10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2026; 22(4):2132-2134. doi:10.7150/ijbs.129092 This issue Cite
Commentary
LAV-BPIFB4 reverses progeria-associated cardiac aging by restoring diastolic function and reducing senescence
1. Futuristic Animal Resource and Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Cheongju, Chungbuk 28116, Republic of Korea.
2. Advanced Bioconvergence Department, KRIBB School, Korea National University of Science and Technology (UST), Daejeon 34113, Republic of Korea.
3. Center for Gene and Cell Therapy, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon 34141, Republic of Korea
# These authors contributed equally to this work.
Received 2025-11-27; Accepted 2025-12-22; Published 2026-1-29
Commentary-article in doi: 10.1038/s41392-025-02416-3
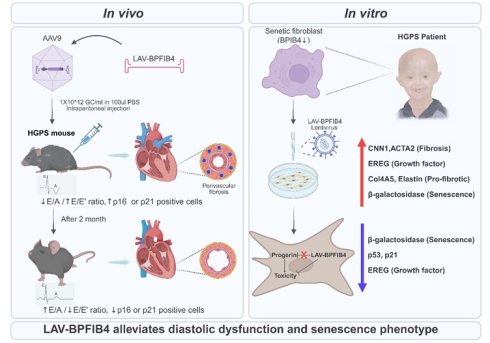
A recent study published in Signal Transduction and Targeted Therapy by Yan Qiu demonstrated that activation of a longevity gene can partially reverse cardiac damage associated with Hutchinson-Gilford progeria syndrome (HGPS) [1]. HGPS is primarily caused by a recurrent point mutation (c.1824 C>T, often designated as G608G) in exon 11 of the LMNA gene, which occurs de novo in most patients [2, 3]. This mutation causes abnormal alternative splicing, impairing the function of a nuclear membrane protein called mutant lamin A or progerin. Progerin accumulates in the cell nucleus, disrupting nucleocytoskeletal coupling and contributing to characteristic symptoms of HGPS, including clinical features resembling premature aging, skeletal deformities, and shortened lifespan. Among the various systemic manifestations, cardiovascular dysfunction, particularly progressive atherosclerosis and cardiac fibrosis, represents the leading cause of mortality in HGPS patients [4]. Consequently, the cardiovascular system has been identified as a primary therapeutic target in progeria research. Over the past decade, extensive efforts have been devoted to improving cardiac outcomes in HGPS, ranging from conventional pharmacological strategies aimed at mitigating vascular pathology [5] to the recent development of CRISPR-based approaches designed to correct the underlying LMNA mutation and selectively eliminate progerin expression [6, 7]. These advances underscore the growing focus on restoring cardiovascular integrity as a central objective of effective therapeutic intervention in HGPS.
Recent studies have introduced a promising paradigm for drug target discovery grounded in the genetics of human extreme longevity [8]. By systematically analyzing the genomes of individuals with exceptional lifespans, researchers have uncovered rare variants within evolutionarily conserved, aging-related pathways. These variants have undergone rigorous functional validation in both cellular and animal models, ultimately establishing a framework for identifying genetic alterations as molecular targets for the development of therapeutics aimed at prolonging healthspan. A compelling example of this approach is the BPI Fold Containing Family B Member 4 (BPIFB4) gene, particularly the longevity-associated variant (LAV; Ile229Val/Asn281Thr/Leu488Phe/Ile494Thr), which has been identified in the homozygous state in supercentenarians, individuals living beyond 110 years of age in good health [9]. Enhanced expression of LAV-BPIFB4 in senescent vascular cells and aged mice not only restores cardiac function and vascularization but also confers substantial anti-aging benefits, thereby mechanistically linking longevity genetics to translational therapeutic applications [10]. Notably, HGPS cells exhibited markedly reduced endogenous BPIFB4 expression, likely attributable to progerin-mediated suppression. Building on these insights, the present study posits that restoring BPIFB4 function may effectively counteract progerin-induced toxicity and ameliorate the cardiovascular dysfunction characteristic of HGPS.
The authors conducted experiments using 26-week-old HGPS mice that exhibited growth retardation compared to age-matched control mice (Figure 1) [1]. Three-dimensional echocardiographic examinations revealed that untreated HGPS mice spontaneously developed left ventricular diastolic dysfunction between 7 and 8 months of age, characterized by a decreased E/A ratio and an elevated E/E' ratio. Conversely, HGPS mice receiving a single intraperitoneal injection of AAV9-LAV-BPIFB4 showed significant recovery of left ventricular diastolic function within 2 months post-transduction, with 2.5-fold enhancement of cardiac BPIFB4 protein expression. Histological analyses demonstrated that AAV9-LAV-BPIFB4 specifically reduced perivascular fibrosis, but not interstitial fibrosis, increased the number of coronary arterioles, and enhanced vascular smooth muscle cell coverage. Two months post-transduction, aortic tissue harvested from 8-month-old progeria mice revealed no detectable atherosclerotic lesions, consistent with the early-stage nature of this HGPS model rather than a direct anti-atherosclerotic effect of LAV-BPIFB4 [1]. Transduction of LAV-BPIFB4 into HGPS patient-derived fibroblasts led to reduced senescence and fibrotic markers, including calponin-1 (CNN1), smooth muscle α-actin (ACTA2), epiregulin (EREG), Col4A5, elastin, and β-galactosidase, without affecting progerin protein accumulation [1]. This was achieved through suppression of the p53-p21 axis and modulation of EGFR-mediated fibrotic signaling.
The novelty of this study lies in identifying LAV-BPIFB4 as a therapeutic modality mechanistically distinct from progerin-elimination approaches [1]. Rather than directly targeting progerin for degradation, LAV-BPIFB4 functions as a cellular resilience activator, restoring nucleolar integrity and function encompassing ribosome biogenesis, ribonucleoprotein assembly, and ribosomal protein interactions [1]. Through this nucleolar-centric mechanism, LAV-BPIFB4 attenuates progerin-induced nucleolar and nuclear stress and nuclear dysfunction, thereby preserving diastolic performance, reducing cardiac fibrosis, and mitigating age-related senescence.
Therapeutic effect of LAV-BPIFB4 on HGPS-induced diastolic dysfunction and cellular senescence. Progeria mice without AAV-mediated LAV-BPIFB4 transduction developed left ventricular diastolic dysfunction, whereas mice treated with AAV9-LAV-BPIFB4 showed functional recovery 2 months post-transduction. Treatment significantly reduced perivascular fibrosis but did not affect interstitial fibrosis, increased coronary arteriole density and vascular smooth muscle cell coverage, while aortic examination revealed no detectable atherosclerotic lesions, consistent with the early-stage nature of this HGPS model. In patient-derived HGPS fibroblasts, LAV-BPIFB4 transduction downregulated senescence and fibrotic markers without altering progerin accumulation. These effects involved inhibition of the p53-p21 pathway and modulation of EGFR-mediated fibrosis. Figure created with BioRender.com.
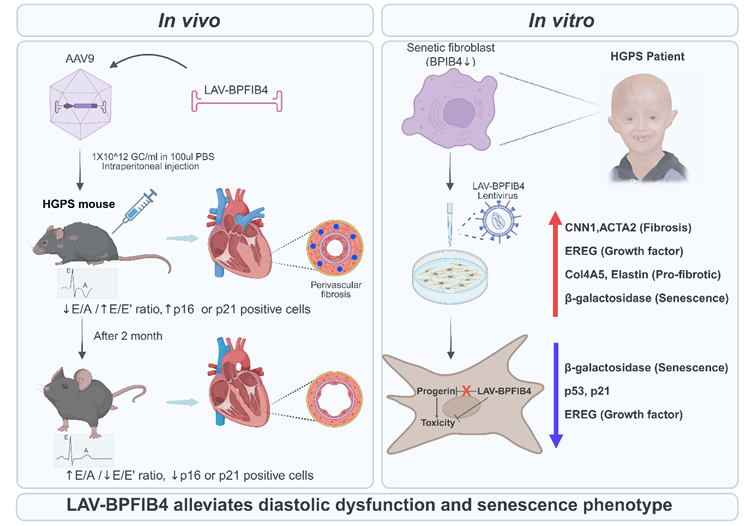
Importantly, this mechanism complements progerin-suppression strategies by enhancing cellular resilience rather than directly reducing progerin levels. While gene-editing interventions correct LMNA mutations or lower progerin burden, LAV-BPIFB4 reinforces nucleolar integrity, ribosome biogenesis, and stress-response capacity [1]. Together, these complementary mechanisms may provide a multi-layered cardioprotective strategy for stabilizing the vulnerable myocardial environments.
In summary, this groundbreaking study reveals that LAV-BPIFB4, a protective variant identified in supercentenarian genetics, reverses cardiac dysfunction in progeria through a resilience-enhancing mechanism rather than direct progerin elimination. By selectively mitigating perivascular fibrosis and fortifying cardiomyocyte stress tolerance while preserving progerin expression, LAV-BPIFB4 establishes a fundamentally new therapeutic paradigm. These findings underscore that the systematic interrogation of human exceptional longevity genetics reveals multiple protective alleles with significant translational potential, not only for rare genetic aging diseases but also for age-related cardiovascular pathologies in the general population.
Abbreviations
HGPS: Hutchinson-Gilford progeria syndrome
LAV: Longevity-associated variant
CNN1: Calponin-1
ACTA2: Smooth muscle α-actin
EREG: Epiregulin.
Acknowledgements
This research was supported by Quantum Platform Program (RS-2025-25460035) through the Korea Joint Quantum Institute of KRISS and the National Research Foundation of Korea (NRF) funded by the Korean government (Ministry of Science and ICT(MSIT)).
Funding
This research was supported by the KRIBB Research Initiative Program (KGM5362521), National Research Foundation (NRF) funded by the Korean government (MSIT) (RS-2021-NR057659, RS-2025-00518480, RS-2025-25460035), National Research Council of Science & Technology (NST) grant by the Korea government (MSIT) (No. GTL24022-000) and the Technology Innovation Program funded by the Ministry of Trade, Industry and Energy (MOTIE-20009707).
Author contributions
U.C., Y.H.P., T.K., K.S.C. and S.U.K. designed, researched, and wrote the manuscript. U.C., Y.H.P., T.K., K.S.C. and S.U.K. participated in the discussion. U.C., Y.H.P., T.K., K.S.C. and S.U.K. supervised and reviewed all the research. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Qiu Y, Cattaneo M, Maciag A, Puca AA, Madeddu P. A longevity-associated variant of the human BPIFB4 gene prevents diastolic dysfunction in progeria mice. Signal Transduct Target Ther. 2025;10:314
2. Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L. et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293-8
3. De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I. et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055
4. Chang W, Wang Y, Luxton GWG, Ostlund C, Worman HJ, Gundersen GG. Imbalanced nucleocytoskeletal connections create common polarity defects in progeria and physiological aging. Proc Natl Acad Sci U S A. 2019;116:3578-83
5. Gordon LB, Shappell H, Massaro J, D'Agostino RB Sr, Brazier J, Campbell SE. et al. Association of Lonafarnib Treatment vs No Treatment with Mortality Rate in Patients with Hutchinson-Gilford Progeria Syndrome. JAMA. 2018;319:1687-95
6. Chae U, Yang HJ, Kim H, Lee SH, Lee DG, Koo JY. et al. Precise progerin targeting using RfxCas13d: A therapeutic avenue for Hutchinson-Gilford progeria syndrome. Mol Ther. 2025;33:4394-413
7. Olsen FJ, Gordon LB, Smoot L, Kleinman ME, Gerhard-Herman M, Hegde SM. et al. Progression of Cardiac Abnormalities in Hutchinson-Gilford Progeria Syndrome: A Prospective Longitudinal Study. Circulation. 2023;147:1782-4
8. Zhang ZD, Milman S, Lin JR, Wierbowski S, Yu H, Barzilai N. et al. Genetics of extreme human longevity to guide drug discovery for healthy ageing. Nat Metab. 2020;2:663-72
9. Malavolta M, Dato S, Villa F, Rango F, Iannone F, Ferrario A. et al. LAV-BPIFB4 associates with reduced frailty in humans and its transfer prevents frailty progression in old mice. Aging (Albany NY). 2019;11:6555-68
10. Cattaneo M, Beltrami AP, Thomas AC, Spinetti G, Alvino VV, Avolio E. et al. The longevity-associated BPIFB4 gene supports cardiac function and vascularization in ageing cardiomyopathy. Cardiovasc Res. 2023;119:1583-95
Author contact
![]() Corresponding authors: Taeho Kwon, Ph.D., e-mail: kwonre.kr (T. K.); Kyung-Sook Chung, Ph.D., e-mail: kschungre.kr (K.S.C.); Sun-Uk Kim, Ph.D., e-mail: sunukre.kr (S.U.K.)
Corresponding authors: Taeho Kwon, Ph.D., e-mail: kwonre.kr (T. K.); Kyung-Sook Chung, Ph.D., e-mail: kschungre.kr (K.S.C.); Sun-Uk Kim, Ph.D., e-mail: sunukre.kr (S.U.K.)

 Global reach, higher impact
Global reach, higher impact