10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2026; 22(1):426-446. doi:10.7150/ijbs.118831 This issue Cite
Review
Unravelling Resistance: Integrating Metabolism, Epigenetics, Immunology, and Proteostasis in Strategies against Kirsten Rat Sarcoma Viral Oncogene Homolog-mutant Colorectal Cancer
1. Department of Medical Oncology, the First Hospital of China Medical University, Shenyang 110001, China.
2. Key Laboratory of Anticancer Drugs and Biotherapy of Liaoning Province, the First Hospital of China Medical University, Shenyang 110001, China.
3. Liaoning Province Clinical Research Center for Cancer, the First Hospital of China Medical University, Shenyang 110001, China.
4. Clinical Cancer Treatment and Research Center of Shenyang, the First Hospital of China Medical University, Shenyang, China.
Received 2025-6-4; Accepted 2025-11-6; Published 2026-1-1
Abstract
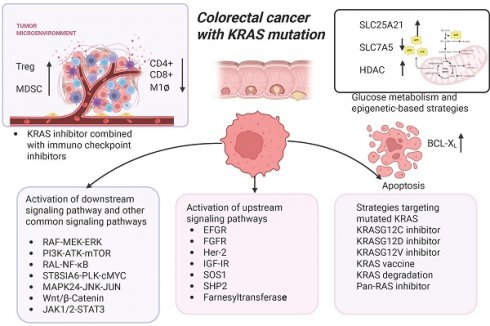
Colorectal cancer (CRC) has a high incidence and mortality rate globally, with approximately 40% of patients harboring Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations. Patients with KRAS mutations exhibit poorer prognoses compared to those with KRAS wild-type tumors, often showing resistance to targeted therapies and chemotherapy. There is an acute need for innovative therapeutic strategies, particularly as KRASG12C inhibitors have been approved by the U.S. Food and Drug Administration (FDA) for second-line therapy in treating non-small cell lung cancer (NSCLC). Yet, their efficacy in CRC remains suboptimal due to the emergence of drug resistance. Current research on KRAS inhibitor resistance in CRC focuses mainly on single biological processes, leaving the complex interplay between cellular systems underexplored. This review synthesizes evidence across four key dimensions — metabolism, epigenetics, immunology, and proteostasis — to reveal multidimensional mechanisms of resistance. How these interconnected pathways work in synergy with signaling aberrations, genetic alterations, and translational modifications to reinforce resistance was investigated. The research also integrates recent advances in multi-targeted strategies, including combinations of KRAS inhibitors with metabolic or epigenetic modulators or immune checkpoint blockade. These findings provide potential strategies for overcoming resistance in KRAS-mutant CRC, addressing a critical gap in precision oncology.
Keywords: KRAS mutation, KRAS inhibitor, colorectal cancer, drug resistance, tumor microenvironment
Introduction
Colorectal cancer (CRC) stands as the third most frequently diagnosed malignancy and accounts for approximately 10% of all new cancer cases globally [1]. Although there are various regimens for CRC, such as surgical intervention, chemoradiotherapy, targeted therapy, and immunotherapy, the mortality rate remains high due to the challenges arising from postoperative recurrence and distant metastasis. Furthermore, a significant proportion (40%) of CRC patients exhibit mutations in the Kirsten rat sarcoma viral oncogene homolog (KRAS). It has been thought to be “undruggable” in the past, bringing great challenges to the treatment of CRC patients. In CRC, approximately 85% of KRAS mutations are concentrated at three key hotspots: codons 12, 13, and 61. The most frequently observed mutation is codon 12, particularly the G12D and G12V subtypes, which are the most prevalent. In contrast, G13D and G12C mutations follow in descending order of frequency [2, 3]. The KRAS gene encodes a protein that is pivotal in regulating various essential cellular functions, including cell proliferation, differentiation, and apoptosis [4]. KRAS mutations lead to sustained activation of KRAS-dependent pathways [5]. Patients harboring KRAS mutations not only show a poorer prognosis and shorter overall survival (OS) but also demonstrate reduced sensitivity to standard treatment [6]. Patients with KRAS mutations also show resistance to receptor tyrosine kinase (RTK) antagonists, including anti-epidermal growth factor receptor (EGFR) monoclonal antibodies. This resistance arises due to KRAS mutations locking the protein in a constitutively active guanosine triphosphate (GTP)-bound state. This renders it independent of upstream RTK signaling (e.g., EGFR) and thus unresponsive to EGFR inhibition [7-12]. Moreover, inhibitors that target the downstream signaling cascade of KRAS, including the mitogen-activated protein kinase (MEK) inhibitor selumetinib, exhibit limited efficacy in CRC patients. Therefore, it is necessary to investigate treatment options for KRAS-mutant CRCs.
For years, KRAS mutation has been considered “undruggable” due to its unique intrinsic characteristics. Ongoing research involves mutant KRAS inhibitors, including sotorasib and adagrasib, which have received approval for the second-line therapy of advanced non-small cell lung cancer (NSCLC) [13, 14]. Nevertheless, these inhibitors exerted suboptimal effects in CRC, with mechanisms of resistance appearing prevalent. Mechanistic studies have primarily focused on isolated pathways, such as mitogen-activated protein kinase (MAPK) reactivation or bypass signaling. However, the complex multidimensional crosstalk among cellular processes is generally overlooked. Specifically, emerging evidence indicates that metabolic reprogramming, epigenetic modifications, immune microenvironment remodeling, and proteostasis dysregulation all contribute to resistance. This critical gap limits our ability to design effective combinatorial strategies.
Therefore, to dissect the molecular basis of KRAS inhibitor resistance in CRC, this review innovatively integrates four critical dimensions and abnormal activation of the MAPK pathway of resistance. By studying their regulatory modalities, the research identifies novel therapeutic mechanisms of resistance and provides ideas for developing rational combination therapies to overcome resistance in KRAS-mutant CRC.
The structure of the KRAS protein
As a constituent of the RAS gene family, the KRAS gene is categorized as a proto-oncogene, first identified in human lung cancer cells in 1982 [15, 16]. The KRAS gene encodes the KRAS protein, a small GTPase that is part of the broader RAS superfamily of proteins, which includes KRAS4A, KRAS4B, HRAS, and NRAS [17-19]. The KRAS protein comprising 188 amino acid residues can be attached to the intracellular membrane through farnesylation, a modification facilitated by farnesyltransferase [20, 21]. Characterized by a molecular weight of 21 kDa, the KRAS protein comprises six β strands and five α helices, with two main domains: a conserved G-domain and highly variable regions at the C-terminus. The G-terminal comprises a switch I exchange region, a switch II exchange region, and a P-ring. It is responsible for transducing downstream signals in combination with downstream signals and is then involved in regulating cell growth and development [22]. The last four amino acid residues at the C-terminal are CAAX, which are the targets of post-translational modifications and are essential in binding to cell membranes [18]. The KRAS protein transitions from an inactive form to an active form by binding to GTP and guanosine diphosphate (GDP), respectively. The activity of KRAS is primarily regulated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). Under physiological conditions, the KRAS protein exists in an inactive state bound to GDP. When cells receive stimuli such as growth factors or other external signals, GEFs become activated. The activated GEFs bind to KRAS and promote the release of GDP, allowing the empty nucleotide binding site to bind GTP. Upon binding GTP, KRAS undergoes a conformational change, transitioning to an active state, which enables interactions with downstream effector molecules and signal transduction [23]. In the GTP-bound state, KRAS enhances its intrinsic GTPase activity through interactions with GAPs. GAPs facilitate the hydrolysis of GTP, leading to the inactivation of KRAS and its return to the GDP-bound state. When the KRAS gene mutates, it disrupts GAP-mediated GTP hydrolysis, bringing KRAS into an activated state. The continuously activated KRAS will continue activating downstream signaling pathways through phosphorylation, stimulating cell migration and proliferation, and ultimately leading to tumorigenesis [24-28].
Frequency and type of KRAS gene mutations
KRAS is the most frequently mutated gene within the RAS family, representing nearly 85% of all RAS gene mutations, with NRAS and HRAS following in prevalence. The KRAS mutation rate reaches the highest level in pancreatic ductal adenocarcinoma (PDAC), approximately 90%, and then in CRC patients with a mutation rate ranging from 30% to 50% [29]. Furthermore, KRAS mutations are found in various other malignancies, including NSCLC, cholangiocarcinoma, and cervical cancer. In CRC, mutations at the 12th codon of the KRAS gene are particularly associated with tumor invasion and metastasis [30], followed by codons 13, 61, 117, and 146 [31]. A retrospective analysis indicated that mutations in the G12C and G12V loci were related to poorer OS [32]. Mutated KRAS can stimulate several downstream signaling pathways. These pathways contain the RAS-RAF-MEK-ERK cascade, the RAS-PI3K-AKT-mTOR cascade [33, 34], and the RAS-(RAL)-NF-kB pathway. All of which contribute to cellular proliferation and survival [35-38]. Among the various KRAS mutants, the KRASG12C protein exhibits the highest intrinsic rate of hydrolysis of GTP. This leads to a greater proportion of KRAS remaining in an inactive conformation, thereby facilitating the binding of allele-specific inhibitors targeting the KRASG12C variant [39].
KRAS inhibitors
KRAS has long been regarded as “undruggable” as it does not contain a classical pocket suitable for the occupation of small molecule inhibitor binding for allosteric inhibitors [17, 24, 40, 41]. The binding affinity of the KRAS protein for guanosine GTP is exceptionally high, operating at the picomolar level. This makes it nearly impossible to develop small molecule inhibitors that compete with GTP binding [42]. In addition, non-selective inhibition of both mutant and wild-type KRAS may result in the inhibition of normal signaling, further leading to potential toxicity, which limited the progress of this type of inhibitor. Research has shown that ablation of the KRAS gene in mouse models leads to myelomonocytic metaplasia, resulting in the inability of the mice to survive [43]. Furthermore, the clinical trial of the pan-KRAS inhibitor BI-1701963 was ultimately discontinued by the sponsor due to the occurrence of fatal interstitial lung disease and limited efficacy, with the best response being stable disease [44]. These studies highlight the importance of evaluating the potential toxic consequences of these non-selective inhibitors in clinical applications.
KRASG12C inhibitors
Approximately 3% of CRC patients have KRASG12C mutations, and research into KRASG12C inhibitors has made certain preliminary progress. Recent research, however, has underpinned significant strides in the development of targeted therapies against mutant KRAS. Shokat et al. discovered a hidden pocket in the mutant KRASG12C-GDP complex (switch-II pockets) adjacent to mutant cysteine 12 [45] (mutation from glycine to cysteine). Hence, binding to mutant cysteine stabilizes KRAS in an inactive GDP-bound state. In contrast, this covalent approach does not suppress wild-type KRAS due to the absence of cysteine in the active site. With the discovery of the switch II pocket of the KRAS protein, research into KRAS inhibitors has made rapid progress. Among these, the development of KRASG12C inhibitors is the most advanced, with several drugs having entered the phase of clinical research. In contrast, inhibitors targeting other mutations, such as KRASG12V inhibitors, remain at a preclinical stage of research.
Sotorasib
Sotorasib is the first marketed KRASG12C inhibitor approved by the U.S. Food and Drug Administration (FDA) [46, 47]. It can bind covalently and irreversibly to cysteine in the switch-II pocket region, preventing GEF-catalyzed nucleotide exchange and inhibiting the activation of downstream signaling cascades. Sotorasib demonstrated vigorous antitumor activity in somatic cells and patients [48]. To assess the therapeutic effects of sotorasib, a Phase-I clinical trial involving 129 patients with advanced solid tumors that possess the KRASG12C mutation was conducted. Among NSCLC patients, the objective response rate (ORR) was 32.2%, with 88.1% of patients achieving disease control. The median progression-free survival (PFS) was 6.3 months. In contrast, CRC patients showed a confirmed response rate of only 7.1%, although 73.8% experienced disease control, with a median PFS of 4.0 months [49]. Furthermore, the CodeBreaK100 study, a Phase-II clinical trial, assessed the efficacy of sotorasib in a cohort of 62 patients diagnosed with KRASG12C-mutant CRC, revealing an ORR of only 9.7% [50]. These findings indicate that sotorasib demonstrates comparatively less efficacy in CRC patients than in NSCLC patients [51].
Adagrasib
Adagrasib is a selective and irreversible inhibitor specifically designed to target the KRASG12C mutations by covalently binding to the mutant cysteine residue [52, 53]. In the study of KRYSTAL-1, a total of 25 patients with KRASG12C-mutant solid tumors were included, of whom 15 patients had NSCLC, eight patients (53.3%) achieved a partial response, two patients were diagnosed with CRC, and one achieved a partial response [54]. In December 2022, the FDA granted authorization for use of adagrasib in the treatment of patients with locally advanced or metastatic NSCLC with KRASG12C mutations who previously received standard systemic therapy [14]. Given the limited number of CRC patients incorporated in the research of KRYSTAL-1, further investigation is warranted to determine definitively the efficacy of adagrasib in this patient population.
Other KRASG12C inhibitors
There are many unapproved KRASG12C inhibitors that have demonstrated antitumor activity in clinical studies and preclinical models. Divarasib is a covalent KRASG12C inhibitor. In a Phase-I clinical trial, divarasib showed better efficacy in NSCLC compared to CRC [55, 56]. Among the NSCLC cohort, a definite ORR was found in 53.4% of patients, with a median PFS of 13.1 months. In contrast, a confirmed ORR of 29.1% was recorded among the CRC patients, and the median PFS was 5.6 months. JDQ443 disrupts downstream signaling by targeting the KRASG12C switch-II P loop and shows enhanced antitumor activity in xenograft models, particularly in combination with inhibitors targeting associated signaling pathways [57]. JNJ-74699157 covalently binds to the KRASG12C-GDP complex to inhibit downstream signaling, but its development is halted due to significant toxicity observed in clinical trials (with 60% of patients experiencing elevated creatine phosphokinase (CPK) levels) [58, 59]. The selective KRASG12C inhibitor 143D shows significant antitumor activity and synergizes with SOS1/EGFR/MEK/ERK inhibitors in both in vitro and in vivo models [60]. Recently, a research team designed a novel small molecule, inspired by natural products, remodels cyclophilin A (CYPA) to bind selectively the active state of KRASG12C, disrupting oncogenic signaling and causing tumor regression in various cancer models [61]. Additionally, AZD4747 targets mutant KRASG12C and penetrates the central nervous system, making it suitable for use in the treatment of tumors with brain metastases [62]. AZD4625 is a highly potent KRASG12C inhibitor with favorable pharmacokinetic properties [63]. The compound 12VC1 can degrade active KRASG12C and KRASG12V, showing potential for non-covalent therapies [64]. Additionally, BI-2852 binds both active and inactive KRAS with nanomolar affinity, showing a novel mechanism of action [65]. Furthermore, numerous registered clinical trials (both ongoing and planned) are exploring the potential of new KRAS inhibitors (Table 1).
Although significant progress has been made in the development of KRASG12C inhibitors, the differences in efficacy across various types of cancer (such as NSCLC and CRC) imply that different genetic backgrounds and tumor microenvironments play important roles in resistance. NSCLC patients respond significantly better to these inhibitors than CRC patients, indicating that specific biomarkers or signaling pathways may affect drug effectiveness. This warrants further investigation. In addition, clinical research data indicate that patients have a PFS of only 4 to 6 months, and clinical responses are not enduring. Further studies are needed to enhance drug efficacy. The results indicated that some inhibitors (such as JNJ-74699157) were discontinued due to their significant toxicity, highlighting the need to evaluate drug safety and tolerability during the development of new drugs. Future research must ensure the safety of treatment methods while improving their efficacy.
Ongoing or planned registered clinical trials of KRAS inhibitors in advanced solid tumors or colorectal cancer listed on clinicaltrials.gov (query date: September 2025)
| Drug | Phase | Cancer type | ClinicalTrials.gov Identifier | State |
|---|---|---|---|---|
| RMC-6291 | Phase1 | Advanced Solid Tumors | NCT05462717 | Recruiting |
| GFH925 | Phase1/2 | Advanced Solid Tumors | NCT05005234 | Recruiting |
| ZG19018 | Phase1/2 | Advanced Solid Tumors | NCT06237400 | Recruiting |
| BEBT-607 | Phase 1 | Advanced Solid Tumors | NCT06117371 | Recruiting |
| D3S-001 | Phase 1 | Advanced Solid Tumors | NCT05410145 | Recruiting |
| HBI-2438 | Phase 1 | Advanced Solid Tumors | NCT05485974 | Recruiting |
| FMC-376 | Phase1/2 | Advanced Solid Tumors | NCT06244771 | Recruiting |
| JAB-21822 | Phase1/2 | Advanced Solid Tumors | NCT05009329 | Recruiting |
| MRTX849 | Phase 1 | Advanced Solid Tumors | NCT05263986 | Active, not recruiting |
| SY-5933 | Phase 1 | Advanced Solid Tumors | NCT06006793 | Recruiting |
| GEC255 | Phase 1 | Advanced Solid Tumors | NCT05768321 | Recruiting |
| LY3537982 | Phase 1 | Advanced Solid Tumors | NCT06235983 | Not yet recruiting |
| HS-10370 | Phase1/2 | Advanced Solid Tumor | NCT05367778 | Recruiting |
| BPI-421286 | Phase 1 | Advanced Solid Tumors | NCT05315180 | Unknown |
| HRS-4626 | Phase 1 | Advanced Solid Tumors | NCT05533463 | Recruiting |
| MRTX-1133 | Phase1/2 | Advanced Solid Tumors | NCT05737706 | Recruiting |
| ASP4396 | Phase1 | Advanced Solid Tumors | NCT06364696 | Not yet recruiting |
| RMC-9805 | Phase1 | Advanced Solid Tumors | NCT06040541 | Recruiting |
Table 1 summarizes ongoing or planned clinical trials exploring the efficacy of KRAS inhibitor monotherapy in solid tumors. Data from ClinicalTrials.gov.
KRASG12D inhibitors
KRASG12D mutations were observed in approximately 10% to 17% of CRC individuals [5, 66]. Nevertheless, in contrast to KRASG12C, selective inhibition of KRASG12D presents greater challenges considering the necessity for inhibitors to exhibit sufficiently high affinity in their binding to KRASG12D. There are currently no approved KRASG12D inhibitors. MRTX1133 is the first small molecule compound developed to bind KRASG12D in a non-covalent manner. Functionally, MRTX1133 reduced cell viability and inhibited crucial signaling molecules in KRASG12D-mutant pancreatic and other cancer cell lines. Although MRTX1133 entered the clinical research stage, work thereon was halted at Phase I due to unstable pharmacokinetic data. HRS-4642, a novel KRASG12D inhibitor, demonstrated promising antitumor activity in a Phase-I trial, with potential synergy observed when combined with carfilzomib. Carfilzomib may act synergistically with HRS-4642 by downregulating Notch4 signaling. In addition, the combination of HRS-4642 and carfilzomib can reshape the TME, promoting the infiltration and activation of immune cells with anti-tumor properties [67]. Weller's team designed a small molecule compound RMC-9805 targeting the mutated KRASG12D. This compound creates a neomorphic protein-protein interface between CYPA, an abundant intracellular chaperone, and active RAS by binding to CYPA, thereby covalently modifying the D12 mutation located in the induced pocket at the interface. RMC-9805 reduced tumor growth in experimental models of lung, pancreatic, and CRC [68]. These findings demonstrate a potential therapeutic strategy for KRASG12D-mutant CRC [69].
KRASG12V inhibitor
KRASG12V mutations were observed in approximately 10% of CRC individuals [70]. Research into KRASG12V inhibitors remains in the preclinical stage. A research team developed a selective siRNA molecule targeting KRASG12V, named EFTX-G12V. EFTX-G12V demonstrated significant anti-tumor effects in a mouse xenograft model harboring the KRASG12V mutations. Furthermore, treatment with EFTX-G12V resulted in a marked upregulation of granzyme B expression within the tumor immune microenvironment. In addition, the team found that the combination of EFTX-G12V and cetuximab improved both the depth and duration of response in in-vivo models of CRC [71].
KRAS vaccine
Cancer vaccines play an essential role in preventing tumor cells from evading immune surveillance by enhancing the body's immune response [72]. Vaccine therapy represents a promising novel approach for targeting KRAS mutations. Recently, Reilly's research team conducted a Phase-I clinical trial to assess the efficacy of a novel vaccine, ELI-0022P, in patients with colorectal and pancreatic cancers containing KRAS mutations. ELI-002 2P is a three-component lymph node-targeted vaccine comprising amphiphile (Amph)-modified G12D and G12R mKRAS long peptides as well as Amph-modified Toll-like receptor 9 agonistic CpG-7909 DNA. Vaccination can robustly re-program the immune microenvironment to develop high-magnitude, functional T cell responses. The regimen was well tolerated, with 84% of patients showing tumor biomarker responses. The median recurrence-free survival was 16.33 months [72, 73]. Furthermore, a seven-peptide product, ELI-002 7P has shown antitumor activity in patients with KRAS-mutant CRC and PDAC, inducing CD4 and CD8 responses in 63.6% of patients [74].
KRAS degradation
In addition to covalently suppressing mutant KRAS proteins, the degradation of the oncogene KRAS using the proteolysis-targeting chimeras (PROTAC) has emerged as an alternative approach to KRAS-mutant cancers [75]. PROTACs are bifunctional molecules including a ligand that recruits E3 ubiquitin ligases, a ligand that binds to the target protein, and a linker connecting these two components. This design directs the targeted protein to the E3 ligase for ubiquitination, followed by subsequent degradation by the proteasome [76, 77]. Yang's research team designed a PROTAC known as YF135, which can induce quick and persistent degradation of endogenous KRASG12C and suppress pERK in KRAS-mutant CRC cell lines [78]. Additionally, Zhou's team designed a compound, 8o, which can induce rapid degradation of KRASG12D and inhibit the proliferation of multiple KRASG12D-mutant cancer cells including PDAC, NSCLC, and gastric cancer cells [79]. Alessio's team discovered a novel pan-RAS inhibitor, ACBI3, which can degrade 13 of the 17 most common oncogenic KRAS alleles. Compared with traditional covalent inhibitors, ACBI3 exerts a more durable inhibitory effect on downstream signaling pathways [80].
Mechanisms of resistance and reversal strategies of KRAS inhibitors in patients with CRC
The KRAS-mutant protein is considered to be constitutively active. KRAS-mutant tumors are independent of RTK activation. However, drug resistance to KRAS inhibitors in CRC involves multiple complex mechanisms, which can be classified into genetic alterations, reactivation of signaling pathways, and proteostasis reprogramming, as detailed below.
Feedback activation of upstream signaling pathways
Reactivation of signaling pathways, particularly through EGFR, is a key resistance mechanism in KRAS-mutant cancers. EGFR/ERBB1 is the most characteristic RAS signaling activator, which acts by binding to the growth factor receptor binding protein 2 (GRB2) [81, 82]. The GRB2 protein recruits and activates SOS1 and SHP2, activating the RAS signaling pathway [83-85]. Co-targeting of KRAS and EGFR monoclonal antibodies was found to downregulate the level of RAS-GTP in both mutant and wild-type types [86].
Studies found that EGFR signaling significantly contributes to resistance against KRASG12C and KRASG12D inhibitors, suggesting that co-targeting KRAS and EGFR monoclonal antibodies may provide a new therapeutic approach to overcome resistance [87, 88]. A study found that MRTX1133 exhibited synergistic anti-tumor activity when combined with pan-HER inhibitors in KRASG12D-mutant CRC cells [89].
Secondary mutations and gene alterations in resistance
Whole-genome sequencing of patients treated with KRAS inhibitors has uncovered various secondary mutations and fusions contributing to drug resistance. Notable alterations include: MET amplification, mutations in RET, BRAF, MAP2K1, and NRAS, as well as oncogenic fusions involving ALK, FGFR3, BRAF, RAF1, RET, and loss-of-function mutations in PTEN, and NF1. It is noteworthy that secondary KRAS mutations are also present in patients with drug resistance, such as KRASG12D, KRASG12V, KRASG13D, among others. The acquired activation of the RTK-RAS-MAPK-PI3K cascade network and the emergence of specific fusion genes further demonstrate the complexity of mechanisms governing resistance faced by KRAS-mutant CRC tumors [90]. These fusion genes encompass CCDC6-RET, EML4-ALK, FGFR3-TACC3, AKAP-BRAF, NRF1-BRAF, RAF1-CCDC176, and RAF1-TRAK1.
Genetic alterations are among the most thoroughly studied mechanisms of resistance in clinical practice, thus serving as actionable targets for personalized therapy. For instance, patients with MET amplification or RET fusions are likely to benefit from a combination therapy with KRAS and MET/RET inhibitors.
Roles of the cell cycle and apoptosis pathways
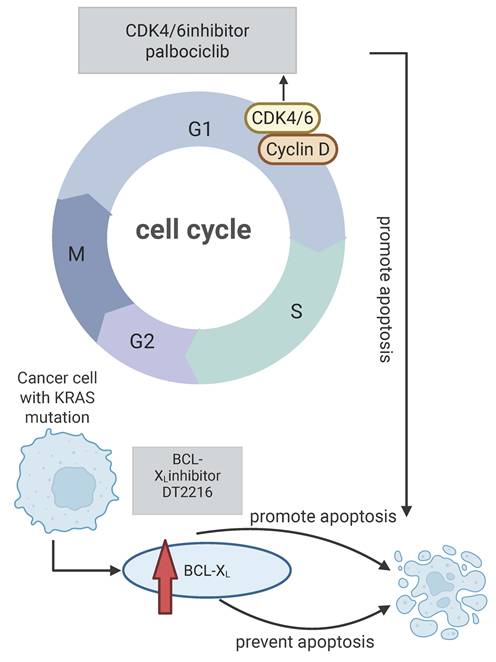
Resistance to KRASG12C inhibitors may involve alterations in key regulators of the cell cycle and apoptosis pathways. CDK4/6 promotes the cell cycle progression by phosphorylating the retinoblastoma gene (RB). A synthetic lethal relationship between CDK4 and KRAS has been identified in NSCLC models [91, 92]. Additionally, high expression of the anti-apoptotic protein BCL-XL has been linked to resistance against KRASG12C inhibitors in KRAS-mutant tumors [93]. Traditional BCL-XL targeting has resulted in severe thrombocytopenia, but the PROTAC DT2216 can selectively degrade BCL-XL without this side effect [94-96]. Sotorasib facilitates the interaction between BCL-XL and BIM. Consequently, when DT2216 degrades BCL-XL, it can enhance the apoptosis of tumor cells alongside sotorasib treatment. Thus, simultaneously targeting BCL-XL and KRASG12C, or co-targeting CDK4 and BCL-XL, may provide a novel strategy to overcome resistance and improve treatment outcomes for KRAS-mutant patients. Figure 1 illustrates the impact of KRAS mutations on apoptosis and the synergistic antitumor activity achieved by combining KRAS inhibitors with cell cycle or anti-apoptotic inhibitors [97].
Proteostasis reprogramming
Protein homeostasis plays a crucial role in cell growth and development. Inactivation of the oncogene KRAS downregulates the heat-shock response and the IRE1α branch of the unfolded protein response (UPR), incurring severe disruption of protein homeostasis. However, cancer cells can employ mechanisms to restore protein homeostasis. Targeting these pathways may enhance cell death and reduce drug resistance. Wang's research team found that the HSP90 inhibitor AUY922 suppresses the growth of KRAS-mutant CRC xenografts by inducing apoptosis associated with endoplasmic reticulum (ER) stress. Mechanistically, AUY922-induced ER stress upregulates BIM transcription in KRAS-mutant CRC. BIM deficiency blocks the inhibitory effect of AUY922 on the growth of KRAS-mutant CRC xenografts. Furthermore, inhibiting GRP78 or XBP-1 - two key pro-survival factors in the ER stress response - enhances the cytotoxic effects of AUY922 [98]. Another research team found that, in KRAS-mutant CRC cell lines, gene ablation of ERN1 enhances cellular sensitivity to MEK inhibition. ERN1 is linked to the JUN pathway through its binding factor TRAF2, which mediates a signaling cascade that activates JUN N-terminal kinase (JNK), promoting cell proliferation. JNK kinase inhibitors exhibit synergistic antitumor activity in combination with MEK inhibitors [99]. Tan's research team found that the expression levels of Nrf2 and GLS1 proteins were significantly elevated in CRC cell lines with acquired resistance to adagrasib. Subsequently, they extracted a natural cyclic peptide, RA-V (a natural cyclopeptide isolated from the roots of Rubia yunnanensis). RA-V can restore the sensitivity of resistant CRC cells to sotorasib. The specific mechanism of action involves RA-V inhibiting Nrf2 protein through ubiquitin-proteasome-dependent degradation, causing the induction of oxidative stress, ER stress, and DNA damage in CRC cell lines. Therefore, RA-V may serve as a novel therapy with which to improve the efficacy of adagrasib [100].
Effects of KRAS mutations on cell cycle and apoptosis. KRAS mutations can cause the level of BCL-XL protein, and then prevent apoptosis, targeting BCL-XL can promote apoptosis and reverse drug resistance, and CDK4/6 inhibitors can promote apoptosis by inhibiting cell cycle progression and have synergistic anti-tumor effects with KRAS inhibitors. Created in BioRender. Li, J. (2025) https://BioRender.com/vzgd3pj.
Other common mechanisms of drug resistance
In addition to the aforementioned mechanisms of resistance, the activation of other pathways is also associated with tumor drug resistance. Figure 2 shows that the activation of several common signaling pathways can lead to resistance to KRAS inhibitors. Polo-like Kinase (PLK1) and c-Myc are overexpressed in resistant cell lines, with PLK1 phosphorylating c-Myc. This activated c-Myc enhances the expression of PLK1 through E-box binding, creating a positive feedback loop that accelerates cell mitosis and tumor proliferation. ST8SIA6-AS1, a long non-coding RNA upregulated in drug-resistant cells, interacts with PLK1 and Aurora A, promoting PLK1 phosphorylation and reinforcing this feedback loop. Targeting ST8SIA6-AS1 can counteract resistance to KRASG12C inhibitors by disrupting the mutual activation of PLK1 and c-Myc signaling [101].
Another study highlighted the role of MAP2K4-JNK-JUN pathway in CRC resistance to KRASG12C inhibitors (Figure 2). MAP2K4 induces RTK-dependent rebound activation of KRAS signaling when MAPK inhibitors are present. JUN activation stimulates several RTKs, notably ERBB2 and ERBB3, reactivating the MAPK pathway and contributing to drug resistance. Combining sotorasib with the MAP2K4 inhibitor HRX-0233 can impede this feedback and enhance the antitumor activity [102, 103].
Johnson et al. found that the YAP1/TAZ pathway promotes resistance in KRAS-mutant patients [104]. YAP1/TAZ can regulate transcription through TEAD, stimulating the ERK-independent gene expression that promotes cell cycle progression and activates PI3K-AKT-mTOR signaling, allowing cells to evade apoptosis [105]. These findings emphasize the complex mechanisms of drug resistance in KRAS-mutant CRC, potentially providing new therapeutic strategies for clinicians. By disrupting these feedback loops and signaling pathways, the efficacy of KRASG12C inhibitors may be enhanced.
Common signaling pathways associated with drug resistance. Activation of the ST8SIA6-AS-PLK-c-MYC, MAPK24-JNK-JUN, Wnt/β-Catenin, and JAK1/2-STAT3 pathways is associated with drug resistance in KRAS mutated tumors, and targeting these pathways can reverse drug resistance. Created in BioRender. Li, J. (2025) https://BioRender.com/vzgd3pj.

The above studies indicate that the resistance mechanisms in KRAS-mutant CRC are dynamic and multifaceted processes, involving feedback activation, acquired genetic changes, and reprogramming of core cellular processes such as protein homeostasis and apoptosis. This suggests that merely targeting KRAS is insufficient. These findings facilitate a shift in treatment paradigms from monotherapy to mechanism-based combination therapies. Future directions should focus on proactively targeting these escape pathways. Additionally, identifying reliable biomarkers to pinpoint the dominant resistance pathways in patients is crucial.
Reversal of KRAS inhibitor resistance by targeting downstream signaling pathways
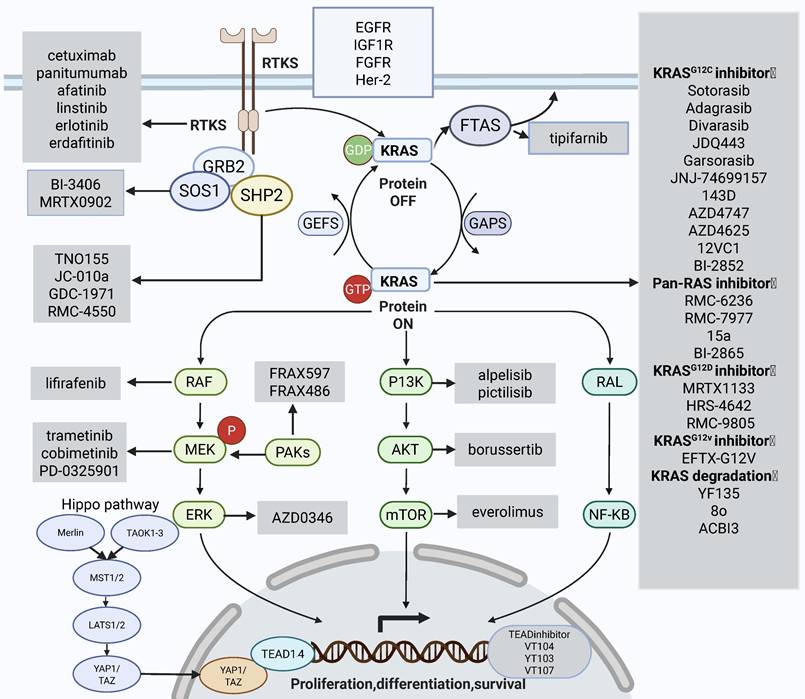
Due to the limited efficacy of KRAS inhibitors as monotherapy, resistance often develops rapidly; therefore, the development of combination therapies is progressing swiftly. As shown in Figure 3, common combination therapies that can alleviate resistance are summarized; these are based on the RAS signaling pathway and focus on its downstream and upstream components.
Targeting the MAPK signaling pathway
A prominent mechanism underpinning drug resistance is the reactivation of the RAS-MAPK pathway [106]. The MEK is an important component of the RAF/MEK/ERK cascade downstream of KRAS [107]. For a long time, MEK inhibitors have failed to achieve any significant efficacy in CRC patients [108]. This phenomenon might stem from feedback phosphorylation of MEK by C-RAF and reactivation of MAPK signaling. However, MEK inhibitors lay a basis for combination therapy, showing synergistic effects when paired with RAF inhibitors [109]. Xi et al. reported that the RAF dimer inhibitor lifirafenib showed strong synergy with MEK inhibitors in suppressing the proliferation of KRAS-mutant CRC cell lines [110]. Other research found that the combination of type II RAF inhibitors with MEK inhibitors could overcome acquired resistance in KRAS-mutant cancers. Additionally, resistance of MEK inhibitors in CRC is found to be correlated with ERK-dependent activation of the RTK-SOS-WTRAS-PI3K signaling pathway [111-113]. MEK inhibitors, when combined with ERK inhibitors, exert a synergistic effect in KRAS-mutant tumors and significantly reverse the resistance caused by MEK inhibitors alone [114]. Research has identified that in CRC cell lines exhibiting resistance to MEK inhibitors, the GRB7-mediated signaling pathway is prominently enriched. PLK1 functions as a major interacting kinase with GRB7; thus, inhibition of PLK1 can suppress the downstream signaling associated with RTKs, thereby reversing drug resistance [115].
Targeting the PI3K-AKT-mTOR signaling pathway
The PI3K/AKT signaling cascade was confirmed to be activated in sotorasib-resistant cell lines, with sotorasib and PI3K inhibitor alpelisib showing synergistic cytotoxicity [116]. Belli et al. found that mTOR inhibitor everolimus and PAK inhibitor exhibit synergistic antitumor activity in KRAS-mutant CRC [117]. Targeting the MAPK signaling pathway and the PI3K-AKT-mTOR pathway will be efficacious [118]. However, these combination strategies may also cause increased toxicity profiles. A Phase-I clinical study showed that the incidence of drug-related adverse events of Grade 3 or higher was 18.1%, limiting the clinical application of the combination [119]. John et al. proposed that borussertib, an allosteric inhibitor of AKT, has a synergistic effect with MEK inhibitors in a preclinical model of the colorectum [120]. Clarke et al. discovered that the MEK inhibitor cobimetinib and PI3K inhibitor pictilisib act synergistically in human CRC cells.
Targeting the Wnt signaling pathway
Previous investigations identified the wingless integrated (Wnt) signaling pathway as a mechanism of resistance to selumetinib [121]. Krishnamurthy et al. initiated a Phase-Ib clinical trial and found that a combination of selumetinib and cyclosporin A, an unconventional modulator of the Wnt signaling pathway, exhibited favorable tolerability and activity in CRC patients. The ORR in patients reached 76.9% [122]. Jai-Hee et al. revealed that the β-catenin pathway inhibitor (NVP-TNS656) could overcome resistance induced by MEK inhibitors in KRAS-mutant CRC cells (Figure 2) [123].
KRAS signaling pathways and combination therapies that can alleviate resistance. The main mechanisms that can reverse drug resistance are: targeting the downstream RAF-MEK-ERK signaling pathway; targeting the PI3K-AKT signaling pathway; targeting YAP/TAZ and its transcription factor TEAD in the Hippo pathway, targeting upstream SHP2, SOS1, FTAS, targeting upstream continuously activated receptor tyrosine kinases. The gray box areas show the main targeted drugs for each signaling pathway. Created in BioRender. Li, J. (2025) https://BioRender.com/vzgd3pj.
Clinical trials evaluating the efficacy of KRAS inhibitor combined with ICIs in CRC patients.
| Study | Phase | Intervention | ORR | mPFS (month) | 3-4TRAE |
|---|---|---|---|---|---|
| NCT04449874 [126] | Ib | Divarasib +Cetuximab | 62.5% | 8.1 | 44.8% |
| NCT04185883 [127] | Ib | Sotorasib+Panitumumab | 30.0% | 5.7 | 27% |
| NCT03785249 [128] | I-II | Adagrasib+ Cetuximab vs. Adagrasib | 46% vs.19% | 6.9 vs. 5.6 | 16% vs. 34% |
| NCT05198934 [129] | III | Sotorasib(960mg/240mg) + Panitumumab vs. standard-care (trifluridine-tipiracil or regorafenib) | 26.4% vs. 5.7% vs.0% | 5.6 vs. 3.9 vs.2.0 | 35.8% vs. 30.2% vs. 43.1% |
| NCT04585035 [130] | II | Garsorasib + Cetuximab vs. Garsorasib | 45.2% vs. 19.2% | 7.5 vs. 5.5 | 19.2% vs.14.3% |
Table 2 summarizes the efficacy and safety of KRAS inhibitors in combination with EGFR monoclonal antibodies.
Data from ClinicalTrials.gov.
Targeting the JAK-STAT signaling pathway
Sandra et al. found that activation of Janus kinase 1/2 (JAK1/2)-dependent signal transducer and activator of transcription 3 (STAT3) can mediate the resistance to MEK inhibitors in KRAS-mutant CRC models. Notably, treatment with MEK inhibitors alone also leads to the activation of c-MET. Inhibition of either c-MET or JAK1/2, using compounds including AZD1480 and TG1038, shows the potential to enhance the sensitivity to MEK inhibitors (Figure 2).
Reversal of KRAS inhibitor resistance by targeting upstream signaling pathways
Several studies indicate that KRASG12C-mutant CRC retains the sensitivity to upstream RTK signaling. Upon the activation of RTK, GRB2 engages with the GEF SOS1 to form a complex that is associated with the cell membrane-bound KRAS protein, thus promoting the activation of KRAS. Consequently, more investigations have focused on the synergistic potential of combining KRAS inhibitors with agents targeting these upstream signaling pathways. Such combinatorial approach has shown promise in improving therapeutic efficacy in KRAS-mutant CRC.
RTKs
RTKs constitute a distinct subclass of tyrosine kinases, which are instrumental in facilitating intercellular communication and regulating a diverse array of intricate biological processes. These include cellular growth, motility, differentiation, and metabolic activities. Upon activation, RTKs recruit and modulate various downstream signaling cascades, such as the RAS/MAPK, PI3K/AKT, and JAK2/STAT pathways [124]. The activation of RTKs has been identified as a significant mechanism of resistance in patients with KRAS-mutant CRC. Clinical studies aimed at overcoming the resistance of KRAS inhibitors are also underway. Robin's team conducted clinical trials to evaluate the efficacy of MEK inhibitors in the downstream signaling pathway of KRAS in combination with pan-HER-2 inhibitors in KRASG12C-mutant tumor groups. Still, the combination therapy was found to confer no apparent benefit in CRC, and the toxicity was severe [125]. In addition, given the feedback activation of EGFR, several clinical researchers are exploring the efficiency of KRASG12C inhibitors combined with EGFR monoclonal antibodies. Multiple clinical studies implied that KRASG12C inhibitors combined with EGFR monoclonal antibodies show promising efficacy in patients with KRAS-mutant CRC, with manageable safety profiles [126-130]. Table 2 summarizes the current five clinical studies on the combination of KRAS inhibitors with EGFR monoclonal antibodies in CRC patients. Future large-scale Phase-III clinical trials should further study the feasibility of combination treatment strategies. These results imply that targeting EGFR significantly improves the resistance of KRASG12C inhibitors in CRC patients.
SHP2 inhibitors
SHP2 is a non-receptor protein tyrosine phosphatase that integrates growth and differentiation signals from RTKs into the RAS/MAPK cascade. SHP2 encoded by the protein tyrosine phosphatase non-receptor type 11 (PTPN11) gene functions downstream of RTKs to facilitate the physiological activation of KRAS [131]. SHP2 also promotes nucleotide exchange in KRAS proteins [132]. SHP2 activates RAS/MAPK and PI3K pathways by promoting KRAS-GTP. Inhibition of SHP2 reduces the circulation of KRASG12C, which, together with SOS1, leads to a shift from an inactive GDP-bound pattern to an active GTP-bound pattern. Therefore, targeting SHP2 can overcome KRAS inhibitor resistance. Chen et al. proposed that SHP2 inhibitors block the feedback activation of downstream signaling pathways mediated by wild-type RAS, which exerts a synergistic effect with KRASG12C inhibitors and significantly suppresses the phosphorylation of downstream ERK [133]. SHP2 inhibitor TNO155 has been reported to show synergistic effects with KRASG12C inhibitors [134]. SHP2 inhibitors block RTK and MAPK-driven tumor cell proliferation [133] and inhibit the activation of RTK-driven feedback after treatment with KRAS inhibitors [135]. The SHP2 inhibitor JC-010a eliminates RTK/SHP2-mediated resistance to selumetinib in KRAS-mutant cancers [136]. Recently, Alexander's team identified a novel SHP2 inhibitor, GDC-1971, which exhibited significant antitumor ability in a mouse xenograft model of NSCLC and exerted a synergistic antitumor effect when combined with KRAS inhibitors [137]. A Phase-I clinical study is now ongoing to assess the potency of divarasib combined with GDC-1971 in patients with KRAS-mutant solid tumors [132].
SOS1 inhibitors
SOS1 as a type of GEF which is positioned upstream of KRAS plays a pivotal role in converting inactive KRAS proteins into their active GTP-bound state. Targeting SOS1 suppresses the activation of the RAS signaling pathway [83]. Furthermore, SOS1 is essential for the negative feedback regulation of the KRAS pathway; thus, utilizing a combination of KRAS inhibitors and SOS1 inhibitors in KRAS-mutant CRC patients may significantly reverse drug resistance. BI-3406, a selective KRAS-SOS1 interaction inhibitor, significantly ameliorates MEK inhibitor resistance in KRASG12C-mutant CRC cell lines and PDX models [138]. In addition, the SOS1 inhibitor MRTX0902, when combined with the KRASG12C inhibitor adagrasib, has shown synergistic antitumor activity in PDX models of KRASG12C-mutant pancreatic cancers. The clinical efficacy of this combination in KRASG12C-mutant CRC patients remains to be confirmed [139]. Furthermore, targeted degradation of SOS1 may yield superior efficacy compared to conventional inhibition. Currently, targeted degradation of SOS1 alone exhibits significant antitumor activity in KRASG12C-mutant CRC and demonstrates synergistic anti-tumor activity with KRASG12C inhibitors [140, 141].
Farnesyltransferase inhibitors
KRAS protein is located on the inner side of the cell membrane. This process requires farnesylation modification, which adds farnesyl groups to protein molecules under the action of farnesyltransferase. Hence, farnesyl transferase inhibitors may affect the activation of downstream signaling pathways mediated by KRAS protein. Marcell et al. identified that KRAS-mutated lung adenocarcinoma is sensitive to farnesyltransferase inhibitors. Alan et al. further confirmed that it has excellent clinical activity in HRAS-driven head and neck squamous cell carcinoma [142-144]. Recently, a combination of additional KRASG1C inhibitors, sotorasib, and farnesyl-transferase inhibitors, tipifarnib, shows synergism in lung, colorectal, and pancreatic adenocarcinoma cellular models [145].
Other strategies
TEAD inhibitors
The Hippo pathway is a crucial growth control pathway conserved across species, negatively regulating the closely associated transcriptional coactivator paralogs YAP1 and TAZ. Overexpression or activation of YAP1 and TAZ can overcome the dependency of cancer cells on mutant KRAS, resulting in the proliferation of cancer cells [146, 147]. Overexpression of YAP1-TAZ exerts proliferative effects on various tumor mutations, which may be related to the resistance to KRAS inhibitors. Several studies found that inhibition of YAP/TAZ or its transcription factor TEAD can overcome resistance to KRASG12C inhibitors. Edwards et al. developed TEAD inhibitors, including VT103, VT104, and VT107. All of which exert synergistic effects when used in conjunction with KRASG12C inhibitors [105, 148-150].
Pan-RAS inhibitors
Dongsung et al. developed a pan-KRAS inhibitor, BI-2865, which can block nucleotide exchange to impede the activation of both wild-type KRAS and multiple KRAS mutants [151]. Additionally, another pan-KRAS inhibitor, RMC-6236, has shown promising antitumor activity [152]. Recently, it has been reported that a novel pan-RAS translation inhibitor 15a also suppresses the proliferation of KRAS mutation-driven tumor cells [153]. Additionally, Mallika's team designed a new small-molecule compound, RMC-7977, with broad inhibition of the active pattern of wild-type and mutants KRAS, HRAS, and NRAS. RMC-7977 exhibited optimal efficacy against RAS-addicted neoplasms characterized by diverse RAS genotypic profiles in preclinical oncology models, showing pronounced activity in models harboring KRAS codon 12 mutations (KRASG12X) [154]. Subsequently, Olive et al. confirmed the significant antitumor efficacy of RMC-7977 in preclinical models of pancreatic cancer and revealed that overexpression of YAP promotes resistance to RMC-7977 in pancreatic cancer [155].
Targeting metabolism-related pathways
Studies discovered that KRAS-mutant cells improve the expression of proteins that participate in glycolysis, the non-oxidative branch of the pentose phosphate pathway, glutamine metabolism, and the phosphoserine-biosynthesis pathway [156, 157].
Targeting glucose metabolism
KRAS-mutant tumors are more reliant on the glycolytic pathway for energy production, driving the conversion of glucose to pyruvate and ultimately to lactate through upregulation of key enzymes in glycolysis [158, 159]. Targeting glycolysis has emerged as a promising strategy for KRAS-driven tumors. Li's research team found that BNBZ induces metabolic reprogramming in KRAS-mutant CRC cells by inhibiting glycolytic flux, thereby suppressing the tumor cell growth. The underlying mechanism primarily involves the inhibition of the key glycolytic enzyme HK2, which leads to impaired cell proliferation and increased production of reactive oxygen species (ROS). Additionally, BNBZ promotes apoptosis by upregulating Bax and downregulating Bcl-2[160]. Johanna found that simultaneous inhibition of the glucose transporter GLUT1 and the ATR/CHK1 cell-cycle checkpoint signaling axis induces S-phase specific genotoxic stress and apoptosis in various KRAS-mutant cell lines, including CRC, both in vitro and in vivo [161].
Targeting glutamine metabolism
Glutamine is critical to the survival and progression of KRAS-mutant CRC. Solute carrier 25 member 21 (SLC25A21) is an oxo-dicarboxylate carrier responsible for transporting 2-oxo adipate (2-OA) and α-ketoglutarate (α-KG) across the inner mitochondrial membrane [162]. The survival and progression of KRAS-mutant CRC depend on glutamine (Gln). Downregulation of SLC25A21 inhibits the mitochondrial export of Gln-derived α-ketoglutarate (α-KG), thereby enhancing glutamine metabolism. This response, characterized by the cessation of α-KG export due to the downregulation of SLC25A21, promotes downstream oxidative decarboxylation reactions and GTP production, leading to sustained KRAS activation in KRAS-mutant CRC, which drives cell proliferation. Increasing the SLC25A21 expression can induce cell death in KRAS-mutant CRC [163]. Another study indicated that SLC7A5 maintains intracellular amino acid levels through transcriptional and metabolic reprogramming following KRAS activation, further supporting the demand of the tumor cells for extensive protein synthesis. Additionally, the loss of SLC7A5 can inhibit the mTOR signaling. Targeting the glutamine transporter SLC7A5 suppresses the proliferation of KRAS-mutant cancers. When combined with mTOR inhibitors, this approach shows a synergistic effect [164]. These findings indicate that targeting metabolic pathways with KRAS inhibitors may reverse drug resistance.
Epigenetic-based treatment strategies
For a long time, epigenetic and genetic alterations have been thought of as two independent mechanisms participating in tumorigenesis [165]. Epigenetics assumes a crucial role in tumor progression [166]. Among them, inhibition of histone deacetylases (HDACs) has been found to promote the effects of RAS pathway inhibitors in numerous tumor types [167, 168]. HDAC-mediated histone deacetylation is a post-translational modification process involving the lysine residues of histones in the nucleosome, which affects the chromatin structure and consequently regulates the gene expression. In malignant tumors, the activity of HDAC is often upregulated to further suppress the expression of tumor suppressor genes, which positions HDACs as attractive therapeutic targets [168]. Selumetinib and the HDAC inhibitor vorinostat show synergistic antitumor activity in KRAS-mutant CRC cell lines [169]. Hendrik et al. found that KRAS-mutant CRC cells induce the expression of hypoxia-inducible factor-1α and 2α. The microtubule agent dolastatin 10 and the Class I HDAC inhibitor largazole suppress the oncogenic KRAS and HIF pathways, inducing tumor regression [170]. Additionally, the HDAC6 inhibitor C1A was found to modulate autophagy substrates in KRAS-positive CRCs, inducing cell death [171]. These findings imply that co-targeting HDACs and KRAS could be a valuable avenue for future research and therapeutic strategies. In addition, studies reported that inhibitors of the histone methyltransferase EZH2 exert a synergistic antitumor effect when combined with RAS pathway inhibitors in KRAS-mutant CRC. The combined use of both can inhibit the WNT pathway, leading CRC to reach a more differentiated cellular state, thereby making it more sensitive to inhibitors [172].
The combination of KRAS inhibitors and immune checkpoint inhibitors
The emergence of immunotherapy has remarkably converted the therapeutic landscape of CRC. Considerable attention has been paid to the collaborative application of immune checkpoint inhibitors (ICIs) in conjunction with drugs that disrupt the activation of oncogenic signaling cascades. Previous studies reported that KRAS-mutant CRC patients are associated with an immunosuppressive tumor microenvironment (TME) (Figure 4).
Regulation of immune cell subsets
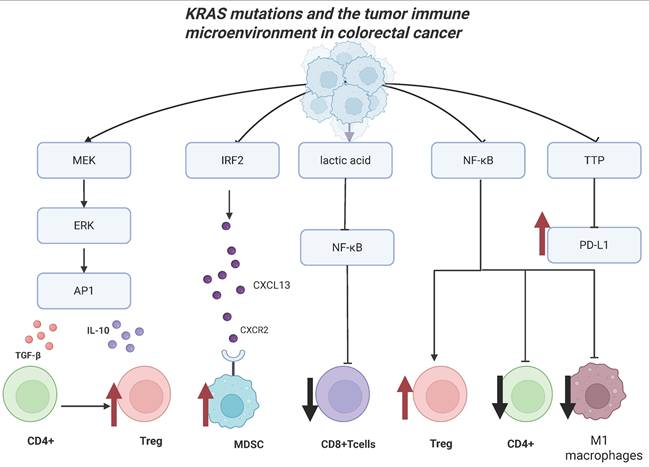
KRAS-mutant CRC sensitizes tumor-specific cytotoxic CD8+ T-cells to activation-induced cell death via NF-κB inactivation (Figure 4) [173]. Liu et al. also discovered that NF-κB and T-cell receptor signaling cascades were significantly suppressed in patients with KRAS mutations. Additionally, Tregs were increased while activated CD4 memory T cell and macrophage M1 could be reduced in KRAS-mutant CRC (Figure 4) [174].
Effect on immune checkpoint molecules
Oncogenic RAS signaling upregulates the expression of PD-L1 mRNA in CRC. This process is regulated by the AU-rich element-binding protein tritetrapeptide TTP, which negatively regulates the expression of PD-L1 mRNA. MEK signaling downstream of oncogenic RAS suppresses the expression of TTP, upregulating the PD-L expression to evade immune surveillance (Figure 4) [175]. Additionally, another study found that KRAS-mutant patients have higher expression of CTLA-4 mRNA and poorer prognosis [176].
Cytokine and chemokine production
The mutant KRAS protein can drive the expression of diverse cytokines and chemokines, thus facilitating the formation of an immune-heterogeneous TME [177]. Specifically, the activation of the MEK-ERK-AP1 pathway by mutated KRAS induces tumor cells to secrete interleukin-10 (IL-10) and transforming growth factor-β1 (TGF-β1). This expedites the transformation of CD4+ T cells into regulatory T cells (Tregs), which inhibit the tumor immunity (Figure 4). Moreover, the oncogenic KRASG12D variant has been found to inhibit the expression of interferon regulatory factor 2 (IRF2), which negatively regulates the CXCL3 expression. KRASG12D-mediated inhibition of IRF2 ultimately causes the high expression of CXCL3, which can bind to CXCR2 on myeloid-derived suppressor cells (MDSCs) and promotes its migration to the TME [178] (Figure 4). Moreover, the TGF-β signaling pathway functions as a mediator of immune escape and tumor invasion in KRAS-mutant CRC [179, 180].
Measures for combination therapy
Given the immunosuppressive nature of KRAS-mutant tumors, combining ICIs with KRAS-targeted therapies holds promise. KRAS inhibitors can also remodel the TME, leading to the formation of pro-inflammatory TME, and resulting in the remodeling of the tumor immune microenvironment in KRAS-mutant cancers [181].
KRAS mutations and the tumor immune microenvironment. Effect of KRAS mutations on tumor microenvironment in colorectal cancer. KRAS-mutated tumor cells can convert CD4+ T cells into regulatory T cells through the MEK-ERK-AP1 signaling pathway; recruiting MDSCs to the tumor microenvironment by upregulating the expression of IRF2; inhibiting NF-κB signaling by upregulating the expression of lactic acid, further reducing the number of CD+ T cells; inhibiting the NF-κB signaling pathway, increasing the number of regulatory T cells, reducing the number of CD4+ T cells and the number of M1 macrophages, Inhibiting the expression of TTP and upregulates the expression of PD-L1. Created in BioRender. Li, J. (2025) https://BioRender.com/vzgd3pj.
Preclinical studies show that the combination of KRAS inhibitors and ICIs significantly improves survival in CRC models. In the KRASG12C-mutant CT26 syngeneic mouse model, adagrasib reduces intra-tumoral MDSCs and enhances the infiltration of M1-polarized macrophages, CD4+ T cells, dendritic cells, and CD8+ T cells. The combination of ICI and KRASG12C inhibitor significantly increases the survival ratio of mice in the subcutaneous model of CT-26 syngeneic CRC [182]. In a KRASG12D-mutant CRC mouse model, treatment with RMC-9805 increases T cell infiltration, reduces the infiltration of M2-like macrophages and MDSCs, significantly decreases the expression of immune checkpoint molecules, and markedly increases the MHC-I expression. RMC-9805 exerts a synergistic antitumor effect with ICIs, significantly inhibiting tumor proliferation [183].
Clinical studies confirmed that KRASG12C inhibitors in conjunction with ICIs elicit substantial antitumor activity in NSCLC patients, achieving an ORR of 63% in individuals with a programmed cell death 1 ligand 1 (PD-L1) tumor proportion score (TPS) of greater than, or equal to, 50%. The median duration of response does not reach (95% CI, 12.6 - NE) [184]. The efficiency of KRASG12C inhibitors combined with ICIs in KRAS-mutant CRC patients has not, at time of writing, been studied. Combining ICIS and KRAS inhibitors warrants further exploration [185].
Hapten-based immunotherapy has the potential to overcome drug resistance [186-188]. Zhang et al. found that the covalent modification of KRAS (G12C) on tumor-specific cysteine residues causes the formation of haptenized peptides that can be presented by MHC-I molecules. These peptides were identified by the recombinant antibody P1A4, which selectively activates a cytolytic T-cell response to target and eliminate tumor cells. This approach provides a novel strategy for reversing drug resistance in cancer therapies [189].
Furthermore, post-translational modifications of proteins significantly affect the TME. Kim et al. found that statins enhance the cross-priming capabilities of dendritic cells, thus promoting immunogenic cell death of CD8+ T cells targeting KRAS-mutant tumors. This indicates that statins have synergistic antitumor effects when used in combination with ICIs [190].
The interaction between mutated KRAS and the immune microenvironment is a key determinant of tumorigenesis, progression, and treatment response. Targeting this pathway can not only directly suppress the proliferation of tumor cells but also enhance antitumor immune responses by remodeling the immune microenvironment. The combination of KRAS inhibitors with ICIs holds promise as a new treatment option for patients with KRAS-mutant CRC.
Discussion
Significant progress has been achieved in the research of KRAS inhibitors. In addition to the two approved KRASG12C inhibitors (sotorasib and adagrasib), non-covalent selective inhibitors targeting KRASG12D (MRTX1133 and HRS-4642), the inhibitor targeting KRASG12V (EFTX-G12V), and pan-RAS inhibitors (BI-2865, RMC-6236, and RMC-7977) are currently at different stages of clinical development. Resistance remains a major concern, as a single inhibitor is inadequate for effective tumor eradication, underscoring the need for novel therapeutics and combination strategies to overcome resistance [191]. Current combination treatment strategies primarily focus on KRASG12C inhibitors. The combination of KRASG12C inhibitors with EGFR monoclonal antibodies may yet become a standard treatment for CRC patients. However, the efficacy of combinations involving SOS1, SHP2, and farnesyltransferase inhibitors has not yet been validated in clinical studies. A preclinical study confirmed that in CRC models resistant to adagrasib, combining adagrasib with SOS1 or SHP2 inhibitors shows comparable efficacy, both surpassing the effectiveness of adagrasib combined with EGFR monoclonal antibodies [192]. Although both therapeutic approaches demonstrate similar efficacy, SOS1 inhibition may mitigate some of the pleiotropic effects associated with SHP2 inhibitors, potentially improving clinical tolerability. Studies in mouse models have validated that KRASG12D inhibitors, KRASG12V inhibitors, and the Pan-RAS inhibitor exhibit synergistic anti-tumor effects when combined with EGFR monoclonal antibodies.
The combination therapy of KRAS inhibitors with ICIs is also being actively investigated [48, 182]. Several ongoing clinical studies are exploring the conjunction of ICIs with KRASG12C inhibitors (Table 3). Recent research utilizing spatial transcriptomics has revealed that the TME in KRAS-mutant CRC patients is suppressed, with specific immune gene signatures linked to poorer OS. The study identified an upregulation of CD40, CTLA4, ARG1, STAT3, IDO, and CD274 in the TME of these patients, which may serve as characteristics of immune suppression [193]. Therefore, the clinical effect of combined treatment is expected to be promising.
Patient selection is critical for analyzing the efficacy of KRAS inhibitors in CRC. Molecular profiling of CRC patients supports precision therapy, as factors like NRAS, BRAF, and PIK3CA mutations can affect treatment responses. Therefore, a comprehensive assessment of multiple biomarkers is essential for developing treatment plans. Next-generation sequencing (NGS) technology provides comprehensive information on the tumor genome, helping to identify mutations associated with resistance [194].
Although many studies have found that targeting metabolism-related pathways may alleviate resistance, treating cancer metabolism as a therapeutic target still faces significant challenges. One major challenge is how to eliminate tumor cells while maintaining cellular metabolic homeostasis. Additionally, tumor heterogeneity and the differences in metabolic profiles among patients highlight the importance of precision medicine. Development of metabolic biomarkers could aid in predicting patient responses to these therapies, thus facilitating more personalized, effective treatment options. Protein homeostasis also plays a crucial role in tumor resistance. Clinical trials discovered that the IRE1α inhibitor ORIN1001 exhibits antitumor activity in various solid tumors, including CRC, highlighting the potential to target protein homeostasis.
In CRC cells, cellular plasticity is often regulated by epigenetic mechanisms, implying that targeting key epigenetic regulators, in addition to traditional oncogenic signaling pathways, may represent a potential therapeutic strategy. Current research has identified that HDAC and EZH2 inhibitors exert synergistic antitumor effects when combined with RAS pathway inhibitors. Further exploration of this area of study is warranted.
Ongoing or planned registered clinical trials of KRAS inhibitors, in combination with other medications in advanced solid tumors or colorectal cancer listed on clinicaltrials.gov (query date: September 2025)
| Drug | Target | Combination | Phase | Cancer type | ClinicalTrials.gov Identifier | State |
|---|---|---|---|---|---|---|
| ASP3082 | EGFR | Cetuximab | Phase 1 | Advanced Solid Tumors | NCT05382559 | Recruiting |
| MRTX849 | PD-1/EGFR | Pembrolizumab, Cetuximab, Afatinib | Phase 1/2 | Advanced Solid Tumors | NCT03785249 | Recruiting |
| JDQ443 | MEK/CDK4/6 EGFR | Trametinib, Ribociclib, cetuximab | Phase Ib/II | Advanced Solid Tumors | NCT05358249 | Recruiting |
| Adagrasib | SHP2/EGFR | BMS-986466 with or without Cetuximab | Phase1/2 | Advanced Solid Tumors | NCT06024174 | Active, not recruiting |
| Sotorasib | EGFR | Panitumumab | Phase2 | Advanced Solid Tumors | NCT05993455 | Active, not recruiting |
| Sotorasib | PD1/L1 | Anti PD-1/L1, Midazolam | Phase 1/2 | Advanced Solid Tumors | NCT03600883 | Active, not recruiting |
| Adagrasib | CDK4/6 | Palbociclib | Phase1 | Advanced Solid Tumor | NCT05178888 | Active, not recruiting |
| QTX3034 | EGFR | Cetuximab | Phase1 | Advanced Solid Tumors | NCT06227377 | Recruiting |
| BI- 1823911 | SOS1 | BI-1701963 Midazolam | Phase1 | Advanced Solid Tumors | NCT04973163 | Active, not recruiting |
| Drug | Target | Combination | Phase | Cancer type | ClinicalTrials.gov Identifier | State |
| IBI315 | EGFR | Cetuximab | Phase 1 | KRAS G12C Mutated Metastatic CRC | NCT05497336 | Unknown status |
| Sotorasib | MEK SHP2 EGFR PD-1 EGFR / / / / PD-L1 CDK4/6 / / SHP2 / / SOS1 PD-1 mTOR | Trametinib, RMC-4630, Afatinib, Pembrolizumab Panitumumab, Carboplatin, pemetrexed, docetaxel, paclitaxel, Atezolizumab, Palbociclib, MVASI® (bevacizumab-awwb), TNO155, FOLFIRI, FOLFOX, BI 1701963, AMG 404, Enerolimus | Phase 1 | Advanced Solid Tumors | NCT04185883 | Active, not recruiting |
Table 3 summarizes ongoing or planned clinical trials exploring the efficacy of KRASG12C inhibitors combined with other treatments in solid tumors. Data from ClinicalTrials.gov.
Despite certain advances in the research into KRAS-mutant CRC, current studies still face several limitations: first, many preclinical studies are based on cell lines or cell-derived xenograft (CDX) models. These models may exhibit significant differences in biological characteristics and drug responses compared to human tumors, thereby limiting the generalizability of their results. Future preclinical research should focus on patient-derived xenograft (PDX) models, and integrate new technologies like organoids, organ-on-a-chip systems, and artificial intelligence to achieve high-throughput precision drug screening [195]. This approach aims to deeply elucidate resistance mechanisms and ultimately match each patient with the most effective personalized treatment. Second, the safety of KRAS inhibitor combination therapies is a major challenge. Current regimens mainly pair them with EGFR monoclonal antibodies; existing Phase I-II studies show manageable safety, but long-term profiles need large-scale trials. Combining with other targeted/immunotherapies is constrained by higher toxicity. A 1,029-patient network meta-analysis found grade 3+ AEs occurred in 26% of patients (monotherapy) vs 35% (combination therapy). Notably, KRASG12C inhibitor combined with ICIs raised this to 50% (with liver toxicity a concern [196]), while Codebreak 100/101 showed sotorasib + anti-PD-(L)1 boosted grade 3-4 TRAEs, mainly elevated transaminases. Future research should focus on exploring low-toxicity combination strategies and identifying predictive biomarkers of adverse events. Finally, the clinical translation process faces numerous challenges. Factors such as patient heterogeneity and the complexity of the TME can significantly impact treatment efficacy. Therefore, determining how to accurately select suitable patients to maximize therapeutic benefits is a crucial direction for future research.
KRAS inhibitors have demonstrated promise in CRC treatment, however, they remain constrained by challenges including patient selection, mechanisms of resistance and TRAEs. The integration of the application of biomarkers with emerging technologies such as next-generation sequencing and spatial transcriptomics holds potentials to enhance the efficacy of KRAS inhibitors; meanwhile, optimizing strategies to reduce adverse reactions can further mitigate treatment risks. Together, these efforts will bring safer, and more effective, new treatment options for CRC patients.
Abbreviations
AP1: activating protein-1; CDK: Cyclin-dependent kinase; CDX: cell-derived xenografts; CsA: cyclosporin A; CYPA: cyclophilin A; CRC: colorectal cancer; EGFR: epidermal growth factor receptor; ERK: Extracellular regulated protein kinases; FDA: Food and Drug Administration; FGFR: Fibroblast Growth Factor Receptor; GAPs: GTPase activates proteins; GDP: Guanosine diphosphate; GEFs: Guaninenucleotide exchange factors; GRB2: growth factor receptor binding protein 2; GTP: Guanosine triphosphate; HVRs: highly variable regions; IGF-IR: Insulin-like Growth Factor 1 Receptor; IL-10: interleukin-10; JAK1/2: Janus kinase1/2; KEAP1: Kelch-like ECH-associated protein 1; KRAS: Kirsten rat sarcoma virus; MAPK: mitogen-activated protein kinase; MEK: mitogen-activated protein kinase kinase; mTOR: mechanistic target of rapamycin kinase; MYC: myc avian myelocytomatosis; NCSCL: non-small cell lung cancer; ORR: objective response rate; OS: overall survival; PAK: P21-activated kinase; PD-L1: programmed cell death 1 ligand 1; PDX: patient-derived xenografts; PDAC: Pancreatic ductal adenocarcinoma; PFS: progression-free survival; PI3K: phosphoinositide-3-kinase; PLK1: Polo-like Kinase 1; PROTAC: Proteolysis-Targeting Chimeras; PTPN11: protein tyrosine phosphatase non-receptor type 11; RB: Retinoblastoma gene; RFS: Recurrence-free survival; RTK: receptor tyrosine kinase; SHP2: Src homology-2 domain-containing protein tyrosine phosphatase-2; SOS1: son of sevenless homolog 1; STAT3: signal transducer and activator of transcription 3; TGF-β1: transform growth factor-β1; TPS: Tumor Proportion Score; TME: tumor microenvironment; Wnt: wingless integrated.
Acknowledgements
The authors would like to thank all the reviewers for their review work.
Funding
Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD 0501603), Shenyang Science and Technology Bureau Assists China Medical University in High Quality Development Special Project (61010204), Scientific Research Project of Liaoning Province Universities (JYTMS20230084), Key Research and Development Project of Liaoning Province (2024JH2/102500023), Natural Science Foundation of Liaoning Province (No. 2021-MS-184).
Author contributions
Jingyi Li: Writing - Original Draft, Investigation, Visualization; Na Song: Validation, Supervision, Investigation, Writing - Review & Editing, Visualization, Funding acquisition; Rui Ma: Writing - Original Draft, Validation, Supervision, Investigation, Writing - Review & Editing, Visualization, Funding acquisition; Xiujuan Qu: Conceptualization, Validation, Supervision, Investigation, Writing - Review & Editing, Visualization, Funding acquisition.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
2. Bteich F, Mohammadi M, Li T, Bhat MA, Sofianidi A, Wei N. et al. Targeting KRAS in Colorectal Cancer: A Bench to Bedside Review. Int J Mol Sci. 2023 24
3. Zhu G, Pei L, Xia H, Tang Q, Bi F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol Cancer. 2021;20:143
4. Ferreira A, Pereira F, Reis C, Oliveira MJ, Sousa MJ, Preto A. Crucial Role of Oncogenic KRAS Mutations in Apoptosis and Autophagy Regulation: Therapeutic Implications. Cells. 2022 11
5. Yaeger R, Chatila WK, Lipsyc MD, Hechtman JF, Cercek A, Sanchez-Vega F. et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell. 2018 33
6. Ciardiello D, Chiarazzo C, Famiglietti V, Damato A, Pinto C, Zampino MG. et al. Clinical efficacy of sequential treatments in KRASG12C-mutant metastatic colorectal cancer: findings from a real-life multicenter Italian study (CRC-KR GOIM). ESMO Open. 2022;7:100567
7. Lièvre A, Bachet J-B, Boige V, Cayre A, Le Corre D, Buc E. et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374-9
8. Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC. et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757-65
9. Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, Veronese S. et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643-8
10. Jimeno A, Messersmith WA, Hirsch FR, Franklin WA, Eckhardt SG. KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: practical application of patient selection. J Clin Oncol. 2009;27:1130-6
11. Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov. 2014;4:1269-80
12. Lièvre A, Bachet J-B, Le Corre D, Boige V, Landi B, Emile J-F. et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992-5
13. Blair HA. Sotorasib: First Approval. Drugs. 2021;81:1573-9
14. Dhillon S. Adagrasib: First Approval. Drugs. 2023;83:275-85
15. McBride OW, Swan DC, Tronick SR, Gol R, Klimanis D, Moore DE. et al. Regional chromosomal localization of N-ras, K-ras-1, K-ras-2 and myb oncogenes in human cells. Nucleic Acids Res. 1983;11:8221-36
16. Chang EH, Gonda MA, Ellis RW, Scolnick EM, Lowy DR. Human genome contains four genes homologous to transforming genes of Harvey and Kirsten murine sarcoma viruses. Proc Natl Acad Sci U S A. 1982;79:4848-52
17. Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: Mission possible? Nat Rev Drug Discov. 2014;13:828-51
18. Chen Z, Otto JC, Bergo MO, Young SG, Casey PJ. The C-terminal polylysine region and methylation of K-Ras are critical for the interaction between K-Ras and microtubules. J Biol Chem. 2000;275:41251-7
19. Pells S, Divjak M, Romanowski P, Impey H, Hawkins NJ, Clarke AR. et al. Developmentally-regulated expression of murine K-ras isoforms. Oncogene. 1997;15:1781-6
20. Stokoe D, Macdonald SG, Cadwallader K, Symons M, Hancock JF. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994;264:1463-7
21. Kohl NE, Wilson FR, Mosser SD, Giuliani E, deSolms SJ, Conner MW. et al. Protein farnesyltransferase inhibitors block the growth of ras-dependent tumors in nude mice. Proc Natl Acad Sci U S A. 1994;91:9141-5
22. Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299-304
23. Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761-74
24. Simanshu DK, Nissley DV, McCormick F. RAS Proteins and Their Regulators in Human Disease. Cell. 2017;170:17-33
25. Khan AQ, Kuttikrishnan S, Siveen KS, Prabhu KS, Shanmugakonar M, Al-Naemi HA. et al. RAS-mediated oncogenic signaling pathways in human malignancies. Semin Cancer Biol. 2019 54
26. Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459-65
27. Liu P, Wang Y, Li X. Targeting the untargetable KRAS in cancer therapy. Acta Pharm Sin B. 2019;9:871-9
28. Scheffzek K, Ahmadian MR, Kabsch W, Wiesmüller L, Lautwein A, Schmitz F. et al. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277(5324):333-8
29. Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72(10):2457-67
30. Guerrero S, Casanova I, Farré L, Mazo A, Capellà G, Mangues R. K-ras codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res. 2000;60:6750-6
31. Haigis KM. KRAS Alleles: The Devil Is in the Detail. Trends Cancer. 2017;3:686-97
32. Jones RP, Sutton PA, Evans JP, Clifford R, McAvoy A, Lewis J. et al. Specific mutations in KRAS codon 12 are associated with worse overall survival in patients with advanced and recurrent colorectal cancer. Br J Cancer. 2017;116:923-9
33. Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46:372-83
34. Danielsen SA, Eide PW, Nesbakken A, Guren T, Leithe E, Lothe RA. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim Biophys Acta. 2015;1855:104-21
35. Lavoie H, Therrien M. Regulation of RAF protein kinases in ERK signalling. Nat Rev Mol Cell Biol. 2015;16:281-98
36. Krygowska AA, Castellano E. PI3K: A Crucial Piece in the RAS Signaling Puzzle. Cold Spring Harb Perspect Med. 2018 8
37. Hofer F, Fields S, Schneider C, Martin GS. Activated Ras interacts with the Ral guanine nucleotide dissociation stimulator. Proc Natl Acad Sci U S A. 1994;91:11089-93
38. Pacold ME, Suire S, Perisic O, Lara-Gonzalez S, Davis CT, Walker EH. et al. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000;103:931-43
39. Hunter JC, Manandhar A, Carrasco MA, Gurbani D, Gondi S, Westover KD. Biochemical and Structural Analysis of Common Cancer-Associated KRAS Mutations. Mol Cancer Res. 2015;13:1325-35
40. Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC. et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell. 2018 173
41. Matikas A, Mistriotis D, Georgoulias V, Kotsakis A. Targeting KRAS mutated non-small cell lung cancer: A history of failures and a future of hope for a diverse entity. Crit Rev Oncol Hematol. 2017 110
42. Papke B, Der CJ. Drugging RAS: Know the enemy. Science. 2017;355:1158-63
43. Zamorano-Dominguez E, Morales-Cacho L, Barrero R, Jiménez-Parrado S, Dhawahir A, Hernández-Porras I. et al. Systemic Kras ablation disrupts myeloid cell homeostasis in adult mice. Proc Natl Acad Sci U S A. 2025;122:e2512404122
44. Gort E, Johnson ML, Hwang JJ, Pant S, Dünzinger U, Riemann K. et al. A phase I, open-label, dose-escalation trial of BI 1701963 as monotherapy and in combination with trametinib in patients with KRAS mutated advanced or metastatic solid tumors. Journal of Clinical Oncology. 2020;38:TPS3651-TPS
45. Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548-51
46. FDA Approves First KRAS Inhibitor. Sotorasib. Cancer Discov. 2021;11:OF4
47. Lanman BA, Allen JR, Allen JG, Amegadzie AK, Ashton KS, Booker SK. et al. Discovery of a Covalent Inhibitor of KRASG12C (AMG 510) for the Treatment of Solid Tumors. J Med Chem. 2020;63:52-65
48. Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D. et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217-23
49. Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI. et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med. 2020;383:1207-17
50. Fakih MG, Kopetz S, Kuboki Y, Kim TW, Munster PN, Krauss JC. et al. Sotorasib for previously treated colorectal cancers with KRASG12C mutation (CodeBreaK100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2022;23:115-24
51. Sotorasib's Benefits in Colorectal Cancer Modest. Cancer Discov. 2022; 12: OF1.
52. Hallin J, Engstrom LD, Hargis L, Calinisan A, Aranda R, Briere DM. et al. The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020;10:54-71
53. Chen C-Y, Lu Z, Scattolin T, Chen C, Gan Y, McLaughlin M. Synthesis of Adagrasib (MRTX849), a Covalent KRASG12C Inhibitor Drug for the Treatment of Cancer. Org Lett. 2023;25:944-9
54. Ou S-HI, Jänne PA, Leal TA, Rybkin II, Sabari JK, Barve MA. et al. First-in-Human Phase I/IB Dose-Finding Study of Adagrasib (MRTX849) in Patients With Advanced KRASG12C Solid Tumors (KRYSTAL-1). J Clin Oncol. 2022;40:2530-8
55. Xu J, Grosslight S, Mack KA, Nguyen SC, Clagg K, Lim N-K. et al. Atroposelective Negishi Coupling Optimization Guided by Multivariate Linear Regression Analysis: Asymmetric Synthesis of KRAS G12C Covalent Inhibitor GDC-6036. J Am Chem Soc. 2022;144:20955-63
56. Xu J, Lim N-K, Timmerman JC, Shen J, Clagg K, Orcel U. et al. Second-Generation Atroposelective Synthesis of KRAS G12C Covalent Inhibitor GDC-6036. Org Lett. 2023;25:3417-22
57. Lorthiois E, Gerspacher M, Beyer KS, Vaupel A, Leblanc C, Stringer R. et al. JDQ443, a Structurally Novel, Pyrazole-Based, Covalent Inhibitor of KRASG12C for the Treatment of Solid Tumors. J Med Chem. 2022;65:16173-203
58. Wang J, Martin-Romano P, Cassier P, Johnson M, Haura E, Lenox L. et al. Phase I Study of JNJ-74699157 in Patients with Advanced Solid Tumors Harboring the KRAS G12C Mutation. Oncologist. 2022 27
59. Patricelli MP, Janes MR, Li L-S, Hansen R, Peters U, Kessler LV. et al. Selective Inhibition of Oncogenic KRAS Output with Small Molecules Targeting the Inactive State. Cancer Discov. 2016;6:316-29
60. Xu L-S, Zheng S-X, Mei L-H, Yang K-X, Wang Y-F, Zhou Q. et al. 143D, a novel selective KRASG12C inhibitor exhibits potent antitumor activity in preclinical models. Acta Pharmacol Sin. 2023;44:1475-86
61. Schulze CJ, Seamon KJ, Zhao Y, Yang YC, Cregg J, Kim D. et al. Chemical remodeling of a cellular chaperone to target the active state of mutant KRAS. Science. 2023;381:794-9
62. Kettle JG, Bagal SK, Barratt D, Bodnarchuk MS, Boyd S, Braybrooke E. et al. Discovery of AZD4747, a Potent and Selective Inhibitor of Mutant GTPase KRASG12C with Demonstrable CNS Penetration. J Med Chem. 2023;66:9147-60
63. Kettle JG, Bagal SK, Bickerton S, Bodnarchuk MS, Boyd S, Breed J. et al. Discovery of AZD4625, a Covalent Allosteric Inhibitor of the Mutant GTPase KRASG12C. J Med Chem. 2022;65:6940-52
64. Teng KW, Tsai ST, Hattori T, Fedele C, Koide A, Yang C. et al. Selective and noncovalent targeting of RAS mutants for inhibition and degradation. Nat Commun. 2021;12:2656
65. Kessler D, Gmachl M, Mantoulidis A, Martin LJ, Zoephel A, Mayer M. et al. Drugging an undruggable pocket on KRAS. Proc Natl Acad Sci U S A. 2019;116:15823-9
66. Myer PA, Lee JK, Madison RW, Pradhan K, Newberg JY, Isasi CR. et al. The Genomics of Colorectal Cancer in Populations with African and European Ancestry. Cancer Discov. 2022;12:1282-93
67. Zhou C, Li C, Luo L, Li X, Jia K, He N. et al. Anti-tumor efficacy of HRS-4642 and its potential combination with proteasome inhibition in KRAS G12D-mutant cancer. Cancer Cell. 2024;42:1286-300.e8
68. Weller C, Burnett GL, Jiang L, Chakraborty S, Zhang D, Vita NA. et al. A neomorphic protein interface catalyzes covalent inhibition of RAS(G12D) aspartic acid in tumors. Science. 2025;389:eads0239
69. Zhou C, Li W, Song Z, Zhang Y, Huang D, Yang Z. et al. LBA33 A first-in-human phase I study of a novel KRAS G12D inhibitor HRS-4642 in patients with advanced solid tumors harboring KRAS G12D mutation. Annals of Oncology. 2023
70. Lee JK, Sivakumar S, Schrock AB, Madison R, Fabrizio D, Gjoerup O. et al. Comprehensive pan-cancer genomic landscape of KRAS altered cancers and real-world outcomes in solid tumors. NPJ Precis Oncol. 2022;6:91
71. Stanland LJ, Huggins HP, Sahoo SS, Porrello A, Chareddy Y, Azam SH. et al. A first-in-class EGFR-directed KRAS G12V selective inhibitor. Cancer Cell. 2025
72. Zhang Y, Ma J-A, Zhang H-X, Jiang Y-N, Luo W-H. Cancer vaccines: Targeting KRAS-driven cancers. Expert Rev Vaccines. 2020;19:163-73
73. Pant S, Wainberg ZA, Weekes CD, Furqan M, Kasi PM, Devoe CE. et al. Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: the phase 1 AMPLIFY-201 trial. Nat Med. 2024;30:531-42
74. Devoe CE, Pant S, Wainberg ZA, Chung V, George TJ, Kasi PM. et al. AMPLIFY-7P, a first-in-human safety and efficacy trial of adjuvant mKRAS-specific lymph node targeted amphiphile ELI-002 7P vaccine in patients with minimal residual disease-positive pancreatic and colorectal cancer. Journal of Clinical Oncology. 2024;42:2636 -
75. Yang F, Wen Y, Wang C, Zhou Y, Zhou Y, Zhang Z-M. et al. Efficient targeted oncogenic KRASG12C degradation via first reversible-covalent PROTAC. Eur J Med Chem. 2022;230:114088
76. Toure M, Crews CM. Small-Molecule PROTACS: New Approaches to Protein Degradation. Angew Chem Int Ed Engl. 2016;55:1966-73
77. Lai AC, Crews CM. Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov. 2017;16:101-14
78. Zeng M, Xiong Y, Safaee N, Nowak RP, Donovan KA, Yuan CJ. et al. Exploring Targeted Degradation Strategy for Oncogenic KRASG12C. Cell Chem Biol. 2020 27
79. Zhou C, Fan Z, Gu Y, Ge Z, Tao Z, Cui R. et al. Design, Synthesis, and Biological Evaluation of Potent and Selective PROTAC Degraders of Oncogenic KRASG12D. J Med Chem. 2024;67:1147-67
80. Popow J, Farnaby W, Gollner A, Kofink C, Fischer G, Wurm M. et al. Targeting cancer with small-molecule pan-KRAS degraders. Science. 2024;385:1338-47
81. Gale NW, Kaplan S, Lowenstein EJ, Schlessinger J, Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993;363:88-92
82. Egan SE, Giddings BW, Brooks MW, Buday L, Sizeland AM, Weinberg RA. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993;363:45-51
83. Hillig RC, Sautier B, Schroeder J, Moosmayer D, Hilpmann A, Stegmann CM. et al. Discovery of potent SOS1 inhibitors that block RAS activation via disruption of the RAS-SOS1 interaction. Proc Natl Acad Sci U S A. 2019;116:2551-60
84. Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018 12
85. Koch WJ, Hawes BE, Allen LF, Lefkowitz RJ. Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by G beta gamma activation of p21ras. Proc Natl Acad Sci U S A. 1994;91:12706-10
86. McFall T, Trogdon M, Guizar AC, Langenheim JF, Sisk-Hackworth L, Stites EC. Co-targeting KRAS G12C and EGFR reduces both mutant and wild-type RAS-GTP. NPJ Precis Oncol. 2022;6:86
87. Amodio V, Yaeger R, Arcella P, Cancelliere C, Lamba S, Lorenzato A. et al. EGFR Blockade Reverts Resistance to KRAS(G12C) Inhibition in Colorectal Cancer. Cancer Discov. 2020;10:1129-39
88. Feng J, Hu Z, Xia X, Liu X, Lian Z, Wang H. et al. Feedback activation of EGFR/wild-type RAS signaling axis limits KRAS(G12D) inhibitor efficacy in KRAS(G12D)-mutated colorectal cancer. Oncogene. 2023;42:1620-33
89. Kilroy-Gehret MK, Wischmeier C, Park S, Choi D, Feroz W, Mishra R. et al. Co-targeting KRASG12D and the HER family is efficacious in colorectal cancer. Carcinogenesis. 2025 46
90. Awad MM, Liu S, Rybkin II, Arbour KC, Dilly J, Zhu VW. et al. Acquired Resistance to KRASG12C Inhibition in Cancer. N Engl J Med. 2021;384:2382-93
91. Puyol M, Martín A, Dubus P, Mulero F, Pizcueta P, Khan G. et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell. 2010;18:63-73
92. Kwong LN, Costello JC, Liu H, Jiang S, Helms TL, Langsdorf AE. et al. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nature Medicine. 2012;18:1503-10
93. Kasper S, Breitenbuecher F, Reis H, Brandau S, Worm K, Köhler J. et al. Oncogenic RAS simultaneously protects against anti-EGFR antibody-dependent cellular cytotoxicity and EGFR signaling blockade. Oncogene. 2013;32:2873-81
94. Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S. et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173-86
95. Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR. et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943-51
96. Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJ. et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood. 2011;118:1663-74
97. Khan S, Wiegand J, Zhang P, Hu W, Thummuri D, Budamagunta V. et al. BCL-XL PROTAC degrader DT2216 synergizes with sotorasib in preclinical models of KRASG12C-mutated cancers. J Hematol Oncol. 2022;15:23
98. Wang CY, Guo ST, Wang JY, Liu F, Zhang YY, Yari H. et al. Inhibition of HSP90 by AUY922 Preferentially Kills Mutant KRAS Colon Cancer Cells by Activating Bim through ER Stress. Molecular Cancer Therapeutics. 2016;15:448-59
99. Šuštić T, van Wageningen S, Bosdriesz E, Reid RJD, Dittmar J, Lieftink C. et al. A role for the unfolded protein response stress sensor ERN1 in regulating the response to MEK inhibitors in KRAS mutant colon cancers. Genome Med. 2018;10:90
100. Jiang Z, Ye S, Wu Y, Zhou C, Cao F, Tan N. Cyclopeptide RA-V from Rubia yunnanensis restores activity of Adagrasib against colorectal cancer by reducing the expression of Nrf2. Pharmacol Res. 2024;206:107252
101. Wang Y, Yao M, Li C, Yang K, Qin X, Xu L. et al. Targeting ST8SIA6-AS1 counteracts KRASG12C inhibitor resistance through abolishing the reciprocal activation of PLK1/c-Myc signaling. Exp Hematol Oncol. 2023;12:105
102. Jansen RA, Mainardi S, Dias MH, Bosma A, van Dijk E, Selig R. et al. Small-molecule inhibition of MAP2K4 is synergistic with RAS inhibitors in KRAS-mutant cancers. Proc Natl Acad Sci U S A. 2024;121:e2319492121
103. Xue Z, Vis DJ, Bruna A, Sustic T, van Wageningen S, Batra AS. et al. MAP3K1 and MAP2K4 mutations are associated with sensitivity to MEK inhibitors in multiple cancer models. Cell Res. 2018;28:719-29
104. Johnson CW, Haigis KM. All Roads Lead to Rome: YAP/TAZ Activity Influences Efficacy of KRASG12C Inhibitors. Cancer Res. 2023;83:4005-7
105. Edwards AC, Stalnecker CA, Jean Morales A, Taylor KE, Klomp JE, Klomp JA. et al. TEAD Inhibition Overcomes YAP1/TAZ-Driven Primary and Acquired Resistance to KRASG12C Inhibitors. Cancer Res. 2023;83:4112-29
106. Tanaka N, Lin JJ, Li C, Ryan MB, Zhang J, Kiedrowski LA. et al. Clinical Acquired Resistance to KRASG12C Inhibition through a Novel KRAS Switch-II Pocket Mutation and Polyclonal Alterations Converging on RAS-MAPK Reactivation. Cancer Discov. 2021;11:1913-22
107. Yoon J, Koo K-H, Choi K-Y. MEK1/2 inhibitors AS703026 and AZD6244 may be potential therapies for KRAS mutated colorectal cancer that is resistant to EGFR monoclonal antibody therapy. Cancer Res. 2011;71:445-53
108. Zimmer L, Barlesi F, Martinez-Garcia M, Dieras V, Schellens JHM, Spano J-P. et al. Phase I expansion and pharmacodynamic study of the oral MEK inhibitor RO4987655 (CH4987655) in selected patients with advanced cancer with RAS-RAF mutations. Clin Cancer Res. 2014;20:4251-61
109. Lamba S, Russo M, Sun C, Lazzari L, Cancelliere C, Grernrum W. et al. RAF suppression synergizes with MEK inhibition in KRAS mutant cancer cells. Cell Rep. 2014;8:1475-83
110. Yuan X, Tang Z, Du R, Yao Z, Cheung S-H, Zhang X. et al. RAF dimer inhibition enhances the antitumor activity of MEK inhibitors in K-RAS mutant tumors. Mol Oncol. 2020;14:1833-49
111. Hayes TK, Neel NF, Hu C, Gautam P, Chenard M, Long B. et al. Long-Term ERK Inhibition in KRAS-Mutant Pancreatic Cancer Is Associated with MYC Degradation and Senescence-like Growth Suppression. Cancer Cell. 2016;29:75-89
112. Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13:928-42
113. Hong A, Piva M, Liu S, Hugo W, Lomeli SH, Zoete V. et al. Durable Suppression of Acquired MEK Inhibitor Resistance in Cancer by Sequestering MEK from ERK and Promoting Antitumor T-cell Immunity. Cancer Discov. 2021;11:714-35
114. Flemington V, Davies EJ, Robinson D, Sandin LC, Delpuech O, Zhang P. et al. AZD0364 Is a Potent and Selective ERK1/2 Inhibitor That Enhances Antitumor Activity in KRAS-Mutant Tumor Models when Combined with the MEK Inhibitor, Selumetinib. Mol Cancer Ther. 2021;20:238-49
115. Yu C, Luo D, Yu J, Zhang M, Zheng X, Xu G. et al. Genome-wide CRISPR-cas9 knockout screening identifies GRB7 as a driver for MEK inhibitor resistance in KRAS mutant colon cancer. Oncogene. 2022;41:191-203
116. Chan C-H, Chiou L-W, Lee T-Y, Liu Y-R, Hsieh T-H, Yang C-Y. et al. PAK and PI3K pathway activation confers resistance to KRASG12C inhibitor sotorasib. Br J Cancer. 2023;128:148-59
117. Belli S, Pesapane A, Servetto A, Esposito D, Napolitano F, Ascione CM. et al. Combined blockade of mTOR and p21-activated kinases pathways prevents tumour growth in KRAS-mutated colorectal cancer. Br J Cancer. 2023;129:1071-82
118. Clarke PA, Roe T, Swabey K, Hobbs SM, McAndrew C, Tomlin K. et al. Dissecting mechanisms of resistance to targeted drug combination therapy in human colorectal cancer. Oncogene. 2019;38:5076-90
119. Shimizu T, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW, Smith LS. et al. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin Cancer Res. 2012;18:2316-25
120. Weisner J, Landel I, Reintjes C, Uhlenbrock N, Trajkovic-Arsic M, Dienstbier N. et al. Preclinical Efficacy of Covalent-Allosteric AKT Inhibitor Borussertib in Combination with Trametinib in KRAS-Mutant Pancreatic and Colorectal Cancer. Cancer Res. 2019;79:2367-78
121. Spreafico A, Tentler JJ, Pitts TM, Tan AC, Gregory MA, Arcaroli JJ. et al. Rational combination of a MEK inhibitor, selumetinib, and the Wnt/calcium pathway modulator, cyclosporin A, in preclinical models of colorectal cancer. Clin Cancer Res. 2013;19:4149-62
122. Krishnamurthy A, Dasari A, Noonan AM, Mehnert JM, Lockhart AC, Leong S. et al. Phase Ib Results of the Rational Combination of Selumetinib and Cyclosporin A in Advanced Solid Tumors with an Expansion Cohort in Metastatic Colorectal Cancer. Cancer Res. 2018;78:5398-407
123. Moon J-H, Hong S-W, Kim JE, Shin J-S, Kim J-S, Jung S-A. et al. Targeting β-catenin overcomes MEK inhibition resistance in colon cancer with KRAS and PIK3CA mutations. Br J Cancer. 2019;120:941-51
124. Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17:58
125. van Geel RMJM, van Brummelen EMJ, Eskens FALM, Huijberts SCFA, de Vos FYFL, Lolkema MPJK. et al. Phase 1 study of the pan-HER inhibitor dacomitinib plus the MEK1/2 inhibitor PD-0325901 in patients with KRAS-mutation-positive colorectal, non-small-cell lung and pancreatic cancer. Br J Cancer. 2020;122:1166-74
126. Desai J, Alonso G, Kim SH, Cervantes A, Karasic T, Medina L. et al. Divarasib plus cetuximab in KRAS G12C-positive colorectal cancer: a phase 1b trial. Nat Med. 2024;30:271-8
127. Kuboki Y, Fakih M, Strickler J, Yaeger R, Masuishi T, Kim EJ. et al. Sotorasib with panitumumab in chemotherapy-refractory KRAS(G12C)-mutated colorectal cancer: a phase 1b trial. Nat Med. 2024;30:265-70
128. Yaeger R, Weiss J, Pelster MS, Spira AI, Barve M, Ou SI. et al. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N Engl J Med. 2023;388:44-54
129. Fakih MG, Salvatore L, Esaki T, Modest DP, Lopez-Bravo DP, Taieb J. et al. Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C. N Engl J Med. 2023;389:2125-39
130. Ruan DY, Wu HX, Xu Y, Munster PN, Deng Y, Richardson G. et al. Garsorasib, a KRAS G12C inhibitor, with or without cetuximab, an EGFR antibody, in colorectal cancer cohorts of a phase II trial in advanced solid tumors with KRAS G12C mutation. Signal Transduct Target Ther. 2025;10:189
131. Ruess DA, Heynen GJ, Ciecielski KJ, Ai J, Berninger A, Kabacaoglu D. et al. Mutant KRAS-driven cancers depend on PTPN11/SHP2 phosphatase. Nat Med. 2018;24:954-60
132. Nichols RJ, Haderk F, Stahlhut C, Schulze CJ, Hemmati G, Wildes D. et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat Cell Biol. 2018;20:1064-73
133. Liu C, Lu H, Wang H, Loo A, Zhang X, Yang G. et al. Combinations with Allosteric SHP2 Inhibitor TNO155 to Block Receptor Tyrosine Kinase Signaling. Clin Cancer Res. 2021;27:342-54
134. Weiss A, Lorthiois E, Barys L, Beyer KS, Bomio-Confaglia C, Burks H. et al. Discovery, Preclinical Characterization, and Early Clinical Activity of JDQ443, a Structurally Novel, Potent, and Selective Covalent Oral Inhibitor of KRASG12C. Cancer Discov. 2022;12:1500-17
135. Lu H, Liu C, Velazquez R, Wang H, Dunkl LM, Kazic-Legueux M. et al. SHP2 Inhibition Overcomes RTK-Mediated Pathway Reactivation in KRAS-Mutant Tumors Treated with MEK Inhibitors. Mol Cancer Ther. 2019;18:1323-34
136. Lu X, Yu R, Li Z, Yang M, Dai J, Liu M. JC-010a, a novel selective SHP2 allosteric inhibitor, overcomes RTK/non-RTK-mediated drug resistance in multiple oncogene-addicted cancers. Cancer Lett. 2024;582:216517
137. Taylor AM, Williams BR, Giordanetto F, Kelley EH, Lescarbeau A, Shortsleeves K. et al. Identification of GDC-1971 (RLY-1971), a SHP2 Inhibitor Designed for the Treatment of Solid Tumors. J Med Chem. 2023;66:13384-99
138. Hofmann MH, Gmachl M, Ramharter J, Savarese F, Gerlach D, Marszalek JR. et al. BI-3406, a Potent and Selective SOS1-KRAS Interaction Inhibitor, Is Effective in KRAS-Driven Cancers through Combined MEK Inhibition. Cancer Discov. 2021;11:142-57
139. Ketcham JM, Haling J, Khare S, Bowcut V, Briere DM, Burns AC. et al. Design and Discovery of MRTX0902, a Potent, Selective, Brain-Penetrant, and Orally Bioavailable Inhibitor of the SOS1:KRAS Protein-Protein Interaction. J Med Chem. 2022;65:9678-90
140. Bian Y, Alem D, Beato F, Hogenson TL, Yang X, Jiang K. et al. Development of SOS1 Inhibitor-Based Degraders to Target KRAS-Mutant Colorectal Cancer. J Med Chem. 2022;65:16432-50
141. Lv Y, Yang Z, Chen Y, Ma X, Guo M, Zhang C. et al. A Potent SOS1 PROTAC Degrader with Synergistic Efficacy in Combination with KRASG12C Inhibitor. J Med Chem. 2024
142. Rao S, Cunningham D, de Gramont A, Scheithauer W, Smakal M, Humblet Y. et al. Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J Clin Oncol. 2004;22:3950-7
143. Sharma S, Kemeny N, Kelsen DP, Ilson D, O'Reilly E, Zaknoen S. et al. A phase II trial of farnesyl protein transferase inhibitor SCH 66336, given by twice-daily oral administration, in patients with metastatic colorectal cancer refractory to 5-fluorouracil and irinotecan. Ann Oncol. 2002;13:1067-71
144. Tabernero J, Rojo F, Marimón I, Voi M, Albanell J, Guix M. et al. Phase I pharmacokinetic and pharmacodynamic study of weekly 1-hour and 24-hour infusion BMS-214662, a farnesyltransferase inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2005;23:2521-33
145. Baranyi M, Molnár E, Hegedűs L, Gábriel Z, Petényi FG, Bordás F. et al. Farnesyl-transferase inhibitors show synergistic anticancer effects in combination with novel KRAS-G12C inhibitors. Br J Cancer. 2024
146. Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q. et al. Yap1 Activation Enables Bypass of Oncogenic Kras Addiction in Pancreatic Cancer. Cell. 2019;179:1239
147. Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X. et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171-84
148. Hagenbeek TJ, Zbieg JR, Hafner M, Mroue R, Lacap JA, Sodir NM. et al. An allosteric pan-TEAD inhibitor blocks oncogenic YAP/TAZ signaling and overcomes KRAS G12C inhibitor resistance. Nat Cancer. 2023;4:812-28
149. Ott CA, Aplin AE. Targeting TEAD-ious resistance. Trends Cancer. 2023;9:780-1
150. Garcia-Rendueles MER, Krishnamoorthy G, Saqcena M, Acuña-Ruiz A, Revilla G, de Stanchina E. et al. Yap governs a lineage-specific neuregulin1 pathway-driven adaptive resistance to RAF kinase inhibitors. Mol Cancer. 2022;21:213
151. Kim D, Herdeis L, Rudolph D, Zhao Y, Böttcher J, Vides A. et al. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature. 2023;619:160-6
152. Drugging RAS. Moving Beyond KRASG12C. Cancer Discov. 2023;13:OF7
153. Li M-L, Dai L-T, Gao Z-Y, Yan J-T, Xu S-M, Tan J-H. et al. Discovery of Novel Coumarin-quinolinium Derivatives as Pan-KRAS Translation Inhibitors by Targeting 5'-UTR RNA G-Quadruplexes. J Med Chem. 2024;67:1961-81
154. Holderfield M, Lee BJ, Jiang J, Tomlinson A, Seamon KJ, Mira A. et al. Concurrent inhibition of oncogenic and wild-type RAS-GTP for cancer therapy. Nature. 2024
155. Wasko UN, Jiang J, Dalton TC, Curiel-Garcia A, Edwards AC, Wang Y. et al. Tumour-selective activity of RAS-GTP inhibition in pancreatic cancer. Nature. 2024
156. Hutton JE, Wang X, Zimmerman LJ, Slebos RJC, Trenary IA, Young JD. et al. Oncogenic KRAS and BRAF Drive Metabolic Reprogramming in Colorectal Cancer. Mol Cell Proteomics. 2016;15:2924-38
157. Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H. et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555-9
158. Amendola CR, Mahaffey JP, Parker SJ, Ahearn IM, Chen W-C, Zhou M. et al. KRAS4A directly regulates hexokinase 1. Nature. 2019;576:482-6
159. Kawada K, Nakamoto Y, Kawada M, Hida K, Matsumoto T, Murakami T. et al. Relationship between 18F-fluorodeoxyglucose accumulation and KRAS/BRAF mutations in colorectal cancer. Clin Cancer Res. 2012;18:1696-703
160. Zheng M, Wu C, Yang K, Yang Y, Liu Y, Gao S. et al. Novel selective hexokinase 2 inhibitor Benitrobenrazide blocks cancer cells growth by targeting glycolysis. Pharmacol Res. 2021;164:105367
161. Erber J, Steiner JD, Isensee J, Lobbes LA, Toschka A, Beleggia F. et al. Dual Inhibition of GLUT1 and the ATR/CHK1 Kinase Axis Displays Synergistic Cytotoxicity in KRAS-Mutant Cancer Cells. Cancer Res. 2019;79:4855-68
162. Fiermonte G, Dolce V, Palmieri L, Ventura M, Runswick MJ, Palmieri F. et al. Identification of the human mitochondrial oxodicarboxylate carrier. Bacterial expression, reconstitution, functional characterization, tissue distribution, and chromosomal location. J Biol Chem. 2001;276:8225-30
163. Hu S-S, Han Y, Tan T-Y, Chen H, Gao J-W, Wang L. et al. SLC25A21 downregulation promotes KRAS-mutant colorectal cancer progression by increasing glutamine anaplerosis. JCI insight. 2023 8
164. Najumudeen AK, Ceteci F, Fey SK, Hamm G, Steven RT, Hall H. et al. The amino acid transporter SLC7A5 is required for efficient growth of KRAS-mutant colorectal cancer. Nat Genet. 2021;53:16-26
165. You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012 22
166. Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of cancer. Science. 2017 357
167. Perurena N, Situ L, Cichowski K. Combinatorial strategies to target RAS-driven cancers. Nat Rev Cancer. 2024;24:316-37
168. Mai A, Massa S, Rotili D, Cerbara I, Valente S, Pezzi R. et al. Histone deacetylation in epigenetics: an attractive target for anticancer therapy. Med Res Rev. 2005;25:261-309
169. Morelli MP, Tentler JJ, Kulikowski GN, Tan A-C, Bradshaw-Pierce EL, Pitts TM. et al. Preclinical activity of the rational combination of selumetinib (AZD6244) in combination with vorinostat in KRAS-mutant colorectal cancer models. Clin Cancer Res. 2012;18:1051-62
170. Bousquet MS, Ma JJ, Ratnayake R, Havre PA, Yao J, Dang NH. et al. Multidimensional Screening Platform for Simultaneously Targeting Oncogenic KRAS and Hypoxia-Inducible Factors Pathways in Colorectal Cancer. ACS Chem Biol. 2016;11:1322-31
171. Kaliszczak M, van Hechanova E, Li Y, Alsadah H, Parzych K, Auner HW. et al. The HDAC6 inhibitor C1A modulates autophagy substrates in diverse cancer cells and induces cell death. Br J Cancer. 2018;119:1278-87
172. Loi P, Schade AE, Rodriguez CL, Krishnan A, Perurena N, Nguyen VTM. et al. Epigenetic and Oncogenic Inhibitors Cooperatively Drive Differentiation and Kill KRAS-Mutant Colorectal Cancers. Cancer Discov. 2024;14:2430-49
173. Liu H, Liang Z, Cheng S, Huang L, Li W, Zhou C. et al. Mutant KRAS Drives Immune Evasion by Sensitizing Cytotoxic T-Cells to Activation-Induced Cell Death in Colorectal Cancer. Adv Sci (Weinh). 2023;10:e2203757
174. Liu J, Huang X, Liu H, Wei C, Ru H, Qin H. et al. Immune landscape and prognostic immune-related genes in KRAS-mutant colorectal cancer patients. J Transl Med. 2021;19:27
175. Coelho MA, de Carné Trécesson S, Rana S, Zecchin D, Moore C, Molina-Arcas M. et al. Oncogenic RAS Signaling Promotes Tumor Immunoresistance by Stabilizing PD-L1 mRNA. Immunity. 2017 47
176. Aktar S, Islam F, Cheng T, Gamage SMK, Choudhury IN, Islam MS. et al. Correlation between KRAS Mutation and CTLA-4 mRNA Expression in Circulating Tumour Cells: Clinical Implications in Colorectal Cancer. Genes (Basel). 2023 14
177. Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447-58
178. Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P. et al. KRAS-IRF2 Axis Drives Immune Suppression and Immune Therapy Resistance in Colorectal Cancer. Cancer Cell. 2019 35
179. Boutin AT, Liao W-T, Wang M, Hwang SS, Karpinets TV, Cheung H. et al. Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev. 2017;31:370-82
180. Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M. et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538-43
181. Mugarza E, van Maldegem F, Boumelha J, Moore C, Rana S, Llorian Sopena M. et al. Therapeutic KRASG12C inhibition drives effective interferon-mediated antitumor immunity in immunogenic lung cancers. Sci Adv. 2022;8:eabm8780
182. Briere DM, Li S, Calinisan A, Sudhakar N, Aranda R, Hargis L. et al. The KRASG12C Inhibitor MRTX849 Reconditions the Tumor Immune Microenvironment and Sensitizes Tumors to Checkpoint Inhibitor Therapy. Mol Cancer Ther. 2021;20:975-85
183. Menard MJ, Chow C, Chen K, Blaj C, Shifrin NT, Courtney H. et al. Abstract 3475: RMC-9805, a first-in-class, mutant-selective, covalent and orally bioavailable KRASG12D(ON) inhibitor, promotes cancer-associated neoantigen recognition and synergizes with immunotherapy in preclinical models. Cancer Research. 2023;83:3475 -
184. Frontline Promise for Adagrasib-Pembrolizumab Combination. Cancer Discov. 2023; 13: OF2.
185. Tani T, Kitajima S, Conway EB, Knelson EH, Barbie DA. KRAS G12C inhibition and innate immune targeting. Expert Opin Ther Targets. 2021;25:167-74
186. Abdel Mouti M, Pauklin S. Chemically modified neoantigen-based immunotherapy for targeting KRASG12C-driven tumors. Trends Pharmacol Sci. 2023;44:255-7
187. Xie N, Shen G, Gao W, Huang Z, Huang C, Fu L. Neoantigens: promising targets for cancer therapy. Signal Transduct Target Ther. 2023;8:9
188. Kishton RJ, Lynn RC, Restifo NP. Strength in Numbers: Identifying Neoantigen Targets for Cancer Immunotherapy. Cell. 2020;183:591-3
189. Zhang Z, Rohweder PJ, Ongpipattanakul C, Basu K, Bohn M-F, Dugan EJ. et al. A covalent inhibitor of K-Ras(G12C) induces MHC class I presentation of haptenated peptide neoepitopes targetable by immunotherapy. Cancer Cell. 2022 40
190. Nam G-H, Kwon M, Jung H, Ko E, Kim SA, Choi Y. et al. Statin-mediated inhibition of RAS prenylation activates ER stress to enhance the immunogenicity of KRAS mutant cancer. J Immunother Cancer. 2021 9
191. Zhao D, Liu Y, Yi F, Zhao X, Lu K. Recent advances in the development of inhibitors targeting KRAS-G12C and its related pathways. Eur J Med Chem. 2023;259:115698
192. Thatikonda V, Lyu H, Jurado S, Kostyrko K, Bristow CA, Albrecht C. et al. Co-targeting SOS1 enhances the antitumor effects of KRASG12C inhibitors by addressing intrinsic and acquired resistance. Nat Cancer. 2024;5:1352-70
193. Tanjak P, Chaiboonchoe A, Suwatthanarak T, Acharayothin O, Thanormjit K, Chanthercrob J. et al. The KRAS-Mutant Consensus Molecular Subtype 3 Reveals an Immunosuppressive Tumor Microenvironment in Colorectal Cancer. Cancers (Basel). 2023 15
194. Fontanges Q, De Mendonca R, Salmon I, Le Mercier M, D'Haene N. Clinical Application of Targeted Next Generation Sequencing for Colorectal Cancers. Int J Mol Sci. 2016 17
195. Zhou L, Huang J, Li C, Gu Q, Li G, Li ZA. et al. Organoids and organs-on-chips: Recent advances, applications in drug development, and regulatory challenges. Med. 2025;6:100667
196. Chen QA, Lin WH, Zhou XX, Cao Z, Feng XL, Gao YB. et al. Outcomes following KRAS(G12C) inhibitor treatment in patients with KRAS(G12C)-mutated solid tumors: A systematic review and meta-analysis. Pharmacol Res. 2024;200:107060
Author contact
![]() Corresponding authors: Rui Ma: The First Hospital of China Medical University, No. 155, Nanjing North Street, Heping District, Shenyang City, Liaoning 110001, China; E-mail: maruiedu.cn. Xiujuan Qu: The First Hospital of China Medical University, No. 155, Nanjing North Street, Heping District, Shenyang City, Liaoning 110001, China; E-mail: xjquedu.cn.
Corresponding authors: Rui Ma: The First Hospital of China Medical University, No. 155, Nanjing North Street, Heping District, Shenyang City, Liaoning 110001, China; E-mail: maruiedu.cn. Xiujuan Qu: The First Hospital of China Medical University, No. 155, Nanjing North Street, Heping District, Shenyang City, Liaoning 110001, China; E-mail: xjquedu.cn.

 Global reach, higher impact
Global reach, higher impact