10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(1):225-241. doi:10.7150/ijbs.75459 This issue Cite
Research Paper
HIF-1α/YAP Signaling Rewrites Glucose/Iodine Metabolism Program to Promote Papillary Thyroid Cancer Progression
Department of Nuclear Medicine, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. Shanghai, China.
*These authors contributed equally to this paper.
Abstract
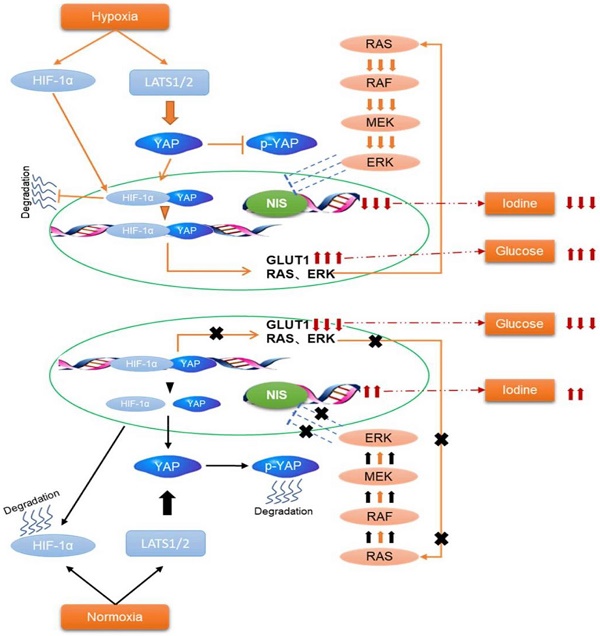
Background: The management of aggressive and progressive metastatic papillary thyroid cancer (PTC) is very difficult. An inverse relationship between radioiodine and F-18 fluorodeoxyglucose (FDG) uptake (''flip-flop'' phenomenon) is described for invasive PTC during dedifferentiation. However, no satisfactory biologic explanation for this phenomenon. Hypoxia is an important microenvironmental factor that promotes cancer progression and glycolysis. The Hippo-YAP is a highly conserved tumor suppressor pathway and contributes to cancer metabolic reprogramming. Thus, we investigated the underlying molecular mechanisms of glucose/iodine metabolic reprogramming in PTC, focusing on the tumor hypoxia microenvironment and Hippo-YAP signaling.
Methods: Immunohistochemistry staining was conducted to evaluate the expressions of hypoxia-inducible factor 1α (HIF-1α), yes-associated protein (YAP), glucose transporters 1 (GLUT1) and sodium iodine symporter (NIS) in matched PTC and the adjacent noncancerous tissues. PTC cell lines were cultured under normoxic (20% O2) and hypoxic (1% O2) conditions and the glycolysis level and NIS expression were measured. Further, we characterized the molecular mechanism of glucose/iodine metabolic reprogramming in PTC cell. Finally, we validated the results in vivo by establishing subcutaneous xenografts in nude mice.
Results: The expression levels of HIF1-α, YAP and GLUT1 were upregulated in PTC tissues and YAP expression was positively associated with HIF-1α, GLUT1 and TNM stages. Meanwhile, the expression of NIS was negatively correlated with YAP. Further, in vitro studies indicated that hypoxia-induced YAP activation was critical for accelerating glycolysis and reducing NIS expression in PTC cells. Inhibition of YAP had the opposite effects in vitro and tumorigenicity in vivo. Hypoxia inhibited the Hippo signaling pathway resulting in the inactivation of YAP phosphorylation, further promoting the nuclear localization of YAP in PTC cells. The mechanism is that hypoxic stress promoted YAP binding to HIF-1α in the nucleus and maintained HIF-1α protein stability. The YAP/HIF-1α complex bound and directly activated the GLUT1 transcription to accelerate glycolysis. Meanwhile, HIF-1α/YAP signaling might indirectly reduce the expression of NIS by promoting the output of MAPK signaling. In vivo studies confirmed the YAP-mediated reprogramming of glucose/iodine metabolism promoted PTC progression.
Conclusions: Collectively, our data revealed a novel regulatory mechanism of the glucose/iodine metabolic program rewritten by HIF-1α/YAP signaling in PTC. Inhibition of HIF-1α/YAP signaling alone or in combination with other potential markers may effectively combat aggressive PTC.
Keywords: Papillary thyroid cancer, Glucose/Iodine metabolism, HIF-1α, YAP, Progression.

 Global reach, higher impact
Global reach, higher impact