Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2005; 1(1):24-33. doi:10.7150/ijbs.1.24 This issue Cite
Research Paper
Diversity of endophytic fungal community of cacao (Theobroma cacao L.) and biological control of Crinipellis perniciosa, causal agent of Witches' Broom Disease
1 Instituto de Biotecnologia, UCS, 95001-970, Caxias do Sul/RS,
2 Almirante Cacau, Agrícola, Comércio e Exportação Ltda. CP 55, 45630-000 Itajuípe - BA,
3 Dept. of Genetics, ESALQ/USP, 13400-970, Piracicaba/SP/Brazil
Received 2004-9-20; Accepted 2004-11-15; Published 2005-2-1
Abstract
The basidiomycete fungus Crinipellis perniciosa (Stahel) Singer is the causal agent of Witches' Broom Disease of Cacao (Theobroma cacao L.) which is the main factor limiting cacao production in the Americas. Pod losses of up to 90% are experienced in affected areas as evidenced by the 50% drop in production in Bahia province, Brazil following the arrival of the C. perniciosa in the area in 1989. The disease has proven particularly difficult to control and many farmers in affected areas have given up cacao cultivation. In order to evaluate the potential of endophytes as a biological control agent of this phytopathogen, the endophytic fungal community of resistant and susceptible cacao plants as well as affected branches was studied between 2001 and 2002. The fungal community was identified by morphological traits and rDNA sequencing as belonging to the genera Acremonium, Blastomyces, Botryosphaeria, Cladosporium, Colletotrichum, Cordyceps, Diaporthe, Fusarium, Geotrichum, Gibberella, Gliocladium, Lasiodiplodia, Monilochoetes, Nectria, Pestalotiopsis, Phomopsis, Pleurotus, Pseudofusarium, Rhizopycnis, Syncephalastrum, Trichoderma, Verticillium and Xylaria. These fungi were evaluated both in vitro and in vivo by their ability to inhibit C. perniciosa. Among these, some were identified as potential antagonists, but only one fungus (Gliocladium catenulatum) reduced the incidence of Witches' Broom Disease in cacao seedlings to 70%.
Keywords: Endophytes, antibiosis, plant resistance, biodiversity, rDNA analysis
1. Introduction
The basidiomycete Crinipellis perniciosa (Stahel) Singer, is the causal agent of Witches' Broom Disease of cacao (Theobroma cacao L.). Witches' Broom is one of the main limiting factors for the cacao production in South America and Caribbean islands, and has been considered as one of the most important pathogen for the crop [10]. The infection process occurs after basidiospore germination and penetration of the germ tube on meristematic tissues and pods [14], starting the biotrophic stage of colonization [13]. This phase is characterized by intercellular growth, thick and monokaryotic hyphae without clamp connections [7, 19]. In the next phase, the saprophytic stage, necrosis and intracellular colonization by dikaryotic hiphae occur [13]. This fungus colonizes meristematic tissues reducing the productivity as well as the life of the host plant. As this fungus colonizes growing plant tissues, the fungicide application is a not an effective treatment, being biological control a potential alternative strategy.
Endophytic fungi colonize their hosts without causing any external disease symptoms [8], except when the host is under stress conditions. Studies on microorganisms from tropical plant species are recently becoming more frequent, since these fungi and bacteria have been studied for biological control and production of compounds with pharmacological properties [4, 15]. Studies on the cacao endophytic community are recent, but practical aspects have been already evaluated [3]. However, more studies on Witches' Broom Disease and endophytic community interaction should be carried out since the understanding of the disease physiology and ecology is crucial for devising better control strategies for the pathogen.
In the present study we isolated endophytic fungi from stems of different cacao plants to further identify possible biocontrol agent for witches' broom disease. The composition and population frequency of the endophytic fungi population was also studied.
2. Material and methods
2.1. Biological material
The diversity of endophytic fungi was estimated in branches of cacao (Theobroma cacao) from at three categories of cacao trees (resistant, healthy susceptible and symptomatic plants). For statistical purposes, these three categories of plants (resistant, healthy and symptomatic) were considered as treatments in the present study. Plants from orchards located in nearest to Itabuna, Bahia State, Brazil were sampled. For each treatment/location, branches from 5 plants (repetitions) were collected during 2001 and 2002. The strains VV1 and VV2 of C. perniciosa belonging to the Almirante Cacau Farm collection were also utilized.
2.2. Surface Disinfection and Endophytic Fungi Isolation
All branches were washed in running tap water and graded by size and surface appearance and any visibly damaged material excluded. The plant tissues were rinsed with 70% ethanol, surface disinfected with sodium hypochloride solution (3% available Cl-) for 3 minutes, rinsed once in 70% ethanol and twice in sterile distilled water. The disinfection process was checked by pressing the disinfected plant material onto Potato Dextrose Agar (PDA). Aliquots of the water from final rinse solutions were also plated on the same media. The surface disinfected branches were used in the fungal isolations as described below.
Endophytic fungi were isolated from cacao trees per categories (resistant, healthy susceptible and diseased plants). A random sample from each tree, consisting of 30 branches was taken. After surface disinfection, each branch was then peeled and cut in 10 fragments (3-4 mm), which were placed onto PDA containing tetracycline (50 μg/ml). After 3-15 days incubation at 28°C the number of pieces showing fungal growth was counted. The hyphal tip of each morphologically different mycelium that emerged from a branch fragment was sub cultured and transferred to PDA slants for later identification. The endophyte incidence (EI) was calculated as the percentage of pieces showing fungal growth. Following incubation, fungal isolates recovered from each plant fragment were selected at random, purified and grouped on the basis of phenotypic characteristics, e.g. colony morphology, colony colour and growth rate. Isolates representing each fungal group of interest were selected for further identification by morphological traits (classic taxonomy) and/or rDNA sequencing.
2.3. Characterization of endophytic microorganisms
Fungal DNA was extracted [16] and the rDNA and ITS region were amplified in a 50 µl final volume containing 1 µl (0.5 – 10.0 ng) of total DNA, 0.2 µM of primers ITS1 (5´-TCCGATGGTGAACCTGCGG-3´) and ITS2 (5´-TCCTCGTTATTGATATGC-3´), 200 µM of each dNTP, 3.75 mM of MgCl2 and 0.05 U of Taq DNA polymerase (Invitrogen) in 20 mM of pH 8.4 Tris-HCl containing 50 mM KCl. A negative control (PCR mixture without DNA) was included in all PCR experiments. The reaction conditions were as follows: 94oC for 4 min followed by 25 cycles of denaturation at 94oC for 30s, annealing at 57oC for 1 min and primer extension at 72oC for 1 min; followed by a final extension at 72oC for 7 min. The reaction products were separated by running 5 µl of the PCR reaction mixture in 1.2% (w/v) agarose gel and staining the bands with ethidium bromide [18]. For identification, the PCR products of at least 20% of isolates were purified using a GFX PCR DNA and gel band purification kit (Amersham Biosciences) and sequenced using the ITS1 primer. Analyses of sequences were performed with the basic sequence alignment BLAST program run against the database (National Center for Biotechnology Information website [http://www.ncbi.nlm.nih.gov]) and the determined sequence were aligned using Clustal X and the distance matrices and phylogenetic trees were calculated [11] and neighbour-joining [17] algorithms, respectively, using PAUP software [21]. The nucleotide sequences obtained in this study have been submitted to the GenBank and assigned accession numbers AY745985 to AY746007, AY753281 to AY753285 and AY753987 to AY754010
2.4. Plants cultivation and endophyte inoculation
To obtain endophyte-free seedlings of T. cacao, seeds from healthy, mature fruit borne on Witches' Broom susceptible plants were collected, surface disinfected for 15 minutes in an aqueous solution of sodium hypochloride and germinated on MS medium [12]. Seedlings were maintained on controlled conditions (25°C and photoperiod of 12 hours). Forty days after germination, seedlings were evaluated to confirm that leaves and stems were endophyte-free. The inoculum containing propagules of endophytes were introduced into seedlings stems with a sterile needle.
For greenhouse germination, seeds of susceptible cacao seeds were planted into pots containing dark soil and were maintained in greenhouse. Sixty days after seed germination, the seedlings were inoculated with suspensions containing the propagules of endophytic fungi.
2.5. Selection of endophytic fungi antagonist to C. perniciosa
The in vitro selection of antagonists against C. perniciosa was carried out on PDA medium. For this, mycelial discs (5 mm) of C. perniciosa were inoculated on Petri dishes (100 mm) containing PDA medium and incubated at 28°C (photoperiod of 12 hours). After 8 days, the endophytic microorganisms were inoculated 50 mm from C. perniciosa colony. The antagonism was detected by formation of an inhibition halo.
For in planta screening for C. perniciosa antagonists, 3 days after inoculation of endophytic fungi, basidiospores of C. perniciosa were introduced into stem of seedlings with a sterile needle. Control plants were inoculated just with PBS buffer ((g/l) NaCl, 8; KCl, 0.2; Na2HPO4, 1.4; KH2PO4, 0.24). The symptoms were evaluated from 10 to 60 days and the data were statistically analysed by the One-way Variance method and the Tukey-Kramer Multiple Comparison Test compared the means.
3. Results
3.1. Isolation of Endophytic Fungi
The diversity of endophytic fungi was assessed in branches of 3 categories (resistant, healthy susceptible and symptomatic) of cacao plants. To avoid contamination and to isolate endophytic fungi only from inner plant tissues, the branches were peeled after surface disinfection. The endophytic fungal community which was isolated from cacao branches included Acremonium sp., Blastomyces sp., Botryosphaeria sp., Cladosporium sp., Colletotrichum gloeosporiodes, Cordyceps sobolifera, Diaporthe phaseolorum, D. helianthi, Fusarium sp., F. chlamydosporum, F. oxysporum, F. polyphialidicum, Geotrichum sp., Gibberella zeae, G. fujikuroi, G. moniliformis, Gliocladium sp., G. catenulatum, Lasiodiplodia theobromae, Monilochoetes sp., Nectria haematococca, Pestalotiopsis microspora, Phomopsis sp., Pleurotus ostreatus, Pseudofusarium purpureum, Rhizopycnis vagum, Syncephalastrum sp., Trichoderma sp., Verticillium sp., V. luteo-album, Xylaria sp. The number of cultivable endophytic fungi that was recovered using PDA medium was not significantly different within the three categories of plant (healthy, resistant and symptomatic) evaluated. The frequency of fungi isolation was 0.42, 0.34 and 0,50 for healthy, resistant and symptomatic plants, respectively.
3.2. Characterization of endophytic fungi from cacao branches
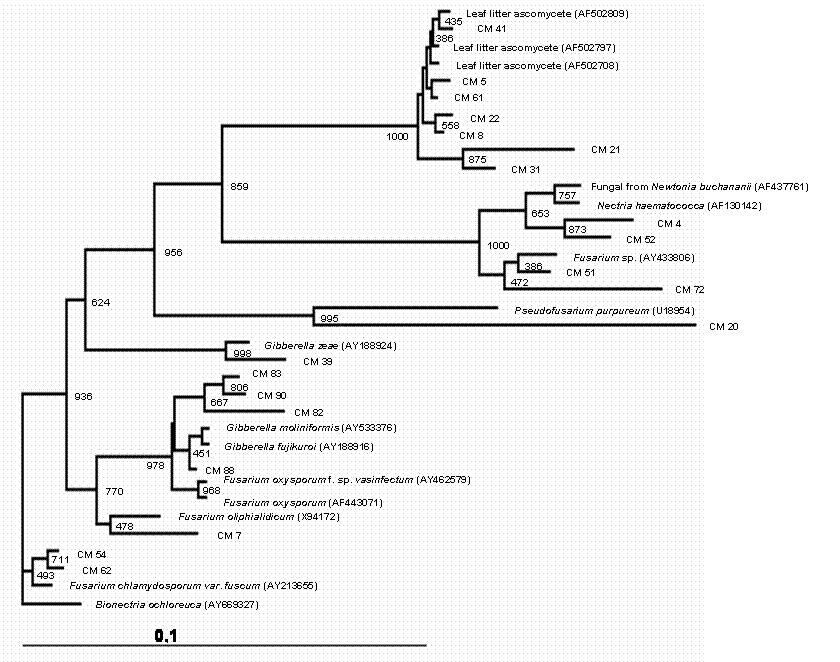
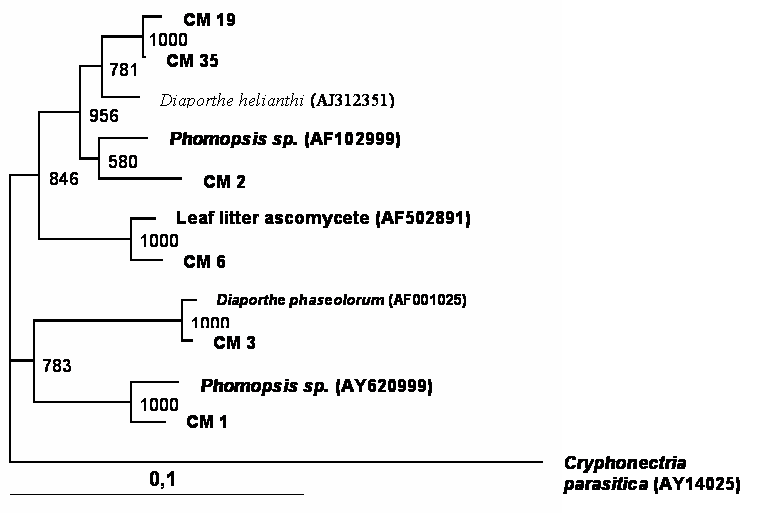
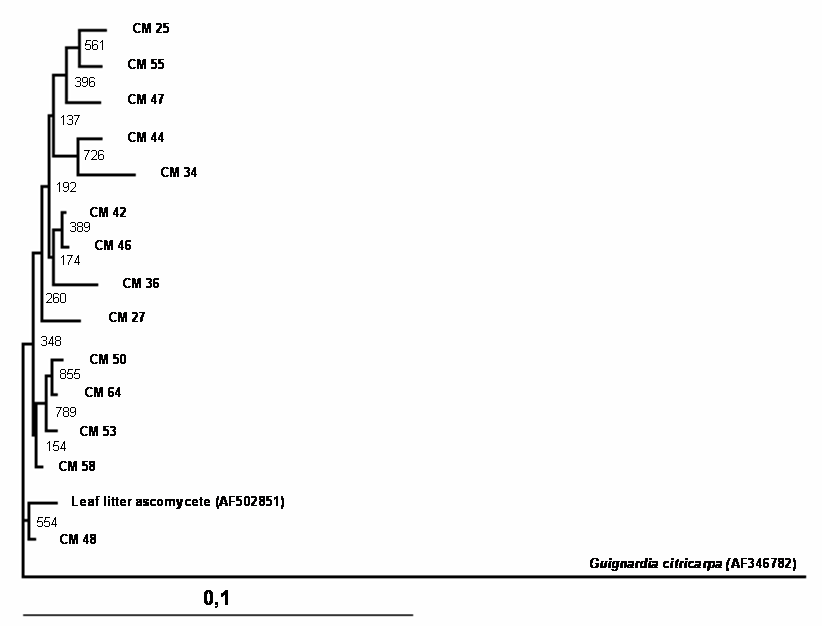
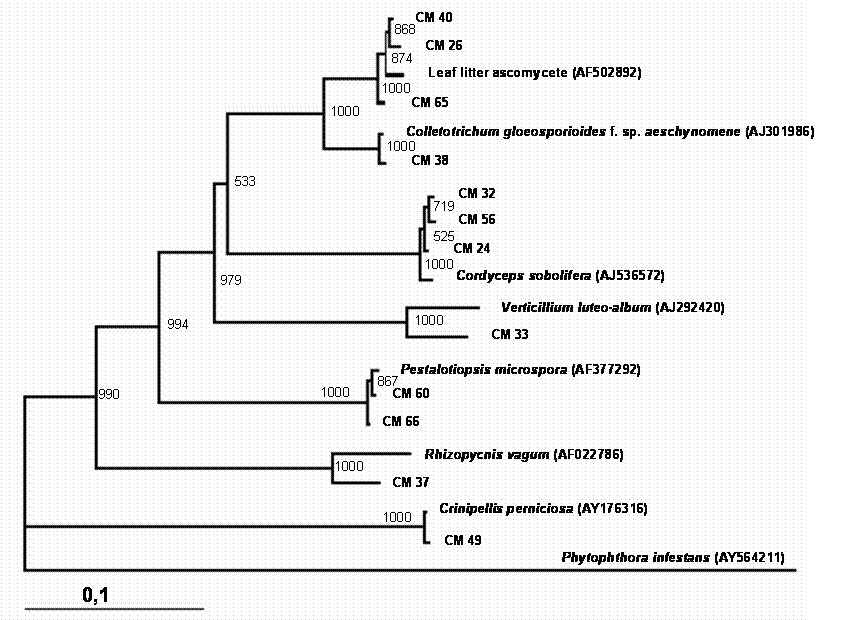
A total of 150 endophytic fungi isolated from stems of cacao were randomly picked up, and this population was partially characterized by rDNA (partial 18S, ITS-1, 5.8S, ITS-2 and partial 23S) sequencing. The results (Table 1) showed that the cultivable endophytic fungi associated with cacao cultivars belong mainly to Ascomycetes group (Figures 1, 2, 3 and 4) being the Botryosphaeriaceae, Valsaceae and Nectriaceae families the most frequent. The fungus Fusarium spp. was the dominant genus and showed the highest diversity (Figure 1). No correlation between fungal groups and plant categories was observed.
Partial sequences of rDNA were aligned and the relationships between endophytic isolates were evaluated by neighbour-joining algorithm (Figures 1, 2, 3 and 4). Using this strategy, some isolates, such as CM27, CM34, CM36, CM42, CM44, CM46, CM50, CM53, CM58 and CM64 (figure 3) and CM26, CM40, CM65 (figure 4) could not be identified.
3.3. Screening of endophytic fungi in vitro against C. perniciosa
A total of 265 endophytic fungi were evaluated in vitro against C. perniciosa. Forty-three isolates (16.22%) were able to inhibit the growth of the causal agent of Witches' Broom Disease of cacao. In general, the frequency of endophytic fungi able to inhibit C. perniciosa decreased from healthy (18.96%)>resistant (16.06%)>symptomatic (14.28%) plants.
3.4. Screening of endophytic fungi able to reduce witches' broom symptoms
Axenic cacao seedlings were inoculated with endophytic fungi and further with C. perniciosa basidiospores. Fourteen isolates were able to inhibit completely the development of witches' broom symptoms in the evaluated seedlings. However, in greenhouse conditions, only a isolated identified as Gliocladium catenulatum was able to reduce in 70.84% the symptoms of Witches' Broom Disease.
4. Discussion
The plant-associated habitat is a dynamic environment in which many factors affect the structure and species composition of the microbial communities that colonize roots, stems, branches and leaves. It has previously been shown that endophytic communities vary spatially in the plant [9] or may be dependent on the interaction with other endophytic or pathogenic microorganisms [1, 2]. These endophytic microorganisms are ubiquitous and may increase the plant fitness by improving tolerance to heavy metals and drought, reducing the herbivory or phytopathogen settling [5] and promoting plant growth [22].
Even though it remains difficult to compare earlier and more recent studies that isolated endophytic fungi, certain trends are apparent with predominant fungal types isolated as endophytes. The genus Fusarium seem to be very ubiquitous and has been isolated from many host plants. In the present study, this genus was isolated in high frequency, while some genus, such as Acremonium, Cordiceps, Pseudofusarium, Syncephalastrum and Trichoderma occurred in very low frequencies throughout the study. However, no correlation was observed between plant categories and endophytic fungal species recovered from cacao branches. Some endophytic isolates were not consistently identified by rDNA sequencing (Figures 3 and 4) and morphological traits, suggesting that these could belong to species not yet described. In fact, it has been suggest that plant from tropical rainforest could be inhabited by a great diversity of endophytic microorganisms, which present a remarkable biological activity [20] and a source of new species. More studies in taxonomic approach should be done to clarify the identification of these isolates. Also, these microorganisms could be a source of novel molecular structures and biologically active compounds.
As shown above, the genus Fusarium that is described as endophyte and/or phytopathogen in many plant species was the dominant group, suggesting that these fungi could play a role in the plant development. In cucumber, nonpathogenic F. oxysporum may induce host resistance against Pythium ultimum through a combination of antibiosis and mycoparasitism, as well as inducing plant defence reactions [6]. However, in the present analysis, the Fusarium isolates were not able to inhibit efficiently the Witches' Broom symptoms both in vitro and greenhouse conditions.
Based on previous report that endophytic fungi are able to protect cacao against phytopathogens [3], we assessed in vitro and in planta interaction between endophytic fungi isolated from cacao and C. perniciosa. This fungus is the causal agent of Witches' Broom Disease of cacao (Theobroma cacao L.) that is one of the main limiting factors for the cacao production in South America and Caribbean islands, and has been considered as one of the most important pathogen for the crop [10]. The infection process occurs after basidiospore germination and penetration of the germ tube on meristematic tissues and pods [14], being this stage a important point for inhibit the infection, since the viability of the spores on the plant tissues is reduced. Although a large proportion of endophytes (16.22%) inhibit the C. perniciosa in pairwise trials on PDA medium, in planta conditions, the proportion was reduced, showing that the pairwise evaluation just select microorganisms able to produce biologically active compounds, but not to select microorganisms to be used in planta conditions. Also, several endophytic isolates were able to inhibit completely the Witches' Broom symptoms in axenic conditions, but only G. catenulatum reduced the symptoms in greenhouse. These result show that this isolate is promising to be used as biological control agent against C. perniciosa in field conditions. Endophyte-mediated protection is greater in mature leaves, which bear less intrinsic defence against fungal pathogens than do young leaves [3]. Also, the authors suggest that host affinity is mediated by leaf chemistry, and protection may be mediated by direct interactions of endophytes with foliar pathogens. Therefore, taking in mind that in the present work the frequency of endophytic isolates, able to control C. perniciosa, decreased as follow in vitro>axenic plant> greenhouse plants, we may suggest that this low frequency in greenhouse plants is due the competition between the target endophytes and indigenous community associated to the host seedlings. Besides, if the inoculated endophyte is not able to establish inside the cacao plant the control will be not possible.
Mutualistic interactions between host and vertically inherited symbionts such as endophytes are easily reconciled with existing theory of species interactions [3]. However, these interactions depend on a complex web relation within plant tissues, including different fungi and bacteria species, as well as plant genotype. Understanding which microbial species are involved, how and when they occur and what are the advantages of these plant interactions, it is possible to use this approach to control several plant disease in cacao. Today, an improved protection program implies the availability of highly efficient biofungicides, therefore, due its remarkable biological properties, the G. catenulatum isolate offers very good prospects for integrated management of Witches' Broom Disease. So, further studies should be focus in analysis and development of an efficient strategy to control C. perniciosa in planta based on this endophytic fungi.
Acknowledgements
This work was supported by a grant from the Foundation for Research Assistance, São Paulo State, Brazil (FAPESP) and by a grant from Almirante Cacau (Lomato Júnior, Bahia, Brazil). We thank FAPESP and CAPES for the Fellowship to W. L. Araújo and C. S. Maki. We also thank Dr. Ricardo Harakava (Instituto Biológico, São Paulo, SP, Brazil) for performing the sequencing.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Araújo WL. et al. Variability and interactions between endophytic bacteria and fungi isolated from leaf tissues of citrus rootstocks. Can J Microbiol. 2001 ;47:229-236
2. Araújo WL. et al. Diversity of endophytic bacterial populations and Citrus plants. Appl Environ Microbiol. 2002 ;10:4906-4914
3. Arnold AE. et al. Fungal endophytes limit pathogen in a tropical tree. Proc Nat Acad Sci. 2003 ;100:15649-15654
4. Azevedo JL. et al. Microrganismos endofíticos e seu papel em plantas tropicais. In: (ed.) Serafini LA. et al. Biotecnologia: avanços na agricultura e na agroindústria. Caxias do Sul: EDUCS. 2002:233-268
5. Azevedo JL. et al. Endophytic microorganisms: a review on insect control and recent advances on tropical plants. Electronic Journal of Biotechnology. 2000 ;3(1):e4-
6. Benhamou N. et al. Ability of nonpathogenic Fusarium oxysporum strain Fo47 to induce resistance against Pythium ulimum infection in cucumber. Appl Environ Microbiol. 2002 ;68:4044-4060
7. Calle H. et al. Histology of witches' broom caused in cacao by Crinipellis perniciosa. Phytopathol. 1982 ;72:1479-1481
8. Carroll GC. Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Ecol. 1998 ;69:2-9
9. Fisher PJ. et al. The distribution of some fungal and bacterial endophytes in maize (Zea mays L.). New Phytol. 1992 ;122:299-305
10. Griffith GW. et al. Autoecology and evolution of the witches' broom pathogen (Crinipellis perniciosa) of cocoa. In: (ed.) Blakeman JP, Williamson B. Ecology of Plant Pathogens. Oxon: CAB International. 1994:245-267
11. Junkes TH, Cantor CR. Evolution of protein molecules. In: (ed.) Munro. Mammalian protein metabolism. New York: Academic Press. 1969:21-132
12. Murashigue T, Skoog F. A revised medium for rapid grown and bioassays with tobacco tissue culture. Physiol Plant. 1962 ;15:473-497
13. Muse RB. et al. Effects of the fungus Crinipellis perniciosa, causal agent of witches' broom disease, on cell and tissue cultures of cocoa (Threobroma cacao L.). Plant Pathol. 1996 ;45:145-154
14. Orchard J. et al. Changes in morphology and measurement of cytokinin levels during the development of witches' broom on cocoa. Plant Pathol. 1994 ;43:65-72
15. Peixoto-Neto PAS. et al. Microorganismos endofíticos. Biotecnologia Ciência & Desenvolvimento. 2002:62-76
16. Raeder U, Broda P. Rapid preparation of DNA from filamentous fungi. Letters Appl Microbiol. 1985 ;1:17-20
17. Saitou N, Nei M. The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 ;4:406-425
18. Sambrook J. et al. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press. 1989
19. Silva SDVM, Matsuoka K. Histologia da interação Crinipellis perniciosa em cacaueiros susceptível e resistente à vassoura de bruxa. Fitopatologia Brasileira. 1999 ;24:54-59
20. Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev. 2003 ;67:491-502
21. Sworfford DL. PAUP*: phylogenetic analysis using parsimony (*and other methods). Massachusetts: Sinauer Associates. 2002
22. Varma A. et al. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. App Environ Microbiol. 1999 ;65:2741-2744
Table and figures
Endophytic fungi isolated from cacao branches.
| Isolates | Most closely related fungal sequence* | Accession nº of closest hit | Identity (%) |
| CM38 | Colletotrichum gloeosporioides | AJ301986 | 99 |
| CM24, CM32, CM56 | Cordyceps sobolifera | AJ536572 | 98 |
| CM49 | Crinipellis perniciosa | AY176316 | 99 |
| CM19, CM35 | Diaporthe helianthi | AJ312351 | 97 |
| CM3 | Diaporthe phaseolorum | AF001025 | 99 |
| CM52 | Fungal isolate from Newtonia buchananii wood | AF437761 | 99 |
| CM54, CM62 | Fusarium chlamydosporum | AY213655 | 99 |
| CM83 | Fusarium oxysporum | AY462579 | 92 |
| CM90 | Fusarium oxysporum | AF443071 | 91 |
| CM7 | Fusarium polyphialidicum | X94172 | 98 |
| CM51, CM72 | Fusarium sp. | AY433806 | 99 |
| CM82 | Gibberella fujikuroi | AY188916 | 93 |
| CM39 | Gibberella zeae | AY188924 | 99 |
| CM63 | Giberella moliniformis | AY533376 | 98 |
| CM25, CM55 | Lasiodiplodia theobromae | AY160201 | 99 |
| CM21 | Leaf litter ascomycete | AF502797 | 98 |
| CM22 | Leaf litter ascomycete | AF502708 | 98 |
| CM26, CM40 | Leaf litter ascomycete | AF502892 | 98 |
| CM27, CM36, CM42, CM46, CM47, CM58 | Leaf litter ascomycete | AF502851 | 98 |
| CM34, CM44, CM48, CM50, CM53, CM64 | Leaf litter ascomycete | AF502851 | 99 |
| CM5, CM41, CM61, CM8, CM31 | Leaf litter ascomycete | AF502809 | 99 |
| CM6 | Leaf litter ascomycete | AF502891 | 97 |
| CM65 | Leaf litter ascomycete | AF502892 | 97 |
| CM4 | Nectria haematococca | AF130142 | 98 |
| CM60, CM66 | Pestalotiopsis microspora | AF377292 | 98 |
| CM1 | Phomopsis sp. | AY620999 | 96 |
| CM2 | Phomopsis sp. | AF102999 | 96 |
| CM20 | Pseudofusarium purpureum | U18954 | 92 |
| CM37 | Rhizopycnis vagum | AF022786 | 91 |
| CM33 | Verticillium luteo-album | AJ292420 | 91 |
Phylogenetic tree showing the relationship between cacao endophytic fungi (Genera Fusarium/Gibberella/Nectria) and other fungal species. The tree was constructed based on the rDNA sequence (ITS1, 5.8S and ITS2) fragment sequence by using neighbour-joining method. The bootstrap analysis was performed with 1000 repetitions. For a description of the endophytic fungi see Table 1.
Phylogenetic tree showing the relationship between cacao endophytic fungi (Genera Phomopsis/Diaporthe) and other fungal species. See legend Figure 1 for details.
Phylogenetic tree showing the relationship between cacao endophytic fungi (Genera Lasiodiplodia/Botryosphaeria) and other fungal species. See legend Figure 1 for details.
Phylogenetic tree showing the relationship between cacao endophytic fungi and other fungal species. See legend Figure 1 for details.
Author biography
Marciano R. Rubini (MSc. University of Caxias do Sul, 2003) is a researcher at University of Caxias do Sul. Current work includes the optimisation of production and formulation processes for entomopathogenic microorganisms. Previous work includes the selection of endophytic microorganisms against Crinipellis perniciosa, causal agent of the Witche's broom of the cocoa tree.
Rute T. Silva-Ribeiro (Ph.D. Federal University of Rio Grande do Sul, 2002) currently works on the biological control of plant disease at University of Caxias do Sul. Research interests include investigation of ecology and biochemistry of antagonistic fungi, epiphytic and endophytic species, and management of organics apple orchards, wineyards, vegetables and ornamental plants.
Alan W. V. Pomella (PhD Universidade de Viçosa, 1999) is a research manager at Almirante Cacau, a company of Masterfoods Inc. USA. Research interests include investigation of cocoa plant diseases focusing on Biological control of Witches´Broom and Black Pod.
Cristina S. Maki (M.S. Londrina State University 1999) is a graduate student in Biology at Londrina State University and Ph.D. student at Dept. of Genetics, ESALQ/USP. Current research includes fungal genetic diversity and fungal-plant interactions. Previously she worked as a research position (1999-2002) at the CNPMS/Embrapa, Sete Lagoas, Minas Gerais, Brazil.
Welington L. Araújo (Ph.D. Dept. of Genetics, ESALQ/USP 2000): Research interests include study of plant-endophytes interactions and effect of genetically modified plants in microbial communities, and the relationship between endophytic bacteria and Xylella fastidiosa, causal agent of Citrus Variegated Chlorosis.
Deise R. dos Santos (Biol. University of Caxias do Sul, 2004) is a researcher at University of Caxias do Sul, Institute of Biotechnology. The research includes the isolation and genetic characterization of lactic bacteria of Serranos cheeses by RAPD. Previously worked with molecular analysis of Colletotrichum spp., at the CNPUV/Embrapa, Bento Gonçalves, Rio Grande do Sul, Brazil.
João L. Azevedo (Ph.D. University of Sheffield, Sheffield, UK, 1971): Research interests include the study of entomopathogenic filamentous fungi and plant-endophytes interactions, the genetic recombination in filamentous fungi and relationship between endophytic bacteria and Xylella fastidiosa, causal agent of Citrus Variegated Chlorosis.
![]() Corresponding address:
Corresponding address:
Dr. Welington L. Araújo, Departamento de Genética, Escola Superior de Agricultura “Luiz de Queiroz”, Universidade de São Paulo, P. O. Box 83, 13400-970 Piracicaba, São Paulo, Brazil. E-mail wlaraujousp.br; Tel. (55-19) 34294251; Fax (55-19) 34336706

 Global reach, higher impact
Global reach, higher impact