10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2026; 22(6):2906-2924. doi:10.7150/ijbs.128203 This issue Cite
Review
Orchestrating Tumor Metastasis: Exosomes as Master Regulators of the Local and Distant Microenvironment
1. School of Medical and Life Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu, China.
2. Translational Chinese Medicine Key Laboratory of Sichuan Province, Sichuan Academy of Chinese Medicine Sciences, Sichuan Institute for Translational Chinese Medicine, Chengdu, Sichuan 610041, China.
Received 2025-11-11; Accepted 2026-2-7; Published 2026-2-18
Abstract
Metastasis remains a critical challenge in oncology and constitutes the leading cause of cancer mortality. Recent studies have revealed that exosomes are involved in every step of the cascades of tumor invasion and metastasis. Therefore, it is necessary to further investigate the exosome-mediated intercellular communication network within the tumor microenvironment to elucidate the mechanisms of cancer metastasis. This review summarizes alterations in the tumor microenvironment at primary and metastatic sites during metastasis, encompassing processes such as epithelial-mesenchymal transition (EMT) induction, extracellular matrix (ECM) remodeling, immune suppression, and angiogenesis. In addition, we examine the role of exosomes in mediating tumor drug resistance and explore the clinical translational potential of exosomes in biomarker detection, drug delivery systems, and cancer vaccines.
Keywords: tumor microenvironment, cancer metastasis, exosomes, cancer therapy
Introduction
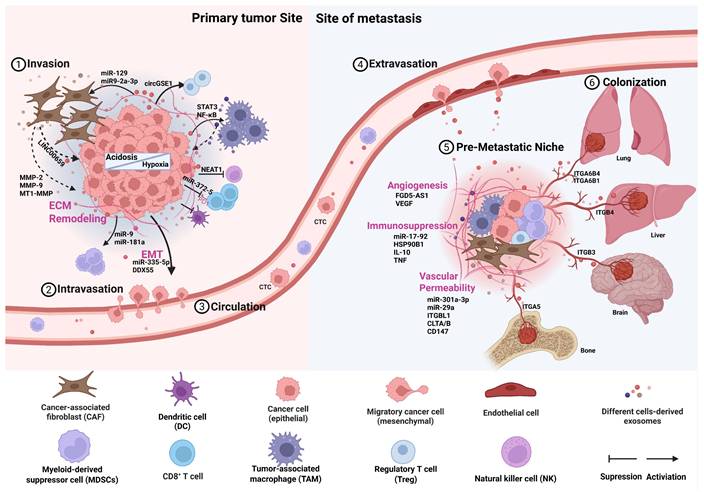
Cancer metastasis—the process by which tumor cells spread from the primary site to distant organs, accounts for approximately 90% of cancer-related mortality[1]. Distant metastasis can be divided into two key stages: (1) Tumor cell invasion at the primary tumor site and migration to secondary sites;(2) the colonization and adaptation of these cells to the microenvironment of distant organs[2]. As the primary tumor proliferates, tumor cells acquire the ability to invade surrounding tissues, migrating from the stromal tissue of the originating organ and infiltrating the bloodstream. These cells become known as circulating tumor cells (CTCs) once they enter the circulatory system. Subsequently, CTCs strive to survive and infiltrate distant organs. Within the pre-metastatic niche of these organs, tumor cells gradually adapt and proliferate, forming metastatic lesions. This complex, multi-step process is known as the metastatic cascade[3].
Research has demonstrated that complex paracrine and endocrine signaling between the tumor cells and their surrounding environment, which coordinates both the local and distant microenvironments, regulates the proliferation and metastasis of malignant tumors. The tumor microenvironment (TME) refers to the cellular environment in which the tumor resides, primarily composed of the extracellular matrix (ECM), various stromal cells (such as endothelial cells, immune cells, and fibroblasts), and numerous tumor-regulating factors[4]. The TME is intricate and continuously evolving, contributing to cancer cell heterogeneity, clonal evolution, and multi-drug resistance, ultimately driving tumor progression and metastasis[5]. Therefore, understanding the communication network between cancer cells, the original tumor site, and distant organs is critical for advancing cancer treatment.
Exosomes were first discovered in the 1980s as a form of extracellular vesicle released by most eukaryotic cells. They have a diameter of 30 to 150 nm and are made up of a membrane with a lipid bilayer that encloses complex molecular contents such as proteins, nucleic acids, and lipids[6]. Over the past decade, the role of exosomes in communication within the tumor microenvironment has attracted increasing attention. Exosomes mediate communication between tumor cells and stromal cells through three distinct pathways: (1) activation of target cell signals via receptor-ligand binding; (2) fusing with the membrane of the target cell to transport their contents; and (3) direct internalization by target cells[7].
A rising amount of evidence reveals that exosomes play an important part in the multi-step process of tumor metastasis. This review explores how stressors, such as hypoxia and acidosis, regulate exosome synthesis and cargo sorting, along with particular alterations of the TME at both initial and distant sites during exosome-mediated tumor metastasis (Figure 1). Furthermore, we examine the clinical significance of exosomes, including their roles in therapeutic resistance, biomarkers, drug delivery, and potential applications in tumor vaccine development.
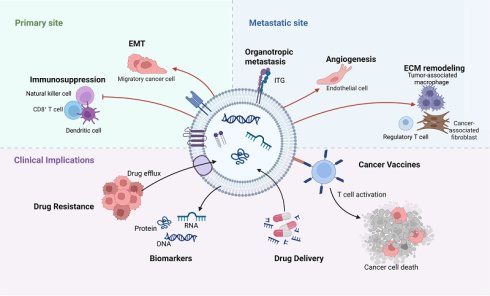
Exosome-mediated alterations in the tumor microenvironment at primary and metastatic sites. This schematic illustrates the cascade of events through which exosomes orchestrate microenvironmental changes supporting metastasis. The cascade is initiated within the primary tumor microenvironment (TME) by key stressors such as hypoxia and acidosis. Tumor cells and stromal cells release exosomes that promote local invasion and migration. These exosomes subsequently enter the circulation and travel to predetermined distant sites. At these sites, exosomes mediate the formation of a pre-metastatic niche by promoting angiogenesis, increasing vascular permeability, recruiting immunosuppressive cells, and remodeling the extracellular matrix (ECM). This prepared and hospitable niche enables the subsequent colonization and outgrowth of circulating tumor cells, ultimately leading to the formation of overt metastatic lesions. (Created with Biorender.com).
Regulation of exosome biogenesis and cargo by the TME
Mechanisms of exosome biogenesis
Exosome biogenesis is closely linked to the endocytic system[8]. Early endosomes (EE) are generated by plasma membrane invagination and mature as late endosomes (LE), which further bud inward to produce intraluminal vesicles (ILVs). Endosomes harboring ILVs are known as multivesicular bodies (MVBs)[9]. Each step in this process involves the sorting of various proteins and bioactive small molecules. The secretion of ILVs relies on two distinct sorting mechanisms: the ESCRT (endosomal sorting complexes required for transport)-dependent pathway and the ESCRT-independent pathway[10]. ESCRT is a sophisticated protein machinery that serves a key function in exosome biogenesis via ILV formation. It is composed of four distinct protein complexes (ESCRT-0 to III) and associated proteins. Initially, the ubiquitin-binding subunit of ESCRT-0, Hrs, recognizes and sorts ubiquitinated proteins to endosomal membrane. ESCRT-0 then recruits ESCRT-I through connecting to tumor susceptibility gene 101. The ESCRT-I and -II complexes then facilitate the inward budding of the endosome around the cluster of ubiquitinated proteins. Finally, the ESCRT-III complex cleaves the buds to form ILVs, which are released from the MVB membrane[11]. Following fusion with the plasma membrane, the bulk of ILVs are discharged into the extracellular space as exosomes of diverse sizes and compositions.
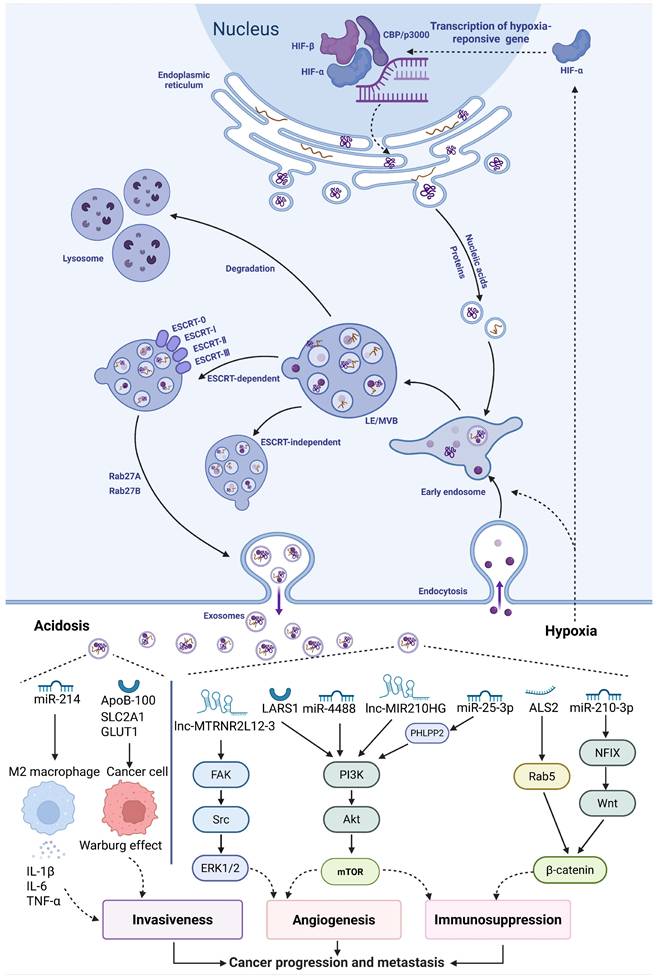
Mechanisms of exosome biogenesis and tumor microenvironment-driven cargo loading. The biogenesis of exosomes is intimately associated with the endosomal system, specifically through the inward invagination of the multivesicular body (MVB) membrane, which gives rise to intraluminal vesicles (ILVs). The fusion of ILV-containing MVBs with the plasma membrane, resulting in the extracellular release of exosomes. The key TME stressors of hypoxia and acidosis remodel the molecular cargo (e.g., proteins, miRNAs, mRNAs) of exosomes, thereby establishing a pro-tumorigenic communication network. (Created with Biorender.com).
Regulation of exosome secretion by the tumor microenvironment
Hypoxia, low pH, and nutrient deprivation are characteristics of the tumor microenvironment (TME), which creates an adaptive environment that encourages tumor growth. Tumor-derived exosomes, as distant messengers of these stress signals, show changes in secretion levels and functional properties, facilitating tumor progression, such as invasion, metastasis, and immune escape (Figure 2). Notably, the concentration of exosomes in the blood of cancer patients is about twice that of healthy individuals (approximately 4×10^15 compared to about 2×10^15 particles), with this increase primarily driven by hypoxic and acidic conditions within the TME[12].
Hypoxia is a major regulator of exosome secretion. Hypoxia is a major regulator of exosome secretion[13]. Early work by King et al. indicated that hypoxic breast cancer cells release more exosomes than cells under normoxic conditions, a process associated with HIF-1α activity[14]. Subsequent studies have demonstrated that hypoxia can regulate the expression and membrane localization of specific small GTPases (e.g., Rab27a, RAB22A) in a HIF-dependent manner, and these Rab proteins are key executors of exosome MVB transport, anchoring, and secretion[15, 16]. Acidosis is a metabolic consequence of hypoxia-driven glycolytic shift. According to the Warburg effect, hypoxia enhances glycolysis in tumor cells, and the accumulation of lactate and bicarbonate produced subsequently leads to the acidification of the tumor microenvironment[17]. Acidosis then serves as an evolutionary selection pressure, causing tumor cells to develop phenotypic plasticity that enables them to adapt to hypoxic and acidic environments. This adaptive change further exacerbates genomic instability in tumor cells and enhances their invasive capabilities[18]. Therefore, acidosis in the TME is closely associated with increased metastatic potential in various malignant tumors and poor prognosis in patients. In melanoma, this acidic environment has been shown to trigger a significant increase in exosome release. The potential mechanism may involve lowering membrane rigidity, which enhances membrane fusion and uptake efficiency, benefiting both exosome secretion and the re-endocytosis of exosomes by recipient tumor cells. Furthermore, Chenhao Jiang and colleagues discovered that tumor cells can take up extracellular lactate through monocarboxylate transporter 1 (MCT1), which facilitates the transport of multivesicular bodies (MVBs) to the plasma membrane, thereby enhancing exosome release. Mechanistically, lactate boosts the activity of the acetyltransferase p300, which catalyzes the acetylation modification of Rab7A.The acetylation of Rab7A inhibits its GTPase activity, thereby promoting the fusion of MVBs with the plasma membrane and exosome release[19].
Regulation of exosomal cargo by the tumor microenvironment
While increasing exosome secretion, the characteristic hypoxic and acidic conditions in the TME drive the increase in exosome heterogeneity and reprogram their cargo composition. For instance, under hypoxic stress, tumor cells selectively package and release exosomes containing the long non-coding RNA SNHG16. Once taken up by recipient cells, the SNHG16 delivered by exosomes acts as a competing endogenous RNA, adsorbing miR-132-3p and relieving its inhibition on the oncogene KIF5A. This signaling axis not only directly promotes cancer cell proliferation and stem cell properties but also induces tumor-associated macrophages to polarize towards the M2 phenotype, thereby jointly creating an immunosuppressive microenvironment[20]. In addition, the stress also regulates metabolic reprogramming through exosomes. Exosomes derived from hypoxic breast cancer cells display a distinct N-glycosylation profile, with significant increases in the expression of patterns such as H4N3F1S2, H3N3F1S0, and H7N4F3S2. These alterations help the cells adapt to hypoxic stress[21]. On the other hand, exosomes from hypoxic prostate cancer cells exhibit triglyceride accumulation, indicating that they may supply energy to recipient cells[22].
Apart from hypoxia, acidosis also notably reprograms the cargo of exosomes. Andreucci et al. found that under in vitro simulated acidic conditions (pH 6.7±0.1), exosomes released by melanoma cells are rich in miR-214, which promotes microenvironmental inflammation and tumor cell migration by activating a pro-inflammatory macrophage phenotype and increasing vascular permeability[23]. Recent research also revealed that exosomes secreted by prostate cancer cells (PC-3AcT) pretreated in acidic medium undergo significant proteomic alterations, with 159 proteins showing differential expression. Among these, the expression level of apolipoprotein B-100 increased approximately 7.6-fold, and it can upregulate glucose transporter proteins SLC2A1/GLUT1 and other glycolysis-related genes, reprogramming normal PC-3 cells into the Warburg phenotype, thereby enhancing their survival and proliferation in the acidic microenvironment[24]. More information on the changes in exosome cargo and function induced by the hypoxic and acidic microenvironment can be found in Table 1. In conclusion, exosomes and the tumor microenvironment establish a dynamic bidirectional interaction network. On one hand, stress signals like hypoxia and acidosis not only quantitatively enhance exosome secretion levels but also qualitatively reprogram their cargo composition. On the other hand, these reprogrammed exosomes deliver specific signaling molecules (such as RNA, proteins, and metabolites), activate several pro-tumor pathways within recipient cells, and remodel the microenvironment into a state more favorable for tumor growth through immune suppression, angiogenesis, and other processes[20, 24]. However, the detailed regulatory mechanisms of selective cargo packaging in exosomes under hypoxia remain unclear. It is critical to study the specific features of exosomes generated from different hypoxic tumor tissues in order to provide novel therapeutic targets for dynamic monitoring of tumor progression.
Exosomes in primary tumor progression
Driving EMT and invasion
The importance of the epithelial-mesenchymal transition (EMT) in the development and metastasis of cancer has gained widespread recognition in recent years[25]. For metastasis to occur, before tumor cells may penetrate the basement barrier and invade the blood stream, they must develop robust migratory and invasion abilities, facilitating their spread to distant locations. This critical step is largely driven by EMT. Mechanistically, EMT causes cancer cells to produce less E-cadherin and more vimentin, leading in cell-cell junction disintegration, loss of apical-basal polarity, and improved migratory and invasion capabilities[26]. This provides the foundation for metastasis and resistance to chemotherapy and radiotherapy. Exosomes facilitate the initiation of EMT and endow tumor cells with invasive properties by modulating various oncogenic signaling pathways.
According to a number of studies, tumor-derived exosomes are essential for controlling the EMT process[27]. For example, exosomes derived from CRC can deliver miR-335-5p, directly targeting and inhibiting the expression of RAS p21 protein activator 1 (RASA1). The p120RasGTPase-activating protein encoded by RASA1 is a key negative regulator of the RAS/MAPK pathway, and its downregulation leads to sustained activation of this pathway, driving EMT and ultimately promoting tumor cell invasion and metastasis[28]. Studies have shown that upregulation of DEAD-box helicase 55 (DDX55) is associated with prognosis in hepatocellular carcinoma (HCC) patients. Exosomes derived from HCC activate the PI3K/Akt/GSK-3β/β-catenin pathway in HCC, driving malignant progression of the disease. Mechanistically, DDX55 interacts with BRD4, together occupying the promoter region of the PIK3CA gene, thereby activating the PI3K/Akt signaling pathway. The activated PI3K/Akt signaling pathway inactivates GSK-3β via phosphorylation, thereby inhibiting GSK-3β-mediated phosphorylation and degradation of β-catenin. This series of events leads to increased stability of β-catenin in both the cytoplasm and nucleus, subsequently inducing transcriptional reprogramming of its downstream target genes, ultimately promoting cell cycle progression and EMT[29]. Exosomes derived from non-tumor cells also trigger the initiation of EMT. For instance, cancer-associated fibroblasts (CAFs) deliver the lncRNA LINC00355 to colorectal cancer cells through exosomes. LINC00355 serves as a molecular sponge for miR-34b-5p, alleviating its inhibition of the CRKL gene and leading to upregulation of CRKL protein expression. Importantly, the CRKL gene, a critical signaling molecule, regulates the Ras/MAPK signaling pathway through its SH2 and SH3 domains, thereby collaboratively enhancing cancer cell proliferation and EMT progression[30]. Tumor-associated macrophages with a pro-invasive phenotype promote tumor progression by secreting exosomes. In both in vivo and in vitro experiments, exosomes carrying high levels of miR-95 act as tumor initiators by activating the target gene JunB in prostate cancer cells, which promotes cancer proliferation, invasion, and EMT[31].
However, the role of exosomes in tumor progression is not unidirectional. Their functional output shows a complex duality: exosomes can drive invasion, yet under specific conditions, they may exert a tumor-suppressive effect. This stark contrast in function is primarily determined by the following factors acting together:(1) Differences in pathological or physiological inducing factors in the body. (2) The different cell origins determine the functional differences[32, 33]. For instance, the gastric-specific protein GKN1, which is produced and secreted by gastric mucosal epithelial cells, can act as an exosome cargo to exert tumor-suppressive effects. Mechanistically, the BRICHOS domain of GKN1 binds strongly to HRas, preventing GTP-bound HRas from interacting with its downstream effector proteins, b-Raf and c-Raf. This action inhibits the activation of the Ras/Raf/MEK/ERK kinase signaling pathway, which significantly suppresses the expression of several EMT-related proteins[34]. Furthermore, exosomes originating from mesenchymal stem cells deliver miR-200a, suppressing ZEB1 and increasing E-cadherin expression, thus blocking the EMT process[35]. Exosomes derived from bone marrow mesenchymal stem cells can exert tumor-suppressive effects by delivering let-7i. The exosomal let-7i inhibits the transcription factor KDM3A, downregulating the expression of its target gene DCLK1, thereby relieving DCLK1's suppression of FXYD3, ultimately effectively curbing the proliferation, migration, and invasion of lung cancer cells[36]. In summary, the function of exosomes depends on the specific molecular cargo they carry, which is dynamically regulated by the microenvironment and the cell of origin. Understanding how these cargoes determine the functional differences of exosomes is crucial for elucidating their contradictory roles in cancer, and it also provides precise targets for exploiting or modulating exosomal function.
Inducing ECM degradation
The extracellular matrix (ECM) is a multi-molecular network composed of a variety of proteins, including collagen, fibrin, proteoglycans, and elastin, that is essential in tumor occurrence, development, and metastasis. Tumor cells at the primary site must extensively remodel the ECM to establish a structural and bioactive environment conducive to invasion and dissemination[37].
Exosomes facilitate ECM protein degradation by activating or delivering matrix metalloproteinases (MMPs) and encouraging the development of invasive pseudopodia[38]. MMPs are a class of soluble or membrane-bound proteases essential for ECM remodeling, expressed by cancer cells and non-tumor cells (primarily CAFs and endothelial cells). Exosomes produced after co-culturing fibroblasts with thyroid tumor cells activate MMP-2 to promote ECM component degradation[39]. Similarly, TNBC-derived exosomes activate MMP-2 and MMP-9, promoting the formation of invasive pseudopodia to facilitate ECM degradation[40]. Exosomes also transfer MMP-2 to convey the invasive pseudopodia activity in tumor cells[41]. Therefore, a synergistic relationship exists between exosomes and invasive pseudopodia. A recent study has demonstrated for the first time that tethered exosomes play a functional role in tumor migration. Exosomes containing MT1-MMP are anchored to the surface of migrating cells by fibronectin, which enhances local ECM degradation and promotes invasion and migration[42].
Cancer-associated fibroblasts (CAFs) are key components of the tumor stroma, remodeling the ECM by depositing ECM proteins, secreting growth factors, and contracting the ECM. Multiple studies have shown that s cancer-derived exosomes can trigger the differentiation of normal fibroblasts (NFs) and MSCs into CAFs, thereby enhancing invasion and migration[43]. For example, cisplatin-treated latent lung adenocarcinoma (LUAD) cells that release exosomes that carry ITGB6 to fibroblasts. This causes CAF differentiation by activating the TGF-β and KLF10 pathways[44]. Exosomal miR-12961 and miR-92a-3p promote the conversion of NFs to CAFs by targeting the MT1G/AKT and KLF4/CH25H axes[45, 46]. Furthermore, Hepatic stellate cells (HSCs) are converted into CAFs by HCC-derived exosome miRNA-21, which inhibits PTEN and activates the downstream PDK1/AKT pathway[47].
It is noteworthy that exosomes secreted by CAFs can also act on tumor cells, promoting tumor progression[48]. CAF-derived exosomes containing LINC00659 interact with miR342-3p to enhance ANXA2 expression, promoting CRC proliferation, invasion and migration[49]. CAF-derived exosomes containing miR-181b-3pand miR-345-5p promote tumor progression[50, 51]. The transformation and activation of CAFs are associated with tumor progression and poor prognosis of various cancers, and exosomes can serve as a potential target to block the interaction between CAFs and the TME, thereby inhibiting CAF-mediated stromal remodeling. These studies suggest that exosomes regulate the extracellular matrix and stromal cells (especially fibroblasts), creating conditions conducive to further tumor cell metastasis.
Mediating immunosuppression
Tumor cells modify immune cell (including lymphocytes, macrophages, myeloid-derived suppressor cells, and granulocytes) in order to avoid immune system identification and elimination, thereby promoting malignant tumor proliferation. As mediators of tumor-stroma crosstalk, exosome-mediated immune suppression operates through a multi-layered regulatory network.
Macrophages are typically categorized into pro-inflammatory M1 and immune-suppressive M2 types. Clinical and experimental data indicate that M1-polarized macrophages exhibit anti-tumor activity, while M2-polarized macrophages promote tumor growth. Tumor-associated macrophages (TAMs) typically exhibit an M2-like phenotype and are crucial for tumor development and growth[52]. Pancreatic neuroendocrine tumor cells produce exosomes rich in CEACAM5 in a hypoxic microenvironment, inducing M2 polarization of TAMs by activating the MAPK signaling pathway[53]. Exosomes from glioma and pancreatic cancer cells containing circ-001422 and lncRNA FGD5-AS1 have been shown to mediate STAT3 acetylation by interacting with p300, thereby activating the STAT3/NF-κB pathway to induce M2 macrophage polarization[54, 55]. Furthermore, miR-200b is significantly overexpressed in ovarian cancer-derived exosomes. This microRNA downregulates KLF6 expression, thereby inducing M2 polarization, which in turn facilitates the proliferation and invasion of ovarian cancer cells[56].
Exosomes from various sources can regulate the expression of PD-L1, thereby directly or indirectly suppressing T cell function. PD-L1 is present on the surface of various tumor cells and immune cells in the TME, and its binding to PD-1 on T cells inhibits T cell activation, making it a crucial target for immune checkpoint inhibitors (ICIs). CAF-derived exosomes containing circHIF1A and zinc finger protein ZNF250 stabilize PD-L1 expression in HCC cells, thereby inducing immune escape[57, 58]. CRC-derived exosomes activate the PTEN/AKT/NF-κB signaling to enhance TAM-mediated suppression of CD8+ T cell. More specifically, knockdown of exosomal miR-372-5p decreases PTEN expression in macrophages, leading to an increase in pAKT/AKT and p-P65/P65 levels, downregulating PD-L1 expression, and suppressing cancer cell proliferation and progression[59]. Furthermore, nasopharyngeal carcinoma cells directly release exosomes carrying PD-L1 protein, which connect to the PD-1 on CD8+ T cells, directly inhibiting their cytotoxic function[60].
Exosomes promote immune evasion by regulating the number and function of natural killer (NK) cells and regulatory T cells (Tregs). For example, exosomes derived from multiple myeloma cells carry lncRNA NEAT1, which downregulates the pre-leukemia transcription factor PBX1 and inhibits NK cell activity, thereby promoting immune evasion in multiple myeloma[61]. Conversely, exosomes from HCC containing circGSE1 activate and induce Tregs, suppressing effector T cell function and creating a tumor microenvironment conducive to immune escape. Mechanistically, TGFBR1 is the receptor for TGFβ1, and Smad3 is an important substrate for TGF-β, contributing to the expansion of Tregs. circGSE1 functions as a sponge for miR-324-5p, activating TGFBR1 and Smad3[62].
Exosomes facilitate a tumor-friendly microenvironment by inducing the activation and expansion of myeloid-derived suppressor cells (MDSCs), thereby promoting tumor metastasis. MDSCs consist of immature myeloid cells (IMCs) and exhibit potent immunosuppressive activity. Exosomal miR-9 and miR-181a stimulate the growth of early MDSCs via stimulating the JAK/STAT signaling pathway[63]. Glioma-derived exosomal lncRNA Agap2-As1 increases MDSC secretion of TGF-β1 by targeting miR-486-3p, thereby participating in immune cell signaling[64]. Overall, these findings systematically reveal the multi-pathway synergistic roles of exosomes in tumor immune regulation. Refining the exosome-mediated immunosuppressive network will aid in the development of immune therapeutic targets and enhance understanding of mechanisms of treatment resistance.
Changes in exosomal cargo and function induced by the hypoxic and acidic microenvironment.
| TME stimulus | Cancer type | Altered cargo | Mechanism | Effect | Ref |
|---|---|---|---|---|---|
| Hypoxia | Breast cancer | lnc-MTRNR2L12-3 | Src/FAK | Angiogenesis | [126] |
| Hypoxia | Breast cancer | miR-143-3p | RICTOR | Migration, invasion | [127] |
| Hypoxia | Breast cancer | lncRNA H19 | DNMT1/ miR-497 | Drug resistance | [128] |
| Hypoxia | Breast cancer | circSTAT3 | miR-671-5p / NOTCH1 | Drug resistance | [129] |
| Hypoxia | Breast cancer | lncRNA MIR210HG | PI3K/Akt/mTOR | Angiogenesis, invasiveness | [130] |
| Hypoxia | Breast cancer | miR-210-3p | NFIX-Wnt/β-catenin | Migration, invasion | [131] |
| Hypoxia | Pancreatic cancer | LARS1 | HIF-1α/LARS1/mTOR | Angiogenesis | [132] |
| Hypoxia | Gastric cancer | LAMB2 | ROCK1/CAV1/Rab11 | Metastasis | [133] |
| Hypoxia | Colorectal cancer | miR-4299 | HIF-1α/miR-4299/ZBTB4 | Proliferation, metastasis | [134] |
| Hypoxia | Hepatocellular carcinoma | circHIF1A | HuR/PD-L1 | Invasion, immune escape | [57] |
| Hypoxia | Glioma | miR-25-3p | PHLPP2/PI3K-AKT/mTOR | Immune escape | [135] |
| Hypoxia | Glioma | PKM2 | ROS | Drug resistance | [136] |
| Hypoxia | Lung cancer | circPLEKHM1 | PABPC1-eIF4G/OSMR | Immune escape | [137] |
| Hypoxia | Lung cancer | ALS2 | Rab5/β-catenin | Angiogenesis | [138] |
| Hypoxia | Esophageal squamous cell carcinoma | circNRIP1 | TRMT6 | Migration, invasion | [139] |
| Hypoxia | Prostate cancer | miR-500a-3p | miR-500a-3p/FBXW7/HSF1 | Metastasis | [140] |
| Acidosis | Melanoma | miR-214 | ↓VE-cadherin | Migration | [23] |
| Acidosis | Melanoma | miR-155, miR-210 | glycolysis | Metastasis | [141] |
| Acidosis | Melanoma | CFL, GSN, HYOU1 | \ | Metastasis | [142] |
| Acidosis | Melanoma | miR-155-5p | SOCS1/JAK2/STAT3 | Angiogenesis | [143] |
| Acidosis | Melanoma | \ | ↓Cisplatin activation | Drug resistance | [144] |
| Acidosis | Prostate cancer | Carbonic anhydrase IX | \ | Metastasis | [145] |
| Acidosis | Hepatocellular carcinoma | miR-21, miR-10b | HIF-1α, HIF-2α | Proliferation, metastasis | [146] |
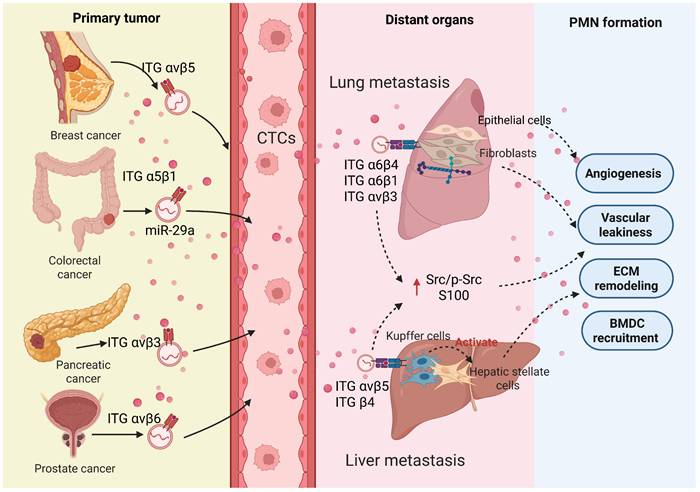
Mechanism of different exosomal integrins-mediated organ-specific metastasis. Primary tumor cells (e.g., from breast, colorectal, pancreatic, and prostate cancers) release exosomes that travel to distinct distant organs (such as liver and lung) via integrin-specific trafficking and target resident cells (e.g., fibroblasts and Kupffer cells). Subsequently, they promote the establishment of the pre-metastatic niche by enhancing vascular permeability, stimulating angiogenesis, remodeling the extracellular matrix (ECM), and recruiting bone marrow-derived cells (BMDCs). (Created with Biorender.com).
Exosome-mediated pre-metastatic niche formation
Deciphering the code of organotropic metastasis
The "seed and soil" hypothesis proposes that there is a selective interaction between tumor cells (the seed) and distant organs (the soil), explaining why tumor cells preferentially metastasize to certain organs (e.g., lungs, liver, brain, and bones)—a phenomenon known as organ-specific metastasis[2, 65]. Growing research indicates that, apart from the intrinsic genetic determinants of tumor cells, organ-specific metastasis is closely linked to integrins present on the surface of exosomes (Figure 3).
Integrins (ITG)are a class of ubiquitously present transmembrane adhesion receptors, serving as key bridges connecting the extracellular matrix to the intracellular cytoskeleton. They precisely regulate cell behavior through bidirectional signaling: the extracellular domain recognizes and binds extracellular matrix proteins, while the intracellular domain interacts with various cytoskeletal proteins and signaling molecules[66]. A study by Ayako Hoshino and colleagues suggests that the uptake of tumor exosomes by resident cells in different organs depends on the specific integrin expression patterns on their surface. For example, exosomal integrins α6β1 and α6β4 promote lung metastasis of breast cancer by activating Src phosphorylation and inducing pro-inflammatory S100 gene expression. Exosomes containing αvβ5, on the other hand, are specifically absorbed by hepatic Kupffer cells, promoting liver metastasis[67]. Similarly, studies have shown that high expression of exosomal ITGA6 and ITGB4 in colorectal cancer is linked to lung organotropism and significantly enhances the proliferation and tubulogenesis of HUVECs[68]. Interestingly, highly invasive cancer cells tend to secrete higher levels of integrins, and these exosomal integrins remodel the invasive phenotype of target cells, creating favorable conditions for metastasis. In epithelial ovarian cancer (EOC), high expression levels of exosome-derived ITGA3 are closely associated with liver metastasis[69]. ITGA3 upregulates the expression of S100A7 in recipient cells, thereby activating the p-ERK/ERK signaling pathway, while knocking down S100A7 reverses this activation effect. The activation of this signaling axis significantly boosts cancer cell migration, invasion, and their colonization in the liver[70]. A study found that ITGA5 and ITGB1 are highly expressed in the serum and ascites of EOC patients and co-localize with asparagine endopeptidase (AEP). When HPMCs take up exosomes carrying the ITGA5/ITGB1/AEP complex, the FAK/Akt/Erk pathway is activated, thereby laying the foundation for peritoneal metastasis[71]. Different types of integrins carried by exosomes that induce organ-specific metastasis and promote PMN formation are depicted in Table 2. These findings collectively highlight the central role of exosomal integrins in mediating organ-specific metastasis. The mechanism may involve two aspects: one is directly enhancing the invasive ability of tumor cells at the primary site, and the second is actively remodeling the microenvironment at the site by selective uptake of exosomes by resident cells of specific distant organs, thereby guiding and promoting metastatic colonization. Future research should focus on elucidating the integrin profiles of metastatic lesions, aiming to provide new targets and decision-making frameworks for precision treatment.
Modulating angiogenesis and vascular permeability
To guarantee the nutrient supply for rapid tumor growth in distant organs, PMN triggers angiogenesis and increases vascular permeability. Normally, endothelial cells are connected by adhesion molecules and tight junctions to limit the passage of proteins and cells, thereby maintaining the vascular barrier. Soluble factors and exosomes secreted by tumor cells impair endothelial cell junctions, thereby increasing vascular permeability and allowing the entry of immune cells, stromal cells, and tumor cells into the PMN[72].
The levels of non-coding RNAs (ncRNAs) in tumor-derived exosomes are crucial for vascular leakage. KLF, a zinc finger transcription factor, regulates endothelial adhesion. Exosomes transport miR-3157-3p and miR-29a to endothelial cells, inhibiting KLF2 and KLF4, which leads to the upregulation of angiogenesis factors (VEGF, MMP2/MMP9) and downregulation of tight junction proteins, thus enhancing vascular permeability in metastatic NSCLC and CRC[73, 74]. Studies have found that the expression of lncRNA FGD5-AS1 in serum exosomes from metastatic thyroid cancer (TC) patients is higher than in adjacent tissues. FGD5-AS1 promotes vascular leakage by regulating the miR-6838-5p/VAV2 pathway[75].
Exosomes promote angiogenesis and remodel the microvascular niche. The heparin-binding VEGF on the exosomal surface has signaling capacity, promoting endothelial cell migration and vascular formation by binding to and activating the tyrosine kinase receptor VEGFR2, without being neutralized by bevacizumab[76]. Interestingly, research found that treatment with exosomes enriched in GTF2H2 derived from HCC decreased VEGFR and MMP2/9 expression in human umbilical vein endothelial cells (HUVECs), inhibiting endothelial viability and tumor migration[77]. Likewise, silencing aminopeptidase N from RCC-derived exosomes reduces endothelial gap formation and angiogenesis in the bone marrow, inhibiting CTC migration to the bone marrow microenvironment[78]. These findings suggest that the mechanisms by which exosomes mediate angiogenesis could serve as potential molecular targets for tumor inhibition. Chronic inflammation is a key driver of tumor progression and metastasis. Exosomes facilitate the release of pro-inflammatory factors by resident cells in distant organs, enhancing vascular permeability. CRC releases exosomes enriched with ITGBL1 into circulation, activating resident fibroblasts in lung and liver metastatic tumors, promoting the secretion of pro-inflammatory cytokines such as IL-6 and IL-8, and inducing the formation of an inflammatory microenvironment[79].
Integrin-mediated organ-specific metastasis.
| Exosomal integrin | PMNSite | Target | Primary Tumor | Mechanism | Ref. |
|---|---|---|---|---|---|
| Integrin α6β4 | Lung | HUVECs | Colorectal cancer | Facilitates proliferation and tubulogenesis | [68] |
| Integrin α6β4 | Lung | Epithelial cells, fibroblasts | Breast cancer | Activates the expression of S100 | [67] |
| Integrin α6β1 | Lung | Fibroblasts | Breast cancer | Activates the expression of S100 | [67] |
| Integrin αvβ3 | Lung | \ | Melanoma | Enhances tumor cell invasion | [147] |
| Integrin α2β1 | Lung | Fibroblasts | Salivary adenoid cystic carcinoma | Induces the expression of p-Smad3 and activate the TGF-β signaling pathway | [86] |
| Integrin αvβ5 | Liver | \ | Colorectal cancer | Activates αvβ5/FAK/NF-κB signaling | [148] |
| Integrin αvβ5 | Liver | Macrophages | Breast cancer | Promote metastatic niche formation | [149] |
| Integrin β4 | Liver | Macrophages | Breast cancer | Enhances tumor cell invasion | [149] |
| Integrin α5 | Bone | Osteoblasts | Breast cancer | Facilitates RUNX2 high-expressing breast cancer cell colonization in bone | [150] |
| Integrin αvβ6 | Bone | \ | Prostate cancer | Initiate the osteolysis | [151] |
| Integrin β3 | Brain | Endothelial cells | Breast cancer, melanoma | Facilitates metastatic niche formation | [67] |
| Integrin β4 | \ | Prostate cancer cells | Prostate cancer | Enhances cell adhesion, migration, and invasion | [152] |
| Integrin αvβ6 | \ | TGFβ | Prostate cancer | Induces migration and invasion | [153] |
| Integrin αvβ3 | \ | Lung | Non-small-cell lung cancer | Induces polarization of M2-like macrophages | [154] |
| Integrin α5β1 | \ | Peritoneal mesothelial cells | Colorectal cancer, Epithelial ovarian cancer | Enhances cell adhesion and migration | [71, 155] |
ECM remodeling in distant organs
During PMN formation, tumors deposit new extracellular matrix components through soluble factors, exosomes, and direct cell-to-cell interactions, which alter the matrix composition[80]. Multiple ECM molecules, such as fibronectin, osteopontin, and multifunctional proteoglycans, specifically deposit in the PMN, providing substrates for the anchoring of CTCs. Meanwhile, the ECM in the PMN is rich in MMPs and LOX family proteins, which, by catalyzing collagen crosslinking, not only enhance matrix stiffness but also promote the homing of bone marrow-derived cells and CTCs to this site[81, 82].
In the PMN of the liver, fibronectin deposition mediated by activated stromal fibroblasts can be observed[81]. As the primary stromal cells in the liver, activated hepatic stellate cells (HSCs) can transform into myofibroblasts and play a key role in cancer progression by secreting cytokines and ECM components. Exosomes participate in the activation of HSCs through various mechanisms. For example, exosomes derived from pancreatic ductal adenocarcinoma (PDAC) deliver the CD44v6/C1QBP complex to the HSC membrane, triggering the phosphorylation of IGF-1signaling molecules and subsequently activating HSCs[83]. Similarly, exosomal miR-188-3p derived from colorectal cancer (CRC) cells can activate HSCs by targeting PHLPP2[84]. Exosomes derived from PDAC can be taken up by Kupffer cells, stimulating the secretion of TGF-β, which in turn activates HSCs, induces significant deposition of fibronectin, and promotes the recruitment of macrophages and neutrophils to the PMN[85]. Additionally, during the early metastatic stage of salivary duct carcinoma, the lungs are the most common site of metastasis. During this process, exosomes from CAFs are taken up by lung fibroblasts via integrin α2β1, thereby activating the TGF-β signaling pathway within the cells and inducing the overexpression of POSTN[86].
Cytokines resident in or newly secreted by the ECM are key drivers in recruiting monocytes and macrophages to the microenvironment. Primary tumors release exosomes rich in integrin β-1-like protein into the circulatory system. These exosomes can directly bind toTNFAIP3, activating the NF-κB signaling pathway, which transforms lung fibroblasts and HSCs into an activated phenotype. Activated cells then secrete pro-inflammatory cytokines such as IL-6 and IL-8, inducing the formation of pro-metastatic ECM[79]. Monocyte chemoattractant protein-1 (MCP-1) and stromal cell-derived factor-1 (SDF-1) play particularly crucial roles in mediating the specific recruitment of monocytes and macrophage polarization. For example, exosomes carrying CXCR4 can bind to SDF-1α expressed on the surface of lymphatic endothelial cells (LECs), thereby enhancing the secretion of MMP-9, MMP-2, and vascular VEGF-C by LECs. These factors collectively promote LEC proliferation and lymph angiogenesis, thereby creating favorable conditions for the colonization of liver cancer cells in lymph nodes[87]. Similarly, exosomal miR-122-5p derived from breast cancer cells can directly target mitogen-activated protein kinase phosphatase-2 (MKP-2) in lung fibroblasts, upregulating the secretion of MCP-1 and SDF-1, thereby driving the lung metastasis of breast cancer[88]. A detailed analysis of the interactions between exosomes and the molecular components of the ECM is crucial for elucidating the mechanisms of ECM remodeling mediated by exosomes and for developing targeted intervention strategies based on these insights.
Recruitment and education of bone marrow-derived cells in immunosuppression
Once exosomes from circulation arrive at distant organs, they recruit and educate bone marrow-derived cells (BMDCs), converting them into immune-suppressive cells that aid tumor progression, thus creating a safe microenvironment for tumor cells. These cells contribute to tumor cell evasion of immune surveillance, thereby establishing an immune-suppressive microenvironment, a key feature in the formation of the PMN[89].
Exosomes trigger the secretion of immune-suppressive cytokines, modulating the local microenvironment at potential metastatic sites. In the gastric cancer peritoneal metastasis model, exosome-induced immune-suppressive PMN is predominantly driven by macrophages. Mechanistically, gastric cancer cells enhance exosome secretion via the METTL3-m⁶A axis. Exosomes transfer miR-17-92 to peritoneal macrophages, inhibiting SRC kinase signaling inhibitor 1, activating SRC proto-oncogene signaling, elevating IL-10 and TNF levels, and reducing IL-1 and IL-6 levels, thereby creating a pro-metastatic immune microenvironment in the peritoneum[90]. Additionally, colorectal cancer-derived exosome HSP90B1 can polarize M1 macrophages to the M2 phenotype and reduce CD8+ T cell activity, promoting liver metastasis[91].
Tumor-derived exosomes are essential for attracting MDSCs. Exosomes from PDAC travel through the bloodstream to the pre-metastatic liver microenvironment, delivering tRF-GluCTC-0005 to hepatic stellate cells, activating the WDR1/YAP signaling axis, recruiting MDSCs, and establishing immune suppression, which then promotes liver metastasis[92]. In addition, during the early stages of tumor development, the migration of bone marrow-derived neutrophils contributes to the immune suppression within the host organ. Primary tumors secrete exosomes that express Lin28B, which promote neutrophil recruitment and polarization to the N2 phenotype, thus creating an immune-suppressive microenvironment characterized by upregulated PD-L2 expression and cytokine dysregulation[93].
Clinical implications and translational applications
Exosome-mediated drug resistance
Drug resistance poses a critical challenge to cancer treatment. Over time, tumor cells develop resistance through genetic and phenotypic alterations within the cells, or as a result of interactions with surrounding cells[94]. Recent studies have found that exosomes mediate the interaction between cancer cells and stromal cells in the tumor microenvironment, thus increasing the complexity of resistance mechanisms and promoting tumor progression[95].
Exosomes deliver bioactive molecules that transfer drug resistance to sensitive cells, facilitating the spread of the resistant phenotype in tumor populations[96]. Paclitaxel, a commonly used first-line chemotherapy agent for breast cancer, is one such example. Paclitaxel-resistant breast cancer cells and their exosomes express higher levels of miR-99b-3p compared to sensitive cells and their exosomes. Further studies confirmed that miR-99b-3p promotes AKT/mTOR phosphorylation by targeting the protein phosphatase 2 catalytic subunit α, thereby conferring paclitaxel resistance to sensitive cells[97]. Similarly, exosomes from breast cancer cells resistant to the mTOR inhibitor rapamycin were shown to significantly activate MAP kinase and AP-1 signaling in sensitive cells, contributing to drug resistance. Notably, DNA methyltransferase 3αmay play a role in suppressing rapamycin resistance[98]. Additionally, Qin et al. demonstrated how pancreatic cancer cells use exosome miR-31-5p to regulate the Hippo/SPARC pathway in pancreatic stellate cells, promoting chemotherapy resistance in pancreatic cancer cells[99].
Exosome-mediated chelation and enhanced drug efflux in tumor cells can significantly reduce or even eliminate the efficacy of chemotherapy drugs by directly encapsulating and expelling them[95]. For example, in ovarian cancer, the cisplatin content in exosomes released by resistant tumor cells is notably higher than in sensitive cells[100]. Additionally, Liu et al.'s review provides a comprehensive summary, highlighting that many chemotherapy drugs (e.g., enzalutamide, cisplatin, 5-Fu, paclitaxel) can be actively transported out of cells through this mechanism, thereby lowering intracellular drug concentrations in tumor cells[96]. In conclusion, these research findings show exosomes' potential as a target for overcoming medication resistance. Understanding exosome-mediated resistance mechanisms will aid in the development of more effective cancer treatments.
Exosomes as potential biomarkers
Current cancer detection methods, such as imaging, pathological diagnosis, and tumor markers, often lack sufficient sensitivity for early-stage lesion detection. Exosomes, with their stable lipid bilayer and low immunogenicity, play distinct roles throughout tumor metastasis. As a result, tumor-specific proteins and RNA from exosomes have the potential to serve as biomarkers for clinical diagnosis, prognosis, and metastasis risk assessment[101]. For instance, Nakamura et al. conducted a whole-genome analysis of blood specimens and demonstrated that specific exosomal miRNAs could be used as biomarkers, improving the diagnostic accuracy of early-stage pancreatic ductal adenocarcinoma[102]. More information on exosomes as biomarkers for diagnosis and prognosis are depicted in Table 3.
It is noteworthy that during the cascade of tumor metastasis, the dynamic evolution of exosomal cargo profiles can be used to infer the real-time progression stage of the tumor. For example, in the early stages of tumorigenesis and local progression, exosome-mediated miR-720 targets and inhibits the expression of StarD13, promoting the proliferation and inhibiting apoptosis of hepatocellular carcinoma cells. Detection of exosomal miR-720 in serum can enable early diagnosis of small hepatocellular carcinoma (< 2cm), showing superior performance compared to AFP or DCP[103]. As the tumor progresses to the invasive and metastatic stages, there is a significant difference in the exosomal cargo profiles between metastatic and non-metastatic patients. has-let-7f-5p is expressed significantly higher in the serum exosomes of pancreatic cancer patients with distant metastasis compared to non-metastatic patients, and can be used to enhance the early detection and risk stratification of pancreatic cancer metastasis[104]. In contrast to patients without metastatic disease, plasma-derived EV miR-6084 levels are significantly reduced in colorectal cancer patients with liver metastasis[105]. Exosome-mediated drug resistance can serve as a dynamic indicator for monitoring treatment response and adjusting strategies in a timely manner. For example, exosomal miR-9-5p derived from CAFs targets CREBRF to activate the MAPK pathway, mediating cisplatin resistance in osteosarcoma and being associated with poor prognosis[106]. By establishing a model linking the multi-omics exosomal profile (including integrin membrane proteome, RNA profile, and proteome) with clinical staging, it is expected that future cancer staging will surpass traditional imaging and pathology-based methods, enabling dynamic, real-time, and non-invasive tumor staging.
Engineered exosomes as drug delivery systems
Unlike synthetic nanoparticles, exosomes are natural nanocarriers with inherent targeting capabilities, equipped with various surface proteins that facilitate participation in cell communication. Additionally, exosomes possess biocompatibility, biodegradability, and the ability to cross multiple biological barriers, providing them with a distinct advantage in drug delivery[107].
Exosome-based therapies, particularly those utilizing tumor suppressor miRNAs, hold significant potential for cancer treatment. For instance, exosomes loaded with miR-29a-3p effectively reduce collagen I levels to reshape the ECM of the PMN and inhibit lung cancer metastasis[108]. Similarly, animal studies have demonstrated that exosome-miR-338-3p inhibits the proliferation of gastric cancer cell lines (MKN45 and HGC27) and their adhesion to mesothelial cells, indicating its therapeutic potential in preventing the peritoneal spread of advanced gastric cancer[109]. However, it is important to note that due to the complexity of the in vivo tumor microenvironment, translating in vitro efficacy to significant in vivo outcomes remains a major challenge, potentially impacting the therapeutic effectiveness of miRNA-loaded exosomes.
Exosomes are capable of encapsulating a variety of drugs, enhancing their therapeutic efficacy and improving cancer treatment outcomes. Zhou et al. developed a liposome/macrophage-derived exosome mixed delivery system that simultaneously loads the phototherapeutic agent IR780 and heme, significantly enhancing the phototherapy effect in skin melanoma[110]. Similarly, Zhang's team created a bone marrow mesenchymal stem cell-derived exosome nanomedicine loaded with galectin-9 siRNA, DOGEM, and indocyanine green, which enhances the tumor-killing ability of CD8⁺ T cells, reduces the proportion of regulatory T cells (Tregs), and improves the immune-suppressive microenvironment in pancreatic cancer[111]. In the case of refractory KRAS-mutant colon cancer, co-delivery of siRNA, 3-bromopyruvate (3BP), and cetuximab (CTX) successfully suppressed KRAS oncogene expression and induced cancer cell apoptosis[112]. Table 4 depicts a further array of cancer treatment strategies founded on exosome delivery platforms. These studies indicate that therapeutic strategies should be aligned with the cellular origin of exosomes[113]. For instance, tumor cell-derived exosomes, leveraging their innate tropism for homologous cancer cells, are often employed to deliver nucleic acid therapeutics (e.g., siRNA, miRNA) back to tumor sites. In contrast, MSC-derived exosomes, capitalizing on their superior biocompatibility and low immunogenicity, are frequently utilized as carriers for chemotherapeutic drugs or targeted siRNAs. Consequently, when engineering novel exosome-based drug delivery systems, close attention must be paid to their source-specific properties in order to develop potent and personalized cancer treatment strategies.
Harnessing exosomes for cancer vaccines
Cancer vaccines represent an emerging form of immunotherapy that activate the immune system by delivering large quantities of tumor antigens alongside adjuvants, thereby enhancing immune responses and improving the effectiveness of immunotherapy[114]. Due to their superior carrier properties and specific immune activation capabilities, exosomes can function as both adjuvants and antigens to induce potent antitumor immune responses. Currently, exosome-based cancer vaccines can be classified into three types: (1) Whole exosomes derived from tumor cells and dendritic cells (DCs), which directly alleviate immune suppression and enhance immunostimulatory characteristics; (2) Exosome-activated dendritic cell vaccines; and (3) Vaccines composed of exosome membrane-loaded nanomaterial cores[115].
In mouse models and human breast cancer organoids, breast cancer-derived exosomes loaded with human neutrophil elastase and histone (a TLR3 agonist) exhibit antitumor and immune-activating activity. This vaccine significantly enhances antigen cross-presentation by cDC1 and stimulates the generation of tumor-reactive CD8⁺ T cells[116]. Furthermore, induced pluripotent stem cells (iPSCs) are capable of producing a variety of tumor antigens, thereby triggering antitumor responses and inhibiting tumor growth. When iPSC-derived exosomes are incubated with dendritic cells (DCs) and pulsed to form a vaccine, they induce tumor cell killing in various cancers, including melanoma, lung cancer, breast cancer, and colorectal cancer. Notably, in mouse models, this vaccine significantly suppressed melanoma growth and generated long-lasting immune memory against tumor cells, preventing relapse[117].
Nevertheless, tumor-derived exosome vaccines currently do not provide adequate antitumor immune responses[115]. The effectiveness of exosome vaccines relies on the immunosuppressive status of cancer patients, and exosomes themselves have weak immunogenicity and limited immune-suppressive functions[118]. Moreover, clinical trials of exosome-based anticancer vaccines are still in the early stages and require further detailed and comprehensive studies to establish their efficacy.
Exosomes as potential biomarkers for the diagnosis and prognosis in different types of cancers.
| Biomarker | Types of cancers | Sources of samples | Clinical applications | Ref |
|---|---|---|---|---|
| circRNA-100338 | Hepatocellular carcinoma (HCC) | serum | risk indicator of pulmonary metastasis and poor survival | [156] |
| lncRNA CASC9 | Hepatocellular carcinoma (HCC) | serum | early diagnosis | [157] |
| miRNA-720 | Hepatocellular carcinoma (HCC) | serum | diagnoses small HCC and evaluate advance | [103] |
| miR-1275 | Hepatocellular carcinoma (HCC) | serum | early diagnosis and prognosis | [158] |
| RAB22A | Multiple myeloma (MM) | serum | evaluates progression and relapse of MM | [159] |
| tsRNA tRF-3004a | Colorectal cancer (CRC) | serum | associated with metastasis, CEA levels, and nerve/vascular invasion | [160] |
| lncRNA DLEU1 | Cervical cancer (CC) | serum | diagnoses, monitors recurrence and metastasis, and evaluates prognosis | [161] |
| miR-9-5p | Osteosarcoma (OS) | serum | early diagnosis | [106] |
| miR-223-5p | Pancreatic ductal adenocarcinoma (PDAC) | serum | diagnoses PDAC patients with sarcopenia | [162] |
| enolase 1 | Breast cancer (BC) | serum | early diagnosis | [163] |
| syndecan-2 | Breast cancer (BC) | serum | predicts chemotherapy response in obese BC patients. | [164] |
| RAB21 | Follicular thyroid carcinoma (FTC) | serum | early diagnosis | [165] |
| Protein tyrosine phosphatase receptor-type O | Lung adenocarcinoma (LUAD) | saliva | assesses the risk of adverse prognosis for early LUAD | [166] |
| miRNA-1307-5p | Oral squamous cell carcinoma (OSCC) | saliva | predicts disease progression and prognosis | [167] |
| circPRMT5 | Head and neck squamous cell carcinoma (HNSCC) | saliva | early diagnosis | [168] |
Exosome-mediated drug delivery systems
| Sources of exosomes | Types of cancers | Therapeutic payloads | Effects | Ref |
|---|---|---|---|---|
| Cancer cell | Colorectal cancer | miRNA206 | Target T cell immune receptor | [169] |
| Cancer cell | Breast cancer | 5-FU | Induced apoptosis | [170] |
| Cancer cell | Breast cancer | 3,3′-diindolylmethane, doxorubicin | Inhibit cancer stem cells-driven EMT | [171] |
| Cancer cell | Breast cancer | LINC02544 siRNA | Inhibit proliferation and metastasis | [172] |
| Cancer cell | Diffuse large B-cell lymphoma | BTK siRNA | Accelerate T cells activation | [173] |
| Mesenchymal stem cell | Glioblastoma | Rapamycin | Modulate blood-brain barrier penetration and VEGF axis | [174] |
| Mesenchymal stem cell | Pancreatic cancer | Gal9 siRNA, DOGEM, ICG | Chemo-photo-immunotherapy | [111] |
| Mesenchymal stem cell | Colorectal cancer | Anti-miR-146b Antisense oligonucleotide | Suppress Smad signaling and EMT | [175] |
| Adipose-derived stem cell | Pancreatic cancer | PD-L1 siRNA | Bind to CD133-positive pancreatic cancer cells and suppress PD-L1 expression | [176] |
| Adipose-derived stem cell | Colon cancer | PIN1 siRNA | Target sAPRIL and inhibit tumor growth and EMT | [177] |
| Serum | Glioblastoma | TanIIA, glycyrrhizic acid | Induce apoptosis | [178] |
| Serum | Colon cancer | PD-1 | Significant tumor growth retardation and immune responses | [179] |
| Mesothelial cell | Gastric cancer | miR-338-3p | Inhibit the proliferation and adhesion | [109] |
| M1 macrophage | Breast cancer | REV, SR780Fe | Activate photodynamic therapy, ferroptosis | [180] |
| HEK293T cells | Colorectal cancer | KRAS siRNA, 3-BP | Restored chemosensitivity | [112] |
Clinical applications of exosomes. The multifaceted role of exosomes in therapeutic innovation, including mediating tumor drug resistance and their clinical translational potential in biomarker detection, drug delivery, and cancer vaccines. (Created with Biorender.com).
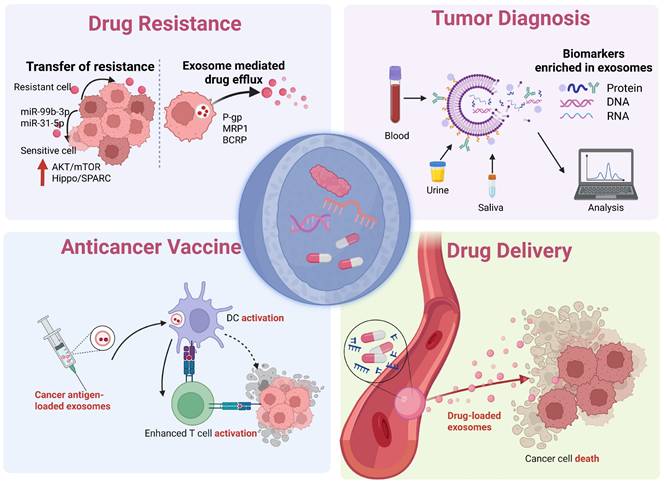
Clinical translation: perspectives and challenges
We have discussed the mechanisms by which exosomes mediate tumor drug resistance and their applications in liquid biopsy for early diagnosis, treatment efficacy monitoring, and metastasis prediction. Furthermore, we have highlighted their potential as drug delivery vehicles and cancer vaccines in the field of immunotherapy (Figure 4). Despite the significant potential of exosome therapies in cancer treatment, several challenges remain in their practical application, necessitating further research and solutions.
The inherent heterogeneity of exosomes is a core bottleneck limiting their translation into clinical applications. This heterogeneity is reflected in multiple dimensions, including their physical size, cargo composition, membrane surface molecular markers, and the source of the producing cells. The heterogeneity of exosomes primarily arises from: (1) biological source heterogeneity, (2) heterogeneity due to physiological and pathological conditions, and (3) subpopulation heterogeneity[119]. This complexity has led to a lack of a widely accepted standardized classification framework in the academic community, which has posed multiple challenges in both research and translation.
Exosomes and their contents, as biomarkers, show significant potential in the early diagnosis, precise staging, and prognostic evaluation of various cancers. However, there is still a lack of an integrated technological platform capable of simultaneously achieving efficient exosome isolation, high-purity enrichment, and detailed molecular characterization[120]. Current mainstream methods (such as differential centrifugation and size exclusion chromatography) often struggle to balance high recovery rates, high purity, and resolution of functional subpopulations, with poor batch-to-batch reproducibility. Although emerging microfluidic technologies facilitate high-throughput analysis, quality control and standardized procedures for their large-scale application have yet to be established[121]. In the realm of engineered drug delivery, the development of exosomes as drug delivery systems faces numerous challenges. First, there is still a lack of efficient targeting ligands to enable the specific delivery of exosomes to tumor tissues while avoiding unnecessary accumulation in normal tissues[122]. Second, the efficient loading and precise evaluation of therapeutic cargo remain significant challenges[123]. The drug-loading efficiency is heavily influenced by the physicochemical properties of the drug and the process conditions, while current evaluation techniques (such as fluorescence labeling) are prone to various interferences, raising concerns about their accuracy[124].
The clinical application of exosomes is still in the early stages of exploration. Rigorous, large-scale, prospective clinical studies based on exosomes remain scarce, with existing evidence mainly derived from small sample retrospective analyses or early exploratory trials. In addition, whether as a drug carrier or a therapeutic tumor vaccine, the efficacy and long-term safety of exosome-based strategies still require systematic evaluation[125]. Therefore, conducting systematic and rigorous clinical studies is crucial for assessing the risks and benefits of exosome-based therapies and, ultimately, for safely and effectively integrating them into the standard cancer treatment paradigm.
Conclusions
In recent years, increasing research has focused on the role of exosomes in cancer metastasis. The intricate bidirectional communication between exosomes and the tumor microenvironment has become a critical challenge in the treatment of cancer metastasis. The changes in the microenvironment dictate the heterogeneity of exosomes. Therefore, further research is essential to explore the regulatory mechanisms underlying the exosome-mediated cell crosstalk.
As discussed herein, exosomes are involved in shaping both local invasion and distant metastasis in the TME, influencing processes such as angiogenesis, immune suppression, and ECM remodeling. However, the changes at local and distant metastatic sites carry distinct implications. For example, at the primary tumor site, exosomes regulate ECM remodeling by degrading ECM components, enhancing tumor cell invasiveness. In contrast, exosomes at distant PMN sites promote ECM formation that supports cell adhesion, enabling circulating tumor cells to survive and proliferate. Whether exosomes regulate changes at different sites through similar signaling pathways requires further investigation. Additionally, the mechanisms behind exosome-mediated organotropism—where tumors metastasize from different primary sites to the same organ—remain to be fully understood.
A thorough investigation of exosomes' roles in tumor chemoresistance is essential for developing strategies to reverse cancer drug resistance. Exosomal drug efflux pumps may be targeted in anti-resistance therapy. Exosomes also show potential in cancer immunotherapy as effective vaccines and targeted drug carriers. Additionally, their application in early cancer detection could lead to higher survival rates. Nevertheless, the clinical application of exosomes still faces several common challenges: (1) The high heterogeneity of exosomes derived from different tissues; (2) The absence of standardized protocols for the isolation and detection of exosomes; (3) The insufficiency of rigorously designed, large-scale clinical trials for their validation.
Abbreviations
TME: tumor microenvironment; TAMs: tumor-associated macrophages; CTCs: cancer circulating tumor cells; EE: early endosomes; LE: late endosomes; MVBs: multivesicular bodies; ILVs: multiple intraluminal vesicles; ESCRT: endosomal sorting complexes required for transport; EMT: epithelial-mesenchymal transition; CRC: colorectal cancer; ECM: extracellular matrix; MMPs: matrix metalloproteinases; NFs: normal fibroblasts; ICIs: immune checkpoint inhibitors; NK: natural killer; PMN: pre-metastatic niches; IMCs: immature myeloid cells; HIFs: Hypoxia-inducible factors; HCC: hepatocellular carcinoma; HNSCC: neck squamous cell carcinoma; CSCs: Cancer stem cells; CAFs: tumor-associated fibroblasts; MDSCs: myeloid-derived suppressor cells; MSCs: Mesenchymal stem cells; BMDCs: bone marrow-derived cells; DCs: dendritic cells; IL4R: nterleukin-4 receptor; TNBC: triple-negative breast cancer; PTX: paclitaxel; DFX: Deferasirox; BBB: blood-brain barrier; IPSCs: induced pluripotent stem cells; CTX: cetuximab; Tregs: regulatory T cells; 3BP: 3-bromopyruvate; DNMT3A: DNA methyltransferase 3α.
Acknowledgements
The work is supported by National Natural Science Foundation of China (82305011).
Author contributions
YZ and LS were involved in the conception of the study. YZ, AL and XZ were involved in writing the article. LS, AZ and YZ critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Competing interests
The authors have declared that no competing interest exists.
References
1. Guan X. Cancer metastases: Challenges and opportunities. Acta Pharmaceutica Sinica B. 2015;5:402-18
2. Bastón E, García-Agulló J, Peinado H. The influence of extracellular vesicles on tumor evolution and resistance to therapy. Physiological Reviews. 2025;105:1173-212
3. Liu Z, Chen J, Ren Y, Liu S, Ba Y, Zuo A. et al. Multi-stage mechanisms of tumor metastasis and therapeutic strategies. Signal Transduction and Targeted Therapy. 2024;9:270
4. El-Tanani M, Rabbani SA, Babiker R, Rangraze I, Kapre S, Palakurthi SS. et al. Unraveling the tumor microenvironment: Insights into cancer metastasis and therapeutic strategies. Cancer Letters. 2024;591:216894
5. Peppicelli S, Calorini L, Bianchini F, Papucci L, Magnelli L, Andreucci E. Acidity and hypoxia of tumor microenvironment, a positive interplay in extracellular vesicle release by tumor cells. Cellular oncology (Dordrecht, Netherlands). 2025;48:27-41
6. Paskeh MDA, Entezari M, Mirzaei S, Zabolian A, Saleki H, Naghdi MJ. et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. JOURNAL OF HEMATOLOGY & ONCOLOGY. 2022 15
7. Huang C, Li JJ, Xie ZC, Hu XJ, Huang Y. Relationship between exosomes and cancer: Formation diagnosis, and treatment. INTERNATIONAL JOURNAL OF BIOLOGICAL SCIENCES. 2025;21:40-62
8. Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J. et al. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduction and Targeted Therapy. 2020;5:145
9. Tang Z, Chen C, Zhou C, Liu Z, Li T, Zhang Y. et al. Insights into tumor-derived exosome inhibition in cancer therapy. European journal of medicinal chemistry. 2025;285:117278
10. Zhou X, Jia Y, Mao C, Liu S. Small extracellular vesicles: Non-negligible vesicles in tumor progression, diagnosis, and therapy. Cancer letters. 2024;580:216481
11. Lee YJ, Shin KJ, Chae YC. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. EXPERIMENTAL AND MOLECULAR MEDICINE. 2024;56:877-89
12. Maqsood Q, Sumrin A, Saleem Y, Wajid A, Mahnoor M. Exosomes in cancer: Diagnostic and therapeutic applications. CLINICAL MEDICINE INSIGHTS-ONCOLOGY. 2024 18
13. Mu YF, Yang MR, Liu JY, Yao Y, Sun HM, Zhuang J. Exosomes in hypoxia: Generation, secretion, and physiological roles in cancer progression. FRONTIERS IN IMMUNOLOGY. 2025 16
14. King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421
15. Guo XY, Song JJ, Liu M, Ou XY, Guo YC. The interplay between the tumor microenvironment and tumor-derived small extracellular vesicles in cancer development and therapeutic response. CANCER BIOLOGY & THERAPY. 2024 25
16. He GP, Peng XQ, Wei SB, Yang S, Li XY, Huang MY. et al. Exosomes in the hypoxic TME: From release, uptake and biofunctions to clinical applications. MOLECULAR CANCER. 2022 21
17. Lee Y-J, Seo CW, Chae S, Lee CY, Kim SS, Shin Y-H. et al. Metabolic reprogramming into a glycolysis phenotype induced by extracellular vesicles derived from prostate cancer cells. Molecular & Cellular Proteomics: MCP. 2025;24:100944
18. Boussadia Z, Lamberti J, Mattei F, Pizzi E, Puglisi R, Zanetti C. et al. Acidic microenvironment plays a key role in human melanoma progression through a sustained exosome mediated transfer of clinically relevant metastatic molecules. Journal of Experimental & Clinical Cancer Research: CR. 2018;37:245
19. Chenhao J, He X, Chen X, Huang J, Liu Y, Zhang J. et al. Lactate accumulation drives hepatocellular carcinoma metastasis through facilitating tumor-derived exosome biogenesis by Rab7A lactylation. Cancer letters. 2025;627:217636
20. Hou YS, Li XM, Zeng LF, Zhang Y. SNHG16-loaded extracellular vesicles from hypoxic NSCLC cells drive M2 macrophage polarization to enhance cancer aggressiveness. MOLECULAR IMMUNOLOGY. 2025;187:66-79
21. Peng BJ, Bartkowiak K, Song FZ, Nissen P, Schlüter H, Siebels B. Hypoxia-induced adaptations of N-glycomes and proteomes in breast cancer cells and their secreted extracellular vesicles. INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES. 2024 25
22. Schlaepfer IR, Nambiar DK, Ramteke A, Kumar R, Dhar D, Agarwal C. et al. Hypoxia induces triglycerides accumulation in prostate cancer cells and extracellular vesicles supporting growth and invasiveness following reoxygenation. Oncotarget. 2015;6:22836-56
23. Andreucci E, Ruzzolini J, Bianchini F, Versienti G, Biagioni A, Lulli M. et al. miR-214-enriched extracellular vesicles released by acid-adapted melanoma cells promote inflammatory macrophage-dependent tumor trans-endothelial migration. CANCERS. 2022 14
24. Lee YJ, Seo CW, Chae S, Lee CY, Kim SS, Shin YH. et al. Metabolic reprogramming into a glycolysis phenotype induced by extracellular vesicles derived from prostate cancer cells. MOLECULAR & CELLULAR PROTEOMICS. 2025 24
25. Celia-Terrassa T, Kang Y. How important is EMT for cancer metastasis? PLoS BIOLOGY. 2024;22:e3002487
26. Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no, maybe? Current Opinion in Cell Biology. 2016;43:7-13
27. Shao Y, Lu B. The crosstalk between circular RNAs and the tumor microenvironment in cancer metastasis. Cancer Cell International. 2020;20:448
28. Sun X, Lin F, Sun W, Zhu W, Fang D, Luo L. et al. Exosome-transmitted miRNA-335-5p promotes colorectal cancer invasion and metastasis by facilitating EMT via targeting RASA1. Mol Ther Nucleic Acids. 2021;24:164-74
29. Yu B, Zhou S, Long D, Ning Y, Yao H, Zhou E. et al. DDX55 promotes hepatocellular carcinoma progression by interacting with BRD4 and participating in exosome-mediated cell-cell communication. Cancer Sci. 2022;113:3002-17
30. Hu J-H, Tang H-N, Wang Y-H. Cancer-associated fibroblast exosome LINC00355 promotes epithelial-mesenchymal transition and chemoresistance in colorectal cancer through the miR-34b-5p/CRKL axis. Cancer gene therapy. 2024;31:259-72
31. Guan H, Peng R, Fang F, Mao L, Chen Z, Yang S. et al. Tumor-associated macrophages promote prostate cancer progression via exosome-mediated miR-95 transfer. Journal of cellular physiology. 2020;235:9729-42
32. Yassine S, Alaaeddine N. Mesenchymal stem cell exosomes and cancer: Controversies and prospects. Advanced biology. 2022;6:e2101050
33. Lin ZJ, Wu YL, Xu YT, Li GQ, Li ZH, Liu T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. MOLECULAR CANCER. 2022 21
34. Yoon JH, Ashktorab H, Smoot DT, Nam SW, Hur H, Park WS. Uptake and tumor-suppressive pathways of exosome-associated GKN1 protein in gastric epithelial cells. Gastric Cancer. 2020;23:848-62
35. Mirzaei S, Gholami MH, Aghdaei HA, Hashemi M, Parivar K, Karamian A. et al. Exosome-mediated miR-200a delivery into TGF-β-treated AGS cells abolished epithelial-mesenchymal transition with normalization of ZEB1, vimentin and Snail1 expression. Environmental research. 2023;231:116115
36. Liu J, Feng Y, Zeng X, He M, Gong Y, Liu Y. Extracellular vesicles-encapsulated let-7i shed from bone mesenchymal stem cells suppress lung cancer via KDM3A/DCLK1/FXYD3 axis. Journal of Cellular and Molecular Medicine. 2021;25:1911-26
37. Yuan Z, Li Y, Zhang S, Wang X, Dou H, Yu X. et al. Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments. Molecular Cancer. 2023;22:48
38. Karampoga A, Tzaferi K, Koutsakis C, Kyriakopoulou K, Karamanos NK. Exosomes and the extracellular matrix: A dynamic interplay in cancer progression. The International Journal of Developmental Biology. 2021;66:97-102
39. Bravo-Miana RdC, Soler MF, Ceschin DG, Royo F, Negretti-Borga DM, Azkargorta M. et al. Extracellular vesicles from thyroid cancer harbor a functional machinery involved in extracellular matrix remodeling. European Journal of Cell Biology. 2022;101:151254
40. Pachane BC, Nunes ACC, Cataldi TR, Micocci KC, Moreira BC, Labate CA. et al. Small extracellular vesicles from hypoxic triple-negative breast cancer cells induce oxygen-dependent cell invasion. International Journal of Molecular Sciences. 2022;23:12646
41. Whitehead CA, Fang H, Su H, Morokoff AP, Kaye AH, Hanssen E. et al. Small extracellular vesicles promote invadopodia activity in glioblastoma cells in a therapy-dependent manner. Cellular Oncology. 2023;46:909-31
42. Palmulli R, Jackson HK, Edgar JR. Tethered exosomes containing the matrix metalloproteinase MT1-MMP contribute to extracellular matrix degradation. JOURNAL OF EXTRACELLULAR VESICLES. 2025 14
43. Liang C, Wang M, Huang Y, Yam JWP, Zhang X, Zhang X. Recent Advances of Small Extracellular Vesicles for the Regulation and Function of Cancer-Associated Fibroblasts. International journal of molecular sciences. 2024 25
44. Feng X, Liu X, Xiang J, Xu J, Yin N, Wang L. et al. Exosomal ITGB6 from dormant lung adenocarcinoma cells activates cancer-associated fibroblasts by KLF10 positive feedback loop and the TGF-β pathway. Translational Lung Cancer Research. 2023;12:2520-37
45. Zhou M, Wang S, Liu D, Zhou J. LINC01915 Facilitates the Conversion of Normal Fibroblasts into Cancer-Associated Fibroblasts Induced by Colorectal Cancer-Derived Extracellular Vesicles through the miR-92a-3p/KLF4/CH25H Axis. ACS Biomaterials Science & Engineering. 2021;7:5255-68
46. Zhou Z, Qu C, Zhou P, Zhou Q, Li D, Wu X. et al. Extracellular vesicles activated cancer-associated fibroblasts promote lung cancer metastasis through mitophagy and mtDNA transfer. Journal of Experimental & Clinical Cancer Research. 2024;43:158
47. Zhou Y, Ren H, Dai B, Li J, Shang L, Huang J. et al. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. Journal of Experimental & Clinical Cancer Research. 2018;37:324
48. Vokurka M, Lacina L, Brabek J, Kolar M, Ng YZ, Smetana K. Cancer-associated fibroblasts influence the biological properties of malignant tumours via paracrine secretion and exosome production. INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES. 2022 23
49. Zhou L, Li J, Tang Y, Yang M. Exosomal LncRNA LINC00659 transferred from cancer-associated fibroblasts promotes colorectal cancer cell progression via miR-342-3p/ANXA2 axis. Journal of Translational Medicine. 2021;19:8
50. Shi W, Liu Y, Qiu X, Yang L, Lin G. Cancer-associated fibroblasts-derived exosome-mediated transfer of miR-345-5p promotes the progression of colorectal cancer by targeting CDKN1A. Carcinogenesis. 2023;44:317-27
51. Jiang Y, Qiu Q, Jing X, Song Z, Zhang Y, Wang C. et al. Cancer-associated fibroblast-derived exosome miR-181b-3p promotes the occurrence and development of colorectal cancer by regulating SNX2 expression. Biochemical and Biophysical Research Communications. 2023;641:177-85
52. Zhou M, He X, Mei C, Ou C. Exosome derived from tumor-associated macrophages: biogenesis, functions, and therapeutic implications in human cancers. BIOMARKER RESEARCH. 2023 11
53. Ye M, Lu F, Gu D, Xue B, Xu L, Hu C. et al. Hypoxia exosome derived CEACAM5 promotes tumor-associated macrophages M2 polarization to accelerate pancreatic neuroendocrine tumors metastasis via MMP9. FASEB JOURNAL. 2024 38
54. Cao W, Zeng Z, Sun J, Chen Y, Kuang F, Luo S. et al. Exosome-derived circ-001422 promotes tumor-associated macrophage M2 polarization to accelerate the progression of glioma. COMMUNICATIONS BIOLOGY. 2024 7
55. He Z, Wang J, Zhu C, Xu J, Chen P, Jiang X. et al. Exosome-derived FGD5-AS1 promotes tumor-associated macrophage M2 polarization-mediated pancreatic cancer cell proliferation and metastasis. CANCER LETTERS. 2022 548
56. Xiong J, He X, Xu Y, Zhang W, Fu F. MiR-200b is upregulated in plasma-derived exosomes and functions as an oncogene by promoting macrophage M2 polarization in ovarian cancer. Journal of Ovarian Research. 2021;14:74
57. Shang H, Lu L, Fan M, Lu YX, Shi XL, Lu HW. Exosomal circHIF1A derived from hypoxic-induced carcinoma-associated fibroblasts promotes hepatocellular carcinoma cell malignant phenotypes and immune escape. INTERNATIONAL IMMUNOPHARMACOLOGY. 2024 138
58. Feng HZ, Liu JM, Jia HX, Bu XQ, Yang WH, Su P. Cancer-associated fibroblasts-derived exosomal ZNF250 promotes the proliferation, migration, invasion, and immune escape of hepatocellular carcinoma cells by transcriptionally activating PD-L1. JOURNAL OF BIOCHEMICAL AND MOLECULAR TOXICOLOGY. 2024 38
59. Wu YL, Xiao YH, Ding YX, Ran RR, Wei K, Tao S. et al. Colorectal cancer cell-derived exosomal miRNA-372-5p induces immune escape from colorectal cancer via PTEN/AKT/NF-κB/PD-L1 pathway. INTERNATIONAL IMMUNOPHARMACOLOGY. 2024 143
60. Yang J, Chen J, Liang H, Yu Y. Nasopharyngeal cancer cell-derived exosomal PD-L1 inhibits CD8+ T-cell activity and promotes immune escape. Cancer Science. 2022;113:3044-54
61. Wang QM, Lian GY, Sheng SM, Xu J, Ye LL, Min C. et al. Exosomal lncRNA NEAT1 Inhibits NK-Cell Activity to Promote Multiple Myeloma Cell Immune Escape via an EZH2/PBX1 Axis. MOLECULAR CANCER RESEARCH. 2024;22:125-36
62. Huang MY, Huang X, Huang N. Exosomal circGSE1 promotes immune escape of hepatocellular carcinoma by inducing the expansion of regulatory T cells. CANCER SCIENCE. 2022;113:1968-83
63. Jiang M, Zhang W, Zhang R, Liu P, Ye Y, Yu W. et al. Cancer exosome-derived miR-9 and miR-181a promote the development of early-stage MDSCs via interfering with SOCS3 and PIAS3 respectively in breast cancer. Oncogene. 2020;39:4681-94
64. Tian Y, Gao X, Yang X, Chen S, Ren Y. Glioma-derived exosome lncrna Agap2-As1 promotes glioma proliferation and metastasis by mediating tgf-β1 secretion of myeloid-derived suppressor cells. HELIYON. 2024 10
65. Chen H, Liu L, Xing G, Zhang D, A N, Huang J. et al. Exosome tropism and various pathways in lung cancer metastasis. Frontiers in Immunology. 2025;16:1517495
66. Pang X, He X, Qiu Z, Zhang H, Xie R, Liu Z. et al. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduction and Targeted Therapy. 2023;8:1
67. Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M. et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329-35
68. Cong F, Huang J, Wu C, Zhong H, Qiu G, Luo T. et al. Integrin α6 and integrin β4 in exosomes promote lung metastasis of colorectal cancer. Journal of Cancer Research and Therapeutics. 2024;20:2082
69. Zhu K, Cheng T, Wang Y. The role of ITGA3 expression in predicting liver metastasis in patients with epithelial ovarian cancer. BMC Medical Genomics. 2025;18:201
70. Yin Z, Ma J, Adu-Amankwaah J, Xie G, Wang Y, Tai W. et al. Exosomal integrin alpha 3 promotes epithelial ovarian cancer cell migration via the S100A7/p-ERK signaling pathway: Exosomal ITGA3 drives EOC migration via S100A7/p-ERK pathway. Acta Biochimica et Biophysica Sinica. 2025;57:1006
71. Li X, Tang M, Zhu Q, Wang X, Lin Y, Wang X. The exosomal integrin α5β1/AEP complex derived from epithelial ovarian cancer cells promotes peritoneal metastasis through regulating mesothelial cell proliferation and migration. Cellular oncology (Dordrecht, Netherlands). 2020;43:263-77
72. Chen Z, Wang Q, Liu J, Wang W, Yuan W, Liu Y. et al. Effects of extracellular vesicle-derived noncoding RNAs on pre-metastatic niche and tumor progression. GENES & DISEASES. 2024;11:176-88
73. Ma Z, Wei K, Yang F, Guo Z, Pan C, He Y. et al. Tumor-derived exosomal miR-3157-3p promotes angiogenesis, vascular permeability and metastasis by targeting TIMP/KLF2 in non-small cell lung cancer. Cell Death & Disease. 2021;12:840
74. Liu K, Dou R, Yang C, Di Z, Shi D, Zhang C. et al. Exosome-transmitted miR-29a induces colorectal cancer metastasis by destroying the vascular endothelial barrier. Carcinogenesis. 2023;44:356-67
75. Liu B, Chen J, Shang F, Lian M, Shen X, Fang J. Tumor-derived exosome FGD5-AS1 promotes angiogenesis, vascular permeability, and metastasis in thyroid cancer by targeting the miR-6838-5p/VAV2 axis. JOURNAL OF ONCOLOGY. 2022. 2022
76. Ko SY, Lee W, Kenny HA, Dang LH, Ellis LM, Jonasch E. et al. Cancer-derived small extracellular vesicles promote angiogenesis by heparin-bound, bevacizumab-insensitive VEGF, independent of vesicle uptake. COMMUNICATIONS BIOLOGY. 2019 2
77. Zhenkun L, Li Y, Ouyang Q, Li X, Huang J. Exosome-derived GTF2H2 from Huh7 cells can inhibit endothelial cell viability, migration, tube formation, and permeability. TISSUE & CELL. 2022 79
78. Masashi T, Sakamoto H, Shibasaki N, Fukui T, Magaribuchi T, Sumiyoshi T. et al. Extracellular vesicles secreted from bone metastatic renal cell carcinoma promote angiogenesis and endothelial gap formation in bone marrow in a time-dependent manner in a preclinical mouse model. FRONTIERS IN ONCOLOGY. 2023 13
79. Ji Q, Zhou L, Sui H, Yang L, Wu X, Song Q. et al. Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation. Nature Communications. 2020;11:1211
80. Wieland E, Rodriguez-Vita J, Liebler SS, Mogler C, Moll I, Herberich SE. et al. Endothelial Notch1 activity facilitates metastasis. Cancer Cell. 2017;31:355-67
81. Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G. et al. Pre-metastatic niches: organ-specific homes for metastases. Nature Reviews Cancer. 2017;17:302-17
82. Wu S, Xing X, Wang Y, Zhang X, Li M, Wang M. et al. The pathological significance of LOXL2 in pre-metastatic niche formation of HCC and its related molecular mechanism. Eur J Cancer. 2021;147:63-73
83. Xie Z, Gao Y, Ho C, Li L, Jin C, Wang X. et al. Exosome-delivered CD44v6/C1QBP complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment. Gut. 2022;71:568-79
84. Li T, Li TY, Liang YH, Yuan YL, Liu Y, Yao Y. et al. Colorectal cancer cells-derived exosomal miR-188-3p promotes liver metastasis by creating a pre-metastatic niche via activation of hepatic stellate cells. JOURNAL OF TRANSLATIONAL MEDICINE. 2025 23
85. Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature Cell Biology. 2015;17:816-26
86. Kong J, Tian H, Zhang F, Zhang Z, Li J, Liu X. et al. Extracellular vesicles of carcinoma-associated fibroblasts creates a pre-metastatic niche in the lung through activating fibroblasts. Molecular Cancer. 2019;18:175
87. Li M, Lu Y, Xu Y, Wang J, Zhang C, Du Y. et al. Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene. 2018;676:101-9
88. Lv Y, Li Y, Zhou J, Liu X, Wang DD, Wang DM. et al. Exosomal miR-122-5p for regulation of secretory functions of fibroblasts and promotion of breast cancer metastasis by targeting MKP-2: An experimental study. CANCER BIOLOGY & THERAPY. 2025 26
89. Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: Communication from a distance. Developmental Cell. 2019;49:347-60
90. Li S, Zhou J, Wang S, Yang Q, Nie S, Ji C. et al. N(6)-methyladenosine-regulated exosome biogenesis orchestrates an immunosuppressive pre-metastatic niche in gastric cancer peritoneal metastasis. Cancer Commun (Lond). 2025;45:941-65
91. Li S, Fu X, Ning D, Liu Q, Zhao J, Cheng Q. et al. Colon cancer exosome-associated HSP90B1 initiates pre-metastatic niche formation in the liver by polarizing M1 macrophage into M2 phenotype. Biology Direct. 2025;20:52
92. Chen W, Peng W, Wang R, Bai S, Cao M, Xiong S. et al. Exosome-derived tRNA fragments tRF-GluCTC-0005 promotes pancreatic cancer liver metastasis by activating hepatic stellate cells. Cell Death & Disease. 2024;15:102
93. Qi M, Xia Y, Wu Y, Zhang Z, Wang X, Lu L. et al. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nature Communications. 2022;13:897
94. Malla R, Bhamidipati P, Samudrala AS, Nuthalapati Y, Padmaraju V, Malhotra A. et al. Exosome-mediated cellular communication in the tumor microenvironment imparts drug resistance in breast cancer. Cancers. 2025 17
95. Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S. et al. Extracellular vesicles-mediated intercellular communication: Roles in the tumor microenvironment and anti-cancer drug resistance. Molecular Cancer. 2019;18:55
96. Liu G, Liu J, Li S, Zhang Y, Ren H. Exosome-mediated chemoresistance in cancers: Mechanisms, therapeutic implications, and future directions. Biomolecules. 2025;15:685
97. Mao HL, Ye RY, Tang GH, Tao S, Wei K, Wu YL. et al. Drug-resistant exosome miR-99b-3p induces macrophage polarization and confers chemoresistance on sensitive cells by targeting PPP2CA. INTERNATIONAL IMMUNOPHARMACOLOGY. 2024 142
98. Shchegolev YY, Sorokin DV, Scherbakov AM, Andreeva OE, Salnikova DI, Mikhaevich EI. et al. Exosomes are involved in the intercellular transfer of rapamycin resistance in the breast cancer cells. BIOIMPACTS. 2023;13:313-21
99. Qin C, Zhao BB, Wang YY, Li ZR, Li TY, Zhao YT. et al. Extracellular vesicles miR-31-5p promotes pancreatic cancer chemoresistance via regulating LATS2-hippo pathway and promoting SPARC secretion from pancreatic stellate cells. JOURNAL OF EXTRACELLULAR VESICLES. 2024 13
100. Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W. et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Molecular Cancer Therapeutics. 2005;4:1595-604
101. Huang Y, Kanada M, Ye J, Deng Y, He Q, Lei Z. et al. Exosome-mediated remodeling of the tumor microenvironment: From local to distant intercellular communication. Cancer Letters. 2022;543:215796
102. Nakamura K, Zhu ZX, Roy S, Jun E, Han HY, Munoz RM. et al. An exosome-based transcriptomic signature for noninvasive, early detection of patients with pancreatic ductal adenocarcinoma: A multicenter cohort study. GASTROENTEROLOGY. 2022;163:1252 -+
103. Kim JM, Kim HS, Kim JS, Han JW, Lee SK, Nam H. et al. Exosomal miRNA-720 as a potential diagnostic and prognostic biomarker for hepatocellular carcinoma. The Korean journal of internal medicine. 2025;40:939-51
104. Ren S, Song L-N, Zhao R, Tian Y, Wang Z-Q. Serum exosomal hsa-let-7f-5p: A potential diagnostic biomarker for metastatic pancreatic cancer detection. World journal of gastroenterology. 2025;31:109500
105. Zhang Y, Yang X, Zhang S, Huang Q, Liu S, Qiu L. et al. MicroRNA-6084 orchestrates angiogenesis and liver metastasis in colorectal cancer via extracellular vesicles. JCI insight. 2025 10
106. Niu X, Tian W, Li Z, Zhang G, Zhang P. Rab27a(+)CAF exosomal miR-9-5p promotes osteosarcoma progression via CREBRF/MAPK signaling pathway. Cellular signalling. 2025;134:111964
107. Zhang TT, Liu ZM, Wei YY, Lu J, He ZH, Wu ZX. et al. Extracellular vesicles as natural nanocarriers: From in vitro engineering to in situ generation in cancer therapy. CHEMICAL ENGINEERING JOURNAL. 2025 510
108. Yan Y, Du C, Duan X, Yao X, Wan J, Jiang Z. et al. Inhibiting collagen I production and tumor cell colonization in the lung <i>via</i> miR-29a-3p loading of exosome-/liposome-based nanovesicles. Acta Pharmaceutica Sinica B. 2022;12:939-51
109. Yamamoto T, Arita T, Konishi H, Nanishi K, Takabatake K, Shimauchi Y. et al. Evaluation of exosome-based delivery of tumor-suppressive microRNAs for novel therapy in peritoneal dissemination of gastric cancer: Evidence from experimental animal models. ANNALS OF SURGICAL ONCOLOGY. 2025;32:8046-59
110. Zhou H, Zhu CX, Li YC, Zhao FY, Feng QX, Liu SG. et al. Exosome/liposome hybrid nanovesicles for enhanced phototherapy and boosted anti-tumor immunity against melanoma. EUROPEAN JOURNAL OF MEDICINAL CHEMISTRY. 2025 289
111. Zhang RX, Zhang YJ, Hao F, Su ZX, Duan X, Song XR. Exosome-mediated triple drug delivery enhances apoptosis in pancreatic cancer cells. APOPTOSIS. 2025;30:1893-911
112. Xiang YR, Liu Q, Liu K, Chen LX, Chen FJ, Li T. et al. An exosome-based nanoplatform for siRNA delivery combined with starvation therapy promotes tumor cell death through autophagy, overcoming refractory KRAS-mutated tumors and restoring cetuximab chemosensitivity. MATERIALS TODAY BIO. 2025 32
113. Chen Z, Xiong M, Tian J, Song D, Duan S, Zhang L. Encapsulation and assessment of therapeutic cargo in engineered exosomes: A systematic review. Journal of Nanobiotechnology. 2024;22:18
114. Khleif SN, Gupta S. Cancer vaccines as enablers of immunotherapy. Nature Immunology. 2025;26:1877-89
115. Zhao G, Wang YN, Xing SJ, Jiang YL, Ding JT, Cai YT. et al. Exosome-based anticancer vaccines: From bench to bedside. CANCER LETTERS. 2024 595
116. Huang L, Rong Y, Tang X, Yi K, Qi P, Hou J. et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Molecular Cancer. 2022;21:45
117. Wang RH, Zhu TC, Hou BZ, Huang X. An iPSC-derived exosome-pulsed dendritic cell vaccine boosts antitumor immunity in melanoma. MOLECULAR THERAPY. 2023;31:2376-90
118. Sheikhhossein HH, Iommelli F, Di Pietro N, Curia MC, Piattelli A, Palumbo R. et al. Exosome-like systems: From therapies to vaccination for cancer treatment and prevention-exploring the state of the art. VACCINES. 2024 12
119. Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B. et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. Journal of Extracellular Vesicles. 2024;13:e12404
120. Tiwari S, Kumar V, Randhawa S, Verma SK. Preparation and characterization of extracellular vesicles. American Journal of Reproductive Immunology. 2021;85:e13367
121. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: Biogenesis, biologic function and clinical potential. Cell & Bioscience. 2019;9:19
122. Zhang M, Hu S, Liu L, Dang P, Liu Y, Sun Z. et al. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduction and Targeted Therapy. 2023;8:124
123. Ming-Kun C, Zi-Xian C, Mao-Ping C, Hong C, Zhuang-Fei C, Shan-Chao Z. Engineered extracellular vesicles: A new approach for targeted therapy of tumors and overcoming drug resistance. Cancer communications (London, England). 2024;44:205-25
124. Kimiz-Gebologlu I, Oncel SS. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. Journal of Controlled Release. 2022;347:533-43
125. Sadeghi S, Tehrani FR, Tahmasebi S, Shafiee A, Hashemi SM. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology. 2023;31:145-69
126. Zheng L, Mo JF, He XS, Xu Q, Bao Y, Wu JY. lnc-MTRNR2L12-3 derived from hypoxic breast cancer cell exosomes facilitates angiogenesis via the src/FAK signaling pathway. MEDICAL ONCOLOGY. 2025 42
127. Lian HY, Yu M, Li Q, Xiao JY, Tang X, Zhang B. et al. Hypoxic breast cancer cell-derived exosomal miR-143-3p targets RICTOR to regulate M2 macrophage polarization, thereby modulating cancer cell invasiveness. HUMAN CELL. 2025 38
128. Tao S, Wang J, Li F, Shi BX, Ren QH, Zhuang YH. et al. Extracellular vesicles released by hypoxia-induced tumor-associated fibroblasts impart chemoresistance to breast cancer cells via long noncoding RNA H19 delivery. FASEB JOURNAL. 2024 38
129. Yang L, Zhao SY, Liu X, Zhang YC, Zhao SR, Fang X. et al. Hypoxic cancer-associated fibroblast exosomal circSTAT3 drives triple negative breast cancer stemness via miR-671-5p/NOTCH1 signaling. JOURNAL OF TRANSLATIONAL MEDICINE. 2025 23
130. Feng LY, Wang YX, Wang XY, Li BC, Liu YF, Zhang WY. et al. Exosomal lncRNA MIR210HG derived from hypoxic TAMs promotes the metastasis of triple negative breast cancer. INTERNATIONAL IMMUNOPHARMACOLOGY. 2025 165
131. Wang M, Zheng Y, Hao Q, Mao GC, Dai ZJ, Zhai Z. et al. Hypoxic BMSC-derived exosomal miR-210-3p promotes progression of triple-negative breast cancer cells via NFIX-wnt/β-catenin signaling axis. JOURNAL OF TRANSLATIONAL MEDICINE. 2025 23
132. Zhao TT, Cai XF, Chen H, Wang ZH, Bu HQ, Lin SZ. Rg3 inhibits hypoxia-induced tumor exosomes from boosting pancreatic cancer vasculogenic mimicry through the HIF-1α/LARS1/mTOR axis. PHYTOMEDICINE. 2025 139
133. Li DY, Jin Y, He X, Deng J, Lu WQ, Yang ZC. et al. Hypoxia-induced LAMB2-enriched extracellular vesicles promote peritoneal metastasis in gastric cancer via the ROCK1-CAV1-Rab11 axis. ONCOGENE. 2024;43:2768-80
134. Wu LP, Xue M, Lai SC, Chen JY, Lin YF, Ding N. et al. Hypoxia derived exosomes promote the proliferation and metastasis of colorectal cancer through the regulation of HIF-1α/miR-4299/ZBTB4. LIFE SCIENCES. 2023 329
135. Xue ZW, Liu JZ, Xing WC, Mu FY, Wu YZ, Zhao JL. et al. Hypoxic glioma-derived exosomal miR-25-3p promotes macrophage M2 polarization by activating the PI3K-AKT-mTOR signaling pathway. JOURNAL OF NANOBIOTECHNOLOGY. 2024 22
136. Li GF, Xiong ZY, Li Y, Yan C, Cheng YY, Wang YW. et al. Hypoxic microenvironment-induced exosomes confer temozolomide resistance in glioma through transfer of pyruvate kinase M2. DISCOVER ONCOLOGY. 2024 15
137. Wang DL, Wang S, Jin MM, Zuo Y, Wang JP, Niu Y. et al. Hypoxic exosomal circPLEKHM1-mediated crosstalk between tumor cells and macrophages drives lung cancer metastasis. ADVANCED SCIENCE. 2024 11
138. Silva P, Hernández N, Tapia H, Gaete-Ramírez B, Torres P, Flores T. et al. Tumor-derived hypoxic small extracellular vesicles promote endothelial cell migration and tube formation via ALS2/Rab5/β-catenin signaling. FASEB JOURNAL. 2024 38
139. Qiao GE, Li CJ, Wang M, Zhang WJ, Shi JJ, Meng B. et al. Hypoxia-induced exosomal circNRIP1 activates cancer-associated fibroblasts to promote esophageal squamous cell carcinoma migration and invasion. BMC GASTROENTEROLOGY. 2025 25
140. Liu ZL, Lin ZM, Jiang MX, Zhu GY, Xiong TY, Cao F. et al. Cancer-associated fibroblast exosomes promote prostate cancer metastasis through miR-500a-3p/FBXW7/HSF1 axis under hypoxic microenvironment. CANCER GENE THERAPY. 2024;31:698-709
141. Shu SL, Yang Y, Allen CL, Maguire O, Minderman H, Sen A. et al. Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Scientific Reports. 2018;8:12905
142. Zaira B, Lamberti J, Mattei F, Pizzi E, Puglisi R, Zanetti C. et al. Acidic microenvironment plays a key role in human melanoma progression through a sustained exosome mediated transfer of clinically relevant metastatic molecules. Journal of Experimental & Clinical Cancer Research. 2018;37:245
143. Zhou X, Yan T, Huang C, Xu Z, Wang L, Jiang E. et al. Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. Journal of Experimental & Clinical Cancer Research. 2018;37:242
144. Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M. et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLOS ONE. 2014 9
145. Logozzi M, Capasso C, Di Raimo R, Del Prete S, Mizzoni D, Falchi M. et al. Prostate cancer cells and exosomes in acidic condition show increased carbonic anhydrase IX expression and activity. Journal of Enzyme Inhibition and Medicinal Chemistry. 2019;34:272-8
146. Tian X-P, Wang C-Y, Jin X-H, Li M, Wang F-W, Huang W-J. et al. Acidic microenvironment up-regulates exosomal miR-21 and miR-10b in early-stage hepatocellular carcinoma to promote cancer cell proliferation and metastasis. Theranostics. 2019;9:1965-79
147. Huilcaman R, Campos A, Contreras P, Simón L, Varas-Godoy M, Grünenwald F. et al. Inclusion of ΑVβ3 integrin into extracellular vesicles in a caveolin-1 tyrosine-14- phosphorylation dependent manner and subsequent transfer to recipient melanoma cells promotes migration, invasion and metastasis. Cell communication and signaling: CCS. 2025;23:139
148. Liang Z, Liu H, Zhang Y, Xiong L, Zeng Z, He X. et al. Cyr61 from adipose-derived stem cells promotes colorectal cancer metastasis and vasculogenic mimicry formation via integrin α(V) β(5). Molecular oncology. 2021;15:3447-67
149. Grigoryeva ES, Tashireva LA, Savelieva OE, Zavyalova MV, Popova NO, Kuznetsov GA. et al. The association of integrins β3, β4, and αVβ5 on exosomes, CTCs and tumor cells with localization of distant metastasis in breast cancer patients. International journal of molecular sciences. 2023 24
150. Li X-Q, Zhang R, Lu H, Yue X-M, Huang Y-F. Extracellular vesicle-packaged CDH11 and ITGA5 induce the premetastatic niche for bone colonization of breast cancer cells. Cancer Research. 2022;82:1560-74
151. Dutta A, Li J, Lu H, Akech J, Pratap J, Wang T. et al. The αvβ6 integrin promotes an osteolytic program through upregulation of MMP2. Cancer research. 2014;74:1598-608
152. Kawakami K, Fujita Y, Kato T, Mizutani K, Kameyama K, Tsumoto H. et al. Integrin β4 and vinculin contained in exosomes are potential markers for progression of prostate cancer associated with taxane-resistance. International Journal of Oncology. 2015;47:384-90
153. Fedele C, Singh A, Zerlanko BJ, Iozzo RV, Languino LR. The αvβ6 integrin is transferred intercellularly via exosomes *. Journal of Biological Chemistry. 2015;290:4545-51
154. Huang L, Wang F, Wang X, Su C, Wu S, Yang C. et al. M2-like macrophage-derived exosomes facilitate metastasis in non-small-cell lung cancer by delivering integrin αVβ3. MedComm. 2023;4:e191
155. Cardeñes B, Clares I, Toribio V, Pascual L, López-Martín S, Torres-Gomez A. et al. Cellular integrin α5β1 and exosomal ADAM17 mediate the binding and uptake of exosomes produced by colorectal carcinoma cells. International journal of molecular sciences. 2021 22
156. Huang X-Y, Huang Z-L, Huang J, Xu B, Huang X-Y, Xu Y-H. et al. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. Journal of Experimental & Clinical Cancer Research. 2020;39:20
157. Wu J, Li X, Yin X, Hu J, Zhou P, Zhong X. et al. Rapid detection of plasma exosomal LncRNA CASC9 for HCC using RT-RPA-CRISPR/Cas12a assay. Journal of pharmaceutical and biomedical analysis. 2025;266:117085
158. Jun Y, Yeng C, Sainan S, Jingwen X, Yu H, Abdul Khalil KB. et al. Serum exosomal miR-1275 as a potential biomarker for the diagnosis and prognostic assessment of hepatocellular carcinoma. Cancer biomarkers: section A of Disease markers. 2025;42:18758592251396221
159. Fan B, Wang L, Wang J. RAB22A as a predictor of exosome secretion in the progression and relapse of multiple myeloma. Aging. 2024;16:4169-90
160. Zhou M, Yu X, Zhang J, Huang Z, Feng S, Xiao X. et al. Plasma-derived exosomal tRF-3004a as a diagnostic biomarker for colorectal cancer. Scientific reports. 2025;15:45558
161. Chen Y, Cui F, Wu X, Zhao W, Xia Q. The expression and clinical significance of serum exosomal-long non-coding RNA DLEU1 in patients with cervical cancer. Annals of medicine. 2025;57:2442537
162. Xu K, Shen R, Zhang L, Gao X, Wang X, Zhang C. et al. Pancreatic cancer-derived extracellular vesicles enriched with miR-223-5p promote skeletal muscle wasting associated with cachexia. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2025;12:e04064
163. Salmond N, Moravcova R, Tam WS, Khanna K, Rogalski JC, Lynn K. et al. Circulating enolase 1 as a diagnostic biomarker for early-stage breast cancer. NPJ precision oncology. 2025;9:326
164. Talat LY, Mohamed G, Ibraheem MH, WalyEldeen AA, Hassan H, Ibrahim SA. SDC2 and FN as cargo proteins in circulating extracellular vesicles in obese breast cancer patients with lymph node metastasis. Scientific reports. 2025;15:32498
165. Kawakami K, Edo N, Morita K, Ishikawa T, Onose H, Fukumori T. et al. Proteomics analysis reveals elevated RAB21 in serum-derived extracellular vesicles from patients with follicular thyroid carcinoma. Oncology letters. 2025;30:444
166. Dong H, Chen S, Cui W, Yang P, Liu F, Cai S. et al. Circulating extracellular vesicle PTPRO methylation: An exploratory biomarker for minimally invasive diagnosis of early-stage lung adenocarcinoma. Cancer cell international. 2026
167. Patel A, Patel S, Patel P, Mandlik D, Patel K, Tanavde V. Salivary exosomal miRNA-1307-5p predicts disease aggressiveness and poor prognosis in oral squamous cell carcinoma patients. International journal of molecular sciences. 2022 23
168. Jiang S, Ou L, Wang Y, Su K, Chen Z, He L. et al. CircPRMT5, a potential salivary biomarker, facilitates the progression of head and neck squamous cell carcinoma via the IGF2BP3-SERPINE1 pathway. International journal of nanomedicine. 2025;20:1597-613
169. Tian JH, Liu M, Yang XJ, Wang T, Zhou RJ, Liu JY. et al. Tumor-derived exosome-based microRNA-206 delivery system as dual modulators of the immune microenvironment and gut microbiota for colorectal cancer therapy. INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES. 2025 321
170. Soudi T, Bagheri F, Shojaosadati SA, Rezaei M, Motamedian E. Combination therapy using carvedilol and 5-fluorouracil loaded exosomes as a novel synergistic strategy against breast cancer. JOURNAL OF DRUG DELIVERY SCIENCE AND TECHNOLOGY. 2025 109
171. Sarkar R, Biswas S, Ghosh R, Samanta P, Pakhira S, Mondal M. et al. Exosome-sheathed porous silica nanoparticle-mediated co-delivery of 3,3'-diindolylmethane and doxorubicin attenuates cancer stem cell-driven EMT in triple negative breast cancer. Journal of nanobiotechnology. 2024;22:285
172. Lian B, Li JY, Tang SH, Li T, Li JP. Targeting LINC02544/miR-497-5p/CAPRIN1 axis via exosome-based siRNA to overcome immunotherapy resistance in triple-negative breast cancer. MOLECULAR MEDICINE. 2025 31
173. Sun RW, Yang YC, Zhang B, Chen JY, Wang Y, Jiang ZH. et al. Tumor-homing exosomes enable targeted delivery of siRNA and isoimperatorin for overcoming BTK inhibitor resistance in DLBCL. MATERIALS TODAY BIO. 2025 35
174. Song LL, Tang YP, Qu YQ, Yun YX, Zhang RL, Wang CR. et al. Exosomal delivery of rapamycin modulates blood-brain barrier penetration and VEGF axis in glioblastoma. JOURNAL OF CONTROLLED RELEASE. 2025 381
175. Yu S, Liao R, Bai L, Guo M, Zhang Y, Zhang Y. et al. Anticancer effect of hUC-MSC-derived exosome-mediated delivery of PMO-miR-146b-5p in colorectal cancer. Drug Delivery and Translational Research. 2024;14:1352-69
176. Yoon YC, Lee D, Park JH, Kim OH, Choi HJ, Kim SJ. Enhancing pancreatic cancer therapy with targeted CD133-exosome delivery of PD-L1 siRNA: A preclinical investigation. PANCREAS. 2025;54:e210-e20
177. Kim H-J, Lee DS, Park JH, Hong H-E, Choi HJ, Kim O-H. et al. Exosome-based strategy against colon cancer using small interfering RNA-loaded vesicles targeting soluble a proliferation-inducing ligand. World journal of stem cells. 2024;16:956-73
178. Cui J, Wang X, Li J, Zhu A, Du Y, Zeng W. et al. Immune exosomes loading self-assembled nanomicelles traverse the blood-brain barrier for chemo-immunotherapy against glioblastoma. ACS Nano. 2023
179. Zhang Y, Zuo BF, Yu ZZ, Zhao KJ, Zhang YL, He K. et al. Complete remission of tumors in mice with neoantigen-painted exosomes and anti-PD-1 therapy. MOLECULAR THERAPY. 2023 31
180. Guo YW, Lv TT, Li ZJ, Wei X, Yang CW, Li W. et al. Acidity-activatable dynamic hybrid nanoplatforms derived from extracellular vesicles of M1 macrophages enhance cancer immunotherapy through synergistic triple immunotherapy. JOURNAL OF NANOBIOTECHNOLOGY. 2024 22
Author contact
![]() Corresponding authors: Linjiang Song, School of Medical and Life Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan 611137, P.R. China. E-mail address: songlinjiangedu.cn. Anqi Zeng, Translational Chinese Medicine Key Laboratory of Sichuan Province, Sichuan Academy of Chinese Medicine Sciences, Sichuan Institute for Translational Chinese Medicine, Chengdu, Sichuan 610041, P.R. China. E-mail address: zeng6002aqcom.
Corresponding authors: Linjiang Song, School of Medical and Life Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan 611137, P.R. China. E-mail address: songlinjiangedu.cn. Anqi Zeng, Translational Chinese Medicine Key Laboratory of Sichuan Province, Sichuan Academy of Chinese Medicine Sciences, Sichuan Institute for Translational Chinese Medicine, Chengdu, Sichuan 610041, P.R. China. E-mail address: zeng6002aqcom.

 Global reach, higher impact
Global reach, higher impact