10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2025; 21(6):2806-2823. doi:10.7150/ijbs.104074 This issue Cite
Review
Integrating Subacute Ruminal Acidosis, Lipopolysaccharide, and Trained Immunity: A Comprehensive Review
1. State Key Laboratory of Animal Nutrition and Feeding, International Calf and Heifer Organization, College of Animal Science and Technology, China Agricultural University, Beijing, 100193, China.
2. College of Animal Science and Technology, Beijing University of Agriculture, Beijing, 102206, China.
3. College of Animal Science, Xinjiang Agricultural University, Urumqi, Xinjiang, 830052, China.
†These authors contributed equally to this work.
Received 2024-9-23; Accepted 2025-2-11; Published 2025-3-31
Abstract
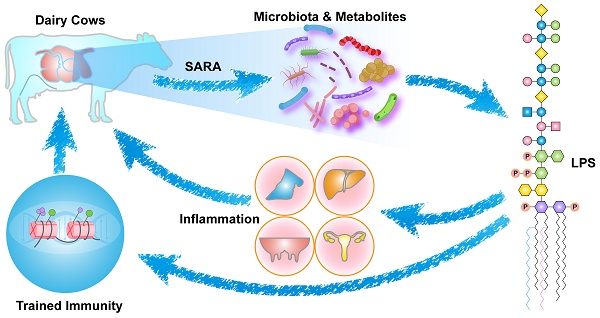
Subacute ruminal acidosis (SARA) has emerged as a prevalent digestive disorder that significantly affects the overall health of ruminants, with notable links to various inflammatory diseases. Throughout the progression of SARA, elevated lipopolysaccharide (LPS) levels in the rumen play a crucial role in initiating the innate immune response. In this review, we evaluate the recent insights into the pathways associated with SARA-induced inflammatory responses, with a specific focus on LPS. It is important to recognize the variation in the immune response activation potential of LPS derived from different bacterial sources. This variability aligns with the widespread detection of LPS in the rumens of ruminants with SARA. Nonetheless, trained immunity is expected to become a novel strategy for the prevention and control of SARA. This mechanism offers a rapid response to secondary stimuli, including LPS, effectively preventing inflammation. Ultimately, this review establishes a comprehensive system integrating SARA, LPS, and trained immunity. Through this integrated approach, we aim to provide innovative solutions to the challenges associated with SARA.
Keywords: subacute ruminal acidosis, ruminant, lipopolysaccharide, inflammation, trained immunity
Introduction
High-quality dairy products play a vital role in human nutrition and disease prevention [1]. Cows represent a significant source of dairy products. High-concentrate (HC) diets are employed in the agricultural industry to improve the efficiency of dairy production. However, these diets contain fermentable carbohydrates, which promote the production and accumulation of volatile fatty acids (VFAs) in the rumen, causing a decrease in rumen pH and leading to subacute ruminal acidosis (SARA). SARA can be considered to occur when pH levels are below 5.6 or 5.8 for a minimum of 3 or 5.4 hours/day, respectively [2,3]. Although ruminants can neutralize excessive VFAs by secreting saliva, the low proportion of neutral detergent fiber hinders the production of sufficient saliva to effectively counteract the negative effects of VFAs [4]. This prolonged reduction in pH distinguishes SARA from acute ruminal acidosis caused by lactic acid accumulation [5,6]. Cows experiencing acute rumen acidosis exhibit a rumen pH below 5.0, as well as increased lactic acid in the rumen and blood, an increased abundance of gram-positive and lactic acid-producing bacteria [7].
SARA-induced inflammatory disease mechanisms. Prolonged consumption of high-concentrate diets disrupts the internal rumen environment in dairy cows, characterized by the accumulation of TVFA, LPS, and HIS, along with a decrease in ruminal pH, milk yield, milk fat and protein, and the lysis of Gram-negative bacteria. These changes exert persistent deleterious effects on rumen epithelial cells, including the disruption of tight junctions, which facilitates the translocation of LPS into systemic circulation. Once LPS reaches various tissues and organs and accumulates beyond a critical threshold, it has the potential to trigger inflammatory responses.
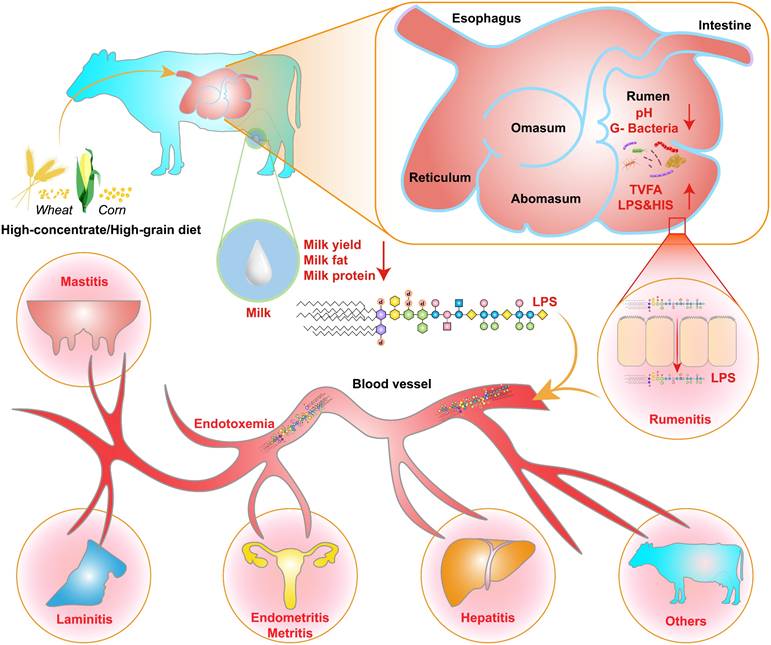
The increase in the ruminal concentration of lipopolysaccharide (LPS) has been well documented for SARA challenges based on HC diets. The change in ruminal conditions caused by these challenges results in lysis and death of gram-negative bacteria, and leads to the release of LPS [8,9]. The high concentration of ruminal LPS overactivated the NF-κB and MAPKs inflammatory pathways in the rumen epithelium and partially induced rumenitis [10]. Furthermore, LPS contribute to hindering the regeneration of epithelial cells and reduces the cell viability of rumen epithelial cells, potentially due to histamine (HIS) accumulation [11-13]. This results in a disruption to the barrier function of the rumen epithelium, resulting in the translocation of LPS [14,15]. This translocation allows LPS to enter the portal circulation and the peripheral circulation [16]. LPS in the systemic circulation first binds to LPS-binding protein (LBP). It is then transported to the surface of immune cells, where it binds to CD14 to form the LPS-LBP-CD14 triple complex. It is then transported to the TLR4-MD2 protein complex, activating TLR4 and triggering an inflammatory response, such as rumenitis, mastitis, endometritis, laminitis, and hepatitis (Figure 1) [17-20]. These diseases can result in substantial economic losses for the dairy industry, including expenses associated with reduction in milk fat and protein, forced culling of cows, treatment costs, milk production losses, veterinary fees, and expenditures on veterinary pharmaceuticals [12,21,22]. Therefore, SARA exerts various adverse effects on dairy cows' health, ultimately limiting the progress of the dairy industry.
In recent years, the concept of “trained immunity” was introduced in the field of immunology [23]. It is a non-specific immune response with “memory” characteristics that occurs in innate immune cells. This process differs from the differentiation of memory immune cells in adaptive immunity. Instead, it is associated with epigenetic reprogramming, metabolic reprogramming, and long-term protection mediated by hematopoietic stem cells [24]. Trained immunity refers to the persistent changes in innate immune cell function induced by certain microbial stimuli and endogenous ligands, ultimately leading to heightened responses to subsequent challenges [25]. Typically, β-glucan components of fungal cell walls or Bacillus Calmette-Guérin (BCG) vaccines are employed as an initial stimulus to induce trained immunity, which enhances host resistance to heterologous stimuli, including LPS and viruses [26]. Indeed, the success of LPS in inducing trained immunity as an initial stimulus has also been demonstrated in studies of microglia [27].
Therefore, LPS plays a crucial role in both SARA and host immunity. In this review, we aim to establish the connection between SARA and trained immunity. Specifically, we present the latest insights into SARA, highlighting potential directions for future research in this field. Additionally, we examine the structural characteristics of LPS from various sources, analyzing and comparing their capacities to initiate immune responses. Importantly, the prevalence of LPS in the rumens of animals with SARA underpins the development of targeted strategies to mitigate both SARA and its associated inflammatory effects. Furthermore, we explore the theory, mechanisms of trained immunity in various animals, discussing ongoing investigations and its potential applications in preventing and controlling of SARA. Finally, we propose a theoretical model that elucidates the complex interplay between SARA, LPS, and trained immunity, offering a comprehensive framework to tackle related inflammatory diseases.
SARA promotes the inflammatory response
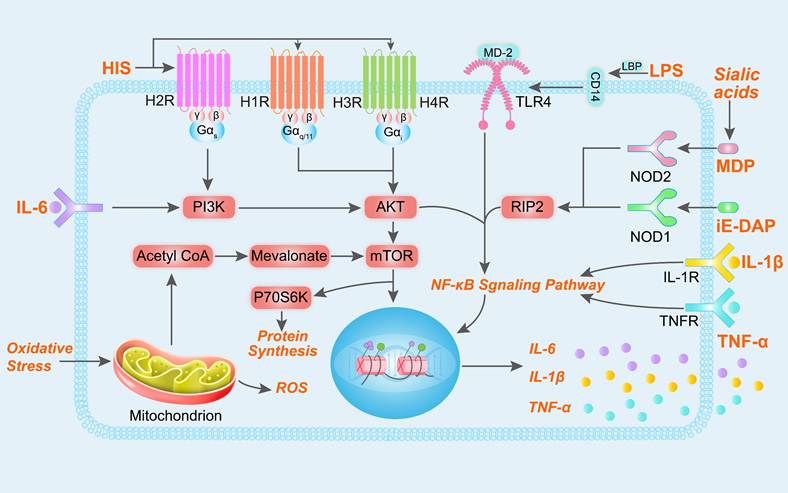
Animals with SARA typically exhibit reduced dry matter intake, decreased milk yield, and diminished milk fat and protein synthesis. Moreover, SARA has been associated with several inflammatory diseases, including rumenitis, mastitis, laminitis, and endometritis [10,12,13,28]. During the pathogenesis of SARA-induced inflammatory disease, there is a notable increase in the levels of certain metabolites in the rumen, such as LPS, HIS, N-γ-d-glutamyl-meso-diaminopimelic acid (iE-DAP) and sialic acids (Figure 2) [4,29-32]. The increase in LPS in the rumen fluid is closely related to the relative abundance of Proteobacteria and Bacteroidetes (such as Escherichia coli, Succinivibrionaceae_UCG-001, Prevotella albensis, Prevotella brevis, and Prevotella ruminicola) [9,33-35]. These gram-negative bacteria undergo lysis in the ruminal low-pH environments, releasing cell wall-bound LPS and activating inflammatory responses through the TLR4/NF-κB signaling pathway [36]. During SARA, the ruminal low-pH environments lead to dysbiosis of the rumen microbiota and increases in histamine-producing bacteria. HIS in the rumen is derived from Allisonella histaminiformans, which is able to grow in environments containing histidine and utilizes histidine as its only energy source [37]. And HIS may participate in the PI3K-AKT-mTOR and NF-κB pathways by binding to HIS receptors [12,38]. The release of inflammatory cytokines may also trigger the significant release of HIS from mast cells and basophils, exacerbating inflammatory reactions. This process may enhance neutrophil adhesion, partly through the inhibition of autophagy [39]. In addition, rumen iE-DAP levels also increased significantly during SARA, which may be related to changes in Proteobacteria, Prevotella, and Streptococcus bovis, as these microbiota are susceptible to death and release large amounts of iE-DAP in low-pH environments [35,40-42]. Studies have shown that iE-DAP can activate the NF-κB pathway by binding to NOD-like receptor 1 (NOD1) and induce inflammation and injury in bovine hepatocytes and mammary epithelial cells [43,44]. It is worth noting that recent studies have identified the interaction of microbial derived muramyl dipeptide (MDP) with NOD-like receptor 2 (NOD2) as important factors in the pathogenesis of mastitis [45]. Sialic acids (both Neu5Gc and Neu5Ac) is important differential metabolite in the rumen of healthy cows and SARA cows, which exacerbate mastitis in mice with gut dysbiosis [28]. This gut dysbiosis facilitated Enterococcus cecorum expansion and promoted MDP release, which induced inflammation by activating the NOD2-RIP2-NF-κB axis [45]. Additionally, elevated levels of multiple metabolites may increase the production of reactive oxygen species, leading to in oxidative stress and cellular damage [46,47].
Overall, the elevation of metabolites such as LPS, HIS, iE-DAP and MDP, and their associated inflammatory responses, is closely associated with the lysis of gram-negative bacteria in low-pH environments. Notably, the structure of LPS is recognized as a crucial factor influencing the efficacy of phage therapy. However, E. coli and its mutants express diverse LPS structures, resulting in varying infection phenotypes in phage therapy [48]. Therefore, we aim to evaluate and elucidate the role of LPS in SARA-induced inflammatory responses, providing insights that may contribute to novel therapeutic strategies.
The role of LPS in SARA-induced inflammatory responses
LPS have garnered initial interest, primarily for their ability to activate the immune system [49]. However, due to structural and source variation among LPS molecules, host immune cells often exhibit specificity, responding primarily to certain serotypes of LPS [50]. Notably, atypical sources of LPS may exert contrasting immunogenic or inhibitory effects on the same immune response [51,52]. This underscores the need to investigate the structural and physiological characteristics of diverse LPS in the rumen of ruminants. Nevertheless, most LPS used in SARA studies originate from E. coli or LPS mixtures extracted from the rumen or digestive tract, with limited focus on specific bacterial sources that may have distinct immunogenic profiles [16,53-55].
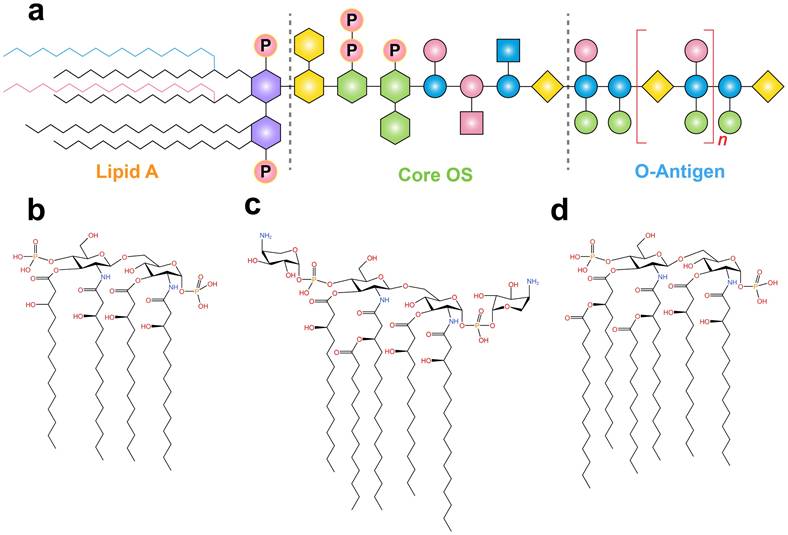
LPS primarily consist of three distinct regions: lipid A, core oligosaccharide (OS), and O antigen (Figure 3). Lipid A is the central component of LPS, responsible for their endotoxicity and pyrogenic properties. The variation in the immune-stimulating potential of LPS has been linked to the strain and degree of lipid A acylation. Two acyltransferases, Kdo2-lipid IVA lauroyltransferase (LpxL) and lauroyl-Kdo2-lipid IVA myristoyltransferase (LpxM), add the fifth and sixth acyl chains to tetraacylated lipid A. Compared to tetraacylated and penta-acylated lipid A, hexa-acylated bis-phosphorylated lipid A exhibits the highest level of immune stimulation in mammals. Conversely, the presence of only four or five acyl chains in LPS inhibits the formation of TLR4/MD-2/LPS heterodimer formation and hinders signal transduction [56]. Furthermore, given the absence of a comprehensive protocol for the detection of rumen LPS, E. coli hexa-acylated LPS is frequently employed in in vitro experiments on rumen tissue to study the inflammatory response of rumen acidosis [57]. Therefore, further investigation into the diversity of LPS types and the degree of lipid A acylation in various biological samples, such as rumen fluid, serum, milk, and different tissues, is crucial for understanding their role in immune responses.
Signaling pathways inflammation and oxidative stress induced by LPS, HIS, iE-DAP and MDP. After binding to their respective receptors, LPS, HIS, iE-DAP, and MDP participate in the PI3K-AKT-mTOR and NF-κB signaling pathways, regulating gene expression and promoting the release of cytokines IL-1β, IL-6, and TNF-α. Mitochondria generate reactive oxygen species (ROS) in response to oxidative stress, and additionally, metabolic byproducts from mitochondrial metabolism can participate in the mTOR signaling pathway and regulate gene expression.
The general chemical structure of LPS and the structural diversity in the lipid A region. (a) The general chemical structure of LPS. The red and blue acyl chains represent two different acyltransferases encoded by genes LpxL and LpxM, respectively. These acyltransferases are responsible for the addition of fifth and sixth acyl chains to tetraacylated endotoxins. (b) Lipid IVA consists of only four primary acyl chains. Lipid A derived from Burkholderia cepacia (c) and E. coli (d). Adapted with permission from Di Lorenzo et al. [49]. Copyright 2022, American Chemical Society.
The O antigen is highly variable encompasses a wide range of serotypes. Although some serotypes of E. coli were eliminated, 181 O-antigens with different structures have been identified in this bacterial species [58]. Additionally, bacteriophages bind to O antigens, enabling their attachment to bacterial core oligosaccharides and facilitating the infection of resistant bacterial strains [59]. E. coli strains F5 and F17, and their mutants, exhibit different O antigens, resulting in varying levels of protection against phage infection [60,61]. The O antigen also determines the sensitivity of Pseudomonas protegens to phage tail-like particles, and protects bacteria from the host immune system [62]. However, there is a lack of targeted research on the role of LPS O antigen in SARA. Therefore, further exploration of the O antigen produced by the rumen core microbiota could lead to the development of targeted therapeutic strategies to against phage infections.
In contrast to the O-antigen, structural changes in the core OS region are relatively minor but can be further categorized into inner core and outer core OS modifications. Typically, the inner core OS contains 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo), while the outer core exhibits more variability [63]. Kdo is an eight-carbon sugar that connects lipid A to the core oligosaccharides [64]. Therefore, differences in the core OS structure of LPS may affect the interaction between MD-2 and TLR4, as well as the homologous and heterologous dimerization of TLR4 [56,65]. Overall, lipid A, core OS, and O antigens play important roles in activating the immune system. The development of vaccines based on their antigenic characteristics is a potential approach for the prevention of related diseases. However, due to the limited development of precise LPS detection techniques, accurately predicting the source of different LPS strains based solely on their structure remains challenging.
The translocation of LPS into the systemic circulation disrupts homeostasis by altering the immune function of dairy cows. This ultimately leads to a pro-inflammatory state, which increases susceptibility to laminitis, ruminitis, endometritis and mastitis [31,33,66,67]. Previous research has indicated a notably elevated concentration of LPS in various biological samples of ruminants prone to SARA or with SARA, including milk, mammary glands, lacteal veins, tail veins, feces and rumen fluids (Table 1). This observation suggested that LPS underwent translocation to these fluids. Similar findings were observed in an investigation on Hu sheep [31,46]. Importantly, when LPS is absorbed into the peripheral blood circulation and reaches a specific concentration, the potential for endotoxemia subsequently arises [17]. Subsequently, LPS and other toxins disseminate throughout the body, eliciting inflammatory responses in various tissues and organs [68]. Low degree endotoxemia causes persistent damage and mastitis by activating the TLR4-cGAS-STING-NF-κB/NLRP3 signaling pathway. Furthermore, low-degree endotoxemia can also aggravate E. coli induced mouse mastitis by activating neuraminidase to damage host alkaline phosphatase [69]. Similarly, the administration of LPS via intramammary injection results in the elevation of acute-phase proteins and modulation of the blood metabolome [70]. LPS modulates the inflammatory response in the breast by activating the TLR4/NF-κB inflammatory signaling pathway, while simultaneously suppressing the synthesis of casein through the PI3K-AKT-mTOR signaling pathway [54,71]. Thus, LPS not only circulates throughout the body but also negatively impacts dairy product quality.
Existing literature on the impact of LPS in SARA primarily focuses on total LPS levels in rumen fluid or serum. As mentioned earlier, validation tests often use LPS derived from a specific bacterium, particularly E. coli, to induce inflammation in cell or mouse models [85]. However, it is crucial to recognize that LPS exhibits strain specificity. In the rumen, LPS is produced through the lysis of diverse gram-negative bacteria rather than being exclusively derived from E. coli. The varied sources of LPS can lead to distinct physiological effects, ranging from promoting to inhibiting inflammation [86]. Early studies have shown that purified LPS from E. coli, Salmonella typhimurium, Pseudomonas aeruginosa, and Klebsiella pneumoniae exhibit different effects on the synthesis of inflammatory mediators such as TNF-α, IL-Iβ, IFN-γ, and IL-10. Recent research further suggests that E. coli-derived LPS can impair intestinal glucose absorption while enhancing insulin and glucagon-like peptide 1 secretion [86]. In contrast, Chlorella-derived LPS has been shown to mitigate glucose abnormalities induced by equivalent doses of E. coli LPS, improve blood sugar regulation in obese mice, and promote beneficial endotoxemia [86]. Similarly, Salmonella-derived LPS may induce a metabolically advantageous state of endotoxemia. These findings underscore the complexity of LPS effects, which cannot be fully captured by measuring endotoxin units or circulating LBPs alone.
Moreover, neutrophils have been found to distinguish between LPS from different bacterial species, such as E. coli, Salmonella, and P. aeruginosa. Interestingly, neutrophils respond specially to LPS derived from E. coli O128:B12 and P. aeruginosa 10, but not to other LPS types [87]. This highlights the unique toxicity of E. coli-derived LPS compared to LPS from other common gram-negative ruminal bacteria, suggesting that E. coli LPS may not accurately represent the effects of ruminal LPS on the host [88-90]. Recent studies also indicate that ruminal pH plays a role in determining LPS profiles. For instance, under low pH conditions, penta-acylated LPS is predominantly derived from Prevotella, whereas hexa-acylated LPS is mainly associated with Succinivibrionaceae_UCG-001, rather than E. coli [34]. This finding underscores the necessity of comparing the virulence of common gram-negative ruminal bacteria, such as Prevotella and Succinivibrionaceae_UCG-001, with E. coli LPS. The microbial sources of LPS likely influence host responses in different ways during SARA. In summary, investigating the mechanisms of LPS derived from specific bacterial sources may provide valuable insights into systemic inflammation caused by SARA in dairy cows. Identifying beneficial LPS variants could further contribute to preventing inflammatory diseases, developing vaccines, improving milk quality, and fostering the healthy growth of the dairy industry.
Trained immunity represents a type of congenital immunological memory
Animal immunity consists of two major components: innate immunity and adaptive immunity. Traditionally, innate immunity has been regarded as lacking the capacity for memory, in contrast to adaptive immunity. However, the conventional understanding that these systems differ solely based on the presence or absence of immune memory has been increasingly challenged [91]. The concept of “trained immunity”, introduced in 2011, describes an intrinsic form of innate immune memory [23]. Notably, trained immunity was first identified in organisms that rely exclusively on innate immunity, such as plants, invertebrates, and bacteria [92,93]. These groups collectively represent the overwhelming majority of Earth's species diversity, comprising approximately 97-99% of all species [94]. In contrast, vertebrates, which account for only 1-3% of total species diversity, possess both innate and adaptive immune systems, enabling them to develop immune memory through adaptive processes. Interestingly, evidence from plants challenges the traditional distinction between innate and adaptive immunity. When plants are infected by microbial pathogens, they develop enhanced systemic immunity against subsequent infections, effectively forming a “memory” of the initial encounter, despite lacking adaptive immunity [95]. This demonstrates that the innate immune systems of plants possess memory-like capabilities, suggesting that similar mechanisms may also exist within the innate immune systems of animals.
Early evidence supporting innate immune memory can be found in a study conducted in the 1960s, which indicated that mice that received the BCG vaccine exhibited protection against subsequent infections caused by Listeria monocytogenes and S. Typhimurium [96]. Following the initial immune response, innate immune cells transition to a resting state. However, through epigenetic modifications, these cells can exhibit increased inflammation and antimicrobial properties upon re-exposure to antigens (Figure 4a) [94,97]. This phenomenon results in an enhanced non-specific response to subsequent infections, ultimately improving the survival rate of the host [91,98,99]. Currently, the initial stimuli employed for trained immunity primarily include BCG, β-glucan, recombinant proteins, cytokines, and peptidoglycan. Similarly, the secondary stimuli for trained immunity mainly include LPS, viruses, bacteria, and β-glucan (Figure 4a) [26,100]. Numerous studies have revealed the distinct features of innate immune cells, such as monocytes, macrophages, natural killer cells, dendritic cells, neutrophils, and microglia, that play crucial roles in trained immunity (Figure 4b) [27,97,101-104]. At the cellular level, different stimuli can induce epigenetic changes in response to TLR agonists, affecting microbe clearance and inflammatory cytokine secretion in in vitro. Notably, this process is requiring glycolysis, mevalonate synthesis, and mTOR activation (Figure 4c).
Research into trained immunity has progressed through various animal experiments (Figure 4d). In a study involving laying hens and broiler chickens, trained immunity of monocytes could be induced by β-glucan, followed by LPS stimulation, leading to increased mRNA levels of IL-1β [105,106]. Similar findings were reported in dogs, where the synthesis of pro-inflammatory and antimicrobial compounds was triggered in monocytes following bacterial stimulation after β-glucan induction [107]. Similarly, evidence of trained immunity has also been identified in bony fish and shrimp [108-111]. Nonetheless, immune training research on pigs has yielded varied results; the use of the BCG vaccine or β-glucan to induce trained immunity in pig monocytes can lead to either a training or tolerance state [112]. However, administration of the BCG vaccine did not restrict the replication of swine influenza A virus or prevent clinical symptoms in this pig model [113]. Alternatively, heat-inactivated Mycobacterium bovis immune stimulators have been found to enhance non-specific protection against Salmonella cholera infections in pigs [114]. This research provides evidence for trained immunity not only in humans and mice, but also in domesticated animals, including ruminants. Newborn goats and calves can exhibit activated trained immunity following the oral administration of yeast-derived β-glucan, which enhances their resistance to subsequent LPS stimulation [115,116]. Studies have also shown that milk and its component, bovine immunoglobulin G (bIgG), may trigger trained immune responses in human monocytes. When re-stimulated with the TLR1/2 ligand, Pam3CSK4, monocytes previously exposed to raw milk exhibited increased IL-6 production compared to non-trained monocytes. Moreover, following stimulation with TLR4 and TLR7/8, prior bIgG training led to increased cytokine production [102,117]. Nonetheless, other milk components may also activate trained immunity [118]. Therefore, investigating whether raw milk and bIgG can elicit trained immunity in bovine monocytes and macrophages represents a promising research direction. Additionally, aerosol BCG vaccination of young calves has been found to induce a "trained" phenotype in circulating PBMCs in in vivo assays. This induction of the trained phenotype was linked to increased Toll-like receptor expression compared to that in PBMCs from unvaccinated control calves. Furthermore, the vaccinated calves exhibited significantly enhanced pro-inflammatory cytokine responses and metabolic reprogramming [119].
SARA and trained immunity
The concept of trained immunity has deepened our understanding of the role of innate immunity in preventing infection and inflammation. Furthermore, this phenomenon has heightened interest in the correlation between innate and adaptive immunity and their intricate interplay to combating infection and inflammation. LPS plays a key role in the SARA-induced inflammatory response as well as the activation of trained immunity. This observation highlights the importance of exploring the relationship between SARA and trained immunity. However, there are currently no reports on the association between SARA and trained immunity. This review aims to identify potential connections between SARA and trained immunity based on existing studies.
The use of Saccharomyces cerevisiae as a feed additive has been associated with the development of trained immunity. S. cerevisiae effectively enhances the growth performance, including weight gain and feed efficiency, in pigs, chickens, and calves [120-122]. It has also been shown to reduce greenhouse gas emissions from ruminants, including carbon dioxide and methane [123,124]. These beneficial effects may be linked to the β-glucans abundant in the cell wall of S. cerevisiae, owing to their immunomodulatory and physiological functions. Notably, supplementation S. cerevisiae and β-glucan has been found to enhance immune responses in chickens against non-specific pathogens, such as Salmonella and Newcastle disease [122,125]. This observation suggests that β-glucan likely serve as a primary stimulus for activating trained immunity in these animals.
The mechanism of trained immunity and related research. (a) Priming stimulus and secondary stimulus for trained immunity. Following the initial stimulation, innate immune cells undergo epigenetic modifications and metabolic reprogramming, releasing a small amount of pro-inflammatory cytokines. Subsequent heterologous stimulation leads to an enhanced immune response and an increased release of pro-inflammatory cytokines. (b) Specific innate immune cells contribute to trained immunity. (c) Common metabolic and epigenetic pathways in trained immunity involve the activation of the mTOR signaling pathway and subsequent glycolysis, initiated by BCG vaccine, β-Glucan, and OxLDL. (d) Trained immunity in animals.
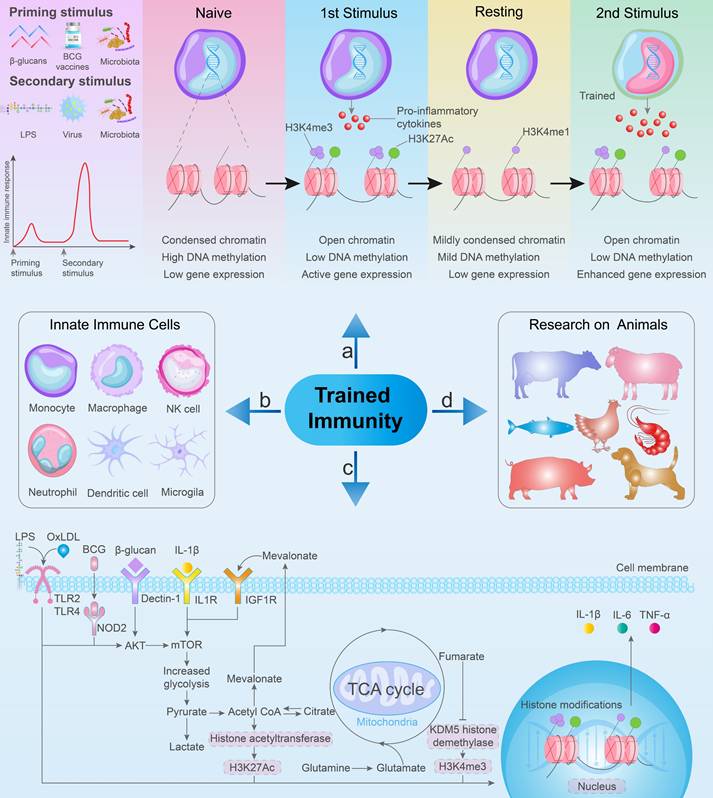
In a study of newborn goats, researchers administered the probiotic Debaryomyces hansenii containing β-glucan orally. This enhanced cellular phagocytosis, nitric oxide production and glycolysis, glucose consumption and lactate production [126]. After LPS stimulation, plasma levels of IL-1β, IL-6 and TNF-α increased, which indicated the successful induction of trained immunity [115]. As a common feed additive for ruminants, the effect of S. cerevisiae containing β-glucan on cows with SARA have been widely studied. These effects include benefits such as increased feeding frequency, milk fat production, anti-inflammatory activity, and elevated rumen pH [127-129]. Furthermore, S. cerevisiae has been shown to enhance adaptive immunity, reduces free LPS concentration, and lowers serum IL-1β levels in cows with SARA [72]. Specifically, supplementation with yeast culture can enhance the activation of innate immune responses in neutrophils. This results in increased glucose utilization through enhanced oxidative bursts in these cells [130]. According to previous reports, neutrophils exposed to β-glucan training may also exhibit anti-tumor effects [131]. A recent study found that neutrophils trained with Shigella exhibit increased bacterial clearance efficiency, although this training differs from BCG- and β-glucan-induced training [132,133]. These observations may provide a mechanistic explanation for the inhibitory effect of S. cerevisiae on rumen LPS concentrations in cows with SARA. Therefore, activating trained immunity in cows via β-glucan may help to mitigate the inflammatory responses associated with SARA.
In addition, findings from studies on trained immunity in calves may also offer valuable insights. Guerra-Maupome et al. and Samuel et al. induced trained immunity in calves using aerosolized BCG and subcutaneous BCG injections, respectively, which was reflected in the enhanced production of pro-inflammatory cytokines following LPS stimulation [119,134]. In vitro studies have shown that β-glucan upregulates the expression of co-stimulatory molecules CD80 and CD86 on the surface of bovine monocytes. Moreover, stimulated cells showed increased IL-8 production and mRNA expression of TNF-α, IL-1β and IL-6 in a dose-dependent manner, indicating evidence for trained immunity in bovine monocytes [135]. However, no relevant research has been conducted on trained immunity in cows with SARA. In cows, SARA is often characterized by reduced ruminal pH and elevated levels of LPS, HIS, and iE-DAP. Elevated levels of TNF-α, IL-1β, and IL-6 have been detected in the milk, rumen fluid, and blood of cows with SARA [10,33,39,73]. These inflammatory cytokines play a crucial role in mediating the inflammatory response of the rumen epithelium through paracrine signaling. This process partially contributes to ruminal inflammation in cows with SARA. Elevated levels of LPS, HIS, iE-DAP and MDP in the rumen and other tissues are closely associated with increased pro-inflammatory factor production and the activation of inflammatory signaling pathways.
The AKT-mTOR signaling pathway plays a crucial role in the activation of trained immunity. BCG, oxLDL, and β-glucan, as the initial stimuli for trained immunity, can engage the AKT-mTOR signaling pathway by binding to NOD2, TLR2/TLR4, and Dectin1 receptors, respectively [136]. Interestingly, LPS, HIS, iE-DAP, and MDP can also bind to receptors such as TLR4, HIS receptors, and NOD1/NOD2, activating the AKT-mTOR and NF-κB signaling pathways. These pathways further trigger a series of epigenetic modifications, including histone modifications and DNA methylation, ultimately leading to changes in gene expression patterns [91,98]. During SARA, elevated levels of stimuli such as LPS, HIS, iE-DAP, and MDP may prompt trained immune cells to mount a faster and more robust response to these challenges, thereby accelerating the initiation of inflammation and driving the secretion of increased amounts of pro-inflammatory cytokines [91,98]. Overall, BCG and β-glucan may activate trained immunity in SARA cows, enhancing non-specific immune responses to LPS from various sources. This activation may help reduce the occurrence of inflammatory reactions. However, further research is necessary to confirm the underlying mechanisms.
A growing body of research shows that LPS exposure can activate trained immunity and enhance the protective functions of innate immune cells against heterologous stimuli. A recent study demonstrated that a single low-dose exposure of the respiratory mucosa to LPS induces sustained changes in the immune phenotype of airway macrophages, promoting trained immunity and providing robust protection against acute pneumococcal infections [137]. LPS exposure can also induce profound and long-lasting remodeling of signaling pathways involved in response to LPS or fungal pathogens. Subsequent exposure to LPS or fungal stimuli may lead to immune tolerance or immune training phenotypes, respectively, depending on the type of secondary stimulus [138]. Thus, given the diverse sources of LPS in the rumen of cows with SARA, certain LPS variants might beneficially activate innate immunity and enhance resistance to toxic LPS. While previous studies have explored the beneficial and toxic effects of LPS from various bacterial sources, research specifically examining the impact of different LPS molecules on SARA remains limited. This raises questions about which bacteria produce beneficial LPS and the role these molecules play in trained immunity. Ultimately, this underscores the need to identify and compare the bacterial LPS sources in cows with SARA and healthy cows.
The induction of trained immunity is closely associated with epigenetic modifications, including changes in chromatin structure and gene expression. This corresponds to the suppression of inflammatory genes during LPS tolerance, allowing for maintained or enhanced expression of genes involved in antibacterial responses [24,139]. Therefore, it is crucial to elucidate the role of LPS in SARA and trained immunity activation. Additionally, investigating how pro-inflammatory factors function within the interplay of innate and adaptive immunity during SARA could advance our understanding of immune training and adaptive responses.
The memory duration of trained immunity is notably shorter than that of classical adaptive immunological memory [24]. Different initial stimuli can exert either beneficial or detrimental effects on immune cells, depending on the context and duration of exposure [140]. SARA in dairy cows primarily occurs during the perinatal and peak lactation periods, with minimal occurrence at other stages. Thus, the transient nature of this prevention strategy corresponds well with the elevated incidence of SARA during these stages. Effective treatment strategies for trained immunity require precise targeting of immune-related molecular mechanisms in select cells within a limited activation window [136]. It is crucial to explore the mechanisms linking SARA and trained immunity, particularly the roles of specific contributing factors at different physiological stages. Prolonged stimulation of mammalian bone marrow progenitor cells has been shown to generate “trained” myelocytes, providing a potential basis for long-term therapeutic interventions [98].
Preventative measures for SARA
To mitigate the economic losses caused by SARA on commercial farms, it is essential to implement effective preventive and corrective measures. Currently, commercial farms have adopted the total mixed rations feeding model, reducing the reliance on high-corn or high-wheat diets. This approach effectively mitigates selective feeding and excessive grain consumption, significantly reducing the occurrence of SARA [141,142]. Furthermore, maintaining a clean and well-ventilated environment alleviates stress levels associated with SARA. Recent studies show that sodium bicarbonate reduces biogenic amines and iE-DAP in the rumen, leading to an increase in rumen pH and improved microbial ecology. Sodium bicarbonate consumption may also alleviate mastitis in ruminants through the NOD1/RIRP2 signaling pathway [18,143,144].
Plant extracts contain bioactive compounds with antioxidant, antibiotic, antiviral, anticancer, antiparasitic, and antifungal properties [145,146]. Extracts derived from sources such as green tea, chestnut shells, bamboo leaves, and grape pomace have been shown to benefit animal intestinal flora, reduce oxidative stress, modulate inflammatory reactions, and enhance immune function [147-152]. Consuming a combination of naringin, glycyrrhetinic acid, cinnamon extract, and other plant extracts has been shown to increase rumen pH, improve the bacterial community, and suppress inflammatory reactions, effectively managing rumen acidosis [153-156]. Most of these plant extracts are flavonoids or polyphenols. Flavonoids are believed to act as epigenetic modulators, mediating changes and participating in disease prevention and treatment [157]. Curcumin, an important polyphenolic compound, has been shown to boost anti-inflammatory, antioxidant, and immune capabilities in post-weaning cows, although its role in SARA cows remains under-researched [158]. Curcumin may act as an epigenetic regulator of histone acetyltransferases and modifications, contributing to its involvement in trained immunity [159]. Resveratrol, another polyphenolic compound, is believed to increase beneficial rumen bacteria, reduce methanogenic bacteria, and promote average daily weight gain in fattening goats [160]. Resveratrol also induce various chemical modifications, such as oxidation, dihydroxylation, and demethylation, enhancing the ability of BCG to induce trained immunity [161,162]. Future studies should explore a broader range of plant extracts and their potential combination with trained immunity research to improve SARA treatment efficacy. This approach not only maximizes the utilization of plant residues but also helps commercial farms achieve cost savings, improve energy efficiency, and enhance overall performance.
S. cerevisiae, Lactobacilli spp., rumen-derived Diutina rugosa, and multi-strain probiotics have been shown to stabilize or increase rumen pH, regulate the intestinal microbiota, and enhance the population of cellulose-degrading microorganisms in the rumen [72,163-167]. Of particular significance is that S. cerevisiae, Lactobacilli spp. and Diutina rugosa all contain β-glucan. This suggests that some β-glucan-containing probiotics may exert their beneficial effects by stimulating trained immunity, thereby reducing rumen LPS concentrations and inflammation [74]. Akkermansia muciniphila is a promising probiotic that protect gut barrier function, reduce inflammatory cytokine levels, and has potential to induce trained immunity and mitigate inflammation [168].
LPS levels in SARA and SARA susceptible ruminants.
| Animal | Rumen fluid | Blood | Milk | Gut or feces | Tissues | Reference |
|---|---|---|---|---|---|---|
| Dairy cows | Control group: 30,768 ± 1035 EU/mL SARA group: 130,589 ± 1675 EU/mL ↑ | Control group: <0.005 EU/mL SARA group: 0.21 ± 0.06 EU/mL ↑ | - | - | - | [10] |
| Dairy cows | - | Hepatic vein and portal vein ↑ | - | - | - | [16] |
| Dairy cows | Control (LC) group: 47,170 EU/mL SARA (HC) group: 79,040 EU/mL ↑ | Jugular vein: Control (LC) group: 470 EU/mL SARA (HC) group: 860 EU/mL ↑ | - | - | - | [19] |
| Dairy cows | - | Mammary veins ↑ | - | - | - | [22] |
| Dairy cows | - | ↑ | - | - | - | [29] |
| Dairy cows | Control group: 19.7 EU/mL SARA (HC) group: 26.8 EU/mL ↑ | Control group: 12.0 EU/mL SARA (HC) group: 17.0 EU/mL ↑ | - | - | - | [30] |
| Sheep | ↑ | ↑ | - | - | Uterus ↑ | [31] |
| Dairy cows | ↑ | Tail vein and lacteal veins ↑ | ↑ | - | Mammary gland ↑ | [33] |
| Dairy cows | - | ↑ | - | - | - | [34] |
| Dairy cows | Control group: 25,704 - 29,492 EU/mL SARA group: 73,283 - 151,985 EU/mL ↑ | Control group: <0.05 EU/mL SARA group: 0.31 - 0.81 EU/mL ↑ | - | - | - | [35] |
| Sheep | ↑ | ↑ | - | - | - | [46] |
| Dairy cows | Control (LG) group: 47.17 × 103 EU/mL SARA (HC) group: 79.04 × 103 EU/mL ↑ | Hepatic vein, portal vein and jugular vein ↑ | - | - | - | [53] |
| Dairy cows | ↑ | - | - | Feces ↑ | - | [72] |
| Goats | Control group: 26.46 × 103 EU/mL SARA (HC) group: 48.37 × 103 EU/mL ↑ | Control group: 0.28 EU/mL SARA (HC) group: 0.69 EU/mL ↑ | - | - | - | [73] |
| Dairy cows | ↑ | - | - | Cecum and feces ↑ | - | [74] |
| Dairy cows | - | Control (LC) group: 7.17 ± 1.25 μg/mL SARA (HC) group: 12.12 ± 1.26 μg/mL ↑ | - | - | - | [75] |
| Dairy cows | ↑ | - | - | - | - | [76] |
| Dairy cows | ↑ | No significance | - | Cecal digesta and feces ↑ | - | [77] |
| Dairy cows | ↑ | - | - | - | - | [78] |
| Dairy cows | Control (LC) group: 4.921 × 105 EU/mL SARA (HC) group: 7.855 × 105 EU/mL ↑ | Portal vein: Control (LC) group: 0.106 EU/mL SARA (HC) group: 0.204 EU/mL ↑ | - | Cecum content: Control (LC) group: 11.960 × 105 EU/g SARA (HC) group: 13.115 × 105 EU/g ↑ | - | [79] |
| Goats | - | - | - | Colon digesta ↑ | - | [80] |
| Goats | Control group: 15.38 × 103 EU/mL SARA group: 21.81 × 103 EU/mL ↑ | Control group: 0.18 × 103 EU/mL SARA group: 0.54 × 103 EU/mL ↑ | - | - | - | [81] |
| Goats | - | ↑ | - | - | - | [82] |
| Sheep | ↑ | Hepatic vein ↑ | - | - | - | [83] |
| Sheep | ↑ | Hepatic vein and portal vein ↑ | - | colon content ↑ | - | [84] |
The interrelationships between SARA, LPS, and trained immunity, alongside key research considerations. Cows with SARA exhibit reduced dry matter intake, diminished milk production, milk fat depression, and decreased rumen pH. Decreased rumen pH leads to disruption of microbial structures and an increase in abnormal metabolites. Elevated levels of LPS in the systemic circulation can cause inflammation and adversely affect cow health. Different types of LPS may be involved as initial stimuli or secondary stimuli in the activation of trained immunity, ultimately improving the health of SARA cows.
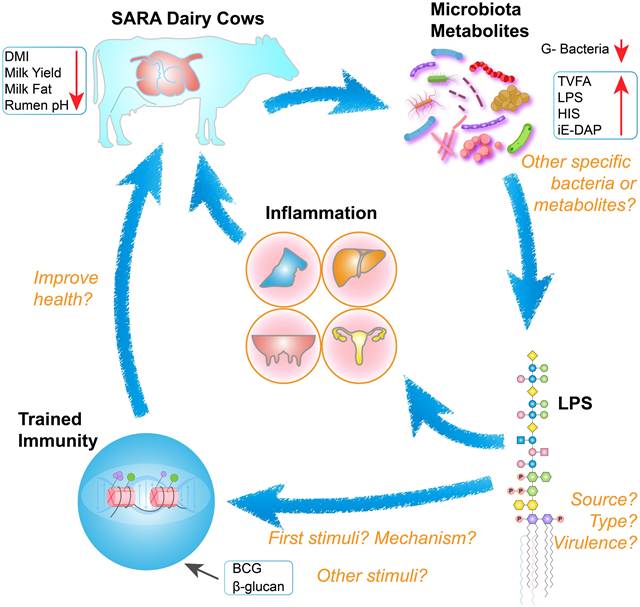
However, its role in the relationship between SARA and trained immunity requires further research. Another study showed that Lactococcus lactis can induce innate immune memory and improve cure rates in cows with chronic mastitis [169]. In conclusion, there is strong evidence suggesting that probiotics can significantly enhance the health and well-being of ruminant animals. However, there is currently no direct evidence that probiotics reduce SARA incidence by activating trained immunity.
Thiamine deficiency is a significant risk factor in lactic acidosis and alcohol use disorder [170,171]. Both animals and humans rely on dietary thiamine [172]. Thiamine supplementation has been shown to increase the abundance of microorganisms and enzymes that break down carbohydrates, while inhibiting lactic acid production [173-176]. Furthermore, thiamine supplementation regulates the Nrf2/NF-κB signaling pathway, enhancing rumen epithelial barrier function. It also improves the structure of intestinal tissue and the microbial environment [124,177,178]. As a result, numerous studies show that thiamine supplementation improves ruminal health by enhancing the microbiota composition and regulating inflammatory signaling pathways.
In summary, several strategies are available to increase ruminal pH and improve ruminal health. Notably, these include dietary supplementation with sodium bicarbonate, probiotics, plant extracts, and thiamine. However, SARA in ruminants primarily cause systemic inflammation, tissue damage, and a reduced milk production and quality. Thus, we propose that the combining strain-specific LPS with trained immunity induction may offer a novel approach to limit systemic inflammation.
Future directions and conclusions
SARA contributes to the onset of inflammatory diseases, particularly by disrupting rumen microbiota. This disruption can lead to mastitis, posing a significant threat to the quality and safety of dairy products [28,179]. HC diets increase the abundance of starch-degrading bacteria, leading to a drop in rumen pH in dairy cows. In low pH environments, gram-negative bacteria undergo lysis, releasing metabolites such as LPS, HIS, and iE-DAP. These metabolites subsequently enter peripheral blood circulation and trigger an inflammatory response. While many studies have examined SARA's occurrence and prevention, few have explored its links to trained immunity. However, the beneficial effects of S. cerevisiae and β-glucan in mitigating SARA and reducing LPS levels offer valuable insights [117]. To our knowledge, this is the first review to explore the potential links between SARA, LPS, and trained immunity (Figure 5).
HC diets are commonly used in research to induce SARA for study purposes. With advancements in commercial ranching management and implementation of effective techniques, the overall well-being of cows has significantly improved. However, variations in the incidence of SARA can still occur even when following the same diet [180]. This variability has been linked to differences in fecal bacteria and fatty acid profiles in pre- and postpartum cows during the perinatal period [181]. Early-lactating Simmental cows also exhibit varying susceptibility to SARA even on identical grain-rich diets, highlighting that dietary factors are not the sole contributors to SARA in ruminants [182]. Interestingly, studies on SARA susceptibility in cows and sheep revealed distinct rumen microbiota compositions. SARA-tolerant animals exhibited a higher proportion of fiber-degrading bacteria, while SARA-susceptible groups had more starch-degrading bacteria [183,184]. This indicates that the balance between fiber- and starch-degrading bacteria is a key determinant of SARA susceptibility and may characterize SARA-tolerant cows. Future research should explore non-dietary factors and further characterize SARA-tolerant cows.
With advancements in omics technologies, the integration of multi-omics approaches has become a powerful strategy for accurately predicting the diagnosis, prognosis, and treatment of various diseases [185]. A notable shift in research focus from single-omics to multi-omics analyses has further enhanced this potential [28,186]. The combined analysis of the microbiome, transcriptome, metabolome, and metagenome has greatly advanced the study of SARA, providing deeper insights into its underlying mechanisms. In particular, these approaches have elucidated the molecular interactions between rumen bacterial communities and their host under HC diet conditions, paving the way for novel molecular regulatory strategies to mitigate the adverse effects of SARA on ruminants.
The activation of animal innate immunity using microbial-derived LPS represents a promising therapeutic approach. LPS from intestinal Bacteroides, which is less potent and more antagonistic than the highly inflammatory LPS by E. coli, may offer reduced efficacy in suppressing inflammatory processes [187]. Specifically, low-acylated lipid A, a component of LPS, has the ability to inhibit TLR4 signaling by competing with high-acylated lipid A, thereby mitigating inflammatory responses [49]. As essential glycolipids, LPS molecules play critical roles in biological processes across diverse organisms. Currently, various techniques are employed to detect LPS, including the rabbit pyrogen test, limulus amebocyte lysate assay, antibody- and aptamer-based biosensors, endotoxin activity assays, and single-molecule nanopore analysis [188-191]. These methods enable the qualitative and quantitative analysis of LPS in biological samples. However, identifying the precise structure and acylation patterns of individual LPS molecules within complex samples remains a significant challenge. Antibody-based biosensors, while offering sensitivity and rapid detection, are costly and time-intensive [189]. The integration of omics technologies has facilitated advancements in LPS detection analysis. By combining these approaches, researchers can gain deeper insights into the structural diversity and biological functions of LPS molecules in rumen fluid, blood, and feces from cows with SARA. This knowledge will support the creation of an LPS database to identify corresponding LPS-producing microorganisms. Developing this database requires elucidating the relationships between microbial LPS in dairy cows, characterizing structural variations in these molecules, and understanding their recognition by the host immune system. Such efforts could significantly enhance our comprehension of the role of LPS in health and disease.
Common stimuli for inducing trained immunity include the BCG vaccine and β-glucan. Recent studies have expanded this list to include non-classical stimuli such as inactivated vitiligo syndrome virus, inactivated Mycobacterium bovis, nanodrugs, and the COVID-19 adenovirus vaccine [97,192-195]. The discoveries mark a significant advancement in immunology, offering innovative strategies for preventing and treating various diseases. In the context of SARA, yeast-derived β-glucan has shown promising potential to enhance immune responses in affected cows, suggesting its utility as a targeted intervention. However, further studies are required to elucidate the mechanisms underpinning immune training in cows, particularly regarding epigenetic modifications and the activation of innate immune cells. The identification of additional non-classical inducers of trained immunity opens a fascinating avenue for research, with potential applications in both veterinary and human medicine. Investigating their effects and mechanisms will likely uncover novel therapeutic strategies to combat inflammation and improve health outcomes.
Recently, numerous studies have elucidated the mechanisms through which SARA induces inflammation. The application of multi-omics technologies shows great promise in identifying targeted therapeutic drugs and addressing the challenges of current SARA treatment strategies. Emerging trained immunity provides new perspectives for comprehensively investigating SARA-related issues, particularly by examining the roles of microorganisms, their metabolites, and host immunity. Specifically, this can be achieved by considering the roles of microorganisms, their metabolites, and host immunity. Future research should integrate studies on SARA, multi-omics approaches, LPS, and immune system interaction networks to advance animal health. These findings could support the sustainable development of the dairy industry and ultimately enhance human well-being.
Abbreviations
BCG: Bacillus Calmette-Guerin vaccine; bIgG: bovine immunoglobulin G; HC: high-concentrate; HIS: histamine; iE-DAP: N-γ-d-glutamyl-meso-diaminopimelic acid; IFN-γ: interferon γ; IL-1β: interleukin 1β; IL-6: interleukin 6; LBP: lipopolysaccharide binding proteins; LC: low-concentrate; LPS: lipopolysaccharide; LpxL: kdo2-lipid IVA lauroyltransferase; LpxM: lauroyl-Kdo2-lipid IVA myristoyltransferase; MAPK: mitogen-activated protein kinases; MD-2: myeloid differentiation factor-2; MDP: muramyl dipeptide; mTOR: mechanistic target of rapamycin; NF-κB: nuclear factor-kappa B; NOD1/2: NOD-like receptor 1/2; Nrf2: nuclear factor erythroid 2-related factor 2; OS: oligosaccharide; PBMC: peripheral blood mononuclear cells; PI3K: phosphatidylinositol 3-kinase; ROS: reactive oxygen species; SARA: subacute ruminal acidosis; TLR2/4: toll-like receptor 2/4; TNF-α: tumor necrosis factor α; VFAs: volatile fatty acids.
Acknowledgements
We thank Mei Ma, Xiaotong Kang, Shuangyi Wang, Tianhao Dong, Tianyu Chen, and Wenli Guo from China Agricultural University (Beijing, China) for their kindly help during this study.
Funding
This work was supported by the National Natural Science Foundation of China (U20A2062), the Key Research and Development Program of Ningxia (2024BBF01006), and the National Center of Technology Innovation for Dairy (2024-KFKT-026).
Author contributions
Guobin Hou: Writing - original draft, Writing - review & editing, Conceptualization, Visualization. Jingjun Wang: Writing - original draft, Writing - review & editing, Conceptualization. Shuai Liu, Duo Gao, Yiming Xu, Yimin Zhuang, Wenzhuo Dong, Yi Yue, Jinni Bai, Shangru Li, Jiaying Ma, Mengmeng Li, Wei Wang, Yajing Wang and Shengli Li: Writing - review & editing. Zhijun Cao: Writing - review & editing, Conceptualization, Supervision and Funding acquisition.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Willett WC, Ludwig DS. Milk and health. N Engl J Med. 2020;382(7):644-54
2. Gozho GN, Plaizier JC, Krause DO, Kennedy AD, Wittenberg KM. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J Dairy Sci. 2005
3. Zebeli Q, Dijkstra J, Tafaj M, Steingass H, Ametaj BN, Drochner W. Modeling the adequacy of dietary fiber in dairy cows based on the responses of ruminal pH and milk fat production to composition of the diet. J Dairy Sci. 2008;91(5):2046-66
4. Fu Y, He Y, Xiang K, Zhao C, He Z, Qiu M. et al. The role of rumen microbiota and its metabolites in subacute ruminal acidosis (SARA)-induced inflammatory diseases of ruminants. Microorganisms. 2022 10(8)
5. Slyter LL. Influence of acidosis on rumen function. Journal of Animal Science. 1976;43(4):910-29
6. Plaizier JC, Krause DO, Gozho GN, McBride BW. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. The Veterinary Journal. 2008;176(1):21-31
7. Hernández J, Benedito JL, Abuelo A, Castillo C. Ruminal acidosis in feedlot: from aetiology to prevention. ScientificWorldJournal. 2014;2014:702572
8. Gozho GN, Krause DO, Plaizier JC. Ruminal lipopolysaccharide concentration and inflammatory response during grain-induced subacute ruminal acidosis in dairy cows. J Dairy Sci. 2007;90(2):856-66
9. Plaizier JC, Mulligan FJ, Neville EW, Guan LL, Steele MA, Penner GB. Invited review: Effect of subacute ruminal acidosis on gut health of dairy cows. J Dairy Sci. 2022;105(9):7141-60
10. Zhao C, Liu G, Li X, Guan Y, Wang Y, Yuan X. et al. Inflammatory mechanism of Rumenitis in dairy cows with subacute ruminal acidosis. BMC Vet Res. 2018;14(1):135
11. Ogata T, Makino H, Ishizuka N, Iwamoto E, Masaki T, Kizaki K. et al. Long-term high-grain diet alters ruminal pH, fermentation, and epithelial transcriptomes, leading to restored mitochondrial oxidative phosphorylation in Japanese Black cattle. Sci Rep. 2020;10(1):6381
12. Chang G, Wang L, Ma N, Zhang W, Zhang H, Dai H. et al. Histamine activates inflammatory response and depresses casein synthesis in mammary gland of dairy cows during SARA. BMC Vet Res. 2018;14(1):168
13. Tian M, Li K, Liu R, Du J, Zou D, Ma Y. Angelica polysaccharide attenuates LPS-induced inflammation response of primary dairy cow claw dermal cells via NF-κB and MAPK signaling pathways. BMC Vet Res. 2021;17(1):248
14. Plaizier JC, Danesh Mesgaran M, Derakhshani H, Golder H, Khafipour E, Kleen JL. et al. Review: Enhancing gastrointestinal health in dairy cows. Animal. 2018;12(s2):s399-s418
15. Aschenbach JR, Zebeli Q, Patra AK, Greco G, Amasheh S, Penner GB. Symposium review: The importance of the ruminal epithelial barrier for a healthy and productive cow. J Dairy Sci. 2019;102(2):1866-82
16. Guo J, Chang G, Zhang K, Xu L, Di Jin, Bilal MS. et al. Rumen-derived lipopolysaccharide provoked inflammatory injury in the liver of dairy cows fed a high-concentrate diet. Oncotarget. 2017;8(29):46769-80
17. Violi F, Cammisotto V, Bartimoccia S, Pignatelli P, Carnevale R, Nocella C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat Rev Cardiol. 2023;20(1):24-37
18. Wang Y, Zhang W, Ma N, Wang L, Dai H, Bilal MS. et al. Overfeeding with a high-concentrate diet activates the NOD1-NF-κB signalling pathway in the mammary gland of mid-lactating dairy cows. Microbial Pathogenesis. 2019;128:390-5
19. Bilal MS, Abaker JA, Ul Aabdin Z, Xu T, Dai H, Zhang K. et al. Lipopolysaccharide derived from the digestive tract triggers an inflammatory response in the uterus of mid-lactating dairy cows during SARA. BMC Vet Res. 2016;12(1):284
20. Chang G, Liu X, Ma N, Yan J, Dai H, Roy AC. et al. Dietary addition of sodium butyrate contributes to attenuated feeding-induced hepatocyte apoptosis in dairy goats. Journal of Agricultural and Food Chemistry. 2018;66(38):9995-10002
21. Egyedy AF, Ametaj BN. Mastitis: Impact of Dry Period, Pathogens, and Immune Responses on Etiopathogenesis of Disease and its Association with Periparturient Diseases. Dairy. 2022;3(4):881-906
22. Ma N, Abaker JA, Wei G, Chen H, Shen X, Chang G. A high-concentrate diet induces an inflammatory response and oxidative stress and depresses milk fat synthesis in the mammary gland of dairy cows. J Dairy Sci. 2022;105(6):5493-505
23. Netea MG, Quintin J, van der Meer JWM. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355-61
24. Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG. et al. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098
25. Netea MG, Joosten LAB. Trained innate immunity: Concept, nomenclature, and future perspectives. J Allergy Clin Immunol. 2024;154(5):1079-84
26. Angulo M, Angulo C. Trained immunity against diseases in domestic animals. Acta Trop. 2022;229:106361
27. Heng Y, Zhang X, Borggrewe M, van Weering HRJ, Brummer ML, Nijboer TW. et al. Systemic administration of β-glucan induces immune training in microglia. J Neuroinflammation. 2021;18(1):57
28. Zhao C, Hu X, Qiu M, Bao L, Wu K, Meng X. et al. Sialic acid exacerbates gut dysbiosis-associated mastitis through the microbiota-gut-mammary axis by fueling gut microbiota disruption. Microbiome. 2023;11(1):78
29. Zhang H, Xue Y, Xie W, Wang Y, Ma N, Chang G. et al. Subacute ruminal acidosis downregulates FOXA2, changes oxidative status, and induces autophagy in the livers of dairy cows fed a high-concentrate diet. J Dairy Sci. 2023;106(3):2007-18
30. Wu Z, Guo Y, Zhang J, Deng M, Xian Z, Xiong H. et al. High-dose Vitamin E supplementation can alleviate the negative effect of subacute ruminal acidosis in dairy cows. Animals. 2023 13(3)
31. Zeng J, Lv J, Duan H, Yang S, Wu J, Yan Z. et al. Subacute ruminal acidosis as a potential factor that induces endometrium injury in sheep. Int J Mol Sci. 2023 24(2)
32. Wang Y, Gao Q, Cheng X, Chang G, Roy AC, Shen X. Determination of γ-D-glutamyl-meso-diaminopimelic acid in rumen fluid of dairy cows by pre-column chiral derivatization-HPLC. Anim Biotechnol. 2022;33(6):1109-17
33. Hu X, Li S, Mu R, Guo J, Zhao C, Cao Y. et al. The rumen microbiota contributes to the development of mastitis in dairy cows. Microbiology Spectrum. 2022;10(1):e02512-21
34. Hou G, You J, Zhuang Y, Gao D, Xu Y, Jiang W. et al. Disorders of acid-base balance promote rumen lipopolysaccharide biosynthesis in dairy cows by modulating the microbiome. Front. Microbiol. 2024;15:2216
35. Khafipour E, Krause DO, Plaizier JC. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J Dairy Sci. 2009;92(3):1060-70
36. Jiang M, Wang K, Huang Y, Zhang X, Yang T, Zhan K. et al. Quercetin alleviates lipopolysaccharide-induced cell oxidative stress and inflammatory responses via regulation of the TLR4-NF-κB signaling pathway in bovine rumen epithelial cells. Toxins (Basel). 2023 15(8)
37. Garner MR, Flint JF, Russell JB. Allisonella histaminiformans gen. nov, sp. nov. A novel bacterium that produces histamine, utilizes histidine as its sole energy source, and could play a role in bovine and equine laminitis. Syst Appl Microbiol. 2002;25(4):498-506
38. Barcik W, Wawrzyniak M, Akdis CA, O'Mahony L. Immune regulation by histamine and histamine-secreting bacteria. Curr Opin Immunol. 2017;48:108-13
39. Wang K, Sun Z, Li Y, Liu M, Loor JJ, Jiang Q. et al. Histamine promotes adhesion of neutrophils by inhibition of autophagy in dairy cows with subacute ruminal acidosis. J Dairy Sci. 2022;105(9):7600-14
40. Chen Y, Penner GB, Li M, Oba M, Le Guan L. Changes in bacterial diversity associated with epithelial tissue in the beef cow rumen during the transition to a high-grain diet. Appl Environ Microbiol. 2011;77(16):5770-81
41. Petri RM, Schwaiger T, Penner GB, Beauchemin KA, Forster RJ, McKinnon JJ. et al. Changes in the rumen epimural bacterial diversity of beef cattle as affected by diet and induced ruminal acidosis. Appl Environ Microbiol. 2013;79(12):3744-55
42. Plaizier JC, Li S, Danscher AM, Derakshani H, Andersen PH, Khafipour E. Changes in Microbiota in Rumen Digesta and Feces Due to a Grain-Based Subacute Ruminal Acidosis (SARA) Challenge. Microb Ecol. 2017;74(2):485-95
43. Roy AC, Wang Y, Zhang H, Roy S, Dai H, Chang G. et al. Sodium butyrate mitigates iE-DAP induced inflammation caused by high-concentrate feeding in liver of dairy goats. Journal of Agricultural and Food Chemistry. 2018;66(34):8999-9009
44. Roy AC, Chang G, Roy S, Ma N, Gao Q, Shen X. γ-d-Glutamyl-meso-diaminopimelic acid induces autophagy in bovine hepatocytes during nucleotide-binding oligomerization domain 1-mediated inflammation. J Cell Physiol. 2021;236(7):5212-34
45. Qiu M, Ye C, Bao L, Wu K, Zhao Y, Zhao X. et al. Elevated muramyl dipeptide by sialic acid-facilitated postantibiotic pathobiont expansion contributes to gut dysbiosis-induced mastitis in mice. J Adv Res. 2024
46. Meng M, Zhao X, Huo R, Li X, Chang G, Shen X. Disodium fumarate alleviates endoplasmic reticulum stress, mitochondrial damage, and oxidative stress induced by the high-concentrate diet in the mammary gland tissue of Hu sheep. Antioxidants. 2023 12(2)
47. Foo J, Bellot G, Pervaiz S, Alonso S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol. 2022;30(7):679-92
48. Dherbey JR, Parab L, Gallie J, Bertels F. Stepwise evolution of E. coli C and ΦX174 reveals unexpected lipopolysaccharide (LPS) diversity. Mol Biol Evol. 2023 40(7)
49. Di Lorenzo F, Duda KA, Lanzetta R, Silipo A, Castro C de, Molinaro A. A journey from structure to function of bacterial lipopolysaccharides. Chem Rev. 2022;122(20):15767-821
50. Stephens M, Weid P-Y von der. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes. 2020;11(3):421-32
51. Li Y, Xu M, Zhai H, Yang C, Yang J, Ke Z. et al. Lipopolysaccharide (LPS) extracted from Bacteroides vulgatus effectively prevents LPS extracted from Escherichia coli from inducing epithelial-mesenchymal transition. Mol Med Rep. 2023 28(4)
52. Sulit AK, Daigneault M, Allen-Vercoe E, Silander OK, Hock B, McKenzie J. et al. Bacterial lipopolysaccharide modulates immune response in the colorectal tumor microenvironment. NPJ Biofilms Microbiomes. 2023;9(1):59
53. Abaker JA, Xu TL, Jin D, Chang GJ, Zhang K, Shen XZ. Lipopolysaccharide derived from the digestive tract provokes oxidative stress in the liver of dairy cows fed a high-grain diet. J Dairy Sci. 2017;100(1):666-78
54. Zhang K, Chang G, Xu T, Xu L, Guo J, Di Jin. et al. Lipopolysaccharide derived from the digestive tract activates inflammatory gene expression and inhibits casein synthesis in the mammary glands of lactating dairy cows. Oncotarget. 2016;7(9):9652-65
55. Jin D, Chang G, Zhang K, Guo J, Xu T, Shen X. Rumen-derived lipopolysaccharide enhances the expression of lingual antimicrobial peptide in mammary glands of dairy cows fed a high-concentrate diet. BMC Vet Res. 2016;12(1):128
56. Pither MD, Illiano A, Pagliuca C, Jacobson A, Mantova G, Stornaiuolo A. et al. Bacteroides thetaiotaomicron rough-type lipopolysaccharide: The chemical structure and the immunological activity. Carbohydr Polym. 2022;297:120040
57. Sarmikasoglou E, Faciola AP. Ruminal Lipopolysaccharides Analysis: Uncharted Waters with Promising Signs. Animals (Basel). 2021 11(1)
58. Liu B, Furevi A, Perepelov AV, Guo X, Cao H, Wang Q. et al. Structure and genetics of Escherichia coli O antigens. FEMS Microbiol Rev. 2020;44(6):655-83
59. Sun L, You J, Li D, Zhang Z, Qin X, Pang W. et al. Variants of a putative baseplate wedge protein extend the host range of Pseudomonas phage K8. Microbiome. 2023;11(1):18
60. Golomidova AK, Naumenko OI, Senchenkova SN, Knirel YA, Letarov AV. The O-polysaccharide of Escherichia coli F5, which is structurally related to that of E. coli O28ab, provides cells only weak protection against bacteriophage attack. Arch Virol. 2019;164(11):2783-7
61. Knirel YA, Ivanov PA, Senchenkova SN, Naumenko OI, Ovchinnikova OO, Shashkov AS. et al. Structure and gene cluster of the O antigen of Escherichia coli F17, a candidate for a new O-serogroup. Int J Biol Macromol. 2019;124:389-95
62. Heiman CM, Maurhofer M, Calderon S, Dupasquier M, Marquis J, Keel C. et al. Pivotal role of O-antigenic polysaccharide display in the sensitivity against phage tail-like particles in environmental Pseudomonas kin competition. ISME J. 2022;16(7):1683-93
63. Forn-Cuní G, Merino S, Tomás JM. Comparative genomics of the aeromonadaceae core oligosaccharide biosynthetic regions. Int J Mol Sci. 2017 18(3)
64. Ovchinnikova OG, Mallette E, Koizumi A, Lowary TL, Kimber MS, Whitfield C. Bacterial β-Kdo glycosyltransferases represent a new glycosyltransferase family (GT99). Proc Natl Acad Sci U S A. 2016;113(22):E3120-9
65. Recio AM, Merino J, Jiménez AG, Alvarez RC, Fresno M, Santamaría SM. Immune evasion through Toll-like receptor 4: The role of the core oligosaccharides from α2-Proteobacteria atypical lipopolysaccharides. Carbohydr Polym. 2023;318:121094
66. Dennis CK-, Aschenbach JR, Griebel PJ, Penner GB. Effects of lipopolysaccharide exposure in primary bovine ruminal epithelial cells. J Dairy Sci. 2020;103(10):9587-603
67. Guo J, Mu R, Li S, Zhang N, Fu Y, Hu X. Characterization of the Bacterial Community of Rumen in Dairy Cows with Laminitis. Genes (Basel). 2021 12(12)
68. Reisinger N, Wendner D, Schauerhuber N, Mayer E. Effect of lipopolysaccharides (LPS) and lipoteichoic acid (LTA) on the inflammatory response in rumen epithelial cells (REC) and the impact of LPS on claw explants. Animals. 2021 11(7)
69. Zhao C, Hu X, Bao L, Wu K, Zhao Y, Xiang K. et al. Gut dysbiosis induces the development of mastitis through a reduction in host anti-inflammatory enzyme activity by endotoxemia. Microbiome. 2022;10(1):205
70. Humer E, Aditya S, Zebeli Q. Innate immunity and metabolomic responses in dairy cows challenged intramammarily with lipopolysaccharide after subacute ruminal acidosis. Animal. 2018;12(12):2551-60
71. Wu T, Wang C, Ding L, Shen Y, Cui H, Wang M. et al. Arginine relieves the inflammatory response and enhances the casein expression in bovine mammary epithelial cells induced by lipopolysaccharide. Mediators Inflamm. 2016;2016:9618795
72. Guo J, Xu L, Khalouei H, Fehr K, Senaratne V, Ghia JE. et al. Saccharomyces cerevisiae fermentation products reduce bacterial endotoxin concentrations and inflammation during grain-based subacute ruminal acidosis in lactating dairy cows. J Dairy Sci. 2022;105(3):2354-68
73. Ma Y, Zhang Y, Zhang H, Wang H, Elmhadi M. Thiamine alleviates high-concentrate-diet-induced oxidative stress, apoptosis, and protects the rumen epithelial barrier function in goats. Front Vet Sci. 2021;8:663698
74. Li S, Yoon I, Scott M, Khafipour E, Plaizier JC. Impact of Saccharomyces cerevisiae fermentation product and subacute ruminal acidosis on production, inflammation, and fermentation in the rumen and hindgut of dairy cows. Animal Feed Science and Technology. 2016;211:50-60
75. Meng M, Li X, Huo R, Ma N, Chang G, Shen X. A high-concentrate diet induces mitochondrial dysfunction by activating the MAPK signaling pathway in the mammary gland of dairy cows. J Dairy Sci. 2023;106(8):5775-87
76. Humer E, Kröger I, Neubauer V, Schedle K, Reisinger N, Zebeli Q. Supplementing phytogenic compounds or autolyzed yeast modulates ruminal biogenic amines and plasma metabolome in dry cows experiencing subacute ruminal acidosis. J Dairy Sci. 2018;101(10):9559-74
77. Li S, Khafipour E, Krause DO, Kroeker A, Rodriguez-Lecompte JC, Gozho GN. et al. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J Dairy Sci. 2012;95(1):294-303
78. Stefanska B, Człapa W, Pruszynska-Oszmałek E, Szczepankiewicz D, Fievez V, Komisarek J. et al. Subacute ruminal acidosis affects fermentation and endotoxin concentration in the rumen and relative expression of the CD14/TLR4/MD2 genes involved in lipopolysaccharide systemic immune response in dairy cows. J Dairy Sci. 2018;101(2):1297-310
79. Ma N, Guo J, Li Z, Xu L, Zhang K, Xu T. et al. Disturbances of Ruminal Microbiota and Liver Inflammation, Mediated by LPS and Histamine, in Dairy Cows Fed a High-Concentrate Diet. Animals (Basel). 2024 14(10)
80. Hou M, Song P, Chen Y, Yang X, Chen P, Cao A. et al. Bile acids supplementation improves colonic mucosal barrier via alteration of bile acids metabolism and gut microbiota composition in goats with subacute ruminal acidosis (SARA). Ecotoxicol Environ Saf. 2024;287:117313
81. He B, Fan Y, Wang H. Lactate uptake in the rumen and its contributions to subacute rumen acidosis of goats induced by high-grain diets. Front Vet Sci. 2022;9:964027
82. Tuniyazi M, Tang R, Hu X, Zhang N, Shen P. Efficacy of Carbonate Buffer Mixture in Preventing Hoof Lamella Injury Associated with Subacute Ruminal Acidosis in Dairy Goats. Vet Sci. 2024 11(9)
83. Zhang H, Shi H, Zhou S, Wei G, Xie W, Meng M. et al. Dietary disodium fumarate supplementation alleviates subacute ruminal acidosis (SARA)-induced liver damage by inhibiting pyroptosis via mitophagy-NLRP3 inflammasome pathway in lactating Hu sheep. Front Immunol. 2023;14:1197133
84. Chen M, Xie W, Zhou S, Ma N, Wang Y, Huang J. et al. A high-concentrate diet induces colonic inflammation and barrier damage in Hu sheep. J Dairy Sci. 2023;106(12):9644-62
85. Munro P, Dufies O, Rekima S, Loubat A, Duranton C, Boyer L. et al. Modulation of the inflammatory response to LPS by the recruitment and activation of brown and brite adipocytes in mice. Am J Physiol Endocrinol Metab. 2020;319(5):E912-E922
86. Anhê FF, Barra NG, Cavallari JF, Henriksbo BD, Schertzer JD. Metabolic endotoxemia is dictated by the type of lipopolysaccharide. Cell Rep. 2021;36(11):109691
87. Pieterse E, Rother N, Yanginlar C, Hilbrands LB, van der Vlag J. Neutrophils discriminate between lipopolysaccharides of different bacterial sources and selectively release neutrophil extracellular traps. Front Immunol. 2016;7:484
88. Netea MG, van Deuren M, Kullberg BJ, Cavaillon J-M, van der Meer JWM. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 2002;23(3):135-9
89. Seydel U, Schromm AB, Blunck R, Brandenburg K. Chemical structure, molecular conformation, and bioactivity of endotoxins. Chem Immunol. 2000;74:5-24
90. Jacobsen S, Andersen PH, Toelboell T, Heegaard PMH. Dose dependency and individual variability of the lipopolysaccharide-induced bovine acute phase protein response. J Dairy Sci. 2004;87(10):3330-9
91. Ochando J, Mulder WJM, Madsen JC, Netea MG, Duivenvoorden R. Trained immunity - basic concepts and contributions to immunopathology. Nat Rev Nephrol. 2023;19(1):23-37
92. Wenig M, Ghirardo A, Sales JH, Pabst ES, Breitenbach HH, Antritter F. et al. Systemic acquired resistance networks amplify airborne defense cues. Nat Commun. 2019;10(1):3813
93. Milutinović B, Kurtz J. Immune memory in invertebrates. Semin Immunol. 2016;28(4):328-42
94. Domínguez-Andrés J, Dos Santos JC, Bekkering S, Mulder WJM, van der Meer JWM, Riksen NP. et al. Trained immunity: adaptation within innate immune mechanisms. Physiol Rev. 2023;103(1):313-46
95. Reimer-Michalski E-M, Conrath U. Innate immune memory in plants. Semin Immunol. 2016;28(4):319-27
96. Blanden RV, Lefford MJ, Mackaness GB. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969;129(5):1079-107
97. Kalafati L, Hatzioannou A, Hajishengallis G, Chavakis T. The role of neutrophils in trained immunity. Immunol Rev. 2023;314(1):142-57
98. Mulder WJM, Ochando J, Joosten LAB, Fayad ZA, Netea MG. Therapeutic targeting of trained immunity. Nat Rev Drug Discov. 2019;18(7):553-66
99. Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R. et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 2016;17(10):2562-71
100. Arts RJW, Novakovic B, Ter Horst R, Carvalho A, Bekkering S, Lachmandas E. et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016;24(6):807-19
101. Ferreira AV, Uijen RF, Bulut O, Jonge MI de, Domínguez-Andrés J, Netea MG. Limited role of the spleen in a mouse model of trained immunity: impact on neutrophilia. J Leukoc Biol. 2022;111(1):9-17
102. Splunter Mv, van Osch TLJ, Brugman S, Savelkoul HFJ, Joosten LAB, Netea MG. et al. Induction of trained innate immunity in human monocytes by bovine milk and milk-derived immunoglobulin G. Nutrients. 2018 10(10)
103. Petit J, Embregts CWE, Forlenza M, Wiegertjes GF. Evidence of trained immunity in a fish: Conserved features in carp macrophages. The Journal of Immunology. 2019;203(1):216-24
104. Burgess SL, Buonomo E, Carey M, Cowardin C, Naylor C, Noor Z. et al. Bone marrow dendritic cells from mice with an altered microbiota provide interleukin 17A-dependent protection against Entamoeba histolytica colitis. mBio. 2014;5(6):e01817
105. Verwoolde MB, van den Biggelaar RHGA, Vries Reilingh G de, Arts JAJ, van Baal J, Lammers A. et al. Innate immune training and metabolic reprogramming in primary monocytes of broiler and laying hens. Dev Comp Immunol. 2021;114:103811
106. Dagan HB, Cohen NA, Gover O, Rozenboim I, Schwartz B, Vetvicka V. Beta-glucans induce cellular immune training and changes in intestinal morphology in poultry. Front Vet Sci. 2022;9:1092812
107. Paris S, Chapat L, Pasin M, Lambiel M, Sharrock TE, Shukla R. et al. β-Glucan-induced trained immunity in dogs. Front Immunol. 2020;11:566893
108. You X, Yang J, Wang Z, Wang Q, Liu Q, Zhang Y. et al. Progress and perspective of trained immunity in teleost fish. Reviews in Aquaculture. 2023;13(1):274
109. Mu D, Yang J, Jiang Y, Wang Z, Chen W, Huang J. et al. Single-cell transcriptomic analysis reveals neutrophil as orchestrator during β-Glucan-induced trained immunity in a Teleost fish. The Journal of Immunology. 2022;209(4):783-95
110. Zang S, Lv L-X, Liu C-F, Zhang P, Li C, Wang J-X. Metabolomic investigation of ultraviolet ray-inactivated white spot syndrome virus-induced trained immunity in marsupenaeus japonicus. Front Immunol. 2022;13:885782
111. Wu Z, Yang Y, Li J, Bossier P, Wei X, Guo Z. et al. β-Glucans in particulate and solubilized forms elicit varied immunomodulatory and apoptosis effects in teleost macrophages in a dosedependent manner. Front Immunol. 2023;14:1243358
112. Byrne KA, Tuggle CK, Loving CL. Differential induction of innate memory in porcine monocytes by β-glucan or bacillus Calmette-Guerin. Innate Immun. 2021;27(6):448-60
113. Loving CL, Hill D, Byrne K. Neonatal bacilllus Calmette-Guerin (BCG) administration did not limit influenza A virus replication or clinical disease in pigs. The Journal of Immunology. 2023;210(1_Supplement):73.09-73.09
114. Vaz-Rodrigues R, Ferreras-Colino E, Ugarte-Ruíz M, Pesciaroli M, Thomas J, García-Seco T. et al. Nonspecific protection of heat-inactivated Mycobacterium bovis against Salmonella choleraesuis infection in pigs. Vet Res. 2022;53(1):31
115. Angulo M, Reyes-Becerril M, Cepeda-Palacios R, Angulo C. Oral administration of Debaryomyces hansenii CBS8339-β-glucan induces trained immunity in newborn goats. Dev Comp Immunol. 2020;105:103597
116. Angulo M, Carlos A. Immunometabolic changes of β-glucan-trained immunity induction and inhibition on neonatal calf immune innate cells. Mol Immunol. 2023;159:58-68
117. Porbahaie M, Savelkoul HFJ, Haan CAM de, Teodorowicz M, van Neerven RJJ. Direct binding of bovine IgG-containing immune complexes to human monocytes and their putative role in innate immune training. Nutrients. 2022 14(21)
118. Hellinga AH, Tsallis T, Eshuis T, Triantis V, Ulfman LH, van Neerven RJJ. In vitro induction of trained innate immunity by bIgG and whey protein extracts. Int J Mol Sci. 2020 21(23)
119. Guerra-Maupome M, Vang DX, McGill JL. Aerosol vaccination with Bacille Calmette-Guerin induces a trained innate immune phenotype in calves. PLoS One. 2019;14(2):e0212751
120. Maggiolino A, Centoducati G, Casalino E, Elia G, Latronico T, Liuzzi MG. et al. Use of a commercial feed supplement based on yeast products and microalgae with or without nucleotide addition in calves. J Dairy Sci. 2023;106(6):4397-412
121. Law K, Johnston LJ, Gomez A, Urriola PE, Kiros TGG, Loughmiller J. 279 Maternal programming of the piglet microbiome through supplementation with a yeast feed additive. Journal of Animal Science. 2023;101(Supplement_2):166-7
122. Chaney WE, McBride H, Girgis G. Effect of a Saccharomyces cerevisiae postbiotic feed additive on Salmonella enteritidis colonization of cecal and ovarian tissues in directly challenged and horizontally exposed layer pullets. Animals. 2023 13(7)
123. Hernández A, Kholif AE, Elghandour MMMY, Camacho LM, Cipriano MM, Salem AZM. et al. Effectiveness of xylanase and Saccharomyces cerevisiae as feed additives on gas emissions from agricultural calf farms. Journal of Cleaner Production. 2017;148:616-23
124. Vallejo-Hernández LH, Elghandour MMY, Greiner R, Anele UY, Rivas-Cáceres RR, Barros-Rodríguez M. et al. Environmental impact of yeast and exogenous xylanase on mitigating carbon dioxide and enteric methane production in ruminants. Journal of Cleaner Production. 2018;189(1):40-6
125. Qui NH, Linh NT. Effects of dietary β-glucan and rice fermented on growth performance, fatty acids, and Newcastle disease immune response in turkey broilers. Saudi J Biol Sci. 2023;30(8):103736
126. Angulo M, Angulo C. Analysis of the potential long-lasting effects of probiotic Debaryomyces hansenii CBS 8339 on trained immunity in newborn goats. Developmental & Comparative Immunology. 2024;30(3):105292
127. Sivinski SE, Meier KE, Mamedova LK, Saylor BA, Shaffer JE, Sauls-Hiesterman JA. et al. Effect of Saccharomyces cerevisiae fermentation product on oxidative status, inflammation, and immune response in transition dairy cattle. J Dairy Sci. 2022;105(11):8850-65
128. Ahmed G, Wang H. Active dry yeast and thiamine in synergistic mode can mitigate adverse effects of in vitro ruminal acidosis model of goats. Animals. 2022 12(18)
129. Vailati-Riboni M, Coleman DN, Lopreiato V, Alharthi A, Bucktrout RE, Abdel-Hamied E. et al. Feeding a Saccharomyces cerevisiae fermentation product improves udder health and immune response to a Streptococcus uberis mastitis challenge in mid-lactation dairy cows. J Anim Sci Biotechnol. 2021;12(1):62
130. Marins TN, Gutierrez Oviedo FA, Costa MLGF, Chen Y-C, Goodnight H, Garrick M. et al. Impacts of feeding a Saccharomyces cerevisiae fermentation product on productive performance, and metabolic and immunological responses during a feed-restriction challenge of mid-lactation dairy cows. J Dairy Sci. 2023;106(1):202-18
131. Kalafati L, Kourtzelis I, Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T. et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell. 2020;183(3):771-785.e12
132. Gomes MC, Brokatzky D, Bielecka MK, Wardle FC, Mostowy S. Shigella induces epigenetic reprogramming of zebrafish neutrophils. Sci Adv. 2023;9(36):eadf9706
133. Darroch H, Keerthisinghe P, Sung YJ, Rolland L, Prankerd-Gough A, Crosier PS. et al. Infection-experienced HSPCs protect against infections by generating neutrophils with enhanced mitochondrial bactericidal activity. Sci Adv. 2023;9(36):eadf9904
134. Samuel BER, Diaz FE, Maina TW, Corbett RJ, Tuggle CK, McGill JL. Evidence of innate training in bovine γδ T cells following subcutaneous BCG administration. Front Immunol. 2024;15:1423843
135. Pedro ARV, Lima T, Fróis-Martins R, Leal B, Ramos IC, Martins EG. et al. Dectin-1-Mediated Production of Pro-Inflammatory Cytokines Induced by Yeast β-Glucans in Bovine Monocytes. Front Immunol. 2021;12:689879
136. Riksen NP, Bekkering S, Mulder WJM, Netea MG. Trained immunity in atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2023
137. Kang A, Ye G, Afkhami S, Aleithan F, Singh K, Dvorkin-Gheva A. et al. LPS-induced lung tissue-resident trained innate immunity provides differential protection against pneumococci and SARS-CoV-2. Cell Rep. 2024;43(10):114849
138. Zuani M de, Dal Secco C, Tonon S, Arzese A, Pucillo CEM, Frossi B. LPS Guides Distinct Patterns of Training and Tolerance in Mast Cells. Front Immunol. 2022;13:835348
139. Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7147):972-8
140. Jeljeli M, Riccio LGC, Doridot L, Chêne C, Nicco C, Chouzenoux S. et al. Trained immunity modulates inflammation-induced fibrosis. Nat Commun. 2019;10(1):5670
141. Abeyta MA, Goetz BM, Mayorga EJ, Rodriguez-Jimenez S, Opgenorth J, Freestone AD. et al. Effects of abomasally infused rumen fluid from corn-challenged donor cows on production, metabolism, and inflammatory biomarkers in healthy recipient cows. J Dairy Sci. 2023
142. Elmhadi ME, Ali DK, Khogali MK, Wang H. Subacute ruminal acidosis in dairy herds: microbiological and nutritional causes, consequences, and prevention strategies. Animal Nutrition. 2022;10:148-55
143. Mao S, Huo W, Liu J, Zhang R, Zhu W. In vitro effects of sodium bicarbonate buffer on rumen fermentation, levels of lipopolysaccharide and biogenic amine, and composition of rumen microbiota. J Sci Food Agric. 2017;97(4):1276-85
144. Wang Y, Liu J, Huang J, Chang G, Roy AC, Gao Q. et al. Sodium butyrate attenuated iE-DAP induced inflammatory response in the mammary glands of dairy goats fed high-concentrate diet. J Sci Food Agric. 2021;101(3):1218-27
145. Li C, Cui Z, Deng S, Chen P, Li X, Yang H. The potential of plant extracts in cell therapy. Stem Cell Res Ther. 2022;13(1):472
146. Armendáriz-Barragán B, Zafar N, Badri W, Galindo-Rodríguez SA, Kabbaj D, Fessi H. et al. Plant extracts: from encapsulation to application. Expert Opin Drug Deliv. 2016;13(8):1165-75
147. Gessner DK, Brock C, Hof LM, Most E, Koch C, Eder K. Effects of supplementation of green tea extract on the milk performance of peripartal dairy cows and the expression of stress response genes in the liver. J Anim Sci Biotechnol. 2020;11:57
148. Gessner DK, Winkler A, Koch C, Dusel G, Liebisch G, Ringseis R. et al. Analysis of hepatic transcript profile and plasma lipid profile in early lactating dairy cows fed grape seed and grape marc meal extract. BMC Genomics. 2017;18(1):253
149. Cheng X, Yang S, Xu C, Li L, Zhang Y, Guo Y. et al. Proanthocyanidins protect against β-hydroxybutyrate-induced oxidative damage in bovine endometrial cells. Molecules. 2019 24(3)
150. Xiong Y, Dong S, Zhao X, Guo KJ, Gasco L, Zoccarato I. Gene expressions and metabolomic research on the effects of polyphenols from the involucres of Castanea mollissima Blume on heat-stressed broilers chicks. Poult Sci. 2016;95(8):1869-80
151. Liu S, Lu Z, Liu C, Chang X, Apudureheman B, Chen S. et al. Castanea mollissima shell polyphenols regulate JAK2 and PPARγ expression to suppress inflammation and lipid accumulation by inhibiting M1 macrophages polarization. Journal of Functional Foods. 2022;92:105046
152. Shu G, Kong F, Xu D, Yin L, He C, Lin J. et al. Bamboo leaf flavone changed the community of cecum microbiota and improved the immune function in broilers. Sci Rep. 2020;10(1):12324
153. Rivera-Chacon R, Castillo-Lopez E, Ricci S, Petri RM, Reisinger N, Zebeli Q. Supplementing a phytogenic feed additive modulates the risk of subacute rumen acidosis, rumen fermentation and systemic inflammation in cattle fed acidogenic diets. Animals. 2022 12(9)
154. López-Campos O, Bodas R, Prieto N, Giráldez FJ, Pérez V, Andrés S. Naringin dietary supplementation at 0.15% rates does not provide protection against sub-clinical acidosis and does not affect the responses of fattening lambs to road transportation. Animal. 2010;4(6):958-64
155. Ahmed MG, Al-Sagheer AA, El-Zarkouny SZ, Elwakeel EA. Potential of selected plant extracts to control severe subacute ruminal acidosis in vitro as compared with monensin. BMC Vet Res. 2022;18(1):356
156. Liu J, Ma B, Hao G, Su D, Wang T, Ding Z. et al. Glycyrrhizin inhibits LPS-induced inflammatory responses in goat ruminal epithelial cells in vitro. BMC Mol Cell Biol. 2023;24(1):28
157. Fatima N, Baqri SSR, Bhattacharya A, Koney NK-K, Husain K, Abbas A. et al. Role of Flavonoids as Epigenetic Modulators in Cancer Prevention and Therapy. Front Genet. 2021;12:758733
158. Novakoski PV, Vitt MG de, Molosse VL, Xavier ACH, Wagner R, Klein B. et al. The addition of curcumin to the diet of post-weaning dairy calves: effects on ruminal fermentation, immunological, and oxidative responses. Trop Anim Health Prod. 2024;56(4):142
159. Hassan F-U, Rehman MS-U, Khan MS, Ali MA, Javed A, Nawaz A. et al. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front Genet. 2019;10:514
160. Meng Q, Li J, Wang C, Shan A. Biological function of resveratrol and its application in animal production: a review. J Anim Sci Biotechnol. 2023;14(1):25
161. Griñán-Ferré C, Bellver-Sanchis A, Izquierdo V, Corpas R, Roig-Soriano J, Chillón M. et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer's disease pathology: From antioxidant to epigenetic therapy. Ageing Res Rev. 2021;67:101271
162. Bulut O, Baydemir I, Kilic G, Domínguez-Andrés J, Netea MG. Resveratrol Potentiates BCG-induced Trained Immunity in Human Monocytes. J Leukoc Biol. 2024
163. Goto H, Qadis AQ, Kim Y-H, Ikuta K, Ichijo T, Sato S. Effects of a bacterial probiotic on ruminal pH and volatile fatty acids during subacute ruminal acidosis (SARA) in cattle. J Vet Med Sci. 2016;78(10):1595-600
164. AlZahal O, Dionissopoulos L, Laarman AH, Walker N, McBride BW. Active dry Saccharomyces cerevisiae can alleviate the effect of subacute ruminal acidosis in lactating dairy cows. J Dairy Sci. 2014;97(12):7751-63
165. Malekkhahi M, Tahmasbi AM, Naserian AA, Danesh-Mesgaran M, Kleen JL, AlZahal O. et al. Effects of supplementation of active dried yeast and malate during sub-acute ruminal acidosis on rumen fermentation, microbial population, selected blood metabolites, and milk production in dairy cows. Animal Feed Science and Technology. 2016;213:29-43
166. Lettat A, Nozière P, Silberberg M, Morgavi DP, Berger C, Martin C. Rumen microbial and fermentation characteristics are affected differently by bacterial probiotic supplementation during induced lactic and subacute acidosis in sheep. BMC Microbiol. 2012;12:142
167. Guo J, Zhang Z, Le Guan L, Yoon I, Plaizier JC, Khafipour E. Postbiotics from Saccharomyces cerevisiae fermentation stabilize microbiota in rumen liquid digesta during grain-based subacute ruminal acidosis (SARA) in lactating dairy cows. J Anim Sci Biotechnol. 2024;15(1):101
168. Peña-Cearra A, Palacios A, Pellon A, Castelo J, Pasco ST, Seoane I. et al. Akkermansia muciniphila-induced trained immune phenotype increases bacterial intracellular survival and attenuates inflammation. Commun Biol. 2024;7(1):192
169. Chaumond E, Peron S, Daniel N, Le Gouar Y, Guédon É, Williams DL. et al. Development of innate immune memory by non-immune cells during Staphylococcus aureus infection depends on reactive oxygen species. Front Immunol. 2023;14:1138539
170. Ricci Z, Romagnoli S. The 11th pitfall: thiamine deficiency. Intensive Care Med. 2018;44(9):1597
171. Pawar RD, Balaji L, Grossestreuer AV, Thompson G, Holmberg MJ, Issa MS. et al. Thiamine supplementation in patients with alcohol use disorder presenting with acute critical illness: A nationwide retrospective observational study. Ann Intern Med. 2022;175(2):191-7
172. Feng X, Yang S, Tang K, Zhang Y, Leng J, Ma J. et al. GmPGL1, a thiamine thiazole synthase, is required for the biosynthesis of thiamine in soybean. Front Plant Sci. 2019;10:1546
173. Zhang Y, Wang C, Peng A, Zhang H, Wang H. Metagenomic insight: Dietary thiamine supplementation promoted the growth of carbohydrate-associated microorganisms and enzymes in the rumen of Saanen goats fed high-concentrate diets. Microorganisms. 2021 9(3)
174. Ma P, Sun C, Liu M, You H, Shen Y, Kang Y. et al. Metagenomic insights into the rumen epithelial integrity responses to the vitamin B1 supplement under high-concentrate diets treatments. Frontiers in Microbiology. 2022;13:1008373
175. Pan XH, Yang L, Xue FG, Xin HR, Jiang LS, Xiong BH. et al. Relationship between thiamine and subacute ruminal acidosis induced by a high-grain diet in dairy cows. J Dairy Sci. 2016;99(11):8790-801
176. Xue F, Pan X, Jiang L, Guo Y, Xiong B. GC-MS analysis of the ruminal metabolome response to thiamine supplementation during high grain feeding in dairy cows. Metabolomics. 2018;14(5):67
177. Zhang H, Peng AL, Zhao FF, Yu LH, Wang MZ, Osorio JS. et al. Thiamine ameliorates inflammation of the ruminal epithelium of Saanen goats suffering from subacute ruminal acidosis. J Dairy Sci. 2020;103(2):1931-43
178. Wen K, Zhao MM, Liu L, Khogali MK, Geng TY, Wang HR. et al. Thiamine modulates intestinal morphological structure and microbiota under subacute ruminal acidosis induced by a high-concentrate diet in Saanen goats. Animal. 2021;15(10):100370
179. Ma N, Chen X, Johnston LJ, Ma X. Gut microbiota-stem cell niche crosstalk: A new territory for maintaining intestinal homeostasis. iMeta. 2022 1(4)
180. Huot F, Claveau S, Bunel A, Santschi DE, Gervais R, Paquet ÉR. Relationship between farm management strategies, reticuloruminal pH variations, and risks of subacute ruminal acidosis. J Dairy Sci. 2023;106(4):2487-97
181. Yang H, Heirbaut S, Jing X, Neve N de, Vandaele L, Jeyanathan J. et al. Susceptibility of dairy cows to subacute ruminal acidosis is reflected in both prepartum and postpartum bacteria as well as odd- and branched-chain fatty acids in feces. J Anim Sci Biotechnol. 2022;13(1):87
182. Khiaosa-ard R, Pourazad P, Aditya S, Humer E, Zebeli Q. Factors related to variation in the susceptibility to subacute ruminal acidosis in early lactating Simmental cows fed the same grain-rich diet. Animal Feed Science and Technology. 2018;238:111-22
183. Zhang ZA, Li F, Ma ZY, Li FD, Wang ZL, Li SR. et al. Variability in chewing, ruminal fermentation, digestibility and bacterial communities between subacute ruminal acidosis-susceptible and acidosis-tolerant sheep. Animal. 2023;17(8):100902
184. Zhang T, Mu Y, Zhang R, Xue Y, Guo C, Qi W. et al. Responsive changes of rumen microbiome and metabolome in dairy cows with different susceptibility to subacute ruminal acidosis. Animal Nutrition. 2022;8:331-40
185. Pan Y, Lei X, Zhang Y. Association predictions of genomics, proteinomics, transcriptomics, microbiome, metabolomics, pathomics, radiomics, drug, symptoms, environment factor, and disease networks: A comprehensive approach. Med Res Rev. 2022;42(1):441-61
186. Mu Y, Qi W, Zhang T, Zhang J, Mao S. Multi-omics analysis revealed coordinated responses of rumen microbiome and epithelium to high-grain-induced subacute rumen acidosis in lactating dairy cows. mSystems. 2022;7(1):e0149021
187. Vatanen T, Kostic AD, d'Hennezel E, Siljander H, Franzosa EA, Yassour M. et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165(4):842-53
188. Steinhagen F, Schmidt SV, Schewe J-C, Peukert K, Klinman DM, Bode C. Immunotherapy in sepsis - brake or accelerate? Pharmacol Ther. 2020;208:107476
189. Virzì GM, Mattiotti M, Cal M de, Ronco C, Zanella M, Rosa S de. Endotoxin in sepsis: Methods for LPS detection and the use of omics techniques. Diagnostics. 2022 13(1)
190. Ikeda T, Kamohara H, Suda S, Nagura T, Tomino M, Sugi M. et al. Comparative evaluation of endotoxin activity level and various biomarkers for infection and outcome of ICU-admitted patients. Biomedicines. 2019;7(3):47
191. Zhu R, Qin F, Zheng X, Fang S, Ding J, Wang D. et al. Single-molecule lipopolysaccharides identification and the interplay with biomolecules via nanopore readout. Biosens Bioelectron. 2023;240:115641
192. Juste RA, Ferreras-Colino E, La Fuente J de, Domínguez M, Risalde MA, Domínguez L. et al. Heat inactivated mycobacteria, alpha-Gal and zebrafish: Insights gained from experiences with two promising trained immunity inductors and a validated animal model. Immunology. 2022;167(2):139-53
193. Leent MvMT, Priem B, Schrijver DP, Dreu A de, Hofstraat SRJ, Zwolsman R. et al. Regulating trained immunity with nanomedicine. Nat Rev Mater. 2022;7(6):465-81
194. Li D, Li W, Zheng P, Yang Y, Liu Q, Hu Y. et al. A "trained immunity" inducer-adjuvanted nanovaccine reverses the growth of established tumors in mice. J Nanobiotechnology. 2023;21(1):74
195. Netea MG, Joosten LA. Beyond adaptive immunity: induction of trained immunity by COVID-19 adenoviral vaccines. J Clin Invest. 2023 133(2)
Author contact
![]() Corresponding author: Zhijun Cao, caozhijunedu.cn, State Key Laboratory of Animal Nutrition and Feeding, International Calf and Heifer Organization, College of Animal Science and Technology, China Agricultural University, Beijing, 100193, China.
Corresponding author: Zhijun Cao, caozhijunedu.cn, State Key Laboratory of Animal Nutrition and Feeding, International Calf and Heifer Organization, College of Animal Science and Technology, China Agricultural University, Beijing, 100193, China.

 Global reach, higher impact
Global reach, higher impact