10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2026; 22(1):258-279. doi:10.7150/ijbs.119630 This issue Cite
Review
Gut Microbiota-Driven Pathways Linking Chronic Stress to Tumor Progression
1. Department of Gastroenterology and Hepatology, Sichuan University-University of Oxford Huaxi Joint Centre for Gastrointestinal Cancer, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu, China.
2. Department of Pathology, Sichuan University-University of Oxford Huaxi Joint Centre for Gastrointestinal Cancer, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu, China.
* These authors contribute equally to this work.
Received 2025-6-15; Accepted 2025-11-8; Published 2026-1-1
Abstract
Chronic stress is increasingly recognized as a critical factor influencing tumor progression, but its underlying mechanisms remain incompletely understood. This review examines the role of gut microbiota as a critical mediator linking chronic stress to tumor progression. Recent evidence suggests that chronic stress triggers gut dysbiosis, characterized by reduced microbial diversity, depletion of beneficial bacteria, and enrichment of potentially harmful species. We summarize the mechanisms by which chronic stress regulates gut microbial dysbiosis, including stress-related hormone signaling, intestinal inflammation, mucosal barrier disruption, and altered gut motility. Additionally, we examine how stress-induced dysbiosis contributes to tumor progression through immune suppression, metabolic reprogramming, enhanced tumor stemness, and potentially through barrier dysfunction, and chronic inflammation. We further discuss potential therapeutic interventions, including specific probiotics, prebiotics and other strategies that may help suppress tumor development by modulating the stress-microbiota-cancer axis. In conclusion, these emerging insights provide a foundation for novel therapeutic strategies that target the stress-microbiome-cancer axis, which may help suppress tumor progression and complement conventional cancer treatments to improve clinical outcomes in cancer patients.
Keywords: Chronic stress, Tumor progression, Gut microbiota, Dysbiosis.
1. Introduction
Cancer is a major public health concern and a leading cause of mortality, with an estimated 20 million new cancer diagnoses and approximately 9.7 million cancer-related deaths worldwide in 2022 [1]. The global cancer burden continues to rise, with increasing incidence and mortality rates in many regions [2]. Chronic stress, characterized by the sustained physiological response to emotional pressures, is now considered not only a psychological concern but also a factor that may influence the course of various diseases [3]. There is growing evidence that psychological stress is prevalent among cancer patients and is more frequently being considered a risk factor for cancer development and progression [4].
Chronic stress is believed to influence the occurrence, development, recurrence, and metastasis of various types of cancer through alterations in the neuroendocrine system [5-7]. Psychological stress can activate the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS), leading to the release of stress hormones such as cortisol, catecholamines (epinephrine and norepinephrine), and other neurotransmitters [8]. These stress mediators can directly or indirectly affect cancer cells and their microenvironment, promoting tumor growth [5, 9], angiogenesis [10, 11], metastasis [7, 12], and immune evasion [13-15]. From a clinical perspective, pharmacological blockade of these hormonal pathways has yielded mixed results. For instance, a recent study reported no overall association between β-blocker use and breast cancer prognosis, except for a potential benefit in triple-negative breast cancer patients [16]. A meta-analysis similarly found that β-blocker use had no significant effect on cancer-specific survival overall, but revealed substantial heterogeneity across cancer types, showing potential benefit in melanoma and breast cancer while even suggesting harm in pancreatic and head-and-neck cancers [17]. These findings imply that the cancer-promoting effects of chronic stress may not be fully explained by neurohormonal signaling alone, and that additional mechanisms in this process need further investigation.
The human gut microbiota, a complex ecosystem of trillions of microorganisms, plays a pivotal role in facilitating bidirectional communication along the gut-brain axis through microbial metabolite signaling and neuroimmune modulation [18]. Chronic stress has been associated with reduced microbial diversity, increased intestinal permeability, and a shift towards a pro-inflammatory gut microbiome profile [19, 20]. Simultaneously, gut microbial dysbiosis is now widely recognized as an important factor promoting tumor development and progression [21], with current research indicating that approximately 20% of cancers are closely associated with microbiome [22]. Given these evidences, we may infer that these stress-induced changes in the gut microbiota could contribute to the development and progression of cancer.
Emerging evidence further positions the gut microbiota as a critical mediator of stress-induced effects on tumor biology [23-25]. In this review, we aim to dissect the mechanisms by which chronic stress influences the gut microbiota and, in turn, how these changes can modulate tumor progression. By understanding the complex interplay between chronic stress, gut microbiota, and cancer, we hope to identify potential therapeutic targets and guide the development of novel interventions that integrate stress reduction and microbiota manipulation to improve cancer outcomes.
2. Epidemiological evidence linking chronic stress and tumor progression
Tumor development is a process involving complex interactions among genetic, environmental, and lifestyle factors [26, 27]. Recent epidemiological studies have suggested a close link between chronic stress and tumor progression. A large-scale meta-analysis by Chida et al. examined the relationship between psychosocial stress and cancer incidence by pooling data from 142 prospective studies. They revealed that individuals experiencing psychosocial stress had a 6% higher likelihood of being diagnosed with cancer compared to those with lower stress levels (hazard ratio = 1.06, 95% confidence interval 1.02-1.11, P = 0.005), providing robust evidence that stress is a risk factor for cancer [28]. Further supporting this, Wang et al. performed another systematic review and concluded that psychosocial stress was associated with an increased incidence of lung cancer, oral cavity cancer, prostate cancer and skin cancer [29]. Moreover, a prospective study involving 3,015 women found that stress levels were significantly associated with the overall cancer risk [30]. In addition to the increased risk of cancer incidence, chronic stress has also been shown to negatively affect cancer outcomes. This is evidenced by increased cancer-specific mortality in patients with breast cancers, lung cancer, colorectal cancer, hematopoietic system cancer, prostate cancer, kidney cancer and bladder cancer who experienced psychosocial stress [29]. Moreover, emotional distress (ED), a common manifestation of psychosocial stress, has been associated with poorer clinical outcomes in patients with advanced non-small-cell lung cancer treated with immune checkpoint inhibitors (ICIs). Compared to patients without ED, patients with ED exhibited significantly shorter median progression-free survival (7.9 vs. 15.5 months), a lower objective response rate (46.8% vs. 62.1%), and a reduced 2-year overall survival rate (46.5% vs. 64.9%) [31].
Although these studies provide valuable insights into the relationship between chronic stress and tumor progression, a comprehensive review summarizing the findings of previous research is necessary to better understand the mechanisms underlying this association and to identify potential areas for future investigation.
3. Chronic stress reshapes the intestinal flora
While previous studies have elucidated various mechanisms by which chronic stress directly promotes tumor progression through neuroendocrine hormones [4, 8], these pathways may not fully explain the relationship between stress and cancer. Growing evidence suggests that the influence of chronic stress extends beyond direct neuroendocrine regulation, involving more complex systemic changes [8]. Among these, the gut microbiota, a key component of the gut-brain axis disturbed under stress [32], may serve as an important bridge connecting chronic stress to tumor biology.
3.1 Chronic stress leads to disturbance of gut microbiota
It is estimated that the human microbiome comprises around 1014 bacterial cells, which is 10 times the number of human cells [33]. Among these, the gastrointestinal microbiota accounts for the majority of the total bacterial population in the human body and is predominantly composed of the phyla Bacteroidetes and Firmicutes [34]. Under normal conditions, the gut microbiota maintains a dynamic balance between beneficial and potentially pathogenic bacteria. However, chronic stress disrupts this balance, leading to alterations in microbial composition and function that critically mediate the impact of stress on host health.
3.1.1 Chronic stress leads to changes in the composition of gut microbiota
In rodent models, chronic stress exposure, such as chronic restraint or social defeat, consistently reduce microbial diversity, marked by declines in α diversity (the species richness and evenness within a single sample), and increased β diversity (the difference in species composition between samples) [35, 36]. These findings are corroborated by a systematic review demonstrating that psychological stress negatively correlated with α diversity indices, including the Shannon, Chao1, and Simpson indices, while being positively associates with β diversity metrics, such as weighted UniFrac distances, further highlight stress-induced structural disruptions in the microbial community [37]. Beyond disruptions in overall diversity, chronic stress drives specific changes in microbial taxa in animal models [38-44], typically characterized by the expansion of pro-inflammatory species and the depletion of beneficial commensals. For instance, in the water avoidance stress (WAS) model, mice exhibited a 2-fold decrease in Bacteroidetes and a 2 to 3-fold increase in Firmicutes and Gammaproteobacteria, suggesting a dysbiotic profile linked to pro-inflammatory potential [45]. Similarly, in the chronic restraint stress model, mice showed an increase in pro-inflammatory taxa such as Peptostreptococcaceae, Helicobacter, Streptococcus, and Enterococcus faecalis [20]. Notably, these shifts are often accompanied by the overgrowth of pathobionts, including increased relative abundance of Muribaculaceae, Enterorhabdus, Marvinbryantia and Candidatus Arthromitus [19]. Concurrently, chronic stress depletes bacteria that are critical for maintaining gut homeostasis. Lactobacillus, a genus known for its anti-inflammatory properties, was significantly reduced under chronic stress conditions [46]. Metagenomic analyses further highlight stress-associated declines in Bacteroides and Alistipes, which are involved in bile acid metabolism and inflammation suppression, while increases in Parasutterella and Rikenellaceae_RC9_gut_group correlate with intestinal dysregulation [47]. Additionally, chronic stress decreases the abundance of Lachnospiraceae and Roseburia, which produce short-chain fatty acids (SCFAs) that are essential for barrier integrity and immune regulation [20]. In addition, numerous studies have reported alterations in gut microbiota under different stress models [48-52], which we have summarized in Table 1.
These preclinical findings are supported by emerging clinical evidence demonstrating parallel microbial alterations in humans exposed to chronic psychosocial stressors [39, 53]. Students experiencing academic stress exhibited reduced fecal lactic acid bacterial levels [54]. Similarly, frontline healthcare workers experiencing psychological stress during the COVID-19 pandemic exhibited gut microbiota dysbiosis with continuously decreasing α-diversity. Beneficial bacteria such as Eubacterium hallii and Lachnospiraceae ND3007 were reduced, both belonging to the Lachnospiraceae family, which are known butyric acid-producing bacteria [55]. Psychiatric cohorts provide additional support, as depressed patients display altered α and β diversity and compositional shifts across multiple taxonomic levels [56-58]. Evidence from gastrointestinal disorders provides additional insight into stress-microbiota interactions. In ulcerative colitis (UC), patients with comorbid depression or anxiety had lower fecal microbial richness and diversity, characterized by an overrepresentation of Lactobacillales, Sellimonas, Streptococcus, and Enterococcus, and depletion of Prevotella and Lachnospira [59]. Another study further demonstrated that the relative abundances of Enterobacterales and Enterococcaceae were positively correlated with anxiety and depression scores in UC patients [60]. In patients with Crohn's disease, those experiencing high levels of stress showed a significant decrease in the phylum Firmicutes and genus Anaerostipes, along with a significant increase in Parabacteroides [61]. These microbial alterations were accompanied by metabolomic dysregulation [59, 61]. Longitudinal evidence from the Swiss IBD Cohort Study further identify this association, among 171 participants in clinical remission, higher perceived stress was linked to significantly lower mucosal microbial α diversity, while anxiety and depressive symptoms correlated with β diversity differences. Taxa from Lachnospiraceae, Fusobacteriaceae, Ruminococcaceae, Veillonellaceae, Alcaligenaceae, Desulfovibrionaceae, and Bacteroidaceae were consistently reduced in individuals with higher psychological distress [62].
Together, these findings indicate that chronic stress consistently reduces microbial diversity, depletes benificial taxa, and favors a pro-inflammatory microbial profile, those changes may amplify systemic inflammation and contribute to cancer-promoting immune dysregulation. A summary of stress-associated alterations in gut microbiota across animal models and human studies is provided in Table 1.
3.1.2 Chronic stress leads to changes in the metabolic function of gut microbiota
Beyond taxonomic disruption, chronic stress profoundly reprograms microbial metabolic networks. One important effect is on tryptophan metabolism in the microbiota-gut-brain axis, where long-term stress shifts the balance toward the kynurenine metabolic pathway [63]. Separately, chronic social defeat in mice decreased the prevalence of pathways associated with the synthesis and metabolism of neurotransmitter precursors and SCFAs [64]. Bile acid metabolism represents another pathway affected by chronic stress. A previous study showed that chronic stress can reshape bile acid metabolism by altering the abundance of Ruminococcaceae, a family central to secondary bile acid production, which may lead to elevated levels of hydrophobic secondary bile acids such as deoxycholic acid [65]. These compounds emerged as drivers of intestinal barrier dysfunction and pro-tumorigenic signaling [66, 67]. Chronic stress also depletes protective microbial metabolites. In murine models, chronic restraint stress exposure significantly reduced the abundance of gut microbial metabolites phosphatidylethanolamine (PE) and hemolytic phosphatidylethanolamine [LysoPE (15:0/0:0)] in mice, and the reduction of these two metabolites supports a link between chronic stress-altered gut microbial metabolites and enhanced colorectal cancer growth and metastasis [68].
Stress-associated alterations in gut microbiota across animal models and human studies.
| Category | Model/Population | Main microbiota changes | References |
|---|---|---|---|
| Animal models | Chronic unpredictable mild stress | ↑ Bacteroidetes; Bacteroidaceae, Helicobacteraceae, Rikenellaceae, Muribaculaceae.↓ Firmicutes; Eggerthellaceae, Bifidobacteriaceae, Lactobacillaceae, Prevotellaceae, Ruminococcaceae, Lachnospiraceae; Lactobacillus, Bacteroides, Bifidobacterium. | [40-44] |
| Animal models | Social stress | ↑ Parabacteroides; Muribaculaceae; Enterorhabdus, Clostridium, Flavobacterium.↓ Marvinbryantia, Candidatus Arthromitus, Lactobacillus, Roseburia, Bacteroides, Turicibacter; Lactobacillus johnsoni. | [19, 39, 46, 48, 151] |
| Animal models | Chronic restraint stress | ↑ Peptostreptococcaceae; Helicobacter, Streptococcus, Oscillibacter, Gordonibacter; Enterococcus faecalis, Citrobacter rodentium.↓ Porphyromonadaceae; Parabacteroides, Ruminococcus, Prevotella, Lactobacillus, Alistipes. | [20, 38, 49-51] |
| Animal models | Water avoidance stress | ↑ Firmicutes, Gammaproteobacteria, Actinobacteria; Ruminococcaceae, Christensenellaceae-R-7; Staphylococcus, Erysipelatoclostridium, Streptococcus.↓ Bacteroidetes, Firmicutes; Proteobacteria, Lachnospiraceae, Muribaculaceae; Lactobacillus, Bifidobacterium. | [45, 52, 95] |
| Animal models | Emotional stress | ↑ Parasutterella and Rikenellaceae_RC9.↓ Bacteroides, Alistipes. | [47] |
| Clinical (psychological stress) | Students under exam stress | ↓ Lactic acid bacteria. | [54] |
| Clinical (psychological stress) | Healthcare workers during COVID-19 experiencing psychological stress | ↓ α-diversity; ↓ Eubacterium hallii, Lachnospiraceae ND3007. | [55] |
| Clinical (psychological stress) | Patients with depression/anxiety | Altered α- and β-diversity. ↑ Thermoanaerobacteriaceae; Eggerthella, Holdemania, Gelria, Turicibacter, Paraprevotella, Anaerofilum; Eggerthella lenta, Flavonifractor plautii.↓Prevotellaceae; Lactobacillus, Prevotella, Dialister; Ruminococcus bromi, Victivallis vadensis, Ruminococcus bicirculans. | [39, 56-58] |
| Clinical (GI disease + stress comorbidity) | UC patients with depression/anxiety | ↓ Richness and diversity.↑Enterobacterales, Enterococcaceae; Lactobacillales, Sellimonas, Streptococcus, Enterococcus.↓ Prevotella, Lachnospira. | [59, 60] |
| Clinical (GI disease + stress comorbidity) | CD patients with stress | ↑ Parabacteroides. ↓Firmicutes; Anaerostipes. | [61] |
| Clinical (GI disease + stress comorbidity) | Swiss IBD cohort | ↓ α-diversity.↓ Lachnospiraceae, Fusobacteriaceae, Ruminococcaceae, Veillonellaceae, Alcaligenaceae, Desulfovibrionaceae, Bacteroidaceae. | [62] |
GI, gastrointestinal; UC, ulcerative colitis; CD, Crohn's disease; IBD, Inflammatory bowel disease.
Human studies corroborate these findings, demonstrating that stress-associated microbiota alterations (e.g., reduced Faecalibacterium and Bacteroides, increased Blautia and Collinsella) correlate with disrupted lipid metabolism [69]. Specifically, diminished fecal cysteine levels, a glutathione precursor, compromise antioxidant defenses, exacerbating oxidative damage to the intestinal epithelium [70]. Extending beyond these metabolic pathways, another study provided additional insights into stress-related dysbiosis effects. Comparative analyses of depressed and non-depressed subjects revealed 279 differentially expressed bacterial synthetic proteins, primarily involved in glucose and amino acid metabolism [71], highlighting how psychological distress impacts microbial protein expression and consequently impacts host physiological function.
Collectively, chronic stress transforms the gut microbiota into a pro-inflammatory and metabolically dysfunctional state. This dual assault, driven by microbial taxonomic imbalance and metabolic perturbation, erodes intestinal barrier function and sustains systemic inflammation, thereby creating a permissive microenvironment for disease progression.
3.2 Mechanisms by which chronic stress promotes dysbiosis of gut microbiota
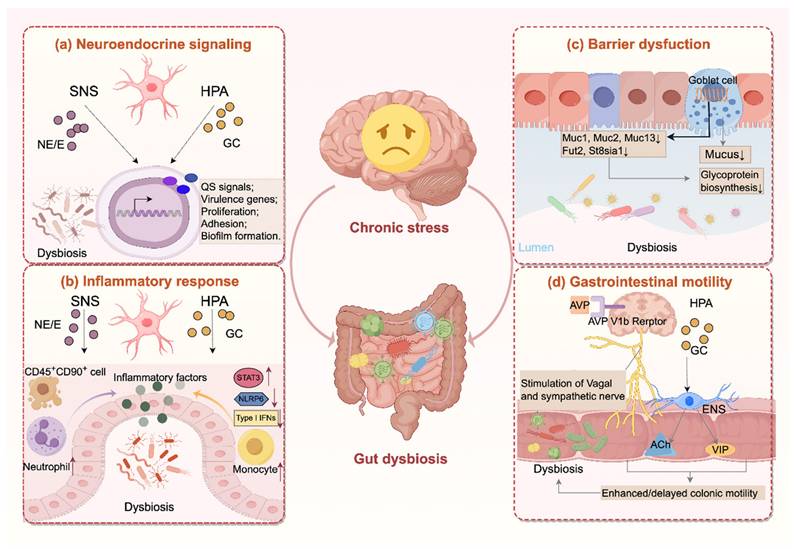
Chronic stress drives the gut microbiota through multiple mechanisms, involving neuroendocrine signaling, inflammatory response, barrier dysfunction, and alterations in gastrointestinal motility. An overview of these pathways is presented in Figure 1.
3.2.1 Chronic stress regulates gut bacteria directly through stress-related hormones
Chronic stress simultaneously activates the HPA axis and the SNS, resulting in sustained release of stress hormones including cortisol and catecholamines. Within the HPA axis, stress stimulation drives the hypothalamus to secrete corticotropin-releasing hormone (CRH), which triggers adrenocorticotropic hormone (ACTH) release from the anterior pituitary and subsequent cortisol secretion from the adrenal cortex [72]. Under physiological conditions, excessive cortisol exerts a negative feedback loop that suppresses CRH and ACTH production [73]. However, chronic stress disrupts this glucocorticoid-mediated feedback system, resulting in persistently elevated or reduced cortisol levels [74, 75]. Meanwhile, sensory information is processed by the prefrontal cortex and transmitted to the amygdala, and the latter relays signals to the dorsomedial hypothalamus (DMH) via the stria terminalis. By sending excitatory signals to brainstem regions including the rostral medullary raphe and rostral ventrolateral medulla, which house sympathetic premotor neurons projecting to the spinal cord, the DMH activates the SNS. These premotor neurons engage sympathetic preganglionic neurons that release acetylcholine, stimulating nicotinic receptors on adrenal medullary chromaffin cells, leading to systemic secretion of epinephrine and norepinephrine [76].
Accumulating evidence suggests that these hormones can directly interact with gut microbiota, modifying their growth and survival, thereby reshaping microbial composition, metabolic function, and pathogenic potential [77, 78]. Although direct evidence for the effect of cortisol on intestinal microbiota remains limited, microbial transcriptomic data from other mucosal sites show that cortisol reprograms bacterial gene expression, upregulating genes related to proteolysis, iron acquisition, and motility [79]. By contrast, catecholamine-bacteria interactions are well characterized. Physiological plasma concentrations of catecholamines typically range from 23-85 pg/mL for epinephrine and 176-386 pg/mL for norepinephrine, but during or following stress these levels may rise sharply, often by 5 to 20-fold [77]. At such stress-relevant concentrations, studies have demonstrated that catecholamines differentially regulate key bacteria. Norepinephrine inhibits Porphyromonas gingivalis growth while decreasing quorum-sensing signals, while enhancing virulence-associated rgpB expression [80]. Both epinephrine and norepinephrine suppress the growth of Eikenella corrodens and Prevotella intermedia, yet promote the proliferation of Fusobacterium nucleatum and Tannerella forsythia [81]. Epinephrine further enhances adhesion, biofilm formation, and virulence across a broad range of pathogens, including Pseudomonas aeruginosa, Enterococcus faecalis, Enterotoxigenic Escherichia, Klebsiella pneumoniae, Staphylococcus aureus and Escherichia coli [82-85]. Mechanistically, catecholamines act as interkingdom signaling molecules sensed by bacterial two-component systems such as QseC/QseS, thereby promoting growth, motility, chemotaxis, biofilm formation and virulence gene expression [86]. Consistently, in vivo studies demonstrate that norepinephrine facilitates bacterial translocation to mesenteric lymph nodes, spleen, and liver [87], highlighting a critical role for stress-induced catecholamines in shaping host-microbe interactions.
Chronic stress disrupts the gut microbiota through multiple pathways. (a) Chronic stress activates the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS), elevating glucocorticoids and catecholamines that regulate quorum sensing (QS), bacterial proliferation, adhesion and virulence gene expression, and biofilm formation. (b) Hormones released during chronic stress exacerbate intestinal inflammation through multiple mechanisms, including activation of pro-inflammatory signaling pathways (e.g., STAT3), inhibition of type I interferon responses, recruitment of immune cells (e.g., neutrophil, monocyte), and disruption of inflammasome activity (e.g., NLRP6). (c) Moreover, chronic stress weakens the mucus barrier by reducing mucin biosynthesis and glycosylation and decreasing goblet cell numbers. (d) In addition, chronic stress alters gut motility through AVP V1b receptor-mediated activation of sympathetic and vagal pathways, and glucocorticoid receptor-dependent Ach/VIP release from enteric neurons, leading to both hypermotility or hypomotility that disrupt microbial stability. Together, these changes drive dysbiosis characterized by reduced diversity, depletion of protective taxa, and overgrowth of pro-inflammatory species. Ach, acetylcholine; AVP, arginine vasopressin; E, epinephrine; ENS, enteric nervous system; GC, glucocorticoid; NE, norepinephrine; VIP, vasoactive intestinal peptide.
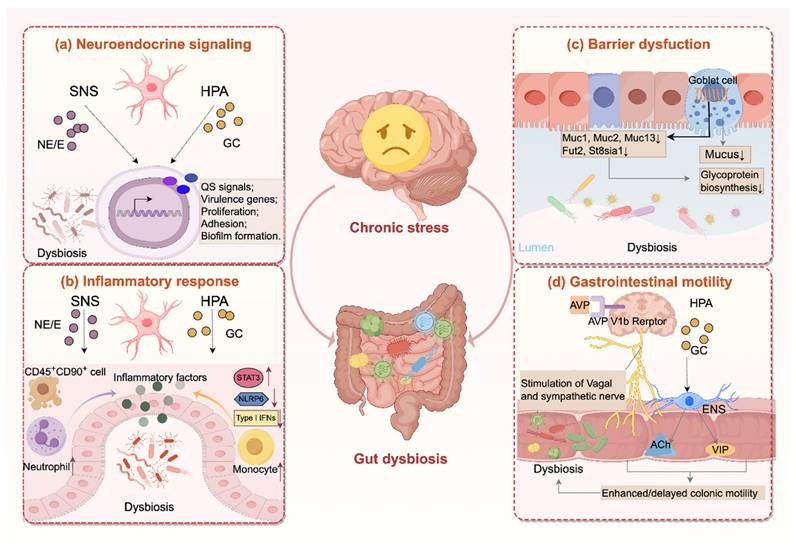
Together, these findings suggest that stress hormones release under chronic stress could act as microbial growth signals, directly reshaping gut microbial composition and pathogenic potential.
3.2.2 Chronic stress alters gut microbiota by fostering a pro-inflammatory environment
Mounting evidence indicates that the gut microbiota is altered under inflammatory condition in gut tract [88]. Since intestinal inflammation destabilizes commensal communities and selectively promotes the expansion of bacterial taxa with genetic adaptations enabling them to exploit nutrient resources enriched under inflammatory conditions [89]. Therefore, as studies have demonstrated that chronic stress establishes an intestinal inflammatory environment, this may contribute to microbial dysbiosis.
At the transcriptional level, stress activates β-adrenergic signaling, linking sympathetic activity to a gene expression program characterized by upregulated pro-inflammatory genes and suppressed type I interferon responses [90]. Consistently, elevated CRH under stress suppresses intestinal NLRP6 inflammasome, thereby aggravating intestinal inflammation and reshaping the microbiota [45]. Stress hormones also perturb intestinal immune cell homeostasis. Stress-induced glucocorticoid triggers apoptosis of CD45+CD90+ cells, impairing IL-22-dependent epithelial repair and allowing overgrowth of pathobionts associated with Crohn's disease [91]. Elevated catecholamines activate β-adrenergic signaling and subsequently the STAT3 pathway, thereby upregulating pro-inflammatory cytokines (IL-1β, IL-6, IL-17A, IL-22) and neutrophil chemokines (CXCL1, CXCL2), which drive neutrophil infiltration and exacerbate intestinal inflammation [92]. The presence of such a systemic low-grade inflammatory state is supported by clinical evidence, as a systematic review of 24 studies reported significantly higher plasma concentrations of TNF-α and IL-6 in patients with major depression [93].
Beyond these pathways, the enteric nervous system (ENS) is another critical mediator of stress-induced inflammatory responses. Persistently elevated glucocorticoid levels generate inflammatory subsets of enteric glial cells (EGCs) that promote monocyte recruitment through CSF1 production and induce inflammation via TNF secretion [94]. In the colon tissue of rats under WAS, EGCs also regulate the activity of nitric oxide synthase (NOS) and cholinergic neurons in the ENS, leading to a significant decrease in the abundance of Firmicutes, Proteobacteria, Lactobacillus, and Lachnospiraceae_NK4A136, while increasing the relative abundance of Actinobacteria Ruminococcaceae_UCG-005 and Christensenellaceae-R-7 [95].
Taken together, these findings suggest that chronic stress induces intestinal inflammation through diverse neuroendocrine and immune mechanisms, and given that intestinal inflammation is a well-established driver of microbial dysbiosis, this may partly explain how chronic stress perturbs gut microbial homeostasis.
3.2.3 Chronic stress disturbed gut microbiota through impairing gut barrier
The intestinal mucus barrier, consisting of a dual-layered system in the colon, serves as a crucial defense against gut microbes, dietary antigens, and other harmful toxins [96, 97]. Beyond its protective role, the mucus layer also shapes the gut microbiota by providing O-glycans that serve as attachment sites and nutrient sources that facilitate bacterial colonization and growth [97, 98]. Consequently, factors that disrupt mucus integrity not only allow closer contact between microbes and epithelial cells but also create a selective environment that favors pathobiont overgrowth.
Chronic stress has been reported to impair the intestinal mucus barrier, significantly reducing the thickness of the colonic mucus layer, accompanied by increased bacterial penetration into the inner mucus layer [19, 99]. Mechanistically, these effects are linked to transcriptional changes in glycoprotein biosynthesis genes (Muc1, Muc13) and mucin glycosylation genes (Fut2, St8sia1), which disrupt normal mucin synthesis and glycosylation [19]. In the duodenum, stress suppresses neuronal activity in the central amygdala (CeA), reducing vagal excitability and thereby inhibiting Brunner's gland mucus secretion, ultimately lowering Lactobacillus colonization [100]. In the colon, stress downregulates Muc2, together with its positive regulator Cdx2, and decreases goblet cell numbers, collectively impairing mucus production [20]. Concurrently, Muc13 expression is also reduced, further compromising mucus barrier [101]. In addition, chronic stress also alters mucin O-glycosylation, disrupting the biochemical structure and reducing the cohesiveness of the mucus layer, thereby weakening the barrier integrity [102].
The ultimate consequence of these barrier disruptions is increased intestinal permeability in both rodents and humans, resulting in systemic low-grade inflammation due to bacterial translocation [103, 104], and enabling bacteria to translocate to extraintestinal organ systems [105]. Collectively, these findings suggest that chronic stress may perturb the gut microbiota, at least in part, through disruption of the mucus barrier.
3.2.4 Chronic stress modulates gastrointestinal motility and thus changes gut microbiota
Gastrointestinal transit time is a major determinant of microbial composition and metabolic activity [106]. Individuals with delayed gastrointestinal transit exhibit a distinct microbial profile, with a marked reduction in Bacteroides abundance and an increase in Firmicutes [107]. Clinical observations in constipated patients show significant reductions in Bifidobacterium and Bacteroides abundance [108], coupled with increased relative proportions of methanogenic archaea compared to healthy controls [109]. These findings emphasize the role of normal intestinal motility as a critical factor in maintaining microbial homeostasis [110]. Studies have demonstrated that chronic stress stimuli can modulate intestinal motility, which may influence the composition and function of gut microbiota. These motility-associated changes in gut microbiota highlight the importance of understanding the specific mechanisms by which chronic stress affects intestinal transit.
Researches reveal that stress exhibits bidirectional effects on gut motility, either accelerating or decelerating intestinal transit. In terms of accelerated colonic motility, chronic psychological stress has been shown to enhance proximal colonic transit through activation of AVP V1b receptors in the brain [111]. Similarly, experimental models simulating chronic stress through maternal separation (MS) and CRH administration increase colorectal motility [112], with CRH modulating colonic activity by regulating both vagal and sympathetic components of the autonomic nervous system [113]. Glucocorticoid receptor signaling pathways also contribute to stress-induced enhancement of colonic motility [114]. Additionally, rats subjected to chronic unpredictable mild stress (CUMS) exhibit increased numbers of enteric neurons (particularly cholinergic and VIP-secreting motor neurons) and glial cells in the ileal submucosal plexus, further accelerating intestinal transit [115]. Conversely, stress can also delay intestinal transit. Clinical evidence indicates that adverse life experiences can influence neurophysiological pathways, exacerbating constipation symptoms [116]. A large cross-sectional study confirmed that anxiety states significantly correlate with increased constipation risk (OR: 1.49) [117]. Animal studies provide further support, demonstrating that CUMS stimulation prolongs gastrointestinal transit time in mice [118, 119]. These stress-induced alterations in gut motility, whether accelerating or slowing transit, modify the intestinal microenvironment by changing factors such as substrate availability in the colon. These changes selectively favor the growth of certain bacterial populations while inhibiting others, ultimately reshaping the composition of the gut microbiome [106].
4. Chronic stress promotes tumor progression by regulating gut microbiota
Chronic stress profoundly reshapes gut microbiota composition and function, raising the critical question of whether these microbial changes could influence tumor development and progression. Although most mechanistic evidence derives from rodent models, which raises important translational limitations, emerging data directly suggest that gut microbiota and their metabolites can regulate tumor progression by impairing anti-tumor immune surveillance, enhancing tumor cell stemness, and driving the accumulation of pro-tumorigenic metabolites (Figure 2).
Microbiota-dependent mechanisms linking chronic stress to tumor progression. (a) Chronic stress depletes beneficial taxa, including Blautia, Lactobacillus johnsonii, Akkermansia muciniphila, and Bifidobacterium, leading to reduced microbial metabolites. These changes collectively enhance tumor cell stemness, impair anti-tumor immunity, promote tumor proliferation, and drive metastasis. (b) Lactobacillus johnsonii depletion reduces protocatechuic acid (PCA), activating Wnt/β-catenin signaling and increasing stemness of colorectal cancer (CRC) cells; loss of Akkermansia muciniphila -derived butyrate impairs HDAC inhibition, stabilizes LRP5, and enhances β-catenin signaling, promoting breast cancer (BC) cell stemness. (c) Blautia depletion reduces acetate levels, impairing acetyl-CoA metabolism in CD8⁺ T cells, thereby suppressing IFN-γ production and anti-tumor cytotoxicity. (d) Loss of Akkermansia muciniphila decreases outer membrane vesicles (OMVs) release, facilitating CRC cell proliferation. (e) Bifidobacterium depletion disrupts oleate hydratase activity, downregulated degradation of oleic acid, which enhances CRC and BC metastasis.

One important example involves stress-sensitive commensals that sustain CD8⁺ T cell immunity. Blautia species are consistently reduced under chronic stress in both breast cancer patients and mouse model, leading to diminished production of acetate, a SCFA essential for CD8⁺ T cell effector function. Supplementation of acetate fuels acetyl-CoA metabolism within CD8⁺ T cells, thereby sustaining IFNγ production and anti-tumor cytotoxicity. Thus, stress-induced depletion of Blautia suppresses this pathway and facilitates breast cancer progression [23]. Notably, Blautia abundance also correlates with improved response to PD-1 blockade, where Blautia enhanced CD8⁺ T cell infiltration and suppressed tumor growth [120, 121]. Lactobacillus johnsonii is another key bacterium found to be depleted in mouse models of colorectal cancer under conditions of chronic stress [24]. Restoration of L. johnsonii or its metabolite protocatechuic acid (PCA) suppresses β-catenin signaling and inhibits tumor stemness [24]. The protective role of Lactobacillus johnsonii extends to other cancer types as well. In papillary thyroid carcinoma (PTC), decreased levels of Lactobacillus johnsonii are observed in tumor tissues of patients with lymph node metastasis, while supplementation with Lactobacillus johnsonii has been shown to inhibit tumor progression by suppressing the Wnt/β-catenin pathway [122]. Beyond its direct impact on tumor cell signaling, Lactobacillus johnsonii is critical for orchestrating a robust anti-tumor immune response. A fasting-mimicking diet, which increases Lactobacillus johnsonii levels, also boosts the infiltration of CD8+ T cells into tumors, leading to tumor inhibition [123]. Mechanistically, phospholipids from Lactobacillus johnsonii can mature bone marrow-derived dendritic cells by upregulating genes related to maturation and migration [124], and it also promotes the synthesis of the metabolite indole-3-propionic acid (IPA), which enhances the efficacy of immune checkpoint blockade (ICB) in multiple tumor types by promoting the differentiation of progenitor exhausted CD8+ T cells [125]. Another critical commensal, Akkermansia muciniphila, is also depleted under chronic stress in both patients and mouse model. In breast cancer, loss of Akkermansia muciniphila-derived butyrate, a histone deacetylase (HDAC) inhibitor that suppresses Wnt/β-catenin signaling via LRP5 destabilization, promotes tumor stemness and accelerates tumor progression [25]. In colorectal cancer, stress-induced depletion of Akkermansia muciniphila impairs the release of protective outer membrane vesicles (OMVs), thereby facilitating tumor cell proliferation [126]. Moreover, Akkermansia muciniphila has also been recognized as a beneficial commensal with tumor-suppressive functions in colorectal cancer by inhibiting AhR/β-catenin signaling [127]. In addition, outer membrane proteins of Akkermansia muciniphila such as Amuc_1100 and Amuc_2172 remodel the tumor microenvironment and promote CD8⁺ T cell immunity [128, 129].
Beyond the depletion of protective taxa, chronic stress also reshapes microbial metabolic network. Elevated glucocorticoid levels during chronic stress inhibit Bifidobacterium growth, impairing its ability to degrade oleic acid. The resulting accumulation of serum oleic acid enhances metastatic potential in both breast and colorectal cancer [130]. Chronic stress also alters bile acid metabolism, marked by increased deconjugation and secondary bile acid synthesis in mice [65]. These changes may be mediated in part by bile salt hydrolase (BSH), an enzyme broadly distributed among gut microbes [131], which regulates bile acid pools. Recent work has highlighted the structural diversity of BSH and its pivotal role in modulating host-microbiota interactions, with emerging implications in cancer progression [132]. In particular, BSH activity in Bacteroides increases unconjugated bile acids, which activate bile acid receptors and β-catenin/CCL28 signaling, driving Treg-mediated immunosuppression and colorectal cancer progression [67]. Together, these findings suggest that stress-induced depletion of protective commensals removes key microbial defenses against tumor progression across cancer types.
In addition to the evidence described above, certain stress-induced alterations may also contribute to the interactions among chronic stress, the gut microbiota, and tumor progression. First, chronic stress promotes intestinal inflammation in a microbiota-dependent manner. Across multiple stress paradigms, stress exacerbates colitis, elevates colonic cytokines and ROS, and induces dysbiosis [133, 134], these effects were reversed in germ-free or broad-spectrum antibiotic-treated mice [135]. Given the well-established link between chronic inflammation and tumorigenesis [136, 137], stress-driven pro-inflammatory microbiota may create a permissive milieu for cancer initiation and progression. Second, stress impairs epithelial barriers. In addition to the mucus barrier, the epithelial tight junctions, another critical component of the gut barrier [138], have also been shown to be disrupted under chronic stress, including occludin, TJP1, and TJP2, thereby increasing intestinal permeability and bacterial translocation [139, 140]. Mechanistically, CRH enhances paracellular and transcellular permeability [141], promotes autophagy and Paneth cell metaplasia [142]. Glucocorticoid signaling is another key contributor, as it reduces the expression of tight junction protein such as occludin both in vitro and in vivo, effects that can be reversed by the corticosteroid receptor antagonist RU-486 [143, 144]. This indicates that the changes occur through elevated corticosteroid levels, and likely via activation of epithelial cell glucocorticoid receptors (GR) [145]. Mast cell activation further contributes to barrier disruptions, as chronic stress induced mast cell hyperplasia and activation [146], and human data further confirm stress-induced intestinal hyperpermeability via CRH-mast cell pathways [147]. Such barrier dysfunction allows pathogenic bacterial translocation and systemic dissemination of microbial metabolites, which may in turn facilitate tumor development in colon and distant organs [148-150]. Third, chronic stress diminishes beneficial taxa such as Lactobacillus and Bifidobacterium [151], which a normally exert anti-inflammatory, antioxidant, and barrier-protective functions [152, 153]. For example, S-layer proteins from Lactobacillus crispatus interact with DC-SIGN on dendritic cells to attenuate mucosal inflammation in the lower female reproductive [154], while the secreted factor p40 from Lactobacillus rhamnosus GG promotes colonic Tregs differentiation and preserves epithelial barrier function under inflammatory stress [155]. The enzymatic protein LPH from Lactobacillus further contributes to intestinal protection by generating muramyl dipeptide to activate NOD2 signaling [156]. Moreover, extracellular vesicles from Lactobacillus paracasei (LpEVs) counteract LPS-induced inflammation by reducing the expression of pro-inflammatory cytokine (IL-1α, IL-1β, IL-2, and TNFα) while upregulating IL-10 and TGFβ [157]. Certain metabolites of Lactobacillus also have direct tumor-suppressive roles, γ-linolenic acid from Lactobacillus plantarum MM89 induce ferroptosis in colorectal cancer cells [158], reuterin from Lactobacillus reuteri disrupts redox homeostasis to inhibit tumor growth [159], and indole derivatives such as IAA, ICA, ILA, and IPA from Lactobacillus modulate Treg differentiation, enhance dendritic cell function, and reprogram CD8⁺ T cell chromatin accessibility to strengthen antitumor immunity and improve responses to immunotherapy [125, 160-162]. Moreover, in hepatocellular carcinoma, acetate produced by Lactobacillus reuteri suppresses IL-17A release from ILC3s by modulating histone acetylation of Sox13 at site K30, thereby inhibiting tumor growth [163]. Their depletion under stress removes critical immunoregulatory pathways, potentially compromising tumor surveillance. Supporting clinical evidence comes from a randomized trial in bladder cancer patients, where oral Lactobacillus casei supplementation significantly reduced recurrence rates [164].
In summary, chronic stress appears to promote tumor progression through microbiota-dependent pathways, including immune suppression, metabolic reprogramming, enhanced tumor stemness, barrier dysfunction, and chronic inflammation. Importantly, these microbiota-dependent effects are not uniform across cancers as discussed above. For instance, Blautia depletion under stress has been linked to impaired CD8⁺ T-cell immunity in breast cancer [23], whereas another study reported that Akkermansia muciniphila constrains stemness in breast cancer [25]. In colorectal cancer under chronic stress, loss of Lactobacillus johnsonii has been shown to promote stemness [24], while other evidence suggests that Akkermansia muciniphila suppresses tumor cell proliferation [126]. These discrepancies likely reflect heterogeneity in baseline microbiota across mice, tumor models, stress paradigms and duration, and cancer types.
Moreover, although accumulating evidence suggests that chronic stress, gut microbiota alterations, and cancer progression are linked, most available data remain associative and preclinical. From a causal inference perspective, temporality is insufficiently established because most human studies are cross-sectional. Evidence for a dose-response relationship is lacking, as no studies have systematically compared different levels or durations of stress. By contrast, mechanistic plausibility is relatively well supported by animal studies demonstrating that stress-sensitive taxa and their metabolites can modulate immune surveillance, barrier integrity, and tumor biology. Current human evidence for the stress-microbiota-cancer axis is largely indirect, consisting of studies that examine stress-associated microbial alterations or associations between specific microbial taxa and cancer risk, while research directly connecting all three components remains limited. Nevertheless, several studies have reported associations between stress and microbiota changes, or between microbiota profiles and cancer outcomes, which are summarized in Table 2. These findings broadly align with mechanisms established in preclinical models and provide supportive, although not yet causal, evidence that stress-induced microbial dysbiosis may contribute to tumorigenesis. Notably, evidence appears relatively more robust for colorectal and breast cancers, where stress-induced microbial changes and tumor-promoting effects have been repeatedly documented, whereas data for other cancer types remain sparse. Future research should include well-designed studies that vary stress exposures, incorporate temporal assessments, and control for baseline microbiota to strengthen causal inference. Expanding clinical evidence through prospective cohorts and intervention trials will also be essential to validate preclinical findings and define cancer-type-specific pathways, thereby advancing translational opportunities within the stress-microbiota-cancer axis.
5. The interplay of confounding factors modulating the stress-microbiota-cancer axis
The pathway linking chronic stress to tumor progression via microbiota disruption does not act in isolation. Instead, it is embedded within a complex network of confounding variables, including diet, host genetics, medication use, and lifestyle, all of which can substantially modulate each component of the axis. Disentangling the specific contribution of stress from these factors is a critical challenge.
5.1 Diet
Among all environmental influences, diet is perhaps the most powerful modulator of gut microbiota composition and function [165]. Western-style diets rich in saturated fats and emulsifiers promote dysbiosis, reduce SCFAs-producing bacteria such as Faecalibacterium prausnitzii, and increase pathobionts, thereby impairing barrier integrity and sustaining low-grade inflammation that can prime tumorigenesis [166, 167]. Conversely, diets rich in fiber and polyphenols, such as the Mediterranean diet, foster resilient microbial communities that produce anti-inflammatory metabolites like butyrate, which directly inhibit cancer cell growth and enhance the efficacy of cancer therapies [167]. Diet also shapes the stress response itself, since diets rich in fiber, omega-3 fatty acids, vitamin D, and iron are associated with reduced psychiatric symptoms (depression, anxiety, and stress), while poor nutritional status may exacerbate stress-induced pathophysiology [168, 169]. Thus, acting as a pivotal link among chronic stress, the gut microbiome, and tumor development, diet plays a dual role in either mitigating or potentiating the adverse consequences of stress. Importantly, dietary background must be considered as a key confounding factor, since it can obscure whether observed microbiota changes are stress-driven or diet-driven.
5.2 Host genetics
Host genetics provides a fundamental framework that influences both microbial communities and host responses to chronic stress. Genome-wide association studies have identified specific host genetic variations associated with the abundance of particular microbial taxa [170]. By regulating mucosal immunity pathways and epithelial barrier related genes, host genetics establishes the baseline for microbial stability [171, 172]. Moreover, genetic variation also affects how individuals respond to stress. Polymorphisms in genes can regulate the HPA axis and neurotransmitter systems, which further contribute to variability in stress reactivity and downstream neuroimmune signaling [173], thereby modifying the extent to which chronic stress perturbs the gut microbiota. Collectively, these mechanisms may help explain the observed heterogeneity in microbiota composition, stress resilience, and cancer outcomes across individuals. Host genetics therefore represents a potential confounding factor in the stress-microbiota-cancer axis, as genetic variability may obscure or amplify the effects attributed to chronic stress. Future studies should integrate host genomics with longitudinal microbiome and metabolome profiling to clarify causal relationships.
5.3 Medication
Pharmacological agents are major contributors to microbiota perturbation. Even transient antibiotic exposure can cause rapid diversity loss and long-term shifts, eliminating microbial taxa that are important for immune homeostasis and stress resilience [174, 175]. Such dysbiosis has been linked to reduced efficacy of cancer immunotherapy, as the microbiota is essential for priming anti-tumor immune responses [176]. While antibiotics provide the clearest example, other commonly used drugs such as proton pump inhibitors (PPIs), antidiabetics (metformin), nonsteroidal anti-inflammatory drugs (NSAIDs) and atypical antipsychotics (AAPs), have also been associated with changes in microbiome composition [177]. Antidepressants alter microbial composition as well, raising the possibility that part of their therapeutic effect may be mediated through the gut-brain axis [178]. Taken together, these findings highlight the importance of obtaining a comprehensive medication history, particularly regarding antibiotic use, for accurately interpreting microbiome data in clinical research and patient management.
5.4 Lifestyle factors
Lifestyle factors strongly influence both microbiota and stress responses. Regular physical exercise enhances microbial diversity, increases SCFA producers, and reduces systemic inflammation, thereby mitigating both stress and cancer risk [179]. Conversely, sleep disruption and circadian misalignment induce dysbiosis, hyperactivate the HPA axis, and sustain a pro-tumorigenic inflammatory milieu [180]. Furthermore, individuals under chronic stress often adopt unhealthy behaviors such as smoking and excessive alcohol consumption [181, 182]. These habits are recognized risk factors for cancer, and they also contribute to microbial dysbiosis and increased intestinal permeability [183-185], thereby amplifying the downstream effects of stress on host physiology. Interactions among stress, poor sleep, and unhealthy habits create feedback loops that synergistically drive tumor development.
Collectively, diet, genetics, medication, and lifestyle are not merely background noise but active participants in the stress-gut microbiota-tumor axis. Failure to account for these confounder risks overestimating the direct effects of stress in human studies. Future investigations must employ rigorous longitudinal designs, incorporate detailed covariate tracking, and apply advanced statistical approaches to disentangle causality. A deeper understanding of these interactions will be essential for developing personalized interventions that target this complex network to improve cancer outcomes.
6. Potential therapeutic strategies targeting the stress-microbiota-cancer axis
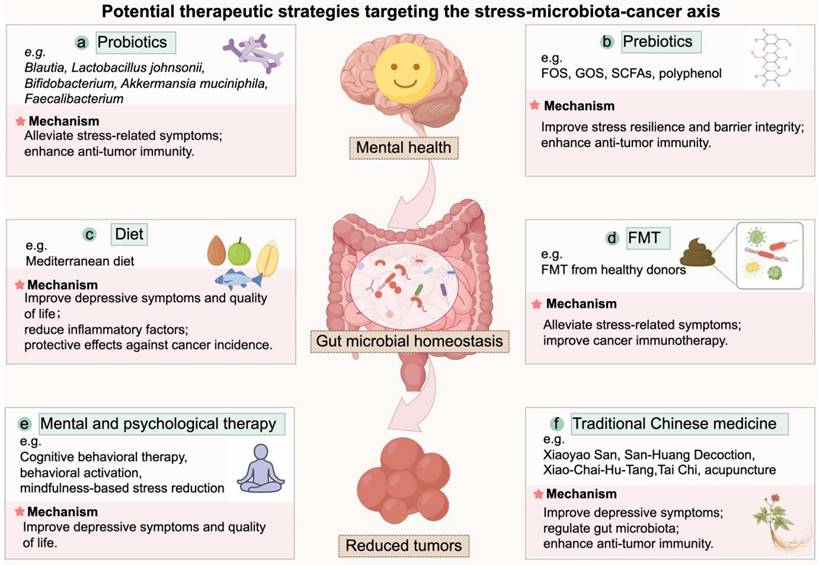
Based on the evidence outlined above, it can be inferred that interventions targeting gut microbiota may serve as a potential strategy to mitigate tumor-promoting effects of chronic stress. Consistent with recent comprehensive reviews, microbiota-directed strategies including probiotics, prebiotics, dietary interventions, and fecal microbiota transplantation (FMT) can reshape the tumor microenvironment and improve therapeutic outcomes in cancers [186, 187]. Meanwhile, mental and psychological therapies and traditional Chinese medicine (TCM)-based approaches may improve patient outcomes through the alleviation of chronic stress and related systemic effects. These strategies warrant further investigation in the context of stress-associated cancer progression, an overview of these potential therapeutic strategies was illustrated in Figure 3.
6.1 Probiotics
As discussed earlier, commensals such as Blautia, Lactobacillus johnsonii, Bifidobacterium, and Akkermansia muciniphila are consistently depleted under chronic stress, and supplementation in animal models restores immune surveillance and suppresses tumor growth [23-25, 130]. In addition to these direct antitumor effects reported above, numerous animal studies report that probiotics alleviate stress-related behavioral and physiological alterations. For instance, pretreatment with Bifidobacterium adolescentis reduced anxiety- and depression-like behaviors induced by chronic restraint stress by remodeling the gut microbiota, suppressing hippocampal inflammation, and upregulating brain-derived neurotrophic factor (BDNF) [188]. Similarly, Bifidobacterium breve CCFM1025 prevents stress-induced emotional disturbances and gastrointestinal dysmotility by restoring microbial balance [189]. Faecalibacterium prausnitzii also prevented anxiety- and depression-like behaviors in CUMS models and protected against sleep deprivation-induced intestinal injury [190, 191]. Importantly, these genera (e.g., Lactobacillus, Bifidobacterium, Faecalibacterium) or their metabolites also display anti-tumor activities in preclinical models. Lactobacillus species act mainly through metabolite-driven pathways, including reuterin-mediated redox disruption, indole derivatives that enhance T cell immunity, and SCFA-mediated suppression of Wnt/β-catenin signaling [125, 158-163]. Bifidobacterium species modulate the tumor microenvironment by boosting host immunity, for example, promoting CD8⁺ T cell responses, activating macrophage or recruiting dendritic cells [192-194]. Faecalibacterium prausnitzii suppresses tumor growth by attenuating inflammation and increasing CD8⁺ T cell infiltration [195]. Thus, probiotics may exert dual benefits by alleviating stress-induced dysfunction and directly modulating tumor biology.
Evidence from human studies provides partial but encouraging support. Most probiotic formulations tested in clinical trials for depression are based on Lactobacillus and Bifidobacterium species [196]. In one randomized controlled trial, a multi-strain probiotic containing Lactobacillus and Bifidobacterium significantly improved mood after four weeks of intervention in individuals with depressive symptoms [197]. Likewise, a meta-analysis of seven randomized controlled trials confirmed that probiotics, particularly Lactobacillus and Bifidobacterium species, reduced subjective stress levels and alleviated stress-related anxiety and depression in humans [198]. However, not all studies reported beneficial effects. For example, an eight-week intervention with Lactobacillus helveticus and Bifidobacterium longum failed to improve mood in individuals with depressive symptoms [199]. These discrepancies suggest that probiotic efficacy may depend on microbial strain, dosage, and intervention duration, and host factors. Supporting this, a meta-analysis concluded that interventions longer than eight weeks and exceeding 10 × 109 CFU were more effective at reducing depressive symptoms [200]. Notably, clinical studies have also provided evidence that probiotics can modulate tumor outcomes. In a randomized trial of non-muscle-invasive bladder cancer, oral supplementation with Lactobacillus casei significantly reduced tumor recurrence [164]. In addition, observational studies in cancer patients receiving immunotherapy have shown that the presence of Bifidobacterium, Lactobacillus is associated with improved responses to PD-1/PD-L1 blockade [201], consistent with their immunostimulatory roles described in preclinical studies [202, 203]. Clinical evidence further indicates that perioperative probiotic supplementation may enhance postoperative recovery in cancer patients by reducing infection rates, alleviating intestinal inflammation, and promoting bowel function [187].
Despite these promising results, several key challenges must be addressed before clinical implementation. As highlighted by Karam et al., although emerging data support the efficacy of probiotic interventions, a deeper understanding of strain-specific functional activities and mechanisms is required before clinical use [204]. Moreover, the ability of orally administered probiotics to stably colonize the gut and retain functional activity remains uncertain, as survival is influenced by lysozyme, gastric acidity, pancreatic and bile juice, and colonization resistance from resident microbiota [205-208]. Safety concerns must also be considered, especially in immunocompromised or elderly cancer patients, as rare cases of probiotic-associated sepsis have been reported, and horizontal transfer of antibiotic resistance genes to commensal or opportunistic pathogens has been documented [209, 210]. The above findings highlight the need for cautious use, strain-level safety profiling, and additional clinical monitoring in vulnerable populations [211]. Finally, translational gaps persist due to differences in microbiota composition and host immunity between animal models and humans [212, 213].
These preclinical and clinical findings suggest that targeted probiotic supplementation represents a promising strategy to mitigate the impact of chronic stress and remodel the tumor microenvironment, thereby counteracting chronic stress-driven tumor promotion. However, before it can be incorporated into clinical management, well-designed clinical trials are needed to clarify optimal strains, dosing regimens, and treatment duration, particularly in the context of stress-associated cancer progression.
Summary of evidence connecting chronic stress, gut microbiota alterations, and cancer progression in animal and human studies.
| Microbe / Metabolite | Change under chronic stress | Cancer types | Direct Mechanism in tumor under chronic stress | Other mechanism that related to tumor progression without stress | References |
|---|---|---|---|---|---|
| Blautia spp. | ↓ (patients, mice) | Breast cancer | ↓ Acetate → impaired acetyl-CoA metabolism in CD8⁺ T cells → ↓ IFN-γ and cytotoxicity. | Enhanced CD8⁺ T cell infiltration andimproved better PD-1 response. | [23, 120, 121] |
| Lactobacillus johnsonii | ↓ (mice) | Colorectal cancer | ↓ PCA metabolite → ↑ Wnt/β-catenin → ↑ tumor stemnes. | Suppresses tumor progression by inhibiting the Wnt/β-catenin pathway; Enhances CD8⁺ T cell infiltration into tumors;Promotes DC maturation via bacterial phospholipids; IPA metabolite promotes progenitor exhausted CD8⁺ T cell differentiation and enhancing immunotherapy efficacy. | [24, 122-125] |
| Akkermansia muciniphila | ↓ (patients, mice) | Breast cancer;Colorectal cancer | ↓ Butyrate acts as HDAC inhibitor → ↓ LRP5 destabilization → ↑ Wnt/β-catenin → ↑ tumor stemnes;↓ OMVs → ↑ tumor cell proliferation. | Suppresses Wnt/β-catenin signaling;Outer membrane proteins (Amuc_1100, Amuc_2172) remodel the TME and enhance CD8⁺ T cell immunity. | [25, 127-129] |
| Bifidobacterium spp. | ↓ (patients, mice) | Colorectal cancer | ↓ Oleic acid degradation → ↑ serum oleic acid → ↑ tumor metastasis. | \ | [130] |
| Bile acid metabolism (BSH activity) | ↑ deconjugation and secondary bile acids in mouse models | \ | \ | Unconjugated bile acids activate bile acid receptors and β-catenin/CCL28 → Treg-mediated immunosuppression. | [65, 67, 131, 132] |
| Pro-inflammaroty microbiota | ↑ (mice) | \ | \ | ↑ colonic cytokines/ROS, closely related to inflammation associated cancer | [133-137] |
DC, dendritic cell; HDAC, Histone deacetylase; IPA, indole-3-propionic acid; OMVs, outer membrane vesicles; PCA, Protocatechuic acid; TME, tumor microenviroment.
Potential therapeutic strategies targeting the stress-microbiota-cancer axis. This schematic summarizes six categories of interventions that modulate the stress-microbiota-cancer axis. (a) Probiotics alleviate stress-related symptoms and enhance anti-tumor immunity. (b) Prebiotics promote beneficial taxa, improve stress resilience, reinforce barrier integrity, and enhance anti-tumor immunity. (c) Dietary interventions improve mental health, reduce inflammation, and lower cancer risk. (d) FMT alleviates stress-related symptoms and improves responses to cancer immunotherapy. (e) Mental and psychological therapies relieve chronic stress and improve depressive symptoms and quality of life of cancer patients. (f) TCMs alleviate stress and modulate gut microbiota and immune function, thereby contributing to anti-tumor effects. FOS, fructooligosaccharides; GOS, galactooligosaccharides; FMT, Fecal microbiota transplantation; SCFA, short-chain fatty acid; TCM, traditional Chinese medicine.
6.2 Prebiotics
In addition to probiotic-based interventions, prebiotics represent another promising strategy, exerting their effects by selectively stimulating the growth or activity of beneficial microorganisms. The International Scientific Association for Probiotics and Prebiotics (ISAPP) recently redefined prebiotics as “a substrate that is selectively fermented by gut microbes, thereby enhancing host health” [214].
Among various candidates, fructooligo-saccharides (FOS) and galactooligosaccharides (GOS) are the most extensively studied in the context of stress and depression [215]. In animal models, GOS alone or combination with FOS suppressed stress-induced corticosterone release, alters hippocampal and hypothalamic gene expression, and increased beneficial SCFAs such as acetate and propionate while reducing isobutyrate [216]. Other prebiotics such as plant-derived polysaccharides also exhibit anxiolytic and antidepressant effects [217]. Moreover, synbiotic formulations (probiotics combined with polyphenol-rich prebiotics) have been shown to mitigate ileal and prefrontal cortical inflammation and ameliorate depressive- and anxiety-like behaviors in mouse models by generating metabolites such as 4-hydroxyphenylpropionic acid and caffeic acid [218]. Beyond stress regulation, prebiotics also provide anti-tumor benefits. In mouse models, oral administration of prebiotics enhanced microbial production of SCFAs, thereby suppressing tumor growth and improving drug sensitivity [219, 220]. Similarly, inulin supplementation enriched Bifidobacterium, increased γδ T cell infiltration in tumors, and inhibited tumor progression [221].
Evidence from human studies, though heterogeneous, is generally favorable. A randomized controlled trial reported that GOS supplementation tended to reduce trait anxiety and improve reaction times in young females with high baseline anxiety [222], while another study showed that GOS supple-mentation alleviated stress-related gastrointestinal dysfunction [223]. Recent meta-analyses further concluded that GOS interventions generally lower anxiety levels, though effect sizes vary across populations [224]. With respect to cancer, however, clinical data on prebiotics remain limited. Most evidence comes from preclinical models, and large-scale clinical trials with cancer-related endpoints are still lacking [225].
When considering prebiotics for potential clinical translation, both dose and safety profile must be taken into account. Previous study indicated that daily intake of ≥ 5 g of FOS or GOS can improve anxiety and depressive symptoms [168]. Besides, prebiotics are generally well tolerated, though higher doses may cause transient, dose-dependent gastrointestinal effects such as bloating, flatulence, and osmotic diarrhea [226]. Careful monitoring may be required when prebiotics are used in vulnerable populations, such as elderly individuals or patients with gastrointestinal disorders.
Collectively, these findings indicate that prebiotics exert beneficial effects on stress responses and show promise in suppressing tumor progression. However, their clinical translation remains limited.
6.3 Dietary interventions
Dietary interventions are powerful modulators of gut microbiota composition and function [227]. In a 20-year prospective cohort of 49,261 Swedish women, higher adherence to a Mediterranean diet in midlife was linked to a significantly lower risk of depression [228]. A randomized controlled trial in young men with moderate to severe depression similarly found that a 12-week Mediterranean diet intervention improved depressive symptoms and quality of life [229]. Individuals with higher compliance to the Mediterranean diet showed significant reductions in inflammatory markers, including C-reactive protein, TNF-α, and IL-6, which may contribute to gastric cancer prevention, however, the magnitude of this effect varied depending on dietary adherence [230]. A recent meta-analysis by Giordano et al. further suggested that adherence to a Mediterranean diet may protect against cancer incidence in older adults [231]. These results suggest that dietary modification may help counteract stress-related immune and metabolic dysregulation relevant to cancer progression.
6.4 Fecal microbiota transplantation
FMT also provides a direct method to restore microbial balance and has been reported to alleviate stress-related symptoms such as anxiety and depression in patients with irritable bowel syndrome [232, 233]. In cancer therapy, two landmark trials showed that FMT restored responsiveness to PD-1 blockade in subsets of melanoma patients [234, 235], highlighting its therapeutic potential in cancer. Although generally considered a safe procedure, FMT may still cause adverse events, most commonly abdominal discomfort, bloating, nausea, and diarrhea, and its potential long-term risks remain insufficiently characterized, warranting further investigation [236]. Together, these findings suggest that FMT, by targeting both stress-related dysbiosis and cancer treatment responses, represents a promising yet still experimental strategy within the stress-microbiota-cancer axis.
6.5 Mental and psychological therapy
Mental and psychological interventions represent an essential component of comprehensive management for stress-associated cancer progression. Modifying patients' responses to stressors can significantly reduce psychological distress and improve quality of life [237, 238]. Among these, cognitive behavioral therapy (CBT) is one of the most extensively studied interventions, with strong evidence supporting its efficacy in alleviating anxiety, depression, and overall psychological distress commonly observed in cancer patients [239]. According to the ASCO guidelines, clinicians are recommended to provide CBT, behavioral activation (BA), mindfulness-based stress reduction (MBSR), structured physical exercise, or other empirically supported psychosocial interventions for patients presenting with moderate depressive symptoms [240]. A recent review by Anabel et al. summarized the impact of stress management interventions on cancer outcomes, highlighting that the effect of psychological therapies on cancer survival remains controversial [4]. Some studies have reported improved survival among breast cancer patients receiving psychological interventions [241, 242], whereas others found no significant survival benefit [243, 244]. To date, most studies have focused on breast cancer, and the applicability of these findings to other cancer types remains uncertain. Within the stress-microbiota-cancer axis, psychological therapies primarily target the stress response. Their benefits for anxiety, depression, and quality of life are well documented [245, 246]. Whether such interventions directly reverse dysbiosis, restore barrier function, or modify tumor‑associated inflammation in cancer populations has not been demonstrated and warrants further study. Collectively, psychological therapies may alleviate chronic stress, enhance patients' resilience, and potentially contribute to better clinical outcomes in cancer.
6.6 Traditional Chinese medicine (TCM) treatments
According to traditional Chinese medicine (TCM) theory, psychological stress is regarded as a key factor contributing to disease development and progression. Both preclinical and clinical studies have demonstrated its therapeutic potential in alleviating stress-related disorders and inhibiting tumor progression [247, 248]. Xiaoyao San (XYS), a classical prescription traditionally used to treat mental disorders, was shown to suppress chronic stress-induced hepatic metastasis in a mouse model of colorectal cancer [249]. In a clinical study, San-Huang Decoction (SHD) alleviated chronic stress caused by long-term endocrine therapy in breast cancer patients, while also inhibiting tumor growth and preventing drug resistance [250]. Moreover, Xiao-Chai-Hu-Tang (XCHT) significantly improved depression scores, systemic inflammation, and gut dysbiosis in cancer patients with depressive symptoms. Experimental evidence revealed that XCHT suppressed tumor growth by downregulating the TLR4/MyD88/NF-κB and IL-6/JAK2/STAT3 signaling pathways and improving the tumor immune microenvironment [251, 252]. In addition to herbal therapies, mind-body interventions rooted in TCM, such as Tai Chi (TC), resistance training (RT), and traditional Chinese acupuncture (TCA), have shown promising benefits. A randomized controlled trial in elderly cancer patients (aged >55 years) demonstrated that a 12-week TC and RT program improved sleep quality, mental health, and cancer-related fatigue [253]. Another clinical study reported that TCA effectively alleviated chronic stress symptoms [254]. Collectively, these findings indicate that TCM-based interventions may concurrently modulate psychological, microbial, and immune pathways, providing a systems-level strategy to disrupt the stress-microbiota-cancer axis.
7. Conclusion
By recognizing the gut microbiota as a pivotal mediator in the stress-cancer axis, this review provides a framework for developing novel therapeutic strategies that target this axis at multiple levels. Future research should focus on identifying specific microbial signatures associated with stress-induced cancer progression and developing personalized interventions based on individual microbiome profiles.
Abbreviations
ACTH: adrenocorticotropic hormone; BA: behavioral activation; BDNF: Brain-derived neurotrophic factor; CBT: cognitive behavioral therapy; CeA: central amygdala; CRH: Corticotropin-releasing hormone; CUMS: Chronic unpredictable mild stress; ENS: Enteric nervous system; ED: Emotional distress; FOS: Fructooligosaccharides; GABA: γ-aminobutyric acid; GF: Germ-free; GOS: Galactooligosaccharides; HDAC: Histone deacetylase; HNSCC: Head and neck squamous cell carcinoma; HPA: Hypothalamic-pituitary-adrenal; IAA: Indole-3-acetic acid; IBD: Inflammatory bowel disease; ICA: Indole-3-carboxylic acid; ICIs: Immune checkpoint inhibitors; IECs: Intestinal epithelial cells; ILA: Indole-3-lactic acid; IPA: Indole-3-propionic acid; MBSR: mindfulness-based stress reduction; MDD: Major depressive disorder; MS: Maternal separation; NOS: Nitric oxide synthase; PCA: Protocatechuic acid; PE: Phosphatidylethanolamine; ROS: Reactive oxygen species; RT: resistance training; SCFAs: Short-chain fatty acids; XCTH: Xiao-Chai-Hu-Tang; SFB: Segmented filamentous bacteria; SHD: San-Huang Decoction; SNS: Sympathetic nervous system; SOC: Social overcrowding; XYS: Xiaoyao San; TC: Tai Chi; TCA: traditional Chinese acupuncture; TCM: traditional Chinese medicine; TEER: Transepithelial electrical resistance; WAS: Water avoidance stress; VOE: Vaso-occlusive episodes; 4NQO: 4-nitroquinoline-1-oxide.
Acknowledgements
Figures were created under the academic license of Figdraw.com.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82403002), the Sichuan Science and Technology Program (No. 2025ZNSFSC1897), and the China Postdoctoral Science Foundation (No. GZC20231810).
Author contributions
Qing Li: Conceptualization and funding acquisition; Qing Li, Siyuan Xia and Xian Zhang: Writing and editing; Siyuan Xia and Yuqiang Liu: Visualization; Xue xiao and Jinlin Yang: Supervision, review and editing.
Consent for publication
All authors have read and approved the final manuscript and consent to its publication.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-63
2. Lin L, Li Z, Yan L, Liu Y, Yang H, Li H. Global, regional, and national cancer incidence and death for 29 cancer groups in 2019 and trends analysis of the global cancer burden, 1990-2019. J Hematol Oncol. 2021;14:197
3. O'Connor DB, Thayer JF, Vedhara K. Stress and Health: A Review of Psychobiological Processes. Annu Rev Psychol. 2021;72:663-88
4. Eckerling A, Ricon-Becker I, Sorski L, Sandbank E, Ben-Eliyahu S. Stress and cancer: mechanisms, significance and future directions. Nat Rev Cancer. 2021;21:767-85
5. Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W. et al. A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature. 2011;477:349-53
6. Lamkin DM, Sloan EK, Patel AJ, Chiang BS, Pimentel MA, Ma JC. et al. Chronic stress enhances progression of acute lymphoblastic leukemia via beta-adrenergic signaling. Brain Behav Immun. 2012;26:635-41
7. Obradovic MMS, Hamelin B, Manevski N, Couto JP, Sethi A, Coissieux MM. et al. Glucocorticoids promote breast cancer metastasis. Nature. 2019;567:540-4
8. Ma Y, Kroemer G. The cancer-immune dialogue in the context of stress. Nat Rev Immunol. 2024;24:264-81
9. Cui B, Luo Y, Tian P, Peng F, Lu J, Yang Y. et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J Clin Invest. 2019;129:1030-46
10. Xie H, Li C, He Y, Griffin R, Ye Q, Li L. Chronic stress promotes oral cancer growth and angiogenesis with increased circulating catecholamine and glucocorticoid levels in a mouse model. Oral Oncol. 2015;51:991-7
11. Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C. et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939-44
12. Wu W, Liu S, Liang Y, Zhou Z, Bian W, Liu X. Stress Hormone Cortisol Enhances Bcl2 Like-12 Expression to Inhibit p53 in Hepatocellular Carcinoma Cells. Dig Dis Sci. 2017;62:3495-500
13. Globig AM, Zhao S, Roginsky J, Maltez VI, Guiza J, Avina-Ochoa N. et al. The beta(1)-adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature. 2023;622:383-92
14. Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B. et al. beta-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8(+) T Cells and Undermines Checkpoint Inhibitor Therapy. Cancer Res. 2017;77:5639-51
15. Qiao G, Chen M, Mohammadpour H, MacDonald CR, Bucsek MJ, Hylander BL. et al. Chronic Adrenergic Stress Contributes to Metabolic Dysfunction and an Exhausted Phenotype in T Cells in the Tumor Microenvironment. Cancer Immunol Res. 2021;9:651-64
16. Scott OW, Tin Tin S, Botteri E. Beta blocker use and breast cancer survival by subtypes: A population-based cohort study. Breast. 2025;81:104474
17. Zhang F, Wang Y, Liu F, Li Y, Liu X, Ren X. et al. Impact of beta blockers on cancer neuroimmunology: a systematic review and meta-analysis of survival outcomes and immune modulation. Front Immunol. 2025;16:1635331
18. Morais LH, Schreiber HLt, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19:241-55
19. Allen JM, Mackos AR, Jaggers RM, Brewster PC, Webb M, Lin CH. et al. Psychological stress disrupts intestinal epithelial cell function and mucosal integrity through microbe and host-directed processes. Gut Microbes. 2022;14:2035661
20. Gao X, Cao Q, Cheng Y, Zhao D, Wang Z, Yang H. et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci U S A. 2018;115:E2960-E9
21. Meyers M, Stoffels CBA, Frache G, Letellier E, Feucherolles M. Microbiome in cancer metastasis: biological insights and emerging spatial omics methods. Front Cell Infect Microbiol. 2025;15:1559870
22. El Tekle G, Garrett WS. Bacteria in cancer initiation, promotion and progression. Nat Rev Cancer. 2023;23:600-18
23. Ye L, Hou Y, Hu W, Wang H, Yang R, Zhang Q. et al. Repressed Blautia-acetate immunological axis underlies breast cancer progression promoted by chronic stress. Nat Commun. 2023;14:6160
24. Cao Q, Zhao M, Su Y, Liu S, Lin Y, Da H. et al. Chronic stress dampens Lactobacillus johnsonii-mediated tumor suppression to enhance colorectal cancer progression. Cancer Res. 2024
25. Cui B, Luo H, He B, Liu X, Lv D, Zhang X. et al. Gut dysbiosis conveys psychological stress to activate LRP5/beta-catenin pathway promoting cancer stemness. Signal Transduct Target Ther. 2025;10:79
26. Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 2017;355:1330-4
27. Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529:43-7
28. Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466-75
29. Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM. et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. 2020;25:1487-99
30. Shen J, Fuemmeler BF, Guan Y, Zhao H. Association of Allostatic Load and All Cancer Risk in the SWAN Cohort. Cancers (Basel). 2022 14
31. Zeng Y, Hu CH, Li YZ, Zhou JS, Wang SX, Liu MD. et al. Association between pretreatment emotional distress and immune checkpoint inhibitor response in non-small-cell lung cancer. Nat Med. 2024;30:1680-8
32. Madison AA, Bailey MT. Stressed to the Core: Inflammation and Intestinal Permeability Link Stress-Related Gut Microbiota Shifts to Mental Health Outcomes. Biol Psychiatry. 2024;95:339-47
33. Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-20
34. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65
35. Kers JG, Saccenti E. The Power of Microbiome Studies: Some Considerations on Which Alpha and Beta Metrics to Use and How to Report Results. Front Microbiol. 2021;12:796025
36. Wang Y, Cui P, Cao M, Ai L, Zeng L, Li X. et al. Chronic restraint stress affects the diurnal rhythms of gut microbial composition and metabolism in a mouse model of depression. BMC Microbiol. 2025;25:38
37. Ma L, Yan Y, Webb RJ, Li Y, Mehrabani S, Xin B. et al. Psychological Stress and Gut Microbiota Composition: A Systematic Review of Human Studies. Neuropsychobiology. 2023;82:247-62
38. Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun. 2010;78:1509-19
39. Zhu X, Sakamoto S, Ishii C, Smith MD, Ito K, Obayashi M. et al. Dectin-1 signaling on colonic gammadelta T cells promotes psychosocial stress responses. Nat Immunol. 2023;24:625-36
40. Siopi E, Chevalier G, Katsimpardi L, Saha S, Bigot M, Moigneu C. et al. Changes in Gut Microbiota by Chronic Stress Impair the Efficacy of Fluoxetine. Cell Rep. 2020;30:3682-90 e6
41. Cheng S, Zhu Z, Li H, Wang W, Jiang Z, Pan F. et al. Rifaximin ameliorates depression-like behaviour in chronic unpredictable mild stress rats by regulating intestinal microbiota and hippocampal tryptophan metabolism. J Affect Disord. 2023;329:30-41
42. Dong Z, Xie Q, Xu F, Shen X, Hao Y, Li J. et al. Neferine alleviates chronic stress-induced depression by regulating monoamine neurotransmitter secretion and gut microbiota structure. Front Pharmacol. 2022;13:974949
43. Duan J, Huang Y, Tan X, Chai T, Wu J, Zhang H. et al. Characterization of gut microbiome in mice model of depression with divergent response to escitalopram treatment. Transl Psychiatry. 2021;11:303
44. Li H, Xiang Y, Zhu Z, Wang W, Jiang Z, Zhao M. et al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J Neuroinflammation. 2021;18:254
45. Sun Y, Zhang M, Chen CC, Gillilland M 3rd, Sun X, El-Zaatari M. et al. Stress-induced corticotropin-releasing hormone-mediated NLRP6 inflammasome inhibition and transmissible enteritis in mice. Gastroenterology. 2013;144:1478-87 87 e1-8
46. Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS. et al. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189
47. Zhang Y, Zhang J, Wu J, Zhu Q, Chen C, Li Y. Implications of gut microbiota dysbiosis and fecal metabolite changes in psychologically stressed mice. Front Microbiol. 2023;14:1124454
48. Szyszkowicz JK, Wong A, Anisman H, Merali Z, Audet MC. Implications of the gut microbiota in vulnerability to the social avoidance effects of chronic social defeat in male mice. Brain Behav Immun. 2017;66:45-55
49. Chen C, Xiao Q, Wen Z, Gong F, Zhan H, Liu J. et al. Gut microbiome-derived indole-3-carboxaldehyde regulates stress vulnerability in chronic restraint stress by activating aryl hydrocarbon receptors. Pharmacol Res. 2025;213:107654
50. Yang HL, Li MM, Zhou MF, Xu HS, Huan F, Liu N. et al. Links Between Gut Dysbiosis and Neurotransmitter Disturbance in Chronic Restraint Stress-Induced Depressive Behaviours: the Role of Inflammation. Inflammation. 2021;44:2448-62
51. Duan C, Wang L, Wang T, Wu Y, Shan N, Wang Y. et al. Alteration of intestinal mucosal immunity and metabolites in mice exposed to chronic restraint stress. Brain Behav Immun. 2025;130:106090
52. Hochuli N, Kadyan S, Park G, Patoine C, Nagpal R. A Gut Microbial Metabolite Alleviates Stress-Induced Neurobehavioral Dysfunction in an Alzheimer's Disease Model. Mol Neurobiol. 2025;62:11666-80
53. Almand AT, Anderson AP, Hitt BD, Sitko JC, Joy RM, Easter BD. et al. The influence of perceived stress on the human microbiome. BMC Res Notes. 2022;15:193
54. Knowles SR, Nelson EA, Palombo EA. Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: a possible mechanism underlying susceptibility to illness. Biol Psychol. 2008;77:132-7
55. Gao F, Guo R, Ma Q, Li Y, Wang W, Fan Y. et al. Stressful events induce long-term gut microbiota dysbiosis and associated post-traumatic stress symptoms in healthcare workers fighting against COVID-19. J Affect Disord. 2022;303:187-95
56. Barandouzi ZA, Starkweather AR, Henderson WA, Gyamfi A, Cong XS. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front Psychiatry. 2020;11:541
57. Kelly JR, Borre Y, C OB, Patterson E, El Aidy S, Deane J. et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109-18
58. Brushett S, Gacesa R, Vich Vila A, Brandao Gois MF, Andreu-Sanchez S, Swarte JC. et al. Gut feelings: the relations between depression, anxiety, psychotropic drugs and the gut microbiome. Gut Microbes. 2023;15:2281360
59. Yuan X, Chen B, Duan Z, Xia Z, Ding Y, Chen T. et al. Depression and anxiety in patients with active ulcerative colitis: crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes. 2021;13:1987779
60. Joo MK, Lee JW, Woo JH, Kim HJ, Kim DH, Choi JH. Regulation of colonic neuropeptide Y expression by the gut microbiome in patients with ulcerative colitis and its association with anxiety- and depression-like behavior in mice. Gut Microbes. 2024;16:2319844
61. Mackner LM, Hatzakis E, Allen JM, Davies RH, Kim SC, Maltz RM. et al. Fecal microbiota and metabolites are distinct in a pilot study of pediatric Crohn's disease patients with higher levels of perceived stress. Psychoneuroendocrinology. 2020;111:104469
62. Humbel F, Rieder JH, Franc Y, Juillerat P, Scharl M, Misselwitz B. et al. Association of Alterations in Intestinal Microbiota With Impaired Psychological Function in Patients With Inflammatory Bowel Diseases in Remission. Clin Gastroenterol Hepatol. 2020;18:2019-29 e11
63. Deng Y, Zhou M, Wang J, Yao J, Yu J, Liu W. et al. Involvement of the microbiota-gut-brain axis in chronic restraint stress: disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes. 2021;13:1-16
64. Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology. 2016;63:217-27
65. Qu Y, Su C, Zhao Q, Shi A, Zhao F, Tang L. et al. Gut Microbiota-Mediated Elevated Production of Secondary Bile Acids in Chronic Unpredictable Mild Stress. Front Pharmacol. 2022;13:837543
66. Li T, Ding N, Guo H, Hua R, Lin Z, Tian H. et al. A gut microbiota-bile acid axis promotes intestinal homeostasis upon aspirin-mediated damage. Cell Host Microbe. 2024;32:191-208 e9
67. Sun L, Zhang Y, Cai J, Rimal B, Rocha ER, Coleman JP. et al. Bile salt hydrolase in non-enterotoxigenic Bacteroides potentiates colorectal cancer. Nat Commun. 2023;14:755
68. Zhao L, Hou X, Feng Y, Zhang Y, Shao S, Wu X. et al. A chronic stress-induced microbiome perturbation, highly enriched in Ruminococcaceae_UCG-014, promotes colorectal cancer growth and metastasis. Int J Med Sci. 2024;21:882-95
69. Song X, Wang Z, Xia Y, Chen Z, Wang G, Yang Y. et al. A Cross Talking between the Gut Microbiota and Metabolites of Participants in a Confined Environment. Nutrients. 2024 16
70. Karl JP, Margolis LM, Madslien EH, Murphy NE, Castellani JW, Gundersen Y. et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol Gastrointest Liver Physiol. 2017;312:G559-G71
71. Chen Z, Li J, Gui S, Zhou C, Chen J, Yang C. et al. Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. Neuroreport. 2018;29:417-25
72. Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R. et al. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol. 2016;6:603-21
73. Nieman LK. Molecular Derangements and the Diagnosis of ACTH-Dependent Cushing's Syndrome. Endocr Rev. 2022;43:852-77
74. Lei AA, Phang VWX, Lee YZ, Kow ASF, Tham CL, Ho YC. et al. Chronic Stress-Associated Depressive Disorders: The Impact of HPA Axis Dysregulation and Neuroinflammation on the Hippocampus-A Mini Review. Int J Mol Sci. 2025 26
75. Feng X, Jia M, Cai M, Zhu T, Hashimoto K, Yang JJ. Central-peripheral neuroimmune dynamics in psychological stress and depression: insights from current research. Mol Psychiatry. 2025
76. Marwaha K, Cain R, Asmis K, Czaplinski K, Holland N, Mayer DCG. et al. Exploring the complex relationship between psychosocial stress and the gut microbiome: implications for inflammation and immune modulation. J Appl Physiol (1985). 2025;138:518-35
77. Boyanova L. Stress hormone epinephrine (adrenaline) and norepinephrine (noradrenaline) effects on the anaerobic bacteria. Anaerobe. 2017;44:13-9
78. Deflorin N, Ehlert U, Amiel Castro RT. Associations of maternal prenatal psychological symptoms and saliva cortisol with neonatal meconium microbiota: A cross-sectional study. Prog Neuropsychopharmacol Biol Psychiatry. 2024;129:110895
79. Duran-Pinedo AE, Solbiati J, Frias-Lopez J. The effect of the stress hormone cortisol on the metatranscriptome of the oral microbiome. NPJ Biofilms Microbiomes. 2018;4:25
80. Saito T, Inagaki S, Sakurai K, Okuda K, Ishihara K. Exposure of P. gingivalis to noradrenaline reduces bacterial growth and elevates ArgX protease activity. Arch Oral Biol. 2011;56:244-50
81. Jentsch HF, Marz D, Kruger M. The effects of stress hormones on growth of selected periodontitis related bacteria. Anaerobe. 2013;24:49-54
82. Cambronel M, Tortuel D, Biaggini K, Maillot O, Taupin L, Rehel K. et al. Epinephrine affects motility, and increases adhesion, biofilm and virulence of Pseudomonas aeruginosa H103. Sci Rep. 2019;9:20203
83. Cambronel M, Nilly F, Mesguida O, Boukerb AM, Racine PJ, Baccouri O. et al. Influence of Catecholamines (Epinephrine/Norepinephrine) on Biofilm Formation and Adhesion in Pathogenic and Probiotic Strains of Enterococcus faecalis. Front Microbiol. 2020;11:1501
84. Niu L, Gao M, Wen S, Wang F, Shangguan H, Guo Z. et al. Effects of Catecholamine Stress Hormones Norepinephrine and Epinephrine on Growth, Antimicrobial Susceptibility, Biofilm Formation, and Gene Expressions of Enterotoxigenic Escherichia coli. Int J Mol Sci. 2023 24
85. Belay T, Sonnenfeld G. Differential effects of catecholamines on in vitro growth of pathogenic bacteria. Life Sci. 2002;71:447-56
86. Hughes DT, Clarke MB, Yamamoto K, Rasko DA, Sperandio V. The QseC adrenergic signaling cascade in Enterohemorrhagic E. coli (EHEC). PLoS Pathog. 2009;5:e1000553
87. Meng J, Chen H, Lv Q, Luo X, Yang K. The Release of Norepinephrine in C57BL/6J Mice Treated with 6-Hydroxydopamine (6-OHDA) is Associated with Translocations in Enteric Escherichia coli via the QseC Histidine Kinase Receptor. Med Sci Monit. 2020;26:e922986
88. Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR. et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15:39-49
89. Zeng MY, Inohara N, Nunez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18-26
90. Cole SW. The Conserved Transcriptional Response to Adversity. Curr Opin Behav Sci. 2019;28:31-7
91. Shaler CR, Parco AA, Elhenawy W, Dourka J, Jury J, Verdu EF. et al. Psychological stress impairs IL22-driven protective gut mucosal immunity against colonising pathobionts. Nat Commun. 2021;12:6664
92. Deng Q, Chen H, Liu Y, Xiao F, Guo L, Liu D. et al. Psychological stress promotes neutrophil infiltration in colon tissue through adrenergic signaling in DSS-induced colitis model. Brain Behav Immun. 2016;57:243-54
93. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK. et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446-57
94. Schneider KM, Blank N, Alvarez Y, Thum K, Lundgren P, Litichevskiy L. et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell. 2023;186:2823-38 e20
95. Lu T, Huang C, Weng R, Wang Z, Sun H, Ma X. Enteric glial cells contribute to chronic stress-induced alterations in the intestinal microbiota and barrier in rats. Heliyon. 2024;10:e24899
96. Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064-9
97. Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69:2232-43
98. Breugelmans T, Oosterlinck B, Arras W, Ceuleers H, De Man J, Hold GL. et al. The role of mucins in gastrointestinal barrier function during health and disease. Lancet Gastroenterol Hepatol. 2022;7:455-71
99. Jaggers RM, DiSabato DJ, Loman BR, Kontic D, Spencer KD, Allen JM. et al. Stressor-Induced Reduction in Cognitive Behavior is Associated with Impaired Colonic Mucus Layer Integrity and is Dependent Upon the LPS-Binding Protein Receptor CD14. J Inflamm Res. 2022;15:1617-35
100. Chang H, Perkins MH, Novaes LS, Qian F, Zhang T, Neckel PH. et al. Stress-sensitive neural circuits change the gut microbiome via duodenal glands. Cell. 2024;187:5393-412 e30
101. Rivet-Noor CR, Merchak AR, Render C, Gay NM, Beiter RM, Brown RM. et al. Stress-induced mucin 13 reductions drive intestinal microbiome shifts and despair behaviors. Brain Behav Immun. 2024;119:665-80
102. Da Silva S, Robbe-Masselot C, Ait-Belgnaoui A, Mancuso A, Mercade-Loubiere M, Salvador-Cartier C. et al. Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: prevention by a probiotic treatment. Am J Physiol Gastrointest Liver Physiol. 2014;307:G420-9
103. Soderholm JD, Yang PC, Ceponis P, Vohra A, Riddell R, Sherman PM. et al. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123:1099-108
104. Martel J, Chang SH, Ko YF, Hwang TL, Young JD, Ojcius DM. Gut barrier disruption and chronic disease. Trends Endocrinol Metab. 2022;33:247-65
105. Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM. et al. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553-60
106. Prochazkova N, Falony G, Dragsted LO, Licht TR, Raes J, Roager HM. Advancing human gut microbiota research by considering gut transit time. Gut. 2023;72:180-91
107. Tian H, Chen Q, Yang B, Qin H, Li N. Analysis of Gut Microbiome and Metabolite Characteristics in Patients with Slow Transit Constipation. Dig Dis Sci. 2021;66:3026-35
108. Kim SE, Choi SC, Park KS, Park MI, Shin JE, Lee TH. et al. Change of Fecal Flora and Effectiveness of the Short-term VSL#3 Probiotic Treatment in Patients With Functional Constipation. J Neurogastroenterol Motil. 2015;21:111-20
109. Attaluri A, Jackson M, Valestin J, Rao SS. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol. 2010;105:1407-11
110. Liu Q, Luo Y, Ke X. Interaction between the Gut Microbiota and Intestinal Motility. Evid Based Complement Alternat Med. 2022;2022:3240573
111. Ataka K, Nagaishi K, Asakawa A, Inui A, Fujimiya M. Alteration of antral and proximal colonic motility induced by chronic psychological stress involves central urocortin 3 and vasopressin in rats. Am J Physiol Gastrointest Liver Physiol. 2012;303:G519-28
112. Murakami T, Kamada K, Mizushima K, Higashimura Y, Katada K, Uchiyama K. et al. Changes in Intestinal Motility and Gut Microbiota Composition in a Rat Stress Model. Digestion. 2017;95:55-60
113. Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33-40
114. Blin J, Gautier C, Aubert P, Durand T, Oullier T, Aymeric L. et al. Psychological stress induces an increase in cholinergic enteric neuromuscular pathways mediated by glucocorticoid receptors. Front Neurosci. 2023;17:1100473
115. Li S, Fei G, Fang X, Yang X, Sun X, Qian J. et al. Changes in Enteric Neurons of Small Intestine in a Rat Model of Irritable Bowel Syndrome with Diarrhea. J Neurogastroenterol Motil. 2016;22:310-20
116. Nehra V, Bruce BK, Rath-Harvey DM, Pemberton JH, Camilleri M. Psychological disorders in patients with evacuation disorders and constipation in a tertiary practice. Am J Gastroenterol. 2000;95:1755-8
117. Li X, Bian C, Dai H, Chen X, Qian H, Zhang D. Association of anxiety status and anxiety duration with constipation in adult Americans: a cross-sectional study using data from the NHANES 2007-2010. BMC Gastroenterol. 2025;25:31
118. Liang F, Liu S, Zhang H, Xiang R, Xie M, He X. et al. Effects of chronic unpredictable mild stress on gut sensation and function in male mice. Stress. 2024;27:2374768
119. Lobo B, Tramullas M, Finger BC, Lomasney KW, Beltran C, Clarke G. et al. The Stressed Gut: Region-specific Immune and Neuroplasticity Changes in Response to Chronic Psychosocial Stress. J Neurogastroenterol Motil. 2023;29:72-84
120. Wang B, Shangguan W, Li W, Xie M, Yu Y, Yang Q. et al. Blautia coccoides and its metabolic products enhance the efficacy of bladder cancer immunotherapy by promoting CD8(+) T cell infiltration. J Transl Med. 2024;22:964
121. Jahanbakhshi S, Bibi A, Hoyd R, Dravillas C, Williams N, Zhang S. et al. Abstract 2377: Gut microbiome modulation by a black raspberry nectar f or improving anti-PD1 therapy effectiveness in pre-clinical cancer mod els. Cancer Research. 85: 2377-.
122. Xie M, Yang T, Liu Q, Ning Z, Feng L, Min X. The influence of Lactobacillus johnsonii on tumor growth and lymph node metastasis in papillary thyroid carcinoma. Commun Biol. 2025;8:419
123. Luo M, Wang Q, Sun Y, Jiang Y, Wang Q, Gu Y. et al. Fasting-mimicking diet remodels gut microbiota and suppresses colorectal cancer progression. NPJ Biofilms Microbiomes. 2024;10:53
124. Cuaycal AE, Teixeira LD, Lorca GL, Gonzalez CF. Lactobacillus johnsonii N6.2 phospholipids induce immature-like dendritic cells with a migratory-regulatory-like transcriptional signature. Gut Microbes. 2023;15:2252447
125. Jia D, Wang Q, Qi Y, Jiang Y, He J, Lin Y. et al. Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer. Cell. 2024;187:1651-65 e21
126. Jin S, Lu Y, Zuo Y, Xu Q, Hao Y, Zuo H. et al. Akkermansia muciniphila ameliorates chronic stress-induced colorectal tumor growth by releasing outer membrane vesicles. Gut Microbes. 2025;17:2555618
127. Zhang L, Ji Q, Chen Q, Wei Z, Liu S, Zhang L. et al. Akkermansia muciniphila inhibits tryptophan metabolism via the AhR/beta-catenin signaling pathway to counter the progression of colorectal cancer. Int J Biol Sci. 2023;19:4393-410
128. Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang C. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut. 2020;69:1988-97
129. Jiang Y, Xu Y, Zheng C, Ye L, Jiang P, Malik S. et al. Acetyltransferase from Akkermansia muciniphila blunts colorectal tumourigenesis by reprogramming tumour microenvironment. Gut. 2023;72:1308-18
130. He Z, Liu C, Gong J, Yu J, Liang D, Lai P. et al. IDDF2024-ABS-0223 Chronic stress promotes cancer metastasis through glucocorticoid-driven bifidobacterium depletion and oleic acid accumulation. Gut. 2024;73:A152-A
131. Song Z, Cai Y, Lao X, Wang X, Lin X, Cui Y. et al. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome. 2019;7:9
132. Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M. et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018 360
133. Watanabe Y, Arase S, Nagaoka N, Kawai M, Matsumoto S. Chronic Psychological Stress Disrupted the Composition of the Murine Colonic Microbiota and Accelerated a Murine Model of Inflammatory Bowel Disease. PLoS One. 2016;11:e0150559
134. Wei W, Liu Y, Hou Y, Cao S, Chen Z, Zhang Y. et al. Psychological stress-induced microbial metabolite indole-3-acetate disrupts intestinal cell lineage commitment. Cell Metab. 2024
135. Delaroque C, Chervy M, Gewirtz AT, Chassaing B. Social overcrowding impacts gut microbiota, promoting stress, inflammation, and dysglycemia. Gut Microbes. 2021;13:2000275
136. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y. et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6:263
137. Liu X, Yin L, Shen S, Hou Y. Inflammation and cancer: paradoxical roles in tumorigenesis and implications in immunotherapies. Genes Dis. 2023;10:151-64
138. Neurath MF, Artis D, Becker C. The intestinal barrier: a pivotal role in health, inflammation, and cancer. Lancet Gastroenterol Hepatol. 2025;10:573-92
139. Kuti D, Winkler Z, Horvath K, Juhasz B, Paholcsek M, Stagel A. et al. Gastrointestinal (non-systemic) antibiotic rifaximin differentially affects chronic stress-induced changes in colon microbiome and gut permeability without effect on behavior. Brain Behav Immun. 2020;84:218-28
140. Jang HM, Kim JK, Joo MK, Shin YJ, Lee CK, Kim HJ. et al. Transplantation of fecal microbiota from patients with inflammatory bowel disease and depression alters immune response and behavior in recipient mice. Sci Rep. 2021;11:20406
141. Keita AV, Soderholm JD, Ericson AC. Stress-induced barrier disruption of rat follicle-associated epithelium involves corticotropin-releasing hormone, acetylcholine, substance P, and mast cells. Neurogastroenterol Motil. 2010;22:770-8 e221-2
142. Wang SL, Shao BZ, Zhao SB, Chang X, Wang P, Miao CY. et al. Intestinal autophagy links psychosocial stress with gut microbiota to promote inflammatory bowel disease. Cell Death Dis. 2019;10:391
143. Zong Y, Zhu S, Zhang S, Zheng G, Wiley JW, Hong S. Chronic stress and intestinal permeability: Lubiprostone regulates glucocorticoid receptor-mediated changes in colon epithelial tight junction proteins, barrier function, and visceral pain in the rodent and human. Neurogastroenterol Motil. 2019;31:e13477
144. Zheng G, Wu SP, Hu Y, Smith DE, Wiley JW, Hong S. Corticosterone mediates stress-related increased intestinal permeability in a region-specific manner. Neurogastroenterol Motil. 2013;25:e127-39
145. Shukla PK, Meena AS, Pierre JF, Rao R. Central role of intestinal epithelial glucocorticoid receptor in alcohol- and corticosterone-induced gut permeability and systemic response. FASEB J. 2022;36:e22061
146. Santos J, Yang PC, Soderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48:630-6
147. Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E. et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293-9
148. Zhang Y, Zhang J, Xia Y, Sun J. Bacterial translocation and barrier dysfunction enhance colonic tumorigenesis. Neoplasia. 2023;35:100847
149. Schneider KM, Mohs A, Gui W, Galvez EJC, Candels LS, Hoenicke L. et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat Commun. 2022;13:3964
150. Wang X, Fang Y, Liang W, Cai Y, Wong CC, Wang J. et al. Gut-liver translocation of pathogen Klebsiella pneumoniae promotes hepatocellular carcinoma in mice. Nat Microbiol. 2025;10:169-84
151. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397-407
152. Li C, Peng K, Xiao S, Long Y, Yu Q. The role of Lactobacillus in inflammatory bowel disease: from actualities to prospects. Cell Death Discov. 2023;9:361
153. Gavzy SJ, Kensiski A, Lee ZL, Mongodin EF, Ma B, Bromberg JS. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes. 2023;15:2291164
154. Decout A, Krasias I, Roberts L, Gimeno Molina B, Charenton C, Brown Romero D. et al. Lactobacillus crispatus S-layer proteins modulate innate immune response and inflammation in the lower female reproductive tract. Nat Commun. 2024;15:10879
155. Kaur H, Ali SA, Short SP, Williams CS, Goettel JA, Washington MK. et al. Identification of a functional peptide of a probiotic bacterium-derived protein for the sustained effect on preventing colitis. Gut Microbes. 2023;15:2264456
156. Gao J, Wang L, Jiang J, Xu Q, Zeng N, Lu B. et al. A probiotic bi-functional peptidoglycan hydrolase sheds NOD2 ligands to regulate gut homeostasis in female mice. Nat Commun. 2023;14:3338
157. Choi JH, Moon CM, Shin TS, Kim EK, McDowell A, Jo MK. et al. Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp Mol Med. 2020;52:423-37
158. Chen Y, Zhang Y, Dai M, Qiu C, Sun Q, Fan T. et al. gamma-Linolenic acid derived from Lactobacillus plantarum MM89 induces ferroptosis in colorectal cancer. Food Funct. 2025;16:1760-71
159. Bell HN, Rebernick RJ, Goyert J, Singhal R, Kuljanin M, Kerk SA. et al. Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell. 2022;40:185-200 e6
160. Wang J, Hao Y, Yang Y, Zhang Y, Xu C, Yang R. Gut microbiota derived indole-3-acetic acid ameliorates precancerous inflammatory intestinal milieu to inhibit tumorigenesis through IL-35. J Immunother Cancer. 2025 13
161. Fong W, Li Q, Ji F, Liang W, Lau HCH, Kang X. et al. Lactobacillus gallinarum-derived metabolites boost anti-PD1 efficacy in colorectal cancer by inhibiting regulatory T cells through modulating IDO1/Kyn/AHR axis. Gut. 2023;72:2272-85
162. Zhang Q, Zhao Q, Li T, Lu L, Wang F, Zhang H. et al. Lactobacillus plantarum-derived indole-3-lactic acid ameliorates colorectal tumorigenesis via epigenetic regulation of CD8(+) T cell immunity. Cell Metab. 2023;35:943-60 e9
163. Hu C, Xu B, Wang X, Wan WH, Lu J, Kong D. et al. Gut microbiota-derived short-chain fatty acids regulate group 3 innate lymphoid cells in HCC. Hepatology. 2023;77:48-64
164. Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur Urol. 1995;27:104-9
165. Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212-5
166. Newsome R, Yang Y, Jobin C. Western diet influences on microbiome and carcinogenesis. Semin Immunol. 2023;67:101756
167. Nakatsu G, Andreeva N, MacDonald MH, Garrett WS. Interactions between diet and gut microbiota in cancer. Nat Microbiol. 2024;9:1644-54
168. Taylor AM, Holscher HD. A review of dietary and microbial connections to depression, anxiety, and stress. Nutr Neurosci. 2020;23:237-50
169. Song J, Zhou B, Kan J, Liu G, Zhang S, Si L. et al. Gut microbiota: Linking nutrition and perinatal depression. Front Cell Infect Microbiol. 2022;12:932309
170. Turpin W, Espin-Garcia O, Xu W, Silverberg MS, Kevans D, Smith MI. et al. Association of host genome with intestinal microbial composition in a large healthy cohort. Nat Genet. 2016;48:1413-7
171. Igartua C, Davenport ER, Gilad Y, Nicolae DL, Pinto J, Ober C. Host genetic variation in mucosal immunity pathways influences the upper airway microbiome. Microbiome. 2017;5:16
172. Blekhman R, Goodrich JK, Huang K, Sun Q, Bukowski R, Bell JT. et al. Host genetic variation impacts microbiome composition across human body sites. Genome Biol. 2015;16:191
173. de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463-75
174. Hong Y, Li H, Chen L, Su H, Zhang B, Luo Y. et al. Short-term exposure to antibiotics begets long-term disturbance in gut microbial metabolism and molecular ecological networks. Microbiome. 2024;12:80
175. Fishbein SRS, Mahmud B, Dantas G. Antibiotic perturbations to the gut microbiome. Nat Rev Microbiol. 2023;21:772-88
176. Elkrief A, Derosa L, Kroemer G, Zitvogel L, Routy B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: a new independent prognostic factor? Ann Oncol. 2019;30:1572-9
177. Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE. et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623-8
178. Gamboa J, Le GH, Wong S, Alteza EAI, Zachos KA, Teopiz KM. et al. Impact of antidepressants on the composition of the gut microbiome: A systematic review and meta-analysis of in vivo studies. J Affect Disord. 2025;369:819-33
179. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555-68
180. Matenchuk BA, Mandhane PJ, Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. 2020;53:101340
181. Lawless MH, Harrison KA, Grandits GA, Eberly LE, Allen SS. Perceived stress and smoking-related behaviors and symptomatology in male and female smokers. Addict Behav. 2015;51:80-3
182. Azagba S, Sharaf MF. The effect of job stress on smoking and alcohol consumption. Health Econ Rev. 2011;1:15
183. Chen G, Shi F, Yin W, Guo Y, Liu A, Shuai J. et al. Gut microbiota dysbiosis: The potential mechanisms by which alcohol disrupts gut and brain functions. Front Microbiol. 2022;13:916765
184. Huang C, Shi G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J Transl Med. 2019;17:225
185. Capurso G, Lahner E. The interaction between smoking, alcohol and the gut microbiome. Best Pract Res Clin Gastroenterol. 2017;31:579-88
186. Chen Z, Jin D, Hu J, Guan D, Bai Q, Gou Y. Microbiota and gastric cancer: from molecular mechanisms to therapeutic strategies. Front Cell Infect Microbiol. 2025;15:1563061
187. Chaturvedi P, Pathak R, Dayal R, Parihar H, Kathireshan AK, Tirumalai PS. Colorectal Cancer Mitigation Through Probiotics: Current Evidence and Future Directions. Curr Microbiol. 2025;82:339
188. Guo Y, Xie JP, Deng K, Li X, Yuan Y, Xuan Q. et al. Prophylactic Effects of Bifidobacterium adolescentis on Anxiety and Depression-Like Phenotypes After Chronic Stress: A Role of the Gut Microbiota-Inflammation Axis. Front Behav Neurosci. 2019;13:126
189. Zhu H, Tian P, Qian X, Gu L, Zhao J, Wang G. et al. Perinatal transmission of a probiotic Bifidobacterium strain protects against early life stress-induced mood and gastrointestinal motility disorders. Food Funct. 2022;13:7520-8
190. Hao Z, Wang W, Guo R, Liu H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology. 2019;104:132-42
191. Wang X, Li Y, Wang X, Wang R, Hao Y, Ren F. et al. Faecalibacterium prausnitzii Supplementation Prevents Intestinal Barrier Injury and Gut Microflora Dysbiosis Induced by Sleep Deprivation. Nutrients. 2024 16
192. Mao YQ, Huang JT, Zhang SL, Kong C, Li ZM, Jing H. et al. The antitumour effects of caloric restriction are mediated by the gut microbiome. Nat Metab. 2023;5:96-110
193. Lin Y, Fan L, Qi Y, Xu C, Jia D, Jiang Y. et al. Bifidobacterium adolescentis induces Decorin(+) macrophages via TLR2 to suppress colorectal carcinogenesis. J Exp Clin Cancer Res. 2023;42:172
194. Li Q, Li Y, Wang Y, Xu L, Guo Y, Wang Y. et al. Oral administration of Bifidobacterium breve promotes antitumor efficacy via dendritic cells-derived interleukin 12. Oncoimmunology. 2021;10:1868122
195. Guo J, Meng F, Hu R, Chen L, Chang J, Zhao K. et al. Inhibition of the NF-kappaB/HIF-1alpha signaling pathway in colorectal cancer by tyrosol: a gut microbiota-derived metabolite. J Immunother Cancer. 2024 12
196. Liu L, Wang H, Chen X, Zhang Y, Zhang H, Xie P. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine. 2023;90:104527
197. Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258-64
198. Zhang N, Zhang Y, Li M, Wang W, Liu Z, Xi C. et al. Efficacy of probiotics on stress in healthy volunteers: A systematic review and meta-analysis based on randomized controlled trials. Brain Behav. 2020;10:e01699
199. Romijn AR, Rucklidge JJ, Kuijer RG, Frampton C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust N Z J Psychiatry. 2017;51:810-21
200. Musazadeh V, Zarezadeh M, Faghfouri AH, Keramati M, Jamilian P, Jamilian P. et al. Probiotics as an effective therapeutic approach in alleviating depression symptoms: an umbrella meta-analysis. Crit Rev Food Sci Nutr. 2023;63:8292-300
201. Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104-8
202. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084-9
203. Si W, Liang H, Bugno J, Xu Q, Ding X, Yang K. et al. Lactobacillus rhamnosus GG induces cGAS/STING- dependent type I interferon and improves response to immune checkpoint blockade. Gut. 2022;71:521-33
204. Karam F, El Deghel Y, Iratni R, Dakroub AH, Eid AH. The Gut Microbiome and Colorectal Cancer: An Integrative Review of the Underlying Mechanisms. Cell Biochem Biophys. 2025;83:2637-50
205. Han S, Lu Y, Xie J, Fei Y, Zheng G, Wang Z. et al. Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey. Front Cell Infect Microbiol. 2021;11:609722
206. Maldonado-Gomez MX, Martinez I, Bottacini F, O'Callaghan A, Ventura M, van Sinderen D. et al. Stable Engraftment of Bifidobacterium longum AH1206 in the Human Gut Depends on Individualized Features of the Resident Microbiome. Cell Host Microbe. 2016;20:515-26
207. Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S. et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell. 2018;174:1388-405 e21
208. Mendonca AA, Pinto-Neto WP, da Paixao GA, Santos DDS, De Morais MA Jr, De Souza RB. Journey of the Probiotic Bacteria: Survival of the Fittest. Microorganisms. 2022 11
209. Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83:1256-64 quiz 446-7
210. Freedman SB, Schnadower D, Tarr PI. The Probiotic Conundrum: Regulatory Confusion, Conflicting Studies, and Safety Concerns. JAMA. 2020;323:823-4
211. Merenstein D, Pot B, Leyer G, Ouwehand AC, Preidis GA, Elkins CA. et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes. 2023;15:2185034
212. Kieser S, Zdobnov EM, Trajkovski M. Comprehensive mouse microbiota genome catalog reveals major difference to its human counterpart. PLoS Comput Biol. 2022;18:e1009947
213. Park JC, Im SH. Of men in mice: the development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp Mol Med. 2020;52:1383-96
214. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ. et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491-502
215. Palepu MSK, Bhalerao HA, Sonti R, Dandekar MP. Faecalibacterium prausnitzii, FOS and GOS loaded synbiotic reverses treatment-resistant depression in rats: Restoration of gut-brain crosstalk. Eur J Pharmacol. 2024;983:176960
216. Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G. et al. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol Psychiatry. 2017;82:472-87
217. He Q, Si C, Sun Z, Chen Y, Zhang X. The Intervention of Prebiotics on Depression via the Gut-Brain Axis. Molecules. 2022 27
218. Westfall S, Caracci F, Zhao D, Wu QL, Frolinger T, Simon J. et al. Microbiota metabolites modulate the T helper 17 to regulatory T cell (Th17/Treg) imbalance promoting resilience to stress-induced anxiety- and depressive-like behaviors. Brain Behav Immun. 2021;91:350-68
219. Li Y, Elmen L, Segota I, Xian Y, Tinoco R, Feng Y. et al. Prebiotic-Induced Anti-tumor Immunity Attenuates Tumor Growth. Cell Rep. 2020;30:1753-66 e6
220. Wang Y, Su X, Liu Y, Hu L, Kang L, Xu C. et al. Improved prebiotic-based "shield" equipped probiotics for enhanced colon cancer therapy by polarizing M1 macrophages and regulating intestinal microbiota. Acta Pharm Sin B. 2025;15:4225-47
221. Boucher E, Plazy C, Richard ML, Suau A, Mangin I, Cornet M. et al. Inulin prebiotic reinforces host cancer immunosurveillance via ɣdelta T cell activation. Front Immunol. 2023;14:1104224
222. Johnstone N, Cohen Kadosh K. A randomised controlled trial of the effects of Galacto-Oligosaccharides on the gut brain-axis of young females. Brain Behav Immun. 2025;129:573-84
223. Hughes C, Davoodi-Semiromi Y, Colee JC, Culpepper T, Dahl WJ, Mai V. et al. Galactooligosaccharide supplementation reduces stress-induced gastrointestinal dysfunction and days of cold or flu: a randomized, double-blind, controlled trial in healthy university students. Am J Clin Nutr. 2011;93:1305-11
224. Yang Y, Zhou B, Zhang S, Si L, Liu X, Li F. Prebiotics for depression: how does the gut microbiota play a role? Front Nutr. 2023;10:1206468
225. Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16:605-16
226. Guarino MPL, Altomare A, Emerenziani S, Di Rosa C, Ribolsi M, Balestrieri P. et al. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients. 2020 12
227. Perrone P, D'Angelo S. Gut Microbiota Modulation Through Mediterranean Diet Foods: Implications for Human Health. Nutrients. 2025 17
228. Yin W, Lof M, Chen R, Hultman CM, Fang F, Sandin S. Mediterranean diet and depression: a population-based cohort study. Int J Behav Nutr Phys Act. 2021;18:153
229. Bayes J, Schloss J, Sibbritt D. The effect of a Mediterranean diet on the symptoms of depression in young males (the "AMMEND: A Mediterranean Diet in MEN with Depression" study): a randomized controlled trial. Am J Clin Nutr. 2022;116:572-80
230. Reytor-Gonzalez C, Zambrano AK, Montalvan M, Frias-Toral E, Simancas-Racines A, Simancas-Racines D. Adherence to the Mediterranean Diet and its association with gastric cancer: health benefits from a Planeterranean perspective. J Transl Med. 2024;22:483
231. Giordano G, Mastrantoni L, Terranova R, Colloca GF, Zuccala G, Landi F. The role of Mediterranean diet in cancer incidence and mortality in the older adults. NPJ Aging. 2024;10:61
232. Kurokawa S, Kishimoto T, Mizuno S, Masaoka T, Naganuma M, Liang KC. et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: An open-label observational study. J Affect Disord. 2018;235:506-12
233. Lin H, Guo Q, Wen Z, Tan S, Chen J, Lin L. et al. The multiple effects of fecal microbiota transplantation on diarrhea-predominant irritable bowel syndrome (IBS-D) patients with anxiety and depression behaviors. Microb Cell Fact. 2021;20:233
234. Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM. et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595-602
235. Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602-9
236. Cymbal M, Chatterjee A, Baggott B. Fecal microbiota transplantation: Current evidence and future directions. Cleve Clin J Med. 2025;92:421-8
237. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30:1335-42
238. Rodin G, Lo C, Rydall A, Shnall J, Malfitano C, Chiu A. et al. Managing Cancer and Living Meaningfully (CALM): A Randomized Controlled Trial of a Psychological Intervention for Patients With Advanced Cancer. J Clin Oncol. 2018;36:2422-32
239. Antoni MH, Moreno PI, Penedo FJ. Stress Management Interventions to Facilitate Psychological and Physiological Adaptation and Optimal Health Outcomes in Cancer Patients and Survivors. Annu Rev Psychol. 2023;74:423-55
240. Andersen BL, Lacchetti C, Ashing K, Berek JS, Berman BS, Bolte S. et al. Management of Anxiety and Depression in Adult Survivors of Cancer: ASCO Guideline Update. J Clin Oncol. 2023;41:3426-53
241. Andersen BL, Yang HC, Farrar WB, Golden-Kreutz DM, Emery CF, Thornton LM. et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450-8
242. Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2:888-91
243. Mulick A, Walker J, Puntis S, Burke K, Symeonides S, Gourley C. et al. Does depression treatment improve the survival of depressed patients with cancer? A long-term follow-up of participants in the SMaRT Oncology-2 and 3 trials. Lancet Psychiatry. 2018;5:321-6
244. Goodwin PJ, Leszcz M, Ennis M, Koopmans J, Vincent L, Guther H. et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345:1719-26
245. Semenenko E, Banerjee S, Olver I, Ashinze P. Review of psychological interventions in patients with cancer. Support Care Cancer. 2023;31:210
246. Arch JJ, Mitchell JL, Genung SR, Judd CM, Andorsky DJ, Bricker JB. et al. Randomized trial of acceptance and commitment therapy for anxious cancer survivors in community clinics: Outcomes and moderators. J Consult Clin Psychol. 2021;89:327-40
247. Parekh HS, Liu G, Wei MQ. A new dawn for the use of traditional Chinese medicine in cancer therapy. Mol Cancer. 2009;8:21
248. Ding W, Wang L, Li L, Li H, Wu J, Zhang J. et al. Pathogenesis of depression and the potential for traditional Chinese medicine treatment. Front Pharmacol. 2024;15:1407869
249. Zhao L, Zhu X, Ni Y, You J, Li A. Xiaoyaosan, a traditional Chinese medicine, inhibits the chronic restraint stress-induced liver metastasis of colon cancer in vivo. Pharm Biol. 2020;58:1085-91
250. Feng M, Wang H, Zhu Z, Yao B, Li Y, Xue J. et al. Sanhuang Decoction Controls Tumor Microenvironment by Ameliorating Chronic Stress in Breast Cancer: A Report of Ninety Cases. Front Oncol. 2021;11:677939
251. Shao S, Jia R, Zhao L, Zhang Y, Guan Y, Wen H. et al. Xiao-Chai-Hu-Tang ameliorates tumor growth in cancer comorbid depressive symptoms via modulating gut microbiota-mediated TLR4/MyD88/NF-kappaB signaling pathway. Phytomedicine. 2021;88:153606
252. Yao W, Hua DM, Zhang YR, Zhao YY, Feng Y, Zhang ZZ. et al. Molecular mechanisms of the Xiao-chai-hu-tang on chronic stress-induced colorectal cancer growth based on an integrated network pharmacology and RNA sequencing approach with experimental validation. BMC Complement Med Ther. 2025;25:135
253. Cheng D, Wang X, Hu J, Dai LL, Lv Y, Feng H. et al. Effect of Tai Chi and Resistance Training on Cancer-Related Fatigue and Quality of Life in Middle-Aged and Elderly Cancer Patients. Chin J Integr Med. 2021;27:265-72
254. Huang W, Howie J, Taylor A, Robinson N. An investigation into the effectiveness of traditional Chinese acupuncture (TCA) for chronic stress in adults: a randomised controlled pilot study. Complement Ther Clin Pract. 2011;17:16-21
Author contact
![]() Corresponding author: Jinlin Yang, MD PhD, Department of Gastroenterology and Hepatology, West China Hospital, Sichuan University, 37 Guoxue Lane, 610041, Chengdu, China. E-mail: yangjinlincn; Xue Xiao, MD PhD, Department of Gastroenterology and Hepatology, West China Hospital, Sichuan University, 37 Guoxue Lane, 610041, Chengdu, China. E-mail: xiaoxuecn.
Corresponding author: Jinlin Yang, MD PhD, Department of Gastroenterology and Hepatology, West China Hospital, Sichuan University, 37 Guoxue Lane, 610041, Chengdu, China. E-mail: yangjinlincn; Xue Xiao, MD PhD, Department of Gastroenterology and Hepatology, West China Hospital, Sichuan University, 37 Guoxue Lane, 610041, Chengdu, China. E-mail: xiaoxuecn.

 Global reach, higher impact
Global reach, higher impact