10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2026; 22(1):327-345. doi:10.7150/ijbs.122960 This issue Cite
Review
The Roles of TOPK in Tumorigenesis and Development: Structure, Mechanisms, Pathways, and Therapeutic Implications
1. Department of Pathophysiology, School of Basic Medical Sciences, Zhengzhou University, Zhengzhou 450001, China.
2. China-US (Henan) Hormel Cancer Institute, Zhengzhou 450008, China.
3. Tianjian Laboratory of Advanced Biomedical Sciences, Zhengzhou 450052, China.
4. Department of Clinical Research and Translational Medicine, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou,450002, China.
5. College of Korean Medicine, Dongshin University, Naju 58245, Republic of Korea.
#Equal contribution.
Received 2025-8-2; Accepted 2025-10-13; Published 2026-1-1
Abstract
TOPK (T-LAK cell-originated protein kinase), also known as PDZ-binding kinase, is a serine/threonine kinase belonging to the mitogen-activated protein kinase family. It is a critical regulator of essential cellular processes, including survival, proliferation, apoptosis, inflammation, and autophagy. As an oncogenic kinase, TOPK is predominantly expressed in actively proliferating cells, where its dysregulation contributes to the pathogenesis of various cancers. Through phosphorylation, TOPK activates key signaling pathways such as ERK/RSK/c-Jun, which in turn promote cancer cell proliferation, migration, and resistance to apoptosis. Furthermore, TOPK has been implicated in the regulation of the tumor microenvironment and immune evasion. This review provides an in-depth examination of the molecular structure of TOPK, the role of TOPK in tumorigenesis, and the underlying mechanisms that support its oncogenic activity. Given its central role in cancer progression, TOPK represents a promising candidate for novel cancer therapies. Additionally, we explore the therapeutic potential of targeting TOPK in cancer treatment, highlighting ongoing research efforts and the challenges in translating TOPK inhibition into clinical practice.
Introduction
PDZ-binding kinase/T-LAK cell-derived protein kinase (PBK/TOPK) encodes a serine/threonine protein kinase that plays as a pivotal role in regulating key cellular processes, including survival, proliferation, growth, apoptosis, and inflammation [1, 2]. Under physiological conditions, TOPK expression is primarily restricted to tissues with high proliferative activity. It is abundantly expressed in testis, particularly in spermatogenic germ cells, and in a few normal tissues such as the placenta, and T-LAK cells [3, 4]. In contrast, its expression is minimal or nearly absent in non-proliferative normal tissues such as the adult brain, except for regions containing rapidly dividing progenitor cells, including the subependymal zone and the outer layer of the early postnatal cerebellum [5]. In pathological contexts, however, TOPK is markedly upregulated in malignant tumor cells. Elevated TOPK expression has been observed across various cancer types, and is often associated with tumor aggressiveness and poor clinical prognosis [4]. As a member of the mitogen-activated protein kinase kinase (MAPKK) family, TOPK exerts its biological functions by phosphorylating downstream targets through its kinase domain, thereby modulating signaling pathways relate to proliferation, apoptosis, inflammation, and autophagy. Given its dual presence in proliferative normal tissues and tumors, TOPK has emerged as a promising biomarker and therapeutic target in oncology. It contributes to tumor progression through multiple mechanisms, including the regulation of signal transduction, cellular metabolism, and immune modulation. Targeted TOPK has shown potential in inhibiting cancer growth, making it an attractive target. Consequently, understanding the molecular mechanisms underlying TOPK activation and regulation is of great significance for elucidating its roles in cancer biology. In this review, we comprehensively discuss the structure, expression patterns, biological functions, molecular mechanism, and inhibitors of TOPK in cancer. A deeper understanding of its multifaceted regulatory roles will not only advance our knowledge of cellular signaling and disease pathogenesis but also pave the way for novel precision medicine approaches and targeted cancer therapies.
The structure of TOPK
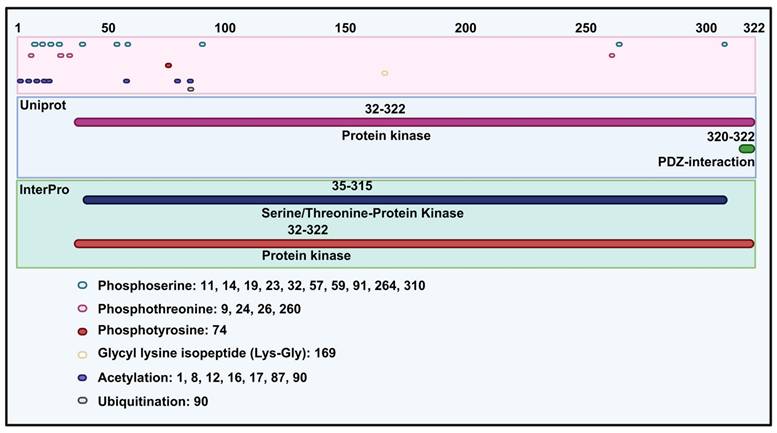
TOPK, a member of MAPKK, shares structural similarities with other kinases in this family, particularly in its serine/threonine kinase subdomains, which are essential for its enzymatic activity. It contains 322 amino acids and has a molecular weight of 36.09 kDa. Specifically, according to the UniProt database (https://www.uniprot.org/) and InterPro database (https://www.ebi.ac.uk/interpro/), TOPK includes a protein kinase domain (32-320 amino acids) and PDZ-interaction region (Figure 1). Structurally, TOPK consists of 11 alpha helices and 11 beta sheets. In addition, molecular modeling reveals that the N-terminal domain is more hydrophobicity than the C-terminal domain, with nonpolar amino acids enriched in the C-domain [6]. With respect to post-translational modification, TOPK is mainly regulated through phosphorylation of serine, threonine, and N6-acetyllysine (Figure 1). The N-terminal region of TOPK contains a conserved phosphorylation site at threonine 9 (Thr9), which is phosphorylated by cdc2/cyclin B during mitosis [7]. Functionally, phosphorylation at Thr9 contributes to cytokinesis, chromosomal segregation, and the regulation of the DNA damage response [3, 8, 9]. Inhibition the kinase activity of TOPK at this site has been shown to suppress tumor growth [10, 11]. Moreover, another phosphorylation site is located at threonine 198 (Thr198), phosphorylated by an unidentified MAPKKK. This site, residing in the ATP-binding pocket, is crucial for the catalytic activity of TOPK and remains phosphorylated throughout the cell cycle [3, 12]. TOPK also contains a C-terminal PDZ-binding motif (T/SXV), enabling interactions with PDZ domain-containing proteins such as hDlg [13], and with proteins like PRC1 to facilitate spindle formation and cytokinesis [14].
Protein structure of TOPK and phosphorylation, glycyl lysine isopeptide, acetylation and ubiquitination site of TOPK.
Regarding conformational changes, different pH conditions induce a transition of PBK between dimers and monomers. Under alkaline conditions, it forms an inactive dimer, with the C-terminal region being structurally conserved and the N-terminal region showing significant variability [15]. Notably, the only available crystal structure of TOPK is a double mutant (T9E and T198E) dimer with a distorted N-leaf conformation [16]. Furthermore, Markov state model (MSM) analysis suggests that wild-type TOPK can partially open even without phosphorylation, while the T9E and Y74E mutations, which accelerate the opening transition, enhance global activity. Based on these findings, two potential phosphorylation regulation pathways are proposed: one where phosphorylation accelerates the open transition of the closed conformation, and another where phosphorylation inhibits the closed-to-open transition [17]. Structural studies using templates such as 2F4J from the Protein Data Bank have provided insights into the 3D conformation of TOPK, its binding sites, and interactions with inhibitors such as HI-TOPK-032 [10, 18]. Overall, the structural features of TOPK highlight its crucial role as a mitotic kinase and its potential as a therapeutic target in cancer treatment, owing to its involvement in oncogenic signaling and tumor progression.
Expression of TOPK in cancer
TOPK, an oncogenic protein, plays a critical role in regulating cell survival, proliferation, growth, apoptosis, and inflammation. Accumulating evidence indicates that TOPK is dysregulated in various cancers, and its expression levels correlate with poor prognosis and tumor aggressiveness. For instance, it is significantly upregulated in several tumor types, including adrenocortical carcinoma (ACC), bladder urothelial carcinoma (BLCA), breast invasive carcinoma (BRCA), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), colon adenocarcinoma (COAD), lymphoid neoplasm diffuse large B-cell lymphoma (DLBC), esophageal carcinoma (ESCA), glioblastoma multiforme (GBM), brain lower grade glioma (LGG), liver hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), ovarian serous cystadenocarcinoma (OV), pancreatic adenocarcinoma (PAAD), rectum adenocarcinoma (READ), skin cutaneous melanoma (SKCM), stomach adenocarcinoma (STAD), thymoma (TYHM), uterine corpus endometrial carcinoma (UCEC), uterine carcinosarcoma (UCS). By contrast, it is downregulated in acute myeloid leukemia (LAML) and testicular cancer (TGCT) according to the GEPIA 2 database (http://gepia2021.cancer-pku.cn/) (Supplement Figure 2A) [19]. Consistently, data from the TIMER 2.0 database (https://compbio.cn/timer2/) show that TOPK/PBK is upregulated in multiple cancers, including BLCA, BRCA, CESC, cholangio carcinoma (CHOL), COAD, ESCA, GBM, head and neck squamous cell carcinoma (HNSC), kidney chromophobe (KICH), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), LIHC, LUAD, LUSC, PAAD, pheochromocytoma and paraganglioma (PCPG), prostate adenocarcinoma (PRAD), SKCM with metastasis, STAD, thyroid carcinoma (THCA), while showing reduced expression in LAML and TGCT (Supplement Figure 2B) [20-22]. Furthermore, our summary of experimental data confirms that TOPK is notably expressed in bladder cancer, breast cancer, cervical cancer, cholangiocarcinoma, chordoma, colorectal cancer, esophageal squamous cell carcinoma (ESCC), gastric cancer, lung adenocarcinoma, lymphoma, oral cancer, osteosarcoma, and ovarian cancer, whereas it is underexpressed in clear hepatocellular carcinoma (Table 1). Notably, according to the research and Kaplan-Meier Plotter database (https://kmplot.com/) show that elevated expression of TOPK has been linked to increased tumor aggressiveness and poor prognosis across multiple cancers (Supplement Figure 2C) [23-27]. In contrast, TOPK expression is reduced in TGCT, consistent with database analyses [28]. In addition to expression levels, analysis of TOPK mutations shows significant correlation with cancer subtypes, such as Basal and LumA of BRCA, COAD, GBM, LIHC, LUAD, READ, and SKCM of primary tumors, according to the TIMER 2.0 database. Moreover, data from cBioPortal database (https://www.cbioportal.org/) reveals that TOPK mutations, included missense, fusion, and insertions, are present in cancers such as renal non-clear cell carcinoma, ovarian epithelial tumor, and glioblastoma (Supplement Figure 1A-B) [29-31]. In conclusion, these findings further highlighting the key role of TOPK in cancer development, both through deregulated expression and genetic alterations (Supplement Figure 1-2).
TOPK as a tumor promoter in cancer
As a mitotic kinase, TOPK regulates the timely progression of cells through the metaphase checkpoint during mitosis, ensuring proper cell cycle progression. Notably, the upregulation of TOPK in cancer is closely associated with tumor progression, including invasiveness, metastasis, and prognosis [23, 50]. It also plays an integral role in chromosome segregation and cytokinesis by phosphorylating multiple targets (Figure 2-4) [51]. In this section, therefore, we explore the expression patterns of TOPK in different cancers, its molecular functions in regulating cell cycle progression, apoptosis, and epithelial-mesenchymal transition (EMT), and its pivotal role in promoting tumor growth and metastasis. Moreover, we discuss the underlying mechanisms which TOPK contributes to cancer progression, such as its interactions with critical signaling pathways and involvement in DNA damage repair, autophagy, and immune responses.
The role of TOPK in different types of cancer
| Cancer Type | Samples (Number of cases) | Quantity of expression | Experiments | Statistical influencing factors (P values) | References | |
|---|---|---|---|---|---|---|
| Bladder cancer | Tissues (76) | Upregulation | QRT-PCR, IHC | Stage (*), grade (*), type of bladder cancer (*) | [32] | |
| Breast cancer | Tissues (202) | Upregulation, with poor prognosis | IHC | Histological (*), TNM (*), lymph node metastasis (*), number of lymph node (*), estrogen receptor (ER *), progesterone receptor (PR *), human epidermal growth factor receptor 2 (HER-2 *), Ki-67 (*), molecular typing (*) | [33] | |
| Triple-negative breast cancer (TNBC) patient tissues before and after of neoadjuvant chemotherapy (NACT) with docetaxel + epirubicin + cyclophosphamide (TEC) drugs (66) | Upregulation, with poor prognosis and poor response to treatment | IHC | The Miller-Payne (MP) system (*) | [34] | ||
| Cervical cancer | Tissues (170) | Upregulation | IHC | Age (*), histological (*), differentiation (*), lymph node metastasis (*), vaginal and cervical invasion (*), TNM (*), tumor size (*), and type of cervical cancer | [35] | |
| Cholangiocarcinoma | Tissues (74) | Upregulation, with the low expression with poor of PBK/TOPK is predicative of poor survival | IHC | Differentiation (*), gender (*) | [36] | |
| Chordoma | Tissues (55) | Upregulation, with poor prognosis | IHC | Recurrence and metastasis (*), disease status (*) | [37] | |
| Colorectal cancer | Tissues (1420, 162 and 269) | Upregulation in both cytoplasm and nucleus | IHC, fluorescent immunohistochemistry | Age (*), differentiation (*), T value (*), tumor location (*), grade (*), stage (*), histological subtype (*), advanced disease stage (*), metastasis (*), low cytoplasm PBK/TOPK expression, negative nuclear PBK/TOPK expression, and low total PBK/TOPK expression were significantly associated with poor overall patient survival, Ki-67 (*), mutation of KRAS/BRAF | [38-40] | |
| ESCC | Tissue (54), ESCC cell lines (15) | Upregulation with poor survival | IHC, | Macroscopic appearance (*), TNM classification of T category (*), macroscopic (*), tumor depth (*), tumor size (*), venous and lymphatic invasion (*) | [41] | |
| Hepatocellular carcinoma | Tissues (33 cases of hepatocellular carcinoma and 10 cases of normal liver tissue) | No expression | IHC | - | [36] | |
| Gastric cancer | Tissues (144) and GC cell lines (5) | Upregulation | IHC, QRT-PCR | Size (*), stage (*), venous invasion (*), TNM classification of pT category (*) and pN category (*), recurrence (*), invasion depth (*), p53 (DO7) | [42] | |
| Tissues (385) | Upregulation with poor survival | IHC, QRT-PCR | Size (*), location, gross type, histological type, stage (*), invasion depth (*), perineural invasion, lymphovascular emboli, lymph node metastasis (*) | [43] | ||
| Lymphoma | Tissues (20) | Upregulation of p-TOPK with short PFS | IHC | Karnofsky performance status (KPS) (*), ocular involvement, deep brain structure involvement, number of lesions (*), chemotherapy and radiotherapy, etc | [44] | |
| Tissues (2), Burkitt lymphoma cell lines (8), other tumor cell lines (10) | Upregulation | PCR, Northern analysis | - | [45] | ||
| Lung adenocarcinoma | Tissues (203) | Upregulation with poor survival | IHC | Age (*), smoking history, tumor size (*), differentiation, necrosis (*), angiolymphatic invasion (*), TOPK IHC score (>3) (*) | [46] | |
| Oral cancer | Tissues (287) | Upregulation, mainly localized in the cytoplasm | IHC | Smoking, betel nut, alcohol consumption, differentiation, stage (*) | [47] | |
| Osteosarcoma | Tissues (66) | Upregulation with poor survival | IHC | Tumor location, histological grade, recurrence, metastasis (*), disease status (*) | [48] | |
| Ovarian cancer | Tissues (163 of EOC and 26 of borderline tumor) | Upregulation with poor progression-free survival and overall survival | IHC | Grade (*), lymph node metastasis, Ki-67 (*) and cytological examination | [49] | |
| Testicular Cancer | GEO datasets (GSE3218 and GSE1818) | Downregulation | - | - | [28] | |
Functional roles and molecular mechanisms of TOPK in cancer development and progression.

Role of TOPK in cell proliferation and tumor growth
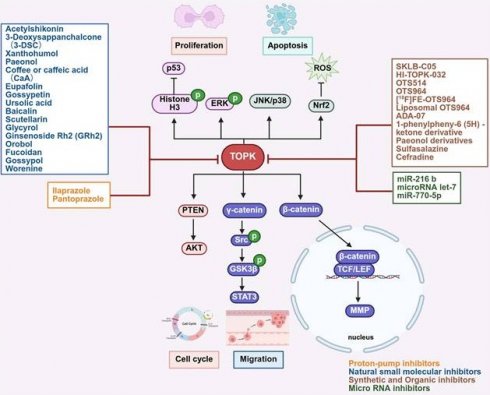
Elevated TOPK expression has been linked to poor outcomes in various cancers (Table 1). For example, in breast cancer, high TOPK expression correlates with adverse clinicopathological features and poor prognosis [33, 34]. Similarly, increased TOPK expression in oral cancer serves as a reliable prognostic marker for patient survival [47]. Consistent with these findings, elevated TOPK expression has also been significantly associated with survival rates in chordoma and lymphoma [37, 44, 45]. In addition, in renal cell carcinoma (RCC), particularly in advanced stages, TOPK overexpression is an independent prognostic factor, making it a potential marker for predicting patient outcomes [52]. Interestingly, evidence indicates that TOPK is elevated in hematologic malignancies but significantly suppressed during TPA-induced differentiation of HL-60 cells, with decreased c-Myc phosphorylation [53]. Functionally, increased expression of TOPK has been shown to enhance cell proliferation in vitro and drive tumor formation in vivo [54]. Mechanistically, TOPK plays a key role in mitotic spindle assembly and function, particularly through phosphorylation at Thr9 by the Cyclin-Dependent Kinase 1 (CDK1)/cyclin B complex (Figure 2). This phosphorylation facilitates the recruitment of CDK1/cyclin B to the mitotic spindle, ensuring proper spindle dynamics during mitosis. The phosphorylation of TOPK is further regulated by CDK1, which inactivates protein phosphatase 1α (PP1α), thereby enabling TOPK activation (Figure 3) [51, 55]. Moreover, TOPK interacts with microtubule-associated proteins (MAPs) to ensure accurate localization of CDK1/cyclin B to the spindle [7]. Beyond this, PBK directly binds to cyclin-dependent kinase 5 (CDK5), and phosphorylation at Thr9 promotes EMT progression and proliferation of prolactinomas, thereby enhancing the stability of PBK. Moreover, phosphorylated p38 levels decrease with increasing Thr9 phosphorylation of TOPK both in vivo and in vitro [56]. Downstream, TOPK activates signaling pathways such as MAPKs and ribosomal S-6 kinase (RSK), which drives cancer cell proliferation and metastasis. It also interacts with transcription factors including AP-1 and NF-κB, linking its kinase activity to cancer progression [57]. In addition, the interaction between TOPK and upstream kinases such as Src increases its stability and activity through phosphorylation at Y74 and Y272, thereby promoting oncogenesis [58]. PBK also upregulates c-Myc expression through phosphorylation of ERK1/2, whereas inhibitors like OTS514 block ERK1/2 phosphorylation and c-Myc transcriptional activity, thereby reversing PBK-induced proliferation and metastasis [59]. Furthermore, the phosphorylation of TOPK by Janus kinase 2 (JAK2) at Tyr74 enhances its activity, driving tumor proliferation in vivo and carcinogenesis in vitro [60]. TOPK also regulates the expression of Y-box binding protein 1 (YB1), a key oncoprotein involved in tumorigenesis. By phosphorylating YB1 at Thr89 and Ser209, TOPK enhances YB1-mediated transcription of eEF1A1, triggering the AKT/mTOR signaling pathway and promoting tumor growth [61]. Similarly, TOPK and PTEN play critical roles in CHFR-mediated mitotic regulation, where TOPK inactivates PTEN and activates the AKT pathway, thus ensuring proper G2/M progression [62]. Moreover, TOPK regulates H₂O₂-mediated signal transduction via its interaction with Prx1 and phosphorylation at Ser32, thereby modulating oxidative stress responses [63]. Importantly, TOPK interacts with the DNA-binding domain of p53, suppressing its transcriptional activity and impairing tumor suppressor functions. In gastrointestinal cancers, TOPK overexpression is linked to cell proliferation and TP53 mutations, further emphasizing its role in oncogenesis [42, 64]. TOPK also stabilizes Nrf2, a key regulator of oxidative stress, thereby promoting cell cycle progression and survival under stress conditions, such as in immature granulocytes [50]. Moreover, TOPK enhances UVB-induced JNK1 activity, which is crucial for cell transformation mediated by H-Ras [65]. LGN/GPSM2 plays a key role in cell division in breast cancer. PBK/TOPK targets Thr450 of LGN/GPSM2 during mitosis, suggesting that the PBK/TOPK-LGN/GPSM2 pathway could be a potential target for breast cancer therapies [66]. PBK further enhances cell proliferation by phosphorylating histone H3 and inhibits colorectal cancer migration and invasion through CDH1 stabilization [67]. Additionally, TOPK/PBK regulates C2H2 zinc finger proteins (ZFPs) during mitosis by phosphorylating C2H2 adaptor sequences in vitro [68].
Regulatory mechanisms and biological functions of TOPK involved in tumorigenesis.
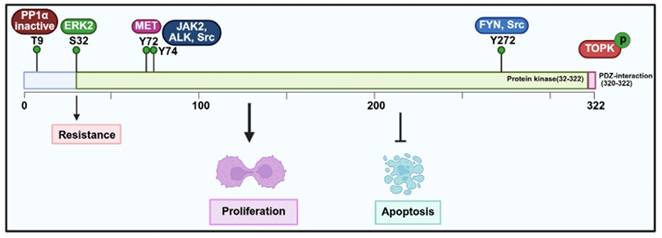
By contrast, inhibiting TOPK in cancer cell lines significantly suppresses tumor growth in vivo [69]. In HCC cells, TOPK inhibition results in cell cycle arrest and reduced colony formation, underscoring its role in cell proliferation and survival [70]. Furthermore, oxidative stress induced by HI-TOPK-032 promotes cell apoptosis by activating the MAPK signaling pathway, highlighting its role of TOPK in regulating NPC growth [71]. Inhibition of TOPK has been shown to suppress tumor growth and enhance treatment sensitivity [72, 73]. Notably, knockdown of TOPK enhances radiosensitivity by altering cell cycle dynamics and increasing radiation-induced damage. Specifically, TOPK depletion disrupts the G1/S transition and G2/M arrest, leading to chromosomal aberrations, multinucleation, and apoptotic cell death [74]. TOPK and maternal embryonic leucine zipper kinase (MELK) together regulate cancer cell proliferation and stem-like properties. Inhibiting either kinase reduces FOXM1 activity, suggesting that TOPK and MELK are promising therapeutic targets for renal cancer. Moreover, combined inhibition with OTS514 (TOPK inhibitor) and OTS167 (MELK inhibitor) enhances antitumor efficacy and may reduce side effects [72].
Role of TOPK in tumor invasion and metastasis
Epithelial-mesenchymal transition (EMT) is a critical process that promotes cancer metastasis by enhancing cell mobility, invasiveness, and resistance to apoptosis [75]. In this context, TOPK plays a central role in promoting EMT, thereby driving tumor invasion and metastasis. Specifically, TOPK facilitates EMT and tumor invasiveness by modulating multiple signaling pathways [76]. Mechanistically, as a mitotic kinase, TOPK amplifies CDK1/cyclin B1-dependent phosphorylation of PRC1 at Thr481, which is essential for efficient cell division [14]. It further regulates EMT in breast cancer through the TGF-β/Smad and NF-κB/Snail signaling pathways [76-79]. In gastric cancer, FYN, a Src family kinase, interacts with TOPK to promote proliferation and metastasis by phosphorylating TOPK at the Y272 and HSPB1 [80, 81]. Moreover, TOPK promotes AKT phosphorylation while reducing PTEN levels, thereby enhancing cell migration and tumor progression through the PI3K/PTEN/AKT pathway [82]. TOPK facilitates the metastasis of ESCC by activating the Src/GSK3β/STAT3 and ERK signaling pathway through γ-catenin [83]. Under hypoxia conditions, TOPK upregulates HIF-1α expression, which promotes EMT and enhances the invasive potential of NSCLC cells [84]. Similarly, in prostate cancer, TOPK activates the β-catenin-TCF/LEF pathway, leading to upregulation of MMP-2 and MMP-9, both of which are associated with tumor invasion and metastasis [77, 85]. At the molecular regulation level, SRSF7 has been shown to enhance GBM cell proliferation and migration partly through m6A modification on PBK mRNA, with two m6A sites on PBK mRNA being directly regulated by SRSF7 [86]. In addition, TOPK interacts with PRPK to regulate colorectal cancer metastasis [87], while PBK overexpression promotes HCC metastasis via the ETV4-uPAR pathway [88]. Finally, TOPK also enhances renal fibrosis through the SGK3/TOPK signaling axis, further driving EMT [89].
Role of TOPK in apoptosis and autophagy
TOPK also plays a crucial role in apoptosis through its interactions with various molecules. For instance, TOPK interacts with histone H2AX, suppressing its phosphorylation in response to arsenic (As³⁺) and thereby promoting apoptosis in cancer cells. This strategy shows potential for melanoma therapy [9]. Moreover, ALK phosphorylates TOPK at the Y74 site to promote cancer cell survival, while dual inhibition of ALK and TOPK significantly increases apoptosis in ALK-positive cancer cells both in vitro and in vivo [90]. Similarly, inhibition of TOPK enhances hydrogen peroxide-induced apoptosis in ovarian granulosa cells by regulating the p53/SIRT1 axis, thereby promoting pro-apoptotic gene expression and cell death [91, 92]. In chordoma, elevated TOPK expression reduced anti-apoptotic proteins such as Mcl-1 and Survivin, while increasing PARP degradation to promote apoptosis [37]. However, resistance mechanisms also exist. For example, ABT-737 induces apoptosis by targeting anti-apoptotic Bcl-2 proteins, disrupting the mitochondrial membrane, and activating caspases, but it also activates TOPK, which in turn upregulates survivin and confers resistance to ABT-737 in cancer cells [93].
Autophagy, a process that maintains cellular homeostasis under stress conditions, also interacts closely with TOPK, and its deregulation contributes to cancer progression [94-96]. Mechanistically, TOPK induces chemotherapy resistance through autophagy. It interacts with ULK1 and inhibits autophagy by phosphorylating ULK1 at Ser469, Ser495, and Ser533, thereby contributes to glioma resistance to temozolomide (TMZ). Inhibition of TOPK increases autophagic vacuoles, which are further enhanced by chloroquine, suggesting that TOPK regulates autophagy initiation [97]. In cervical squamous cell carcinoma (CSCC), TOPK overexpression activates NF-κB and promotes autophagy, accelerating cancer progression, whereas knockdown of TOPK reduces NF-κB pathway activity and autophagy, thereby limiting migration and invasion [98]. Likewise, in glioblastoma, TOPK regulates HDAC1 activity to activate NF-κB and contributing to the malignant phenotype [99]. Additionally, TOPK blocks paclitaxel-induced autophagic cell death in H460 non-small cell lung cancer cells. Knockdown of TOPK increases apoptosis, autophagy, and p53 levels, enhancing paclitaxel-mediated apoptosis [100]. Through the ERK/mTOR axis, TOPK further promotes autophagy and enhances cisplatin resistance. TOPK expression can also be transcriptionally regulated by EVI1, which directly targets the TOPK promoter [101].
Role of TOPK in ferroptosis and anoikis
Ferroptosis, a regulated form of necrotic cell death, plays a role in cancer progression [102]. Recent studies have shown that CLDN6 triggers Nrf2-mediated ferroptosis by regulating the TOPK-dependent AKT/GSK3 β/FYN axis and recruiting TOPK to the cell membrane for degradation by the UPS [103]. Anoikis, a specialized form of programmed cell death caused by the loss of extracellular matrix attachment, represents another barrier to metastasis [104]. Interestingly, TOPK has been linked to anoikis resistance in colorectal cancer (CRC). In this context, CXCL8 enhances resistance to apoptosis by activating AKT and ERK, with TOPK acting downstream of AKT. Thus, simultaneous inhibition of AKT, TOPK, and ERK may provide therapeutic strategies for CRC [90, 105].
Role of TOPK in DNA damage repair
Oncogene activation disrupts DNA replication, thereby affecting replication fork progression and cell cycle timing [106]. In line with this, TOPK inhibition impairs the DNA damage response, leading to increased DNA damage and decreasing cell survival [8]. During the G1 phase, TOPK helps regulate phosphorylation events that are essential for maintaining genomic stability [14]. Depletion of TOPK also results in replication fork stalling and collapse under stress. Furthermore, TOPK interacts with DNA damage response proteins such as CHK1 and Cdc25c, and its inhibition increases cancer cells' vulnerability to genotoxic stress, thereby enhancing sensitivity to radiation therapy [23, 107]. Additionally, TOPK regulates p38α activity by phosphorylating MKP1, which stabilizes MKP1 and suppresses p38α signaling, ultimately supporting tumor growth under DNA damage conditions [108]. Consistently, knockdown of TOPK impairs p38 activation and reduces cell motility in response to growth factors, further underscoring its role in DNA damage response and tumor progression [109, 110].
Role of TOPK in cardiovascular and metabolic regulation
TOPK also regulates metabolic pathways during cancer cell progression. By interacting with signaling molecules, it reprograms cellular metabolism to support the energy and biosynthetic demands of rapidly proliferating cancer cells. For example, in mice and cells exposed to high glucose, Sevoflurane Postconditioning (SPostC) reduces myocardial injury, apoptosis, and oxidative stress, which is accompanied by increased phosphorylation of TOPK, PTEN, and AKT. Inhibition of TOPK or the PI3K/AKT pathway blocks these protective effects, indicating that SPostC protects the heart through TOPK-mediated PTEN/PI3K/AKT activation [111]. In pancreatic β-cells, ectopic expression of TOPK increases ERK1/2 phosphorylation, thereby promoting insulin secretion. TOPK expression is also epigenetically regulated by JunD and HDAC3, and inhibition of JunD improves β-cell proliferation and glucose tolerance in diabetic mice [112, 113]. During pregnancy, TOPK upregulation is linked to impaired glucose tolerance and reduced β-cell proliferation when its kinase activity is inhibited [114]. Additionally, PBK/TOPK mediates geranylgeranylation signaling to promote breast cancer cell proliferation [115].
Role of TOPK in inflammatory and the tumor microenvironment
TOPK mediates lipopolysaccharide (LPS)-induced migration and invasion of breast cancer cells by activating the TLR4 signaling pathway, which increases TOPK expression. Mechanistically, depletion of TOPK reduces LPS-induced phosphorylation of p38 MAPK and suppresses NF-κB and MMP9 promoter activity, thereby inhibiting cancer cell migration and invasion. Elevated levels of TOPK and TLR4 in high-grade breast cancer tissues further support their involvement in tumor progression, positioning TOPK as a promising therapeutic target for inflammation-driven cancer metastasis [78]. Furthermore, TOPK regulates inducible nitric oxide synthase (iNOS) and nitric oxide production in response to LPS, acting as a key effector in the LPS/TLR4-mediated signaling cascade. It promotes IκBα phosphorylation and NF-κB activation, which are critical for iNOS transcription. Knockdown of TOPK or inhibition of the NF-κB binding site decreases transcriptional activity in response to LPS [116]. In addition, the PI3K/AKT/PBK pathway is involved in neutrophil extracellular trap (NET) formation, as observed in co-cultures of neutrophils and OSCC cells. TOPK contributes to enhanced NETs formation through changes in the PI3K/AKT/PBK pathway protein expression [117, 118]. Similarly, in psoriasis, TOPK regulates neutrophil chemokines such as CXCL1, CXCL2, and CXCL8 by activating STAT3 and NF-κB p65 in keratinocytes, thereby promoting neutrophil infiltration and psoriasis progression [119]. Inhibition of TOPK alleviates psoriasis-like dermatitis by regulating neutrophils infiltration [120]. The tumor microenvironment (TME) represents a major barrier to cancer therapy, with cancer-associated fibroblasts (CAFs) driving tumor progression, drug resistance, and immune suppression. In kidney renal clear cell carcinoma, TOPK overexpression is strongly associated with reduced cytotoxic immune cell infiltration, increased immune checkpoint expression, and enhanced infiltration of immunosuppressive cells such as MDSCs and CAFs, underscoring its prognostic and therapeutic potential [121, 122]. Moreover, TOPK enhances microglia/macrophage M2 polarization by inhibiting HDAC1/HDAC2 activity, potentially contributing to its neuroprotective effects against cerebral ischemia-reperfusion injury [99]. Taken together, these findings confirm the involvement of TOPK in inflammatory processes.
The expression of TOPK regulated by transcription factors
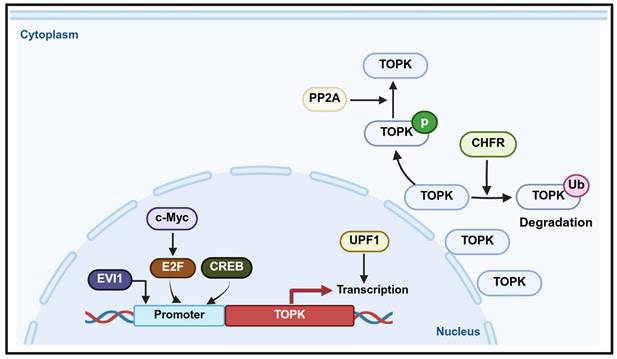
PBK/TOPK plays a crucial role in determining cell fate, with its transcription regulated by several transcription factors and signaling pathways (Figure 4). For instance, E2F1, a key cell cycle transcription factor, collaborates with c-Myc to activate PBK/TOPK transcription, thereby promoting cell cycle progression [123-125]. In addition, CREB/ATF binds to the PBK/TOPK promoter to upregulate its expression [126]. During mitosis, CDK1/cyclin B1 inactivates PP1α, leading to TOPK activation through autophosphorylation, which highlights the role of CDK1/cyclin B1 in regulating TOPK during cell division [51]. In leukemia, TOPK is regulated by PP2A and BCR/ABL, where PP2A associates with and dephosphorylates TOPK, while BCR/ABL upregulates its expression. Conversely, inhibition of BCR/ABL by imatinib or activation of PP2A reduces TOPK phosphorylation, suggesting that TOPK functions as a downstream target of BCR/ABL signaling [127]. Up-frameshift protein 1 (UPF1) promotes PBK transcription, as PBK transcriptional activity decreases upon UPF1 knockdown and increases with UPF1 overexpression. Consequently, UPF1 regulates PBK mRNA and protein levels and modulates FOXO1 expression through the PBK signaling axis [128].
Regulation of the TOPK promoter and transcriptional activity.
Targeting TOPK for cancer therapy
The oncogenic roles of TOPK highlight its potential as a therapeutic target to inhibit cancer progression and improve patient outcomes. In this section, we review the effects of targeting TOPK and its role in chemotherapy resistance.
Inhibition of TOPK could arrest cancer growth
Targeting TOPK or suppressing its expression can be a promising strategy for cancer treatment. For example, inhibitors such as atorvastatin or GGTase I inhibitors (GGTI-298) suppress proliferation of ER-negative breast cancer MDA-MB-231 cells by targeting PBK/TOPK, indicating PBK/TOPK as a downstream effector of geranylgeranyl signaling. Additionally, PBK/TOPK inhibition reduces MDA-MB-231 cell proliferation, while YAP, a key Hippo signaling effector, regulates PBK expression, linking TOPK to Hippo-YAP signaling [115]. In prostate cancer, TOPK induces androgen receptor splice variant ARv7 expression, promoting androgen independence. Silencing TOPK sensitizes prostate cancer cells to androgen receptor-targeted therapies, demonstrating its role in ARv7 regulation [129]. Similarly, in high-grade lymphomas, PBK/TOPK overexpression forms part of a c-Myc-E2F1-PBK axis, and targeting this pathway reduces lymphoma cell growth and survival [124]. In medulloblastoma, LIN28B regulates TOPK and its inhibition reduces tumor viability and growth [130]. In chronic myeloid leukemia (CML), TOPK is regulated by BCR/ABL, and its inhibition with OTS514 suppresses cell proliferation and colony formation, underscoring its therapeutic relevance [127]. In Ewing sarcoma, inhibiting the EWS-FLI1 fusion oncogene reduces PBK/TOPK expression, suggesting it is a directly target gene [131]. Moreover, PRPK, a cancer-related protein, is phosphorylated by TOPK. Knockdown of TOPK reduces PRPK phosphorylation, conferring resistance to SSL-induced skin cancer in mice. Inhibiting PRPK reduces skin hyperplasia, angiogenesis, and cutaneous squamous cell carcinoma by blocking PRPK activation and lowering expression of proliferation and tumor markers such as cyclin D1, COX-2, and MMP-9 [132]. At the molecular level, TOPK interacts with JNK-interacting protein 1 to enhance JNK1 activity, which is essential for AP-1 transcription and cell transformation induced by UVB or H-Ras [65]. Interestingly, TOPK has been identified as a potential target, showing promise for inhibiting SARS-CoV-2 [133].
Targeted TOPK could affect the cancer immune response
TOPK also modulates immune responses in cancer. In CRC, TOPK expression negatively correlates with immunosuppressive cells like Tregs and M2 macrophages, but positively associating with cytotoxic T cell-related genes [134]. In RCC, TOPK activates PD-L1 expression, promoting immune evasion and inhibiting CD8+ T cell infiltration. Its inhibition enhances CD8+ T cell infiltration and improves anti-PD-L1 treatment efficacy [135]. In skin cancer, a dual-target system incorporating the TOPK inhibitor OTS964 and albendazole (ABZ) demonstrate synergistic cytotoxic effects [136]. In addition, TOPK inhibition enhances IFN-γ and TNF-α production in NK-92MI cells, thereby improving antitumor activity [137]. In ESCC, TOPK regulates the tumor microenvironment by promoting immune cell invasion and microsatellite instability [138]. Similarly, TOPK, CCNA2, and KIF4A collectively regulate the tumor microenvironment in HCC, influencing immune cell invasion and microsatellite instability (MSI) [139]. Furthermore, in CAR T-cell therapy, TOPK inhibition enhances T cell proliferation, memory formation, and immune checkpoint regulation [140]. Immune infiltration analysis also showed that TOPK-induced immune escape may involve altered antigen presentation, dendritic cells, and CD8+ T cell infiltration [141]. Mechanistically, TOPK phosphorylates MSL1, enhancing its interaction with MSL2, MSL3, and KAT8, which enriches the MSL complex at the CD276 promoter. This increases histone H4 K16 acetylation and activates CD276 transcription, a molecule regulated in NPC and correlates with immune infiltration, suggesting that the PBK/MSL1/CD276 signaling axis contributes to immune evasion [142].
Targeted TOPK could enhance cancer cells sensitivity to chemotherapy
TOPK is also implicated in chemotherapy resistance. For instance, methylseleninic acid (MSeA) overcomes gefitinib resistance in NSCLC through the asparagine-MET-TOPK axis [143], while ERK2-mediated phosphorylation of TOPK enhances RCC cell sensitivity to sorafenib [52]. In NSCLC, the COX2-TXA2 pathway activates MET and TOPK phosphorylation, promoting gefitinib resistance [144]. Moreover, combining TOPK inhibitors with targeted therapies improves therapeutic sensitivity and delays drug resistance. TOPK interacts with c-Jun to influence the response of lung cancer to EGFR inhibitors gefitinib, with silencing TOPK enhancing drug sensitivity [145]. The TOPK inhibitor pantoprazole, in combination with celecoxib and gefitinib, induced apoptosis in gefitinib-resistant lung cancer cells and suppressed tumor growth [144]. In addition, TOPK enhances doxorubicin resistance by positively regulating NF-κB activity in TRAIL signaling, thereby promoting anti-apoptotic gene expression [146]. MicroRNA-216b (miR-216b) downregulates TOPK in lung adenocarcinoma, increasing oxaliplatin sensitivity [147]. In HCC, PBK mediates oxaliplatin resistance by regulating PTEN, while in ovarian cancer, PBK interacts with TRIM37 to promote PARPi resistance in ovarian cancer [148, 149]. Notably, combining HI-TOPK-032 with alectinib, a first-line therapy for ALK-positive lung cancer, improves treatment sensitivity [150]. Finally, TOPK ablation sensitizes cells to TRAIL-induced apoptosis [151], while, CHIP, an E3 ubiquitin ligase, reduces PBK stability through the ubiquitin-protease pathway, inhibiting ERK pathway and suppressing NSCLC radio-resistance [152, 153]. In glioblastoma, PBK inhibition improves radiotherapy efficacy by regulating CCNB2, a key factor in tumorigenesis and radio-resistance [154]. Collectively, these findings demonstrate that targeting TOPK not only suppresses tumor growth but also modulates immune responses and reverses drug resistance. Thus, TOPK inhibition (either as monotherapy or in combination with existing targeted drugs) holds significant promise for clinical translation and may ultimately improve cancer patient outcomes.
Inhibitors of TOPK in cancer treatment
Targeted therapy is a promising approach in drug design, and the upregulation of TOPK has been linked to cancer diagnosis and prognosis, making it an important therapeutic target. To date, several effective TOPK inhibitors have been developed and can be categorized into four main groups: synthetic and organic compounds, proton pump inhibitors, natural small molecular inhibitors and microRNAs (Figure 5, Table 2-5). Notable inhibitors include HI-TOPK-032 [10], OTS514 [155] and OTS964 [156], alongside organic compounds like sulfasalazine [157] and proton pump inhibitors like pantoprazole [158] and ilaprazole [159], some of which are already FDA-approved drugs. In this section, we provide a comprehensive summary of the mechanisms of action of various TOPK inhibitors, offering valuable insights and potential strategies for their future clinical application in cancer therapy.
Synthetic and biologically derived inhibitors of TOPK
Given its critical role in tumorigenesis, metastasis, and poor prognosis, targeting TOPK has emerged as a viable therapeutic strategy. HI-TOPK-032, OTS514, OTS964, and SKLB-C05 are representative synthetic inhibitors of TOPK (Table 2). For instance, HI-TOPK-032, developed by our group, specifically targets TOPK and suppresses diverse tumor types by inhibiting ERK and RSK phosphorylation through AP-1 or p53 pathways. In mouse xenograft models, treatment with HI-TOPK-032 (1-10 mg/kg) inhibited tumor growth by more than 60% without evident toxicity [10, 140]. Similarly, the TOPK inhibitor OTS514 suppresses cancer cell proliferation by downregulating E2F target genes and inhibiting FOXM1 and MELK activities, thereby inducing cell cycle arrest and apoptosis through the p53 signaling pathway. It exhibits potent growth-inhibitory effects in multiple tumor types, including oral squamous carcinoma and small-cell lung cancer [155, 160]. OTS964, another TOPK inhibitor, causes cell division defects and apoptosis [74, 156]. Additionally, its radiolabeled derivative [18F] FE-OTS964 has been developed as an imaging agent [161], while liposomal OTS964 shown enhanced fluorescence when bound to albumin [162]. ADA-07 binds TOPK to inhibit ERK1/2, p38, JNK phosphorylation, and AP-1 activity [163]. SKLB-C05 inhibits colorectal carcinoma growth and metastasis by downregulating TOPK-mediated signaling, and blocks the FAK/Src-MMPs pathway [164]. Moreover, structural optimization has led to phenanthridinone derivatives with stronger anti-TOPK activity than OTS964 [165]. OTS964, itself has been reported to suppress tumor growth in ovarian and lung cancers [49, 156], and to enhance radiosensitivity [166, 167]. However, acquired resistance to OTS964 remains a major challenge. Other compounds, such as paeonol derivatives, inhibit TOPK-related signaling by modulating MAPK and NF-κB pathways, as well as DNA damage-related proteins such as H2AX and STAT3 [168]. Among repurposed drugs, sulfasalazine inhibits TOPK, reduces p-AKT, and suppresses tumor proliferation and metastasis through the PI3K/AKT pathway, with significant in vivo antitumor effects at concentrations up to 150 μM [157]. Cefradine, another agent, directly binds TOPK and inhibits T-LAK cell-derived protein kinase. Importantly, cefradine showed no cytotoxicity in HaCat or JB6 cells, while treatment at 100 mg/kg markedly reduced epidermal thickness and attenuated immune cell infiltration in mice [169].
Targeting TOPK with proton pump inhibitors
Proton pump inhibitors (PPI) are widely prescribed drugs [170, 171]. Interestingly, pantoprazole and ilaprazole also function as TOPK inhibitors (Table 3). In preclinical models, pantoprazole at 100 mg/kg significantly suppressed xenograft tumor growth, while oral administration of ilrazole at 75 and 150 mg/kg inhibited tumor growth without causing hepatotoxicity, nephrotoxicity, or weight loss [158, 159]. In addition, pantoprazole sensitizes gefitinib-resistant NSCLC cells to apoptosis by directly targeting TOPK [144].
Schematic representation of TOPK inhibitors and their mechanisms of action in cancer.

Synthetic and organic inhibitors of TOPK
| Inhibitors | Structure | Mechanism of action | Types of cancer | Classification | References |
|---|---|---|---|---|---|
| HI-TOPK-032 | 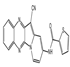 | Inhibition of phosphorylation of ERK and ERK direct downstream proteins ERK and RSK, or through AP-1 or p53 signaling pathways | Colorectal cancer | [10] | |
| OTS514 | 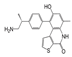 | FOXM1 and MELK activities were inhibited, and cell cycle arrest and apoptosis were induced | Small cell lung cancer | [155] | |
| OTS514 inhibited the proliferation of OSCC cells by down-regulating the expression of E2F target genes, and induced cell apoptosis by mediating the p53 signaling pathway | Oral squamous carcinoma | [160] | |||
| OTS964 | 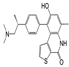 | Inhibition of TOPK activity inhibits tumor growth by inducing defects in cell division and inducing apoptosis | Lung cancer | [156] | |
| [18F] FE-OTS964 | 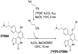 | The first TOPK inhibitor for imaging purposes | Glioblastoma | [161] | |
| Liposomal OTS964 | - | The simple method of association of OTS964 with liposomes, when bound to albumin, relies on enhanced OTS964 fluorescence | [162] | ||
| SKLB-C05 | 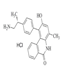 | SKLB-C05 accelerated apoptosis by targeting members of the Bcl-2 family, including Mcl-1, Bcl-2 and Bax, with the expression of both cyclin B1 and CDK1 inhibited | Colorectal cancer | Synthetic compounds | [164] |
| ADA-07 | 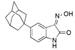 | ADA-07 directly binds to TOPK and inhibits TOPK kinase activity, and inhibits ERK1/2, p38 and JNKs phosphorylation, thereby inhibiting AP-1 activity | Skin cancer | [163] | |
| 1-phenylpheny-6 (5H) -ketone derivative | 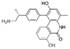 | The growth of cancer cells was inhibited by apoptosis and the activity of TOPK was specifically inhibited. | Colorectal cancer | [165] | |
| Paeonol derivatives | _ | Paeonol derivatives interacts with TOPK, regulates its downstream pathways MAPK and NF-κB, and inhibits the expression of DNA damage-related protein H2AX and proliferation-related protein STAT3 | Psoriasiform skin inflammation | [168] | |
| Sulfasalazine | 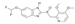 | Sulfasalazine directly binds TOPK to reduce the level of p-AKT, and affects the PI3K/AKT signaling pathway, inhibit the proliferation and metastasis | Thyroid cancer | Organic compounds (FDA approved) | [157] |
| Cefradine | 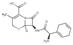 | Cefradine can directly bind to TOPK, block solar UV-induced skin inflammation by directly inhibiting T-LAK cell-derived protein kinase | Skin inflammation and skin cancer | Organic compounds (FDA approved) | [169] |
Small-molecule inhibitors of TOPK from natural sources
Natural compounds, owing to their diverse chemical structures, can modulate multiple cellular pathways by inhibiting proliferation, inducing apoptosis, or regulating inflammation. Importantly, they also enhance drug sensitivity, suppress tumor growth and metastasis, and show synergistic effects with conventional therapies [172-175]. Among them, acetylshikonin, a bioactive compound from Lithospermum erythrorhizon root, directly binds the ATP pocket of TOPK, inducing G1 arrest, inhibiting colon cancer cell proliferation, and promoting apoptosis. Mechanistically, it blocks TOPK-mediated signaling by reducing the phosphorylation of ERK, RSK, c-Jun, as well as NF-κB activity. In vivo, acetylshikonin (120 or 160 mg/kg) significantly reduced tumor growth without affecting body weight [1]. Likewise, 3-Deoxysappanchalcone (3-DSC) induces G2/M cell cycle arrest and apoptosis in colon and skin cancers by targeting TOPK [2, 176]. Xanthohumol, a principal prenylated chalcone from hops, also directly targets TOPK and inhibits its activity, thereby reducing phosphorylation of histone H3 and AKT in vitro and in vivo, which contributes to its anticancer effects [177]. Other natural agents also exhibit inhibitory effects on TOPK. For example, paeonol reduces SUV-induced inflammation by targeting TOPK [178]. Caffeic acid and coffee inhibit colon cancer metastasis by suppressed MEK1/TOPK and ERK/AP-1 signaling [179]. Eupafolin decreased histone H3 and Ki67 expression while enhancing caspase 3 activity in tumor tissues [180]. Gossypetin inhibits PBK/TOPK activity and downregulates the phosphorylation of PBK/TOPK and p38 MAPK [181]. Ursolic acid (UA), a triterpene found in apples, rosemary, and basil, inhibits TOPK phosphorylation in a concentration-dependent manner, activating the p53-p21 pathway and inducing G1 cell cycle arrest in breast cancer and colorectal cancer [182]. Additionally, gossopol promotes differentiation through the PBK/TOPK pathway [183]. Other flavonoids, such as baicalin, scutellarin, and glycyrol, have also been reported to inhibit TOPK activity [184-190]. Beyond oncology, natural inhibitors may also regulate inflammation conditions through TOPK inhibition. For example, worenine, a compound from the rhizome of Coptis chinensis, inhibits TOPK activity and alleviates M5- and IMQ-induced psoriasis-like dermatitis by reducing neutrophil infiltration [120].
Proton pump inhibitors of TOPK
| Inhibitors | Structure | Mechanism of action | Types of cancer | References | ||
|---|---|---|---|---|---|---|
| Pantoprazole | 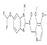 | Direct binding to TOPK to inhibit TOPK activity in vitro and in vivo strongly reduced the phosphorylation of histone H3 (Ser10) | Colorectal cancer | FDA-approved | [158] | |
| Ilaprazole | 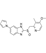 | Direct binding of TOPK to inhibit TOPK activity resulted in strongly reduced phosphorylation of histone H3 (Ser10), a downstream substrate of TOPK, in cancer cells | Lung cancer, colon cancer, human ovarian cancer, and pancreatic cancer cells | FDA-approved | [159] | |
Natural small molecular inhibitors of TOPK
| Inhibitors | Structure | Mechanism of action | Types of cancer | Source | References |
|---|---|---|---|---|---|
| Acetylshikonin | 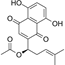 | Direct inhibition of TOPK activity, interacting with the ATP-binding pocket of TOPK, inhibits cell proliferation by inducing cell cycle arrest in G1 phase, stimulates apoptosis, and increases the expression of apoptotic biomarkers in colorectal cancer cell lines | Colorectal cancer | The main bioactive compounds present in the roots of Lithospermum erythrorhizon | [1] |
| 3-Deoxysappanchalcone (3-DSC) | 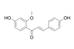 | 3-DSC inhibits colon cancer cell growth and induces G2/M cell cycle arrest and apoptosis by directly targeting the TOPK-mediated signaling pathway | Colorectal cancer, Skin Cancer | An ingredient of Caesalpinia sappan L. is a natural oriental medicine | [2, 176] |
| Xanthohumol | 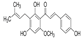 | Direct interaction with TOPK reduced the phosphorylation of TOPK kinase activity and its downstream signals histone H3 and Akt | Non-small cell lung cancer | The major isoprenylated chalcones isolated from hops | [177] |
| Paeonol | 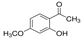 | Paeonol has a protective effect on SUV-induced inflammation by targeting TOPK | Inflammation of the skin | Isolated from traditional Chinese herbs, such as danpi, paeoniflora root, and Japanese wild peony | [178] |
| Coffee or caffeic acid (CaA) | 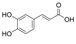 | Targeting MEK1 and TOPK inhibited colon cancer metastasis and tumor cell transformation, inhibited ERKs phosphorylation, AP-1 and NF-κB transactivation, and subsequently inhibited TPA, EGF and H-ras-induced tumor transformation in JB6 P+ cells | Colorectal cancer | [179] | |
| Eupafolin | 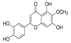 | Eupafolin treatment decreased histone H3 and Ki-67 and increased caspase-3 in tumor tissues | Esophageal cancer | The main active ingredient extracted from the traditional Chinese medicine Artemisia vulgaris L. | [180] |
| Gossypetin | 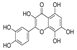 | Gossypetin inhibited the activity of PBK/TOPK and inhibited the phosphorylation of PBK/TOPK, p38 MAPK, ERK1/2 and H2AX induced by solar UV | Basal cell carcinoma of the skin | Flavonoids derived from hibiscus, a traditional Chinese medicine | [181] |
| Ursolic acid | 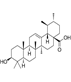 | Ursolic acid could inhibit mouse minute-2 protein (MDM2) and T-LAK cell-derived protein kinase (TOPK), two negative regulators of p53, leading to ursolic acid-induced p53 activation | Breast cancer, colon cancer | apple, rosemary, and holy basil | [182] |
| Gossypol | 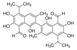 | Gossypol could induce differentiation in leukemic cell lines by specifically inhibiting the phosphorylation of PBK/TOPK without affecting total protein levels | Leukemia | Cottonseed (gossypium) plants | [183] |
| Baicalin | 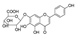 | Direct binding to PBK/TOPK inhibits TOPK activity, and PBK/TOPK downstream signaling molecules histone H3 and ERK2 are also reduced | Lung cancer | The main bioactive component extracted from the root of Baical Skullcap | [184] |
| Scutellarin |  | Direct binding to TOPK in vitro, inhibit the activity of TOPK, and inhibit the phosphorylation of extracellular regulated protein kinase 1/2 (ERK1/2) and histone H3 in cells | Melanoma | The active ingredient extracted from Erigeron breviscapus (Vant.) Hand-Mazz. | [185] |
| Glycyrol | 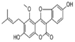 | Glycyrol binds strongly to TOPK protein and inhibits its kinase activity, leading to activation of the apoptotic signaling pathway | Lung cancer | Representative coumarin compounds isolated from licorice | [186] |
| Inhibition of iron cell apoptosis alleviates acute kidney injury | Acute kidney injury | [190] | |||
| Ginsenoside Rh2 (GRh2) |  | GRh2 could directly bind to PBK/TOPK, inhibit the activity of PBK/TOPK, inhibit the phosphorylation of extracellular regulated protein kinase 1/2 (ERK1/2) and (H3) in colorectal cancer cells, and inhibit the growth of xenograft tumors | Colorectal cancer | The main bioactive ingredient in American ginseng, a commonly used herbal medicine | [187] |
| Orobol |  | Direct binding to TOPK and inhibits TOPK kinase activity in an ATP-independent manner | Squamous cell carcinoma of the skin | Derived from soybean, it is present in trace amounts in natural and fermented foods | [188] |
| Fucoidan | 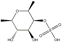 | Fucoidan directly targeting TOPK and suppressing the TOPK/ERK1/2/MSK1 signaling axis, fucoidan effectively prevented EGF-induced tumor cell transformation in both ex vivo and in vitro models | Colon cancer | Fucus evanescens | [189] |
| Worenine | 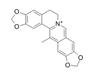 | Worenine inhibits TOPK activity to alleviate psoriasis-like dermatitis induced by M5 | Psoriasiform dermatitis | Coptis chinensis | [120] |
MicroRNA inhibitors of TOPK
| Inhibitors | Mechanism of action | Types of cancer | References |
|---|---|---|---|
| miR-216 b | MiR-216b was negatively correlated with the expression of TOPK, and it could down-regulate the level of TOPK by binding to the 3' untranslated region (3' UTR) of TOPK | Colorectal cancer | [147] |
| miR-216b-3p | Overexpression of miR-216b-3p may increase the expression of p53 and p21 and prevent p38 MAPK activation | Adenocarcinoma of the lung | [192] |
| microRNA let-7 | MicroRNA let-7 inhibits PBK to reduce cell proliferation | Medulloblastoma | [130] |
| miR-770-5p | Overexpression of miR-770-5p increases apoptosis by directly targeting PDZ-binding kinase (PBK) and ultimately sensitizing the radiation response in vitro and in vivo | MCF7, A549 and HCT-116 cells | [193] |
Targeting TOPK with microRNA inhibitors
MiRNAs regulate diverse processes in cancer, including proliferation, apoptosis, invasion, and angiogenesis [191]. Notably, miR-216b downregulates TOPK by binding to its 3' untranslated region (3' UTR) [147], whereas miR-216b-3p suppresses lung adenocarcinoma cell proliferation by targeting TOPK, leading to increased p53 and p21 expression and inhibition p38 MAPK activation [192]. Additionally, let-7 inhibits PBK to reduce cell proliferation [130], and miR-770-5p sensitizes cells to radiation by targeting TOPK and increasing apoptosis (Table 5) [193].
Conclusions
In summary, as one of the kinases in the MAPKK family, TOPK plays a crucial role in cell cycle regulation and mitotic progression, influencing a wide range of cellular functions and activating key signaling pathways, such as MAPK and PI3K/AKT. Its overexpression is strongly associated with tumor growth, metastasis, drug resistance, and it is notably upregulated in most cancer tissues. Collectively, these findings suggest that TOPK drives tumorigenesis and development by regulation cell proliferation, kinase-mediated signaling, immune modulation, and metabolic pathways. However, its biological role appears to be context-dependent, varying across cancer types and potentially shaped by tissue-specific or mutational factors. Although database analyses indicate that TOPK is highly expressed in multiple cancers, including ACC, BLCA, CESC, LGG and TYHM, experimental validation remains limited, and the precise oncogenic mechanisms in these malignancies are still unclear. Moreover, the role of TOPK in metabolic regulation and post-translational modifications has scarcely been investigated. Future studies should therefore aim to bridge these gaps by employing advanced approaches such as single-cell transcriptomics, spatial transcriptomics, metabolomics, and proteomics of post-translational modifications. From a therapeutic perspective, targeting TOPK presents a promising strategy in cancer treatment. As targeted therapies continue to advance, inhibiting kinase phosphorylation to disrupt aberrant signaling cascades remains a central focus of anticancer drug development. Nevertheless, despite extensive preclinical evidence demonstrating the important of TOPK in tumor growth and progression, and the availability of multiple inhibitors capable of suppressing its kinase activity, there are currently no specific TOPK-targeted drugs approved for clinical use. Therefore, further investigation is required to evaluate their therapeutic efficacy in patients, and combining strategies with other clinal drugs may offer synergistic benefits. In addition, the role of TOPK in chemotherapy resistance warrants deeper exploration to better understand its contribution to treatment failure and to identify novel approaches for overcoming resistance. Overall, more focused and integrative research is essential to fully elucidate the biological functions of TOPK and to realize its potential as a viable therapeutic target in cancer therapy.
Supplementary Material
Supplementary figures.
Acknowledgements
Funding
This work was supported by grants from the National Natural Science Foundation of China (NSFC 82403140, 82303651), the China Postdoctoral Science Foundation Funded Project (No. 2023M743180), Project of Zhongyuan Scholar Talent (No. 234000510008), Major Science and Technology Projects in Henan Province (No. 221100310100) and the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (No. 2022R1A5A2029546).
Authorship contributions
Mengyu Zhao: Formal analysis, Validation, Writing - review & editing. Min Zhang: Writing - review & editing. Mengrui Liang: Visualization, Formal analysis. Xinru Wang: Formal analysis. Shengjie Wu: Formal analysis. Qipan Feng: Formal analysis, Validation. Xueli Tian: Formal analysis. Ding Ding: Formal analysis. Xiang Li: Supervision. Kangdong Liu: Supervision, Project administration. Mee-Hyun Lee: Supervision, Writing - review & editing, Writing-original draft, Funding acquisition, Conceptualization. Ran Zhao: Supervision, Writing-review & editing, Writing-original draft, Funding acquisition. Zigang Dong: Writing - review & editing, Writing-original draft, Conceptualization, Funding acquisition. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zhao R, Choi BY, Wei L, Fredimoses M, Yin F, Fu X. et al. Acetylshikonin suppressed growth of colorectal tumour tissue and cells by inhibiting the intracellular kinase, T-lymphokine-activated killer cell-originated protein kinase. British Journal of Pharmacology. 2020;177:2303-19
2. Zhao R, Huang H, Choi BY, Liu X, Zhang M, Zhou S. et al. Cell growth inhibition by 3-deoxysappanchalcone is mediated by directly targeting the TOPK signaling pathway in colon cancer. Phytomedicine. 2019;61:152813
3. Fujibuchi T, Abe Y, Takeuchi T, Ueda N, Shigemoto K, Yamamoto H. et al. Expression and phosphorylation of TOPK during spermatogenesis. Development, Growth & Differentiation. 2005;47:637-44
4. Huang H, Lee MH, Liu K, Dong Z, Ryoo Z, Kim MO. PBK/TOPK: An Effective Drug Target with Diverse Therapeutic Potential. Cancers (Basel). 2021;13:2232
5. Dougherty JD, Garcia ADR, Nakano I, Livingstone M, Norris B, Polakiewicz R. et al. PBK/TOPK, a Proliferating Neural Progenitor-Specific Mitogen-Activated Protein Kinase Kinase. The Journal of Neuroscience. 2005;25:10773-85
6. Kirubakaran P, Karthikeyan M, Singh KD, Nagamani S, Premkumar K. In silico structural and functional analysis of the human TOPK protein by structure modeling and molecular dynamics studies. Journal of Molecular Modeling. 2012;19:407-19
7. Matsumoto S, Abe Y, Fujibuchi T, Takeuchi T, Kito K, Ueda N. et al. Characterization of a MAPKK-like protein kinase TOPK. Biochemical and Biophysical Research Communications. 2004;325:997-1004
8. Ayllón V, O'Connor R. PBK/TOPK promotes tumour cell proliferation through p38 MAPK activity and regulation of the DNA damage response. Oncogene. 2006;26:3451-61
9. Zykova TA, Zhu F, Lu C, Higgins L, Tatsumi Y, Abe Y. et al. Lymphokine-Activated Killer T-Cell-Originated Protein Kinase Phosphorylation of Histone H2AX Prevents Arsenite-Induced Apoptosis in RPMI7951 Melanoma Cells. Clinical Cancer Research. 2006;12:6884-93
10. Kim DJ, Li Y, Reddy K, Lee MH, Kim MO, Cho YY. et al. Novel TOPK inhibitor HI-TOPK-032 effectively suppresses colon cancer growth. Cancer Res. 2012;72:3060-8
11. Roh E, Han Y, Reddy K, Zykova TA, Lee MH, Yao K. et al. Suppression of the solar ultraviolet-induced skin carcinogenesis by TOPK inhibitor HI-TOPK-032. Oncogene. 2020;39:4170-82
12. Abe Y, Matsumoto S, Kito K, Ueda N. Cloning and Expression of a Novel MAPKK-like Protein Kinase, Lymphokine-activated Killer T-cell-originated Protein Kinase, Specifically Expressed in the Testis and Activated Lymphoid Cells. Journal of Biological Chemistry. 2000;275:21525-31
13. Gaudet S, Branton D, Lue RA. Characterization of PDZ-binding kinase, a mitotic kinase. Proc Natl Acad Sci U S A. 2000;97:5167-72
14. Abe Y, Takeuchi T, Kagawa-Miki L, Ueda N, Shigemoto K, Yasukawa M. et al. A Mitotic Kinase TOPK Enhances Cdk1/cyclin B1-dependent Phosphorylation of PRC1 and Promotes Cytokinesis. Journal of Molecular Biology. 2007;370:231-45
15. Dong C, Tang X, Xie Y, Zou Q, Yang X, Zhou H. The crystal structure of an inactive dimer of PDZ-binding kinase. Biochemical and Biophysical Research Communications. 2016;476:586-93
16. Fakhouri LI, Al-Shar'i NA. The design of TOPK inhibitors using structure-based pharmacophore modeling and molecular docking based on an MD-refined homology model. Mol Divers. 2022;26:2679-702
17. Wang H, Zhu X, Zhao Y, Zang Y, Zhang J, Kang Y. et al. Markov State Models Underlying the N-Terminal Premodel of TOPK/PBK. J Phys Chem B. 2022;126:10662-71
18. Han Z, Li L, Huang Y, Zhao H, Luo Y. PBK/TOPK: A Therapeutic Target Worthy of Attention. Cells. 2021;10:371
19. Li C, Tang Z, Zhang W, Ye Z, Liu F. GEPIA2021: integrating multiple deconvolution-based analysis into GEPIA. Nucleic Acids Res. 2021;49:W242-w6
20. Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q. et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:W509-w14
21. Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS. et al. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017;77:e108-e10
22. Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J. et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17:174
23. Herbert KJ, Ashton TM, Prevo R, Pirovano G, Higgins GS. T-LAK cell-originated protein kinase (TOPK): an emerging target for cancer-specific therapeutics. Cell Death Dis. 2018;9:1089
24. Győrffy B. Discovery and ranking of the most robust prognostic biomarkers in serous ovarian cancer. Geroscience. 2023;45:1889-98
25. Posta M, Győrffy B. Pathway-level mutational signatures predict breast cancer outcomes and reveal therapeutic targets. Br J Pharmacol. 2025;182:5734-47
26. Győrffy B. Integrated analysis of public datasets for the discovery and validation of survival-associated genes in solid tumors. Innovation (Camb). 2024;5:100625
27. Győrffy B. Transcriptome-level discovery of survival-associated biomarkers and therapy targets in non-small-cell lung cancer. Br J Pharmacol. 2024;181:362-74
28. Zhang C, Zhang W, Cui H, Zhang B, Miao P, Yang Q. et al. Role of Hub Genes in the Occurrence and Development of Testicular Cancer Based on Bioinformatics. Int J Gen Med. 2022;15:645-60
29. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401-4
30. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1
31. de Bruijn I, Kundra R, Mastrogiacomo B, Tran TN, Sikina L, Mazor T. et al. Analysis and Visualization of Longitudinal Genomic and Clinical Data from the AACR Project GENIE Biopharma Collaborative in cBioPortal. Cancer Res. 2023;83:3861-7
32. Singh PK, Srivastava AK, Dalela D, Rath SK, Goel MM, Bhatt ML. Expression of PDZ-binding kinase/T-LAK cell-originated protein kinase (PBK/TOPK) in human urinary bladder transitional cell carcinoma. Immunobiology. 2014;219:469-74
33. Qiao L, Ba J, Xie J, Zhu R, Wan Y, Zhang M. et al. Overexpression of PBK/TOPK relates to poor prognosis of patients with breast cancer: a retrospective analysis. World Journal of Surgical Oncology. 2022;20:316
34. Wang K, Chai J, Xu J, Wei J, Li P, Liu Y. et al. TOPK: A new predictor of the therapeutic response to neoadjuvant chemotherapy and prognosis in triple-negative breast cancer. Pathology - Research and Practice. 2021;226:153603
35. Qiong Luo BL, Shuguang Liu, Yaowen Chen, Wenjie Sheng, Peixin Lin, Wenxia Li, Haili Zhu, Hong Shen. Expression of PBK/TOPK in cervical cancer and cervical intraepithelial neoplasia. Int J Clin Exp Pathol. 2014:8059-64
36. He F, Yan Q, Fan L, Liu Y, Cui J, Wang J. et al. PBK/TOPK in the differential diagnosis of cholangiocarcinoma from hepatocellular carcinoma and its involvement in prognosis of human cholangiocarcinoma. Human Pathology. 2010;41:415-24
37. Thanindratarn P, Dean DC, Nelson SD, Hornicek FJ, Duan Z. T-LAK cell-originated protein kinase (TOPK) is a Novel Prognostic and Therapeutic Target in Chordoma. Cell Proliferation. 2020;53:e12901
38. Su TC, Chen CY, Tsai WC, Hsu HT, Yen HH, Sung WW. et al. Cytoplasmic, nuclear, and total PBK/TOPK expression is associated with prognosis in colorectal cancer patients: A retrospective analysis based on immunohistochemistry stain of tissue microarrays. PLoS One. 2018;13:e0204866
39. Nagano-Matsuo A, Inoue S, Koshino A, Ota A, Nakao K, Komura M. et al. PBK expression predicts favorable survival in colorectal cancer patients. Virchows Arch. 2021;479:277-84
40. Zlobec I, Molinari F, Kovac M, Bihl MP, Altermatt HJ, Diebold J. et al. Prognostic and predictive value of TOPK stratified by KRAS and BRAF gene alterations in sporadic, hereditary and metastatic colorectal cancer patients. Br J Cancer. 2010;102:151-61
41. Ohashi T, Komatsu S, Ichikawa D, Miyamae M, Okajima W, Imamura T. et al. Overexpression of PBK/TOPK Contributes to Tumor Development and Poor Outcome of Esophageal Squamous Cell Carcinoma. Anticancer Research. 2016;36:6457-66
42. Ohashi T, Komatsu S, Ichikawa D, Miyamae M, Okajima W, Imamura T. et al. Overexpression of PBK/TOPK relates to tumour malignant potential and poor outcome of gastric carcinoma. British Journal of Cancer. 2016;116:218-26
43. Kwon CH, Park HJ, Choi YR, Kim A, Kim HW, Choi JH. et al. PSMB8 and PBK as potential gastric cancer subtype-specific biomarkers associated with prognosis. Oncotarget. 2016;7:21454-68
44. Koh M, Hayakawa Y, Akai T, Hayashi T, Tomita T, Nagai S. et al. Novel biomarker, phosphorylated T-LAK cell-originated protein kinase (p-TOPK) can predict outcome in primary central nervous system lymphoma. Neuropathology. 2018;38:228-36
45. Simons-Evelyn M, Bailey-Dell K, Toretsky JA, Ross DD, Fenton R, Kalvakolanu D. et al. PBK/TOPK Is a Novel Mitotic Kinase Which Is Upregulated in Burkitt's Lymphoma and Other Highly Proliferative Malignant Cells. Blood Cells, Molecules, and Diseases. 2001;27:825-9
46. Wei DC, Yeh YC, Hung JJ, Chou TY, Wu YC, Lu PJ. et al. Overexpression of T-LAK cell-originated protein kinase predicts poor prognosis in patients with stage I lung adenocarcinoma. Cancer Science. 2012;103:731-8
47. Chang C-F, Chen S-L, Sung W-W, Hsieh M-J, Hsu H-T, Chen L-H. et al. PBK/TOPK Expression Predicts Prognosis in Oral Cancer. International Journal of Molecular Sciences. 2016;17:1007
48. Thanindratarn P, Wei R, Dean DC, Singh A, Federman N, Nelson SD. et al. Expression of Concern: T-LAK cell-originated protein kinase (TOPK): an emerging prognostic biomarker and therapeutic target in osteosarcoma. Molecular Oncology. 2021;15:3721-37
49. Yuji Ikeda J-HP, Takashi Miyamoto, Naofumi Takamatsu, Taigo Kato, Akiko Iwasa, Shuhei Okabe, Yuichi Imai, Keiichi Fujiwara, Yusuke Nakamura, Kosei Hasegawa. T-LAK cell-originated protein kinase (TOPK) as a prognostic factor and a potential therapeutic target in ovarian cancer. Clin Cancer Res. 2016:6110-7
50. Liu Y, Liu HUI, Cao H, Song BIN, Zhang WEN, Zhang W. PBK/TOPK mediates promyelocyte proliferation via Nrf2-regulated cell cycle progression and apoptosis. Oncology Reports. 2015;34:3288-96
51. Park JH, Nishidate T, Nakamura Y, Katagiri T. Critical roles of T-LAK cell-originated protein kinase in cytokinesis. Cancer Science. 2010;101:403-11
52. Sun H, Zheng J, Xiao J, Yue J, Shi Z, Xuan Z. et al. TOPK/PBK is phosphorylated by ERK2 at serine 32, promotes tumorigenesis and is involved in sorafenib resistance in RCC. Cell Death & Disease. 2022;13:450
53. Nandi A, Tidwell M, Karp J, Rapoport AP. Protein expression of PDZ-binding kinase is up-regulated in hematologic malignancies and strongly down-regulated during terminal differentiation of HL-60 leukemic cells. Blood Cells Mol Dis. 2004;32:240-5
54. Yang YF, Pan YH, Cao Y, Fu J, Yang X, Zhang MF. et al. PDZ binding kinase, regulated by FoxM1, enhances malignant phenotype via activation of β-Catenin signaling in hepatocellular carcinoma. Oncotarget. 2017;8:47195-205
55. Stauffer S, Zeng Y, Zhou J, Chen X, Chen Y, Dong J. CDK1-mediated mitotic phosphorylation of PBK is involved in cytokinesis and inhibits its oncogenic activity. Cellular Signalling. 2017;39:74-83
56. Fang Q, Liu C, Nie D, Guo J, Xie W, Zhang Y. Phosphorylation of PBK at Thr9 by CDK5 correlates with invasion of prolactinomas. CNS Neurosci Ther. 2024;30:e14629
57. Hai Huang, Mee-Hyun Lee, Kangdong Liu, Zigang Dong, Zeayoung Ryoo, Kim MO. PBK/TOPK: An Effective Drug Target with Diverse Therapeutic Potential. Cancers. 2021;13:2232
58. Xiao J, Duan Q, Wang Z, Yan W, Sun H, Xue P. et al. Phosphorylation of TOPK at Y74, Y272 by Src increases the stability of TOPK and promotes tumorigenesis of colon. Oncotarget. 2016;7:24483-94
59. Ma H, Han F, Yan X, Qi G, Li Y, Li R. et al. PBK promotes aggressive phenotypes of cervical cancer through ERK/c-Myc signaling pathway. J Cell Physiol. 2021;236:2767-81
60. Wang K, Wei J, Ma J, Jia Q, Liu Y, Chai J. et al. Phosphorylation of PBK/TOPK Tyr74 by JAK2 promotes Burkitt lymphoma tumor growth. Cancer Letters. 2022;544:215812
61. Wu W, Xu J, Gao D, Xie Z, Chen W, Li W. et al. TOPK promotes the growth of esophageal cancer in vitro and in vivo by enhancing YB1/eEF1A1 signal pathway. Cell Death & Disease. 2023;14:364
62. Shinde SR, Gangula NR, Kavela S, Pandey V, Maddika S. TOPK and PTEN participate in CHFR mediated mitotic checkpoint. Cellular Signalling. 2013;25:2511-7
63. Zykova TA, Zhu F, Vakorina TI, Zhang J, Higgins LA, Urusova DV. et al. T-LAK Cell-originated Protein Kinase (TOPK) Phosphorylation of Prx1 at Ser-32 Prevents UVB-induced Apoptosis in RPMI7951 Melanoma Cells through the Regulation of Prx1 Peroxidase Activity. Journal of Biological Chemistry. 2010;285:29138-46
64. Hu F, Gartenhaus RB, Eichberg D, Liu Z, Fang HB, Rapoport AP. PBK/TOPK interacts with the DBD domain of tumor suppressor p53 and modulates expression of transcriptional targets including p21. Oncogene. 2010;29:5464-74
65. Oh SM, Zhu F, Cho YY, Lee KW, Kang BS, Kim HG. et al. T-lymphokine-activated killer cell-originated protein kinase functions as a positive regulator of c-Jun-NH2-kinase 1 signaling and H-Ras-induced cell transformation. Cancer Res. 2007;67:5186-94
66. Fukukawa C, Ueda K, Nishidate T, Katagiri T, Nakamura Y. Critical roles of LGN/GPSM2 phosphorylation by PBK/TOPK in cell division of breast cancer cells. Genes, Chromosomes and Cancer. 2010;49:861-72
67. Koshino A, Nagano A, Ota A, Hyodo T, Ueki A, Komura M. et al. PBK Enhances Cellular Proliferation with Histone H3 Phosphorylation and Suppresses Migration and Invasion with CDH1 Stabilization in Colorectal Cancer. Front Pharmacol. 2021;12:772926
68. Rizkallah R, Batsomboon P, Dudley GB, Hurt MM. Identification of the oncogenic kinase TOPK/PBK as a master mitotic regulator of C2H2 zinc finger proteins. Oncotarget. 2015;6:1446-61
69. Joel M, Mughal AA, Grieg Z, Murrell W, Palmero S, Mikkelsen B. et al. Targeting PBK/TOPK decreases growth and survival of glioma initiating cells in vitro and attenuates tumor growth in vivo. Molecular Cancer. 2015;14:121
70. Chen F, Li R, Wang C, Cao L, Wang Y, Yu L. T-LAK cell-originated protein kinase is essential for the proliferation of hepatocellular carcinoma SMMC-7721 cells. Cell Biochem Funct. 2013;31:736-42
71. Wang MY, Lin ZR, Cao Y, Zheng LS, Peng LX, Sun R. et al. PDZ binding kinase (PBK) is a theranostic target for nasopharyngeal carcinoma: driving tumor growth via ROS signaling and correlating with patient survival. Oncotarget. 2016;7:26604-16
72. Kato T, Inoue H, Imoto S, Tamada Y, Miyamoto T, Matsuo Y. et al. Oncogenic roles of TOPK and MELK, and effective growth suppression by small molecular inhibitors in kidney cancer cells. Oncotarget. 2016;7:17652-64
73. Zhang H, Dong QQ, Shu HP, Tu YC, Liao QQ, Yao LJ. TOPK Activation Exerts Protective Effects on Cisplatin-induced Acute Kidney Injury. Curr Med Sci. 2022;42:742-53
74. Pirovano G, Ashton TM, Herbert KJ, Bryant RJ, Verrill CL, Cerundolo L. et al. TOPK modulates tumour-specific radiosensitivity and correlates with recurrence after prostate radiotherapy. British Journal of Cancer. 2017;117:503-12
75. Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol. 2018;13:395-412
76. Lee Y-J, Park J-H, Oh S-M. TOPK promotes epithelial-mesenchymal transition and invasion of breast cancer cells through upregulation of TBX3 in TGF-β1/Smad signaling. Biochemical and Biophysical Research Communications. 2020;522:270-7
77. Brown-Clay JD, Shenoy DN, Timofeeva O, Kallakury BV, Nandi AK, Banerjee PP. PBK/TOPK enhances aggressive phenotype in prostate cancer via β-catenin-TCF/LEF-mediated matrix metalloproteinases production and invasion. Oncotarget. 2015;6:15594-609
78. Seol MA, Park JH, Jeong JH, Lyu J, Han SY, Oh SM. Role of TOPK in lipopolysaccharide-induced breast cancer cell migration and invasion. Oncotarget. 2017;8:40190-203
79. Lee Y-J, Park J-H, Oh S-M. Activation of NF-κB by TOPK upregulates Snail/Slug expression in TGF-β1 signaling to induce epithelial-mesenchymal transition and invasion of breast cancer cells. Biochemical and Biophysical Research Communications. 2020;530:122-9
80. Elias D, Ditzel HJ. Fyn is an important molecule in cancer pathogenesis and drug resistance. Pharmacological Research. 2015;100:250-4
81. Peng S, Yin Y, Zhang Y, Zhu F, Yang G, Fu Y. FYN/TOPK/HSPB1 axis facilitates the proliferation and metastasis of gastric cancer. Journal of Experimental & Clinical Cancer Research. 2023;42:80
82. Shih MC, Chen JY, Wu YC, Jan YH, Yang BM, Lu PJ. et al. TOPK/PBK promotes cell migration via modulation of the PI3K/PTEN/AKT pathway and is associated with poor prognosis in lung cancer. Oncogene. 2011;31:2389-400
83. Jiang Y, Zhang J, Zhao J, Li Z, Chen H, Qiao Y. et al. TOPK promotes metastasis of esophageal squamous cell carcinoma by activating the Src/GSK3β/STAT3 signaling pathway via γ-catenin. BMC Cancer. 2019;19:1264
84. Park J-H, Moon M, Kim J-S, Oh S-M. TOPK mediates hypoxia-induced epithelial-mesenchymal transition and the invasion of nonsmall-cell lung cancer cells via the HIF-1α/snail axis. Biochemical and Biophysical Research Communications. 2021;534:941-9
85. Daniele A, Zito AF, Giannelli G, Divella R, Asselti M, Mazzocca A. et al. Expression of metalloproteinases MMP-2 and MMP-9 in sentinel lymph node and serum of patients with metastatic and non-metastatic breast cancer. Anticancer Res. 2010;30:3521-7
86. Cun Y, An S, Zheng H, Lan J, Chen W, Luo W. et al. Specific Regulation of m(6)A by SRSF7 Promotes the Progression of Glioblastoma. Genomics Proteomics Bioinformatics. 2023;21:707-28
87. Zykova TA, Zhu F, Wang L, Li H, Bai R, Lim DY. et al. The T-LAK Cell-originated Protein Kinase Signal Pathway Promotes Colorectal Cancer Metastasis. EBioMedicine. 2017;18:73-82
88. Yang QX, Zhong S, He L, Jia XJ, Tang H, Cheng ST. et al. PBK overexpression promotes metastasis of hepatocellular carcinoma via activating ETV4-uPAR signaling pathway. Cancer Lett. 2019;452:90-102
89. Shu H, Wang Y, Zhang H, Dong Q, Sun L, Tu Y. et al. The role of the SGK3/TOPK signaling pathway in the transition from acute kidney injury to chronic kidney disease. Frontiers in Pharmacology. 2023;14:1169054
90. Xiao YC, Yang ZB, Cheng XS, Fang XB, Shen T, Xia CF. et al. CXCL8, overexpressed in colorectal cancer, enhances the resistance of colorectal cancer cells to anoikis. Cancer Lett. 2015;361:22-32
91. JUNG-HWAN PARK S-AP, YOUNG-JU LEE, NA-RAE JOO, JONGdAE SHIN, SANG-MUK OH. TOPK inhibition accelerates oxidative stress-induced granulosa cell apoptosis via the p53/SIRT1 axis. Molecular Medicine. 2020:1923-37
92. Joo NR, Park SA, Park JH, Oh SM. TOPK inhibits TNF-α-induced granulosa cell apoptosis via regulation of SIRT1/p53. Biochem Biophys Res Commun. 2023;664:128-35
93. Zhang E, Yin S, Lu X, Ye L, Fan L, Hu H. Glycycoumarin Sensitizes Liver Cancer Cells to ABT-737 by Targeting De Novo Lipogenesis and TOPK-Survivin Axis. Nutrients. 2018;10:353
94. Pimentel JM, Zhou JY, Wu GS. Autophagy and cancer therapy. Cancer Lett. 2024;605:217285
95. Lane JD, Korolchuk VI, Murray JT, Zachari M, Ganley Ian G. The mammalian ULK1 complex and autophagy initiation. Essays in Biochemistry. 2017;61:585-96
96. Wong P-M, Puente C, Ganley IG, Jiang X. The ULK1 complex. Autophagy. 2014;9:124-37
97. Lu H, Xiao J, Ke C, Ni X, Xiu R, Tian Q. et al. TOPK inhibits autophagy by phosphorylating ULK1 and promotes glioma resistance to TMZ. Cell Death & Disease. 2019;10:583
98. Li J, Zhang Z-c, Yuan X-s, Tang S-s, Wang T, Liu H-f. et al. TOPK Affects Autophagy of Skin Squamous Cell Carcinoma by Regulating NF-KB Pathway through HDAC1. Disease Markers. 2022;2022:1-11
99. Han Z, Zhao H, Tao Z, Wang R, Fan Z, Luo Y. et al. TOPK Promotes Microglia/Macrophage Polarization towards M2 Phenotype via Inhibition of HDAC1 and HDAC2 Activity after Transient Cerebral Ischemia. Aging Dis. 2018;9:235-48
100. Park JH, Park SA, Lee YJ, Park HW, Oh SM. PBK attenuates paclitaxel-induced autophagic cell death by suppressing p53 in H460 non-small-cell lung cancer cells. FEBS Open Bio. 2020;10:937-50
101. Ma H, Li Y, Wang X, Wu H, Qi G, Li R. et al. PBK, targeted by EVI1, promotes metastasis and confers cisplatin resistance through inducing autophagy in high-grade serous ovarian carcinoma. Cell Death Dis. 2019;10:166
102. Liang D, Minikes AM, Jiang X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell. 2022;82:2215-27
103. Qi D, Lu Y, Qu H, Dong Y, Jin Q, Sun M. et al. CLDN6 triggers NRF2-mediated ferroptosis through recruiting DLG1/PBK complex in breast cancer. Cell Death Dis. 2025;16:122
104. Han HJ, Sung JY, Kim SH, Yun UJ, Kim H, Jang EJ. et al. Fibronectin regulates anoikis resistance via cell aggregate formation. Cancer Lett. 2021;508:59-72
105. Hu G, Li J, Zeng Y, Liu L, Yu Z, Qi X. et al. The anoikis-related gene signature predicts survival accurately in colon adenocarcinoma. Sci Rep. 2023;13:13919
106. Gaillard H, García-Muse T, Aguilera A. Replication stress and cancer. Nature Reviews Cancer. 2015;15:276-89
107. Herbert KJ, Puliyadi R, Prevo R, Rodriguez-Berriguete G, Ryan A, Ramadan K. et al. Targeting TOPK sensitises tumour cells to radiation-induced damage by enhancing replication stress. Cell Death & Differentiation. 2020;28:1333-46
108. Li S, Zhu F, Zykova T, Kim MO, Cho YY, Bode AM. et al. T-LAK Cell-originated Protein Kinase (TOPK) Phosphorylation of MKP1 Protein Prevents Solar Ultraviolet Light-induced Inflammation through Inhibition of the p38 Protein Signaling Pathway. Journal of Biological Chemistry. 2011;286:29601-9
109. Schieven GL. The p38α Kinase Plays a Central Role In Inflammation. Current Topics in Medicinal Chemistry. 2009;9:1038-48
110. Pang SG, Zhang X, Li ZX, He LF, Chen F, Liu ML. et al. TOPK Inhibition Enhances the Sensitivity of Colorectal Cancer Cells to Radiotherapy by Reducing the DNA Damage Response. Curr Med Sci. 2024;44:545-53
111. Gao S, Wang R, Dong S, Wu J, Perek B, Xia Z. et al. Inactivation of TOPK Caused by Hyperglycemia Blocks Diabetic Heart Sensitivity to Sevoflurane Postconditioning by Impairing the PTEN/PI3K/Akt Signaling. Oxidative Medicine and Cellular Longevity. 2021;2021:1-18
112. Yang S-Y, Lee J-J, Lee J-H, Lee K, Oh Seung H, Lim Y-M. et al. Secretagogin affects insulin secretion in pancreatic β-cells by regulating actin dynamics and focal adhesion. Biochemical Journal. 2016;473:1791-803
113. Ma J, Xing B, Cao Y, He X, Bennett KE, Tong C. et al. Menin-regulated Pbk controls high fat diet-induced compensatory beta cell proliferation. EMBO Mol Med. 2021;13:e13524
114. Cao Y, Feng Z, He X, Zhang X, Xing B, Wu Y. et al. Prolactin-regulated Pbk is involved in pregnancy-induced β-cell proliferation in mice. J Endocrinol. 2021;252:107-23
115. Dou X, Wei J, Sun A, Shao G, Childress C, Yang W. et al. PBK/TOPK mediates geranylgeranylation signaling for breast cancer cell proliferation. Cancer Cell International. 2015;15:27
116. Park J-H, Jeong Y-J, Won HK, Choi S-Y, Park J-H, Oh S-M. Activation of TOPK by lipopolysaccharide promotes induction of inducible nitric oxide synthase through NF-κB activity in leukemia cells. Cellular Signalling. 2014;26:849-56
117. Dąbrowska D, Jabłońska E, Garley M, Ratajczak-Wrona W, Iwaniuk A. New Aspects of the Biology of Neutrophil Extracellular Traps. Scand J Immunol. 2016;84:317-22
118. Garley M, Jabłońska E, Miltyk W, Grubczak K, Surażyński A, Ratajczak-Wrona W. et al. Cancers Cells in Traps? The Pathways of NETs Formation in Response to OSCC in Humans-A Pilot Study. Cancer Control. 2020;27:1073274820960473
119. Zeng F, Du S, Wu M, Dai C, Li J, Wang J. et al. The oncogenic kinase TOPK upregulates in psoriatic keratinocytes and contributes to psoriasis progression by regulating neutrophils infiltration. Cell Commun Signal. 2024;22:386
120. Lu H, Huang Y, Ni X, Ma T, Chang T, Liu M. et al. TOPK promotes the development of psoriasis and worenine alleviates psoriasiform dermatitis by inhibiting TOPK activity. J Eur Acad Dermatol Venereol. 2024;38:851-63
121. Rimal R, Desai P, Daware R, Hosseinnejad A, Prakash J, Lammers T. et al. Cancer-associated fibroblasts: Origin, function, imaging, and therapeutic targeting. Adv Drug Deliv Rev. 2022;189:114504
122. Zheng Z, Xiong R, Sui X, Li L, Sun H, Shao C. TOPK promotes immune suppression in kidney renal clear cell carcinoma and emerges as a prognostic and therapeutic target. BMC Cancer. 2025;25:1334
123. Denechaud P-D, Fajas L, Giralt A. E2F1, a Novel Regulator of Metabolism. Frontiers in Endocrinology. 2017;8:311
124. Hu F, Gartenhaus RB, Zhao XF, Fang H-B, Minkove S, Poss DE. et al. c-Myc and E2F1 drive PBK/TOPK expression in high-grade malignant lymphomas. Leukemia Research. 2013;37:447-54
125. Chen J-H, Liang Y-X, He H-C, Chen J-Y, Lu J-M, Chen G. et al. Overexpression of PDZ-binding kinase confers malignant phenotype in prostate cancer via the regulation of E2F1. International Journal of Biological Macromolecules. 2015;81:615-23
126. Nandi AK, Rapoport AP. Expression of PDZ-binding kinase (PBK) is regulated by cell cycle-specific transcription factors E2F and CREB/ATF. Leuk Res. 2006;30:437-47
127. Uchida E, Suwa S, Yoshimoto R, Watanabe K, Kasama T, Miura O. et al. TOPK is regulated by PP2A and BCR/ABL in leukemia and enhances cell proliferation. Int J Oncol. 2019;54:1785-96
128. Wang P, Li T, Fang L, Chen D, Qi H, Gu C. UPF1 regulates FOXO1 protein expression by promoting PBK transcription in non-small cell lung cancer. Biochem Biophys Res Commun. 2023;666:10-20
129. Alhawas L, Amin KS, Salla B, Banerjee PP. T-LAK cell-originated protein kinase (TOPK) enhances androgen receptor splice variant (ARv7) and drives androgen-independent growth in prostate cancer. Carcinogenesis. 2021;42:423-35
130. Shahab SW, Roggeveen CM, Sun J, Kunhiraman H, McSwain LF, Juraschka K. et al. The LIN28B-let-7-PBK pathway is essential for group 3 medulloblastoma tumor growth and survival. Molecular Oncology. 2023;17:1784-802
131. Herrero-Martín D, Osuna D, Ordóñez JL, Sevillano V, Martins AS, Mackintosh C. et al. Stable interference of EWS-FLI1 in an Ewing sarcoma cell line impairs IGF-1/IGF-1R signalling and reveals TOPK as a new target. British Journal of Cancer. 2009;101:80-90
132. Roh E, Lee M-H, Zykova TA, Zhu F, Nadas J, Kim H-G. et al. Targeting PRPK and TOPK for skin cancer prevention and therapy. Oncogene. 2018;37:5633-47
133. Agrawal P, Sambaturu N, Olgun G, Hannenhalli S. A Path-Based Analysis of Infected Cell Line and COVID-19 Patient Transcriptome Reveals Novel Potential Targets and Drugs Against SARS-CoV-2. Front Immunol. 2022;13:918817
134. Lee D-H, Jeong Y-J, Won J-Y, Sim H-I, Park Y, Jin H-S. PBK/TOPK Is a Favorable Prognostic Biomarker Correlated with Antitumor Immunity in Colon Cancers. Biomedicines. 2022;10:299
135. Li J, Sun H, Fu M, Zheng Z, Xu C, Yang K. et al. TOPK mediates immune evasion of renal cell carcinoma via upregulating the expression of PD-L1. iScience. 2023;26:107185
136. Movahedi F, Liu J, Sun B, Cao P, Sun L, Howard C. et al. PD-L1-Targeted Co-Delivery of Two Chemotherapeutics for Efficient Suppression of Skin Cancer Growth. Pharmaceutics. 2022;14:1488
137. Deng M, Yang R, Sun Q, Zhang J, Miao J. Small-molecule inhibitor HI-TOPK-032 improves NK-92MI cell infiltration into ovarian tumours. Basic & Clinical Pharmacology & Toxicology. 2024;134:629-42
138. Cao S, Li M, Cui Z, Li Y, Niu W, Zhu W. et al. Establishment and validation of the prognostic risk model based on the anoikis-related genes in esophageal squamous cell carcinoma. Ann Med. 2024;56:2418338
139. Zhang HY, Zhu JJ, Liu ZM, Zhang YX, Chen JJ, Chen KD. A prognostic four-gene signature and a therapeutic strategy for hepatocellular carcinoma: Construction and analysis of a circRNA-mediated competing endogenous RNA network. Hepatobiliary Pancreat Dis Int. 2024;23:272-87
140. Zhang Q, Zheng F, Chen Y, Liang CL, Liu H, Qiu F. et al. The TOPK Inhibitor HI-TOPK-032 Enhances CAR T-cell Therapy of Hepatocellular Carcinoma by Upregulating Memory T Cells. Cancer Immunol Res. 2024;12:631-43
141. Ma H, Zhang J, Shi Y, Wang Z, Nie W, Cai J. et al. PBK correlates with prognosis, immune escape and drug response in LUAD. Sci Rep. 2023;13:20452
142. Wang MY, Qi B, Wang F, Lin ZR, Li MY, Yin WJ. et al. PBK phosphorylates MSL1 to elicit epigenetic modulation of CD276 in nasopharyngeal carcinoma. Oncogenesis. 2021;10:9
143. Cui J, Zhao S, Chen H, Fu Y, Han K, Yin S. et al. Methylseleninic acid overcomes gefitinib resistance through asparagine-MET-TOPK signaling axis in non-small cell lung cancer cells. Biochemical Pharmacology. 2023;215:115690
144. Xiao J, Wang F, Lu H, Xu S, Zou L, Tian Q. et al. Targeting the COX2/MET/TOPK signaling axis induces apoptosis in gefitinib-resistant NSCLC cells. Cell Death Dis. 2019;10:777
145. Ying Li1 ZY, Weijie Li, Shudi Xu, Mengjie Niu, Shengli Zhang, Lintao Jia, Tao Wang, Ting Wang, Shengqing Li. TOPK promotes lung cancer resistance to EGFR tyrosine kinase inhibitors by phosphorylating and activating c-Jun. oncotarget. 2016: 7(6):6748-64.
146. Park J-H, Yoon D-S, Choi H-J, Hahm D-H, Oh S-M. Phosphorylation of IκBα at Serine 32 by T-lymphokine-activated Killer Cell-originated Protein Kinase Is Essential for Chemoresistance against Doxorubicin in Cervical Cancer Cells. Journal of Biological Chemistry. 2013;288:3585-93
147. Zou J, Kuang W, Hu J, Rao H. miR-216b promotes cell growth and enhances chemosensitivity of colorectal cancer by suppressing PDZ-binding kinase. Biochemical and Biophysical Research Communications. 2017;488:247-52
148. Cao H, Yang M, Yang Y, Fang J, Cui Y. PBK/TOPK promotes chemoresistance to oxaliplatin in hepatocellular carcinoma cells by regulating PTEN. Acta Biochim Biophys Sin (Shanghai). 2021;53:584-92
149. Ma H, Qi G, Han F, Peng J, Yuan C, Kong B. PBK drives PARP inhibitor resistance through the TRIM37/NFκB axis in ovarian cancer. Exp Mol Med. 2022;54:999-1010
150. Xiao J, Zhang L, Yi H, Zou L, Mo J, Xue F. et al. Inhibiting ALK-TOPK signaling pathway promotes cell apoptosis of ALK-positive NSCLC. Cell Death Dis. 2022;13:828
151. Kwon HR, Lee KW, Dong Z, Lee KB, Oh SM. Requirement of T-lymphokine-activated killer cell-originated protein kinase for TRAIL resistance of human HeLa cervical cancer cells. Biochem Biophys Res Commun. 2010;391:830-4
152. Wang T, Wang W, Wang Q, Xie R, Landay A, Chen D. The E3 ubiquitin ligase CHIP in normal cell function and in disease conditions. Ann N Y Acad Sci. 2020;1460:3-10
153. Tan B, Zhang J, Wang W, Ma H, Yang Y. Tumor-suppressive E3 ubiquitin ligase CHIP inhibits the PBK/ERK axis to repress stem cell properties and radioresistance in non-small cell lung cancer. Apoptosis. 2023;28:397-413
154. Mao P, Bao G, Wang YC, Du CW, Yu X, Guo XY. et al. PDZ-Binding Kinase-Dependent Transcriptional Regulation of CCNB2 Promotes Tumorigenesis and Radio-Resistance in Glioblastoma. Transl Oncol. 2020;13:287-94
155. Park JH, Inoue H, Kato T, Zewde M, Miyamoto T, Matsuo Y. et al. TOPK (T-LAK cell-originated protein kinase) inhibitor exhibits growth suppressive effect on small cell lung cancer. Cancer Sci. 2017;108:488-96
156. Matsuo Y, Park JH, Miyamoto T, Yamamoto S, Hisada S, Alachkar H. et al. TOPK inhibitor induces complete tumor regression in xenograft models of human cancer through inhibition of cytokinesis. Sci Transl Med. 2014;6:259ra145
157. Zou L, Gao Z, Zeng F, Xiao J, Chen J, Feng X. et al. Sulfasalazine suppresses thyroid cancer cell proliferation and metastasis through T-cell originated protein kinase. Oncol Lett. 2019;18:3517-26
158. Zeng X, Liu L, Zheng M, Sun H, Xiao J, Lu T. et al. Pantoprazole, an FDA-approved proton-pump inhibitor, suppresses colorectal cancer growth by targeting T-cell-originated protein kinase. Oncotarget. 2016;7:22460-73
159. Zheng M, Luan S, Gao S, Cheng L, Hao B, Li J. et al. Proton pump inhibitor ilaprazole suppresses cancer growth by targeting T-cell-originated protein kinase. Oncotarget. 2017;8:39143-53
160. Kato M, Ota A, Ono T, Karnan S, Hyodo T, Rahman ML. et al. PDZ-binding kinase inhibitor OTS514 suppresses the proliferation of oral squamous carcinoma cells. Oral Dis. 2024;30:223-34
161. Pirovano G, Roberts S, Brand C, Donabedian PL, Mason C, de Souza PD. et al. [(18)F]FE-OTS964: a Small Molecule Targeting TOPK for In Vivo PET Imaging in a Glioblastoma Xenograft Model. Mol Imaging Biol. 2019;21:705-12
162. Gilabert-Oriol R, Sutherland BW, Anantha M, Pallaoro A, Bally MB. Liposomal OTS964, a TOPK inhibitor: a simple method to estimate OTS964 association with liposomes that relies on enhanced OTS964 fluorescence when bound to albumin. Drug Deliv Transl Res. 2019;9:1082-94
163. Gao G, Zhang T, Wang Q, Reddy K, Chen H, Yao K. et al. ADA-07 Suppresses Solar Ultraviolet-Induced Skin Carcinogenesis by Directly Inhibiting TOPK. Molecular Cancer Therapeutics. 2017;16:1843-54
164. Gao T, Hu Q, Hu X, Lei Q, Feng Z, Yu X. et al. Novel selective TOPK inhibitor SKLB-C05 inhibits colorectal carcinoma growth and metastasis. Cancer Letters. 2019;445:11-23
165. Hu Q-F, Gao T-T, Shi Y-J, Lei Q, Liu Z-H, Feng Q. et al. Design, synthesis and biological evaluation of novel 1-phenyl phenanthridin-6(5H)-one derivatives as anti-tumor agents targeting TOPK. European Journal of Medicinal Chemistry. 2019;162:407-22
166. Yang Y, Teng QX, Wu ZX, Wang JQ, Lei ZN, Lusvarghi S. et al. PBK/TOPK inhibitor OTS964 resistance is mediated by ABCB1-dependent transport function in cancer: in vitro and in vivo study. Mol Cancer. 2022;21:40
167. Yang Y, Wu ZX, Wang JQ, Teng QX, Lei ZN, Lusvarghi S. et al. OTS964, a TOPK Inhibitor, Is Susceptible to ABCG2-Mediated Drug Resistance. Front Pharmacol. 2021;12:620874
168. Wu J, Zhu RD, Cao GM, Du JC, Liu X, Diao LZ. et al. Discovery of novel paeonol-based derivatives against skin inflammation in vitro and in vivo. Journal of Enzyme Inhibition and Medicinal Chemistry. 2022;37:817-31
169. Fan X, Duan Q, Ke C, Zhang G, Xiao J, Wu D. et al. Cefradine blocks solar-ultraviolet induced skin inflammation through direct inhibition of T-LAK cell-originated protein kinase. Oncotarget. 2016;7:24633-45
170. Hálfdánarson Ó, Pottegård A, Björnsson ES, Lund SH, Ogmundsdottir MH, Steingrímsson E. et al. Proton-pump inhibitors among adults: a nationwide drug-utilization study. Therap Adv Gastroenterol. 2018;11:1756284818777943
171. Lassalle M, Le Tri T, Bardou M, Biour M, Kirchgesner J, Rouby F. et al. Use of proton pump inhibitors in adults in France: a nationwide drug utilization study. Eur J Clin Pharmacol. 2020;76:449-57
172. Naeem A, Hu P, Yang M, Zhang J, Liu Y, Zhu W. et al. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules. 2022;27:8367
173. Zhao Y, Lu L, Chen X, Yin Q. Natural compounds targeting ferroptosis in ovarian cancer: Research progress and application potential. Pharmacol Res. 2025;215:107729
174. Takahashi H, Kawaguchi M, Kitamura K, Narumiya S, Kawamura M, Tengan I. et al. An Exploratory Study on the Anti-inflammatory Effects of Fucoidan in Relation to Quality of Life in Advanced Cancer Patients. Integr Cancer Ther. 2018;17:282-91
175. Cheon C, Ko SG. A Phase I Study to Evaluate the Safety of the Herbal Medicine SH003 in Patients With Solid Cancer. Integr Cancer Ther. 2020;19:1534735420911442
176. Fu X, Zhao R, Yoon G, Shim JH, Choi BY, Yin F. et al. 3-Deoxysappanchalcone Inhibits Skin Cancer Proliferation by Regulating T-Lymphokine-Activated Killer Cell-Originated Protein Kinase in vitro and in vivo. Front Cell Dev Biol. 2021;9:638174
177. Zhao S, Cui J, Cao L, Han K, Ma X, Chen H. et al. Xanthohumol inhibits non-small cell lung cancer via directly targeting T-lymphokine-activated killer cell-originated protein kinase. Phytotherapy Research. 2023;37:3057-68
178. Xue P, Wang Y, Zeng F, Xiu R, Chen J, Guo J. et al. Paeonol suppresses solar ultraviolet-induced skin inflammation by targeting T-LAK cell-originated protein kinase. Oncotarget. 2017;8:27093-104
179. Kang NJ, Lee KW, Kim BH, Bode AM, Lee H-J, Heo Y-S. et al. Coffee phenolic phytochemicals suppress colon cancer metastasis by targeting MEK and TOPK. Carcinogenesis. 2011;32:921-8
180. Fan X, Tao J, Cai X, Fredimoses M, Wu J, Jiang Z. et al. Eupafolin Suppresses Esophagus Cancer Growth by Targeting T-LAK Cell-Originated Protein Kinase. Frontiers in Pharmacology. 2019;10:1248
181. Wang L, Zhang Z, Ge R, Zhang J, Liu W, Mou K. et al. Gossypetin Inhibits Solar-UV Induced Cutaneous Basal Cell Carcinoma Through Direct Inhibiting PBK/TOPK Protein Kinase. Anticancer Agents Med Chem. 2019;19:1029-36
182. Zhang X, Song X, Yin S, Zhao C, Fan L, Hu H. p21 induction plays a dual role in anti-cancer activity of ursolic acid. Exp Biol Med (Maywood). 2016;241:501-8
183. Wang WQ, Li R, Bai QX, Liu YH, Zhang WP, Wang JH. et al. Gossypol-Induced Differentiation in Human Leukemia HL-60 Cells. Int J Biomed Sci. 2006;2:395-401
184. Diao X, Yang D, Chen Y, Liu W. Baicalin suppresses lung cancer growth by targeting PDZ-binding kinase/T-LAK cell-originated protein kinase. Bioscience Reports. 2019;39:BSR20181692
185. Mu X, Wang L, Zhang Z, Ge R, Zhang J, Liu W. et al. Scutellarin Suppresses RPMI7951 Melanoma Cell Proliferation by Targeting TOPK. Anticancer Agents Med Chem. 2021;21:640-8
186. Lu S, Ye L, Yin S, Zhao C, Yan M, Liu X. et al. Glycyrol exerts potent therapeutic effect on lung cancer via directly inactivating T-LAK cell-originated protein kinase. Pharmacological Research. 2019;147:104366
187. Yang J, Yuan D, Xing T, Su H, Zhang S, Wen J. et al. Ginsenoside Rh2 inhibiting HCT116 colon cancer cell proliferation through blocking PDZ-binding kinase/T-LAK cell-originated protein kinase. Journal of Ginseng Research. 2016;40:400-8
188. Roh E, Kim J-E, Zhang T, Shin SH, Kim B-G, Li J. et al. Orobol, 3′-hydroxy-genistein, suppresses the development and regrowth of cutaneous SCC. Biochemical Pharmacology. 2023;209:115415
189. Vishchuk OS, Sun H, Wang Z, Ermakova SP, Xiao J, Lu T. et al. PDZ-binding kinase/T-LAK cell-originated protein kinase is a target of the fucoidan from brown alga Fucus evanescens in the prevention of EGF-induced neoplastic cell transformation and colon cancer growth. Oncotarget. 2016;7:18763-73
190. Cao L, Han K, Fan L, Zhao C, Yin S, Hu H. Glycyrol Alleviates Acute Kidney Injury by Inhibiting Ferroptsis. International Journal of Molecular Sciences. 2024;25:2458
191. Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199-227
192. Chai Y, Xue H, Wu Y, Du X, Zhang Z, Zhang Y. et al. MicroRNA-216b-3p inhibits lung adenocarcinoma cell growth via regulating PDZ binding kinase/T-LAK-cell-originated protein kinase. Experimental and Therapeutic Medicine. 2018;15:4822-8
193. Lee HC, Her N-G, Kang D, Jung SH, Shin J, Lee M. et al. Radiation-inducible miR-770-5p sensitizes tumors to radiation through direct targeting of PDZ-binding kinase. Cell Death & Disease. 2017;8:e2693-e
Author contact
![]() Corresponding authors: Tel.: +86-371-65587008, fax: +86-371-65587670; E-mail addresses: mhyun_leenet (Mee-Hyun Lee), zhaoran2020edu.cn (Ran Zhao), and dongzgedu.cn (Zigang Dong).
Corresponding authors: Tel.: +86-371-65587008, fax: +86-371-65587670; E-mail addresses: mhyun_leenet (Mee-Hyun Lee), zhaoran2020edu.cn (Ran Zhao), and dongzgedu.cn (Zigang Dong).

 Global reach, higher impact
Global reach, higher impact