10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2020; 16(16):3100-3115. doi:10.7150/ijbs.48066 This issue Cite
Research Paper
NDRG2 ablation reprograms metastatic cancer cells towards glutamine dependence via the induction of ASCT2
1. The State Key Laboratory of Cancer Biology, Department of Biochemistry and Molecular Biology, The Fourth Military Medical University, Xi'an, 710032, China.
2. State Key Laboratory of Military Stomatology &National Clinical Research Center for Oral Diseases&Shaanxi Clinical Research Center for Oral Diseases, Department of Oral and Maxillofacial Surgery, School of Stomatology, The Fourth Military Medical University, No. 145 Changle Xi Road, Xi'an, 710032, China.
3. Department of Oral and Maxillofacial Surgery, Hospital of Stomatology, Jiamusi University, Jiamusi, 154002, China.
4. State Key Laboratory of Military Stomatology &National Clinical Research Center for Oral Diseases & Shaanxi International Joint Research Center for Oral Diseases, Department of Oral Anatomy and Physiology and TMD, the Fourth Military Medical University, Xi'an 710032, China.
5. Shaanxi University of Chinese Medicine, Xianyang, 712046, China.
6. Department of Gastroenterology, the First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, 710061, China.
7. Department of General Surgery, Tangdu Hospital, Air Force Medical University, Xi'an, 710032, China.
8. Xi'an Peihua University, Xi'an, 710125, China.
#These authors contributed equally to this work.
Abstract
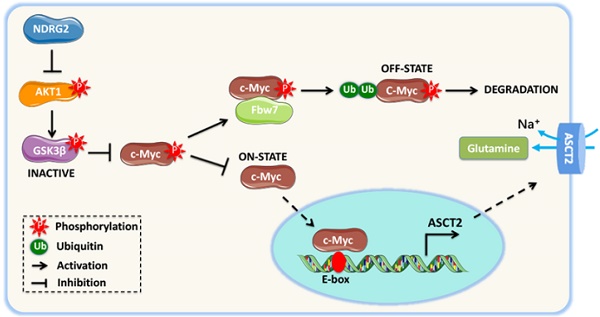
Background: Metastasis is the most common cause of lethal outcome in various types of cancers. Although the cell proliferation related metabolism rewiring has been well characterized, less is known about the association of metabolic changes with tumor metastasis. Herein, we demonstrate that metastatic tumor obtained a mesenchymal phenotype, which is obtained by the loss of tumor suppressor NDRG2 triggered metabolic switch to glutamine metabolism.
Methods: mRNA-seq and gene expression profile analysis were performed to define the differential gene expressions in primary MEC1 and metastatic MC3 cells and the downstream pathways of NDRG2. NDRG2 regulation of Fbw7-dependent c-Myc stability were determined by immunoprecipitation and protein half-life assay. Luciferase reporter and ChIP assays were used to determine the roles of Akt and c-Myc in mediating NDRG2-dependent regulation of ASCT2 in in both tumor and NDRG2-knockout MEF cells. Finally, the effect of the NDRG2/Akt/c-Myc/ASCT2 signaling on glutaminolysis and tumor metastasis were evaluated by functional experiments and clinical samples.
Results: Based on the gene expression profile analysis, we identified metastatic tumor cells acquired the mesenchymal-like characteristics and displayed the increased dependency on glutamine utilization. Further, the gain of NDRG2 function blocked epithelial-mesenchymal transition (EMT) and glutaminolysis, potentially through suppression of glutamine transporter ASCT2 expression. The ASCT2 restoration reversed NDRG2 inhibitory effect on EMT program and tumor metastasis. Mechanistic study indicates that NDRG2 promoted Fbw7-dependent c-Myc degradation by inhibiting Akt activation, and subsequently decreased c-Myc-mediated ASCT2 transcription, in both tumor and NDRG2-knockout MEF cells. Supporting the biological significance, the reciprocal relationship between NDRG2 and ASCT2 were observed in multiple types of tumor tissues, and associated with tumor malignancy.
Conclusions: NDRG2-dependent repression of ASCT2 presumably is the predominant route by which NDRG2 rewires glutaminolysis and blocks metastatic tumor survival. Targeting glutaminolytic pathway may provide a new strategy for the treatment of metastatic tumors.
Keywords: NDRG2, ASCT2, c-Myc, glutaminolysis, mucoepidermoid carcinoma, EMT

 Global reach, higher impact
Global reach, higher impact