Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2016; 12(11):1279-1288. doi:10.7150/ijbs.13453 This issue Cite
Research Paper
Blockage of epithelial to mesenchymal transition and upregulation of let 7b are critically involved in ursolic acid induced apoptosis in malignant mesothelioma cell
College of Korean Medicine, Kyung Hee University, Seoul, 130-701, Republic of Korea
* Equally contributed
Received 2015-8-4; Accepted 2016-8-12; Published 2016-10-18
Abstract
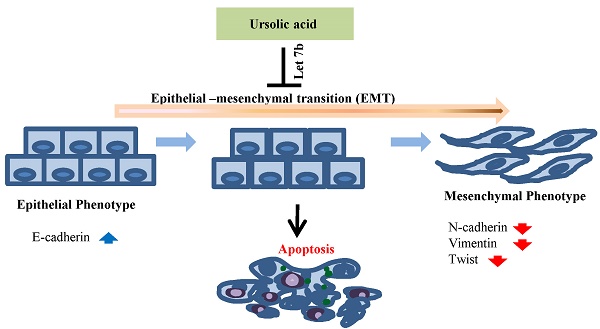
Malignant pleural mesothelioma (MPN), which is caused by asbestos exposure, is one of aggressive lung tumors. In the present study, we elucidated the anti-tumor mechanism of ursolic acid in malignant mesotheliomas. Ursolic acid significantly exerted cytotoxicity in a time and dose dependent manner in H28, H2452 and MSTO-211H mesothelioma cells and inhibited cell proliferation by colony formation assay in a dose-dependent fashion. Also, ursolic acid treatment accumulated the sub-G1 population, attenuated the expression of procapase 9, cyclin D1, pAKT, p-glycogen synthase kinase 3-alpha/beta (pGSK3α/β), β-catenin and nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) and also cleaved caspase 3 and poly (ADP-ribose) polymerase (PARP) in mesothelioma cells. Furthermore, ursolic acid treatment blocked epithelial and mesenchymal transition (EMT) molecules by activating E-cadherin as an epithelial marker and attenuating Vimentin, and Twist as mesenchymal molecules. Interestingly, miRNA array revealed that 23 miRNAs (>2 folds) including let-7b and miRNA3613-5p, miRNA134 and miRNA196b were significantly upregulated while 33 miRNAs were downregulated in ursolic acid treated H2452 cells. Furthermore, overexpression of let 7b using let-7b mimics enhanced the antitumor effect of ursolic acid to attenuate the expression of procaspases 3, pro-PARP, pAKT, β-catenin and Twist and increase sub-G1 accumulation in H2452 mesothelioma cells. Overall, our findings suggest that ursolic acid induces apoptosis via inhibition of EMT and activation of let7b in mesothelioma cells as a potent chemotherapeutic agent for treatment of malignant mesotheliomas.
Keywords: Ursolic acid, mesothelioma, apoptosis, epithelial and mesenchymal transition, miRNA array, let 7b
Introduction
Malignant mesothelioma is a very aggressive tumor originated from mesothelial cells lining body cavities after asbestos exposure. The overall survival is less than 1 year following diagnosis [1]. Approximately 60% of the human malignant mesothelioma patients at the diagnosis resulted in the chemotherapy failure due to chemoresistance and adverse effects induced by activation of PI3K/Akt/mTOR, osteopontin/Akt, Mcl-1/Bcl-xL pathways [2]. Thus, recently many natural products such as isothiocyanate [3] and resveratrol [4] are highlighted with less toxicity for combination treatment with classical anticancer agents.
Epithelial-mesenchymal transition (EMT) plays a pivotal role in cancer progression and metastasis of mesotheliomas by losing epithelial phenotypes such as cell polarity and gaining the mesenchymal characteristics with mortality [5, 6]. MicroRNAs such as miR-1, miR-184, miR-34, miR-34a [7], miR-34b/c [8], miR-625-3P, miRNA-103, miR-126[9], miR-1254 [10] and has- miR-29c [11] as non-coding RNAs, post-transcriptionally regulate gene expression and affect development, differentiation, and apoptosis in several cancers including malignant mesotheliomas. Also, recent studies showed that several microRNAs mediate EMT transition via PTEN/Akt signaling pathway in mesotheliomas [12-14].
Ursolic acid (UA) is an active pentacyclic triterpene acid contained in many medicinal plants, such as Eriobotrya japonica, Rosmarinus offıcinalis and Glechoma hederaceae and several fruits including apples and rosemary [15]. Many studies showed the multi-biological functions of ursolic acid, including anti-oxidant and anti-inflammatory activity [16]. Also, ursolic acid has anti-cancer activity by inhibiting proliferation and stimulating apoptosis in prostate cancer [17, 18], colon cancer [19, 20], breast cancer [21], ovarian cancer [22], leukemia [23] and melanoma [24].
Nevertheless, the critical roles of EMT and microRNA were never investigated in ursolic acid treated mesothelioma cells until now. Thus, in the present study, the underlying antitumor mechanism of ursolic acid was elucidated in mesothelioma cells in association with the roles of EMT and let7b following microRNA array.
Material and methods
Cell culture and Chemicals
H28, H2452 and MSTO-211H mesothelioma cells were obtained from the American Type Culture Collection (ATCC) and cultured in RPMI 1640 (Welgene, Daegu, Korea) supplemented with 10% fetal bovine serum (FBS) (Welgene, Daegu, Korea) and 1 % penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified 5% CO2 atmosphere. Ursolic acid, apigenin and gallotannin were obtained from Sigma Aldrich (Sigma Aldrich, St. Louis, MO, USA). Galbanic acid was isolated from the gum resin of F. assafoetida purchased from Hanil Herbal Shop (Seoul, Korea). 1,2,3,4,6-penta-O-galloyl-beta-d-glucose (PGG) was isolated from the gallnut of Rhus chinensis MILL as described previously [25, 26].
Cytotoxicity assay
To comparatively check the cytotoxicity of various compounds such as ursolic acid, apigenin, galbanic acid, gallotannin, decursin and PGG against H28, H2452 and MSTO-211H mesothelioma cells, MTT colorimetric assay (Sigma, St. Louis, MO, USA) was applied. Mesothelioma cells were seeded onto 96-well microplates at a density of 1 × 104 cells per well and then treated with various compounds, respectively. After incubation, MTT working solution (5 mg/ml in PBS) was added and incubated at 37°C for 2 h. The optical density (OD) was then measured at 570 nm using a Sunrise microplate reader (TECAN, Männedorf, Switzerland). Cell viability was calculated as the percentage of viable cells treated with compounds versus untreated control cells as follows: Cell viability (%) = [OD (Treatment) - OD (Blank)] / [OD (Control) - OD (Blank)] × 100.
Cell cycle analysis
Mesothelioma cells (2.5 × 105) were treated with ursolic acid (0, 20 or 40 μM) for 24 h and then fixed in 75% ethanol at -20°C, resuspended in PBS containing RNase A (1 mg/ml). After washing, fixed cells were incubated for 1 h at 37°C and propidium iodide (PI; 50 μg/ml) was incubated for 30 min at room temperature in the dark. CellQuest Software with a FACS Calibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ) was applied to check the DNA contents of the stained cells.
Colony formation assay
H28 and H2452 mesothelioma cells treated by ursolic acid were seeded onto six well plates in the complete medium containing 0.3%-0.4% agar. H28 and H2452 cells were cultured for 14 days and stained with crystal violet. Cell colonies were photographed and enumerated under an inverted microscope.
Real time -quantitative real time polymerase chain reaction (RT-qPCR)
To detect the mRNA level of cyclin D1, Twist, and pre-let-7b in ursolic acid-treated mesothelioma cells, a quantitative real-time PCR was used. Total RNA from ursolic acid treated mesothelioma was used to construct the template cDNA with the reverse transcription kit (Promega, WI, USA). RT-qPCR was used with the LightCycler TM instrument (Roche Applied Sciences, Indianapolis, USA) according to the manufacturer's protocol. GAPDH was used to normalize the expression of genes of interest. The primers used to be as follows: cyclin D1 forward primer 5'-TCCTCTCCAAAATGCCAGAG -3', reverse primer 5'- GGCGGATTGGAAATGAACTT-3', Twist forward primer 5'- GGCTCAGCTACGCCT TCTC-3', reverse primer 5'-CCTTCTCTGGAAACAATGACATCT-3', pre-let7b forward primer 5'-GGGTGAGGTAGTAGGTTGTGTG-3', reverse primer 5'-CAGGGAAGGCAGTAGGTTGT-3', GAPDH forward primer: 5′-CCACTCCTCCACCTT TGAC-3′, reverse primer: 5′-ACCCTGTTGCTGTAGCCA-3′.
Western blot analysis
Cell lysates from ursolic acid treated mesothelioma cells (H28, H2452, MSTO-211H) were prepared on ice in lysis buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF, and 1X protease inhibitor cocktail (Roche, Mannhein, Germany). Protein concentrations were determined by Bradford assay (Bio-Rad, Hercules, CA, USA). Proteins were separated by electrophoresis on 4-12% NuPAGE Bis-Tris gels (Novex, Carlsbad, CA, USA) and then transferred to a Hybond ECL transfer membrane. Antibodies of E-cahderin, Twist, cyclin D1 (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), Vimentin, N-cadherin, NFkB, Akt, pAKT, β-catenin, pGSK3α/β (Cell signaling, USA) and β-actin (Sigma, St. Louis, MO, USA) were incubated on the membrane overnight. After washing, the horseradish peroxidase (HRP)-conjugated secondary anti-mouse or rabbit antibodies (AbD Serotec, Kidlington, UK) were incubated for 1 h and protein bands were visualized by chemiluminescence (ECL) system (Amersham Pharmacia, Piscataway, NJ).
microRNA array
Total RNAs were extracted from H28 cells in the presence or absence of ursolic acid (20μM) for 24 h using QIAzol (Invitrogen, Carlsbad, CA, USA). MicroRNA array was performed as described previously [27].
microRNA transfection assay
The let7b mimic or inhibitor and control vector (200 nM) (Genolution, Korea) were transfected into H2452 cells using lipofetamine 2000 (Invitrogen, Carlsbad, CA, USA) reagent according to the manufacture's protocol. Two days after transfection, H2452 cells exposed to ursolic acid (20μM) for 24 h were harvested for protein expression analysis.
Statistical analyses
Statistical analyses were conducted using SigmaPlot version 12 (Systat Software Inc., San Jose, CA, USA). All data are expressed as means ± standard deviation (SD).
Results
Ursolic acid exerted the cytotoxic and anti-proliferative activity in H28, H2452 and MSTO-211H mesothelioma cells
To compare the cyotoxicity of various natural compounds (apigenin, galbanic acid, gallotanin, decursin, PGG and ursolic acid) in H28 and H2452 mesothelioma cell lines, MTT assay was performed. As shown in Figure 1A, ursolic acid showed significant cytotoxicity in H28 and H2452 cells compared with other compounds.
Ursolic acid exerted the cytotoxic and antiproliferative activity in H28, H2452 and MSTO-211H mesothelioma cells. (A) Cytotoxicity of H28 and H2452 mesothelioma cells at different concentrations (0, 10, 20, 40, or 80 μM) by several compounds such as apigenin, galbanic acid, gallotannin, decursin, PGG, and ursolic acid for 24 h by MTT assay. (B) Time - and dose - dependent cytotoxicity of ursolic acid in H28, H2452 and MSTO-211H for 24h, 48h and 72h by MTT assay. Each value represents the mean ± SD (n =
= 3). (C) The inhibitory effect of usrsolic acid on colony formation in H2452 and H28 cells. Different concentrations (0, 10, 20, 40 μM) of ursolic acid were treated in H2452 and H28 cells for 14 days and colony formation assay was performed. **p<0.01 ***p<0.001 vs untreated control.
3). (C) The inhibitory effect of usrsolic acid on colony formation in H2452 and H28 cells. Different concentrations (0, 10, 20, 40 μM) of ursolic acid were treated in H2452 and H28 cells for 14 days and colony formation assay was performed. **p<0.01 ***p<0.001 vs untreated control.
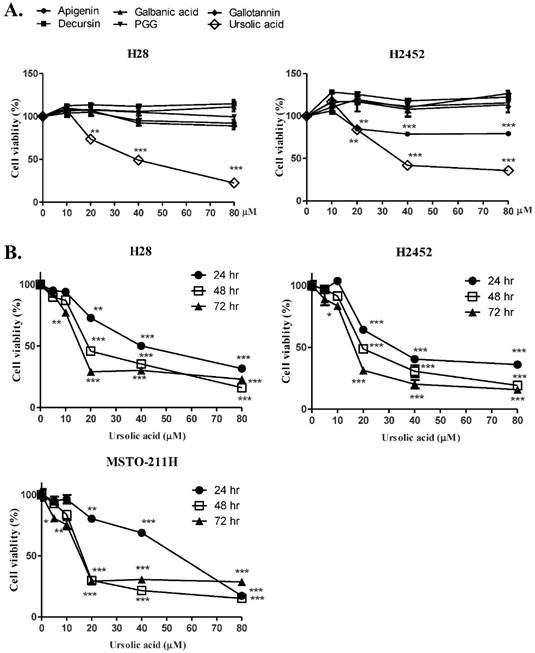
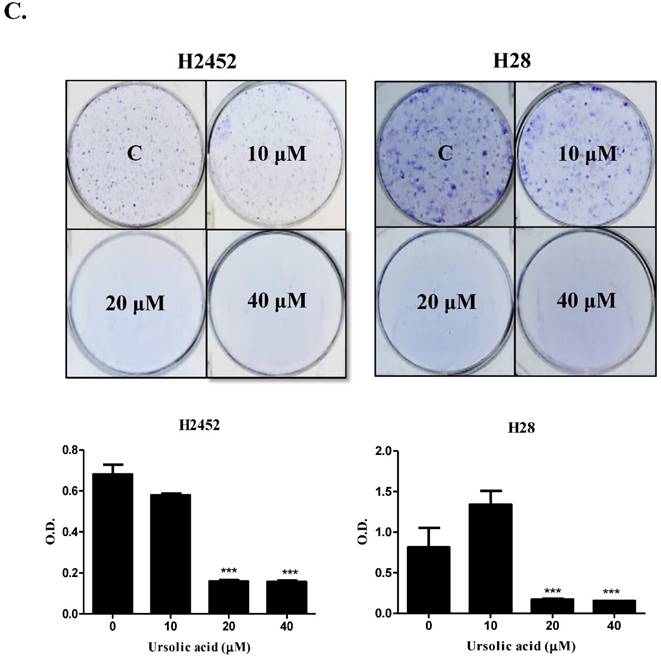
Furthermore, ursolic acid significantly showed cytotoxicity in a time or dose dependent manner in three malignant mesothelioma cells (H28, H2452 or MSTO-211H) as shown in Figure 1B. Consistently, the colony forming assay revealed that ursolic acid inhibited the proliferation of H2452 and H28 mesothelioma cells as shown in Figure 1C.
Ursolic acid increased sub G1 portion and regulated apoptosis related proteins in mesothelioma cells
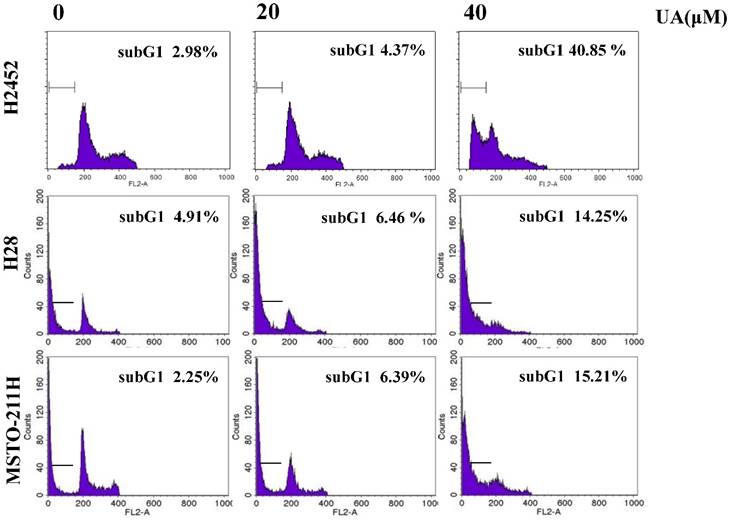
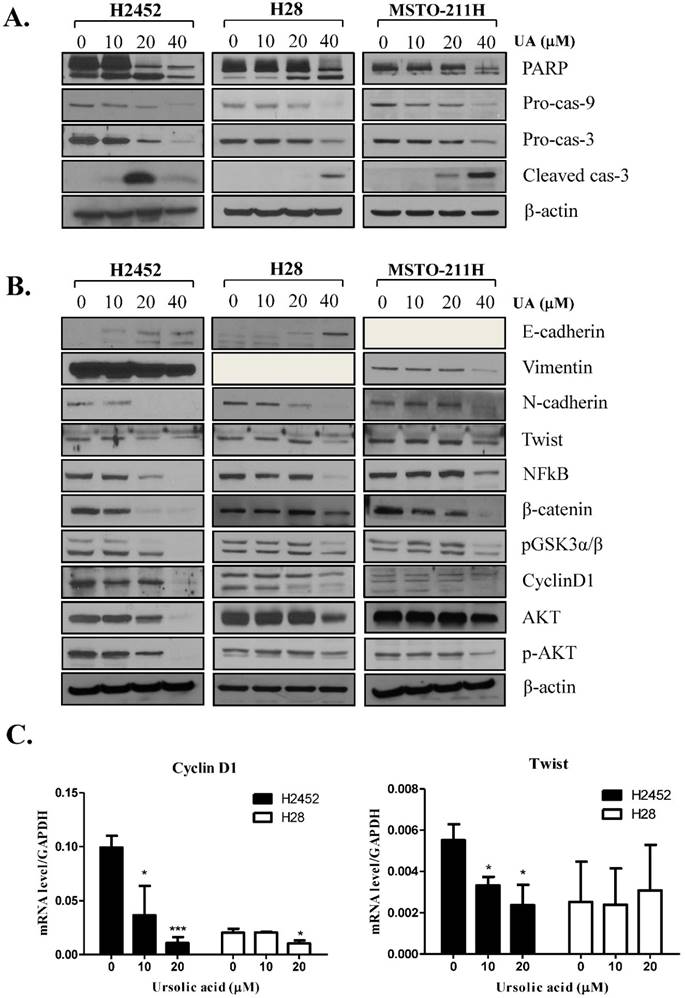
To confirm whether or not cytotoxicity was mediated by apoptosis in ursolic acid treated mesothelioma cells, cell cycle analysis was performed. As shown in Figure 2, ursolic acid significantly accumulated the sub-G1 portion in H2452, H28 and MSTO-211H cells compared to untreated control. To confirm the effect of ursolic acid on apoptosis related proteins, Western blotting was carried out. Here, ursolic acid induced the cleavages of PARP and caspase 3 and also attenuated the expression of procaspase -3 and -9 in a dose dependent manner in H2452, H28 and MSTO-211H cells (Figure 3A).
Ursolic acid regulated EMT related molecules in mesothelioma cells
It was well documented that EMT molecules are involved in apoptosis in several cancers [28]. As shown in Figure 3B, E-cadherin, an epithelial molecule, was markedly induced in a dose dependent manner in ursolic acid treated H2452 and H28 cells but not undetected in MSTO-211H cells. In contrast, the expression level of Vimentin, N-cadherin, Twist, β-catenin, pGSK3α/β such as mesenchymal markers were decreased by ursolic acid in H2452 and MSTO-211H cells, while Vimentin was not detected in H28 cells. Furthermore, the expression of survival genes such as cyclin D1, p-AKT and NFkB was attenuated in ursolic acid treated mesothelioma cells. Consistently, RT-qPCR revealed that mRNA levels of cyclin D1 and Twist were abrogated in ursolic acid treated H2452 and H28 cells. But mRNA expression of Twist was not affected by ursolic acid in H28 cells (Figure 3C).
Upregulation of let7b played a critical role in the apoptotic effect of ursolic acid in H2452 cells
Previous studies showed that microRNAs regulate apoptosis in several cancer cells [8, 29].
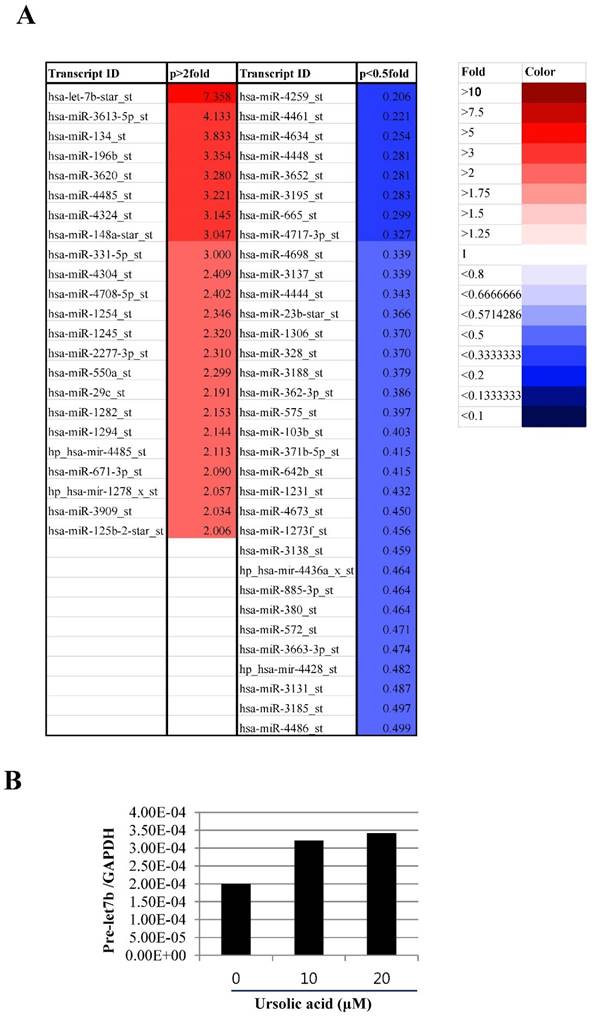
Therefore, to examine the role of microRNA in ursolic acid induced apoptosis in mesothelioma cells, microRNA array was performed in ursolic treated H2452 cells. As shown in Figure 4A, 33 microRNAs including let 7b (7.358 fold), miR 3613-5p (4.133 fold), miR 134 (3.833 fold), miR 196b (3.354 fold) were upregulated in ursolic treated H2452 cells, while 33 miRNAs were downregulated. Among them, we found microRNA let 7b was the most potent in ursolic treated H2452cells. Consistently, we validated that let7b was upregulated in ursolic acid treated H28 cells by using qRT-PCR (Figure 4B).
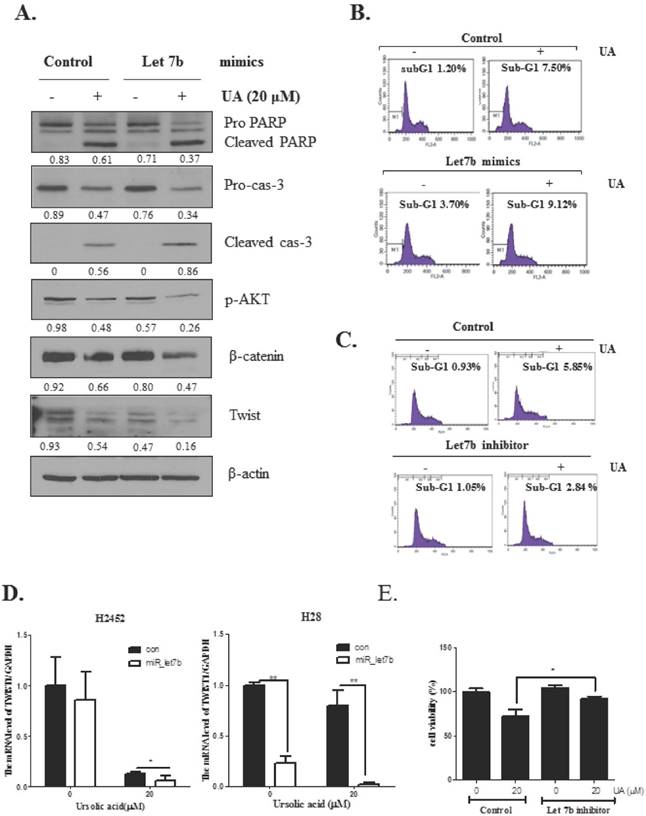
To verify whether or not let 7b is critically involved in apoptosis and EMT induced by ursolic acid, let-7b mimic was transfected into H2452 cells and treated with ursolic acid (20 μM). As shown in Figure 5A, let7b mimic enhanced apoptotic effect of ursolic acid to induce cleavages of PARP and caspase 3 and suppress the expression of p-AKT, β-catenin and Twist in H2452 cells. Likewise, FACS analysis showed that let 7b mimic enhanced accumulation of subG1 portion in ursolic and, while let 7b inhibitor blocked the sub-G1 accumulation in H2452 cells by ursolic acid treatment (Figure 5C). Furthermore, let 7b mimic attenuated the mRNA expression of Twist was in ursolic acid treated H2452 and H28 cells (Figure 5D). Additionally, to confirm the role of let 7b in UA induced cytotoxicity, we performed MTT assay using let 7b inhibitor. Our data showed that let 7b inhibitor blocked the cytotoxicity by ursolic acid treatment in H2452 cells as shown in Figure 5E.
Ursolic acid increased sub G1 portion in mesothelioma cells. Ursolic acid (0, 20, 40 μM) was treated to H2452, H28 and MSTO-211H cells for 24h and stained with PI using Flow cytometry. CellQuest Software with a FACSCalibur flow cytometer was applied to check the DNA contents of the stained cells.
Ursolic acid regulated apoptosis related proteins and EMT molecules in H2452, H28 and MSTO-211H cells. (A) Effect of ursolic acid on apoptosis related proteins in H2452, H28 and MSTO-211H cells. Ursolic acid treated mesothelioma cells were subjected to Western blotting for PARP, cleaved caspase-3, pro-caspase -9 and -3, and β-Actin. (B) The effect of ursolic acid on EMT (Twist, E-cadherin, N-cadherin, β-catenin, pGSKα/β) and survival proteins (cyclin D1, p-AKT, NFkB) by Western blotting. Ursolic acid was treated mesothelioma cells for 24 h and subjected to Western blotting for EMT and survival proteins. (C) mRNA levels of Twist and cyclin D1 in ursolic acid treated H2452 and H28 cells by real-time PCR. GAPDH was used to normalize the target gene. Ursolic acid was treated H2452 and H28 cells for 24 h and subjected to Western blotting for Twist and cyclin D1. *p<0.05, ***p<0.001 vs untreated control.
Ursolic acid modulated the microRNA expression in H2452 mesothelioma cells. (A) The microRNA differential profile in ursolic acid treated H2452 cells. H2452 cells were exposed to ursolic acid for 24 h and microRNA array was performed as shown in Materials and Methods. Red color p >2 fold, Blue color p <0.5 fold. (B) Upregulation of let 7b by ursolic acid treatment in H2452 cells. RT-qPCR was used to verify the level of pre let7b expression. GAPDH was used as an internal control.
Overexpression of let7b enhanced the antitumor effect of ursolic acid to increase sub G1 population and cleave PARP and caspase 3, and also attenuate the expression of p-AKT, Twist and β-catenin in H2452 cells. (A) Effect of let7b mimic on PARP, caspase 9, caspase 3, Twist and β-catenin in ursolic acid treated H2452 cells. (B) Effect of let7b mimic on sub G1 population in ursolic acid treated H2452 cells by FACS analysis. (C) Effect of let7b inhibitor on sub G1 population in ursolic acid treated H2452 cells by FACS analysis. (D) Effect of let 7b mimic on mRNA level of Twist in ursolic acid treated H2452 and H28 cells. RT-qPCR was performed to check the mRNA level of Twist. GAPDH was used to normalize the target gene. * p<0.05, **p<0.01 vs untreated control. (E) Effect of let 7b inhibitor on cytotoxicity of ursolic acid in H2452 cells. After transfection with let 7b inhibitor in H2452 cells, the cells were exposed to ursolic acid for 24 h and then MTT assay was performed. * p<0.05 vs untreated control.
Discussion
Malignant pleural mesothelioma(MPN) is an aggressive cancer arising from the mesothelial lining cells of the pleura associated with asbestos with poor prognosis [30]. In the current study the underlying antitumor mechanism of ursolic acid was investigated in H28, H2452 and MSTO-211H mesothelioma cells in association with EMT and microRNA let 7b, ursolic acid significantly showed cytoxic and anti-proliferative activity in H28, H2452, MSTO-211H mesothelioma cells, implying that ursolic acid can be a chemotherapeutic agent for MPN. Also, ursolic acid increased sub-G1 population in three mesothelioma cells, indicating that the cytotoxic and anti-proliferative effects of ursolic acid are induced via apoptosis induction.
There are accumulating evidences that apoptosis is induced via caspase9/3 activation for mitochondrial dependent pathway [31, 32] and inhibition of anti-apoptotic survival genes [33] such as cyclin D1, pAKT, pGSK3α/β, β-catenin and NFkB. In our work Western blotting revealed that ursolic acid cleaved PARP and caspase 3 and attenuated the expression of procaspase 9, cyclin D1, pAKT, pGSK3α/β, β-catenin and NFkB in three mesothelioma cells, demonstrating that apoptosis induced by ursolic acid was mediated via caspase activation and survival gene inhibition. Similarly, previous studies showed that ursolic acid induced apoptosis via inhibition of the Wnt5/β-catenin pathway in prostate cancer cells [17] and also inhibited the cell growth via AMPK [33], ER stress [34] or Akt/NF-kB [35] in human bladder cancer cells carcinoma cells.
EMT is a biological process that epithelial cells are converted into mesenchymal cells with the ability of the metastasis and carcinogenesis of cancer cells [36, 37]. Additionally, it is well documented that activation of EMT is involved in carcinogenesis and metastasis in lung cancers [28, 38, 39]. Here, loss of E-cadherin and increased expression of N-cadherin, Vimentin and Twist are the representative phenotypes of EMT [37, 40]. In the current study, ursolic acid blocked the EMT process by activating E-cadherin and blocking the expression of N-cadherin, Vimentin and Twist in three mesothelioma cells, implying that anti-tumor activity of ursolic acid is associated with inhibition of EMT process in mesothelioma cells. Consistent with our data, Liu et al reported that ursolic acid can inhibit EMT by suppressing AEG1 in A549 cells [18] and Zhang et al showed evidences that ursolic acid suppressed the growth of SKOV3 and sphere cells [22]. Also, upregulation of beta-catenin and pGSKα/β is well known to be correlated with EMT process and potency of proliferation [41] and NF-kB plays an important role for induction of EMT process and metastasis [42]. In the current study, our data showed that ursolic acid blocks the EMT process by activation of E-cadherin and inhibition of N-cadherin, Vimentin and Twist in H28, H2452 and MSTO-211H mesothelioma cells. Furthermore, ursolic acid attenuated the expression of NFkB, p-GSK, beta-catenin, cyclin D1, and p-AKT, implying that ursolic acid inhibits the proliferation of mesothelioma cells by inhibition of EMT molecules and survival genes.
There are accumulating evidences that microRNAs are involved in carcinogenesis and metastasis [8, 11, 12]. Our microRNA array revealed that microRNAs including let 7b (7.358 fold), miR 3613 (4.133 fold), miR 134 (3.833 fold), miR 196b (3.354 fold) were upregulated in ursolic treated H2452 cells, while 33 miRNAs were downregulated. Consistently, there are evidences that let7b modulated proliferation, inhibited cyclin D1 and increased cisplatin-induced G1 arrest and apoptosis [43] in gastric cancer [44], multiple myeloma [28, 45] and melanoma cells [46]. Also, Li and his colleagues showed that upregulation of let 7b by natural agents, 3,3'-diindolylmethane (DIM) or isoflavone, resulted in reversal of EMT transition in gemcitabine-resistant pancreatic cancer cells [47]. In our study, let7b mimic enhanced apoptotic effect of ursolic acid to induce cleavages of PARP and caspase 3 and suppress the expression of p-AKT, β-catenin and Twist in H2452 cells. Also, let 7b mimic enhanced subG1 accumulation and let 7b inhibitor suppressed subG1 accumulation and cytotoxicity in ursolic treated H2452 cells, demonstrating the critical role of let7b in apoptosis induced by ursolic acid in H2452 mesothelioma cells.
In summary, ursolic acid exerted cytotoxic and anti-proliferative effects and induced apoptosis via caspas9/3 activation and reversal of EMT by enhancing E-cadherin and attenuating Twist, N-cadherin, and Vimentin such as mesenchymal markers. Of note, ursolic acid upregulated microRNA let7b leading to apoptosis induction by inhibition of procaspase 3, pro-PARP, pAKT, pGSKα/β, β-catenin and Twist and enhancement of sub-G1 accumulation in H2452 mesothelioma cells. Overall, our findings provide scientific evidences that inhibition of EMT and activation of let7b are critically involved in ursolic acid induced apoptosis in mesothelioma cells.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (No. 2014R1A2A1A10052872 and 2015R1C1A1A02036842).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Yates DH, Corrin B, Stidolph PN, Browne K. Malignant mesothelioma in south east England: clinicopathological experience of 272 cases. Thorax. 1997;52:507-12
2. Barbone D, Yang TM, Morgan JR, Gaudino G, Broaddus VC. Mammalian target of rapamycin contributes to the acquired apoptotic resistance of human mesothelioma multicellular spheroids. The Journal of biological chemistry. 2008;283:13021-30
3. Denis I, Cellerin L, Gregoire M, Blanquart C. Cisplatin in combination with Phenethyl Isothiocyanate (PEITC), a potential new therapeutic strategy for malignant pleural mesothelioma. Oncotarget. 2014;5:11641-52
4. Lee YJ, Lee YJ, Im JH, Won SY, Kim YB, Cho MK. et al. Synergistic anti-cancer effects of resveratrol and chemotherapeutic agent clofarabine against human malignant mesothelioma MSTO-211H cells. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2013;52:61-8
5. Berx G, Raspe E, Christofori G, Thiery JP, Sleeman JP. Pre-EMTing metastasis? Recapitulation of morphogenetic processes in cancer. Clinical & experimental metastasis. 2007;24:587-97
6. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420-8
7. Ghawanmeh T, Thunberg U, Castro J, Murray F, Laytragoon-Lewin N. miR-34a expression, cell cycle arrest and cell death of malignant mesothelioma cells upon treatment with radiation, docetaxel or combination treatment. Oncology. 2011;81:330-5
8. Maki Y, Asano H, Toyooka S, Soh J, Kubo T, Katsui K. et al. MicroRNA miR-34b/c enhances cellular radiosensitivity of malignant pleural mesothelioma cells. Anticancer research. 2012;32:4871-5
9. Santarelli L, Strafella E, Staffolani S, Amati M, Emanuelli M, Sartini D. et al. Association of MiR-126 with soluble mesothelin-related peptides, a marker for malignant mesothelioma. PloS one. 2011;6:e18232
10. Foss KM, Sima C, Ugolini D, Neri M, Allen KE, Weiss GJ. miR-1254 and miR-574-5p: serum-based microRNA biomarkers for early-stage non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2011;6:482-8
11. Pass HI, Goparaju C, Ivanov S, Donington J, Carbone M, Hoshen M. et al. hsa-miR-29c* is linked to the prognosis of malignant pleural mesothelioma. Cancer Res. 2010;70:1916-24
12. Liu Z, Wang J, Guo C, Fan X. microRNA-21 mediates epithelial-mesenchymal transition of human hepatocytes via PTEN/Akt pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2015;69:24-8
13. Drasin DJ, Guarnieri AL, Neelakantan D, Kim J, Cabrera JH, Wang CA. et al. TWIST1-Induced miR-424 Reversibly Drives Mesenchymal Programming while Inhibiting Tumor Initiation. Cancer Res. 2015;75:1908-21
14. Dong P, Konno Y, Watari H, Hosaka M, Noguchi M, Sakuragi N. The impact of microRNA-mediated PI3K/AKT signaling on epithelial-mesenchymal transition and cancer stemness in endometrial cancer. Journal of translational medicine. 2014;12:231
15. Taniguchi S, Imayoshi Y, Kobayashi E, Takamatsu Y, Ito H, Hatano T. et al. Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochemistry. 2002;59:315-23
16. Ikeda Y, Murakami A, Ohigashi H. Ursolic acid: an anti- and pro-inflammatory triterpenoid. Molecular nutrition & food research. 2008;52:26-42
17. Park JH, Kwon HY, Sohn EJ, Kim KA, Kim B, Jeong SJ. et al. Inhibition of Wnt/beta-catenin signaling mediates ursolic acid-induced apoptosis in PC-3 prostate cancer cells. Pharmacological reports: PR. 2013;65:1366-74
18. Liu K, Guo L, Miao L, Bao W, Yang J, Li X. et al. Ursolic acid inhibits epithelial-mesenchymal transition by suppressing the expression of astrocyte-elevated gene-1 in human nonsmall cell lung cancer A549 cells. Anticancer Drugs. 2013;24:494-503
19. Wang W, Zhao C, Jou D, Lu J, Zhang C, Lin L. et al. Ursolic acid inhibits the growth of colon cancer-initiating cells by targeting STAT3. Anticancer research. 2013;33:4279-84
20. Andersson D, Liu JJ, Nilsson A, Duan RD. Ursolic acid inhibits proliferation and stimulates apoptosis in HT29 cells following activation of alkaline sphingomyelinase. Anticancer research. 2003;23:3317-22
21. Yeh CT, Wu CH, Yen GC. Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating c-Jun N-terminal kinase, Akt and mammalian target of rapamycin signaling. Molecular nutrition & food research. 2010;54:1285-95
22. Zhang J, Wang W, Qian L, Zhang Q, Lai D, Qi C. Ursolic acid inhibits the proliferation of human ovarian cancer stem-like cells through epithelial-mesenchymal transition. Oncol Rep. 2015;34:2375-84
23. Li G, Zhou T, Liu L, Chen J, Zhao Z, Peng Y. et al. Ezrin dephosphorylation/downregulation contributes to ursolic acid-mediated cell death in human leukemia cells. Blood cancer journal. 2013;3:e108
24. Mahmoudi M, Rabe SZ, Balali-Mood M, Karimi G, Tabasi N, Riahi-Zanjani B. Ursolic acid induced apoptotic cell death following activation of caspases in isolated human melanoma cells. Cell biology international. 2015;39:230-6
25. Chai Y, Lee HJ, Shaik AA, Nkhata K, Xing C, Zhang J. et al. Penta-O-galloyl-beta-D-glucose induces G1 arrest and DNA replicative S-phase arrest independently of cyclin-dependent kinase inhibitor 1A, cyclin-dependent kinase inhibitor 1B and P53 in human breast cancer cells and is orally active against triple negative xenograft growth. Breast cancer research: BCR. 2010;12:R67
26. Zhang Y, Shaik AA, Xing C, Chai Y, Li L, Zhang J. et al. A synthetic decursin analog with increased in vivo stability suppresses androgen receptor signaling in vitro and in vivo. Investigational new drugs. 2012;30:1820-9
27. Sohn EJ, Won G, Lee J, Lee S, Kim SH. Upregulation of miRNA3195 and miRNA374b Mediates the Anti-Angiogenic Properties of Melatonin in Hypoxic PC-3 Prostate Cancer Cells. Journal of Cancer. 2015;6:19-28
28. Fassina A, Cappellesso R, Guzzardo V, Dalla Via L, Piccolo S, Ventura L. et al. Epithelial-mesenchymal transition in malignant mesothelioma. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:86-99
29. Wang Z, Li Y, Ahmad A, Azmi AS, Kong D, Banerjee S. et al. Targeting miRNAs involved in cancer stem cell and EMT regulation: An emerging concept in overcoming drug resistance. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2010;13:109-18
30. Ray M, Kindler HL. Malignant pleural mesothelioma: an update on biomarkers and treatment. Chest. 2009;136:888-96
31. Creagh EM. Caspase crosstalk: integration of apoptotic and innate immune signalling pathways. Trends Immunol. 2014;35:631-40
32. Wu H, Che X, Zheng Q, Wu A, Pan K, Shao A. et al. Caspases: a molecular switch node in the crosstalk between autophagy and apoptosis. Int J Biol Sci. 2014;10:1072-83
33. Zheng QY, Jin FS, Yao C, Zhang T, Zhang GH, Ai X. Ursolic acid-induced AMP-activated protein kinase (AMPK) activation contributes to growth inhibition and apoptosis in human bladder cancer T24 cells. Biochemical and biophysical research communications. 2012;419:741-7
34. Zheng QY, Li PP, Jin FS, Yao C, Zhang GH, Zang T. et al. Ursolic acid induces ER stress response to activate ASK1-JNK signaling and induce apoptosis in human bladder cancer T24 cells. Cell Signal. 2013;25:206-13
35. Gai L, Cai N, Wang L, Xu X, Kong X. Ursolic acid induces apoptosis via Akt/NF-kappaB signaling suppression in T24 human bladder cancer cells. Mol Med Rep. 2013;7:1673-7
36. Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192-206
37. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178-96
38. Shih JY, Yang PC. The EMT regulator slug and lung carcinogenesis. Carcinogenesis. 2011;32:1299-304
39. Casarsa C, Bassani N, Ambrogi F, Zabucchi G, Boracchi P, Biganzoli E. et al. Epithelial-to-mesenchymal transition, cell polarity and stemness-associated features in malignant pleural mesothelioma. Cancer Lett. 2011;302:136-43
40. Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49:287-92
41. Jiang YG, Luo Y, He DL, Li X, Zhang LL, Peng T. et al. Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int J Urol. 2007;14:1034-9
42. Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H. et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569-81
43. Guo Y, Yan K, Fang J, Qu Q, Zhou M, Chen F. Let-7b expression determines response to chemotherapy through the regulation of cyclin D1 in glioblastoma. J Exp Clin Cancer Res. 2013;32:41
44. Yu J, Feng J, Zhi X, Tang J, Li Z, Xu Y. et al. Let-7b inhibits cell proliferation, migration, and invasion through targeting Cthrc1 in gastric cancer. Tumour Biol. 2015;36:3221-9
45. Xu H, Liu C, Zhang Y, Guo X, Liu Z, Luo Z. et al. Let-7b-5p regulates proliferation and apoptosis in multiple myeloma by targeting IGF1R. Acta Biochim Biophys Sin (Shanghai). 2014;46:965-72
46. Schultz J, Lorenz P, Gross G, Ibrahim S, Kunz M. MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell research. 2008;18:549-57
47. Li Y, VandenBoom TG 2nd, Kong D, Wang Z, Ali S, Philip PA. et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer research. 2009;69:6704-12
Author contact
![]() Corresponding author: Sung-Hoon Kim, K.M.D., Ph.D., Cancer Preventive Material Development Research Center, College of Oriental Medicine, Kyung Hee University, 1 Hoegi-dong, Dongdaemun-gu, Seoul 130-701, South Korea. Tel: 82-2-961-9233; Fax: 82-2-964-1064; E-mail: sungkim7ac.kr
Corresponding author: Sung-Hoon Kim, K.M.D., Ph.D., Cancer Preventive Material Development Research Center, College of Oriental Medicine, Kyung Hee University, 1 Hoegi-dong, Dongdaemun-gu, Seoul 130-701, South Korea. Tel: 82-2-961-9233; Fax: 82-2-964-1064; E-mail: sungkim7ac.kr

 Global reach, higher impact
Global reach, higher impact