10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2025; 21(6):2725-2746. doi:10.7150/ijbs.106382 This issue Cite
Review
FMRFamide-like peptides (FaLPs) - an overview of diverse physiological roles in insects and other arthropods
1. Department of Animal Physiology and Developmental Biology, Adam Mickiewicz University, Poznań, Poland, Uniwersytetu Poznańskiego 6 Str., 61-614 Poznań, Poland.
2. Competence Centre for Plant Health, Free University of Bozen-Bolzano, Bozen-Bolzano, Italy.
Received 2024-11-5; Accepted 2025-2-14; Published 2025-3-31
Abstract
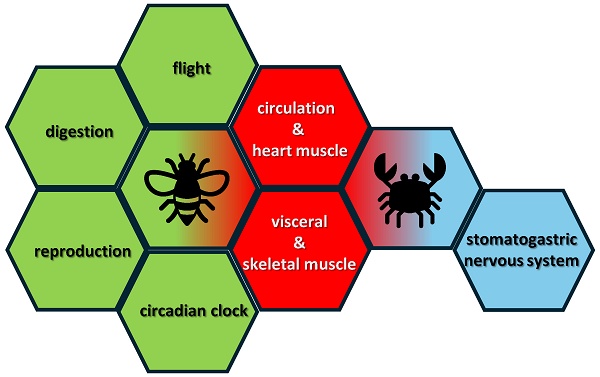
FMRFamide-like peptides (FaLPs) are neuropeptides that play a pivotal role in regulating various physiological processes in insects and other arthropods including behaviour, reproduction, and homeostasis. FaLPs mostly act through G-protein coupled receptor and influence muscle activity by modulating Ca2+ influx. Historically, the function described for these neuropeptides was primarily associated with myostimulatory activity. After more than three decades of research, it is now well established that FaLPs are implicated in the regulation of circadian rhythms, affecting locomotor activity and phase changes in response to environmental cues. During reproduction, FaLPs influence contractile activity in both the male and female reproductive systems. They also participate in physiological processes such as diapause induction, sleep modulation, and flight regulation in insects. Similarly, in crustaceans, FaLPs regulate the circulatory system, stomatogastric nervous system, and muscle contractions. Nowadays, it is also known how the physiological properties of FaLPs in arthropods share similarities with mammalian RFamide peptides, which are involved in a wide range of functions, including muscle contraction, feeding, reproduction, and stress responses, mediated through various RFamide receptors. Therefore, summarizing the investigated physiological functions in arthropods may be relevant also for future research aiming to test their activity in other organisms such as mammalians.
Keywords: insects, neuropeptides, GPCR, muscle contraction, physiology, mass spectrometry, -RFamide
Introduction
Neuropeptides play a pivotal role in regulating nearly all physiological processes in various arthropods by acting as neurotransmitters, neuromodulators, or (neuro)hormones. Among these peptides, FMRFamide-like peptides (FaLPs) are a significant group known for their multidirectional influence on arthropod physiology. The term FMRFamide refers to a sequence of four amino acids (Phe-Met-Arg-Phe-NH2) initially identified as a cardioacceleratory agent in molluscs [1]. When searching the literature, especially from the first decade of this century, readers can be slightly puzzled by the two terms used interchangeably to describe this family of peptides, especially in insects. In insects, FMRFa itself has not been identified, the FaLPs are N-terminus extended FMRFamides (Table 1). Prior to the recommendation of Coast and Schooley on a consensus insect peptide nomenclature [2], other families of peptides with similar C-terminus sequences such as -FLRFa (myosuppressins, MS), -HM/LRFa/HLRFa (sulfakinins, SK), -RP/VRFa (neuropetides F; NPF), -RLRFa (short neuropeptide F; sNPF), were often described as FMRFamide-related peptides (FaRPs) or FMRFamide-like peptides (FaLPs). Nowadays, these peptides are assigned to separate families because they are encoded by different genes and activate distinct receptors [2]. Hence, this review will focus on the FaLPs as defined by Coast and Schooley and which activate the FMRFa receptors. In other subphyla such as arachnids and crustaceans the nomenclature seems to be the same, however in some studies based on mainly on mass spectrometry FaLPs may still embrace sNPF, MS and SK [2, 3].
The physiological properties of FaLPs have been studied in various arthropod species. However, the majority of studies on FaLPs have involved mainly pancrustaceans with special emphasis on insects, and crustaceans (Table 3). Since, there is only a handful of studies on FaLPs in myriapods and chelicerates, we only briefly include these groups in our review.
First research performed on insects and crustaceans revealed at that time that they are myoactive neurohormones that show species and tissue specificity, causing both inhibition and stimulation of heart and visceral organ contraction [4-6].
Examples of amino acid sequences of FaLPs from different arthropod species and humans.
| Species | Acronym | Amino acid sequence |
|---|---|---|
| Rhodnius prolixus | Rhopr-FMRFa | IKDNFIRFa |
| Drosophila melanogaster | Drome-FMRFa-1 | SVQDNFMHFa |
| Drome-FMRFa-2 | DPKQDFMRFa | |
| Drome-FMRFa-3 | TPAEDFMRFa | |
| Drome-FMRFa-4 | SDNFMRFa | |
| Drome-FMRFa-5 | SPKQDFMRFa | |
| Drome-FMRFa-6 | PDNFMRFa | |
| Drome-FMRFa-7 | SAPQDFVRSa | |
| Drome-FMRFa-8 | MDSNFIRFa | |
| Tenebrio molitor | Tenmo-FMRFa-1 | NNNNFLRFa |
| Tenmo-FMRFa-2 | SGKTEKNDHFIRFa | |
| Tenmo-FMRFa-3 | SKQDFLRFa | |
| Tenmo-FMRFa-4 | DQHRVVRDRSGNYLRFa | |
| Tenmo-FMRFa-5 | GGSNFMRFa | |
| Tenmo-FMRFa-6 | NSNFLRFa | |
| Schistocerca gregaria | Schgr-FMRFa-1 | DADAEAAAVDDDGAGGEGDGDGELGLVQTTPRSNFLRLa |
| Schgr-FMRFa-2 | AGGAHSAFLRLa | |
| Schgr-FMRFa-3 | DRASSGFLRLa | |
| Schgr-FMRFa-4 | GSERNFLRFa | |
| Schgr-FMRFa-5 | SGMADPLTRHDRNFIRFa | |
| Schgr-FMRFa-6 | SGRSGTAQHQAGAEPALWAALLDPRLAVLPAPAGADDADVDGAAGSGRGRVSTRSFLRLa | |
| Periplaneta americana* | Peram-FMRF-1 | AEEQNQPPPV |
| Peram-FMRF-2 | RRCSNRNFVRLa | |
| Peram-FMRF-3 | GHDFDQDDVNSSGEKDESLVRIa | |
| Peram-FMRF-4 | GGRSNDNFIRFa | |
| Peram-FMRF-5 | GGKNDNFIRFa | |
| Peram-FMRF-6 | GGKQDNFIRFa | |
| Peram-FMRF-7 | DRSDNFIRFa | |
| Peram-FMRF-8 | GKTDNFIRFa | |
| Peram-FMRF-9 | GRSDNFIRFa | |
| Peram-FMRF-10 | GKSDNFIRFa | |
| Peram-FMRF-11 | ARPDNFIRFa | |
| Peram-FMRF-12 | GKQDFIRFa | |
| Peram-FMRF-13 | GNSNFVRFa | |
| Peram-FMRF-15 | GGKSGSNFIRFa | |
| Peram-FMRF-16 | ARPSSNFIRLa | |
| Peram-FMRF-18 | GRPSNNFVRFa | |
| Peram-FMRF-19 | pQTEDDDKFVRLS | |
| Peram-FMRF-20 | SGNSNELRRGKL | |
| Peram-FMRF-21 | TDRNFIRLa | |
| Peram-FMRF-22 | SGPSYDEKEQENEDGNSVRLa | |
| Peram-FMRF-23 | SENPSNSRNFIRLa | |
| Peram-FMRF-24 | ALDQNLLVDEHLMRFa | |
| Carcinus maenas | RNFLRFa | |
| pQGNFLRFa | ||
| NRSFLRFa | ||
| SRNYLRFa | ||
| NRNFLRFa | ||
| DRNFLRFa | ||
| APRNFLRFa | ||
| GNRNFLRFa | ||
| APQGNFLRFa | ||
| GAHKNFLRFa | ||
| GLSRNYLRFa | ||
| DGNRNFLRFa | ||
| APQRNFLRFa | ||
| YGNRSFLRFa | ||
| SENRNFLRFa | ||
| Homarus americanus | GYSDRNYLRF | |
| NFLRF | ||
| NRNFLRF | ||
| DQNRNFLRF | ||
| GAHKNYLRF | ||
| GNRNFLRF | ||
| GDRNFLRF | ||
| FSHDRNFLRF | ||
| APSKNFLRF | ||
| Daphnia pulex | Dappu-FIRFa-1 | SLRSNFIRFa |
| Dappu-FIRFa-2 | SALNKNFIRFa | |
| Tigriopus californicus | NFLRF | |
| DYREFVRF | ||
| DDFLRF | ||
| SNDFLRF | ||
| Echinogammarus veneris | AFPRSFLRFa | |
| pEPSDLHFIRFa | ||
| AFPVNFSRFa | ||
| pEPSDLNFIRFa | ||
| Latrodectus hesperus | pQHNIMRFa | |
| PAGGHNLIHFa | ||
| pEDTSKTPQHSFLYFa | ||
| GHTIMRFa | ||
| DHNMMRFa | ||
| pEGHSMMYFa | ||
| DGHSMLYFa | ||
| GPGHAILSFa | ||
| YGTDTWHSMMNFa | ||
| SENPWDHSTMHFa | ||
| DVDDPWHNMMSFa | ||
| SDILWDHNTMHFa | ||
| SGNPWDQHSTLHFa | ||
| DSHSMIHFa | ||
| NHNLLRFa | ||
| TEGSRKGSHAMIHFa | ||
| SEPWENHNTMHFa | ||
| LTPWESHNTMHFa | ||
| SELWENHNTMHFa | ||
| SEDPWESHNTMHFa | ||
| SDPWENHSTLHFa | ||
| Homo sapiens | NPFF | SQAFLFQPQRFa |
| NPAF | AGEGLNSQFWSLAAPQRa | |
| RFRP-1 | MPHSFANLPLRFa | |
| RFRP-3 | VPNLPQRFa | |
| QRFP43 | EDEGSEATGFLPAAGEKTSGPLGNLAEELNGYSRKKGGFSFRFa | |
| QRFP26 | TSGPLGNLAEELNGYSRKKGGFSFRFa | |
| PrRP31 | SRTHRHSMEIRTPDINPAWYASRGIRPVGRFa | |
| PrRP20 | TPDINPAWYASRGIRPVGRFa | |
| Kisspeptin-54 | GTSLSPPPESSGSPQQPGLSAPHSRQIPAPQGAVLVQREKDLPNYNWNSFGLRa | |
| Kisspeptin-10 | YNWNSFGLRFa |
To maintain consistency with the existing databases the nomenclature of single analogue has been retain (FMRFa instead of FaLPs). Note the C-terminal typical F-L/M/I/V-R-L/Fa sequence. * List according to [44], note Peram-FMRF-1, 19, 20 are not amidated
The influence of FaLPs extends beyond muscle contraction to encompass broader aspects of insect physiology, including the regulation of circadian rhythms, feeding behaviour, and reproductive functions [7]. Their widespread distribution and coexpression with other neuropeptides, such as sulfakinins [8], suggest a multifaceted role in the regulation of insect physiology, making them a subject of interest for understanding the intricate network of peptidergic signalling in invertebrates. Furthermore, FaLPs in crustaceans and insects can be considered as analogues (similar function, but different origin) or homologues (common origin, but possibly different functions) of mammalian RFamide neuropeptides. At present it is difficult to decide this issue unequivocally. Thus, knowledge about FaLPs biology can be used to better understand the mechanism of action of neuropeptides in general, not only in insects. These findings may be useful in the future in translational research, e.g., in the search for new solutions for the treatment of animal and even human health disorders.
Until now only few, excellent reviews were published on the influence of FaLPs (designated sometimes as FaRPs) on various aspects of insect physiology [9-12]. However, they were published several years ago and focused not only on FaLPs but also on other RFamides (myossupressins, sulfakinins, short neuropeptides F). For this reason, here, we summarize the current knowledge exclusively about FMRFa-like peptides (FaLPs), including their biosynthesis, mode of action, and role as crucial components of insect physiology regulation, with their effects permeating through various systems and processes. Moreover, we compare the properties of insect FaLPs with crustaceans ones, showing their conserved role as myoactive factors which directly or indirectly influence circulatory system and regulate feeding. Finally, we compare the known function in insects with those known for RFamides in mammalians and indicate possible similarities in regulation of chosen physiological processes.
Identification and precursor structure
FaLPS in insects
Neuropeptide precursors can be separated into single-copy and multi-copy precursors. The latter group, which includes FaLP precursors, contains a variable number of para-copies of the same neuropeptide family, typically activating the same G protein-coupled receptor (GPCR), with some exceptions, such as pyrokinin precursors, which activate different receptors [13]. Generally, each neuropeptide family contains a typical C-terminal consensus sequence of five amino acids, which may vary slightly between species in the case of a single copy [14] but also within the same genus for multi-copy precursors. Usually, the number of para-copies is consistent within the same group, but in some instances, it can vary dramatically within the same genus [15].
FMRFamide was originally identified in the sunray venus clam Macrocallista nimbosa by Edman degradation [1]. In insects, the first FaLP neuropeptide was characterized in Drosophila melanogaster [16, 17]. Subsequently, these peptides have been identified in nearly all invertebrates and are now recognized as one of the most widely distributed neuropeptide families among arthropods and metazoans. Thus far, only N-terminally extended FaLPs have been identified, mainly by mass spectrometry.
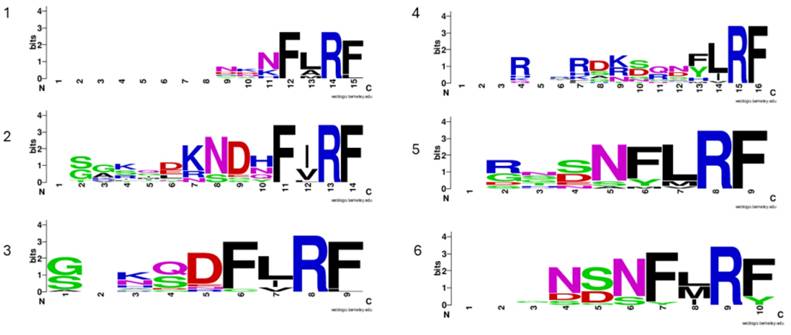
Subsequent investigations showed that FMRFamide precursors exhibit a variable number of para-copies across taxa (Table 1), often accompanied by slightly different N-terminal consensus motifs. In hemimetabolous insects, the number of para-copies varies both across and within groups: in the silverfish Thermobia domestica (n = 12; [18]; in Periplaneta americana (n = 24; [19]; in Zootermopsis nevadensis (n = 10; [20]; in Schistocerca gregaria (n = 6; [21]; in Locusta migratoria (n = 5; [22]; in Carausius morosus (n = 8; [23]; in Rhodnius prolixus (n = 1; [24]; and in Acyrthosiphon pisum (n = 4; [25]. In holometabola insects, there is a general reduction in the number of para-copies with Apis mellifera (n = 6; [26]; Bombus terrestris (n = 6; [27]; Manduca sexta (n = 6; [28]; Bombyx mori (n = 4; [29]; Spodoptera frugiperda (n = 5; [29]; Galleria mellonella (n = 5; [30]; Carabus violaceus (n = 6; [31]; Hylobius abietis (n = 6; [32]; Tribolium castaneum (n = 6; [33]; Tenebrio molitor (n =6; [34], and Zophobas atratus (n = 6; [34]. In a recent investigation of the neuropeptidome in Coleoptera, Veenstra [33] reported a variable number of FaLPs para-copies ranging from four to six. Moreover, aligning homologous sequences among different species and groups is difficult both with automatic approaches and with manual editing of the alignments (Figure 1).
Despite the considerable increase in the number of species sequenced using high-throughput techniques in recent years, only relatively few studies have confirmed the peptide sequences of FaLPs neuropeptide family across the central nervous system (CNS) of insects using mass spectrometer such as MALDI (Matrix-Assisted Laser Desorption/Ionization) and/or Orbitrap [15, 21, 23, 25, 31, 32, 34-43].
Sequence conservation of the six putative bioactive FaLPs peptides (1-6) in Coleoptera. All precursors were aligned using MAFFT and manually reviewed to correctly align cleavage sites. In the weblogo only putative bioactive peptides with amidated C-terminal were included. Species included are: Aethina tumida XP_019869081.1; Aleochara curtula (SRR921563); Anoplophora glabripennis (XP_023310731.1); Aquatica sp. (AQULA_011885-PA); Dendroctonus ponderosae (XP_019753890.1); Coccinella septempunctata (GCA_907165205.1); Harmonia axyridis (GCA_914767665.1); Hycleus sp. (Hpha006160); Hypothenemus hampei (GCA_013372445.1); Ignelater luminosus (ILUMI_09630-PA); Leptinotarsa decemlineata (XP_023026284.1); Nicrophorus vespilloides (XP_017784347.1); Oryctes borbonicus (GCA_902654985.2); Photinus pyralis (PPYR_06929-PA); Pogonus chalceus (JU434250.1); Tenebrio molitor (ON110508); Tribolium castaneum (GCA_031307605.1).
Collectively, these studies have provided crucial insight into both the localization of neuropeptide neurons in the CNS and the processing of precursors. Historically, the distribution of FaLPs in the CNS was investigated using immunohistochemistry (IHC). However, IHC has limitations for neuropeptide families such as FaLPs since several neuropeptide families share a similar C-terminal consensus sequence (-RFamides). These include FaLPs and the above-mentioned MS, SK, sNPF, and NPF families. Indeed, it is likely that the antisera produced against RFamide peptides exhibit cross-reactivity among all these neuropeptide families in the same tissue. Studies employing a combination of IHC and direct tissue profiling initially reported a tagmata-specific distribution of neuropeptides [44]. FaLPs are predominantly expressed in posterolateral cells (PLCs) of the thoracic ganglia, with axons projecting into neurohemal organs, the thoracic perisympathetic organs (tPSO), in which mature and bioactive peptides are likely to be stored and released. In most insects, MALDI direct tissue profiling of tPSO contains almost only ion signals of FMRFamide-like peptides. Furthermore, Liessem et al [23] demonstrated, through direct tissue profiling of 16 single PLCs, that the ion intensity of the different para-copies varies considerably, but the pattern remains consistent across different preparations, indicating no alternative processing of these neuropeptide precursors, as was reported for pyrokinins (PKs) [31, 34, 45]. FaLPs have also been identified in the cerebral ganglion of P. americana, particularly at higher concentrations in the antennal nerve and at lower concentrations in the antennal lobes [42]. More recently, using mass spectrometry imaging (MSI), the distribution of FMRFamide-like peptides was shown in sections of the major neurohemal organ of insects, which is connected to the cerebral ganglion and gnathal ganglion, the retrocerebral complex (RCC) of P. americana [19]. Although, the ion signals of these peptides in RCC are of relatively low intensity compared to those in tPSO, MSI effectively distinguished the distribution of these peptides from other RFamide peptides, such as MS, which is typically abundant in RCC. Additionally, MSI single spectra confirmed a similar pattern of ion signals matching the FMRFamide-like peptide para-copies observed in PLCs, suggesting identical processing of all para-copies in the tPSO as well in the RCC of the analysed insects. Another crucial aspect that can be investigated through direct tissue profiling of tissues abundant in FaLPs is the processing of precursors and postprocessing modifications such as pyroglutamate, which are important for preserving the structure and stability of a few peptides. In C. morosus, partial use of internal cleavage sites and unusual cleavage sites of the signal peptide have been reported in the FaLPs precursor [23]. The former case occurs in those multicopy precursors when a single monobasic cleavage site, such as a single Arg residue (IFG-RGK), is situated between two consecutive potentially bioactive peptides. The latter could result in the production of either a longer precursor and/or spacer peptide after the signal peptide or an N-terminal extended bioactive neuropeptide. Similar occurrences have also been documented for other neuropeptide precursors, such as pheromone biosynthesis activating neuropeptide (PBAN), in moths [46]. Incomplete processing of the FaLPs precursor was also observed in Coleoptera. It is noteworthy that in Coleoptera species such as Carabus violaceus, the PLCs do not project into a single median tPSO but instead project through transverse nerves into two separated lateral tPSOs posterior to the ganglia [31]. Similar thoracic neurohemal organs are found in tenebrionid species such as T. molitor and Z. atratus [47]. Mass spectra of the tPSO organs of C. violaceus (Carabidae), H. abietis (Curculionidae), and Z. atratus (Tenebrionidae) revealed N-terminal extended forms, likely resulting from a less efficient cleavage at the Arg residue [31, 32, 34]. Currently, it is unclear whether these forms exhibit distinct bioactivity, but it is important to highlight that the combination of IHC and mass spectrometry (especially MSI) is capable of producing the most comprehensive information about both the site of expression and processing of neuropeptides.
FaLPs in Crustacea
Among crustaceans, FaLPs are localized in both the central and peripheral nervous systems, playing a critical role in regulating several physiological processes. Until 2003, twelve crustacean FaLPs had been identified, mostly in decapods such as Callinectes sapidus [48], Homarus americanus [49], Cancer borealis [50], Procambarus clarkii [51], and Macrobrachium rosenbergii [52]. These neuropeptides were identified in several tissues throughout the nervous systems of different species, including the pericardial organs, the stomatogastric nervous system, and the eyestalk. In the following years, this number grew considerably. Nineteen FMRFamide-related peptides were identified in H. americanus [53], 25 FMRFamide-like peptides were identified in C. maenas using a combination of ESI-Q-TOF and direct tissue/off-line HPLC fractionation coupled with MALDI-FTMS analysis [54], and 14 FaLPs were identified in Litopenaeus vannamei through transcriptomic and mass spectrometry analysis [55]. The authors identified most of the FaLPs in the VNC using a combination of two mass spectrometry approaches, as well as in the brain and eyestalk ganglia, the latter including the sinus gland. These neuropeptides are typically between 7 and 10 amino acids in length, excluding N-terminal extended forms (Table 1). For clarity, it should be again mentioned that at that time, several RFamide families, including MS, NPF, sNPF, and SK, were still partially grouped together. Among the RFamide families, MS, sNPF, and NPF are usually single-copy peptides or occasionally contain two putative bioactive peptides. Two bioactive peptides are typically encoded by the SK gene, with the bioactive peptides presenting post-translational modifications such as sulfation (-SO3) and/or N-terminal blocked forms like pyroglutamate. The so-called FaLPs are the only multicopy precursors, usually expressing more than three bioactive neuropeptides. In decapods, likely the most studied group among Crustacea, the precursor organization is relatively conserved, similar to that of coleopterans. Moreover, following the aforementioned studies, neuropeptides in other species were subsequently described, mostly using in silico predictions, such as in Penaeus monodon [56]. In decapods, the precursors contain N-terminally extended YLRFamide, FLRFamide, and FIRFamide. In other crustaceans, the number of mature neuropeptides decreased considerably. In the water flea Daphnia pulex (Cladocera), the firfamide gene encodes two FIRFamides, confirmed by mass spectrometry [57]. Similarly, in the copepods Calanus finmarchicus and Tigriopus californicus, one transcript was predicted in silico to encode two FIRFamides, and two transcripts were predicted to encode four putative mature FaLPs (three FLRFamides and one FVRFamide), respectively [58, 59]. In the former species, C. finmarchicus, an FMRF receptor sequence was also predicted using homology searches with known receptors (see below for more details). In amphipods, a FaLP gene was predicted to encode six peptides in Echinogammarus veneris (three FL/IRFamides), as well as a second transcript that encodes eight peptides, including an RYamide-like peptide (NNRNLLRYamide; see [60] for details). This latter RYamide peptide is also present in several other amphipod FaLP precursors, such as Gammarus chevreuxi (comp62867_c0_seq1), suggesting a common feature in amphipods and ruling out errors in transcriptome assembly. Therefore, the FaLP precursors in crustaceans, like those in insects, contain a variable number of putative bioactive peptides, ranging from 2-4 in Cladocera and Copepoda to as many as 9 in decapods [53-55]. According to functional experiments conducted in D. melanogaster [61], all the above-mentioned peptides should be capable of activating the FaLPs receptor. In D. melanogaster, it was shown that out of the eight possible putative bioactive peptides, all but one (FVRSamide) activate the receptor, including the one with an Arginine-to-Histidine replacement (FMHFamide). More recently, similar to what has already been described in insects, neuropeptide identification and distribution in decapods have also been investigated using mass spectrometry imaging (MSI). The cardiac ganglion of C. borealis was analyzed using a combination of MALDI-TOF-TOF imaging and Orbitrap [62]. In addition to identifying a large number of peptides from several different neuropeptide families, including FaLPs, the study revealed the complexity of the cardiac ganglion's modulatory circuits. The authors suggest that future studies should combine the precise identification of neuropeptides via MSI with more detailed immunohistochemical resolution and localization to elucidate the specific functions of neuropeptides in the studied organ, as several aspects remain largely unknown. When comparing MSI with IHC, the main limitation associated with MSI is the fine-scale resolution, particularly in samples of relatively small size, as is common in arthropods. Currently, the primary limitation to achieving higher resolution is related to the spraying conditions: matrix crystals of about 20 μm prevent the use of smaller laser spot sizes (e.g., 15 μm). Although smaller laser spot sizes (5-10 μm) have been tested, they failed to generate a complete peptidome [19]. On the other hand, MSI allows for the study of the co-occurrence of different neuropeptide families (including all para-copies of multicopy neuropeptides like FaLPs) and, through spatial segmentation analysis, the identification of tissues or compartments within organs associated with specific neuropeptide families. This capability, along with the reproducibility of MSI, could expand our current knowledge of the biological activity and functions of several neuropeptide families. Finally, despite the great accuracy in identifying immunoreactive cells and tissues using IHC, unless this approach is coupled with mass spectrometry, it is extremely difficult to distinguish between RFamide peptides in tissues where more than one neuropeptide family co-occurs.
FaLPs in Chelicerata and Myriapoda
Among other arthropods, considerably fewer studies have been conducted on chelicerates. In this group, most research has focused solely on in silico predictions of both neuropeptide precursors and receptors. It is important to note that ligands and receptors are thought to co-evolve. However, in many of these groups, the ligands appear to have undergone greater variability compared to the receptors (see next section). In Limulus polyphemus, only a few genuine FaLP neuropeptides with FIRFamide have been identified, while the majority of other peptides have a MIRFamide C-terminus or others such as TIRFamide (Figure S1). Among mites, such as Ixodes scapularis, the FaLP N-terminal sequences appear to have evolved into YLHFamide, ILHFamide, IMHFamide, and MLHFamide (Figure S1), with a replacement of the arginine residues with histidine, as previously reported in D. melanogaster [61]. In the groups Scorpiones and Araneae (Arachnopulmonata), most of the information has been obtained using in silico prediction. In Arachnida, the western black widow spider Latrodectus hesperus is used as a model to search for neuropeptides in other species [2, 63]. In L. hesperus, more than 20 FaLP peptides with C-termini IMRFamide, MMYFamide, and MIHFamide were identified. In Scorpiones, in silico predicted peptides with a C-terminus consensus sequence similar to other chelicerates were identified. The FMRFamide gene of Centruroides sculpturatus encodes several peptides with C-termini IMRFamide, LIRFamide, MIHFamide, LMHFamide, LIHFamide, and IMHFamide (Figure S1). Finally, in Myriapoda, the predicted FaLP precursor gene of Hanseniella nivea is similar to that of Pancrustacea, encoding several FLRFamide peptides (Figure S1). Most of the above-mentioned peptides need to be corroborated by both mass spectrometry analyses and functional studies in the future.
Receptor structure
FaLP receptor in insects
The signalling pathways of FaLPs have been studied in a several species, including A. gambiae [5], R. prolixus [4], Baculum extradentatum [6] and D. melanogaster [64]. The FaLP receptor (FaLPR) in insects was initially discovered and characterized in D. melanogaster in 2002 [61, 65]. This discovery paved the way for further characterization and structure-activity studies in the fly over the next decade [66, 67]. Like other insect neuropeptide receptors, FaLPRs belong to GPCRs composed of seven transmembrane (TM) helixes (Figure 2). These helices are connected by six loops, three of which are extracellular while three intracellular interact with the G protein. The structure includes also an N-terminal extracellular segment and an intracellular C-terminus. Ligands can attach to transmembrane helix binding sites or to extracellular loops and N-termini [68]. In insects, neuropeptides act through GPCRs [69] followed by the generation of soluble second messengers such as cyclic adenosine monophosphate (cAMP) [70-72] or inositol 1,4,5-trisphosphate (IP3) [73, 74]. IP3 binds to and activates the endoplasmic reticulum (ER)-localized Ca2+ channel, the IP3 receptor (IP3R), resulting in the release of calcium from ER stores [75]. FaLPRs, among others, are involved in this signalling cascade [73, 74, 76]. In other arthropods the FaLPs act by the same mechanism, activating their GPCRs. The analyses conducted by Rasmussen et al. [77] showed that FaLPRs belong to subfamily A of the GPCRs rhodopsin-like family in which ligands bind to the binding site within transmembrane helices. Thus, the receptors for FaLPs contain typical rhodopsin-like amino acid patterns, e.g., GN in helix 1, LX3Din helix 2, D(E)RY(F) in helix 3, CNL(I)X2 in helix 6 and NFX2Y in helix 7, as shown in T. molitor and Z. atratus [78]. These sequences comprise molecular switches that are responsible among others for propagation of a hydrogen-bond network from the ligand binding pocket to the intracellular side of the receptor upon activation, are essential in holding the receptor in an inactive state and preventing access to the G protein-binding site through interactions between TM3 and TM6 or are important in binding the agonist in order to induce structural changes near TM5 and TM6 which lead to receptor activation (for details see [77]). However, in other invertebrates FaLPs can also act through other types of receptors. In mollusc, Aplysia californica it can directly bind and activate the ionotropic receptor for sodium (FMRFamide-activated sodium channels (FaNaCs))[79]. Although peptide-activated ion channels are rare, authors of another study from 2022, do not dismiss the possibility that other FaLP/FMRFa-gated channels can be found in other clades [80]. However, this needs thorough and deep research. To date, using at least in silico methods, FaLPRs have been identified in most insects, including flies, mosquitoes, moths, kissing bugs, leaf bugs, and beetles [68, 77, 78, 81].
Alignment of five insect, two chelicerata and one crustacean FMRFa-like peptide receptors from Diploptera punctata (DippuFaLPsR, KAJ9593213.1), Schostocerca americana (SchamFaLPsR, XP_046985417.1), Anopheles gambiae (AngeFaLPsR, AAQ73620.1), Tenebrio molitor (TenmoFaLPsR, KAJ3627863.1), Drosophila melanogaster (DromeFaLPsR, NP_647758.1), Limulus polyphemus (XP_022239599.1), Argiope bruennichi (XP_055950503.1) and Panulirus argus (Christie 2020). The analysis was conducted using the DeepTMHMM server [93], accessed in January 2025. Colour black indicates seven transmembrane helices.

Receptor localisation
In most of the research, the distribution of FaLPRs was studied for the first time and the most in D. melanogaster [65], followed by mosquitos [5] and beetles [78]. Meeusen et al. [65] demonstrated that Drome-FaLPsR is present in the nervous tissues, guts, ovaries, trachea, and fat body of fruit flies. This indicates that FaLPs signalling is involved in the regulation of numerous physiological processes. Studies on two beetles, T. molitor and Z. atratus, showed similar spatial distributions to D. melanogaster, revealing FaLPRs in the central nervous system (brain, ventral nerve cord (VNC), RCC), heart, hindgut, and reproductive organs of both males and females (ejaculatory duct and oviduct) [78]. Together with the fact that the application of synthetic peptides affects the contractile activity of the above-mentioned organ muscles, it can be concluded that signalling through FaLPRs modulates the activity of these organs. On the other hand, in A. gambiae in the abdomen, the expression of FaLPR was very low, whereas samples from the head and thorax of this insect showed high receptor abundance [5]. The fact that in mosquitos, the receptor in the abdomen is present in low quantities is unusual since, FaLPs immunoreactivity was observed in the abdominal ganglia, midgut, and other visceral organs as well as in abdominal neural processes in D. melanogaster, R. prolixus, S. gregaria, Phormia regina and other insects [4, 82, 83], suggesting that the FaLPRs are also highly expressed in the abdomen of A. gambiae. This might be due to the specific way that mosquitoes digest food in comparison to beetles or flies.
FaLP receptor in crustaceans and chelicerates
FaLP receptors in crustaceans exhibit a widespread distribution throughout their nervous systems, including the brain, ventral nerve cord, and stomatogastric nervous system [58, 84]. It should be noted, however, that in the case of studies on Calanus finmarchicus, although the authors claim to have identified a true member of the FaLP receptor family, they were only able to predict five transmembrane domains (whereas GPCRs are typically characterized by 7 TMs) [58]. Additionally, in certain crustaceans, such as Panulirus argus, there are two isoforms of the FaLPs receptor. Unlike other receptor groups where diversity arises from multiple genes, the diversity of FaLP receptors appears to result from alternative splicing of a single gene [84]. Consequently, more detailed studies are required to precisely characterize these receptors in crustaceans.
In Chelicerata studies focusing on the identification of FaRP receptors are scarce. However, the distribution of FaLPs themselves seems to be similar as in crustaceans. For example, studies on the tropical wandering spider Cupiennius salei have shown that FaLPs are expressed in all ganglia of the CNS, with varying expression levels among different neurons [85]. The presence of FaLPs throughout the nervous system implies that their receptors should be similarly distributed to mediate the neuromodulatory effects of these peptides. However, further research is necessary to map the precise localization of FaLPRs and fully understand their functional roles.
In general, when available databases in NCBI were searched for FaLPR, they have been found in different arthropods groups (Supplementary material 1). Phylogenetic analysis showed similarities between the receptors. The phylogenetic tree constructed using different FaLPs receptors is in agreement with recent arthropod phylogeny, supporting the idea that receptors co-evolved with species while maintaining physiological functions (Supplementary material 1). Among arthropods, the only group in which it was not possible to identify a FaLPR was the Parasitiformes (e.g., Ixodes). Since a precursor sequence for the FaLPs was identified in this group, it is evolutionarily unlikely that the receptor was lost. In this case, one might be tempted to suggest that the receptor was lost as a consequence of the parasitic lifestyle associated with mites. However, this hypothesis would be unlikely, especially for a neuropeptide family that is widely distributed. Therefore, it is more likely that the receptor was not sequenced, or that the receptor sequence is highly derived and cannot be identified using a homology search strategy. Alternatively, it is also possible that the FaLPs neuropeptide of the Parasitiformes can activate other RFamide receptors, but this needs further investigation.
Physiological properties of FaLPs in insects
Heart and skeletal muscle contractions
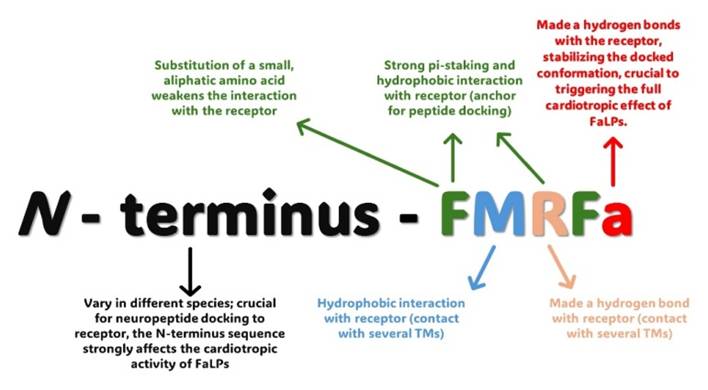
One of the well-known physiological properties of FaLPs in insects is their myotropic activity on the insect heart and visceral/skeletal muscles [9, 94]. Generally, direct and indirect regulation of muscle contractions seems to be a major physiological activity of FaLPs [78]. Notably, the first documented property of these neuropeptides was the cardioacceleratory action of the FMRFamide peptide on the heart of M. nimbosa [1]. Further research carried out on insects indicated that the cardioregulatory effects of extended FMRFamides may be dependent on the tested insect species, the amino acid sequence of the bioanalogue, and the applied concentrations [9, 78, 94]. Research on this issue showed that the tested analogues of FMRFamides evoked a stimulatory or inhibitory effect but also a biphasic response characterized by an initial increase in heart rate, which results in a cardioinhibitory effect. For example, FMRFamide injection increased the heart rate of the mosquito larva A. gambiae [95]. Additionally, Hillyer et al. [5] revealed that the injection of FMRFamide at a concentration of 10-6 M increased the heart rate of adult A. gambiae. However, the application of this peptide at a concentration of 10-2 M led to a significant decrease in the anterograde and retrograde heart rate in the mosquito [5]. Interestingly, a high concentration of FMRFamide influences the proportion of anterograde and retrograde contractions by twofold increasing the percentage of retrograde contractions after FMRFamide injection at a concentration of 10-2 M [5]. Similar results were observed after the application of SALDKNFMRFamide; however, the authors postulated that the cardioinhibitory effects are nonphysiological because they occur at concentrations much higher than those normally observed in insect haemolymph [5]. A similar dose dependent, bimodal action of FaLPs was also observed in other insects, for example, in the locust S. gregaria research by Cuthbert & Evans [96] showed that the application of one of the tested FaLP analogues (YGGFMRFamide) at high concentrations (above 10-7 M) caused an immediate increase in the heart rate of S. gregaria. After this period, the heart rate of the locust decreased. A cardioinhibitory effect was observed until YGGFMRFamide was removed from the superfusate [96]. Interestingly, at lower concentrations (up to 10-7 M), the cardioaccelerator effect of FaLPs was observed in S. gregaria only in the case of hearts characterized by regular beating [96] The cardioacceleratory property of FaLPs was also observed in beetles. Research by Marciniak et al. [78] showed that the application of FMRF6 (NSNFLRFamide) led to a dose-dependent increase in the heartbeat frequency of the semi-isolated heart of Z. atratus. In contrast to that of Z. atratus, the heart of T. molitor reacts differently, and the application of FMRF6 elicits an immediate cardioinhibitory effect, but statistically significant changes in heart rate were observed only at higher concentrations (10-5 M). Structure-activity studies have shown that C-terminal amidation seems to be crucial for the cardiostimulatory action of FMRFamides [97]. Intriguingly, the amidation of the N-terminus increases the potency of FMRFamide-like peptides [96]. Further results presented by Cuthbert & Evans [96] also support the supposition about the significance of the N-terminus structure for the cardioregulatory action of FaLPs. For example, the threshold value for the lobster FMRFamide extended analogue (TNRNFLRFamide) was 1000 times lower (10-10 M) than that for the locust FaLPs. Moreover, the effect elicited by lobster peptides on the locust heart was much stronger. Furthermore, the results obtained by Duve et al. indicate that even single amino acid replacement can be crucial for the cardioregulatory properties of FaLPs. For example, Calvi-FMRF6 (ASGQDFMRFamide) compared to Calvi-FMRF5 (APGQDFMRFamide), is not active. This result suggested that the substitution of serine with proline at the second position is crucial for the cardioacceleratory activity of FaLPs [97]. Authors proposed that these differences in the physiological activity of FaLPs could also be related to their activation of different receptors by those neuropeptides. This supposition was partially confirmed by Johnson et al. [98], who showed that DPKQDFMRFamide elicits effects that are mediated by both the FaLPs receptor and a myosuppressin receptor. These results can explain why cardioacceleratory effects are usually not very strong and are sometimes missing. In silico research by Maynard et al. study in detailed docking of the FMRFa and other FMRFa-like peptides to the FMRF receptor and their cardiotropic activity on D. melanogaster heart [66]. The authors mostly focused on the importance of C-terminus and N-terminus modifications during interaction of FaLPs with their receptor (Figure 3) [66]. However, further research is needed to fully evaluate the mode of action of FaLPs on the heart of this insect. It should also be mentioned that the close connection between FaLPs signalling and the regulation of heart physiology was also confirmed by the results of immunohistochemical studies showing the localization of nerves exhibiting FMRFamide-like immunoreactivity. For example, research by Calvin and Lange [99] on the stick insect Baculum extradentatum revealed FMRFamide-like immunoreactive staining inter alia in nerve branches of segmental nerves projecting to the heart and nerves innervating the heart ostia [99]. For example, research by Calvin and Lange [99] on the stick insect Baculum extradentatum revealed FMRFamide-like immunoreactive staining inter alia in nerve branches of segmental nerves projecting to the heart and nerves innervating the heart ostia [99].
FMRFamide-like peptides are not only important for the regulation of heart contractility. Investigations concerning the myotropic activity of these neuropeptides indicate that FaLPs are crucial for the regulation of motoneuron activity and skeletal muscles. For example, research by Evans & Myers [100] conducted on the locust S. gregaria showed that FMRFamide and related peptides modulate the twitch tension of the extensor-tibiae muscle by stimulating the slow excitatory motor neuron (SETi). Furthermore, the effect of FMRFamide on the tension induced by SETi was additive to the stimulatory effect elicited by octopamine and proctolin [100]. Interestingly, FMRFamide did not affect the tension induced by the fast motor neuron (FETi) [100]. Further research on this issue showed that the stimulatory effect of FMRFamide on slow muscle fibres, similar to its effect on heart contractility, is dose dependent [100, 101]. Additionally, similar observations were reported for FMRFamides identified in D. melanogaster. Research by Hewes et al. [102] showed that different FMRFamide bioanalogues processed from FaLPs precursor significantly affect the twitch tension of Drosophila larval body wall muscles [102]. Intriguingly, for the mixtures of tested FMRFamide analogues, the muscle response was stronger than that for the FMRFamide analogue alone [102]. Additionally, one of the tested analogues, DPKQDFMRFamide, increased the amplitude of the excitatory junctional current (EJP), which may suggest that FaLPs improve synaptic transmission [102]. The effect of DPKQDFMRFamide on Drosophila muscle was also confirmed, for example, by Dunn and Mercier [103], who showed that DPKQDFMRFamide selectively modulates the amplitude of the EJP associated with motor neurons innervating the ventral longitudinal muscles of D. melanogaster. The effect of FaLPs on insect muscle contractions was also confirmed at the organismal level. Research by Klose et al. [73] proved that the application of DPKQDFMRFamide enhances the fictive locomotion of Drosophila flies. On the other hand, the FMRFamide analogues F(D-MET)RFamide and YGGFMRFamide did not significantly affect the neurally evoked contractions or resting tension of the coxal depressor muscles of the cockroach P. americana [104].
The structure-activity studies performed, for example, by Walther et al. [105] and Evans & Myers [100], showed that the C-terminal sequence is essential for the activity of skeletal muscles. Walther et al. [105] showed that FMRFamide and YGGFMRFamide activity is maintained when methionine is replaced by another neutral amino acid but not when it is replaced by an acidic one. Additionally, research by Cuthbert and Evans [96] showed that FLRFamide and FMRYamide can induce submaximal effects at concentrations of 10-5 M. Furthermore, FPRFamide, RMRFamide, FGRFamide, and pQGRFamide (anthoRFamide, an anthozoan peptide) did not affect the twitch tension of the extensor tibiae muscle in the locust S. gregaria. In addition, FMRF analogues without C-terminal amidation did not have stimulatory effects on locust muscles too [96]. Research by Evans and Myers [100] revealed that the C-terminal dipeptide RFamide is also not active. These authors also noted the important role of the N-terminal sequence in shaping muscle activity via FaLPs. Similar to research conducted on insect hearts, the extended form of YGGFMRFamide is much more potent than FMRFamide [96, 100].
Summary of the interactions between insects FaLPs with FaLPs receptor based on the in silico analysis performed by Maynard et al. [66].
The role of FaLPs in the functioning of skeletal muscles was also confirmed by immunohistochemical staining. Several investigations have shown that antibodies against FaLPs or RFamides bind to neurons that innervate insect skeletal muscles [104]. Furthermore, research conducted on the cockroach P. americana revealed numerous stained cell bodies in the methathoracic ganglion and peripheral nerves that contain motor and sensory neurones. Axons of these neurones, for example the femur-tibia, joint in P. americana. Additionally, Myers and Evans [82] found FMRF-like immunoreactive (using bovine pancreatic polypeptide (BPP) antiserum) neurons associated with muscles in the locust S. gregaria. Authors have shown that immunoreactive nerves project to spiracle muscles or dorsal longitudinal flight muscles [82].
Despite wide knowledge concerning the role of FaLPs in the activity of insect muscle, the strict mode of the regulatory action of these neuropeptides has not been fully characterized. Recent research has shown that FaLPs act via GPCR receptors [67]. However, as we mentioned previously, FMRFamide may activate not only specific FaLPs receptors but also receptors for myosuppressin [98]. These results are supported by those of Klose et al. [73], who demonstrated that DPKQDFMRFamide enhanced synaptic transmission in D. melanogaster neuromuscular junctions through activation of the FaLP receptor and Dromyosuppressin Receptor-2 (DmsR-2). On the other hand, Milakovic et al. [67] showed that the frequency of muscle contractions in Drosophila larvae induced by DPKQDFMRFamide was significantly decreased in individuals with reduced expression of the gene encoding the FaLPs receptor. However, Milakovic et al. [67] did not suggest the involvement of myosuppressin receptors in the induction of myotropic effects after the application of DPKQDFMRFamide.
After activation of the FaLPs receptor, the myotropic activity of FaLPs is closely associated with Ca2+ influx. Research by Clark et al. [106] showed that the application of nifedipine and nicardipine, which are potent blockers of L-type channels, completely abolished the effect of DPKQDFMRFamide on Drosophila muscles. However, T-type channel blockers did not cause a similar effect. The close connection between the Ca2+ and FaLP modes of action was also postulated by Klose et al. [73], who revealed that the application of DPKQDFMRFamide increased the presynaptic Ca2+ response and the quantal content of the released transmitter.
Moreover, Dunn and Mercier [103] showed that calmodulin-dependent protein kinase II (CamKII) is an important effector protein in FaLPs signalling. However, Milakovic et al. obtained different results and negated the role of CamKII in the myomodulatory action of FaLP [67]. Interestingly, in both studies, the authors used the same model organism (D. melanogaster, larvae from the third stage of the wandering stage) and used a similar method of confirmation of CamKII involvement in FaLP signalling, including analysis of the effect of the same neuropeptide (DPKQDFMRFamide) and CamKII inhibitor (KN-93). The only significant difference is the fact that Dunn and Mercier [103] tested presynaptic effects by evoked EPJ recorded in the ventral longitudinal muscles, but Milakovic et al. [67] focused on the postsynaptic effect and analysis of muscle tonus and contractions. As Milakovic et al. suggested the postsynaptic and presynaptic effects of a modulatory substance might be regulated by different intracellular signalling pathways and receptors. Furthermore, based on the literature and their own research, authors postulated a possible model of DPKQDFMRFamide activity based on the fact that this peptide activates the G protein, whose subunits act directly on L-type channels to stimulate Ca2+ influx. Ca2+ influx subsequently elicits skeletal muscle contraction via the calcium-induced release of calcium from the sarcoplasmic reticulum [67]. The involvement of other second messengers, such as cyclic nucleotides, IP3, arachidonic acid, and phospholipase C (PLC), was negated in research conducted on S. gregaria and D. melanogaster [67, 100, 101]. However, the effects of some second messengers, such as linoleic acid, have still not been tested.
Circadian clock regulation
FaLPs have been implicated in the regulation of circadian rhythms in insects. Soechler et al. [107] revealed the presence of FaLPs in the accessory medulla (AMe), which is known as the circadian clock that controls circadian locomotor activity rhythms in Leucophea maderae. FMRFamide-like immunoreactivity was found in four of the six soma groups associated with the AMe, as well as in most neuropils of the protocerebrum. In the same study, the injection of Peram-FMRFa-7 (DRSDNFIRFamide), previously identified in P. americana [44], caused time-dependent phase changes in the locomotor activity rhythms of L. maderae cockroaches at circadian times 4 and 8 (CT 4 and CT 8). The change caused by Peram-FMRFa-7 at CT 4 was also positively correlated with the dose of this peptide, as phase delays decreased with decreasing amounts of the peptide injected. These results suggest that this peptide could be responsible for activity changes during the early day [107].
It was also shown that FaLPs and the FaLPR are involved in modulating the sleep induced by cellular stress. Drosophila mutants for FaLPs or its receptors were shown to have a reduced recovery sleep caused by heat stress or infection with Serratia marcescens and became infected more quickly than controls [108].
FaLPs are also supposed to play an important role in insect diapause. Hao et al. [109] have detected FaLPs encoded by the Flrf gene under a short and long photoperiod in the CNS of L. migratoria. The presence of two of them (GSERNFLRFa and DRNFIRFa) was detected only in the group of insects treated with a short photoperiod, suggesting their role in the induction of photoperiodic diapause. Their results showed that the Flrf gene promotes diapause in locusts [109]. They also proposed that FaLPs expressed from the Flrf gene are regulated by the photoperiod and subsequently activate downstream FOXO and ROS to induce diapause of L. migratoria, but specific mechanisms of this regulation are still unknown.
Reproduction
FaLPs activity also occurs in the insect reproductive system. In the case of male insects, transcripts for extended FMFRamide receptors have been identified in various parts of this system in R. prolixus, with the highest expression in the vas deferens and the ejaculatory duct [110]. The same authors also reported FMRFamide-like immunoreactivity in other reproductive organs, such as seminal vesicles and accessory gland ducts, of this insect. The presence of the receptor for extended FMRFamide was also confirmed in the ejaculatory ducts of two beetle species, T. molitor and Z. atratus [78].
Further in vitro tests on the ejaculatory duct, transparent accessory gland, and seminal vesicle muscles confirmed these results, showing a concentration relationship between the activity of AKDNFIRFamide and an increase in contractile activity, expressed as an increase in the frequency of contractions [110]. Marciniak's research in which NSNFLRFamide was applied to the muscles of the ejaculatory duct of the beetle T. molitor showed a high (over 70%) but irregular increase in the frequency of contractions [78].
In females, FaLPs immunoreactivity (FLI) have been detected in the calyx of kissing bugs, but their presence in the ovaries and ovarioles has not been confirmed [4]. However, processes related to FMRF-like immunoreactivity were observed in the oviduct, both lateral and common parts, bursa copulatrix, and spermatheca [4]. Similar effects were observed in L. migratoria, in which FLI was observed in the muscles of spermatheca [111]. This was partially confirmed in T. molitor and Z. atratus, in which the presence of the receptor for extended FMRFamide was confirmed [78].
Physiological bioassays confirmed the described results. The application of AKDNFIRFamide and GNDNFMRFamide to organs such as ovaries and oviducts caused a dose-dependent increase in muscle tone [4], similar to what was observed in the case of L. migratoria and YGGFMRFamide, which led to an increase in the contraction of oviduct muscles [111]. The application of NSNFLRFamide on the oviduct of the beetle T. molitor caused an increase in the frequency of muscle contractions [78]. After the application of FaLPs to organs such as the ovaries, the presence of receptor transcripts for these neuropeptides has not been demonstrated [4].
Other physiological properties
FaLPs are also involved in energy balance regulation and digestion and can alter the activity of digestive enzymes. Hill and Orchard [112] revealed that endogenous FaLPs, GQERNFLRFamide and AFIRFamide, similar to locusta acronym MS (previously designated as SchistoFLRFamide), increased total amylase and α-glucosidase activity in vitro in the midgut lumen of L. migratoria. However, GQERNFLRFamide and AFIRFamide did not cause significant changes in the total activity of digestive enzymes in tissue extracts at the tested concentration of 10-8 M [112]. In a previous study, GQERNFLRFamide was shown to have no effect on midgut muscle contractions [113]. It has also been shown that FMRFamide and its cognate receptor FMRFR mediate the fat loss effect of dietary cysteine in D. melanogaster. When cysteine consumption is increased, FaLP triggers lipid degradation by activating FaLPR and the downstream PKA pathway in fat body cells, while at the same time, in the CNS of flies, the peptide and receptor reduce the sensitivity of sweet-sensing gustatory neurons and hence food consumption [114]. FaLPRs have also been associated with the larval to pupal transition under nutrient-limiting conditions [74].
Other investigations have shown that FaLPRs are also responsible for the modulation of insect flight. D. melanogaster mutants of the gene encoding Drome-FMRFR exhibit significant flight deficits associated with dopaminergic cells. The downregulation of expression of gene for the receptor by specific RNAi in adult central dopaminergic neurons results in the progressive loss of sustained flight [76]. In addition to the regulation of visceral muscle activity, FaLPs and their receptors have been associated with an escape response to intense light in Drosophila larvae [73]. Exposure to bright light is a stress factor, and a quick reaction protects larvae from desiccation. The receptor, as well as a receptor for dromyosuppressin, is required for this behaviour, as the knockdown of these receptors causes a significant reduction in locomotor activity during the escape response [73]. Knockdown of the FaLPs receptor in D. melanogaster causes a reduction in heat stress- or infection-induced sleep and reduces startle-induced locomotor activity [108, 115, 116].
Physiological properties of FaLPs in crustaceans
As mentioned above FaLPs in crustaceans have been isolated from diverse neurosecretory and nervous tissues, such as pericardial organs, eyestalks, the brain, and the thoracic, stomatogastric and commissural ganglia [48, 49, 51-54, 117-127].
The physiological activity of the crustacean FaLPs have been studied in different species, including the lobster H. americanus, the crab C. sapidus, C. magister, Cancer productus, C. borealis, Carcinus means, the crayfish P. clarkii, the giant freshwater prawn M. rosenbergii, and the giant tiger prawn P. monodon (Table 2). FaLPs have been shown to regulate many systems in these animals, including circulatory system, stomatogastric nervous system, and muscle system [12, 128, 129].
Circulatory system: influence of FaLPs on the heart
The first FaLPs identified in crustacean, lobster H. americanus pericardial organs were TNRNFLRFa (F1) and SDRNFLRFa (F2) [49]. These peptides were tested on C. means heart and have positive chronotropic and negative inotropic effects with reduced stroke volume, arterial pulse pressure and ventricular pressure [130]. Mercier and Russens [131] used an isolated heart from P. clarkii to test TNRNFLRFa and SDRNFLRFa. An increase in the rate and amplitude of heartbeats was observed. McGaw et al. [132] and McMahon [133] tested SDRNFLRFa and TNRNFLRFa in vitro and in vivo on the C. magister heart. Both peptides caused cardioexcitation when they were semi-isolated and, in contrast, had cardioinhibitory effects in vivo. The TNRNFLRFa peptide was also tested in H. americanus hearts and was shown to increase the rate and strength of the heartbeat [134]. The SDRNFLRFa peptide tested on H. americanus ostial valve muscle caused an increase in the amplitude of electrically evoked contractions [135]. Similar to H. americanus peptides, two P. clarkii peptides (DRNFLRFa - DF2 and NRNFLRFa - NF1) were found to increase the heart rate and amplitude of the semi-isolated P. clarkii heart [51, 136]. Krajniak [48] isolated and identified GYNRSFLRFa from crab C. sapidus. The peptide caused a dose-dependent increase (10-9-10-5 M) in crab heart rate. Fort et al. [137] examined the effects of TNRNFLRFa, SDRNFLRFa, and GYNRSFLRFa on C. sapidus heart. All the tested FaLPs showed both chronotropic and inotropic positive effects. In 2006, Cruz-Bermudez et al. identified and sequenced the neuropeptide GAHKNYLRF from C. borealis, C. productus, and C. magister [121]. This peptide increased the frequency of bursts and the number of spikes per burst of the isolated cardiac ganglion in C. borealis. When one sums up the effects of different FaLPs on the crustacean's circulatory system it is rather clear that these peptides pose cardioaccelerator activity (Table 2) which is similar to insects (Table 3). To date any tests were done whether these peptides are influenced in regulation of metabolism. However, regulation of heart activity highly likely indirectly influence other physiological processes in crustaceans.
Summary of known physiological properties of FaLPs in crustaceans
| Function | Neuropeptide | Species | References |
|---|---|---|---|
| Cardiotropic effects: | |||
| positive chronotropic and negative inotropic effects on heart | TNRNFLRFa (F1) SDRNFLRFa (F2) | Carcinus means | [130] |
| increase in the rate and amplitude of heartbeats | TNRNFLRFa (F1) SDRNFLRFa (F2) | Procambarus clarkii | [131] |
| DRNFLRFa (DF2) NRNFLRFa (NF1) | Homarus americanus | [51, 136] | |
| cardioexcitation in semi-isolated heart and cardioinhibition in vivo | TNRNFLRFa (F1) SDRNFLRFa (F2) | Cancer magister | [132, 133] |
| increase in the rate and strength of the heartbeat | TNRNFLRFa (F1) | Homarus americanus | [134] |
| increase in the amplitude of electrically evoked contractions of ostial valve muscle | SDRNFLRFa (F2) | Homarus americanus | [135] |
| dose-dependent increase of heart rate | GYNRSFLRFa | Callinectes sapidus | [48] |
| chrono- and inotropic positive effects | TNRNFLRFa (F1) SDRNFLRFa (F2) GYNRSFLRFa | Callinectes sapidus | [137] |
| increase the frequency of bursts and the number of spikes per burst | GAHKNYLRFa | Cancer borealis | [121] |
| Motility of the digestive system: | |||
| activation of gastric motor patterns | TNRNFLRFa (F1) SDRNFLRFa (F2) | Cancer borealis | [50] |
| activation of pyloric and gastric mill | TNRNFLRFa (F1) SDRNFLRFa (F2) | Cancer borealis | [50, 139, 142] |
| Homarus americanus | [140] | ||
| Procambarus clarkii | [141] | ||
| Myotropic effects: | |||
| increase in nerve-evoked tension and increase of postsynaptic potentials | TNRNFLRFa (F1) SDRNFLRFa (F2) | Procambarus clarkii | [143] |
| rhythmic depolarizations and contractions | YGGFMRFa | Palaemon serratus | [144] |
| tonic contractions of muscle without nerve activation | TNRNFLRFa (F1) | Homarus americanus | [134] |
| increase of amplitude of excitatory junctional potentials | NRNFLRFa DRNFLRFa | Procambarus clarkii | [136] |
Comparison of known physiological properties of FaLPs in insects and crustaceans

Stomatogastric nervous system: influence of FaLPs
Skiebe [138] reviewed the peptidergic modulation of the stomatogastric nervous system in crustaceans. Thus, this review only briefly describes the functions of FaLPs in the stomatogastric nervous system. In 1993, Weimann and Marder isolated and sequenced two FaLPs, TNRNFLRFa and SDRNFLRFa, from extracts of the stomatogastric nervous system of the crab C. borealis, which were previously found in the pericardial organs of H. americanus [50]. Both peptides activated gastric motor patterns in the crab. Pyloric and gastric mill activation was also observed in several other studies [50, 139-142]. As with the circulatory system FaLPs cause a stimulatory effect on gastrointenstinal tract in crustaceans. However, it is not known whether they activate the receptor, or this is somehow indirect effects. Increased activity of digestive tract can be related to increased activity of circulatory system what in turn may help in absorption and distribution of nutrients.
Muscle system: influence of FaLPs
FaLPs modulate somatic muscle contractions and neuromuscular transmission in crustaceans in the same manner as they do in insects (Table 3). Mercier et al. [143] tested TNRNFLRFa and SDRNFLRFa in the phasic extensor muscles of the abdomen of P. clarkii and reported an increase in nerve-evoked tension and excitatory postsynaptic potentials. Meyrand and Marder [144] tested FaLPs on the pyloric dilator muscle of the shrimp Palaemon serratus and observed sequences of rhythmic depolarizations and contractions. Worden et al. [134] again examined the TNRNFLRFa in nerve-muscle preparations from H. americanus. This peptide induced tonic contractions of the exoskeletal muscle in the absence of nerve activity, which suggests direct action on the dactyl opener muscle. Skerrett et al. [136] examined NRNFLRFa and DRNFLRFa in the neuromuscular junctions of the deep abdominal extensor muscles of P. clarkii. The two peptides increased the amplitude of excitatory junctional potentials in the muscles tested.
What is really known about physiological properties of FaLPs in other arthropods?
As was already described, the number of reports regarding the identification of FaLPs transcripts in tissues of various Arthropod species, other than insects or crustaceans, is increasing [2, 85, 117, 145-147]. However, information about the physiological properties of these peptides has been still very limited.
Ticks
FaLPs were shown, using the immunocytochemical method, to be broadly distributed in the synganglion of two tick species Ornithodoros parkeri and Dermacentor varisbilis [148]. The results of the analysis of synganglion transcriptomes of other tick species (Ixodes scapularis, Rhipicephalus microplus) seem to confirm these findings [146, 149]. It was also revealed that FaLPs were present in neurons that innervate the coxal muscles in O. parkeri, suggesting their involvement in locomotor control [148] what was also proved in other arthropods.
Spiders
Using the polyclonal antibody against FMRFamide-like peptides, wide distribution of FaLPs were observed in the central nervous system, especially in the visual neuropils of the spider Cupiennius salei [147, 150]. They were also found in mechanosensory neurons and nerve fibres projecting to each leg ganglion in C. salei [147]. Fabien-Fine and co-workers showed that in some central and mechanosensory neurons of C. salei FaLPs co-loaclizedlocalized with acetylcholine [147]. Furthermore, groups of neurons in which FaLPs co-localized with glutamate were observed in this species [85]. This suggests that these molecules can be co-released and FaLPs could be engaged in synaptic transmission as a neurotransmitter and/or a neuromodulator.
Horseshoe crab
FaLPs immunoreactivity has been found in the central and peripheral nervous system and muscles of the horseshoe crab Limulus polyphemus [151-153]. The extensive distribution of these peptides in nervous tissue indicates a possible role of these peptides in synaptic transmission or modulation of pathways in the CNS.
The crustacean FaLPs (TNRNFLRFamide; SDRNFLRFamide, GYNRSFLRFamide) were tested on the heart of L. polyphemus and caused chronotropic and inotropic stimulation of the heartbeat in a dose-dependent manner. This was also show in crustaceans (see above). They also increased the excitability and contractility of the cardiac muscle fibers. TNRNFLRFamide and SDRNFLRFamide enhanced excitation of cardiac ganglion neurons by increasing the burst rate [154]. FaLPs fractions isolated from the brain of L. polyphemus also caused an increase in the frequency of the heart rate as well as the amplitude of contractions of the heart of the horseshoe crab [151, 154]. The results of another study suggest that FaLPs can be released by the intestinal nerve axons and regulate the motility of the gut of L. polyphemus. The crustacean FaLPs relaxed the gut of L. polyphemus, and also decreased its contraction activity induced by proctolin. The effect of the intestine relaxation was also obtained by applying FaLPs fractions from HPLC on the L. polyphemus midgut preparations [153].
Similar physiological properties of mammalian RFamide peptides (upper row) and insect FaLPs (lower row). FMRFamide-like peptides (FaLPs) are neuropeptides that play a pivotal role in regulating various physiological processes in insects and other arthropods including behaviour, reproduction, and homeostasis. FaLPs mostly act through G-protein coupled receptor and influence muscle activity by modulating Ca2+ influx. Historically, the function described for these neuropeptides was primarily associated with myostimulatory activity. After more than three decades of research, it is now well established that FaLPs are implicated in the regulation of circadian rhythms, affecting locomotor activity and phase changes in response to environmental cues. During reproduction, FaLPs influence contractile activity in both the male and female reproductive systems. They also participate in physiological processes such as diapause induction, sleep modulation, and flight regulation in insects. Similarly, in crustaceans, FaLPs regulate the circulatory system, stomatogastric nervous system, and muscle contractions. Nowadays, it is also known how the physiological properties of FaLPs in arthropods share similarities with mammalian RFamide peptides, which are involved in a wide range of functions, including muscle contraction, feeding, reproduction, and stress responses, mediated through various RFamide receptors. Therefore, summarizing the investigated physiological functions in arthropods may be relevant also for future research aiming to test their activity in other organisms such as mammals.
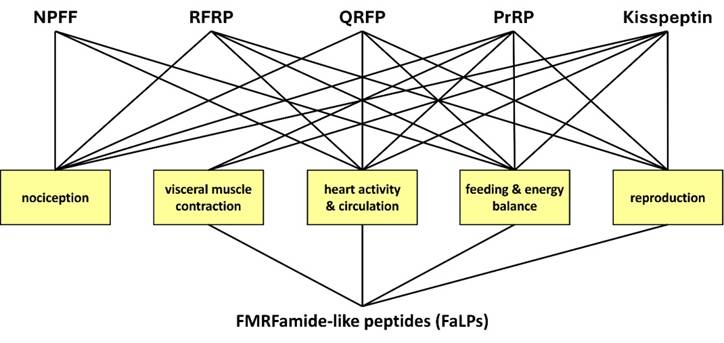
Similarities between insects FaLPs and mamallian peptides from -RF family
In mammals, five genes encode RFamide peptides. These peptides are expressed in the brain and some peripheral tissues [155]. The first two mammalian RFamide-like peptides, the neuropeptides FF and AF, have been isolated from the bovine brain [156]. Currently, the increasing number of RFamide peptides found in mammals can be subdivided into five groups: the neuropeptide FF (NPFF) group, RF-amide related peptide (RFRP) group, pyroglutamylated RF-amide peptide (QRFP) group, kisspeptin/metastin group and prolactin-releasing peptide (PrRP) group [157].
RFamide peptides, similarly as FaLPs in insects, are widely distributed within the central and peripheral nervous system. Moreover, they have been detected in many peripheral tissues and organs, such as the spleen, lungs, heart, pancreas, adrenal glands, placenta, pituitary gland, thymus, and macrophages [155, 157-163]. The distribution of members of different RF-amide peptide groups varies and shows species specificity.
The activity of RFamide peptides results from the ability to activate a wide group of RFamide peptide receptors. Several of these proteins have been identified, including NPFFR1, NPFFR2, QRFPR, prolactin-releasing peptide receptor (PrRPR), and Kiss1R. All of these receptors are GPCRs and commonly activate Gi/o signal transduction pathways [159]. The selectivity of receptors for binding a specific ligand is quite high, and RFRP activates NPFFR1, NPFF interacts with NPFFR2, QRFP interacts with QRFPR, PrRP interacts with PrRPR, and kisspeptin binds to Kiss1R. Nevertheless, some RFamide peptides can activate more than one type of receptor, where NPFFR1 and NPFFR2 can be activated by all RF peptides but with various efficiencies, whereas QRFPR, PrRPR, and Kiss1R cannot, and their affinity for endogenous peptide ligands is very strict [158].
The wide distribution of RFamide peptides and their receptors within the nervous system and peripheral tissue confirms their important role in the regulation of many physiological processes. This is also the case for FaLPs. RFa peptides play an equally important role in the central processing of visceral autonomic signals related to feeding, energy balance, generation of central cardiovascular responses, muscle contraction, reproduction, nociception, neuroendocrine regulation, response to stresses, and behaviour [158, 161]. Such wide physiological activity is like that of insects and other arthropods, especially in terms of regulation of muscle contraction activity, feeding and reproduction (Figure 3). Further studies revealing not only physiological but also structural similarities on the level of precursors and receptors between FaLPs and mammalian RFamides are needed. On the other hand, the current knowledge about the physiological functions of FaLPs is discontinuous. Different cell specific processing of the precursor has not been shown thus further studies about the role of different bioanalogues in the precursor is of interest, especially when only one receptor is present.
As neuropeptides are mainly considered as activators of G-protein coupled receptors, the FMRFa is a notable example of a neuropeptide which can directly activate also a ionotropic receptors [79, 80]. An example are FMRFa-gated sodium channels (FaNaC). They belong to the epithelial sodium channel/degenerin (ENaC/DEG) superfamily of cation channels [79, 80]. This superfamily includes seven families, three of which were first described in vertebrates: ENaC, Acid-Sensing Ion Channels (ASICs), and Brain-Liver-Intestine Sodium Channel (BLINaC)/Human Intestine Sodium Channel (hINaC). Other ENaC/DEG families were reported in invertebrates: the Degenerins from C. elegans, the Drosophila PPK channels, the FMRFamide-gated Sodium Channel (FaNaC) from mollusks and annelids, the FLR-1 receptor that was only identified in C. elegans and Hydra-RFamides I and II activated sodium channels [164-166]. The ability to activate the ENaC/DEG channels by FaLPs were demonstrated for mollusks, annelids, Hydrozoa and partially for mammals. When the invertebrate FaNaC from Helix aspersa were expressed in rat hippocampus slice, it was shown that the application of FMRFamide is able to activate the sodium channel current across the cell membrane of neurons and produces large prolonged depolarizations and bursts of action potentials. Nevertheless, mammalian neuropeptides neuropeptide FF and RFRP-1, which have amidated RF terminus, did not affect HaFaNaC-expressing neurons [167]. However, the binding of FMRFa or similar RFamides to ASICs has been shown to enhance proton-gated currents [168]. To date, there is no evidence that FaLPs can activate the insect ENaC/DEG belonging to pickpocket (PPK) receptors which mediate diverse functions such as the detection of mechano- and chemo-sensory stimuli [164]. Of course, that does not mean there is a lack of this activity of FaLPs in insects. It rather shows new perspectives for further studies and a deeper glimpse into the evolutionary history of FaLPs as well as their receptors.
Conclusions and future directions
Nowadays, it is possible to identify and compare genetic sequences of both neuropeptide precursors and receptors using publicly available datasets. Unfortunately, these sequences do not reveal much about the processed peptides that likely activate the corresponding receptors. In this context, more studies based on mass spectrometry analyses will improve our understanding far more than those based on transcriptome data. However, the greatest limitation is the lack of comprehensive studies on the functions of this group of neuropeptides. Current knowledge about the physiological functions of FaLPs peptides in insects and other arthropods is indeed still discontinuous. Besides the fact that few physiological functions are shared within Pancrustacea (mainly myotropic), there may be species-specific functions in each group (Table 3). To date, significantly less studies have been performed on neurohormonal regulation (also including FaLPs) of physiological processes in crustaceans, so it is extremely challenging to compare the exact physiological properties of these peptides between different arthropod groups. However, the reduced genetic differences in FaLPs genes between crustaceans and insects strongly support the homologous origin of these genes. In Chelicerates and Myriapods, detailed studies on FaLPs are scarce, and it is likely that these peptides perform similar functions in those two groups of arthropods as they do in insects and crustaceans. Nevertheless physiological studies in these groups are needed. This is even more important if FaLPs will be considered, together with other insect neuropeptides, as molecules used to design ecofriendly bioinsecticides [169, 170]. To evaluate physiological properties of FaLPs the strategy might involve use factors which affect biostability of peptides, such as additional chemical groups attached to the neuropeptide molecule, e.g., polyethyleneglycol (PEG). So far, several different insect neuropeptide analogues with enhanced biostability have been invented [171-176]. For example, PEG-stabilised pyrokinin analogues have been shown to be effective as pest control agents targeting aphids [171].
In insects the exact physiological functions of FaLPs are also not precisely known. So far it is confirmed that FaLPs poses myotropic properties on different visceral muscles and are involved in regulation of cardiac functioning. This is probably due to the fact that historically most of the neuropeptide studies focused on the evaluation of the myotropic activity. However, myoactive properties of FaLPs might indirectly influence other important processes such as reproduction, digestion and feeding. Apart from single studies the exact mechanism in not addressed. Similarly possible direct or indirect (due to myotropic functions) involvement of FaLPs in metabolism needs to checked. This is even more important when we take into account that FaLPs can be involved in the regulation of the circadian clock. In this context also interaction of FaLPs with other neuropeptides should be checked with usage of available techniques such as RNAi or CRISPR/CAS.
Finally, thus far, only one type of receptor, from the GPCR family, has been found in insects and other arthropods. Some studies suggest that FaLPs can also activate the ionotropic receptor [79]. So far it has been proved in mollusks, however, it might also be possible in arthropods. It is even more possible if one considers the neuromodulatory and myomodulatory effects of FaLPs, which seem to be highly conserved across different groups of arthropods.
Another unaddressed question is related to evolutionary relations between FaLPs and other RF peptides (especially mammalian RFa peptides). The differences in RFamide peptide gene sequence in mammals and the shared physiological functions with Pancrustacea suggest evolutionary convergence among these gene groups between vertebrates and invertebrates. This convergence may have evolved from a common ancestor of the two lineages. For instance, in tunicates, RFamide peptides have been described and show a conserved role in the regulation of muscle contraction. Therefore, this suggests that, at least for FaLPs peptides, both the genes and the main physiological functions are homologous between invertebrates and vertebrates. At the same time, other physiological functions are likely to evolve because of convergence, and are thus analogous, both within FaLPs and other -RFamide peptides.
Supplementary Material
Supplementary figures.
Acknowledgements
The study was supported by a project funded by the National Science Center no. 2021/B/NZ3/00221. AI-assisted software (Curie module provided by AJE service) was used to improve the readability and language of the manuscript. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Price DA, Greenberg MJ. Structure of a molluscan cardioexcitatory neuropeptide. Science. 1977;197:670-1
2. Christie AE, Chi M. Neuropeptide discovery in the Araneae (Arthropoda, Chelicerata, Arachnida): Elucidation of true spider peptidomes using that of the Western black widow as a reference. Gen Comp Endocrinol. 2015;213:90-109
3. Christie AE, Stemmler EA, Dickinson PS. Crustacean neuropeptides. Cell Mol Life Sci. 2010;67:4135-69
4. Sedra L, Lange AB. The female reproductive system of the kissing bug, Rhodnius prolixus: arrangements of muscles, distribution and myoactivity of two endogenous FMRFamide-like peptides. Peptides. 2014;53:140-7
5. Hillyer JF, Estevez-Lao TY, de la Parte LE. Myotropic effects of FMRFamide containing peptides on the heart of the mosquito Anopheles gambiae. Gen Comp Endocrinol. 2014;202:15-25
6. Lange AB, Calvin A, da Silva R. Neuropeptides modulate the heart of the stick insect Baculum extradentatum. Ann N Y Acad Sci. 2009;1163:448-50
7. Nässel DR, Zandawala M. Recent advances in neuropeptide signaling in Drosophila, from genes to physiology and behavior. Prog Neurobiol. 2019;179:101607
8. Nässel DR. Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog Neurobiol. 2002;68:1-84
9. Orchard I, Lange A, Bendena W. FMRFamide-related peptides: a multifunctional family of structurally related neuropeptides in insects. 2001.
10. Nichols R. Signaling pathways and physiological functions of Drosophila melanogaster FMRFamide-related peptides. Annu Rev Entomol. 2003;48:485-503
11. Orchard I, Lange AB. FMRFamide-like peptides (FLPs). The handbook of biologically active peptides. 2013;3:237-46
12. Walker RJ, Papaioannou S, Holden-Dye L. A review of FMRFamide- and RFamide-like peptides in metazoa. Invert Neurosci. 2009;9:111-53
13. Cazzamali G, Torp M, Hauser F, Williamson M, Grimmelikhuijzen CJP. The Drosophila gene CG9918 codes for a pyrokinin-1 receptor. Biochem Biophys Res Commun. 2005;335:14-9
14. Blaser M, Predel R. Evolution of Neuropeptide Precursors in Polyneoptera (Insecta). Front Endocrinol (Lausanne). 2020;11:197
15. Wegener C, Gorbashov A. Molecular evolution of neuropeptides in the genus. Genome Biology. 2008;9:R131
16. Nambu JR, Murphy-Erdosh C, Andrews PC, Feistner GJ, Scheller RH. Isolation and characterization of a Drosophila neuropeptide gene. Neuron. 1988;1:55-61
17. Schneider LE, Taghert PH. Isolation and Characterization of a Drosophila Gene That Encodes Multiple Neuropeptides Related to Phe-Met-Arg-Phe-Nh2 (Fmrfamide). Proc Natl Acad Sci U S A. 1988;85:1993-7
18. Derst C, Dircksen H, Meusemann K, Zhou X, Liu S, Predel R. Evolution of neuropeptides in non-pterygote hexapods. BMC Evol Biol. 2016;16:51
19. Ly A, Ragionieri L, Liessem S, Becker M, Deininger SO, Neupert S. et al. Enhanced Coverage of Insect Neuropeptides in Tissue Sections by an Optimized Mass-Spectrometry-Imaging Protocol. Anal Chem. 2019;91:1980-8
20. Veenstra JA. The contribution of the genomes of a termite and a locust to our understanding of insect neuropeptides and neurohormones. Frontiers in Physiology. 2014;5:454
21. Ragionieri L, Verdonck R, Verlinden H, Marchal E, Vanden Broeck J, Predel R. Schistocerca neuropeptides - An update. J Insect Physiol. 2022;136:104326
22. Hou L, Jiang F, Yang P, Wang X, Kang L. Molecular characterization and expression profiles of neuropeptide precursors in the migratory locust. Insect Biochem Mol Biol. 2015;63:63-71
23. Liessem S, Ragionieri L, Neupert S, Buschges A, Predel R. Transcriptomic and Neuropeptidomic Analysis of the Stick Insect, Carausius morosus. Journal of Proteome Research. 2018;17:2192-204
24. Tsang PW, Orchard I. Distribution of FMRFamide-related peptides in the blood-feeding bug Rhodnius prolixus. J Comp Neurol. 1991;311:17-32
25. Huybrechts J, Bonhomme J, Minoli S, Prunier-Leterme N, Dombrovsky A, Abdel-Latief M. et al. Neuropeptide and neurohormone precursors in the pea aphid, Acyrthosiphon pisum. Insect Mol Biol. 2010;19:87-95
26. Hummon AB, Richmond TA, Verleyen P, Baggerman G, Huybrechts J, Ewing MA. et al. From the Genome to the Proteome: Uncovering Peptides in the <i>Apis</i> Brain. Science. 2006;314:647-9
27. Sadd BM, Barribeau SM, Bloch G, de Graaf DC, Dearden P, Elsik CG. et al. The genomes of two key bumblebee species with primitive eusocial organization. Genome Biology. 2015;16:76
28. Kanost MR, Arrese EL, Cao X, Chen Y-R, Chellapilla S, Goldsmith MR. et al. Multifaceted biological insights from a draft genome sequence of the tobacco hornworm moth, Manduca sexta. Insect Biochem Mol Biol. 2016;76:118-47
29. Shi Y, Li J, Li L, Lin G, Bilal AM, Smagghe G. et al. Genomics, transcriptomics, and peptidomics of Spodoptera frugiperda (Lepidoptera, Noctuidae) neuropeptides. Arch Insect Biochem Physiol. 2021;106:e21740
30. Luo LL, Lin Y, Linghu JH, Gong W, Luo YH, Liu M. et al. Genomics, transcriptomics, and peptidomics of the greater wax moth Galleria mellonella neuropeptides and their expression in response to lead stress. Insect Sci. 2024;31:773-791
31. Ragionieri L, Predel R. The neuropeptidome of Carabus (Coleoptera, Adephaga: Carabidae). Insect Biochem Mol Biol. 2020;118:103309
32. Pandit AA, Ragionieri L, Marley R, Yeoh JGC, Inward DJG, Davies SA. et al. Coordinated RNA-Seq and peptidomics identify neuropeptides and G-protein coupled receptors (GPCRs) in the large pine weevil Hylobius abietis, a major forestry pest. Insect Biochem Mol Biol. 2018;101:94-107
33. Veenstra JA. Coleoptera genome and transcriptome sequences reveal numerous differences in neuropeptide signaling between species. Peerj. 2019;7:e7144
34. Marciniak P, Pacholska-Bogalska J, Ragionieri L. Neuropeptidomes of Tenebrio molitor L. and Zophobas atratus Fab. (Coleoptera, Polyphaga: Tenebrionidae). J Proteome Res. 2022;21:2247-60
35. Verleyen P, Huybrechts J, Sas F, Clynen E, Baggerman G, De Loof A. et al. Neuropeptidomics of the grey flesh fly, Neobellieria bullata. Biochem Biophys Res Commun. 2004;316:763-70
36. Neupert S, Predel R. Mass spectrometric analysis of single identified neurons of an insect. Biochem Biophys Res Commun. 2005;327:640-5
37. Li B, Predel R, Neupert S, Hauser F, Tanaka Y, Cazzamali G. et al. Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum. Genome Res. 2008;18:113-22
38. Ons S, Richter F, Urlaub H, Pomar RR. The neuropeptidome of Rhodnius prolixus brain. Proteomics. 2009;9:788-92
39. Rahman MM, Fromm B, Neupert S, Kreusch S, Predel R. Extended FMRFamides in dipteran insects: conservative expression in the neuroendocrine system is accompanied by rapid sequence evolution. Gen Comp Endocrinol. 2009;162:52-8
40. Audsley N, Matthews HJ, Down RE, Weaver RJ. Neuropeptides associated with the central nervous system of the cabbage root fly, Delia radicum (L). Peptides. 2011;32:434-40
41. Zoephel J, Reiher W, Rexer K-H, Kahnt J, Wegener C. Peptidomics of the agriculturally damaging larval stage of the cabbage root fly Delia radicum (Diptera: Anthomyiidae). 2012; 7: e41543.
42. Neupert S, Fusca D, Schachtner J, Kloppenburg P, Predel R. Toward a single-cell-based analysis of neuropeptide expression in Periplaneta americana antennal lobe neurons. J Comp Neurol. 2012;520:694-716
43. Zeng H, Qin Y, Du E, Wei Q, Li Y, Huang D. et al. Genomics- and Peptidomics-Based Discovery of Conserved and Novel Neuropeptides in the American Cockroach. J Proteome Res. 2021;20:1217-28
44. Predel R, Neupert S, Wicher D, Gundel M, Roth S, Derst C. Unique accumulation of neuropeptides in an insect: FMRFamide-related peptides in the cockroach, Periplaneta americana. Eur J Neurosci. 2004;20:1499-513
45. Neupert S, Marciniak P, Kohler R, Nachman RJ, Suh CP, Predel R. Different processing of CAPA and pyrokinin precursors in the giant mealworm beetle Zophobas atratus (Tenebrionidae) and the boll weevil Anthonomus grandis grandis (Curculionidae). Gen Comp Endocrinol. 2018;258:53-9
46. Ragionieri L, Ozbagci B, Neupert S, Salts Y, Davidovitch M, Altstein M. et al. Identification of mature peptides from pban and capa genes of the moths Heliothis peltigera and Spodoptera littoralis. Peptides. 2017;94:1-9
47. Lubawy J, Marciniak P, Rosinski G. Identification, Localization in the Central Nervous System and Novel Myostimulatory Effect of Allatostatins in Tenebrio molitor Beetle. Int J Mol Sci. 2020;21:3510
48. Krajniak KG. The identification and structure-activity relations of a cardioactive FMRFamide-related peptide from the blue crab Callinectes sapidus. Peptides. 1991;12:1295-302
49. Trimmer BA, Kobierski LA, Kravitz EA. Purification and characterization of FMRFamidelike immunoreactive substances from the lobster nervous system: isolation and sequence analysis of two closely related peptides. J Comp Neurol. 1987;266:16-26
50. Weimann JM, Marder E, Evans B, Calabrese RL. The effects of SDRNFLRFamide and TNRNFLRFamide on the motor patterns of the stomatogastric ganglion of the crab Cancer borealis. J Exp Biol. 1993;181:1-26
51. Mercier AJ, Orchard I, TeBrugge V, Skerrett M. Isolation of two FMRFamide-related peptides from crayfish pericardial organs. Peptides. 1993;14:137-43
52. Sithigorngul P, Saraithongkum W, Jaideechoey S, Longyant S, Sithigorngul W. Novel FMRFamide-like neuropeptides from the eyestalk of the giant freshwater prawn Macrobrachium rosenbergii. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 1998;120:587-95
53. Ma M, Chen R, Sousa GL, Bors EK, Kwiatkowski MA, Goiney CC. et al. Mass spectral characterization of peptide transmitters/hormones in the nervous system and neuroendocrine organs of the American lobster Homarus americanus. Gen Comp Endocrinol. 2008;156:395-409
54. Ma M, Bors EK, Dickinson ES, Kwiatkowski MA, Sousa GL, Henry RP. et al. Characterization of the Carcinus maenas neuropeptidome by mass spectrometry and functional genomics. Gen Comp Endocrinol. 2009;161:320-34
55. Ma M, Gard AL, Xiang F, Wang J, Davoodian N, Lenz PH. et al. Combining in silico transcriptome mining and biological mass spectrometry for neuropeptide discovery in the Pacific white shrimp Litopenaeus vannamei. Peptides. 2010;31:27-43
56. Christie AE. Expansion of the Litopenaeus vannamei and Penaeus monodon peptidomes using transcriptome shotgun assembly sequence data. Gen Comp Endocrinol. 2014;206:235-54
57. Dircksen H, Neupert S, Predel R, Verleyen P, Huybrechts J, Strauss J. et al. Genomics, Transcriptomics, and Peptidomics of Daphnia pulex Neuropeptides and Protein Hormones. Journal of Proteome Research. 2011;10:4478-504
58. Christie AE, Roncalli V, Wu L-S, Ganote CL, Doak T, Lenz PH. Peptidergic signaling in Calanus finmarchicus (Crustacea, Copepoda): In silico identification of putative peptide hormones and their receptors using a de novo assembled transcriptome. Gen Comp Endocrinol. 2013;187:117-35
59. Christie AE. Prediction of the peptidomes of Tigriopus californicus and Lepeophtheirus salmonis (Copepoda, Crustacea). Gen Comp Endocrinol. 2014;201:87-106
60. Christie AE. Identification of the first neuropeptides from the Amphipoda (Arthropoda, Crustacea). Gen Comp Endocrinol. 2014;206:96-110
61. Cazzamali G, Grimmelikhuijzen CJ. Molecular cloning and functional expression of the first insect FMRFamide receptor. Proc Natl Acad Sci U S A. 2002;99:12073-8
62. DeLaney K, Li L. Neuropeptidomic Profiling and Localization in the Crustacean Cardiac Ganglion Using Mass Spectrometry Imaging with Multiple Platforms. J Am Soc Mass Spectrom. 2020;31:2469-78
63. Christie AE. In silico characterization of the neuropeptidome of the Western black widow spider Latrodectus hesperus. Gen Comp Endocrinol. 2015;210:63-80
64. Merte J, Nichols R. Drosophila melanogaster FMRFamide-containing peptides: redundant or diverse functions? Peptides. 2002;23:209-20
65. Meeusen T, Mertens I, Clynen E, Baggerman G, Nichols R, Nachman RJ. et al. Identification in Drosophila melanogaster of the invertebrate G protein-coupled FMRFamide receptor. Proceedings of the national academy of sciences. 2002;99:15363-8
66. Maynard BF, Bass C, Katanski C, Thakur K, Manoogian B, Leander M. et al. Structure-activity relationships of FMRF-NH2 peptides demonstrate A role for the conserved C terminus and unique N-terminal extension in modulating cardiac contractility. PLoS One. 2013;8:e75502
67. Milakovic M, Ormerod KG, Klose MK, Mercier AJ. Mode of action of a Drosophila FMRFamide in inducing muscle contraction. J Exp Biol. 2014;217:1725-36
68. Hauser F, Cazzamali G, Williamson M, Park Y, Li B, Tanaka Y. et al. A genome-wide inventory of neurohormone GPCRs in the red flour beetle Tribolium castaneum. Front Neuroendocrinol. 2008;29:142-65
69. Hewes RS, Taghert PH. Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res. 2001;11:1126-42
70. Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS. et al. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213-9
71. Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33-7
72. Chen X, Ganetzky B. A neuropeptide signaling pathway regulates synaptic growth in Drosophila. J Cell Biol. 2012;196:529-43
73. Klose MK, Dason JS, Atwood HL, Boulianne GL, Mercier AJ. Peptide-induced modulation of synaptic transmission and escape response in Drosophila requires two G-protein-coupled receptors. J Neurosci. 2010;30:14724-34
74. Jayakumar S, Richhariya S, Reddy OV, Texada MJ, Hasan G. Drosophila larval to pupal switch under nutrient stress requires IP3R/Ca(2+) signalling in glutamatergic interneurons. Elife. 2016;5:e17495
75. Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009;1793:933-40
76. Ravi P, Trivedi D, Hasan G. FMRFa receptor stimulated Ca2+ signals alter the activity of flight modulating central dopaminergic neurons in Drosophila melanogaster. PLoS Genet. 2018;14:e1007459
77. Rasmussen M, Leander M, Ons S, Nichols R. Conserved molecular switch interactions in modeled cardioactive RF-NH2 peptide receptors: Ligand binding and activation. Peptides. 2015;71:259-67
78. Marciniak P, Witek W, Szymczak M, Pacholska-Bogalska J, Chowanski S, Kuczer M. et al. FMRFamide-Related Peptides Signaling Is Involved in the Regulation of Muscle Contractions in Two Tenebrionid Beetles. Frontiers in Physiology. 2020;11:456
79. Liu F, Dang Y, Li L, Feng H, Li J, Wang H. et al. Structure and mechanism of a neuropeptide-activated channel in the ENaC/DEG superfamily. Nat Chem Biol. 2023;19:1276-85
80. Dandamudi M, Hausen H, Lynagh T. Comparative analysis defines a broader FMRFamide-gated sodium channel family and determinants of neuropeptide sensitivity. J Biol Chem. 2022;298:102086
81. Gao H, Li Y, Wang M, Song X, Tang J, Feng F. et al. Identification and Expression Analysis of G Protein-Coupled Receptors in the Miridae Insect Apolygus lucorum. Front Endocrinol (Lausanne). 2021;12:773669
82. Myers CM, Evans PD. The distribution of bovine pancreatic polypeptide/FMRFamide-like immunoreactivity in the ventral nervous system of the locust. J Comp Neurol. 1985;234:1-16
83. Nichols R, McCormick JB, Lim IA, Starkman JS. Spatial and temporal analysis of the Drosophila FMRFamide neuropeptide gene product SDNFMRFamide: Evidence for a restricted expression pattern. Neuropeptides. 1995;29:205-13
84. Christie AE. Identification of putative neuropeptidergic signaling systems in the spiny lobster, Panulirus argus. Invertebr Neurosci. 2020;20:2
85. Tarr EA, Fidler BM, Gee KE, Anderson CM, Jager AK, Gallagher NM. et al. Distribution of FMRFamide-related peptides and co-localization with glutamate in Cupiennius salei, an invertebrate model system. Cell Tissue Res. 2019;376:83-96
86. Larsson A. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 2014;30:3276-8
87. Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14:587-9
88. Chernomor O, Von Haeseler A, Minh BQ. Terrace aware data structure for phylogenomic inference from supermatrices. Syst Biol. 2016;65:997-1008
89. Minh BQ, Dang CC, Vinh LS, Lanfear R. QMaker: Fast and Accurate Method to Estimate Empirical Models of Protein Evolution. Syst Biol. 2021;70:1046-60
90. Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A. et al. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol Biol Evol. 2020;37:1530-4
91. Hoang DT, Chernomor O, Haeseler Av, Minh BQ, Vinh LS. UFBoot2: Improving the Ultrafast Bootstrap Approximation. bioRxiv. 2017: 153916.
92. Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst Biol. 2010;59:307-21
93. Hallgren J, Tsirigos K, Pedersen MD, Almagro Armenteros JJ, Marcatili P, Nielsen H. et al. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv. 2022
94. Chowanski S, Lubawy J, Urbanski A, Rosinski G. Cardioregulatory Functions of Neuropeptides and Peptide Hormones in Insects. Protein Pept Lett. 2016;23:913-31
95. Duttlinger A, Mispelon M, Nichols R. The structure of the FMRFamide receptor and activity of the cardioexcitatory neuropeptide are conserved in mosquito. Neuropeptides. 2003;37:120-6
96. Cuthbert BA, Evans PD. A comparison of the effects of FMRFamide-like peptides on locust heart and skeletal muscle. J Exp Biol. 1989;144:395-415
97. Duve H, Elia AJ, Orchard I, Johnsen AH, Thorpe A. The effects of CalliFMRFamides and other FMRFamide-related neuropeptides on the activity of the heart of the blowfly Calliphora vomitoria. J Insect Physiol. 1993;39:31-40
98. Johnson EC, Bohn LM, Barak LS, Birse RT, Nässel DR, Caron MG. et al. Identification of Drosophila neuropeptide receptors by G protein-coupled receptors-β-arrestin2 interactions. J Biol Chem. 2003;278:52172-8
99. Calvin A, Lange AB. The association of the FMRFamide-related peptide family with the heart of the stick insect, Baculum extradentatum. Open Access Insect Physiology. 2010:1-10
100. Evans PD, Myers CM. The modulatory actions of FMRFamide and related peptides on locust skeletal muscle. J Exp Biol. 1986;126:403-22
101. Evans PD, Robb S, Cuthbert BA. Modulation of neuromuscular transmission in the locust by FMRFamide-like peptides. Modulation of Synaptic Transmission and Plasticity in Nervous Systems: Springer. 1988 p. 305-18
102. Hewes RS, Snowdeal EC, Saitoe M, Taghert PH. Functional Redundancy of FMRFamide-Related Peptides at theDrosophila Larval Neuromuscular Junction. J Neurosci. 1998;18:7138-51
103. Dunn TW, Mercier AJ. Synaptic modulation by a Drosophila neuropeptide is motor neuron-specific and requires CaMKII activity. Peptides. 2005;26:269-76
104. Elia A, Orchard I. Peptidergic innervation of leg muscles of the cockroach, Periplaneta americana (L.), and a possible role in modulation of muscle contraction. Journal of Comparative Physiology A. 1995;176:425-35
105. Walther C, Schiebe M, Voigt K. Synaptic and non-synaptic effects of molluscan cardioexcitatory neuropeptides on locust skeletal muscle. Neurosci Lett. 1984;45:99-104
106. Clark J, Milakovic M, Cull A, Klose MK, Mercier AJ. Evidence for postsynaptic modulation of muscle contraction by a Drosophila neuropeptide. Peptides. 2008;29:1140-9
107. Soehler S, Neupert S, Predel R, Stengl M. Examination of the role of FMRFamide-related peptides in the circadian clock of the cockroach Leucophaea maderae. Cell Tissue Res. 2008;332:257-69
108. Lenz O, Xiong J, Nelson MD, Raizen DM, Williams JA. FMRFamide signaling promotes stress-induced sleep in Drosophila. Brain Behav Immun. 2015;47:141-8
109. Hao K, Ullah H, Jarwar AR, Nong X, Tu X, Zhang Z. Functional identification of an FMRFamide-related peptide gene on diapause induction of the migratory locust, Locusta migratoria L. Genomics. 2020;112:1821-8
110. Lange AB, Kisana A, Leyria J, Orchard I. The Male Reproductive System of the Kissing Bug, Rhodnius prolixus Stål, 1859 (Hemiptera: Reduviidae: Triatominae): Arrangements of the Muscles and the Myoactivity of the Selected Neuropeptides. Insects. 2023;14:324
111. Clark J, Lange AB. Evidence for the association of FMRFamide-related peptides with the spermatheca of Locusta migratoria. Peptides. 2002;23:613-9
112. Hill SR, Orchard I. In vitro analysis of the digestive enzymes amylase and alpha-glucosidase in the midguts of Locusta migratoria L. in response to the myosuppressin, SchistoFLRFamide. J Insect Physiol. 2005;51:1-9
113. Lange AB, Orchard I. The effects of SchistoFLRFamide on contractions of locust midgut. Peptides. 1998;19:459-67
114. Song T, Qin W, Lai Z, Li H, Li D, Wang B. et al. Dietary cysteine drives body fat loss via FMRFamide signaling in Drosophila and mouse. Cell Res. 2023;33:434-47
115. Kiss B, Szlanka T, Zvara Á, Žurovec M, Sery M, Kakaš Š. et al. Selective elimination/RNAi silencing of FMRF-related peptides and their receptors decreases the locomotor activity in Drosophila melanogaster. Gen Comp Endocrinol. 2013;191:137-45
116. Schoofs L, De Loof A, Van Hiel MB. Neuropeptides as Regulators of Behavior in Insects. Annu Rev Entomol. 2017;62:35-52
117. Christie AE, Chi M, Lameyer TJ, Pascual MG, Shea DN, Stanhope ME. et al. Neuropeptidergic Signaling in the American Lobster Homarus americanus: New Insights from High-Throughput Nucleotide Sequencing. PLoS One. 2015;10:e0145964
118. Cape SS, Rehm KJ, Ma M, Marder E, Li L. Mass spectral comparison of the neuropeptide complement of the stomatogastric ganglion and brain in the adult and embryonic lobster, Homarus americanus. J Neurochem. 2008;105:690-702
119. Chen R, Jiang X, Conaway MC, Mohtashemi I, Hui L, Viner R. et al. Mass spectral analysis of neuropeptide expression and distribution in the nervous system of the lobster Homarus americanus. J Proteome Res. 2010;9:818-32
120. Christie AE, Pascual MG. Peptidergic signaling in the crab Cancer borealis: Tapping the power of transcriptomics for neuropeptidome expansion. Gen Comp Endocrinol. 2016;237:53-67
121. Cruz-Bermúdez ND, Fu Q, Kutz-Naber KK, Christie AE, Li L, Marder E. Mass spectrometric characterization and physiological actions of GAHKNYLRFamide, a novel FMRFamide-like peptide from crabs of the genus Cancer. J Neurochem. 2006;97:784-99
122. Fu Q, Kutz KK, Schmidt JJ, Hsu YW, Messinger DI, Cain SD. et al. Hormone complement of the Cancer productus sinus gland and pericardial organ: an anatomical and mass spectrometric investigation. J Comp Neurol. 2005;493:607-26
123. Huybrechts J, Nusbaum MP, Bosch LV, Baggerman G, Loof AD, Schoofs L. Neuropeptidomic analysis of the brain and thoracic ganglion from the Jonah crab, Cancer borealis. Biochem Biophys Res Commun. 2003;308:535-44
124. Li L, Kelley WP, Billimoria CP, Christie AE, Pulver SR, Sweedler JV. et al. Mass spectrometric investigation of the neuropeptide complement and release in the pericardial organs of the crab, Cancer borealis. J Neurochem. 2003;87:642-56
125. Ma M, Sturm RM, Kutz-Naber KK, Fu Q, Li L. Immunoaffinity-based mass spectrometric characterization of the FMRFamide-related peptide family in the pericardial organ of Cancer borealis. Biochem Biophys Res Commun. 2009;390:325-30
126. Sithigorngul P, Pupuem J, Krungkasem C, Longyant S, Chaivisuthangkura P, Sithigorngul W. et al. Seven novel FMRFamide-like neuropeptide sequences from the eyestalk of the giant tiger prawn Penaeus monodon. Comp Biochem Physiol B Biochem Mol Biol. 2002;131:325-37
127. Sithigorngul P, Saraithongkum W, Longyant S, Panchan N, Sithigorngul W, Petsom A. Three more novel FMRFamide-like neuropeptide sequences from the eyestalk of the giant freshwater prawn Macrobrachium rosenbergii. Peptides. 2001;22:191-7
128. Mercier AJ, Friedrich R, Boldt M. Physiological functions of FMRFamide-like peptides (FLPs) in crustaceans. Microsc Res Tech. 2003;60:313-24
129. Krajniak KG. Invertebrate FMRFamide related peptides. Protein Pept Lett. 2013;20:647-70
130. Wilkens JL, McMahon BR. Intrinsic properties and extrinsic neurohormonal control of crab cardiac hemodynamics. Experientia. 1992;48:827-34
131. Mercier AJ, Russenes RT. Modulation of Crayfish Hearts by FMRFamide-related Peptides. Biol Bull. 1992;182:333-40
132. McGaw IJ, Wilkens JL, McMahon BR, Airriess CN. Crustacean cardioexcitatory peptides may inhibit the heart in vivo. J Exp Biol. 1995;198:2547-50
133. McGaw IJ, McMahon BR. The FMRFamide-related peptides F1 and F2 alter hemolymph distribution and cardiac output in the crab Cancer magister. Biol Bull. 1995;188:186-96
134. Worden MK, Kravitz EA, Goy MF. Peptide F1, an N-terminally extended analog of FMRFamide, enhances contractile activity in multiple target tissues in lobster. J Exp Biol. 1995;198:97-108
135. Wilkens JL, Shinozaki T, Yazawa T, ter Keurs HE. Sites and modes of action of proctolin and the FLP F2 on lobster cardiac muscle. J Exp Biol. 2005;208:737-47
136. Skerrett M, Peaire A, Quigley P, Mercier A. Physiological effects of two FMRFamide-related peptides from the crayfish Procambarus clarkii. J Exp Biol. 1995;198:109-16
137. Fort TJ, Brezina V, Miller MW. Regulation of the crab heartbeat by FMRFamide-like peptides: multiple interacting effects on center and periphery. J Neurophysiol. 2007;98:2887-902
138. Skiebe P. Neuropeptides are ubiquitous chemical mediators: Using the stomatogastric nervous system as a model system. J Exp Biol. 2001;204:2035-48
139. Jorge-Rivera JC, Marder E. TNRNFLRFamide and SDRNFLRFamide modulate muscles of the stomatogastric system of the crab Cancer borealis. J Comp Physiol A. 1996;179:741-51
140. Marder E, Richards KS. Development of the peptidergic modulation of a rhythmic pattern generating network. Brain Res. 1999;848:35-44
141. Tierney AJ, Blanck J, Mercier J. FMRFamide-like peptides in the crayfish (Procambarus clarkii) stomatogastric nervous system: distribution and effects on the pyloric motor pattern. The Journal of experimental biology. 1997 200 Pt 24: 3221-33
142. Swensen AM, Marder E. Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J Neurosci. 2000;20:6752-9
143. Mercier AJ, Schiebe M, Atwood HL. Pericardial peptides enhance synaptic transmission and tension in phasic extensor muscles of crayfish. Neurosci Lett. 1990;111:92-8
144. Meyrand P, Marder E. Matching neural and muscle oscillators: control by FMRFamide-like peptides. J Neurosci. 1991;11:1150-61
145. Veenstra JA, Rombauts S, Grbić M. In silico cloning of genes encoding neuropeptides, neurohormones and their putative G-protein coupled receptors in a spider mite. Insect Biochem Mol Biol. 2012;42:277-95
146. Egekwu N, Sonenshine DE, Bissinger BW, Roe RM. Transcriptome of the Female Synganglion of the Black-Legged Tick Ixodes scapularis (Acari: Ixodidae) with Comparison between Illumina and 454 Systems. PLOS ONE. 2014;9:e102667
147. Fabian-Fine R, Anderson CM, Roush MA, Johnson JAG, Liu H, French AS. et al. The distribution of cholinergic neurons and their co-localization with FMRFamide, in central and peripheral neurons of the spider Cupiennius salei. Cell Tissue Res. 2017;370:71-88
148. Zhu XX, Zhang WY, Oliver JH. Immunocytochemical mapping of FMRFamide-like peptides in the argasid tick Ornithodoros parkeri and the ixodid tick Dermacentor variabilis. Exp Appl Acarol. 1995;19:1-9
149. Waldman J, Xavier MA, Vieira LR, Logullo R, Braz GRC, Tirloni L. et al. Neuropeptides in Rhipicephalus microplus and other hard ticks. Ticks and Tick-borne Diseases. 2022;13:101910
150. Schmid A, Becherer C. Distribution of histamine in the CNS of different spiders. Microsc Res Tech. 1999;44:81-93
151. Watson WH, Groome JR, Chronwall BM, Bishop J, O'Donohue TL. Presence and distribution of immunoreactive and bioactive FMRFamide-like peptides in the nervous system of the horseshoe crab, limulus polyphemus. Peptides. 1984;5:585-92
152. Groome JR. Distribution and partial characterization of FMRFamide-like peptides in the horseshoe crab, Limulus polyphemus. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology. 1993;104:79-85
153. Groome JR, deTschaschell M, Watson WH 3rd. Peptidergic regulation of the Limulus midgut. J Comp Physiol A. 1992;170:631-43
154. Groome JR, Townley MA, Watson WH 3rd. Excitatory actions of FMRFamide-related peptides (FaRPs) on the neurogenic Limulus heart. Biol Bull. 1994;186:309-18
155. Panula P, Aarnisalo AA, Wasowicz K. Neuropeptide FF, a mammalian neuropeptide with multiple functions. Prog Neurobiol. 1996;48:461-87
156. Yang HY, Iadarola MJ. Modulatory roles of the NPFF system in pain mechanisms at the spinal level. Peptides. 2006;27:943-52
157. Ayachi S, Simonin F. Involvement of mammalian RF-amide peptides and their receptors in the modulation of nociception in rodents. Front Endocrinol (Lausanne). 2014;5:158
158. Quillet R, Ayachi S, Bihel F, Elhabazi K, Ilien B, Simonin F. RF-amide neuropeptides and their receptors in Mammals: Pharmacological properties, drug development and main physiological functions. Pharmacol Ther. 2016;160:84-132
159. Findeisen M, Rathmann D, Beck-Sickinger AG. RFamide Peptides: Structure, Function, Mechanisms and Pharmaceutical Potential. Pharmaceuticals. 2011;4:1248-80
160. Anderson GM. Pituitary actions of RFamide peptides: a critique of the evidence. The Open Neuroendocrinology Journal. 2011;4:51-63
161. Jhamandas JH, Goncharuk V. Role of neuropeptide FF in central cardiovascular and neuroendocrine regulation. Front Endocrinol (Lausanne). 2013;4:8
162. Nguyen T, Marusich J, Li JX, Zhang Y. Neuropeptide FF and its receptors: therapeutic applications and ligand development. J Med Chem. 2020;63:12387-402
163. Wang Y, Zuo Z, Shi J, Fang Y, Yin Z, Wang Z. et al. Modulatory role of neuropeptide FF system in macrophages. Peptides. 2024;174:171164
164. Latorre-Estivalis JM, Almeida FC, Pontes G, Dopazo H, Barrozo RB, Lorenzo MG. Evolution of the Insect PPK Gene Family. Genome Biology and Evolution. 2021;13:evab185
165. Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735-67
166. Golubovic A, Kuhn A, Williamson M, Kalbacher H, Holstein TW, Grimmelikhuijzen CJ. et al. A peptide-gated ion channel from the freshwater polyp Hydra. J Biol Chem. 2007;282:35098-103
167. Schanuel SM, Bell KA, Henderson SC, McQuiston AR. Heterologous expression of the invertebrate FMRFamide-gated sodium channel as a mechanism to selectively activate mammalian neurons. Neuroscience. 2008;155:374-86
168. Askwith CC, Cheng C, Ikuma M, Benson C, Price MP, Welsh MJ. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron. 2000;26:133-41
169. Audsley N, Down RE. G protein coupled receptors as targets for next generation pesticides. Insect Biochem Mol Biol. 2015;67:27-37
170. Zhang Y, Liu Y, Wu X, Lu X, Wang M, Ye D. et al. A Novel Peptidomimetic Insecticide: Dippu-AstR-Based Rational Design and Biological Activity of Allatostatin Analogs. J Agric Food Chem. 2024;72:11341-50
171. Nachman RJ, Hamshou M, Kaczmarek K, Zabrocki J, Smagghe G. Biostable and PEG polymer-conjugated insect pyrokinin analogs demonstrate antifeedant activity and induce high mortality in the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidae). Peptides. 2012;34:266-73
172. Nachman RJ, Mahdian K, Nässel DR, Isaac RE, Pryor N, Smagghe G. Biostable multi-Aib analogs of tachykinin-related peptides demonstrate potent oral aphicidal activity in the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidae). Peptides. 2011;32:587-94
173. Smagghe G, Mahdian K, Zubrzak P, Nachman RJ. Antifeedant activity and high mortality in the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidae) induced by biostable insect kinin analogs. Peptides. 2010;31:498-505
174. Shi Y, Li H, Nachman RJ, Liu T-X, Smagghe G. Insecticidal efficacy and risk assessment of different neuropeptide analog combinations against the peach-potato aphid following topical exposure. Pest Manage Sci. 2023;79:226-33
175. Orchard I, Leyria J, Al-Dailami AN, Nachman RJ, Lange AB. Functional characterization of the kinin receptor in the Chagas disease vector Rhodnius prolixus; activity of native kinins and potent biostable Aib-containing insect kinin analogs. Peptides. 2024;172:171135
176. Xiong C, Kaczmarek K, Zabrocki J, Pietrantonio PV, Nachman RJ. Evaluation of Aib and PEG-polymer insect kinin analogs on mosquito and tick GPCRs identifies potent new pest management tools with potentially enhanced biostability and bioavailability. Gen Comp Endocrinol. 2019;278:58-67
Author contact
![]() Corresponding author: Paweł Marciniak, PhD; pmarcinedu.pl.
Corresponding author: Paweł Marciniak, PhD; pmarcinedu.pl.

 Global reach, higher impact
Global reach, higher impact