Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2020; 16(13):2405-2413. doi:10.7150/ijbs.38925 This issue Cite
Review
Interleukin 22 in Liver Injury, Inflammation and Cancer
1. The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, China.
2. The Second Hospital of Anhui Medical University, Hefei, Anhui, 230601, China.
3. The First Affiliated Hospital of University of Science and Technology of China, Hefei, Anhui, 230022, China.
Received 2019-8-1; Accepted 2020-6-16; Published 2020-6-29
Abstract
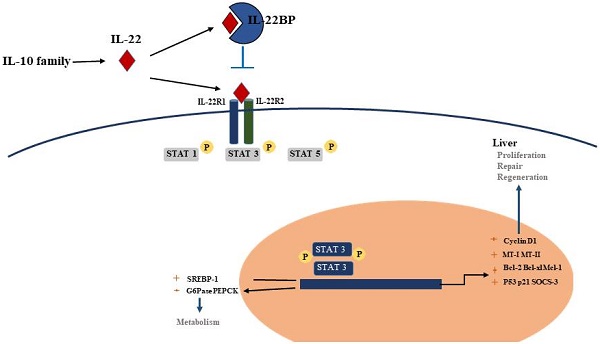
Interleukin 22(IL-22), a member of the IL-10 cytokine family and is an emerging CD4+Th cytokine that plays an important role in anti-microbial defense, homeostasis and tissue repair. We are interested in IL-22 as it has the double function of suppressing or encouraging inflammation in various disease models including hepatic inflammation. As a survival factor for hepatocytes, IL-22 plays a protective role in many kinds of liver diseases, such as hepatitis, liver fibrosis, or hepatocellular carcinoma (HCC) by binding to the receptors IL-22R1 and IL-10R2. Overexpression of IL-22 reduces liver fibrosis by attenuating the activation of hepatic stellate cell (the main cell types involved in hepatic fibrosis), and down-regulating the levels of inflammatory cytokines. Administration of exogenous IL-22 increases the replication of hepatocytes by inhibiting cell apoptosis and promoting mitosis, ultimately plays a contributing role in liver regeneration. Furthermore, treatment with IL-22 activates hepatic signal transducer and activator of transcription 3 (STAT3), ameliorates hepatic oxidative stress and alcoholic fatty liver, effectively alleviate the liver damage caused by alcohol and toxicant. In conclusion, the hepatoprotective functions and liver regeneration promoting effect of IL-22 suggests the therapeutic potential of IL-22 in the treatment of human hepatic diseases.
Keywords: IL-22, Liver injury, Inflammation, HCC, Liver regeneration.
Introduction
Interleukin-22 (IL-22) was first identified by Dumoutier et al. in 2000 in the secretome of IL-9-stimulated thymic lymphomas [1, 2]. Due to the similarity in the gene and protein structure with IL-10, IL-22 was classified as a member of the IL-10 cytokine family, which also includes other cytokines such as IL-10, IL-19, IL-20, IL-24, IL-26 and the type III interferons [3]. The IL-10 family can be further subdivided into three groups based on their function; IL-10 itself, the IFN-λ subfamily and the IL-20 subfamily.
IL-22, which acts on a variety of tissues and organs such as the intestines, lungs, liver, kidney, thymus, pancreas and skin, has a lot of functions; It is worth mentioning that IL-22 acts as either a anti-inflammatory or proinflammatory cytokine in many disease models such as psoriasis, ulcerative colitis, systemic lupus erythematosus and other inflammatory diseases [3]. In the liver, hepatocytes are the primary target of IL-22, and IL-22 is thought to induce hepatic production of acute-phase proteins. In liver injury, IL-22 usually induces proteins involved in protection and regeneration, such as anti-apoptotic proteins bcl-2/bcl-xl, cyclin D1, c-myc, and CDK4. Thus, IL-22 has considerable protective effects in a variety of different experimental models of liver injury, including alcohol-induced liver damage, liver ischemia-reperfusion injury. IL-22 treatment can provide critical protection against liver toxicity and liver damage caused by many different types of toxic substances [4-6]. During liver inflammation, IL-22 can also provide protection to hepatocytes [7]. These facts indicate that IL-22 will be a potential new target for the treatment of liver disease in the future. In this review, we summarize our current understanding of the involvement of IL-22 in liver injury, inflammation and cancer.
IL-22
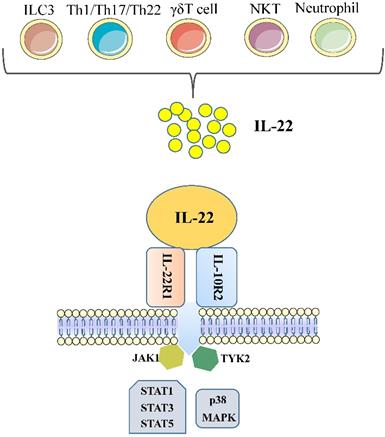
IL-22 is a cytokine largely secreted by lymphoid cells; encompass the innate and adaptive immune systems, such as group 3 innate lymphoid cells (ILC3), αβ T cells, γδ T cells and NKT cells [8]. In the absence of overt infection or inflammation, ie, steady state, the main source of IL-22 is ILC3, which are present in large numbers in the mucosae of the large and the small intestine [9]. Among αβ T cells, IL-22-producing human T cells are mainly Th1, Th17, and Th22 cells; approximately 33% of IL-22-producing CD4+ T cells are Th1 cells, 50% are Th22 cells, and 15% are Th17 cells [3, 7]. Some reports have also found that neutrophils produce IL-22 [10, 11].
Although the production of IL-22 is limited to hematopoietic cells, mainly the lymphoid system, the IL-22 receptor( IL-22R) complex appears to be restricted to non-hematopoietic cells. Functional IL-22R is a heterodimer composed of IL-22R1 and IL-10R2, the latter is shared with various cytokine receptor complexes of the IL-10 family [12, 13]. IL-10R2 is commonly expressed, while IL-22R1 is believed to only be expressed in the epidermal cells of various organs (such as bronchial, liver, pancreas and intestinal) and stroma cells [14, 15]. In the liver, hepatocytes express both IL-10R2 and IL-22R1; Many diseases, such as psoriasis, graft-versus-host disease, liver and pancreas damage, ulcerative colitis and tumours are closely related to the IL-22 -IL-22R1 system, this point has been demonstrated in the experimental data in recent years [12]. Upon binding of IL-22 to the IL-22 receptor-associated Jak1/Tyk2 kinases are activated resulting in phosphorylation of these receptors and activator of transcription (STAT)1, STAT3, STAT5 proteins. While STAT3 phosphorylation is the primary mediator of IL-22 signaling, STAT1 and STAT5 also show phosphorylation to some extent. STAT3 induces the expression of many genes involved in several signalling pathways, including pathways involved with apoptosis, the cell cycle and others. In addition to the STAT signaling, the IL-22-IL-22R1-IL-10R2 complex also results in activation of the mitogen-activated protein kinases (MAPK) and p38 pathways [12, 16-18] (Figure 1). One of the key regulators of IL-22 signaling is IL-22 binding protein (IL-22BP), which is a soluble form of the IL-22R1 subunit. Il-22BP binds IL-22 where it overlaps IL-22R1, thus interfering directly with the binding of the membrane-bound receptor. The affinity of IL22 to this soluble receptor (IL22BP) is 1, 000-fold higher than that of the membrane receptor (IL22R1). The effects of IL22 are inhibited directly by binding of IL22 to IL22BP. IL22BP was expressed in lung, pancreas, skin and other tissues as well as in DC, MQ and epithelial cells [7, 19, 52].
Cellular sources and effects of IL-22. IL-22 is produced mainly by cells of the hematopoietic system. During steady-state, ILC3 are the main producers of IL-22 in the mucosae of the large and the small intestine. During infections, Th1/Th17/Th22 cells will expand and produce amounts of IL-22. In addition, γδT cells and NKT cells have also been shown to capable of producing IL-22. Some reports have also found that neutrophils produce IL-22. IL-22 is a member of the IL-10 family of cytokines, all of which share common features in their receptors. Functional IL-22R is a heterodimer composed of IL-22R1 and IL-10R2. Each of these receptors signals through the Jak1/Tyk2-STAT pathway, although there is evidence that IL-22 can also signal through MAPK and p38 pathways. (ILC, innate lymphoid cell; Th, T helper cell; NKT, natural killer T cell; IL, interleukin; STAT, signal transducer and activator of transcription; MAPK, mitogen-activated protein kinases).
IL-22 and liver injury
Alcoholic liver injury
Chronic alcohol consumption is a major cause of chronic liver disease, causing a wide range of disorders, from fatty liver disease to liver fibrosis and cirrhosis [20]. Alcoholic liver disease (ALD) is a complicated process and causes a wide range of liver damage. The pathogenesis mainly includes direct hepatotoxicity caused by ethanol and its metabolites, oxidative stress caused by ethanol metabolism, elevation of pro-inflammatory cytokines and chemokines, activation of innate immune system, and other mechanisms. In the early stages of ALD, controlled diet and nutritional interventions have proven to be beneficial in curing most patients with mild ALD, but cannot cure severe forms of ALD. Corticosteroids, which were first utilised in the early 1970s for the treatment of severe alcoholic liver disease, are still the only drugs currently used for the treatment of this disease. However, hormone therapy may lead to many negative side effects, including the inhibition of liver regeneration and the promotion of bacterial infections [21, 22].
In the models of alcoholic liver injury (chronic-binge ethanol feeding, acute ethanol feeding), IL-22 has undoubtedly a protective effect on alcoholic fatty liver and related liver damage [6, 23, 24]. IL-22 ameliorats steatosis and hepatocellular damage via the activation of STAT3 in hepatocytes during early alcoholic liver injury; and activation of STAT3 subsequently leads to the upregulation of anti-oxidative (related to MT-1 and MT-2 gene), anti-apoptotic (related to Bcl-2, Bcl-xL and Mcl-1 gene) and anti-bacterial genes (related to lipocalin 2 gene) (Figure 2). It also leads to the downregulation of transcription factors related to lipid biosynthesis. In vivo or in vitro, treatment of IL-22 can promote hepatocyte proliferation or liver regeneration, respectively [25]. It has been clearly demonstrated that IL-22 was able to preventing the occurrence of bacterial infections and acute kidney injury (AKI), which are the main causes of death in patients with alcoholic hepatitis (AH) [22]. In a mouse AKI model, IL-22 treatment was used to improve the survival and regeneration of renal tubular epithelial cells, which significantly improved the ischemia and reperfusion injury; On the other hand, IL-22 plays an important role in the host defence against invading pathogens by stimulating epithelial cells to produce antimicrobial proteins. Administration of IL-22 combine with steroid may be beneficial in the treatment of severe alcoholic hepatitis as IL-22 can negate the side effects of hormone therapy and contribute to the regeneration of the liver. A recent study from Dr. Schnabl's lab showed that ethanol-associated dysbiosis reduced intestinal IAA levels, activated AHR, and decreased intestinal IL-22 expression, leading to a decrease in REG3G expression. The result is that bacterial translocation to the liver and steatohepatitis. Bacteria engineered to produce IL-22 can induce REG3G expression to reduce ethanol-induced steatohepatitis [26].
Nonalcoholic fatty liver injury
Nonalcoholic fatty liver disease (NAFLD) ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), cirrhosis, and even hepatocellular carcinoma. Prevalence of NAFLD in Asia is currently about 25%, similar to many western countries [27]. Although progress has been made in the potential mechanisms of NAFLD and in the identification and development of new therapeutic targets, many problems remain to be resolved and there are no approved therapeutic drugs [28]. IL-22 plays a particular role in alleviating the progression of NAFLD [29].
Blueberries combined with probiotics (BP) have anti-inflammatory and anti-apoptotic properties and may be potential candidates for NAFLD treatment. Using rat NAFLD model, Zhu J et al. [30] found that IL-22 participated in BP therapy by activating JAK1/STAT3 signaling pathway and inhibiting apoptotic factor BAX. In the presence of IL-22, BP could significantly reduce the accumulation of liver lipid droplets and triglyceride (TG), while in the absence of IL-22, lipid droplets and TG levels were significantly increased. Administration of IL-22 relieved metabolic syndrome in obese mice, resulting in reduction of weight and epididymal fat-pad mass, improvement of glucose and insulin tolerance, and regulation expression of lipogeniic genes [31]. Additionally, IL-22 reduced the elevation of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels induced by HFD, and partially inhibited the upregulation of lipid-related genes involved in liver lipid synthesis. Activation of JAK1/STAT3 signaling pathways might account for the protective action of IL-22 against fatty liver [32, 33].
Toxic liver injury
Many toxic substances cause different types of liver damage, both acute injury and chronic injury. Researchers typically use the chemical substances concanavalin A (ConA), Carbon tetrachloride (CCl4), lipopolysaccharide (LPS)/D-galactosamine(GaIN) to induce liver injury in mice and thereby simulate different types of human liver injuries [34-36]. IL-22 has been shown to activate a variety of signal transduction pathways, including the activator of transcription factor (STAT) pathway, the Janus kinase-signal transducer pathway and the mitogen-activated protein kinase pathway in hepatic cells. IL-22 plays a protective role in T cell-mediated hepatitis by activating STAT3; Overexpression of IL-22 can significantly increase the activity of STAT3 and the induction of anti-apoptotic proteins such as Bcl-xL, Bcl-2 and Mcl-1 [37] (Figure 2).
Just as we expected, as a survival factor for hepatocytes, IL-22 has a direct protective effect on acute liver injury induced by various chemical substances [38, 39]. Hydrodynamic gene delivery of IL-22 prevented liver damage in ConA, CCl4 and Fas agonist liver injury models [4]. Results have shown that the expression of IL-22 protein in the liver was significantly induced after injection of ConA. The blockade of IL-22 by neutralising antibodies can aggravate the liver damage induced by ConA and that treatment with recombinant IL-22 protein is sufficient to reduce the damage [37, 40]. In a mouse model of liver injury induced by CCl4, the expression of IL-22 protein was also modestly increased [4, 37]. The protective effect of IL-22 on liver injury induced by GaIN/LPS is also evident. Xing et al. revealed that IL-22 activates STAT3 after liver injury, increasing the expression of HO-1 and Ref-1, which both play a role in antioxidation. IL-22 treatment is also able to decrease the serum levels of ALT, total bilirubin (T.Bil) activity and improve liver histological markers, which reduces the mortality caused by GaIN/LPS [41]. In acetaminophen (APAP)-induced liver injury (AILI) model, IL-22 pretreatment protected mice from APAP-mediated hepatotoxicity. Similarly, the protection was also dependent on STAT3. What's interesting is that short-term acute IL-22 exposure protects mice against AILI through STAT3 activation; however, chronic constitutive overexpression of IL-22 exacerbates AILI by increasing Cyp2E1 and toxic reactive APAP metabolite production [42]. Mo R's lab revealed that enhanced AMP-activated kinase (AMPK)-dependent autophagy contributes to protective effects of IL-22 against APAP-induced liver injury. IL-22 administration distinctly upregulated hepatic LC3-II and p-AMPK; reduced serum ALT/AST, liver necrosis, and hepatic reactive oxygen species in APAP-challenged mice. When p-AMPK was blocked with AMPK inhibitor, IL-22-mediated LC3-II conversion and protection against APAP-induced cytotoxicity were decreased [43].
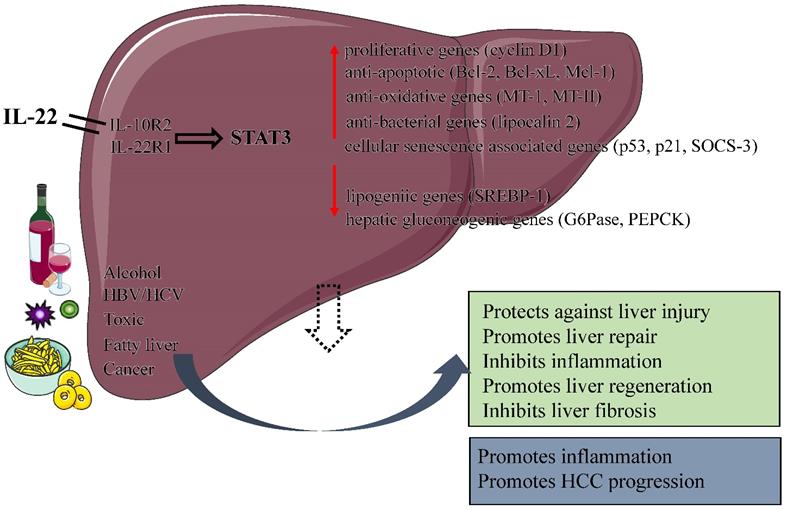
Protective and pathological effects of IL-22. IL-22 plays a number of protective roles in the liver by increasing the expression of anti-apoptotic, anti-oxidative, proliferative and anti-bacterial genes, including protecting against liver injury, Inhibiting liver fibrosis, promoting liver repair and regeneration. In addition, the function of IL-22 is dual nature: a pro-inflammatory activity in acute viral hepatitis infection; an anti-inflammatory activity in chronic viral hepatitis infection. IL-22 itself does not initiate HCC development but may promote HCC progression. (IL, interleukin; STAT 3, signal transducer and activator of transcription 3; HBV, Hepatitis B virus; HCV, Hepatitis C virus; SOCS-3, suppressor of cytokine signaling-3; HCC, Hepatocellular carcinoma ).
IL-22 and inflammation
Hepatitis B
Hepatitis B virus (HBV) infection is a global public health threat, and is the major cause of hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC) [44]. The outcome of HBV infection mainly depends on the balance between host and virus. The occurrence, development and pathogenesis of HBV infection is a complex process involving host innate and adaptive immunity, which often affects the efficacy of anti-HBV drugs [45]. T-cell populations able to produce IL-22 are higher in patients with HBV hepatitis than in healthy subjects [46, 47], such as regulatory T, Thelper (Th)17, and Th22 cells. Similarly, the expression of IL-22 was significantly increased in patients with hepatitis B and the levels of IL-22 in serum or in the liver are closely related to the severity of liver disease in patients with Hepatitis B viral infection [48].
The role of IL-22 in HBV induced-liver inflammation remains controversial. It is possible that IL-22 has paradoxical dual nature of the anti-inflammatory or proinflammatory activity in HBV hepatitis. In acute HBV infection IL-22 may play a proinflammatory role by amplifying immune cell infiltration and clearance of the virus. In HBV transgenic mice model, injection of IL-22 increased the expression of pro-inflammatory genes in liver, and the transplantation of splenocytes from HBV-immunized mice into HBV transgenic mice significantly ameliorated the severity of liver injury in mice by neutralization of IL-22 [47, 49]. However, during chronic HBV infection, IL-22 may be mainly protective in some situations, yet pathogenic in others. In livers of mice and patients with chronic HBV infection, IL22 produced by inflammatory cells promoted proliferation of liver stem/progenitor cells (LPCs) via the activation of STAT3 [48]. Depletion of IL-22 significantly inhibited the expression of chemokines and recruitment of inflammatory cells into the liver, suggesting that IL-22 may be an important mediator by increasing the expression of chemokines to recruit inflammatory cells into the liver. Although IL-22 plays such an important role, IL-22 does not directly inhibit virus replication [47]. In fact, chronic HBV infection is a complex process that includes immune tolerance (high HBV and low ALT), immune activity (high HBV and high ALT), and immune inactivity (low HBV and low ALT). IL-22 expression may correlate with high or low ALT levels, suggesting that the balance between IL-22's protective and pro-inflammatory effects may be tipped during chronic HBV infection [50].
Hepatitis C
Hepatitis C virus (HCV) threatens the quality of life of more than 350 million people worldwide. An effective immune response clears about 20% of HCV infections. However, without an adequate immune response, the virus will continue to replicate and cause a chronic infiltration of inflammatory cells into the liver [51]. Similar to hepatitis B, IL22-producing Th17 cells also increased significantly in the liver of patients with chronic hepatitis C [52]. The expression of hepatic IL-22 mRNA was higher in HCV hepatitis than that in cholestatic liver disease, although the serum IL-22 level was not significantly different between HCV hepatitis and the normal control group. Due to IL-22 has no significant effect on the expression of IFN-α/β and antiviral proteins MxA and 2', 5'-oligoadenylates synthesis, it has no relevant antiviral activity in vitro models of HCV replication and infection [53]. Additionly, serum IL-22 levels were dramatically higher in the antiviral treatment group than in the untreated group, and its levels increased gradually with the improvement of treatment effect, suggests that IL-22 serum level can be used as a predictor of the efficacy of antiviral therapy in HCV patients [54]. However, the role of IL-22 in the process of HCV fibrosis is still controversial. Sertorio M et al. demonstrated that high levels of IL-22 can alleviate liver fibrosis and portal hypertension in hepatitis C patients, while IL-22BP (IL-22 binding protein, the physiological inhibitor of IL-22) increases the risk to develop severe fibrosis [55]. On the other hand, Wu LY et al. found that most IL-22 producing (+) cells in the liver were located in the liver fibrosis area of HCV patients with cirrhosis, and the increase in the number were positively correlated with the fibrosis staging score. IL-22 may participate in the fibrosis formation of HCV-related liver fibrosis by promoting the proliferation and activation of hepatic stellate cells (HSCs), inhibiting cell apoptos [56].
IL-22 and liver fibrosis
Fibrosis is an internal reaction to chronic injury. Its function is to maintain the integrity of the organ despite widespread necrosis or apoptosis [57]. Liver fibrosis is a result of chronic liver injury, and it is characterised by the activation of HSCs and an accumulation of extracellular matrix proteins [58]. Long-term liver damage may cause fibrosis to develop into cirrhosis. Liver fibrosis can be caused by many diseases, such as chronic hepatitis B or C infection, autoimmune diseases, biliary tract disease, alcoholic fatty hepatitis as well as non-alcoholic fatty hepatitis [57]. IL-22 plays a role in various liver diseases, including liver inflammation, liver fibrosis and cirrhosis [25, 49, 59]. HSCs are the predominant type of cell that induces liver fibrosis after liver injury. HSCs express high levels of IL-10R2 and IL-22R1. Both in vivo and in vitro experiments demonstrate that IL-22 can inhibit the apoptosis of HSCs and promote their survival via the induction of the anti-apoptotic genes Bcl-2 and Bcl-xl. Surprisingly, IL-22 overexpression, either by IL-22 transgenic mice or exogenous administration of adenovirus expressing IL-22, is able to reduce hepatic fibrosis and accelerate the resolution of liver fibrosis during recovery [59]. HSCs are activated after hepatic injury, then begin to express alpha smooth muscle actin (α-SMA) and produce a large quantity of collagen, eventually lead to fibrosis of the liver. The level of α-SMA expression decreased after treatment with IL-22, as well as increase the expression of proteins related to cellular senescence, including p53 at serine suppressor 15 (ser15 p-p53), p53, p21 and SOCS3 (suppressor of cytokine signalling 3). Administration of IL-22 increased the number of HSCs associated with SA-β-gal (senescence-associated-galactosidase). In addition, the signal transducer and activator of transcription 3 (STAT3) will be activated by IL-22, which promotes the senescence of HSCs by p53- and p21-dependent pathways, and knockdown of STAT3 will prevent IL-22 induced the senescence of HSCs. In other words, IL-22 can improve hepatic fibrosis by inducing the senescence of HSCs [59].
IL-22 and liver regeneration
The liver is the only solid organ system capable of regeneration after tissue injury in mammals [60]. Liver regeneration occurs both in the acute recovery of liver mass after resection and in the maintenance of liver mass after chronic injury, is an active area of research currently. Liver regeneration is a highly organised and orderly process of tissue growth caused by the loss of liver tissue; therefore, there is a large number of genes involved in the process of liver regeneration [61].
IL-22 is closely related to the proliferation of many types of cells, including epithelial cells, hepatic cells and other cell types [62, 63]. The expression of IL-22 in the serum and IL-22Ra mRNA in hepatic of mice were significantly increased after 70% hepatectomy under general anaesthesia. Although administration of exogenous IL-22 did not increase the replication of hepatocytes before the partial hepatectomy, anti-IL-22 antibody treatment prior to surgery was able to significantly reduce hepatic cell replication [63]. This suggests IL-22 may promote the regeneration of liver by increasing the replication of hepatocytes.
Treatment with IL-22 significantly increased the number of hepatocytes by inhibiting cell apoptosis and promoting mitosis to promote liver regeneration [64]. Previous studies have shown that HepG2 cells stably transfected with IL-22 cDNA express high levels of cyclinD1 and c-myc; further in vivo and in vitro researches have demonstrated that the overexpression of cyclin D1 contributes to the replication and growth of hepatocytes, furthermore, c-myc plays an important role in IL-22 induced liver cell replication, suggesting that both cyclinD1 and c-myc may promote mitotic activity in hepatocytes. Just as we expected, the stimulating effects of IL-22 on liver regeneration are related to STAT3 activation [65]. The levels of IL-6 in the liver may increases with the activation of STAT3 after a partial hepatectomy, suggestes that IL-6 and STAT3 are part of an early mechanism of liver cell proliferation.
There are two different forms of liver regeneration: hepatocyte regeneration and liver progenitor cells (LPCs) regeneration. LPC regeneration is largely responsible for liver regeneration after liver injury [66, 67]. Progenitor cells have a high degree of regulation to regulate replication, as well as the ability to differentiate to achieve accurate tissue repair [61]. In severe liver injury or chronic liver injury, if the mature liver cells cannot proliferate to repair the damage, LPC-mediated repair mechanisms will be used to maintain liver function. LPCs are able to differentiate into hepatocytes and bile duct epithelial cells [68-71], meanwhile express both IL-10R2 and IL-22R1. It has been proved that IL-22 has the ability to promote the proliferation of LPCs in a STAT3- dependent manner. Consequently, IL-22 can not only stimulate the proliferation of mature liver cells, but can also promote liver repair and regeneration in patients with severe or chronic liver injury by targeting LPCs [48]. Hepatic IL-22 expression was increased in HBV patients and correlated with severity of liver inflammation and proliferation of LPCs. Proliferation of LPCs was increased significantly after 3, 5-diethoxycarbonyl-1, 4-dihydrocollidine (DDC) diet in overexpressed an IL-22 transgene specifically in liver (IL-22TG) mice. STAT3 was activated in LPCs isolated from DDC-fed IL-22TG mice. Proliferation of LPCs was decreased in liver with deletion of STAT3. IL-22R1 and IL-10R2 were also detected on LPCs isolated from DDC-fed wild-type mice. Treatment with IL-22 on LPCs activated STAT3 to induce these cells proliferation, but IL-22 had no effect on proliferation of LPCs with deletion of STAT3.
IL-22 and Hepatocellular carcinoma
Hepatocellular carcinoma accounts for the majority of primary liver cancers. Worldwide, liver cancers are the fourth most common cause of cancer-related death and rank sixth in terms of incident cases [72]. The 5-year survival rate for patients with liver cancer is only 18%, making it the second most deadly cancer after pancreatic cancer [73]. Most HCC occurs in patients with underlying liver disease, mainly as a result of viral hepatitis or alcohol abuse. Currently, there are many treatments for HCC, such as surgical treatment, chemotherapy, radiotherapy, transcatheter chemoembolization/transcatheter chemoembolization (TACE), but for advanced patients, liver transplantation may be the only effective method. Unfortunately, due to the lack of donor, immune rejection, surgical injury and high cost, liver transplantation is not an ideal treatment [74, 75]. The tumour and inflammatory microenvironment are considered to be the main battlefield between antitumor immunity and tumor promotion; it is very meaningful for the further understanding of the HCC with the new exploration of tumor microenvironment [76].
We know that IL-22 is a protective factor for the liver, by promoting the regeneration of tissue, increasing the function of the barrier, reducing the chronic inflammation and preventing the occurrence of cancer. In fact, IL-22 plays a dual role in the occurrence of HCC; On the one hand, it protects the liver from damage by contributing to the survival of normal hepatic cells; On the other hand, IL-22 can promotes the survival of damaged liver cells (which can be the precursor of hepatocellular carcinoma), ultimately leading to the occurrence of hepatocellular carcinoma [3]. Compared with peripheral lymphocytes, IL-22 expression was significantly upregulated in human HCC tumor infiltrating leukocytes (TILs) [77]. Moreover, IL-22 expression in Edmondson Grade III-IV HCC patients was observably higher than that in Grade I-II. IL-22 expression and STAT3 activation continued to increase in liver tissue in mice with chronic hepatitis and HCC models. Phosphorylation of STAT3 and up-regulation of downstream genes CyclinD1, Bcl-2, Bcl-XL, and vascular endothelial growth factor (VEGF) promote tumor growth and metastasis. In the diethylnitrosamine-induced HCC model, tumor formation was significantly reduced in IL-22 knockout mice. In conclusion, IL-22 plays a pro-tumor and anti-apoptosis role in HCC. More significantly, Oliver Waidmann [78] found that serum IL-22 levels is associated with disease severity in patients with advanced cirrhosis, and high serum IL-22 level is closely related to the short overall survival of patients with HCC verified by assessment of serum IL-22 level for 156 patients with HCC. IL-22 serum levels may reflect increased aggressiveness of liver cancer disease and act as a negative prognostic indicator in patients with HCC. It is important to note that although IL-22 is able to promote the proliferation and survival of liver cancer cells, IL-22 transgenic mice (with a high level of IL-22) cannot spontaneously induce hepatocellular carcinoma, suggesting that IL-22 itself does not lead to the development of HCC [5].
Conclusions
Liver diseases remain a major global threat to human health [79]. Cytokines are attractive therapeutic targets in liver diseases because of their importance in regulating immune and inflammatory responses [80-82]. For instance, IL-6 have been shown to play an important role in alleviating steatosis and hepatocyte injury by activating STAT3 in hepatocytes during early alcoholic liver injury [83, 84]. Although the hepatoprotective function of IL-6 has been fully demonstrated, the ubiquitous expression of IL-6 receptor makes many potential side effects of IL-6 in the treatment of liver diseases, which limits its clinical application [85]. As an emerging CD4+Th cytokine, IL-22 plays an overwhelming hepatoprotective and regenerative roles in various liver diseases with the activation of STAT3 pathway [3]. IL-22 has a protective effect on liver cells, can protects against liver injury, hepatitis, and liver fibrosis caused by a variety of reasons, and can promote liver regeneration after partial hepatectomy (Figure 2). What is worth mentioning is that IL-22 receptor expression is restricted to epithelial cells, which suggests that IL-22 treatment would likely produce fewer side effects than IL-6. IL-22 is expected to be a new attractive candidate for human liver diseases therapy.
A phase I trial of the recombinant human IL-22-Fc fusion protein (F-652) published by Tang et al. [86] shows minimal side effects in healthy volunteers. F-652 was well-tolerated in the first-in-man study following IV dosing. F-652 shown potent bioactivities, and did not induce pro-inflammatory cytokines/chemokines. Unlike the conventional anti-inflammation new drug, F-652 may be a first-in-class drug that plays an important role in protecting the survival and regeneration of tissues under immunological attack. A phase IIa trial is currently under way to determine whether IL-22-Fc treatment is also tolerated in patients with severe alcoholic hepatitis, and a phase IIb trial has been proposed for the treatment of severe alcoholic hepatitis [87]. We look forward to the results of these clinical trials and hope that IL-22 therapy may benefit some patients with severe alcoholic hepatitis.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dumoutier L, Van Roost E, Colau D, Renauld JC. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci U S A. 2000;97(18):10144-9
2. Dumoutier L, Van Roost E, Ameye G. et al. IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun. 2000;1(8):488-94
3. Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: Immunobiology and Pathology. Annu Rev Immunol. 2015;33:747-85
4. Pan H, Hong F, Radaeva S, Gao B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol. 2004;1(1):43-9
5. Park O, Wang H, Weng H. et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology. 2011;54(1):252-61
6. Kong X, Feng D, Mathews S, Gao B. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J Gastroenterol Hepatol. 2013;28(Suppl 1):56-60
7. Shabgah AG, Navashenaq JG, Shabgah OG. et al. Interleukin-22 in human inflammatory diseases and viral infections. Autoimmun Rev. 2017;16(12):1209-1218
8. Hernandez P, Gronke K, Diefenbach A. A catch-22: Interleukin-22 and cancer. Eur J Immunol. 2018;48(1):15-31
9. Savage AK, Liang HE, Locksley RM. The Development of Steady-State Activation Hubs between Adult LTi ILC3s and Primed Macrophages in Small Intestine. J Immunol. 2017;199:1912-1922
10. Dyring-Andersen B, Honoré TV, Madelung A. et al. Interleukin (IL)-17A and IL-22-producing neutrophils in psoriatic skin. Br J Dermatol. 2017;177(6):e321-e322
11. Lee YS, Yang H, Yang JY. et al. Interleukin-1 (IL-1) signaling in intestinal stromal cells controls KC/ CXCL1 secretion, which correlates with recruitment of IL-22- secreting neutrophils at early stages of Citrobacter rodentium infection. Infect Immun. 2015;83(8):3257-67
12. Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov. 2014;13(1):21-38
13. Trevejo-Nunez G, Elsegeiny W, Conboy P. et al. Critical Role of IL-22/IL22-RA1 Signaling in Pneumococcal Pneumonia. J Immunol. 2016;197(5):1877-83
14. Hu H, Li L, Yu T, et al. Interleukin-22 receptor 1 upregulation and activation in hypoxic endothelial cells improves perfusion recovery in experimental peripheral arterial disease.Biochem Biophys Res Commun. 2018; 505(1):60-66
15. Akil H, Abbaci A, Lalloué F. et al. IL22/IL-22R pathway induces cell survival in human glioblastoma cells. PLoS One. 2015;10(3 e):0119872
16. Calautti E, Avalle L, Poli V. Psoriasis: A STAT3-Centric View. Int J Mol Sci. 2018 19(1)
17. Bai L, Fang H, Xia S. et al. STAT1 activation represses IL-22 gene expression and psoriasis pathogenesis. Biochem Biophys Res Commun. 2018;501(2):563-569
18. Wang S, Yao Y, Yao M. et al. Interleukin-22 promotes triple negative breast cancer cells migration and paclitaxel resistance through JAK-STAT3/MAPKs/AKT signaling pathways. Biochem Biophys Res Commun. 2018;503(3):1605-1609
19. Hebert KD, Mclaughlin N, Galeas-Pena M. et al. Targeting the IL-22/IL-22BP axis enhances tight junctions and reduces inflammation during influenza infection. Mucosal Immunol. 2020Jan;13(1):64-74
20. Dunn W, Shah VH. Pathogenesis of Alcoholic Liver Disease. Clin Liver Dis. 2016;20(3):445-56
21. Xu M, Chang B, Mathews S, Gao B. New drug targets for alcoholic liver disease. Hepatol Int. 2014;2:475-80
22. Gao B, Shah VH. Combination therapy: New hope for alcoholic hepatitis? Clin Res Hepatol Gastroenterol. 2015;39(Suppl 1):S7-S11
23. Xiang X, Feng D, Hwang S. et al. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J Hepatol. 2019 Nov 28
24. Arab JP, Sehrawat TS, Simonetto DA. et al. An Open Label, Dose Escalation Study To Assess The Safety And Efficacy Of IL-22 Agonist F-652 In Patients With Alcoholic Hepatitis. Hepatology. 2019 Nov 27
25. Ki SH, Park O, Zheng M. et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52(4):1291-300
26. Hendrikx T, Duan Y, Wang Y. et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut. 2019;68(8):1504-1515
27. Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67(4):862-873
28. Gluchowski NL, Becuwe M, Walther TC, Farese RV Jr. Lipid droplets and liver disease: from basic biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2017;14(6):343-355
29. Hwang S, He Y, Xiang X. et al. Interleukin-22 ameliorates neutrophil-driven nonalcoholic steatohepatitis through multiple targets. Hepatology. 2019 Nov 9
30. Zhu J, Zhou M, Zhao X. et al. Blueberry, combined with probiotics, alleviates non-alcoholic fatty liver disease via IL-22-mediated JAK1/STAT3/BAX signaling. Food Funct. 2018;9(12):6298-6306
31. Wang X, Ota N, Manzanillo P. et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514(7521):237-41
32. Yang L, Zhang Y, Wang L. et al. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol. 2010;53(2):339-47
33. Zai W, Chen W, Wu Z. et al. Targeted Interleukin-22 Gene Delivery in the Liver by Polymetformin and Penetratin-Based Hybrid Nanoparticles to Treat Nonalcoholic Fatty Liver Disease. ACS Appl Mater Interfaces. 2019;11(5):4842-4857
34. Hammerich L, Warzecha KT, Stefkova M. et al. Cyclic adenosine monophosphate-responsive element modulator alpha overexpression impairs function of hepatic myeloid-derived suppressor cells and aggravates immune-mediated hepatitis in mice. Hepatology. 2015;61(3):990-1002
35. Zhao L, Jin Y, Donahue K. et al. Tissue Repair in the Mouse Liver Following Acute Carbon Tetrachloride Depends on Injury-Induced Wnt/β-Catenin Signaling. Hepatology. 2019;69(6):2623-2635
36. Wang W, Zhang Y, Li H. et al. Protective Effects of Sesquiterpenoids from the Root of Panax ginseng on Fulminant Liver Injury Induced by Lipopolysaccharide/d-Galactosamine. J Agric Food Chem. 2018;66(29):7758-7763
37. Radaeva S, Sun R, Pan HN. et al. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39(5):1332-42
38. Zhang HB, Luo HC, Xin XJ, Zeng AZ. Up-regulated Reg proteins induced by Interleukin-22 treatment ameliorate acute liver injury in rat model. Int J Clin Exp Med. 2015;8(1):1253-8
39. Zhou H, Xie G, Mao Y. et al. Enhanced Regeneration and Hepatoprotective Effects of Interleukin 22 Fusion Protein on a Predamaged Liver Undergoing Partial Hepatectomy. J Immunol Res. 2018;2018:5241526
40. Zhang YM, Liu ZR, Cui ZL. et al. Interleukin-22 contributes to liver regeneration in mice with concanavalin A-induced hepatitis after hepatectomy. World J Gastroenterol. 2016;22(6):2081-91
41. Xing WW, Zou MJ, Liu S. et al. Hepatoprotective effects of IL-22 on fulminant hepatic failure induced by d-galactosamine and lipopolysaccharide in mice. Cytokine. 2011;56(2):174-9
42. Feng D, Wang Y, Wang H. et al. Acute and chronic effects of IL-22 on acetaminophen-induced liver injury. J Immunol. 2014;193(5):2512-8
43. Mo R, Lai R, Lu J. et al. Enhanced autophagy contributes to protective effects of IL-22 against acetaminophen-induced liver injury. Theranostics. 2018;8(15):4170-4180
44. Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392(10161):2313-2324
45. Gehring AJ, Protzer U. Targeting Innate and Adaptive Immune Responses to Cure Chronic HBV Infection. Gastroenterology. 2019;156(2):325-337
46. Cobleigh MA, Robek MD. Protective and pathological properties of IL-22 in liver disease: implications for viral hepatitis. Am J Pathol. 2013;182(1):21-8
47. Zhang Y, Cobleigh MA, Lian JQ. et al. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology. 2011;141(5):1897-906
48. Feng D, Kong X, Weng H. et al. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology. 2012;143(1):188-98.e7
49. Gao W, Fan YC, Zhang JY, Zheng MH. Emerging Role of Interleukin 22 in Hepatitis B Virus Infection: a Double-edged Sword. J Clin Transl Hepatol. 2013;1(2):103-8
50. Xiang X, Gui H, King NJ. et al. IL-22 and non-ELR-CXC chemokine expression in chronic hepatitis B virus-infected liver. Immunol Cell Biol. 2012;90(6):611-9
51. Ashraf M, Iman K, Khalid MF. et al. Evolution of efficacious pangenotypic hepatitis C virus therapies. Med Res Rev. 2019;39(3):1091-1136
52. Foster RG, Golden-Mason L, Rutebemberwa A, Rosen HR. Interleukin (IL)-17/IL-22-producing T cells enriched within the liver of patients with chronic hepatitis C viral (HCV) infection. Dig Dis Sci. 2012;57(2):381-9
53. Dambacher J, Beigel F, Zitzmann K. et al. The role of interleukin-22 in hepatitis C virus infection. Cytokine. 2008;41(3):209-16
54. F GM, Attia FM, Saleh RM. et al. Interleukin-22 and Chemokine Interferon γ -Inducible-10 (IP-10) Levels in Chronic Hepatitis C Patients and Treatment Response to Pegylated Interferon and Ribavirin Therapy. Egypt J Immunol. 2017;24(2):83-91
55. Sertorio M, Hou X, Carmo RF. et al. IL-22 and IL-22 binding protein (IL-22BP) regulate fibrosis and cirrhosis in hepatitis C virus and schistosome infections. Hepatology. 2015;61(4):1321-31
56. Wu LY, Liu S, Liu Y. et al. Up-regulation of interleukin-22 mediates liver fibrosis via activating hepatic stellate cells in patients with hepatitis C. Clin Immunol. 2015;158(1):77-87
57. Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123(5):1887-901
58. Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27-42
59. Kong X, Feng D, Wang H. et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56(3):1150-9
60. Cordero-Espinoza L, Huch M. The balancing act of the liver: tissue regeneration versus fibrosis. J Clin Invest. 2018;128(1):85-96
61. DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest. 2013;123(5):1861-6
62. Kumar P, Rajasekaran K, Palmer JM. et al. IL-22: An Evolutionary Missing-Link Authenticating the Role of the Immune System in Tissue Regeneration. J Cancer. 2013;4(1):57-65
63. Ren X, Hu B, Colletti LM. IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2010;298(1):G74-80
64. Kudira R, Malinka T, Kohler A. et al. P2X1-regulated IL-22 secretion by innate lymphoid cells is required for efficient liver regeneration. Hepatology. 2016;63(6):2004-17
65. Brand S, Dambacher J, Beigel F. et al. IL-22-mediated liver cell regeneration is abrogated by SOCS-1/3 overexpression in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;292(4):G1019-28
66. Boulter L, Lu WY, Forbes SJ. Differentiation of progenitors in the liver: a matter of local choice. J Clin Invest. 2013;123(5):1867-73
67. Van Haele M, Roskams T. Hepatic Progenitor Cells: An Update. Gastroenterol Clin North Am. 2017;46(2):409-420
68. Michalopoulos GK. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65(4):1384-1392
69. Zhang J, Zhao X, Liang L. et al. A decade of progress in liver regenerative medicine. Biomaterials. 2018;157:161-176
70. Michalopoulos GK. Liver regeneration: alternative epithelial pathways. Int J Biochem Cell Biol. 2011;43(2):173-9
71. Tanaka M, Itoh T, Tanimizu N, Miyajima A. Liver stem/progenitor cells: their characteristics and regulatory mechanisms. J Biochem. 2011;149(3):231-9
72. Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450-1462
73. Jemal A, Ward EM, Johnson CJ. et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017 109(9)
74. Dutta R, Mahato RI. Recent advances in hepatocellular carcinoma therapy. Pharmacol Ther. 2017;173:106-117
75. Zhou K, Fountzilas C. Outcomes and Quality of Life of Systemic Therapy in Advanced Hepatocellular Carcinoma. Cancers (Basel). 2019 11(6)
76. Ringelhan M, Pfister D, O'Connor T. et al. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19(3):222-232
77. Jiang R, Tan Z, Deng L. et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011;54(3):900-9
78. Waidmann O, Kronenberger B, Scheiermann P. et al. Interleukin-22 serum levels are a negative prognostic indicator in patients with hepatocellular carcinoma. Hepatology. 2014;59(3):1207
79. Wang FS, Fan JG, Zhang Z. et al. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099-108
80. Del Campo JA, Gallego P, Grande L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J Hepatol. 2018;10(1):1-7
81. Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2016;64(3):955-65
82. Hong JT, Son DJ, Lee CK. et al. Interleukin 32, inflammation and cancer. Pharmacol Ther. 2017;174:127-137
83. Naseem S, Hussain T, Manzoor S. Interleukin-6: A promising cytokine to support liver regeneration and adaptive immunity in liver pathologies. Cytokine Growth Factor Rev. 2018;39:36-45
84. Xiang DM, Sun W, Ning BF. et al. The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut. 2018;67(9):1704-1715
85. Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol. 2012;27(Suppl 2):89-93
86. Tang KY, Lickliter J, Huang ZH. et al. Safety, pharmacokinetics, and biomarkers of F-652, a recombinant human interleukin-22 dimer, in healthy subjects. Cell Mol Immunol. 2019;16(5):473-482
87. Gao B, Xiang X. Interleukin-22 from bench to bedside: a promising drug for epithelial repair. Cell Mol Immunol. 2019;16(7):666-667
Author contact
![]() Corresponding author: Dian Zhou, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, China. Email: ayfy_zhoudiancom
Corresponding author: Dian Zhou, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, China. Email: ayfy_zhoudiancom

 Global reach, higher impact
Global reach, higher impact