain barrier in the early stages of life, which permits NPs to penetrate and potentially induce neurological dysfunction [45,46]. An in vivo study on maternal rats revealed neurological developmental disorders resulting from the exposure of fetal brain tissue to NPs during early developmental stages [45]. Another study discovered that NPs transferred to fetuses during gestation affect the function and composition of neural cells and alter the brain histology of neural stem cells cultured from offspring [53]. Moreover, NPs exposure was associated to small intestine histological alterations, which were related to impairment of superoxide dismutase (SOD) and malondialdehyde (MDA) levels and activation of ferroptotic pathway [47] (Figure 2). Also, recent research indicated that offspring's hearts are more susceptible to damage from MNPs compared to the fully developed adult hearts [54]. Indeed, a significant reduction in mitochondrial membrane potential and an altered cellular metabolism, which coincided with a marked decrease in electrophysiological activity during the early stages of contractility has been observed in in vitro experiments conducted on neonatal rat ventricular cardiomyocytes [46,55].
Polystyrene MPs exposure in male mice increased testicular oxidative stress and induced the activation of c-Jun NH2-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) pathways, which resulted in decreased sperm number and motility, as well as suppressed metabolism-related enzymes lactate dehydrogenase (LDH) and succinate dehydrogenase (SDH) activity [56]. Moreover, a synergic effect of polystyrene NPs and lipopolysaccharide (LPS) has been detected, demonstrated by reduced sperm count, lower testosterone levels and steroidogenic acute regulatory (StAR) expression levels, along with severe inflammatory response and oxidative stress [57]. Ma et al. assessed the toxic effect of dibutyl phthalate-charged NPs on the blood-testis barrier in mice and in vitro. In this study, NPs induced spermatogenesis impairment and disrupted blood-testis barrier, testified by decreased sperm quality, reduced ZO-1 and occludin and increased matrix metalloproteinases (MMPs) expression [58] (Figure 2). Furthermore, in toxicological studies on rodents and cell cultures MNPs have been shown to produce considerable damages, including abnormal sperm structure and reduced quality [59]. In addition, mice exposed to MPs and NPs demonstrated an impairment of sperm motility, linearity and velocity, especially when exposed to 25-30 nm and 1-5 µm particles [60].
On the other hand, studies demonstrated that exposure to polystyrene MPs can lead to ovary dysfunction with reduced follicles development and embryos number and deleterious impact on female fertility and reproduction [61,62]. For instance, recent studies elucidated the harmful effect of MPs and NPs exposure on the ovarian tissue which can lead to a lower number of developing follicles, to a reduced number and diameter of small uterine arteries provoking a deleterious effect on female fertility and ovarian reserve [63,64,65]. MPs induced granulosa cells apoptotic and pyroptotic cell death, activating NOD-like receptor protein 3 (NLRP3)/caspase signaling and impairing Wnt pathway, as well as inducing TLR4/ NADPH Oxidase (NOX)2 pathway activation and uterine fibrosis [65] (Figure 2). Indeed, this aspect has also been confirmed in different works where the increased ROS levels is related to the reduction of antioxidant enzymes following MNPs exposure [63,66]. The effects on NPs on the reproductive system have also been investigated on swine granulosa cells, reporting increased cell proliferation with disrupted 17-β estradiol and progesterone secretion and increased oxidative stress, deeply altering ovary follicles development and function [62] (Figure 2).
Effects of MNPs on reproduction. Image showing how MNPs impair ovary-related sexual hormones secretion, reduce follicles development, increase TLR4/NOX2 and NLRP3/caspase pathways, thus triggering apoptosis and pyroptosis and reduce Wnt signaling. MNPs alter spermatogenesis via JNK and p38, and decreasing LDH and SDH activity, moreover, disrupt blood-testis barrier, reducing ZO-1 and occluding expression and increasing MMPs. MNPs affect embryonal development being associated to lower birth weight, impaired cell motility and invasion, altering neural and intestinal development and increasing oxidative stress and inflammation. TLR4—Toll-like receptor 4; NOX2—NADPH Oxidase 2; NLRP3— NOD-like receptor protein 3; JNK—c-Jun NH2-terminal kinase; LDH—Lactate dehydrogenase; SDH—Succinate dehydrogenase; ZO-1—zonula occludens 1; MMPs—matrix metalloproteinases; SOD—Superoxide dismutase; MDA—Malondialdehyde; ROS—reactive oxygen species.
To this end, evidence on the effects of MNPs on the reproductive system appear to be extremely polymorphic, considering the possible effects on future generations occurring since the moment of fecundation. Different studies already indicate the accumulation of MPs and NPs in both human male and human female genital tracts, as well as in fetal tissues, and several studies in mice also evidenced MNPs involvement in developmental alterations, including central nervous system development.
Undoubtedly, the ability of MNPs to induce oxidative stress in the reproductive system correlates at molecular level with apoptotic and pyroptotic cell death, via NLRP3/caspase signaling, with the activation of JNK/MAPK pathways, along with mitochondrial damage, altered cell membrane integrity and autophagy initiation and autophagosome production. These cellular events alter signaling pathways pivotal to an adequate function of these tissues could be correlated to hormonal dysfunction and increased germinal tumor development. Possibly, further studies and trials will allow to assess these effects in human models and possibly find countermeasures.
Effects of MNPs on cardiovascular system
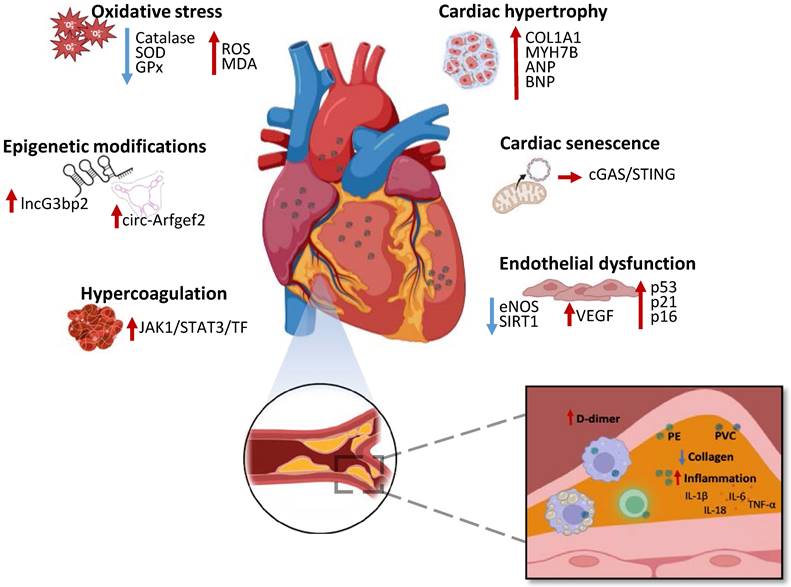
The effects of MNPs have been recently investigated and demonstrated in the cardiovascular system, being associated to different alterations widening from cardiac dysfunction to atherosclerosis [22]. In this scenario, Marfella et al. is a landmark study linking MNPs to serious human health problems (NCT05900947) [67]. In this observational study, 304 patients undergoing carotid endarterectomy were enrolled, with 257 patients who completed the study follow-up. The excised atheromas were examined and the level of polyethylene and polyvinyl chloride MNPs was quantified. The results of enzyme-linked immunosorbent assays and immunohistochemistry evidenced increased IL-18, IL-1β, IL-6 and TNF-α, decreased collagen and increased cluster of differentiation (CD)3 and CD68 plaque content (Figure 3). During the follow-up, the number of myocardial infarctions, stroke and any-cause death was compared in patients who displayed MNPs-associated plaques versus MNPs clean plaques patients. Notably, the results of the study evidenced higher mortality and morbidity rates in patients whose atheromas were infiltrated with MNPs (NCT05900947) [67] (Figure 3). Significantly, a successive human-based study, analyzing thrombi collected by 30 patients undergoing stroke, myocardial infarction and deep vein thrombosis related surgery, provided sustaining evidence detecting MNPs, above all polyethylene, in 80% of samples, which were associated to higher patients D-dimer levels and greater disease severity [68]. Moreover, Liu et al. evaluated the concentration of MPs in coronary and carotid arteries plaques and in the non-atherosclerotic aorta. The concentration of MPs was reported to be significantly higher in the atheroma associated arteries specimen compared to non-pathologic aorta, supporting the role of plastic particles in cardiovascular atherosclerotic [69].
Nonetheless, the effects of MNPs on human cardiovascular system still need to be comprehensively explained, with numerous studies in vitro and in vivo suggesting an even vaster toxicity. Mice- and human-derived cardiac organoids exposed to different concentration of MPs demonstrated increased oxidative stress, inflammation, apoptotic cell death and collagen accumulation, which resulted associated to hypertrophic-related genes expression, such as myosin, heavy chain 7B (MYH7B), atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), Collagen, type I, alpha 1 (COL1A1) [70] (Figure 3). In mice models, administration of MPs determined upregulation of circular RNA circ-Arfgef2 and long non-coding RNA lncG3bp2 N6-methyladenosine (m6A) methylation modifications and expression, which has been suggested as a potential mechanism of MPs cardiotoxicity [71] (Figure 3). Polyethylene terephthalate MPs administration to mice led to capillary congestion, myocardial fiber breakage and fibrosis, associated to cardiomyocytes apoptotic cell death [72]. Contextually, in vitro myocardial cells treated with MPs demonstrated ROS accumulation, elevated MDA level with decreased catalase, SOD and glutathione peroxidase (GPx) decreased activity [72] (Figure 3).
NPs long term exposure on cardiac cells in vitro and in vivo models determined cell senescence and dysfunction via mitochondrial destabilization with leakage of mitochondrial DNA (mtDNA) in the cytoplasm and activation of cyclic GMP-AMP synthase (cGAS)/Stimulator of interferon genes (STING) pathway [73] (Figure 3). Endothelial cells treated with NPs underwent premature senescence, demonstrated by the observation of senescence markers expression, such as p53, p21 and p16. Particularly, NPs related senescence has been associated to downregulated endothelial nitric oxide synthase (eNOS), impairing normal vasal motility, as well as increased NOXs and suppressed sirtuin (SIRT)1 expression [74] (Figure 3). Aortic endothelial cells treated with NPs displayed disrupted redox state associated to increased metabolic activity and Vascular Endothelial Growth Factor (VEGF) expression, negatively affecting vasal homeostasis [75] (Figure 3). Wang et al. demonstrated that polystyrene NPs mice exposure results in vascular endothelial damage with the activation of janus kinase 1 (JAK1)/Signal transducer and activator of transcription (STAT)3/tissue factor (TF) signaling and dysfunctional prothrombotic state [76] (Figure 3). Mice and RAW264.7 macrophages exposed to polystyrene NPs exhibited lipid accumulation, altered redox status and inflammation, associated to atherosclerotic plaques formation [77]. In conclusion, MNPs can affect multiple pathways related to cardiac fibrosis and dysfunction, endothelial and coagulative impairment, along with increased atheroma formation, suggesting their molecular role in determining negative cardiovascular events. The recent landmark study published by Marfella et al. contributed to confirm the existence of toxic effects exerted by MNPs in humans, raising awareness all over the world. In addition, MNPs entering the cardiovascular system, could be accumulating also in cardiomyocytes and platelets, implying the presence of other pathways determining negative cardiovascular outcomes related to MNPs in humans. Nonetheless, the molecular mechanisms responsible of these effects are still to be defined with novel studies in vivo and in vitro. Possibly, upon accumulation in cardiac tissue, MNPs trigger oxidative stress trough ROS production, activating the NLRP3 inflammasome and JAK1/STAT3 pathways, which promote inflammation, endothelial dysfunction, thrombosis and atherogenesis, ultimately leading to increased risk of myocardial infarction and stroke.
Effects of MNPs on the cardiovascular system and representation of human atherosclerotic plaque with MNPs. MNPs determine oxidative stress with increase of ROS and MDA levels along with decrease of catalase, SOD and GPx activity, cardiac hypertrophy with COL1A1, MYH7B, ANP and BNP upregulation, cardiac senescence with mitochondrial DNA leakage and activation of cGAS/STING pathway, alteration of cardiac epigenetics, endothelial dysfunction associated to eNOS and SIRT1 downregulation and VEGF, p53, p21 and p16 upregulation and an hypercoagulative state with JAK1/STAT3/TF pathway activation. Atheromas from patients with high levels of PE and PVC show decreased collagen, increased proinflammatory cytokines IL-6, IL-1β, IL-18, TNF-α, along with increased inflammatory cells and increased D-dimer plasmatic levels. ROS—Reactive oxygen species; MDA—Malondialdehyde; SOD—Superoxide dismutase; GPx—Glutathione peroxidase; COL1A1—Collagen type I alpha 1; MYH7B—Myosin heavy chain 7B; ANP—atrial natriuretic peptide; BNP—Brain natriuretic peptide; cGAS—Cyclic GMP-AMP synthase; STING—Stimulator of interferon genes; eNOS—Endothelial nitric oxide synthase; SIRT1—Sirtuin 1; VEGF—Vascular Endothelial Growth Factor; JAK1—Janus kinase 1; STAT3—Signal transducer and activator of transcription 3; TF—Tissue factor; PE—Polyethylene; PVC—Polyvinyl chloride; IL-1 β—interleukin-1β; IL-6— interleukin-6; IL-18— interleukin-18; TNF-α—tumor necrosis factor-α.
Effects of MNPs on the digestive system and gut microbiota
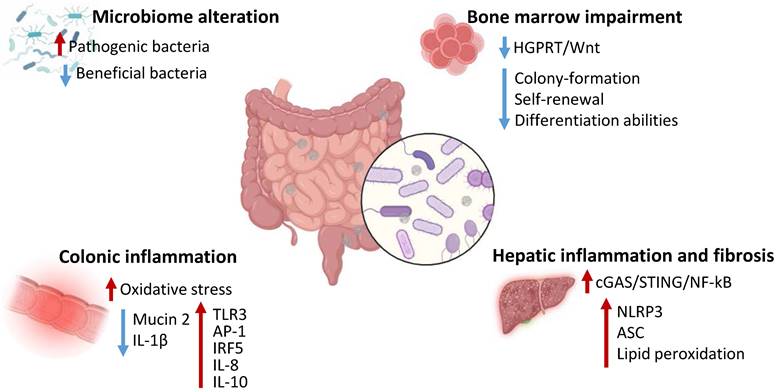
The effects of MNPs exposure on human digestive system have not been clarified yet, with no trials or clear evidence indicating specific toxic mechanisms in human models. Nonetheless, accumulating studies have clearly reported the ability of MNPs to influence and modify gut microbiota composition with effects at a local, as well as systemic, level [19]. For instance, a recent study indicated that humans highly exposed to MPs exhibited abundance of pathogenic Klebsiella and Helicobacter and reduced beneficial Bacteroides in the nose compared to lowly exposed subjects. Furtherly, humans overexposed to MPs displayed disease associated Bifidobacterium, Streptococcus, and Sphingomonas abundance along with reduced healthy Ruminococcus Torques group, Dorea, Fusobacterium, and Coprococcus [78]. The effects of MNPs on the gut microbiota and digestive system health have been investigated in mice models. Mice exposed to polyethylene MPs displayed alterations in gut microbial number, species and diversity. Particularly, MPs exposure determined an increase in Staphylococcus and reduction of Parabacteroides abundance and was correlated to increased IL-1α serum levels, with intestinal inflammatory aspects and higher TLR3, activator protein (AP)-1 and interferon regulatory factor (IRF)5 expression [79]. Furtherly, Jing et al. observed an association between abnormal gut microbiota and bone marrow alterations, with decreased colony-formation, self-renewal and differentiation abilities and increased lymphocyte abundance in mice treated with MPs and NPs [80]. Moreover, long-term MPs administration or fecal transplantation of microbiota from MPs-treated mice negatively affected bone marrow health and was associated to reduced Rikenellaceae and hypoxanthine intestinal content and subsequent decreased survival and proliferation of hematopoietic stem cells via inhibition of hypoxanthine-guanine phosphoribosyltransferase (HGPRT)/Wnt signaling [81]. Interestingly, human patient who underwent hematopoietic stem cells transplantation exhibited a negative correlation between MPs levels and transplanted patients' survival time, as well as a positive correlation between patient survival and Rikenellaceae and hypoxanthine intestinal content [81]. Zhang et al. indicated that the hepatic damage correlated to MPs could be attributed to microbial alterations. Mice treated with MPs exhibited systemic and hepatic inflammation and fibrosis along with intestinal barrier disruption, colonic inflammation accompanied by decrease in probiotics Akkermansia, Mucispirillum, and Faecalibaculum and increased pathogenic Tuzzerella. Particularly, the elimination of gut microbiota or the administration of an antibiotic cocktail reduced MPs-associated systemic inflammation as well as hepatic inflammation [82]. Other studies have also evidenced direct toxic effects of MNPs on the gastrointestinal system independently from gut microbiota. Mice models exposed to polypropylene MPs for 4 weeks demonstrated damaged and dysfunctional hepatic structure [83]. Furtherly, the hepatotoxic effect of MPs has been related to activation of pyroptotic pathway, activation of NLRP3 inflammasomes and apoptosis associated speck-like protein (ASC) and increased lipid peroxidation, accompanied by ferroptosis trigger [83]. MPs could determine hepatocytes nuclear and mitochondrial DNA damage with subsequent activation of cyclic cGAS/STING/NF-κB pathway and production of pro-inflammatory cytokines, finally leading to liver dysfunction and fibrosis [84]. Mice and intestinal organoids exposed to benzo [a] pyrene-loaded polystyrene MPs displayed increased Notch signaling activation, related to the increased ROS production. The overactivation of Notch in these models resulted in disrupted colonic mucosal barrier and tight junctions' injury, with increased inflammation, autophagy and bacterial translocation [85]. Mice exposed for 30 days to MPs exhibited decreased colonic mucin 2 production and IL-1β along with increased IL-8 and IL-10 levels. These results were accompanied by decreased Firmicutes and increased Bacteroides number as well as enhanced microbiota amino acids metabolism [86]. NPs exhibited higher effects compared to MPs in inducing gut macrophage activation in mice, triggering IL-1 dependent inflammation and affecting the gut-brain axis, resulting in microglial activation and T Helper 17 differentiation thus correlating with cognitive and short-term memory impairment [87]. Overall, several studies in vivo models indicated the ability of MPs and NPs to alter digestive function, with greater effects on the gut microbial composition. The alterations of the microbiota have been correlated to both intestinal and extraintestinal dysfunctions, including hematopoiesis and neural function, possibly indicating MNPs-associated microbiota effects as main players in mediating MNPs effects. Overall, MNPs can cause damage to the digestive system by activating NF-kB and TLR molecular pathways, inducing inflammation, oxidative stress and compromising the intestinal barrier by reducing tight junction proteins, promoting dysbiosis and chronic diseases. Nonetheless, studies are required to confirm and unravel the effects of MNPs on humans.
Effects of micro- and nanoplastics on human cells and tissues of respiratory, endocrine and immune systems.
| System | Sample/Model | Potential MNPs Effects | Reference |
|---|---|---|---|
| Respiratory System | Human lung epithelial cells BEAS-2B | Decrease α1-antitrypsin levels | 90,91,93 |
| Induce IL-6 and IL-8 expression | |||
| Human alveolar type II epithelial A549 and BEAS-2B | Induce senescence | 92,93 | |
| Increase ROS generation and pro-inflammatory state | |||
| A549 | Induce apoptosis increasing BAX, caspase-3, caspase-8, caspase-9 and cytochrome c | 93 | |
| Induce cell cycle S phase arrest | |||
| BEAS-2B and human pulmonary alveolar epithelial cells HPAEpiC | Decrease cell viability and induce redox imbalance | 94 | |
| Increase levels of MMP9 and surfactant protein A | |||
| Immunitary System | Human monocytes | Pro-inflammatory cytokines release | 96 |
| Hematopoietic stem cells | Decrease survival and proliferation | 81 | |
| Human Peripheral blood mononuclear cell PBMCs | Induce IL-6 and TNF-α production | 97 | |
| Renal system | Human kidney HK-2 cells | Increase ROS levels | 99 |
| Increase Bad and decrease Bcl-2 protein levels | |||
| Increase ER stress-and autophagy | |||
| Increase MAPK and AKT/mTOR signaling pathways | |||
| HK-2 cells | Increase extracellular vesicles | 100 | |
| Embryonic kidney cells HEK 293 | Decrease cell viability | 101 | |
| Increase oxidative stress | |||
| Decrease ATP production | |||
| Increase TNF, IL-6, caspase 3 and caspase 9 gene expression |
Effects of MNPs on the digestive system and gut microbiota. MNPs determine alterations of gut microbiota, increasing pathogenic bacteria and decreasing beneficial bacterial content, impair bone marrow function inhibiting HGPRT/Wnt pathway and decreasing hematopoietic stem cells function, induce colonic inflammation increasing oxidative stress and inflammatory proteins expression and reducing mucin 2 and IL-1β levels, trigger hepatic inflammation and fibrosis via activation of cGAS/STING/NF-kB pathway and upregulating NLRP3, ASC and lipid peroxidation. HGPRT - hypoxanthine-guanine phosphoribosyltransferase; IL - Interleukin; TLR - Toll-like receptor; AP-1 - activator protein; IRF - interferon regulatory factor; cGAS - cyclic GMP-AMP synthase; STING - Stimulator of interferon genes; NF - nuclear factor; NLRP - NOD-like receptor protein 3; ASC - apoptosis associated speck-like protein.
Discussion
To date, plastic represents a fundamental part of human life, whose social importance strongly contrasts with its enormous negative environmental and health impact. Increasing evidence is assessing the redox imbalance and toxic effects related to plastics overproduction and over disposal, particularly associated to the continuous release and accumulation on MNPs. Clinical results from Marfella et al. are the first clear evidence on the effects of MNPs in human health, possibly setting the basis for the assessment of MNPs exposure as an independent cardiovascular risk factor [56]. Nonetheless, evidences, in vitro and in vivo, are suggesting that the consequences of MNPs in human health could be even more vast, ranging from chronic nervous diseases to gastrointestinal function alterations, as well as fertility and fetus development impairment [88]. In recent years, increasing studies are focusing on the accumulation of MNPs other districts also evaluating their effects in vitro and in vivo. For instance, MPs have been identified via autoptic studies in human lungs, even though their effects in humans have not been assessed [89]. In mice models, MPs inhalation has been associated to nasal and lung microbial dysbiosis and experiments in normal human lung epithelial BEAS-2B cells evidenced that MPs can cause cytotoxic and inflammatory effects correlated to reactive oxygen species production and decreased α1-antitrypsin levels, increasing the risk for chronic obstructive pulmonary disease [90,91]. MPs can induce senescence of human lung derived A549 and BEAS-2B epithelial cells increasing ROS levels, as well as inducing pro-inflammatory IL-6 and IL-8 cytokines production [91,92,93]. In A549 cells, MPs determine the activation of the apoptotic mechanism via Bax, caspase 3, 8 and 9 and cytochrome C upregulation, along with cell cycle S phase arrest [93]. Yang et al. indicated that MPs can cause redox imbalance and increase levels of MMP9 and surfactant protein A expression in BEAS-2b and human pulmonary alveolar epithelial cells HPAEpiC cells [94]. In addition, polystyrene MPs were reported to negatively interact with porcine lung surfactant structure altering its phase behavior, surface tension, and membrane structure, as well as increasing ROS production in lung fluids [95]. Irregular polyvinyl chloride NPs in vitro treatment of primary human monocytes and monocyte-derived dendritic cells induced high pro-inflammatory cytokines release [96]. In human peripheral blood mononuclear cells MPs exposure was able to increase IL-6 and TNF-α production [97]. Also, MPs are able to reduce S100A8 expression, thus inducing spleen damage and immune suppression [98]. Additionally, studies reported MNPs toxic effects in renal proximal tubular epithelial HK-2. Specifically, MNPs exposure causes oxidative stress, induce apoptosis upregulating Bad and reducing Bcl-2 levels, endoplasmic reticulum stress, autophagy, activation of MAPK and mammalian target of rapamycin (mTOR) signaling and increased extracellular vesicles release [99,100]. Moreover, in renal HEK293 cells NPs treatment increased ROS, MDA and LDH levels, while decreasing ATP production and upregulating inflammatory cytokines TNF and IL-6, along with caspase 3 and 9 gene expression [101]. Comprehensive, MNPs can cause damage in various systems by triggering common mechanisms such as inflammation, oxidative stress and disruption of cellular barriers. They activate immune responses and impair organs homeostasis, leading to chronic inflammation, cellular dysfunction and microbiome disturbance, contributing to long-term health effects.
Even though different techniques have been developed to detect MNPs in various human tissues, standardized protocols for quantifying MPs in biological samples are still lacking [102,103]. There is a substantial gap in epidemiological studies concerning the health effects of MNPs on humans, with most existing research relying on animal studies or in vitro experiments. Comprehensive research is crucial to fully understand the health effects and exposure routes of MNPs in humans [103,104]. Current methodologies for sampling, isolating, detecting, quantifying, and characterizing MNPs are inadequate, suffering from poor standardization and quality control. Despite this, evidence suggests that MPs are prevalent in food, drinking water, and air. Due to methodological shortcomings and focus on larger particles, existing evaluations probably underestimate human exposure and frequently ignore smaller particles [105,106]. Indeed, an important question to be reported is the hypothetic different impact of MPs and NPs in human subjects. Human exposure to NPs is still considered a “scientific challenge”, due to the lack of adequate methods and materials available, as well as a standardization across the analytical procedures utilized [107]. Nonetheless, several studies evidenced the peculiar and higher harm of NPs in other biological systems. NPs can penetrate biological barriers more easily due to their small size, thus overpassing cell membranes and the blood-brain barrier [108,109]. NPs have a higher surface area to volume ratio, which increases their chemical reactivity and potential for generating ROS, leading to oxidative stress and cellular damage [110]. In addition, they can interact with cellular components, leading to genotoxicity and disruption of cellular processes [111]. Comparative studies suggest that NPs might pose a greater risk due to their ability to cause systemic effects, including impacting the gut-brain axis and inducing inflammation [87,112]. Hopefully, the development of advanced methods to identify and characterize NPs and their effects in human subjects will be soon provided. In the next years, new studies will be able to unravel the uncertainties related to human-plastic relationship, hopefully laying the foundations for a more conscious administration of plastics.
In this scenario, many efforts are focusing on finding adequate means to manage plastics in the best and toxic-less way. In a recent work, three ways to counteract “plastics pandemic” have been investigated, with their potentialities and limitations. Particularly, mechanical recycling, enzymes-based recycling and the production of bioplastics [113]. Recycling represents a fundamental part of a circular economy in which plastics would be environmentally affordable [113]. Mechanical recycling would be useful to reduce plastics overproduction, still this process could also lead to a down cycling, rendering plastics unrecyclable [113]. The development of enzyme-based recycling processes surely presents high potentialities, but is graved by high developmental costs and requires more energy and gases compared to mechanical recycling, along with a high selectivity the requires specific studies and production processes [113,114]. As for bioplastics, many studies are focusing on the production of various bio-based types of polymers, such as cellulose or lignin reinforced plastics as well as bacterially biosynthesized bioplastics [115]. Bioplastics have been defined as biodegradable materials, able to degrade within months in the environment without producing toxic residues, with the bigger categories represented by polyhydroxyalkanoates and polylactic acid [114].
In the same context, it is acquiring increasing appeal the evaluation of methods to efficiently biodegrade and dispose of plastics already accumulated in human's body, possibly mitigating and dissecting MNPs toxic effects. Studies have demonstrated the potential of the microbiota to degrade MNPs. Specifically, microbes can colonize and introduce hydrophilic functional groups on MNPs surface, thus increasing their susceptibility to degradation via oxidoreductase and hydrolase enzymes. Finally, the products of these reactions can be used as carbon sources by microbes and degraded in oxidative metabolites [116]. Interestingly, the administration of probiotics has been shown to improve gut microbiota alterations and intestinal leakage induced by MNPs exposure, along with reduction of inflammatory biomarkers [117]. In addition, the intake of probiotics could eliminate common plastic toxic ingredients, including bisphenol A [118]. The results of Marfella et al. prompted the necessity to define new strategies not only in the prevention but also in the treatment of MNPs toxic effects, due to their ability to increase cardiovascular mortality rates. Hopefully, new methods to reduce the toxic biological effects of accumulated MNPs will be soon provided.
Overall, the further development of these strategies as well as the definition of new ones, along with a more conscious management of plastics production is surely the bases to put an end to plastics pollution crisis and protect environmental and human health.
Abbreviations
MPs: Microplastics; NPs: Nanoplastics; MNPs: Micro- and nanoplastics; ROS: Reactive Oxygen Species; ATSDR: Agency for Toxic Substances and Disease Registry; PERK: protein kinase R-like endoplasmic reticulum kinase; NF-κB: nuclear factor-κB; TNF-α: tumor necrosis factor-α; IL: interleukin; TLR: toll-like receptor; iNOS: inducible nitric oxide synthase; ZO-1: zonula occludens 1; MBP: myelin basic protein; MOG: myelin oligodendrocyte glycoprotein; ATP: adenosine triphosphate; BMI: Body Mass Index; IUGR: Intrauterine Growth Restriction; HUVECs: human umbilical vein endothelial cells; SOD: superoxide dismutase; MDA: malondialdehyde; JNK: c-Jun NH2-terminal kinase; MAPK: p38 mitogen-activated protein kinase; LDH: lactate dehydrogenase; SDH: succinate dehydrogenase; LPS: lipopolysaccharide; StAR: steroidogenic acute regulatory; MMPs: matrix metalloproteinases; NLRP3: NOD-like receptor protein 3; NOX: NADPH Oxidase; CD: cluster of differentiation; MYH7B: myosin, heavy chain 7B; ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; COL1A1: Collagen, type I, alpha 1; m6A: N6-methyladenosine; GPx: glutathione peroxidase; mtDNA: mitochondrial DNA; cGAS: cyclic GMP-AMP synthase; STING: stimulator of interferon genes; eNOS: endothelial nitric oxide synthase; SIRT: sirtuin; VEGF: Vascular Endothelial Growth Factor; JAK1: janus kinase 1; STAT: Signal transducer and activator of transcription; TF: tissue factor; AP: activator protein; IRF: interferon regulatory factor; HGPRT: hypoxanthine-guanine phosphoribosyltransferase; ASC: apoptosis associated speck-like protein; Akt: protein kinase B; mTOR: mammalian target of rapamycin.
Acknowledgements
Funding
This research was funded by FORMULAMI Project, Funder: Ministero dello Sviluppo Economico, Number: F/310110/04/X56.
Author contributions
Isabella Donisi: conceptualization, writing and original draft preparation, writing, review and editing, visualization.
Antonino Colloca: writing and original draft preparation, writing, review and editing, visualization.
Camilla Anastasio: visualization.
Maria Luisa Balestrieri: conceptualization, writing and original draft preparation, writing, review and editing, visualization, supervision, funding acquisition.
Nunzia D'Onofrio: conceptualization, writing and original draft preparation, writing, review and editing, visualization, supervision.
All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Yang X, Man YB, Wong MH, Owen RB, Chow KL. Environmental health impacts of microplastics exposure on structural organization levels in the human body. Sci Total Environ. 2022;825:154025
2. Landrigan P, Symeonides C, Raps H, Dunlop S. The global plastics treaty: why is it needed? Lancet. 2023;402(10419):2274-6
3. Dube E, Okuthe GE. Plastics and Micro/Nano-Plastics (MNPs) in the Environment: Occurrence, Impact, and Toxicity. Int J Environ Res Public Health. 2023;20(17):6667
4. Liu Y, Ben Y, Che R, Peng C, Li J, Wang F. Uptake, transport and accumulation of micro- and nano-plastics in terrestrial plants and health risk associated with their transfer to food chain - A mini review. Sci Total Environ. 2023;902:166045
5. Rathod NB, Xavier KAM, Özogul F, Phadke GG. Impacts of nano/micro-plastics on safety and quality of aquatic food products. Adv Food Nutr Res. 2023;103:1-40
6. Li P, Liu J. Micro(nano)plastics in the Human Body: Sources, Occurrences, Fates, and Health Risks. Environ Sci Technol. 2024;58(7):3065-78
7. Kiran BR, Kopperi H, Venkata Mohan S. Micro/nano-plastics occurrence, identification, risk analysis and mitigation: challenges and perspectives. Rev Environ Sci Biotechnol. 2022;21(1):169-203
8. Vega-Herrera A, Garcia-Torné M, Borrell-Diaz X, Abad E, Llorca M, Villanueva CM. et al. Exposure to micro(nano)plastics polymers in water stored in single-use plastic bottles. Chemosphere. 2023;343:140106
9. Bastyans S, Jackson S, Fejer G. Micro and nano-plastics, a threat to human health? Emerg Top Life Sci. 2022;6(4):411-22
10. Liu Y, Cao Y, Li H, Liu H, Bi L, Chen Q. et al. A systematic review of microplastics emissions in kitchens: Understanding the links with diseases in daily life. Environ Int. 2024;188:108740
11. Hu M, Palić D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020;37:101620
12. Yang S, Li M, Kong RYC, Li L, Li R, Chen J. et al. Reproductive toxicity of micro- and nanoplastics. Environ Int. 2023;177:108002
13. Kadac-Czapska K, Ośko J, Knez E, Grembecka M. Microplastics and Oxidative Stress-Current Problems and Pro-spects. Antioxidants (Basel). 2024;13(5):579
14. Ma YB, Xie ZY, Hamid N, Tang QP, Deng JY, Luo L. et al. Recent advances in micro (nano) plastics in the environment: Distribution, health risks, challenges and future prospects. Aquat Toxicol. 2023;261:106597
15. Gao B, Shi X, Li S, Xu W, Gao N, Shan J. et al. Size-dependent effects of polystyrene microplastics on gut meta-genome and antibiotic resistance in C57BL/6 mice. Ecotoxicol Environ Saf. 2023;254:114737
16. Dusza HM, Katrukha EA, Nijmeijer SM, Akhmanova A, Vethaak AD, Walker DI. et al. Uptake, Transport, and Toxicity of Pristine and Weathered Micro- and Nanoplastics in Human Placenta Cells. Environ Health Perspect. 2022;130(9):97006
17. Liang B, Zhong Y, Huang Y, Lin X, Liu J, Lin L. et al. Underestimated health risks: polystyrene micro- and nano-plastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part Fibre Toxicol. 2021;18(1):20
18. Zhu X, Wang C, Duan X, Liang B, Genbo Xu E, Huang Z. Micro- and nanoplastics: A new cardiovascular risk factor? Environ Int. 2023;171:107662
19. Han SW, Choi J, Ryu KY. Recent progress and future directions of the research on nanoplastic-induced neurotoxicity. Neural Regen Res. 2024;19(2):331-335
20. Kumar R, Manna C, Padha S, Verma A, Sharma P, Dhar A. et al. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere. 2022;298:134267
21. Landrigan PJ, Raps H, Cropper M, Bald C, Brunner M, Canonizado EM. et al. The Minderoo-Monaco Commission on Plastics and Human Health. Ann Glob Health. 2023;89(1):23
22. Zhao B, Rehati P, Yang Z, Cai Z, Guo C, Li Y. The potential toxicity of microplastics on human health. Sci Total Environ. 2024;912:168946
23. Yang D, Zhu J, Zhou X, Pan D, Nan S, Yin R. et al. Polystyrene micro- and nano-particle coexposure injures fetal thalamus by inducing ROS-mediated cell apoptosis. Environ Int. 2022;166:107362
24. Vojnits K, de León A, Rathore H, Liao S, Zhao M, Gibon J. et al. ROS-dependent degeneration of human neurons induced by environmentally relevant levels of micro- and nanoplastics of diverse shapes and forms. J Hazard Mater. 2024;469:134017
25. Campen M, Nihart A, Garcia M, Liu R, Olewine M, Castillo E. et al. Bioaccumulation of Microplastics in Decedent Human Brains Assessed by Pyrolysis Gas Chromatography-Mass Spectrometry. Res Sq [Preprint]. 2024:rs.3.rs-4345687.
26. Li G, Liu X, Sun X, Huang L, Kuang W, Ou J. et al. Polystyrene microplastics induce anxiety via HRAS derived PERK-NF-κB pathway. Environ Int. 2024;185:108543
27. Antunes J, Sobral P, Martins M, Branco V. Nanoplastics activate a TLR4/p38-mediated pro-inflammatory response in human intestinal and mouse microglia cells. Environ Toxicol Pharmacol. 2023;104:104298
28. Li C, Chen X, Du Z, Geng X, Li M, Yang X. et al. Inhibiting ferroptosis in brain microvascular endothelial cells: A potential strategy to mitigate polystyrene nanoplastics-induced blood-brain barrier dysfunction. Environ Res. 2024;250:118506
29. Zhang Y, Tian L, Chen J, Liu X, Li K, Liu H. et al. Selective bioaccumulation of polystyrene nanoplastics in fetal rat brain and damage to myelin development. Ecotoxicol Environ Saf. 2024;278:116393
30. Liang B, Huang Y, Zhong Y, Li Z, Ye R, Wang B. et al. Brain single-nucleus transcriptomics highlights that poly-styrene nanoplastics potentially induce Parkinson's disease-like neurodegeneration by causing energy metabolism disorders in mice. J Hazard Mater. 2022;430:128459
31. Liu Z, Sokratian A, Duda AM, Xu E, Stanhope C, Fu A. et al. Anionic nanoplastic contaminants promote Parkin-son's disease-associated α-synuclein aggregation. Sci Adv. 2023;9(46):eadi8716
32. Jeong A, Park SJ, Lee EJ, Kim KW. Nanoplastics exacerbate Parkinson's disease symptoms in C. elegans and human cells. J Hazard Mater. 2024;465:133289
33. Huang Y, Liang B, Li Z, Zhong Y, Wang B, Zhang B. et al. Polystyrene nanoplastic exposure induces excessive mitophagy by activating AMPK/ULK1 pathway in differentiated SH-SY5Y cells and dopaminergic neurons in vivo. Part Fibre Toxicol. 2023;20(1):44
34. Wei Z, Wang Y, Wang S, Xie J, Han Q, Chen M. Comparing the effects of polystyrene microplastics exposure on re-production and fertility in male and female mice. Toxicology. 2022;465:153059
35. Blackburn K, Green D. The potential effects of microplastics on human health: What is known and what is unknown. Ambio. 2022;51(3):518-530
36. Montano L, Raimondo S, Piscopo M, Ricciardi M, Guglielmino A, Chamayou S. et al. First evidence of microplastics in human ovarian follicular fluid: an emerging threat to female fertility. medRxiv. 2024 24305264
37. Qin X, Cao M, Peng T, Shan H, Lian W, Yu Y. et al. Features, Potential Invasion Pathways, and Reproductive Health Risks of Microplastics Detected in Human Uterus. Environ Sci Technol. 2024;58(24):10482-10493
38. Pontecorvi P, Ceccarelli S, Cece F, Camero S, Lotti LV, Niccolai E. et al. Assessing the impact of polyethylene nano/microplastic exposure on human vaginal keratinocytes. Int. J. Mol. Sci. 2023;24:11379
39. Hu CJ, Garcia MA, Nihart A, Liu R, Yin L, Adolphi N. et al. Microplastic presence in dog and human testis and its potential association with sperm count and weights of testis and epididymis. Toxicol Sci. 2024:kfae060.
40. Zhao Q, Zhu L, Weng J, Jin Z, Cao Y, Jiang H. et al. Detection and characterization of microplastics in the human testis and semen. Sci Total Environ. 2023;877:162713
41. Demirelli E, Tepe Y, Oğuz U, Aydın H, Kodat M, Tok DS. et al. The first reported values of microplastics in prostate. BMC Urol. 2024;24(1):106
42. Chen Y, Cheng C, Xu W, Cui Y, Tian Y, Jiang Y. et al. Occurrence, toxicity and removal of polystyrene microplastics and nanoplastics in human sperm. Environ Chem Lett. 2024 22, 2159-2165
43. Garcia MA, Liu R, Nihart A, El Hayek E, Castillo E, Barrozo ER. et al. Quantitation and identification of micro-plastics accumulation in human placental specimens using pyrolysis gas chromatography mass spectrometry. Toxicol Sci. 2024;199(1):81-88
44. Amereh F, Amjadi N, Mohseni-Bandpei A, Isazadeh S, Mehrabi Y, Eslami A. et al. Placental plastics in young women from general population correlate with reduced foetal growth in IUGR pregnancies. Environ Pollut. 2022;314:120174
45. Fournier SB, D'Errico JN, Adler DS, Kollontzi S, Goedken MJ, Fabris L. et al. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part. Fibre Toxicol. 2020;17(1):55
46. Zhu L, Xie C, Chen L, Dai X, Zhou Y, Pan H. et al. Transport of microplastics in the body and interaction with biological barriers, and controlling of microplastics pollution. Ecotoxicol Environ Saf. 2023;255:114818
47. Zurub RE, Cariaco Y, Wade MG, Bainbridge SA. Microplastics exposure: implications for human fertility, pregnancy and child health. Front Endocrinol (Lausanne). 2024;14:1330396
48. Fu Y, Fan M, Xu L, Wang H, Hu Q, Jin Y. Amino-Functionalized Polystyrene Nano-Plastics Induce Mitochondria Damage in Human Umbilical Vein Endothelial Cells. Toxics. 2022;10(5):215
49. Lu YY, Li H, Ren H, Zhang X, Huang F, Zhang D. et al. Size-dependent effects of polystyrene nanoplastics on autophagy response in human umbilical vein endothelial cells. J Hazard Mater. 2022;421:126770
50. Jeong B, Baek JY, Koo J, Park S, Ryu YK, Kim KS. et al. Maternal exposure to polystyrene nanoplastics causes brain abnormalities in progeny. J Hazard Mater. 2022;426:127815
51. Tang J, Bu W, Hu W, Zhao Z, Liu L, Luo C. et al. Ferroptosis Is Involved in Sex-Specific Small Intestinal Toxicity in the Offspring of Adult Mice Exposed to Polystyrene Nanoplastics during Pregnancy. ACS Nano. 2023;17(3):2440-9
52. Zaheer J, Kim H, Ko IO, Jo EK, Choi EJ, Lee HJ. et al. Pre/post-natal exposure to microplastic as a potential risk factor for autism spectrum disorder. Environ Int. 2022;161:107121
53. Jeong B, Baek JY, Koo J, Park S, Ryu YK, Kim KS. et al. Maternal exposure to polystyrene nanoplastics causes brain abnormalities in progeny. J. Hazard. Mater. 2022;426:127815
54. Liang J, Ji F, Lee A, Abdullah A, Qin W, Zhu T. et al. Micro/nano-plastics impacts in cardiovascular systems across species. Sci Total Environ. 2024;942:173770
55. Roshanzadeh A, Oyunbaatar NE, Ganjbakhsh SE, Park S, Kim DS, Kanade PP. et al. Exposure to nanoplastics impairs collective contractility of neonatal cardiomyocytes under electrical synchronization. Biomaterials. 2021;278:121175
56. Xie X, Deng T, Duan J, Xie J, Yuan J, Chen M. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol Environ Saf. 2020;190:110133
57. Li Y, Liu Y, Chen Y, Yao C, Yu S, Qu J. et al. Combined effects of polystyrene nanoplastics and lipopolysaccharide on testosterone biosynthesis and inflammation in mouse testis. Ecotoxicol Environ Saf. 2024;273:116180
58. Ma T, Liu X, Xiong T, Li H, Zhou Y, Liang J. Polystyrene nanoplastics aggravated dibutyl phthalate-induced blood-testis barrier dysfunction via suppressing autophagy in male mice. Ecotoxicol Environ Saf. 2023;264:115403
59. Amereh F, Babaei M, Eslami A, Fazelipour S, Rafiee M. The emerging risk of exposure to nano (micro)plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge. Environ Pollut. 2020;261:114158
60. Lin Z, Li Z, Ji S, Lo HS, Billah B, Sharmin A. et al. Size-dependent deleterious effects of nano- and microplastics on sperm motility. Toxicology. 2024;506:153834
61. Afreen V, Hashmi K, Nasir R, Saleem A, Khan MI, Akhtar MF. Adverse health effects and mechanisms of micro-plastics on female reproductive system: a descriptive review. Environ Sci Pollut Res Int. 2023;30(31):76283-96
62. Basini G, Bussolati S, Andriani L, Grolli S, Ramoni R, Bertini S. et al. Nanoplastics impair in vitro swine granulosa cell functions. Domest Anim Endocrinol. 2021;76:106611
63. An R, Wang X, Yang L, Zhang J, Wang N, Xu F. et al. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology. 2021;449:152665
64. Huang T, Zhang W, Lin T, Liu S, Sun Z, Liu F. et al. Maternal exposure to polystyrene nanoplastics during gestation and lactation induces hepatic and testicular toxicity in male mouse offspring. Food Chem Toxicol. 2022;160:112803
65. Wu H, Xu T, Chen T, Liu J, Xu S. Oxidative stress mediated by the TLR4/NOX2 signalling axis is involved in polystyrene microplastic-induced uterine fibrosis in mice. Sci Total Environ. 2022;838(Pt 2):155825
66. Liu T, Hou B, Zhang Y, Wang Z. Determination of biological and molecular attributes related to polystyrene microplastic-induced reproductive toxicity and its reversibility in male mice. Int J Environ Res Public Health. 2022;19(21):14093
67. Marfella R, Prattichizzo F, Sardu C, Fulgenzi G, Graciotti L, Spadoni T. et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N Engl J Med. 2024;390(10):900-10
68. Wang T, Yi Z, Liu X, Cai Y, Huang X, Fang J. et al. Multimodal detection and analysis of microplastics in human thrombi from multiple anatomically distinct sites. EBioMedicine. 2024;103:105118
69. Liu S, Wang C, Yang Y, Du Z, Li L, Zhang M. et al. Microplastics in three types of human arteries detected by py-rolysis-gas chromatography/mass spectrometry (Py-GC/MS). J Hazard Mater. 2024;469:133855
70. Zhou Y, Wu Q, Li Y, Feng Y, Wang Y, Cheng W. Low-dose of polystyrene microplastics induce cardiotoxicity in mice and human-originated cardiac organoids. Environ Int. 2023;179:108171
71. Zhang M, Shi J, Zhou J, Song L, Ding J, Deng HP. et al. N6-methyladenosine methylation mediates non-coding RNAs modification in microplastic-induced cardiac injury. Ecotoxicol Environ Saf. 2023;262:115174
72. Lu T, Li D, Yuan X, Wang Z, Shao Z, Feng X. et al. Potential Effects of Orally Ingesting Polyethylene Terephthalate Microplastics on the Mouse Heart. Cardiovasc Toxicol. 2024;24(3):291-301
73. Wang K, Du Y, Li P, Guan C, Zhou M, Wu L. et al. Nanoplastics causes heart aging/myocardial cell senescence through the Ca2+/mtDNA/cGAS-STING signaling cascade. J Nanobiotechnology. 2024;22(1):96
74. Shiwakoti S, Ko JY, Gong D, Dhakal B, Lee JH, Adhikari R. et al. Effects of polystyrene nanoplastics on endothe-lium senescence and its underlying mechanism. Environ Int. 2022;164:107248
75. Basini G, Grolli S, Bertini S, Bussolati S, Berni M, Berni P. et al. Nanoplastics induced oxidative stress and VEGF production in aortic endothelial cells. Environ Toxicol Pharmacol. 2023;104:104294
76. Wang X, Jia Z, Zhou X, Su L, Wang M, Wang T. et al. Nanoplastic-induced vascular endothelial injury and coagulation dysfunction in mice. Sci Total Environ. 2023;865:161271
77. Wen J, Sun H, Yang B, Song E, Song Y. Long-term polystyrene nanoplastic exposure disrupt hepatic lipid metabo-lism and cause atherosclerosis in ApoE-/- mice. J Hazard Mater. 2024;466:133583
78. Zhang X, Wang H, Peng S, Kang J, Xie Z, Tang R. et al. Effect of microplastics on nasal and intestinal microbiota of the high-exposure population. Front public health. 2022;10:1005535
79. Li B, Ding Y, Cheng X, Sheng D, Xu Z, Rong Q. et al. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere. 2020;244:125492
80. Jing J, Zhang L, Han L, Wang J, Zhang W, Liu Z. et al. Polystyrene micro-/nanoplastics induced hematopoietic damages via the crosstalk of gut microbiota, metabolites, and cytokines. Environ int. 2022;161:107131
81. Jiang L, Ye Y, Han Y, Wang Q, Lu H, Li J. et al. Microplastics dampen the self-renewal of hematopoietic stem cells by disrupting the gut microbiota-hypoxanthine-Wnt axis. Cell discovery. 2024;10(1):35
82. Zhang K, Yang J, Chen L, He J, Qu D, Zhang Z. et al. Gut Microbiota Participates in Polystyrene Microplastics-Induced Hepatic Injuries by Modulating the Gut-Liver Axis. ACS nano. 2023;17(15):15125-15145
83. Mu Y, Sun J, Li Z, Zhang W, Liu Z, Li C. et al. Activation of pyroptosis and ferroptosis is involved in the hepato-toxicity induced by polystyrene microplastics in mice. Chemosphere. 2022;291(Pt 2):132944
84. Shen R, Yang K, Cheng X, Guo C, Xing X, Sun H. et al. Accumulation of polystyrene microplastics induces liver fi-brosis by activating cGAS/STING pathway. Environ Pollut. 2022;300:118986
85. Shaoyong W, Jin H, Jiang X, Xu B, Liu Y, Wang Y. et al. [a] pyrene-loaded aged polystyrene microplastics promote colonic barrier injury via oxidative stress-mediated notch signalling. J Hazard Mater. 2023;457:131820
86. Sun H, Chen N, Yang X, Xia Y, Wu D. Effects induced by polyethylene microplastics oral exposure on colon mucin release, inflammation, gut microflora composition and metabolism in mice. Ecotoxicol Environ Saf. 2021;220:112340
87. Yang Q, Dai H, Cheng Y, Wang B, Xu J, Zhang Y. et al. Oral feeding of nanoplastics affects brain function of mice by inducing macrophage IL-1 signal in the intestine. Cell reports. 2023;42(4):112346
88. Ali N, Katsouli J, Marczylo EL, Gant TW, Wright S, Bernardino de la Serna J. The potential impacts of micro- and nano plastics on various organ systems in humans. EBioMedicine. 2024;99:104901
89. Amato-Lourenço LF, Carvalho-Oliveira R, Júnior GR, Dos Santos Galvão L, Ando RA, Mauad T. Presence of airborne microplastics in human lung tissue. J Hazard Mater. 2021;416:126124
90. Zha H, Xia J, Li S, Lv J, Zhuge A, Tang R. et al. Airborne polystyrene microplastics and nanoplastics induce nasal and lung microbial dysbiosis in mice. Chemosphere. 2023;310:136764
91. Dong CD, Chen CW, Chen YC, Chen HH, Lee JS, Lin CH. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J Hazard Mater. 2020;385:121575
92. Jin W, Zhang W, Tang H, Wang P, Zhang Y, Liu S. et al. Microplastics exposure causes the senescence of human lung epithelial cells and mouse lungs by inducing ROS signaling. Environ int. 2024;185:108489
93. Xu M, Halimu G, Zhang Q, Song Y, Fu X, Li Y. et al. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci Total Environ. 2019;694:133794
94. Yang S, Cheng Y, Chen Z, Liu T, Yin L, Pu Y. et al. In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicol Environ Saf. 2021;226:112837
95. Shi W, Cao Y, Chai X, Zhao Q, Geng Y, Liu D. et al. Potential health risks of the interaction of microplastics and lung surfactant. J Hazard Mater. 2022;429:128109
96. Weber A, Schwiebs A, Solhaug H, Stenvik J, Nilsen AM, Wagner M. et al. Nanoplastics affect the inflammatory cytokine release by primary human monocytes and dendritic cells. Environ Int. 2022;163:107173
97. Han S, Bang J, Choi D, Hwang J, Kim T, Oh Y. et al. Surface pattern analysis of microplastics and their impact on human-derived cells. ACS Appl Polym Mater. 2020;2:4541-4550
98. Wang J, Wang X, Zhang C, Zhou X. Microplastics induce immune suppression via S100A8 downregulation. Ecotoxicol Environ Saf. 2022;242:113905
99. Wang YL, Lee YH, Hsu YH, Chiu IJ, Huang CC, Huang CC. et al. The Kidney-Related Effects of Polystyrene Microplastics on Human Kidney Proximal Tubular Epithelial Cells HK-2 and Male C57BL/6 Mice. Environ Health Perspect. 2021;129(5):57003
100. Wang YL, Huang CC, Zheng CM, Liu WC, Lee YH, Chiu HW. Polystyrene microplastic-induced extracellular vesicles cause kidney-related effects in the crosstalk between tubular cells and fibroblasts. Ecotoxicol Environ Saf. 2024;273:116098
101. Li Y, Li Y, Li J, Song Z, Zhang C, Guan B. Toxicity of polystyrene nanoplastics to human embryonic kidney cells and human normal liver cells: Effect of particle size and Pb2+ enrichment. Chemosphere. 2023;328:138545
102. Liu S, Guo J, Liu X, Yang R, Wang H, Sun Y. et al. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: A pilot prospective study. Sci Total Environ. 2023;854:158699
103. Damaj S, Trad F, Goevert D, Wilkesmann J. Bridging the Gaps between Microplastics and Human Health. Microplastics. 2024;3:46-66
104. Masseroni A, Rizzi C, Urani C, Villa S. Nanoplastics: Status and Knowledge Gaps in the Finalization of Environmental Risk Assessments. Toxics. 2022;10(5):270
105. Wright SL, Ulke J, Font A, Chan KLA, Kelly FJ. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ Int. 2020;136:105411
106. Catarino AI, Macchia V, Sanderson WG, Thompson RC, Henry TB. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ Pollut. 2018;237:675-684
107. Yee MS, Hii LW, Looi CK, Lim WM, Wong SF, Kok YY. et al. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials (Basel). 2021;11(2):496
108. Mattsson K, Johnson EV, Malmendal A, Linse S, Hansson LA, Cedervall T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci Rep. 2017:7(1);11452.
109. Kopatz V, Wen K, Kovács T, Keimowitz AS, Pichler V, Widder J. et al. Micro- and Nanoplastics Breach the Blood-Brain Barrier (BBB): Biomolecular Corona's Role Revealed. Nanomaterials (Basel). 2023Apr;13(8):1404
110. Revel M, Châtel A, Mouneyrac C. Micro(nano)plastics: A threat to human health? Curr. Opin. Environ. Sci. Health. 2018;1:17-23
111. Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z. et al. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018;619-620:1-8
112. Ramsperger AFRM, Narayana VKB, Gross W, Mohanraj J, Thelakkat M, Greiner A. et al. Environmental exposure enhances the internalization of microplastic particles into cells. Sci Adv. 2020;6(50):eabd1211
113. Kwon D. Three ways to solve the plastics pollution crisis. Nature. 2023;616(7956):234-237
114. Clark RA, Shaver MP. Depolymerization within a Circular Plastics System. Chem Rev. 2024;124(5):2617-50
115. White MFM, Wallace S. A New PETase from the Human Saliva Metagenome and Its Functional Modification via Ge-netic Code Expansion in Bacteria. Angew Chem Int Ed Engl. 2023;62(12):e202216963
116. Wang J, Wang Y, Li Z, Wang J, Zhao H, Zhang X. Gut microbiota, a key to understanding the knowledge gaps on micro-nanoplastics-related biological effects and biodegradation. Sci. Total Environ. 2024;944:173799
117. Bazeli J, Banikazemi Z, Hamblin MR, Sharafati Chaleshtori R. Could probiotics protect against human toxicity caused by polystyrene nanoplastics and microplastics? Front Nutr. 2023;10:1186724
118. Kyrila G, Katsoulas A, Schoretsaniti V, Rigopoulos A, Rizou E, Doulgeridou S. et al. Bisphenol a removal and degradation pathways in microorganisms with probiotic properties. J Hazard Mater. 2021;413:125363
Author contact
![]() Corresponding author: marialuisa.balestrieriit (M.L.B.).
Corresponding author: marialuisa.balestrieriit (M.L.B.).

 Global reach, higher impact
Global reach, higher impact